Introduction
Metastatic prostate cancer presenting at initial
diagnosis as a large pelvic mass with generalized lymphadenopathy
is rare; without prostate-specific antigen (PSA) screening, this
can easily be mistaken for lymphoma (1). The occurrence of acute inferior vena
cava syndrome, due to the progression of metastatic lymph nodes
around the abdominal aorta and inferior vena cava, is also rare,
with only three cases (2,3) documented in two references and no
records regarding its natural progression. The present study
describes a case of metastatic prostate adenocarcinoma, which
manifested as generalized lymphadenopathy and ultimately led to
death of the patient from acute inferior vena cava syndrome. A
literature review in 2005 revealed that, in the MEDLINE database,
there were only nine patients with prostate cancer who had
initially presented with generalized lymphadenopathy (4). Most researchers consider Gleason
scores a notable determinant of adenocarcinoma prognosis (1,5,6). The
presence of generalized lymph node metastases does not influence
the response of prostate cancer to androgen deprivation therapy
(ADT) (5). To the best of our
knowledge, the present study is the first to report on a case where
metastatic lesions exhibited only a partial response to ADT in this
type of prostate adenocarcinoma. Furthermore, this case is the
first to detail the natural progression of metastatic prostate
cancer with inferior vena cava syndrome.
Case report
A 71-year-old male patient presented with bilateral
waist pain persisting for ~20 days and was admitted to the West
China School of Public Health and West China Fourth Hospital
(Chengdu, China), as a result, in February 2023. Although the
patient experienced minor discomfort due to frequent urination,
they had no notable difficulty urinating. Upon physical
examination, substantial enlargement of the prostate and
supraclavicular lymph nodes was observed. A series of routine tests
was performed, including a routine urine and blood test, liver and
kidney function tests, electrolyte level detection and coagulation
profiling. Additionally, etiological screenings for pathogens, such
as human immunodeficiency virus, syphilis and hepatitis B were
performed. Each test yielded no marked abnormalities; however, the
creatinine level was 133 µmol/l (the normal reference range, 57–111
µmol/l). Computed tomography (CT) with intravenous contrast of the
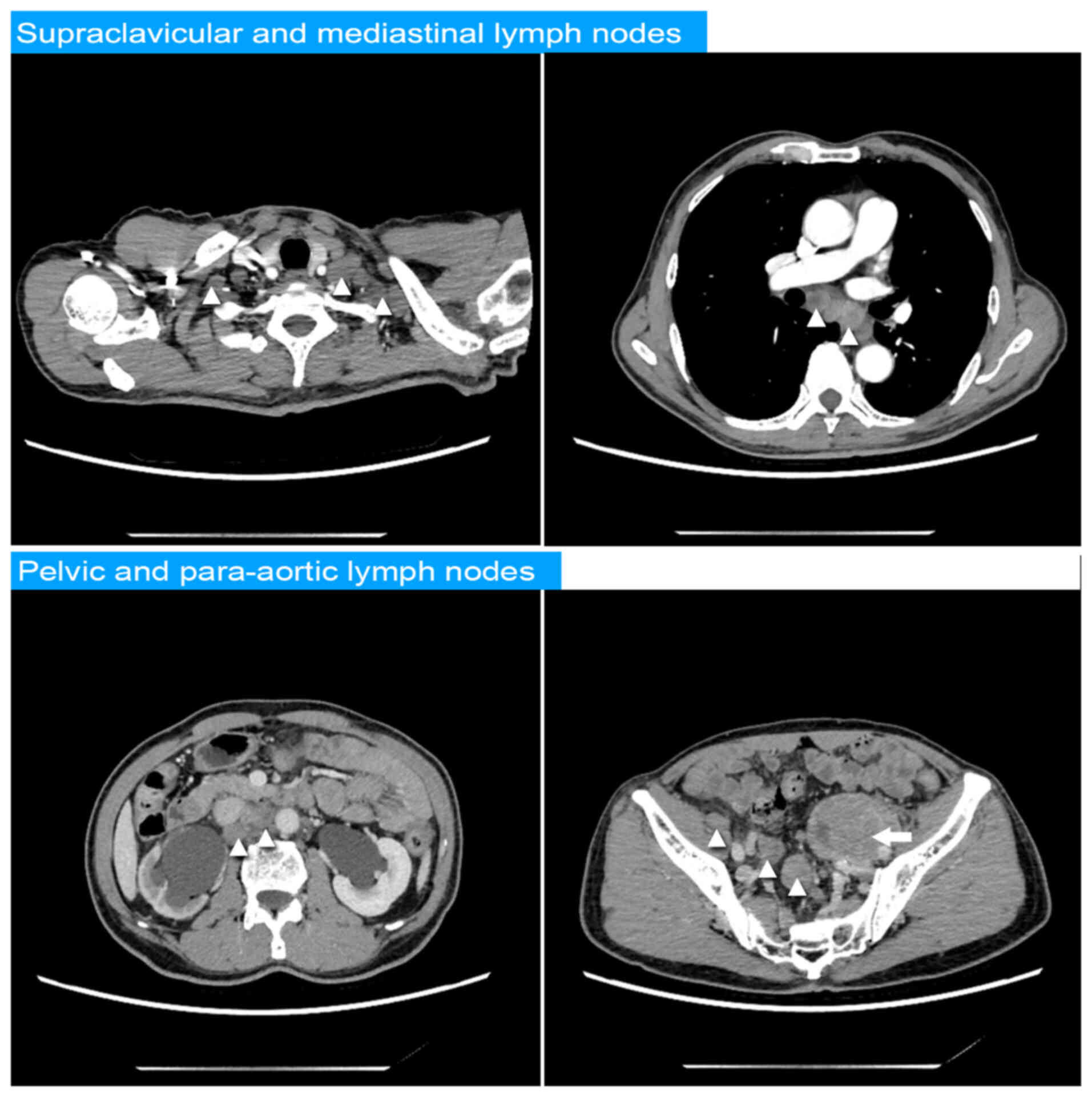
chest and abdomen revealed multiple enlarged lymph nodes in the
mediastinum, the bilateral supraclavicular fossa, inguinal regions,
and surrounding the abdominal aorta, inferior vena cava, and both
iliac arteries and veins. A mass was discerned between the prostate
