Introduction
Primary central nervous system lymphoma (PCNSL) is a
rare and aggressive form of extranodal lymphoma (1). It is classified as a type of
non-Hodgkin's lymphoma. Although it is a hematological malignancy,
patients that are primarily diagnosed with PCNSL usually present to
the neurosurgery department first with intracranial lesions. For a
definitive diagnosis, a tissue sample from a brain biopsy needs to
be obtained by a neurosurgeon. Due to community referral patterns,
patients with PCNSL may be treated at either a neurosurgical
department or hematology/oncology department.
High-dose methotrexate (MTX)-based chemotherapy is
generally accepted as the standard induction treatment for PCNSL
(1). However, the optimal induction
regimen, including the optimal combination therapy to add to
high-dose MTX, remains uncertain. In addition, no consolidation
therapy for use after MTX-based chemotherapy has been established.
Recently, intensified chemotherapy, including high-dose
chemotherapy with autologous stem cell transplantation (ASCT), has
tended to be considered for consolidation therapy in such cases.
However, the treatments that can be provided depend on the
availability of certain facilities and equipment. For example, ASCT
requires specialist equipment for harvesting and preserving
hematopoietic stem cells and medical staff capable of performing
transplantation procedures. Therefore, treatment methods may vary
depending on the department providing the treatment, e.g.,
neurosurgery and hematology departments may differ in the
treatments they can provide for PCNSL. Such differences in the
available treatment options among departments may result in
different outcomes, just as treatment outcomes for acute
lymphoblastic leukemia (ALL) in adolescents and young adults (AYA)
differ between pediatric and hematology departments (2). However, the outcomes of PCNSL
treatment have not been compared between neurosurgery and
hematology/oncology departments.
In chemotherapy for hematological malignancies, it
is important to maintain an appropriate therapeutic intensity to
achieve better outcomes. Several studies have demonstrated that
maintaining an appropriate relative dose intensity (RDI) during
R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine,
and prednisone) chemotherapy improved survival in newly diagnosed
diffuse large B-cell lymphoma (DLBCL) patients (3,4).
Recently, the RDI of MTX was reported to have an important
prognostic impact in patients with PCNSL (5). However, the difference in the RDI of
MTX between treating departments has not been assessed.
Different treatments may be selected by different
departments, depending on the expertise of the medical staff, which
may lead to differences in treatment outcomes among treating
departments. At our hospital, PCNSL patients were treated by the
neurosurgery or hematology/oncology department, depending on the
year in which they were diagnosed. In this study, we compared the
outcomes of PCNSL patients treated at our neurosurgery and
hematology/oncology departments based on the RDI of MTX.
Materials and methods
Patients
This was a single-center retrospective study,
conducted at Wakayama Medical University Hospital. Consecutive
patients with newly diagnosed PCNSL who received high-dose
MTX-based therapy between January 2011 and December 2021 were
selected via a review of electronic medical records. Patients who
received radiotherapy alone or palliative chemotherapy were
excluded from this analysis. The selected patients had untreated
histologically-proven DLBCL. A PCNSL patient with natural
killer/T-cell lymphoma histology was excluded. Patients with PCNSL
that were diagnosed between January 2011 and March 2018 were
treated at the neurosurgery department (Fig. S1). These patients visited the
neurosurgery department for a mass lesion in the brain, underwent a
brain biopsy to obtain a definitive diagnosis, and continued to be
treated at the neurosurgery department. When patients required
multi-drug combination chemotherapy after relapse, they were
transferred to and treated at the hematology/oncology department.
On the other hand, patients that were diagnosed with PCNSL between
April 2018 and December 2021 were treated at the
hematology/oncology department from the initial treatment onwards.
These patients were referred to the hematology/oncology department
once a definitive diagnosis had been made by the neurosurgery
department. To assess the impact of the treating department on
survival, the patients were classified into those treated at the
neurosurgery department and those treated at the
hematology/oncology department.
All patients underwent positron emission
tomography-computed tomography (PET-CT) or CT (i.e., patients who
could not undergo PET-CT) for staging in addition to brain
gadolinium-enhanced magnetic resonance imaging (MRI) before the
initial treatment. Prognostic factors were evaluated using the
Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic model and
the International Extranodal Lymphoma Study Group (IELSG) model
(6,7).
Treatments
Induction therapy was performed with three different
regimens: high-dose MTX; rituximab and MTX (R-MTX); and rituximab,
MTX, procarbazine, and vincristine (R-MPV). The high-dose MTX
treatment regimen consisted of MTX (3.5 mg/m2) and 15 mg
leucovorin (15 mg) every 4 h, starting 24 h after the
administration of MTX. In the R-MTX regimen, the high-dose MTX
regimen was administered, but rituximab (375 mg/m2) was
added the day before the MTX infusion. In some patients, the first
dose of rituximab was administered after MTX. The R-MPV regimen was
modified from the original regimen reported by the MSKCC (8). The modified regimen consisted of
rituximab (375 mg/m2) on day 1, vincristine (1.4
mg/m2, capped at 2 mg/body) on day 2, and procarbazine
(100 mg/m2) orally on days 2 to 8 in addition to the
high-dose MTX regimen, starting on day 2. Procarbazine was only
administered during odd cycles. The dose of MTX was reduced at the
attending doctor's discretion, based on the patient's performance
status and the presence/absence of renal impairment. Treatment
decisions, including regarding the dose reduction of chemotherapy
agents, were made in treatment meetings held at each
department.
