Introduction
Encapsulated papillary carcinoma (EPC) of the
breast, which is observed in only 0.5–1% of all malignant cases
worldwide (1), is regarded as a
transition between ductal carcinoma in situ (DCIS) and
invasive carcinoma (2). According
to the histological features, EPC can be divided into three
subtypes: Pure EPC, EPC associated with DCIS and EPC associated
with invasive carcinoma (3).
Microscopically, similar to other types of papillary
intraductal carcinoma, EPCs arise in a cystically dilated duct and
lack myoepithelial cells (MECs), both in the fibrovascular cores
and at the periphery (4). The
absence of MECs and reported cases of metastatic tumors indicate
that these tumors represent low-grade invasive carcinomas with an
expansile growth pattern (5).
However, the presence of continuous and intense collagen IV
expression at the periphery is considered highly suggestive of a
non-invasive carcinoma that is confined within an intact basement
membrane (6). EPC without an
adjacent DCIS or any invasive component has a very favorable
prognosis with adequate local therapy; however, the presence of
associated DCIS or invasive components confers a higher risk of
local recurrence (7).
Notably, the outcomes of EPC cases associated with
invasive carcinoma remain unclear. Several retrospective
clinicopathological studies have demonstrated that EPC has a
favorable prognosis with suitable local therapy alone, regardless
of whether they are in situ or not (8,9).
However, evidence from other studies has indicated that EPC
associated with invasive carcinoma behaves aggressively, and these
tumors should be staged and treated as invasive breast cancer,
especially in cases with an invasive component outside the tumor
capsule (7,10). To the best of our knowledge, no
clearly defined guidelines on treatments for EPC have been
established thus far, due to its low incidence rate.
To better understand the pathology of EPC of the
breast and to investigate the therapeutic role of treatment
modalities, including surgery, chemotherapy, radiotherapy and
hormonal therapy, the present study aimed to compare the
clinicopathological features and survival of patients with
non-invasive EPC and invasive EPC admitted to The Third Hospital of
Nanchang City (Nanchang, China), and to provide preliminary
guidelines for standard treatment recommendations of this rare
clinical entity.
Materials and methods
Patient cohort
From the institutional database, all patients that
were diagnosed with EPC and admitted to the Prevention and Cure
Center of Breast Disease, The Third Hospital of Nanchang City
between January 2015 and December 2021 were included in this
retrospective clinicopathological study. All microscopy slides of
these cases were confirmed as EPC by two pathologists
independently. Cases with/without an adjacent DCIS or any invasive
components were included. Data such as age, sex, menopausal status,
primary complaint and treatments were also collected.
Pathological examination
All available hematoxylin and eosin
(H&E)-stained EPC slides were reviewed by two independent
pathologists. Tumor morphologies, including nuclear grade, mitotic
rate, and presence and extent of associated in situ and
invasive carcinoma were assessed using recently described criteria
(11). Immunohistochemical analyses
were performed on 4-µm paraffin-embedded tissue slides, which were
initially fixed with 10% formalin at room temperature for 6 h, as
previously described (12). First,
the slides were incubated at 65°C for 2 h and then deparaffinized
twice, 5 min each time. Antigen retrieval was performed in antigen
retrieval solution (10 mmol/l Tris; 1 mmol/l EDTA; pH 9.0) at 100°C
for 5 min and 2% sheep serum (Beyotime Institute of Biotechnology)
was added for blocking at room temperature for 30 min. The slides
were then incubated with primary antibody at 4°C overnight, and
with the horseradish peroxidase-labeled secondary antibody and DAB
for 1 h at 37°C. Tumor immunoreactivity was evaluated by two
pathologists independently. Moreover, immunohistochemistry (IHC),
as well as fluorescence in situ hybridization (FISH), was
used to determine the status of human epidermal growth factor
receptor-2 (HER2) according to the updated 2018 American Society of
Clinical Oncology-College of American Pathologists recommendations
for HER2 testing in breast cancer (13). FISH analyses were performed as
previously reported (14).
Follow-up
All patients were followed-up in the Prevention and
Cure Center of Breast Disease, The Third Hospital of Nanchang City.
During the follow-up period, routine physical and radiological
examinations were performed to monitor recurrence. All of the
patients were followed up by telephone communication and the date
of the last follow-up was October 1, 2022. In total, in the present
study, 46 patients with EPC were included; one of which was
diagnosed with bilateral primary EPC. Follow-up information was
available from 42 patients.
Statistical analysis
Data were analyzed using SPSS version 21.0 (IBM
Corp.). All continuous data, such as tumor size and age were
compared using unpaired Student's two-sided t-test. In addition, a
Pearson's χ2 test or Fisher's exact test was used to
evaluate the categorical variables, including clinicopathological
features, such as sex, axillary nodal invasion and hormone receptor
(HR) status. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical and pathological
findings
Descriptive characteristics and clinical findings of
the patients included in the present study are listed in Table I. Between January 2015 and December
2021, 46 patients with EPC were admitted to the Third Hospital of
Nanchang City. Consistent with previous studies (15,16),
the majority of patients with EPC were female, with only one
70-year-old male patient diagnosed in 2017 in the present case
series. The age of onset ranged from 41–88 years, with a median age
of 62.1 years. As shown in Table I,
a total of 22 cases (46.8%) were pure EPC, 6 cases (12.8%) were EPC
associated with DCIS and 19 cases (40.4%) were EPC associated with
invasive carcinoma.
 | Table I.Clinicopathological characteristics of
patients with EPCa. |
Table I.
