Introduction
Colon cancer is the third most common cancer type
globally and the third-leading cause of cancer death (1). The traditional prognostic factors
based on morphological features or blood markers are not sufficient
to stratify the risk of post-operative tumor progression (2). Although various genomic or molecular
biomarkers, ranging from tissue markers to serum-derived markers,
have been developed for more exact prediction of tumor recurrence
(3), novel biomarkers are still
needed to screen out the patients with poor therapeutic response,
as well as those at high risk of tumor progression.
Ki67 is broadly used to evaluate cell proliferation
and aggressiveness in various malignant tumors (4,5).
Although the expression levels of Ki67 are higher in malignant
tumors compared with normal tissues in the majority of solid tumor
types, the prognostic value of Ki67 is still controversial
(6). In colon cancer, some reports
have mentioned that high Ki67 expression was associated with poor
prognosis (7,8), while others reported the opposite
conclusion, showing that high Ki67 expression reflected improved
clinical outcome (9,10). Even high-quality meta-analyses have
reported contradicting conclusions (11,12),
depending on the evidence they relied on.
To investigate the prognostic significance of Ki67
in colon cancer, the associations of Ki67 expression levels with
clinicopathological variables and survival data from 312 patients
with colon cancer were analyzed. All cases were divided into four
grades based on 25% intervals of the Ki67-positive cell percentage
in immunohistochemical staining to identify an optimal cut-off
value for prognostic evaluation.
Patients and methods
Patient recruitment
A total of 312 consecutive patients with stage I–III
colon cancer treated at Peking University Cancer Hospital (Beijing,
China) between January 2004 and December 2010 were retrospectively
included. Radical surgery was performed in all patients with or
without adjuvant chemotherapy. Adjuvant chemotherapy with the
regimens of FOLFOX, XELOX or capecitabine alone was performed for
patients with lymph node metastasis and those with high-risk
microsatellite stable stage II tumors, including patients with
perforated tumors, pT4N0 lesions, vascular invasion and/or bowel
obstruction (13), following the
NCCN guidelines of colon cancer (Version 3.2022). Within the 195
patients who underwent chemotherapy, 35 patients underwent
capecitabine alone, while 160 patients accepted combined