The treatment response was assessed using
gadolinium-enhanced MRI after induction therapy and consolidation
therapy and was defined as a complete response (CR) or partial
response (PR) (9). The tumor burden
was estimated using the sum of the products of the longest
perpendicular diameters (SPD), which was calculated by multiplying
the two longest perpendicular diameters for up to five target
lesions. Adverse events were classified according to the National
Cancer Institute's Common Terminology Criteria for Adverse Events
(CTCAE), version 5.0. Adverse events were assessed during MTX-based
induction therapy. Since it is not possible to determine adverse
events per treatment for leukoencephalopathy, the adverse event
rate for leukoencephalopathy was calculated for the entire course
of treatment.
Assessment of MTX-based therapy
The dosage, number of courses, and adverse events
were evaluated for high-dose MTX-based therapy, including R-MTX and
R-MPV. The RDI of MTX was calculated as the ratio of the delivered
dose of MTX to the reference dose per unit of time. The reference
dose intensity was based on 3.5 g/m2 MTX being
administered every 2 weeks. For patients in whom the high-dose MTX
regimen was discontinued, the RDI was calculated based on the
number of courses administered. The relative treatment intensity of
MTX was calculated by multiplying the RDI of MTX by the number of
MTX-based chemotherapy courses, as previously reported in a study
of older DLBCL patients based on relative treatment intensity
(10). For example, if three
courses of MTX involving a dose of 3.5 g/m2 were
administered every two weeks without postponement, the relative
treatment intensity for MTX would be three. Delayed MTX clearance
was defined as a serum MTX concentration of ≥0.1 mmol/l at 72 h
after the start of the high-dose MTX therapy.
Statistical analyses
Bivariate analyses of categorical variables were
conducted using Fisher's exact test. To analyze continuous
variables, the Mann-Whitney U-test or Student's unpaired t-test was
used. The primary endpoint was overall survival (OS). The secondary
endpoint was progression-free survival (PFS). OS was calculated
from diagnosis to death or the last follow-up. PFS was calculated
from disease progression, relapse, death, or the last follow-up. OS
and PFS were estimated using the Kaplan-Meier method and were
compared using the log-rank test. Data were censored at the date of
the last follow-up or September 30, 2022. The Cox proportional
hazards regression model was used in the univariate and
multivariate analyses of PFS. Clinical factors that exhibited
P-values of <0.20 in the univariate analyses were subjected to
multivariate analysis and selected in a stepwise-deleted manner.
The stepwise method allows the automatic inclusion and exclusion of
variables based on their significance levels (P-values). Age was
divided into two groups; over and under 60 years old. The
consolidation therapies described in Table I were divided into three groups:
radiotherapy, chemotherapy, and none. The chemotherapy group
included the patients treated with whole-brain radiotherapy (WBRT)
combined with high-dose cytarabine (HD-Ara-C), autologous stem cell
transplantation (ASCT) following high-dose chemotherapy, or
tirabrutinib. The grades of adverse events were also assessed.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using the EZR
software package, version 1.55 (Jichi Medical University Saitama
Medical Center, Saitama, Japan), or GraphPad PRISM, version 9 (San
Diego, CA, USA) (11).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Total (n=26) | Neurosurgery
(n=15) | Hematology/oncology
(n=11) | P-value |
|---|
| Date of initial
treatment | Jan. 2011-Dec.
2021 | Jan. 2011-Mar.
2018 | Apr. 2018-Dec.
2021 |
|
| Median age, years
(range) | 62 (31–83) | 65 (31–72) | 62 (48–83) | 0.38 |
| Male, n (%) | 18 (69) | 11 (73) | 7 (63) | 0.68 |
| ECOG PS ≥2, n
(%) | 15 (58) | 10 (67) | 5 (46) | 0.43 |
| KPS <70, n
(%) | 11 (42) | 7 (47) | 4 (37) | 0.70 |
| MSKCC prognostic
model, n (%) |
|
|
| 0.65 |
| Class
1 | 4 (16) | 3 (20) | 1 (9) |
|
| Class
2 | 11 (42) | 5 (33) | 6 (55) |
|
| Class
3 | 11 (42) | 7 (47) | 4 (36) |
|
| IELSG prognostic
model, n (%) |
|
|
| 0.26 |
| Low | 7 (27) | 5 (33) | 2 (18) |
|
|
Intermediate | 9 (35) | 3 (20) | 6 (55) |
|
|
High | 10 (38) | 7 (47) | 3 (27) |
|
| CSF infiltration, n
(%) |
| 0 (0) | 2 (18) | 0.17 |
| Intraocular
infiltration, n (%) | 4 (16) | 1 (6) | 3 (27) | 0.28 |
| EGFR, mean
(range) | 81.3
(49.8–109.4) | 86.7
(56.3–107.6) | 73.9
(49.8–109.4) | 0.056 |
| Induction therapy,
n (%) |
|
|
| <0.001 |
|
HD-MTX | 15 (58) | 15 (0) | 0 (0) |
|
|
R-MTX | 3 (12) | 0 (0) | 3 (27) |
|
|
R-MPV | 8 (30) | 0 (0) | 8 (73) |
|
| Consolidation
therapy, n (%) |
|
|
| <0.001 |
|
WBRT | 11 (42) | 11 (73) | 0 (0) |
|
| WBRT
and HD-AraC | 2 (8) | 0 (0) | 2 (18) |
|
|
ASCT | 3 (11) | 0 (0) | 3 (27) |
|
|
Tirabrutinib | 2 (8) | 0 (0) | 2 (18) |
|
| Local
RT | 1 (4) | 0 (0) | 1 (10) |
|
|
None | 7 (27) | 4 (27) | 3 (27) |
|
Results
Patient characteristics
Twenty-six patients were analyzed. There were 15
(58%) and 11 (42%) patients in the neurosurgery and
hematology/oncology groups, respectively. The clinical
characteristics of each group are summarized in Table I. The median age of all patients at
diagnosis was 62 years (range: 31–83). Eleven patients (42%)
presented with a Karnofsky Performance Status (KPS) of <70.
