Introduction
Breast cancer is the most prevalent malignant tumor
affecting women (1). The incidence
of breast cancer is currently increasing worldwide and is projected
to surpass 3 million new cases annually by 2040 (2). Related studies have demonstrated that
the incidence of breast cancer in China is rising, but the survival
rate is not high, with a 5-year survival rate of 40–60% (3,4).
Despite an increased understanding of the disease and advancements
in treatment strategies, breast cancer remains the leading cause of
cancer-related mortality among women worldwide (5). The majority of breast cancer-related
deaths are primarily attributed to metastasis. Approximately 12% of
patients with breast cancer will develop metastatic disease
(6). The most important parameters
that influence development of metastases include tumor size,
histological grade, lymphovascular spread, nodal involvement,
presence of hormonal receptors and human epidermal growth factor
receptor-2 (HER2) status (7).
Although there are various sites for metastasis, such as the lungs,
liver, bones, brain and lymph nodes, bladder metastasis is
exceedingly rare (8). Metastatic
bladder cancer includes involvement of lymph nodes beyond the
pelvis or other visceral organs. The prognosis at this stage is
poor, with <10% of patients surviving over 5 years after the
diagnosis (9).
For patients with metastatic breast cancer, 30–60%
of lesions are in the bones, 4–10% in the brain, 15–32% in the
liver and 21–32% in the lungs (10); bladder metastases are rare and
reported in the literature occasionally. Due to the rarity of
bladder metastasis from breast cancer, large-scale retrospective or
prospective cohort studies are not possible. Currently, global
coverage mainly consists of clinical research in the form of case
reports or autopsy reports. Previous literature based on autopsies
has reported a disease incidence rate as low as 2% (11). Conversely, there is a high incidence
of direct invasion of the bladder by malignant tumors originating
from peripheral organs (12).
Furthermore, there is a lack of relevant studies investigating the
treatment and its correlation with the pathological classification
of primary breast lesions. The present study describes the case of
a patient with bladder metastasis from breast cancer, providing a
comprehensive account of the clinical and pathological
characteristics, progression and course of treatment. Additionally,
a concise review of cases reported in the existing literature is
provided.
Case report
A 58-year-old woman with a diagnosis of breast
cancer and a performance status score of 0, underwent a left
radical mastectomy at Cancer Hospital of Henan University
(Zhengzhou, China) in August 2013. The post-operative pathology
revealed invasive ductal carcinoma (IDC) in the left breast, along
with metastasis to 10 of the 16 ipsilateral axillary lymph nodes
dissected. Immunohistochemical analysis (data extracted from the
medical records) revealed the positive expression of estrogen
receptor (ER), progesterone receptor (PR), HER2 and Ki-67 antigen
(1%). The post-operative pathological stage was determined as IIIc
(pT2N3M0) according to the 8th edition of the American Joint
Committee on Cancer staging system (13). Adjuvant chemotherapy with 80 mg
doxorubicin intravenous (iv) + 1.0 g cyclophosphamide (iv) on day 1
every 3 weeks for 4 cycles, followed by 130 mg docetaxel on day 1
every 3 weeks for 4 cycles, was administered after surgery. This
was followed by 40 Gy/20 fractions of local radiotherapy.
Subsequently, maintenance therapy with tamoxifen (10 mg, oral,
twice a day) was administered for 4 years. In February 2018,
multiple bone metastases were detected in the thoracic vertebrae,
ribs and clivus via a bone scan due to chest pain. Local
radiotherapy was performed on the thoracic spine, followed by oral
anastrozole treatment at a dose of 1 mg once daily for 2 years.
In April 2020, a routine follow-up examination with
computed tomography (CT) examination revealed progressive bone
metastases in the neck, chest and lumbar vertebrae. Subsequently,
endocrine treatment with fulvestrant (500 mg, every 4 weeks) was
initially performed until September 2021. In September 2021, a
urological ultrasound examination indicated hydronephrosis in the
right kidney and no apparent abnormalities in the bladder. A CT
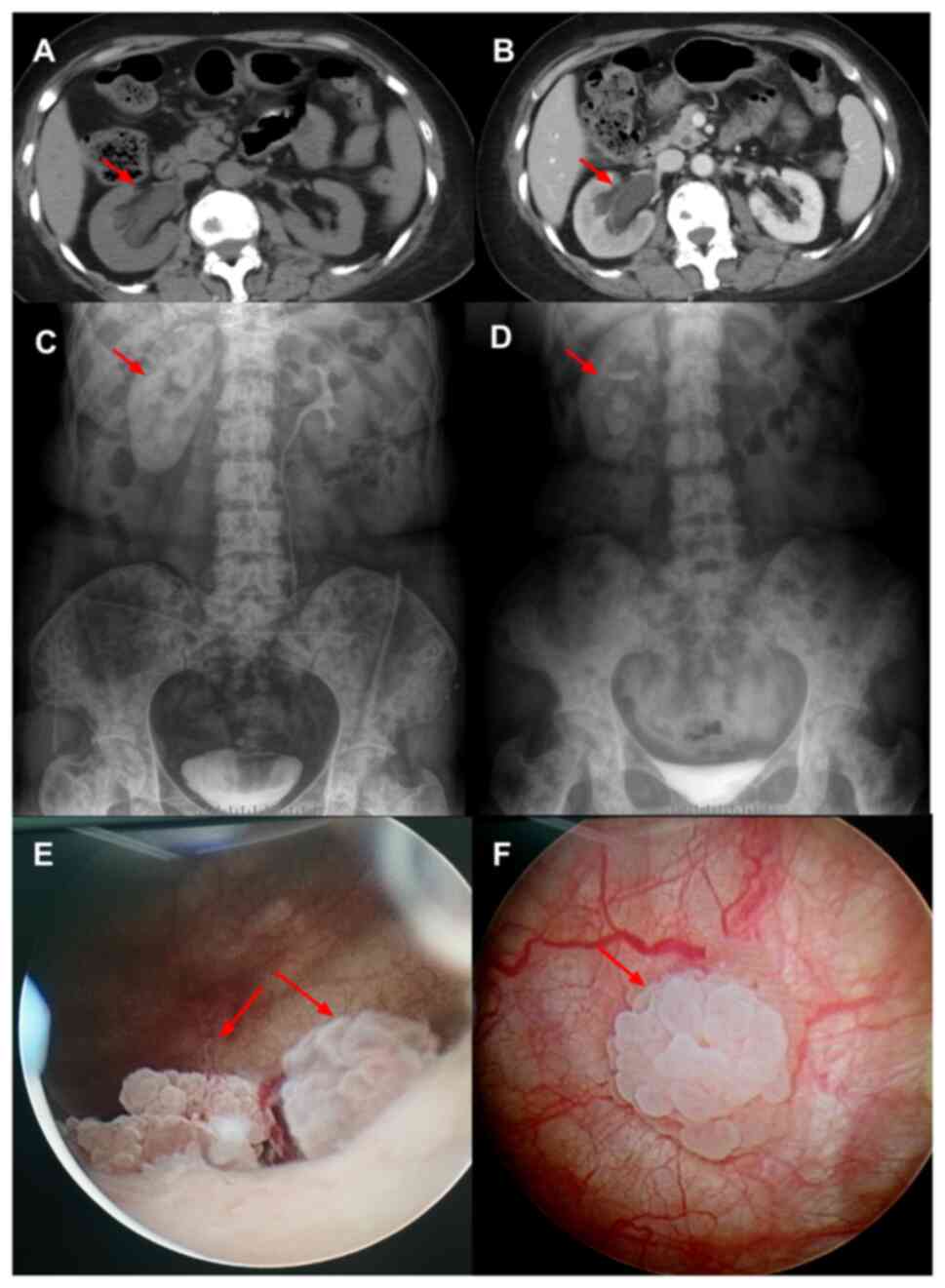
examination (Fig. 1A and B)
revealed dilation and hydronephrosis of the right renal pelvis,
calyces and upper ureter, and the bladder wall exhibited roughness.