chemotherapy (FOLFOX or XELOX). All patients were given adjuvant
chemotherapy for 6 months after surgery, equally 8–12 cycles.
Patients were followed up regularly every 6 months post-surgery,
with physical examination, serum CEA testing, chest radiography,
computed tomography and colonoscopy once per year. The follow-up
lasted 5 years, and patients missing in follow-up were
excluded.
Detection of tissue Ki67
Sections (5-µm-thick) were cut from
paraffin-embedded blocks of tumor tissue. Immunohistochemistry
staining was performed as previously reported (14). Repaired tissue sections were
incubated with the Ki67 primary monoclonal antibody (dilution,
1:200; cat. no. ZM-0166; OriGene Technologies, Inc.). The Dako REAL
EnVision Detection System (cat. no. K5007; Agilent Technologies,
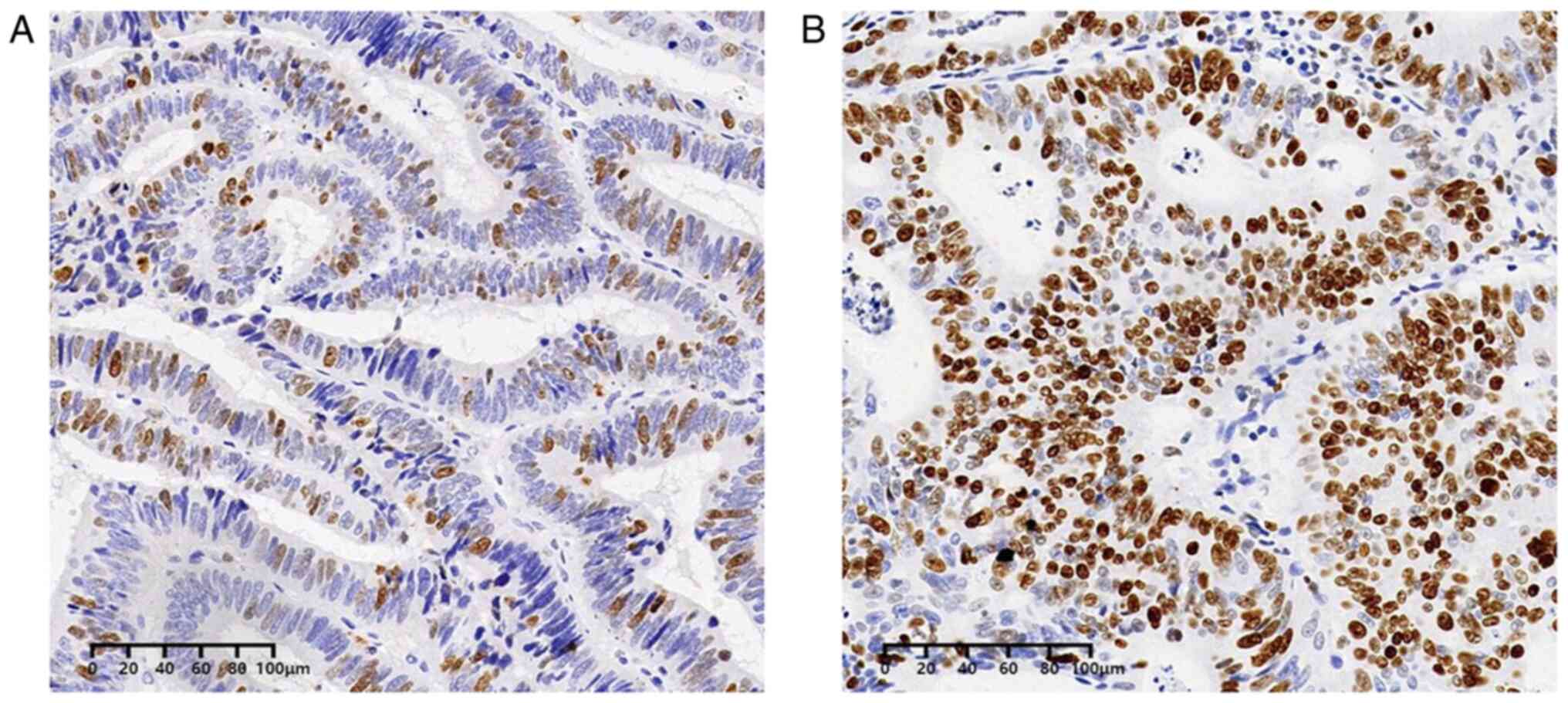
Inc.) was used for staining and detection. Ki67 expression was
defined as follows: +, >0 and ≤25%; ++, >25 and ≤50%; +++,
>50 and ≤75%; and ++++, >75%; among which, + and ++ were
defined as low expression, while +++ and ++++ were defined as high
expression (Fig. 1), as reported
previously (15).
Statistical analysis
Categorical variables such as clinicopathological
characteristics are presented as patient numbers and percentages.
The association between Ki67 expression and clinicopathological
variables was analyzed using the χ2 test. The 5-year
disease-free survival (DFS) rate and overall survival (OS) rate
were analyzed using Kaplan-Meier survival curves with log-rank
tests based on different Ki67 expression level. Multivariate
analysis was performed using a Cox proportional hazard model with
the Enter-method to detect which factors independently affected DFS
or OS. SPSS software (version 21; IBM Corp.) was used for
statistical analysis. P<0.05 (two-tailed test) was considered to
indicate a statistically significant difference.
Results
Clinicopathological
characteristics
A total of 312 patients of the 409 CRC cases were
enrolled, including 178 men (57.1%) and 134 women (42.9%), with a
median age of 67 years (range, 28–83 years). The number of patients
with a Ki67 expression level of +, ++, +++ and ++++ was 53 (17%),
91 (29.2%), 90 (28.8%) and 78 (25%), respectively. All
clinicopathological parameters are listed in Table I. High Ki67 expression was
significantly associated with poor histological differentiation of
the tumor (P<0.05), while no other associations were observed
(Table II).
 | Table I.Clinicopathological characteristics of
included patients. |
Table I.