Clinicopathological characteristics of
patients with EPCa.
| Characteristic | Value |
|---|
| Age at diagnosis, n
(%) |
|
| <60
years | 15 (32.6) |
| 60-80
years | 27 (58.7) |
| >80
years | 4 (8.7) |
| Median
age, years (range) | 62 (41–88) |
| Sex, n (%) |
|
|
Male | 1 (2.2) |
|
Female | 45 (97.8) |
| Menopausal
statusb, n (%) |
|
|
Postmenopausal | 33 (73.3) |
|
Premenopausal or
perimenopausal | 12 (26.7) |
| Family
history, n (%) |
|
|
Yes | 3 (6.5) |
| No | 43 (93.5) |
| Clinical
presentationc, n
(%) |
|
|
Mass | 38 (82.6) |
| Nipple
discharge | 5 (10.9) |
| Laterality, n
(%) |
|
|
Left | 24 (52.2) |
|
Right | 21 (45.7) |
|
Bilateral | 1 (2.2) |
| Site, n (%) |
|
|
Central | 28 (59.6) |
|
Peripheral | 15 (31.9) |
|
Unknown | 4 (8.5) |
| Subtype, n (%) |
|
| Pure
EPC | 22 (46.8) |
| EPC
associated with DCIS | 6 (12.8) |
| EPC
associated with invasion | 19 (40.4) |
| Graded, n (%) |
|
|
Low/intermediate | 42 (89.4) |
|
High | 5 (10.6) |
| T
stagee,f, n (%) |
|
|
Tis | 0 (0) |
| T1 | 13 (32.5) |
| T2 | 24 (60.0) |
| T3 | 3 (7.5) |
| T4 | 0 (0) |
| N
stagee,f, n (%) |
|
| N0 | 43 (91.5) |
| N1 | 4 (8.5) |
| N2 | 0 (0) |
| N3 | 0 (0) |
In the present study, the most common clinical
manifestation of EPC was a painless and palpable lump in the breast
(82.9%). Nipple discharge was present in 5 cases (10.8%). Of the
cases, 24 tumors (52.2%) were located in the left breast and 21
tumors (45.7%) were located in the right breast. Notably, one
61-year-old woman (2.2%) presented with synchronous bilateral EPC
tumors. Upon palpation, the tumor size ranged from 0.8–6.1 cm
(median, 2.5 cm). Ultrasonography revealed that most of the EPC
lesions presented with a solid or mixed cystic nodule, which
displayed a heterogeneous echo structure and the border was often
obscured or irregular in shape. In 7 cases (14.9%), ipsilateral
axillary node enlargement with no abnormal blood flow signal was
also observed. Screening mammograms also depicted a
well-circumscribed, round-to-oval and lobulated mass in 31 cases
(66.0%). Clustered microcalcifications were also found in 6 cases
(12.8%).
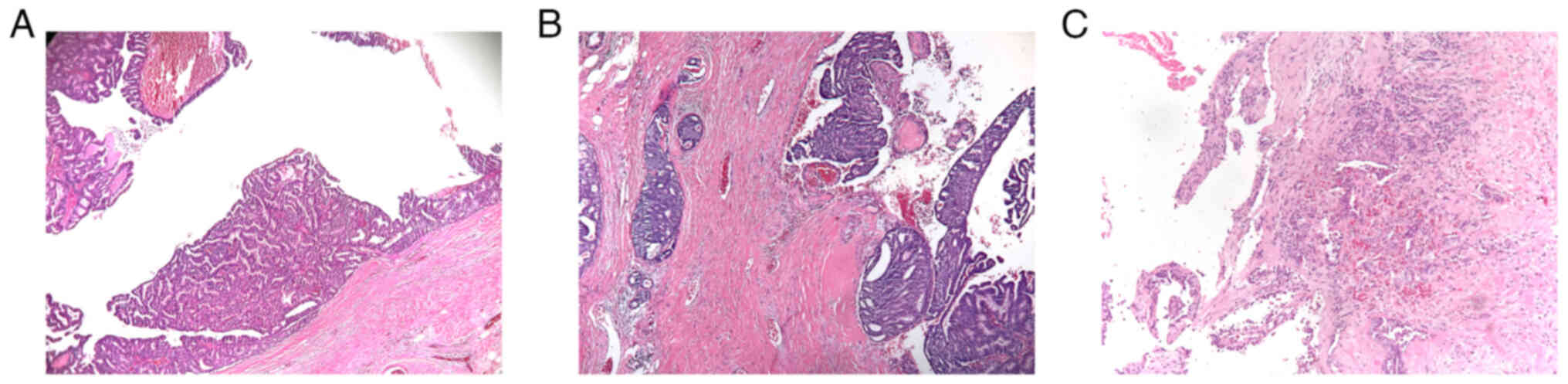
Histopathological examination of EPC showed a
well-defined lesion of papillary carcinoma within a dilated duct
comprised of fibrovascular cores covered by single or multiple
layers of neoplastic cells, surrounded by a fibrous capsule. The
surrounding fibrous capsule was thick and may have been accompanied
by inflammatory cell infiltration (Fig.
