Introduction
The pancreaticobiliary duodenal junction (PBDJ) is
the area where the pancreatic duct (PD), bile duct and duodenum are
connected, including the head of pancreas, the pancreatic segment
of the common bile duct (CBD) and the intraduodenal segment, the
descending and horizontal parts of duodenum, and the soft tissue
around the pancreatic head (1).
This site has a complex anatomical structure and an important
physiological function. Digestive fluids such as bile, pancreatic
juice and gastrointestinal fluid converge at the PBDJ, referred to
as the ‘confluence of three rivers’ (2). The PBDJ is susceptible to a range of
conditions, such as stones, inflammation and tumors, which can lead
to obstructive jaundice, cholangitis and pancreatitis (3). Due to the intricate anatomy and
diverse pathology of this area, early diagnosis and precise
treatment of malignant tumors in the PBDJ are challenging.
Additionally, the progression of these diseases may be associated
with the unique anatomical basis of the region (4). Thus, understanding and delineating the
anatomical configurations of the PBDJ is significant for preventing
and treating such conditions at their source.
Despite the rapid advancements in medical imaging
technologies providing a variety of high-precision methods for the
diagnosis of malignant tumors at the PBDJ, numerous challenges and
limitations remain. Firstly, the complex anatomical layout of this
region complicates image interpretation, particularly in the early
tumor stages where lesions are small and poorly defined, increasing
the risk of missed or misdiagnosed cases (5). Secondly, the different imaging
modalities have their advantages; for instance, computed tomography
(CT) excels in demonstrating tumor morphology, density and
relationships with surrounding structures but has limited soft
tissue resolution (6). By contrast,
magnetic resonance imaging (MRI) offers superior delineation of
soft tissue details but requires longer examination times and
notable patient cooperation (7).
Emerging artificial intelligence (AI)-assisted diagnostic tools and
three-dimensional visualization technology (3DVT) show potential
but are still in the early stages of development, necessitating
further validation of their accuracy, stability and widespread
applicability (8).
Furthermore, despite the application of several
treatment approaches for malignant tumors of the PBDJ, such as
surgical intervention, radiotherapy, chemotherapy, immunotherapy,
targeted therapy and neoadjuvant therapy, which have yielded
certain results (including enhanced surgical resection rates,
diminished recurrence risks, specific inhibition of cancer cell
proliferation and metastasis, and extended overall survival time
for patients), numerous challenges remain (9–12). The
complexity of the surgical procedures, the prevalence of
complications and the slow postoperative recovery restrict
treatment options for certain patients. Additionally, the efficacy
of radiotherapy and chemotherapy may be limited by drug side
effects or the emergence of tumor resistance (13). Therefore, tailoring personalized and
precise therapeutic strategies based on individual patient
differences continues to be one of primary focuses of current
clinical research.
The diagnosis and treatment of malignant tumors at
the PBDJ is a complex task which requires continuous investigation
and innovation in order to overcome the limitations of current
technologies, and enhance both the accuracy of diagnosis and the
effectiveness of treatment. The present review aimed to
comprehensively discuss the commonly used imaging techniques in the
diagnosis of malignant tumors at this anatomical site, along with
other novel methodologies, with the intention of providing a
scientifically sound reference for clinicians and patients alike;
thereby collectively advancing the standards of care in this
field.
Preliminary study on the anatomy of the
PBDJ
The pancreatic head is located within the concave
surface of the duodenum, to the right of the midline. It is ~1 cm
thick with its lower portion extending downward and leftward in a
hook-like fashion encircling the posterior aspect of the mesenteric
vessels (14). The pancreatic head
is surrounded by the duodenum on its superior, inferior and right
sides, with the area in contact with the duodenum slightly recessed
inward (15). The anterior side of
the pancreatic head is mostly adjacent to the beginning of the
transverse colon and its mesentery; the superior portion is covered
by the posterior wall of the omental sac, whilst the inferior
portion is covered by the membrane extending from the transverse
colon mesentery and is adjacent to the small intestine (16). The hepatic artery travels along the
superior margin of the pancreas, directed rightwards. Posteriorly,
the pancreatic head is adjacent to the medial border of the upper
half of the right kidney, the right renal vessels, the inferior
vena cava, the terminal section of the left renal vein and the
right diaphragmatic foot (17).
When a pancreatic head tumor is large, it may compress the inferior
vena cava or the portal vein, resulting in lower limb edema or
ascites.
The CBD begins at the junction of the cystic duct
and the common hepatic duct, terminating at the major duodenal
papilla, with a length range of 4–8 cm (18). It is divided into the supraduodenal,
retroduodenal, pancreatic and intramural segments. During its
descent, the CBD is initially positioned posterior to the
pancreatic head, with its terminal part passing through the head,
which is a common site for obstructive jaundice due to pancreatic
head cancer (PHC) invasion (19).
Prior to entering the duodenum, the CBD expands to form the
ampullary structure, known as ampulla of Vater, where ampullary
cancer (AC) may occur, representing another frequent site of lower
segment obstruction of the CBD (20).
The duodenum, which is the initial section of the
small intestine, connects superiorly to the stomach and inferiorly
to the jejunum, measuring ~25 cm in length and forming a ‘C’ shape
that encircles the pancreatic head (21). In PHC, this ‘C’-shaped loop may
become enlarged or distorted. The duodenum is divided into four
parts: i) Superior; ii) descending; iii) horizontal; and iv)
ascending, each with distinct clinical significance (22). The medial side of the descending
part of the duodenum is closely associated with the pancreatic
head, CBD and PD opening to the major duodenal papilla in the
middle of its posterior medial side. With the development of
duodenal surgery, variations of the duodenum are increasingly
common (23). For instance, the
horizontal part of the duodenum may be positioned anteriorly to the
descending portion or ascend to the right side. The terminal
portion may terminate on the right side or traverse behind the
pancreas and mesenteric vessels to ultimately connect with the
duodenojejunum flexure on the left side. Such variations arise due
to abnormal rotation (24).
The PD is located within the substance of the
pancreas, originating from the tail of the pancreas and traversing
its entire length to the right edge of the pancreatic head
(22). Typically, it merges with
the CBD to form the ampulla of Vater, which subsequently opens into
the major duodenal papilla, or the PD may have a separate opening
(17). The diameter range of the PD
near the duodenum is 2–3 mm. Occasionally, a small duct can be
observed in the pancreatic head running above the PD, opening onto
a smaller papilla adjacent to the major duodenal papilla, known as
the accessory PD, which has an occurrence rate of ~50% (25). Among the abdominal organs, the PBDJ
is regarded as the most intricate and delicate structure. This
region involves three distinct organs: i) The biliary tract; ii)
the pancreas; and iii) the intestines, which collectively receive
precise regulation from the nervous and endocrine systems,
justifying its consideration as a closely linked structural and
functional entity (26). Lesions at
the PBDJ can have varying origins, but their pathogenesis,
pathological changes and clinical manifestations often interrelate,
necessitating a comprehensive approach in diagnosis and treatment
(3). Once the PBDJ is compromised,
the leakage and mixing of bile and pancreatic juice can activate
pancreatic enzymes, triggering a severe corrosive ‘chain reaction’
that leads to extensive erosion of surrounding tissues, and even
hemorrhage, necrosis, infection and abscess formation in the
abdominal cavity or retroperitoneal tissues, which can be
life-threatening in severe cases (27,28).
Biological characteristics of PBDJ
tumors
Common manifestations of malignant
tumors at the PBDJ
Malignant tumors at the PBDJ encompass PHC, distal
bile duct cancer (DBDC) and duodenal cancer (DC), which typically
leads to biliary obstruction, dilation and gallbladder enlargement.
PHC may also manifest as localized PD destruction with distal
dilation (29). Most patients
present with a notable mass and often exhibit involvement of
mesenteric vessels, lymph node or surrounding organ metastasis
(30). Furthermore, levels of
specific serological and secretory markers may significantly
increase, triggered either by the tumor itself or due to
biliopancreatic duct obstruction (31).
Biological characteristics of PHC
Based on an extensive analysis of pancreatic cancer
cases, it has been shown that PHC primarily exhibits an invasive
multifocal growth pattern (32–34).
The risk factors for its development include, but are not limited
to, age, smoking history, alcohol abuse, obesity, diabetes, genetic
predisposition, dysbiosis of gut microbiota and chronic
pancreatitis (35,36). As a highly malignant
gastrointestinal tumor, PHC is characterized by its insidious
onset, rapid progression, high postoperative recurrence rates and
insensitivity to both chemotherapy and radiotherapy, leading to a
low 5-year survival rate (37). The
degree of tumor differentiation is inversely associated with its
malignant potential, with poorly differentiated tumors being more
prone to metastasis and vascular invasion.
The biological characteristics of PHC are manifested
as follows: First, the pancreatic head itself lacks a capsule,
which facilitates intraductal spread and invasion of adjacent
organs and blood vessels (38),
such as the celiac trunk, hepatic artery, superior mesenteric
artery, splenic artery, abdominal aorta, portal venous system and
inferior vena cava, resulting in tumors that are unresectable or
inadequately resected. Electron microscopy has revealed that nerve
fibers within the pancreas are predominantly unmyelinated, allowing
cancer cells to easily disrupt the perineurium, nerve fibers and
their synaptic membranes, leading to central-side neural metastasis
and the formation of intra-pancreatic multicentric lesions. When
the main (M)PD is obstructed, tumor cells can implant and grow
retrogradely in the ducts (39,40).
Second, lymphatic and hematogenous metastasis may be at early
stages. Due to the abundance of peripancreatic lymphatic tissue,
lymph node metastasis occurs early and has a high incidence
(41,42). The complex mechanisms underlying
lymph node metastasis are not fully understood; however, research
has reported that microRNA-1231 in exosomes derived from bone
marrow mesenchymal stem cells inhibit the invasion, metastasis and
tumor microenvironment of PHC (43). Third, PHC exhibits neurotropic
growth and the characteristic of invasive spread along perineural
sheaths. Nerves are protected by three layers of connective tissue:
i) The epineurium; ii) perineurium; and iii) endoneurium, with
interstitial spaces between these layers providing pathways for
cancer cell invasion. Selvaggi et al (44) and Wang et al (45) define neural infiltration as the
presence of tumor cells in any layer of the three-layer nerve
sheath or tumor cells surrounding >1/3 of the nerve tissue
within a lesion. PHC demonstrates a neural invasion rate of
80–100%, which is a critical factor contributing to postoperative
recurrence and poor prognosis, severely affecting the outcomes of
curative surgeries (46–48).
Biological characteristics of
DBDC
DBDC originates from bile duct epithelial cells and
is classified as a primary malignant tumor of the biliary system,
located in the extrahepatic bile duct region below the point where
cystic duct merges with common hepatic duct. The incidence of bile
duct cancer is relatively low, accounting for only ~3% of
gastrointestinal malignancies, whilst DBDC represents 20–30% of
bile duct cancers (49). Research
has reported that DBDC is characterized by infiltrative multifocal
growth and shares numerous biological features with PHC, including
pathological findings that exhibit biliopancreatic morphological
changes which contribute to its poor prognosis (50). However, the surgical resection rate
and prognosis for DBDC are superior to those for PHC, potentially
attributable to the following: i) The tendency for DBDC to cause
biliary obstruction, with jaundice symptoms appearing early,
facilitating early diagnosis and radical surgical intervention; and
ii) its unique biological characteristics, such as differing
mutation patterns of the KRAS, P16 and P53 genes compared with
pancreatic cancer (51–53), higher tumor differentiation and less
infiltration into the duodenum with lymph node metastasis tending
to occur later with a migratory pattern distinctly different from
that of PHC often confined to lymph nodes near the distal bile duct
(54,55). Moreover, Kwon et al (56) reported that lymphovascular invasion
and tumor (T)-node-metastasis staging are independent risk factors
affecting patient prognosis.
