Introduction
Tailgut cyst is a rare congenital cystic lesion
occurring in the retrorectal space between the rectum and the
sacrum and above the retrorectal fascia (1). This lesion is presumed to arise from
the remnant of the primitive tailgut and is hypothesized to exist
due to incomplete regression of the tail extension of the hindgut
(1). Less than 200 patients with
tailgut cyst have been reported in the English-language literature
(1). This cyst mainly affects
middle-aged females. Lower back or lower abdominal pain, or urinary
symptoms are the common presentations, and incidental detection of
the lesion without any symptoms is also not uncommon (1,2). The
cysts are usually multilocular and smooth muscle bundles are
irregularly distributed in numerous cases (1,2). The
wall is covered by several types of epithelia, including columnar,
ciliated, keratinizing or non-keratinizing squamous, and
transitional types (1,2).
Of note, the development of neoplastic lesions
arising from tailgut cyst has been reported (2,3). A
recent meta-analysis showed that 26.6% (51 of 196 patients) had a
neoplastic lesion in a tailgut cyst, although this result is likely
to be biased for frequency of the reported cases of neoplastic
transformation (3). In a
retrospective analysis of 70 consecutive patients with tailgut
cyst, the rate of the neoplastic lesion was ~9% (2). Various types of neoplastic lesion may
occur in tailgut cyst, and adenocarcinoma, not otherwise specified,
or mucinous adenocarcinoma and neuroendocrine tumors are the most
common types of the neoplastic lesions (2,3). These
results suggest that the histopathology of the neoplastic lesions
reflects the type of epithelium within tailgut cysts. However, due
to the rarity of the tumor, the histogenesis of the neoplastic
lesions arising from tailgut cyst has remained elusive. In the
present article, the clinicopathological features of tailgut cyst
were retrospectively analysed, in particular regarding the
development of neoplastic lesions.
Materials and methods
Patient selection
Consecutive patients with tailgut cyst who underwent
surgical resection at the Department of Surgery at Kansai Medical
University Hospital between January 2014 and December 2019 were
included in the present study. The diagnostic criteria for tailgut
cyst were as follows: A cystic lesion is present in the retrorectal
space between the rectum and the sacrum and above the retrorectal
fascia on imaging and intraoperative findings, and,
histopathologically, the cystic wall is covered by columnar,
ciliated, non-keratinizing or keratinizing squamous, or
transitional epithelia, usually surrounded by smooth muscle bundles
without a teratomatous component, according to review articles
(1,2). Accordingly, four patients with tailgut
cyst were included in this study.
Histopathological analyses were performed on the
epithelial component in the cystic wall and the neoplastic lesions.
Thereafter, immunohistochemical analysis was performed in Patient
4.
This retrospective, single-institution study was
conducted according to the principles of the Declaration of
Helsinki and the study protocol was approved by the Institutional
Review Board of Kansai Medical University Hospital (Hirakata,
Japan; approval no. 2019088). All data were anonymized. The
institutional review board waived the requirement for informed
consent because of the retrospective study design, as medical
records and archived samples were used with no risk to the
participants. Furthermore, the present study did not include
minors. For information regarding this study, the opt-out method
waived the requirement for informed patient consent when using
patient samples in research. A consent form for publication in
Japanese was signed by each patient.
Histopathological analysis
Surgically resected specimens were fixed with 10%
buffered formalin for 24–48 h. These specimens were dehydrated in
ethanol and xylene at room temperature and embedded in paraffin
(60°C). Following the preparation of 4-µm sections, they were
stained with haematoxylin and eosin at room temperature according
to a standard protocol. Two researchers (TK and MI) independently
evaluated the histopathological features of all the slides under a
microscope.
Immunohistochemical analysis
The tumor from Patient 4 was immunohistochemically
analyzed. The 4-µm tumor tissue sections underwent
immunohistochemical staining using autostainers (Ultra System;
Roche Diagnostics; or Autostainer link 48; DAKO; Agilent
Technologies, Inc.), according to the manufacturers' instructions.
Sections were incubated with a rabbit monoclonal antibody against
caudal type homeobox 2 (CDX2) (cat. no. EPR2764Y; pre-diluted;
Roche Diagnostics), a mouse monoclonal antibody against cytokeratin
(CK)-7 (cat. no. OV-TL12/30; pre-diluted; DAKO; Agilent
Technologies, Inc.), a mouse monoclonal antibody against CK-20
(cat. no. Ks20.8; pre-diluted; DAKO; Agilent Technologies, Inc.)
and a mouse monoclonal antibody against p53 (cat. no. DO-7; diluted
1:50; DAKO; Agilent Technologies, Inc.) for 20 min at room
temperature. Secondary antibodies were pre-diluted [Optiview DAB
Universal Kit (cat. no. 518-111427; Roche Diagnostics) and
Envision™ FLEX (cat. no. K8000; DAKO; Agilent Technologies, Inc.]
and were used to incubate the sections for 8 min at room
temperature. Two researchers (TK and MI) independently evaluated
the immunohistochemical staining under a microscope.
Results
Patient characteristics
Table I summarises
the clinicopathological features of the patients of the present
study. A total of one female and three male patients were included
in this study. No symptoms were present in two patients, and
discomfort of the perineal region was described in one patient
(Patient 1). The remaining patient presented with constipation and
urinary retention (Patient 4). Only one patient (Patient 2) had a
past history of surgery for a tailbone tumor at the age of 1 year
(detailed information was not available).
 | Table I.Clinicopathological features of
patients with tailgut cyst of the present cohort. |
Table I.
