Introduction
Multiple myeloma (MM) is characterized by a
proliferation of plasma cells (PCs) with a strong dependence on the
bone marrow (BM) microenvironment. However, in up to one-third of
patients with MM, the PC proliferation can escape the cellular
microenvironment influences resulting in soft-tissue plasmacytomas.
The most frequent mechanism resulting in soft-tissue plasmacytomas
is direct growth from skeletal tumors by disruption of the cortical
bone, while the remainder result from hematogenous dissemination
without involving bony structures (1). Extramedullary involvement or
extramedullary disease (EMD) is an aggressive form of multiple
myeloma (MM) and it serves as an independent adverse prognostic
indicator for MM (2). According to
the timing of its occurrence, EMD can be categorized into the
following two subtypes: i) Primary EMD (pEMD), which occurs
concurrently with the onset of MM; and ii) secondary EMD (sEMD),
which occurs in an advanced stage of MM, after relapse or
progression (3). The prognosis for
EMD is poor, with the median overall survival for patients
undergoing extramedullary relapse being <6 months (4). At present, there is an absence of a
stratified prognostic system or an ideal treatment strategy backed
by evidence-based medical research, as they do not meet clinical
requirements. Therefore, it is difficult to recommend one treatment
strategy over another (2). The
present study reports the case of a patient with extramedullary
plasmacytoma at progression, who was treated with a CV-MED regimen
(chidamide, bortezomib, mitoxantrone hydrochloride liposome,
etoposide and dexamethasone). This protocol may be a promising
therapy for obtaining an early response in patients with EMD.
Case report
In February 2023, a 59-year-old female patient was
referred to Peking University Third Hospital (Beijing, China) due
to intermittent bone pain for the past year. The patient reported
that the pain was primarily concentrated in the lower and middle
sternum, bilateral costal areas, and thoracic and lumbar vertebrae,
and worsened after physical activity. Although the patient had been
treated with zoledronic acid and calcium carbonate, the bone pain
persisted and progressively worsened, which limited mobility.
Following the patient's admission to the Peking University Third
Hospital, laboratory tests demonstrated the following: White blood
count (WBC), 6.15×109/l (normal range,
4.0–10.0×109/l); hemoglobin (Hb) level, 91 g/l (normal
range, 110–160 g/l); platelet (PLT) count, 134×109/l
(normal range, 100–300×109/l); calcium level, 2.69
mmol/l (normal range, 2.2–2.65 mmol/l); creatinine level, 103
µmol/l (normal range, 60–110 µmol/l); albumin level, 37 g/l (normal
range, 40–55 g/l); total protein level, 64.9 g/l (normal range,
65–85 g/l); and β2-microglobulin level, 13.09 ng/ml (normal range,
1–3 ng/ml). Furthermore, electrophoresis of serum immune-fixation
(agarose gel electrophoresis; fully automated Hydrasys System;
Sebia) demonstrated a light chain monoclonal band indicative of M
protein (11.3%; 7.3 g; Table I).
Quantitative analysis of serum light chains demonstrated elevated λ
chain levels (993 mg/dl; normal range, 269–638 mg/dl) and reduced κ
chain levels (190 mg/dl; normal range, 574–1276 mg/dl).