and the posterior wall of the bladder, along with bilateral renal
hydronephrosis. Additionally, another mass measuring 8.9×4.1×6.3 cm
was discovered adjacent to the left iliac vessels, as shown in
Fig. 1. No signs of bone or
visceral metastasis were observed. A cystoscopic examination
revealed tumor invasion on the neck and trigone of the bladder, and
both ureteral orifices were unobservable. The protruding mass was
characterized by a smooth urothelial mucous membrane covering its
surface. The absence of discernible bladder tumor characteristics,
coupled with the anomalous prostate morphology and enhancement
properties in the CT scan, primarily raised suspicions of prostate
cancer infringing upon the neck and trigone of the bladder. PSA
screening was subsequently performed and the total PSA level was
>100 ng/ml (normal reference range, 0–4 ng/ml).
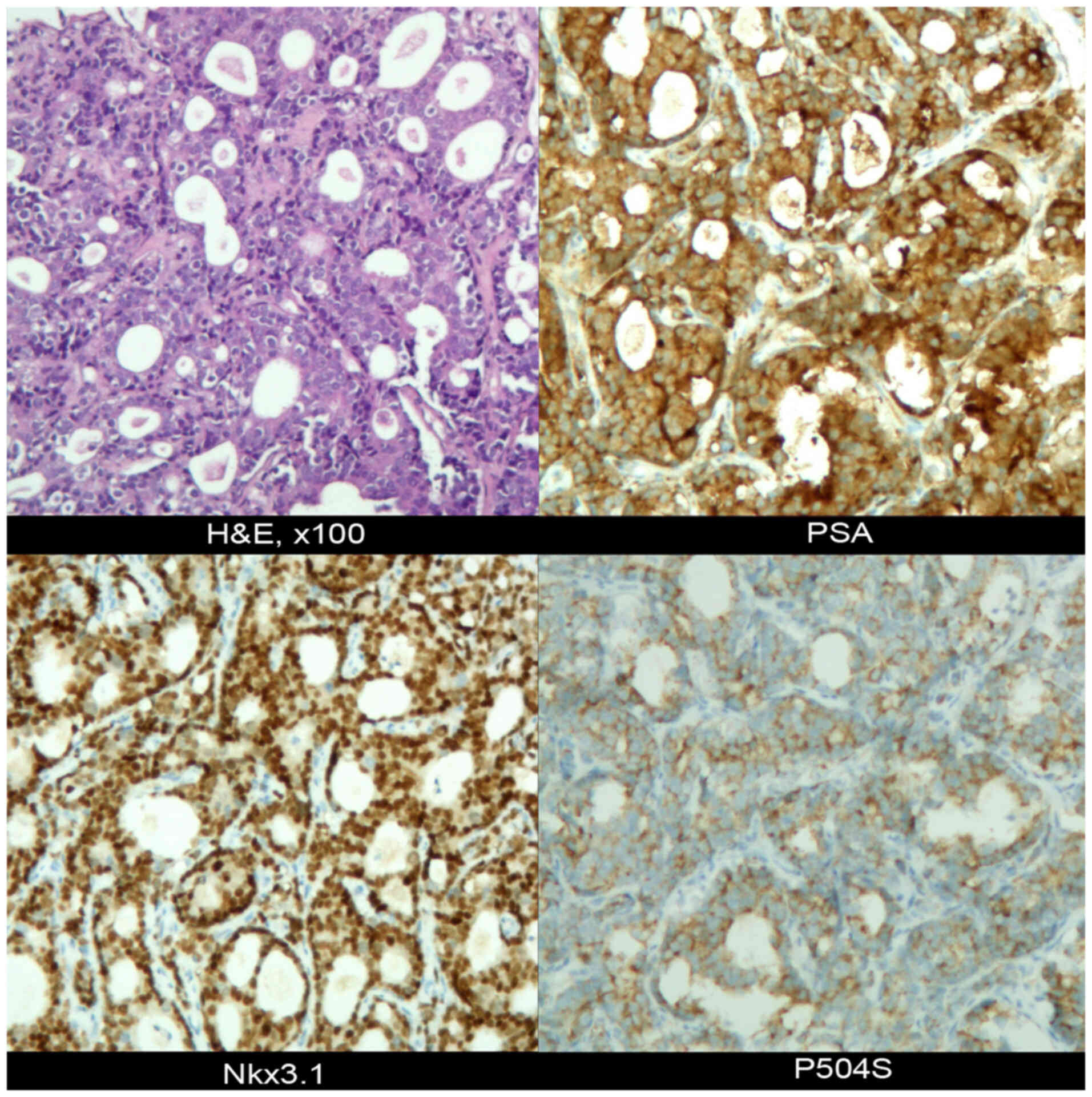
A biopsy of the large pelvic mass near the left
iliac vessels was conducted, whereupon the puncture specimen
revealed the presence of prostate-derived adenocarcinoma based on
the findings of immunohistochemical (IHC) analysis. IHC analysis,
performed as previously described (7), yielded the following profile: PSA (+),
P504S (+), Nkx3.1 (+), INSM1 (−), CgA (low +), Syn (low +), P53
(+), ERG (−), Ki-67 (+, ~30%) (Fig.
