Introduction
Lung cancer causes 25% of cancer-related deaths and
has a high incidence rate despite significant advances in therapies
made in recent years, representing 11.6% of new cancer cases
worldwide (1,2). Genomic instability is one of the main
factors leading to tumor occurrence, progression and poor outcomes
(2). Xeroderma pigmentosum group C
(XPC) is a DNA damage recognition factor and a promoter of
nucleotide excision repair that plays an important role in
maintaining DNA stability (3). XPC
is a key factor in the development of a number of tumor types
including lung cancer (4–7). Our previous study revealed that a
reduction in XPC expression can markedly promote the stem cell
properties of lung cancer cells and increase the proliferation and
migration of lung cancer cells, and a low XPC expression in
patients with lung cancer is associated with poor prognosis
(8). Zhang et al (9) showed that the loss of XPC could
increase the amount of oxidatively damaged DNA and promote
Kras-mediated lung cancer development. The underlying mechanisms
through which XPC affects lung cancer development remain not
understood; more specifically, the mediator responsible for these
processes has yet to be identified.
IFN-γ in the blood comes from monocytes,
macrophages, endothelial cells and tumor cells, and considered a
diagnostic and prognostic biomarker in lung cancer (10,11).
Studies have shown that IFN-γ can mediate lung cancer inhibition
via programmed death-ligand 1 expression (12,13).
IFN-γ-induced endoplasmic reticulum stress impairs autophagy and
triggers apoptosis in lung cancer cells (14).
A total of >90% of lung cancer-related deaths are
due to metastasis of lung cancer cells (15). Cytological studies have shown that
the expression of both XPC and IFN-γ is closely related to the
metastasis of lung cancer (8,16).
A study by Wu et al (17) revealed that XPC expression levels
were linked to prognosis and metastasis in patients with lung
cancer. In a study by Ahn et al (18) investigating the relationship between
the expression level of IFN-γ, and the prognosis and metastasis
status of patients with adenocarcinoma, it was found that patients
with low in vitro production of IFN-γ by peripheral immune
cells had a markedly lower 1-year overall survival rate than
patients with high IFN-γ production.
However, the expression of XPC and IFN-γ in lung
cancer tissue has yet to be studied. In the present study, 140 lung
cancer tissue samples were collected, and the correlation between
the expression of XPC and IFN-γ in lung cancer tissue and patient
prognosis was analysed. In addition, the differences in the
expression of XPC and IFN-γ were investigated according to the
histopathological characteristics of different patients with lung
cancer.
Materials and methods
The Cancer Genome Atlas (TCGA)
analysis
Using the relevant clinical data of TCGA database,
the relationship between the expression of XPC and the
clinicopathological characteristics of patients with lung cancer
was statistically analysed. There are no data on IFN-γ in TCGA
database. The present study complied with the National Institutes
of Health TCGA Human Guidelines Subject Protection and Data Access
Policy, and the results shown are in whole or part based upon data
generated by the TCGA Research Network: cancer.gov/tcga (19).
Specimens
Wax blocks of lung adenocarcinoma tissues from
patients with lung cancer with a pathological diagnosis were
collected from Weifang Second People's Hospital (Weifang, China)
between January 2011 and December 2015. Confirmation of diagnosis
was conducted by two independent pathologists using H&E
staining. For this, tissues were fixed using 4% paraformaldehyde at
25°C for 24 h and embedded in paraffin. Sections were cut into 5-µm
thick slices and mounted on adhesive slides using a sectioning
machine. The slices were then baked at 60°C for 2 h. Subsequently,
the tissue sections were rehydrated using xylene and a gradient of
100-70% alcohol, stained with hematoxylin at room temperature for 7
min and eosin at room temperature for 1 min. Finally, the sections
were dehydrated in an increasing series of alcohol (70–100%) and
sealed with a neutral resin. Observations were conducted using a
light microscope under a 20× objective and 10× eyepiece. Tumor
specimens were collected from 140 patients, from which 48
paracancerous samples were also obtained, and made into one tissue
microchip by Shanghai Outdo Biotech Co., Ltd. The tissue microchip
was used for subsequent experiments. Patient prognosis and survival
time were obtained via telephone follow-up.
The present study protocols were reviewed and
approved by the ethics committee of Weifang Second People's
Hospital (YX2020-001-01). The study complied with the relevant
regulations of the Declaration of Helsinki and was conducted under
the supervision of the hospital ethics committee.
Tissue microarray and
immunohistochemistry (IHC)
Immunohistochemical staining with XPC and IFN-γ
antibodies was performed on a microchip to analyse the protein
expression in NSCLC tissues. The tissues embedded in paraffin,
which were used for HE staining in the previous step, were
processed into a tissue chip using a tissue microarray spotter. The
tissue chip was then baked at 55°C for 4 h to fix the tissue.
Subsequently, the tissue chip was sliced into 4-µm wax slices using
a tissue slicer and affixed to adhesive slides. After xylene and
gradient alcohol treatment to rehydrate the tissues, the tissues
were repaired by microwave heating and boiling in Citrate Antigen
Repair Solution (3.23 g/l; pH, 6; cat. no. ZLI-9064; Beijing
Zhongshan Jinqiao Biotechnology Co.) for 30 min, followed by
incubation with 3% hydrogen peroxide for 5 min at room temperature,
and then incubation with 3% goat serum (cat. no. SL038; Sorabio)
for 1 h. Subsequently, the tissues were incubated with either XPC
Rabbit Polyclonal Primary Antibody (1:50; cat. no. ab155025; Abcam)
or IFN-γ Rabbit Polyclonal Primary Antibody (1:1,000; cat. no.
ab25101; Abcam) overnight (>12 h) at 4°C. The tissue sections
were then incubated with an HRP-conjugated goat-conjugated
anti-rabbit secondary antibody (1:500; cat. no. ab6721; Abcam) for
4 h at room temperature. Hematoxylin staining (cat. no. H8070;
Sorabio) at room temperature for 7 min. Finally, sections were
dehydrated in an increasing series of alcohol (70–100%) and sealed
with a neutral resin. The IHC images were collected using a
Panoramic MIDI light microscope (3DHISTECH, Ltd.), and the
percentage of positive IHC signals was analyzed using Density Quant
version 2.3 (3DHISTECH, Ltd.). According to the IHC staining
intensity, samples were grouped according to the percentage of
positive cells. A positive rate >90% was considered to indicate
high expression and was marked as XPChigh or
IFN-γhigh; a positive rate <90% was considered to
indicate low expression and was marked as XPClow or
IFN-γlow.