Additionally, 24-h urine light chain analysis showed elevated λ
chain levels of 33 mg/dl (normal range, <5 mg/dl) and κ chain
levels of <1.85 mg/dl (normal range, <1.85 mg/dl). Finally,
serum immunoglobulin analysis demonstrated IgG, IgA, IgM and IgE
levels of 2.58 g/l (normal range, 6.94–16.18 g/l), 0.084 g/l
(normal range, 0.7–3.8 g/l), 0.08 g/l (normal range, 0.6–2.63 g/l)
and 0.21 g/l (normal range, ≤100 g/l), respectively. Positron
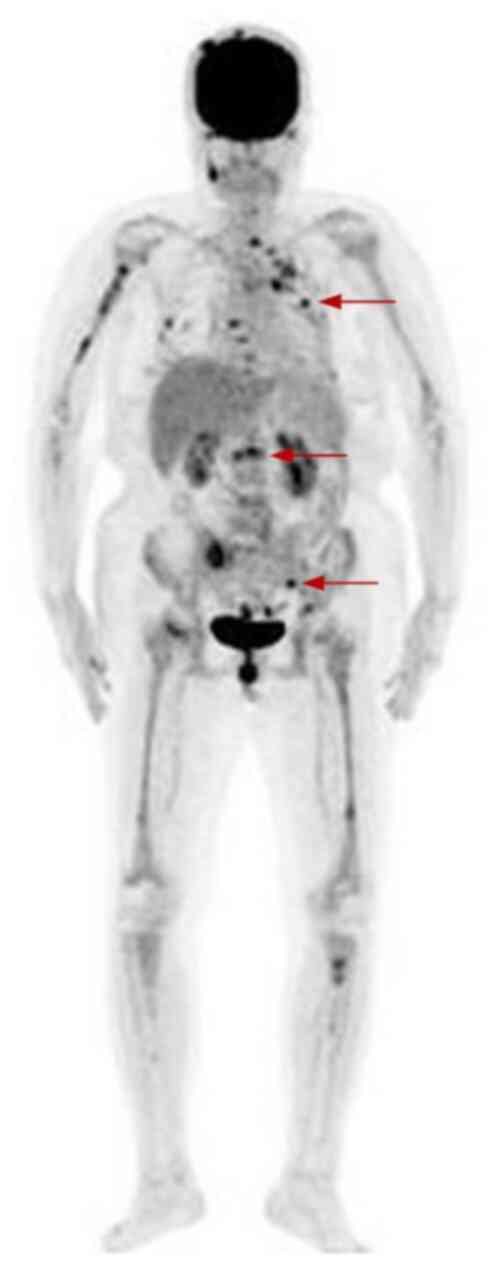
emission tomography-computed tomography (CT) scans demonstrated
notable bone erosion in the sacrum, ilium and lumbar vertebrae,
accompanied by metabolic activity (Fig.
1). Following bone marrow aspiration and biopsy,
hypocellularity with 13% plasma cells was identified, 9% of which
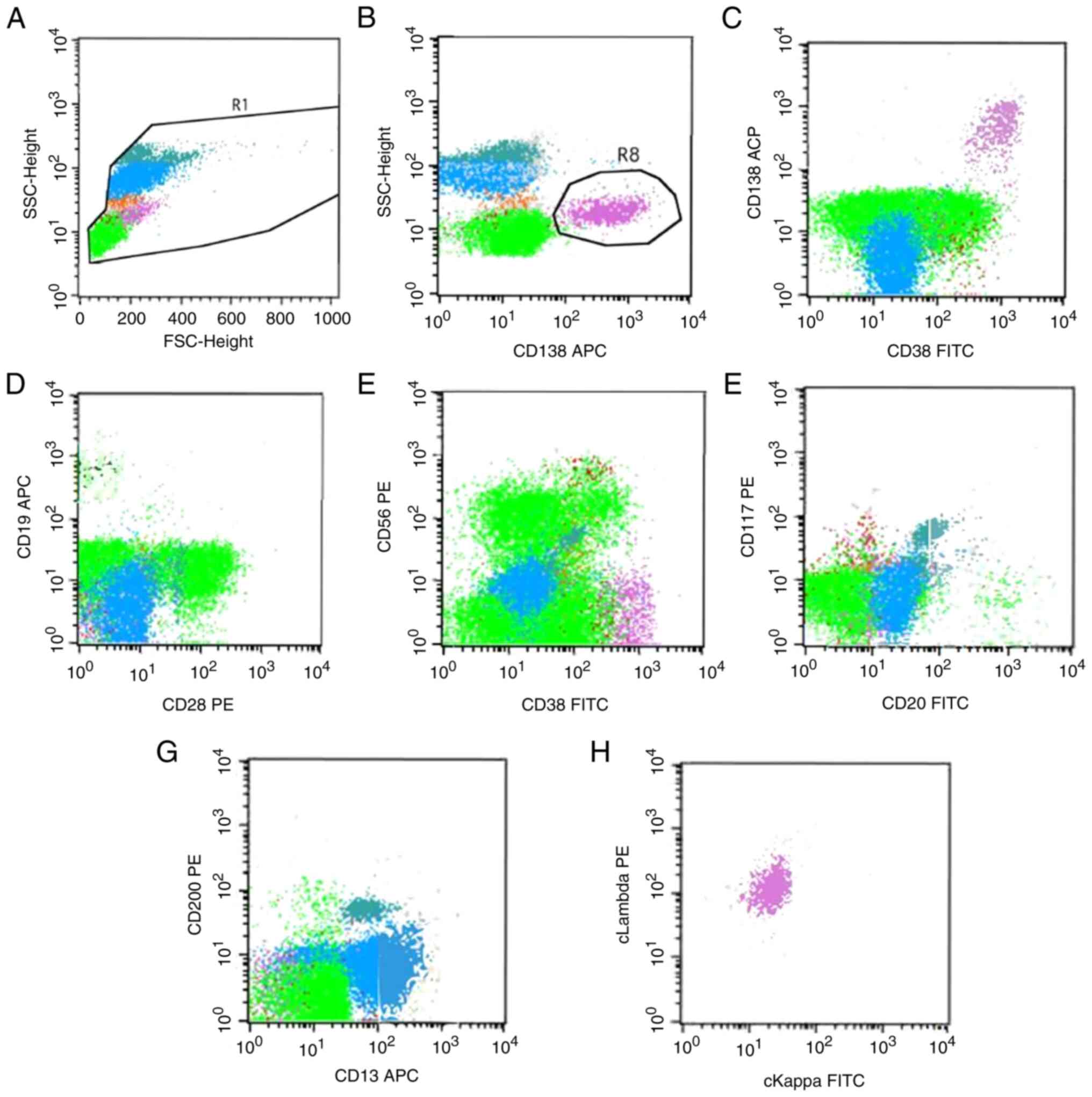