2). In addition, hematoxylin and eosin (H&E) staining was
performed according to a previously described protocol (7). Subsequently, bilateral renal drainage,
and ADT treatment with bicalutamide (50 mg, orally administered,
daily) and goserelin (10.8 mg, subcutaneous injection, once every
12 weeks) were commenced. An ultrasound-guided biopsy of the
prostate, conducted at a different institution (Sichuan Modern
Hospital, Chengdu, China), yielded a Gleason score of 4+5,
according to the findings of H&E and IHC staining; this follows
the 2014 International Society of Urological Pathology guidelines
on Gleason Grading of Prostatic Carcinoma (8) (Fig.
S1). After 3 months of treatment, the PSA levels declined to
6.96 ng/ml; however, they increased slightly to 7.85 ng/ml within 6
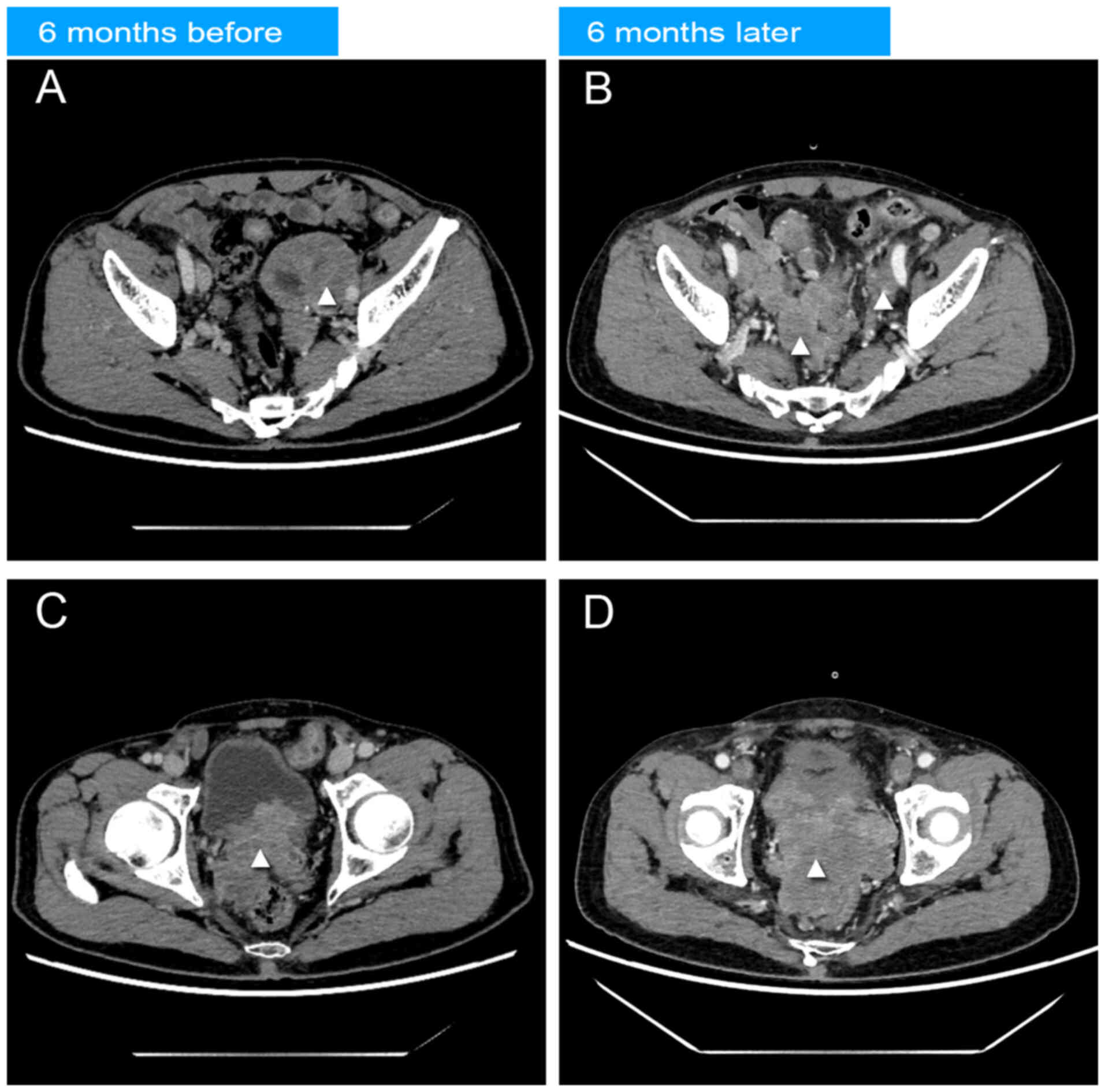
months. A follow-up abdominal CT scan with contrast revealed a
notable reduction in the pelvic mass, but enlargement of the
prostate lesion area and lymph nodes around the right iliac vessels
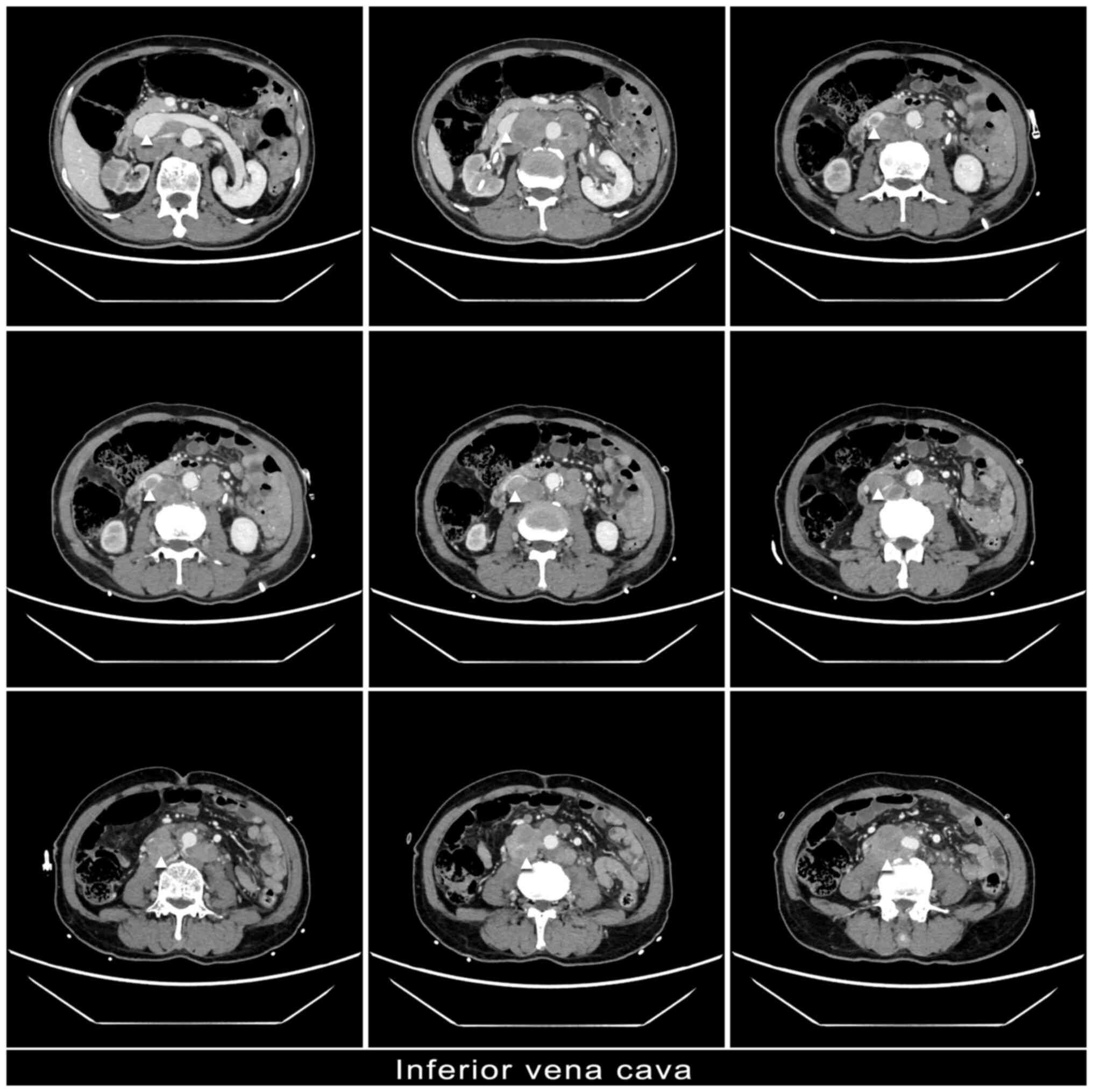
(Fig. 3). In the venous phase of CT
imaging, there was no enhancement observed in the segment of the
inferior vena cava, spanning from the plane of the right renal vein
to the bilateral external iliac veins. Additionally, multiple
enlarged lymph nodes compressed these veins, indicating potential
thrombosis within the lumen (Fig.