Statistical analysis
SPSS (version 22; IBM Corp.) was used to perform a
χ2-test and analyze the expression of XPC and IFN-γ in
NSCLC tissues, and the relationship between the expression of XPC
or IFN-γ and the clinicopathological characteristics of patients
with NSCLC. The association between XPC and IFN-γ was calculated
using Pearson's correlation coefficient. A Kaplan-Meier survival
curve was used to perform survival analysis, and the log-rank test
was applied to evaluate statistical significance. A unpaired t-test
was used to compare two groups of TCGA data, while one-way ANOVA
was used to analyze the TCGA data from ≥3 groups. The log-rank test
was used for survival curve analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
XPC is expressed at low levels in lung
adenocarcinoma tissues in TCGA database
The expression of XPC, a nucleic acid splice repair
gene, was significantly different in various cancers (Fig. 1A). In the present study, the
relationship between the expression of XPC in 502 lung cancer
tissues in TCGA database and patient prognosis was first analyzed.
The results showed that patients with high XPC expression had a
better prognosis than those patients with low XPC expression;
however, the difference was not significant (P=0.083; Fig. 1B).
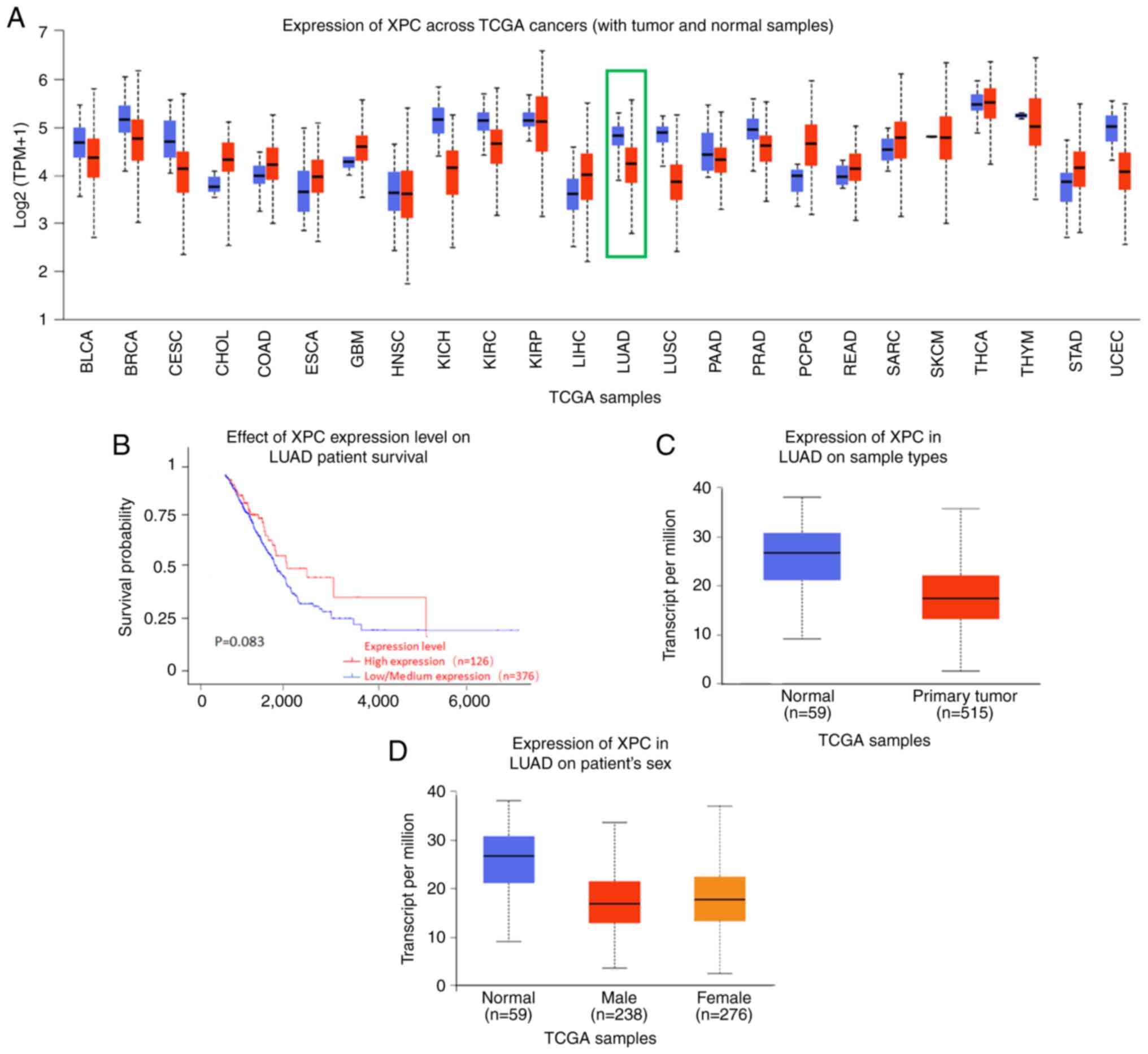 | Figure 1.Expression of XPC in TCGA cohort. (A)
Expression of XPC across TCGA cancer cohorts; the green box
represents XPC in LUAD. There are 515 cases of tumor tissue in red
and 59 cases of normal tissue in blue. (B) Effect of the XPC
expression level on LUAD patient survival. (C) Expression of XPC in
