Introduction
Gastric cancer (GC) is the fifth most prevalent
cancer globally and the fourth leading cause of all cancer-related
deaths; in 2020, GC caused 769,000 deaths worldwide (1). Furthermore, GC has the highest
incidence rate in East Asia; however, its age-adjusted occurrence
has decreased over the past quarter-century (2). Nevertheless, there is a rise in the
number of new cases in Japan, attributed to the aging society
(2,3).
Mismatch repair (MMR) deficiency (dMMR) plays an
important role in the oncogenic process and in determining the
properties of cancer cells in various cancer types (4,5). In
addition, the detection of dMMR holds significant diagnostic value
for Lynch syndrome (6) and serves
as a predictor of the efficacy of immune checkpoint inhibitors
(ICI) (7). The microsatellite
instability (MSI) test and immunohistochemistry (IHC) for MMR
proteins are established methods for determining the dMMR status; a
positive outcome is determined by the presence of high-frequency
microsatellite instability (MSI-H) or MMR protein loss,
respectively (6). The concordance
rate between these two tests exceeds 90% (8) in colorectal cancer (CRC). However,
some studies have demonstrated discrepancies between them, which
might be attributable to the methods used (e.g., microsatellite
marker, antibody), the type of cancer, and the genes responsible
(9,10). For example, numerous Lynch syndromes
identified in patients with uterine cancer are reportedly caused by
the MSH6 gene, a gene prone to yielding false negative
results in MSI testing, causing discrepancies in the results
obtained from IHC testing (9).
Therefore, we posit that procuring datasets detailing the
concordance rates between these tests for each cancer type, coupled
with an understanding of the causes of discordance, holds the
potential to enhance screening accuracy for Lynch syndrome and
facilitate the appropriate application of immune checkpoint
inhibitors. GC is classified as one of the cancers associated with
Lynch syndrome according to the Revised Bethesda guidelines
(11); it is also the third most
frequent MSI-H cancer among unresectable/recurrent solid cancers,
followed by endometrial cancer and small intestine cancer in Japan
(12). This underscores the
importance of conducting dMMR testing for GC in clinical settings.
However, the comprehensive investigation of the concordance rate
between these two testing methods and cases of discordance in GC
remains a relatively sparse area of study (13,14).
To our knowledge, till date, none of the studies have directly
compared the results of IHC with those of MSI testing using the
Promega panel, which is used worldwide and serves as a companion
diagnostic for ICI in Japan (12).
Therefore, we performed MSI testing using the Promega panel and
conducted IHC for MMR proteins, which allowed the elucidation of
their concordance rate. Furthermore, discordant cases were explored
in detail.
Materials and methods
Patients
A total of 489 consecutive patients who had
undergone gastrectomy at the Department of Digestive Tract and
General Surgery, Saitama Medical Center, Saitama Medical
University, between April 2005 and May 2016, were included in the
analyses. Clinicopathological data were obtained from the medical
records of the patients. The study was approved by the local ethics
committee of Saitama Medical Center, Saitama Medical University
(approval numbers: 860, 924-VIII, 925, and 926-V), and Saitama
Cancer Center (approval number: 1079).
Histological evaluation
All tissue samples were fixed in neutralized 10%
formalin after resection and embedded in paraffin using standard
procedures. Subsequently, serial sections of 4- and 10-µm thickness
were prepared from each specimen. The 4-µm-thick sections were used
for hematoxylin-eosin staining and IHC (15), whereas the 10-µm-thick sections were
used for DNA extraction. The 489 GC cases were pathologically
diagnosed according to the Japanese Classification of Gastric
Carcinoma (16). The main
histological subtypes were as follows: well-differentiated tubular
adenocarcinoma (tub1), moderately differentiated tubular
adenocarcinoma (tub2), papillary adenocarcinoma (pap), solid-type,
poorly differentiated adenocarcinoma (por1), nonsolid-type,
poorly-differentiated adenocarcinoma (por2), signet-ring cell
carcinoma (sig), and mucinous carcinoma (muc). Histological
subtypes, that are not predominant, characterized by the
pathologist as comprising over 10% of the tumor were defined as
mixed components. In addition, gastric carcinomas were divided into
differentiated and undifferentiated types according to the Nakamura
classification (17). The
differentiated and undifferentiated types are almost equivalent to
the intestinal and diffuse types in Lauren's classification.
Immunohistochemistry (IHC)
IHC was performed using the 4-µm-thick GC sections
and a DAKO EnVision FLEX system (Agilent Technologies, Santa Clara,
CA, USA) according to the manufacturer's protocol (15). The anti-hMLH1 (clone ES05, DAKO,
dilution 1:50), anti-hMSH2 (clone FE11, DAKO, 1:50), anti-hMSH6
(clone EP49, DAKO, 1:50), and anti-hPMS2 (clone EP51, DAKO, 1:40)
antibodies were used for detecting MMR proteins.
A case was denoted as MMR-D if a defect was present
in one or more MMR proteins in tumor cell nuclei, whereas it was
denoted as MMR-P if all MMR proteins were normal in tumor cell
nuclei.
DNA extraction
Genomic DNA for MSI testing was extracted from the
10-µm-thick formalin-fixed paraffin-embedded specimens prepared
from the resected tumors and corresponding normal gastric tissue
using the QIAamp DNA FFPE tissue kit (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's instructions.
Microsatellite instability test
To assess MSI status, DNA from normal and tumor
tissues was evaluated using an MSI test kit (FALCO Biosystems,
Kyoto, Japan) as previously reported (12). This kit utilizes the same marker
regions as the Promega Panel. MSI testing was performed by a single
Clinical Laboratory Improvement Amendments-certified and College of
American Pathologists-accredited laboratory (FALCO Biosystems).
In the cases of discordant MSI and IHC findings,
in-house MSI analysis was performed using seven microsatellite
markers (BAT25, BAT25, NR21, NR24, D2S123, D5S346, and D17S250).
Using a fluorescence-based PCR method, amplified products obtained
with primers of these marker regions were analyzed using the
GenomeLab GeXP Genetic Analysis System (Beckman Coulter Inc., Brea,
CA, USA) and CEQ8000 software (Beckman Coulter Inc.) as described
previously (18).
The MSI status was classified as MSI-H in the
presence of two or more unstable markers, MSI-low (MSI-L) in the
presence of only one unstable marker, and microsatellite stable
(MSS) in the absence of unstable markers. The markers used in each
MSI test are listed in Table SI.
