Introduction
Mortality caused by cancers including colorectal
cancer (CRC) has continued to decline recently because of earlier
detection and improved treatment options. Incidence rates, however,
increased for CRC in young adults, which is first leading cause of
cancer death in men and second in women (1). Despite continued overall declines, CRC
is rapidly shifting to diagnosis at a younger age, in the left
colon or rectum, and at a more advanced or metastatic stage
(2). Chemotherapy regimens for
metastatic CRC are classified as first-, second-, and third-line
treatment such as 5-fluorouracil (5-FU), epidermal growth factor
(EGF) receptor (EGFR) inhibitor, and src kinase inhibitor,
respectively (3).
CDH1 gene located on chromosome 16q22.1 is composed
of 16 exons. It encodes the E-cadherin protein. Frequent mutations
of CDH1 gene have been reported mostly in gastric cancers (4,5), in
which most variations have been identified in exons 4–12 encoding
the extracellular domain. Based on cell-cell adhesion properties,
E-cadherin is known as a tumor suppressor protein in experimental
cancer researches. E-cadherin, however, might promote proliferation
via interaction with EGFR, resulting in EGF-dependent ERK
activation in CRC (6–8). Lack or dysfunction of the
transmembrane domain of E-cadherin might trigger cell proliferation
or epithelial-mesenchymal transition (EMT) by activating β-catenin.
The loss or dysfunction on extracellular domain of E-cadherin might
accelerate malignant transformation by ruining EGFR/E-cadherin
complex (9,10).
A three-dimensional (3D) spheroid formation model is
a basic tool for cancer research (11), because it resembles in vivo
solid tumors by anchorage-independent EMT (12). In this experimental setting, cells
initially aggregate and then form compact spheroids via E-cadherin
(12), which is inversely
correlated with EMT similar to malignant transformation (13). Although E-cadherin is frequently
downregulated with carcinogenesis, cell-cell adherence between
cancer cells is disturbed after adding a soluble fragment of
E-cadherin, leading to malignant transformation (14,15).
Contrary to the well-known tumor suppressor role of E-cadherin
(9,10), the extracellular domain of
E-cadherin can act as EGF to EGFR. Therefore, the extracellular
domain of E-cadherin has been suggested as a possible oncogene
(14,16,17).
Growth factors or fetal bovine serum (FBS) could be
supplemented in a spheroid formation model, although the efficiency
could differ between cell lines (12,18,19).
We have previously revealed that spheroid formation is related to
soluble E-cadherin and pan-RAS in CRC cells, including
5-FU-resistance acquired cell line, but not related to any marker
for cancer stem cells or drug resistance (19). Increased soluble E-cadherin during
spheroid formation has only been observed in FBS-supplemented
environment, not in growth factor-supplemented environment
irrespective of 5-FU resistance. Therefore, application of
exogenous E-cadherin in FBS-supplemented spheroid formation is
needed to reveal whether soluble E-cadherin is a result or a cause
of spheroidogenesis.
The extracellular domain of E-cadherin (around 80
kDa) is a soluble fragment. A commercial E-cadherin protein (around
64.4 kDa) could be collectively regarded as a soluble E-cadherin
(19). Exogenous soluble E-cadherin
(around 1 µg/ml) is known to contribute to ultraviolet-induced skin
carcinogenesis via association with human epidermal growth factor
receptor (14). Treatment with
DECMA-1 (antibody against the ectodomain of E-cadherin) at 1 mg/kg
can suppress breast carcinogenesis in an in vivo model
(20). Although germline variants
in CDH1 are associated with elevated risks of diffuse gastric
cancer (21), whether they could
increase the risk of CRC has not been reported yet. Effects of
E-cadherin on FBS-supplemented spheroid formation and associated
mechanisms have not been suggested either. Thus, this study aimed
to investigate whether exogenous soluble E-cadherin or DECMA-1
could affect spheroidogenesis of CRC cells and determine possible
mechanisms involved.
Materials and methods
Reagents and antibodies
Reagents and antibodies used in this study are
summarized in Table I.
 | Table I.Reagents and antibodies used in the
experiment. |
Table I.
Reagents and antibodies used in the
experiment.
| A, Reagents |
|---|
|
|---|
| Name (cat.
no.) | Company | Purpose/action |
|---|
| MTT (M6494) | Thermo Fisher
Scientific | Cell viability |
| 5-FU (F6627);
erlotinib (S2156) | Sigma-Aldrich
(Merck KGaA) | Anti-cancer
drugs |
| Saracatinib
(11497) | Cayman
Chemical |
|
| T-PER™ protein
extraction reagent (78510) | Thermo Fisher
Scientific | Preparation for
western blotting |
| Protease inhibitor
cocktail (ab201111); | Abcam | Preparation for
western blotting |
| phosphatase
inhibitor cocktail I (ab201112); |
|
|
| phosphatase
inhibitor cocktail II (ab201113) |
|
|
| Pierce™ BCA protein
assay kit (23227) | Thermo Fisher
Scientific | Preparation for
western blotting |
| PD98059 (9900) | Cell Signaling
Tech | MEK-ERK
inhibitor |
| Hematoxylin
(H-3404) | Vector
Laboratories |
Counter-staining |
| Human E-cadherin
protein (ab235682) | Abcam | Recombinant
E-cadherin protein for |
|
|
|
spheroidogenesis |
| DECMA-1
(ab11512) | Abcam | Anti-E-cadherin
antibody |
|
|
| (intercellular
junction marker) |
|
|
| for
spheroidogenesis |
| Human E-cadherin
SimpleStep ELISA Kit (ab233611) | Abcam | Soluble E-cadherin
detection |
|
| B,
Antibodies |
|
| Name (cat.
no.) | Company |
Purpose/action |
|
| Phosphor-ERK
(4370) | Cell Signaling
Tech | Primary
antibody |
| EGFR (ab52894) | Abcam | Primary
antibody |
| ERK (sc-93);
β-catenin (sc-7199); | Santa Cruz
Biotechnology | Primary
antibody |
| pan-RAS
(sc-166691); β-actin (sc-47778); |
|
|
| GAPDH
(sc-47724) |
|
|
| Anti-mouse IgG
(PI-2000); anti-rabbit IgG (PI-1000) | Vector
Laboratories | Secondary
antibody |
| ImmPRESS Reagent
kit (MP-7401) | Vector
Laboratories |
Immunocytochemistry |
Cell culture
Wild-type (WT) and CDH1 knock-out (KO) HCT116 cells
were purchased from SYNTHEGO (Synthego Engineered cells; Redwood
city, CA, USA). CDH1 KO was performed using the CRISPR/Cas-9 system
targeting the exon 3, corresponding to the precursor of CDH1. Guide
RNA sequences were ‘UUACAGUCAAAAGGCCUCUA’, ‘CUUUCUGUAGGUGGAGUCCC’,
and ‘AACAUACCUGAUGGGGCGGG’.