1). According to the Nottingham Grading System in primary
breast cancer, 5 EPC cases were high nuclear grade (10.6%), and
they were all EPC associated with invasive carcinoma. Subsequent
immunohistochemical analyses demonstrated that the EPC cases were
primarily estrogen receptor (ER)- and progesterone receptor
(PR)-positive (ER, 91.5%; PR, 80.9%). Notably, all 4 ER-negative
cases were diagnosed with EPC associated with invasive carcinoma.
As for the 9 PR-negative cases, 2 were pure EPC, 2 were EPC
associated with DCIS and 5 were EPC associated with invasive
carcinoma. According to the American Society of Clinical
Oncology-College of American Pathologists Guideline for HER2
testing in breast cancer (17),
cancer with HER2 overexpression refers to those patients who are
HER2 IHC (3+), or HER2 IHC (2+) and FISH (+). Therefore, the
majority of lesions were HER2-negative breast cancer (97.9%) in the
present study, except for only one HER2 (2+) patient who had
invasive EPC and was positive in the subsequent FISH analysis
(Table II). Myoepithelial markers,
such as p63, α smooth muscle antigen (α-SMA) and cytokeratin
(CK)5/6, were negative in the majority of lesions (p63, 85.1%
negative; α-SMA, 76.6% negative; CK5/6, 87.2% negative), which
indicated that myoepithelial cells were often absent both in the
fibrovascular cores and at the periphery of the tumor nodules of
EPC (Fig. 2). A summary of the
immunohistochemical analysis of the cohort is shown in Table II.
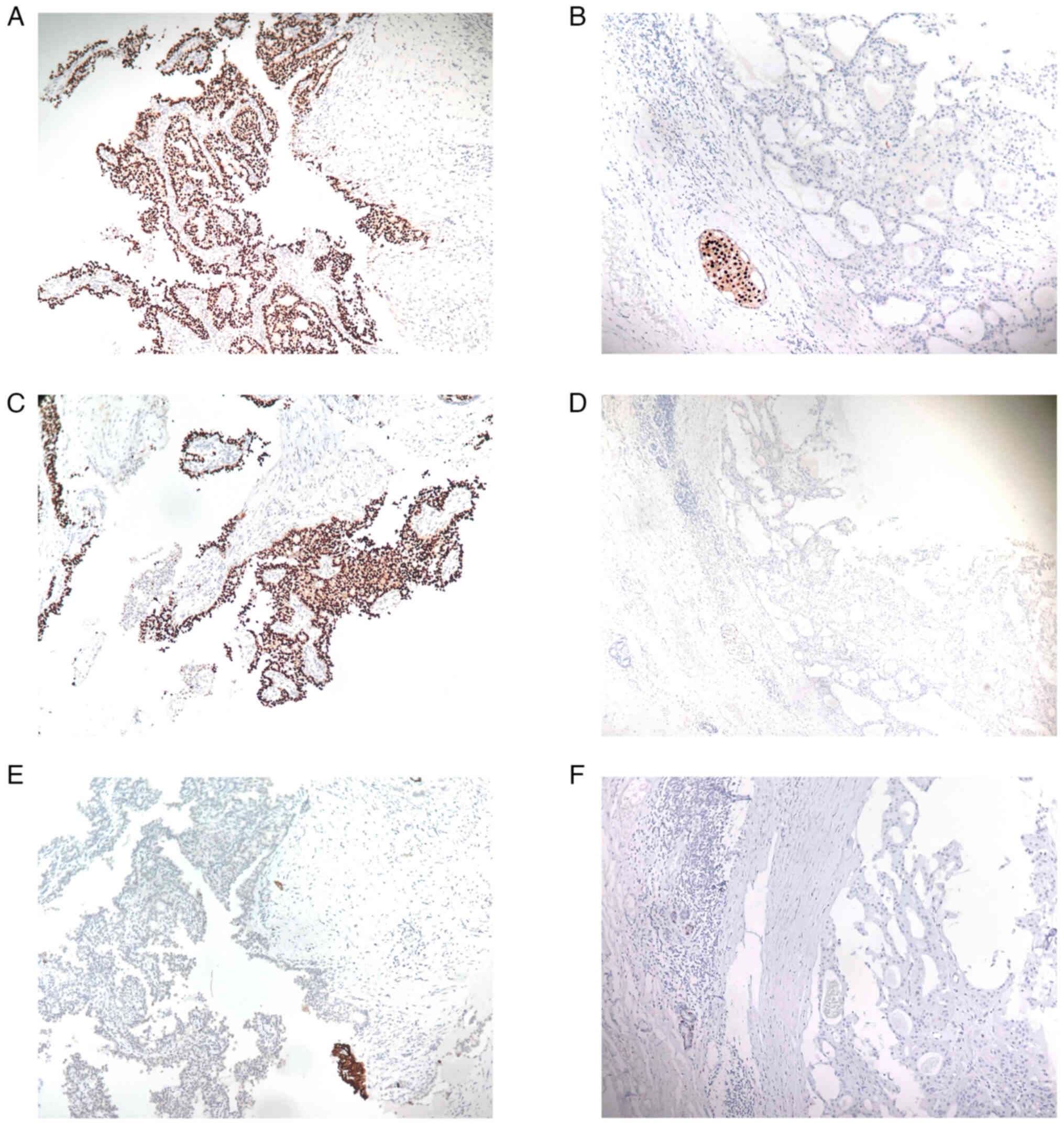 | Figure 2.Immunohistochemical analysis of EPC.