Clinicopathological characteristics of
included patients.
| Clinicopathological
parameters | No. (%) |
|---|
| Median age
(range) | 67 (28–83) |
| Sex (M:F) | 178:134 |
| Location |
|
|
Right | 122 (39.1) |
| Left | 163 (52.2) |
|
Middle | 27 (8.7) |
| Histological
differentiation |
|
| Well | 24 (7.7) |
|
Moderate | 262 (84) |
| Poor | 13 (4.2) |
| Mucinous
and signet | 13 (4.2) |
| TNM stage |
|
| I | 27 (8.7) |
| II | 140 (44.9) |
| III | 145 (46.5) |
| Vascular
invasion | 68 (21.9) |
| Ki67 expression |
|
| + | 53 (17) |
| ++ | 91 (29.2) |
| +++ | 90 (28.8) |
| ++++ | 78 (25) |
 | Table II.Association between Ki67 expression
and clinicopathological parameters. |
Table II.
Association between Ki67 expression
and clinicopathological parameters.
|
| Ki67 expression |
|
|---|
| Clinicopathological
parameters |
|
|
|---|
| Low (%) | High (%) | P value |
|---|
| Sex |
|
| 0.541 |
| Male | 85 (59) | 93 (55.4) |
|
|
Female | 59 (41) | 75 (44.6) |
|
| Age |
|
| 0.351 |
|
<60 | 32 (22.2) | 45 (26.8) |
|
|
≥60 | 112 (77.8) | 123 (73.2) |
|
| Tumor location |
|
| 0.641 |
|
Right | 60 (41.7) | 62 (36.9) |
|
|
Left | 73 (50.7) | 90 (53.6) |
|
|
Middle | 11 (7.6) | 16 (9.5) |
|
| Histological
differentiation |
|
| 0.01 |
|
Well | 13 (9) | 11 (6.5) |
|
|
Moderate | 117 (81.3) | 145 (86.3) |
|
|
Poor | 3 (2.1) | 10 (6) |
|
|
Mucinous and signet-ring | 11 (7.6) | 2 (1.2) |
|
| T stage |
|
| 0.157 |
|
T1-2 | 12 (8.3) | 22 (13.1) |
|
| T3 | 109 (75.7) | 129 (76.8) |
|
| T4 | 23 (16) | 17 (10.1) |
|
| N stage |
|
| 0.388 |
| N0 | 72 (50) | 95 (56.5) |
|
| N1 | 35 (24.3) | 40 (23.8) |
|
| N2 | 37 (25.7) | 33 (19.6) |
|
| Vascular
invasion |
|
| 0.752 |
|
Yes | 30 (21.1) | 38 (22.6) |
