Introduction
Esophageal cancer is a highly malignant tumor of the
digestive system, characterized by high incidence and mortality
rates. According to the World Health Organization (WHO), in 2020,
53.7% of the global esophageal cancer cases (324,000 new cases) and
55.4% of the associated deaths (301,000 cases) were reported in
China, thus China ranked first in the world in terms of both new
cases and associated deaths. In light of current data, the
prognosis of esophageal cancer is not ideal. Based on data from 60
countries and regions around the world, the age-standardized 5-year
survival rate of esophageal cancer is only 10.0–30.0% (1). As a result, esophageal cancer poses a
major threat to human health.
Numerous clinical studies have clarified the status
of synchronous radiotherapy in the treatment of locally advanced
esophageal cancer (2–4). Due to their efforts, the survival rate
of patients has greatly improved. However, the 5-year survival rate
remains at a low level. More than two-thirds of patients are
diagnosed at advanced stages, where surgical options are no longer
viable. Immune checkpoint inhibitors (ICIs), as novel therapeutic
tools in clinical practice, have gradually changed the treatment
paradigm of esophageal cancer by exhibiting durable response rates
in some refractory tumors. Over the last 5 years, numerous large
randomized controlled studies have actively explored the efficacy
and safety of immunotherapy combined with chemotherapy in
esophageal squamous cell carcinoma (ESCC) in first-line clinical
treatment (5–9). However, survival benefits are still
limited, highlighting the need for further exploration of improved
treatment strategies.
Sintilimab is a human IgG4 monoclonal antibody that
binds to programmed cell death receptor-1 (PD-1) in order to block
the interaction of PD-1 with its ligands [programmed cell
death-ligand 1 (PD-L1) and polyclonal antibody to mitochondrial
ribosomal protein L2] and consequently help to restore the
endogenous antitumor T-cell response (10). Due to its potential advantages,
sintilimab has been investigated extensively in China, and was
recently approved by the State Drug Administration of China for the
treatment of classical Hodgkin's lymphoma, natural killer/T cell
lymphoma, non-small cell lung cancer (NSCLC) and hepatocellular
carcinoma. To explore its broader applications, sintilimab is
undergoing phase I, II and III development for use in various solid
tumors, including glioblastoma, osteosarcoma and pancreatic
adenocarcinoma, in China (11–13).
Based on the results of the ORIENT-15 study, sintilimab in
combination with chemotherapy has been approved for first-line
treatment of ESCC (14).
When combined with anti-angiogenesis agents, such as
bevacizumab and ramucirumab, ICIs have exhibited synergistic
antitumor effects in preclinical studies (15,16).
Recombinant human-endostatin (rh-endostatin) is a broad-spectrum
anti-angiogenic drug, which is a novel recombinant human vascular
endothelial inhibitor drug independently developed in China.
Rh-endostatin inhibits endothelial cell migration, impedes
angiogenesis and reduces tumor blood supply, thereby suppressing
tumor growth and metastasis. Additionally, the drug enhances the
local tumor microenvironment and induces tumor cell death,
potentially prolonging patient survival (17). It has also been reported that
rh-endostatin combined with conventional chemotherapy can improve
therapeutic outcomes in numerous malignant tumors, including lung,
breast and colorectal cancer (18–21).
However, its combination with chemotherapy for advanced ESCC
remains underexplored.
Considering the potential therapeutic benefits of
rh-endostatin combined with sintilimab and chemotherapy, the
present study collected clinicopathological data from 31 patients
with unresectable locally advanced or metastatic ESCC treated with
rh-endostatin combined with sintilimab, paclitaxel liposome and
platinum (TP regimen) as a first-line treatment. Subsequently, the
overall effective rate, incidence of adverse events (AEs) and
survival times were assessed.
The present study aimed to provide effective
combination medication regimens and supportive evidence to improve
the efficiency of first-line treatment for advanced ESCC, improve
the prognosis of patients and provide a theoretical basis for the
clinical exploration of novel treatment regimens.
Patients and methods
Study design
The present retrospective analysis included data
from 31 patients with unresectable locally advanced or metastatic
esophageal cancer who received treatment with rh-endostatin in
combination with sintilimab and the TP regimen at the First
Affiliated Hospital of Nanjing Medical University (Nanjing, China)
between January 2019 and December 2023. The objective was to assess
the clinical efficacy and AEs associated with this regimen.