Clinicopathological features of
patients with tailgut cyst of the present cohort.
| Patient no. | Sex | Age, years | Size, mm | Symptoms | Past history | Serum tumor
markersa | Magnetic resonance
imaging findings | Method of
excision | Ciliated
epithelium | Squamous
epithelium | Neoplastic
lesion | Outcome |
|---|
| 1 | M | 38 | 90×60×55 | Perineal
discomfort | None | CEA, 1.7 ng/ml;
CA19-9, 12.3 U/ml | Multiloculated
lesion. | Kraske procedure | + | +
(non-keratinizing) | Dysplasia | No recurrence seven
years after surgery |
|
|
|
|
|
|
|
| Hypointense on T1WI,
hyperintense on T2WI |
|
|
|
|
|
| 2 | F | 52 | 40×35×35 | None | Tailbone tumor (1
year old) | CEA, 3.4, ng/ml;
CA19-9, 14.1 U/ml | Cystic lesion
accompanied by daughter cyst. | Kraske procedure | + | + (keratinizing) | None | No recurrence at two
years and six months after surgery |
|
|
|
|
|
|
|
| Hypointense on T1WI,
hyperintense on T2WI |
|
|
|
|
|
| 3 | M | 39 | 40×35×35 | None | Vertebral
compression | CEA, 2.7 ng/ml;
CA19-9, 26.9 U/ml | Focal cystic
lesion. | Kraske procedure | + | +
(non-keratinizing) | None | No recurrence two
years |
|
|
|
|
|
| fracture by falling
accident |
| Hypointense on T1WI,
hyperintense on T2WI |
|
|
|
| after surgery |
| 4 | M | 69 | 150×100×88 | Constipation and
urinary retention | None | CEA, 1.9 ng/ml;
CA19-9, 14.2 U/ml | Large cyst with
numerous small septa with a mass with a solid component. | Hartmann's
procedure | + | +
(non-keratinizing) | Adenocarcinoma, not