Furthermore, it revealed no contrast agent concentration in the
ureter during excretion phase. A lower degree of arteriovenous
enhancement was observed in the right kidney in the parenchymal
phase compared with that on the contralateral side. Intravenous
pyelography (Fig. 1C and D)
demonstrated poor function of the right kidney, and a liquid level
of contrast medium was found in part of the renal pelvis with
pressure release after 50 min. There was no gross hematuria and no
urinary symptoms, such as frequent urination, urgency or dysuria,
were observed. The urine occult blood test yielded negative
results. Cystoscopy (September 2021; Fig. 1E and F) revealed that the ureteral
orifice was normal, while the right ureteral cavity was narrow at 1
cm in diameter; in addition, two raised masses were observed behind
the ureteral orifice on the left side of the bladder, with one mass
measuring ~1.0×0.8 cm and another measuring ~1.2×0.8 cm. The
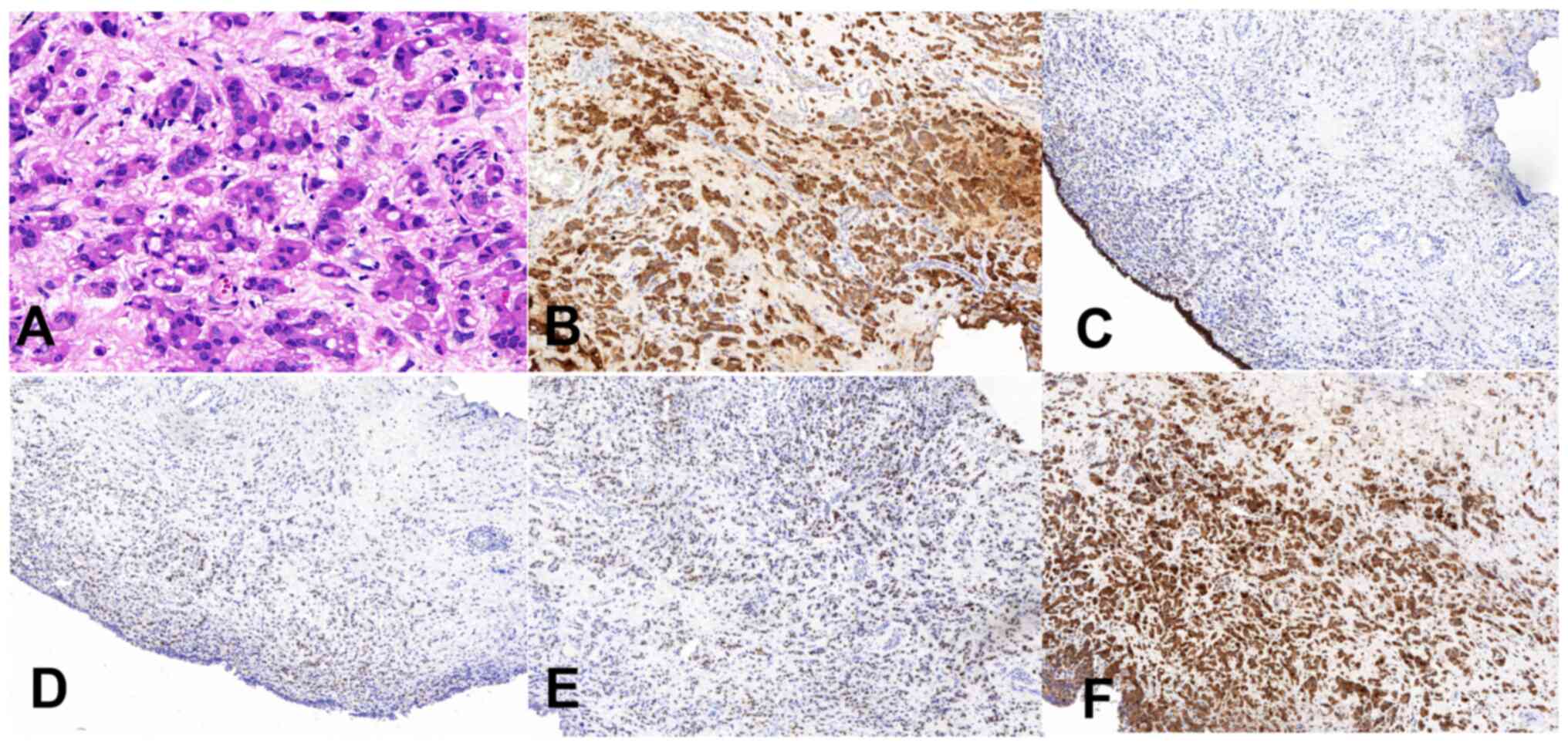
post-operative pathological analysis (Fig. 2A) revealed the infiltrative growth
of adenocarcinoma cells within the lamina propria of the bladder
mucosa, consistent with metastatic breast invasive carcinoma. The
protocol for histopathological staining was as follows: Tissue
samples from the operation were fixed with 3.7% neutral
formaldehyde solution for 24 h at room temperature, followed by
routine dehydration and embedding in paraffin. The paraffin blocks
were cut into sections with a thickness of 4 µm, stained with eosin
staining solution for 5 min at room temperature and observed by
conventional light microscopy.
Paraffin sections were immunohistochemically stained
using the EnVision two-step method. Immunohistochemistry (IHC) was
performed on formalin-fixed (10% formalin for 48 h at room
temperature) and paraffin-embedded tissue sections (4 µm thick).
IHC was performed automatically using the BenchMark Ultra platform
(Ventana Medical Systems, Inc.) according to the manufacturer's
instructions. the protocol for immunohistochemistry (IHC) staining
was as follows: 4-µm-thick tumor sections from a paraffin block
were deparaffinized and rehydrated in a descending alcohol series
(80, 90, 95 and 100%) and water. For quenching of endogenous
peroxidase activity, the slides were incubated with 3% hydrogen
peroxide(H2O2) solution in methanol for 30
min at room temperature. slides were rinsed three times with 0.01 M
PBS, then treated with antigen retrieval reagent (CC1, Roche, LTD.)
in 100°C for 60 min. Slides were washed three times with 0.01 M PBS
(pH 7.4; 10min/wash) at room temperature and were then blocked with
10% goat non-immune serum (SP KIT-B1; Fuzhou Maixin Biotech Co.,
Ltd.) for 30 min at room temperature. Sections were rinsed three
times with 0.01 M PBS (pH 7.4; 10 min/wash), and were then
incubated with these monoclonal primary antibodies from Ventana
Medical Systems, Inc. at 37°C for 32 min: CD138 (1:200; KIT-9921),
cytokeratin (CK)7 (1:200; KIT-7604), CD38 (1:200;KIT-3351), p63
(1:200; KIT-9922), P504S (1:200; KIT-6627), CK20 (1:200; KIT-5630),
HER-2 (1:200; KIT-0048), GATA-3 (1:200; KIT-3535), p53 (1:200;
KIT-5001), Ki-67 (1:200; KIT-5002), E-cadherin (1:200; KIT-5020),
ER (1:200; KIT-5010), PR (1:200; KIT-5011) and mammaglobin (1:200;
KIT-3433). Primary antibodies were diluted with PBS. Slides were
washed three times with 0.01 M PBS (5 min/wash), then incubated
with ultra View Universal HRP(ready to use; Roche, Ltd.) for 8 min
at 37°C. Ultra View Universal DAB (ready to use; K01773; Roche,
Ltd.) with 3% H2O2 was used as the chromogen
for 8 min at 37°C, and sections were counterstained with Mayer's
hematoxylin for 8 min at room temperature. Subsequently, slides
were sealed with Permount Mounting Medium and observed under a
light microscope (Olympus BX143) with 20× and 40× magnifications.
The immunohistochemical analysis demonstrated positive staining for
CD138 and CK7 (Fig. 2B), negative
staining for CD38, p63, P504S, paired box-8, CK20 and HER2 (1+),
and positive staining for GATA binding protein 3 (Fig. 2E), p53 (minority), Ki-67 (20% of
hotspots), E-cadherin (partial) (Fig.
2C), ER (moderate intensity, ~30%) (Fig. 2D), PR (moderate intensity, ~5%) and
mammaglobin (Fig. 2F). The patient
received a 3-month treatment with palbociclib (125 mg, every day
for 21 days and discontinued for 7 days) + fulvestrant (500 mg,
every 4 weeks), which had to be discontinued due to severe
myelosuppression caused by palbociclib. Due to the absence of a
standardized treatment regimen for third-line and subsequent
therapies, particularly in hormone receptor-positive patients
following resistance to endocrine therapy, the patient was
subsequently administered an 8 mg lenvatinib by mouth once
daily(qd) + 500 mg fulvestrant by intramuscular injection every 3
weeks (q3w) combination until January 2023. However, meningeal
metastasis occurred and despite receiving one cycle of chemotherapy
with albumin-paclitaxel (300 mg on day 1) + carboplatin (0.6 g on
day 1) + bevacizumab (600 mg on day 1) and repeated every 3 weeks
the patient exhibited a poor response and succumbed to tumor
progression in March 2023.