LUAD tissues based on sample type. (D) Expression of XPC in LUAD
based on patient sex. LUAD, lung adenocarcinoma; TCGA, The Cancer
Genome Atlas; XPC, xeroderma pigmetosum group C; BLCA, bladder
urothelial carcinoma; BRCA, breast invasive carcinoma; CESC,
cervical squamous cell carcinoma and endocervical adenocarcinoma;
CHOL, cholangio carcinoma; COAD, colon adenocarcinoma; ESCA,
esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and
neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC,
kidney renal clear cell carcinoma; KIRP, kidney renal papillary
cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung
adenocarcinoma; LUSC, lung squamous cell carcinoma; PAAD,
pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; PCPG,
pheochromocytoma and paraganglioma; READ, rectum adenocarcinoma;
SARC, sarcoma; SKCM, skin C=cutaneous melanoma; THCA, thyroid
carcinoma; THYM, thymoma; STAD, stomach adenocarcinoma; UCEC,
uterine corpus endometrial carcinoma. |
In addition, the expression of XPC in 574 lung
cancer tissues including 515 lung cancer tissues and 59 adjacent
normal tissues was analysed. Box plots revealed that the expression
of XPC in lung tumor tissues was significantly different; compared
with the XPC expression in normal tissues, XPC expression was
significantly lower in lung tumor tissues (Fig. 1C). In addition, the expression of
XPC in lung tumor tissues was not related to sex (Fig. 1D).
Expression of XPC and IFN-γ is
positively correlated in the tumor tissues of patients with
NSCLC
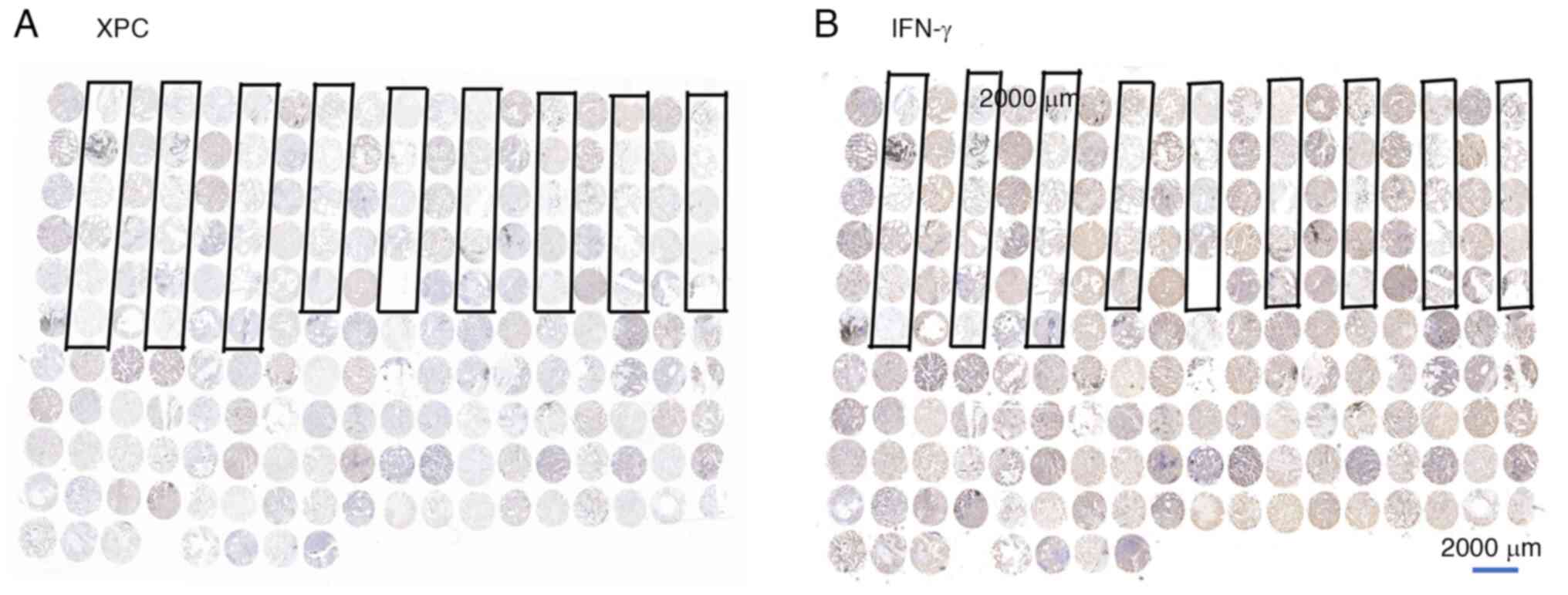
Immunohistochemical images of samples from all
patients are presented in Fig. 2.