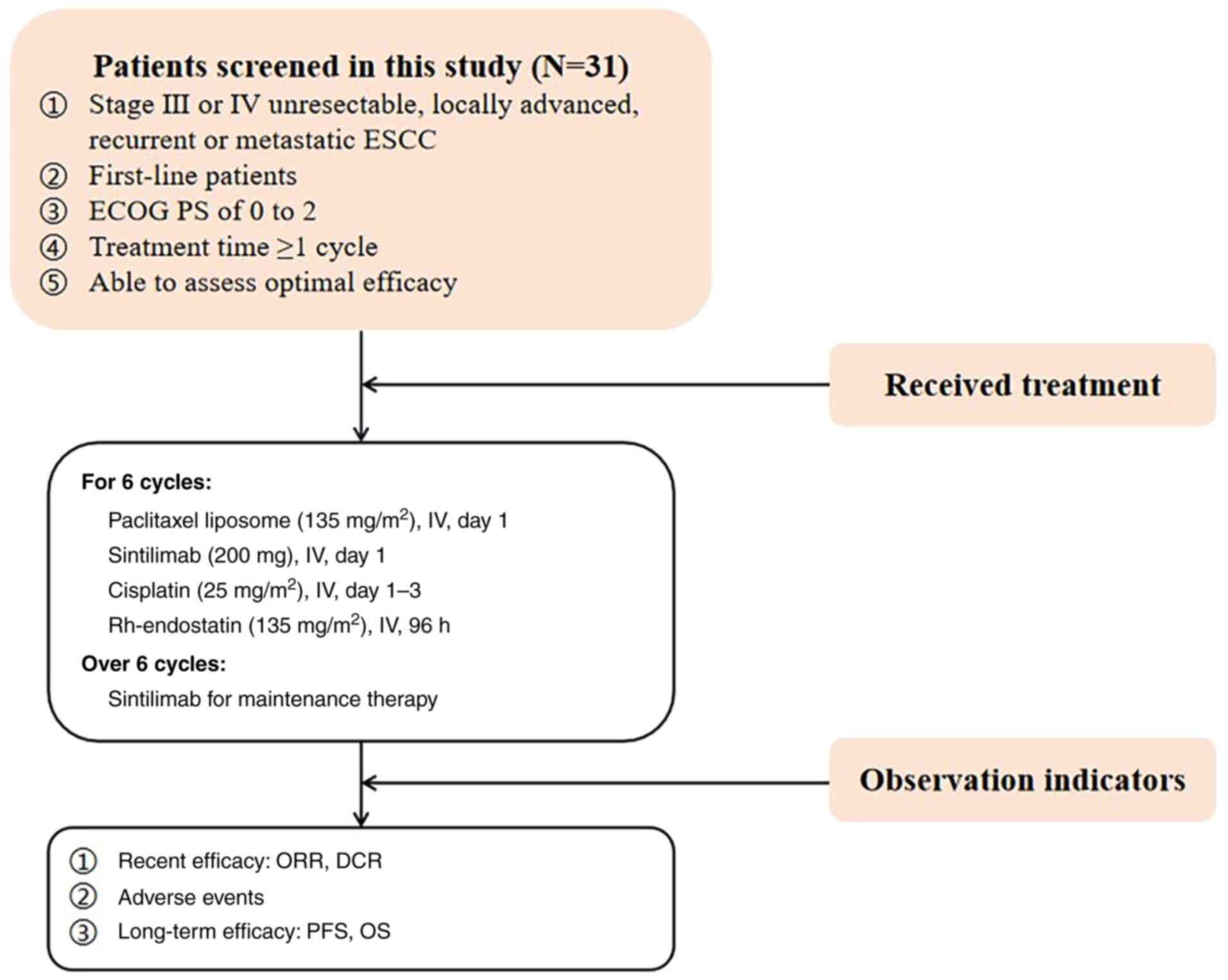
Patient eligibility
A total of 31 patients with unresectable locally
advanced or metastatic esophageal cancer treated with rh-endostatin
in combination with sintilimab and the TP regimen on a first-line
basis were retrospectively included. All the patients included in
this study provided written informed consent agreeing to the use of
their clinical data and biological samples.
The inclusion criteria were as follows: i) Patients
with histologically confirmed unresectable locally advanced,
recurrent or metastatic ESCC; ii) patients who were underwent
first-line treatment with rh-endostatin in combination with
sintilimab and the TP regimen; iii) patients with at least one
evaluable lesion according to Response Evaluation Criteria in Solid
Tumors (RECIST 1.1) classification (22); iv) patients with an expected
survival time ≥3 months; and v) patients with adequate heart, lung,
liver and kidney function.
The exclusion criteria were as follows: i) Patients
who were severely malnourished (weight <40 kg or body mass index
<18), or who required tube feeding; ii) patients who had major
surgery within 30 days prior to study treatment; iii) patients with
other malignant tumors that have not been cured within 2 years
(except for patients with a combination of other early-stage tumors
that have been treated radically and are assessed by the
investigator to be at low risk of recurrence in the short term);
iv) patients with active infections who still require systemic
therapy 7 days prior to administration of the first dose of
treatment in this study; v) patients who are allergic to the drugs
or related ingredients involved in this study; vi) patients who are
currently enrolled in other interventional clinical studies; vii)
patients who have not recovered from prior antitumor-related AEs to
CTCAE Grade 1 prior to the first dose; viii) patients with a
tendency or history of thrombosis or bleeding, regardless of
severity, within 60 days prior to the first dose; and ix) patients
with any serious or unstable medical condition or mental illness;
patients with known active alcohol or drug abuse or dependence.
Data collection
The patients in this study were selected from the
biobank of the First Affiliated Hospital of Nanjing Medical
University. All donors of clinical data had provided informed
written consent, agreeing that the related information could be
used for all medical research. Medical records for this study were
collected and obtained in January 2024. During the survival
follow-up process, follow-up surveys were conducted via telephone
with patients who had previously used the drug or with their family
members.
Treatment plan
All included patients received rh-endostatin
combined with sintilimab and the TP regimen. Based on the dosage
applied in previous studies, the continuous intravenous infusion of
cisplatin (25 mg/m2) was administrated consecutively
from the first day to the third day, with paclitaxel liposome (135
mg/m2) administrated on day 1, sintilimab (200 mg) on
day 1 and continuous intravenous infusion of rh-endostatin (135
mg/m2) on day 1 for 96 h (14). All treatments were applied
intravenously every 3 weeks as one cycle, with 6 cycles completed.
Following the completion of 6 cycles, maintenance therapy with
sintilimab was administered until disease progression occurred
(Fig. 1).
Baseline and follow-up assessment
Baseline assessments included age, sex, pathological
type, stage, metastasis, PD-L1 tumor proportion score (TPS)
(23), Eastern Cooperative Oncology
Group performance status (ECOG PS) (24) and smoking status. AEs were monitored
weekly during treatment. Efficacy was assessed every two treatment
cycles based on the RECIST 1.1 guidelines (22).
The follow-up assessment included recent efficacy,
AEs and long-term efficacy. The recent efficacy of patients was
classified as complete response (CR), partial response (PR), stable
disease (SD) and progressive disease (PD), and the total efficacy
rate was calculated as CR + PR, according to the WHO solid tumor
efficacy evaluation criteria (25).