Primer information for in-house MSI analysis is shown in Table SII (primer information for the MSI
test kit is not available). Reassessment of MSI test results was
carried out by KA and OS.
MLH1 promoter methylation
analysis
In the case of MSI-H or MMR-D GC, the methylation
status of the MLH1 promoter region was analyzed using the
real-time PCR-based method MethyLight. The methylation status of a
sample was considered positive at a cut-off percentage of
methylated reference volume >10%, following a previous report
(19). Primer information for
MLH1 promoter methylation analysis is shown in Table SII. The Methylight method is a
semiquantitative analysis of C to T conversion at target sites
using bisulfite-treated DNA. Therefore, primers are also used only
for sequences after bisulfite treatment.
Statistical analysis
All data were analyzed using SPSS version 22 (SPSS
Inc, Chicago, IL, USA). Comparisons among continuous and
categorical variables were made using the Mann-Whitney and Fisher's
exact tests, respectively. Fisher's exact test was performed
separately to determine whether MSI-H GC is more frequently in the
elderly (>70), in female, in the lower region, in type 2, and in
early stage (stage I and II) compared to MSS. The tests are 2×2 for
each category (or categories) and for the other, MSI-H and MSS.
P-values <0.01 (two-sided) were considered statistically
significant.
Results
Patient characteristics and MSI
status
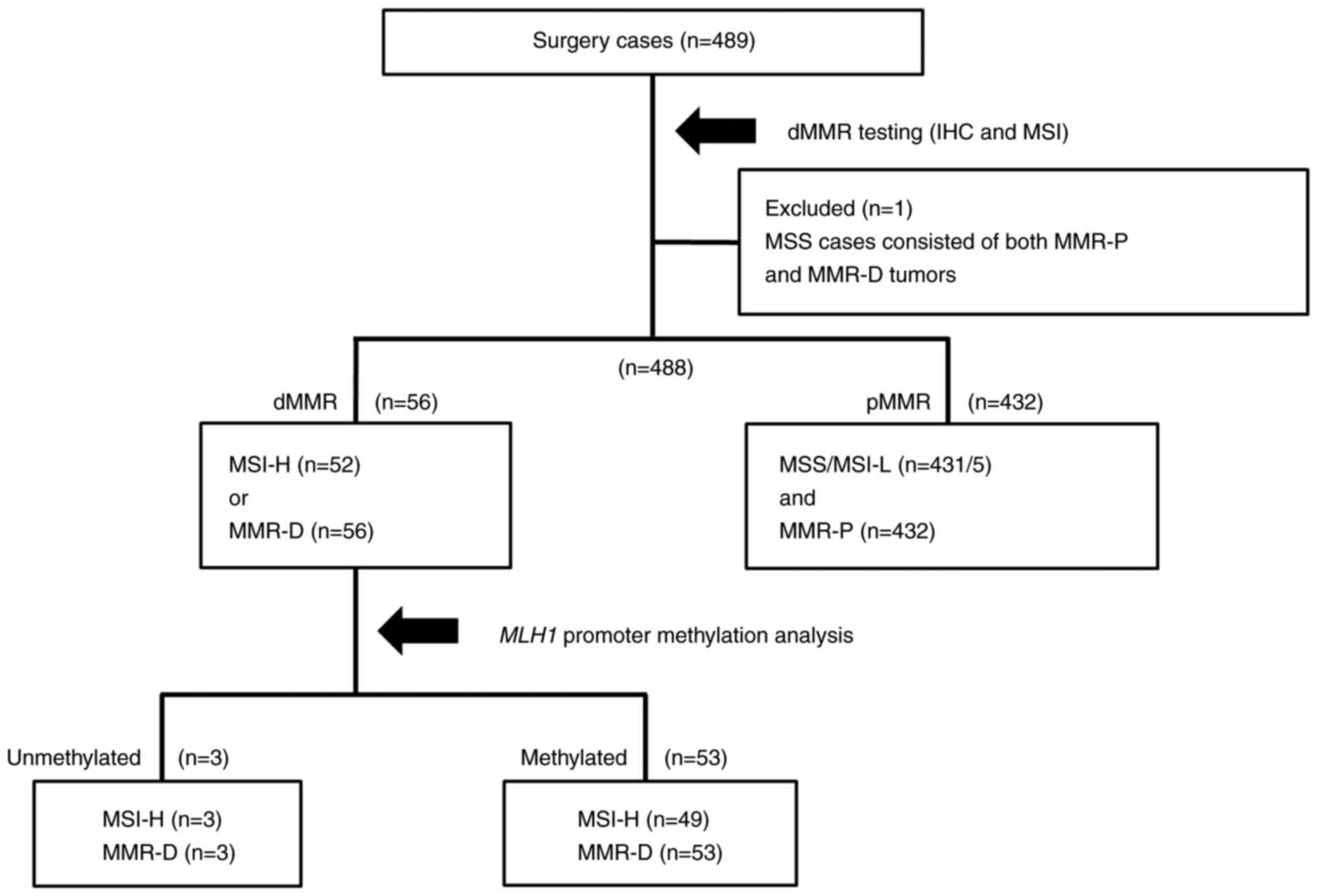
The flowchart of dMMR testing is shown in Fig. 1. Cases with MMR-D or MSI-H were
considered dMMR, whereas those with MMR-P and MSS/MSI-L were
considered proficient MMR (pMMR). One case was excluded because it
contained biphasic regions of MMR-P and MMR-D within the tumor and
could not be evaluated separately for MSI status in the two regions
(Fig. S1), leaving a total of 488
cases. Of the 488 GC cases, 52 (10.6%), 5 (1.0%), and 431 (88.3%)
were determined to be MSI-H, MSI-L, and MSS, respectively,
according to the MSI test; in contrast, 56 (11.5%) and 432 (88.5%)
were MMR-D and MMR-P, respectively, according to IHC. In our
previous study, germline genetic testing for MMR genes to diagnose
Lynch syndrome was performed on three dMMR and Unmethylated cases
(Fig. 1). As a result, a pathogenic
variant of MLH1 gene was detected in one case, and this
patient was diagnosed with Lynch syndrome (15).
The clinicopathological characteristics of MSI
status are shown in Table I.
Similar to MMR-D (15), MSI-H GC
occurred more frequently at >70 vs. ≤70 years and Lower region,
and was often Type 2 vs. the other regions and other types,
respectively, and was often Stage I and II vs. stages III–IV,
compared with the MSS/MSI-L group (Table I); however, there was no association
with histological classifications. Stage IV cases encompass
instances determined to be Stage IV through postoperative
pathology, cases of palliative surgery, and cases of debulking
surgery performed considerably earlier. None of the patients
underwent neoadjuvant therapy.
 | Table I.Clinicopathological findings. |
Table I.