According to the MSKCC prognostic model, 4 (16%) and 11 (42%)
patients were classified into classes 1 and 2, respectively. Age
did not differ significantly between the neurosurgery and
hematology/oncology groups (P=0.38). In addition, there was no
significant difference in the distribution of the MSKCC or IELSG
prognostic scores between the two groups. The mean estimated
glomerular filtration rate (eGFR) before the initial treatment was
81.3 ml/min/1.73 m2 (range: 49.8–109.4) for all
patients. Although the difference was not significant, the patients
treated at the hematology/oncology department tended to have lower
eGFR than those treated at the neurosurgery department
(P=0.056).
Treatments administered at each
department
Different treatment strategies were followed at each
department (Fig. S1). All
treatment plans were discussed at departmental meetings. The
treatment regimens administered as induction and consolidation
therapies at each department are summarized in Table I. In this study, three types of
high-dose MTX-based regimens were administered to PCNSL patients as
induction therapies. All PCNSL patients treated at the neurosurgery
department received the high-dose MTX regimen. In the
hematology/oncology department, 3 (27%) and 8 (73%) patients
received the R-MTX and R-MPV regimens, respectively.
After achieving a CR or PR, the patients received
consolidation therapy. At the neurosurgery department, 11 patients
(73%) were treated with WBRT as consolidation therapy. On the other
hand, two patients (18%) received WBRT at the hematology/oncology
department. In these two cases, one patient's dose of WBRT was
reduced, and a high-dose cytarabine (Ara-C) regimen was
administered to both patients (8).
The proportion of patients who received WBRT as consolidation
therapy was significantly lower among the patients treated at the
hematology/oncology department than among those treated at the
neurosurgery department (P=0.045).
At the hematology/oncology department, 3 patients
(27%) received high-dose chemotherapy and underwent autologous
hematopoietic stem cell transplantation (auto-HSCT). The high-dose
chemotherapy regimen included intravenous busulfan and thiotepa at
previously reported doses (12).
Two patients who could not continue receiving the high-dose MTX
regimen after achieving a PR due to toxicities caused by the
chemotherapy were treated with oral tirabrutinib, a
second-generation Bruton's tyrosine kinase (BTK) inhibitor, as
consolidation or maintenance therapy at the hematology/oncology
department (13).
The treatments administered after relapse or
progression are shown in Fig. S2.
At the neurosurgery department, 4 patients underwent retreatment
with the high-dose MTX regimen. One of the patients treated at the
hematology/oncology department developed a relapse in their eye and
was treated with radiotherapy (delivered to the ocular lesion) and
oral tirabrutinib without further recurrence.
MTX treatment delivery