The tumors in the ampullary region of Vater have
diverse origins, with marked differences in biological
characteristics, pathological features and prognosis among PHC,
DBDC, AC and DC. Zheng-Pywell and Reddy (57) and Williams et al (58) reported that patients with PHC have
the worst prognosis, followed by those with DBDC; AC prognosis is
moderate, whilst patients with duodenal papilla cancer have the
best prognosis, suggesting that the site of origin of the tumor is
a critical factor affecting patient outcomes. Pathologically, DBDC
can be categorized into sclerotic, nodular, papillary and
infiltrative types (59).
Early-stage cholangiocarcinoma is further subdivided into elevated,
superficial and depressed types. Histologically, the main
classifications include papillary adenocarcinoma, tubular
adenocarcinoma, mucinous carcinoma, squamous cell carcinoma and
undifferentiated carcinoma, with papillary adenocarcinoma and
tubular adenocarcinoma accounting for >90% of cases (60). Although papillary adenocarcinoma has
a relatively favorable prognosis, it tends to spread along the bile
duct mucosal surface.
Biological characteristics of DC
Primary DC specifically refers to malignant tumors
originating from the epithelial cells of the duodenum and confined
to several parts of the duodenum excluding the pancreatic head, the
distal CBD and the ampulla of Vater. Such tumors are relatively
rare in clinical practice, accounting for ~0.3% of gastrointestinal
tumors and 30–45% of small intestine malignancies (61,62).
Due to their mild and non-specific clinical manifestations, early
diagnosis is challenging, leading to missed and misdiagnosed cases.
However, advances in endoscopic detection and imaging technologies
have improved the early diagnosis rates of primary duodenal tumors
(63).
Zhao et al (64) performed a retrospective analysis of
clinical data from 94 cases of primary duodenal malignancies
between January 2014 and December 2019, which included 60 cases of
adenocarcinoma (63.8%), 32 cases of stromal tumors (34.1%) and two
cases of lymphoma (2.1%). To identify factors associated with
prognosis, the authors performed a Kaplan-Meier analysis and
reported that pancreatic invasion is associated with the prognosis
of patients with adenocarcinoma. By contrast, the location of the
tumor, complications, depth of infiltration, and the distance from
the mesangial side of the tumor to the duodenal papilla are not
associated with patient prognosis.
From a pathological perspective, the macroscopic
morphology of DC is diverse, with the polypoid type being the most
common, followed by the ulcerative, constrictive and diffuse
infiltrative types. The pathological types of adenocarcinoma are
varied, encompassing poorly differentiated adenocarcinoma,
well-differentiated adenocarcinoma, papillary adenocarcinoma and
mucinous adenocarcinoma (65).
Depending on the relative position of the tumor to major duodenal
papilla, cancers around the papilla often present as infiltrative
ulcerative or polypoid types, whilst tumors above the papilla
predominantly exhibit polypoid forms. Those below tend to be
constrictive. The pathogenic factors and mechanisms of DC remain
unclear, but they may be associated with bile acid forming
carcinogenic cholanthracene and methylcholanthracene under the
influence of intestinal bacteria, as well as with abnormalities in
bile and pancreatic secretions and imbalances in the acid-base
levels of duodenal fluids leading to mucosal damage (66). Certain studies have suggested
dietary factors, such as refined carbohydrates, lack of dietary
fiber and diets high in sugar and fat, especially those with
excessive red meat consumption and insufficient fruit and vegetable
intake, may be risk factors for the occurrence of DC, similar to
those associated with colorectal cancer (67,68).
Research by Kakushima et al (69) further emphasized smoking and
Helicobacter pylori infection as common high-risk factors
among male and female patients.
Imaging diagnosis of PBDJ tumors
Ultrasound (US)
US diagnostic technology encompasses surface US and
endoscopic (E)US. As tumors at the PBDJ often display no
characteristic manifestations in the early stages, most clinical
cases commonly present with progressive jaundice, significant
weight loss, abdominal distension and dull pain, typically
indicating middle-to-late-stage disease (70). Therefore, it is essential to focus
on relevant clinical signs while remaining vigilant for this
condition, employing auxiliary examination methods for timely and
accurate diagnosis. This approach is crucial for minimizing
misdiagnosed and missed cases, formulating precision treatment
plans, enhancing the rate of radical resection and improving
prognosis (71). As a widely used
preliminary screening tool, US has the advantages of being
non-invasive, rapid, cost-effective and easy to perform. However,
its imaging quality is frequently compromised by intestinal gas
interference, which limits clear visualization of the PBDJ
(6). Despite these limitations, US
remains the preferred examination for patients with a high
suspicion of tumors at the PBDJ, as it can initially reveal tumor
location, size and degree of dilatation in the bile and PD. Color
Doppler flow imaging further enhances diagnostic capability by
demonstrating the relationship between the tumor and adjacent blood
vessels, thereby assisting in the preoperative assessment of tumor
resectability (60). Water window
ultrasonography using patient-ingested water to fill the
gastrointestinal tract, serving as an acoustic window that
effectively reduces gas interference and enhances the delineation
of mass boundaries, size and involvement of neighboring organs,
thus improving diagnostic accuracy and tumor staging abilities
(72,73). Double contrast-enhanced
ultrasonography (DCEUS) uses oral or injected gastrointestinal
echogenic agents alongside intravenous US contrast agents. This
method not only clearly depicts the morphology, size and boundaries
of lesions and their relationships with surrounding tissues, but
also reveals the vascular supply characteristics of tumors
improving the detection rate of tumors at the PBDJ (5,74,75).
Research data indicate that DCEUS markedly enhances the visibility
of lesions compared with conventional US and gastroduodenal water
window ultrasonography (76,77).
However, the application of DCEUS and the water-window technique is
relatively limited given the convenience of the clinical operation
and diagnostic accuracy offered by CT and MRI.
EUS leverages its probe to establish close contact
with lesions through the intestinal wall within the
gastrointestinal cavity, thus overcoming the limitations of
conventional US which is often hindered by intestinal gas. This
modality offers enhanced soft tissue resolution, enabling the clear
visualization of the relationship between tumors at the PBDJ and
adjacent structures, which is beneficial for preoperative tumor
staging assessment (78,79). The sensitivity of EUS in T staging
surpasses that of both US and CT (80,81),
making it a crucial tool for guiding fine-needle (FN) aspiration
(FNA) and FN biopsy (FNB) to achieve cytological and histological
diagnoses (82). Research has
indicated that the sensitivity for diagnosing pancreatic
malignancies using EUS-FNA and -FNB is 71 and 82%, respectively,
with both techniques achieving a specificity of 100% (83). In evaluating the etiology of biliary
strictures, the overall diagnostic accuracy of EUS-guided tissue
sampling exceeds that of endoscopic retrograde
cholangiopancreatography (ERCP)-guided tissue sampling,
particularly for strictures caused by pancreatic lesions; however,
in the case of primary malignant biliary obstruction, the
difference between the two methods is not significant as confirmed
by multiple studies (82,84–86).
The advantage of EUS lies in its ability to
visualize the intestinal cavity and the duodenal papilla directly,
facilitating procedures such as biopsies and fluid collections,
thereby providing a wealth of information for comprehensive
diagnostics (79). In areas that
are challenging to access endoscopically, percutaneous FNA cytology
can serve as a supplementary method to obtain qualitative
diagnostic evidence of malignant cells. Ultimately, a definitive
diagnosis often necessitates surgical exploration to thoroughly
assess the lesion nature and extent, the involvement of surrounding
organs and distant lymph node metastasis, providing critical
information for surgical decision-making and technique
selection.
CT
Multi detector (MD)CT is a crucial imaging technique
for diagnosing tumors at the PBDJ due to its rapid scanning speed,
and superior spatial and density resolution. Through
post-processing technologies such as multi planar reconstruction
and curved planar reformation, MDCT can provide a more intuitive
and stereoscopic visualization of tumors and the surrounding
anatomy, enhancing the detection rates and diagnostic accuracy of
lesions (87). Research has
reported a sensitivity of 100% for MDCT in assessing the
resectability of tumors at the PBDJ, with an overall accuracy of
84.4%, thereby solidifying its core position in this domain
(88). Moreover, the introduction
of spectral CT technology, which not only reflects the anatomical
structure of lesions, but also reveals their functional
characteristics, provides new directions for the diagnosis and
differential diagnosis of tumors.
Liang et al (89) reported that low kilovolt
monoenergetic images from dual-energy CT markedly improves both the
subjective and objective quality of images in patients with
pancreatic cancer as well as the consistency in tumor measurements,
whilst combining iodine maps enhances the detectability of isodense
pancreatic cancers. However, it is noteworthy that despite its
promising prospects, in-depth research on the application of
spectral CT in the diagnosis and differential diagnosis of tumors
at the PBDJ is currently lacking. Therefore, there is an urgent
need for more exploratory studies in the future to fully uncover
its potential clinical applications.
Direct CT signs of tumors at the PBDJ include soft
tissue density masses in the ampulla, thickening of the duodenal
wall or intraluminal soft tissue shadows, and thickening of the
wall or intraluminal soft tissue shadows at the end of CBD
(90). Indirect signs manifest as
atrophy of the distal pancreatic parenchyma, dilation of PD,
dilation of the intrahepatic and extrahepatic bile ducts, and
enlargement of the gallbladder (91). When the lesions are large and
extensive, it becomes challenging to identify the origin of primary
lesions, and certain CT signs can have auxiliary diagnostic
significance.