(A) ER-positive, (B) ER-negative, (C) PR-positive, (D) PR-negative,
(E) CK5/6-negative and (F) p63-negative samples. The majority of
EPC cases were ER- and/or PR-positive. Due to the absence of the
myoepithelial layer within the papillae and around the tumor, p63
and CK5/6 immunostaining were often negative. Magnification, ×200.
EPC, encapsulated papillary carcinoma; ER, estrogen receptor; PR,
progesterone receptor; CK5/6, cytokeratin 5/6. |
 | Table II.Immunohistochemical analysis of the
cohort. |
Table II.
Immunohistochemical analysis of the
cohort.
| Immunohistochemical
markers | n, (%) |
|---|
| ER status |
|
|
Positive | 43 (91.5) |
|
Negative | 4 (8.5) |
| PR status |
|
|
Positive | 38 (80.9) |
|
Negative | 9 (19.1) |
| HER2 status |
|
|
(−) | 15 (31.9) |
|
(1+) | 17 (36.2) |
|
(2+) | 15 (31.9) |
|
(3+) | 0 (0) |
| Ki67 level |
|
|
≤14% | 25 (53.2) |
|
>14% | 22 (46.8) |
| p63 status |
|
|
Positive | 6 (12.8) |
|
Negative | 40 (85.1) |
|
Unknown | 1 (2.1) |
| α-SMA status |
|
|
Positive | 8 (17.0) |
|
Negative | 36 (76.6) |
|
Unknown | 3 (6.4) |
| CK5/6 status |
|
|
Positive | 6 (12.8) |
|
Negative | 41 (87.2) |
|
Unknown | 0 (0) |
| Calponin
status |
|
|
Positive | 5 (10.6) |
|
Negative | 38 (80.9) |
|
Unknown | 4 (8.5) |
| Molecular
subtype |
|
|
Luminal | 43 (91.5) |
| HER2
overexpression | 1 (2.1) |
|
TNBC | 3 (6.4) |
Diagnosis, treatments and
survival
To establish a diagnosis, an ultrasound-guided core
needle biopsy was performed in 26 cases prior to surgery. Of the 26
cases, 6 cases were diagnosed as pure EPC, 2 cases were diagnosed
as EPC with DCIS, 2 cases were diagnosed as EPC with invasive
carcinoma, 3 cases were diagnosed as invasive carcinoma, 10 cases
were diagnosed as papilloma neoplasm, 2 cases were diagnosed as EPC
suspicious for invasion and 1 case was diagnosed as atypical ductal
hyperplasia. Subsequently, surgical excision was advised in all of
these cases. Breast-conserving surgery/lumpectomy was performed in
16 cases (34.0%) and mastectomy was performed in 31 cases (66.0%).
Sentinel lymph node biopsy (SLNB) was performed in all 47 cases to
evaluate the axillary lymph node status and 4 (8.5%) of the cases
were found to exhibit axillary lymph node involvement. Notably, all
of the patients who had positive sentinel lymph nodes were
diagnosed with EPC associated with invasive carcinoma.
Additionally, all of these patients underwent subsequent axillary
lymph node dissection (ALND) (Table
III). Due to the observation that a substantial proportion of
invasive EPC cases were axillary lymph node-positive (21.1%), SLNB
should be considered in this unique subgroup.
 | Table III.Treatments and clinical outcomes of
patients with EPC. |
Table III.
Treatments and clinical outcomes of
patients with EPC.
| Treatments and
outcomes | n (%) |
|---|
|
Surgerya |
|
| BCS or
Lumpectomy | 16 (34.0) |
|
Mastectomy | 31 (66.0) |
| Surgery of axillary
lymph nodesa |
|
|
SLNB | 47 (100) |
|
ALNDb | 4 (8.5) |
| Axillary lymph node
metastasis |
|
|
Yes | 4 (8.7) |
| No | 42 (91.3) |
| Chemotherapy |
|
|
Yes | 4 (8.7) |
| No | 42 (91.3) |
| Radiotherapy |
|
|
Yes | 18 (39.1) |
| No | 28 (60.9) |
| Endocrine
therapy |
|
|
SERMs | 11 (23.9) |
|
AIs | 30 (65.2) |
|
OFS | 1 (2.2) |
| HER2-targeted
therapy |
|
|
Yes | 1 (2.2) |
| No | 45 (97.8) |
| Clinical
outcome |
|
| Local
recurrence | 2 (4.3) |
| Distant
metastasis | 1 (2.2) |
Concerning the postoperative treatments, tumor size,
histological grade and the molecular subtyping of the invasive
tumor cells should be considered when frankly invasive carcinoma is
present in association with an EPC. In the present study,
radiotherapy, chemotherapy, as well as endocrine therapy, were
administered. Among all of the patients, 18 of them (39.1%)
received adjuvant radiotherapy. Additionally, 4 patients (8.7%) who
were originally diagnosed with axillary lymph node metastasis
received systemic chemotherapy. Notably, these 4 patients all had
invasive EPC and their invasive components were all HR-negative or
HER2-positive. Moreover, one 41-year-old woman diagnosed with EPC
associated with invasive carcinoma received adjuvant HER2-targeted
therapy for 1 year as the invasive tumor cells were HER2-positive.