Cells were cultured in McCoy's 5A medium
supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin,
and 100 mg/ml streptomycin at 5% CO2, 37°C, and
humidified atmosphere conditions. All cell culture reagents were
obtained from Corning Inc. (Corning, NY, USA).
Cell viability assay
Cell proliferation was determined using MTT assay.
Briefly, cells were plated in a 96-well plate (2×103
cells/well in 200 µl of media). Starting from the day of seeding
(day 0), cell viability was checked for 4 days consecutively. Each
day, 20 µl of MTT reagent was added to each well. After 3 h,
formazan crystals were dissolved in dimethylsulfoxide. The
absorbance was read at 595 and 620 nm using a VERSAmax microplate
reader (Molecular Devices Korea LLC.; Seoul, Republic of Korea).
Absorbance values of treated cells were compared to those of
vehicle-treated cells, representing 100% cell viability.
Cells were seeded in triplicate wells of 96-well
plates, and treated with 5-FU, erlotinib, saracatinib (0, 0.1, 1,
10, and 100 µM/each), E-cadherin (0, 0.1, 1, 10, and 100 ng/ml),
DECMA-1 (0, 1, 10, 100, and 1,000 ng/ml), or PD98059 (0, 1, 10, 20,
and 50 µM). After cells were incubated for 3 days, cell viability
was assessed as described above.
Spheroid formation
Ultra-low attachment 96-well plates were purchased
from Corning Inc. (Corning) to create an anchorage-independent
environment. Cells were cultured with conventional culture media as
described above. After adding E-cadherin (0, 1, 10, 100, and 1,000
ng/ml), DECMA-1 (0, 1, 10, 100, and 1,000 ng/ml), or PD98059 (0, 1,
10, 20, and 50 µM), cells were cultured in round bottom plates
(Corning, #7007) at a density of 2×102 cells/well.
Long-term changes of spheroidogenesis were evaluated using flat
bottom plates (Corning, #3474) at a density of 2×103
cells/well. Spheroid formation was checked for morphometry on day 5
in round bottom plates or on days 7, 14, and 21 in flat bottom
plates.
Western blot analysis
WT and CDH1 KO cells were cultured in conventional
2D monolayer for 3 days with or without PD98059 (20 µM) and in
spheroid formation culture in flat bottom plates for 7, 14, and 21
days. Cells and spheroids were harvested in T-PERTM
protein extraction reagent including 1% protease inhibitor
cocktail, 0.5% phosphatase inhibitor cocktail I, and 0.5%
phosphatase inhibitor cocktail II. Protein concentrations were
assessed by BCA protein assay according to the manufacturer's
instructions (19).
Cell lysates (30 µg protein/lane) were subjected to
8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis,
transferred to a polyvinylidene difluoride membrane (#162-0176;
Bio-Rad Laboratories, Hercules, CA, USA), and then blocked with 5%
skimmed milk at room temperature for 1h. These membranes were
incubated with primary antibodies (diluted 1:1,000; 1:2,000 for
β-actin and GAPDH) at 4°C overnight and appropriate secondary
antibodies (diluted 1:2,000) at room temperature for 1h. Protein
bands were detected using the AzureTM c300 and
quantified using the AzureSpot analysis software (version 14.2;
Azure Biosystems, Inc., Dublin, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed according to the manufacturer's
instruction (19). Briefly,
conventional 2D monolayer and spheroid formation culture media
samples (50 µl/each) were added into each well to bind E-cadherin
antibody cocktail (50 µl/each) followed by incubation at room
temperature for 1 h. After wells were washed with a washing buffer,
TMB development solution (100 µl/each) was added into each well
followed by incubation for 10 min at room temperature. A stop
solution (100 µl/each) was then added and optical density was
measured at 450 nm using a VERSAmax microplate reader.
Immunocytochemistry
One and three weeks after spheroid formation
culture, formed spheres (>70 µm) were collected using a cell
strainer with a pore size of 70 µm (Corning Inc., #CLS431751) and
fixed with 4% paraformaldehyde for 24 h at 4°C. The
paraffin-embedded sections (4 µm-thick) were cut with a microtome
and prepared for immunocytochemistry as previously described
(19). These sections were then
blocked with 10% normal horse serum for 1 h at room temperature.
Incubation with an anti-E-cadherin antibody (diluted 1:100) was
performed at 4°C overnight and an anti-rabbit secondary antibodies
(ready-to-use) for 1h at room temperature. The binding was
visualized using 3,3′-diaimnobenzidine, and nuclei were
counterstained with hematoxylin (#H-3404, Vector) for 1 min at room
temperature. Immunolabelled images were directly captured using a
DP22 digital camera and BX-51 light microscope (Olympus, Tokyo,
Japan).
Statistical analysis
All data were compiled from a minimum of three
replicate experiments. Data are expressed as mean values ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference as determined using Student's t-test or
two-way analysis of variance (ANOVA) followed by a Bonferroni
post-hoc test. MS Excel 2016 was used for statistical analysis.