The objective response rate (ORR) and disease control rate (DCR)
can be calculated from the CR, PR, SD and PD. The ORR was defined
as the percentage of patients achieving CR and PR. The DCR was
defined as the percentage of patients achieving CR, PR and SD. The
AE evaluation was performed within 5 months after the
administration of the drug according to the RECIST 1.1 guidelines.
Treatment-related AEs (TRAEs) were graded according to the National
Cancer Institute-Common Terminology Criteria Adverse Events 4.0
guidelines (26). For long-term
efficacy, survival statistics were obtained for the 31 patients by
telephone follow-up or outpatient follow-up. The survival time was
defined as the time from the initial chemotherapy to death or the
follow-up cut-off date.
Statistical analysis
Statistical analysis was performed using SPSS 24.0;
measurements are expressed as median (Q3-Q1), and comparisons were
made using Wilcoxon's rank-sum test and Kruskal-Wallis test. Counts
are expressed as percentages, and the test level was α=0.05. The
median follow-up period, progression-free survival (PFS) time and
overall survival (OS) time were analyzed by the Kaplan-Meier
method.
A clinical heterogeneity analysis was performed
using Wilcoxon's rank-sum test and Kruskal-Wallis test, with the
overall population stratified into groups based on different
subgroup characteristics. Wilcoxon's rank-sum test was used to
compare quantitative efficacy between two groups and the
Kruskal-Wallis test was used for multiple groups. The specific
dataset consisted of the PFS time and the total follow-up time of
the groups. P<0.05 was used to indicate a statistically
significant difference.
Results
Baseline characteristics
A total of 31 patients with unresectable locally
advanced or metastatic esophageal cancer were included in the
present study. Of these, 83.9% (26/31) were male and 16.1% (5/31)
were female, with a median age of 68 years (range, 56–77 years).
All patients had histologically confirmed ESCC, and metastasis
occurred in the liver (5 cases), lung (5 cases), bone (1 case) and
peritoneum (1 case) (Table I).
 | Table I.Baseline characteristics of included
patients (n=31). |
Table I.
Baseline characteristics of included
patients (n=31).
| Baseline
characteristics | n (%) |
|---|
| Age, years |
|
|
<65 | 7 (22.6) |
|
≥65 | 24 (77.4) |
| Sex |
|
|
Male | 26 (83.9) |
|
Female | 5 (16.1) |
| Pathological
type |
|
|
Adenocarcinoma | 0 (0.0) |
|
Squamous carcinoma | 31 (100.0) |
|
Others | 0 (0.0) |
| Stage |
|
|
III | 17 (54.8) |
| IV | 14 (45.2) |
| No. of metastatic
sites |
|
|
<2 | 14 (45.2) |
| ≥2 | 17 (54.8) |
| Liver
metastasis |
|
|
Absent | 26 (83.9) |
|
Present | 5 (16.1) |
| Bone
metastasis |
|
|
Absent | 30 (96.8) |
|
Present | 1 (3.2) |
| Lung
metastasis |
|
|
Absent | 26 (83.9) |
|
Present | 5 (16.1) |
| Peritoneal
metastasis |
|
|
Absent | 30 (96.8) |
|
Present | 1 (3.2) |
| PD-L1 tumor
proportion score |
|
|
<10% | 12 (38.7) |
|
≥10% | 2 (6.5) |
| Could
not be evaluated | 17 (54.8) |
| ECOG PS |
|
| 0-1
points | 29 (93.5) |
| 2
points | 2 (6.5) |
| Smoking status |
|
|
Never | 14 (45.2) |
|
Former/current | 17 (54.8) |
Clinical efficacy
The data cut-off date for the analysis was July 17,
2024, with a median follow-up duration of 13.067 months (95% CI,
10.267–15.866 months). In the overall sample, a total of 31
patients were eligible for efficacy analysis. For the whole cohort,
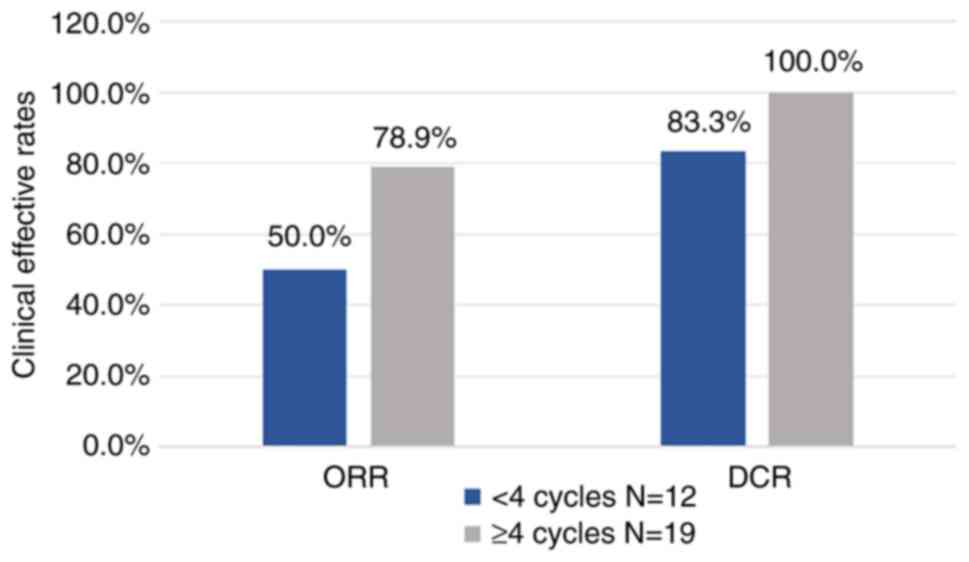
the ORR was 67.7% and the DCR was 93.5%. The data showed that of
the 12 patients who received <4 treatment cycles, 0% achieved
CR, 50.0% achieved PR, 33.3% achieved SD and 16.7% achieved PD.