Furthermore, adjuvant endocrine therapy was administered in 42
patients (91.3%). In the present study, all of the postmenopausal
patients with EPC were given aromatase inhibitors (AIs), and all
the premenopausal patients with EPC were given selective estrogen
receptor modulators (SERMs), such as tamoxifen. Detailed treatments
of this case series are listed in Table III.
Due to of the aggressive biological features and
metastatic potential of invasive EPC, the clinicopathological
characteristics of non-invasive EPC were compared with invasive EPC
(Table IV). The results indicated
that invasive EPC was positively associated with a larger tumor
size, ER-negative status and axillary lymph node metastasis when
compared with non-invasive EPC. Although not statistically
significant, it should be noted that a PR-negative status was more
often observed in EPC associated with invasive carcinoma.
 | Table IV.Comparison of non-invasive EPC with
invasive EPC. |
Table IV.
Comparison of non-invasive EPC with
invasive EPC.
| Clinicopathological
characteristics | Non-invasive
EPCa | Invasive EPC | P-value |
|---|
| Age,
yearsb |
|
|
|
| Mean ±
SD | 61.37±13.19 | 62.65±9.05 | 0.731 |
| Agec |
|
| 0.440 |
| ≤50
years | 6 (22.2) | 2 (10.5) |
|
| >50
years | 21 (77.8) | 17 (89.5) |
|
| Sexc |
|
| 0.413 |
|
Male | 0 (0) | 1 (5.3) |
|
|
Female | 27 (100) | 18 (94.7) |
|
| Tumor size,
cmb,d |
|
|
|
| Mean ±
SD | 2.27±0.96 | 3.33±1.31 | 0.005e |
| Tumor
sizec,d |
|
|
|
| <2
cm | 7 (30.4) | 5 (29.4) | 0.241 |
| 2-5
cm | 16 (69.6) | 9 (52.9) |
|
| >5
cm | 0 (0) | 3 (17.7) |
|
| Gradec |
|
| 0.144 |
|
Low/Intermediate | 26 (96.3) | 15 (78.9) |
|
|
High | 1 (3.7) | 4 (21.1) |
|
| Axillary
metastasisc |
|
| 0.024f |
|
Present | 0 (0) | 4 (21.1) |
|
|
Absent | 27 (100) | 15 (78.9) |
|
| ER
statusc |
|
| 0.024f |
|
Positive | 27 (100) | 15 (78.9) |
|
|
Negative | 0 (0) | 4 (21.1) |
|
| PR
statusc |
|
| 0.133 |
|
Positive | 24 (88.9) | 13 (68.4) |
|
|
Negative | 3 (11.1) | 6 (31.6) |
|
| HER2
statusc |
|
| 0.413 |
|
Positive | 0 (0) | 1 (5.3) |
|
|
Negative | 27 (100) | 18 (94.7) |
|
Follow-up information was available for 42 patients,
with a mean follow-up of 31.5 months (range, 11.0–67.0 months).
Routine physical and radiological examinations at the follow-up
were performed to monitor recurrence. Until October 1, 2022, 40
cases were free of any recurrence on clinical examination and
radiological imaging, while 2 patients experienced local recurrence
or distant metastasis. One of these patients developed ipsilateral
breast recurrence ~28 months after breast-conserving surgery, and
they received a subsequent mastectomy in combination with ALND; the
pathological examination showed a pure EPC with high nuclear grade
and no regional lymph node metastasis. The immunohistochemical
results demonstrated the recurrent EPC tumor was positive for HR
and negative for HER2. However, the patient refused post-operative
radiotherapy after primary breast-conserving surgery. In the other
case, the patient was diagnosed with invasive EPC, and received
mastectomy together with SLNB and adjuvant endocrine therapy. A
total of 41 months after the primary surgery, routine
ultrasonography found an enlarged ipsilateral supraclavicular fossa
lymph node and metastatic adenocarcinoma was found in the
ultrasound-guided core needle biopsy. Notably, the
immunohistochemical staining revealed the metastatic lesion in the
supraclavicular fossa was HR-negative and HER2-negative, although
the corresponding primary tumor was classified as luminal A
subtype. The computed tomography scan also suggested multiple
pulmonary metastases and therefore chemotherapy was administered.
In the follow-up, the 2 patients were still alive. The other
patients (95.2%) were still in good health with no evidence of
relapse or metastasis.