|
| No | 112 (78.9) | 130 (77.4) |
|
Prognosis analysis for Ki67
expression
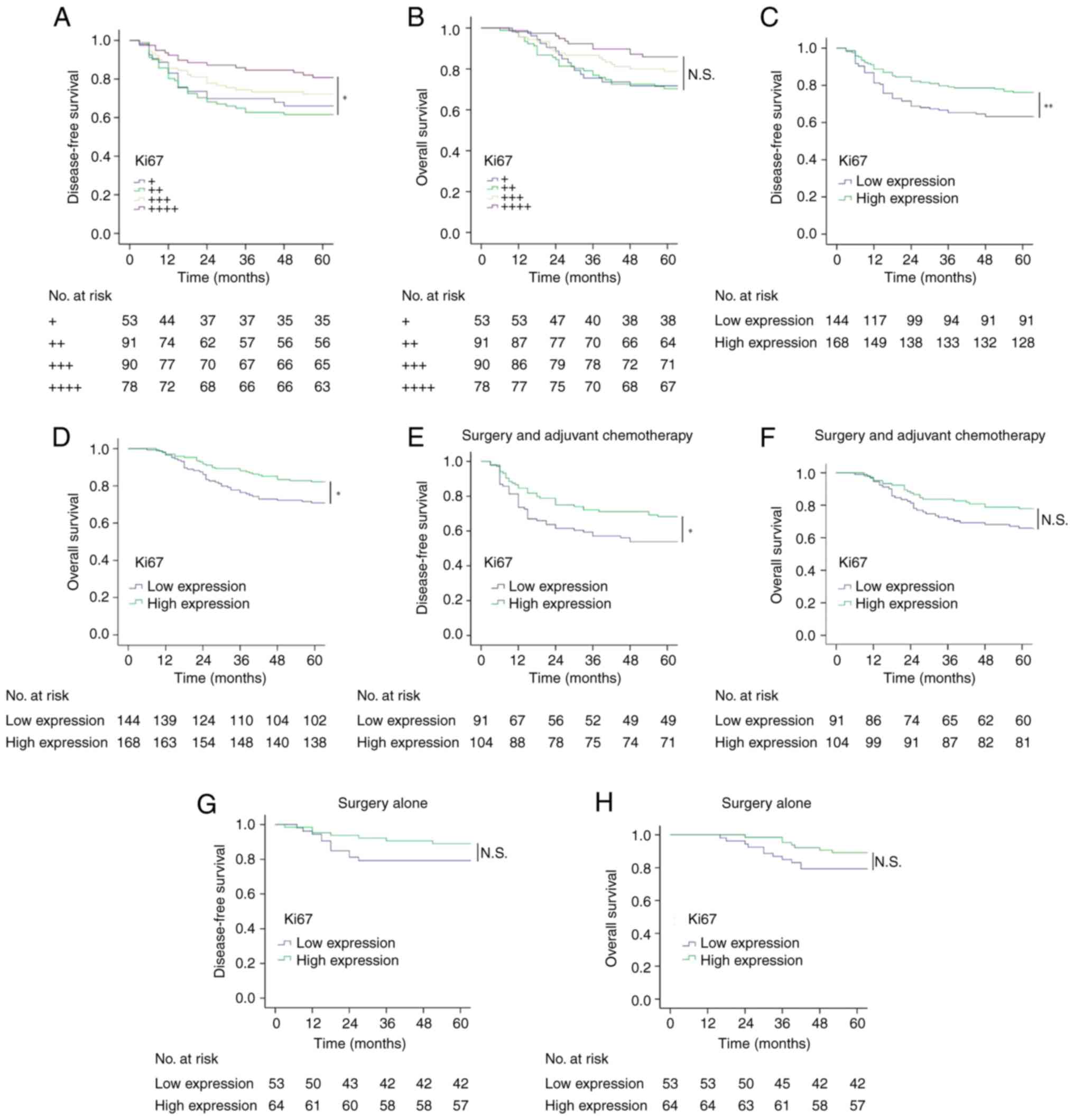
The 5-year DFS and OS rates for all included
patients were 70.2% (219/312) and 76.9% (240/312), respectively.
The expression level of Ki67 was associated with DFS and OS.
Patients in the ++++ group had higher DFS and OS rates than those
in the + or ++ group. The same trend was also observed based on the
low- or high-expression classification. In patients treated with
surgery and adjuvant chemotherapy, high Ki67 expression was
associated with improved DFS but not OS, whereas in patients
treated with surgery alone, there was no statistical association
between Ki67 expression and DFS or OS (Fig. 2). In relation to the administration
of chemotherapy, there was no statistical association between
chemotherapy protocols and DFS or OS (Fig. S1). These results exclude the
affection of chemotherapy protocols to survival of patients treated
with surgery and adjuvant chemotherapy.
Multivariate analysis of DFS
To identify independent prognostic factors for tumor
progression, multivariate analysis using a Cox proportional hazards
regression model (Enter method) was performed. Ki67 expression
level and other variables, including tumor location, histological
differentiation, pathological T and N stage, vascular invasion, and
adjuvant chemotherapy, were analyzed. The results suggested that
pathological T stage and N stage were independent prognostic
factors, whereas the Ki67 did not pass multivariate analysis to be
an independent prognostic factor (Table III).
 | Table III.Multivariate analysis of DFS by Cox
proportional hazards regression (Enter method). |
Table III.
