Introduction
Hepatocellular carcinoma (HCC) is the sixth most
commonly diagnosed cancer and the third most common cause of
cancer-related death worldwide. Furthermore, HCC ranks fifth in the
global incidence rate and second in the mortality rate of men;
therefore, it attracts attention from individuals worldwide
(1). There are numerous causes of
liver cancer, and the risk factors vary depending on the
geographical location, which include hepatitis B and C virus
infections, alcoholic liver disease and aflatoxin intake (2). With the continuous development of
medical technology globally, significant progress has been made in
the treatment of HCC. Liver resection and radiofrequency ablation
can be used to treat early liver cancer (3,4).
However, the majority of patients diagnosed with HCC are already at
the intermediate or advanced stage of disease. For unresectable
liver cancer, there are various palliative treatment methods, which
are selected according to the tumor stage and patient liver
function and mainly include transcatheter arterial
chemoembolization (TACE), targeted therapy and immunotherapy
(5,6). Therefore, TACE combined with tyrosine
kinase inhibitors (TKIs) and camrelizumab immunotherapy has become
the focus of attention for researchers.
The therapeutic effect of TACE on advanced liver
cancer is satisfactory, but it can lead to the formation of tumor
blood vessels in the long run (7).
As anti-angiogenic drugs, TKIs have a highly selective effect on
vascular endothelial growth factor receptor-2 (VEGFR-2), which can
effectively inhibit tumor angiogenesis and tumor proliferation, so
as to accurately compensate for the formation of tumor blood
vessels caused by TACE (8).
Camrelizumab, the first approved programmed cell death protein-1
(PD-1) inhibitor for advanced liver cancer in China, demonstrated
positive efficacy in a multicenter phase II trial (9). TACE has become the first-line
treatment for advanced HCC (10),
and TKIs, such as apatinib, sorafenib and lenvatinib, can prolong
the overall survival of patients with HCC (11). However, exploring novel targeted
therapies and immunotherapy drugs and combining these drugs with
established treatments has been shown to improve survival rates
(12).
TKIs, the main representative type of
anti-angiogenic drugs, include sorafenib, lenvatinib and apatinib.
Lenvatinib was approved as a first-line treatment for HCC in 2018,
and a clinical trial has shown that, compared with sorafenib,
lenvatinib can effectively improve the overall survival (OS) of
patients (13). In addition,
apatinib, a novel drug, is more selective than sorafenib in
targeting VEGFR-2 (14).
TACE is widely accepted as the standard treatment
for mid to late-stage HCC (15–17).
TACE can cause necrosis of most tumor cells; however, hypoxia in
tumor tissues during this process can lead to an increase in the
level of VEGF, which in turn causes tumor angiogenesis and
ultimately leads to tumor growth or metastasis (18). Therefore, anti-angiogenic drugs are
particularly important in systemic therapy as they can block
hypoxia inducible factor-1 α/the VEGF pathway, inhibiting tumor
growth or metastasis and improving patient prognosis (18,19).
TACE combined with TKIs (anti-angiogenic drugs) is a novel
treatment method for patients with HCC. A randomized multicenter
prospective trial by Kudo et al (20) reported that this combination therapy
significantly improved progression-free survival (PFS).
Immune checkpoint inhibitors (ICIs) are the main
therapeutic agents for HCC, and PD-1 can be combined with VEGF
inhibitors to increase the immune response (21–23).
Camrelizumab is a humanized anti-PD-1 monoclonal antibody, and its
efficacy in combination with TKIs, such as apatinib, has been
confirmed in the RESCUE assay for advanced HCC (9,24).
There have been relevant studies on the efficacy of TACE combined
apatininib with or without camrelizumba in the treatment of
unresectable HCC, and the results showed that the triple therapy
with camrelizumba could prolong the overall survival of patients
(25,26). As such, the efficacy and safety of a
new triple therapy, TACE + TKIs + camrelizumab (T-T-C), have been
widely studied. However, in order to provide evidence for clinical
decision-making, the present study collected data on T-T-C and TACE
+ TKIs (T-T) to explore the efficacy and safety of these combined
treatment regimens in unresected HCC.
Materials and methods
Search strategy
The analysis was performed in accordance with the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
statement (27). The present review
is registered on the PROSPERO website (https://www.crd.york.ac.uk/PROSPERO/) under
registration no. CRD42024501473. The available literature was
retrieved through an electronic search of the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase
(https://www.embase.com/) and Cochrane Library
(https://www.cochranelibrary.com/)
databases (Table SI). The main key
words searched were as follows: ‘liver neoplasms’, ‘carcinoma,
hepatocellular’, ‘hepatic*’, ‘carcinoma’, ‘cancer’, ‘tumor’,
‘lenvatinib’, ‘apatinib’, ‘sorafenib’, ‘tyrosinekinase inhibitors’,
‘chemoembolization’, ‘transcatheter arterial chemoembolization’ and
‘camrelizumab’. No language restrictions or limitations were
imposed on the search strategy.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i)
Clinically or pathologically confirmed unresectable HCC with at
least one measurable lesion; ii) Barcelona Clinical Liver Cancer
(BCLC) stage B or C (4); iii) an
Eastern Cooperative Oncology Group performance score (ECOG PS) of 0
or 1 (28); iv) patients aged
>18 years old; v) there was at least one target lesion with a
measurable diameter and arterial strengthening according to the
modified Response Evaluation Criteria in Tumors (mRECIST) (29); and vi) Child-Pugh class A or B
(30).
The exclusion criteria were as follows: i) Presence
of other malignant tumors besides HCC; ii) other treatments, such
as radiofrequency ablation and anhydrous alcohol injection, were
received during treatment; iii) absence of a control group; iv) the
study was a systematic review, meta-analysis, letter or conference
abstract; v) incomplete data; vi) patients with vital organ
dysfunction; and vii) Child-Pugh class C.
Data extraction
After searching for relevant literature in the
databases and organizing literature using Endnote X9 (Bld 12062)
(Clarivate) software, two researchers extracted and organized the
data using Excel version 2016 (Microsoft Corporation). When two
researchers extracted data that were different, a third researcher
was used to re-extract the problematic data. The following data
were extracted from the included literature: i) Name of the main
author, year of publication and country of research; ii) sex and
age of the patients; iii) research design, treatment plan and
number of participants in the experimental and control groups; iv)
α-fetoprotein level in the blood, tumor size, Child-Pugh class,
BCLC stage and ECOG PS; and v) objective response rate (ORR),
disease control rate (DCR), OS and PFS.
Quality assessment
In the present study, the Newcastle-Ottawa Scale
(NOS) was used to conduct quality assessment. NOS is a commonly
used quality assessment tool for observational studies.
Observational studies were evaluated by three modules with a total
of eight items. Specifically, the modules included population
selection, comparability and exposure/outcome evaluation. NOS uses
the semi-quantification principle of the star system to evaluate
the quality of the literature. Excepting comparability in which a
maximum of 2 stars can be awarded, items can be rated up to 1 star.
In the present study, the quality of the included literature was
independently evaluated by two researchers, with a maximum possible
quality score of 9, in which 1–4 indicated low quality and 5–9
indicated high quality (31).
The certainty of the evidence was also independently
evaluated by two authors according to the Grading of
Recommendations Assessment, Development, and Evaluation (GRADE)
assessment (32). GRADE was used to
score each outcome, and the overall quality level of the evidence
was divided into high, medium, low and very low. Finally, GRADEpro
version GDT software (www.gradepro.org) was used to summarize the assessment
results (Table SII).