Discussion
EPC is used to define papillary carcinomas, which
are well-defined lesions surrounded by a fibrous capsule, that lack
myoepithelial cells in the periphery and the papillae (4). Previous studies have demonstrated that
EPC tends to affect elderly women and always presents as a
subareolar mass and/or with nipple discharge (18,19).
Consistent with these reports, the age of the patients in the
present study ranged from 41–88 years, with a median age of 62.1
years. The mean age at initial diagnosis of non-invasive EPC and
EPC associated with invasive carcinoma was 61.4 and 62.7 years,
respectively. These observations may seem inconsistent with
previous studies, which typically had a mean age at initial
diagnosis of invasive EPC that was lower when compared with the
non-invasive counterparts (7,15).
However, it should be acknowledged that a larger sample size is
required to further obtain a meaningful result and it should be
noted that the sample size in the present study was larger than
that of the two aforementioned studies. Previous studies have
demonstrated that male patients comprise 2–7% of EPC cases
(2,3). This is inconsistent with the present
report where a 70-year-old male patient was diagnosed with EPC
associated with invasive breast cancer. Unlike several previous
case reports (20,21), the male patient in the present case
series did not have a significant family history of breast
cancer.
The clinical manifestation of EPC mimics a benign
breast tumor, as the most common symptom is a palpable breast mass
(22,23). As for the tumor size, cases of EPC
associated with invasive carcinoma were larger when compared with
the non-invasive counterparts. Of note, it was not unusual for
patients with EPC to complain of nipple discharge in the present
case series (10.6%), and 4 out of 5 patients that presented with
nipple discharge had EPC associated with invasion.
Microscopically, although the majority of EPC cases
were of a low or intermediate grade according to the Nottingham
Grading System in primary breast cancer (24), a substantial amount of tumors showed
histological features associated with aggressive behavior, such as
high-grade features with a high mitotic count (25,26) or
HR negativity. In the present study, 5 EPC cases with high nuclear
grade (10.6%) were all invasive EPC and 3 of these exhibited
regional lymph node metastases. Therefore, taken together, these
findings further indicated that high-grade features may allow EPC
to metastasize easily, and these tumors should be staged and
treated as non-specific invasive carcinoma.
In the immunohistochemical staining of myoepithelial
markers performed in the present study, p63, α-SMA and CK5/6 were
negative in the majority of cases, which suggested that
myoepithelial cells were often absent in EPC. Although EPC was
initially perceived as a rare subtype of in situ carcinoma,
the observations of the present study further supported the notion
that EPC may be a minimally invasive form of carcinoma with an
expansile growth pattern and indolent behavior, or part of a wide
spectrum of lesions, ranging from in situ to invasive
carcinoma. Due to the lack of myoepithelial cells (27), it has been described that a subset
of EPC cases have invasive potential and are able to develop local
and/or distant metastases (5,28). The
results of the present study also indicated that EPC associated
with invasive carcinoma may behave aggressively and should be
managed with caution. As for HR and HER2 status, only 4 cases were
HR-negative (8.5%), while the majority of EPC cases were
HER2-negative (97.9%). Only one of the 15 HER2 (2+) cases was
positive in the subsequent FISH analysis. Notably, those patients
who were HR-negative or HER2-positive all had EPC associated with
invasive carcinoma. The majority of EPC cases in the present study
had a luminal A or luminal B phenotype, except for 3 patients with
triple-negative breast cancer and 1 patient with HER2-positive
cancer. Subsequently, the clinicopathological characteristics of
patients with non-invasive EPC were compared with those with
invasive EPC. Markedly, invasive EPC was positively associated with
tumor size and axillary nodal metastasis. Additionally, a
HR-negative status was more often observed in cases of invasive
EPC. Therefore, when frankly invasive carcinoma is present in
association with EPC, it is recommended to stage and manage
invasive EPC based on the characteristics of the invasive
component.
To the best of our knowledge, no evidence-based
guidelines have yet been established for EPC management given its
low incidence rate. Once the tumor is diagnosed with EPC
post-biopsy, the primary treatment is based on complete surgical
excision, including breast-conserving surgery or mastectomy
(3,15). However, differentiating EPC from
other papillary breast lesions is difficult when using preoperative
core needle biopsies and having to rely on surgical excision to
obtain accurate pathological diagnoses (10). Moreover, it has been suggested that
pathologists are often confused regarding the displaced fragments
of tumor tissue outside the fibrous capsule and true invasion
(29). Additionally, a newly
proposed variant of invasive lobular carcinoma may sometimes
morphologically mimic EPC growth patterns (30,31).
In the present study, an ultrasound-guided core needle biopsy was
performed in 26 cases before surgery and most of the cases were
diagnosed as papillary neoplasm on core needle biopsy and surgical
excision was suggested to obtain a clear diagnosis. In the present
study, only 1 case of EPC associated with invasion was clearly
diagnosed based on core needle biopsy.
Currently, whether SLNB can be omitted when pure EPC
was clearly diagnosed before axillary surgery is still contested.
However, some researchers have proposed SLNB as a suitable surgical
option when invasive EPC is present given its potentially
beneficial role in both prognosis and treatments (3,10).
Unfortunately, as aforementioned, preoperative diagnosis of whether
EPC occurs with invasion or not is a significant challenge.
