Introduction
Benign esophageal tumors are uncommon, accounting
for <10% of all esophageal tumors. Esophageal leiomyoma is
considered the most common benign tumor of the esophagus, and its
incidence ranges from 0.005 to 5.1% (1). Histological analysis is required for a
definitive diagnosis; however, numerous diagnostic tools, such as
endoscopy, computed tomography (CT) and 18F-fluorodeoxyglucose
positron emission tomography (18F-FDG-PET) are also used for
differentiation from other malignant tumors, such as esophageal
cancer (2). Notably, 18F-FDG-PET is
used to determine whether a mass is benign or malignant, as benign
masses may also form lesions in the esophagus and extra-esophageal
organs and may be misdiagnosed as malignant tumors. Notably, few
previous studies report on the use of 18F-FDG-PET in cases of
esophageal leiomyoma involving lesions in other organs (3). The present study describes a patient
who was considered to have esophageal cancer with adrenal
metastasis based on PET-CT findings. The esophageal lesion tissue
was surgically resected, and the results showed that it was
esophageal leiomyoma combined with adrenal adenoma. If a correct
diagnosis could have been made preoperatively, unnecessary surgical
treatment could have been avoided.
Case report
Patient
A 57-year-old male patient was referred to Jinan
Central Hospital (Jinan, China) in March 2024. During a routine
