Introduction
Platelets play a role in various malignancies,
particularly in hematogenous metastases (1–3). In a
previous in vivo study, we reported that platelets form
complexes with gastric cancer (GC) cells and enhance their
malignant behavior through direct contact. Malignant enhancement of
GC cells has not been observed in environments affected by the
molecules and exosomes secreted by platelets (4). Therefore, GC cell-platelet adhesion is
crucial for enhancing the malignant potential.
Various molecules on both platelets and cancer cells
have been reported to play crucial roles in cancer cell-platelet
adhesion. C-type lectin-like receptor 2 (CLEC-2) is expressed on
platelets and stimulate the Src- and Syk-mediated platelet
activation (5–7). The CLEC-2 ligand podoplanin (PDPN) is
expressed in various malignant cells, lymphatic endothelial cells,
and renal podocytes (8,9). Glycoprotein VI (GPVI) is also a major
collagen receptor on platelets and plays an important role in
collagen-induced platelet activation and aggression (10,11).
Moreover, some studies have reported that this molecule can induce
hematogenous metastases in breast and colorectal cancer by
interacting with galectin-3 (Gal-3) on cancer cells (12). Integrin αIIbβ3 is another membrane
protein abundantly expressing on the surface of platelets and is an
essential molecule for platelet activation and aggregation via
interactions with fibrinogen and other substances (13). Furthermore, in mouse models,
inhibitors of integrin αIIbβ3 have been reported to be useful in
suppressing cancer metastasis (14).
In clinical practice, some studies, including ours,
have demonstrated that substantial intraoperative blood loss
increases postoperative peritoneal dissemination in patients with
GC (15). We hypothesized that
intraoperative free GC cell-platelet contact could enhance
peritoneal dissemination. These findings prompted us to investigate
the potential therapeutic applications for suppressing peritoneal
dissemination by targeting cancer cell interactions with platelets
in GC. In this study, we examined the molecules responsible for
cancer cell-platelet interactions and their therapeutic
applications in inhibiting the development of peritoneal
dissemination in GC. We successfully demonstrated that the
GPVI-Gal-3 interaction could be involved in the promotion of GC
cell metastasis by platelets, providing a promising therapeutic
target for the prevention of peritoneal metastasis in patients with
GC.
Materials and methods
Cell lines
Two human GC cell lines were used: NUGC-3 (RRID:
CVCL_1612) and MKN74 (RRID: CVCL_2791). These cells were purchased
from the Japanese Collection of Research Bioresources Cell Bank
(Osaka, Japan) and were cultured in RPMI 1640 medium (Thermo Fisher
Scientific Inc., Waltham, MA, USA) supplemented with 100 U/ml
penicillin (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), 100
µg/ml streptomycin (Sigma-Aldrich, Merck KGaA), and 10% fetal
bovine serum (Thermo Fisher Scientific Inc.). The cells were grown
in a 5% carbon dioxide atmosphere at 37°C.
YTN16 cells were obtained from the Department of
Gastrointestinal Surgery, Graduate School of Medicine, University
of Tokyo, Japan. YTN16 is a mouse GC cell line established from p53
heterozygous knockout C57BL/6 mice (16). YTN16 cells were cultured in
high-glucose Dulbecco's modified Eagle medium (Sigma-Aldrich Japan,
Tokyo, Japan) containing 1.0 ml/l MITO (Coning Japan, Tokyo,
Japan), 10 ml/l L-glutamine, 10 ml/l penicillin/streptomycin, and
10% fetal bovine serum (FBS), on plastic dishes coated with type I
collagen solution (Iwaki Scitech Div. AGC Techno Glass Co. Ltd.
Shizuoka, Japan) in a 5% carbon dioxide atmosphere at 37°C.
Mesenchymal stem cells were provided by the Cell
Bank, RIKEN BioResource Center (Tsukuba, Japan), and were used as a
noncancer cell line for quantitative reverse
transcription-polymerase chain reaction.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cocultured GC cells
(NUGC-3, MKN74, and YTN16) using the miRNeasy Mini Kit (Qiagen,
Hilden, Germany) according to the manufacturer's instructions.
Subsequently, a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.) was used to measure the total RNA concentration
and 1 µg total RNA was reverse-transcribed using the HighCapacity
cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc.) as
per the manufacturer's instructions. Transcript levels were
quantified using the specific primer sets listed below and SYBR
Green Master Mix (Thermo Fisher Scientific Inc.). Total RNA levels
were quantified using RT-qPCR according to standard procedures. The
RT-qPCR conditions were as follows: Preheating for 10 min at 95°C;
repeating 40 cycles at 95°C for 15 sec and 60°C for 60 sec. The
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were
used as internal controls for normalization, and Gal-3 mRNA levels
were demonstrated using the 2-ΔΔCq method (17).
Primer sequences were designed using Primer3Plus
(https://www.bioinformatics.nl/cgibin/primer3plus/primer3plus.cgi)
from the most conserved region of each sequence obtained from the
National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/nuccore).
Each experiment was performed in triplicates.
The following primers were used for RT-qPCR assay:
Human Gal-3 [forward, 5′-CACCTGCACCTGGAGTCTAC-3′ and reverse,
5′-GCACTTGGCTGTCCAGAAGA-3′]; GAPDH [forward,
5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′]. Moreover, the primer sequences of
mouse Gal-3 were quoted from the previous report [forward,
5′-CAGGAAAATGGCAGACAGCTT-3′ and reverse, 5′-CCCATGCACCCGGATATC-3′]
(18).
Platelet preparation
Human platelets (hPLTs) were obtained from healthy
human volunteers, and washed platelets were purified from whole
blood as previously reported (19).
Mouse platelets (mPLTs) were obtained from C57BL/6 male mice (Japan
SLC Inc., Hamamatsu, Japan), and washed platelets were purified
from whole blood, as previously reported (20,21).
The platelets were used in various experiments
immediately after extraction. Human and mouse GC cells were
cocultured with hPLTs and mPLTs, respectively, in the functional
assays described below. This study was conducted with permission
from the Ethics Committee and Committee of Laboratory Animal
Experimentation at the University of Yamanashi, and all experiments
were performed from the viewpoint of animal welfare (approval nos.