Clinicopathological findings.
| Parameter | MSS/MSI-L (n=436;
89.3%) | MSI-H (n=52;
10.7%) | P-value |
|---|
| Age, years |
|
|
|
|
<41 | 9 | 0 |
|
|
41-50 | 20 | 0 |
|
|
51-60 | 63 | 1 |
|
|
61-70 | 153 | 15 |
|
|
71-80 | 148 | 26 |
|
|
81-90 | 37 | 10 |
|
|
>90 | 6 | 0 |
|
| Age, median
(range) | 69 (22–99) | 76 (56–87) |
<0.01a |
| Sex, n (%) |
|
|
<0.01b |
|
Female | 99 (22.7) | 29 (55.8) |
|
|
Male | 337 (77.3) | 23 (44.2) |
|
| Tumor location, n
(%) |
|
|
<0.01b |
|
Upper | 145 (33.3) | 5 (9.6) |
|
|
Middle | 132 (30.1) | 8 (15.4) |
|
|
Lower | 159 (36.5) | 39 (75) |
|
| Macroscopic type, n
(%) |
|
|
<0.01b |
| Type
0 | 33 (7.6) | 2 (3.8) |
|
| Type
1 | 30 (6.9) | 1 (1.9) |
|
| Type
2 | 120 (27.5) | 31 (59.6) |
|
| Type
3 | 170 (39.0) | 16 (30.8) |
|
| Type
4 | 63 (14.4) | 0 (0) |
|
| Type
5 | 20 (4.6) | 2 (3.8) |
|
| Histological
classification, n (%) |
|
|
>0.99b |
|
Differentiated type | 221 (50.7) | 28 (53.8) |
|
|
Undifferentiated type | 215 (49.3) | 24 (46.2) |
|
|
Immunohistochemistry, n (%) |
|
|
<0.01b |
|
MMR-P | 432 (99.1) | 0 (0) |
|
|
MMR-D | 4 (9) | 52 (100) |
|
| TNM stage, n
(%) |
|
|
<0.01b |
| Stage
I | 34 (7.8) | 12 (23.1) |
|
| Stage
II | 118 (27.1) | 19 (36.5) |
|
| Stage
III | 176 (40.4) | 19 (36.5) |
|
| Stage
IV | 108 (24.8) | 2 (3.8) |
|
Comparison of MSI and IHC tests for
MMR proteins
A comparative analysis of the results of MSI testing
and IHC revealed the presence of four discordant cases. MSI and IHC
results demonstrated concordance in 484 of the 488 cases (99.2%).
The sensitivity and specificity of the MSI test in relation to IHC
were 92.9 and 100%, respectively (Table II). The positive rates for BAT25,
BAT26, NR21, NR24, and Mono27 in MSI-H GCs were 94.2% (49/52), 100%
(52/52), 92.3% (48/52), 92.3% (48/52), and 100% (52/52),
respectively (Table III). Thus,
most MSI-H cases showed positivity for all five markers (45/52:
86.5%, Table SIII); however, the
combination of BAT26 and MONO27 enabled the identification of MSI-H
GCs with 100% sensitivity and 99.5% specificity.
 | Table II.Performance of MSI test versus IHC
test as reference test. |
Table II.
Performance of MSI test versus IHC
test as reference test.
| Group | MMR-D | MMR-P | Total |
|---|
| MSI-H | 52 | 0 | 52 |
| MSS/MSI-L | 4 | 432 | 436 |
| Total | 56 | 432 | 488 |
 | Table III.Number of unstable cases per
loci in MSI-H and MSI-L. |
Table III.
Number of unstable cases per
loci in MSI-H and MSI-L.
| Group | BAT25 | BAT26 | NR21 | NR24 | MONO27 |
|---|
| MSI-H | 49/52 | 52/52 | 48/52 | 48/52 | 52/52 |
| MSI-L | 3/5 | 1/5 | 0/5 | 0/5 | 1/5 |
Histological subtypes and MSI
status
According to classification by predominant
histological subtype, 80.8% of MSI-H GCs were classified as tub2 or
por1 (Table IV). 451 cases
contained some kind of mixed components in addition to the
predominant histological subtype (Table SIV). Within tub2 and por1
predominant subtypes, upon analyzing mixed components, MSI-H GCs
exhibited the presence of mainly both tub2 and por1 (regardless of
which was predominant, Table
SV).
 | Table IV.Correlation between histological
subtypes and MSI status. |
Table IV.
Correlation between histological
subtypes and MSI status.