4). Consequently, on the basis of ADT treatment, the medication
regimen was amended to goserelin (10.8 mg, subcutaneous injection,
once every 12 weeks), abiraterone (1,000 mg, orally administered,
daily), prednisone (5 mg, orally administered, twice a day) and
docetaxel (120 mg, intravenously, every 3 weeks) with an
anticoagulant (rivaroxaban; 20 mg, orally administered, once
daily). After one treatment cycle, the PSA levels decreased to 5.34
ng/ml. However, the patient developed severe leg swelling,
accompanied by a rapid and significant enlargement of the cervical
lymph nodes after 2 weeks of this treatment. It was recommended
that the patient undergo a re-biopsy of the prostate and enlarged
cervical lymph nodes, and interventions by a vascular surgeon were
suggested; however, the patient declined due to previously
unsatisfactory treatment outcomes and their limited financial
resources. Within the next 3 weeks, the patient succumbed to severe
pulmonary infection that exacerbated inferior vena cava
syndrome-induced heart failure, ultimately causing circulatory
collapse.
Discussion
The lymph nodes in the pelvic region are the most
common sites for lymphatic metastasis in prostate cancer, followed
by the periaortic lymph nodes. By contrast, the involvement of
cervical and supraclavicular lymph nodes is rare (9). A literature review in 2005 revealed
that in the MEDLINE database, there were only nine patients with
prostate cancer who had initially presented with generalized
lymphadenopathy (4). Furthermore,
the present literature review, which used the PubMed database
(pubmed.ncbi.nlm.nih.gov) to identify cases of prostate
adenocarcinoma that initially presented with generalized
lymphadenopathy over the past 20 years, identified 19 cases
(5,10–22).
The mechanism of lymph node metastasis may proceed as follows: The
prostatic venous plexus, along with veins in the chest, abdomen and
pelvis, may facilitate metastasis to the pelvic and periaortic
regions. The potential pathway for metastasis to mediastinal and
supraclavicular lymph nodes may involve upwards spread to the
cisterna chyli and thoracic duct (1). Previous studies have suggested that
neuroendocrine differentiation of prostate cancer, such as small
cell and large cell prostate cancer, may initially present with
generalized lymphadenopathy, with or without visceral metastases at
diagnosis (23–25). Notably, metastatic prostate cancer
presenting as generalized lymphadenopathy needs to be
differentiated from malignant lymphoma. In the present case, at the
initial consultation, the patient exhibited generalized
lymphadenopathy without accompanying fever or weight loss.
Alongside the enhanced CT findings at the initial visit, the
differential diagnosis suggested metastatic urothelial carcinoma.
Since bladder cystoscopy showed no typical characteristics of a
urothelial tumor in the patient, prostate cancer was suspected, due
to the mass protruding from the prostate into the bladder, which
led to the invasion of the bladder trigone and the inability to
identify the bilateral ureteral orifices. In addition, PSA
screening indicated that the primary lesion may have originated
from the prostate. Nevertheless, the large pelvic mass isolated
from the prostate and adjacent to the left iliac vessels required
further examination. Consequently, a biopsy of this mass was
performed.
The final pathological findings confirmed that the
primary prostate cancer had metastasized to the left iliac lymph
node, resulting in a sizeable mass. Previous studies have
documented some instances of subclavian vein thrombosis resulting
from metastatic lymph node enlargement (26) and episodes of acute superior vena
cava syndrome (27). Acute inferior
vena cava syndrome is rare in metastatic prostate cancer; to the
best of our knowledge, there have been only three documented cases
(2,3). Kasimis et al (3) reported on the clinical features of two
instances of inferior vena cava syndrome, but did not document the
natural progression. Makusha et al (2) applied vascular stents to the patient
with acute inferior vena cava syndrome, enabling effective relief
of the obstruction and restoration of blood circulation.