Statistical analysis
The main endpoints assessed in the present study
were OS and PFS. OS was defined as the time from randomization
until death from any cause. PFS was defined as the time from
randomization to disease progression or death from any cause.
Survival outcomes were reported using hazard ratios (HRs) and 95%
confidence intervals (CIs).
The secondary endpoints included ORR and DCR, where
ORR was defined as complete and partial remission and DCR was
defined as the sum of the complete remission, partial remission and
disease stability. ORR and DCR were reported using risk ratios
(RRs) and 95% CIs. Tumor response was evaluated according to
mRECIST (29).
Q-statistics and I2 were used to analyze
and evaluate heterogeneity, low heterogeneity was indicated when
I2<50% or P>0.05. In the meta-analysis, the random
effects model was used. Sensitivity analysis was used to evaluate
the stability of the outcomes, and publication bias was evaluated
using Egger's tests. All analyses were conducted using Stata/MP
version 17.0 (StataCorp LLC).
Results
Study selection
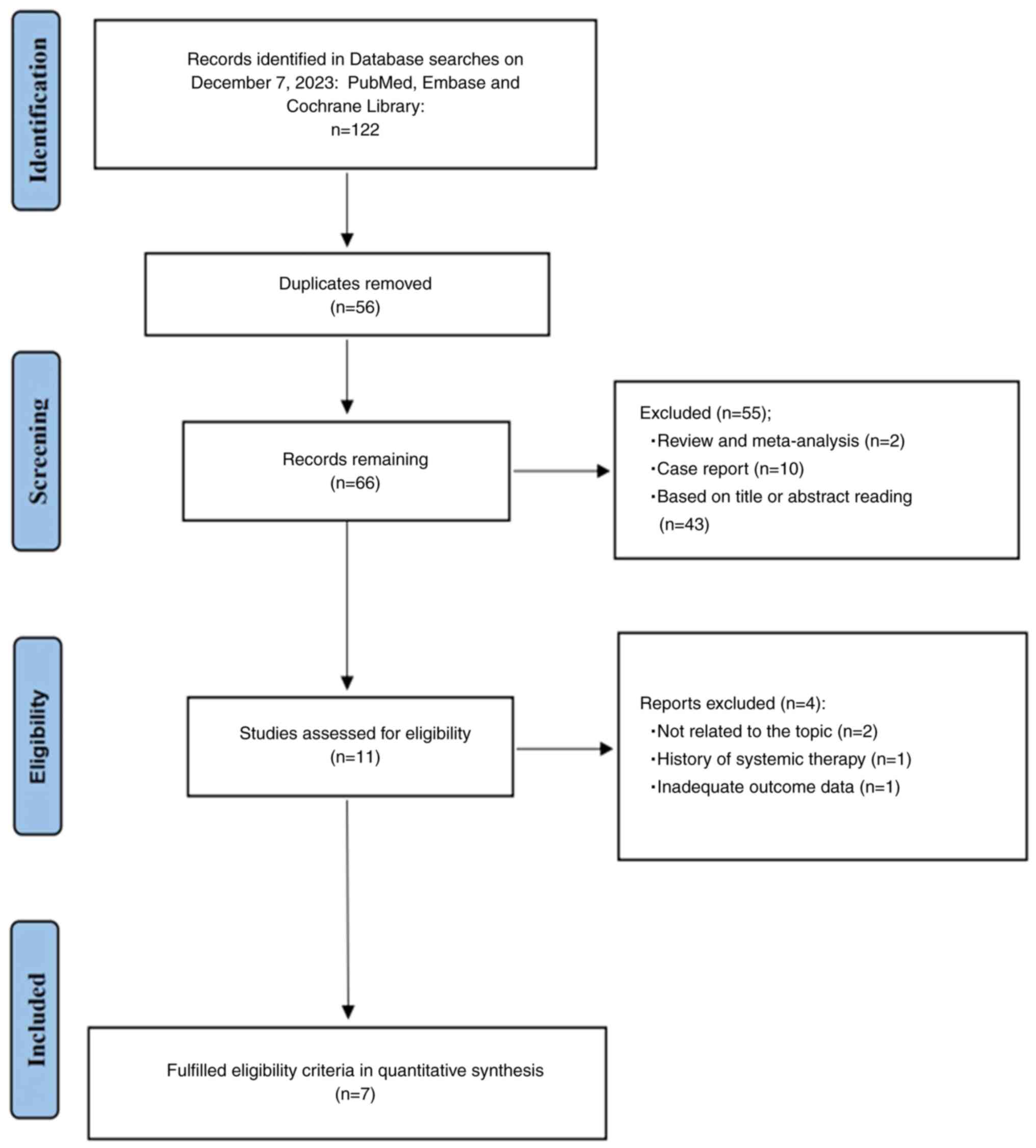
After searching the primary databases, 122 relevant
studies were identified (Fig. 1).
The Endnote version X9 software was used to organize the
literature, and 56 duplicate studies were both automatically and
manually removed. The titles and abstracts of the remaining
articles were carefully read and 11 studies were included. The text
of these 11 studies were read in detail and 7 studies were finally
included in the meta-analysis (25,26,33–37).
Study characteristics and quality
assessment
The meta-analysis included 7 articles, all of which
were from China and were retrospective cohort studies. A total of
1,798 patients with HCC were included, of whom 960 were treated
with T-T and 830 were treated with T-T-C. Table I summarizes the characteristics of
the 7 included studies. The NOS was used to evaluate the quality of
the 7 retrospective cohort studies, all of which were considered
high quality (Table II).
 | Table I.Demographic characteristics of the
included studies. |
Table I.
Demographic characteristics of the
included studies.
| First author,
year | Country | Study design | Treatment
strategy | No. of
patients | Age, years | Male/female, n | AFP, ng/ml:n | Tumor size, cm | Child-Pugh class
A/B, n | BCLC stage B/C,
n | ECOG PS 0/1/2/3,
n | (Refs.) |
|---|
| Duan et
al, | China | RCS | TACE + A | 477 |
52.9±9.6a | 382/95 |
≤400/>400:162/345 |
12.39±4.68a | 193/284 | 75/402 | 256/212/0/0 | (25) |
| 2023 |
|
| TACE + A + C | 483 |
52.6±9.2a | 399/84 |
≤400/>400:198/285 |
11.89±5.06a | 193/290 | 85/398 | 283/200/0/0 |
|
| Liu et
al, | China | RCS | TACE + A | 39 |
<60/≥60:18/21 | 36/3 |
<400/≥400:23/16 | <5/≥5:
21/18 | 35/4 | 24/15 | 24/15/0/0 | (26) |
| 2023 |
|
| TACE + A + C | 37 | <60/≥60:
25/12 | 32/5 |
<400/≥400:20/17 | <5/≥5:
15/22 | 32/5 | 19/18 | 27/10/0/0 |
|
| Pan et
al, | China | RCS | TACE + S | 85 |
63.44±6.099a | 68/17 |
>400/<400:20/65 |
6.989±3.9296a | 63/22 | 50/35 | 52/23/9/1 | (33) |
| 2023 |
|
| TACE + S + C | 150 |
61.25±10.576a | 133/17 |
>400/<400:42/108 |
6.272±3.8188a | 112/38 | 94/56 | 94/38/16/2 |
|
| Sun et
al, | China | RCS | TACE + L | 52 |
51.77±9.791a | 46/6 |
>400/≤400:21/31 |
7.65±4.86a | 43/9 | 17/35 | 22/30/0/0 | (34) |
| 2022 |
|
| TACE + L + C | 31 |
54.84±9.249a | 25/6 |
>400/≤400:12/19 |
8.31±4.80a | 24/7 | 11/20 | 19/12/0/0 |
|
| Sun et
al, | China | RCS | TACE + TKIs | 190 |
51.9±10.1a | 166/24 |
>400/≤400:99/91 |
8.7±4.4a | 157/33 | NA | 81/109/0/0 | (35) |
| 2023 |
|
| TACE + TKIs +
C | 70 |
53.8±10.4a | 58/12 |
>400/≤400:40/30 |
8.5±4.8a | 57/13 | NA | 47/23/0/0 |
|
| Xiang et
al, | China | RCS | TACE + L | 49 |
51.7±11.2a | 45/4 |
<400/≥400:29/20 |
10.6±2.7a | 41/8 | 22/27 | 38/11/0/0 | (36) |
| 2023 |
|
| TACE + L + C | 33 |
51.0±12.2a | 28/5 |
<400/≥400:14/19 |
11.8±3.5a | 25/8 | 10/23 | 22/11/0/0 |
|
| Zhu et
al, | China | RCS | TACE + A | 68 | <60/≥60:
41/27 | 58/10 |
<200/≥200:35/33 | <10/≥10:
47/21 | 56/12 | 26/42 | 34/34/0/0 | (37) |
| 2022 |
|
| TACE + A + C | 34 | <60/≥60:
23/11 | 29/5 |
<200/≥200:21/13 | <10/≥10:
27/7 | 30/4 | 13/21 | 19/15/0/0 |
|
 | Table II.Assessment of the cohort studies
using the Newcastle-Ottawa scale. |
Table II.