In summary, the histological subtypes of the primary
breast cancer and bladder metastases in the patient exhibited
marked concordance, the interval between the breast cancer
diagnosis and the onset of bladder metastases was 8 years and the
progression-free survival (PFS) and overall survival (OS) times
following the diagnosis of bladder metastasis were 16 and 18
months, respectively.
Discussion
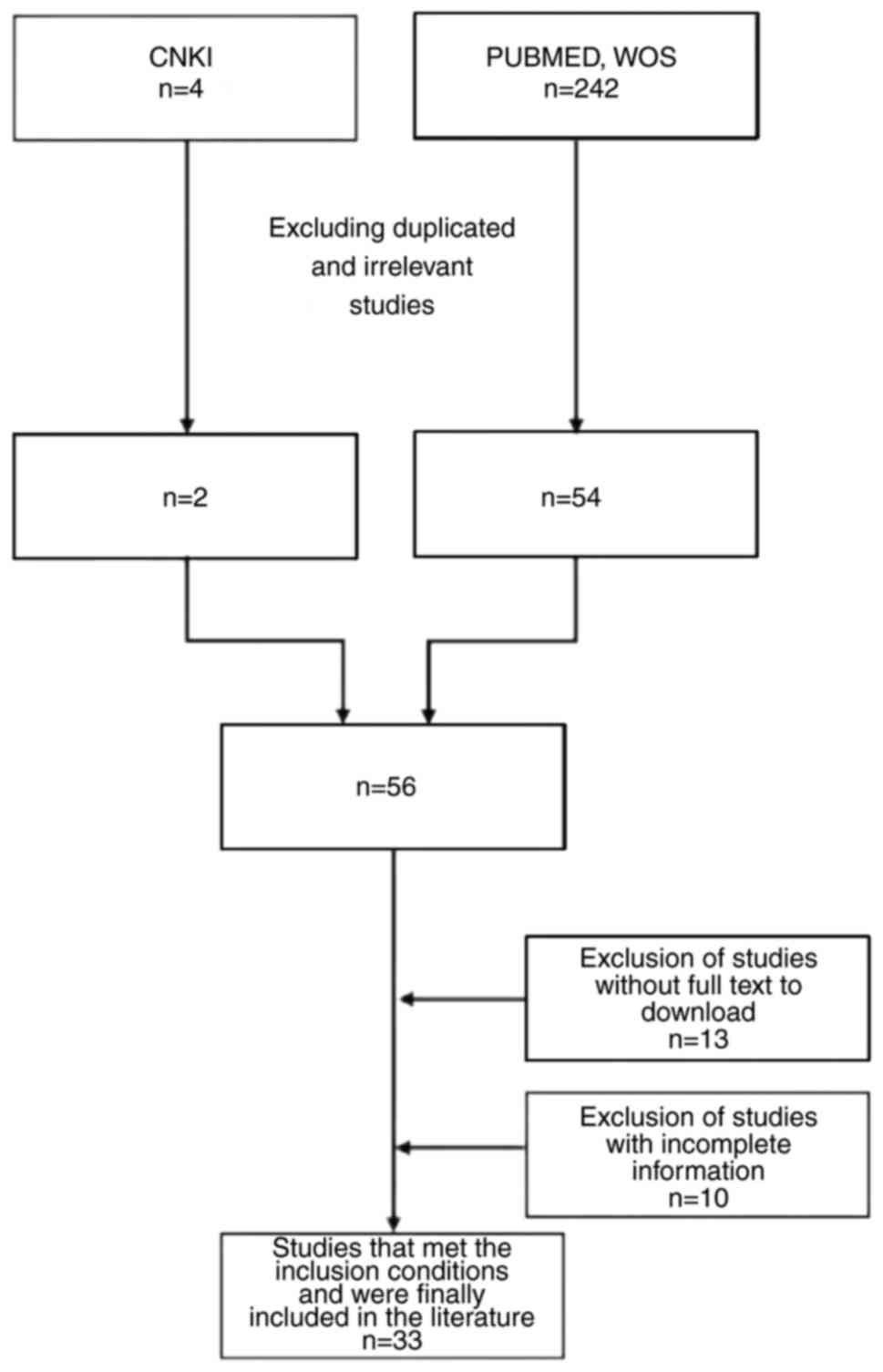
A comprehensive literature search was conducted in
the Chinese database, China National Knowledge Infrastructure
(https://www.cnki.net/), utilizing ‘breast cancer
and bladder metastasis’ as the search terms in Chinese from the
beginning of database construction until June 30, 2022. Out of the
four studies retrieved, two were excluded based on their titles and
abstracts. Additionally, relevant studies in the English language
were retrieved from PubMed (https://pubmed.ncbi.nlm.nih.gov) and Web of Science
(webofscience.com) using the search terms ‘breast cancer AND
bladder AND metastasis’ from the beginning of database construction
until June 30, 2022. The initial search led to the identification
of 242 studies, with 54 being preliminarily deemed as relevant
following a thorough examination. Subsequently, two criteria were
applied and 33 studies were selected out of the 54 studies. The
inclusion criteria were as follows: i) Case reports or serial case
studies related to breast cancer with bladder metastasis; and ii)
provision of complete general information including sex, age and
duration from the diagnosis of breast cancer to the appearance of
bladder metastasis. The literature screening process is presented
in Fig. 3.
A total of 54 patients were included in the 33
articles, coupled with this present case, resulting in a cohort of
55 female patients diagnosed with bladder metastases originating
from breast cancer for the present literature review (Table I). The median age of onset was 65
years (range, 40–91 years) (14,15),
while the median interval time from breast cancer diagnosis to the
appearance of bladder metastases was 5.6 years (range, 0–28 years)
(16,17). Out of the 55 patients, 44 patients
presented with definitive pathological types of primary breast
cancer, consisting of 23 cases (12,16,18–25)
classified as IDC (52.3%), 18 cases(14–16,25–33)
classified as invasive lobular carcinoma (ILC) (40.9%) and 3 cases
categorized as mixed-type (6.8%) (18,34,35).
An analysis of the pathological information obtained from primary
lesions and bladder metastases revealed 100% pathological
consistency in the 36 cases with clear reports. Furthermore, there
was a consistent presence of ER results in 33 out of 37 cases
(89.2%), while PR results exhibited consistency in 25 out of 30
cases (83.3%). Notably, concordance in HER2 expression was 100% in
the 20 patients with clearly reported HER2 results. ER, PR and HER2
were all reported in 11 cases (12,14,15,19,26–29,36,37),
and the expression of all three was completely consistent in 9
cases (81.8%) (15,26,28,29,32,36).
These data are summarized in Table
II.
 | Table I.Clinical presentations of breast
carcinoma metastatic to the bladder in literature cases (n=55). |
Table I.
Clinical presentations of breast
carcinoma metastatic to the bladder in literature cases (n=55).
