Introduction
Breast cancer (BC) is one of the most prevalent
malignant tumors in women, representing 30% of all female cancers
(1). The disease can be divided
into unilateral BC (UBC) and bilateral BC (BBC). The overall
morbidity rate of BBC is low; however, with the improvement in BC
diagnostics and therapeutics and the prolongation of postoperative
survival times in recent years, the cumulative occurrence of this
disease has increased annually. BBC can be divided into synchronous
BBC (SBBC) and metachronous BBC (MBBC) according to the interval
between the first and second tumor diagnosis (2). The incidence of SBBC is 1.39% among
the general population (3), and the
rate of remote metastasis is significantly higher than that of UBC
(4). Hormone receptors are
biomarkers for characterizing the prognosis of patients with BC and
predicting treatment responses (5);
for example, a transition from positive to negative estrogen
receptor (ER) status after neoadjuvant chemotherapy is associated
with a poor prognosis (6). A
retrospective study found that the 5-year disease-free survival
(DFS) and 5-year overall survival (OS) estimates for patients with
persistent ER positivity after neoadjuvant chemotherapy were 88 and
92%, respectively, meanwhile the survival for patients with
ER-positive conversion (85 and 83%) differed significantly
(7). ER, progesterone receptor
(PR), and human epidermal growth factor receptor-2 (HER2) have been
established as strong predictors of the effects of various
molecular targeted therapies, such as endocrine and anti-HER2
treatments (8). Selecting the
optimal therapeutic regimen based on the molecular subtypes of SBBC
is challenging, particularly given the inconsistency in molecular
subtypes.
Materials and methods
Immunohistochemical (IHC)
staining
The surgically resected tissues were fixed with 10%
neutral buffer formalin and dehydrated with gradient alcohol, then
immersed in the embedding molds filled with paraffin at 58°C and
solidified in the freezer. IHC staining was performed on 4 µm-thick
paraffin sections of the tumor tissues according to the
manufacturer's instructions. The ready-to-use primary antibodies
for ER, PR, HER2, CK5/6, P63, Ki-67, P120, CK34βE12 and E-cadherin
(cat. nos. Kit-0012, Kit-0013, Kit-0043, MAB-0744, MAB-0694,
MAB-0672, MAB-0621, Kit-0020 and MAB-0738, respectively) were
purchased from MXB Biotechnologies Co., Ltd. The sections were
incubated with the primary antibodies for 2 h at 37°C. An
EliVision™ plus Polyer HRP kit (cat. no. Kit-9901; MXB
Biotechnologies Co., Ltd.) was used for secondary antibody
incubation and chromogenic reaction. IHC staining sections were
observed under an optical microscope (BX53; Olympus Corp.), and the
representative images were collected.
Fluorescence in situ hybridization
(FISH)
FISH assay was performed on 3 µm-thick paraffin
sections of the tumor tissues according to the instructions of the
human HER2 gene amplification detection kit (cat. no. FP-001; Wuhan
HealthCare Biotechnology Co., Ltd.), which contains a HER2 gene
probe (300 kb Spectrum Orange-directly labelled DNA probe for
17q12-21.1) with a probe for centromeric enumeration of chromosome
17 (CEP17; Spectrum Green-directly labelled fluorescent DNA probe).
The paraffin was removed from the sections with a 15-min wash in
dewaxing agent at 68°C. The sections were dehydrated in 100%
ethanol for 5 min, immersed in permeabilization buffer for 20 min
at 90°C, and rinsed in purified water for 1 min. After incubation
in a protease solution at 37°C for 15 min, the enzymatic reaction
was stopped by placing the sections in 2X saline sodium citrate
(SSC) wash buffer twice for 5 min. Subsequently, the sections were
dehydrated through graded alcohols, and 10 µl dual HER2/CEP17 probe
was applied to the sections. The probe and target tissue were then
co-denatured for 5 min at 85°C and allowed to hybridize for 2 h at
42°C using a hybridization instrument (ThermoBrite; Leica
Biosystems). The sections were washed in 2X SSC/0.1% NP-40 for 2
min at 68°C, rinsed in purified water for 1 min at 37°C, and
counterstained with 10 µl 4′,6-diamidino-2-phenylindole (DAPI).
FISH analysis (×1,000 magnification) was performed under a
fluorescence microscope (BX53; Olympus Corp.).
Whole-exome sequencing (WES)
The research team submitted the application for
project review and the research protocol to the Ethics Committee of
the First Affiliated Hospital of Dali University (Dali, China)
before the initiation of the project. The Ethics Committee held a
meeting to validate the reasonableness and safety of the project
and approved the use of biospecimens obtained in clinical diagnosis
and treatment to conduct the research (approval no. DYF20230309).
After obtaining ethical approval for using patient samples, the
authors commissioned BGI Technology Co., Ltd. (Shenzhen, China) to
perform WES on bilateral tumor tissues. The genomic DNA in the
tissue samples was extracted using a DNA extraction kit (cat. no.
940-000972-00; MGI Tech Co., Ltd.). The concentration of DNA
samples was quantified by Qubit fluorimeter (Thermo Fisher
Scientific, Inc.), and the integrity of DNA samples was detected by
1% agarose gel electrophoresis and visualized by ethidium bromide.
The DNA samples were mechanically fragmented to 200–300 bp using an
ultrasonic cell disruption system (Covaris, LLC) and purified with
an Agencourt AMPure XP kit (cat. no. A63881; Beckman Coulter,
Inc.). Qualified samples can be used for DNA library preparation,
and the fragment size and concentration of DNA library were
examined with a DNA 1000 kit (cat. no. 5067-1504; Agilent
Technologies, Inc.) on a 2100 Bioanalyzer instrument (Agilent
Technologies, Inc.). The loading concentration of the final library
was 10 pM for DNA sequencing. The exome microarray (Agilent_V6;
Agilent Technologies, Inc.) was applied to capture the DNA library,
and paired end sequencing (PE150) was finally performed using a
DNBSEQ instrument (MGI Tech Co., Ltd.). The MuTect2 tool [version
4.1.4.1; genomic analysis toolkit (GATK) team], the Funcotator tool
(version 4.1.4.1; GATK team), the FACETS (9) software (version 0.6.2; Memorial Sloan
Kettering Cancer Center), and MuSic (10) software (version 0.4; Washington
University) were used to analyze the sequencing data.
Literature retrieval
A systematic literature search was conducted in the
PubMed database (https://pubmed.ncbi.nlm.nih.gov/) to identify all
relevant studies published up to September 2023. The following
search strategy was applied to obtain relevant titles and
abstracts: ‘BC’ AND ‘bilateral’ AND (‘discordant’ OR ‘discordance’
OR ‘heterogeneous’). A manual search of all references cited in
full-text papers was performed to identify additional studies for
inclusion.
Results
Case presentation
The patient was a 72-year-old woman who found a
right breast mass without any persistent pain 2 months earlier and
was referred to the local hospital in July 2022. Mammography showed
a right breast mass (category 4B) without redness, swelling, or
rupture and no chills, fever, nausea, or vomiting. The patient was
hospitalized at the First Affiliated Hospital of Dali University on
July 25, 2022 (Dali, China) for further diagnostics and treatment.
The physical examination revealed symmetric breasts with no redness
of the surface skin, no nipple indentation, and no abnormal
elevation or depression on the surface of the mammary glands. A
2.5×2 cm lump was palpated in the right breast, with medium
quality, no compression pain, activity investigated, poorly defined
border, and no nipple discharge on extrusion. An ~3×2 cm enlarged
lymph node was detected in the right axilla, no lump was palpated
in the left breast, and no enlarged lymph node was detected in the
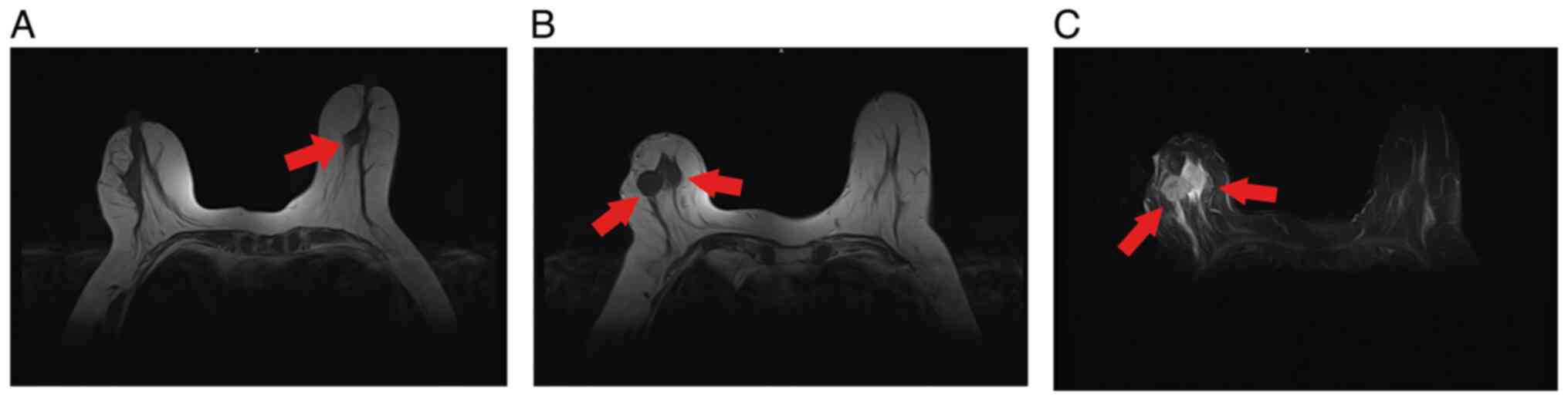
left axilla. Magnetic resonance imaging (MRI) showed two small
shallowly lobulated nodules in the left breast, and multiple
nodules in the right breast with flocculent abnormal signals around
the lesions. Local infiltration was possible (Fig. 1).
Bilateral modified radical mastectomy for BC and
anterior sentinel lymph node biopsy were performed on day 9 after
hospital admission. The left mastectomy specimen was found in the
inner lower quadrant 2 cm from the nipple with a 1 cm diameter
scooped area. No definite mass was observed around the excavation
area, and the rest of the mastectomy tissue was grayish yellow,
grayish-white, solid and medium textured, with an axillary fat size
of 9×8×3 cm. A total of 19 nodules, 0.4–1.3 cm in diameter, were
found within it. The excised right breast specimen showed a mass
measuring 5.5×3×2 cm in size in the outer upper quadrant 2 cm from
the nipple, which was grayish, solid, hard, and poorly demarcated
on the cut surface. The rest of the breast tissue was grayish,
grayish reddish, substantial, and medium in texture. The axillary
fat measured 10×9×2.5 cm, and 18 nodes measuring 0.8–3 cm in
diameter were detected in its interior.