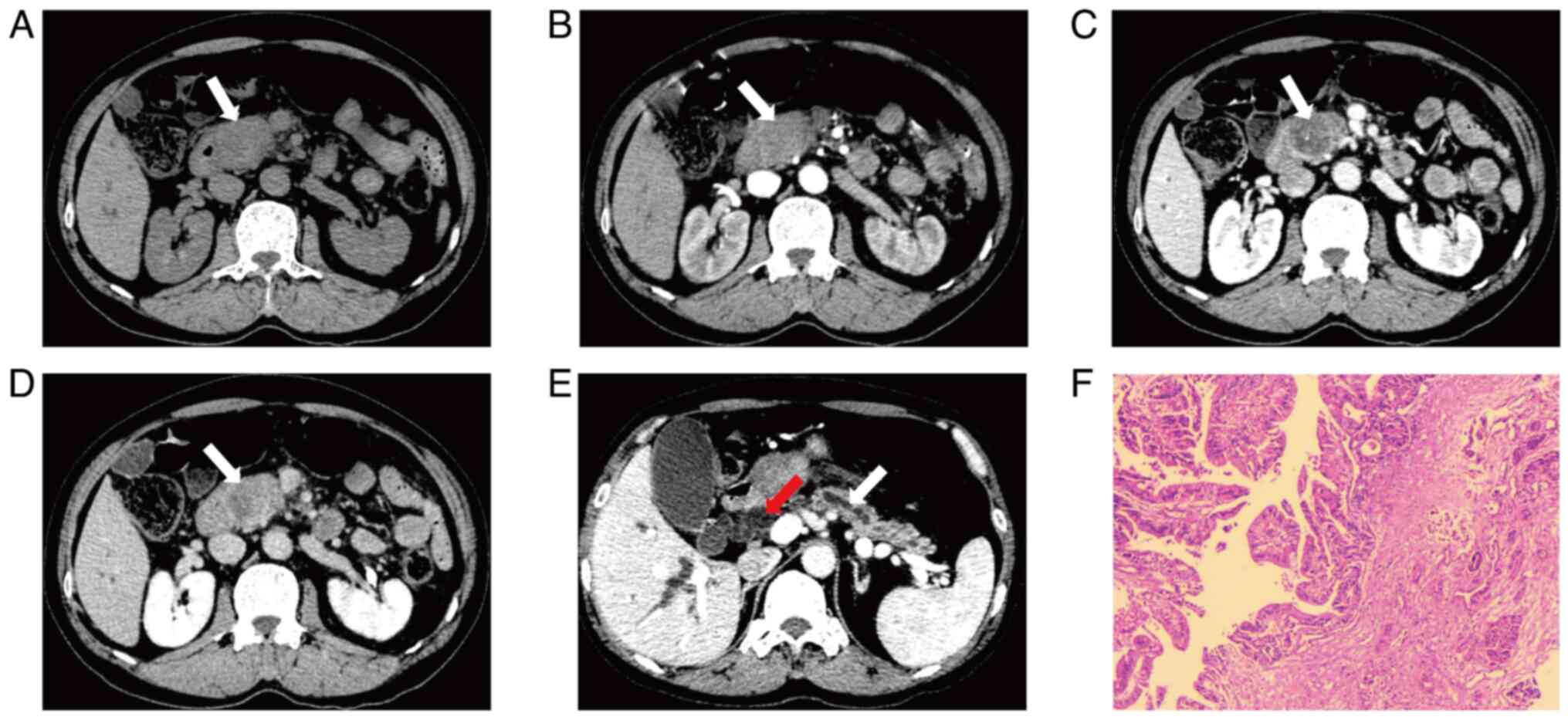
Zhao et al (92) reported the imaging differences
between PHC, cholangiocarcinoma, AC and benign lesions through MDCT
image analysis. Cholangiocarcinoma is characterized by small
lesions with significant wall thickening, markedly dilated
intrahepatic and extrahepatic bile ducts and gallbladder along with
significant delayed enhancement; PHC typically presents as large
lesions with high necrosis rates, extensive invasion, notable
double-duct signs and mild early enhancement in the arterial phase,
with enhancement less than that of normal pancreas; and AC shows
intermediate enhancement, whilst benign lesions generally exhibit
no significant enhancement. Moreover, key points for
differentiating pancreatic cancer also include patient age >50
years, ill-defined tumor borders and pancreatic atrophy (Fig. 1) (93). For AC, the presence of a mass in the
ampullary region, asymmetric narrowing of the distal CBD, dilation
of the intrahepatic bile duct, dilation of the PD, thickening of
the duodenal wall and delayed enhancement, are indicative of
diagnosis (94). Early-stage DBDC
may present with bile duct obstruction symptoms and simple bile
duct dilation without PD dilation. The degree of bile duct wall
thickening and morphological analysis assist in distinguishing
between cholangitis and cholangiocarcinoma (95): Dilation of the CBD due to
inflammatory narrowing often appears tapered, with wall thickening
of <1.5 mm, whilst exceeding this threshold suggests a
neoplastic condition.
Radiomics, a cutting-edge technology at the
forefront of the integration of AI and medical imaging, is capable
of extracting rich and quantifiable features from raw imaging data
and linking them to potential biological behaviors. By analyzing
these features through AI algorithms, it provides critical
information for precise diagnosis and prognostic evaluation
(96). Lee et al (95) combined contrast-enhanced CT imaging
with clinical presentations to construct a predictive nomogram
using indicators such as ampullary masses, enhancement
characteristics and the degree of bile duct dilation and jaundice,
thus effectively distinguishing between benign and malignant
ampullary strictures and enhancing clinical decision support. The
authors focused on the imaging assessment of MPD truncation and
related abnormalities, combining the abnormal parenchyma outline of
MPD truncation, the location of truncation (head or neck), the
presence of acute pancreatitis and elevated cancer antigen 19-9 (CA
19-9) levels to develop a novel nomogram for early diagnosis of
occult pancreatic malignancies (97). Jang et al (98) identified independent predictive
factors for ampullary tumor lesions, including Vater ampulla mass,
Vater ampulla size >12 mm, total bilirubin >1.2 mg/dl and age
≤63 years. The nomogram developed based on these factors
demonstrates a diagnostic accuracy of 93.9%. Histogram parameter
analysis of MDCT during arteriovenous phases revealed the optimal
performance of venous phase percentiles in differentiating between
PHC and DC, with whole focus CT histogram analysis notably
enhancing diagnostic capabilities for tumors at the PBDJ (99).
Based on histological characteristics, PBDJ tumors
are classified into intestinal-type and pancreatobiliary-type, with
most studies indicating that intestinal-type tumors have a better
prognosis (100,101). Ivanovic et al (102) made marked strides in the
differential diagnosis of intestinal-type and pancreatobiliary-type
AC using MDCT technology, achieving high sensitivity (85.7%),
specificity (83.3%) and accuracy (84.4%). The study findings
suggested that the features of intestinal-type AC include nodular
morphology, duodenal papilla bulging, free duodenopancreatic groove
appearance and no involvement of the pancreaticoduodenal artery.
The pancreatobiliary-type tends to exhibit infiltrative growth,
retraction of papilla, invasion of the CBD and MPD, fixed
duodenopancreatic groove appearance and involvement of the
pancreaticoduodenal artery. These characteristics are particularly
evident under conditions of marked duodenal distension,
highlighting the unique advantages of MDCT in distinguishing
histological subtypes of AC. Bi et al (103), through a meticulous CT radiomics
analysis combined with logistic regression algorithm models,
precisely differentiated between intestinal- and
pancreatobiliary-type malignant tumors at the PBDJ, exhibiting
exceptional model performance [sensitivity, 90%; specificity, 93%;
accuracy, 88%; area under the curve (AUC), 0.96], highlighting the
potential application of preoperative CT radiomics in
differentiation and the differences in enhancement patterns between
the two types.
Enhanced CT is a crucial technology for diagnosing
tumors at the PBDJ, demonstrating superior efficacy compared with
that of US, and it also allows for assessment of distant
metastases. However, for cases of missed microlesions or lesions of
uncertain origin, it is necessary to combine it with enhanced MRI
or biopsy pathology to refine the diagnosis.
MRI
MRI has been firmly established as a conventional
imaging diagnostic tool, with its non-invasive and radiation-free
characteristics revolutionizing medical diagnostics. However,
patients with intra-body metallic foreign objects or implants need
to avoid MRI to prevent interference or risks (91). For tumors at the PBDJ, non-invasive
screening modalities such as US, CT and MRI are preferred, as these
technologies can visually demonstrate biliary and PD obstruction
and dilation (104). Diffusion
weighted imaging (DWI) indirectly reflects cell density and tissue
microstructural characteristics by quantifying the diffusion of
water molecules, with tumors at the PBDJ often showing restricted
diffusion (105).
Currently, enhanced MRI in conjunction with magnetic
resonance cholangiopancreatography (MRCP) and DWI are primarily
used for diagnosing and assessing PBDJ tumors (106). MRCP uses the long T2 relaxation
time characteristics of the bile and pancreatic juice to highlight
the biliary and PD systems through a heavily T2-weighted imaging
technique, creating images similar to ERCP, facilitating
observation of lesions (107).
Research has validated that MRCP and ERCP exhibit comparable
efficacy in distinguishing biliary strictures (108). Long-segment asymmetrical
strictures with irregular margins suggest cholangiocarcinoma,
whilst the opposite points towards benign conditions (108). The double duct sign, the degree of
biliary dilation and gradual tapering or sudden narrowing of the
duct are challenges for differential diagnosis, consistent with
findings by Suthar et al (109). Further emphasis on the combined
application of MRCP and CT has been presented by Wang et al
(110), who proposed a scoring
model based on the length of stricture, angle of distal biliary
stricture, double duct sign and low density in the arterial phase,
enhancing the diagnostic accuracy for benign and malignant distal
biliary strictures.
Quantitative MRI analysis also demonstrates
proficiency in differentiating PHC, intrapancreatic
cholangiocarcinoma and AC. For instance, AC often shows the
narrowest confluence angle of the pancreaticobiliary duct and the
minimal distance between the terminus of the dilated
pancreaticobiliary duct and the major duodenal papilla (111). MRI findings for PHC include
enlargement of the pancreatic head, extraluminal mass in the
biliary duct, ductal dilation above the lesion, a large confluence
angle of the pancreaticobiliary duct and mild delayed enhancement
post-contrast (3,112). DBDC typically presents with
thickening of the bile duct wall, intraluminal small masses and
‘rat tail’ type narrowing (113).
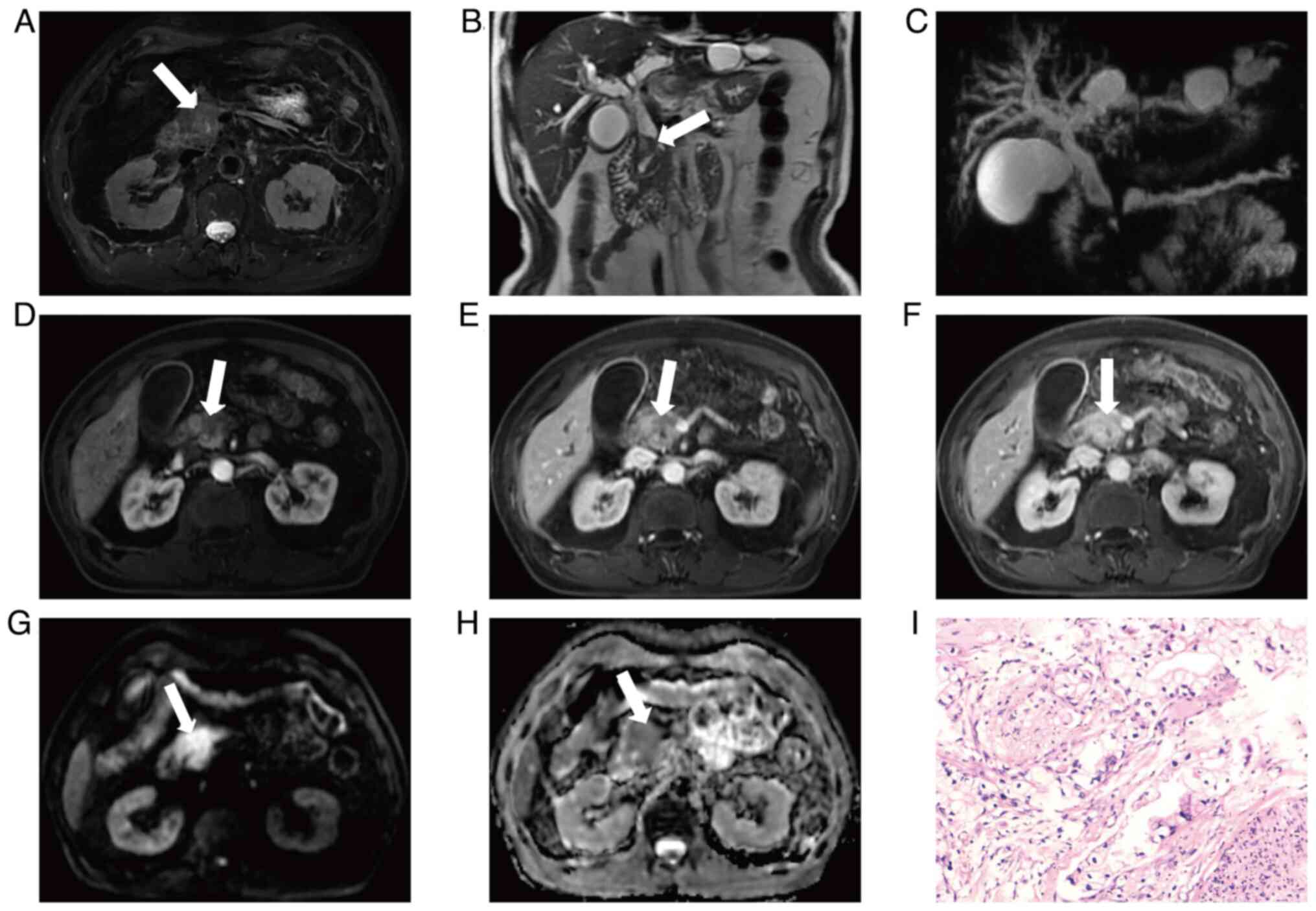
Additionally, DC and AC exhibit unique MRI manifestations, such as
small masses in the duodenal lumen, blunt dilation at the end of
the bile duct and thickening of the ampullary duct wall with a beak
shape of the distal bile duct (Fig.