| First author
(year) | Age, years | Primary breast
lesion (pathology/ER/PR/HER-2 status) | Metastases of the
bladder (pathology/ER/PR/HER-2 status) | First sign of
bladder metastasis | Cystoscopic
findings | Time from diagnosis
of breast cancer to bladder metastasis, years | Incorporation of
other site metastases | Treatment for
breast cancer after bladder metastasis | Treatment
outcome | Survival or
follow-up time after bladder metastasis, months | (Refs.) |
|---|
| Pontes and | 68 | NR | NR | Abdominal | Right | 1.0 | Lung and | Chemotherapy | NR | 2 | (38) |
| Oldford |
|
|
| pain, | ureteral |
| lymph nodes |
|
|
|
|
| (1970) |
|
|
| hematuria, | orifice mass |
|
|
|
|
|
|
|
|
|
|
| leg cramps |
|
|
|
|
|
|
|
|
| 57 | NR | NR | Backache | Cauliflower- | 4.0 | NR | Radiation | NR | 1 |
|
|
|
|
|
|
| like mass on |
|
| therapy |
|
|
|
|
|
|
|
|
| the right |
|
|
|
|
|
|
|
|
|
|
|
| side wall |
|
|
|
|
|
|
| Haid et
al | 45 | NR | NR | Intermittent | Two | 5.5 | Bone, liver, | No | NR | 1 | (30) |
| (1980) |
|
|
| painless | irregular |
| meninges |
|
|
|
|
|
|
|
|
| gross | masses |
|
|
|
|
|
|
|
|
|
|
| hematuria |
|
|
|
|
|
|
|
|
| 83 | ILC/NR/NR/NR | ILC/NR/NR/NR | Urinary | Multiple | 2.7 | None | Radiation | NR | 13 |
|
|
|
|
|
| incontinence, | nodules in |
|
| therapy |
|
|
|
|
|
|
|
| increased | the bladder |
|
|
|
|
|
|
|
|
|
|
| nocturia | wall with |
|
|
|
|
|
|
|
|
|
|
|
| limited |
|
|
|
|
|
|
|
|
|
|
|
| volume |
|
|
|
|
|
|
|
| 76 | NR | NR | Microscopic | NR | 2.7 | Bone, | Chemotherapy | NR | 1 |
|
|
|
|
|
| hematuria |
|
| meninges, |
|
|
|
|
|
|
|
|
| and lethargy |
|
| lung, liver, |
|
|
|
|
|
|
|
|
|
|
|
| bone marrow |
|
|
|
|
|
| 71 | NR | NR | Left adnexal | Walnut size | 3.2 | Lymph nodes |
Chemoradiotherapy | NR | >7 |
|
|
|
|
|
| mass | lump |
| in the left |
|
|
|
|
|
|
|
|
|
|
|
| axilla and |
|
|
|
|
|
|
|
|
|
|
|
| clavicle |
|
|
|
|
| Silverstein | 66 | NR/+/NR/NR | NR/+/NR/NR | Frequent | Raised | 14.0 | None | Chemotherapy | NR | 24 | (40) |
| et al
(1987) |
|
|
| urination, | lesion of the |
|
|
|
|
|
|
|
|
|
|
| urgency, | right lateral |
|
|
|
|
|
|
|
|
|
|
| dysuria, | wall, |
|
|
|
|
|
|
|
|
|
|
| intermittent | smooth |
|
|
|
|
|
|
|
|
|
|
| abdominal | surface |
|
|
|
|
|
|
|
|
|
|
| pain |
|
|
|
|
|
|
|
|
| 54 | IDC/-/-/NR | NR | Gross | Extensive | 0.6 | Brain, bone |
Chemoradiotherapy | NR | 12 |
|
|
|
|
|
| hematuria | nodules in |
|
|
|
|
|
|
|
|
|
|
| and dysuria | the trigone |
|
|
|
|
|
|
|
|
|
|
|
| of the right |
|
|
|
|
|
|
|
|
|
|
|
| lateral wall |
|
|
|
|
|
|
|
|
|
|
|
| of the |
|
|
|
|
|
|
|
|
|
|
|
| bladder |
|
|
|
|
|
|
| Rigatti et
al | NR | IDC | NR | Bladder | Unilateral | 5.3 | Lungs, lymph | NR | NR | NR | (39) |
| (1991) |
|
|
| irritation, | wall uplift |
| nodes |
|
|
|
|
|
|
|
|
| urinary |
|
|
|
|
|
|
|
|
|
|
|
| incontinence |
|
|
|
|
|
|
|
|
| NR | IDC | NR | NR | NR | 13.7 | NR | NR | NR | NR |
|
| Williams | 79 | NR | NR | Frequent | Large mass | 6.0 | NR | No |
| <1 | (42) |
| et al
(1992) |
|
|
| urination, | in bladder |
|
|
|
|
|
|
|
|
|
|
| increased | vault |
|
|
|
|
|
|
|
|
|
|
| nocturia |
|
|
|
|
|
|
|
| Berger et
al | 78 | IDC/-/-/NR | NR | Microscopic | Mass in the | 6.5 | NR | NR | NR | NR | (31) |
| (1992) |
|
|
| hematuria | right lateral |
|
|
|
|
|
|
|
|
|
|
|
| wall, |
|
|
|
|
|
|
|
|
|
|
|
| posterior |
|
|
|
|
|
|
|
|
|
|
|
| wall and |
|
|
|
|
|
|
|
|
|
|
|
| bladder |
|
|
|
|
|
|
|
|
|
|
|
| neck |
|
|
|
|
|
|
|
| 70 | NR | NR | Uroschesis, | NR | 6.0 |
Supraclavicular | Chemoradiothe | NR | <12 |
|
|
|
|
|
| aginal mass, |
|
| lymph | rapy |
|
|
|
|
|
|
|
| gross |
|
| nodes, brain, |
|
|
|
|
|
|
|
|
| hematuria |
|
| bone |
|
|
|
|
|
| 65 | ILC/+/-/NR | NR | Gross | NR | 5.7 |
Retroperitoneal | Chemotherapy | NR | <12 |
|
|
|
|
|
| hematuria |
|
| lymph nodes, |
|
|
|
|
|
|
|
|
|
|
|
| liver |
|
|
|
|
| Schneidaue | 54 | NR | NR | Dysuria, | Diffuse | 2.0 | NR | NR | NR | NR | (43) |
| et al
(1995) |
|
|
| intermittent | bullous |
|
|
|
|
|
|
|
|
|
|
| painless | edema of |
|
|
|
|
|
|
|
|
|
|
| hematuria | the bladder |
|
|
|
|
|
|
|
|
|
|
|
| base and |
|
|
|
|
|
|
|
|
|
|
|
| posterior |
|
|
|
|
|
|
|
|
|
|
|
| lateral wall |
|
|
|
|
|
|
| Elia et
al | 87 | IDC/+/+/NR | IDC/-/+/NR | Urgent | Small polyp | 5.0 | None | Endocrine | Effective |
| (21) |
| (1999) |
|
|
| urination | in the left |
|
| therapy |
|
|
|
|
|
|
|
|
| ureteral |
|
|
|
|
|
|
|
|
|
|
|
| orifice |
|
|
|
|
|
|
| Poulakis | 65 | IDC/+/+/NR | IDC/+/+/NR | Frequent | Multiple | 4.0 | None | Chemotherapy | Effective | >60 | (22) |
| et al
(2001) |
|
|
| urination, | tumors in |
|
| + endocrine |
|
|
|
|
|
|
|
| urgent | the bladder |
|
| therapy |
|
|
|
|
|
|
|
| urination and | wall |
|
|
|
|
|
|
|
|
|
|
| increased |
|
|
|
|
|
|
|
|
|
|
|
| nocturia |
|
|
|
|
|
|
|
| Feldman | 61 | ILC+IDC/-/-/NR | ILC/+/NR/NR | Gross | Nothing | 10.0 | NR | Radiation | NR | >9 | (34) |
| et al
(2002) |
|
|
| hematuria | abnormal, |
|
| therapy |
|
|
|
|
|
|
|
|
| but CT |
|
|
|
|
|
|
|
|
|
|
|
| shows an |
|
|
|
|
|
|
|
|
|
|
|
| irregular |
|
|
|
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
| Soon et
al | 87 | ILC/+/+/- | Same as the
primary | Abdominal | Thickening | 17.0 | None | Endocrine | Effective | 26.4 | (32) |
| (2004) |
|
| lesion | pain and | of the |
|
| therapy |
|
|
|
|
|
|
|
| gross | bladder wall |
|
|
|
|
|
|
|
|
|
|
| hematuria |
|
|
|
|
|
|
|
| Gatti et
al | 49 | IDC +
ILC/-/+/NR | NR | NR | Ulcerated | 5.0 | NR | Chemotherapy | NR | 11 | (18) |
| (2005) |
|
|
|
| mass |
|
|
|
|
|
|
| Kleinmann | 65 | IDC/NR/NR/NR | NR | Painless | Papillary | 2.4 | NR | NR | NR | NR | (23) |
| et al
(2005) |
|
|
| gross | tumor of the |
|
|
|
|
|
|
|
|
|
|
| hematuria | right |
|
|
|
|
|
|
|
|
|
|
|
| ureteral |
|
|
|
|
|
|
|
|
|
|
|
| orifice |
|
|
|
|
|
|
| Lawrentschuk | 74 | ILC/+/-/NR | ILC/+/-/NR | Increased | - | NR | Bone | NR | NR | NR | (33) |
| et al
(2005) |
|
|
| nocturia |
|
|
|
|
|
|
|
|
| 44 | IDC/+/+/NR | IDC/+/-/NR | Lower back | Groin pain | NR | NR | Chemotherapy | NR | NR |
|
|
|
|
|
| pain | and |
|
|
|
|
|
|
|
|
|
|
|
| hydronephrosis |
|
|
|
|
|
|
| Ryan et
al | 71 | NR | ILC/+/-/- | Urinary | A hard | 22.0 | Ovary | Chemotherapy | NR | >3 | (41) |
| (2006) |
|
|
| incontinence | infiltrating |
|
| + endocrine |
|
|
|
|
|
|
|
|
| mass in the |
|
| therapy |
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
| Zagha and | 67 | IDC/+/+/NR | IDC/-/-/NR | Intermittent | Ulcerated | 7.9 | None | Surgery + | NR | NR | (20) |