were immature (data not shown). Flow cytometric analysis showed
1.53% aberrant plasma cells (Fig.
2), and the patient was diagnosed with MM of the λ light chain
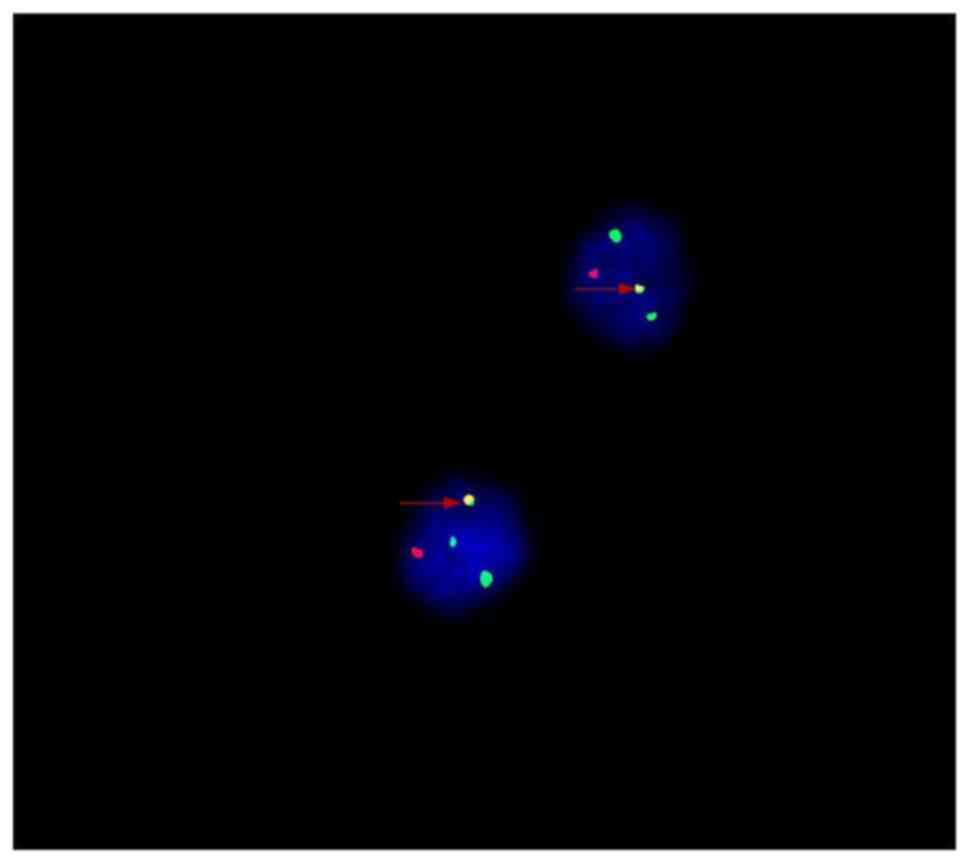
type. Furthermore, fluorescence in situ hybridization (FISH)
analysis was performed utilizing the IGH/CCND1
Translocation, Dual Fusion Probe from Cytocell on CD138-purified
interphase cells according to the manufacturer's instructions, and
the results demonstrated that ~80% of the cells (data not shown)
carried IgH-CCND1 fusion genes (Fig. 3). Chromosomal karyotype analysis
identified a t(11;14)(q13;q32) translocation. The patient was
diagnosed with stage IIa MM, according to the Durie-Salmon system
(5), stage III MM according to the
International Staging System (ISS) and stage II MM according to the
revised ISS (6).