Considering the rapid progression of the disease and the
ineffectiveness of therapies, the patient in this case chose a
relatively palliative course of medication treatment:
anticoagulation. The swelling in the lower extremities and scrotum
progressively worsened over the next 3 weeks, accompanied by
limited mobility, a large amount of skin exudation, infection and
ulceration. Eventually, the patient died of circulatory failure
induced by severe pulmonary infection and non-congestive heart
failure.
An initial diagnosis of prostate cancer, accompanied
by generalized lymphadenopathy, typically characterizes a cluster
of markedly heterogeneous tumors. The limited data currently
available suggest that these could include a variety of
pathological types, such as adenocarcinoma (20), the co-existence of adenocarcinoma
and small cell carcinoma (28),
small cell carcinoma (23,29), large cell carcinoma (30), and the co-existence of
adenocarcinoma and giant cell carcinoma (25). Notably, data on the prognosis of
these cases are scarce. Nevertheless, most researchers (1,5,6)
consider the Gleason score in adenocarcinoma as a significant
determinant of prognosis. The presence of generalized lymph node
metastases does not influence the response to hormonal therapy,
according to the previous studies (1,5,6).
According to the present literature review, Gleason scores mainly
range between 7 and 10 (1,5,6). In
addition, neuroendocrine differentiated types of prostate cancer
are often associated with generalized lymphadenopathy, and they
exhibit no response to hormonal therapy, demonstrating a reaction
to platinum-based chemotherapy instead (31). The prognosis and survival rates of
these types of prostate cancer are poor (23). In the present case, a portion of the
tumor tissue demonstrated a response to endocrine therapy. The
enhanced CT scans, performed 6 months prior to and following
endocrine therapy, demonstrated a notable decrease in the pelvic
mass around the left iliac artery. Concurrently, a notable increase
in metastatic lesions was observed surrounding the right iliac
artery and the abdominal aorta, alongside an enlargement of the
primary prostate malignancy. A total of 6 months into the regimen,
the PSA levels of the patient decreased from >100 to 7.85 ng/ml;
however, imaging findings showed a marked progression of disease.
Considering that the existing literature indicates a positive
response to endocrine therapy in cases of prostate adenocarcinoma
with generalized lymphadenopathy (5,6), the
present study may be the first to report on a case where only some
of the metastatic lesions in this type of prostate adenocarcinoma
exhibited a response to ADT treatment. Current PEACE-1 (32) and ARASENS (33) studies have stipulated that the
combination of ADT, neoadjuvant hormonal therapy and docetaxel may
improve metastatic prostate cancer overall survival. In the present
case, based on the ADT treatment with goserelin, the treatment plan
was adjusted to goserelin, abiraterone and docetaxel with the PSA
levels decreasing to 5.34 ng/ml, thus exhibiting a PSA response.
However, the left cervical lymph nodes rapidly increased in size,
thus suggesting a heterogeneity in the malignant lesions and
indicating that the treatment plan was ineffective for this
potentially heterogeneous tumor. In prostate cancer, the most
common type of differentiation is into neuroendocrine
differentiated tumors. Notably, it is unclear if the primary tumor
lesion was a heterogeneous tumor before or after ADT treatment. The
patient refused to undergo a further cervical lymph node or
prostate biopsy. Without further pathological support,
platinum-based chemotherapy, which has been reported to benefit
neuroendocrine differentiated tumors (31), was not administered. Eventually, the
patient suffered from rapid progression of metastatic lymph nodes
around the inferior vena cava, leading to inferior vena cava
syndrome, and died from circulatory failure induced by a severe
pulmonary infection and heart failure.