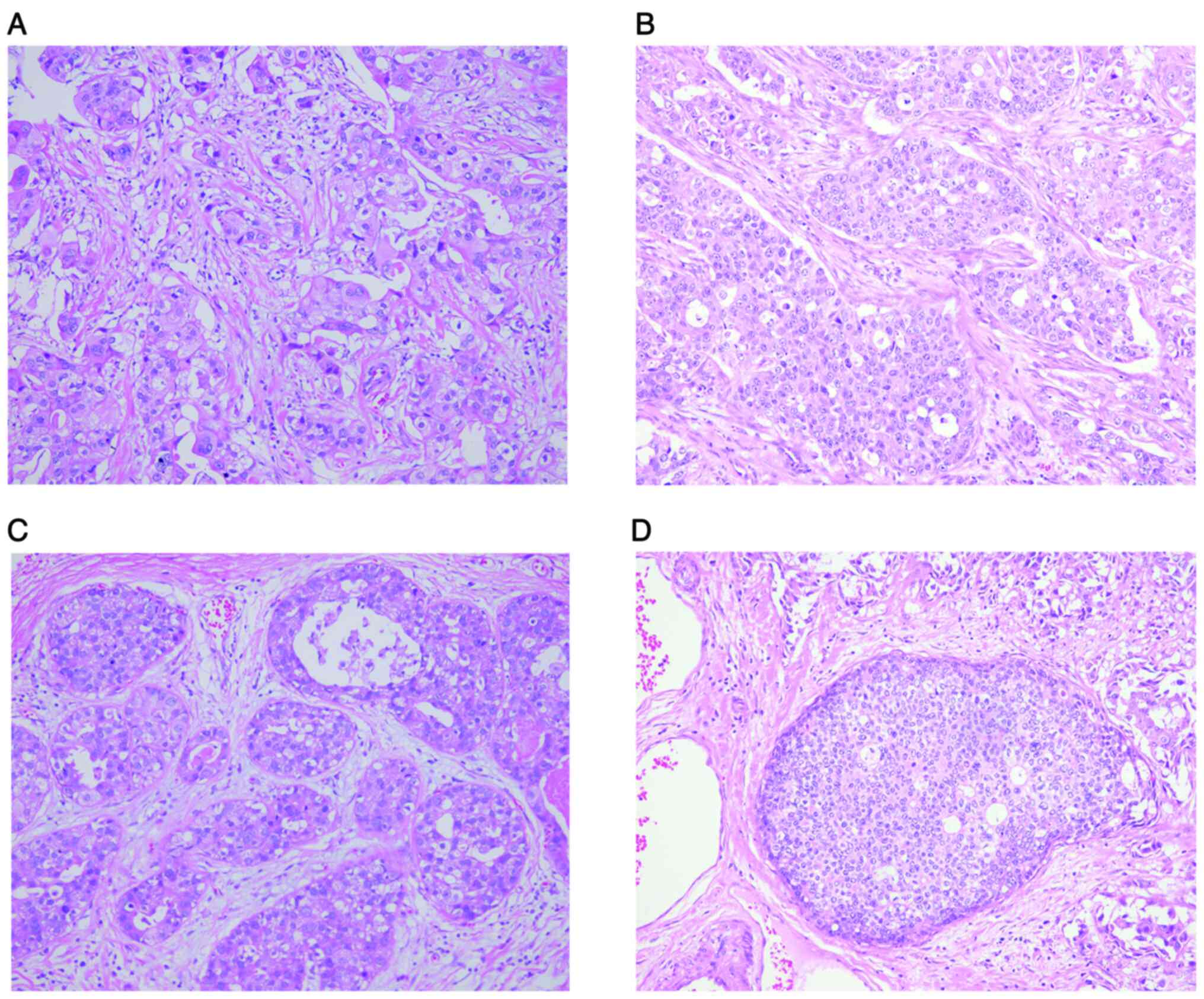
The tumor was diagnosed as invasive ductal carcinoma
with high grade intraductal carcinoma. Microscopically, the tumor
showed infiltrative growth; the tumor cells in invasive ductal
carcinoma area of bilateral breasts were arranged in a trabecular
or nested pattern with reduced interstitial stroma, abundant
eosinophilic cytoplasm, vacuolated nuclei of varying sizes,
extensive nuclear division, and remarkable heterogeneity; and a
small number of lymphocytes were infiltrated in the interstitial
stroma (Fig. 2A and B). The dilated
mammary duct and the tumor cells with high proliferative activity
in the high-grade intraductal carcinoma area of the left breast is
shown in Fig. 2C; necrotic material
with calcification could be observed in the center of the lumen,
and there were large polymorphic cells around the necrotic area;
the tumor cells with irregular nuclei are arranged disorderly. In
the high-grade intraductal carcinoma area of the right breast, the
slightly enlarged tumor cells were typically arranged in either
solid or cribriform pattern, with round or oval nuclei of uniform
size (Fig. 2D). The surgical
pathology report revealed fibro-adenopathy in the tissue
surrounding the tumor with no cancer in the peripheral margins,
base and nipple of the left breast and no carcinoma metastasis in
the ipsilateral axillary lymph nodes; the tissue in the right
breast showed fibro-adenopathy in the peripheral tissue surrounding
the tumor, and the peripheral margins, base and nipple were not
cancerous. A total of 7 out of 14 axillary lymph nodes were
positive for metastasis, and 4 other carcinomatous nodes were
visualized.
IHC staining revealed that the left invasive ductal
carcinoma was negative for ER, PR, cytokeratin5/6 (CK5/6), and P63,
but positive for HER2 (2+), Ki-67 (10%+), P120, CK34βE12 and
E-cadherin (Fig. 3A-I).
Myoepithelial cells were positive for CK5/6 and P63 in the
intraductal carcinoma area (Fig. 3J and
K). FISH assays further confirmed HER2 gene amplification in
the invasive ductal carcinoma area of the left breast (Fig. 3L). The right invasive ductal
carcinoma was positive for ER (90%+), PR (20%+), Ki-67 (50%+),
P120, CK34βE12 and E-cadherin, but negative for HER2, CK5/6 and P63
(Fig. 4A-I). In the right
intraductal carcinoma area, myoepithelial cells were positive for
P63 and CK5/6, while tumor cells were negative for HER2 but highly
positive for ER and PR (Fig. 4J-N).
The patient was diagnosed with bilateral simultaneous BC of
discordant molecular types: The molecular subtype of the left BC
was HER2 positive [HR(−)], and the molecular subtype of the right
BC was luminal B [HER2(−)]. The tumor-node-metastasis staging was
based on the 8th edition of the American Joint Committee on Cancer
(AJCC) BC staging system (2), with
T1bN0M0 IA in the left breast and T3N2M0 IIIA in the right
breast.
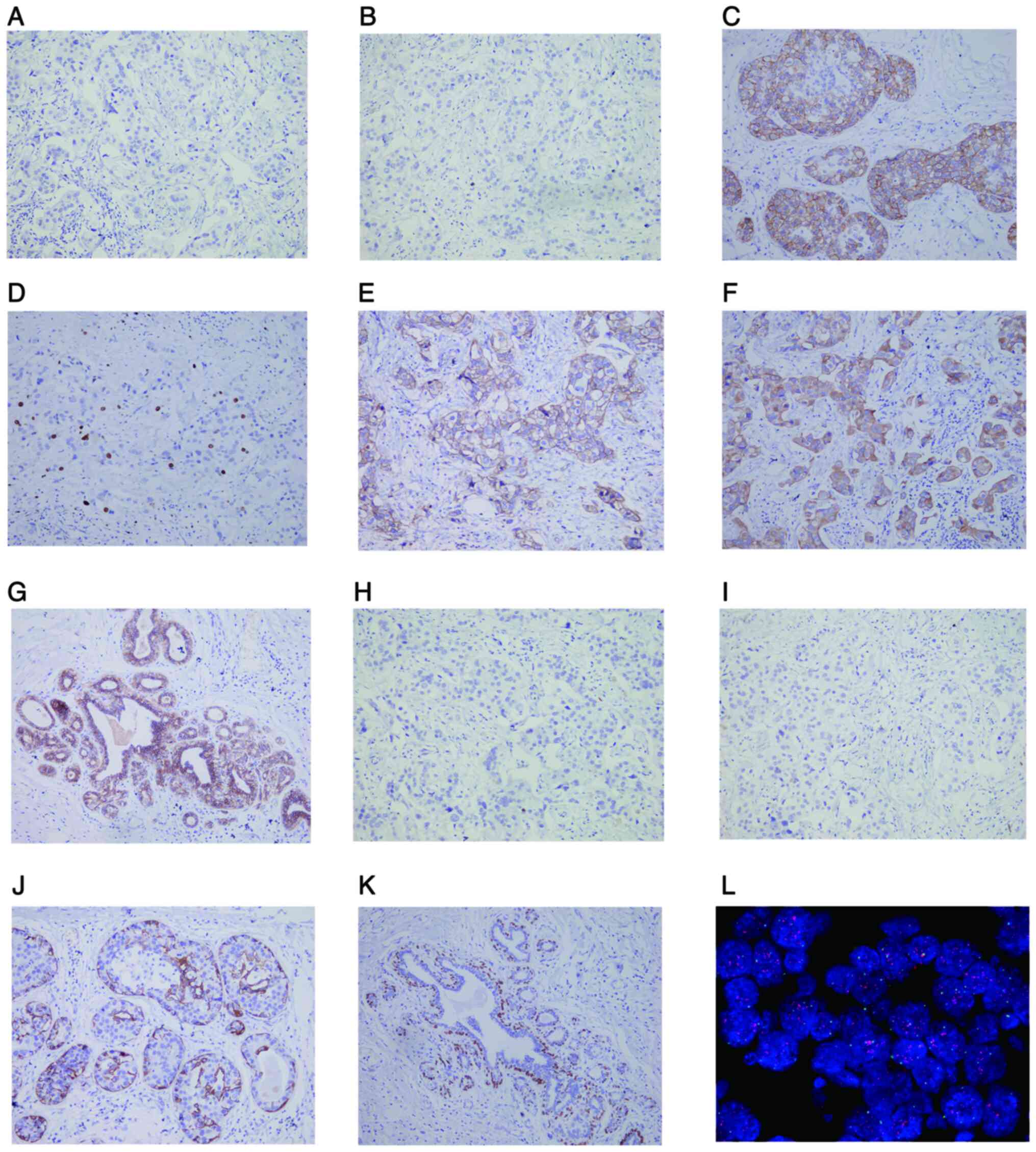 | Figure 3.IHC staining (magnification, ×200)
and FISH assay (magnification, ×1,000) in the left breast cancer
tissues. IHC staining showed that the left invasive ductal
carcinoma cells were negative for (A) ER, (B) PR, (H) CK5/6 and (I)
P63 but positive for (C) HER2, (D) Ki-67, (E) P120, (F) CK34βE12