otherwise specified, with dysplasia | Cystic lesion
recurred two years after surgery, and no adenocarcinoma
present |
|
|
|
|
|
|
|
| Hypointense on T1WI,
hyperintense on T2WI |
|
|
|
|
|
Laboratory tests demonstrated that the serum tumor
markers carcinoembryonic antigen and carbohydrate antigen 19-9 were
within the normal limits in all cases (Table I).
Magnetic resonance imaging (MRI) showed that all
lesions were hypointense on T1-weighted imaging (T1WI) and
hyperintense on T2WI. MRI T2WI sagittal images of cases 1–4 are
presented in Fig. 1A-D,
respectively. The cystic lesion in case 4 was enlarged laterally on
the left in the MRI-axial image. A solid mass was present along
with a large cystic lesion accompanying numerous septa in Patient 4
(Fig. 1D).
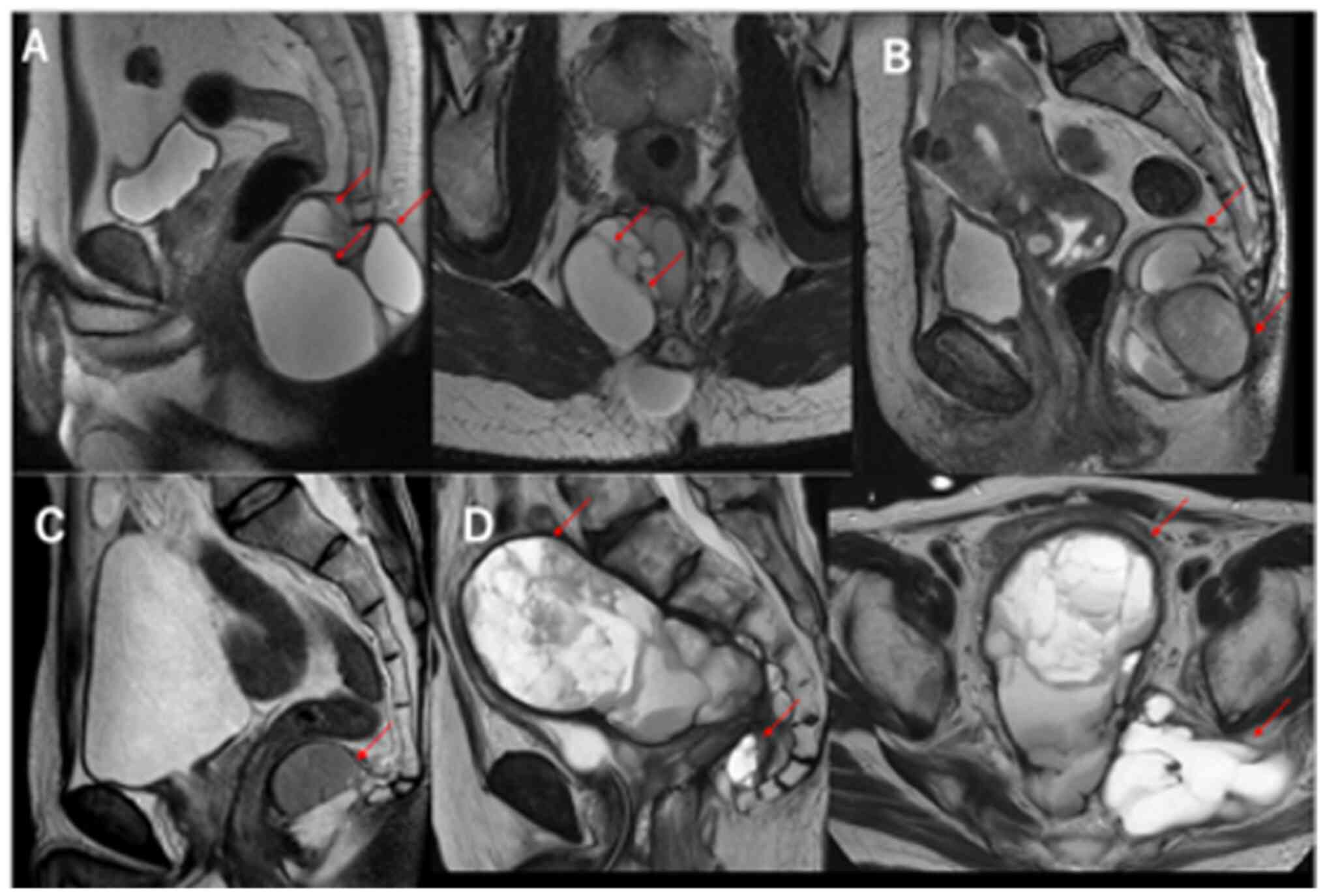 | Figure 1.Magnetic resonance imaging of tailgut
cysts. (A) Well-circumscribed multilocular lesion, hyperintense on
T2W1, in the retrorectal space (Patient 1, arrows). (B) Cystic
lesion accompanied by daughter cyst, hyperintense on T2WI (Patient
2, arrows). (C) Focal cystic lesion, hyperintense on T2WI (Patient
3, arrow). (D) Large cyst with numerous small septa with a mass
with a solid component, hyperintense on T2WI, in the presacral
space (Patient 4, arrows). T2WI, T2-weighted imaging. |
With regard to the surgical technique, the lesions
in three patients were resected using the Kraske procedure with a
combined tailbone resection in the jack-knife position. Hartmann's
procedure was needed in one patient (Patient 4), as the lesion