| Histological
subtypes | MSS/MSI-L, n
(%) | MSI-H, n (%) | Ratio of MSI-H,
% |
|---|
| Pap | 12 (2.8) | 0 (0) | 0.0 |
| Tub1 | 54 (12.4) | 6 (11.5) | 10.0 |
| Tub2 | 151 (34.6) | 22 (42.3) | 12.7 |
| Por1 | 100 (22.9) | 20 (38.5) | 16.7 |
| Por2 | 86 (19.7) | 2 (3.8) | 2.3 |
| Sig | 22 (5.0) | 1 (1.9) | 4.3 |
| Muc | 11 (2.5) | 1 (1.9) | 8.3 |
Cases with discordant results between
MSI testing and IHC
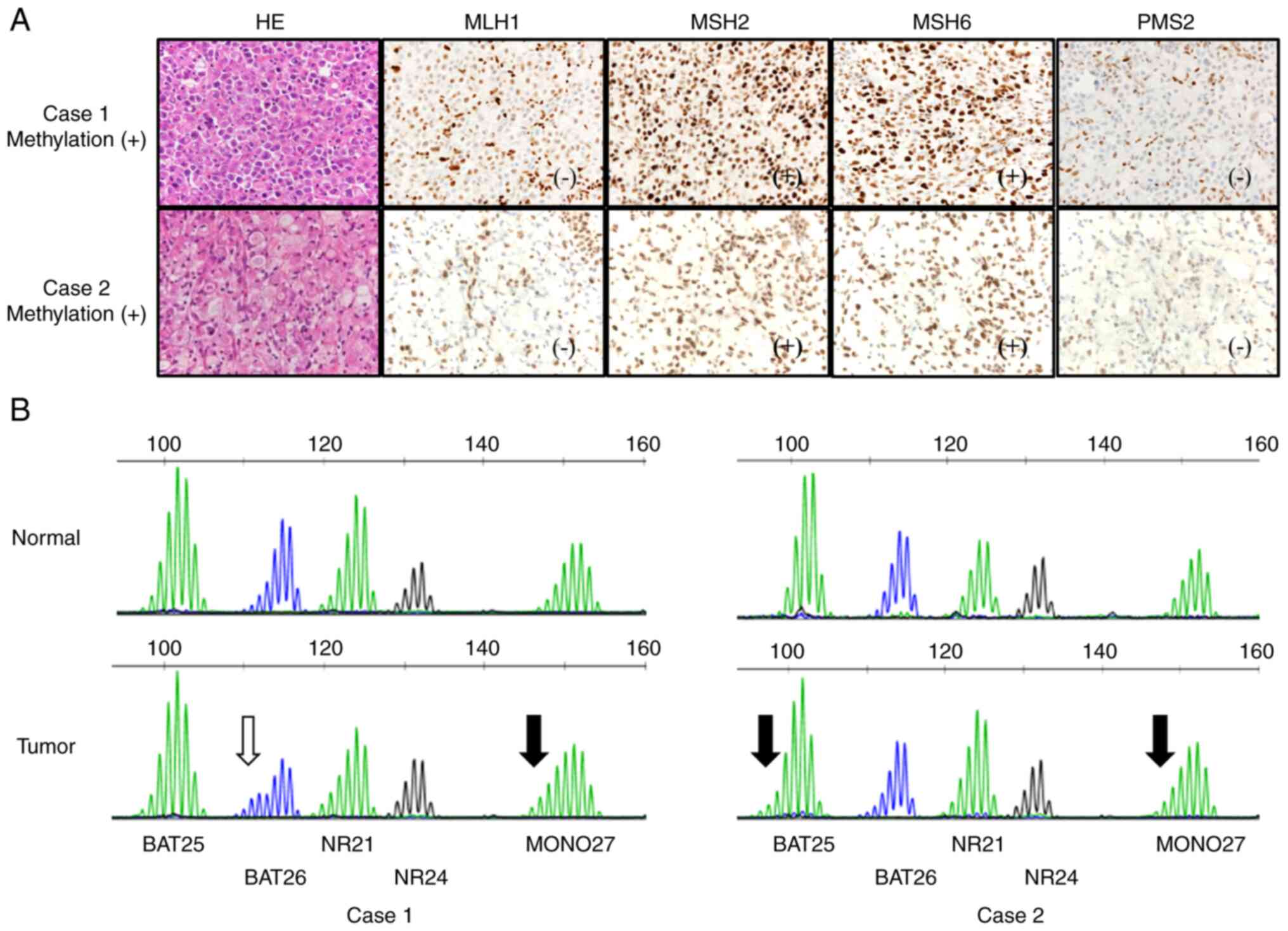
Four GC cases showed discrepancies between MSI
testing (Promega panel) and IHC results. For all cases, the MSI
waveform was visually reassessed by two genetics-specialized
doctors (KA and OS) proficient in observing such patterns; two
cases may be considered MSI-H. The MSI test waveforms and IHC
results for these cases are shown in Fig. 2. In Case 1, MLH1 was sparsely
negative, PMS2 was negative, MLH1 promoter region
methylation was positive, and the histological subtype was por1.
Initially determined to be MSI-L owing to the presence of
instability solely in BAT26, a reassessment prompted suspicion of
MSI-H because of the instability also observed in MONO27. In Case
2, MLH1 and PMS2 were negative, MLH1 promoter region
methylation was positive, and the histological subtype was sig.
Initially determined to be MSS, a subsequent reassessment based on
visual inspection indicated instability of BAT25 and MONO27. Thus,
these two cases may be classified as MSI-H.
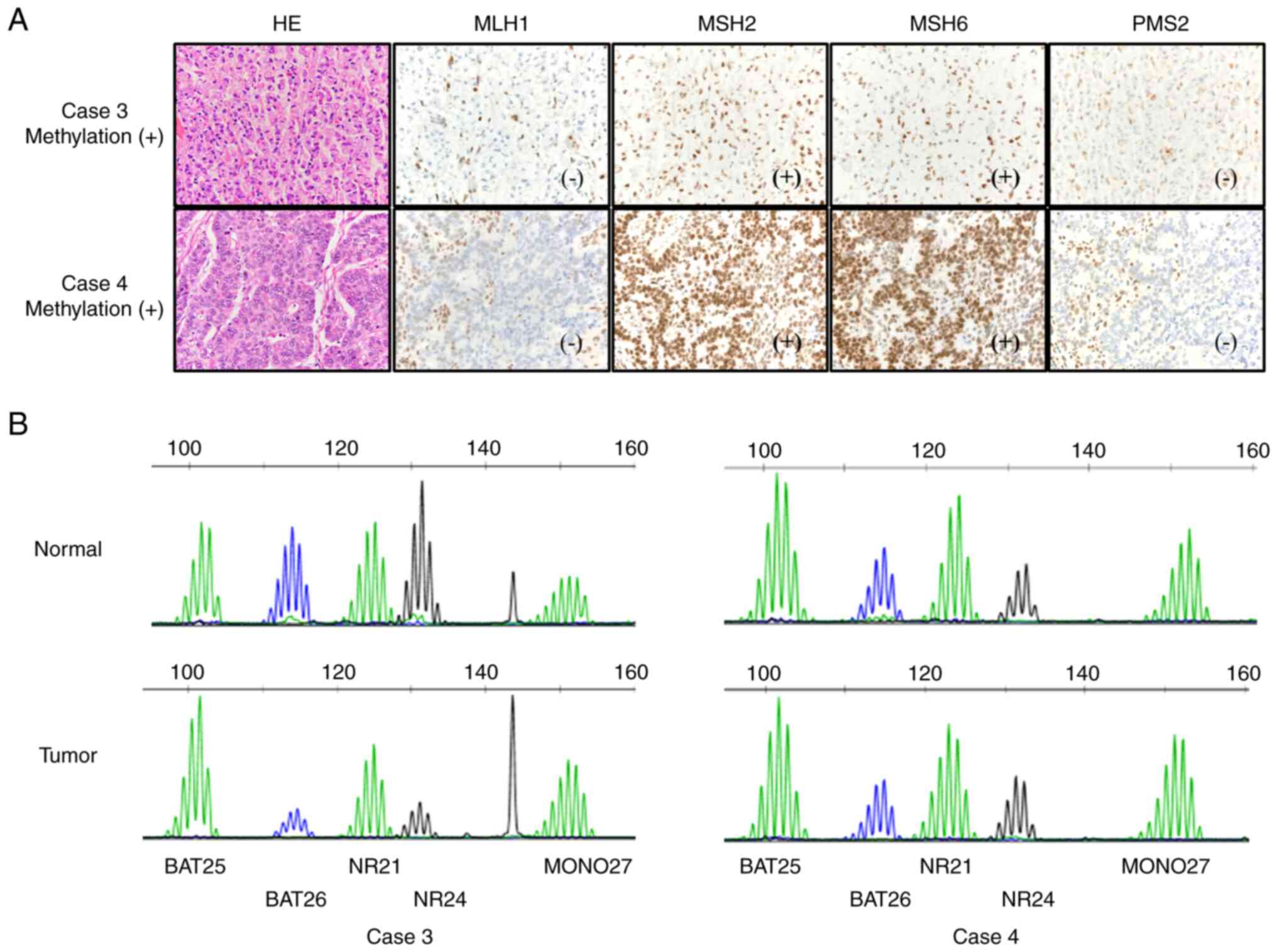
Other cases remained as MSS even after visual
inspection (Fig. 3). Next, an
in-house MSI test involving three dinucleotide repeat markers
(D2S123, D5S346, and D17S250) was performed for these two cases. In
Case 3, MLH1 and PMS2 were negative, MLH1 promoter region
methylation was positive, and the histological subtype was sig.