Assessment of the cohort studies
using the Newcastle-Ottawa scale.
|
| Selection | Comparability |
Exposure/outcome |
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author,
year | Represent
activeness of the cohort ★ | Selection of the
control cohort | Assessment of
exposure ★ | Outcome not present
at the start ★ | Comparability of
cohorts ★★ | Assessment of the
outcome ★ | Length of the
follow-up ★ | Adequacy of the
follow-up ★ | Total score (out of
9) | (Refs.) |
|---|
| Duan et al,
2023 | ★ | ★ | ★ | ★ | ★ | ★ |
| ★ | 7 | (25) |
| Liu et al,
2023 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | (26) |
| Pan et al,
2023 | ★ | ★ | ★ | ★ | ★ | ★ |
|
| 6 | (33) |
| Sun et al,
2022 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | (34) |
| Sun et al,
2023 | ★ | ★ | ★ | ★ | ★★ | ★ |
| ★ | 8 | (35) |
| Xiang et al,
2023 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | (36) |
| Zhu et al,
2022 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | (37) |
Clinical outcomes
OS and PFS
Except for the study by Pan et al (33), which did not report PFS, all studies
reported information regarding OS and PFS. The comprehensive
results of the OS meta-analysis showed that, compared with T-T
combination therapy, T-T-C combination therapy had significant
benefits in terms of the OS rate (HR, 0.38; 95% CI, 0.29–0.50;
I2=61.5%; P=0.016; Fig.
2A). Using a random-effects model, I2=61.5% showed
slightly high heterogeneity. In terms of the PFS rate, T-T-C
combination therapy had significant benefits compared with T-T
combination therapy (HR, 0.37; 95% CI, 0.30–0.46;
I2=44.5%; P=0.109; Fig.
2B), with low heterogeneity.
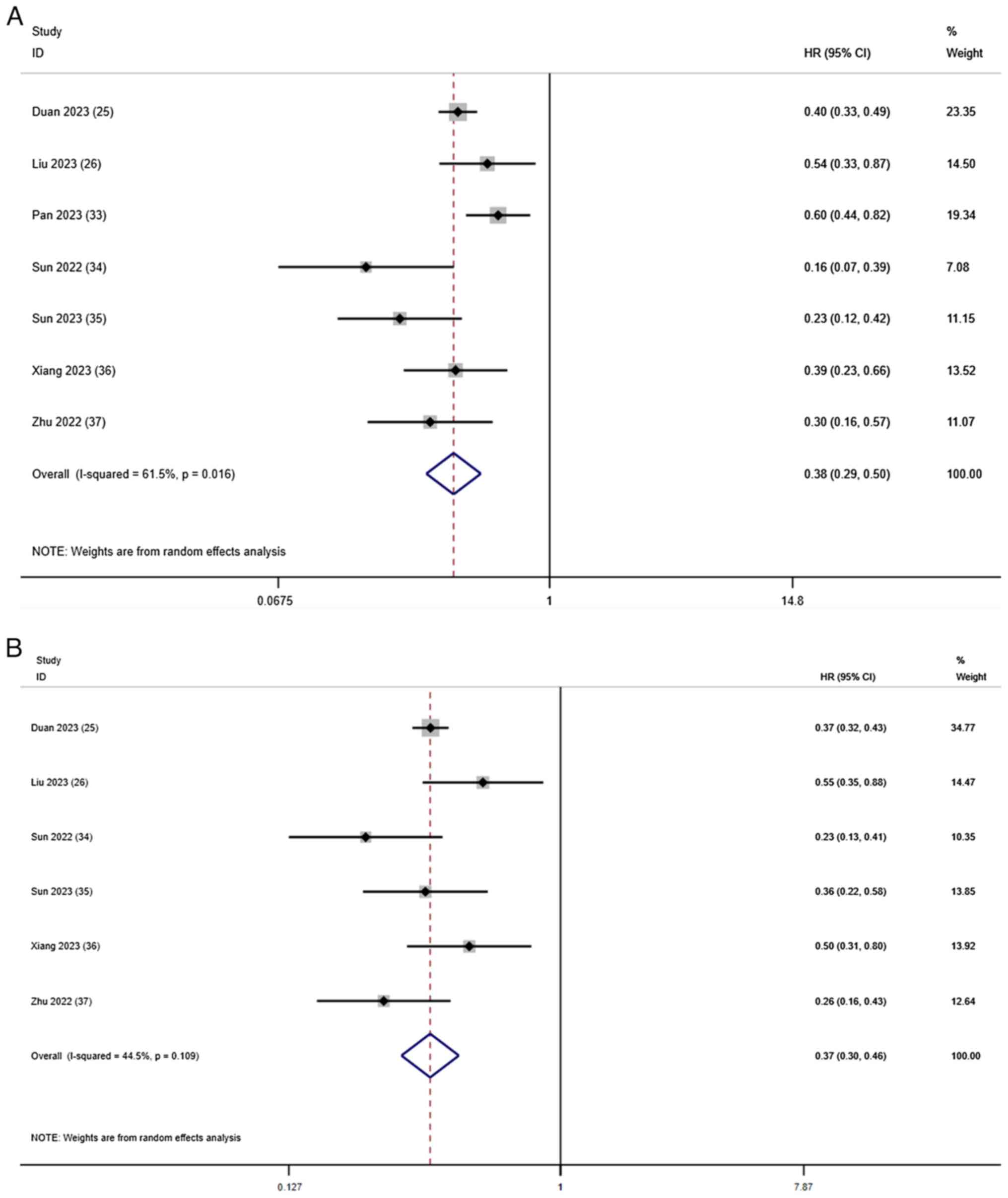 | Figure 2.Forest plots for the comparison of
(A) OS and (B) PFS. Heterogeneity for OS: χ2, 15.59
(P=0.016); I2, 61.5%. Test for overall effect of OS: Z,
6.90 (P<0.001). Heterogeneity for PFS: χ2, 9.01
(P=0.109); I2, 44.5%. Test for overall effect of PFS: Z,
8.91 (P<0.001). CI, confidence interval; HR, hazard ratio; OS,
overall survival; PFS, progression-free survival. |
Tumor response
Evaluation of the tumor response after treatment
based on the ORR and DCR was reported in all 7 studies. A
random-effects model was used to merge the RRs for ORR (RR, 0.82;
95% CI, 0.69–0.96; I2=25.1%; P=0.237; Fig. 3A) and DCR (RR, 0.96; 95% CI,
0.89–1.03; I2=0.0%; P=0.969; Fig. 3B). These results indicated that,
compared with T-T, T-T-C improved the tumor response in patients
with HCC.