health examination, a thoracoabdominal CT scan showed a mass with a
size of ~5.1×2.8 cm in the lower segment of the esophagus.
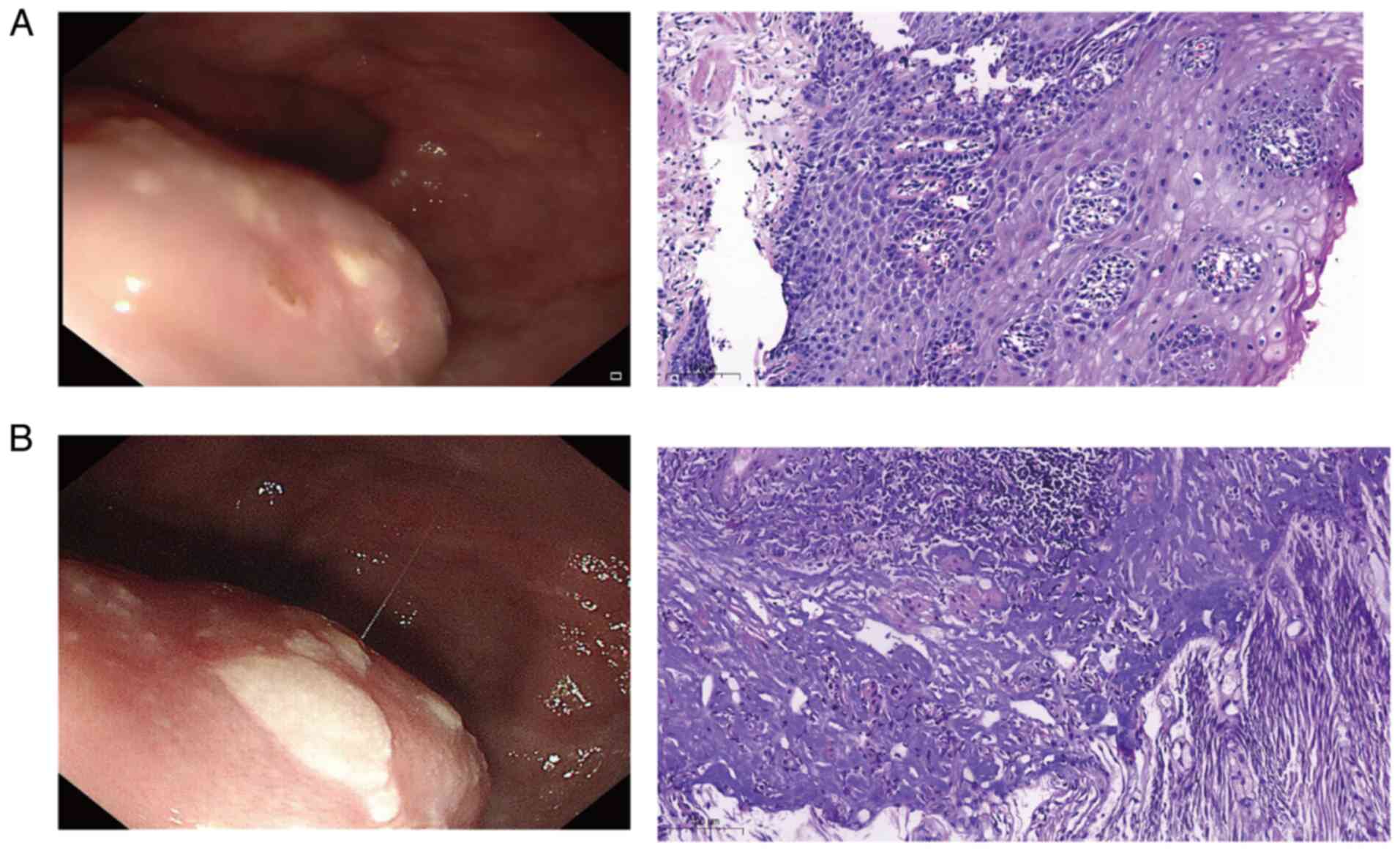
Pathological analysis of a gastroscopic biopsy revealed chronic
mucosal inflammation, squamous epithelial papillary hyperplasia and
localized granulation tissue hyperplasia in the lamina propria
(Fig. 1A), indicating a benign
result. Due to the potential for misdiagnosis using endoscopic
sampling, malignancy could not be confirmed. Thus, 18F-FDG-PET was
performed to determine whether the lower esophageal mass was benign
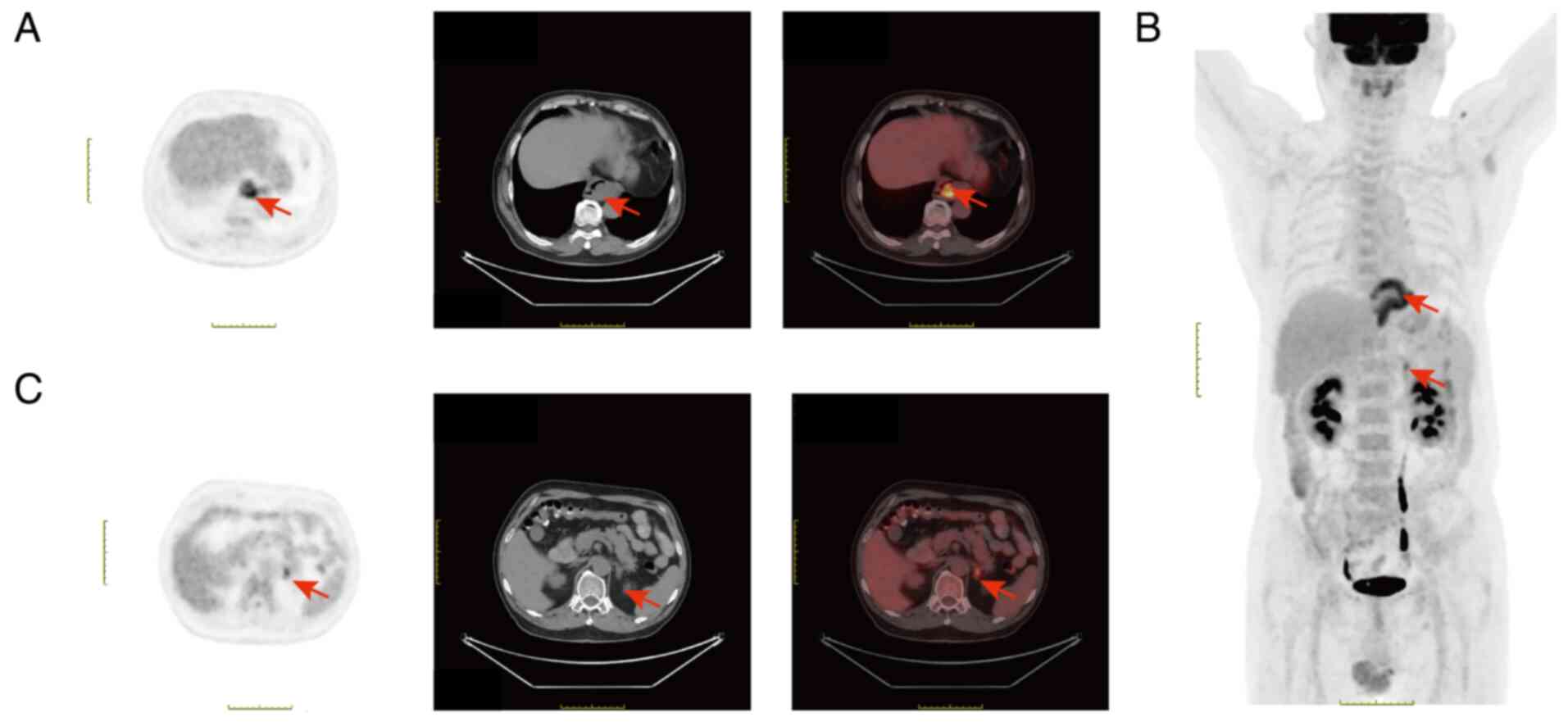
or malignant. PET/CT imaging revealed irregular thickening of the
esophageal wall at the T8-T10 vertebral level, with an intraluminal
soft-tissue mass of ~5.6×2.7×6.6 cm and a maximum standardized
uptake value (SUV) of 6.5. A nodular lesion was also observed in
the left adrenal gland, with heterogeneous density and FDG uptake.
The lesion exhibited a maximum SUV value of 4.1 (delayed maximum
SUV, 5; Fig. 2A-C). Based on the
results obtained using PET/CT analysis, the lesion was diagnosed as
a malignant esophageal tumor with adrenal metastasis. Thus, the
results obtained during the initial gastroscopic biopsy were
considered a false-negative. A repeat gastroscopy revealed a
2.0×2.5-cm submucosal tumor-like elevation in the lower esophagus.