The area encircled by the black box represents a sample of
paracancerous tissue. Based on the calculation method of Pearson's
correlation coefficient mentioned by Akoglu H (20), after Pearson's correlation
coefficient, the results showed a correlation between XPC and IFN-γ
in the tissues of patients with NSCLC. To investigate whether the
expression of XPC was associated with that of IFN-γ in the tissues
of patients with NSCLC, immunohistochemical analysis using cancer
tissues from 140 patients with NSCLC was performed. There was a
significant positive correlation between the expression of XPC and
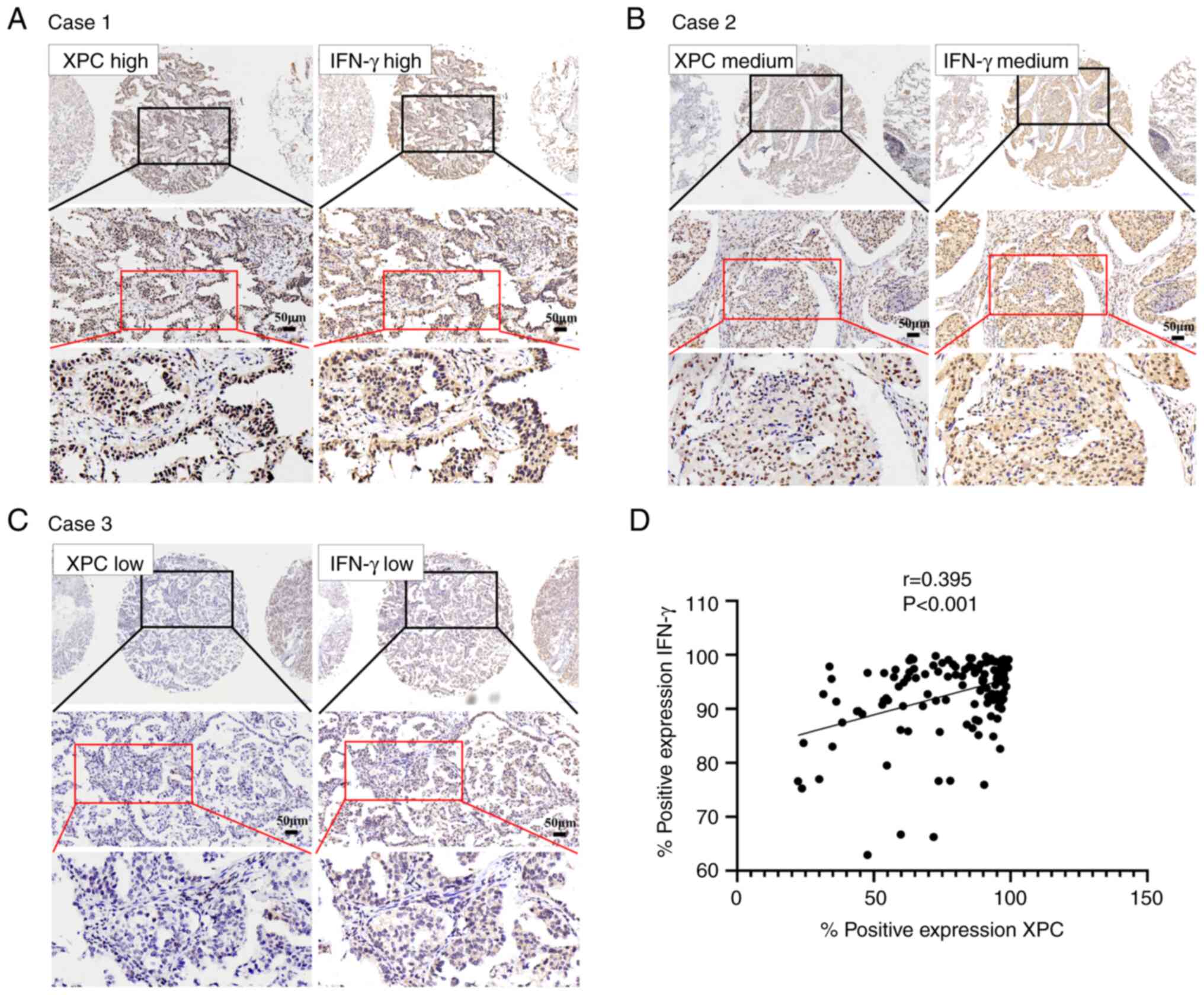
that of IFN-γ. In case 1, XPC and IFN-γ were expressed at high
levels (Fig. 3A). In case 2, both
XPC and IFN-γ were moderately expressed (Fig. 3B). In case 3, the expression levels
of XPC and IFN-γ were weak (Fig.
3C). The expression of XPC and IFN-γ in NSCLC tissues was
significantly positively correlated (r=0.395; P<0.01; Fig. 3D).