and (G) E-cadherin. IHC staining showed that the myoepithelial
cells in the left high-grade intraductal carcinoma area were
positive for (J) CK5/6 and (K) P63. (L) HER2 gene amplification in
the left invasive ductal carcinoma was detected by FISH. The red
signal shows focal amplification of HER2 gene, and the green signal
shows the centromeric enumeration of chromosome 17. IHC,
immunohistochemistry; FISH, fluorescence in situ
hybridization; ER, estrogen receptor; PR, progesterone receptor;
CK5/6, cytokeratin5/6; HER2, human epidermal growth factor
receptor-2. |
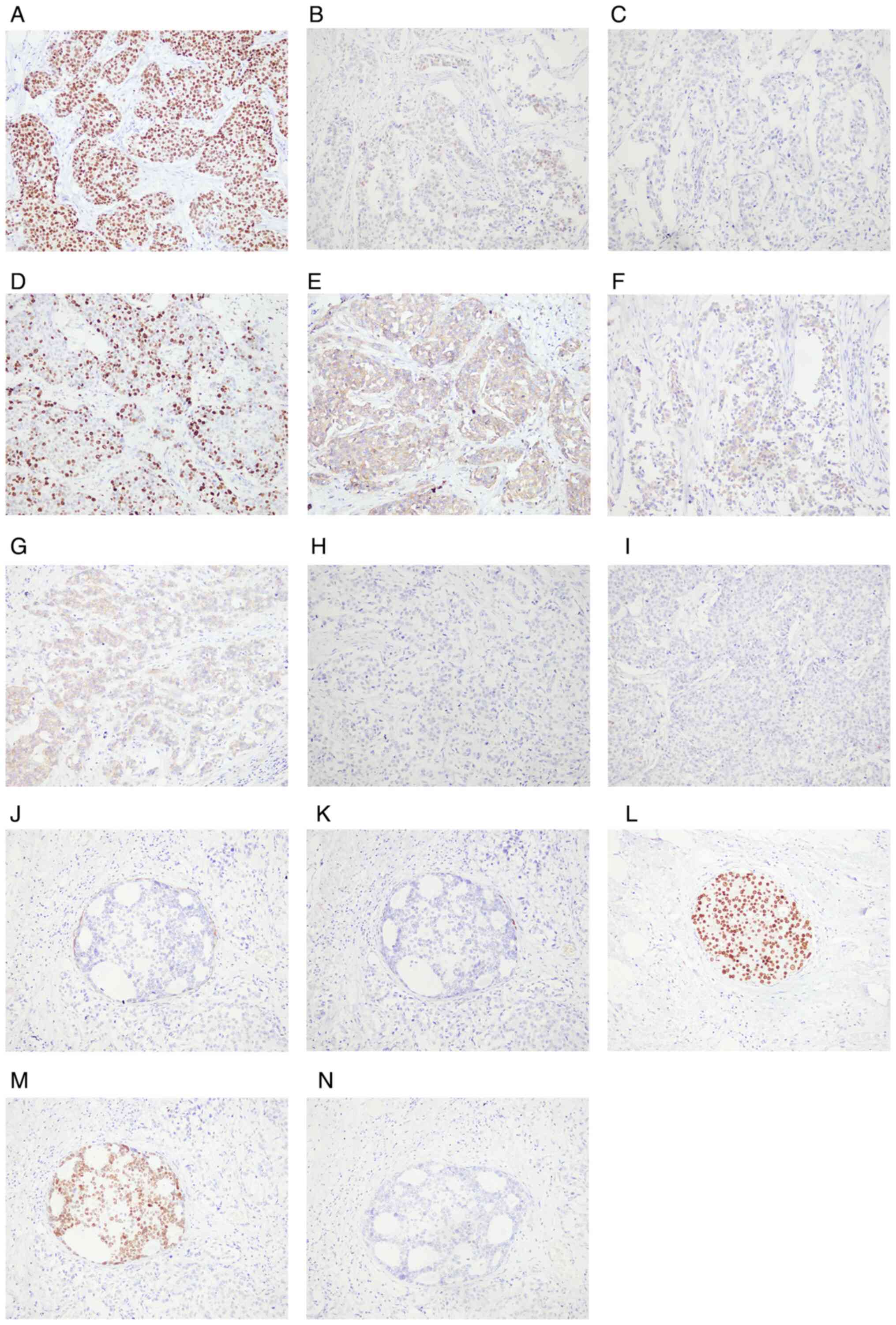 | Figure 4.IHC staining (magnification, ×200) of
the right breast cancer tissues. IHC staining showed that the right
invasive ductal carcinoma cells were positive for (A) ER, (B) PR,
(D) Ki-67, (E) P120, (F) CK34βE12 and (G) E-cadherin but negative
for (C) HER2, (H) CK5/6 and (I) P63. IHC staining showed that in
the right intraductal carcinoma area, myoepithelial cells were
positive for (J) CK5/6 and (K) P63, while tumor cells were positive
for (L) ER and (M) PR but negative for (N) HER2. IHC,
immunohistochemistry; ER, estrogen receptor; PR, progesterone
receptor; HER2, human epidermal growth factor receptor-2; CK5/6,
cytokeratin5/6. |
Because this patient did not undergo pathological
examination through ultrasound-guided percutaneous biopsy before
surgery, although the tumor size and regional lymph node
involvement could be determined, it was not available to determine
the specific subtype of BC as well as some microscopic metastasis
lesions before surgery. For example, M1 staging was defined by the
distant metastasis detected through clinical and imaging
examinations or metastasis lesion >0.2 mm under microscopy
through histopathological examinations. The identification of BC
subtypes requires detection of immunohistochemical markers,
including ER, PR, HER2 and Ki-67, on the histopathological
sections. Therefore, the description of case presentation in the
study was based on this.
After the surgery, a multidisciplinary diagnosis and
treatment team helped to determine the further adjuvant therapeutic
options for the patient. The patient's scars healed well, with no
erythema, tenderness, nodules, or palpable abnormal masses, and no
enlarged lymph nodes were detected bilaterally in the axillae,
supraclavicular region, or neck. She then underwent the first
postoperative chemotherapy on September 2, 2022, with an
albumin-bound paclitaxel + trastuzumab + pertuzumab (THP) regimen.
On September 13, 2022, a specialist examination revealed infection
with necrotic corruption and pus in the left breast incision. The
patient received anti-infective therapy, and received chemotherapy
with trastuzumab + pertuzumab (HP) regimen on September 22, 2022.
From October 2022 to January 2023, the patient received four times
of THP regimens, with 5–10 years of endocrine therapy and targeted
therapy prescribed. During the follow-up period from January 2023
to April 2024, the patient continued to receive a combination
therapy of trastuzumab, pertuzumab and letrozole. The patient has
been in favorable physical condition without tumor recurrence up to
now.