 | Table I.Electrophoretic analysis of
immunoglobulins calculated as the serum protein type/total serum
protein ratio. |
Table I.
Electrophoretic analysis of
immunoglobulins calculated as the serum protein type/total serum
protein ratio.
| Serum protein
type | Percentage, % | Normal range, % |
|---|
| Albumin | 62.3 | 57.2–69.3 |
| α1-globulin | 3.8 | 1.2–3.2 |
| α2-globulin | 12.2 | 3.8–6.4 |
| β-globulin | 8.5 | 5.4–11.4 |
| γ-globulin | 13.2 | 13.7–22.0 |
| IgG-IF | Negative | Negative |
| IgA-IF | Negative | Negative |
| IgM-IF | Negative | Negative |
| κ-IF | Negative | Negative |
| λ-IF | Monoclonal
band-positive | Negative |
| M protein | 11.3 | None |
Along with supportive treatment (such as
gastroprotective agents and calcium additives), the patient was
treated between March and May 2023 with two cycles of VRD (2.0 mg
bortezomib on days 1, 4, 8 and 11; 40 mg dexamethasone on days 1,
4, 8 and 11; and 25 mg lenalidomide on days 1–21; 28-day cycle) and
100 mg/day aspirin to prevent thromboembolism. Upon reevaluation of
the bone marrow, complete remission was achieved in terms of
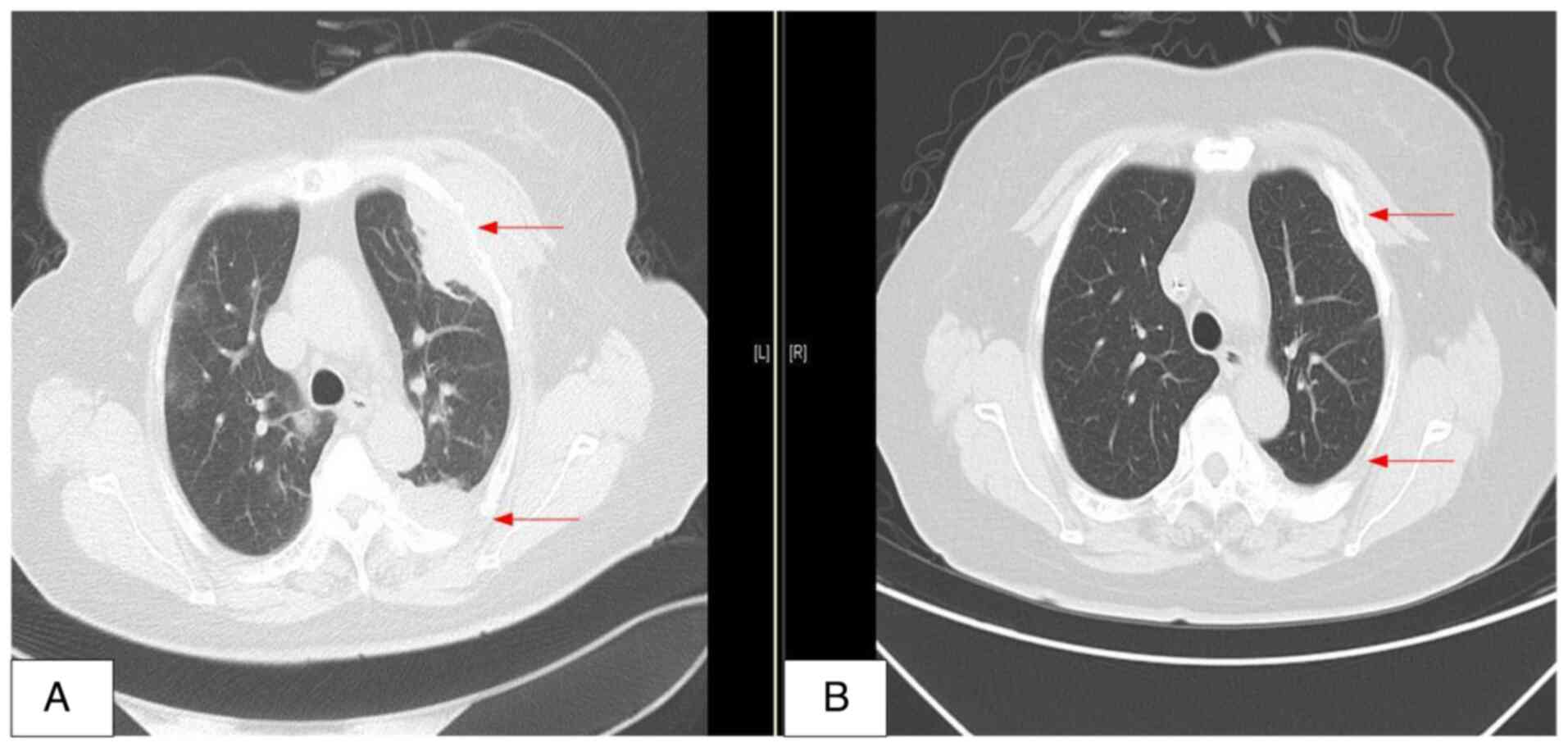
morphology, immunology and cytogenetics. However, a chest CT scan
demonstrated a mass in the left chest wall (Fig. 4A) and a CT-guided needle biopsy was
performed on the extramedullary mass located beneath the pleura.
Immunohistochemical analysis by the Department of Pathology
according to standard procedures demonstrated the following
results: CD20(−), CD3(−), Ki-67(+, 60%), CD138(+), CD38(+),
MUM1(+), CD56(−), CyclinD1(+), κ(−) and λ(+), which verified the
diagnosis of EMD (Fig. 5).
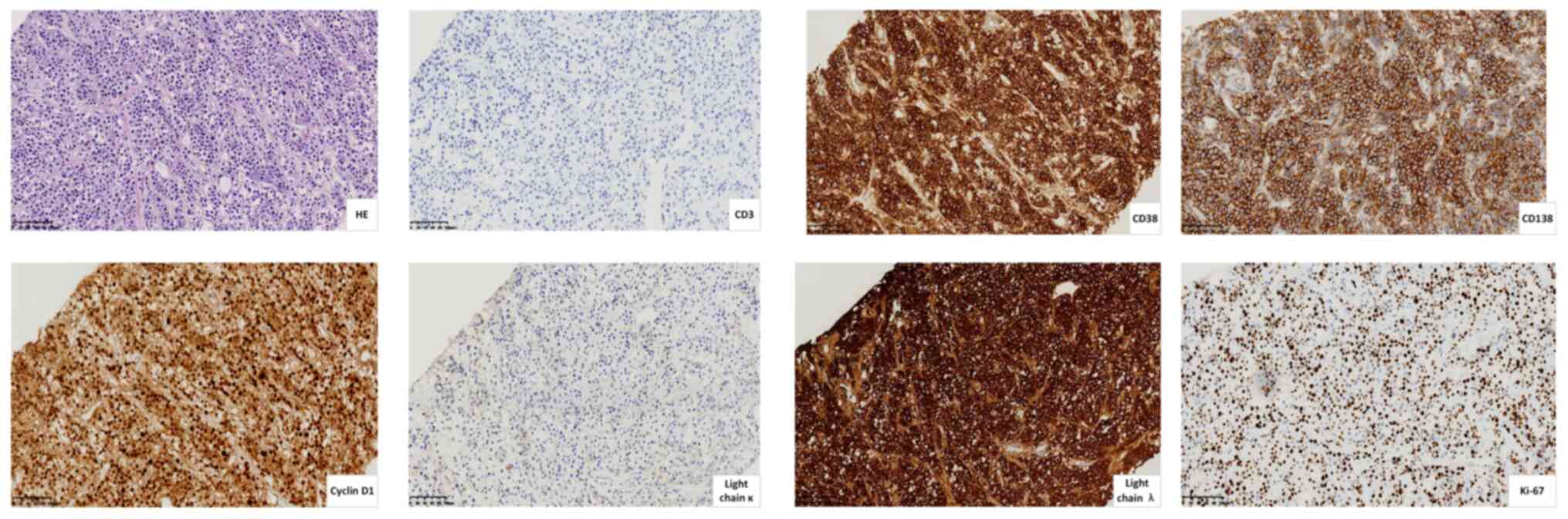 | Figure 5.Immunostaining of biopsy specimens
from an extramedullary mass beneath the pleura (scale bar, 100 µm).