Additionally, of the 19 individuals who received ≥4 treatment
cycles, 10.5% achieved CR, 68.4% achieved PR and 21.1% achieved SD.
For patients with <4 treatment cycles, the ORR reached 50%, and
the DCR reached 83.3%. Compared with fewer treatment cycles, both
ORR and DCR were improved with ≥4 treatment cycles, reaching 78.9
and 100.0%, respectively (Table
II; Fig. 2).
 | Table II.Results of clinical efficacy. |
Table II.
Results of clinical efficacy.
| Best response | Overall (n=31) | <4 cycles
(n=12) | ≥4 cycles
(n=19) |
|---|
| CR, n (%) | 2 (6.5) | 0 (0.0) | 2 (10.5) |
| PR, n (%) | 19 (61.3) | 6 (50.0) | 13 (68.4) |
| SD, n (%) | 8 (25.8) | 4 (33.3) | 4 (21.1) |
| PD, n (%) | 2 (6.5) | 2 (16.7) | 0 (0.0) |
| ORR, % | 67.7 | 50.0 | 78.9 |
| DCR, % | 93.5 | 83.3 | 100.0 |
In subgroup analyses, patients were grouped
according to characteristics, including age, sex, stage, TPS, ECOG
PS and smoking status. Each subgroup member had a corresponding PFS
time and total follow-up time, and Wilcoxon's rank-sum test or
Kruskal-Wallis test was performed between subgroups. No significant
differences were found between the different subgroups based on
age, sex, stage, PD-L1 TPS, ECOG PS or smoking status in terms of
PFS (Table III). The OS in some
subgroups was not reached, making statistical comparison
infeasible.
 | Table III.Subgroup analysis of PFS and OS in
the 31 patients. |
Table III.
Subgroup analysis of PFS and OS in
the 31 patients.
| Variable | Patients, n
(%) | PFS,
monthsa | OS,
monthsa | P-value (PFS) |
|---|
| Age, years |
|
|
| 0.422 |
|
<65 | 7 (22.6) | 4.267
(3.400–15.767) | NR |
|
|
≥65 | 24 (77.4) | 8.300
(4.333–23.067) | 23.067
(5.733-NR) |
|
| Sex |
|
|
| 0.259 |
|
Male | 26 (83.9) | 5.733
(4.267–23.067) | 23.067
(9.767-NR) |
|
|
Female | 5 (16.1) | 12.633
(9.933–15.767) | NR |
|
| Stage |
|
|
| 0.171 |
|
III | 17 (54.8) | 11.967
(4.800–23.067) | 23.067
(5.733-NR) |
|
| IV | 14 (45.2) | 5.367
(3.400–10.367) | NR |
|
| PD-L1 tumor
proportion score |
|
|
| 0.814 |
|
<10% | 12 (38.7) | 9.933
(4.333–12.633) | 13.067
(4.333-NR) |
|
|
>10% | 2 (6.5) | 4.333
(4.333–8.300) | NR |
|
| Could
not be evaluated | 17 (54.8) | 8.100
(4.067–23.067) | 23.067
(23.067-NR) |
|
| ECOG PS |
|
|
| 0.634 |
| 0
points | 14 (45.2) | 9.033
(4.267–23.067) | 23.067
(23.067-NR) |
|
| ≥1
points | 17 (54.8) | 5.733
(4.333–12.633) | NR |
|
| Smoking status |
|
|
| 0.937 |
|
Never | 14 (45.2) | 8.100
(4.800-NR) | NR |
|
|
Former/current | 17 (54.8) | 9.933
(3.367–15.767) | 23.067
(13.067-NR) |
|
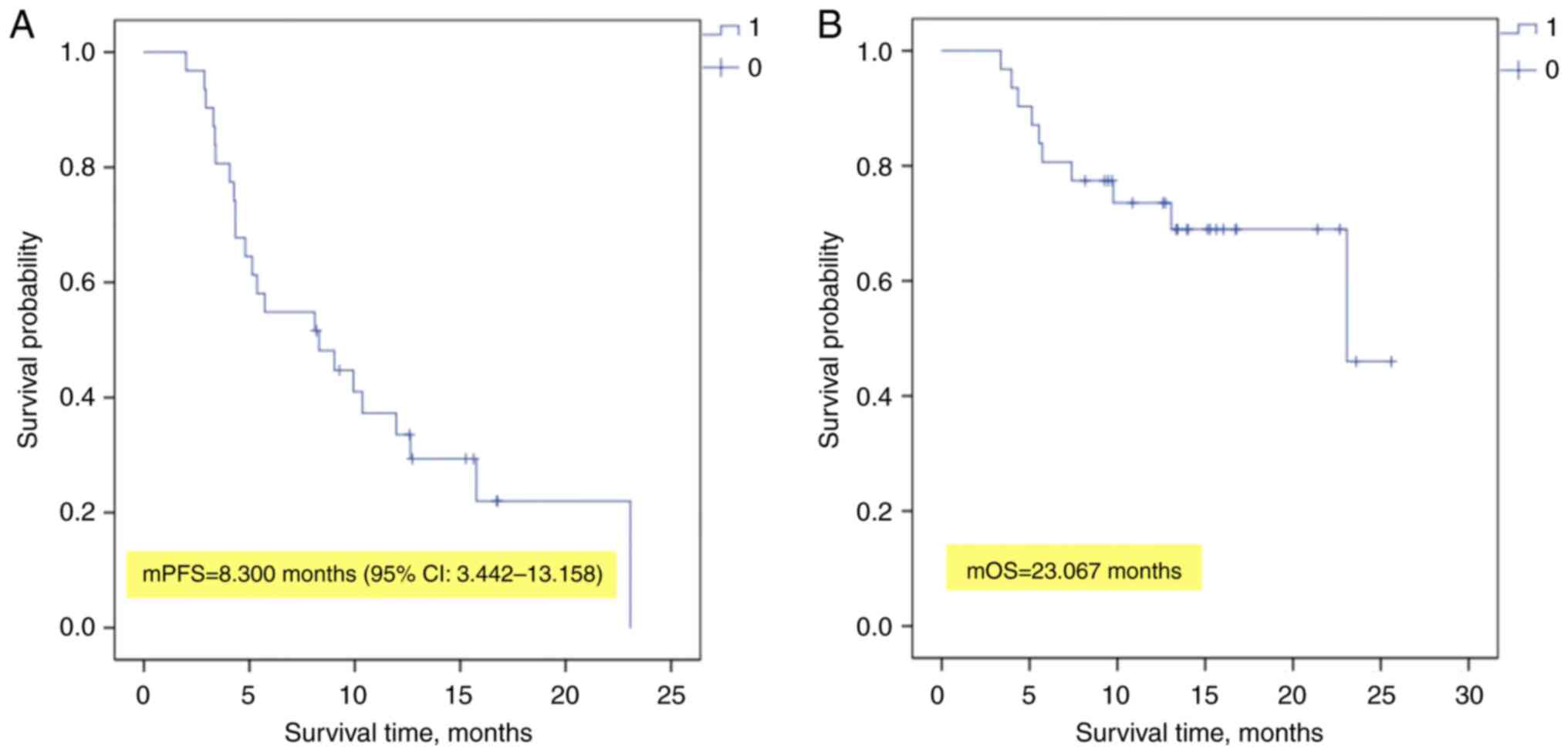
For the entire cohort, the median PFS time was 8.30
months (95% CI, 3.442–13.158 months) and the median OS time reached
23.07 months (Fig. 3).
