|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordan JD, Kennedy EB, Abou-Alfa GK, Beg
MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, et al:
Systemic therapy for advanced hepatocellular carcinoma: ASCO
Guideline. J Clin Oncol. 38:4317–4345. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nault J, Sutter O, Nahon P, Ganne-Carrié N
and Séror O: Percutaneous treatment of hepatocellular carcinoma:
State of the art and innovations. J Hepatol. 68:783–797. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cabibbo G, Enea M, Attanasio M, Bruix J,
Craxì A and Cammà C: A meta-analysis of survival rates of untreated
patients in randomized clinical trials of hepatocellular carcinoma.
Hepatology. 51:1274–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH,
An JH, Lee HC and Lim YS: Efficacy and safety of transarterial
chemoembolization plus external beam radiotherapy vs sorafenib in
hepatocellular carcinoma with macroscopic vascular invasion: A
Randomized clinical trial. JAMA Oncol. 4:661–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Xia D, Bai W, Wang E, Sun J, Huang
M, Mu W, Yin G, Li H, Zhao H, et al: Development of a prognostic
score for recommended TACE candidates with hepatocellular
carcinoma: A multicentre observational study. J Hepatol.
70:893–903. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Yao Q, Zhang H, Guo X, Liu J, Shi
Q, Huang S and Xiong B: Combining transcatheter arterial
embolization with iodized oil containing Apatinib inhibits HCC
growth and metastasis. Sci Rep. 10:29642020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin S, Ren Z, Meng Z, Chen Z, Chai X,
Xiong J, Bai Y, Yang L, Zhu H, Fang W, et al: Camrelizumab in
patients with previously treated advanced hepatocellular carcinoma:
A multicentre, open-label, parallel-group, randomised, phase 2
trial. Lancet Oncol. 21:571–580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogasawara S, Ooka Y, Koroki K, Maruta S,
Kanzaki H, Kanayama K, Kobayashi K, Kiyono S, Nakamura M, Kanogawa
N, et al: Switching to systemic therapy after locoregional
treatment failure: Definition and best timing. Clin Mol Hepatol.
26:155–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kudo M, Cheng AL, Park JW, Park JH, Liang
PC, Hidaka H, Izumi N, Heo J, Lee YJ, Sheen IS, et al: Orantinib
versus placebo combined with transcatheter arterial
chemoembolisation in patients with unresectable hepatocellular
carcinoma (ORIENTAL): A randomised, double-blind,
placebo-controlled, multicentre, phase 3 study. Lancet
Gastroenterol Hepatol. 3:37–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z,
Xu A, Chen X, Zhou C, Ren Z, et al: Apatinib as second-line or
later therapy in patients with advanced hepatocellular carcinoma
(AHELP): A multicentre, double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol.
6:559–568. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
European Association for the Study of the
Liver. Electronic address, . simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver: EASL Clinical
Practice Guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong J, Kong J, Pan B, Ke S, Dong S, Li X,
Zhou A, Zheng L and Sun WB: Insufficient radiofrequency ablation
promotes angiogenesis of residual hepatocellular carcinoma via
HIF-1α/VEGFA. PLoS One. 7:e372662012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kudo M, Han G, Finn RS, Poon RT, Blanc JF,
Yan L, Yang J, Lu L, Tak WY, Yu X, et al: Brivanib as adjuvant
therapy to transarterial chemoembolization in patients with
hepatocellular carcinoma: A randomized phase III trial. Hepatology.