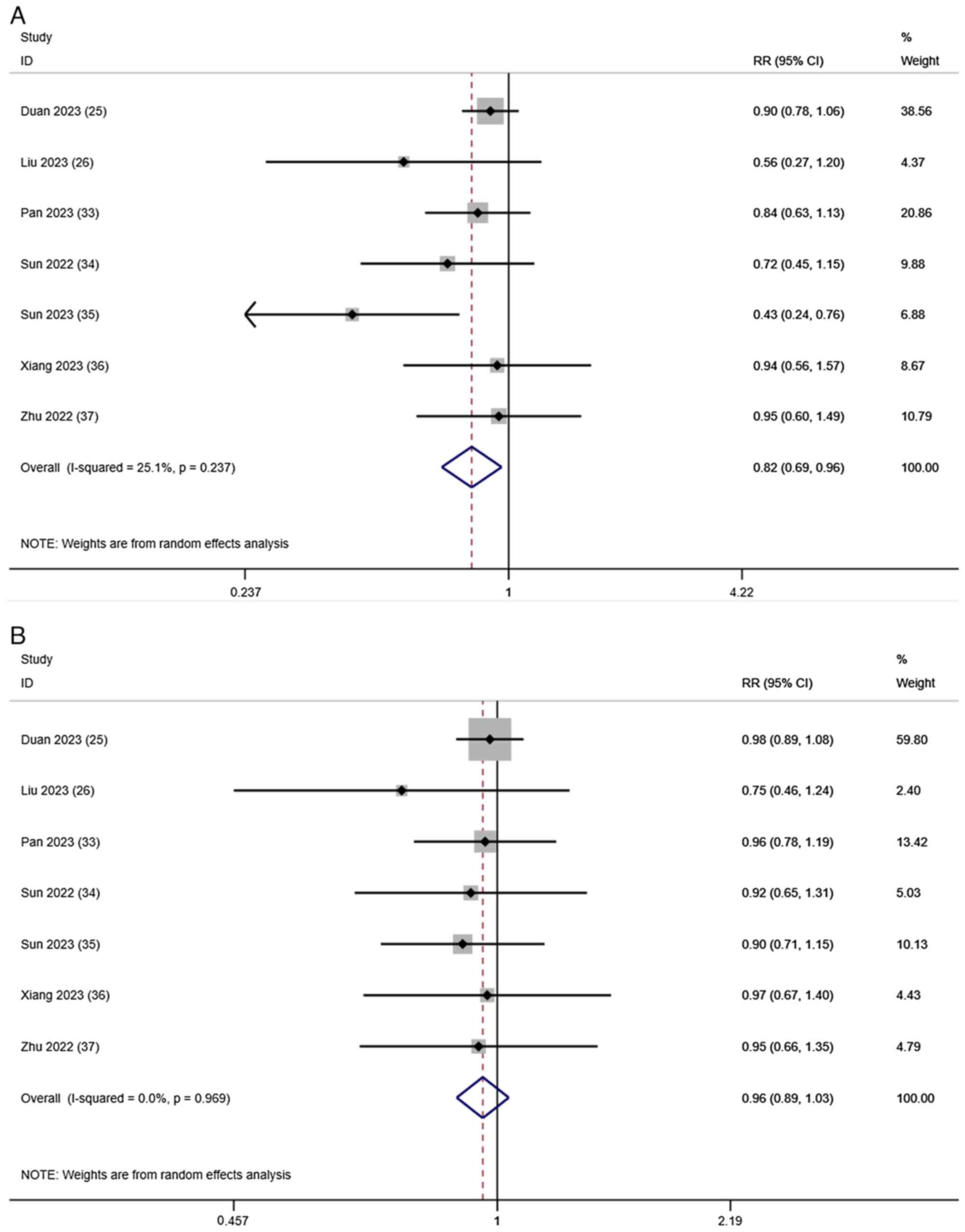 | Figure 3.Forest plots for the comparison of
the (A) ORR and (B) DCR. Heterogeneity for ORR: χ2, 8.01
(P=0.237); I2, 25.1%. Test for overall effect of ORR: Z,
2.42 (P=0.016). Heterogeneity for DCR: χ2, 1.35
(P=0.969); I2, 0.0%. Test for overall effect of DCR: Z,
1.09 (P=0.275). CI, confidence interval; DCR, disease control rate;
ORR, objective response rate; RR, risk ratio. |
AEs
All studies reported AEs. Table III summarizes the seven most
common grade 3 AEs. The most common AEs in the T-T-C group were
hypertension (8.92%), nausea and vomiting (7.78%) and pain (7.08%).
The most common AEs in the T-T group were hypertension (9.42%),
pain (9.06%) and nausea and vomiting (7.93%). Compared with the
T-T-C treatment group, the T-T treatment group had a significantly
increased incidence of diarrhea (RR, 1.97; 95% CI, 0.69–5.61), hand
and foot skin reactions (RR, 1.19; 95% CI, 0.82–1.72) and pain (RR,
0.74; 95% CI, 0.45–1.21) (Table
III). However, this result was not statistically
significant.
 | Table III.Summary of the treatment related
grade 3/4 adverse events. |
Table III.
Summary of the treatment related
grade 3/4 adverse events.