Multivariate analysis of DFS by Cox
proportional hazards regression (Enter method).
| Variable | HR | 95% CI | P-value |
|---|
| T stage |
|
| 0.008 |
| T1 | 1 |
|
|
| T2 | 1.012 | 0.953-1.632 |
|
| T3 | 3.876 | 1.845-6.723 |
|
| T4 | 6.343 | 3.002-9.194 |
|
| N stage |
|
| 0.002 |
| N0 | 1 |
|
|
| N1 | 1.528 | 0.733-3.187 |
|
| N2 | 3.266 | 1.59-6.71 |
|
| Ki67 |
|
| 0.341 |
| + | 1 |
|
|
| ++ | 1.057 | 0.581-1.925 |
|
|
+++ | 0.881 | 0.464-1.647 |
|
|
++++ | 0.597 | 0.289-1.232 |
|
| Vascular
invasion |
|
| 0.329 |
| No | 1 |
|
|
|
Yes | 1.298 | 0.769-2.191 |
|
| Histological
differentiation |
|
| 0.42 |
|
Well | 1 |
|
|
|
Moderate | 1.529 | 0.641-3.649 |
|
|
Poor | 0.844 | 0.223-3.191 |
|
|
Mucinous and Signet | 2.092 | 0.697-6.276 |
|
| Tumor location |
|
| 0.398 |
|
Right | 1 |
|
|
|
Left | 0.762 | 0.478-1.213 |
|
|
Middle | 1.118 | 0.545-2.295 |
|
| Adjuvant
chemotherapy |
|
| 0.651 |
|
Yes | 1 |
|
|
| No | 1.199 | 0.546-2.632 |
|
Discussion
Ki67 has been well established as a pathologic
proliferation marker in cancer, which was first identified in
Hodgkin lymphoma cell nuclei 40 years ago (16). The function of Ki67 is complicated
and has not yet been completely revealed. Based on current
knowledge, Ki67 is a key protein for the formation of the
perichromosomal layer, which is a ribonucleoprotein sheath coating
the condensed chromosomes to prevent aggregation of mitotic
chromosomes, during mitosis (5).
During interphase, Ki67 maintains the normal distribution of
heterochromatin antigens (17). The
role of Ki67 in carcinogenesis has been well established that it
promotes cell proliferation and tumor growth (18); and high Ki67 expression is
associated with poor prognosis in numerous types of malignant
tumors (19,20).
The prognostic value of Ki67 in colorectal cancer is
still controversial. A meta-analysis including 34 studies and 6,180
patients with colorectal cancer by Luo et al (11) suggested that high Ki67 expression
was associated with decreased DFS and OS, especially in patients
with colon cancer who underwent surgery alone, but was not
associated with prognosis in patients treated with surgery and
adjuvant chemotherapy. Interestingly, another meta-analysis
including 8,293 patients based on 30 studies by Xiong et al
(12) reported that high Ki67
expression was associated with improved prognosis in patients
treated with surgery and adjuvant therapy but worse prognosis in
patients treated with surgery alone. The differences in conclusions
between the two meta-analyses may have been due to the different
clinical evidence selected for analysis. There are increasing
reports demonstrating that high Ki67 expression is associated with
improved response to adjuvant chemotherapy (10,12).
For example, Fluge et al (21) investigated Ki67 expression in 409
patients with CRC, reporting that high Ki67 expression was
associated with improved relapse-free survival not in all patients
but only in the patients who received chemotherapy. Similarly,
other studies have also provided evidence suggesting that high
expression levels of Ki67 are associated with improved response to
adjuvant chemoradiotherapy in CRC (22), although contradictory evidence also
exists (15,23). Due to the inconsistencies among
reports, more well-designed studies are required to clarify the
prognostic value of Ki67 expression in colon cancer. The present
study demonstrated that high Ki67 expression was associated with
improved DFS for patients who were treated with surgery and
adjuvant chemotherapy but not for those treated with surgery alone,
suggesting that Ki67 is a potential predictive marker for
therapeutic outcome, which should be further investigated for the
development of precision medicine. On the other hand, the
evaluation criteria of Ki67 is crucial in the present study. The
assessment of Ki67 varies among different studies. Some researchers
used global positive percentage (10), while others used ‘hot spot’ field
counting (24). A high-quality
international study published in 2016 validated the analytical
variability of different Ki67 evaluation criteria, finding that the
global percentage method has the highest interlaboratory agreement,
whereas the hot-spot methods is marginally acceptable (25). In the present study, all marked
cells in the fields were evaluated including the moderate and
strong positive cells. The present study also suggested that the
50% cutoff value was suitable to stratify patients with colon
cancer into postoperative tumor progression risk groups, which is
supported by other reports (8,26).
Unlike other studies, the present study divided
patients into two groups depending on the therapy they received.