Differential variations in BBC
tissues
WES was performed to explore possible different
molecular alterations between the bilateral tumor tissues. By
comparing the mutations in the left and right BC tissues, the
differential single nucleotide variants (SNVs), insertions and
deletions (InDels), and cell copy number variations (CNVs) in the
bilateral tumor tissues of discordant molecular subtypes were
obtained. SNVs are widely found in the human genome and are
associated with many phenotypic differences and susceptibility to
drugs or diseases. InDels refer to insertions and deletions of
small fragments in the genome. InDels in a coding region or splice
site can alter protein translation. The MuTect2 tool (version
4.1.4.1; GATK team) was employed to find SNV and InDel sites and
annotated them using the Funcotator tool (version 4.1.4.1; GATK
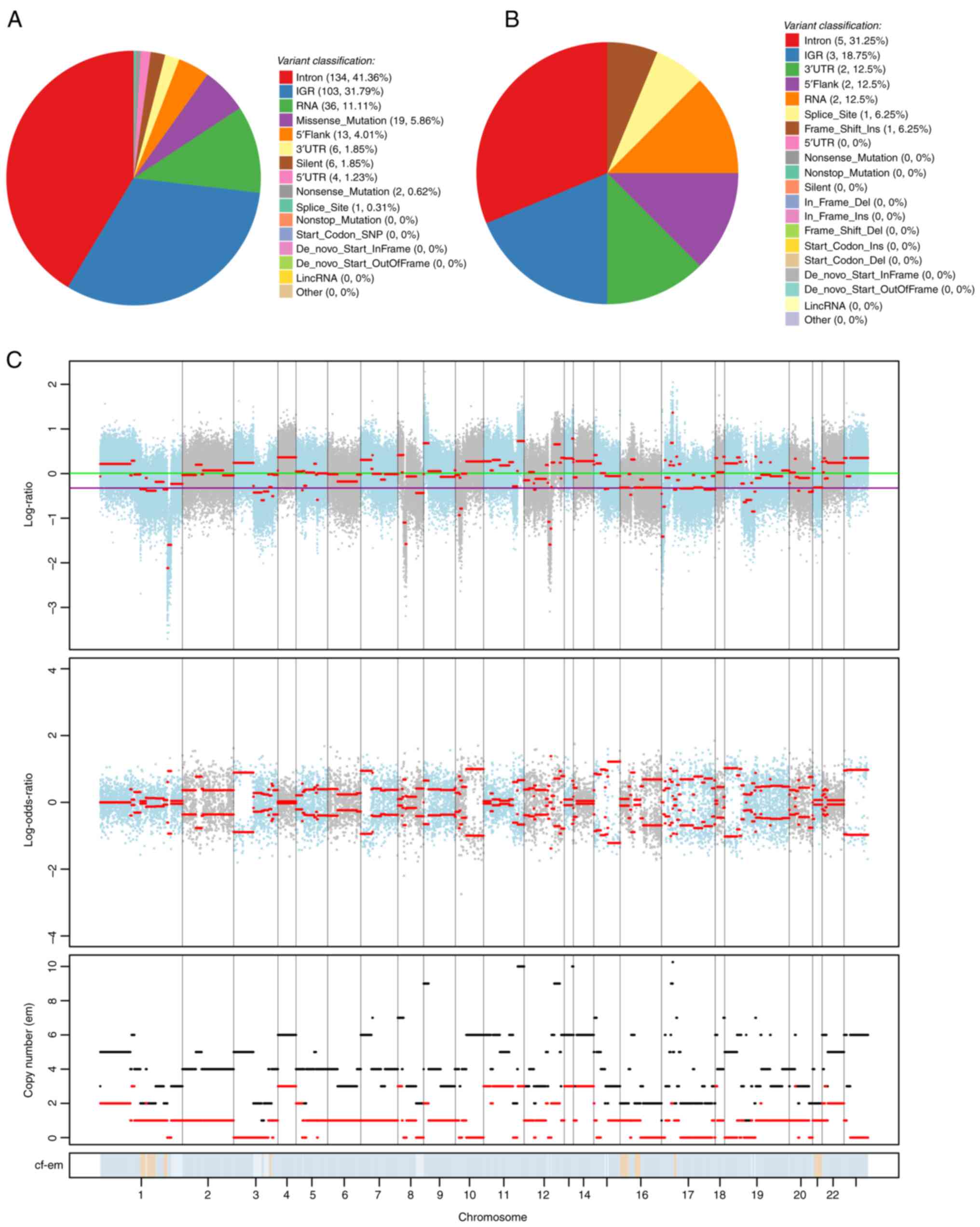
team). A total of 324 differential SNVs, annotated on 189 genes,
were found. The SNVs were primarily distributed in introns (41.36%)
and intergenic regions (31.79%), and there were 19 missense
mutations (5.86%) that did not influence initiation or termination
codons (Fig. 5A). A total of 16
differential InDels were found, annotated on 12 genes, which were
mainly distributed in gene introns (intron, 31.25%), intergenic
regions (intergenic region, 18.75%), 3′-non-coding regions [3′
untranslated region (UTR), 12.5%], and 5′-flanking regions
(5′Flank), with one insertion causing a code-shift mutation that
did not affect the initiation and termination codons (Fig. 5B). CNV manifests as an increase or
decrease in the copy number of genomic fragments, and deletions and
amplifications at the chromosome level have become a focus in tumor
research. FACETS software (version 0.6.2) (9) was used to compare CNVs in the left and
right BCs. A total of 159 differential CNVs were identified, of
which 34 were deletions and 125 were duplications. The comparison
of copy variation multiplicity at different chromosomal locations
is shown in Fig. 5C, indicating
that the overall copy number variation was higher in right BC than
in left BC.
Variation identification of cancer
susceptibility and driver genes in BBC
Susceptibility genes mediate inherited diseases or
result in acquired susceptibility to diseases under appropriate
environmental stimuli. Potential cancer susceptibility genes were
screened by comparing the mutated genes using the Cancer Gene
Census (CGC) database (https://cancer.sanger.ac.uk/census). The following 8
key mutated tumor susceptibility genes were selected: ALK, BRCA1,
FAT1, HNF1A, KDR, PTCH1, SDHA and SETBP1 (Table I). KDR demonstrated missense
mutations and structural interaction variants, while the remainder
contained missense mutations (Table
I). Mutations in these genes in the CGC database (11) are associated with susceptible tumor
types, such as neuroblastoma, BC, ovarian cancer, pancreatic
cancer, melanoma, hepatic adenoma, hepatocellular carcinoma, skin
basal cell carcinoma, medulloblastoma, paraganglioma and
neuroepithelial tumors (Table
I).
 | Table I.WES profile of cancer susceptibility
gene variations in the bilateral cancers. |
Table I.
WES profile of cancer susceptibility
gene variations in the bilateral cancers.
| Genes | Chromosome | Position | Transcript ID | Ref. | Alteration | Variant
classification | Sequencing | Coding | CGC-Cancers |
|---|
| ALK | Chr2 | 29221210 |
ENST00000453137 | G | T | Missense
variant | c.237C>A | p. Phe79Leu | Neuroblastoma |
| BRCA1 | Chr17 | 43071077
43092418 |
ENST00000471181 | T | C | Missense
variant | c.4900A>G;
c.3113A>G | p. Ser1634Gly; p.
Glu1038Gly | Breast cancer;
ovarian cancer |
| FAT1 | Chr4 | 186621601
186709436 |
ENST00000614102 | T G | C A | Missense
variant | c.4991A>G
c.392C>T | p. Asn1664Ser p.
Ala131Val | Pancreatic
cancer |
| HNF1A | Chr12 | 120999418 |
ENST00000541395 | T | C | Missense
variant | c.1652T>C | p. Leu551Ser | Hepatic adenoma;
hepatocellular carcinoma |
| KDR | Chr4 | 55106807
55113391 |
ENST00000263923 | T C | A T | Missense variant
Structural interaction variant | c.1416A>T
c.889G>A | p. Gln472His | Melanoma |
| PTCH1 | Chr9 | 95447312 |
ENST00000331920 | G | A | Missense
variant | c.3944C>T | p. Pro1315Leu | Skin basal cell
carcinoma; medulloblastoma |
| SDHA | Chr5 | 224372 |
ENST00000264932 | T | C | Missense
variant | c.163T>C | p. Tyr55His | Paraganglioma |
| SETBP1 | Chr18 | 44876688 |
ENST00000426838 | G | A | Missense
variant | c.664G>A | p. Ala222Thr | Neuroepithelial
tumors |
Cancer is the consequence of an accumulation of gene
mutations; however, not all gene mutations in cancer cells are
involved in the occurrence and development of cancer. Some of the
mutations involved in this process are referred to as driver
mutations, and the genes in which they are located are named driver
genes. The driver genes in this case were identified by comparing
the differential gene mutations in the BBC tissues with the known
driver genes reported in the IntOGen database (https://www.intogen.org/), CGC database and literature
(11–15). A total of 10 key mutated tumor
driver genes were screened, including BRCA1, EBF1, MET, NF2, NUMA1,
RALGAPA1, ROBO2, SMYD4, UBR5 and ZNF844 (Table II). In particular, the SMYD4
mutation was missense, and the ZNF844 mutation was located in the
3′UTR; the remaining mutations were located in intronic regions
(Table II). The IntOGen and CGC
databases revealed that these gene mutations could drive the
development of BC, ovarian cancer, cervical cancer, colorectal
adenocarcinoma, lymphoma, melanoma and other tumors. Among them,
BRCA1 and NUMA1 have been identified as key driver genes in BC
(Table II).
 | Table II.WES profile of known driver gene
variations in the bilateral breast cancers. |
Table II.
WES profile of known driver gene
variations in the bilateral breast cancers.
| Genes | Chromosome | Position | Ref. | Alteration | Variant
classification | Coding |
IntOGen-Cancers | CGC-Cancers | Literature |
|---|
| BRCA1 | Chr17 | 43063183 | - |
CCAGCCAATAACGGAATTATTAAAAACTTATTTTAACAGAAGGCAGGTAAGA | Intron | - | Breast cancer;
Ovarian cancer | Ovarian cancer | High confidence
driver |
| EBF1 | Chr5 | 158924607 | G | C | Intron | - | Large B-cell
lymphoma; lung squamous cell carcinoma, Non-Hodgkin lymphoma | Lipoma | - |
| MET | Chr7 | 116743773
116743776 | T C | A T | Intron | - | - | Papillary renal;
head and neck squamous cell | Oncogene |
| NF2 | Chr22 | 29658418 | C | A | Intron | - | Cervical cancer;
head and neck cancer; mesothelioma; ovarian cancer; pancreatic
adenocarcinoma; renal cell carcinoma; hepatocellular carcinoma;
skin squamous cell carcinoma | Meningioma;
acoustic neuroma; renal cell carcinoma | - |
| NUMA1 | Chr11 | 72035654 | A C | G T | Intron | - | Breast cancer | - | High confidence
driver |
| RALGAPA1 | Chr14 | 35771120 | A | G | Intron | - | - | - | Candidate
driver |
| ROBO2 | Chr3 | 76485224
76485246 | G G | T A | Intron | - | - | Colorectal
adenocarcinoma; melanoma | - |
| SMYD4 | Chr17 | 1801002 | C | A | Missense
mutation | p. R131I | - | - | Candidate
driver |
| UBR5 | Chr8 | 102346741 | T | C | Intron | - | - | Mantle cell
lymphoma; gastric cancer; colorectal cancer | - |
| ZNF844 | Chr19 | 12077126 | G | A | 3′UTR | - | - | - | High confidence
driver |
Analysis of significantly mutated
genes (SMGs) and mutated sample classification in BBC
SMGs are genes with a mutation frequency that is
significantly higher than the background mutation rate. MuSic
software (version 0.4) (10) was
used to analyze the differential high frequency mutations in the
BBC tissues, and the convolution test was used to conduct a
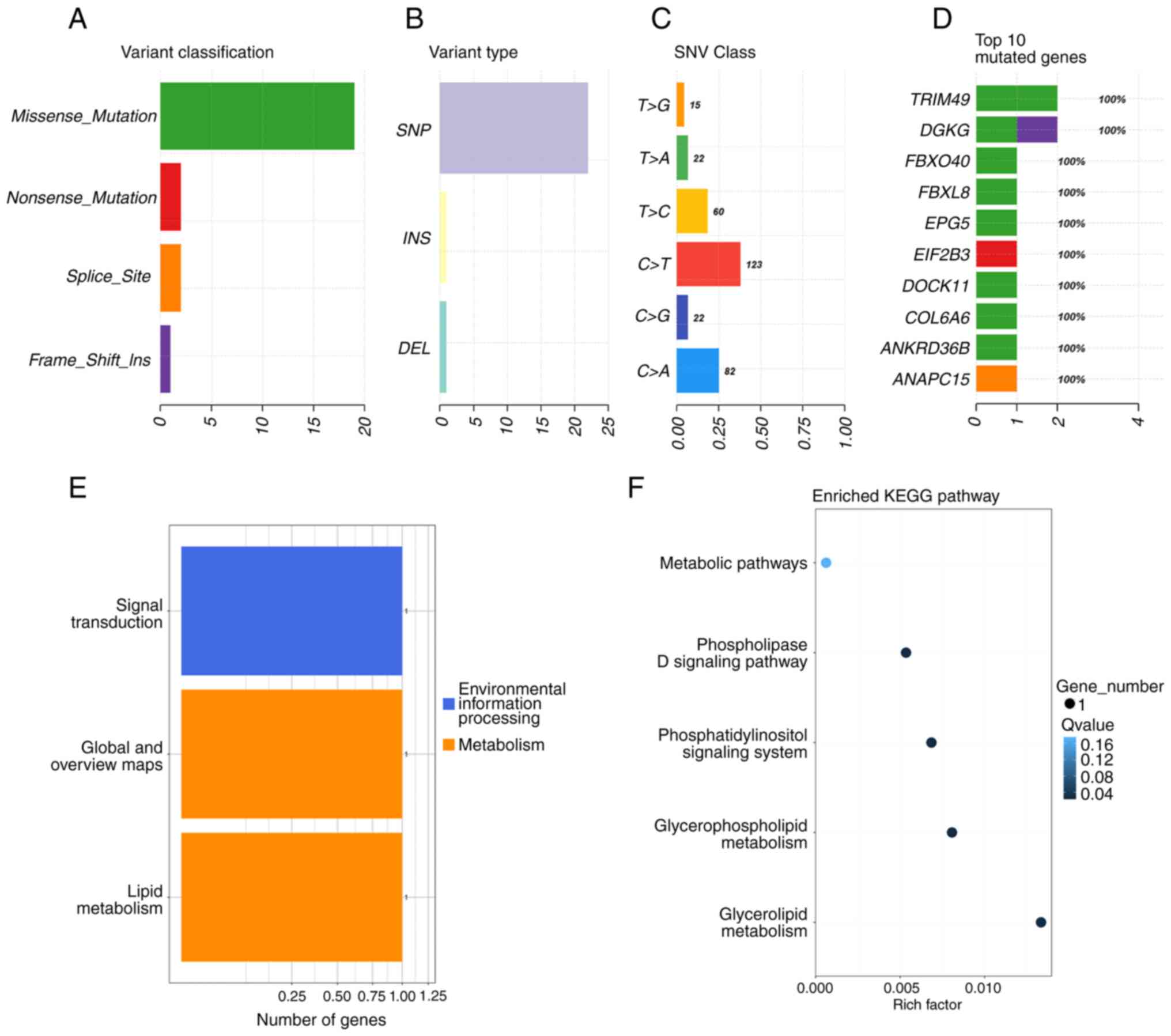