invaded into the rectum and the parietal peritoneal reflex
developed.
Histopathological and
immunohistochemical characteristics
Macroscopic examination of the cut surface of the
surgically resected specimens revealed multilocular lesions in all
tumors. The cyst wall was composed of fibrous tissue and/or fatty
tissue, and irregularly arranged smooth muscle bundles were
occasionally observed. The cyst wall was covered by squamous and
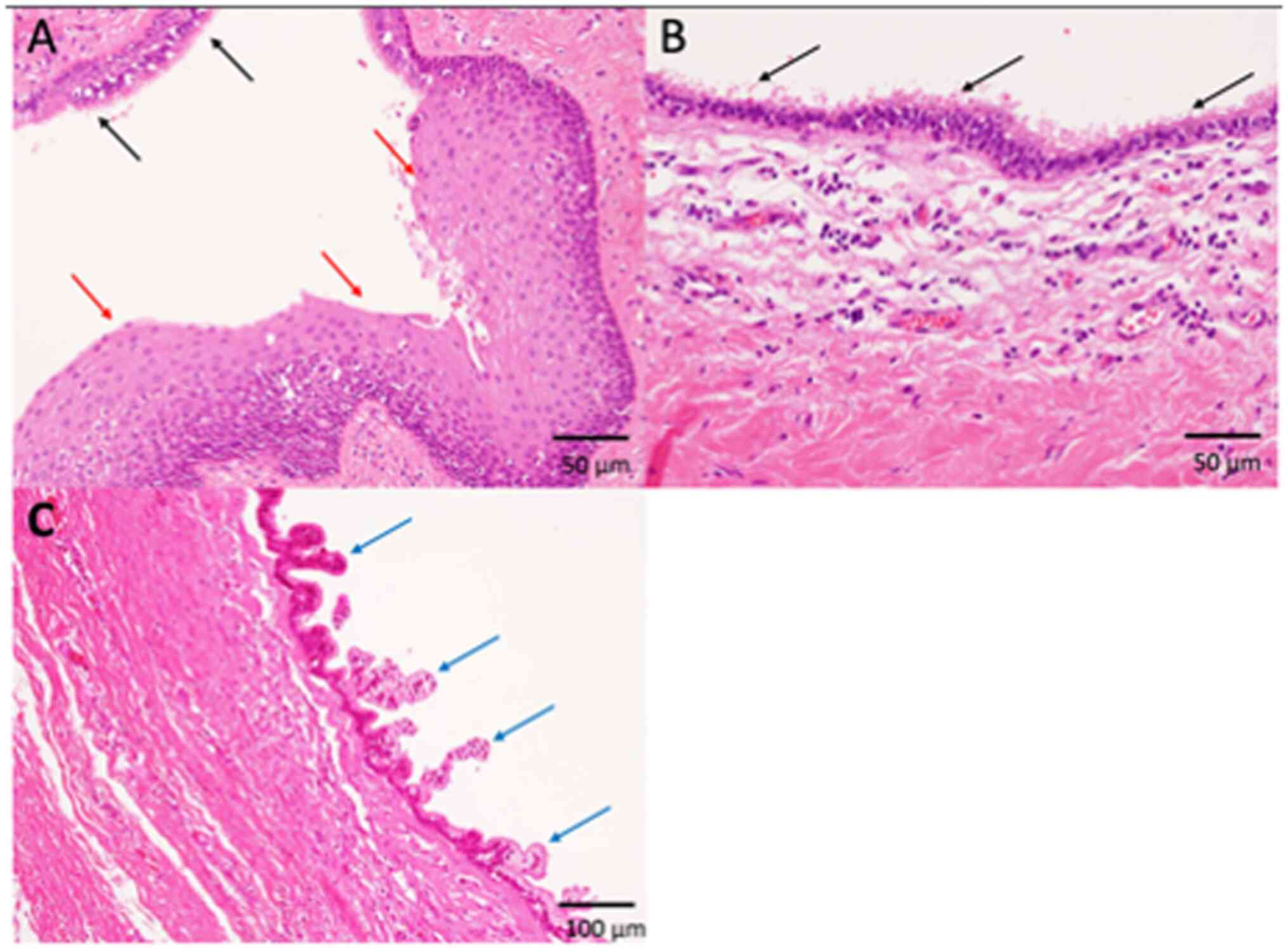
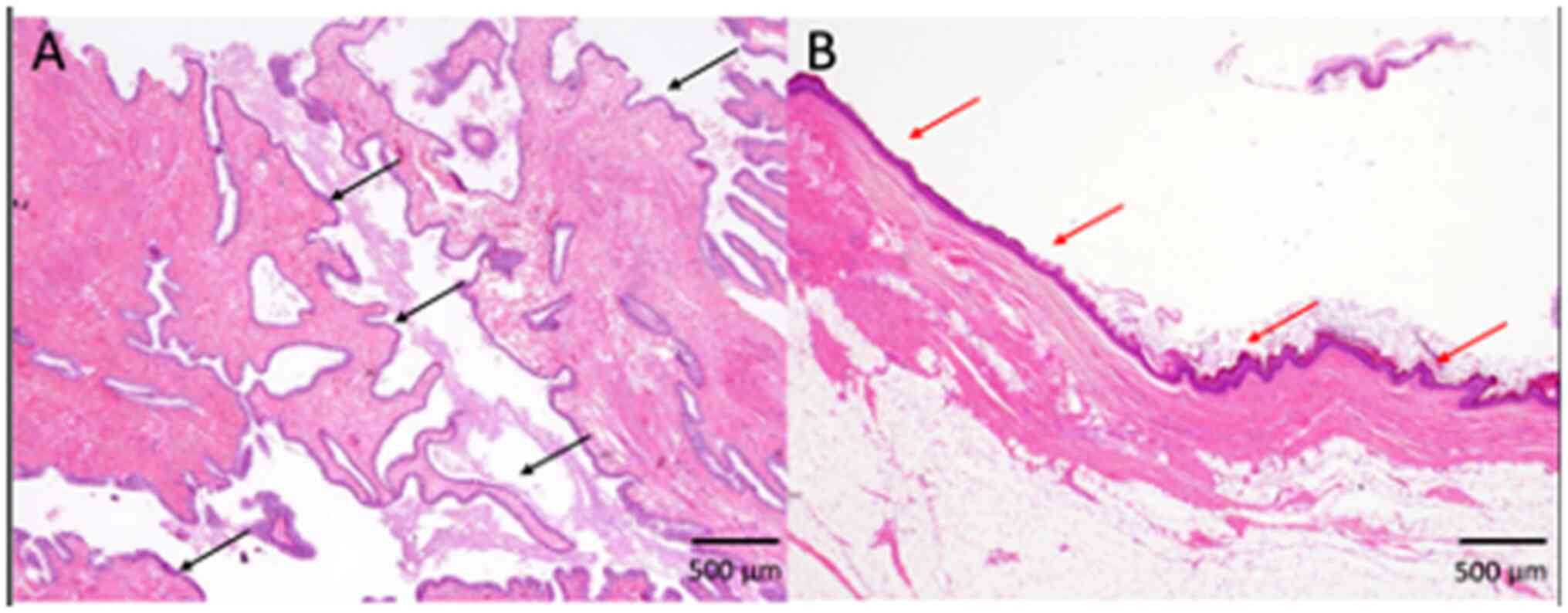
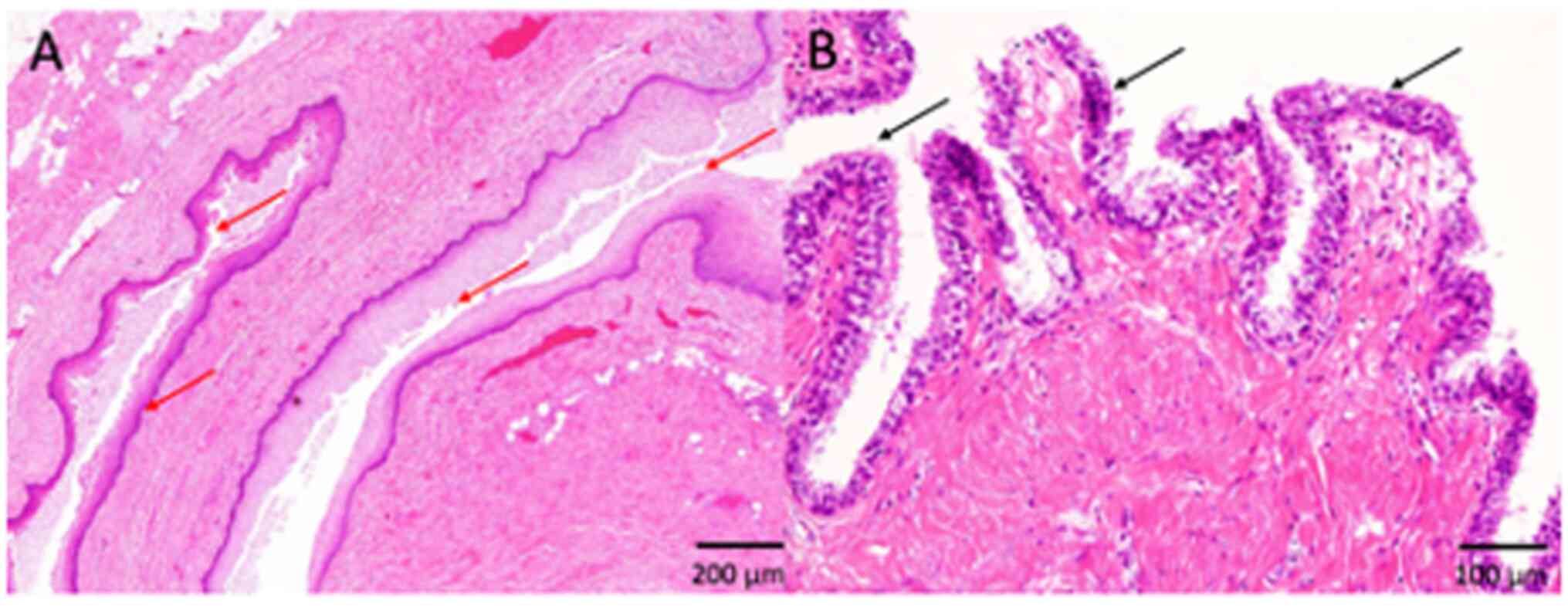
ciliated epithelia in all four tumors (100%) (Fig. 2, Fig.
3, 4; Table I). Keratinization was noted in one
tumor (25%) (Fig. 2), and the
remaining three tumors had non-keratinizing squamous epithelium
(75%) (Figs. 3 and 4; Table
I). These epithelial cells showed no atypia, and no mitotic
figures were observed (Fig. 2,
Fig. 3, 4). No columnar epithelia were noted in any
tumor. Neither skin appendage components, such as hair and
sebaceous glands, nor any other teratomatous components, were
present in any of the patients. According to these
histopathological features and the location of the lesion, a
diagnosis of tailgut cyst was made in all four patients.
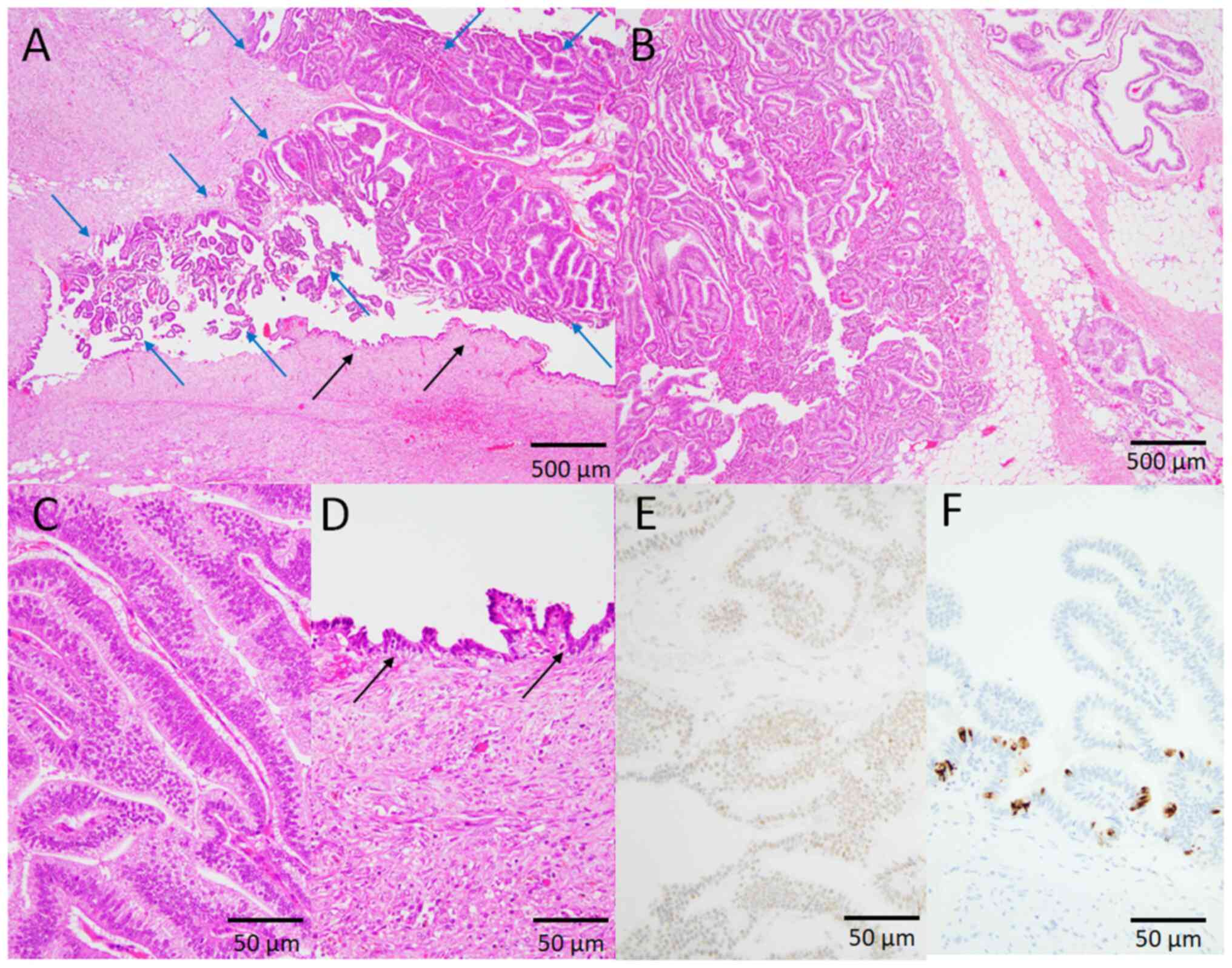
In two patients (50%), the development of the
neoplastic lesions was continuous with the above-mentioned cystic
wall. Dysplastic change was observed in one patient (Patient 1).
Piling-up proliferation of the cuboidal glandular cells with mildly