| Hamawy |
|
|
| gross | mass in the |
|
| endocrine |
|
|
|
| (2007) |
|
|
| hematuria | vault of the |
|
| therapy |
|
|
|
|
|
|
|
|
| bladder |
|
|
|
|
|
|
| Lin and | 68 | IDC/-/-/+ | IDC/+/+/+ | Lower back | Multiple | 3.0 | None | Chemotherapy | Effective | 2 | (24) |
| Chen |
|
|
| pain, | nodules in |
|
| + trastuzumab |
|
|
|
| (2007) |
|
|
|
hydronephrosis, | the bladder |
|
|
|
|
|
|
|
|
|
|
| occult | wall |
|
|
|
|
|
|
|
|
|
|
| blood, |
|
|
|
|
|
|
|
|
|
|
|
| oliguria |
|
|
|
|
|
|
|
| Xiao et
al | 46 | ILC/+/+/NR | NR/+/NR/NR | Dysuria, | Nodules in | NR | Lung, liver | NR | NR | 0.5 | (25) |
| (2012) |
|
|
| hydronephrosis | bladder |
|
|
|
|
|
|
|
|
|
|
|
| neck region |
|
|
|
|
|
|
|
| 53 | ILC/+/+NR | NR/+/NR/NR | Hematuria | Nodules | NR | None | NR | NR | Lost to |
|
|
|
|
|
|
| around the |
|
|
|
| follow-up |
|
|
|
|
|
|
| bladder |
|
|
|
|
|
|
|
|
|
|
|
| neck and |
|
|
|
|
|
|
|
|
|
|
|
| urethra |
|
|
|
|
|
|
|
| 76 | IDC/+/+/NR | NR | Dysuria | Nodule | NR | Lung, liver | NR | NR | <1 |
|
| Luczyńska | 53 | ILC/NR/NR/NR | ILC/NR/NR/NR | CA153 | Left ureteral | 6.0 | Local | Chemotherapy | Effective | NR | (44) |
| et al
(2010) |
|
|
| increased | mass |
| recurrence |
|
|
|
|
| Shah et
al | 45 | ILC/+/-/NR | ILC/+/-/NR | Oliguria, | Thickness | At the | Peritoneum | Chemotherapy | Effective | 6 | (17) |
| (2011) |
|
|
| urinary tract | of the | same |
|
|
|
|
|
|
|
|
|
| infection, | bladder wall | time as |
|
|
|
|
|
|
|
|
|
| foot and | increased | the |
|
|
|
|
|
|
|
|
|
| ankle edema | and | primary |
|
|
|
|
|
|
|
|
|
|
| irregular | breast |
|
|
|
|
|
|
|
|
|
|
|
| cancer |
|
|
|
|
|
| Reichman | 68 | NR | ILC/+/+/NR | Urinary | Lesions in | 23.0 | None | Endocrine | Effective | 10 | (45) |
| et al
(2012) |
|
|
| incontinence, | the trigone |
|
| therapy |
|
|
|
|
|
|
|
| urinary tract | and bladder |
|
|
|
|
|
|
|
|
|
|
| infection | wall |
|
|
|
|
|
|
| Ghaida | 64 | IDC/+/+/- | IDC/+/-/NR | Urinary | Thickening | 5.0 | None | Chemotherapy | Ineffective | 12 | (19) |
| et al
(2013) |
|
|
| incontinence, | of the |
|
|
|
|
|
|
|
|
|
|
| urinary tract | bladder wall |
|
|
|
|
|
|
|
|
|
|
| infection |
|
|
|
|
|
|
|
| Zhai et
al | 40 | ILC/+/+/+ | Same as the
primary | Left lower | Urethral | 3.0 | Bone | Chemotherapy | Effective | NR | (14) |
| (2013) |
|
| lesion | abdominal | stricture, |
|
|
|
|
|
|
|
|
|
|
| pain | cystitis |
|
|
|
|
|
|
| Nieder et
al | 91 | ILC/+/-/- | Same as the
primary | Anemia, | Bladder | 4.5 | None | Endocrine | Effective | 12 | (15) |
| (2014) |
|
| lesion | renal | mass |
|
| therapy + |
|
|
|
|
|
|
|
| dysfunction, | rupture and |
|
| radiation |
|
|
|
|
|
|
|
| hydronephrosis | bleeding |
|
| therapy |
|
|
|
| Cormio | 45 | ILC/+/+/- | Same as the
primary | No | The urethral | 8.0 | Bone and | Chemotherapy | Effective | NR | (26) |
| et al
(2014) |
|
| lesion |
| opening is |
| lymph nodes |
|
|
|
|
|
|
|
|
|
| surrounded |
|
|
|
|
|
|
|
|
|
|
|
| by a mass |
|
|
|
|
|
|
| Al | 50 | ILC/+/+/- | ILC/-/-/- | Edema of the | The | 9.0 | Bone and | NR | NR | NR | (27) |
| Ibraheemi |
|
|
| left lower | posterior |
| pleural |
|
|
|
|
| (2016) |
|
|
| extremity | wall of the |
| effusion |
|
|
|
|
|
|
|
|
|
| bladder is |
|
|
|
|
|
|
|
|
|
|
|
| thickened |
|
|
|
|
|
|
| Yoneyama | 83 | IDC/+/+/+ | Same as the
primary | Left lower | Large mass | 15.0 | Bone | Chemotherapy | NR | NR | (28) |
| et al
(2018) |
|
| lesion | abdominal | in the |
|
|
|
|
|
|
|
|
|
|
| pain, anuria, | bladder |
|
|
|
|
|
|
|
|
|
|
| general |
|
|
|
|
|
|
|
|
|
|
|
| edema |
|
|
|
|
|
|
|
| De Rose | 57 | IDC/+/+/- | Same as the
primary | Lower | Thickening | 5.0 | Lymph node | Targeted | Effective | NR | (36) |
| et al
(2019) |
|
| lesion | backache, | of the |
|
| therapy + |
|
|
|
|
|
|
|
| dysuria | bladder wall |
|
| endocrine |
|
|
|
|
|
|
|
|
|
|
|
| therapy |
|
|
|
| Wang et
al | 57 | IDC/-/NR/+ | Same as the
primary | Kidney | Ureteral | 8.1 | Bone |
Chemoradiotherapy | NR | 23 | (16) |
| (2019) |
|
| lesion | failure | obstruction |
|
|
|
|
|
|
|
| 83 | IDC/+/NR/- | Same as the
primary | Asymptomatic | Thickening | 28.0 | Bone, brain | Chemotherapy | NR | 7 |
|
|
|
|
| lesion | with only a | of the |
|
|
|
|
|
|
|
|
|
|
| CT scan | bladder wall |
|
|
|
|
|
|
|
|
|
|
| revealing |
|
|
|
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
|
|
|
|
|
| thickening |
|
|
|
|
|
|
|
|
| 51 | IDC/-/NR/+ | Same as the
primary | Urgent | Bladder | 5.8 | Bone | Palliative | NR | 1 |
|
|
|
|
| lesion | urination | wall mass |
|
| treatment |
|
|
|
|
| 62 | ILC/+/NR/- | Same as the
primary | NR | NR | 3.4 | Pleura | Endocrine | NR | NR |
|
|
|
|
| lesion |
|
|
|
| therapy |
|
|
|
|
| 66 | IDC/+/NR/NR | Same as the
primary | Hematuria | Bladder | 6.0 | None | Chemotherapy | NR | 12 |
|
|
|
|
| lesion |
| wall mass |
|
|
|
|
|
|
|
| 57 | ILC/+/NR/- | Same as the
primary | Asymptomatic | Bladder | 5.4 | Bone, liver, |
Chemoradiotherapy | NR | 12 |
|
|
|
|
| lesion | with only a | wall mass |
| skin |
|
|
|
|
|
|
|
|
| CT scan |
|
|
|
|
|
|
|
|
|
|
|
| revealing |
|
|
|
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
|
|
|
|
|
| thickening |
|
|
|
|
|
|
|
|
| 67 | IDC/+/NR/- | Same as the
primary | Asymptomatic, | Bladder | 3.8 | Ampullar |
Chemoradiotherapy | NR | 4 |
|
|
|
|
| lesion | with | wall mass |
| region |
|
|
|
|
|
|
|
|
| only a CT |
|
|
|
|
|
|
|
|
|
|
|
| scan |
|
|
|
|
|
|
|
|
|
|
|
| revealing |
|
|
|
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
|
|
|
|
|
| thickening |
|
|
|
|
|
|
|
|
| 68 | IDC/+/NR/- | Same as the
primary | Asymptomatic, | Bladder | 8.2 | Pelvis | Chemotherapy | NR | 2 |
|
|
|
|
| lesion | with only a | wall mass |
|
|
|
|
|
|
|
|
|
|
| CT scan |
|
|
|
|
|
|
|
|
|
|
|
| revealing |
|
|
|
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
|
|
|
|
|
| thickening |
|
|
|
|
|
|
|
|
| 77 | IDC/+/NR/- | Same as the
primary | Hematuria | Bladder | 6.2 | Bone | NR | NR | NR |
|
|
|
|
| lesion |
| wall mass |
|
|
|
|
|
|
|
| 54 | ILC/+/NR/- | Same as the
primary | Asymptomatic, | Bladder | 4.3 | Bone, liver | Chemotherapy | NR | >5 |
|
|
|
|
| lesion | with only a | wall mass |
|
|
|
|
|
|
|
|
|
|
| CT scan |
|
|
|
|
|
|
|
|
|
|
|
| revealing |
|
|
|
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
|
|
|
|
|
| thickening |
|
|
|
|
|
|
|
|
| 74 | ILC/+/NR/- | Same as the
primary | Asymptomatic, | Bladder | 9.0 | Bone | Chemotherapy | NR | >5 |