The results demonstrated that the prognostic value of Ki67 was
different for patients treated with surgery alone compared with
those treated with surgery and chemotherapy. Although high Ki67
expression was not observed to be associated with poor survival in
the surgery alone group in the present study, which was
inconsistent with the results of a single study (27), the patients with high Ki67
expression in the surgery and chemotherapy group had improved
therapeutic outcomes, suggesting that tumors with high
proliferative activity exhibited increased sensitivity to
chemotherapy (22,28). High-expression of Ki67 suggests
active proliferation and mitosis of tumor, so this kind of tumor in
inclined to be more sensitive to chemotherapy. However, the colon
cancer is highly heterogeneous, patients of different race and
countries often respond variously to the same treatment. On the
other hand, acquired chemo-resistance after therapy is another
factor affecting clinical outcome, which would ‘dilute’ the
contribution of Ki67 to prognosis. Therefore, the conclusions from
different studies are usually inconsistent, more clinical evidences
are needed to demonstrate the prognostic value of Ki67 in colon
cancer. Based on our findings, Ki67 is a potential prognostic
marker for outcome prediction for patients with colon cancer
receiving adjuvant chemotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The work is supported by National Natural Science Foundation of
China (grant no. 32170590) and Guangdong University Scientific
Research Project Funding (grant no. 2021ZDZX2062).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CD and YP designed the study and enrolled patients.
QL, DR, LW and JF collected patients' information and analyzed
data. WD and DM provided technological support for
immunohistochemistry work. CD and YP confirmed the authenticity of
the raw data. All authors participated in writing the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The research was approved by the Ethics Committee of
Southern University of Science and Technology (Shenzhen, China) and
Peking University Cancer Hospital (Beijing, China). All patients
provided their signed informed consent for the use of their tissue
samples and medical records for research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sveen A, Kopetz S and Lothe RA:
Biomarker-guided therapy for colorectal cancer: Strength in
complexity. Nat Rev Clin Oncol. 17:11–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martins BA, de Bulhoes GF, Cavalcanti IN,
Martins MM, de Oliveira PG and Martins AMA: Biomarkers in
colorectal cancer: The role of translational proteomics research.
Front Oncol. 9:12842019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang C, Zhang J, Ding M, Xu K, Li L, Mao L
and Zheng J: Ki67 targeted strategies for cancer therapy. Clin
Transl Oncol. 20:570–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrowsky H, Sturm I, Graubitz O, Kooby
DA, Staib-Sebler E, Gog C, Köhne CH, Hillebrand T, Daniel PT, Fong
Y and Lorenz M: Relevance of Ki-67 antigen expression and K-ras
mutation in colorectal liver metastases. Eur J Surg Oncol.
27:80–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weber JC, Nakano H, Bachellier P,
Oussoultzoglou E, Inoue K, Shimura H, Wolf P, Chenard-Neu MP and
Jaeck D: Is a proliferation index of cancer cells a reliable
prognostic factor after hepatectomy in patients with colorectal
liver metastases? Am J Surg. 182:81–88. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roxburgh CS, Richards CH, Macdonald AI,
Powell AG, McGlynn LM, McMillan DC, Horgan PG, Edwards J and Shiels
PG: The in situ local immune response, tumour senescence and
proliferation in colorectal cancer. Br J Cancer. 109:2207–2216.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Melling N, Kowitz CM, Simon R, Bokemeyer
C, Terracciano L, Sauter G, Izbicki JR and Marx AH: High Ki67
expression is an independent good prognostic marker in colorectal
cancer. J Clin Pathol. 69:209–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo ZW, Zhu MG, Zhang ZQ, Ye FJ, Huang WH
and Luo XZ: Increased expression of Ki-67 is a poor prognostic
marker for colorectal cancer patients: A meta analysis. BMC Cancer.
19:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong DD, Lin XG, He RQ, Pan DH and Gan
TQ: Ki67/MIB-1 predicts better prognoses in colorectal cancer
patients received both surgery and adjuvant radio-chemotherapy: A
meta-analysis of 30 studies. Int J Clin Exp Med. 10:1788–1804.
2017.
|
|
13
|
Chan GHJ and Chee CE: Making sense of
adjuvant chemotherapy in colorectal cancer. J Gastrointest Oncol.