|
|
| Rate of events,
% |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Adverse events | No. of studies | TACE + TKIs | TACE + TKIs +
C | RR (95% CI) | P-value | I2 |
|---|
| Hypertension | 6 | 9.42 | 8.92 | 1.15
(0.85–1.56) | 0.998 | 0 |
| Pain | 5 | 9.06 | 7.08 | 0.74
(0.45–1.21) | 0.856 | 0 |
| Hand-foot skin
reaction | 5 | 6.30 | 5.18 | 1.19
(0.82–1.72) | 0.739 | 0 |
| Diarrhea | 4 | 3.25 | 0.88 | 1.97
(0.69–5.61) | 0.988 | 0 |
| Fatigue | 4 | 3.83 | 3.43 | 1.09
(0.46–2.59) | 0.606 | 0 |
| Nausea and
vomiting | 4 | 7.93 | 7.78 | 0.88
(0.44–1.76) | 0.963 | 0 |
Sensitivity analysis and publication
bias
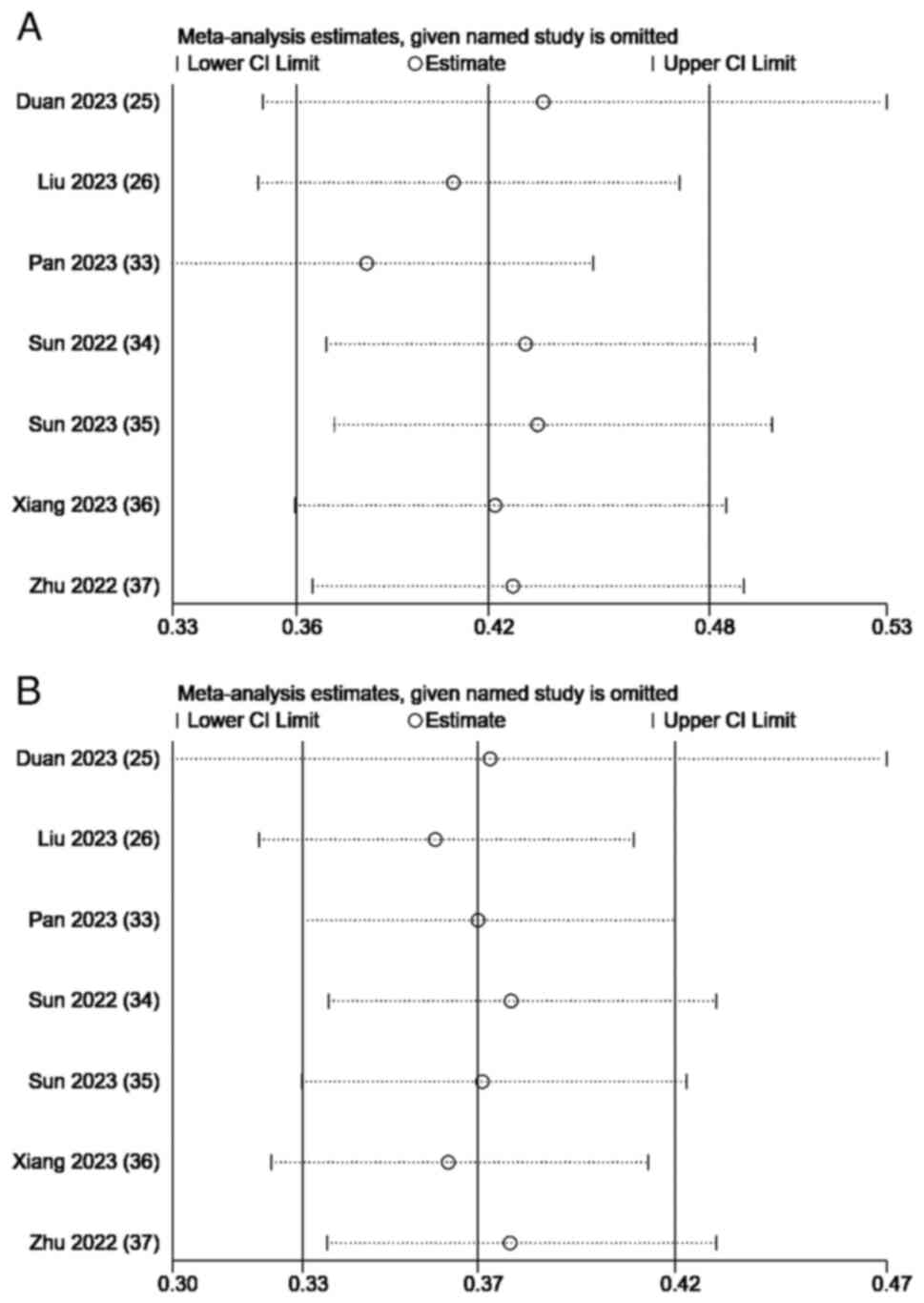
A sensitivity analysis of the combined survival
outcomes was performed. Omitting 1 study at a time resulted in a
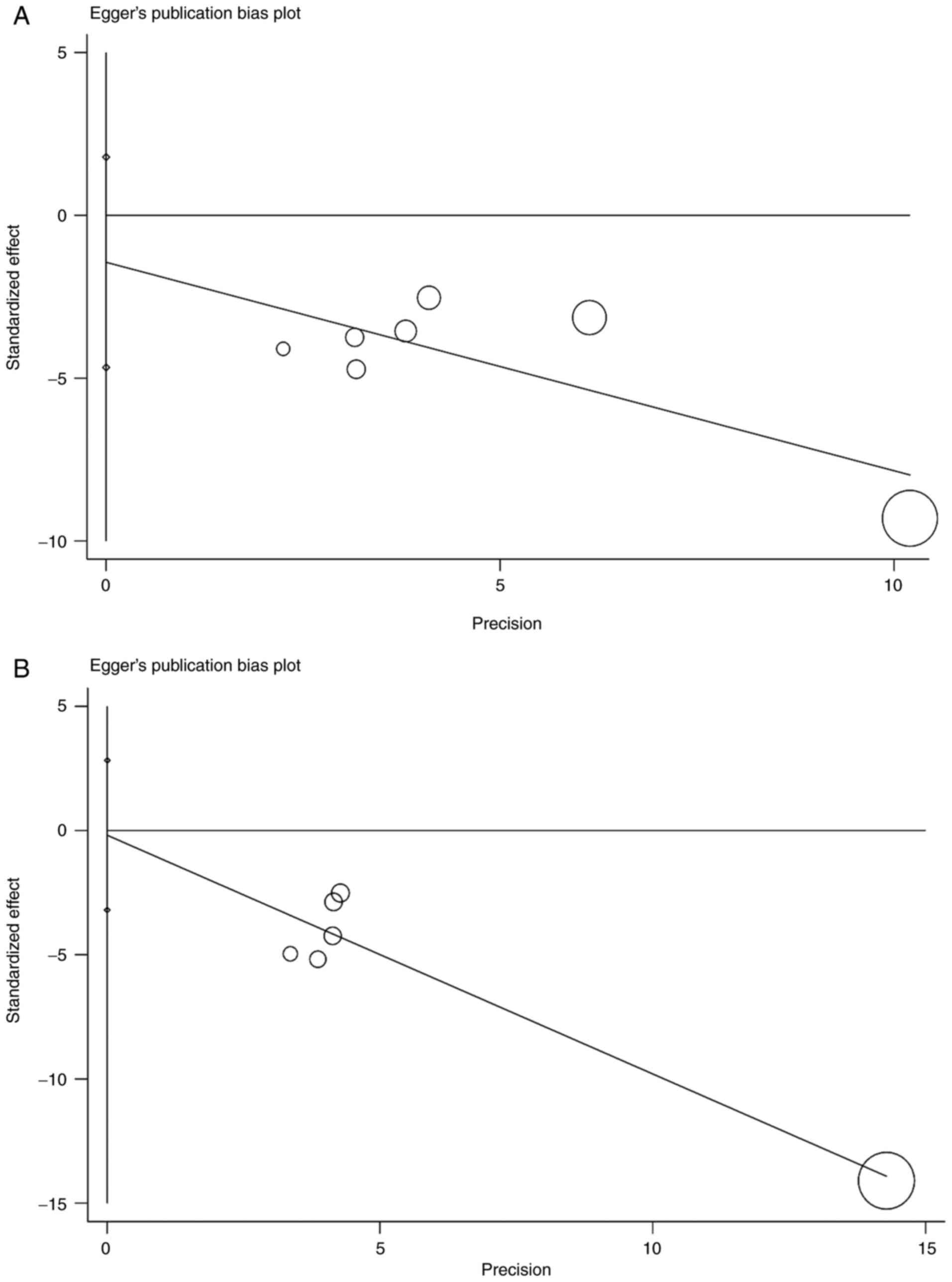
consistent OS and PFS without notable fluctuations (Fig. 4). The Egger's test for OS (P=0.303)
and PFS (P=0.869) indicated no potential publication bias (Fig. 5).