|
|
|
|
| lesion | only | wall mass |
|
|
|
|
|
|
|
|
|
|
| revealed a |
|
|
|
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
|
|
|
|
|
| mass by CT |
|
|
|
|
|
|
|
|
|
|
|
| scan |
|
|
|
|
|
|
|
| Gitau et
al | 54 | ILC/+/+/- | NR | Abdominal | Multiple | 7.0 | Bone, skin | Chemotherapy | Ineffective | 9 | (37) |
| (2020) |
|
|
| distension, | masses in |
|
|
|
|
|
|
|
|
|
|
| hematuria, | the bladder |
|
|
|
|
|
|
|
|
|
|
| ascites |
|
|
|
|
|
|
|
| Khan et
al | 63 | IDC/+/+/- | IDC/+/+/NR | Lower | Thickening | 17.0 | None | Targeted | Effective | NR | (12) |
| (2021) |
|
|
| abdominal | of the |
|
| therapy + |
|
|
|
|
|
|
|
| pain, | bladder wall |
|
| endocrine |
|
|
|
|
|
|
|
| hematuria |
|
|
| therapy |
|
|
|
| Mohammed | 80 | ILC/+/+/- | Same as the
primary | Urinary | Thickening | 4.0 | None | Endocrine | Effective | 24 | (29) |
| et al
(2021) |
|
| lesion | incontinence | of the |
|
| therapy |
|
|
|
|
|
|
|
|
| bladder wall |
|
|
|
|
|
|
| Wang et
al | 65 | IDC + | ILC/NR/NR/NR | Hydronephro | No | 4.0 | Bone | NR | NR | NR | (35) |
| (2022) |
| ILC/NR/NR/NR |
| sis of the | abnormal |
|
|
|
|
|
|
|
|
|
|
| right kidney |
|
|
|
|
|
|
|
| Present | 58 | IDC/+/+/- | Same as the
primary | Uronephrosis | Bladder | 8.0 | Bone | Endocrine | Effective | >9 | - |
| study |
|
| lesion |
| wall mass |
|
| therapy + |
|
|
|
|
|
|
|
|
|
|
|
| targeted |
|
|
|
|
|
|
|
|
|
|
|
| therapy |
|
|
|
 | Table II.Clinical characteristics of patients
with breast cancer and bladder metastasis. |
Table II.
Clinical characteristics of patients
with breast cancer and bladder metastasis.
| Clinical
characteristics | Value |
|---|
| Sex, n (%) |
|
|
Female | 55 (100.00) |
| Median age (range),
years | 65 (40–91) |
| Median time from
diagnosis of breast cancer to bladder metastasis (range),
years | 5.6 (0–28) |
| Median survival or
follow-up time after diagnosis of bladder metastasis (range),
months | 9 (0.5–60) |
| Complete
pathological records of primary lesions, n | 44 |
| IDC
(%) | 23 (52.3) |
| ILC
(%) | 18 (40.9) |
| IDC +
ILC (%) | 3 (6.8) |
| Pathological
consistency between primary and metastatic lesions | Consistent
rate |
| Pathology was
reported in all cases (n=36) | 36 (100.0) |
| ER was reported in
all cases (n=37) | 33 (89.2) |
| PR was reported in
all cases (n=30) | 25 (83.3) |
| HER-2 was reported
in all cases (n=20) | 20 (100.0) |
|
Pathology/ER/PR/HER-2 status was reported
in all cases (n=11) | 9 (81.8) |
| Report the combined
metastasis site in detail, n | 46 |
| Bone
(%) | 20 (43.5) |
| Liver
(%) | 7 (15.2) |
| Lymph
node (%) | 7 (15.2) |
| Lung, n
(%) | 5 (10.9) |
| Brain
or meninges, n (%) | 5 (10.9) |
|
Elsewhere, n (%) | 7 (15.2) |
The main initial symptoms exhibited by the 55
patients were related to the urinary system, including frequent
urination, urgency, dysuria, urinary incontinence, nocturia and
gross hematuria. A small subset of patients presented with lower
back pain, and edema in the lower limbs, feet and ankles (27,28).
Notably, some patients exhibited no overt urinary system symptoms,
and signs were detected only through a CT examination (16). Cystoscopy revealed the thickening of
the bladder wall, a mass within the bladder wall or a mass at the
ureteral orifice. In addition to the involvement of the bladder,
combined metastasis to other sites was observed in 46 patients.
Specifically, bone metastasis was reported in 20 cases (43.5%),
while liver or lymph node metastases were found in 7 cases (15.2%)
each (25,26,30,31,36,38,39).
Metastases to the lungs, brain and meninges were observed in 5
cases (10.9%) each (25,30,38–40),
whereas other sites including the peritoneum (17), ovaries (41), pleural effusion (27), pleura (16), bone marrow (30), ampullary region (16) and skin (16) were involved in only one case each
(totaling 7 cases) (Table II).
Following a diagnosis of bladder metastases,
appropriate treatment measures should be implemented based on the
size of the lesions, the clinical characteristics and the
pathological typing. The majority of the reported cases spanned
from 1970 to 2022 (35,38), with a distribution of 16 cases prior
to 2000 (21,30,31,38–40,42,43),
14 cases between 2001 and 2010 (18,20,22–24,32–34,41,44), 7
cases between 2011 and 2017 (14,15,17,19,25,26,45),
and 18 cases after 2018 (12,16,28,29,35–37).
Prior to the advent of molecular classification for breast cancer,
radiotherapy and chemotherapy were the primary treatments. However,
subsequent to the year 2000, endocrine therapy, as well as targeted
therapies such as trastuzumab (anti-HER2 therapy) were incorporated
into the treatment regimens. In those cases for which treatment
outcomes were reported following the diagnosis of bladder
metastases, the median time until final mortality or follow-up
cut-off was 9 months (Tables I and
II).
Breast cancer has emerged as the most prevalent
malignancy worldwide, with ~2.3 million new cases reported in 2020.
These figures accounted for ~25% of all female malignancies and
surpassed the incidence of lung cancer for the first time (46). As a result, breast cancer currently
stands as the foremost cause of mortality among female
malignancies. Despite advancements in screening technology,
scientific education and diagnosis, this issue continues to pose a
significant challenge to the health of women.
Of all patients with breast cancer, ~45% develop
metastases to various organs, including the lungs, brain, liver,
bones, lymph nodes, skin and other sites (47). Nevertheless, the occurrence of
bladder metastasis is extremely uncommon. Bladder carcinoma usually
manifests primarily as urothelial carcinoma, which has the second
highest prevalence among urinary system malignancies (48,49).
Bladder metastasis constitutes a small fraction of malignant tumors
within bladder carcinoma, reaching reported incidences as low as
2.3% (50). Differentiating between
primary and metastatic bladder cancer poses a significant
diagnostic challenge. The majority of cases involving this organ
are linked to the direct infiltration by adjacent tumor types such
as colorectal, cervical and prostate carcinomas (28,51).
Due to the rarity of bladder metastasis from breast
cancer, there is a dearth of large-scale cohort studies and
epidemiological data pertaining to this particular form of
malignancy. Previous investigations have primarily focused on
postmortem examinations for determining incidence rates, which
range between 0.06 and 7% (19,38,52,53).