Results of the pathological analysis revealed mild acute and
chronic inflammation of the mucosa, focal atypical squamous
epithelial hyperplasia and smooth muscle hyperplasia in the
submucosa, with no tumor cells (Fig.
1B); thus, the mass was considered benign. In addition, the
mass exhibited a clear outline and was localized, with no
definitive histological diagnosis of malignancy. As the patient was
willing to undergo surgery, esophageal mass resection was
considered feasible.
During surgery, a 5.0×3.0-cm tumor was observed in
the esophagus, and rapid intra-operative pathology was used to
confirm the presence of a leiomyoma. Based on this, no further
esophageal tissue resection procedures were performed, and the left
adrenal mass was resected with the assistance of the Department of
Urology (Fig. 3A and B).
Post-operative pathological analysis was also used for confirmation
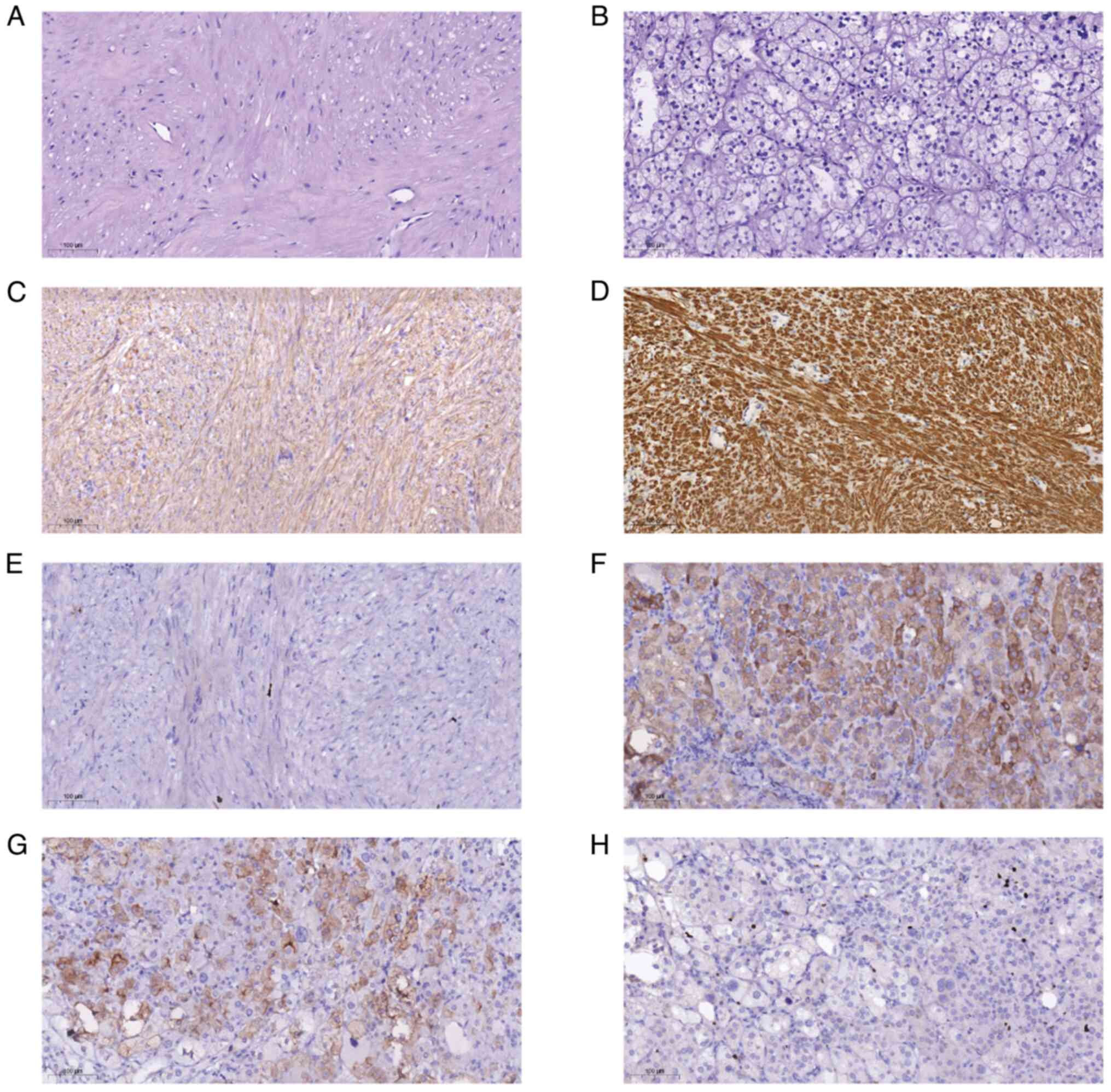
of an esophageal leiomyoma. The results of the immunohistochemical
analysis revealed the positive expression of desmin, smooth muscle
actin (SMA) and Ki-67 (~1%) (Fig.
3C-E). The patient was also diagnosed with an adrenocortical
adenoma with positive expression of inhibin-a, synaptophysin and
Ki-67 (~5%; Fig. 3F-H). The present
case demonstrated that esophageal leiomyoma may be accompanied by
primary adrenal cortical adenoma, which was initially misdiagnosed