2) (114,115). Enhanced MRI combined with analysis
of minimum apparent diffusion coefficient (ADC) demonstrate good
diagnostic efficacy in distinguishing between intestinal- and
pancreatobiliary-type cancers. As indicated by Bi et al
(105), its sensitivity,
specificity and AUC values are 70.4%, 78.6%, and 0.807,
respectively. Furthermore, Nalbant et al (106) reported the application value of
MRI and MRCP in a preliminary diagnosis of a mass at the PBDJ,
highlighting that oval filling defects suggest the likelihood of
intestinal-type tumors, whilst progressive enhancement of the mass,
irregular narrowing of the distal CBD, PD truncation, involvement
of the gastroduodenal artery, lymphadenopathy, and a low ADC value
are more indicative of pancreatcobiliary-type. When utilizing MRI
for the evaluation of tumors at the PBDJ, a multi-sequence and
-phenomenon assessment is necessary, considering the
pathophysiological characteristics of the different tumor types,
inferring potential signs that may arise and providing as much
imaging diagnostic information as possible to aid clinical
decision-making in treatment strategies.
ERCP
ERCP allows for direct visualization of lesions on
the medial walls of the duodenum and the ampullary region through
the injection of contrast agents (116). It facilitates the examination of
pancreatic and bile duct structures, and it enables biopsy
collection for pathological evaluation. Furthermore, it can be
utilized for interventional treatments such as stent placement to
alleviate jaundice in patients with advanced and inoperable
conditions (85). When tumors are
small and undetectable by other imaging modalities, ERCP is
particularly effective for early diagnosis. The preferred method
for diagnosing duodenal tumors is endoscopy, which not only allows
for visual assessment of the tumor size, location and morphological
characteristics, but facilitates biopsy for histopathological
confirmation (117). A study
indicated that the accuracy of endoscopic biopsy for diagnosing
ampullary tumors is 81.9% (118).
However, it may be challenging to identify tumors located in the
horizontal and ascending portions, often necessitating the use of
duodenal double contrast barium studies to enhance diagnostic
rates. Notably, ERCP exhibits a diagnostic accuracy of ≤100% for
ampullary tumors, notably surpassing that of US, CT and MRCP
(104). Nevertheless, due to its
limitations in assessing the spatial relationships of tumors to
adjacent tissues and the extent of invasion into surrounding
structures, additional imaging studies are needed for a
comprehensive evaluation to ensure diagnostic completeness and
accuracy (119).
Positron emission tomography
(PET)/CT
18F-Fluorodeoxy-glucose (18F-FDG) PET/CT is a
diagnostic technique that integrates functional metabolism with
anatomical structure imaging. It effectively distinguishes between
benign and malignant lesions by capturing the glycolytic activity
of malignant cells, demonstrating efficacy particularly in the
diagnosis of pancreatic malignancies (120). In this process, 18F-FDG, a glucose
analogue, is transported into the cells via glucose transporters,
where it is phosphorylated into 18F-FDG-6-phosphate by hexokinase.
Due to the high expression of transporters and kinases in malignant
tumor cells, 18F-FDG tends to be retained within the cells,
resulting in high metabolic hotspots on PET/CT. Whilst PET/CT
cannot replace pancreatic CT or MRI as the first-line examination,
it serves as an advantageous adjunct, especially in the exclusion
and detection of distant metastases, particularly in cases with
larger primary lesions, suspected regional lymph node metastases
and notably elevated CA 19-9 levels (121,122).
Chronic mass pancreatitis is a specific type of
chronic pancreatitis characterized by long-term inflammation
leading to damage of the pancreatic parenchyma and fibrotic tissue
proliferation, potentially forming a mass in the pancreatic head
(123). Currently, CT is widely
used as a routine imaging modality for anatomical assessment and
tumor staging; however, its capacity for differential diagnosis is
limited when faced with the highly similar clinical presentations
and imaging characteristics of chronic mass pancreatitis and
pancreatic cancer (124). The
standardized uptake value (SUV) is an important semi-quantitative
indicator for diagnosing pancreatic cancer, with SUV values being
markedly higher in patients with pancreatic cancer compared with
those in patients with chronic pancreatitis (125). Notably, although 18F-FDG PET/CT
exhibits high sensitivity in diagnosing pancreatic cancer, it also
encounters issues with false positives, such as in cases of active
pancreatitis, peritoneal fibrosis and lymphocytic infiltration, and
false negatives such as in low-density cancer cells and tumors with
high fluid content. Therefore, it is necessary to conduct a
comprehensive evaluation incorporating clinical manifestations,
laboratory tests and other factors (126).
Overall, PET/CT demonstrates superior diagnostic
efficacy compared with enhanced CT in the differential diagnosis of
pancreatic cancer and chronic mass pancreatitis, providing a richer
and more accurate imaging basis for clinical decision-making.
3DVT imaging diagnosis
Tumors at the PBDJ, regardless of their benign or
malignant nature, should be primarily treated with surgical
intervention once diagnosed. Formulating a surgical plan
necessitates a comprehensive consideration of the tumor location,
size, infiltration range, vascular relationships, metastasis and
the physical condition of the patient (127). Due to the unique anatomical
positioning of these tumors, surgical complexity tends to be high,
making preoperative assessment critically important. Traditional
two-dimensional imaging techniques such as US, MDCT and MRI can
provide information about the lesion and adjacent structures;
however, due to the relatively sparse blood supply to the pancreas
and distal bile duct, imaging clarity is often limited, possibly
leading to errors in assessing tumor resectability (128,129).
To overcome the limitations of two-dimensional
imaging, 3DVT has emerged and is gradually being applied in the
diagnosis and treatment of tumors at the PBDJ. This technology
relies on a 3D visualization system for abdominal medical imaging,
allowing for a comprehensive evaluation of the tumor morphology,
position, the state of pancreatobiliary duct obstruction and its
spatial relationships with surrounding major blood vessels. Current
research focuses on the consistency between 3D assessment results
and intraoperative realities, aiming to optimize surgical planning,
shorten operative duration and reduce the risk of injury to major
vessels during surgery, which holds significant clinical importance
(130). 3D imaging not only
provides a clear depiction of anatomical structures, but also
integrates dynamic simulation and real-time interactive
functionalities, substantially enhancing diagnostic accuracy and
the scientific rigor of surgical planning (131–133). In the field of oncology, 3DVT is
particularly vital, granting physicians the precision to closely
examine tumors and their surrounding environments (134,135). Specifically, this technology
reconstructs two-dimensional CT images into 3D models that closely
match the structures of the abdominal organs of the patient,
allowing for an intuitive, spatial and comprehensive separation of
the tumor in 3D images. This facilitates a swift and accurate
assessment of the relationships between the pancreatic head, distal
bile duct or ampullary tumors and vasculature, providing robust
support for surgeons in evaluating tumor resectability and
formulating personalized treatment strategies (136,137).
The resection of tumors located in the head and
body-tail of the pancreas is recognized as one of the most complex
procedures in upper gastrointestinal surgery, often facing
challenges related to vascular variations during surgery,
particularly those involving the portal vein and the hepatic artery
(138). Research indicates that 3D
visualization systems are effective in demonstrating the origins
and branches of vessels, as well as the relationships between
tumors, organs and vessels, achieving a diagnostic sensitivity,
specificity and accuracy of 100% for identifying hepatic artery
variations. The clarity of the images produced rivals that of
angiography, thereby providing individualized preoperative guidance
for patients with hepatic artery anomalies undergoing
pancreaticoduodenectomy (139).
Miyamoto et al (140)
further expanded the application scope of 3DVT, using it to clearly
present anatomical variations of peripancreatic vessels and changes
induced by tumors, thus minimizing surgical trauma and shortening
the operation time through preoperative simulations. Addressing one
of the severe complications associated with
pancreaticoduodenectomy, pancreatic fistulas, Miyamoto et al
(141) proposed that preoperative
measurement of the residual pancreatic volume using 3DVT can
predict the risk of fistula occurrence, offering a scientific basis
for preventing complications. Furthermore, the cinematic rendering
technique, an advanced post-processing technology within 3D
visualization, leverages unique illumination models to generate
higher quality images, significantly enhancing detail
representation (142). This
technology exhibits distinctive advantages in depicting tumor
location, adjacent relationships, modes of enhancement and internal
characteristics such as necrosis and cystic changes. It is also
able to simulate endoscopic views, thereby providing positive
support for the qualitative diagnosis of lesions and planning of
therapeutic strategies (143).
The blood supply to the lower segment of the CBD
primarily originates from the right hepatic artery and the
pancreaticoduodenal artery. Inadequate vascular management can
markedly increase the risks of complications such as bleeding and
anastomotic leaks. The application of 3DVT in surgical procedures
for tumors at the PBDJ markedly enhances the visualization of
lesion structures, facilitates precise surgical planning and
ensures smoother handling of complex cases, ultimately improving
the R0 resection rate (144,145). Furthermore, 3D pancreaticobiliary
duct models demonstrate considerable potential in accurately
assessing complex pathological anatomy, aiding differential
diagnoses, and informing surgical planning by overcoming the
limitations of traditional CT and MRCP techniques, particularly for
patients with tumors at the PBDJ (146,147).
In the realm of diagnosis and treatment of
hepatobiliary diseases, 3DVT also serves a crucial role (8,148–150). Zhang et al (151) reported that this technology has a
notably higher positive predictive value for diagnosing portal vein
invasion in hilar cholangiocarcinoma compared with subjective
assessments based on CT scans; this provides a quantitative basis
for the preoperative determination of resection extent and the
surgical approach. Guo et al (152) explored the efficacy of 3DVT in
guiding hepatic resection for complex intrahepatic stones,
reporting that 3DVT offers a precise preoperative diagnosis of
complex intrahepatic bile duct stones, demonstrating improved
safety, feasibility and effectiveness compared with conventional
imaging modalities. Zhao et al (153) performed a comparative study
between two-dimensional medical imaging and 3DVT in evaluating
tumor resectability, reporting accuracy rates of 85.9% for
conventional imaging and 97.2% for 3DVT. This indicated that 3DVT
predicts tumor resectability more accurately in preoperative
evaluations. Moreover. 3DVT effectively addresses the limitations
of two-dimensional imaging in abdominal CT, particularly in
showcasing intricate details of the surgical area when dealing with
affected organs and their surrounding complex structures, allowing
surgeons to assess the relationships fully and spatially between
tumors and adjacent blood vessels and lymph nodes, thereby
optimizing surgical strategy selection. However, it is noteworthy
that this technology is currently limited to spatial configuration
reconstruction and does not yet provide the functional information
necessary for differential diagnoses (154).
The present review systematically summarized and
analyzed the advantages and limitations of several imaging
techniques in the diagnosis of tumors at the PBDJ. Additionally,
based on current advancements, the present review made
forward-looking predictions and outlooks on future trends. This is
presented in Table I (7,75,78,83,89,103,104,106,108, 143,153,155–157).
 | Table I.Evaluation and value of imaging
techniques in the diagnosis of malignant tumors at the
pancreaticobiliary duodenal junction. |
Table I.