Including the present case, a total of 55 cases were
included in the present literature review. The main clinical
characteristics observed were as follows: All patients were female,
with a median age of onset at 65 years and a median interval time
of 5.6 years between breast cancer diagnosis and the development of
bladder metastasis. Previous study have reported that bladder
metastasis in breast cancer predominantly originates from breast
ILC (19). However, the findings of
the present study revealed that bladder metastasis caused by IDC
accounted for 52%, while ILC accounted for 40.9%. As more case
reports accumulate, this conclusion may gain further validity and
may also be influenced by the higher incidence rate of IDC in
breast cancer (46,54–57)
between the primary tumor and metastases. According to the National
Comprehensive Cancer Network guidelines (58), it is recommended that pathological
biopsies be performed for precise guidance in treating metastatic
lesions. Roulot et al (57)
demonstrated that the concordance rates of ER, PR and HER2 between
primary breast cancer and metastatic lesions were 80, 67 and 92%,
respectively. Similarly, Santinelli et al (59) reported that the concordance rate of
HER2 between primary breast cancer and metastatic lesions was
71.4%. The case described in the present study exhibited a notable
pathological consistency between the primary breast lesion and
bladder metastasis, consistent with the majority of cases included
in the present study. The present study demonstrated a perfect 100%
concordance with regard to the HER2 status, along with substantial
concordance in ER (88.9%) and PR (83.3%). These findings provide
valuable insight for guiding treatment decisions in patients for
whom obtaining another biopsy is not feasible.
The symptoms caused by bladder metastases vary from
asymptomatic manifestations to gross hematuria, obstructive
uropathy and renal failure (16,20)
metastasis (40). The present
literature review indicated that urinary symptoms, including
frequent urination, urgency, dyspnea, urinary incontinence,
nocturia and gross hematuria, were the predominant clinical
manifestations; however, the patient in the present case report did
not exhibit any apparent urinary symptoms upon diagnosis; only
hydronephrosis was found during the regular review, and a further
examination confirmed bladder metastasis. A minority of patients
initially presented with symptoms such as lower back pain or edema
in the legs or ankles. In a small number of cases, no apparent
urinary symptoms were exhibited and only an abnormal bladder signal
was detected by a CT examination. Notably, these clinical
manifestations do not differ from those observed in other types of
bladder tumors. Furthermore, cystoscopy revealed similar findings
to those observed in other types of bladder tumors, most commonly
the thickening of the bladder wall and masses on the ureteral
orifice.
The primary treatment for breast cancer with bladder
metastases involves a comprehensive therapy tailored to the tumor
size, clinical features and pathological immuno-typing of the
metastases. The cases reported in the present study spanned from
1970 to 2022 (35,38), with the majority of the earlier
cases managed with chemotherapeutic interventions due to the
absence of established molecular classifications for breast cancer
(30,31,38,40).
Subsequently, commencing from 2000 onwards, alongside radiotherapy
and chemotherapy, endocrine therapy and anti-HER2 therapy, were
also incorporated (12,15,16,20,22,24,29,32,36,41,45).
The patient in the present case report was diagnosed with bladder
metastasis in 2021 and received a range of novel therapeutic
agents, including palbociclib and fulvestrant.
It has been reported that bladder metastases from
breast cancer are associated with worse prognosis than bone
metastases (60,61) This disparity may be attributed to
the fact that bladder metastases are typically diagnosed
synchronously with or subsequent to other metastases. In the
present study, the highest incidence of combined bladder metastases
with other sites was observed in the bones (43.5%), followed by the
liver, lymph nodes, lungs, brain and other sites. In the present
study, the median time from the diagnosis of bladder metastasis
until mortality or the end of follow-up was only 9 months (ranging
from 0.5 to 60 months), which was potentially influenced by
incomplete data reporting. Some patients were still alive at the
time of the reporting of the case but were subsequently lost to
follow-up.
In conclusion, bladder metastasis from breast cancer
is a rare phenomenon that currently lacks well-defined
epidemiological and pathological characteristics. However, the
utilization of advanced diagnostic methods and imaging techniques
in recent years has contributed to an increased number of reported
cases. Previous studies have reported bladder metastasis from
breast cancer; however, these studies are limited by small sample
sizes and a lack of comparative analysis between primary and
metastatic lesions in terms of pathology. The present study
provides a comprehensive summary of the distinctive features
associated with bladder metastasis in patients with breast cancer.
In summary, bladder metastasis mainly originates from IDC, the
median time from breast cancer diagnosis to bladder metastasis is
5.6 years, the pathological subtypes and immunohistochemical
classifications of ER, PR and HER2 exhibit a significant
concordance between them, and the median time from the diagnosis of
bladder metastasis until mortality is 9 months. The primary breast
cancer in the present patient case was identified as IDC, and the
ER, PR and HER-2 subtypes of both the breast lesions and bladder
metastases were concordant, aligning with the findings from the
literature. The time interval between the diagnosis of breast
cancer and the onset of bladder metastases in this patient was 8
years. Furthermore, the OS time after the diagnosis of bladder
metastasis was 18 months, which is significantly longer than has
been reported in the literature. This discrepancy could potentially
be attributed to the utilization of new targeted therapies.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Research Plan of Henan
Provincial Department of Science and Technology (grant no.
202102310456).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
TK contributed to the conception and the design of
the study. HZ and TK wrote the manuscript and were involved in
analysis and interpretation of data. DL and LC obtained and
analyzed the patient information and contributed to manuscript
drafting and critical revisions of the intellectual content. YZ and
XZ treated the patient and performed the literature review. YG
conducted cystoscopy. ML performed the histological examination of
the tumor. HL, DL, LC and TK confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Third People's Hospital of Zhengzhou (ethical
approval no. SY20230067). The patient provided initial written
informed consent to participate.
Patient consent for publication
Written informed consent was obtained initially from
the patient for publication of the data and images in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Katsura C, Ogunmwonyi I, Kankam HK and
Saha S: Breast cancer: Presentation, investigation and management.
Br J Hosp Med (Lond). 83:1–7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng Z, Wei J, Lu X, Zheng H, Zhong X, Gao
W, Chen Y and Jing J: Treatment and survival patterns of Chinese
patients diagnosed with breast cancer between 2005 and 2009 in
Southwest China: An observational, population-based cohort study.
Medicine (Baltimore). 95:e38652016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lou Z, Fei X, Christakos G, Yan J and Wu
J: Improving spatiotemporal breast cancer assessment and prediction
in Hangzhou City, China. Sci Rep. 7:31882017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trayes KP and Cokenakes S: Breast cancer
treatment. Am Fam Physician. 104:171–178. 2021.PubMed/NCBI
|
|
6
|
Malinaric R, Balzarini F, Granelli G,
Ferrari A, Trani G, Ambrosini F, Mantica G, Panarello D, De Rose AF
and Terrone C: From women to women-hematuria during therapy for
metastatic breast cancer, what to suspect and when to be alarmed;
Bladder metastasis from breast cancer-our experience and a
systematic literature review. Front Oncol. 12:9769472022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei S and Siegal GP: Metastatic
organotropism: An intrinsic property of breast cancer molecular
subtypes. Adv Anat Pathol. 24:78–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanguedolce F, Landriscina M, Ambrosi A,
Tartaglia N, Cianci P, Di Millo M, Carrieri G, Bufo P and Cormio L:
Bladder metastases from breast cancer: Managing the unexpected. a
systematic review. Urol Int. 101:125–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashley S, Choudhury A, Hoskin P, Song Y
and Maitre P: Radiotherapy in metastatic bladder cancer. World J
Urol. 42:472024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q,
Wei W, Zhang Y and Sun S: Breast cancer subtypes predict the
preferential site of distant metastases: A SEER based study.
Oncotarget. 8:27990–27996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bates AW and Baithun SI: Secondary
neoplasms of the bladder are histological mimics of nontransitional
cell primary tumours: Clinicopathological and histological features
of 282 cases. Histopathology. 36:32–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan NAJ, Abdallah M and Tirona MT:
Hormone Receptor Positive/HER2 negative breast cancer with isolated
bladder metastasis: A rare case. J Investig Med High Impact Case
Rep. 9:232470962110221862021.PubMed/NCBI
|
|
13
|
Zhu H and Doğan BE: American Joint
Committee on Cancer's Staging System for Breast Cancer, Eighth
Edition: Summary for Clinicians. Eur J Breast Health. 17:234–238.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhai ZX SJJ and Wang ZP: Invasive lobular
carcinoma of breast with metastatic tumor of bladder and bone: A
case report and literature review. J Contemp Urol Reprod Oncol.