10:1183–1192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabattini E, Bisgaard K, Ascani S, Poggi
S, Piccioli M, Ceccarelli C, Pieri F, Fraternali-Orcioni G and
Pileri SA: The EnVision++ system: A new immunohistochemical method
for diagnostics and research. Critical comparison with the APAAP,
ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol.
51:506–511. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong G, Zhang G, Liu J, Zheng Z, Chen Y,
Niu P and Xu X: Cutoff of 25% for Ki67 expression is a good
classification tool for prognosis in colorectal cancer in the AJCC8
stratification. Oncol Rep. 43:1187–1198. 2020.PubMed/NCBI
|
|
16
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobecki M, Mrouj K, Camasses A, Parisis N,
Nicolas E, Llères D, Gerbe F, Prieto S, Krasinska L, David A, et
al: The cell proliferation antigen Ki-67 organises heterochromatin.
Elife. 5:e137222016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mrouj K, Andres-Sanchez N, Dubra G, Singh
P, Sobecki M, Chahar D, Ghoul EA, Aznar AB, Prieto S, Pirot N, et
al: Ki-67 regulates global gene expression and promotes sequential
stages of carcinogenesis. Proc Natil Acad Sci USA.
118:e20265071182021. View Article : Google Scholar
|
|
19
|
Soliman NA and Yussif SM: Ki-67 as a
prognostic marker according to breast cancer molecular subtype.
Cancer Biol Med. 13:496–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin B, Paesmans M, Mascaux C, Berghmans
T, Lothaire P, Meert AP, Lafitte JJ and Sculier JP: Ki-67
expression and patients survival in lung cancer: Systematic review
of the literature with meta-analysis. Br J Cancer. 91:2018–2025.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fluge O, Gravdal K, Carlsen E, Vonen B,
Kjellevold K, Refsum S, Lilleng R, Eide TJ, Halvorsen TB, Tveit KM,
et al: Expression of EZH2 and Ki-67 in colorectal cancer and
associations with treatment response and prognosis. Br J Cancer.
101:1282–1289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kikuchi M, Mikami T, Sato T, Tokuyama W,
Araki K, Watanabe M, Saigenji K and Okayasu I: High Ki67, Bax, and
thymidylate synthase expression well correlates with response to
chemoradiation therapy in locally advanced rectal cancers: Proposal
of a logistic model for prediction. Br J Cancer. 101:116–123. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allegra CJ, Paik S, Colangelo LH, Parr AL,
Kirsch I, Kim G, Klein P, Johnston PG, Wolmark N and Wieand HS:
Prognostic value of thymidylate synthase, Ki-67, and p53 in
patients with Dukes' B and C colon cancer: A national cancer
institute-national surgical adjuvant breast and bowel project
collaborative study. J Clin Oncol. 21:241–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swiderska-Chadaj Z, Markiewicz T, Grala B
and Lorent M: Content-based analysis of Ki-67 stainedmeningioma
specimens for automatic hot-spot selection. Diagn Pathol.
11:932016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leung SCY, Nielsen TO, Zabaglo L, Arun I,
Badve SS, Bane AL, Bartlett JMS, Borgquist S, Chang MC, Dodson A,
et al: Analytical validation of a standardized scoring protocol for
Ki67: Phase 3 of an international multicenter collaboration. NPJ
Breast Cancer. 2:160142016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin IY, Sung NY, Lee YS, Kwon TS, Si Y,
Lee YS, Oh ST and Lee IK: The expression of multiple proteins as
prognostic factors in colorectal cancer: Cathepsin D, p53, COX-2,
epidermal growth factor receptor, C-erbB-2, and Ki-67. Gut Liver.
8:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu XS, Xi HQ and Chen L: Lgr5 is a
potential marker of colorectal carcinoma stem cells that correlates
with patient survival. World J Surg Oncol. 10:2442012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alba E, Lluch A, Ribelles N, Anton-Torres
A, Sanchez-Rovira P, Albanell J, Calvo L, García-Asenjo JAL,
Palacios J, Chacon JI, et al: High proliferation predicts
pathological complete response to neoadjuvant chemotherapy in early
breast cancer. Oncologist. 21:150–155. 2016. View Article : Google Scholar : PubMed/NCBI
|