AEs
The AEs are outlined in Table IV. Among the 31 patients with ESCC
included in the safety analysis, 24 (77.4%) experienced at least
one TRAE, with 35.5% (11/31) reporting grade ≥3 TRAEs. The most
common TRAEs were anemia (83.9%; 26/31), thrombocytopenia (38.7%;
12/31) and neutropenia (32.3%; 10/31). The most serious AEs,
including grade ≥3 events, were neutropenia and anemia; each was
observed in 9.7% (3/31) of patients. No unexpected AEs were
observed during the study.
 | Table IV.Profile of AEs in the 31
patients. |
Table IV.
Profile of AEs in the 31
patients.
| AEs | Any grade | Grade ≥3 |
|---|
| Hematological
adverse event |
|
|
|
Neutropenia | 10 (32.3) | 3 (9.7) |
|
Thrombocytopenia | 12 (38.7) | 2 (6.5) |
|
Anemia | 26 (83.9) | 3 (9.7) |
| Non-hematological
adverse events |
|
|
| Nausea
or vomiting | 0 (0.0) | 0 (0.0) |
|
Rash | 1 (3.2) | 0 (0.0) |
|
Fatigue | 0 (0.0) | 0 (0.0) |
|
Hemorrhage | 0 (0.0) | 0 (0.0) |
| Immune-related
adverse events |
|
|
|
Reactive cutaneous capillary
endothelial proliferation | 0 (0.0) | 0 (0.0) |
|
Immune-mediated
pneumonitis | 1 (3.2) | 1 (3.2) |
| Liver
injury | 4 (12.9) | 0 (0.0) |
| Kidney
injury | 2 (6.5) | 1 (3.2) |
|
Hypothyroidism | 5 (16.1) | 0 (0.0) |
|
Diarrhea and colitis | 0 (0.0) | 0 (0.0) |
| Cardiac
creatine kinase level change | 3 (9.7) | 1 (3.2) |
| Hair
loss | 9 (29.0) | 0 (0.0) |
Discussion
The present retrospective study evaluated the
efficacy and safety of rh-endostatin in combination with sintilimab
and chemotherapy in patients with advanced ESCC. The exploratory
findings suggested that, compared with the chemotherapy combined
with immunotherapy regimens, rh-endostatin in combination with
sintilimab and chemotherapy exhibited favorable efficacy in
improving survival and treatment outcomes in patients with locally
advanced or metastatic ESCC, with a manageable safety profile.
In the ORIENT-15 study, the PFS and OS times of the
treatment modality involving sintilimab combined with chemotherapy
were found to be 7.2 and 16.7 months, respectively. This indicated
that, compared with the traditional radiotherapy and chemotherapy
regimens, sintilimab combination chemotherapy markedly prolonged
OS, improved PFS and increased the overall remission rate in
patients with locally advanced, recurrent or metastatic ESCC.
Additionally, this combination therapy had a manageable safety
profile compared with placebo-based chemotherapy, regardless of the
PD-L1 expression level (14).
Therefore, sintilimab in combination with chemotherapy is
recommended as a novel first-line standard treatment for locally
advanced, recurrent or metastatic ESCC by the Chinese Society of
Clinical Oncology guidelines (27).
Vascular endothelial growth factor (VEGF) serves a
crucial role in the development of ESCC; it is upregulated in the
tumor microenvironment, leading to the formation of abnormal and
incomplete blood vessel networks around the tumor, which limits
immune cell infiltration. The blood vessels formed under the
influence of VEGF are often structurally abnormal, resulting in
impaired blood flow. These VEGF-induced vessels typically exhibit
leakage, fluid accumulation and irregular barrier functions. The
abnormal vasculature also contributes to increased interstitial
pressure within the tumor microenvironment. As a result, poor blood
flow, leakage and high interstitial pressure hinder the effective
penetration of immune cells into the tumor tissue, thereby
weakening the antitumor response of the immune system (28,29).
Targeting the aforementioned mechanisms, recent
studies have shown that combination of anti-angiogenic treatment
with immunotherapy can improve antitumor efficacy (30,31).