Representative images of HE, CD3(−), CD138(+), CD38(+), Cyclin
D1(+), light chain κ(−), light chain λ(+) and Ki-67(+, 60%)
staining. +, positive; -, negative; HE, hematoxylin and eosin. |
As the patient was diagnosed with sEMD, treatment
with three cycles of the CV-MED chemotherapy regimen was
administered. Each cycle of CV-MED therapy consisted of 10 mg
chidamide three times a week on days 1–21, 1.3 mg/m2
bortezomib on days 1, 4, 8 and 11, 12 mg/m2 mitoxantrone
hydrochloride liposome on day 1, 50 mg/m2 etoposide on
days 1–3 and 20 mg dexamethasone on days 1, 4, 8 and 11 of a 28-day
cycle. The most severe adverse event was hematological toxicity,
and the patient developed febrile and grade IV myelosuppression,
with a WBC count of 0.7×109/l, an Hb level of 105 g/l
and a PLT count of 24×109/l. The symptoms resolved after
systemic treatment (such as platelet transfusions, rehydration
therapy and intravenous antibiotics). No obvious mucositis,
neuropathy or other complications were observed. After three cycles
of CV-MED chemotherapy, further chest CT scans demonstrated that
the mass in the left chest wall was notably reduced in size, thus
demonstrating the beneficial effects of the CV-MED regimen
(Fig. 4B). Following autologous
hematopoietic stem cell harvesting and a maintenance regimen
incorporating daratumumab, the patient has been followed up at
intervals of 1–2 months. To date, there has been no recurrence of
the extramedullary lesion.
Discussion
The incidence of sEMD ranges from 6–20%, and
resistance to chemotherapy and poor patient prognosis has been
reported (7). He et al
(8) reported that multiple
extramedullary bone-related and/or extramedullary extraosseous
lesions are independent factors for a poor prognosis in patients
with MM. Even in the era of novel drugs, there are currently no
universally accepted international or domestic guidelines for sEMD.
To the best of our knowledge, no prospective therapeutic studies on
patients with sEMD have specifically been reported. Consequently,
it is difficult to recommend a particular treatment strategy for
EMD over another. Nonetheless, EMD is regarded as a high-risk
disease and aggressive treatment approaches, if feasible, are
typically recommended (1).
Since a large proportion of patients with EMD would
have already received bortezomib-based front-line therapy, which is
commonly combined with immunomodulatory drugs (IMiDs), effective
treatment strategies for relapse commonly include lymphoma-like
regimens, such as cisplatin, doxorubicin, cyclophosphamide and
etoposide (PACE), dexamethasone, cyclophosphamide, etoposide and
cisplatin (DCEP), or dexamethasone, carmustine, etoposide,
doxorubicin or 1iposomal doxorubicin and melphalan (Dexa-BEAM)
(9). Alkylating agents and
cytotoxic therapies are potent frontline treatments for
extramedullary involvement in MM, especially for para-skeletal (PS)
involvement. Wu et al (10)
reported a partial remission rate of 50.0% with plasmacytomas in
patients who received initial conventional therapy. According to
the Mayo Stratification for Myeloma and Risk-Adapted Therapy
guidelines, patients with MM who are eligible for autologous stem
cell transplant with EMD are recommended to undergo two cycles of
VDT-PACE therapy followed by sequential autologous hematopoietic
stem cell transplantation (11).