Case 4 was similar to Case 3; however, the histological type was
tub2 (Fig. 3). In Case 3, the
in-house MSI test detected changes in the dinucleotide repeats
(D5S346 and D2S123). Conversely, in Case 4, the evaluation of NR24
was revised as ‘unstable’, whereas D2S123 was changed. Thus, both
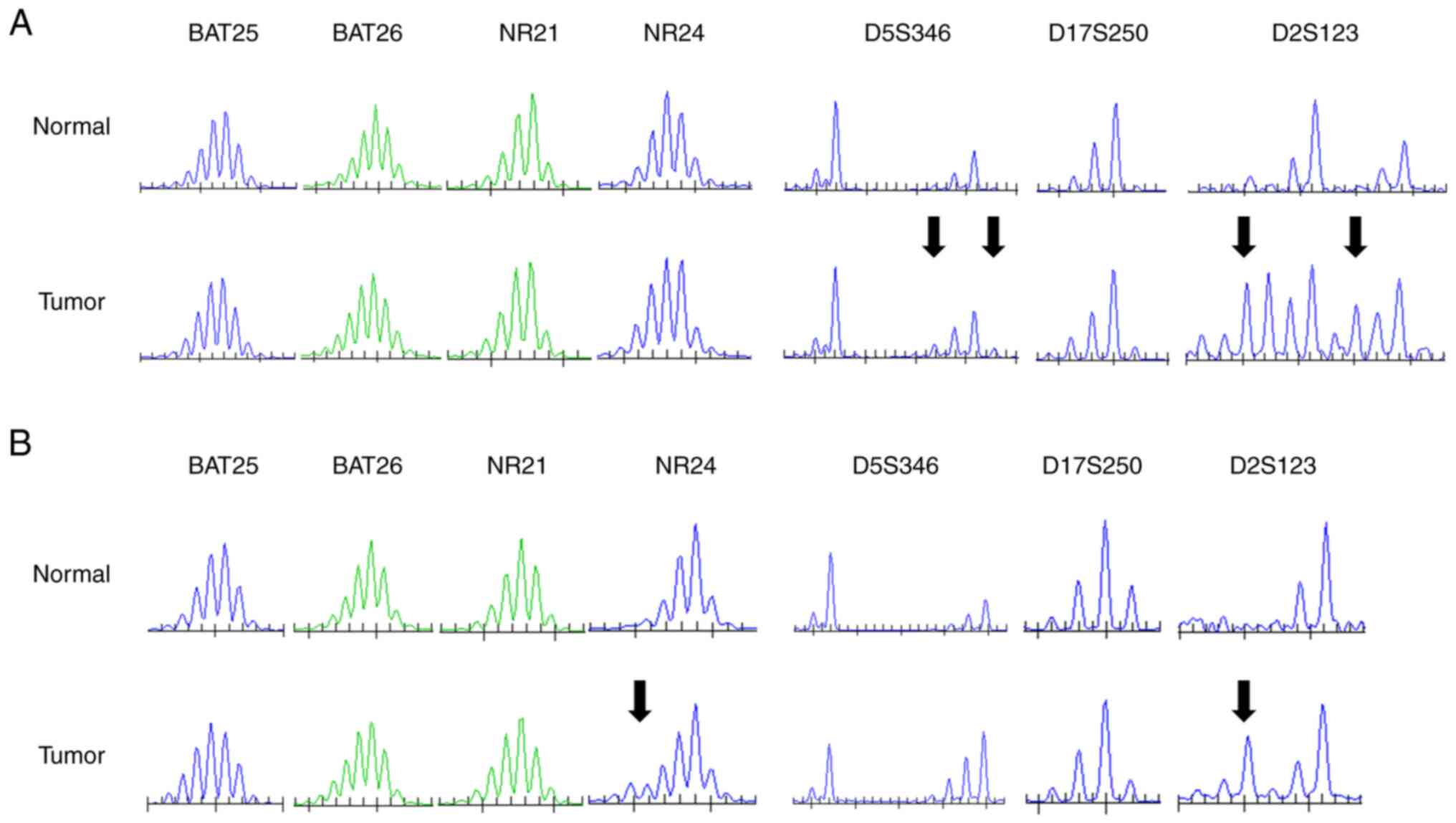
might be possibly classified as MSI-H (Fig. 4). Several MSS cases were analyzed
using the in-house MSI tests; however, no dinucleotide repeats
changes were observed (Fig.
S2).