to moderately enlarged nuclei containing small nucleoli and
intracytoplasmic mucin was observed (Fig. 2). No destructive or invasive
neoplastic growth was observed. In another patient (Patient 4),
infiltrative papillotubular neoplastic growth was observed
(Fig. 5A and B). The columnar
neoplastic cells had large round to oval nuclei and clear to
slightly eosinophilic cytoplasm (Fig.
5C). Dysplastic changes with mildly to moderately enlarged
nuclei without invasive neoplastic growth were observed around the
invasive component (Fig. 5A and D).
Immunohistochemically, the nuclei of carcinoma cells diffusely
expressed p53 (Fig. 5E) and CDX2.
CK-20-positive (cytoplasmic) carcinoma cells were scattered
(Fig. 5F), but no CK-7-positive
carcinoma cells were found (data not shown). Therefore, a diagnosis
of adenocarcinoma, not otherwise specified, arising in tailgut
cyst, was made.
Clinical follow-up results
Of the four patients, three experienced no
recurrence more than two years after the surgery at the outpatient
clinic and on the postoperative computed tomography (CT) images.
However, in Patient 4, the cystic lesion recurred two years after
the surgery and showed a tendency to enlarge on MRI several months
later; thus, abdominal perineal excision was performed. Pathology
results of the resected specimen showed hematoma, but no
adenocarcinoma component was present. No recurrence was noted one
year after the second surgery in Patient 4 at the outpatient clinic