Doxorubicin is recommended as a first-line therapy
for patients with MM and is also widely used to treat
extramedullary plasmacytoma (EMP) (2). A review of 40 patients with relapsed
and refractory MM, who were treated with a median of 5 courses
(range, 3–9 courses) of liposomal doxorubicin chemotherapy after
developing sEMD, showed a short-term efficacy of 68.0 and 46.7% in
the PS plasmacytoma and EMD groups, respectively (12). Mitoxantrone hydrochloride liposomes
belong to the anthracene class of antitumor compounds, which is
similar to liposomal doxorubicin. The high accumulation of the
liposomes in tumor tissues is considered a key characteristic in
preclinical investigations (13).
Previous clinical trials have shown promising results for
mitoxantrone in patients with relapsed and refractory peripheral
T-cell lymphoma (14) and solid
tumors (15). Therefore,
mitoxantrone hydrochloride liposomes were selected as a substitute
for doxorubicin in the present study due to their high accumulation
in tumor tissues in lymphoma, resulting in the establishment of the
MED-based regimen.
According to a pilot study presented at the 65th
American Society of Hematology meeting, 15 patients with EMPs
underwent a median of two cycles of liposomal mitoxantrone-based
therapy. The specific regimens administered included the following:
The mitoxantrone hydrochloride liposome-bortezomib-dexamethasone
(MVD) triplet regimen (3/15 patients; 20.0%), the MVD plus
lenalidomide regimen (M-VRD) (3/15 patients; 20.0%), the MVD plus
IMiDs and etoposide regimen (6/15 patients; 40.0%), and the MVD
plus other cytotoxic agents (cyclophosphamide or cisplatin) regimen
(3/15 patients; 20.0%). The overall response rate for EMP was
53.3%. The study showed promising results regarding the early
response and efficacy of the MVD-based regimen in MM with EMP
(16). Additionally, previous in
vitro research has shown that chidamide could have antimyeloma
effects by inhibiting the proliferation and invasion of MM cells,
as well as by inducing apoptosis (17). Clinically, the successful treatment
of relapsed or refractory MM with a regimen of chidamide,
bortezomib and dexamethasone has been previously reported (18). For the present study, it was thus
sought to integrate chidamide with an MVD-based protocol, and the
CV-MED regimen was ultimately selected as the preferred treatment
approach. Moreover, due to the significant hematological toxicity
associated with the chemotherapy protocol, and the development of
grade IV myelosuppression and febrile neutropenia after treatment,
it was anticipated that the patient might not tolerate weekly
administrations of the CD38 monoclonal antibody (mAb). As a result,
the chemotherapy regimen temporarily excluded the CD38 mAbs.
In conclusion, the present case report indicated
that a chemotherapy regimen based on mitoxantrone hydrochloride
liposomes, bortezomib and chidamide could be an effective approach
for treating patients with EMD. However, further studies are
required to evaluate the efficacy and safety of this treatment.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Special Fund of the
National Clinical Key Specialty Construction Program (2023;
China).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JG, XZ and FD designed the study and analyzed
patient data. HJ advised on patient treatment. JG, FD, XZ and HJ
confirm the authenticity of all the raw data. All authors agree to
be accountable for all aspects of the work. All authors read and
approved the final version of the manuscript and agreed on the
journal to which the article has been submitted.
Ethics approval and consent to
participate
The present study was approved by the Peking
University Third Hospital Medical Science Research Ethics Committee
(approval no. 002-02) and conducted according to the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosiñol L, Beksac M, Zamagni E, Van de
Donk NWCJ, Anderson KC, Badros A, Caers J, Cavo M, Dimopoulos MA,
Dispenzieri A, et al: Expert review on soft-tissue plasmacytomas in
multiple myeloma: Definition, disease assessment and treatment
considerations. Br J Haematol. 194:496–507. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plasma Cell Disease Group, Chinese Society
of Hematology, Chinese Medical Association; Chinese Myeloma
Committee-Chinese Hematology Association, . Consensus for the
diagnosis and management of extramedullary plasmacytoma in China
(2024). Zhonghua Xue Ye Xue Za Zhi. 45:8–17. 2024.(In Chinese).