Results
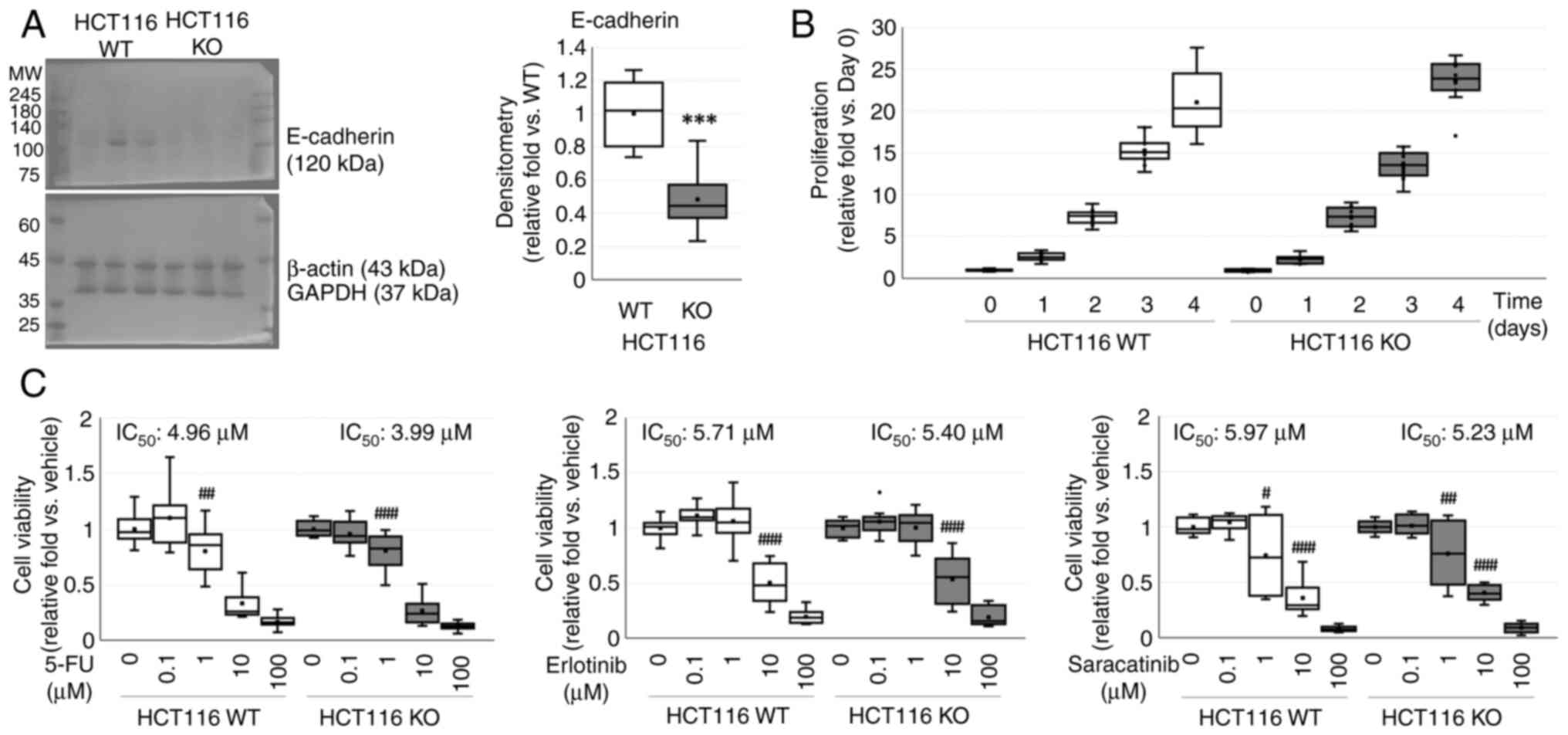
Characteristics of WT and CDH1 KO
HCT116 cells
E-cadherin in CDH1 KO cells was decreased
0.48±0.05-fold (P<0.001) compared to that in WT HCT116 cells
(Fig. 1A). WT and CDH1 KO HCT116
cells showed similar proliferation curves in a time-dependent
manner (P<0.001), but no difference between cell types (P=0.760)
(Fig. 1B).
Effects of anticancer drugs on cell viability were
assessed using the MTT assay (Fig.
1C). Cell viability was decreased in a dose-dependent manner
(P<0.001 for both) in both WT and CDH1 KO HCT116 cells with
similar IC50 values, but no differences between cell
types against 5-FU (P=0.065), erlotinib (P=0.516), and saracatinib
(P=0.802).