The clinical significance of the present case report
is to document a case of metastatic prostate cancer initially
presenting as generalized lymphadenopathy, and to recommend
vigilance regarding the concurrent presence of heterogeneous
tumors, specifically those with neuroendocrine differentiation. To
the best of our knowledge, the present study is the first to report
on a case where only some metastatic lesions in this type of
prostate adenocarcinoma exhibited a response to ADT treatment. The
present study indicated that in the initial evaluation of the
efficacy of endocrine therapy, combining PSA detection with imaging
evaluation should be recommended, and that imaging assessment is
indispensable in this type of prostate cancer. Furthermore, when
there is no response, or only a partial response, of lesions to
endocrine therapy, it is recommended that a re-biopsy be conducted
of the prostate or metastatic lesions. Genetic testing of tumor
tissue or circulating tumor cells may also provide relevant
information. In particular, a re-biopsy of different sites can
provide more detailed and comprehensive pathological results than
prior specimens, such as enlarged cervical lymph nodes or prostate
tissues in this case. A re-biopsy at the identical site within a
short space of time has no clinical value. Furthermore, to the best
of our knowledge, the present case is the first to detail the
natural progression of metastatic prostate cancer with inferior
vena cava syndrome. This pathophysiological transformation resulted
in an unfavorable survival prognosis for the patient, requiring
immediate intervention. The present study provides an important
prognostic and therapeutic reference for similar clinical
conditions, and therefore may have clinical value. Finally, the
literature review, from a pathological perspective, indicated that
this specific type of prostate cancer is often comprised of a group
of highly heterogeneous tumors. This insight provides a theoretical
basis for our improved understanding of this specific type of
prostate cancer, and the formulation of effective treatment
strategies and subsequent follow-up plans. The currently available
data are limited; therefore, more detailed and similar studies on
this specific disease in the future may verify the aforementioned
conclusions.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WW, CG, RW, XL, YL, GC, RR and FL were involved in
data collection. WW, XL and RW contributed to the review of case
materials, analyzed data and drafted the manuscript. CG developed
the patient treatment strategies and established the follow-up
protocols. WW, XL, RW and CG confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of The Declaration of Helsinki. The performance and publication of
this study was approved by the Ethics Committee of West China
School of Public Health and West China Fourth Hospital, Sichuan
University (Chengdu, China; approval no. HXSY-EC-2024058). Written
informed consent was obtained from the patient's family.
Patient consent for publication
Written informed consent was obtained from the
patient's family for publication of the data and images in this
case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oyan B, Engin H and Yalcin S: Generalized
lymphadenopathy: A rare presentation of disseminated prostate
cancer. Med Oncol. 19:177–199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makusha LP, Kulon M, Pucar D and Young CR:
Inferior vena cava syndrome on skeletal scintigraphy secondary to
metastatic prostate cancer. World J Nud Med. 19:324–326. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kasimis BS and Spiers AS: Inferior vena
cava obstruction. A complication of prostate cancer. Arch Intern
Med. 139:1056–1057. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heresi GA, Wang J, Taichman R, Taichman R,
Chirinos JA, Regalado JJ, Lichtstein DM and Rosenblatt JD:
Expression of the chemokine receptor CCR7 in prostate cancer
presenting with generalized lymphadenopathy: Report of a case,
review of the literature, and analysis of chemokine receptor
expression. Urol Oncol. 23:261–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karatzas AD, Zachos I, Mitrakas L,
Dimitropoulos K, Samarinas M, Gravas S, Oeconomou A and Tzortzis V:
Generalized lymphadenopathy of prostate adenocarcinoma origin. A
case series. Urology. 91:e3–e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krpina K, Markić D, Rahelić D, Ahel J,
Rubinić N and Španjol J: 10-year survival of a patient with
metastatic prostate cancer: Case report and literature review. Arch
Ital Urol Androl. 87:252–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hameed O, Sublett J and Humphrey PA:
Immunohistochemical stains for p63 and alpha-methylacyl-CoA
racemase, versus a cocktail comprising both, in the diagnosis of
prostatic carcinoma: A comparison of the immunohistochemical
staining of 430 foci in radical prostatectomy and needle biopsy
tissues. Am J Surg Pathol. 29:579–587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
international society of urological pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–25. 2005. View Article : Google Scholar
|
|
9
|
Chan G and Domes T: Supraclavicular
lymphadenopathy as the initial presentation of metastatic prostate
cancer: A case report and review of literature. Can Urol Assoc J.
7:E433–E435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turkbey B, Basaran C, Boge M,
Karcaaltincaba M and Akata D: Unusual presentation of prostate
cancer with generalized lymphadenopathy and unilateral leg edema.
JBR-BTR. 91:211–213. 2008.PubMed/NCBI
|
|
11
|
Lad M, Sharma A and Patten DK: A
surprising diagnosis: Metastatic prostate cancer causing cervical
lymphadenopathy. BMJ Case Rep. 11:bcr20132016302014. View Article : Google Scholar
|
|
12
|
Hematpour K, Bennett CJ, Rogers D and Head
CS: Supraclavicular lymph node: Incidence of unsuspected metastatic
prostate cancer. Eur Arch Otorhinolaryngol. 263:872–874. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uemura M, Hirai T, Kanno N, Nishimura K,
Mizutani S, Miyoshi S, Yoshida K and Kawano K: Prostatic carcinoma
presenting as neck lymph node metastases: Report of two cases.
Hinyokika Kiyo. 47:755–758. 2001.(In Japanese). PubMed/NCBI
|
|
14
|
Tan-Shalaby J: Prostate carcinoma
presenting with bulky mediastinal and cervical lymphadenopathy. BMJ
Case Rep. 22:bcr20130086432013. View Article : Google Scholar
|
|
15
|
Kosugi S, Mizumachi S, Kitajima A,
Igarashi T, Hamada T, Kaya H, Kurihara K, Ogasawara K, Sakata H,
Yamamoto M, et al: Prostate cancer with supraclavicular
lymphadenopathy and bulky abdominal tumor. Intern Med.