60:1697–1707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shigeta K, Matsui A, Kikuchi H, Klein S,
Mamessier E, Chen IX, Aoki S, Kitahara S, Inoue K, Shigeta A, et
al: Regorafenib combined with PD1 blockade increases CD8 T-cell
infiltration by inducing CXCL10 expression in hepatocellular
carcinoma. J Immunother Cancer. 8:e0014352020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J,
Shao G, Zhang Y, Xu L, Yin T, et al: Camrelizumab in combination
with apatinib in patients with advanced hepatocellular carcinoma
(RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer
Res. 27:1003–1011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
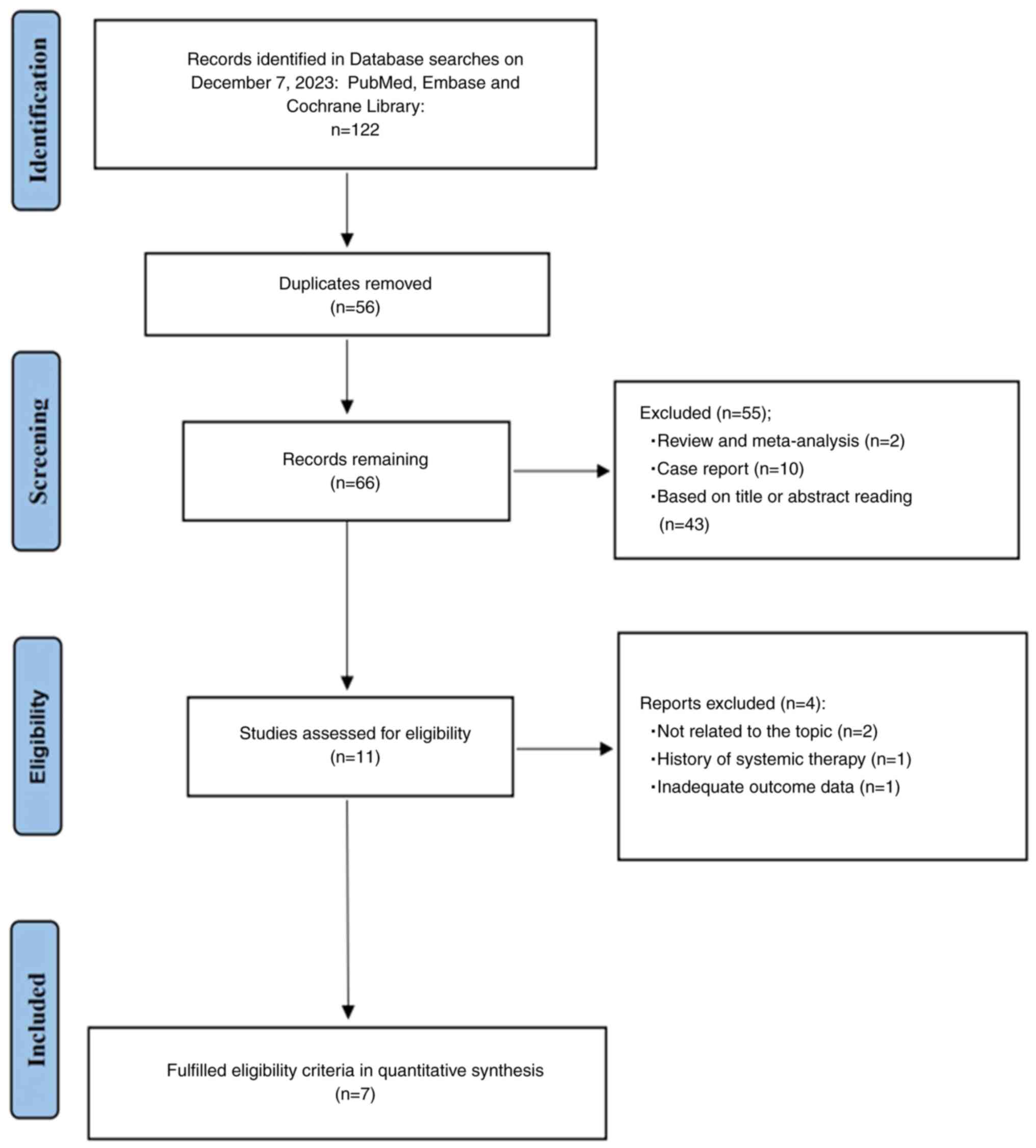