2159 and A2-14). Platelets were adjusted to a final concentration
of 100,000 per µl and used in all experiments.
Migration and invasion assays
Migration and invasion assays were performed using
Falcon Cell Culture Inserts with 8-µm pore membranes (Corning Inc.,
Corning, NY, USA) and BioCoat Matrigel (BD Bioscience, Franklin
Lakes, New Jersey, USA), similar to our previous report (4). Briefly, the optimal cell number of
1×105 NUGC-3 cells, 5×105 MKN74 cells, and
2×105 YTN16 cells were seeded in the upper chambers in
an FBS-free medium with or without platelets, and a 10% FBS medium
was added to the lower chambers. For the suppression or stimulation
experiments, the cells were treated with various agents (inhibitors
or stimulators) and platelets. After a 24-h incubation period,
cells that had not migrated or invaded the pores were removed using
cotton swabs. Migrated or invaded cells were fixed and stained with
Diff-Quick staining reagent (Sysmex, Kobe, Japan) or Hoechst
fluorescent dye (Thermo Fisher Scientific Inc.). Cells were counted
in four independent fields at 100× magnification using a BZ-X710
All-in-One fluorescence microscope (Keyence Corp., Osaka, Japan)
and the BZ-X Analyzer Software (Keyence Corp.). Each assay was
performed in triplicate.
Reagents
The inhibitory effects of various agents on
platelet-contact-induced malignant potential were also evaluated.
The Gal-3 inhibitor GB1107 was purchased from MedChemExpress
(Monmouth Junction, NJ, USA), and a 10 µM solution was used. rat
antimouse-GPVI monoclonal therapeutic antibody JAQ1 (M011-0) and
its negative control, rat IgG, were purchased from EMFRET Analytics
& Co. KG (Wurzburg, Germany). They were then diluted in
phosphate-buffered saline (PBS) at a concentration of 10 µg/ml as
working solutions for preincubation with platelets for 10 min
before each assay. Samples were diluted in PBS. The
Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP), an integrin-blocking peptide, was
purchased from MedChemExpress and a 500-µM solution was used.
Cobalt hematoporphyrin (Co-HP; 1.53 µM) a CLEC-2 suppressant, was
prepared in our laboratory as previously described (22).
In vivo peritoneal dissemination mouse
model
We used 6-week-old male C57BL/6 mice (Japan SLC
Inc., Hamamatsu, Japan) as the peritoneal dissemination mouse model
since the commercially available anti-GPVI antibody is the only
available antimouse monoclonal antibody. They were housed in a
clean, temperature-controlled cage environment with a 12-h
light-dark cycle. The mice were provided with free access to a
regular laboratory chow diet and water. The experiment consisted of
five groups of six mice, randomly allocated to each group by animal
technicians who were not directly involved in the study. The
researchers were blinded to the treatment groups. A total of
2×105 YTN16 cells in 500 µl of Hanks' balanced salt
solution [HBSS(−), Fujifilm Wako Pure Chemical Corporation, Osaka,
Japan] were then intraperitoneal injected into mice on day 0 in the
non-treatment (NT) group. In the mPLT group, we injected mouse
platelets into YTN16 cells. The mPLTs were adjusted to a final
concentration of 100,000/µl and were used in all experiments. In
suppression experiments, mice were injected with the inhibitors
JAQ1, GB1107, or both, in addition to YTN16 cells and mPLTs, into
the peritoneal cavity. After intraperitoneal injection, the mice
were housed in separate cages. Physical condition and body weight
were monitored weekly. After 5 weeks, the mice were sacrificed and
their peritoneal dissemination was examined. For anesthesia, a
mixture of medetomidine hydrochloride (0.3 mg/kg), midazolam (4
mg/kg), and butorphanol tartrate (5 mg/kg) was diluted with saline
to a volume that would provide a dose of 5 µl/g body weight and
administered by intraperitoneal injection (23–25).
Anesthesia was performed based on the aforementioned doses.
However, if the depth of anesthesia was deemed inadequate by
assessment (e.g., loss of the postural reaction and righting
reflex, the eyelid reflex, the pedal withdrawal reflex in the
forelimbs and hind limbs, and tail pinch reflex), the dosage was
increased to ensure adequate anesthetic depth. Peritoneal
dissemination was evaluated as the endpoint of tumor excision from
the host. The number and weight of tumors were measured and
subjected to further analysis. All of the mice were euthanized at
the end of the experiment. Mice were sacrificed by using
CO2 inhalation, and death was confirmed by the absence
of breathing and heartbeat. The CO2 flow rate was set to
displace 30% of the cage volume/minute. All the animal experiments
were approved by the Institutional Animal Care and Use Committee of
the University of Yamanashi, Japan (approval no. A2-14).
Image analysis of the adhesion between
GC cells and platelets
NUGC-3 cells cocultured with platelets were observed
under a laser confocal microscope (LSM-10; Olympus Corp., Tokyo,
Japan). Platelets were incubated with JAQ1 for 15 min before being
cocultured with NUGC-3 cells (platelets treated with IgG under the
same conditions were used as the control).
PlasMem Bright Red (P505, Dojindo Molecular
Technologies, Inc., Tokyo, Japan) and APC Mouse Anti-Human CD42b
(BD Pharmingen, San Diego, CA, USA) were used to stain the NUGC-3
cells and platelet membranes, respectively. All reagents were used
at concentrations recommended by the manufacturer.
Statistical analysis
All quantitative values are represented as mean ±
standard error or median and were statistically analyzed using
unpaired Student's t-test and the Mann-Whitney U-test. To account
for multiple comparisons, we employed one-way ANOVA followed by
Dunnett's post-hoc test when we assumed equal-variance condition,
comparing each group with mPLT group. We also employed
Kruskal-Wallis test followed by the Steel test when we assumed
unequal-variance condition, comparing each group with mPLT group.
Statistical significance was set at P<0.05. All statistical
analyses were conducted using EZR (Saitama Medical Center, Jichi
Medical University, Saitama, Japan), a graphical user interface for
R (R Foundation for Statistical Computing, Vienna, Austria)
(26), and JMP 17 (SAS Institute
Inc., Cary, NC, USA).