On the one hand, antiangiogenic drugs not only reverse the
immunosuppressive effect caused by serum VEGF, but also normalize
the tumor vascular system, promote the exudation and perivascular
accumulation of CD8+ cytotoxic T lymphocytes expressing
γ-interferon (IFN-γ), and may induce or increase active T-cell
transit to specific tumor antigens or other effector cells of the
immune system. On the other hand, ICIs can restore the
immune-supportive microenvironment, normalize the tumor vascular
system by activating effector T cells and upregulating the
secretion of IFN-γ, increase the infiltration and killing function
of effector T cells, facilitate the delivery of drugs, reduce the
dosage of ICIs and decrease the risk of AEs (32–36).
Overall, the combination of anti-angiogenesis treatment and
immunotherapy holds promise for improving the immune
microenvironment while normalizing tumor vasculature, thereby
enhancing therapeutic efficacy.
The Empower study, presented at the 2022 American
Society of Clinical Oncology annual meeting, explored the efficacy
of sintilimab in combination with recombinant human vascular
endothelial inhibitor plus standard platinum-containing two-agent
chemotherapy (pemetrexed + carboplatin/cisplatin) for the
first-line treatment of advanced non-squamous NSCLC in
driver-negative NSCLC (37). The
combination therapy resulted in an ORR of 53% and a DCR of 93%.
Most AEs were grade 1–2 AEs, suggesting that the combination of
recombinant human vascular endothelial inhibitor, immunotherapy and
chemotherapy offers promising efficacy and safety (18). The favorable safety and tolerability
of the combination mode in the Empower study further supports the
exploration and application of the combination regimen of
immunological and antiangiogenic drugs.
In order to improve antitumor efficacy, the present
retrospective study was theoretically based on anti-angiogenesis
treatment combined with immunotherapy. The present study aimed to
explore a potentially optimal treatment regimen by adding the
anti-angiogenic agent rh-endostatin to a chemotherapy and
immunotherapy protocol. The regimen seemed to have a promising
clinical efficacy. Thus, the present study retrospectively analyzed
the data from these patients. Although the analysis was
retrospective, rigorous screening was performed in the patient
selection process, and the treatment process was subjected to
rigorous quality control, closely aligning with the requirements of
a prospective trial.
In the short-term efficacy assessment, improved ORR
and DCR were observed. The study demonstrated that the ORR and DCR
were 67.7 and 93.5% respectively, while in the ORIENT-15 study, the
ORR and DCR were 66.1 and 90.2% respectively. This indicated that
the present results seemed to show an upward trend. However, this
may be related to the relatively small sample size in the present
study, and thus it should be interpreted with caution. In patients
receiving longer cycles of treatment, the improvements in ORR and
DCR seemed to be more marked. Specifically, the ORR was 78.9% for
those receiving ≥4 cycles of treatment, compared with 67.7% for the
overall cohort, and the DCR was 100.0% in patients receiving ≥4
cycles, compared with 93.5% in the overall cohort. These findings
suggest that the treatment duration may be associated with
therapeutic efficacy. Preliminary results indicated that the
adequate ≥4-cycle long regimen of rh-endostatin may be associated
with more significant survival gain. However, the safety of
combination therapy with rh-endostatin in full cycles still
deserves to be explored.
In addition to short-term efficacy assessment, it is
also important to assess the long-term survival of patients. The
median PFS time of patients treated with rh-endostatin combined
with sintilimab and chemotherapy was 8.30 months (95% CI,
3.442–13.158 months), and the median OS time reached 23.07 months.
In the ORIENT-15 study, the PFS and OS of the treatment modality
involving sintilimab combined with chemotherapy were found to be
7.2 and 16.7 months, respectively. It was observed that
rh-endostatin-combined treatment tended to extend PFS and OS.
However, as the present results were from a retrospective study, a
head-to-head comparison could not be conducted. Therefore, further
prospective large-scale clinical trials are still needed for
confirmation.
In terms of safety, the incidence of at least one
TRAE associated with any level of treatment (sintilimab, placebo,
cisplatin, paclitaxel or 5-fluorouracil) in the ORIENT-15 study was
98% (321/327). The incidence of TRAEs of rh-endostatin combined
with sintilimab and chemotherapy in the present study was 77.4%
(24/31), while the incidence of all adverse reactions was lower
than that in the ORIENT-15 study. Anemia, thrombocytopenia and
neutropenia were the most common AEs. However, the majority of
these events were mild to moderate, with severe AEs being rare. The
incidence of grade ≥3 AEs was 35.5% (11/31). In the ORIENT-15
study, the incidence of grade ≥3 TRAEs was 59.9% (196/327) in the
sintilimab chemotherapy group. By contrast, the present study
exhibited a significant decrease. The reduced incidence of these
AEs may be associated with the lower dose of paclitaxel used in the
present study. The chemotherapy regimen used in the present study
had a dose of 135 mg/m2 of paclitaxel liposome, which is
in the low-dose range of paclitaxel liposome (135–175
mg/m2) (38), and the
combined application of rh-endostatin can reduce the chemotherapy
dose, which is conducive to the reduction of AEs of chemotherapy.
The present data suggest that the addition of rh-endostatin
treatment to sintilimab combination chemotherapy is beneficial on
trend, both in terms of efficacy and safety, and that this can also
lower the chemotherapy dose and reduce the incidence of toxic side
effects; however, this needs to be further confirmed by prospective
clinical trials with large samples.
In the subgroup analysis, there were no significant
differences in PFS across subgroups based on age, sex, stage, PD-L1
TPS, ECOG PS or smoking status. This suggests that the response to
treatment was similar across different subgroups, and the subgroup
characteristics studied (age, sex, stage, PD-L1 TPS, ECOG PS and
smoking status) are not key factors influencing treatment efficacy.
Therefore, the treatment strategy exhibited broad effectiveness
across different patient populations.
The present study also had certain limitations.