Evaluation and value of imaging
techniques in the diagnosis of malignant tumors at the
pancreaticobiliary duodenal junction.
| First author/s,
year | Technique | Advantages | Disadvantages | Possible directions
for improvement | (Refs.) |
|---|
| Zhang et al,
2016 and | US | Non-invasive,
convenience, | Susceptible to
gastrointestinal gas | Develop DCEUS
technology to improve small | (75,155) |
| Swaraj et
al, 2023 |
| real-time
observation capabilities | interference,
limited detection rates for | lesion detection.
Integrate with CT and MRI for |
|
|
|
| of lesions and low
economic cost. | small tumors and
high operator | advanced multimodal
imaging Enhance training |
|
|
|
|
| dependence. | for US
diagnosticians. |
|
| Trikudanathan et
al, | EUS | High-resolution
imaging, reduction | The technical
operational difficulty is | Developing higher
frequency US probes and new | (78,83) |
| 2014 and Oppong
et al, |
| of interference
actors, real-time | high, with
associated risks of complica- | image processing
techniques to improve image |
|
| 2020 |
| fguidance for
puncture biopsies and | tions, prolonged
examination times and | quality and
diagnostic accuracy, multi-modal |
|
|
|
| assessment of tumor
resectability. | reliance on the
operator's expertise. | integration and
optimizing patient preparation. |
|
| Liang et al,
2022 and | CT | High-resolution
imaging, multi- | Radiation exposure
risks, limited | Develop low-dose
scanning technologies, | (89,103) |
| Bi et al,
2022 |
| stage scanning
technologies and | detection rates for
small lesions and | apply spectral CT
and promote multimodal |
|
|
|
| extensive
post-processing capabili- | insufficient soft
tissue contrast. | integration
alongside AI-assisted diagnostics, |
|
|
|
| ties with broad
applicability. |
| such as
radiomics. |
|
| Chen et al,
2019 and | MRI | No radiation, high
soft tissue | Spatial resolution
is limited, exami- | Improvements in MRI
hardware and software, | (7,106) |
| Nalbant et
al, 2023 |
| resolution,
multi-sequence imaging, | nation durations
are lengthy, motion | development of new
imaging technologies and |
|
|
|
| advantages of MRCP
and func- | artifacts may
affect results, costs are | sequences,
promotion of multimodal integration |
|
|
|
| tional imaging
capabilities. | relatively high and
contraindications | and applications of
radiomics. |
|
|
|
|
| for
examinations. |
|
|
| Chen et al,
2008 and | ERCP | Intuitive
visualization of the | The invasive nature
of the procedure | It is necessary to
elevate technical levels, | (104,108) |
| Park et al,
2004 |
| pancreaticobiliary
anatomy allows | incurs a higher
risk of complications | optimize the
materials of devices, integrate |
|
|
|
| simultaneous
biliary drainage or | and is limited to
certain patient | other examination
methods and enhance posto- |
|
|
|
| biopsy. | populations |
perative.management. |
|
| Reddy et al,
2022 and | PET/CT | Comprehensive
assessment of | Costs are high,
radiation exposure is | Solutions include
reducing radiation doses, | (156,157) |
| Wen et al,
2020 |
| systemic tumor
metastasis and | significant,
sensitivity to certain low- | integrating other
examination methods and |
|
|
|
| effective
differentiation between | metabolism tumors
is lacking and | developing specific
tracers. |
|
|
|
| benign and
malignant tumors. | detection of
lesions in hollow organs has |
|
|
|
|
|
| blind spots. |
|
|
| Barat et al,
2024 and | 3DVT | Provides intuitive
three- | High-quality raw
imaging data required, | Need to lower
costs, simplify operational | (143,153) |
| Zhao et al,
2018 |
| dimensional data on
tumor mor- | high technical
costs, complex processing | processes,
integrate with other imaging |
|
|
|
| phology, location
and relationship | workflows, and
further data required to | technologies,
pursue ongoing research and |
|
|
|
| with surrounding
structures | validate its
effectiveness and reliability. | innovation such as
exploring new three- |
|
|
|
| enhance diagnostic
precision, and |
| dimensional
visualization algorithms and |
|
|
|
| assists in surgical
planning and |
| reconstruction
techniques, along with performing |
|
|
|
| treatment. |
| more clinical
studies. |
|
Conclusions
In summary, due to its complex anatomical structure
and significant physiological functions, the PBDJ serves as a
convergence point for several digestive fluids such as bile,
pancreatic juice and gastrointestinal secretions, which results in
it being a high-incidence area for malignant tumors and a key
pathological basis. However, the etiological factors and specific
mechanisms underlying tumors in this region remain to be
elucidated, and there are numerous challenges in clinical
diagnosis. Given the low sensitivity of PBDJ tumors to
radiotherapy, chemotherapy, immunotherapy and targeted therapy,
surgical intervention has become the preferred treatment strategy
(158–161). Several imaging diagnostic methods
each have their advantages and disadvantages when evaluating tumors
at the PBDJ. Therefore, the judicious selection and combination of
these techniques are crucial for enhancing tumor detection rates
and diagnostic accuracy. Currently, accurately distinguishing the
tissue origin of tumors at this junction, whether intestinal type
or biliary-pancreatic type, using technologies such as US, MDCT,
MRI, ERCP and PET-CT remains challenging, with limited
differentiation capability. Consequently, there is a need for
in-depth exploration and validation of radiomics and 3DVT to
optimize the diagnostic and assessment strategies for tumors in
this region.
Acknowledgements
The authors would like to thank Ms. Yanfen Tang
(Department of Radiology, Affiliated Nanhua Hospital, University of
South China, Hengyang, China) for supplying the imaging materials
(Figs. 1 and 2) utilized in this review. The authors
confirm that all images are original and, to the best of their
knowledge, have not been published elsewhere.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82170192) and the Provincial Natural
Science Foundation of Hunan (grant no. 2023JJ30377).
Availability of data and materials
Not applicable.
Authors' contributions
WYY participated in gathering and arranging the
literature and drafting the paper. PSH conducted the analysis for
Figs. 1 and 2 and contributed to the manuscript
revisions. CHZ offered guidance and revised the manuscript
throughout the entire process. All authors have read and approved
the final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamisawa T, Kaneko K, Itoi T and Ando H:
Pancreaticobiliary maljunction and congenital biliary dilatation.
Lancet Gastroenterol Hepatol. 2:610–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ono A, Arizono S, Isoda H and Togashi K:
Imaging of pancreaticobiliary maljunction. Radiographics.
40:378–392. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nikolaidis P, Hammond NA, Day K, Yaghmai
V, Wood CG III, Mosbach DS, Harmath CB, Taffel MT, Horowitz JM,
Berggruen SM and Miller FH: Imaging features of benign and
malignant ampullary and periampullary lesions. Radiographics.
34:624–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zulfiqar M, Chatterjee D, Yoneda N,
Hoegger MJ, Ronot M, Hecht EM, Bastati N, Ba-Ssalamah A, Bashir MR
and Fowler K: Imaging features of premalignant biliary lesions and
predisposing conditions with pathologic correlation. Radiographics.
42:1320–1337. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu D, Yang K, Li Y, Ye X, Zhang H, Long
Q, Ding X, Dong F and Xu J: Differential diagnostic value of
periampullary mass: A nomogram established by random forest based
on clinical characteristics and contrast-enhanced ultrasound. J
Clin Ultrasound. 50:918–928. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Hawary MM, Kaza RK and Francis IR:
Optimal imaging modalities for the diagnosis and staging of
periampullary masses. Surg Oncol Clin N Am. 25:239–253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen XP, Liu J, Zhou J, Zhou PC, Shu J, Xu
LL, Li B and Su S: Combination of CEUS and MRI for the diagnosis of
periampullary space-occupying lesions: A retrospective analysis.
BMC Med Imaging. 19:772019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang C, Zhang P and Qi X: Digital and
intelligent liver surgery in the new era: Prospects and dilemmas.
EBioMedicine. 41:693–701. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rizzo A and Brandi G: Neoadjuvant therapy
for cholangiocarcinoma: A comprehensive literature review. Cancer
Treat Res Commun. 27:1003542021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rizzo A and Brandi G: Pitfalls,
challenges, and updates in adjuvant systemic treatment for resected
biliary tract cancer. Expert Rev Gastroenterol Hepatol. 15:547–554.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Federico A, Mosca M, Pagani R, Carloni
R, Frega G, De Giglio A, Rizzo A, Ricci D, Tavolari S, Di Marco M,
et al: Immunotherapy in pancreatic cancer: why do we keep failing?
A focus on tumor immune microenvironment, Predictive biomarkers and
treatment outcomes. Cancers (Basel). 14:24292022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guven DC, Sahin TK, Erul E, Rizzo A, Ricci
AD, Aksoy S and Yalcin S: The association between albumin levels
and survival in patients treated with immune checkpoint inhibitors:
A systematic review and meta-analysis. Front Mol Biosci.
9:10391212022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Federico A, Tateo V, Parisi C, Formica
F, Carloni R, Frega G, Rizzo A, Ricci D, Di Marco M, Palloni A and
Brandi G: Hacking pancreatic cancer: Present and future of
personalized medicine. Pharmaceuticals (Basel). 14:6772021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Padilla-Thornton AE, Willmann JK and
Jeffrey RB: Adenocarcinoma of the uncinate process of the pancreas:
MDCT patterns of local invasion and clinical features at
presentation. Eur Radiol. 22:1067–1074. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Loi M, Magallon-Baro A, Suker M, van Eijck
C, Sharma A, Hoogeman M and Nuyttens J: Pancreatic cancer treated
with SBRT: Effect of anatomical interfraction variations on dose to
organs at risk. Radiother Oncol. 134:67–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaballah AH, Kazi IA, Zaheer A, Liu PS,
Badawy M, Moshiri M, Ibrahim MK, Soliman M, Kimchi E and Elsayes
KM: Imaging after pancreatic surgery: Expected findings and
postoperative complications. Radiographics. 44:e2300612024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bello HR, Sekhar A, Filice RW, Radmard AR
and Davarpanah AH: Pancreaticoduodenal groove: Spectrum of disease
and imaging features. Radiographics. 42:1062–1080. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sah SK, Panth H and Wang YX: Morphometric
analysis of common bile duct: A cadaveric study. J Biomed Res
Environ Sci. 2:64–68. 2021. View Article : Google Scholar
|
|
19
|
Bhutia KD, Lachungpa T and Lamtha SC:
Etiology of obstructive jaundice and its correlation with the
ethnic population of Sikkim. J Family Med Prim Care. 10:4189–4192.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okano K, Oshima M, Suto H, Ando Y, Asano
E, Kamada H, Kobara H, Masaki T and Suzuki Y: Ampullary carcinoma
of the duodenum: Current clinical issues and genomic overview. Surg
Today. 52:189–197. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou C, Zhang H, Wang X and Yang Z: The
‘Hand as Foot’ teaching method in the duodenum anatomy. Asian J
Surg. 45:1768–1769. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu X, Niu R and Wu Y: The ‘Hand as Foot’
teaching method in pancreas-duodenum anatomy. Asian J Surg.