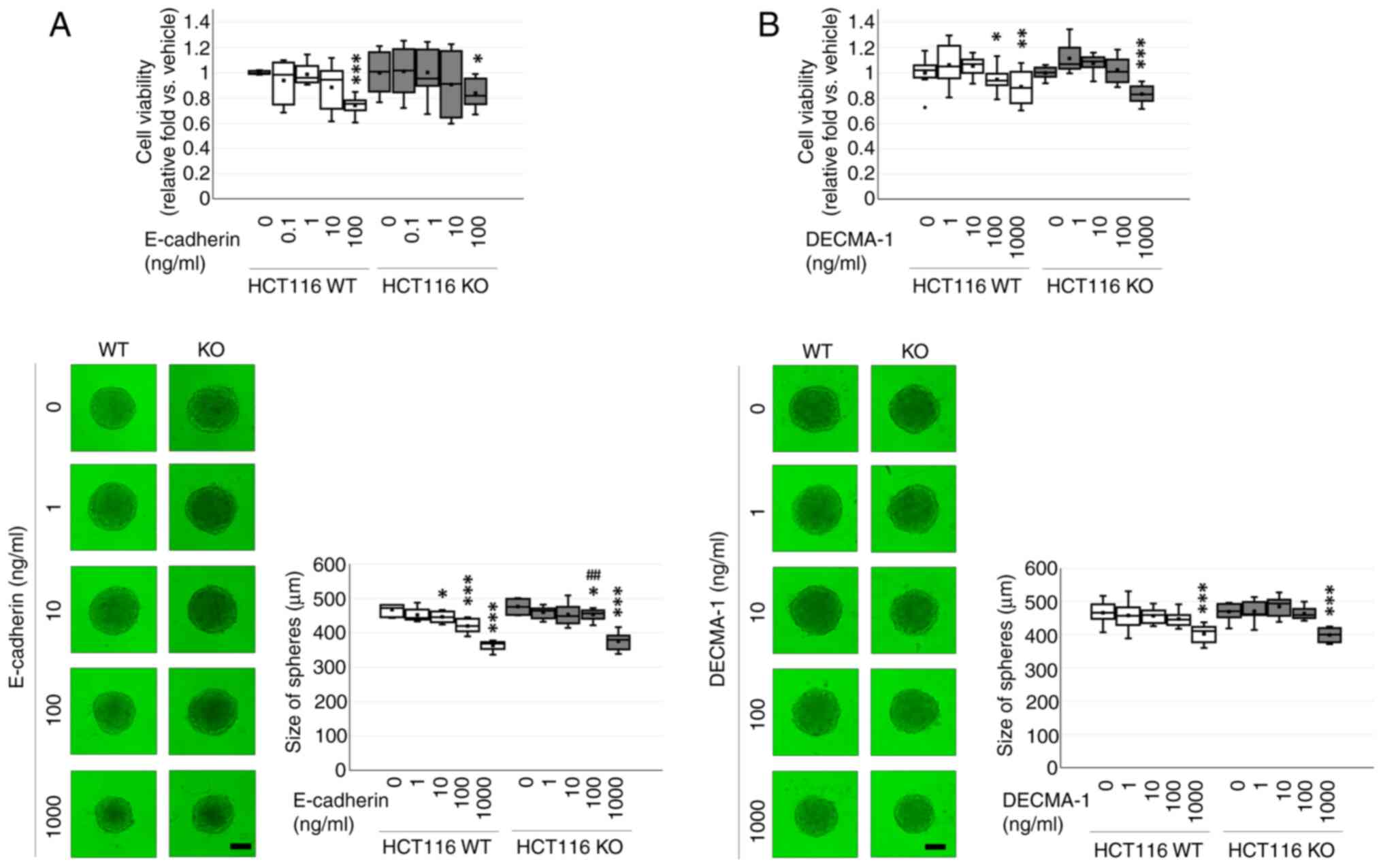
Effects of exogenous E-cadherin and
anti-E-cadherin antibody on WT and CDH1 KO HCT116 cells
Cell viability was decreased after exogenous
E-cadherin treatment in a dose-dependent manner (P=0.001) in both
WT and CDH1 KO HCT116 cell, but no differences between cell types
(P=0.248). Exogenous E-cadherin at 100 ng/ml decreased cell
viability of WT (0.7±0.03-fold, P<0.001) and CDH1 KO
(0.84±0.04-fold; P=0.019) HCT116 cells. Exogenous E-cadherin
inhibited spheroid formation in a dose-dependent manner
(P<0.001) and between WT and CDH1 KO HCT116 cell types
(P=0.003). Significant difference between cells was observed only
at 100 ng/ml (P=0.002) of WT (0.90±0.02-fold) and CDH1 KO
(0.95±0.01-fold) HCT116 cells (Fig.
2A).
Cell viability was decreased after exogenous DECMA-1
treatment in a dose-dependent manner (P<0.001) in both WT and
CDH1 KO HCT116 cells, but no differences between cell types
(P=0.427). Exogenous DECMA-1 at 100 ng/ml decreased viability of WT
(0.87±0.03-fold; P=0.002) and CDH1 KO (0.82±0.03-fold; P=0.042)
HCT116 cells without causing an inter-cellular difference.
Exogenous DECMA-1 inhibited spheroid formation in a dose-dependent
manner (P<0.001) in both WT and CDH1 KO HCT116 cells, but no
differences between cell types (P=0.086). Exogenous DECMA-1 at 1000
ng/ml suppressed spheroidogenesis of WT (0.86±0.01-fold;
P<0.001) and CDH1 KO (0.85±0.02-fold; P<0.001) HCT116 cells
(Fig. 2B).
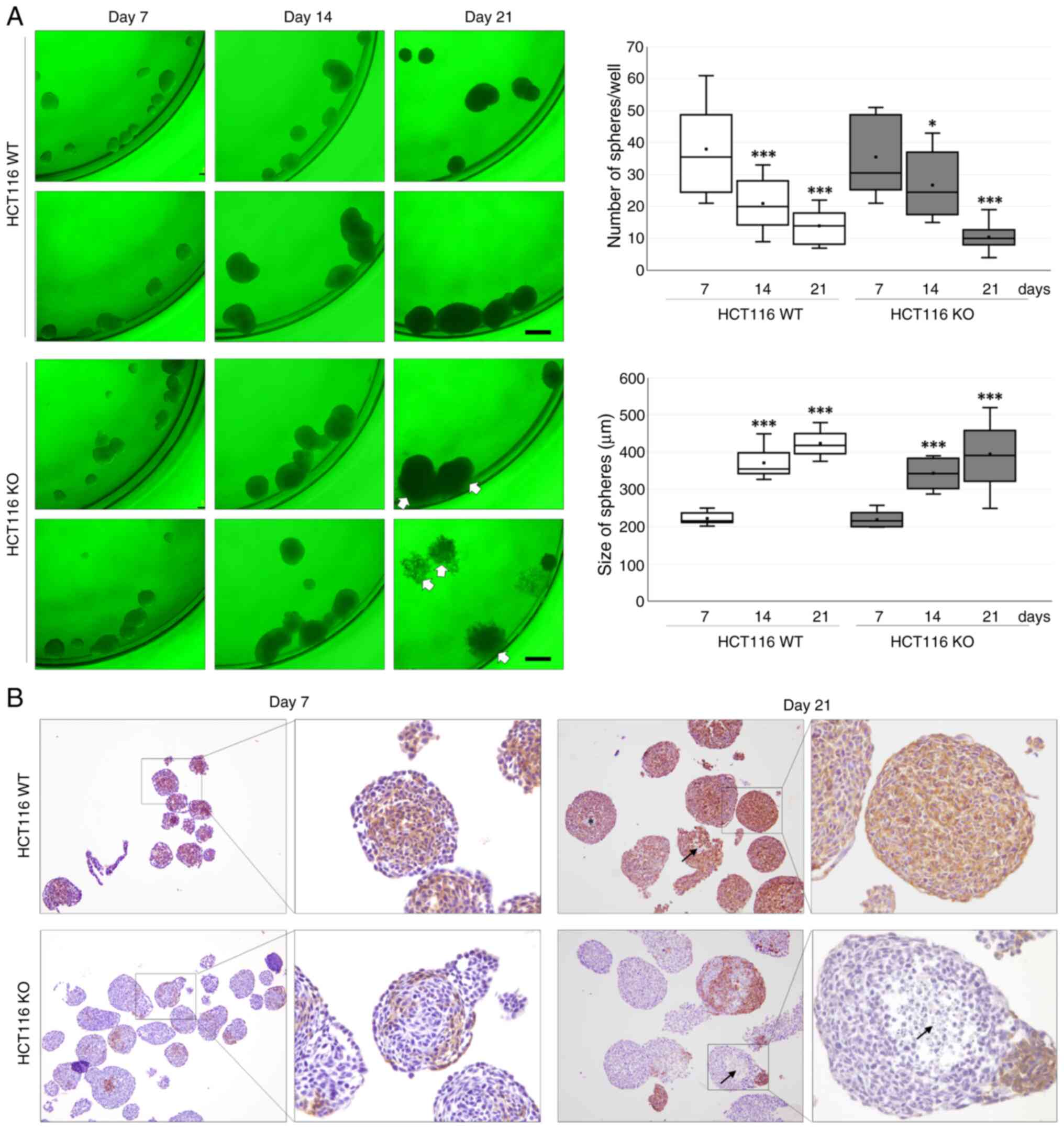
Long-term spheroidogenesis of WT and
CDH1 KO HCT116 cells
Long-term spheroidogenesis was evaluated until day
21 in WT and CDH1 KO HCT116 cells. Although the number of spheroids
was decreased in a time-dependent manner (P<0.001) in both WT
and CDH1 KO HCT116 cells, there was no difference between cell
types (P=0.970). The size of spheroids was increased in a
time-dependent manner (P<0.001) in both WT and CDH1 KO HCT116
cells, but no differences between cell types (P=0.055). Compared to
WT HCT116 cells, CDH1 KO HCT116 cells showed some scattering or