XPC and IFN-γ expression is associated
with a better prognosis in patients with NSCLC
Given the correlation between the expression of XPC
and IFN-γ in NSCLC tissues, the relationship between the
co-expression of XPC and IFN-γ and the prognosis of patients with
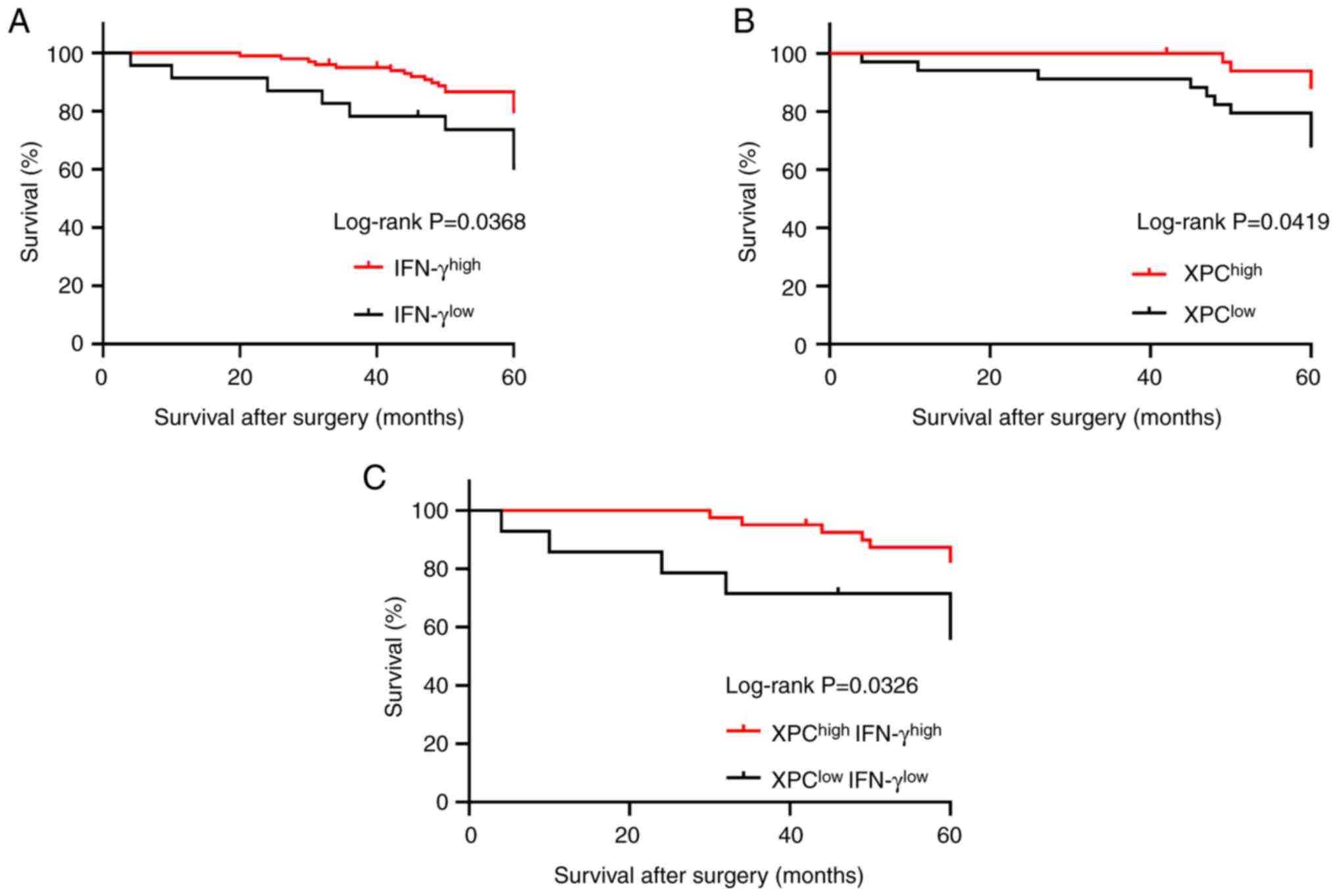
NSCLC was analyzed. Our previous study revealed that high XPC
expression was positively correlated with a good prognosis in
patients (8). High expression of
IFN-γ was positively correlated with good patient prognosis
(log-rank; P=0.0368, Fig. 4A),
whereas analysis of XPC expression alone also showed a significant
positive correlation between XPC expression and good patient
prognosis (log-rank; P=0.0419, Fig.
4B). In addition, based on the expression of XPC and IFN-γ,
patients were divided into the
XPChighIFN-γhigh and
XPClowIFN-γlow groups and Kaplan-Meier
survival analysis was carried out. The survival time of patients in
the XPChighIFN-γhigh group was significantly
longer than that of patients in the
XPClowIFN-γlow group (log-rank, P=0.0326;
Fig. 4C).
IFN-γ expression is associated with
tumor metastasis in patients with NSCLC
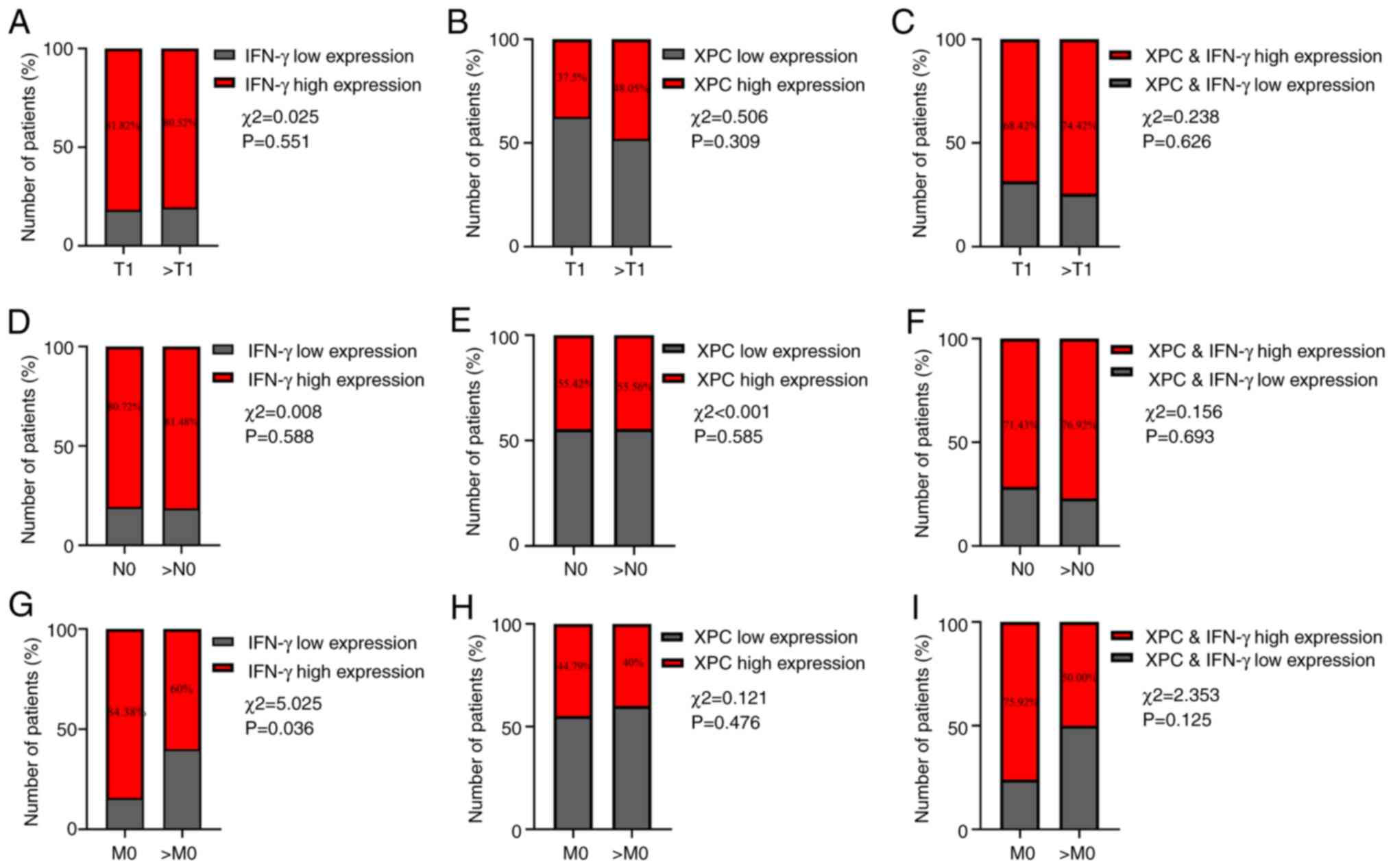
To further study the significance of XPC and IFN-γ
expression in the occurrence, metastasis and recurrence of NSCLC,
the association of XPC and IFN-γ expression with the TNM stage was
investigated (21). There was no
significant difference in the expression of IFN-γ at different
tumor stages or N-phases (Fig. 5),
but there was a significant difference in the expression of IFN-γ
with tumor metastasis (P=0.036; Fig.
5H). However, XPC expression was not strongly associated with
patient TNM stage.
Discussion
Lung cancer can be divided into SCLC and NSCLC
according to histological classification (22). NSCLC is the most common subtype of
lung cancer and can be divided into adenocarcinoma, squamous cell
cancer and large-cell lung cancer. With its high incidence and
recurrence, a better understanding of NSCLC is required for lung
cancer treatment (23–26).
Genomic instability is the primary factor promoting
the occurrence and malignant transformation of cancer (27). XPC is a protein that recognizes
damage and is involved in the nucleotide excision repair pathway
(7,28,29).
Studies have shown that low XPC expression is associated with poor
outcomes in patients with NSCLC (8,30).
However, a study by Teng et al (31) showed that upregulation of XPC
expression could increase the resistance of lung cancer cells to
the chemotherapeutic drug cisplatin through the PI3K/AKT signaling
pathway. This phenomenon has not been validated in animal models or
clinical studies. It is hypothesized that the complex tumor
microenvironment and immune microenvironment in tumor tissues can
affect the physiological function of tumors. Cytological and animal
experiments have shown that reduced XPC expression can promote lung
cancer cell proliferation and migration (32).
IFN-γ is a key moderator of cellular immunity and is
secreted by activated T lymphocytes, gd cells and natural killer