The median number of high-dose MTX courses
administered was 3 (range: 2–5) and 6 (range: 3–7) in the
neurosurgery and hematology/oncology groups, respectively. The
initial dose of MTX administered was 3.5 g/m2 for all
patients treated at the neurosurgery department. On the other hand,
3 (27%) of the patients treated at the hematology/oncology
department had their initial MTX doses reduced, with two patients
receiving 2 g/m2 and one receiving 3 g/m2.
The median volume of supplemental fluid required for hydration was
3,000 ml (in all patients) and 2,000 ml (range: 1,500-3,000) in the
patients treated at the neurosurgery and hematology/oncology
departments, respectively. At both departments, the fluid used for
hydration included sodium bicarbonate to alkalize the patients'
urine. Urinary pH was monitored in the patients treated at the
hematology/oncology department, and sodium bicarbonate was added if
a patient's urinary pH fell below 7.0. Acetazolamide was
administered regularly for diuresis to the patients treated at the
hematology/oncology department. Among the patients treated at the
neurosurgery department, delayed MTX clearance was observed in 33
of 44 (75%) high-dose MTX courses. In the hematology/oncology
department, delayed MTX clearance occurred in 22 of 60 (37%)
high-dose MTX-based courses, which was significantly less frequent
than was seen in the patients treated at the neurosurgery
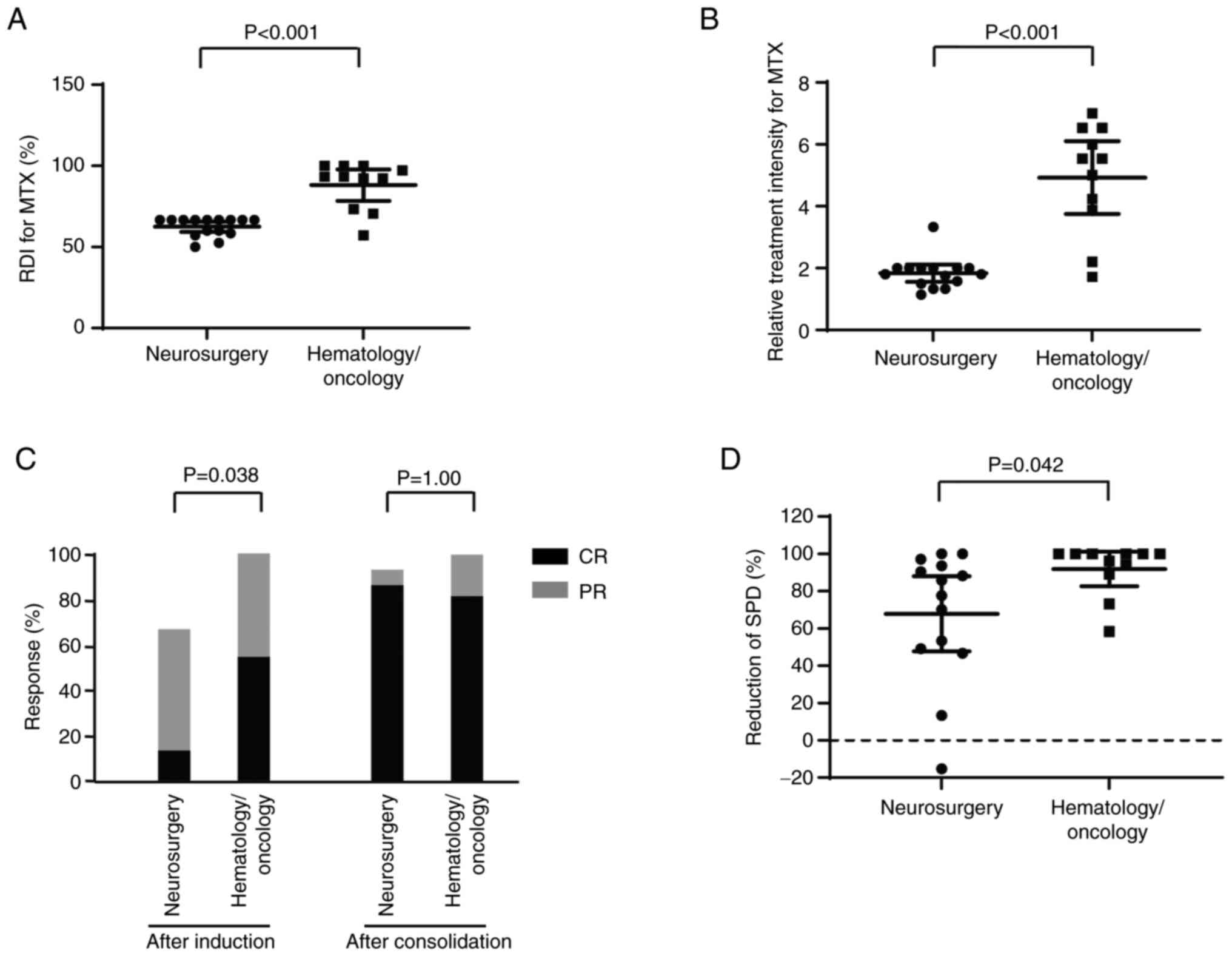
department (P<0.001). The median RDI of MTX was 67% (50–67) and
93% (57–100) in the neurosurgery and hematology/oncology groups,
respectively (P<0.001, Fig. 1A).
The mean relative treatment intensity of MTX was significantly
higher in the hematology/oncology group than in the neurosurgery
group (1.8 vs. 4.9, P<0.001, Fig.
1B).
Response rate
The response rates after induction and consolidation
therapy were assessed. The overall response rate to high-dose
MTX-based induction therapy was 81% among all patients. The CR rate
after induction therapy was 13 and 55% in the neurosurgery and
hematology/oncology groups, respectively (Fig. 1C). The proportion of patients that
achieved a CR after high-dose MTX-based therapy was significantly
higher in the hematology/oncology group than in the neurosurgery
group (P=0.038). After consolidation therapy, including WBRT, there
was no significant difference in the CR rate between the two groups
(neurosurgery group: 87% vs. hematology/oncology group: 82%,
P>0.999, Fig. 1C). The tumor
volume reduction rate, as determined by measuring the SPD, after
high-dose MTX-based therapy was significantly higher in the
hematology/oncology group than in the neurosurgery group (P=0.042,
Fig. 1D).
Survival analysis
Among all patients, the median duration of the
follow-up period was 2.9 years (range: 0.1–11.3). There was no
significant difference in the duration of the follow-up period
between the neurosurgery and hematology/oncology groups (median:
3.4 vs. 2.5 years, respectively; P=0.45). The estimated two-year OS