PubMed/NCBI
|
|
3
|
Du J: Progress in diagnosis and treatment
of extramedullary plasma cell tumors. J Intern Med Concepts Pract.
15:302–307. 2020.(In Chinese).
|
|
4
|
Touzeau C and Moreau P: How I treat
extramedullary myeloma. Blood. 127:971–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma. Correlation of measured myeloma cell
mass with presenting clinical features, response to treatment, and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jagosky MH and Usmani SZ: Extramedullary
disease in multiple myeloma. Curr Hematol Malig Rep. 15:62–71.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He J, Yue X, He D, Zhao Y, Yang Y, Zheng
G, Zhang E, Han X, Wu W, Yang L, et al: Multiple
extramedullary-bone related and/or extramedullary extraosseous are
independent poor prognostic factors in patients with newly
diagnosed multiple myeloma. Front Oncol. 11:6680992021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bladé J, Beksac M, Caers J, Jurczyszyn A,
von Lilienfeld-Toal M, Moreau P, Rasche L, Rosiñol L, Usmani SZ,
Zamagni E and Richardson P: Extramedullary disease in multiple
myeloma: A systematic literature review. Blood Cancer J. 12:452022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu P, Davies FE, Boyd K, Thomas K, Dines
S, Saso RM, Potter MN, Ethell ME, Shaw BE and Morgan GJ: The impact
of extramedullary disease at presentation on the outcome of
myeloma. Leuk Lymphoma. 50:230–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dingli D, Ailawadhi S, Bergsagel PL, Buadi
FK, Dispenzieri A, Fonseca R, Gertz MA, Gonsalves WI, Hayman SR,
Kapoor P, et al: Therapy for relapsed multiple myeloma: Guidelines
from the mayo stratification for myeloma and risk-adapted therapy.
Mayo Clin Proc. 92:578–598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen M, Li X, Zhang JJ, Tang R, Zhan XK,
Fan SB, Zhao FY and Huang ZX: A clinical study on the treatment of
relapsed and refractory multiple myeloma with secondary
extramedullary lesions using a liposome containing doxorubicin
regimen. Chin J Clin Oncol. 49:512–518. 2022.(In Chinese).
|
|
13
|
Gao Y, Huang H, Wang X, Bai B, Huang Y,
Yang H, Zhan Y, Li Y, Li Y, Zhou M, et al: Safety and efficacy of
mitoxantrone hydrochloride liposome in patients with relapsed or
refractory peripheral T-cell lymphoma and extranodal NK/T-cell
lymphoma: A prospective, single-arm, open-label, multi-center,
phase I Clinical trial. Blood. 136 (Suppl 1):36–37. 2020.
View Article : Google Scholar
|
|
14
|
Cai Q, Xia Y, Wang L, Huang H, Wang J, Cai
J and Tian X: Combination of mitoxantrone hydrochloride liposome
with tislelizumab in patients with relapsed or refractory NK/T cell
lymphoma: A phase Ib/I clinical trial. Blood. 142:44702023.
View Article : Google Scholar
|
|
15
|
Mei KC, Liao YP, Jiang J, Chiang M,
Khazaieli M, Liu X, Wang X, Liu Q, Chang CH, Zhang X, et al:
Liposomal delivery of mitoxantrone and a cholesteryl indoximod
prodrug provides effective chemo-immunotherapy in multiple solid
tumors. ACS Nano. 14:13343–13366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jian Y, Liu N, Zhang Y, Gao W, Chen WM,
Zhu HH and Wu Y: Mitoxantrone hydrochloride liposome, bortezomib,
and dexamethasone- based regimen in multiple myeloma patients with
extramedullary plasmacytoma: A pilot study. Blood. 142:66502023.
View Article : Google Scholar
|
|
17
|
Cao HY, Li L, Xue SL and Dai HP:
Chidamide: Targeting epigenetic regulation in the treatment of
hematological malignancy. Hematol Oncol. 41:301–309. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang DY, Cui YS, Liu YZ, Liu LN, Song YP
and Fang BJ: Successful treatment of one case with relapsed
refractory multiple myeloma by chidamide in combination with
bortezomib and dexamethasone. Zhonghua Xue Ye Xue Za Zhi.
37:4632016.(In Chinese). PubMed/NCBI
|