Results
Investigation of responsible molecules
in the GC cells-platelet interaction
The primary objective of this study was to identify
the mechanisms involved in cancer cell-platelet adhesion in GCs
that could serve as potential therapeutic targets. Therefore, we
examined the inhibitory effects of molecules that play important
roles in the direct interaction between GC cells and platelets.
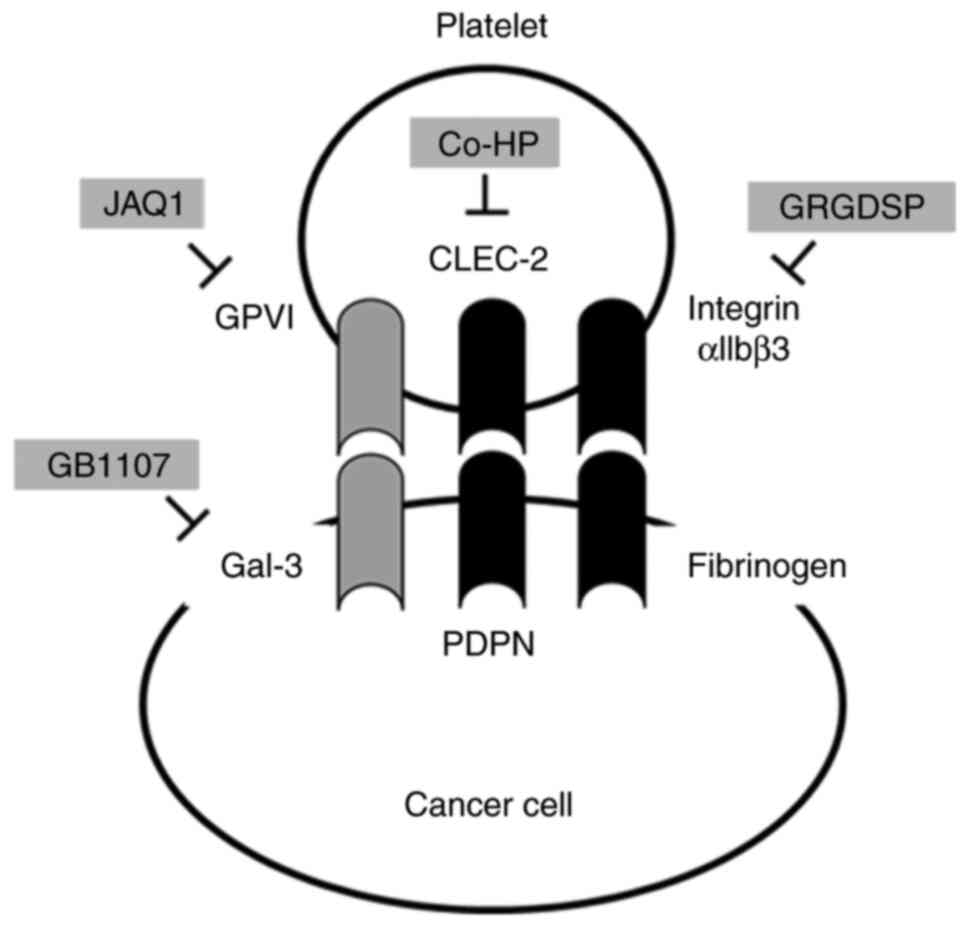
Various candidate molecules, such as platelet GPVI and its ligands
Gal-3 (12), CLEC-2, PDPN (7,27–29),
integrin, and fibrinogen (30,31),
were examined for their involvement in GC cell-platelet
interactions (Fig. 1). Human
platelets were cocultured with GC cells in all inhibitory
experiments except for the anti-GPVI experiment. In the suppression
analysis of GPVI, mPLT was used instead of hPLT for coculture with
GC cells because of the characteristic features of the rat
antimouse-GPVI monoclonal therapeutic antibody (JAQ1). The
expression of Gal-3 in NUGC-3, MKN74, and YTN16 cells was confirmed
by RT-qPCR (Fig. S1). In
exploratory analyses, various candidate molecules were examined for
their inhibitory effects on migratory ability, which was most
markedly enhanced by GC cell-platelet interactions in our previous
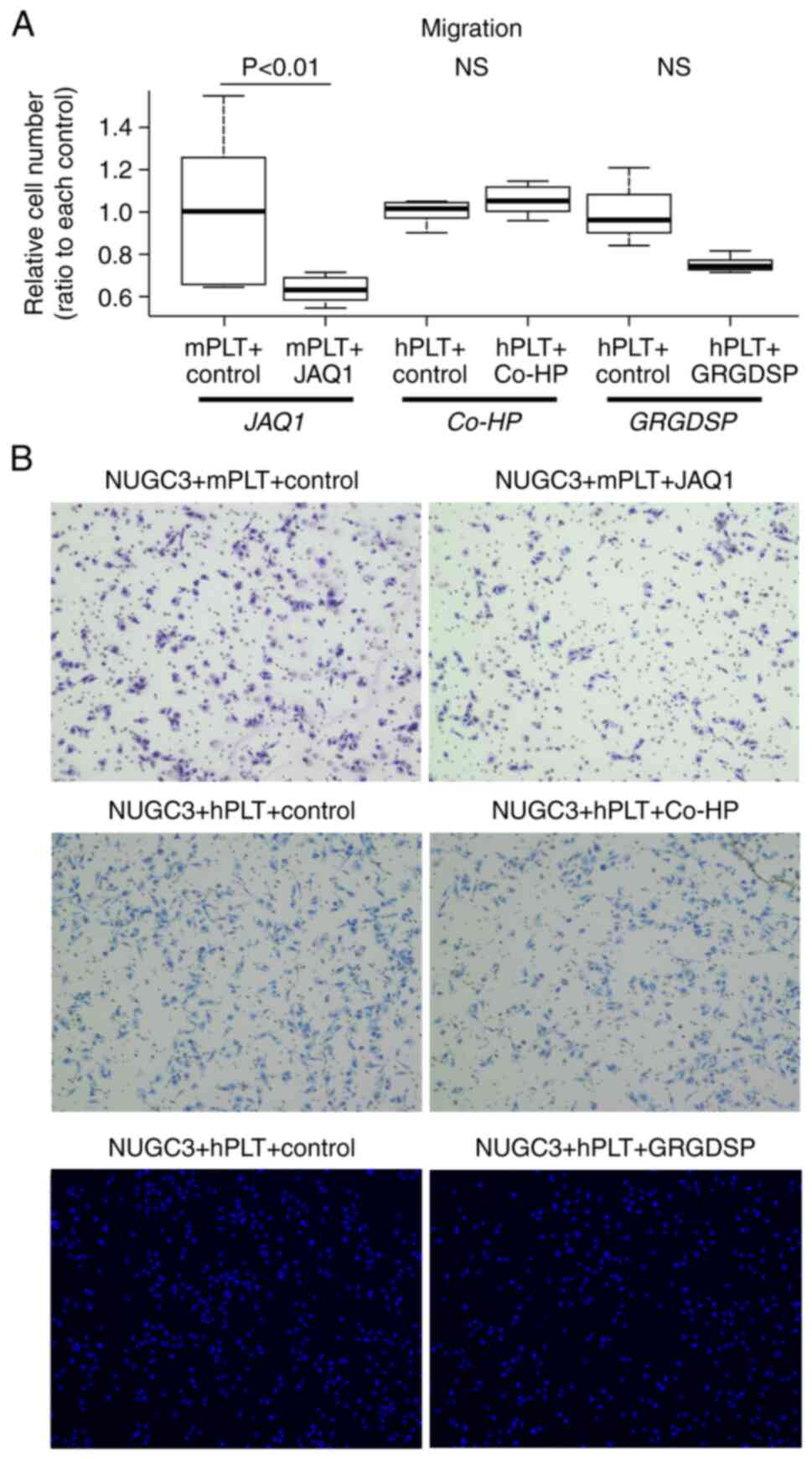
study (4). Consequently, JAQ1
markedly decreased the platelet-induced enhancement of migratory
ability by 40% (P<0.01). However, neither Co-HP, a CLEC-2
suppressant, nor Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP), an
integrin-blocking peptide, significantly suppressed this enhanced
migratory ability (Fig. 2A and B).
The effect of JAQ1 was also examined in MKN74 cells, which showed a