as esophageal malignancy with adrenal metastasis using results of
the PET analysis. The patient did not undergo other treatment after
surgery, but recovered well and was followed up after 1 month.
Pathological assessment
Histopathology
Following proper tissue sampling, the tumor tissue
blocks were immersed in a solution composed of 10% formaldehyde in
0.01 M phosphate-buffered saline (PBS) and fixed at room
temperature for 2 h. Next, the tissue blocks were transferred to
the Tissue-Tek VIP® 6 AI Tissue Processor (Sakura
Finetek USA, Inc.) and then embedded in paraffin. The paraffin
blocks were sliced into 5-µm thick sections. These sections were
first dewaxed using xylene and then rehydrated in a series of
ethanol solutions with decreasing concentrations (95, 90, 80 and
75%) and finally in water. The sections were then put into Harris
hematoxylin staining solution and stained at room temperature for 5
min. Subsequently, the sections were differentiated with 0.3%
acidic ethanol. Next, the sections were treated with 0.6% ammonia
water at room temperature for 5 sec, and then the samples were
incubated with eosin staining solution at room temperature for 1 to
3 min. The sections were dehydrated using ethanol and xylene at
room temperature. Finally, the slides were mounted with neutral gum
and observed under a Pathology Slide Scanner (Pannoramic SCAN II;
3DHISTECH Ltd.).