statistical test for each mutation type. The high-frequency mutated
genes mainly contained missense mutations, among which SNV was the
most common mutation, with C > T and C > A as the main forms
(Fig. 6A-C). The top 10 high
frequency mutated genes were TRIM49, DGKG, FBXO40, FBXL8, EPG5,
EIF2B3, DOCK11, COL6A6, ANKRD36B and ANAPC15 (Fig. 6D). The most important biochemical
processes and signal transduction pathways involved in high
frequency mutated genes were identified through significant
functional categories and Kyoto Encyclopedia of Genes and Genomes
pathway enrichment analyses. These high frequency mutated genes
were related to 3 functional categories, comprising signal
transduction, global and overview maps, and lipid metabolism
(Fig. 6E). In addition, the high
frequency mutated genes were mainly involved in metabolic pathways
such as glycerolipid metabolism, glycerophospholipid metabolism,
the phosphatidylinositol signaling system and the phospholipase D
signaling pathway (Fig. 6F).
Discussion
In 2020, BC accounted for 11.7% of all female cancer
cases worldwide and previously surpassed lung cancer as the most
common cancer in women (16).
Breasts are paired organs in humans; BC can simultaneously or
heterochronously occur in the bilateral breast because both sides
can be exposed to the same internal and external carcinogenic
factors and there is lymphatic transportation between the mammary
glands. A retrospective analysis revealed that only 14.3% of
patients with SBBC display bilateral breast symptoms at first
diagnosis. By contrast, at least 28.6% were asymptomatic bilateral
cases, highlighting the importance of population-based mammography
in detecting asymptomatic or occult BBCs that may be overlooked
(17). Furthermore, there is no
uniformity in the definition of the time interval between
occurrences, and the cutoff time for synchronization described in
the literature is usually between 3 and 6 months (18). For the first time, the 8th edition
of the AJCC BC Guidelines (19)
defined BBCs with an interval of ≤4 months from the initial
diagnosis as SBBC, while those with an interval of >4 months
between diagnoses were considered MBBC. Huo et al (20) argued that it is more reasonable to
adopt <12 months as the diagnostic criterion for concurrent
SBBC, as this cutoff might better reflect the biological
characteristics of the tumor.
Researchers have summarized 4 standards for the
diagnosis of bilateral primary BC (21): i) Secondary tumors found as in
situ lesions or as in situ carcinoma continuation into
invasive carcinoma; ii) the histological types of bilateral tumors
are completely different; iii) the histological differentiation of
the secondary tumors is significantly higher than that of the
primary side; and iv) the contralateral side reoccurs more than 5
years after the primary side's operation, with no local recurrence,
lymphatic metastasis, or distant metastasis. Regarding the
diagnostic criterion of Chaudary et al (21) of ‘completely different histologic
types bilaterally’, a number of studies (22,23)
have shown that the proportion of patients with SBBC with
consistent histologic types of BBCs ranges from 44 to 73%, while
that of patients with consistent histologic grading is 69%. Gong
et al (24) also found that
the histologic type consistency of SBBC (93%) was significantly
higher than that of MBBC (59%) in a large-scale study of Korean
patients with BC. However, this diagnostic standard has certain
limitations, and some academics have supplemented it as follows
(25,26): i) The same type of histology is not
necessarily non-primary; ii) primary BC is mostly located in the
upper outer quadrant of the breast within the intrinsic breast
tissues, whereas metastatic BC is generally located in the inner
quadrant, near the midline of the chest, or in the axillary caudal
adipose tissues; iii) primary lesions tend to be solitary, and
metastatic lesions tend to be multiple; and iv) cases that have
distant metastases at the time of diagnosis are excluded.
Only 6 studies of SBBC with discordant molecular
subtypes were found by a review of the literature in the PubMed
database up to September 2023 (Table
III), suggesting that SBBC with discordant molecular subtypes
is extremely rare. The age at diagnosis in these studies ranged
from 35 to 60 years. The patient in the present study is the oldest
reported to date. Hormone receptor status was discordant in 5 cases
(27–31) and concordant in 1 case (32). Concordant HER2 status was observed
in only 2 cases (27,28). A total of 4 patients underwent long
term follow up (27,30–32), 3
were relapse free at 15, 20 and 78 months after diagnosis (27,31,32),
and 1 succumbed 71 months after the initiation of treatment
(30).
 | Table III.Published case reports of SBBC with
discordant molecular subtypes. |
Table III.
Published case reports of SBBC with
discordant molecular subtypes.
| First author,
year | Age, years | Sex | Side of breast | TNM stage | ER | PR | HER2 | Ki-67, % | Molecular
subtype | Treatment | Follow-up | (Refs.) |
|---|
| Ojo et al,
2023 | 45 | F | Left | N/A | - | - | - | 90 |
Triple-negative | Neoadjuvant therapy
(chemotherapy and trastuzumab), bilateral MRM and SLN, and
evaluation for possible radiotherapy. | 15 months of
follow-up, with no signs of recurrence | (32) |
|
|
|
| Right | N/A | - | - | 2+ | 90 | HER2 positive (HR
negative) |
|
|
|
| Aranda-Gutierrez
et al, 2020 | 35 | F | Left | T2N1M0 | + | + | - | 30 | Luminal A | Adjuvant therapy
(chemotherapy and hormone therapy), and adjuvant radiotherapy. | 78 months of
follow-up, with no signs of recurrence | (27) |
|
|
|
| Right | T2N0M0 | - | - | - | 20 |
Triple-negative |
|
|
|
| Dhadlie et
al, 2018 | 58 | F | Left | N/A | + | + | 3+ | N/A | HER2 positive (HR
positive) | Neoadjuvant therapy
(chemotherapy and hormone therapy). Surgical and adjuvant
management to be determined based on response to neoadjuvant
therapy. | N/A | (28) |
|
|
|
| Right | N/A | - | - | 3+ | N/A | HER2 positive (HR
negative) |
|
|
|
| Copur et al,
2017 | 48 | F | Left | T2N0M0 | + | + | 1+ | N/A | HER2 positive (HR
positive) | Neoadjuvant therapy
(chemotherapy), bilateral MRM with right ALND and left SLN, and
adjuvant therapy (radiotherapy and unspecified
hormonetherapy). | 20 months of
follow-up, with no signs of recurrence | (31) |
|
|
|
| Right | T2N1M0 | - | - | - | N/A |
Triple-negative |
|
|
|
| Esclovon et
al, 2016 | 59 | F | Left | N/A | + | + | - | 1 | Luminal A | Neoadjuvant therapy
(chemotherapy and trastuzumab), bilateral MRM and right SLN, and
adjuvant therapy (unspecified hormone therapy). | N/A | (29) |
|
|
|
| Right | N/A | - | - | 3+ | 67 | HER2 positive (HR
negative) |
|
|
|
| Hayashi et
al, 2013 | 60 | F | Left | T4bN1M0 | - | - | + | N/A | HER2 positive (HR
negative) | Neoadjuvant therapy
(chemotherapy and trastuzumab), bilateral MRM with bilateral ALND,
and adjuvant therapy (trastuzumab and letrozole). | The patient died
after 71 months of follow-up, free of recurrence | (30) |
|
|
|
| Right | T4bN0M0 | + | + | - | N/A | Luminal A |
|
|
|
During treatment, SBBC can be regarded as two
independent tumors occurring simultaneously. The principle of SBBC
treatment is similar to that of UBC, adopting different surgical
methods according to the clinical stage of each side of the BC with
supplementary chemotherapy, radiation therapy, endocrine therapy
and molecular targeting therapy with individualized comprehensive
treatment for the more serious side after surgery if the
pathological types and immunohistochemical test results of the two
sides differ (4). Treatment
according to the molecular subtype of SBBC involves more complex
strategies but will significantly improve the prognosis of patients
with BBC (33).