46:1448–1449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pickhardt PJ and Bhalla S: Intestinal
malrotation in adolescents and adults: Spectrum of clinical and
imaging features. AJR Am J Roentgenol. 179:1429–1435. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson LN, Moran SK, Bhargava P, Revels
JW, Moshiri M, Rohrmann CA and Mansoori B: Fluoroscopic evaluation
of duodenal diseases. Radiographics. 42:397–416. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JY, Huang J and Yang ZY: Abdominal
pain after subtotal gastrectomy: A first report of accessory
pancreatic fistula. J Pain Res. 13:431–435. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Apurva Abdul Sattar RS, Ali A, Nimisha
Kumar Sharma A, Kumar A, Santoshi S and Saluja SS: Molecular
pathways in periampullary cancer: An overview. Cell Signal.
100:1104612022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perri G, Bortolato C, Marchegiani G,
Holmberg M, Romandini E, Sturesson C, Bassi C, Sparrelid E,
Ghorbani P and Salvia R: Pure biliary leak vs pancreatic fistula
associated: Non-identical twins following pancreatoduodenectomy.
HPB (Oxford). 24:1474–1481. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pecorelli N, Capretti G, Sandini M,
Damascelli A, Cristel G, De Cobelli F, Gianotti L, Zerbi A and
Braga M: Impact of sarcopenic obesity on failure to rescue from
major complications following pancreaticoduodenectomy for cancer:
Results from a multicenter study. Ann Surg Oncol. 25:308–317. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skórzewska M, Kurzawa P, Ciszewski T, Pelc
Z and Polkowski WP: Controversies in the diagnosis and treatment of
periampullary tumours. Surg Oncol. 44:1018532022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amr B, Shahtahmassebi G, Briggs CD, Bowles
MJ, Aroori S and Stell DA: Assessment of the effect of interval
from presentation to surgery on outcome in patients with
peri-ampullary malignancy. HPB (Oxford). 18:354–359. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan IA, Singh N, Gunjan D, Nayak B, Dash
NR, Pal S, Lohani N, Yadav R, Gupta S and Saraya A: Serum
miR-215-5p, miR-192-5p and miR-378a-5p as novel diagnostic
biomarkers for periampullary adenocarcinoma. Pathol Res Pract.
260:1554172024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Groot VP, Gemenetzis G, Blair AB,
Rivero-Soto RJ, Yu J, Javed AA, Burkhart RA, Rinkes IHMB, Molenaar
IQ, Cameron JL, et al: Defining and predicting early recurrence in
957 patients with resected pancreatic ductal adenocarcinoma. Ann
Surg. 269:1154–1162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Groot VP, Rezaee N, Wu W, Cameron JL,
Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL and He J:
Patterns, timing, and predictors of recurrence following
pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg.
267:936–945. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wood LD, Canto MI, Jaffee EM and Simeone
DM: Pancreatic cancer: Pathogenesis, screening, diagnosis, and
treatment. Gastroenterology. 163:386–402.e1. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El Hakim BA, Caid N, Saoudi L, Benemla R,
Ramoul R, Rekkache S and Smaili F: Clinical characteristic of
pancreatic cancer. Ann Oncol. 29 (Suppl 5):S502018. View Article : Google Scholar
|
|
37
|
Hessmann E, Buchholz SM, Demir IE, Singh
SK, Gress TM, Ellenrieder V and Neesse A: Microenvironmental
determinants of pancreatic cancer. Physiol Rev. 100:1707–1751.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malsy M, Hackl C, Graf B, Bitzinger D and
Bundscherer A: The effects of analgesics on the migration of
pancreatic cancer cells. In Vivo. 36:576–581. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morita S, Onaya H, Kishi Y, Hiraoka N and
Arai Y: Multiple intraglandular metastases in a patient with
invasive ductal carcinoma of the pancreas. Intern Med.
54:1753–1756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakase A, Koizumi T, Fujita N, Ono H and
Matsumoto Y: Studies of the growth and infiltration of experimental
tumor of the pancreas in rabbits. Am J Surg. 133:590–592. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burke EE, Marmor S, Virnig BA, Tuttle TM
and Jensen EH: Lymph node evaluation for pancreatic adenocarcinoma
and its value as a quality metric. J Gastrointest Surg.
19:2162–2170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shang S, Wang J, Chen S, Tian R, Zeng H,
Wang L, Xia M, Zhu H and Zuo C: Exosomal miRNA-1231 derived from
bone marrow mesenchymal stem cells inhibits the activity of
pancreatic cancer. Cancer Med. 8:7728–7740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Selvaggi F, Melchiorre E, Casari I,
Cinalli S, Cinalli M, Aceto GM, Cotellese R, Garajova I and Falasca
M: Perineural invasion in pancreatic ductal adenocarcinoma: From
molecules towards drugs of clinical relevance. Cancers (Basel).
14:57932022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang J, Chen Y, Li X and Zou X: Perineural
invasion and associated pain transmission in pancreatic cancer.
Cancers (Basel). 13:45942021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang MW, Tao LY, Jiang YS, Yang JY, Huo
YM, Liu DJ, Li J, Fu XL, He R, Lin C, et al: Perineural invasion
reprograms the immune microenvironment through cholinergic
signaling in pancreatic ductal adenocarcinoma. Cancer Res.
80:1991–2003. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bapat AA, Hostetter G, Von Hoff DD and Han
H: Perineural invasion and associated pain in pancreatic cancer.
Nat Rev Cancer. 11:695–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan X, Sivakumar S, Bednarsch J,
Wiltberger G, Kather JN, Niehues J, de Vos-Geelen J, Valkenburg-van
Iersel L, Kintsler S, Roeth A, et al: Nerve fibers in the tumor
microenvironment in neurotropic cancer-pancreatic cancer and
cholangiocarcinoma. Oncogene. 40:899–908. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen
JB, Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zaccari P, Cardinale V, Severi C, Pedica
F, Carpino G, Gaudio E, Doglioni C, Petrone MC, Alvaro D,
Arcidiacono PG and Capurso G: Common features between neoplastic
and preneoplastic lesions of the biliary tract and the pancreas.
World J Gastroenterol. 25:4343–4359. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Felsenstein M, Hruban RH and Wood LD: New
developments in the molecular mechanisms of pancreatic
tumorigenesis. Adv Anat Pathol. 25:131–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Banales JM, Cardinale V, Carpino G,
Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes
SJ, Fouassier L, et al: Expert consensus document:
Cholangiocarcinoma: Current knowledge and future perspectives
consensus statement from the European network for the study of
cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol.
13:261–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kato Y, Takahashi S, Gotohda N and Konishi
M: The likely sites of nodal metastasis differs according to the
tumor extent in distal bile duct cancer. J Gastrointest Surg.
20:1618–1627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Min SK, You Y, Choi DW, Han IW, Shin SH,
Yoon S, Jung JH, Yoon SJ and Heo JS: Prognosis of pancreatic head
cancer with different patterns of lymph node metastasis. J
Hepatobiliary Pancreat Sci. 29:1004–1013. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kwon HJ, Kim SG, Chun JM, Lee WK and Hwang
YJ: Prognostic factors in patients with middle and distal bile duct
cancers. World J Gastroenterol. 20:6658–6665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zheng-Pywell R and Reddy S: Ampullary
cancer. Surg Clin North Am. 99:357–367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Williams JL, Chan CK, Toste PA, Elliott
IA, Vasquez CR, Sunjaya DB, Swanson EA, Koo J, Hines OJ, Reber HA,
et al: Association of histopathologic phenotype of periampullary
adenocarcinomas with survival. JAMA Surg. 152:82–88. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakeeb A, Pitt HA, Sohn TA, Coleman J,
Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ and Cameron
JL: Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and
distal tumors. Ann Surg. 224:463–475. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Khan SA, Davidson BR, Goldin R, Pereira
SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC,
Thursz MR and Wasan H; British Society of Gastroenterology, :
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
Consensus document. Gut. 51 (Suppl 6):VI1–VI9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jiang S, Zhao R, Li Y, Han X, Liu Z, Ge W,
Dong Y and Han W: Prognosis and nomogram for predicting
postoperative survival of duodenal adenocarcinoma: A retrospective
study in China and the SEER database. Sci Rep. 8:79402018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nishio K, Kimura K, Murata A, Ohira G,
Shinkawa H, Kodai S, Amano R, Tanaka S, Shimizu S, Takemura S, et
al: Comparison of clinicopathological characteristics between
resected ampullary carcinoma and carcinoma of the second portion of
the duodenum. World J Gastrointest Surg. 14:1219–1229. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Burasakarn P, Higuchi R, Nunobe S, Kanaji
S, Eguchi H, Okada KI, Fujii T, Nagakawa Y, Kanetaka K, Yamashita
H, et al: Limited resection vs pancreaticoduodenectomy for primary
duodenal adenocarcinoma: A systematic review and meta-analysis. Int
J Clin Oncol. 26:450–460. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao Z, Zhang J, Li C, Liu T and Li W:
Surgical treatment and survival analysis of primary duodenal
malignant tumor: A retrospective cohort study. J Gastrointest
Oncol. 13:1733–1745. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xue Y, Vanoli A, Balci S, Reid MM, Saka B,
Bagci P, Memis B, Choi H, Ohike N, Tajiri T, et al:
Non-ampullary-duodenal carcinomas: Clinicopathologic analysis of 47
cases and comparison with ampullary and pancreatic adenocarcinomas.
Mod Pathol. 30:255–266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang S, Cui Y, Zhong B, Xiao W, Gong X,
Chao K and Chen M: Clinicopathological characteristics and survival
analysis of primary duodenal cancers: A 14-year experience in a
tertiary centre in South China. Int J Colorectal Dis. 26:219–226.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yabuuchi Y, Yoshida M, Kakushima N, Kato
M, Iguchi M, Yamamoto Y, Kanetaka K, Uraoka T, Fujishiro M and Sho
M; Japan Duodenal Cancer Committee, : Risk factors for
non-ampullary duodenal adenocarcinoma: A systematic review. Dig
Dis. 40:147–155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Overman MJ, Hu CY, Kopetz S, Abbruzzese
JL, Wolff RA and Chang GJ: A population-based comparison of
adenocarcinoma of the large and small intestine: Insights into a
rare disease. Ann Surg Oncol. 19:1439–1445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kakushima N, Ono H, Yoshida M, Takizawa K,
Tanaka M, Kawata N, Ito S, Imai K, Hotta K, Ishiwatari H and
Matsubayashi H: Characteristics and risk factors for sporadic
non-ampullary duodenal adenocarcinoma. Scand J Gastroenterol.
52:1253–1257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Woo SM, Ryu JK, Lee SH, Yoo JW, Park JK,
Kim YT, Jang JY, Kim SW, Kang GH and Yoon YB: Recurrence and
prognostic factors of ampullary carcinoma after radical resection:
Comparison with distal extrahepatic cholangiocarcinoma. Ann Surg
Oncol. 14:3195–3201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Secchi M, Leonardo R, Esteban M, Mario C
and Alejandro A: Periampullary malignant tumors. Management and
prognostic. Pancreatology. 17:S162017. View Article : Google Scholar
|
|
72
|
Ma Y, Jiang Q, Zhang Z, Xiao P, Yan Y, Liu
J, Li Q and Wang Z: Diagnosis of hirschsprung disease by
hydrocolonic sonography in children. Eur Radiol. 32:2089–2098.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Limberg B: Diagnosis and staging of
colonic tumors by conventional abdominal sonography as compared
with hydrocolonic sonography. N Engl J Med. 327:65–69. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang L, Wang X, Kou H, He H, Lu M, Zhou L
and Yang Y: Comparing single oral contrast-enhanced ultrasonography
and double contrast-enhanced ultrasonography in the preoperative
Borrmann classification of advanced gastric cancer. Oncotarget.