Firstly, this investigation was a small sample size, single-center,
retrospective analysis. With a sample size of 31 patients and the
study being conducted solely at one institution, it is highly
susceptible to the influence of random factors. This may
potentially compromise the reliability and generalizability of the
research findings. Secondly, the median follow-up duration in the
present study was 13.067 months (95% CI, 10.267–15.866 months). The
relatively short follow-up period may not be adequate to capture a
sufficient number of target events, such as mortality and
recurrence. This can introduce inaccuracies in the analysis of
survival rates and treatment efficacy. Furthermore, short-term
follow-up may impede the accurate assessment of safety. For
instance, immune-related AEs (irAEs) are prevalent adverse
reactions among patients with cancer undergoing ICI therapy. irAEs
are characterized by latency and chronic persistence. Short-term
follow-up may fail to detect these persistent or progressively
deteriorating conditions. Additionally, the symptoms of irAEs are
often non-specific and can be easily confounded with those of other
common diseases or adverse reactions induced by treatment. As a
result, short-term follow-up may overlook certain subtle symptoms,
potentially leading to misdiagnosis or missed diagnosis. This could
lead to an underestimation of the incidence of AEs in the
statistical data.
In future studies, the following adjustments can be
made to address the aforementioned limitations. Firstly, a
prospective randomized controlled clinical trial could be set up to
compare the clinical efficacy of rh-endostatin combined with
sintilimab and chemotherapy against that of sintilimab combined
with chemotherapy. Secondly, the follow-up time could be extended.
The optimal follow-up time should be as long as 2 years, or even
longer if possible. Thirdly, individualized monitoring programs
could be added. Monitoring programs can be developed based on
patients' underlying diseases and their previous adverse reaction
histories. irAEs often involve multiple organ systems, and thus,
timely and comprehensive assessments are crucial for the early
detection of irAEs.
It is therefore necessary to conduct larger sample
size, multicenter, long-term, randomized, controlled clinical
trials to further validate the efficacy and safety of rh-endostatin
in combination with sintilimab and chemotherapy for the first-line
treatment of locally advanced or metastatic ESCC, and to confirm
its long-term effectiveness and toxicity.
In conclusion, rh-endostatin combined with
sintilimab and chemotherapy has a positive efficacy and safety in
the first-line treatment of locally advanced or metastatic ESCC,
suggesting that this regimen may be a potential treatment option
for patients with advanced ESCC. Further randomized controlled
trials are needed to study the efficacy and safety of rh-endostatin
in combination with sintilimab and chemotherapy for advanced
ESCC.
Acknowledgements
Not applicable.
Funding
This study was funded by the Natural Science Foundation of
Jiangsu Province Academy of Traditional Chinese Medicine (grant no.
2024YZRKXJJ20), the National Natural Science Foundation of China
(grant no. 82073164), the Natural Science Foundation of Chongqing,
China (grant no. CSTB2024NSCQ-MSX0914) and the Qijiang District
Science and Technology Plan Project (grant no. 2024082).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SC and LF provided materials and contributed to the
study conception and design. XS was responsible for writing the
draft manuscript and conducting the analysis. YC and TW performed
data analysis and interpretation. PL and RW contributed to the
acquisition of data. LL participated in data analysis and study
design. SC and LF confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was granted by the First Affiliated
Hospital of Nanjing Medical University (Nanjing, China; approval
no. 2024-SR-415). Written informed consent was gathered from the
participants prior to initiation of the study.
Patient consent for publication
In the present study, the patient, parent, guardian
or next of kin as appropriate provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2017 Oesophageal Cancer Collaborators,
. The global, regional, and national burden of oesophageal cancer
and its attributable risk factors in 195 countries and territories,
1990–2017: A systematic analysis for the Global Burden of Disease
Study 2017. Lancet Gastroenterol Hepatol. 5:582–597. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medical Affairs Bureau of the National
Health and Health Commission of the People's Republic of China, .
Esophageal cancer diagnosis and treatment guidelines (2022
edition). Zhonghua Wei Chang Wai Ke Za Zhi. 21:1247–1268. 2022.(In
Chinese).
|
|
3
|
Sasaki Y and Kato K: Chemoradiotherapy for
esophageal squamous cell cancer. Jpn J Clin Oncol. 46:805–810.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe M, Otake R, Kozuki R, Toihata T,
Takahashi K, Okamura A and Imamura Y: Recent progress in
multidisciplinary treatment for patients with esophageal cancer.
Surg Today. 50:12–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab
combination therapy in advanced esophageal squamous-cell carcinoma.
N Engl J Med. 386:449–462. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song Y, Zhang B, Xin D, Kou X, Tan Z,
Zhang S, Sun M, Zhou J, Fan M, Zhang M, et al: First-line
serplulimab or placebo plus chemotherapy in PD-L1-positive
esophageal squamous cell carcinoma: A randomized, double-blind
phase 3 trial. Nat Med. 29:473–482. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Kato K, Raymond E, Hubner RA, Shu Y,
Pan Y, Park SR, Ping L, Jiang Y, Zhang J, et al: Tislelizumab plus
chemotherapy versus placebo plus chemotherapy as first-line
treatment for advanced or metastatic oesophageal squamous cell
carcinoma (RATIONALE-306): A global, randomised,
placebo-controlled, phase 3 study. Lancet Oncol. 24:483–495. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st Randomized clinical trial.
JAMA. 326:916–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoy SM: Sintilimab: First global approval.
Drugs. 79:341–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y, Liao L, Du K, Mo J, Zou X, Liang J,
Chen J, Tang W, Su L, Wu J, et al: Clinical activity and safety of
sintilimab, bevacizumab, and TMZ in patients with recurrent
glioblastoma. BMC Cancer. 24:1332024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li F, Zhang DB, Ma Y, Song Y and Duan XL:
Effects of combined sintilimab and chemotherapy on progression-free
survival and overall survival in osteosarcoma patients with
metastasis. Pak J Med Sci. 40:648–651. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou SQ, Wan P, Zhang S, Ren Y, Li HT and
Ke QH: Programmed cell death 1 inhibitor sintilimab plus concurrent
chemoradiotherapy for locally advanced pancreatic adenocarcinoma.