The Chinese Society of Clinical Oncology BC
Guidelines 2023 (34) state that
the neoadjuvant treatment of HER2(+) BC in stage I should be TCbHP
(1A), THP*6 (2A) and THP*4 (1B) regimens, respectively (T,
paclitaxel; Cb, carboplatin; H, trastuzumab; P, pertuzumab).
Adjuvant therapy after neoadjuvant therapy for HER2(+) BC can
involve preoperative anti-HER2 therapy using trastuzumab alone; HP
(2A) is recommended for pathologic complete remission (pCR) grade
I. Patients who have not reached pathologic complete remission
(non-pCR) are first advised to use TDM1 (1A), followed by HP (2A);
preoperative anti-HER2 therapy using trastuzumab in combination
with pertuzumab can be applied. Patients with grade I showing a pCR
are recommended to receive HP (2A), while those without a pCR are
recommended to receive T-DM1 (2A) or HP (2A). Neoadjuvant endocrine
therapy may be considered for hormone dependent patients who
require preoperative neoadjuvant therapy but are not candidates for
chemotherapy, are temporarily ineligible for surgery or do not
require immediate operation, and are insensitive to neoadjuvant
chemotherapy, with AI (1A, an aromatase inhibitor) or AI + CDK4/6
inhibitor (2B) being recommended for grade I in postmenopausal
women with hormone receptor positive (HR+) BC. 1A, 1B, 2A and 2B
represent recommended levels of therapeutic regimens, with level 1
prioritizing over level 2 and Class A prioritizing over Class
B.
Postoperative adjuvant chemotherapy regimens for
HER2 negative BC are usually based on anthracyclines, such as
doxorubicin/cyclophosphamide (AC) and epirubicin/cyclophosphamide
(EC); due to the cardiotoxicity of anthracyclines, the left
ventricular ejection fraction must be evaluated at least once every
3 months when using anthracyclines. Another option is a sequential
regimen of anthracyclines and paclitaxel, such as AC followed by
paclitaxel (once a week), AC followed by docetaxel (once every 3
weeks), dose-intensive AC followed by paclitaxel (once every 2
weeks) and dose-intensive AC followed by paclitaxel (once a week).
AI can be recommended as an adjuvant endocrine therapy regimen to
all postmenopausal ER− and/or PR+ patients,
with 5 years of extended AI treatment recommended for stage III
patients. For patients with hormone
receptor-positive/HER2− advanced BC, endocrine therapy
combined with CDK4/6 inhibitor or endocrine therapy-based therapy
remains the superior treatment option (34).
According to the reported molecular phenotypes of
discordant SBBC, 5 patients received neoadjuvant chemotherapy, and
only two did not undergo bilateral modified radical mastectomy.
Regarding adjuvant therapy, all patients received chemotherapy, 2
received radiotherapy, 1 was evaluated for radiotherapy, and 3
received concurrent hormone therapy. The remaining three started
trastuzumab monotherapy, and one subsequently received letrozole
for 5 years. Neoadjuvant therapy has traditionally been applied to
locally advanced or inoperable tumors to improve surgical outcomes
(32). However, neoadjuvant therapy
is now increasingly used in early-stage disease to assess the tumor
response and guide future adjuvant therapy (35). In the case of the present study,
after bilateral modified radical mastectomy and sentinel lymph node
biopsy, chemotherapy was administered with THP and HP regimens,
followed by 5–10 years of endocrine therapy and continued targeted
therapy prescribed.
Certain large-sample studies (36–38)
have revealed that SBBC has a poorer prognosis than UBC. The
prognosis of SBBC varies with different intervals. SBBC with an
interval of 3–12 months has the poorest prognosis because it
responds poorly to adjuvant therapy and even develops resistance to
therapy. When the interval was set at 6 months, SBBC and MBBC
showed similar survival ratios (37). Mejdahl et al (39) used competing risk modeling to
demonstrate that the combined effects of having two cancers
resulted in higher mortality rates and poorer prognoses than those
observed in patients with UBC. Liu et al (40) developed an animal model of SBBC and
found that the majority of micro-metastases in the lungs comprised
cells derived from the primary tumor, suggesting a high degree of
metastatic cross-seeding, which could contribute to intratumor
heterogeneity and treatment resistance. Different hormone receptor
statuses exhibit variable responses to hormone therapy, with
ER(+)/PR(+) tumors being the most responsive, and tumors with
ER(−)/PR(+) or ER(+)/PR(−) mixed receptor status show a reduced
response to treatment due to mixed receptor status and intrinsic
resistance to hormone therapy (29); HER2 is expressed in 15–20% of
primary BCs, and HER2(+) BCs have the poorest prognoses among BCs
(41). In a large population based
retrospective study, Ding et al (42) revealed that molecular subtypes were
associated with a poor prognosis in patients with SBBC but not in
those with MBBC.
There are known risk factors for BBC, including
younger age, family history, BC susceptibility gene 1/2 (BRCA1/2)
mutations, lobular histologic type and multicentricity (27). Patients with enhanced BC
susceptibility usually present at an earlier stage of development
with BBC or >1 cancer in an individual or a family (17). Scholars have highlighted that there
is a certain association between family history and BBC, where the
risk of developing the disease increases by 2–4 times when a lineal
descendant experiences BC (43,44).
Therefore, after the diagnosis of SBBC in this patient, it is
advisable to remain alert to the risk of cancer in the patient's
first-degree relatives, perform early genetic screening, and
increase the family members' awareness of the importance of
self-screening.
As SBBC with discordant molecular subtypes is rare,
and its molecular pathogenesis has not yet been fully elucidated,
no prior case studies involving analyses of the genetic mutations
in SBBC were found. In the present study, analysis of genetic
mutations and identification of cancer driver genes were attempted
by WES. Among the 10 key mutated tumor driver genes obtained, BRCA1
and NUMA1 have been identified as the key drivers of BC
tumorigenesis.
The BRCA1 gene on human chromosome 17q21 encodes a
tumor suppressing protein comprising 1,863 amino acids (45). Early studies have revealed that
BRCA1 is critical for the maintenance of genomic stability
(46–48), which is sustained by its
participation in multiple aspects of the cellular response to DNA
double strand breaks, including cell cycle control, chromatin
remodeling, homologous recombination repair and nonhomologous end
joining (49). The prevalence of
germline and somatic BRCA1 mutations in BC is 7.8 and 3.4%,
respectively (50). Moreover, 2~50%
of hereditary BC cases result from germline mutations in
BRCA1/BRCA2 genes, which are associated with early onset BC. The
cumulative risk of BC by the age of 80 years has been estimated to
be 72% among BRCA1 mutation carriers (51). The NCCN recommends an annual
mammographic examination of BRCA1 mutation carriers with breast MRI
screening up to the age of 75 years (52). In a high-risk Chinese cohort study
(53), BRCA mutation carriers were
found to be more likely to have lymph node involvement after a BC
diagnosis. Despite adjusting for clinical prognostic factors, these
patients had significantly worse BC specific outcomes, suggesting
that BRCA mutations represent an independent factor contributing to
poor prognosis. In a multifactorial analysis of the 6 risk factors
for hereditary BC in a cohort of all high-risk individuals in the
aforementioned study, the predominance ratio of germline mutations
in BBCs was 3.27, which was significant (53); thus, it may be necessary to pay
special attention to those with BBC even in the absence of family
history or very young age of onset. The current patient had BRCA1
somatic mutations, suggesting that she and her family should be
further tested for BRCA1 germline mutations to guide PARP inhibitor
targeted therapy and assess genetic risk.
Nuclear mitotic apparatus protein (also referred to
as NUMA1 and NMP-22) is a hyper-molecular mass nuclear matrix
protein first discovered and named by Lydersen and Pettijohn
(54). The NUMA1 gene is located on
chromosome 11q13.4 and encodes a 236 kDa protein essential for
normal mitotic spindle organization (55). NUMA1 organizes the spindle pole in
mitosis and controls spindle orientation; it is also essential for
the establishment of higher order chromatin organization during
epithelial cell differentiation and DNA repair by homologous
recombination (56). The
interaction of NUMA1 with p53 is enhanced following DNA damage, and
NUMA1 upregulates p53-mediated transcription of target genes
(57). NUMA1 prevents 53BP1
accumulation at DNA breaks in breast carcinoma (58). NUMA1 has been reported to be
associated with acute promyelocytic leukemia (APL), and NUMA1-RARα
(retinoic acid receptor α) t(11;17) (q13;q21) translocation has
been observed in very rare cases of APL translocation (59). NUMA1 plays an oncogenic role in
esophageal squamous cell carcinoma by regulating the ASK1-JNK
signaling pathway (60). NUMA1
alternative splicing induces enhanced cell proliferation and
centrosome amplification in nontumorigenic mammary epithelial cells
(61). Salvador et al
(58) found that NUMA1 levels are
highly heterogeneous within and between tumors, and NUMA1
expression was significantly correlated with distal metastasis free
survival in patients by Kaplan Meier analysis of microarray
datasets; however, high NUMA1 expression predicted longer OS times
in patients in a cohort of The Cancer Genome Atlas. In a
large-scale association study, Kammerer et al (62) identified a non-synonymous single
nucleotide polymorphism (SNP; A794G) in NUMA1 that was correlated
more strongly with BC risk than the initial marker SNP, and they
concluded that mutations in the NUMA1 gene might be responsible for
the observed increased BC risk.
Other important genes that were obtained in the
present study function as oncogene or tumor suppressor, and are
also closely involved in the development of BC by regulating cell
division, apoptosis, angiogenesis, tumor stem cell self-renewal and
immune cell infiltration. EBF1 (early B-cell factor 1) is a
transcription factor with multiple effects on cell differentiation
and metabolic processes (63). A
number of studies have suggested that EBF1 is an important
regulator of specific methylation and gene expression programs in
BC subtypes (64). Qiu et al
(65) demonstrated that EBF1 is
highly expressed in triple negative BC (TNBC) cells and that the
knockdown of EBF1 blocks the growth and invasiveness of TNBC cells.
Importantly, the absence of EBF1 also triggers extensive mitosis
and the remodeling of cellular metabolism.