Immunohistochemistry
Tumor sections (5-µm thick) were sliced from the
paraffin block, dewaxed and rehydrated. Subsequently, the slides
were rinsed three times with 0.01 M PBS. Next, the slides were
placed in a pressure cooker and treated with an antigen retrieval
reagent (0.01 M citrate buffer solution, pH 6.0) for 10 min. The
slides were washed again three times with 0.01 M PBS (pH 7.4, with
each wash lasting 5 min) at room temperature. To inhibit the
activity of endogenous peroxidase, a peroxidase blocking agent (kit
cat. no. PV-9000; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China) was added and incubated for 30 min at room
temperature in the dark. Permeabilization with 0.5% Triton (cat.
no. T8200; Beijing Solarbio Science & Technology Co., Ltd.) was
performed for 10 min. Next, 5% goat serum (cat. no. G1208; Wuhan
Servicebio Technology Co., Ltd.) was used to block the slides at
room temperature for 30 min to avoid non-specific binding. The
sections were washed again three times with 0.01 M PBS (Ph 7.4, 5
min per wash), and then incubated with monoclonal primary
antibodies, including anti-desmin (diluted at 1:400; cat. no.
ab32362; Abcam), anti-inhibin-a (diluted at 1:250; cat. no.
ab203824; Abcam), anti-synaptophysin (diluted at 1:2,000; cat. no.
17785-1-AP; Proteintech Group, Inc.), anti-Ki-67 (diluted at
1:8,000; cat. no. 27309-1-AP; Proteintech Group, Inc.) and
anti-α-SMA (diluted at 1:3,000; cat. no. ab7817; Abcam) at 4°C for
12 h. The primary antibodies were diluted with PBS. The slides were
washed three times with 0.01 M PBS (5 min per wash) and then
incubated with Enhanced Enzyme-labeled Goat Anti-Mouse/Rabbit IgG
Polymer (undiluted; kit cat. no. PV-9000; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) at 37°C for 30 min. Diaminobenzidine was
used as the chromogen, and the sections were counterstained with
Mayer's hematoxylin for 2 min at room temperature. Subsequently,
the slides were sealed with neutral gum, and observed and captured
under a Pathology Slide Scanner (Pannoramic SCAN II; 3DHISTECH
Ltd.).
Discussion
It is crucial that esophageal leiomyoma is
differentiated from malignant esophageal cancer, cysts or
esophageal strictures. The prognosis of esophageal leiomyoma is
generally good. After complete resection, patients are usually
cured and have a normal life expectancy. By contrast, esophageal
cancer is a malignant tumor with a relatively poor prognosis.
Leiomyoma is a benign muscular abnormality that commonly occurs in
the middle and distal third of the esophagus, leading to thickening
of the esophageal wall and subsequent esophageal luminal narrowing.
Common clinical symptoms include difficulty in swallowing,
vomiting, and retrosternal pain due to luminal narrowing and
esophageal dysmotility (4). In the
present study, the patient presented with none of the
aforementioned clinical symptoms. Submucosal tumors (SMTs) are
protrusive lesions originating from the muscularis mucosae,
submucosa or muscularis propria, and these may also be extraluminal
lesions. Notably, SMTs that are <2 cm in size are often
asymptomatic and are incidentally found during endoscopic
examination. SMTs exhibit differing levels of incidence in
different parts of the gastrointestinal (GI) tract, with the
majority of SMTs affecting the upper GI tract (5). In total, ~66% of SMTs occur in the
stomach, and the remaining cases affect the esophagus, duodenum and
colon (6). The predilection site of
different types of SMTs is associated with their histopathological
characteristics; thus the location of SMTs is of clinical
diagnostic significance. Leiomyoma is a common pathological type of
esophageal SMT, accounting for 60–80% of all esophageal SMTs.
Furthermore, leiomyoma is more common in the middle and lower third
of the esophagus (7,8), and the present case is reflective of a
typical SMT presentation.