Discussion
Determining dMMR status holds immense significance
for immunotherapy effectiveness and Lynch syndrome diagnosis. The
two major methods for making this assessment are the MSI test and
IHC for MMR proteins. However, the concordance rate between the
results of these methods in GC remains unclear. In the present
study, we investigated this aspect in consecutive series of 488 GC
cases, constituting the largest series for a comparative study
involving MSI and IHC. Our findings revealed a concordance rate of
99.4%, with sensitivity and specificity of 92.9 and 100%,
respectively. This rate surpasses the rates reported in previous
studies (13,14), which may be attributable to the
different antibodies and microsatellite markers used in each study,
thereby affecting the results. The Bethesda panel (11), comprising two mononucleotide markers
and three dinucleotide markers, has been a common choice in
previous studies. However, subsequent research has demonstrated
that five mononucleotide markers (such as in the Promega panel)
exhibit more sensitivity and are preferable (12). In our study, all five markers were
positive in 86.5% (45/52) of MSI-H cases; particularly, BAT26 and
MONO27 showed 100% sensitivity. Therefore, all MSI-H GCs can be
identified using only these two markers, streamlining the MSI test
in GC. In this regard, incorporating MONO27 may have increased the
sensitivity of the MSI test in this study and contributed to the
high concordance rate observed with the IHC test, as MONO27 was not
included in previous studies (13,14).
In contrast, the frequency of MSI-L was 1.0% (5/489), consistent
with the results of previous studies that indicated lower
frequencies of MSI-L in MSI tests comprising mononucleotide markers
(20–22), as opposed to those encompassing
dinucleotide markers (23,24).
In this study, the frequency of MSI-H was 10.7%
among all GC cases. According to investigations conducted in East
Asian countries using universal tumor screening of consecutive
patients, the frequency of MSI-H GC ranged from 8.2% to 17.7%
(14,25,26).
The frequency of MSI-H GC in our study was slightly lower than the
frequencies reported in previous studies in Japan (25,26),
which may be attributed to the male-female ratio and age
distribution, in addition to the different markers used for
identifying MSI-H GC, as discussed previously. Previous reports
have indicated that MSI-H GC is more common in elderly women and in
the lower stomach (25), consistent
with the trends observed in this study.
Regarding histopathological findings, the Por1
subtype exhibited the highest frequency in MSI-H GCs (20/120,
16.7%) among histological subtypes; however, within MSI-H GC, the
tub2 subtype was most common (22/52, 42.3%). MSI-H cancers are
enriched in the por1 subtype in GC and CRCs (25,27,28).
Regardless of their individual predominance, it is plausible that
MSI-H GCs possess an inherent tendency to contain components of
both tub2 and por1 (28). MSI-H GCs
have been reported to be frequently found in the pap subtype
(25); however, our results depict
a scarcity of cases of the pap subtype and no MSI-H GCs in this
subtype. This discrepancy might be related to the fact that GC of
the papillary type is often resected endoscopically, owing to its
propensity for early-stage presentation (29). The relationship between histological
subtypes and dMMR status may need to be analyzed in a larger number
of cases and not limited to surgical specimens. Recent studies
using Artificial intelligence to predict MSI from histopathological
images may potentially compensate for errors in MSI and IHC tests
(30).
There were four cases with discordant results
between the MSI and IHC tests. All of these cases exhibited MSS or
MSI-L/MMR-D patterns with a loss of MLH1/PMS2 and MLH1
promoter hyper-methylation. The high incidence of MSS and loss of
MLH1/PMS2 expression in these discordant cases were similar to
those reported in previous studies (31). The MSI test is prone to false
negatives depending on the amount and proportion of tumor in the
specimen and the type of causative MMR gene (8,9,32).
Among these discordant cases, three were of poorly differentiated
GCs, whereas one was classified as well-differentiated.
Additionally, none of the cases showed a single loss of MSH6
or PMS2, which are the genes prone to false negatives in MSI
tests (8). To explore the
underlying causes of these discordant results, we conducted a close
reevaluation of the waveforms of the MSI test. As a result, two of
the cases were potentially regarded as MSI-H. However, the other
two cases remained as MSS. Additional MSI tests that included three
dinucleotide markers were conducted to further confirm the results.
As a result, instability was detected in the dinucleotide repeat
region (mainly D2S123). Mononucleotide markers are generally more
sensitive in the West (21,22). However, compared with data from
Western countries, several reports from Asia suggest that the
dinucleotide region is often unstable in GCs and that D2S123 is
particularly sensitive (33,34).
Although the involvement of Helicobacter pylori is suspected
as a hallmark of GC in East Asia, its status as a causative factor
remains uncertain because some reports have shown no relationship
between H. pylori and MSI (35). Furthermore, the changes observed
during the evaluation of NR24 in in-house MSI tests may be due to
the reagents and equipment used. Specifically, in the MSI test
(Promega panel), all regions were amplified using multiplex PCR and
measured together. Conversely, the in-house MSI test was conducted
using PCR with only NR21 and NR24, which may have led to the
increased sensitivity observed.
The high intratumor heterogeneity of GC may also be
related to this discrepancy (36).
In uterine cancer, a heterogeneous MLH1 promoter methylation
state is exhibited within tumor tissues (37) and there are cases of MSS even with
the loss of MLH1/PMS2 and MLH1 promoter hypermethylation (38). Cases of MLH1 promoter
hypermethylation with small changes in mononucleotide markers and
large changes in dinucleotide markers have also been reported
(39). These trends are similar to
those observed in the discordant cases in the present study and are
presumed to be possible phenomena in cases of MLH1 promoter
hypermethylation. The concordance rate observed in this study
indicates that the Promega panel can yield results similar to those
achieved through the IHC test. However, the appropriate selection
of dinucleotide markers may improve the determination of dMMR
status in Japanese GC.
The current study had some limitations. First,
limited number of cases with sufficient DNA for MSI testing may
have caused selection bias. Second, the sample size of dMMR cases
was small (52 MSI-H and 56 MMR-D GCs). Nevertheless, the paucity of
reports detailing the concordance rate between the two tests for
ICI application underscores the significance of our comparative
findings for clinical practice utilization.
In recent years, the combined positive score derived
from IHC utilizing PD-L1 antibody has emerged as a frequent
biomarker for guiding the application of ICI in GCs (40). Moreover, research findings have
indicated that the efficacy of ICI monotherapy is comparable to
that of ICI plus chemotherapy in MSI-H GCs (41,42),
and the combination of nivolumab and ipilimumab may be as highly
effective as in MSI-H CRC (43,44).
Therefore, the importance of dMMR decision tests for GC is poised
to escalate in the future.