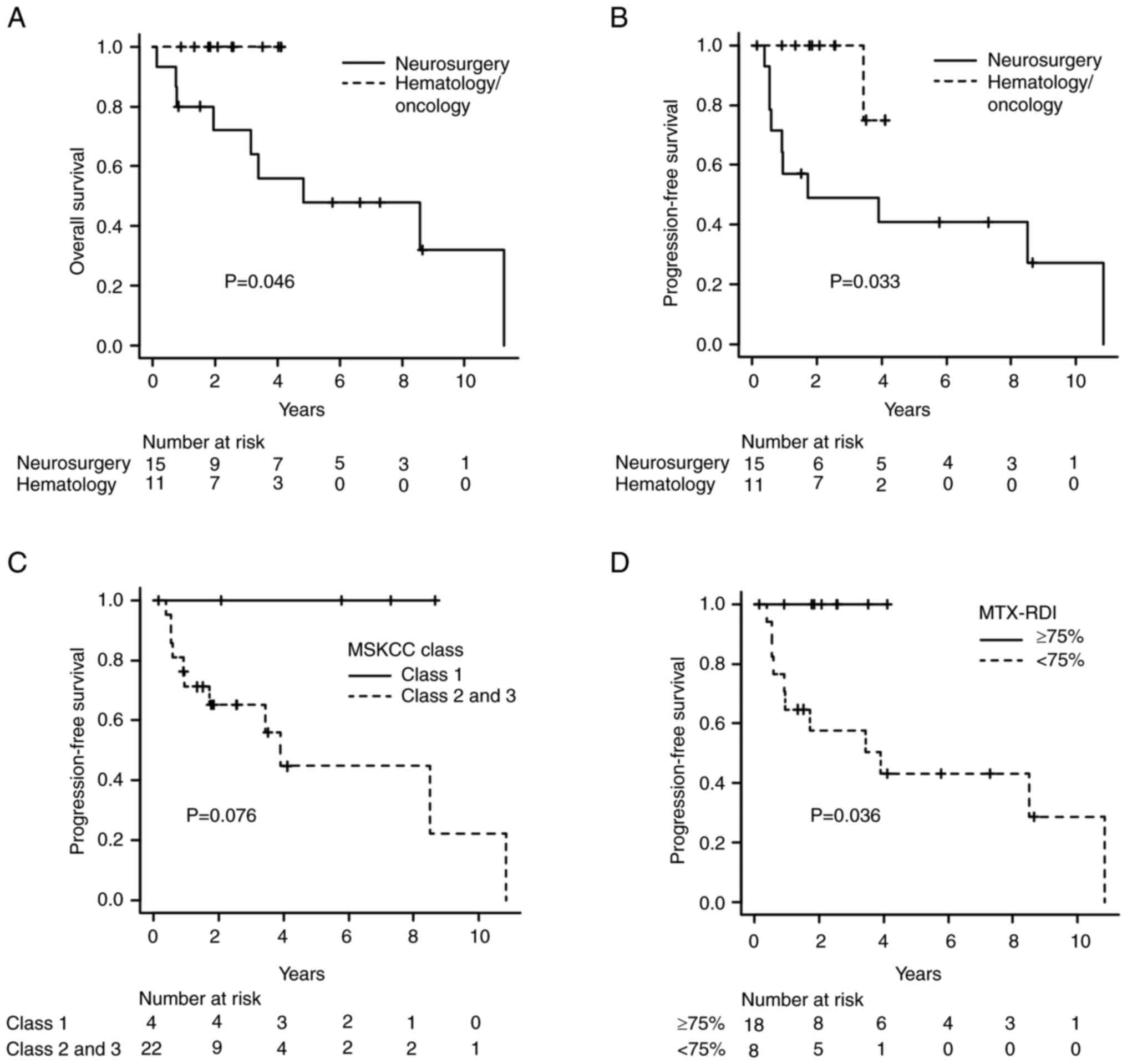
and PFS rates of all patients were 83.3% [95% confidence interval
(CI): 60.9–93.5] and 71.1% (95%CI: 48.5–85.1), respectively. The OS
rate for the patients treated at the hematology/oncology department
was significantly higher than that for those treated at the
neurosurgery department (two-year OS, neurosurgery group: 72% vs.
hematology/oncology group: 100%; P=0.046, Fig. 2A). The two-year PFS rate was 49 and
100% in the neurosurgery and hematology/oncology groups,
respectively (P=0.033, Fig. 2B).
Although the difference was not significant, the patients in
classes 2 and 3 according to the MSKCC prognostic model tended to
have worse OS and PFS rates than those in class 1 (PFS: P=0.076,
Figs. 2C and S3). The PFS rate was significantly higher
in the patients in whom an MTX RDI of ≥75% was achieved than in
those in whom the RDI of MTX was <75% (P=0.036, Fig. 2D). Seven patients died from relapse
during the study observation period. One of the patients treated at
the neurosurgery department died of pneumonia (a case of
treatment-related mortality) during induction therapy. None of the
patients treated at the hematology/oncology department suffered
treatment-related mortality during induction or consolidation
therapy, including auto-HSCT.
Multivariate analysis was performed to evaluate the
factors that affected survival, including the types of induction
and consolidation therapies. Due to the small number of deaths
available for the analysis of OS, multivariate analysis of the
factors that affected PFS was conducted instead. A higher KPS and
being treated at the neurosurgery department were found to be
associated with an increased risk of relapse (Table II).
 | Table II.Univariate and multivariate analyses
of the factors associated with progression-free survival. |
Table II.
Univariate and multivariate analyses
of the factors associated with progression-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Age ≥60 years | 2.37
(0.50–11.2) | 0.28 |
|
|
| Female sex | 0.34
(0.069–1.63) | 0.18 |
|
|
| KPS <70 | 3.04
(0.79–11.7) | 0.045 | 4.85
(1.02–23.0) | 0.047 |
| Induction
therapy | 0.36
(0.11–1.19) | 0.094 |
|
|
| Consolidation
therapy | 0.50
(0.18–1.37) | 0.18 |
|
|
| Neurosurgery
department | 7.08
(0.88–57.2) | 0.066 | 9.61
(1.14–81.1) | 0.038 |
Toxicities
The toxicities of the high-dose MTX-based induction
therapy are summarized in Table
III. The incidence of cytopenia, including leukopenia,
neutropenia, and anemia, was higher in the hematology/oncology
group than in the neurosurgery group. However, there was no
significant difference in the incidence of infections, including
pneumonia, between the two departments. Although the difference was
not significant, the incidence of leukoencephalopathy tended to be
higher among the patients treated at the neurosurgery department
who received WBRT after high-dose MTX therapy.
 | Table III.Adverse events related to the
high-dose MTX-based regimen. |
Table III.