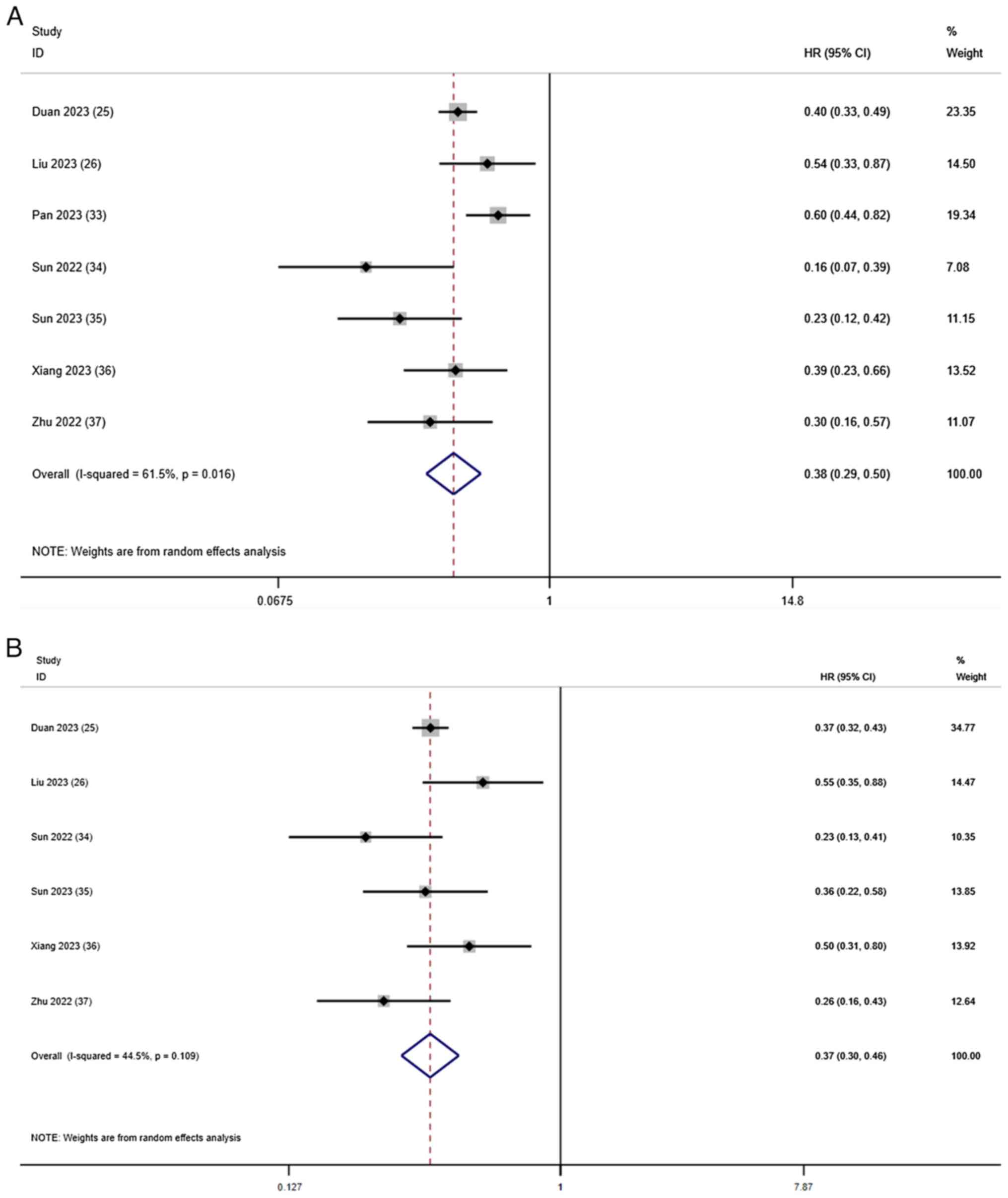
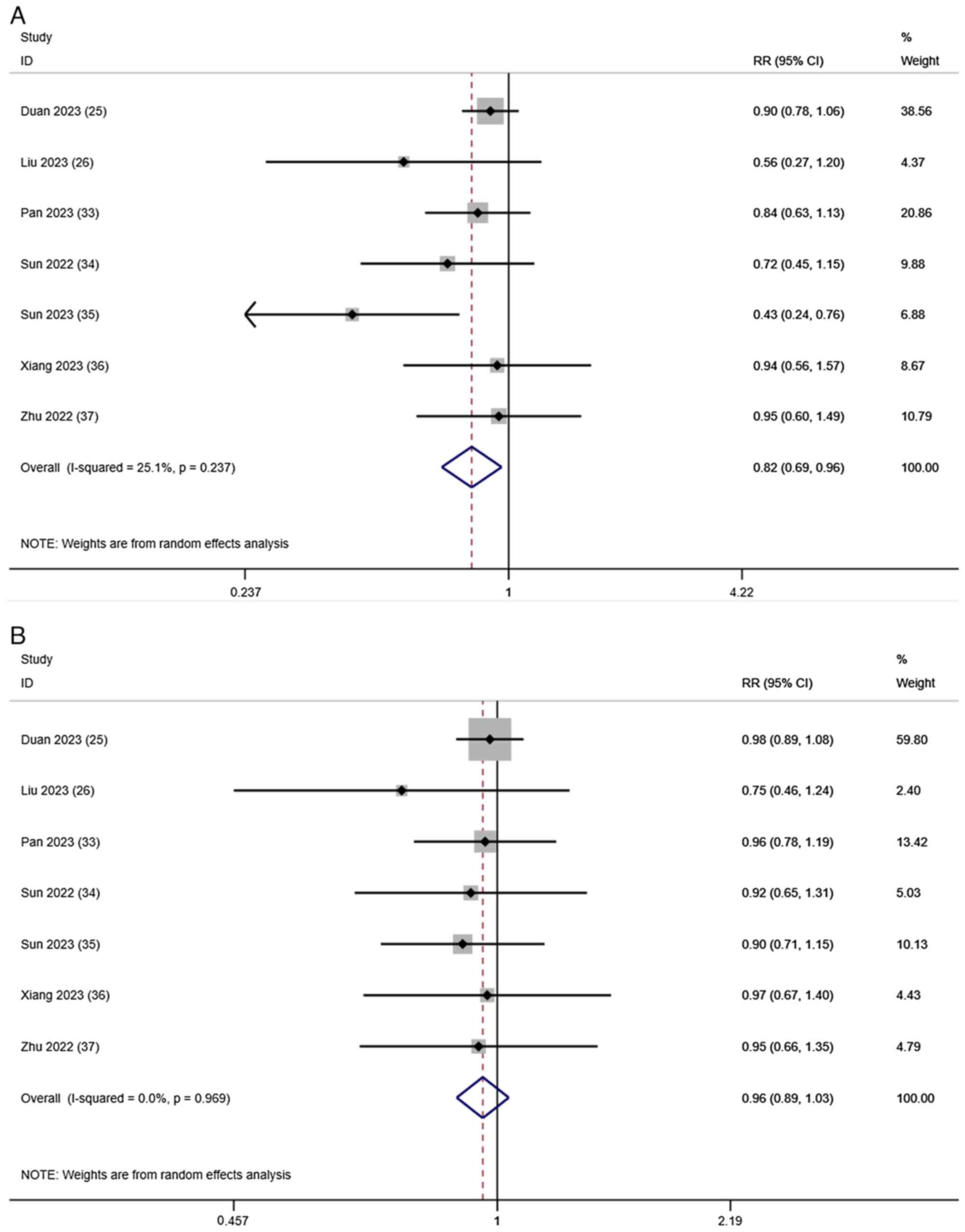
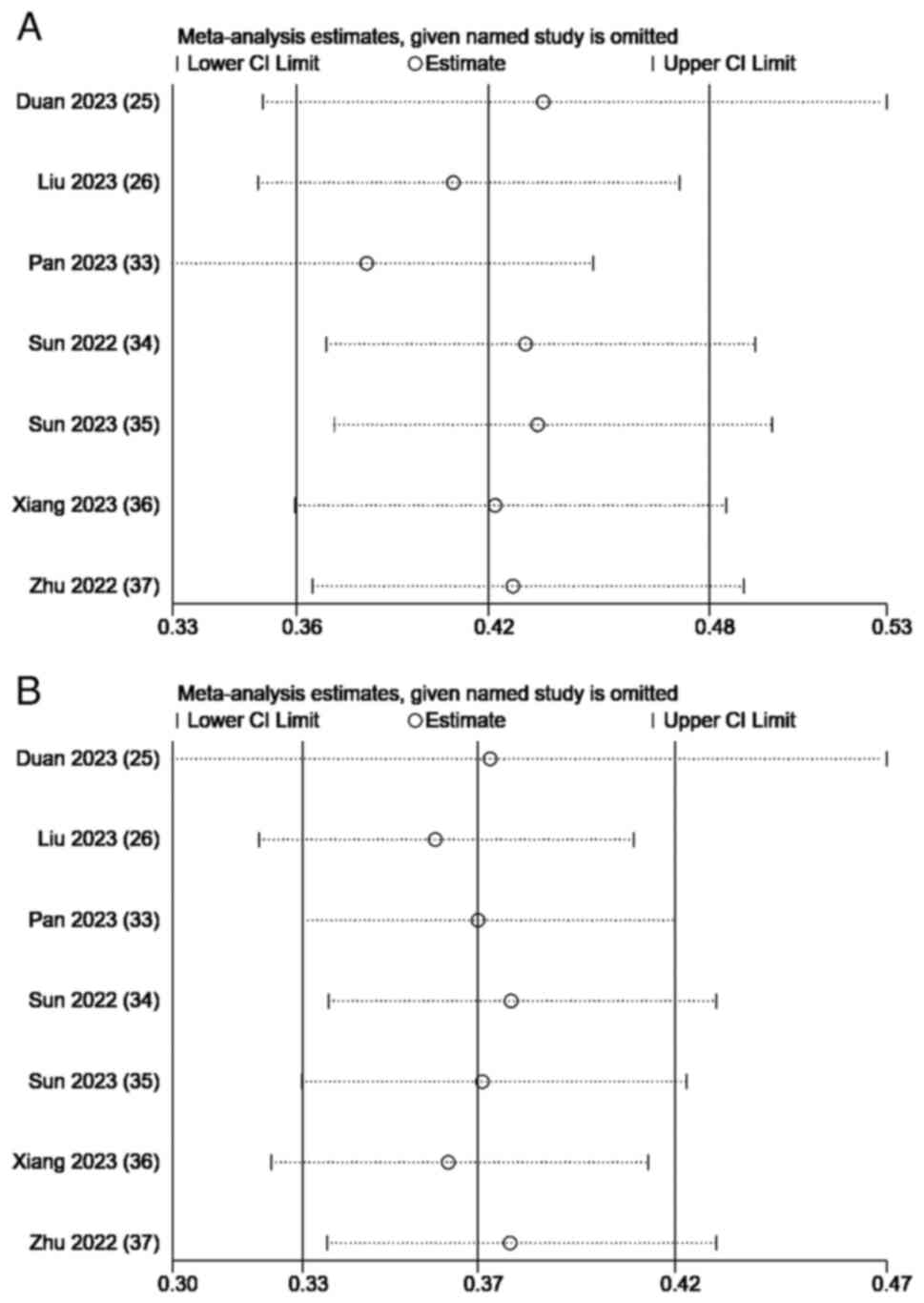
25
|
Duan X, Li H, Kuang D, Chen P, Zhang K, Li
Y, He X, Xing C, Wang H, Liu Y, et al: Transcatheter arterial
chemoembolization plus apatinib with or without camrelizumab for
unresectable hepatocellular carcinoma: A multicenter retrospective
cohort study. Hepatol Int. 17:915–926. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Yu Q, Gu T, Qu P, Ma X, Zhou S, Lu
T, Pan D and Han Z: Transarterial Chemoembolization plus Apatinib
with or without Camrelizumab for the Treatment of Advanced
HBV-related Hepatocellular Carcinoma. J Gastrointestin Liver Dis.