inability to maintain the shape of spheroids (Fig. 3A).
Morphological features were evaluated by performing
immunostaining of E-cadherin (Fig.
3B). E-cadherin was immunostained in spheroids of WT HCT116
cells from day 7 to day 21. In spheroids from CDH1 KO HCT116 cells,
E-cadherin immunostaining was only observed for a few cells. Some
central area was neither immunostained with E-cadherin nor stained
with hematoxylin for nuclei, suggesting necrotic changes.
As compared with 2D monolayer culture, spheroid
cultures showed significant increase of E-cadherin in a
time-dependent manner (P<0.001) and between WT and CDH1 KO
HCT116 cells (P<0.001). Although E-cadherin was increased by
about 4 times in spheroids as compared to that in 2D culture of WT
HCT116 cells, it was increased by about 2 times in spheroid
cultures of CDH1 KO HCT116 cells (Fig.
4A and Table II).
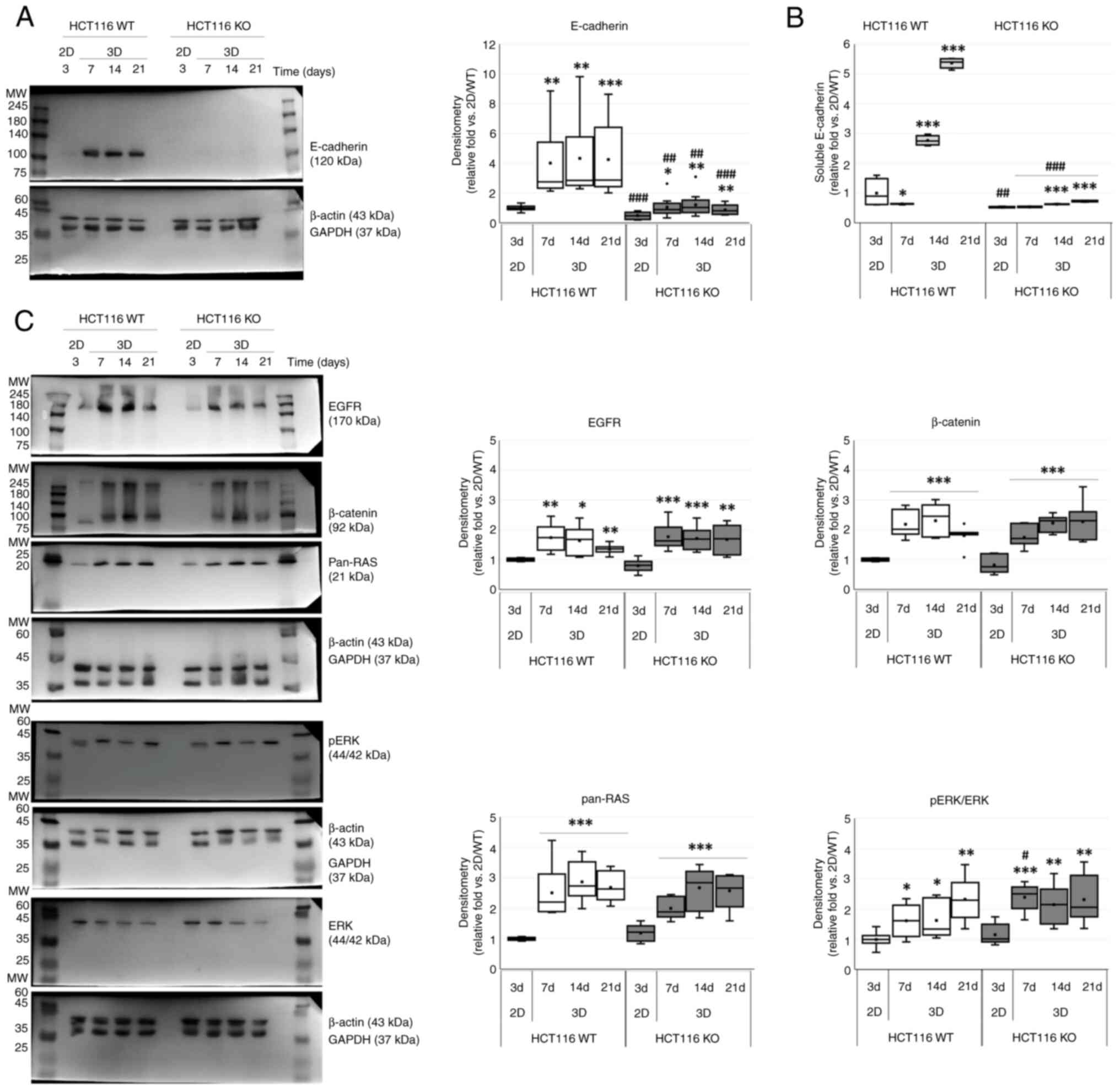 | Figure 4.Characteristics of
long-term-maintained spheroids of WT and KO HCT116 cells. (A)
Western blotting showing significant increase of E-cadherin in
spheroids (3D) as compared to conventional 2D culture in both cell
lines. (B) ELISA on culture media showing time-dependent increases
of soluble E-cadherin in spheroid culture, with such increases
being prominent in WT HCT116 cells. (C) Western blotting showing
significant increases of EGFR, β-catenin, pan-RAS, and ERK
activation in spheroid culture as compared to 2D cultures in both
cell lines. *P<0.05, **P<0.01, ***P<0.001 vs. 2D;
#P<0.05, ##P<0.01,
###P<0.001 vs. WT at same time. WT, wild-type; KO,
knock-out; 2D, 2-dimensional; 3D, 3-dimensional. |
 | Table II.Densitometric comparison of spheroid
formation between WT and CDH1 KO HCT116 colorectal cancer
cells. |
Table II.
Densitometric comparison of spheroid
formation between WT and CDH1 KO HCT116 colorectal cancer
cells.
|
|
|
| Spheroids |
|---|
|
|
|
|
|
|---|
| Protein | Cell type | 2D | Day 7 | Day 14 | Day 21 |
|---|
| E-cadherin | WT | 1.00±0.17 | 4.01±0.73
(bP=0.001) | 4.34±0.85
(bP=0.002) | 4.24±0.74
(bP=0.001) |
|
| KO | 0.49±0.08 | 1.05±0.21