cells (33,34). Studies have indicated that IFN-γ
acts as an antitumor factor by activating the immune system and
triggering tumor cell apoptosis (35–37).
It has also been shown that IFN-γ protects tumor cells during
immune checkpoint blockade (ICB) therapy by inducing
CD4+ T cell apoptosis and facilitating tumor cell
evasion from CD8+ T cell cytotoxicity (38). It is noteworthy that IFN-γ exerts
this function as a modulatory effect during the application of ICB.
In general, the biological effects of IFN receptor signaling are
regulated by three main factors: i) Expression profile of IFN
itself; ii) profile of the receptor; and iii) expression of the
target gene (39). Activated IFNs
can directly initiate gene transcription and multiple downstream
signaling pathways, leading to a variety of cellular responses,
such as cell cycle arrest and apoptosis in tumor cells (40). Moreover, the prognostic and
pathological characteristics of patients with lung cancer need to
be studied further to determine the clinical significance of
IFN-γ.
Both XPC and IFN-γ play important roles in cancer
progression. However, the correlation between XPC and IFN-γ has not
been studied. Our previous study revealed that low XPC expression
could not only promote the proliferation and migration of lung
cancer cells but also increase the stem cell characteristics of
lung cancer cells (8). Song et
al (41) reported that low
expression of IFN-γ could confer stem cell properties to lung
cancer cells through the ICAM1-PI3K-AKT-NOTCH1 pathway, while high
expression of IFN-γ could induce apoptosis in NSCLC through
activation of the JAK1-STAT1-caspase pathway. Low levels of XPC and
IFN-γ can promote the characteristics of lung cancer stem cells. To
further investigate the correlation between XPC and IFN-γ
expression in lung cancer tissues in the present study, the
expression of XPC and IFN-γ was investigated in the tumor tissues
of 140 patients with NSCLC; it was found that the expression of XPC
and IFN-γ in NSCLC was significantly positively correlated.
Moreover, patients with high IFN-γ expression had a favorable
prognosis. The TNM staging results indicated a decreased proportion
of patients with low IFN-γ expression at stage M0, suggesting
distant metastasis of tumor cells. As previously discussed, both
XPC and IFN-γ can significantly influence the stemness of lung
cancer cells during tumor progression. Through TNM staging
analysis, it was discovered that patients with high XPC and INF-γ
expression were more likely to develop distal metastases. It is
hypothesized that this difference may be linked to the increased
stemness of the tumor cells mentioned earlier. The significant
correlation between changes in the expression of the two genes
suggested a potential link between them in this process. Although
the survival curves indicated that the expression of both IFN-γ and
XPC significantly affects patient prognosis, the sample size
limited further investigations of the correlation between the two.