significant inhibition of migration (Fig. S2). Notably, the inhibitory effect
of JAQ1 on platelet adhesion to GC cells was confirmed by imaging
experiments using fluorescent staining, providing robust support
for our findings of phenotypic changes in GC cells (Fig. S3).
Inhibition of GPVI-Gal-3 in the
platelet-induced enhancement of malignant potential
Based on these findings, we focused on inhibiting
GPVI-Gal-3 contact in platelet-induced enhancement of the malignant
potential of GC cells and examined their suppressive efficacy in
vitro. YTN16, a mouse GC cell line, was used in the subsequent
experiments because the anti-GPVI antibody is a mouse monoclonal
antibody. Similar to human GC cells, the migratory and invasive
abilities were significantly increased upon coculture with mPLT in
the YTN16 experiments (P<0.001; Fig.
3A and B). JAQ1 demonstrated a more marked suppression of
enhanced malignant potential in the migration and invasion assays
than GB1107 (55% reduction, P=0.004; Fig. 3A and 63% reduction, P=0.003;
Fig. 3B). In addition, the effect
of GB1107 was limited to the invasion assays (Fig. 3B). The administration of JAQ1 and
GB1107 demonstrated the most effective inhibitory effect (69%
reduction, P=0.003; Fig. 3A, and
75% reduction, P=0.002; Fig. 3B).
Images of the migration and invasion assays are shown in Fig. 3C and D. Taken together, these in
vitro findings indicate that JAQ1 and GB1107 inhibit cancer
cell-platelet interactions and could be potential therapeutic
targets for the inhibition of GC peritoneal metastasis.
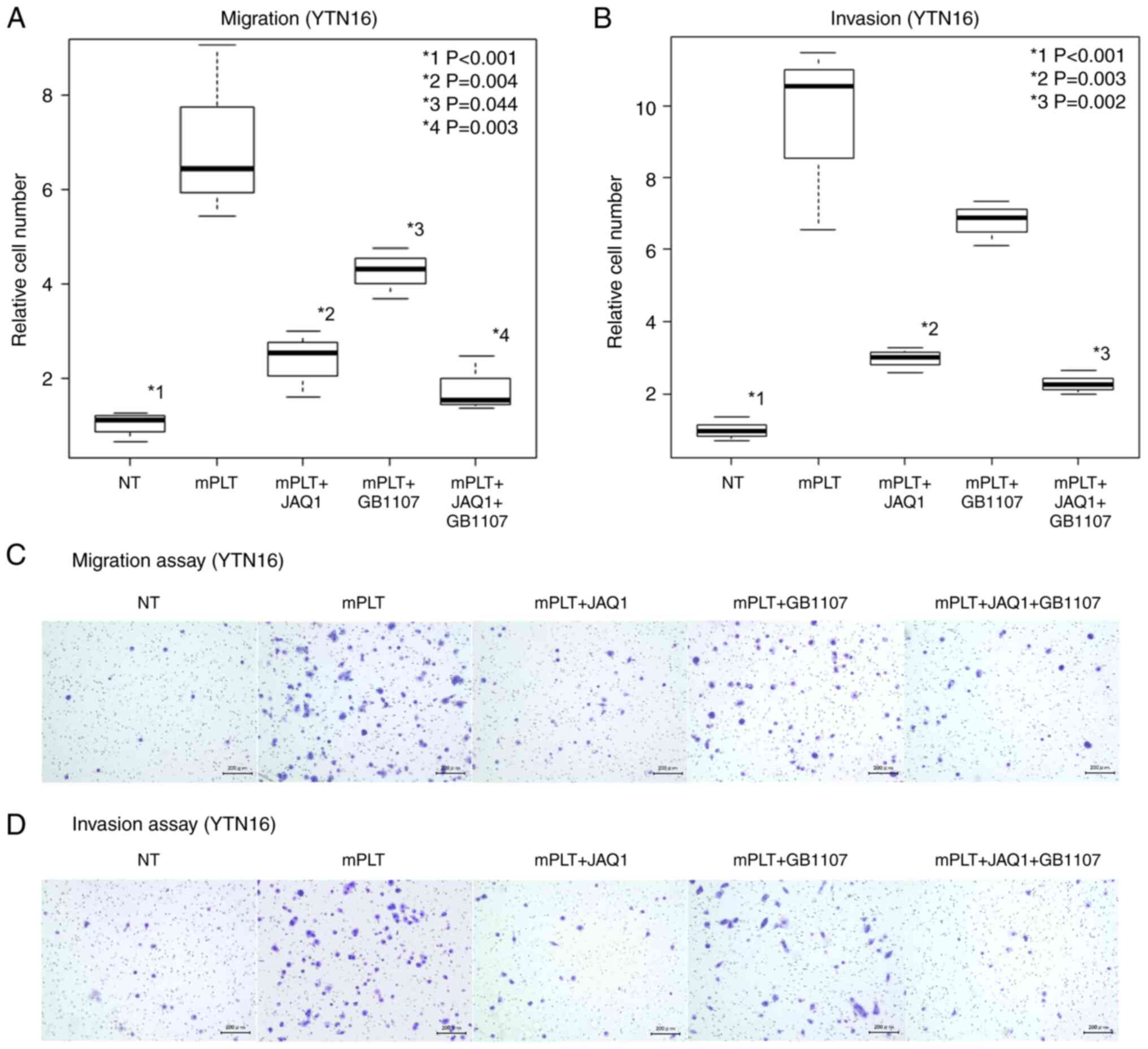 | Figure 3.Inhibitory effect of GPVI/galectin-3
inhibitors on tumor development in mouse cell lines (YTN16).