Our data show a high number of false positive
results for the MSI test. However, even with IHC testing, false
positives and false negatives can occur due to factors such as
specific mutations, treatment, sample condition, and proficiency of
the pathologist (8–10). Prior IHC testing, but if dMMR is
suspected based on other clinicopathological information, such as
older age, female, mixed tub2 and por1 subtypes, etc., the MSI test
should still be performed. In conclusion, the concordance rate
between the IHC and MSI tests was very high in the context of dMMR
determination. However, the IHC test may have a higher ability to
detect than the Promega panel. The underlying difference could stem
from genetic or geographical differences, heterogeneity in the MLH1
promoter methylation status or the type of marker used in the MSI
test. If MSI testing is used, it is advisable to consider the
utilization of a combination of dinucleotide markers, including
D2S123, and mononucleotide markers.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by the Japan Agency for Medical
Research and Development (AMED; grant no. JP18kk0205004) and JSPS
KAKENHI (grant nos. JP18K07339 and JP22K07266).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY, HI and KA conceived and designed the study. TI,
OS and HI collected the gastric cancer cases. TI, OS and NK
collected the clinical information. GY, MK, AT, KI and KA performed
a dMMR test. TA performed the histological examination. GY, TI, OS
and NK analyzed the data. GY, MK, AT, KI and KA drafted the paper.
NK, TA, HI and KA reviewed all the data and revised the final
paper. GY, TI, OS, AT, HI and KA confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The study was approved by the local ethics committee
of Saitama Medical Center, Saitama Medical University (approval
nos. 860, 924-VIII, 925 and 926-V), and Saitama Cancer Center
(approval no. 1079). Informed consent to be included in the study
was obtained from all patients by written form (860, 925 and 926-V)
and opt-out (924-VIII and 1079). All procedures followed were in
accordance the Helsinki Declaration of 1964 and later versions.
Patient consent for publication
Informed consent to be included in the study was
obtained from all patients by written form and opt-out.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Gou Yamamoto, ORCID 0000-0003-4709-7340; Dr
Kiwamu Akagi, ORCHID 0000-0002-9637-9187.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
MMR
|
mismatch repair
|
|
dMMR
|
mismatch repair deficiency
|
|
MSI
|
microsatellite instability
|
|
IHC
|
immunohistochemistry
|
|
MSI-H
|
MSI-high
|
|
CRC
|
colorectal cancer
|
|
MSI-L
|
MSI-low
|
|
MSS
|
microsatellite stable
|
|
pMMR
|
proficient MMR
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Honda M, Wong SL, Healy MA, Nakajima T,
Watanabe M, Fukuma S, Fukuhara S and Ayanian JZ: Long-term trends
in primary sites of gastric adenocarcinoma in Japan and the United
States. J Cancer. 8:1935–1942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katanoda K, Hori M, Saito E, Shibata A,
Ito Y, Minami T, Ikeda S, Suzuki T and Matsuda T: Updated trends in
cancer in Japan: Incidence in 1985–2015 and mortality in
1958–2018-A sign of decrease in cancer incidence. J Epidemiol.
31:426–450. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cortes-Ciriano I, Lee S, Park WY, Kim TM
and Park PJ: A molecular portrait of microsatellite instability
across multiple cancers. Nat Commun. 8:151802017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Germano G, Amirouchene-Angelozzi N, Rospo
G and Bardelli A: The clinical impact of the genomic landscape of
mismatch repair-deficient cancers. Cancer Discov. 8:1518–1528.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hampel H, Frankel WL, Martin E, Arnold M,
Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J,
et al: Screening for the Lynch syndrome (hereditary nonpolyposis
colorectal cancer). N Engl J Med. 352:1851–1860. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guyot D'Asnières De Salins A, Tachon G,
Cohen R, Karayan-Tapon L, Junca A, Frouin E, Godet J, Evrard C,
Randrian V, Duval A, et al: Discordance between immunochemistry of
mismatch repair proteins and molecular testing of microsatellite
instability in colorectal cancer. ESMO Open. 6:1001202021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Shi C, Eisenberg R and
Vnencak-Jones CL: Differences in microsatellite instability
profiles between endometrioid and colorectal cancers: A potential
cause for false-negative results? J Mol Diagn. 19:57–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mangold E, Pagenstecher C, Friedl W,
Fischer HP, Merkelbach-Bruse S, Ohlendorf M, Friedrichs N, Aretz S,
Buettner R, Propping P and Mathiak M: Tumours from MSH2 mutation
carriers show loss of MSH2 expression but many tumours from MLH1
mutation carriers exhibit weak positive MLH1 staining. J Pathol.
207:385–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised Bethesda guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akagi K, Oki E, Taniguchi H, Nakatani K,
Aoki D, Kuwata T and Yoshino T: Real-world data on microsatellite
instability status in various unresectable or metastatic solid
tumors. Cancer Sci. 112:1105–1113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beghelli S, de Manzoni G, Barbi S,
Tomezzoli A, Roviello F, Di Gregorio C, Vindigni C, Bortesi L,
Parisi A, Saragoni L, et al: Microsatellite instability in gastric
cancer is associated with better prognosis in only stage II
cancers. Surgery. 139:347–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seo HM, Chang YS, Joo SH, Kim YW, Park YK,
Hong SW and Lee SH: Clinicopathologic characteristics and outcomes
of gastric cancers with the MSI-H phenotype. J Surg Oncol.
99:143–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito T, Suzuki O, Kamae N, Tamaru JI, Arai
T, Yamaguchi T, Akagi K, Eguchi H, Okazaki Y, Mochiki E and Ishida
H: Comprehensive analysis of DNA mismatch repair-deficient gastric
cancer in a Japanese hospital-based population. Jpn J Clin Oncol.
51:886–894. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura K, Sugano H and Takagi K:
Carcinoma of the stomach in incipient phase: Its histogenesis and
histological appearances. Gan. 59:251–258. 1968.PubMed/NCBI
|
|
18
|
Ishikubo T, Nishimura Y, Yamaguchi K,
Khansuwan U, Arai Y, Kobayashi T, Ohkura Y, Hashiguchi Y, Tanaka Y
and Akagi K: The clinical features of rectal cancers with
high-frequency microsatellite instability (MSI-H) in Japanese
males. Cancer Lett. 216:55–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto A, Yamaguchi T, Suzuki O, Ito T,
Chika N, Kamae N, Tamaru JI, Nagai T, Seki H, Arai T, et al:
Prevalence and molecular characteristics of DNA mismatch repair
deficient endometrial cancer in a Japanese hospital-based
population. Jpn J Clin Oncol. 51:60–69. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murphy KM, Zhang S, Geiger T, Hafez MJ,
Bacher J, Berg KD and Eshleman JR: Comparison of the microsatellite
instability analysis system and the Bethesda panel for the
determination of microsatellite instability in colorectal cancers.