This limitation led to the hypothesis that a more pronounced
synergistic effect was not observed. Upon analyzing the data from
public databases, significant variability in XPC expression in
patients was observed, with standard errors >20% in some cases.
While this variability is attributed to individual patient
differences, it could still potentially influence patient
prognosis. In future studies, the number of clinical samples will
be increased and the association between the JAK/STAT pathway, and
IFN-γ and tumor cell stemness will be investigated to elucidate the
combined impact of the two on tumor cell stemness. These findings
could lead to future studies investigating the interaction between
XPC and IFN-γ and the mechanism through which XPC and IFN-γ affect
the occurrence and recurrence of lung cancer.
In conclusion, the current study is the first to
report the prognostic significance of the combination of XPC and
IFN-γ detected by IHC in patients with lung cancer. The present
study provides novel evidence that links XPC and IFN-γ expression
in lung cancer. Evaluation of the expression of XPC and IFN-γ may
lead to the identification of novel prognostic factors and
therapeutic strategies for lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81871892), the Science and
Technology Development Project of Weifang (grant nos. 2021YX070 and
2022ZJ1059), the Science and Technology Plan of Shandong Health
Committee (grant no. 2019 WSA07011) and the Scientific Project of
Weifang Health Commission (grant no. WFWSJK-2022-220).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YoW and WW were responsible for collecting and
preparing pathological specimens and conducting the IHC staining.
HW, LQ and MZ conducted patient visits and compiled data. YZ, YuW
and CH designed the experiments and provided guidance for the
writing of the manuscript. MQ and GW developed the experimental
protocol, funded the study and reviewed the reliability of the
data. MQ and GW confirm the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The protocols for the present study were reviewed
and approved by The Ethics Committee of Weifang Second People's
Hospital (Weifang, China; YX2020-001-01); all patients provided
written informed consent. All analyses were conducted in accordance
with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu F, Wang L and Zhou C: Lung cancer in
China: Current and prospect. Curr Opin Oncol. 33:40–46. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bade BC and Dela Cruz CS: Lung Cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shell SM, Hawkins EK, Tsai MS, Hlaing AS,
Rizzo CJ and Chazin WJ: Xeroderma pigmentosum complementation group
C protein (XPC) serves as a general sensor of damaged DNA. DNA
Repair (Amst). 12:947–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou H, Saliba J, Sandusky GE and Sears
CR: XPC protects against smoking- and carcinogen-induced lung
adenocarcinoma. Carcinogenesis. 40:403–411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobaisi F, Sulpice E, Barette C, Fayyad N,
Fauvarque MO, Badran B, Fayyad-Kazan M, Fayyad-Kazan H, Gidrol X
and Rachidi W: Isoconazole and clemizole hydrochloride partially
reverse the xeroderma pigmentosum C phenotype. Int J Mol Sci.
22:81562021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Errico M, Parlanti E, Teson M, de Jesus
BM, Degan P, Calcagnile A, Jaruga P, Bjørås M, Crescenzi M, Pedrini
AM, et al: New functions of XPC in the protection of human skin
cells from oxidative damage. EMBO J. 25:4305–4315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fayyad N, Kobaisi F, Beal D, Mahfouf W,
Ged C, Morice-Picard F, Fayyad-Kazan M, Fayyad-Kazan H, Badran B,
Rezvani HR and Rachidi W: Xeroderma pigmentosum C (XPC) mutations
in primary fibroblasts impair base excision repair pathway and
increase oxidative DNA damage. Front Genet. 11:5616872020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Ma S, Ding Z, Yang Y, Wang H, Yang
K, Cai X, Li H, Gao Z and Qu M: XPC protein improves lung
adenocarcinoma prognosis by inhibiting lung cancer cell stemness.
Front Pharmacol. 12:7079402021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, He N, Gu D, Wickliffe J, Salazar
J, Boldogh I and Xie J: Genetic Evidence for XPC-KRAS interactions
during lung cancer development. J Genet Genomics. 42:589–596. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abolfathi H, Sheikhpour M, Shahraeini SS,
Khatami S and Nojoumi SA: Studies in lung cancer cytokine
proteomics: A review. Expert Rev Proteomics. 18:49–64. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marrugal A, Ojeda L, Paz-Ares L,
Molina-Pinelo S and Ferrer I: Proteomic-Based approaches for the
study of cytokines in lung cancer. Dis Markers. 2016:21386272016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X
and Li L: IFN-ү-mediated inhibition of lung cancer correlates with
PD-L1 expression and is regulated by PI3K-AKT signaling. Int J
Cancer. 143:931–943. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning
W, Zeng H, Zhang N, Du W, Chen C and Huang JA: PD-L1 induced by
IFN-ү from tumor-associated macrophages via the JAK/STAT3 and
PI3K/AKT signaling pathways promoted progression of lung cancer.
Int J Clin Oncol. 22:1026–1033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang C, Weng T, Hu S, Yuan Z, Xiong H,
Huang B, Cai Y, Li L and Fu X: IFN-ү-induced ER stress impairs
autophagy and triggers apoptosis in lung cancer cells.
Oncoimmunology. 10:19625912021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasim F, Sabath BF and Eapen GA: Lung
cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kgokolo MCM, Anderson K, Siwele SC, Steel
HC, Kwofie LLI, Sathekge MM, Meyer PWA, Rapoport BL and Anderson R:
Elevated levels of soluble CTLA-4, PD-1, PD-L1, LAG-3 and TIM-3 and
systemic inflammatory stress as potential contributors to immune
suppression and generalized tumorigenesis in a cohort of South
African xeroderma pigmentosum patients. Front Oncol. 12:8197902022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu YH, Wu TC, Liao JW, Yeh KT, Chen CY and
Lee H: p53 dysfunction by xeroderma pigmentosum group c defects
enhance lung adenocarcinoma metastasis via increased Mmp1
expression. Cancer Res. 70:10422–10432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahn SS, Kwon M, Sung M, Jung SM, Lee SW,
Park YB, Kim ST and Song JJ: Ex Vivo Interferon gamma production by
peripheral immune cells predicts survival in lung adenocarcinoma.