9:8716–8724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang T, Su ZZ, Wang P, Wu T, Tang W, Xu
EJ, Ju JX, Quan XY and Zheng RQ: Double contrast-enhanced
ultrasonography in the detection of periampullary cancer:
Comparison with B-mode ultrasonography and MR imaging. Eur J
Radiol. 85:1993–2000. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li T, Lu M, Song J, Wu P, Cheng X and
Zhang Z: Improvement to ultrasonographical differential diagnosis
of gastric lesions: The value of contrast enhanced sonography with
gastric distention. PLoS One. 12:e01823322017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Maconi G, Radice E, Bareggi E and Porro
GB: Hydrosonography of the gastrointestinal tract. AJR Am J
Roentgenol. 193:700–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Trikudanathan G, Njei B, Attam R, Arain M
and Shaukat A: Staging accuracy of ampullary tumors by endoscopic
ultrasound: Meta-analysis and systematic review. Dig Endosc.
26:617–626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kitano M, Yoshida T, Itonaga M, Tamura T,
Hatamaru K and Yamashita Y: Impact of endoscopic ultrasonography on
diagnosis of pancreatic cancer. J Gastroenterol. 54:19–32. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Archibugi L, Petrone MC, Tamburrino D,
Crippa S, Dabizzi E, Mariani A, Nicoletti R, Doglioni C, Capurso G,
Falconi M, et al: EUS versus CT scan in establishing the T stage in
surgically resected pancreatic cancer based on the new TNM 8th
edition. Pancreatology. 18 (Suppl):S130–S131. 2018. View Article : Google Scholar
|
|
81
|
Chen CH, Yang CC, Yeh YH, Chou DA and Nien
CK: Reappraisal of endosonography of ampullary tumors: Correlation
with transabdominal sonography, CT, and MRI. J Clin Ultrasound.
37:18–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
De Moura DTH, Moura EGH, Bernardo WM, De
Moura ETH, Baraca FI, Kondo A, Matuguma SE and Almeida Artifon EL:
Endoscopic retrograde cholangiopancreatography versus endoscopic
ultrasound for tissue diagnosis of malignant biliary stricture:
Systematic review and meta-analysis. Endosc Ultrasound. 7:10–19.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Oppong KW, Bekkali NLH, Leeds JS, Johnson
SJ, Nayar MK, Darné A, Egan M, Bassett P and Haugk B: Fork-tip
needle biopsy versus fine-needle aspiration in endoscopic
ultrasound-guided sampling of solid pancreatic masses: A randomized
crossover study. Endoscopy. 52:454–461. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yeo SJ, Cho CM, Jung MK, Seo AN and Bae
HI: Comparison of the diagnostic performances of same-session
endoscopic ultrasound- and endoscopic retrograde
cholangiopancreatography-guided tissue sampling for suspected
biliary strictures at different primary tumor sites. Korean J
Gastroenterol. 73:213–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jo JH, Cho CM, Jun JH, Chung MJ, Kim TH,
Seo DW, Kim J and Park DH; Research Group for Endoscopic
Ultrasonography in KSGE, : Same-session endoscopic
ultrasound-guided fine needle aspiration and endoscopic retrograde
cholangiopancreatography-based tissue sampling in suspected
malignant biliary obstruction: A multicenter experience. J
Gastroenterol Hepatol. 34:799–805. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
De Moura DTH, Ryou M, De Moura EGH,
Ribeiro IB, Bernardo WM and Thompson CC: Endoscopic
ultrasound-guided fine needle aspiration and endoscopic retrograde
cholangiopancreatography-based tissue sampling in suspected
malignant biliary strictures: A meta-analysis of same-session
procedures. Clin Endosc. 53:417–428. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Raman SP and Fishman EK: Abnormalities of
the distal common bile duct and ampulla: Diagnostic approach and
differential diagnosis using multiplanar reformations and 3D
imaging. AJR Am J Roentgenol. 203:17–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hashemzadeh S, Mehrafsa B, Kakaei F,
Javadrashid R, Golshan R, Seifar F, Hajibonabi F and Salmannezhad
Khorami F: Diagnostic accuracy of a 64-slice multi-detector CT scan
in the preoperative evaluation of periampullary neoplasms. J Clin
Med. 7:912018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liang H, Zhou Y, Zheng Q, Yan G, Liao H,
Du S, Zhang X, Lv F, Zhang Z and Li YM: Dual-energy CT with virtual
monoenergetic images and iodine maps improves tumor conspicuity in
patients with pancreatic ductal adenocarcinoma. Insights Imaging.
13:1532022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wang FB, Ni JM, Zhang ZY, Zhang L, Wu WJ,
Wang D, Ji Y and Gong L: Differential diagnosis of periampullary
carcinomas: Comparison of CT with negative-contrast CT
cholangiopancreatography versus MRI with MR
cholangiopancreatography. Abdom Imaging. 39:506–517. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li B, Zhang L, Zhang ZY, Ni JM, Lu FQ, Wu
WJ and Jiang CJ: Differentiation of noncalculous periampullary
obstruction: Comparison of CT with negative-contrast CT
cholangiopancreatography versus MRI with MR
cholangiopancreatography. Eur Radiol. 25:391–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao DZ, Guo Y, Sun YP, Liu HM, Zhang Z,
Ma QL, Wang YS and Chen CL: Multi-detector spiral CT diagnosis of
common bile duct ampullary carcinoma. Eur Rev Med Pharmacol Sci.
21:3549–3553. 2017.PubMed/NCBI
|
|
93
|
Jang SK, Kim JH, Joo I, Jeon JH, Shin KS,
Han JK and Choi BI: Differential diagnosis of pancreatic cancer
from other solid tumours arising from the periampullary area on
MDCT. Eur Radiol. 25:2880–2888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Angthong W, Jiarakoop K and Tangtiang K:
Differentiation of benign and malignant ampullary obstruction by
multi-row detector CT. Jpn J Radiol. 36:477–488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee JE, Choi SY, Lee MH, Lim S, Min JH,
Hwang JA, Lee S and Kim JH: Differentiating between benign and
malignant ampullary strictures: A prediction model using a nomogram
based on CT imaging and clinical findings. Eur Radiol.
32:7566–7577. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lambin P, Leijenaar RTH, Deist TM,
Peerlings J, De Jong EEC, Van Timmeren J, Sanduleanu S, Larue RTHM,
Even AJG, Jochems A, et al: Radiomics: The bridge between medical
imaging and personalized medicine. Nat Rev Clin Oncol. 14:749–762.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lim CY, Min JH, Hwang JA, Choi SY and Ko
SE: Assessment of main pancreatic duct cutoff with dilatation, but
without visible pancreatic focal lesion on MDCT: A novel diagnostic
approach for malignant stricture using a CT-based nomogram. Eur
Radiol. 32:8285–8295. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jang SY, Kim JS, Baek SY, Lee HA and Lee
JK: Proposed nomogram predicting neoplastic ampullary obstruction
in patients with a suspected ampulla of Vater lesion on CT. Abdom
Radiol (NY). 46:3128–3138. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lu J, Hu D, Tang H, Hu X, Shen Y, Li Z,
Peng Y and Kamel I: Assessment of tumor heterogeneity:
Differentiation of periampullary neoplasms based on CT whole-lesion
histogram analysis. Eur J Radiol. 115:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Radojkovic M, Stojanovic M, Radojković D,
Jeremic L, Mihailovic D and Ilic I: Histopathologic differentiation
as a prognostic factor in patients with carcinoma of the
hepatopancreatic ampulla of Vater. J Int Med Res. 46:4634–4639.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zimmermann C, Wolk S, Aust DE, Meier F,
Saeger HD, Ehehalt F, Weitz J, Welsch T and Distler M: The
pathohistological subtype strongly predicts survival in patients
with ampullary carcinoma. Sci Rep. 9:126762019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ivanovic AM, Alessandrino F, Maksimovic R,
Micev M, Ostojic S, Gore RM and Mortele KJ: Pathologic subtypes of
ampullary adenocarcinoma: Value of ampullary MDCT for noninvasive
preoperative differentiation. AJR Am J Roentgenol. 208:W71–W78.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bi L, Yang L, Ma J, Cai S, Li L, Huang C,
Xu J, Wang X and Huang M: Dynamic contract-enhanced CT-based
radiomics for differentiation of pancreatobiliary-type and
intestinal-type periampullary carcinomas. Clin Radiol. 77:e75–e83.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen WX, Xie QG, Zhang WF, Zhang X, Hu TT,
Xu P and Gu ZY: Multiple imaging techniques in the diagnosis of
ampullary carcinoma. Hepatobiliary Pancreat Dis Int. 7:649–653.
2008.PubMed/NCBI
|
|
105
|
Bi L, Dong Y, Jing C, Wu Q, Xiu J, Cai S,
Huang Z, Zhang J, Han X, Liu Q and Lv S: Differentiation of
pancreatobiliary-type from intestinal-type periampullary carcinomas
using 3.0T MRI. J Magn Reson Imaging. 43:877–886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Nalbant MO, Inci E, Akinci O, Aygan S,
Gulturk U and Boluk Gulsever A: Evaluation of imaging findings of
pancreatobiliary and intestinal type periampullary carcinomas with
3.0T MRI. Acad Radiol. 30:1846–1855. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Dillman JR, Patel RM, Lin TK, Towbin AJ
and Trout AT: Diagnostic performance of magnetic resonance
cholangiopancreatography (MRCP) versus endoscopic retrograde
cholangiopancreatography (ERCP) in the pediatric population: A
clinical effectiveness study. Abdom Radiol (NY). 44:2377–2383.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Park MS, Kim TK, Kim KW, Park SW, Lee JK,
Kim JS, Lee JH, Kim KA, Kim AY, Kim PN, et al: Differentiation of
extrahepatic bile duct cholangiocarcinoma from benign stricture:
Findings at MRCP versus ERCP. Radiology. 233:234–240. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Suthar M, Purohit S, Bhargav V and Goyal
P: Role of MRCP in differentiation of benign and malignant causes
of biliary obstruction. J Clin Diagn Res. 9:TC08–TC012.
2015.PubMed/NCBI
|
|
110
|
Wang GX, Ge XD, Zhang D, Chen HL, Zhang QC
and Wen L: MRCP combined with CT promotes the differentiation of
benign and malignant distal bile duct strictures. Front Oncol.
11:6838692021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu DS, Chen WX, Wang XD, Acharya R and
Jiang XH: Pancreaticobiliary duct changes of periampullary
carcinomas: Quantitative analysis at MR imaging. Eur J Radiol.
81:2112–2117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang L, Sanagapalli S and Stoita A:
Challenges in diagnosis of pancreatic cancer. World J
Gastroenterol. 24:2047–2060. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang S, Liu X and Zhao J, Liu Y, Liu S,
Liu Y and Zhao J: Computer auxiliary diagnosis technique of
detecting cholangiocarcinoma based on medical imaging: A review.
Comput Methods Programs Biomed. 208:1062652021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Dusunceli Atman E, Erden A, Ustuner E,
Uzun C and Bektas M: MRI findings of intrinsic and extrinsic
duodenal abnormalities and variations. Korean J Radiol.