Results of the (A) migration and (B) invasion assays and (C and D)
their microscopic images show that JAQ1 significantly suppresses
both the platelet-induced enhancement of migratory and invasive
abilities of YTN16 cells (P=0.004, vs. mPLT group, and P=0.003, vs.
mPLT group, respectively; magnification, ×100). Furthermore, the
Gal-3 inhibitor GB1107 tended to suppress this enhanced ability,
especially in migration assays (P=0.044 vs. mPLT group, and P=0.071
vs. mPLT group, respectively; magnification, ×100). Gal-3,
galectin-3; hPLT, human platelets; mPLT, mouse platelets. |
Therapeutic effect in peritoneal
dissemination by inhibition of GC cells-platelet interaction
To determine the potential clinical relevance of the
impact of JAQ1- and GB1107-mediated inhibition of GC cell-platelet
interactions, we conducted in vivo experiments using the
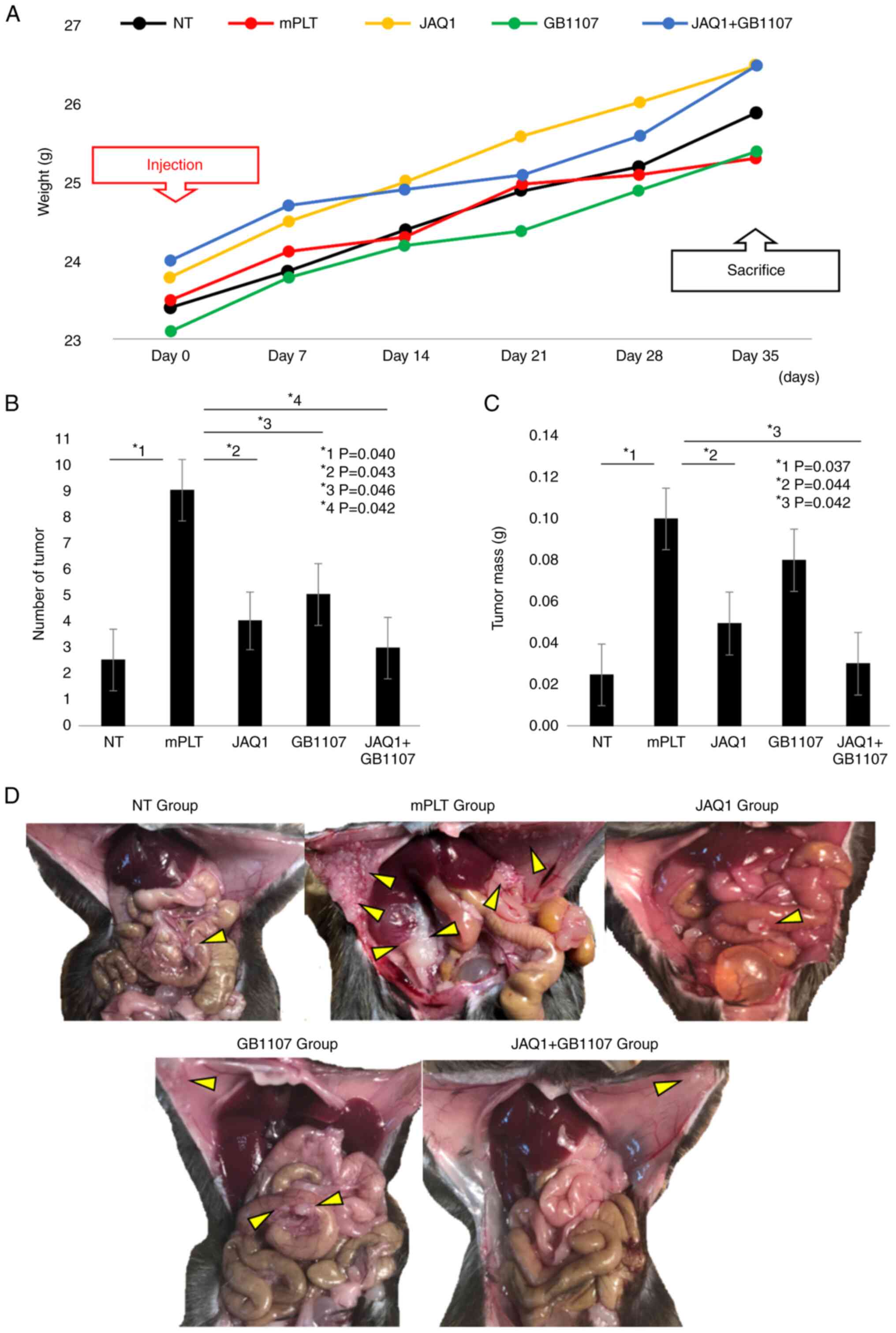
mouse model. In the mouse model, the body weight changes are shown
in Fig. 4A. The addition of
platelets along with YTN16 resulted in a significant increase in
the number of peritoneal tumors and total tumor weight (P=0.040 and
P=0.037, respectively) (Fig. 4B and
C). Coadministration of JAQ1 resulted in a marked decrease in
the number of peritoneal tumors and tumor weight compared to those
in the mPLT group (56 and 57% reduction, P=0.043 and P=0.044,
respectively) (Fig. 4B and C). The
GB1107 group showed a similar tendency, although the difference in
tumor weight was insignificant (Fig.