Clin Lung Cancer. 20:e299–e308. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carrot-Zhang J, Yao X, Devarakonda S,
Deshpande A, Damrauer JS, Silva TC, Wong CK, Choi HY, Felau I,
Robertson AG, et al: Whole-genome characterization of lung
adenocarcinomas lacking alterations in the RTK/RAS/RAF pathway.
Cell Rep. 34:1087842021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akoglu H: User's guide to correlation
coefficients. Turk J Emerg Med. 18:91–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asamura H, Nishimura KK, Giroux DJ,
Chansky K, Hoering A, Rusch V and Rami-Porta R; Members of the
IASLC Staging and Prognostic Factors Committee of the Advisory
Boards, and Participating Institutions, : IASLC lung cancer staging
project: The new database to inform revisions in the Ninth Edition
of the TNM classification of lung cancer. J Thorac Oncol.
18:564–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Villalobos P and Wistuba II: Lung cancer
biomarkers. Hematol Oncol Clin North Am. 31:13–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao C, Kong N, Zhang F, Zhou L, Xu M and
Wu L: Development and validation of the potential biomarkers based
on m6A-related lncRNAs for the predictions of overall survival in
the lung adenocarcinoma and differential analysis with cuproptosis.
BMC Bioinformatics. 23:3272022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruiz-Cordero R and Devine WP: Targeted
therapy and checkpoint immunotherapy in lung cancer. Surg Pathol
Clin. 13:17–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodriguez-Canales J, Parra-Cuentas E and
Wistuba II: Diagnosis and molecular classification of lung cancer.
Cancer Treat Res. 170:25–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo C, Lei M and Zhang Y, Zhang Q, Li L,
Lian J, Liu S, Wang L, Pi G and Zhang Y: Systematic construction
and validation of an immune prognostic model for lung
adenocarcinoma. J Cell Mol Med. 24:1233–1244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lundin A and Driscoll B: Lung cancer stem
cells: Progress and prospects. Cancer Lett. 338:89–93. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugasawa K: XPC: Its Product and
Biological Roles. Molecular Mechanisms of Xeroderma Pigmentosum.
Advances in Experimental Medicine and Biology. Ahmad SI and Hanaoka
F: Volume 637. Springer; New York, NY: 2008, PubMed/NCBI
|
|
29
|
Nemzow L, Lubin A, Zhang L and Gong F:
XPC: Going where no DNA damage sensor has gone before. DNA Repair
(Amst). 36:19–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui T, Srivastava AK, Han C, Yang L, Zhao
R, Zou N, Qu M, Duan W, Zhang X and Wang Q-E: XPC inhibits NSCLC
cell proliferation and migration by enhancing E-Cadherin
expression. Oncotarget. 6:10060–10072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Teng X, Fan XF, Li Q, Liu S, Wu DY, Wang
SY, Shi Y and Dong M: XPC inhibition rescues cisplatin resistance
via the Akt/mTOR signaling pathway in A549/DDP lung adenocarcinoma
cells. Oncol Rep. 41:1875–1882. 2019.PubMed/NCBI
|
|
32
|
Nasrallah NA, Wiese BM and Sears CR:
Xeroderma pigmentosum complementation group C (XPC): Emerging roles
in non-dermatologic malignancies. Front Oncol. 12:8469652022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Medler TR, Murugan D, Horton W, Kumar S,
Cotechini T, Forsyth AM, Leyshock P, Leitenberger JJ, Kulesz-Martin
M, Margolin AA, et al: Complement C5a fosters squamous
carcinogenesis and limits T cell response to chemotherapy. Cancer
Cell. 34:561–578.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weichselbaum RR, Liang H, Deng L and Fu
YX: Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev
Clin Oncol. 14:365–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burke F, East N, Upton C, Patel K and
Balkwill FR: Interferon gamma induces cell cycle arrest and
apoptosis in a model of ovarian cancer: Enhancement of effect by
batimastat. EJC. 33:1114–1121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schoenborn JR and Wilson CB: Regulation of
interferon-gamma during innate and adaptive immune responses. Adv
Immunol. 96:41–101. 2007.PubMed/NCBI
|
|
37
|
Shin EC, Ahn JM, Kim CH, Choi Y, Ahn YS,
Kim H, Kim SJ and Park JH: IFN-gamma induces cell death in human
hepatoma cells through a TRAIL/death receptor-mediated apoptotic
pathway. Int J Cancer. 93:262–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Minn AJ and Wherry EJ: Combination cancer
therapies with immune checkpoint blockade: Convergence on
interferon signaling. Cell. 165:272–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galani V, Kastamoulas M, Varouktsi A,
Lampri E, Mitselou A and Arvanitis DL: IFNs-signaling effects on
lung cancer: An up-to-date pathways-specific review. Clin Exp Med.
17:281–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee KS, Chung WY, Park JE, Jung YJ, Park
JH, Sheen SS and Park KJ: Interferon-ү-Inducible chemokines as
prognostic markers for lung cancer. Int J Environ Res Public
Health. 18:93452021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song M, Ping Y, Zhang K, Yang L, Li F,
Zhang C, Cheng S, Yue D, Maimela NR, Qu J, et al: Low-Dose IFNү
induces tumor cell stemness in tumor microenvironment of non-small
cell lung cancer. Cancer Res. 79:3737–3748. 2019. View Article : Google Scholar : PubMed/NCBI
|