Discussion
HCC is one of the most common solid malignancies
worldwide, accounting for ~90% of primary liver cancer cases, and a
large proportion of patients with HCC are diagnosed at an advanced
stage (12,38). Significant advances have been made
in the treatment of HCC. In the past few years, TACE, a first-line
treatment for HCC, has been shown to cause tumor cell ischemia and
hypoxia by injecting embolic agents into the artery, leading to
tumor destruction. However, TACE can promote the release of VEGF
and cause tumor angiogenesis, leading to tumor growth or metastasis
(39,40). Therefore, anti-VEGF treatment can be
introduced to inhibit tumor angiogenesis. Bevacizumab was one of
the first anti-VEGF drugs approved by the Food and Drug
Administration for the treatment of human cancer and can
effectively inhibit angiogenesis (41,42).
TKIs can simultaneously target multiple anti-angiogenic receptor
sites, thereby blocking the kinase activity of the receptor, thus
achieving the effect of inhibiting angiogenesis (43). TKIs (lenvatinib, sorafenib and
apatinib) are recommended as the first-line treatment for advanced
HCC. A single-arm phase II clinical trial that enrolled patients
with advanced HCC who received apatinib demonstrated a total ORR
and DCR of 30.4 and 65.2%, respectively, and median OS and PFS
times of 13.8 (95% CI, 5.3–22.3) and 8.7 months (95% CI, 5.9–11.1),
respectively, confirming the efficacy of apatinib in patients with
advanced HCC (44). In addition, a
clinical trial conducted by Kudo et al (20) showed that lenvatinib was comparable
to sorafenib in terms of OS. In the treatment of HCC, PD-1
inhibitors can restore the ability of the immune system to kill
tumor cells by blocking the PD-1/PD-L1 cell signaling pathway
(45). Camrelizumab is a humanized
PD-1 monoclonal antibody. The RESCUE trial showed that camrelizumab
combined with apatinib was effective for treating advanced HCC
(24). However, the PD-1 inhibitors
nivolumab and pembrolizumab did not significantly improve OS in
patients with HCC in a phase III trial of monotherapy (46). Therefore, combination treatment
programs have become a research focus. A study analyzing the
effects of TACE with or without apatinib in patients with advanced
HCC showed median OS and PFS times of 8.5 and 2.5 months,
respectively, in the TACE group alone, whereas the median OS and
PFS times in the TACE-apatinib group were 17.0 and 7.0 months,
respectively, which suggested that apatinib improved patient
outcomes (47). A Phase III
randomized clinical trial by Peng et al (48), which divided patients with advanced
HCC into the lenvatinib + TACE or lenvatinib alone groups, showed
that the median OS time was significantly longer in the lenvatinib
+ TACE group (17.8 vs. 11.5 months; RR, 0.45; P<0.001), and the
median PFS time was 10.6 months in the lenvatinib + TACE group and
6.4 months in the lenvatinib alone group (HR, 0.43; P<0.001).
Thus, it was concluded that combination therapy has a better
therapeutic effect than TACE treatment alone.
Owing to the notable efficacy of combination
therapy, many triple therapy trials have also been conducted. A
meta-analysis comparing TACE combined with camrelizumab and TACE
alone in the treatment of advanced HCC showed an ORR and DCR of
46.13 and 77.19%, respectively. However, the ORR and DCR in the
present study were 84 and 96%, respectively, indicating that triple
therapy with TKIs could improve the ORR and DCR of patients
(49). The results of a
retrospective study comparing TACE + sorafenib with or without ICIs
suggested that the PFS and OS times were both prolonged in the TACE
+ sorafenib + ICI group compared with the TACE + sorafenib group
(median PFS time: 16.26 vs. 7.30 months, P<0.001; median OS
time: 23.3 vs. 13.8 months, P=0.012) (50), which was similar to the results of
the present study. A retrospective systematic review showed that
T-T-C was beneficial for the treatment of unresectable HCC
(51). However, due to the small
number of clinical trials, there are no clear clinical trial
results for this triple therapy.
In the present meta-analysis, the T-T and T-T-C
regimens were compared. The results suggested that T-T-C
combination therapy resulted in an improved OS, PFS, ORR and DCR
compared with T-T combination therapy. A study by Zou et al
(52) included 160 patients with
advanced liver cancer, all receiving TACE + lenvatinib with or
without PD-1 inhibitors, and focused on patient outcomes. The
results showed that the triple therapy significantly extended the
median OS (23.5 vs. 18.3 months; P=0.0002) and PFS (7.5 vs. 4.3
months; P<0.0001) times compared with the double therapy. In
addition, a retrospective study comparing TACE + lenvatinib with or
without pembrolizumab showed that the pembrolizumab + lenvatinib +
TACE group had significantly prolonged median OS (18.1 vs. 14.1
months; P=0.004) and PFS (9.2 vs. 5.5 months; P=0.006) times
(53). These results were similar
to those of the present meta-analysis. A similar meta-analysis
comparing the TACE + TKIs group with the TACE + TKIs + ICI group in
the treatment of HCC showed that triple therapy could effectively
improve the ORR of the overall patient population and prolong the
median PFS and OS times, but the PFS heterogeneity in the study was
high (I2=66%) (54). The
source of the heterogeneity was determined through sensitivity
analysis and, after excluding an article by Zheng et al
(50), the heterogeneity changed to
I2=0. The OS heterogeneity was also high in the present
meta-analysis (I2=61.5%; P=0.016), and the results
remained robust after sensitivity analysis. However, due to the
small sample size, meta-regression could not be performed to
determine the source of heterogeneity. Therefore, the number of
included articles should be increased in further analyses.
Although a relevant study has shown that T-T
combination therapy has a positive effect on patients with advanced
HCC (55), it is still weaker than
triple therapy with T-T-C. The reasons can be summarized as
follows: TACE can cause local necrosis of tumors, cause tumor
tissues to release antigens, trigger anticancer immune responses,
increase the expression of PD-1 and improve tumor recognition
ability. Anti-VEGF therapy can be introduced to inhibit tumor
angiogenesis, reduce VEGF-mediated immunosuppression in the tumor
and its microenvironment, and promote T cell infiltration (56,57).
Studies have shown that triple therapy can significantly improve
tumor control and patient survival (58–60),
and these results were similar to the present results.
Although, as aforementioned, the effect of this
triple therapy can be satisfactory, certain patients cannot be
treated with TACE, which mainly includes patients in the following
categories: i) Patients with decompensated cirrhosis (Child-Pugh B
8 or higher); ii) patients with an extensive tumor with massive
replacement of both lobes; iii) patients with severely reduced
portal vein flow (such as non-tumoral portal vein occlusion or
hepatofugal blood flow); iv) patients with technical
contraindications to hepatic intra-arterial treatment (such as
untreatable arterio-venous fistula); and v) patients with renal
insufficiency (creatinine ≥2 mg/dl or creatinine clearance ≤30
ml/min) (61). Therefore, before
carrying out treatment, the various indicators of the patient must
first be evaluated to ensure that the patient meets the treatment
requirements.
Regarding AEs, grade 3/4 AEs in the T-T-C and T-T
groups were analyzed and consistent results were found for the top
four AEs in both groups, which were hypertension, nausea and
vomiting, pain, and hand and foot skin reactions. Since TKI and
TACE treatment were included in both groups, the primary cause of
AEs may be related to TKI and TACE use. These results were
consistent with those of previous studies (23,62).