Esophageal leiomyomas are often PET-negative (FDG
uptake is not increased and SUV is generally <2.5); however,
results obtained using PET analysis in the present study
demonstrated that esophageal leiomyomas may exhibit increased
18F-FDG uptake. In addition, the results of the post-operative
histological examination confirmed the diagnosis of esophageal
leiomyoma. False-positive PET/CT results for esophageal leiomyoma
are rare, with few previous reports describing esophageal
leiomyomas with increased FDG uptake (9,10). In
the present case, the results of the PET/CT analyses demonstrated
elevated FDG uptake in both the esophageal mass and the adrenal
mass. Thus, the patient in the present case was initially diagnosed
with an esophageal malignancy with adrenal metastasis. By contrast,
post-operative pathological results revealed esophageal leiomyoma
with a left adrenal adenoma. To the best of our knowledge, the
present study is the first to report the case of a patient with
this diagnosis. Thus, we hypothesized that the misdiagnosis may be
a result of similarities with esophageal cancer observed during
imaging, as this often presents as thickening of the esophageal
wall and the formation of soft-tissue masses during CT analysis. In
the present study, the results of the CT analysis highlighted key
characteristics of esophageal cancer, leading to a misdiagnosis.
Moreover, the misdiagnosis may be a result of increased FDG uptake.
Notably, FDG is a glucose analog, and due to high levels of
metabolism, tumor cells often absorb higher levels of FDG. However,
its uptake in the body is not absolutely specific. Cells in various
physiological and pathological states may take up FDG (11). During surgery, a large number of
tortuous blood vessels were observed on the mucosal surface of the
esophageal mass. The presence of these blood vessels may have
promoted the local aggregation of FDG (12), thus resulting in the high SUV value
that was used for the diagnosis. Moreover, an SUV value of >2.5
obtained during PET analysis is often indicative of a malignant
tumor. Although the maximum SUV value range of FDG metabolism in
esophageal leiomyoma is between 0 and 7.1, the SUV values of the
majority of esophageal leiomyomas are <2.5 (13). However, the SUV value of the
esophageal tumor in the present case reached 6.5, which may have
led to the misdiagnosis. Thus, an overlap in SUV values between
esophageal malignant tumors and esophageal leiomyoma may lead to
complexities in obtaining accurate diagnoses. Based on the
aforementioned reasons, during the process of tumor diagnosis, one
should not rely solely on the imaging findings of PET-CT and the
SUV value. Instead, a variety of factors, such as results from
biopsy or other imaging tools, need to be comprehensively
considered to reduce the risk of misdiagnosis. However, the
muscular layer biopsy of the esophagus performed via endoscopic
ultrasound is fraught with potential risks, such as bleeding and
esophageal perforation. Generally, it is not recommended for
clinical use. Some other imaging diagnostic tools, such as
endoscopy, endoscopic ultrasonography, CT and magnetic resonance
imaging (MRI), can be recommended for further differential
diagnosis.
The specific mechanism underlying the increased FDG
uptake of esophageal leiomyoma is yet to be fully understood;
however, FDG also accumulates in uterine leiomyoma. Notably, FDG
uptake is associated with the increased expression of basic
fibroblast growth factor, transforming growth factor-β,
granulocyte-macrophage colony-stimulating factor and Ki-67
(14). Results of previous studies
revealed that high levels of metabolism in leiomyoma may be
associated with high concentrations of growth factors that promote
the increased proliferation of smooth muscle cells (15,16).
Increased expression of the aforementioned cytokines may lead to
increased vascularization, cell proliferation and cellular
degeneration, which may lead to increased FDG uptake. The results
of the present study revealed high levels of FDG uptake in the
tumor; however, the results of the histological analysis did not
reveal excessive proliferation of blood vessels or degenerated
cells. Results of the immunohistochemical analysis also revealed
weak positive Ki-67 expression (~1%), and this cytokine is a key
marker of cell proliferation. In addition, the results of the
present study revealed increased FDG uptake in the adrenal gland,
and the post-operative histological diagnosis confirmed a primary
adrenocortical adenoma. At present, the association between the
high FDG uptake of adrenocortical adenoma and esophageal leiomyoma
remains to be fully elucidated.
As an important imaging examination method, PET has
several advantages, such as the ability to detect abnormal
metabolic lesions throughout the body at an early stage, and it is
of great significance for tumor staging and efficacy evaluation.