World J Clin Oncol. 15:859–866. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): Multicentre, randomised, double blind, phase 3 trial.
BMJ. 377:e0687142022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohm JE, Gabrilovich DI, Sempowski GD,
Kisseleva E, Parman KS, Nadaf S and Carbone DP: VEGF inhibits
T-cell development and may contribute to tumor-induced immune
suppression. Blood. 101:4878–4886. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu H, Chen Y, Tan S, Wu S, Huang Y, Fu S,
Luo F and He J: The research Progress of antiangiogenic therapy,
immune therapy and tumor microenvironment. Front Immunol.
13:8028462022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Wang JW, Liu YY, Yu QT, Zhang YP,
Li K, Xu LY, Luo SX, Qin FZ, Chen ZT, et al: Long-term results of a
randomized, double-blind, and placebo-controlled phase III trial:
Endostar (rhendostatin) versus placebo in combination with
vinorelbine and cisplatin in advanced non-small cell lung cancer.
Thorac Cancer. 4:440–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pu X, Wang Q, Liu L, Chen B, Li K, Zhou Y,
Sheng Z, Liu P, Tang Y, Xu L, et al: Rh-endostatin plus
camrelizumab and chemotherapy in first-line treatment of advanced
non-small cell lung cancer: A multicenter retrospective study.
Cancer Med. 12:7724–7733. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Zhong P, Zhu X, Fu S, Li S, Peng
S, Liu Y, Lu Z and Chen L: Immunotherapy combined with
rh-endostatin improved clinical outcomes over immunotherapy plus
chemotherapy for second-line treatment of advanced NSCLC. Front
Oncol. 13:11372242023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Yao Q, Huang M, Wang B, Zhang J,
Wang T, Ming Y, Zhou X, Jia Q, Huan Y, et al: A randomized Phase
III trial of neoadjuvant recombinant human endostatin, docetaxel
and epirubicin as first-line therapy for patients with breast
cancer (CBCRT01). Int J Cancer. 142:2130–2138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Jin F, Jiang S, Cao J, Meng Y, Xu
Y, ChunmengWan g, Chen Y, Yang H, Kong Y, et al: Rh-endostatin
combined with chemotherapy in patients with advanced or recurrent
mucosal melanoma: retrospective analysis of real-world data. Invest
New Drugs. 40:453–460. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuchida Y and Therasse P: Response
evaluation criteria in solid tumors (RECIST): New guidelines. Med
Pediatr Oncol. 37:1–3. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chinese Anti-Cancer Association, . Chinese
expert consensus on PD-L1 immunohistochemical detection criteria
for non-small cell lung cancer. Chin J Lung Cancer. 23:733–740.
2020.
|
|
24
|
Mischel AM and Rosielle DA: Eastern
Cooperative Oncology Group Performance Status #434. J Palliat Med.
25:508–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events v3.0 (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfFebruary
6–2024
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bureau of Medical Administration and
National Health Commission of the People's Republic of China, .
Standardization for diagnosis and treatment of esophageal cancer
(2022 edition). Chin J Dig Surg. 21:1247–1268. 2022.(In
Chinese).
|
|
28
|
Wang B, Zhao Q, Zhang Y, Liu Z, Zheng Z,
Liu S, Meng L, Xin Y and Jiang X: Targeting hypoxia in the tumor
microenvironment: A potential strategy to improve cancer
immunotherapy. J Exp Clin Cancer Res. 40:242021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan KA and Kerbel RS: Improving
immunotherapy outcomes with anti-angiogenic treatments and vice
versa. Nat Rev Clin Oncol. 15:310–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian L, Goldstein A, Wang H, Ching Lo H,
Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, et
al: Mutual regulation of tumour vessel normalization and
immunostimulatory reprogramming. Nature. 544:250–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmidt EV: Developing combination
strategies using PD-1 checkpoint inhibitors to treat cancer. Semin
Immunopathol. 41:21–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Voron T, Colussi O, Marcheteau E, Pernot
S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N,
Tanchot C, et al: VEGF-A modulates expression of inhibitory
checkpoints on CD8(+) T cells in tumors. J Exp Med. 212:139–148.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allen E, Jabouille A, Rivera LB,
Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D,
Michael IP and Bergers G: Combined antiangiogenic and anti-PD-L1
therapy stimulates tumor immunity through HEV formation. Sci Transl
Med. 9:eaak96792017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Manning EA, Ullman JG, Leatherman JM,
Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM and
Emens LA: A vascular endothelial growth factor receptor-2 inhibitor
enhances antitumor immunity through an immune-based mechanism. Clin
Cancer Res. 13:3951–3959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fuming Qiu, Fan J, Shao M, Yao J, Zhao L,
Zhu L, Li B, Fu Y, Li L, Yang Y, et al: Efficacy of two - and
three-cycle neoadjuvant sinadilizumab plus platinum-based
double-agent chemotherapy in patients with resectable non-small
cell lung cancer (neoSCORE): A randomized, single-center, two-arm
Phase II trial. J Clin Oncol. 40:8500. 2022. View Article : Google Scholar
|
|
38
|
Huizing MT, Keung AC, Rosing H, van der
Kuij V, ten Bokkel Huinink WW, Mandjes IM, Dubbelman AC, Pinedo HM
and Beijnen JH: Pharmacokinetics of paclitaxel and metabolites in a
randomized comparative study in platinum-pretreated ovarian cancer
patients. J Clin Oncol. 11:2127–2135. 1993. View Article : Google Scholar : PubMed/NCBI
|