In addition, among the six AEs included in the present study, the
incidence of each AE was higher in the T-T group than in the T-T-C
group, which was inconsistent with the results of a similar
meta-analysis (63). Furthermore,
Xu et al (24) showed that
camrelizumab combined with apatinib reduced proteinuria and
prolonged vascular normalization. In addition, a study has shown
that anti-angiogenic drugs combined with PD-1 can reduce the
incidence of AEs (64). Therefore,
we concluded that the lower incidence of AEs in the T-T-C group may
be due to the effect of camrelizumab alone or camrelizumab combined
with TKIs, which can reduce the incidence of related AEs. However,
the efficacy of this combination regimen in reducing AEs remains
unclear, and further studies are needed to confirm this hypothesis.
In summary, under the premise of ensuring patient safety and
controlling AEs, the T-T-C triple therapy may have an improved
curative effect on patients.
However, the present study had certain limitations.
First, the number of articles included was relatively small, the
sample size was small and the included articles were all from
China, which lacked representativeness and comprehensiveness.
Therefore, more articles should be included in future
meta-analyses, particularly those analyzing samples from other
countries, to increase the comprehensiveness. Second, all the
articles included in the present study were retrospective cohort
studies, which may have a certain selection bias and affect the
final results. Third, the TKIs selected in all the included
articles were different, and different TKIs may bring different
curative effects and ultimately lead to different survival rates of
patients; however, different TKIs were not analyzed separately due
to the small number of available studies. Fourth, among all the
included articles, some contained shorter follow-up times, which
may have caused some valuable observations to be missed. Finally,
the quality of life of the patient can affect the outcome of
different treatment modalities; however, relevant data for this
could not be found.
In summary, the results of the present systematic
review and meta-analysis indicated that, in patients with advanced
HCC, T-T-C combination therapy demonstrated a notable advantage in
terms of OS, PFS, ORR, DCR and manageable AEs. However, further
evidence of this is needed from a larger number of randomized
controlled trials.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
The present study was supported by The Bureau of Science and
Technology of Nanchong City (grant no. 22SXQT0052).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JY, DY, SW, LY and PY contributed to the conception
and design of the study. Data collection was performed by JY and
DY. Statistical analysis was performed by SW and LY. Interpretation
of the data was performed by JY and DY. JY and PY drafted and
revised the manuscript. JY and PY confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordan JD, Kennedy EB, Abou-Alfa GK, Beg
MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, et al:
Systemic therapy for advanced hepatocellular carcinoma: ASCO
Guideline. J Clin Oncol. 38:4317–4345. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nault J, Sutter O, Nahon P, Ganne-Carrié N
and Séror O: Percutaneous treatment of hepatocellular carcinoma:
State of the art and innovations. J Hepatol. 68:783–797. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cabibbo G, Enea M, Attanasio M, Bruix J,
Craxì A and Cammà C: A meta-analysis of survival rates of untreated
patients in randomized clinical trials of hepatocellular carcinoma.
Hepatology. 51:1274–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH,
An JH, Lee HC and Lim YS: Efficacy and safety of transarterial
chemoembolization plus external beam radiotherapy vs sorafenib in
hepatocellular carcinoma with macroscopic vascular invasion: A
Randomized clinical trial. JAMA Oncol. 4:661–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Xia D, Bai W, Wang E, Sun J, Huang
M, Mu W, Yin G, Li H, Zhao H, et al: Development of a prognostic
score for recommended TACE candidates with hepatocellular
carcinoma: A multicentre observational study. J Hepatol.
70:893–903. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Yao Q, Zhang H, Guo X, Liu J, Shi
Q, Huang S and Xiong B: Combining transcatheter arterial
embolization with iodized oil containing Apatinib inhibits HCC
growth and metastasis. Sci Rep. 10:29642020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin S, Ren Z, Meng Z, Chen Z, Chai X,
Xiong J, Bai Y, Yang L, Zhu H, Fang W, et al: Camrelizumab in
patients with previously treated advanced hepatocellular carcinoma:
A multicentre, open-label, parallel-group, randomised, phase 2
trial. Lancet Oncol. 21:571–580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogasawara S, Ooka Y, Koroki K, Maruta S,
Kanzaki H, Kanayama K, Kobayashi K, Kiyono S, Nakamura M, Kanogawa
N, et al: Switching to systemic therapy after locoregional
treatment failure: Definition and best timing. Clin Mol Hepatol.
26:155–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kudo M, Cheng AL, Park JW, Park JH, Liang
PC, Hidaka H, Izumi N, Heo J, Lee YJ, Sheen IS, et al: Orantinib
versus placebo combined with transcatheter arterial
chemoembolisation in patients with unresectable hepatocellular
carcinoma (ORIENTAL): A randomised, double-blind,
placebo-controlled, multicentre, phase 3 study. Lancet
Gastroenterol Hepatol. 3:37–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z,
Xu A, Chen X, Zhou C, Ren Z, et al: Apatinib as second-line or
later therapy in patients with advanced hepatocellular carcinoma
(AHELP): A multicentre, double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol.
6:559–568. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
European Association for the Study of the
Liver. Electronic address, . simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver: EASL Clinical
Practice Guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong J, Kong J, Pan B, Ke S, Dong S, Li X,
Zhou A, Zheng L and Sun WB: Insufficient radiofrequency ablation
promotes angiogenesis of residual hepatocellular carcinoma via
HIF-1α/VEGFA. PLoS One. 7:e372662012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kudo M, Han G, Finn RS, Poon RT, Blanc JF,
Yan L, Yang J, Lu L, Tak WY, Yu X, et al: Brivanib as adjuvant
therapy to transarterial chemoembolization in patients with
hepatocellular carcinoma: A randomized phase III trial. Hepatology.
60:1697–1707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shigeta K, Matsui A, Kikuchi H, Klein S,
Mamessier E, Chen IX, Aoki S, Kitahara S, Inoue K, Shigeta A, et
al: Regorafenib combined with PD1 blockade increases CD8 T-cell
infiltration by inducing CXCL10 expression in hepatocellular
carcinoma. J Immunother Cancer. 8:e0014352020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J,
Shao G, Zhang Y, Xu L, Yin T, et al: Camrelizumab in combination
with apatinib in patients with advanced hepatocellular carcinoma
(RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer
Res. 27:1003–1011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan X, Li H, Kuang D, Chen P, Zhang K, Li
Y, He X, Xing C, Wang H, Liu Y, et al: Transcatheter arterial
chemoembolization plus apatinib with or without camrelizumab for
unresectable hepatocellular carcinoma: A multicenter retrospective
cohort study. Hepatol Int. 17:915–926. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Yu Q, Gu T, Qu P, Ma X, Zhou S, Lu
T, Pan D and Han Z: Transarterial Chemoembolization plus Apatinib
with or without Camrelizumab for the Treatment of Advanced
HBV-related Hepatocellular Carcinoma. J Gastrointestin Liver Dis.
32:182–189. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
Alshahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (Eastern Cooperative Oncology Group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DH, Son JH and Kim TW: New scoring
systems for severity outcome of liver cirrhosis and hepatocellular
carcinoma: Current issues concerning the Child-Turcotte-Pugh score
and the Model of End-Stage Liver Disease (MELD) score. Taehan Kan
Hakhoe Chi. 9:167–179. 2003.(In Korean). PubMed/NCBI
|
|
31
|
Wells G, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. 2021.
|
|
32
|
Balshem H, Helfand M, Schünemann HJ, Oxman
AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S
and Guyatt GH: GRADE guidelines: 3. Rating the quality of evidence.