and on the postoperative CT images.
Discussion
In the present study, the clinicopathological
characteristics of tailgut cyst were retrospectively analysed. In
the present case series, two of four patients developed neoplastic
lesions (dysplasia and adenocarcinoma in each).
Various neoplastic and inflammatory lesions may
occur in the retrorectal space and the incidence of tumorous
lesions is reported to be up to 1 in 40,000 of the population
(4). Several types of neoplastic
lesion can occur in this region. According to the results of
reviewing 1,708 patients with retrorectal lesions, ~70% were benign
lesions and the remaining ~30% were malignant tumors (5). The most common lesion in this site was
congenital tumors (60.5%), including tailgut cyst, followed by
neurogenic tumors (19.1%) and osseous tumors (3.1%) (5). The high frequency of the cystic lesion
is one of the characteristic features of lesions in the retrorectal
space. Certain histological types of cystic lesion have been
reported in this site (2). The most
common cystic lesion is developmental cysts, including tailgut
cyst, epidermoid cyst, dermoid cyst and duplication cyst.
Non-developmental cysts, such as anal gland cyst and parasitic
cyst, may also develop. Furthermore, neoplastic lesions with cystic
change, including schwannoma, gastrointestinal stromal tumor and
aneurysmal bone cyst, are also reported in the retrorectal space
(2).
Tailgut cyst is the most common retrorectal cyst,
accounting for ~50% of them (2).