(aP=0.014; | 1.22±0.24
(bP=0.008; | 0.88±0.10
(bP=0.003; |
|
|
| (fP<0.001) | eP=0.001) | eP=0.003) | fP<0.001) |
| EGFR | WT | 1.00±0.05 | 1.74±0.46
(bP=0.006) | 1.64±0.50
(aP=0.013) | 1.35±0.17
(bP=0.001) |
|
| KO | 0.80±0.22 | 1.77±0.45
(cP<0.001) | 1.71±0.41
(cP<0.001) | 1.68±0.49
(bP=0.001) |
| β-catenin | WT | 1.00±0.05 | 2.19±0.46
(cP<0.001) | 2.31±0.53
(cP<0.001) | 1.81±0.35
(cP<0.001) |
|
| KO | 0.83±0.29 | 1.75±0.36
(cP<0.001) | 2.68±0.27
(cP<0.001) | 2.28±0.65
(cP<0.001) |
| Pan-RAS | WT | 1.00±0.05 | 2.51±0.85
(cP<0.001) | 2.86 0.64
(cP<0.001) | 2.69±0.49
(cP<0.001) |
|
| KO | 1.19±0.28 | 2.00±0.35
(cP<0.001) | 2.67±0.67
(cP<0.001) | 2.57±0.57
(cP<0.001) |
| pERK/ERK | WT | 1.00±0.27 | 1.62±0.55
(aP=0.016) | 1.62±0.62
(aP=0.029) | 2.32±0.72
(bP=0.003) |
|
| KO | 1.16±0.35 | 2.40±0.45
(cP<0.001; | 2.15±0.66
(bP=0.005) | 2.31±0.80
(bP=0.007) |
|
|
|
| dP=0.012) |
|
|
Concentrations of E-cadherin in cultured media of WT
and CDH1 KO HCT116 cells for 2D monolayer and spheroid cultures
were estimated by ELISA (Fig. 4B).
ELISA results showed significant increase of soluble E-cadherin in
a time-dependent manner (P<0.001) and between WT and CDH1 KO
HCT116 cell types (P<0.001). The ratio of soluble E-cadherin in
WT HCT116 cells was found to be significantly increased at 14
(2.76±0.05-fold vs. 1.19±0.03-fold; P<0.001) and 21
(5.36±0.05-fold vs. 1.39±0.05-fold; P<0.001) days after
incubation in WT and CDH1 KO HCT116 cells as compared with 2D
monolayer culture condition.
E-cadherin related proteins such as EGFR, β-catenin,
pan-RAS, and ERK were further checked in spheroidogenesis. The
proteins examined were significantly increased in spheroids as
compared to those under 2D culture conditions in a time-dependent
manner (P<0.001 for both) in both WT and CDH1 KO HCT116 cells,
but there was no difference between cell types on EGFR (P=0.618),
β-catenin (P=0.597), and pan-RAS (P=0.253), except ERK activation
(P=0.037). ERK activation was significantly increased between cell
types at 7 days after spheroidogenesis (P=0.012) (Fig. 4C; Table
II).