16:1240–1252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kim JH, Kim MJ, Chung JJ, Lee WJ, Yoo HS
and Lee JT: Differential diagnosis of periampullary carcinomas at
MR imaging. Radiographics. 22:1335–1352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Baiu I and Visser B: Endoscopic retrograde
cholangiopancreatography. JAMA. 320:20502018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nakagawa K, Sho M, Fujishiro M, Kakushima
N, Horimatsu T, Okada KI, Iguchi M, Uraoka T, Kato M, Yamamoto Y,
et al: Clinical practice guidelines for duodenal cancer 2021. J
Gastroenterol. 57:927–941. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yamamoto K, Itoi T, Sofuni A, Tsuchiya T,
Tanaka R, Tonozuka R, Honjo M, Mukai S, Fujita M, Asai Y, et al:
Expanding the indication of endoscopic papillectomy for T1a
ampullary carcinoma. Dig Endosc. 31:188–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hanada K, Shimizu A, Kurihara K, Ikeda M,
Yamamoto T, Okuda Y and Tazuma S: Endoscopic approach in the
diagnosis of high-grade pancreatic intraepithelial neoplasia. Dig
Endosc. 34:927–937. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ergul N, Gundogan C, Tozlu M, Toprak H,
Kadıoglu H, Aydin M and Cermik TF: Role of (18)F-fluorodeoxyglucose
positron emission tomography/computed tomography in diagnosis and
management of pancreatic cancer; comparison with multidetector row
computed tomography, magnetic resonance imaging and endoscopic
ultrasonography. Rev Esp Med Nucl Imagen Mol. 33:159–164.
2014.PubMed/NCBI
|
|
121
|
Yeh R, Dercle L, Garg I, Wang ZJ, Hough DM
and Goenka AH: The role of 18F-FDG PET/CT and PET/MRI in pancreatic
ductal adenocarcinoma. Abdom Radiol (NY). 43:415–434. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Pu Y, Wang C, Zhao S, Xie R, Zhao L, Li K,
Yang C, Zhang R, Tian Y, Tan L, et al: The clinical application of
18F-FDG PET/CT in pancreatic cancer: A narrative review.
Transl Cancer Res. 10:3560–3575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wolske KM, Ponnatapura J, Kolokythas O,
Burke LMB, Tappouni R and Lalwani N: Chronic pancreatitis or
pancreatic tumor? A problem-solving approach. Radiographics.
39:1965–1982. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Krishnaraju VS, Kumar R, Mittal BR, Sharma
V, Singh H, Nada R, Bal A, Rohilla M, Singh H and Rana SS:
Differentiating benign and malignant pancreatic masses: Ga-68 PSMA
PET/CT as a new diagnostic avenue. Eur Radiol. 31:2199–2208. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ruan Z, Jiao J, Min D, Qu J, Li J, Chen J,
Li Q and Wang C: Multi-modality imaging features distinguish
pancreatic carcinoma from mass-forming chronic pancreatitis of the
pancreatic head. Oncol Lett. 15:9735–9744. 2018.PubMed/NCBI
|
|
126
|
Gnanasegaran G, Agrawal K and Wan S:
18F-Fluorodeoxyglucose-PET-computerized tomography and
non-fluorodeoxyglucose PET-computerized tomography in hepatobiliary
and pancreatic malignancies. PET Clin. 17:369–388. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Michalski CW, Liu B, Heckler M, Roth S,
Sun H, Heger U, Büchler MW and Hackert T: Underutilization of
surgery in periampullary cancer treatment. J Gastrointest Surg.
23:959–965. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang JZ, Yang CX, Gao S, Bu JF, Li QQ,
Wang HL, Yang KN, Tong SS, Qian LJ, Zhang J, et al:
Three-dimensional visualization and evaluation of hilar
cholangiocarcinoma resectability and proposal of a new
classification. World J Surg Oncol. 21:2392023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lin C, Gao J, Zheng H, Zhao J, Yang H, Lin
G, Li H, Pan H, Liao Q and Zhao Y: Three-dimensional visualization
technology used in pancreatic surgery: A valuable tool for surgical
trainees. J Gastrointest Surg. 24:866–873. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Rozenblatt-Rosen O, Regev A, Oberdoerffer
P, Nawy T, Hupalowska A, Rood JE, Ashenberg O, Cerami E, Coffey RJ,
Demir E, et al: The human tumor atlas network: Charting tumor
transitions across space and time at single-cell resolution. Cell.
181:236–249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fang C, An J, Bruno A, Cai X, Fan J,
Fujimoto J, Golfieri R, Hao X, Jiang H, Jiao LR, et al: Consensus
recommendations of three-dimensional visualization for diagnosis
and management of liver diseases. Hepatol Int. 14:437–453. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Higuchi K, Kaise M, Noda H, Kirita K,
Koizumi E, Umeda T, Akimoto T, Omori J, Akimoto N, Goto O, et al:
Three-dimensional visualization improves the endoscopic diagnosis
of superficial gastric neoplasia. BMC Gastroenterol. 21:2422021.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ahmed TM, Rowe SP, Fishman EK, Soyer P and
Chu LC: Three-dimensional CT cinematic rendering of adrenal masses:
Role in tumor analysis and management. Diagn Interv Imaging.
105:5–14. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Almagro J, Messal HA, Zaw Thin M, van
Rheenen J and Behrens A: Tissue clearing to examine tumour
complexity in three dimensions. Nat Rev Cancer. 21:718–730. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kang SH, Won Y, Lee K, Youn SI, Min SH,
Park YS, Ahn SH and Kim HH: Three-dimensional (3D) visualization
provides better outcome than two-dimensional (2D) visualization in
single-port laparoscopic distal gastrectomy: A propensity-matched
analysis. Langenbecks Arch Surg. 406:473–478. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jang W, Song JS, Kim SH and Yang JD:
Comparison of compressed sensing and gradient and spin-echo in
breath-hold 3D MR cholangiopancreatography: Qualitative and
quantitative analysis. Diagnostics (Basel). 11:6342021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen Z, Xue Y, Wu Y, Duan Q, Zheng E, He
Y, Li G, Song Y and Sun B: Feasibility of 3D breath-hold MR
cholangiopancreatography with a spatially selective radiofrequency
excitation pulse: Prospective comparison with parallel imaging
technique and compressed sensing method. Acad Radiol. 29:e289–e295.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Huber T, Huettl F, Tripke V, Baumgart J
and Lang H: Experiences with three-dimensional printing in complex
liver surgery. Ann Surg. 273:e26–e27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang J, Fang CH, Fan YF, Xiang N, Liu J,
Zhu W, Bao SS and Wang HZ: To assess the benefits of medical image
three-dimensional visualization system assisted
pancreaticoduodenctomy for patients with hepatic artery variance.
Int J Med Robot. 10:410–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Miyamoto R, Oshiro Y, Nakayama K and
Ohkohchi N: Impact of three-dimensional surgical simulation on
pancreatic surgery. Gastrointest Tumors. 4:84–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Miyamoto R, Oshiro Y, Sano N, Inagawa S
and Ohkohchi N: Three-dimensional remnant pancreatic volumetry
predicts postoperative pancreatic fistula in pancreatic cancer
patients after pancreaticoduodenectomy. Gastrointest Tumors.
5:90–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Javed AA, Young RWC, Habib JR,
Kinny-Köster B, Cohen SM, Fishman EK and Wolfgang CL: Cinematic
rendering: Novel tool for improving pancreatic cancer surgical
planning. Curr Probl Diagn Radiol. 51:878–883. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Barat M, Pellat A, Terris B, Dohan A,
Coriat R, Fishman EK, Rowe SP, Chu L and Soyer P: Cinematic
rendering of gastrointestinal stromal tumours: A review of current
possibilities and future developments. Can Assoc Radiol J.
75:359–368. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cai W, Fan Y, Hu H, Xiang N, Fang C and
Jia F: Postoperative liver volume was accurately predicted by a
medical image three dimensional visualization system in hepatectomy
for liver cancer. Surg Oncol. 26:188–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Mise Y, Hasegawa K and Kokudo N: Response
to Comment on ‘the virtual hepatectomy changed the practice of
liver surgery: More details, more significance’. Ann Surg.
270:e332019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Allan A, Kealley C, Squelch A, Wong YH,
Yeong CH and Sun Z: Patient-specific 3D printed model of biliary
ducts with congenital cyst. Quant Imaging Med Surg. 9:86–93. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Bati AH, Guler E, Ozer MA, Govsa F,
Erozkan K, Vatansever S, Ersin MS, Elmas ZN and Harman M: Surgical
planning with patient-specific three-dimensional printed
pancreaticobiliary disease models-cross-sectional study. Int J
Surg. 80:175–183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mise Y, Hasegawa K, Satou S, Shindoh J,
Miki K, Akamatsu N, Arita J, Kaneko J, Sakamoto Y and Kokudo N: How
has virtual hepatectomy changed the practice of liver surgery?:
Experience of 1194 virtual hepatectomy before liver resection and
living donor liver transplantation. Ann Surg. 268:127–133. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Uchida M: Recent advances in 3D computed
tomography techniques for simulation and navigation in
hepatobiliary pancreatic surgery. J Hepatobiliary Pancreat Sci.
21:239–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Liu Y, Wang Q, Du B, Wang X, Xue Q and Gao
W: A meta-analysis of the three-dimensional reconstruction
visualization technology for hepatectomy. Asian J Surg. 46:669–676.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhang J, Guo X, Wang H, Zhang J, Liu P,
Qiao Q and Wang X: The application of three-dimensional
visualization in preoperative evaluation of portal vein invasion in
hilar cholangiocarcinoma. Cancer Manag Res. 12:9297–9302. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Guo Q, Chen J, Pu T, Zhao Y, Xie K, Geng X
and Liu F: The value of three-dimensional visualization techniques
in hepatectomy for complicated hepatolithiasis: A propensity score
matching study. Asian J Surg. 46:767–773. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Zhao D, Lau WY, Zhou W, Yang J, Xiang N,
Zeng N, Liu J, Zhu W and Fang C: Impact of three-dimensional
visualization technology on surgical strategies in complex hepatic
cancer. Biosci Trends. 12:476–483. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Song JS, Kim SH, Kuehn B and Paek MY:
Optimized breath-hold compressed-sensing 3D MR
cholangiopancreatography at 3T: Image quality analysis and clinical
feasibility assessment. Diagnostics (Basel). 10:3762020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Swaraj S, Mohapatra M, Sathpathy G,
Yalamanchi R, Sen K, Menon SM, Madhesia A, Kumaraswamy SM, Radha
Krishna K and Bobde DV: Diagnostic performance of ultrasonography
versus magnetic resonance cholangiopancreatography in biliary
obstruction. Cureus. 15:e339152023.PubMed/NCBI
|
|
156
|
Reddy S CT, Mohan VSK, Jeepalem SM,
Manthri R, Kalawat T, Reddy V V, Hulikal N and Devi B VL: Utility
of 18F-FDG PET/CT in management of pancreatic and periampullary
masses-prospective study from a tertiary care center. Indian J Surg
Oncol. 13:288–298. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wen G, Gu J, Zhou W, Wang L, Tian Y, Dong
Y, Fu L and Wu H: Benefits of 18F-FDG PET/CT for the
preoperative characterisation or staging of disease in the
ampullary and duodenal papillary. Eur Radiol. 30:5089–5098. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Deng S, Luo J, Ouyang Y, Xie J, He Z,
Huang B, Bai F, Xiao K, Yin B, Wang J, et al: Application analysis
of omental flap isolation and modified pancreaticojejunostomy in
pancreaticoduodenectomy (175 cases). BMC Surg. 22:1272022.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gugenheim J, Crovetto A and Petrucciani N:
Neoadjuvant therapy for pancreatic cancer. Updates Surg. 74:35–42.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Meijer LL, Alberga AJ, de Bakker JK, van
der Vliet HJ, Le Large TYS, van Grieken NCT, de Vries R, Daams F,
Zonderhuis BM and Kazemier G: Outcomes and treatment options for
duodenal adenocarcinoma: A systematic review and meta-analysis. Ann
Surg Oncol. 25:2681–2692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Gössling GCL, Zhen DB, Pillarisetty VG and
Chiorean EG: Combination immunotherapy for pancreatic cancer:
Challenges and future considerations. Expert Rev Clin Immunol.
18:1173–1186. 2022. View Article : Google Scholar : PubMed/NCBI
|