46:1135–1138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haraoka M, Takamuki M, Toyonaga Y, Tanaka
M, Hanazawa K, Sakamoto Y and Horie S: Prostate cancer of unknown
primary origin with multiple lymph nodes metastasis; a case report.
Nihon Hinyokika Gakkai Zasshi. 105:212–217. 2014.(In Japanese).
PubMed/NCBI
|
|
17
|
Platania M, Bajetta E, Guadalupi V,
Buzzoni R and Colecchia M: Prostate adenocarcinoma presenting with
supraclavicular node enlargement: Report of a case. Tumori.
94:769–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin YY, Lin DS, Kang BH and Lin YS: Neck
mass as the first presentation of metastatic prostatic
adenocarcinoma. J Chin Med Assoc. 74:570–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Diego Rodríguez E, del Valle Schaan JI,
Baños JL, García BM, Rodríguez RH, Martín JA, Gómez MA, Edreira AR,
Peña AV, Velázquez MA and Rodríguez AH: Massive lymphatic
involvement secondary to prostatic adenocarcinoma. Actas Urol Esp.
24:836–839. 2000.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garg PK, Jain BK, Dubey IB and Sharma AK:
Generalized lymphadenopathy: Physical examination revisited. Ann
Saudi Med. 33:298–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang F, Dávila S, Ovalles V, Mejías E,
Rodríguez O and Rodríguez R: Cervical adenopathy presentation of
adenocarcinoma of prostate. Actas Urol Esp. 31:1193–1195. 2007.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimizu K, Nakano S, Okada Y, Nagahama K,
Okubo K and Yasuhara Y: A case of prostate cancer with high levels
of prostate-specific antigen undetected by prostate biopsy.
Hinyokika Kiyo. 65:75–80. 2019.(In Japaese). PubMed/NCBI
|
|
23
|
Wang J, Liu X, Wang Y and Ren G: Current
trend of worsening prognosis of prostate small cell carcinoma: A
population-based study. Cancer Med. 8:6799–6806. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okoye E, Choi EK, Divatia M, Miles BJ,
Ayala AG and Ro JY: De novo large cell neuroendocrine carcinoma of
the prostate gland with pelvic lymph node metastasis: A case report
with review of literature. Int J Clin Exp Pathol. 7:9061–9066.
2014.PubMed/NCBI
|
|
25
|
Lopez-Beltran A, Eble JN and Bostwick DG:
Pleomorphic giant cell carcinoma of the prostate. Arch Pathol Lab
Med. 129:683–685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biyani CS, Basu S, Bottomley DM and Shah
TK: Prostatic adenocarcinoma masquerading as lymphoma and
presentation with axillary-subclavian vein thrombosis. Urol Oncol.
21:3–6. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yun HD and Ershler WB: Superior vena cava
syndrome as a presentation of metastatic prostate cancer. BMJ Case
Rep. 3:bcr20120064802012. View Article : Google Scholar
|
|
28
|
Hashimoto Y, Kimura G, Tsuboi N and
Akimoto M: A case of prostatic small cell carcinoma. Hinyokika
Kiyo. 46:425–427. 2000.(In Japanese). PubMed/NCBI
|
|
29
|
Tsukino H, Nagano M, Takehara T, Hamasuna
R, Hasui Y and Osada Y: Small cell carcinoma of the prostate: A
case report. Hinyokika Kiyo. 47:113–116. 2001.(In Japanese).
PubMed/NCBI
|
|
30
|
Shun Z, Cheng W, Su-Bo Q, Yu D, Chen W,
Huang-Qi D, Wei-Min X and Hai-Bo S: Large cell neuroendocrine
carcinoma of prostate: A case report. Journal of Shanghai Jiao Tong
University (Medical Science). 40:1562–1570. 2020.
|
|
31
|
Kimura H, Uegaki M, Aoyama T, Kawai J,
Hamano T and Hashimura T: Carboplatin plus irinotecan induced
partial response in a patient with small cell carcinoma of the
prostate; a case report. Hinyokika Kiyo. 60:39–43. 2014.(In
Japanese). PubMed/NCBI
|
|
32
|
Fizazi K, Foulon S, Carles J, Roubaud G,
McDermott R, Fléchon A, Tombal B, Supiot S, Berthold D, Ronchin P,
et al: Abiraterone plus prednisone added to androgen deprivation
therapy and docetaxel in de novo metastatic castration-sensitive
prostate cancer (PEACE-1): A multicentre, open-label, randomised,
phase 3 study with a 2×2 factorial design. Lancet. 399:1695–1707.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smith MR, Hussain M, Saad F, Fizazi K,
Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B,
Montesa-Pino Á, et al: Darolutamide and survival in metastatic,
hormone-sensitive prostate cancer. N Eng J Med. 386:1132–1142.
2022. View Article : Google Scholar
|