32:182–189. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
Alshahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (Eastern Cooperative Oncology Group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DH, Son JH and Kim TW: New scoring
systems for severity outcome of liver cirrhosis and hepatocellular
carcinoma: Current issues concerning the Child-Turcotte-Pugh score
and the Model of End-Stage Liver Disease (MELD) score. Taehan Kan
Hakhoe Chi. 9:167–179. 2003.(In Korean). PubMed/NCBI
|
|
31
|
Wells G, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. 2021.
|
|
32
|
Balshem H, Helfand M, Schünemann HJ, Oxman
AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S
and Guyatt GH: GRADE guidelines: 3. Rating the quality of evidence.
J Clin Epidemiol. 64:401–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan S, Zheng J and Shi C: Analysis and
prediction of the efficacy and influencing factors of camrelizumab
combined with TACE and sorafenib in the treatment of advanced
hepatocellular carcinoma. J Cancer Res Clin Oncol. 149:12479–12487.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang
W, Zhu L, Guo Y, Gui Y, Liu F, et al: Safety and efficacy of
lenvatinib combined with camrelizumab plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
two-center retrospective study. Front Oncol. 12:9829482022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun T, Ren Y, Sun B, Chen L, Zhu L, Zhang
L and Zheng C: The Feasibility of TACE Combined with TKIs Plus PD-1
Antibody for Advanced HCC. J Hepatocell Carcinoma. 10:447–457.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H
and Huang M: TACE combined with lenvatinib and camrelizumab for
unresectable multiple nodular and large hepatocellular carcinoma
(>5 cm). Technol Cancer Res Treat. 22:153303382312003202023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu D, Ma K, Yang W, Zhou HF, Shi Q, Ren
JW, Xie YG, Liu S, Shi HB and Zhou WZ: Transarterial
chemoembolization plus apatinib with or without camrelizumab for
unresected hepatocellular carcinoma: A two-center propensity score
matching study. Front Oncol. 12:10575602022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Esagian SM, Kakos CD, Giorgakis E, Burdine
L, Barreto JC and Mavros MN: Adjuvant transarterial
chemoembolization following curative-intent hepatectomy versus
hepatectomy alone for hepatocellular carcinoma: A systematic review
and meta-analysis of Randomized controlled trials. Cancers (Basel).
13:29842021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rizzo A, Ricci AD and Brandi G:
Immune-based combinations for advanced hepatocellular carcinoma:
Shaping the direction of first-line therapy. Future Oncol.
17:755–757. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giuliano S and Pagès G: Mechanisms of
resistance to anti-angiogenesis therapies. Biochimie. 95:1110–1119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mukherji SK: Bevacizumab (Avastin). AJNR
Am J Neuroradiol. 31:235–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mou L, Tian X, Zhou B, Zhan Y, Chen J, Lu
Y, Deng J, Deng Y, Wu Z, Li Q, et al: Improving outcomes of
tyrosine kinase inhibitors in hepatocellular carcinoma: New data
and ongoing trials. Front Oncol. 11:7527252021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hou Z, Zhu K, Yang X, Chen P, Zhang W, Cui
Y, Zhu X, Song T, Li Q, Li H and Zhang T: Apatinib as first-line
treatment in patients with advanced hepatocellular carcinoma: A
phase II clinical trial. Ann Transl Med. 8:10472020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mazzolini GD and Malvicini M:
Immunostimulatory monoclonal antibodies for hepatocellular
carcinoma therapy. Trends and perspectives. Medicina (B Aires).
78:29–32. 2018.PubMed/NCBI
|
|
46
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A Randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qiu Z, Shen L, Chen S, Qi H, Cao F, Xie L
and Fan W: Efficacy of apatinib in transcatheter arterial
chemoembolization (TACE) refractory intermediate and advanced-stage
hepatocellular carcinoma:A propensity score matching analysis.
Cancer Manag Res. 11:9321–9330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, Randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tashrifwala FAA, Karmani VK, Haider I,
Syeda AZ, Noorani A, Mustafa MS, Dave T and Hafeez H: Efficacy of
transarterial chemoembolization combined with camrelizumab in the
treatment of hepatocellular carcinoma: A systematic review and
meta-analysis. Cureus. 15:e486732023.PubMed/NCBI
|
|
50
|
Zheng L, Fang S, Wu F, Chen W, Chen M,
Weng Q, Wu X, Song J, Zhao Z and Ji J: Efficacy and Safety of TACE
combined with sorafenib plus immune checkpoint inhibitors for the
treatment of intermediate and advanced TACE-Refractory
hepatocellular carcinoma: A retrospective study. Front Mol Biosci.