The characteristic histopathological features of tailgut cyst are
as follows: i) The cysts are usually multilocular and contain
mucinous material; ii) irregular distribution of smooth muscle
bundles is noted within the cyst wall; and iii) various types of
epithelia, including keratinizing or non-keratinizing squamous,
columnar, ciliated and transitional types, may be present (2). In a retrospective histopathological
review of a series of 70 consecutive cases of tailgut cyst,
transitional between squamous and columnar epithelium (71%),
ciliated columnar epithelium (7%), non-ciliated columnar epithelium
(24%), keratinizing squamous epithelium (27%), non-keratinizing
squamous epithelium (37%) and more than one epithelial type (7%)
were observed as the type of epithelia of the cyst wall (2). Neither skin appendage components, such
as hair and sebaceous glands, nor other teratomatous components,
are present in tailgut cyst by definition. The pathological
diagnosis of tailgut cyst may be straightforward according to the
above-mentioned histological features. Imaging studies are useful
for detection of the lesion in the retrorectal space and may also
provide important information for the selection of the optimal
surgical approach. The characteristic computed tomography finding
of tailgut cyst is a well-circumscribed multilocular hypodense
lesion without calcification, and MRI shows hypointense on T1WI and
hyperintense on T2WI (1). The
existence of a multilocular cyst, which is usually filled with
mucoid content, is a useful diagnostic criterion for tailgut cyst
(1). The management of tailgut cyst
comprises complete surgical resection-either open, laparoscopic or
robot-assisted surgery (1,6–8). The
appropriate surgical approach depends on the location and size of
the lesion, and its relation with the surrounding structures
(1,6,7).
One of the important issues in the management of
tailgut cyst is the development of neoplastic lesions within the
cyst. Due to the rarity of the lesion, the accurate frequency of
the development of neoplastic lesions has not been determined
(1–3), although a recent analysis of
consecutive patients demonstrated that the rate of neoplastic
lesions was ~9% (2). The most
common histological type is adenocarcinoma (not otherwise specified
or mucinous), following neuroendocrine tumor (2,3). In
the present study, 50% of tailgut cyst cases had neoplastic
lesions, including dysplasia. This rate of the neoplastic lesion is
higher than that of the previous analysis (2), and there may be bias due to the
single-centre nature of the study and small cohort. Thus, there was
an absence of statistical analysis and generalization of the
frequency of the neoplastic lesions in patients with tailgut cyst
was in the present study (discussed later). The histogenesis of the
neoplastic lesion in tailgut cyst remains unresolved, although
histopathological subtypes may reflect the type of epithelium
present in tailgut cyst (2,3). Wang et al (9) summarized the clinicopathological
features of adenocarcinoma arising from tailgut cyst. According to
the results of these 24 patients, most of the patients were
middle-aged females (female/male ratio, 10:1; this ratio of female
predominance in patients with adenocarcinoma is thought to be
higher than that of the whole population of tailgut cyst cases).
The chief complaints of these patients were abdominal mass and
pain, perianal disease and change of stool habits (9). Cysts with thickened and irregularly
enhanced cystic wall boundaries on imaging examinations suggest a
malignant potential (9). Of note,
the presence of dysplastic glandular epithelia within a
pre-existing cystic wall of tailgut cyst around the invasive
adenocarcinoma component has been reported in a patient (10). The present study comprised a small
case series, in which one patient (Patient 1) had dysplastic
glandular epithelia and another patient (Patient 4) had invasive
adenocarcinoma with a dysplastic component. Thus, dysplasia is
regarded as a precursor lesion of invasive adenocarcinoma occurring
in tailgut cyst. These results suggest that adenocarcinoma arising
in tailgut cyst shows a dysplasia-carcinoma sequence (10), although no molecular analysis has
been performed on the lesions of dysplasia or adenocarcinoma
arising from tailgut cyst. The dysplasia-carcinoma sequence may be
important for the development of neoplastic lesions, particularly
adenocarcinoma, in tailgut cyst; therefore, additional molecular
study with a larger cohort may be needed to clarify the
pathogenesis of neoplastic lesions arising from tailgut cyst.
Immunohistochemical analysis demonstrated that adenocarcinoma in
Case 4 of the present study was of the colorectal phenotype
(CDX2-positive, CK-7-negative and CK-20-positive), although the
immunohistochemical phenotype varies in a previous report (2). Furthermore, overexpression of p53 was
noted in the adenocarcinoma component and this finding was
consistent with a previous analysis (3).
One of the noteworthy features of the neoplastic
lesions in tailgut cyst is a high frequency of occurrence of
neuroendocrine tumor (3,11,12).
Review of 29 patients with neuroendocrine tumor arising in tailgut
cyst showed that middle-aged females were preferentially affected
(63%) (12). Chief complaints of
these patients were pain, bowel change and urinary problems
(12). Pre- and post-operative
metastases were noted in 11 and 22% of patients, respectively
(12). Imaging studies,
particularly MRI, can detect multicystic lesions with solid
components in the retrorectal space in these patients. A rare
neuroendocrine tumor arising from tailgut cyst with liver
metastasis in a 77-year-old male was previously reported (13). In that case, genomic analysis
revealed a germline frameshift in breast cancer type-1 associated
protein 1 and transcriptional analysis suggested that
enteroendocrine L cells in the tailgut are a putative cell of
origin of neuroendocrine tumor in tailgut cyst (13). Furthermore, ghrelin-producing
neuroendocrine tumors arising from tailgut cyst have also been
reported (14). Ghrelin is normally
present in the endocrine cells of the gastric mucosa and activates
growth hormone release and appetite. The origin and/or mechanism of
neuroendocrine tumor development in tailgut cysts, and the reason
for its high frequency, remain elusive, although it is suggested
that tailgut cyst should be regarded as a precancerous lesion or
that its embryonic origin may be related to tumor development
(11). In addition, neuroendocrine
tumors, even in well-differentiated types, have metastatic
potential; therefore, careful observation is required in these
patients. Accordingly, due to the high frequency of neoplastic
lesions, tailgut cyst should be treated by complete surgical
resection, even in asymptomatic patients (8,9,11,14).