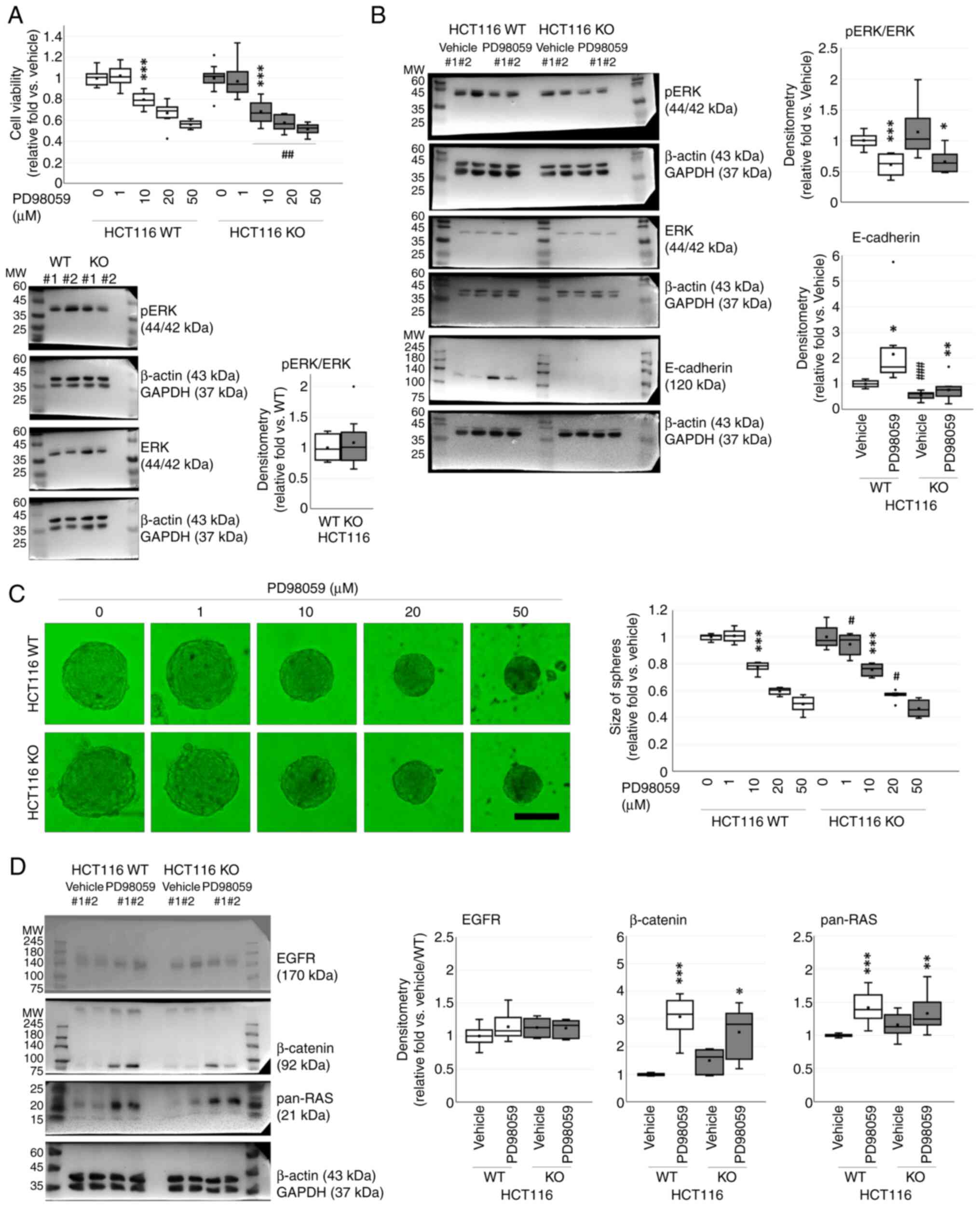
PD98059-induced E-cadherin on
spheroidogenesis of WT and CDH1 KO HCT116 cells
As a significant change on ERK activation was
observed in spheroidogenesis, a MEK-ERK inhibitor (PD98059) was
adopted. Cell viability was decreased after PD98059 treatment in a
dose-dependent manner (P<0.001) and between WT and CDH1 KO
HCT116 cells (P<0.001). Cell viability was significantly
decreased by PD98059 at a concentration of 10 µM (0.80±0.02-fold
vs. 0.68±0.03-fold; P<0.001 for both). Intercellular difference
in viability was observed for groups treated with PD98059 at 10
(P=0.002), 20 (P=0.005), or 50 µM (P=0.008) (Fig. 5A).
There was no significant difference in ERK
activation between WT and CDH1 KO (1.08±0.12-fold; P=0.283) HCT116
cells (Fig. 5A). ERK activation was
significantly decreased after PD98059 (20 µM; P<0.001)
treatment, but there was no difference between cell types
(P=0.388). However, E-cadherin was significantly increased depend
on PD98059 treatment (P=0.004) and between WT and CDH1 KO HCT116
cells (P<0.001). Although E-cadherin was lower in CDH1 KO HCT116
cells as compared to WT HCT116 cells, PD98059 considerably
increased E-cadherin (Fig. 5B and
Table II).
PD98059 inhibited spheroid formation in a
dose-dependent manner (P<0.001) and between WT and CDH1 KO
HCT116 cells (P=0.014) At 10 µM, it significantly decreased
spheroid formation in both cells (P<0.001 for both).
Intercellular difference was observed at 1 µM (1.01±0.05-fold vs.
0.95±0.08-fold; P=0.034) and 20 µM (0.60±0.01-fold vs.
0.57±0.01-fold; P=0.032) of PD98059 (Fig. 5C).
The related proteins suggested in spheroidogenesis
were further checked. β-catenin and pan-RAS were significantly
increased after PD98059 treatment (P<0.001 for both), but there
was no difference between WT and CDH1 KO HCT116 cells on β-catenin
(P=0.913) and pan-RAS (P=0.698). However, EGFR did not change after
PD98059 treatment (P=0.346) or between cell types (P=0.451)
(Fig. 5D; Table III).
 | Table III.Densitometric comparison of effects
of PD98059 treatment on WT and CDH1 KO HCT116 colorectal cancer
cells. |
Table III.