4C). The administration of JAQ1 and GB1107 almost completely
suppressed the platelet-induced enhancement of tumor development
during peritoneal dissemination (67 and 70% reduction,
respectively; P=0.042) (Fig. 4B-D).
These results highlight the possibility that inhibitors of GC cell
development through JAQ1/GB1107-based GC cell-platelet interactions
could provide significant therapeutic benefits for patients with
peritoneal metastases of GC, one of the most refractory
diseases.
Discussion
The prognosis of peritoneal dissemination from GC is
poor, and treatments are still being explored (32,33).
Several risk factors for peritoneal dissemination have been
reported. We previously reported that a large amount of
intraoperative bleeding increases the recurrence pattern of
peritoneal dissemination in patients with GC (15). Kamei et al also demonstrated
that the amount of intraoperative blood loss was significantly
correlated with peritoneal recurrence, and large blood loss was an
independent risk factor for peritoneal recurrence in multivariate
analysis (34). Intraoperative
blood loss is often clinically accompanied by blood transfusion and
is sometimes considered to have an immunosuppressive effect.
However, both studies demonstrated that intraoperative blood loss
did not correlate with other recurrence patterns such as nodal or
hematogenous metastases. These findings may be attributed to the
local effects of intraoperative bleeding in the peritoneal cavity.
Numerous studies have demonstrated the presence of free cancer
cells in the peritoneal cavity of patients with advanced GC.
Exfoliated cancer cells may have a greater opportunity to
perioperatively contact the blood components in the peritoneal
cavity. Among the various blood components, platelets have been
reported to promote hematogenous metastasis by interacting with
circulating tumor cells in some cancers. Therefore, we hypothesized
that intraperitoneally free cancer cells have a chance to contact
platelets via intraoperative bleeding and subsequently increase the
potential for peritoneal metastasis due to enhanced malignant
potential through cancer cell-platelet interactions. In previous
studies, platelets were found to be present around cancer cells and
on the surfaces of fibroblasts after binding to podoplanin
(35,36). Although interactions between cancer
cells, platelets, and fibroblasts are also important in the
microenvironment of peritoneal dissemination, we showed that the
malignant behavior of GC cells, especially their migratory and
invasive abilities, was drastically enhanced by direct contact with
platelets, partially via epithelial-mesenchymal transition-related
mechanisms (4).
In this study, we investigated the molecules
involved in enhancing the malignant potential associated with
direct contact between GC cells and platelets. First, we examined
the molecules potentially responsible for direct adhesion. In
vitro analysis revealed that JAQ1, an antiplatelet agent
against GPVI, inhibited the migration and invasion of GC cells.
However, no inhibitory effect was observed by the blockade of other
membrane molecules, such as CLEC-2 and integrin αIIbβ3. Moreover,
GB1107, a known ligand of GPVI, inhibited the platelet-induced
enhancement of malignant potential in the migration assay. The
Gal-3 inhibitor GB1107 reportedly inhibits the migration and
invasion of thyroid cancer cell lines (37), indicating the existence of a
Gal-3-specific direct pathway for the enhancement of malignancy in
cancer cells.
To confirm these in vivo results, we
investigated the inhibitory effects of antibodies and additional
effects of platelets on GC cells using a mouse peritoneal
dissemination model. Instead of human GC cell lines or nude mice, a
mouse GC cell line derived from C57BL/6 mice was injected
intraperitoneally into the syngeneic mice to confirm its effects
under physiological conditions. As expected from the results of the
in vitro analyses, peritoneal dissemination was
significantly increased by simultaneous administration of platelets
and GC cells. These results indicate that platelets may promote
peritoneal dissemination during intraoperative bleeding when free
cancer cells are present in the peritoneal cavity. Therefore,
surgeons should make the greatest effort to minimize the contact
between platelets and GC cells. Surgeons must reduce intraoperative
bleeding and immediately arrest the hemorrhage. Moreover,
thermocoagulation of hemorrhage-adherent areas, where
platelet-exfoliating GC cell complexes potentially exist, may
effectively prevent postoperative peritoneal dissemination.
In vivo analyses also clearly demonstrated
that the platelet-induced enhancement of peritoneal dissemination
was markedly decreased by the additional administration of each
GPVI and Gal-3 inhibitor and was almost completely suppressed by
coadministration compared to a single administration. These results
suggest that the GPVI-Gal-3 interaction between platelets and GC
cells is critical for peritoneal dissemination and is a promising
therapeutic target for dismal recurrence patterns in patients with
GC. The GPVI-Gal-3 interaction plays an important role in the
initial steps of the metastatic process; therefore, inhibition
occurs during GC surgery. GPVI plays a role in hemostasis and the
immune system; however, its hemostatic ability must be maintained
during surgery. On that point, the GPVI signaling shares several
factors in platelet activation pathways with other
hemostasis-related molecules, such as CLEC-2, and the other
molecules can function as hemostasis-related molecules even when
the function of GPVI of platelets is suppressed.