J Clin Epidemiol. 64:401–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan S, Zheng J and Shi C: Analysis and
prediction of the efficacy and influencing factors of camrelizumab
combined with TACE and sorafenib in the treatment of advanced
hepatocellular carcinoma. J Cancer Res Clin Oncol. 149:12479–12487.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang
W, Zhu L, Guo Y, Gui Y, Liu F, et al: Safety and efficacy of
lenvatinib combined with camrelizumab plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
two-center retrospective study. Front Oncol. 12:9829482022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun T, Ren Y, Sun B, Chen L, Zhu L, Zhang
L and Zheng C: The Feasibility of TACE Combined with TKIs Plus PD-1
Antibody for Advanced HCC. J Hepatocell Carcinoma. 10:447–457.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H
and Huang M: TACE combined with lenvatinib and camrelizumab for
unresectable multiple nodular and large hepatocellular carcinoma
(>5 cm). Technol Cancer Res Treat. 22:153303382312003202023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu D, Ma K, Yang W, Zhou HF, Shi Q, Ren
JW, Xie YG, Liu S, Shi HB and Zhou WZ: Transarterial
chemoembolization plus apatinib with or without camrelizumab for
unresected hepatocellular carcinoma: A two-center propensity score
matching study. Front Oncol. 12:10575602022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Esagian SM, Kakos CD, Giorgakis E, Burdine
L, Barreto JC and Mavros MN: Adjuvant transarterial
chemoembolization following curative-intent hepatectomy versus
hepatectomy alone for hepatocellular carcinoma: A systematic review
and meta-analysis of Randomized controlled trials. Cancers (Basel).
13:29842021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rizzo A, Ricci AD and Brandi G:
Immune-based combinations for advanced hepatocellular carcinoma:
Shaping the direction of first-line therapy. Future Oncol.
17:755–757. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giuliano S and Pagès G: Mechanisms of
resistance to anti-angiogenesis therapies. Biochimie. 95:1110–1119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mukherji SK: Bevacizumab (Avastin). AJNR
Am J Neuroradiol. 31:235–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mou L, Tian X, Zhou B, Zhan Y, Chen J, Lu
Y, Deng J, Deng Y, Wu Z, Li Q, et al: Improving outcomes of
tyrosine kinase inhibitors in hepatocellular carcinoma: New data
and ongoing trials. Front Oncol. 11:7527252021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hou Z, Zhu K, Yang X, Chen P, Zhang W, Cui
Y, Zhu X, Song T, Li Q, Li H and Zhang T: Apatinib as first-line
treatment in patients with advanced hepatocellular carcinoma: A
phase II clinical trial. Ann Transl Med. 8:10472020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mazzolini GD and Malvicini M:
Immunostimulatory monoclonal antibodies for hepatocellular
carcinoma therapy. Trends and perspectives. Medicina (B Aires).
78:29–32. 2018.PubMed/NCBI
|
|
46
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A Randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qiu Z, Shen L, Chen S, Qi H, Cao F, Xie L
and Fan W: Efficacy of apatinib in transcatheter arterial
chemoembolization (TACE) refractory intermediate and advanced-stage
hepatocellular carcinoma:A propensity score matching analysis.
Cancer Manag Res. 11:9321–9330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, Randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tashrifwala FAA, Karmani VK, Haider I,
Syeda AZ, Noorani A, Mustafa MS, Dave T and Hafeez H: Efficacy of
transarterial chemoembolization combined with camrelizumab in the
treatment of hepatocellular carcinoma: A systematic review and
meta-analysis. Cureus. 15:e486732023.PubMed/NCBI
|
|
50
|
Zheng L, Fang S, Wu F, Chen W, Chen M,
Weng Q, Wu X, Song J, Zhao Z and Ji J: Efficacy and Safety of TACE
combined with sorafenib plus immune checkpoint inhibitors for the
treatment of intermediate and advanced TACE-Refractory
hepatocellular carcinoma: A retrospective study. Front Mol Biosci.
7:6093222021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ke Q, Xin F, Fang H, Zeng Y, Wang L and
Liu J: The significance of transarterial chemo(embolization)
combined with tyrosine kinase inhibitors and immune checkpoint
inhibitors for unresectable hepatocellular carcinoma in the Era of
systemic therapy: A systematic review. Front Immunol.
13:9134642022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zou X, Xu Q, You R and Yin G: Correlation
and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor
in the treatment of hepatocellular carcinoma with portal vein tumor
thrombus based on immunological features. Cancer Med.
12:11315–11333. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang
F, Zhuang W, Chen X, Chen H, Xu B, et al: Lenvatinib plus TACE with
or without pembrolizumab for the treatment of initially
unresectable hepatocellular carcinoma harbouring PD-L1 expression:
A retrospective study. J Cancer Res Clin Oncol. 148:2115–2125.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu J, Wang P, Shang L, Zhang Z, Tian Y,
Chen X, Ma Y and Shao H: TACE plus tyrosine kinase inhibitors and
immune checkpoint inhibitors versus TACE plus tyrosine kinase
inhibitors for the treatment of patients with hepatocellular
carcinoma: A meta-analysis and trial sequential analysis. Hepatol
Int. 18:595–609. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gong A and Li X: The efficacy and safety
of Apatinib combined with TACE in the treatment of hepatocellular
carcinoma: A meta-analysis. World J Surg Oncol. 20:692022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khan KA and Kerbel RS: Improving
immunotherapy outcomes with anti-angiogenic treatments and vice
versa. Nat Rev Clin Oncol. 15:310–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Q, Gao J, Di W and Wu X:
Anti-angiogenesis therapy overcomes the innate resistance to
PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models.
Cancer Immunol Immunother. 69:1781–1799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cai M, Huang W, Huang J, Shi W, Guo Y,
Liang L, Zhou J, Lin L, Cao B, Chen Y, et al: Transarterial
chemoembolization combined with lenvatinib Plus PD-1 inhibitor for
advanced hepatocellular carcinoma: A retrospective cohort study.
Front Immunol. 13:8483872022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Teng Y, Ding X, Li W, Sun W and Chen J: A
retrospective study on therapeutic efficacy of transarterial
chemoembolization combined with immune checkpoint inhibitors plus
lenvatinib in patients with unresectable hepatocellular carcinoma.
Technol Cancer Res Treat. 21:153303382210751742022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ,
Wang SJ, Zhou JY, Li YN, Qiu FN, Li B and Yan ML: Lenvatinib
Combined with Anti-PD-1 antibodies plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
Multicenter retrospective study. J Hepatocell Carcinoma.
8:1233–1240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Raoul JL, Sangro B, Forner A, Mazzaferro
V, Piscaglia F, Bolondi L and Lencioni R: Evolving strategies for
the management of intermediate-stage hepatocellular carcinoma:
Available evidence and expert opinion on the use of transarterial
chemoembolization. Cancer Treat Rev. 37:212–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang XG, Sun YY, Wang HQ, Li DS, Xu GH and
Huang XQ: Efficacy and safety of transarterial chemoembolization
combining sorafenib with or without immune checkpoint inhibitors in
previously treated patients with advanced hepatocellular carcinoma:
A propensity score matching analysis. Front Oncol. 12:9143852022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu J, Wei S, Yang L, Yu J, Yan D and Yi
P: Efficacy and safety of transarterial chemoembolization plus
lenvatinib with or without programmed death-1 inhibitors in the
treatment of unresectable hepatocellular carcinoma: A systematic
review and meta-analysis. J Cancer Res Clin Oncol. 149:14451–14461.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao
C, Jia Y, Shi J, Zhang L, Liu X, et al: Low-Dose apatinib optimizes
tumor microenvironment and potentiates antitumor effect of
PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 7:630–643.
2019. View Article : Google Scholar : PubMed/NCBI
|