Therefore, all of the patients received SLNB in the present study
and only 4 patients with invasive EPC exhibited axillary lymph node
metastases (8.7%). Notably, a significant portion of the patients
with invasive EPC showed metastasis in the present cohort (21.1%).
Thus, to provide additional information for clear diagnosis, risk
stratification and appropriate treatment for EPC, diagnostic
imaging modalities, such as digital mammography, contrast-enhanced
ultrasound and magnetic resonance imaging, are now considered to be
of utmost importance (32,33).
Apart from surgical excision, the therapeutic role
of adjuvant radiotherapy, chemotherapy, endocrine therapy, as well
as HER2-targeted therapy, in EPC remains unclear. In principle,
adjuvant treatment of EPC should be based on the malignant
potential of the invasive tumor cells rather than the in
situ components. Thus, previous publications have recommended
adjuvant radiotherapy, chemotherapy and endocrine therapy in
patients with EPC associated with invasive carcinoma (7,34). In
the present study, 18 patients received adjuvant radiotherapy, 4
patients diagnosed with axillary lymph node metastases received
chemotherapy and 1 patient received 1 year of HER2-targeted
therapy. Moreover, the majority of patients with EPC received
standard hormone therapy. Of note, all the postmenopausal patients
with EPC were subsequently given AIs, although whether AIs were
associated with superior benefits was unclear when compared with
SERMs in postmenopausal patients with non-invasive carcinoma
(35). Collectively, the findings
further support that lumpectomy/mastectomy in combination with SLNB
is a reliable therapeutic choice for patients with EPC. Adjuvant
chemotherapy, radiotherapy, as well as endocrine therapy, should be
considered in select patients, especially in cases of EPC
associated with invasion, which display aggressive histological and
biological features.
In conclusion, EPC, which most frequently affects
elderly women, has a relatively excellent prognosis. Due to the
lack of myoepithelial cells, EPC has metastatic potential although
it is considered to be a malignant tumor in situ with
indolent behavior. The present study further confirmed that EPC
associated with invasive carcinoma has aggressive biological
features, especially in lesions associated with unfavorable
clinical or pathological characteristics, such as HR-negative
and/or high nuclear grade. Local resection, as well as SLNB, should
be considered in this population.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation
of China (grant nos. 81860467 and 82060482) and the Key Science and
Technology Support Project of Nanchang (grant no. 2019-258-13).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX, WQ, NY and YC conceived and designed the study.
LX and QM wrote the manuscript. QL contributed to writing and
revising the manuscript. YG and CG performed the statistical
analysis. CG, QM, QL and LL collected and analyzed the data. WQ
performed the pathological examination and provided experimental
support. NY and YC drafted the manuscript and revised it critically
for important intellectual content. LX and YC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Boards of The Third Hospital of Nanchang City (Nanchang,
China) (approval no. K-ky2023004). Written informed consent was
obtained from the patients at the time of tissue collection
according to The Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salemis NS and Mourtzoukou D: Encapsulated
papillary carcinoma of the breast. Breast J. 27:280–283. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
George K, Anna Z, Evanthia K and Vassilios
K: Encapsulated papillary carcinoma of the breast: An overview. J
Cancer Res Ther. 9:564–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steponavičienė L, Gudavičienė D, Briedienė
R, Petroška D and Garnelytė A: Diagnosis, treatment, and outcomes
of encapsulated papillary carcinoma: A single institution
experience. Acta Med Litu. 25:66–75. 2018.PubMed/NCBI
|
|
4
|
Masood S: The significance of accurate
diagnosis of encapsulated papillary carcinoma of the breast by core
needle biopsy. Breast J. 27:207–208. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitahara M, Hozumi Y, Takeuchi N, Ichinohe
S, Fujiwara S, Machinaga M, Saitoh H and Iijima T: Distant
metastasis after surgery for encapsulated papillary carcinoma of
the breast: A case report. Case Rep Oncol. 13:1196–1201. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mulligan AM and O'Malley FP: Metastatic
potential of encapsulated (intracystic) papillary carcinoma of the
breast: A report of 2 cases with axillary lymph node
micrometastases. Int J Surg Pathol. 15:143–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tariq MU, Idress R, Qureshi MB and Kayani
N: Encapsulated papillary carcinoma of breast; a
clinicopathological study of 25 cases and literature review with
emphasis on high grade variant. Ann Diagn Pathol. 49:1516132020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jackson CR, Felty CC, Marotti JD,
Rosenkranz KM and Muller KE: Encapsulated papillary carcinoma with
and without frank invasion: Comparison of clinicopathologic
features and role of axillary staging. Breast J. 27:209–215. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grabowski J, Salzstein SL, Sadler GR and
Blair S: Intracystic papillary carcinoma: A review of 917 cases.
Cancer. 113:916–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashmi AA, Iftikhar SN, Munawar S, Shah A,
Irfan M and Ali J: Encapsulated papillary carcinoma of breast:
Clinicopathological features and prognostic parameters. Cureus.