J Mol Diagn. 8:305–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deschoolmeester V, Baay M, Wuyts W, Van
Marck E, Van Damme N, Vermeulen P, Lukaszuk K, Lardon F and
Vermorken JB: Detection of microsatellite instability in colorectal
cancer using an alternative multiplex assay of quasi-monomorphic
mononucleotide markers. J Mol Diagn. 10:154–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathiak M, Warneke VS, Behrens HM, Haag J,
Böger C, Krüger S and Röcken C: Clinicopathologic characteristics
of microsatellite in gastric carcinomas revisited: Urgent need for
standardization. Appl Immunohistochem Mol Morphol. 25:12–24. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong SP, Min BS, Kim TI, Cheon JH, Kim NK,
Kim H and Kim WH: The differential impact of microsatellite
instability as a marker of prognosis and tumour response between
colon cancer and rectal cancer. Eur J Cancer. 48:1235–1243. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JY, Shin NR, Kim A, Lee HJ, Park WY,
Kim JY, Lee CH, Huh GY and Park DY: Microsatellite instability
status in gastric cancer: A reappraisal of its clinical
significance and relationship with mucin phenotypes. Korean J
Pathol. 47:28–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arai T, Sakurai U, Sawabe M, Honma N, Aida
J, Ushio Y, Kanazawa N, Kuroiwa K and Takubo K: Frequent
microsatellite instability in papillary and solid-type, poorly
differentiated adenocarcinomas of the stomach. Gastric Cancer.
16:505–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shigeyasu K, Nagasaka T, Mori Y, Yokomichi
N, Kawai T, Fuji T, Kimura K, Umeda Y, Kagawa S, Goel A and
Fujiwara T: Clinical significance of MLH1 methylation and CpG
island methylator phenotype as prognostic markers in patients with
gastric cancer. PLoS One. 10:e01304092015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kazama Y, Watanabe T, Kanazawa T, Tanaka
J, Tanaka T and Nagawa H: Poorly differentiated colorectal
adenocarcinomas show higher rates of microsatellite instability and
promoter methylation of p16 and hMLH1: A study matched for T
classification and tumor location. J Surg Oncol. 97:278–283. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arai T, Matsuda Y, Aida J, Takubo K and
Ishiwata T: Solid-type poorly differentiated adenocarcinoma of the
stomach: Clinicopathological and molecular characteristics and
histogenesis. Gastric Cancer. 22:314–322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin SY, Kim JH, Kook MC, Park DY, Ryu KW,
Choi IJ, Noh SH, Kim H and Lee YC: Clinicopathologic features of
submucosal papillary gastric cancer differ from those of other
differentiated-type histologies. Gut Liver. 15:44–52. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alam MR, Abdul-Ghafar J, Yim K, Thakur N,
Lee SH, Jang HJ, Jung CK and Chong Y: Recent applications of
artificial intelligence from histopathologic image-based prediction
of microsatellite instability in solid cancers: A systematic
review. Cancers (Basel). 14:25902022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugimoto R, Endo M, Osakabe M, Toya Y,
Yanagawa N, Matsumoto T and Sugai T: Immunohistochemical analysis
of mismatch repair gene proteins in early gastric cancer based on
microsatellite status. Digestion. 102:691–700. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de la Chapelle A and Hampel H: Clinical
relevance of microsatellite instability in colorectal cancer. J
Clin Oncol. 28:3380–3387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sepulveda AR, Santos AC, Yamaoka Y, Wu L,
Gutierrez O, Kim JG and Graham DY: Marked differences in the
frequency of microsatellite instability in gastric cancer from
different countries. Am J Gastroenterol. 94:3034–3038. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huo X, Xiao X, Zhang S, Du X, Li C, Bai Z
and Chen Z: Characterization and clinical evaluation of
microsatellite instability and loss of heterozygosity in
tumor-related genes in gastric cancer. Oncol Lett. 21:4302021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu G, Qin L, Zhang X, Ye G and Huang T:
Epigenetic silencing of the MLH1 promoter in relation to the
development of gastric cancer and its use as a biomarker for
patients with microsatellite instability: A systematic analysis.
Cell Physiol Biochem. 45:148–162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ho SWT and Tan P: Dissection of gastric
cancer heterogeneity for precision oncology. Cancer Sci.
110:3405–3414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Varley KE, Mutch DG, Edmonston TB,
Goodfellow PJ and Mitra RD: Intra-tumor heterogeneity of MLH1
promoter methylation revealed by deep single molecule bisulfite
sequencing. Nucleic Acids Res. 37:4603–4612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pai RK, Plesec TP, Abdul-Karim FW, Yang B,
Marquard J, Shadrach B and Roma AR: Abrupt loss of MLH1 and PMS2
expression in endometrial carcinoma: Molecular and morphologic
analysis of 6 cases: Molecular and morphologic analysis of 6 cases.
Am J Surg Pathol. 39:993–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ta RM, Hecht JL and Lin DI: Discordant
loss of mismatch repair proteins in advanced endometrial
endometrioid carcinoma compared to paired primary uterine tumors.
Gynecol Oncol. 151:401–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Bragagnoli AC, et al: First-line nivolumab plus chemotherapy versus
chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pietrantonio F, Randon G, Di Bartolomeo M,
Luciani A, Chao J, Smyth EC and Petrelli F: Predictive role of
microsatellite instability for PD-1 blockade in patients with
advanced gastric cancer: A meta-analysis of randomized clinical
trials. ESMO Open. 6:1000362021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chao J, Fuchs CS, Shitara K, Tabernero J,
Muro K, Van Cutsem E, Bang YJ, De Vita F, Landers G, Yen CJ, et al:
Assessment of pembrolizumab therapy for the treatment of
microsatellite instability-high gastric or gastroesophageal
junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and
KEYNOTE-062 clinical trials. JAMA Oncol. 7:895–902. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kawakami H, Hironaka S, Esaki T, Chayama
K, Tsuda M, Sugimoto N, Kadowaki S, Makiyama A, Machida N, Hirano
H, et al: An investigator-initiated phase 2 study of nivolumab plus
low-dose ipilimumab as first-line therapy for microsatellite
instability-high advanced gastric or esophagogastric junction
cancer (NO LIMIT, WJOG13320G/CA209-7W7). Cancers (Basel).
13:8052021. View Article : Google Scholar : PubMed/NCBI
|