7:6093222021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ke Q, Xin F, Fang H, Zeng Y, Wang L and
Liu J: The significance of transarterial chemo(embolization)
combined with tyrosine kinase inhibitors and immune checkpoint
inhibitors for unresectable hepatocellular carcinoma in the Era of
systemic therapy: A systematic review. Front Immunol.
13:9134642022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zou X, Xu Q, You R and Yin G: Correlation
and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor
in the treatment of hepatocellular carcinoma with portal vein tumor
thrombus based on immunological features. Cancer Med.
12:11315–11333. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang
F, Zhuang W, Chen X, Chen H, Xu B, et al: Lenvatinib plus TACE with
or without pembrolizumab for the treatment of initially
unresectable hepatocellular carcinoma harbouring PD-L1 expression:
A retrospective study. J Cancer Res Clin Oncol. 148:2115–2125.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu J, Wang P, Shang L, Zhang Z, Tian Y,
Chen X, Ma Y and Shao H: TACE plus tyrosine kinase inhibitors and
immune checkpoint inhibitors versus TACE plus tyrosine kinase
inhibitors for the treatment of patients with hepatocellular
carcinoma: A meta-analysis and trial sequential analysis. Hepatol
Int. 18:595–609. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gong A and Li X: The efficacy and safety
of Apatinib combined with TACE in the treatment of hepatocellular
carcinoma: A meta-analysis. World J Surg Oncol. 20:692022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khan KA and Kerbel RS: Improving
immunotherapy outcomes with anti-angiogenic treatments and vice
versa. Nat Rev Clin Oncol. 15:310–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Q, Gao J, Di W and Wu X:
Anti-angiogenesis therapy overcomes the innate resistance to
PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models.
Cancer Immunol Immunother. 69:1781–1799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cai M, Huang W, Huang J, Shi W, Guo Y,
Liang L, Zhou J, Lin L, Cao B, Chen Y, et al: Transarterial
chemoembolization combined with lenvatinib Plus PD-1 inhibitor for
advanced hepatocellular carcinoma: A retrospective cohort study.
Front Immunol. 13:8483872022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Teng Y, Ding X, Li W, Sun W and Chen J: A
retrospective study on therapeutic efficacy of transarterial
chemoembolization combined with immune checkpoint inhibitors plus
lenvatinib in patients with unresectable hepatocellular carcinoma.
Technol Cancer Res Treat. 21:153303382210751742022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ,
Wang SJ, Zhou JY, Li YN, Qiu FN, Li B and Yan ML: Lenvatinib
Combined with Anti-PD-1 antibodies plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
Multicenter retrospective study. J Hepatocell Carcinoma.
8:1233–1240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Raoul JL, Sangro B, Forner A, Mazzaferro
V, Piscaglia F, Bolondi L and Lencioni R: Evolving strategies for
the management of intermediate-stage hepatocellular carcinoma:
Available evidence and expert opinion on the use of transarterial
chemoembolization. Cancer Treat Rev. 37:212–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang XG, Sun YY, Wang HQ, Li DS, Xu GH and
Huang XQ: Efficacy and safety of transarterial chemoembolization
combining sorafenib with or without immune checkpoint inhibitors in
previously treated patients with advanced hepatocellular carcinoma:
A propensity score matching analysis. Front Oncol. 12:9143852022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu J, Wei S, Yang L, Yu J, Yan D and Yi
P: Efficacy and safety of transarterial chemoembolization plus
lenvatinib with or without programmed death-1 inhibitors in the
treatment of unresectable hepatocellular carcinoma: A systematic
review and meta-analysis. J Cancer Res Clin Oncol. 149:14451–14461.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao
C, Jia Y, Shi J, Zhang L, Liu X, et al: Low-Dose apatinib optimizes
tumor microenvironment and potentiates antitumor effect of
PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 7:630–643.
2019. View Article : Google Scholar : PubMed/NCBI
|