12:e112822020.PubMed/NCBI
|
|
11
|
Patel A, Hoda RS and Hoda SA: Papillary
breast tumors: Continuing controversies and commentary on WHO's
2019 criteria and classification. Int J Surg Pathol. 30:124–137.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto Y, Hayashi Y, Sakaki H and
Murakami I: Downregulation of fascin induces collective cell
migration in triple-negative breast cancer. Oncol Rep. 50:1502023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jacobs TW, Gown AM, Yaziji H, Barnes MJ
and Schnitt SJ: Comparison of fluorescence in situ hybridization
and immunohistochemistry for the evaluation of HER-2/neu in breast
cancer. J Clin Oncol. 17:1974–1982. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hassan Z, Boulos F, Abbas J, El Charif MH,
Assi H and Sbaity E: Intracystic papillary carcinoma: Clinical
presentation, patterns of practice, and oncological outcomes.
Breast Cancer Res Treat. 182:317–323. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Xu Y, Ye H, Qin S, Hou F and Liu W:
Encapsulated papillary carcinoma of the breast: A
clinicopathological study of 49 cases. Curr Probl Cancer.
42:291–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz CJ, Boroujeni AM,
Khodadadi-Jamayran A, Heguy A, Snuderl M, Jour G, Cotzia P and
Darvishian F: Molecular analysis of encapsulated papillary
carcinoma of the breast with and without invasion. Hum Pathol.
111:67–74. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Solanki MH, Derylo AF, Visotcky AM and
Jorns JM: Encapsulated and solid papillary carcinomas of the
breast: Tumors in transition from in situ to invasive? Breast J.
25:539–541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Romics L Jr, O'Brien ME, Relihan N,
O'Connell F and Redmond HP: Intracystic papillary carcinoma in a
male as a rare presentation of breast cancer: A case report and
literature review. J Med Case Rep. 3:132009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brents M and Hancock J: Ductal carcinoma
in situ of the male breast. Breast Care (Basel). 11:288–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rehman B, Mumtaz A, Sajjad B, Urooj N,
Khan SM, Zahid MT, Mannan H, Chaudhary MZ, Khan A and Parvaiz MA:
Papillary carcinoma of breast: Clinicopathological characteristics,
management, and survival. Int J Breast Cancer. 2022:54278372022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morgan S, Dodington D, Wu JM and
Turashvili G: Solid papillary carcinoma and encapsulated papillary
carcinoma of the breast: Clinical-pathologic features and basement
membrane studies of 50 cases. Pathobiology. 88:359–373. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galea MH, Blamey RW, Elston CE and Ellis
IO: The Nottingham prognostic index in primary breast cancer.
Breast Cancer Res Treat. 22:207–219. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Wu H, Teng L, Zhang H, Lu J and
Liang Z: High-grade encapsulated papillary carcinoma of the breast
is clinicopathologically distinct from low/intermediate-grade
neoplasms in Chinese patients. Histol Histopathol. 34:137–147.
2019.PubMed/NCBI
|
|
26
|
Rakha EA, Varga Z, Elsheik S and Ellis IO:
High-grade encapsulated papillary carcinoma of the breast: An
under-recognized entity. Histopathology. 66:740–746. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Athanasiou A, Khomsi F, de Joliniere B and
Feki A: Encapsulated papillary carcinoma: A case report and review
of the literature. Front Surg. 8:7438812022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yee C, Drost L, Niglas M, Chow E and
Vesprini D: Early local recurrence in a patient with encapsulated
papillary carcinoma of the breast. Clin Breast Cancer.
18:e447–e448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang HJ, Kwon SY, Kim A, Kim WG, Kim EK,
Kim AR, Kim C, Min SK, Park SY, Sung SH, et al: A multicenter study
of interobserver variability in pathologic diagnosis of papillary
breast lesions on core needle biopsy with WHO classification. J
Pathol Transl Med. 55:380–387. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng L, Saluja K and Guo T: Invasive
lobular carcinoma mimicking encapsulated papillary carcinoma with a
literature review: A rare variant detected serendipitously. Int J
Surg Pathol. 30:912–920. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Motanagh SA and Muller KE: Invasive
lobular carcinoma with papillary features: A newly described
variant that poses a difficult histologic differential diagnosis.
Breast J. 26:1231–1233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kurtoğlu Özçağlayan Tİ and Öznur M:
Digital mammography, ultrasound and magnetic resonance imaging
characteristics in differential diagnosis of papillary carcinoma
subtypes of the breast and diagnostic challenges. Eur J Breast
Health. 18:172–181. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang CY, Guan PS, You QQ, Yuan HX and Wang
WP: Contrast-enhanced ultrasound combined with ultrasonic
elastography to diagnose encapsulated papillary carcinoma: A case
report. Clin Hemorheol Microcirc. 82:391–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tariq N, Mamoon N, Usman M, Ali Z and
Nazir I: Encapsulated papillary carcinoma (EPC) of breast: A
clinical, pathological and immunohistochemical analysis of eight
cases. J Pak Med Assoc. 66:1490–1493. 2016.PubMed/NCBI
|
|
35
|
Lazzeroni M, Dunn BK, Pruneri G,
Jereczek-Fossa BA, Orecchia R, Bonanni B and DeCensi A: Adjuvant
therapy in patients with ductal carcinoma in situ of the breast:
The Pandora's box. Cancer Treat Rev. 55:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|