The tyrosine kinase c-Met, also called MET, is a
plasma membrane protein that transduces signals from the
extracellular matrix to the cytoplasm. Dysregulation of MET
signaling has been identified in various malignant and premalignant
lesions and is involved in the uncontrolled survival, growth,
angiogenesis and metastasis of cancer cells (66). A broad range of mechanisms may lead
to aberrant MET signaling in BC, including activating gene
mutations, gene amplification, protein overexpression, increased
ligand dependent paracrine stimulation and autocrine signaling
acquisition (67). MET
overexpression has been reported in 14–53.6% of patients with BC
and is a significant adverse predictor of relapse-free survival and
OS times in patients with BC. In addition, MET may influence the
prognosis of HR(+) patients by mediating resistance to endocrine
therapy, especially in the HR(+)/HER2(−) subgroup, in a
HER2-independent manner (66).
The neurofibromin 2 (NF2) gene encodes two
transcripts, NF2-1 and NF2-2, containing 595 amino acid residues
and NF2-2 contains 590 amino acid residues, respectively (68). NF2 expression is decreased in BC
tissues compared with that in adjacent normal tissues, and low
expression of NF2 associates with tumor stage, while overexpression
of NF2 inhibits the formation of cellular clones and stemness
(69).
ROBO is considered tumor suppressor because it is
frequently inactivated in various tumors, and the SLIT/ROBO
signaling pathway is reportedly involved in BC development and
metastasis. Overexpression of SLIT/ROBO induces its tumor
suppressive effects possibly by inactivating the β-catenin/LEF/TCF
and PI3K/Akt signaling pathways or by altering
β-catenin/E-cadherin-mediated cell-cell adhesion in BC cells
(70). SLIT2 negatively regulates
WNT signaling through ROBO2 signaling in a subpopulation of basal
cells, restricting mammary stem cell renewal (71).
SMYD4 is located on human chromosome 17p13.3 and
serves as a potential tumor suppressor in BC. SMYD4 has been found
to significantly inhibit breast tumorigenesis by suppressing the
expression of platelet derived growth factor receptor α (72). Han et al (73) found that miR-1307-3p significantly
inhibits breast stem cell renewal by targeting SET and SMYD4
expression in BC, exerting oncogenic effects.
UBR5, a HECT structural domain E3 ubiquitin ligase,
is an attractive therapeutic target for invasive BC, in which
CDC73, a critical substrate of UBR5, is involved in regulating the
expression of β-catenin and E-cadherin, apoptosis of tumor cells,
and CD8(+) T-cell infiltration mechanisms that impede the profound
tumorigenic and metastatic activity of UBR5 in TNBC (74).
In conclusion, the diagnostics and treatment
optimization strategies for SBBC with discordant molecular subtypes
are complex. In the present study, a 72-year-old woman patient with
a heterogeneous molecular subtype of SBBC was reported, who
presented with a HER2(+) [HR(−)] tumor in the left breast and a
hormone sensitive [HER2(−)] tumor in the right breast was reported.
The patient underwent systemic chemotherapy, followed by 5–10 years
of endocrine therapy and continued targeted therapy prescribed. To
the best of our knowledge, this patient is the oldest patient among
the reported SBBC cases with discordant molecular subtypes. In the
present study, the patient did not undergo pathological examination
through ultrasound-guided percutaneous biopsy before surgery, so
the molecular subtype and TNM stage of the tumors were determined
after surgery. It would be more rational if a multidisciplinary
therapeutic regimen was determined before surgery. WES revealed
differential gene variations in the BBC tissues and identified 8
cancer susceptibility genes and 10 important cancer driver genes,
including BRCA1 and NUMA1, which may be associated with the
occurrence of SBBC and targeted therapy options. These findings may
offer prognosis assessment and therapeutic guidance for patients
with SBBC and provide a basis for the necessity of self-examination
of the patients' immediate family members. Since SBBC with
discordant molecular subtypes is extremely rare, WES was conducted
in only one case in the present study, which may lead to certain
limitations for genetic analysis, and validation is needed in more
cases for future study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82160582), the Yunnan Fundamental
Research Project (grant no. 202201AT070003) and the Scientific
Research Foundation of The First Affiliated Hospital of Dali
University (grant no. DYFGG2022-01).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The data generated in the
present study may be found in the NCBI Sequence Read Archive under
accession nos. SRR28840132 and SRR28840133 or under the following
URLs: https://www.ncbi.nlm.nih.gov/sra/?term=SRR28840132 and
https://www.ncbi.nlm.nih.gov/sra/?term=SRR28840133.
Authors' contributions
SHH and BG contributed to manuscript writing, data
collection and data analysis. ZJL contributed to the pathological
diagnosis. YCY contributed to data collection. BG contributed to
the study design, project supervision, administrative support and
manuscript revision. SHH and BG confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Dali University
(Dali, China; approval no. DYF20230309). Written informed consent
to participate in the present study was obtained from the
patient.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia H, Zheng Y, Wang P, Wei Z, Li X, Fu G
and Wang C: A retrospective study on the clinicopathologic
characteristics and outcomes of 179 cases of synchronous and
metachronous bilateral breast cancer in China. Clin Breast Cancer.
22:e341–e349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin M, Zhang X, Zhu L, Niu S and Chen Q: A
case of simultaneous primary bilateral breast cancer and literature
review. J Xinxiang Med Coll. 7:623–625. 2018.(In Chinese).
|
|
5
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thürlimann B and Senn HJ; Panel members, : Thresholds for
therapies: Highlights of the St Gallen international expert
consensus on the primary therapy of early breast cancer 2009. Ann
Oncol. 20:1319–1329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S, Chen CM, Yu KD, Zhou RJ and Shao
ZM: Prognostic value of a positive-to-negative change in hormone
receptor status after neoadjuvant chemotherapy in patients with
hormone receptor-positive breast cancer. Ann Surg Oncol.
19:3002–3011. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Liu X, Yu K, Sun X, Xu S, Qiu P,
Lv Z, Zhang X, Guo A and Xu Y: Impact of hormone receptor, HER2,
and Ki-67 status conversions on survival after neoadjuvant
chemotherapy in breast cancer patients: A retrospective study. Ann
Transl Med. 10:932022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwase H: Current topics and perspectives
on the use of aromatase inhibitors in the treatment of breast
cancer. Breast Cancer. 15:278–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arora A, Shen R and Seshan VE: FACETS:
Fraction and allele-specific copy number estimates from tumor
sequencing. Methods Mol Biol. 2493:89–105. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dees ND, Zhang Q, Kandoth C, Wendl MC,
Schierding W, Koboldt DC, Mooney TB, Callaway MB, Dooling D, Mardis
ER, et al: MuSiC: Identifying mutational significance in cancer
genomes. Genome Res. 22:1589–1598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sondka Z, Bamford S, Cole CG, Ward SA,
Dunham I and Forbes SA: The COSMIC cancer gene census: Describing
genetic dysfunction across all human cancers. Nat Rev Cancer.
18:696–705. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martínez-Jiménez F, Muiños F, Sentís I,
Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, Mularoni L, Pich O,
Bonet J, Kranas H, et al: A compendium of mutational cancer driver
genes. Nat Rev Cancer. 20:555–572. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bailey MH, Tokheim C, Porta-Pardo E,
Sengupta S, Bertrand D, Weerasinghe A, Colaprico A, Wendl MC, Kim
J, Reardon B, et al: Comprehensive characterization of cancer
driver genes and mutations. Cell. 173:371–385.e18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamborero D, Gonzalez-Perez A,
Perez-Llamas C, Deu-Pons J, Kandoth C, Reimand J, Lawrence MS, Getz
G, Bader GD, Ding L and Lopez-Bigas N: Comprehensive identification
of mutational cancer driver genes across 12 tumor types. Sci Rep.
3:26502013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang KL, Liu YL, Hsu YY and Kuo WL:
Retrospective analysis of clinicopathological features and familial
cancer history of synchronous bilateral breast cancer. Healthcare
(Basel). 9:12032021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holm M, Tjønneland A, Balslev E and Kroman
N: Prognosis of synchronous bilateral breast cancer: A review and
meta-analysis of observational studies. Breast Cancer Res Treat.
146:461–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huo D, Melkonian S, Rathouz PJ, Khramtsov
A and Olopade OI: Concordance in histological and biological
parameters between first and second primary breast cancers. Cancer.
117:907–915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaudary MA, Millis RR, Hoskins EO, Halder
M, Bulbrook RD, Cuzick J and Hayward JL: Bilateral primary breast
cancer: A prospective study of disease incidence. Br J Surg.
71:711–714. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Padmanabhan N, Subramanyan A and
Radhakrishna S: Synchronous bilateral breast cancers. J Clin Diagn
Res. 9:XC05–XC08. 2015.PubMed/NCBI
|
|
23
|
Londero AP, Bernardi S, Bertozzi S,
Angione V, Gentile G, Dri C, Minucci A, Caponnetto F and Petri R:
Synchronous and metachronous breast malignancies: A cross-sectional
retrospective study and review of the literature. Biomed Res Int.
2014:2507272014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong SJ, Rha SY, Jeung HC, Roh JK, Yang WI
and Chung HC: Bilateral breast cancer: Differential diagnosis using
histological and biological parameters. Jpn J Clin Oncol.
37:487–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kan X: Bilateral primary breast cancer
(review). Foreign Medicine (Oncology). 1:15–18. 1980.(In
Chinese).
|
|
26
|
Robbins GF and Berg JW: Bilateral primary
breast cancer: A prospective clinicopathological study. Cancer.
17:1501–1527. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aranda-Gutierrez A, Gomez-Picos A,
Ferrigno AS, Moncada-Madrazo M and Diaz-Perez H: Molecular subtype
discordance in a young woman with synchronous bilateral breast
cancer: A case report. Cureus. 12:e72422020.PubMed/NCBI
|
|
28
|
Dhadlie S, Whitfield J and Hendahewa R:
Synchronous bilateral breast cancer: A case report of heterogeneous
estrogen receptor status. Int J Surg Case Rep. 53:102–106. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esclovon JW, Ponder M, Aydin N and Misra
S: Challenges of treating incidental synchronous bilateral breast
cancer with differing tumour biology. BMJ Case Rep.
2016:bcr20162162012016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayashi M, Yamamoto Y, Takata N and Iwase
H: A case of synchronous bilateral breast cancer with different
pathological responses to neoadjuvant chemotherapy with different
biological character. Springerplus. 2:2722013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Copur MS, Ramaekers R, Gauchan D, Crockett
D and Clark D: Synchronous bilateral breast cancer with discordant
histology. Oncology (Williston Park). 31:274–277.