This study had some limitations. First, JAQ1, an
anti-GPVI therapeutic antibody used in this study, was developed
for mouse platelets but not human platelets. Although the
inhibitory effect of JAQ1 was confirmed in vitro using human
GC cell lines, owing to its cross-antigenicity, we only evaluated
its effect on mouse GC cell lines using a mouse peritoneal
dissemination model in vivo. Second, both antibodies, JAQ1
and GB1107, were administered intraperitoneally in the mouse model
used in this study; however, oral or intravenous administration may
be more suitable to completely inhibit GC cell-platelet complex
formation. Third, an exhaustive investigation of the side effects
of anti-GPVI, mainly in terms of its hemostatic ability, is
required for clinical applications. A similar strategy may be
applied to patients with various types of cancer; however, the
combination of the responsible molecules should be investigated for
each type of cancer.
In conclusion, both anti-GPVI and anti-Gal-3
inhibitors suppressed the platelet-induced enhancement of malignant
potential in GC cells in vitro, and inhibition of this
interaction completely suppressed the platelet-induced enhancement
of peritoneal dissemination in vivo. Thus, GPVI-Gal-3
interaction is a promising therapeutic target for preventing
peritoneal dissemination in patients with GC.
Supplementary Material
Supporting Data
Acknowledgments
The authors would like to thank Ms. Arisa Ogihara
(University of Yamanashi, Yamanashi, Japan) for their technical
assistance.
Funding
The present study was partially supported by the Japan Society
for the Promotion of Science (JSPS KAKENHI grant nos. 20K17642 and
20K09031).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
TN, RS, SF, KaS, SM, KT, KeS, HAk, YK, HAm, HK, NT,
TS, HS, MY, SN, TT, KSI and DI contributed substantially to the
conception and design of this study. TN and RS confirm the
authenticity of all the raw data. SF, KaS, SM, KT and KeS were
responsible for data acquisition, analysis and interpretation. HAk,
YK, HAm, HK, HS, MY, SN and TT were responsible for
conceptualization, methodology and data curation. TN, RS, NT and TS
were responsible for conceptualization, project administration,
enrolment of patients, investigation and writing of the manuscript.
KSI and DI were responsible for supervision. The work reported in
this paper was performed by the authors unless otherwise specified.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee and
Committee of Laboratory Animal Experimentation at the University of
Yamanashi (approval nos. 2159 and A2-14). This study was conducted
in accordance with the ethical standards of The Declaration of
Helsinki and its amendments (38).
Written informed consent for the use of samples was obtained from
all volunteers. All animals were treated in compliance with ARRIVE
2.0 guidelines (39) and the Guide
for the Care and Use of Laboratory Animals (National Institutes of
Health) (40).
Patient consent for publication
Consent for publication was obtained from all
healthy volunteers.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CLEC-2
|
C-type lectin-like receptor 2
|
|
Co-HP
|
cobalt hematoporphyrin
|
|
Gal-3
|
galectin-3
|
|
GC
|
gastric cancer
|
|
GPVI
|
glycoprotein VI
|
|
GRGDSP
|
Gly-Arg-Gly-Asp-Ser-Pro
|
|
hPLTs
|
human platelets
|
|
mPLTs
|
mouse platelets
|
|
NT
|
non-treatment
|
|
PDPN
|
podoplanin
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rothwell PM, Wilson M, Price JF, Belch
JFF, Mead TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shirai T, Inoue O, Tamura S, Tsukiji N,
Sasaki T, Endo H, Satoh K, Osada M, Sato-Uchida H, Fujii H, et al:
C-type lectin-like receptor 2 promotes hematogenous tumor
metastasis and prothrombotic state in tumor-bearing mice. J Thromb
Haemost. 15:513–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito R, Shoda K, Maruyama S, Yamamoto A,
Takiguchi K, Furuya S, Hosomura N, Akaike H, Kawaguchi Y, Amemiya
H, et al: Platelets enhance malignant behaviours of gastric cancer
cells via direct contacts. Br J Cancer. 124:570–573. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki-Inoue K, Fuller GL, García A, Eble
JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing
GD, et al: A novel Syk-dependent mechanism of platelet activation
by the C-type lectin receptor CLEC-2. Blood. 107:542–549. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki-Inoue K, Inoue O and Ozaki Y: Novel
platelet activation receptor CLEC-2: From discovery to prospects. J
Thromb Haemost. 9 (Suppl 1):S44–S55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki-Inoue K: Roles of the
CLEC-2-podoplanin interaction in tumor progression. Platelets. 1–7.
2018.(Epub ahead of print). PubMed/NCBI
|
|
8
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler
E, Alitalo K and Kerjaschki D: Angiosarcomas express mixed
endothelial phenotypes of blood and lymphatic capillaries:
Podoplanin as a specific marker for lymphatic endothelium. Am J
Pathol. 154:385–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujita N and Takagi S: The impact of
Aggrus/podoplanin on platelet aggregation and tumour metastasis. J
Biochem. 152:407–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moroi M, Jung SM, Okuma M and Shinmyozu K:
A patient with platelets deficient in glycoprotein VI that lack
both collagen-induced aggregation and adhesion. J Clin Invest.
84:1440–1445. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugiyama T, Okuma M, Ushikubi F, Sensaki
S, Kanaji K and Uchino H: A novel platelet aggregating factor found
in a patient with defective collagen-induced platelet aggregation
and autoimmune thrombocytopenia. Blood. 69:1712–1720. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mammadova-Bach E, Gil-Pulido J,
Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, Stegner D,
Remer K, Nurden P, Nurden AT, et al: Platelet glycoprotein VI
promotes metastasis through interaction with cancer cell-derived
galectin-3. Blood. 135:1146–1160. 2020.PubMed/NCBI
|
|
13
|
Ma YQ, Qin J and Plow EF: Platelet
integrin alpha(IIb)beta(3): Activation mechanisms. J Thromb
Haemost. 5:1345–1352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Liu Y, Gao Y, Shen J, Zheng S,
Wei M and Zeng X: Modified heparins inhibit integrin
alpha(IIb)beta(3) mediated adhesion of melanoma cells to platelets
in vitro and in vivo. Int J Cancer. 125:2058–2065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arita T, Ichikawa D, Konishi H, Komatsu S,
Shinozaki A, Hiramoto H, Hamada J, Shoda K, Kawaguchi T, Hirajima
S, et al: Increase in peritoneal recurrence induced by
intraoperative hemorrhage in gastrectomy. Ann Surg Oncol.