There are certain limitations in the present
article. First, and most importantly, it was a retrospective and
single-centre observational study and comprised a small number of
patients with tailgut cyst. This led to bias in the frequency of
development of neoplastic lesion within tailgut cyst and the
absence of a statistical comparison in the present study.
Furthermore, molecular analysis is required to reveal the
histogenesis of the neoplastic lesions in tailgut cyst. However,
molecular analysis was not performed in the present study. Thus,
additional clinicopathological and molecular studies with a larger
cohort may be needed to clarify the histogenesis of neoplastic
lesions in tailgut cyst.
In conclusion, the present study provided a
clinicopathological analysis of an additional four patients with
tailgut cyst. The frequency of the development of neoplastic
lesions was high and a dysplasia-carcinoma sequence may be present
in the development of the neoplastic lesions in tailgut cyst. Thus,
complete surgical resection and accurate histopathological
diagnosis are important.
Acknowledgements
None.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conception and design of the study: TK and MI;
histopathological analysis: TK and MI; acquisition and analysis of
data: TK, MI, HM, TY, MasH, MadH, YH and MS; drafting of the
manuscript and preparation of tables and figures: TK and MI. TK and
MI confirm the authenticity of all the raw data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki, and the study protocol was approved by
the Institutional Review Board of the Kansai Medical University
Hospital (Hirakata, Japan; protocol no. 2019088). All data are
completely anonymized. The present study did not include any
minors. For information regarding this study, the opt-out method
waived the requirement for informed patient consent when using
patient samples in research.
Patient consent for publication
A consent form for publication in Japanese was
signed by each patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CDX2
|
caudal-type homeobox-2
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
Mastoraki A, Giannakodimos I, Panagiotou
K, Frountzas M, Chrysikos D, Kykalos S, Theodoropoulos GE and
Schizas D: Epidemiology, diagnostic approach and therapeutic
management of tailgut cysts: A systematic review. Int J Clin Pract.
75:e145462021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown IS, Sokolova A, Rosty C and Graham
RP: Cystic lesions of the retrorectal space. Histopathology.
82:232–241. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicoll K, Bartrop C, Walsh S, Foster R,
Duncan G, Payne C and Carden C: Malignant transformation of tailgut
cysts is significantly higher than previously reported: Systematic
review of cases in the literature. Colorectal Dis. 21:869–878.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jao SW, Beart RW Jr, Spencer RJ, Reiman HM
and Ilstrup DM: Retrorectal tumors. Mayo Clinic experience,
1960–1979. Dis Colon Rectum. 28:644–662. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baek SK, Hwang GS, Vinci A, Jafari MD,
Jafari F, Moghadamyeghaneh Z and Pigazzi A: Retrorectal tumors: A
comprehensive literature review. World J Surg. 40:2001–2015. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Solís-Peña A, Ngu LWS, Kraft Carré M,
Gomez Jurado MJ, Vallribera Valls F, Pellino G and Espin-Basany E:
Robotic abdominal resection of tailgut cysts-A technical note with
step-by-step description. Colorectal Dis. 24:793–796. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rompen IF, Scheiwiller A, Winiger A,
Metzger J and Gass JM: Robotic-Assisted laparoscopic resection of
tailgut cysts. JSLS. 25:e2021.000352021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patsouras D, Pawa N, Osmani H and Phillips
RK: Management of tailgut cysts in a tertiary referral centre: A
10-year experience. Colorectal Dis. 17:724–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YS, Guo QY, Zheng FH, Huang ZW, Yan
JL, Fan FX, Liu T, Ji SX, Zhao XF and Zheng YX: Retrorectal
mucinous adenocarcinoma arising from a tailgut cyst: A case report
and review of literature. World J Gastrointest Surg. 14:1072–1081.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andea AA and Klimstra DS: Adenocarcinoma
arising in a tailgut cyst with prominent meningothelial
proliferation and thyroid tissue: Case report and review of the
literature. Virchows Arch. 446:316–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee A, Suhardja TS, Nguyen TC and Teoh WM:
Neuroendocrine tumour developing within a long-standing tailgut
cyst: Case report and review of the literature. Clin J
Gastroenterol. 12:539–551. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwata E, Orosz Z, The J, Reynolds J,
Whitwell D, Tanaka Y and Athanasou NA: Neuroendocrine tumor arising
in a tailgut cyst: A rare presacral tumor. Int J Surg Pathol.
27:336–342. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erdrich J, Schaberg KB, Khodadoust MS,
Zhou L, Shelton AA, Visser BC, Ford JM, Alizadeh AA, Quake SR, Kunz
PL and Beausang JF: Surgical and molecular characterization of
primary and metastatic disease in a neuroendocrine tumor arising in
a tailgut cyst. Cold Spring Harb Mol Case Stud. 4:a0030042018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
La Rosa S, Boni L, Finzi G, Vigetti D,
Papanikolaou N, Tenconi SM, Dionigi G, Clerici M, Garancini S and
Capella C: Ghrelin-producing well-differentiated neuroendocrine
tumor (carcinoid) of tailgut cyst. Morphological,
immunohistochemical, ultrastructural, and RT-PCR study of a case
and review of the literature. Endocr Pathol. 21:190–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|