Adverse events related to the
high-dose MTX-based regimen.
|
| Neurosurgery
department | Hematology/oncology
department | P-value |
|---|
|
|
|
|
|
|---|
| Toxicity | All grades (%) | Grade 3 (%) | Grade 4 (%) | Grade 5 (%) | All grades (%) | Grade 3 (%) | Grade 4 (%) | Grade 5 (%) | All grades | Grade 3–5 |
|---|
| Leukopenia | 6 (40) | 2 (13) | 0 (0) | 0 (0) | 10 (91) | 6 (55) | 0 (0) | 0 (0) | 0.014 | 0.038 |
| Neutropenia | 6 (40) | 1 (7) | 0 (0) | 0 (0) | 10 (91) | 3 (27) | 0 (0) | 0 (0) | 0.014 | 0.28 |
| Anemia | 5 (33) | 1 (7) | 0 (0) | 0 (0) | 10 (91) | 3 (27) | 0 (0) | 0 (0) | 0.0052 | 0.28 |
|
Thrombocytopenia | 3 (20) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.24 | 1.00 |
| Oral mucositis | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 2 (18) | 0 (0) | 0 (0) | 0 (0) | 0.56 | 1.00 |
| Hepatic
toxicity | 9 (60) | 1 (7) | 0 (0) | 0 (0) | 7 (64) | 1 (9) | 0 (0) | 0 (0) | 1.00 | 1.00 |
| Renal toxicity | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (27) | 0 (0) | 0 (0) | 0 (0) | 0.28 | 1.00 |
| Pneumonia | 2 (13) | 1 (7) | 0 (0) | 1 (7) | 2 (18) | 2 (18) | 0 (0) | 0 (0) | 1.00 | 1.00 |
| Infection other
than pneumonia | 4 (27) | 0 (0) | 0 (0) | 0 (0) | 2 (18) | 0 (0) | 0 (0) | 0 (0) | 1.00 | 1.00 |
| Deep vein
thrombosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (27) | 3 (27) | 0 (0) | 0 (0) | 0.064 | 0.064 |
|
Leukoencephalopathya | 14 (93) | 3 (20) | 0 (0) | 0 (0) | 7 (63) | 0 (0) | 0 (0) | 0 (0) | 0.13 | 0.24 |
Discussion
The optimal induction and consolidation therapies
for PCNSL have not been established. Therefore, the treatment
options employed may vary depending on the treating department. In
this study, the outcomes of PCNSL patients treated at our
hematology/oncology department were better than those of PCNSL
patients treated at our neurosurgery department. The patients
treated at the hematology/oncology department received combination
therapy for the induction regimen, and their treatment involved a
higher RDI of MTX. The number of high-dose MTX-based courses also
differed, resulting in a higher relative treatment intensity for
MTX in the hematology/oncology group. A higher CR rate and a more
significant reduction in the SPD were achieved after induction
therapy in the hematology/oncology group than in the neurosurgery
group. For consolidation therapy, the patients treated at the
neurosurgery department only received WBRT, while those treated at
the hematology/oncology department received a variety of therapies,
including auto-HSCT and tirabrutinib maintenance therapy.
PCNSL is a hematological malignancy. Hematological
malignancies are sensitive to chemotherapy, and sufficiently
intensive chemotherapy may improve the outcomes of patients with
hematological malignancies. Treating-department-related differences
in outcomes have been reported in the treatment of AYA with ALL.
Among AYA with ALL, the patients treated with pediatric protocols
exhibited better outcomes than those treated with adult protocols
(14,15). Pediatric protocols include greater
cumulative doses of steroids, vincristine, and L-asparaginase. In
addition to differences in protocol design and dose intensity,
potential variations in the degree of adherence to the scheduled
drug treatment outlined in the chosen treatment protocol between
pediatric and adult oncologists may also affect outcomes (2,16). In
the latter studies, treatment by adult oncologists tended to be
more spaced out between chemotherapy courses than treatment by
pediatric oncologists, which led to differences in treatment
intensity. In our study, comparing the outcomes of PCNSL patients
treated at the hematology/oncology department with those of PCNSL
patients treated at the neurosurgery department, we found
differences in the relative dose and treatment intensity of MTX
during induction therapy and in the choices of consolidation
therapy.
The RDI is an indicator of the intensity of
chemotherapy (17). It has been
reported to have an important impact on survival outcomes in DLBCL
patients treated with R-CHOP-based chemotherapy (3,4). The
RDI of MTX was also reported to have a prognostic impact in PCNSL
patients (5). The latter study
demonstrated that an MTX RDI of >75% was associated with
increased OS. However, no previous studies used the RDI of MTX to
compare treatment intensity across treating departments. In our
study, the patients treated at the hematology/oncology department
received HD-MTX at a higher RDI of MTX and achieved better OS. In
addition to the differences in RDI, the number of courses
administered also differed between the two departments. A previous
study of DLBCL suggested that relative treatment intensity may be
valuable for assessing treatment intensity in retrospective studies
in which different numbers of chemotherapy courses were
administered (10). In this study,
the PCNSL patients treated at the hematology/oncology department
received high-dose MTX therapies at significantly higher treatment
intensities than those treated at the neurosurgery department.
Moreover, 63% of the patients in the hematology/oncology group
received combined treatment with high-dose MTX and an alkylating
agent. Some alkylating agents exhibit high CNS bioavailability
(18). Furthermore, the addition of
rituximab to MTX-based chemotherapy can improve survival,
particularly when the blood-brain barrier is disrupted (1). The combination of high-dose MTX with
other agents, including alkylating agents and rituximab, increases
the treatment intensity and improves the treatment response
(1,19,20).
Differences in treatment intensity during induction therapy may
affect CR rates and outcomes in addition to MTX dose intensity.
The consolidation therapies for PCNSL vary and
include WBRT, high-dose non-myeloablative chemotherapy,
myeloablative chemotherapy with auto-HSCT, medical maintenance, and
observation. WBRT was used as the basis of such treatment in the
past. Recently, myeloablative chemotherapy with auto-HSCT was
reported to be effective as a consolidation therapy, especially in
PCNSL patients aged 60 years or younger (21,22).