22:758–764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto M, Nomura S, Hosoi A, Nagaoka K,
Iino T, Yasuda T, Saito T, Matsushita H, Uchida E, Seto Y, et al:
Established gastric cancer cell lines transplantable into C57BL/6
mice show fibroblast growth factor receptor 4 promotion of tumor
growth. Cancer Sci. 109:1480–1492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huan Y, Caixia L and Wei Z: Expression of
galectin-3 in mouse endometrium and its effect during embryo
implantation. Reprod Biomed Online. 24:116–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satoh K, Fukasawa I, Kanemaru K, Yoda S,
Kimura Y, Inoue O, Ohta M, Kinouchi H and Ozaki Y: Platelet
aggregometry in the presence of PGE(1) provides a reliable method
for cilostazol monitoring. Thromb Res. 130:616–621. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki-Inoue K, Inoue O, Frampton J and
Watson SP: Murine GPVI stimulates weak integrin activation in
PLCgamma2-/-platelets: Involvement of PLCgamma1 and PI3-kinase.
Blood. 102:1367–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue O, Hokamura K, Shirai T, Osada M,
Tsukiji N, Hatakeyama K, Umemura K, Asada Y, Suzuki-Inoue K and
Ozaki Y: Vascular smooth muscle cells stimulate platelets and
facilitate thrombus formation through platelet CLEC-2: Implications
in atherothrombosis. PLoS One. 10:e01393572015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsukiji N, Osada M, Sasaki T, Shirai T,
Satoh K, Inoue O, Umetani N, Mochizuki C, Saito T, Kojima S, et al:
Cobalt hematoporphyrin inhibits CLEC-2-podoplanin interaction,
tumor metastasis, and arterial/venous thrombosis in mice. Blood
Adv. 2:2214–2225. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawai S, Takagi Y, Kaneko S and Kurosawa
T: Effect of three types of mixed anesthetic agents alternate to
ketamine in mice. Exp Anim. 60:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narikiyo K, Mizuguchi R, Ajima A, Shiozaki
M, Hamanaka H, Johansen JP, Mori K and Yoshihara Y: The claustrum
coordinates cortical slow-wave activity. Nat Neurosci. 23:741–753.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olajide OJ, Gbadamosi IT, Yawson EO,
Arogundade T, Lewu FS, Ogunrinola KY, Adigun OO, Bamisi O, Lambe E,
Arietarhire LO, et al: Hippocampal degeneration and behavioral
impairment during alzheimer-like pathogenesis involves glutamate
excitotoxicity. J mol Neurosci. 71:1205–1220. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki-Inoue K: Platelets and
cancer-associated thrombosis: Focusing on the platelet activation
receptor CLEC-2 and podoplanin. Blood. 134:1912–1918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hwang BO, Park SY, Cho ES, Zhang X, Lee
SK, Ahn HJ, Chun KS, Chung WY and Song NY: Platelet
CLEC2-podoplanin axis as a promising target for oral cancer
treatment. Front Immunol. 12:8076002021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasaki T, Shirai T, Tsukiji N, Otake S,
Tamura S, Ichikawa J, Osada M, Satoh K, Ozaki Y and Suzuki-Inoue K:
Functional characterization of recombinant snake venom rhodocytin:
rhodocytin mutant blocks CLEC-2/podoplanin-dependent platelet
aggregation and lung metastasis. J Thromb Haemost. 16:960–972.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang J, Li X, Shi X, Zhu M, Wang J, Huang
S, Huang X, Wang H, Li L, Deng H, et al: Platelet integrin αIIbβ3:
Signal transduction, regulation, and its therapeutic targeting. J
Hematol Oncol. 12:262019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Obermann WMJ, Brockhaus K and Eble JA:
Platelets, constant and cooperative companions of sessile and
disseminating tumor cells, crucially contribute to the tumor
microenvironment. Front Cell Dev Biol. 9:6745532021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kitayama J, Ishigami H, Yamaguchi H,
Sakuma Y, Horie H, Hosoya Y, Lefor AK and Sata N: Treatment of
patients with peritoneal metastases from gastric cancer. Ann
Gastroenterol Surg. 2:116–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang B, Rouvelas I and Nilsson M: Gastric
and gastroesophageal junction cancer: Risk factors and prophylactic
treatments for prevention of peritoneal recurrence after curative
intent surgery. Ann Gastroenterol Surg. 6:474–485. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kamei T, Kitayama J, Yamashita H and
Nagawa H: Intraoperative blood loss is a critical risk factor for
peritoneal recurrence after curative resection of advanced gastric
cancer. World J Surg. 33:1240–1246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyashita T, Tajima H, Gabata R, Okazaki
M, Shimbashi H, Ohbatake Y, Okamoto K, Nakanuma S, Sakai S, Makino
I, et al: Impact of extravasated platelet activation and
podoplanin-positive cancer-associated fibroblasts in pancreatic
cancer stroma. Anticancer Res. 39:5565–5572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaguchi T, Fushida S, Kinoshita J,
Okazaki M, Ishikawa S, Ohbatake Y, Terai S, Okamoto K, Nakanuma S,
Makino I, et al: Extravasated platelet aggregation contributes to
tumor progression via the accumulation of myeloid-derived
suppressor cells in gastric cancer with peritoneal metastasis.
Oncol Lett. 20:1879–1887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JJ, Hsu YC, Li YS and Cheng SP:
Galectin-3 inhibitors suppress anoikis resistance and invasive
capacity in thyroid cancer cells. Int J Endocrinol.
2021:55834912021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
World Medical Association, . World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Percie du Sert N, Ahluwalia A, Alam S,
Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U,
Emerson M, et al: Reporting animal research: Explanation and
elaboration for the ARRIVE guidelines 2.0. PLoS Biol.
18:e30004112020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
National Research Council (US), .
Committee for the update of the guide for the care and use of
laboratory animals: Guide for the Care and Use of Laboratory
Animals. 8th edition. National Academies Press; Washington, DC:
2011
|