3122017.PubMed/NCBI
|
|
32
|
Ojo AS, Shittu A, Amadife S, Jackson D,
Grantham M, Ali A and Sarma R: Synchronous bilateral breast cancer
with discordant receptor status: Treating one patient but two
diseases. World J Oncol. 14:224–229. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCart Reed AE, Kutasovic JR, Lakhani SR
and Simpson PT: Invasive lobular carcinoma of the breast:
Morphology, biomarkers and 'omics. Breast Cancer Res. 17:122015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J and Jiang Z: Chinese society of
clinical oncology breast cancer (CSCO BC) guidelines in 2022:
Stratification and classification. Cancer Biol Med. 19:769–773.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Montemurro F, Nuzzolese I and Ponzone R:
Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert
Opin Pharmacother. 21:1071–1082. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hartman M, Czene K, Reilly M, Adolfsson J,
Bergh J, Adami HO, Dickman PW and Hall P: Incidence and prognosis
of synchronous and metachronous bilateral breast cancer. J Clin
Oncol. 25:4210–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan B, Xu Y, Zhou YD, Yao R, Wu HW, Zhu
QL, Wang CJ, Mao F, Lin Y, Shen SJ and Sun Q: The prognostic
comparison among unilateral, bilateral, synchronous bilateral, and
metachronous bilateral breast cancer: A meta-analysis of studies
from recent decade (2008–2018). Cancer Med. 8:2908–2918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jobsen JJ, van der Palen J, Ong F,
Riemersma S and Struikmans H: Bilateral breast cancer, synchronous
and metachronous; differences and outcome. Breast Cancer Res Treat.
153:277–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mejdahl MK, Wohlfahrt J, Holm M, Balslev
E, Knoop AS, Tjønneland A, Melbye M and Kroman N: Breast cancer
mortality in synchronous bilateral breast cancer patients. Br J
Cancer. 120:761–767. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu S, Nyström NN, Kelly JJ, Hamilton AM,
Fu Y and Ronald JA: Molecular imaging reveals a high degree of
cross-seeding of spontaneous metastases in a novel mouse model of
synchronous bilateral breast cancer. Mol Imaging Biol. 24:104–114.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang WT and Zhu XZ: The introduction of
2012 WHO classification of tumours of the breast. Zhonghua Bing Li
Xue Za Zhi. 42:78–80. 2013.(In Chinese). PubMed/NCBI
|
|
42
|
Ding S, Sun X, Lu S, Wang Z, Chen X and
Shen K: Association of molecular subtype concordance and survival
outcome in synchronous and metachronous bilateral breast cancer.
Breast. 57:71–79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H and Shan CP: Research progress on
risk factors of bilateral primary breast cancer. Shandong Medical
Journal. 56:99–101. 2016.(In Chinese).
|
|
44
|
Wadasadawala T, Lewis S, Parmar V,
Budrukkar A, Gupta S, Nair N, Shet T, Badwe R and Sarin R:
Bilateral breast cancer after multimodality treatment: A report of
clinical outcomes in an asian population. Clin Breast Cancer.
18:e727–e737. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miki Y, Swensen J, Shattuck-Eidens D,
Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM,
Ding W, et al: A strong candidate for the breast and ovarian cancer
susceptibility gene BRCA1. Science. 266:66–71. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Roy R, Chun J and Powell SN: BRCA1 and
BRCA2: Different roles in a common pathway of genome protection.
Nat Rev Cancer. 12:68–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu B, Kim St and Kastan MB: Involvement of
Brca1 in S-phase and G(2)-phase checkpoints after ionizing
irradiation. Mol Cell Biol. 21:3445–3450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deng CX: BRCA1: Cell cycle checkpoint,
genetic instability, DNA damage response and cancer evolution.
Nucleic Acids Res. 34:1416–1426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huen MSY, Sy SMH and Chen J: BRCA1 and its
toolbox for the maintenance of genome integrity. Nat Rev Mol Cell
Biol. 11:138–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shao C, Wan J, Lam FC, Tang H, Marley AR,
Song Y, Miller C, Brown M, Han J and Adeboyeje G: A comprehensive
literature review and meta-analysis of the prevalence of pan-cancer
BRCA mutations, homologous recombination repair gene mutations, and
homologous recombination deficiencies. Environ Mol Mutagen.
63:308–316. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kuchenbaecker KB, Hopper JL, Barnes DR,
Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE,
Milne RL, Andrieu N, et al: Risks of breast, ovarian, and
contralateral breast cancer for BRCA1 and BRCA2 mutation carriers.
JAMA. 317:2402–2416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Daly MB, Pilarski R, Yurgelun MB, Berry
MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Garber
JE, et al: NCCN guidelines insights: Genetic/familial High-risk
assessment: Breast, ovarian, and pancreatic, version 1.2020. J Natl
Compr Canc Netw. 18:380–391. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang YA, Jian JW, Hung CF, Peng HP, Yang
CF, Cheng HS and Yang AS: Germline breast cancer susceptibility
gene mutations and breast cancer outcomes. BMC Cancer. 18:3152018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lydersen BK and Pettijohn DE:
Human-specific nuclear protein that associates with the polar
region of the mitotic apparatus: Distribution in a human/hamster
hybrid cell. Cell. 22:489–499. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kilpivaara O, Rantanen M, Tamminen A,
Aittomäki K, Blomqvist C and Nevanlinna H: Comprehensive analysis
of NuMA variation in breast cancer. BMC Cancer. 8:712008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vidi PA, Liu J, Salles D, Jayaraman S,
Dorfman G, Gray M, Abad P, Moghe PV, Irudayaraj JM, Wiesmüller L
and Lelièvre SA: NuMA promotes homologous recombination repair by
regulating the accumulation of the ISWI ATPase SNF2h at DNA breaks.
Nucleic Acids Res. 42:6365–6379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ohata H, Miyazaki M, Otomo R,
Matsushima-Hibiya Y, Otsubo C, Nagase T, Arakawa H, Yokota J,
Nakagama H, Taya Y and Enari M: NuMA is required for the selective
induction of p53 target genes. Mol Cell Biol. 33:2447–2457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Salvador Moreno N, Liu J, Haas KM, Parker
LL, Chakraborty C, Kron SJ, Hodges K, Miller LD, Langefeld C,
Robinson PJ, et al: The nuclear structural protein NuMA is a
negative regulator of 53BP1 in DNA double-strand break repair.
Nucleic Acids Res. 47:2703–2715. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wells RA, Catzavelos C and Kamel-Reid S:
Fusion of retinoic acid receptor alpha to NuMA, the nuclear mitotic
apparatus protein, by a variant translocation in acute
promyelocytic leukaemia. Nat Genet. 17:109–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yin S, Zhao S, Li J, Liu K, Ma X, Zhang Z,
Wang R, Tian J, Liu F, Song Y, et al: NUMA1 modulates apoptosis of
esophageal squamous cell carcinoma cells through regulating
ASK1-JNK signaling pathway. Cell Mol Life Sci. 80:2112023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sebestyén E, Singh B, Miñana B, Pagès A,
Mateo F, Pujana MA, Valcárcel J and Eyras E: Large-scale analysis
of genome and transcriptome alterations in multiple tumors unveils
novel cancer-relevant splicing networks. Genome Res. 26:732–744.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kammerer S, Roth RB, Hoyal CR, Reneland R,
Marnellos G, Kiechle M, Schwarz-Boeger U, Griffiths LR, Ebner F,
Rehbock J, et al: Association of the NuMA region on chromosome
11q13 with breast cancer susceptibility. Proc Natl Acad Sci USA.
102:2004–2009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Boller S and Grosschedl R: The regulatory
network of B-cell differentiation: A focused view of early B-cell
factor 1 function. Immunol Rev. 261:102–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fernandez-Jimenez N, Sklias A, Ecsedi S,
Cahais V, Degli-Esposti D, Jay A, Ancey PB, Woo HD,
Hernandez-Vargas H and Herceg Z: Lowly methylated region analysis
identifies EBF1 as a potential epigenetic modifier in breast
cancer. Epigenetics. 12:964–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Qiu Z, Guo W, Dong B, Wang Y, Deng P, Wang
C, Liu J, Zhang Q, Grosschedl R, Yu Z, et al: EBF1 promotes
triple-negative breast cancer progression by surveillance of the
HIF1α pathway. Proc Natl Acad Sci USA. 119:e21195181192022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yan S, Jiao X, Zou H and Li K: Prognostic
significance of c-Met in breast cancer: A meta-analysis of 6010
cases. Diagn Pathol. 10:622015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ho-Yen CM, Jones JL and Kermorgant S: The
clinical and functional significance of c-Met in breast cancer: A
review. Breast Cancer Res. 17:522015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ahronowitz I, Xin W, Kiely R, Sims K,
MacCollin M and Nunes FP: Mutational spectrum of the NF2 gene: A
meta-analysis of 12 years of research and diagnostic laboratory
findings. Hum Mutat. 28:1–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang Z, Zhou Z, Wang Z and Cui Y: NF2
inhibits proliferation and cancer stemness in breast cancer. Open
Med (Wars). 15:302–308. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gu F, Ma Y, Zhang J, Qin F and Fu L:
Function of Slit/Robo signaling in breast cancer. Front Med.
9:431–436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Harburg G, Compton J, Liu W, Iwai N, Zada
S, Marlow R, Strickland P, Zeng YA and Hinck L: SLIT/ROBO2
signaling promotes mammary stem cell senescence by inhibiting Wnt
signaling. Stem Cell Reports. 3:385–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hu L, Zhu YT, Qi C and Zhu YJ:
Identification of Smyd4 as a potential tumor suppressor gene
involved in breast cancer development. Cancer Res. 69:4067–4072.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Han S, Zou H, Lee JW, Han J, Kim HC, Cheol
JJ, Kim LS and Kim H: miR-1307-3p stimulates breast cancer
development and progression by targeting SMYD4. J Cancer.
10:441–448. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xiang G, Wang S, Chen L, Song M, Song X,
Wang H, Zhou P, Ma X and Yu J: UBR5 targets tumor suppressor CDC73
proteolytically to promote aggressive breast cancer. Cell Death
Dis. 13:4512022. View Article : Google Scholar : PubMed/NCBI
|