However, auto-HSCT requires special equipment for hematopoietic
stem cell harvesting and preservation and medical staff who
specialize in stem cell collection and transplantation. In
neurological diseases other than PCNSL, there are no indications
for auto-HSCT, while in hematological disease auto-HSCT is the
standard treatment for relapsed malignant lymphoma and
initially-diagnosed multiple myeloma. However, treatment experience
may influence treatment decisions. In a retrospective study
conducted in a neurosurgery department, no patients received
auto-HSCT as a consolidation therapy (23). Some patients treated at our
hematology/oncology department received BTK inhibitors as
consolidation therapy, especially patients who could not continue
receiving treatment with cytotoxic agents due to their frailty
(13). Different treatment
departments have different specialties, and different hospital
settings have been reported to lead to different treatment patterns
(24). In addition to differences
in the RDI of MTX in induction therapy, differences in the
treatment options available for consolidation therapy may affect
the outcomes of PCNSL patients.
This study had several limitations. First, it was a
single-center retrospective study. The treatments employed for
PCNSL may vary according to the treating hospital and country. The
MATRix regimen, which includes thiotepa, is widely used for
induction therapy. However, in Japan thiotepa is not covered by the
national health insurance system, except for use during auto-HSCT.
In this study, the induction therapy did not include thiotepa.
Second, the treatment era differed between the neurosurgery and
hematology/oncology groups. Until March 2018, PCNSL was diagnosed
by the neurosurgery department, and the same department managed the
initial treatment. However, combination chemotherapy after relapse
was administered by the hematology department. In 2018, discussions
between the two departments concluded that greater consistency
between the initial treatments and the treatments administered for
relapsed disease was desirable. As a result, the hematology
department has also been responsible for the initial treatment
since April 2018. Although the types of induction and consolidation
therapies were not found to be significantly related to the risk of
relapse in the multivariate analysis, the differences between the
treatment eras may still have affected the results due to advances
in supportive care and other factors. Third, the treatment
intensity of the induction therapies was assessed based on the RDI
of MTX; therefore, the analysis did not include changes in
treatment intensity due to the use of combinations of chemotherapy
agents. Patients treated at an MTX RDI of ≥75% tended to receive
combination chemotherapies as induction therapies (Table SI). Although prospective
comparative studies of the additional therapies used in conjunction
with MTX are lacking, the use of MTX in combination with other
alkylators, including procarbazine, is recommended (1,25). In
addition to the MTX RDI, combination therapy may have contributed
to the increased intensity of induction therapy at our
hematology/oncology department.
In conclusion, the PCNSL patients treated at our
hematology/oncology department had better outcomes than those
treated at our neurosurgery department, although it should be noted
that these groups were treated in different periods. The higher RDI
of MTX and use of combination therapies during induction therapy
may have been responsible for the better prognoses seen in the
patients treated at the hematology/oncology department. As with the
differences in outcomes observed between pediatrics and hematology
departments for AYA with ALL, the treatment outcomes of PCNSL
patients may differ between neurosurgery and hematology/oncology
departments. Further multicenter studies are warranted to elucidate
the outcomes of PCNSL patients treated at neurosurgery and
hematology/oncology departments.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Japan Society for the Promotion
of Science (JSPS) (KAKENHI grant no. 21K16248 to HH).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH designed the study, acquired, analyzed and
interpreted the data, and wrote the original manuscript. TO
designed the study, acquired and interpreted the data, and
critically revised the manuscript. JF, YH, SM and TM acquired and
analyzed the data and critically revised the manuscript. NN and TS
designed the study, critically revised the manuscript and
supervised the study. HH and TO confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Wakayama Medical University (approval no. 3629). All
procedures performed in studies involving human participants were
carried out in accordance with the ethical standards of the
relevant institutional and/or national research committees and with
the 1964 Declaration of Helsinki and its later amendments or
comparable ethical standards. Informed consent was obtained in the
form of opt-out via a website.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schaff LR and Grommes C: Primary central
nervous system lymphoma. Blood. 140:971–979. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stock W: Adolescents and young adults with
acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ
Program. 2010:21–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirakawa T, Yamaguchi H, Yokose N, Gomi S,
Inokuchi K and Dan K: Importance of maintaining the relative dose
intensity of CHOP-like regimens combined with rituximab in patients
with diffuse large B-cell lymphoma. Ann Hematol. 89:897–904. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bataillard EJ, Cheah CY, Maurer MJ,
Khurana A, Eyre TA and El-Galaly TC: Impact of R-CHOP dose
intensity on survival outcomes in diffuse large B-cell lymphoma: A
systematic review. Blood Adv. 5:2426–2437. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farhi J, Laribi K, Orvain C, Hamel JF,
Mercier M, Galy AS, Clavert A, Rousselet MC, Tanguy-Schmidt A,
Hunault-Berger M and Moles-Moreau MP: Impact of front line relative
dose intensity for methotrexate and comorbidities in
immunocompetent elderly patients with primary central nervous
system lymphoma. Ann Hematol. 97:2391–2401. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferreri AJ, Blay JY, Reni M, Pasini F,
Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi
A, et al: Prognostic scoring system for primary CNS lymphomas: The
international Extranodal lymphoma study group experience. J Clin
Oncol. 21:266–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abrey LE, Ben-Porat L, Panageas KS,
Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta
M and DeAngelis LM: Primary central nervous system lymphoma: The
Memorial Sloan-Kettering cancer center prognostic model. J Clin
Oncol. 24:5711–5715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morris PG, Correa DD, Yahalom J, Raizer
JJ, Schiff D, Grant B, Grimm S, Lai RK, Reiner AS, Panageas K, et
al: Rituximab, methotrexate, procarbazine, and vincristine followed
by consolidation reduced-dose whole-brain radiotherapy and
cytarabine in newly diagnosed primary CNS lymphoma: Final results
and long-term outcome. J Clin Oncol. 31:3971–3979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abrey LE, Batchelor TT, Ferreri AJ,
Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A,
Soussain C, DeAngelis LM, et al: Report of an international
workshop to standardize baseline evaluation and response criteria
for primary CNS lymphoma. J Clin Oncol. 23:5034–5043. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiroi T, Hosoi H, Kuriyama K, Murata S,
Morimoto M, Mushino T, Nishikawa A, Tamura S and Sonoki T: An
evaluation based on relative treatment intensity in older patients
treated with reduced-dose R-THP-COP therapy for diffuse large
B-cell lymphoma: A multicenter retrospective cohort study. J
Geriatr Oncol. 14:1013962023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondo E, Ikeda T, Goto H, Nishikori M,
Maeda N, Matsumoto K, Kitagawa H, Noda N, Sugimoto S and Hara J:
Pharmacokinetics of thiotepa in high-dose regimens for autologous
hematopoietic stem cell transplant in Japanese patients with
pediatric tumors or adult lymphoma. Cancer Chemother Pharmacol.