5:308–309. 2013.
|
|
15
|
Nieder C and Pawinski A: A case of
recurrent breast cancer with solitary metastasis to the urinary
bladder. Case Rep Oncol Med. 2014:9315462014.PubMed/NCBI
|
|
16
|
Wang G, Zhou C, Conklin C, Hayes MM,
Villamil CF, Ostry A and Jones EC: Metastatic breast carcinoma to
the urinary bladder-a report of 11 cases including a tumor to tumor
metastasis. Virchows Arch. 474:333–339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shah KG, Modi PR and Rizvi J: Breast
carcinoma metastasizing to the urinary bladder and retroperitoneum
presenting as acute renal failure. Indian J Urol. 27:135–136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gatti G, Zurrida S, Gilardi D, Bassani G,
dos Santos GR and Luini A: Urinary bladder metastases from breast
carcinoma: Review of the literature starting from a clinical case.
Tumori. 91:283–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghaida RA, Ayoub H, Nasr R, Issa G and
Bulbul M: Bladder metastasis from primary breast cancer: A case
report and literature review. Cent European J Urol. 66:177–184.
2013.PubMed/NCBI
|
|
20
|
Zagha RM and Hamawy KJ: Solitary breast
cancer metastasis to the bladder: An unusual occurrence. Urol
Oncol. 25:236–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elia G, Stewart S, Makhuli ZN, Krenzer BE,
Mathur S, Simon HM and Mehdi S: Metastatic breast cancer diagnosed
during a work-up for urinary incontinence: A case report. Int
Urogynecol J Pelvic Floor Dysfunct. 10:39–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poulakis V, Witzsch U, de Vries R and
Becht E: Metastatic breast carcinoma to the bladder: 5-year
followup. J Urol. 165:9052001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kleinmann N, Mor Y, Laufer M, Duvdevani M,
Fridman E and Ramon J: A solitary metastasis of breast cancer to
the urinary bladder. Breast J. 11:4972005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin WC and Chen JH: Urinary bladder
metastasis from breast cancer with heterogeneic expression of
estrogen and progesterone receptors. J Clin Oncol. 25:4308–4310.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao GQ, Chow J and Unger PD: Metastatic
tumors to the urinary bladder: Clinicopathologic study of 11 cases.
Int J Surg Pathol. 20:342–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cormio L, Sanguedolce F, Di Fino G,
Massenio P, Liuzzi G, Ruocco N, Bufo P and Carrieri G: Asymptomatic
bladder metastasis from breast cancer. Case Rep Urol.
2014:6725912014.PubMed/NCBI
|
|
27
|
Al Ibraheemi AA: Case report of metastatic
invasive breast lobular carcinoma to the urinary bladder. Int J
Hematol Oncol Stem Cell Res. 10:51–55. 2016.PubMed/NCBI
|
|
28
|
Yoneyama K, Nakagawa M and Hara A: Bladder
metastasis from primary breast cancer: A case report. Surg Case
Rep. 4:732018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohammed S and Bua AA: Metastatic breast
cancer to the urinary bladder in the caribbean. Case Rep Oncol.
14:1586–1590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haid M, Ignatoff J, Khandekar JD, Graham J
and Holland J: Urinary bladder metastases from breast carcinoma.
Cancer. 46:229–232. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berger Y, Nissenblatt M, Salwitz J and
Lega B: Bladder involvement in metastatic breast carcinoma. J Urol.
147:137–139. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soon PS, Lynch W and Schwartz P: Breast
cancer presenting initially with urinary incontinence: A case of
bladder metastasis from breast cancer. Breast. 13:69–71. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lawrentschuk N, Chan Y and Bolton DM:
Metastatic breast cancer to the bladder. Breast J. 11:1432005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feldman PA, Madeb R, Naroditsky I,
Halachmi S and Nativ O: Metastatic breast cancer to the bladder: A
diagnostic challenge and review of the literature. Urology.
59:1382002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang D, Jing T, Hui X and Yue W: A case
report for Postoperative bladder metastasis of breast cancer. Chin
J of Endourology. 16:89–90. 2022.
|
|
36
|
De Rose AF, Balzarini F, Mantica G,
Toncini C and Terrone C: Late urinary bladder metastasis from
breast cancer. Arch Ital Urol Androl. 91:60–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gitau SN, Njau A and Mwanzi S: Urinary
bladder metastasis from breast cancer: A rare cause of hematuria.
BJR Case Rep. 6:201900482020.PubMed/NCBI
|
|
38
|
Pontes JE and Oldford JR: Metastatic
breast carcinoma to the bladder. J Urol. 104:839–842. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rigatti P, Broglia L, Montorsi F,
Maffezzini M, Sironi M, Radice F, Abelli P, Pace M, Diamantini S,
Veronesi AM, et al: Breast cancer metastases of the urinary
bladder. Int J Tissue React. 13:159–163. 1991.PubMed/NCBI
|
|
40
|
Silverstein LI, Plaine L, Davis JE and
Kabakow B: Breast carcinoma metastatic to bladder. Urology.
29:544–547. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ryan PD, Harisinghani M, Lerwill MF and
Kaufman DS: Case records of the Massachusetts General Hospital.
Case 6-2006. A 71-year-old woman with urinary incontinence and a
mass in the bladder. N Engl J Med. 354:850–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Williams JR, Stott MA and Moisey CU:
Bilateral hydronephrosis secondary to breast carcinoma
metastasising to the bladder. Br J Urol. 69:97–98. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schneidau T, Stroumbakis N, Choudhury M,
Eshgi M and Mallouh C: Metastatic breast cancer to the bladder: A
case report. Int Urol Nephrol. 27:297–300. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luczyńska E, Pawlik T, Chwalibóg A, Anioł
J and Ryś J: Metastatic breast cancer to the bladder case report
and review of literature. J Radiol Case Rep. 4:19–26.
2010.PubMed/NCBI
|
|
45
|
Reichman G, Michielsen DP and De Grève J:
Tamoxifen to treat urge-incontinence from an isolated bladder
metastasis of a primary breast cancer. Cent European J Urol.
65:36–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang R, Zhu Y, Liu X, Liao X, He J and Niu
L: The Clinicopathological features and survival outcomes of
patients with different metastatic sites in stage IV breast cancer.
BMC Cancer. 19:10912019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alfred Witjes J, Max Bruins H, Carrión A,
Cathomas R, Compérat E, Efstathiou JA, Fietkau R, Gakis G, Lorch A,
Martini A, et al: European Association of Urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2023
Guidelines. Eur Urol. 85:17–31. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Karaosmanoglu AD, Onur MR, Karcaaltincaba
M, Akata D and Ozmen MN: Secondary tumors of the urinary system: An
imaging conundrum. Korean J Radiol. 19:742–751. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lodh B, Sinam RS and Singh KA:
Adenocarcinoma of urinary bladder in patient with primary gastric
cancer: An unusual synchronous distant metastasis. Indian J Cancer.
53:53–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Klinger ME: Secondary tumors of the
genito-urinary tract. J Urol. 65:144–153. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Goldstein AG: Metastatic carcinoma to the
bladder. J Urol. 98:209–215. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barroso-Sousa R and Metzger-Filho O:
Differences between invasive lobular and invasive ductal carcinoma
of the breast: Results and therapeutic implications. Ther Adv Med
Oncol. 8:261–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rakha EA and Ellis IO: Lobular breast
carcinoma and its variants. Semin Diagn Pathol. 27:49–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Makki J: Diversity of breast carcinoma:
Histological subtypes and clinical relevance. Clin Med Insights
Pathol. 8:23–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Roulot A, Héquet D, Guinebretière JM,
Vincent-Salomon A, Lerebours F, Dubot C and Rouzier R: Tumoral
heterogeneity of breast cancer. Ann Biol Clin (Paris). 74:653–660.
2016.PubMed/NCBI
|
|
58
|
Bevers TB, Niell BL, Baker JL, Bennett DL,
Bonaccio E, Camp MS, Chikarmane S, Conant EF, Eghtedari M, Flanagan
MR, et al: NCCN Guidelines® Insights: Breast cancer
screening and diagnosis, version 1.2023. J Natl Compr Canc Netw.
21:900–909. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Santinelli A, Pisa E, Stramazzotti D and
Fabris G: HER-2 status discrepancy between primary breast cancer
and metastatic sites. Impact on target therapy. Int J Cancer.
122:999–1004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mao QZ, Rong S, Xia MD, Li HZ and Zang MF:
Bladder secondary carcinoma in female. Beijing Medicine.
26:312–314. 2004.
|
|
61
|
Karjol U, Jonnada P, Cherukuru S and
Chandranath A: Bladder metastasis from breast cancer: A systematic
review. Cureus. 12:e74082020.PubMed/NCBI
|