However, the present found that esophageal leiomyoma adenoma and
adrenal cortical adenoma can be a potential cause of a
false-positive PET diagnosis, which increases the difficulty in
diagnosing leiomyoma. To avoid unnecessary surgical interventions,
instead of relying solely on PET, esophageal leiomyoma and adrenal
cortical adenoma should be diagnosed by means of a comprehensive
assessment that incorporates endoscopy, endoscopic ultrasound, CT,
MRI and the pathological examination of tissue samples.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Shandong Province
Medical and Health Development Plan (grant no. 202304020860) and
the Jinan Municipal Health Commission Science and Technology
Development Plan Project (grant no. 2024302003).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ and HL contributed to the conception and the
design of the study. XS obtained and analyzed the patient
information, and contributed to manuscript drafting and critical
revisions of the intellectual content. XS and LL performed analysis
and interpretation of the PET-CT data. DF and YH performed the
histological examination of the tissue. LZ, HL and XS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of the
article was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang W, Rice TW and Goldblum JR:
Esophageal leiomyoma: Experience from a single institution. Dis
Esophagus. 26:167–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Conca F, Rosso N, López Grove R, Savluk L,
Santino JP and Ulla M: Esophageal tumors: The keys to diagnosis by
pneumo-computed tomography. Radiologia (Engl Ed). 65:546–553. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An YS and Kim DY: 18F-fluorodeoxyglucose
PET/CT in a patient with esophageal and genital leiomyomatosis.
Korean J Radiol. 10:632–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beji H, Bouassida M, Kallel Y, Tormane MA,
Mighri MM and Touinsi H: Leiomyoma of the esophagus: A case report
and review of the literature. Int J Surg Case Rep. 94:1070782022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou P, Li Z and Qin X: Chinese consensus
on endoscopic diagnosis and managment of gastrointestinal
submucosal tumors (version 2023). Chin J Dig Endosc. 253–263.
2023.
|
|
6
|
Deprez PH, Moons LMG, O'Toole D, Gincul R,
Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M,
Vieth M, Borbath I, et al: Endoscopic management of subepithelial
lesions including neuroendocrine neoplasms: European Society of
Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 54:412–429.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee LS, Singhal S, Brinster CJ, Marshall
B, Kochman ML, Kaiser LR and Kucharczuk JC: Current management of
esophageal leiomyoma. J Am Coll Surg. 198:136–146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Li Y, Wang F, Wang W and Zhang L:
Video-assisted thoracoscopic surgery for esophageal leiomyoma: A
Ten-year single-institution experience. J Laparoendosc Adv Surg
Tech A. 28:1105–1108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nero LD, Moscatelli A, Fazio V, Pellegatta
G, Bongioanni F, Sambuceti G, Savarino V and Giannini EG: Positive
PET in a patient with esophageal leiomyoma. Am J Gastroenterol.
111:7672016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyoshi K, Naito M, Ueno T, Hato S and Ino
H: Abnormal fluorine-18-fluorodeoxyglucose uptake in benign
esophageal leiomyoma. Gen Thorac Cardiovasc Surg. 57:629–632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahman WT, Wale DJ, Viglianti BL, Townsend
DM, Manganaro MS, Gross MD, Wong KK and Rubello D: The impact of
infection and inflammation in oncologic 18F-FDG PET/CT imaging.
Biomed Pharmacother. 117:1091682019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao Y, Li YM, He ZX, Civelek AC and Li XF:
Likely common role of hypoxia in driving 18F-FDG uptake
in cancer, myocardial ischemia, inflammation and infection. Cancer
Biother Radiopharm. 36:624–631. 2021.PubMed/NCBI
|
|
13
|
Dendy M, Johnson K and Boffa DJ: Spectrum
of FDG uptake in large (>10 cm) esophageal leiomyomas. J Thorac
Dis. 7:E648–E651. 2015.PubMed/NCBI
|
|
14
|
Meirelles GS, Ravizzini G, Yeung HW and
Akhurst T: Esophageal leiomyoma: A rare cause of false-positive FDG
scans. Clin Nucl Med. 31:342–344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ak I, Ozalp S, Yalçin OT, Zor E and
Vardareli E: Uptake of 2-[18F]fluoro-2-deoxy-D-glucose in uterine
leiomyoma: Imaging of four patients by coincidence positron
emission tomography. Nucl Med Commun. 25:941–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Memisoglu E, Agarwal B, Akduman I, Prather
C, Collins B and Civelek AC: Multimodality diagnostic imaging of
diffuse esophageal leiomyomatosis. J Comput Assist Tomogr.
30:100–104. 2006. View Article : Google Scholar : PubMed/NCBI
|