84:849–860. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okamura T, Hosoi H, Matsufusa T, Akagi Y,
Iwamoto R, Kosako H, Murata S, Mushino T, Murata SI and Sonoki T:
Tirabrutinib maintenance therapy for a patient with high-dose
methotrexate-ineligible primary central nervous system lymphoma.
Ann Hematol. 101:1379–1381. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boissel N, Auclerc MF, Lheritier V, Perel
Y, Thomas X, Leblanc T, Rousselot P, Cayuela JM, Gabert J, Fegueux
N, et al: Should adolescents with acute lymphoblastic leukemia be
treated as old children or young adults? Comparison of the French
FRALLE-93 and LALA-94 trials. J Clin Oncol. 21:774–780. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stock W, La M, Sanford B, Bloomfield CD,
Vardiman JW, Gaynon P, Larson RA and Nachman J; Children's Cancer
Group, : Cancer and Leukemia Group B studies: What determines the
outcomes for adolescents and young adults with acute lymphoblastic
leukemia treated on cooperative group protocols? A comparison of
children's cancer group and cancer and leukemia group B studies.
Blood. 112:1646–1654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiffer CA: Differences in outcome in
adolescents with acute lymphoblastic leukemia: A consequence of
better regimens? Better doctors? Both? J Clin Oncol. 21:760–761.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lepage E, Gisselbrecht C, Haioun C, Sebban
C, Tilly H, Bosly A, Morel P, Herbrecht R, Reyes F and Coiffier B:
Prognostic significance of received relative dose intensity in
non-Hodgkin's lymphoma patients: Application to LNH-87 protocol.
The GELA (Groupe d'Etude des Lymphomes de l'Adulte). Ann Oncol.
4:651–656. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferreri AJ, Cwynarski K, Pulczynski E,
Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosée PL, Schorb
E, et al: Chemoimmunotherapy with methotrexate, cytarabine,
thiotepa, and rituximab (MATRix regimen) in patients with primary
CNS lymphoma: Results of the first randomisation of the
international Extranodal lymphoma study group-32 (IELSG32) phase 2
trial. Lancet Haematol. 3:e217–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasenda B, Ferreri AJ, Marturano E, Forst
D, Bromberg J, Ghesquieres H, Ferlay C, Blay JY, Hoang-Xuan K,
Pulczynski EJ, et al: First-line treatment and outcome of elderly
patients with primary central nervous system lymphoma (PCNSL)-a
systematic review and individual patient data meta-analysis. Ann
Oncol. 26:1305–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoang-Xuan K, Bessell E, Bromberg J,
Hottinger AF, Preusser M, Rudà R, Schlegel U, Siegal T, Soussain C,
Abacioglu U, et al: Diagnosis and treatment of primary CNS lymphoma
in immunocompetent patients: Guidelines from the European
association for neuro-oncology. Lancet Oncol. 16:e322–332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferreri AJM, Cwynarski K, Pulczynski E,
Fox CP, Schorb E, La Rosée P, Binder M, Fabbri A, Torri V,
Minacapelli E, et al: Whole-brain radiotherapy or autologous
stem-cell transplantation as consolidation strategies after
high-dose methotrexate-based chemoimmunotherapy in patients with
primary CNS lymphoma: Results of the second randomisation of the
international Extranodal lymphoma study group-32 phase 2 trial.
Lancet Haematol. 4:e510–e523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Houillier C, Taillandier L, Dureau S, Lamy
T, Laadhari M, Chinot O, Moluçon-Chabrot C, Soubeyran P, Gressin R,
Choquet S, et al: Radiotherapy or autologous stem-cell
transplantation for primary CNS lymphoma in patients 60 years of
age and younger: Results of the intergroup ANOCEF-GOELAMS
randomized phase II PRECIS study. J Clin Oncol. 37:823–833. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sasaki N, Kobayashi K, Saito K, Shimizu S,
Suzuki K, Lee J, Yamagishi Y, Shibahara J, Takayama N, Shiokawa Y
and Nagane M: Consecutive single-institution case series of primary
central nervous system lymphoma treated by R-MPV or high-dose
methotrexate monotherapy. Jpn J Clin Oncol. 50:999–1008. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel AM, Ali O, Kainthla R, Rizvi SM,
Awan FT, Patel T, Pan E, Maher E, Desai NB, Timmerman R, et al:
Primary central nervous system lymphoma: A real-world comparison of
therapy access and outcomes by hospital setting. Neurooncol Pract.
9:183–192. 2022.PubMed/NCBI
|
|
25
|
Calimeri T, Steffanoni S, Gagliardi F,
Chiara A and Ferreri AJM: How we treat primary central nervous
system lymphoma. ESMO Open. 6:1002132021. View Article : Google Scholar : PubMed/NCBI
|