Densitometric comparison of effects
of PD98059 treatment on WT and CDH1 KO HCT116 colorectal cancer
cells.
| Protein | Cell type | Vehicle | PD98059 |
|---|
| pERK/ERK | WT | 1.00±0.11 | 0.61±0.16
(cP<0.001) |
|
| KO | 1.14±0.40 | 0.66±0.18
(aP=0.022) |
| E-cadherin | WT | 1.00±0.12 | 2.15±1.26
(aP=0.012) |
|
| KO | 0.55±0.16 | 0.76±0.18
(eP=0.004) |
|
|
| (fP<0.001) |
|
| EGFR | WT | 1.00±0.16 | 1.14±0.22 |
|
| KO | 1.13±0.16 | 1.12±0.13 |
| β-catenin | WT | 1.00±0.04 | 3.08±0.74
(cP<0.001) |
|
| KO | 1.50±0.43 | 2.52±0.92
(bP=0.005) |
| Pan-RAS | WT | 1.00±0.02 | 1.58±0.24
(cP<0.001) |
|
| KO | 1.18±0.17 | 1.45±0.28
(eP=0.005) |
|
|
| (bP=0.001) |
|
Discussion
This study determined whether exogenous E-cadherin
could affect spheroidogenesis of CRC cells in FBS-supplemented
environment. First of all, we set CDH1 KO condition targeting the
precursor exon 3 to compare general characteristics of HCT116
cells. CDH1 KO did not affect cell proliferation, chemotherapeutic
responses against respective first- to third-line regimens, or
spheroidogenesis as compared to WT HCT116 cells. The addition of
exogenous soluble E-cadherin or DECMA-1 did not cause significant
changes between WT and CDH1 KO HCT116 cells. Subtle differences in
long-term maintenance of spheroids were observed. Some spheroids
from CDH1 KO HCT116 cells did not maintain their shapes
consistently at day 21. However, total number and mean size of
spheroids were not considerably changed between WT and CDH1 KO
HCT116 cells.
Controversy over E-cadherin on spheroidogenesis has
been continued. Cells can aggregate and then form spheroids via
E-cadherin during the initial spheroidogenesis in any environment
(12). Although the expression of
E-cadherin (120 kDa) was decreased during spheroid formation
(22,23), E-cadherin might also induce EMT of
CRC cells (24). Based on decreased
E-cadherin and increased soluble E-cadherin in FBS-supplemented
spheres (19), we have previously
suggested that soluble E-cadherin might promote growth of spheroids
and be an essential component of spheroidogenesis in
FBS-supplemented environment. This hypothesis can be reinforced by
previous studies showing that soluble E-cadherin could be an
oncogene like EGF in spheroidogenesis (14–16) as
well as conventional cultures (14). Contrary to expectations, the
addition of E-cadherin protein or DECMA-1 did not show any
significant effect on spheroidogenesis of HCT116 cells irrespective
of CDH1 KO.
The activation of ERK was not different between WT
and CDH1 KO HCT116 cells, although it was increased in
spheroidogenesis. When ERK activity was inhibited by PD98059,
proliferation of both WT and CDH1 KO HCT116 cells was also
inhibited in a dose-dependent manner. While E-cadherin was
significantly increased by PD98059 treatment, it did not lead to
significant changes in spheroidogenesis of WT or CDH1 KO HCT116
cells. As compared to spheroidogenesis, PD98059 did not affect EGFR
expression. EGF-dependent ERK activation (6–8) might
be a relevant pathway in CRC. However, the possibility of soluble
E-cadherin as an oncogene like EGF was not supported in this
study.
ERK might promote EMT-like phenotype from E-cadherin
to N-cadherin shift (25,26). The condition of an activated ERK
with E-cadherin suppressed is known to promote proliferation and/or
metastasis in CRC (27–33). Opposite results on ERK and
E-cadherin have been reported (34–38),
similar to results of the present study. When ERK activation was
inhibited by PD98059, the proliferation of both WT and CDH1 KO
HCT116 cells was inhibited with E-cadherin expression increased.
Although ERK activation might have no effect on EMT in CRC
(39), it decreased proliferation
and inhibited invasion of CRC when E-cadherin was stabilized
(40). In this context,
overexpression of E-cadherin (41)
or soluble E-cadherin (24) can
stimulate EGFR and EGF-dependent ERK activation, resulting in
cancer stem cell properties such as spheroid formation. However,
when ERK activity was inhibited by PD98059, spheroidogenesis was
also inhibited by PD98059 in a dose-dependent manner regardless
whether E-cadherin expression was induced or CDH1 was knocked
out.
To suggest details of prognostic factors for highly
aggressive soft tissue sarcomas, inflammatory markers were
investigated. Although clinical trial did not reveal significant
differences between inflammatory markers of remission and
non-remission cases (42), the
tumor microenvironment would be different from tumors. E-cadherin
might be involved in the pathogenesis of inflammatory breast
cancer, but not that of non-inflammatory breast cancer, in which a
positive association between E-cadherin loss and poor prognosis has
been suggested (43,44). While reduced or loss of E-cadherin
in localized tumor cells is correlated with proliferation, an
increased level of soluble E-cadherin in circulation might be a
marker for the extent of damaged skin caused by tumor and/or
inflammation (45) or
bacteria-induced diseases (46).
Overexpression of soluble cell adhesion molecules, such as soluble
E-cadherin, in body fluids can trigger inflammation and
pro-carcinogenic programming leading to tumor induction and
metastasis (46). Although the
relationship among soluble E-cadherin, colon cancer and
inflammation has not been thoroughly investigated, the potential
value of E-cadherin for liquid biopsy in cancers has been suggested
since soluble E-cadherin is significantly elevated in patients
suffering from epithelial cancers including CRCs (47,48).
Although soluble E-cadherin becomes hard to generalize the value
due to lack of specificity and sensitivity as compared to existing
tumor markers, such as carcinoembryonic antigen, it has been
suggested as an alternative diagnostic biomarker for monitoring
advanced CRC (49,50). Soluble E-cadherin can diffuse into
the extracellular environment including blood and/or urine and act
as a paracrine and/or autocrine signaling molecule (15). In this study, soluble E-cadherin was
significantly increased in long-term maintained spheres with an
FBS-supplemented environment, not in GF-supplemented environment as
previously reported (19). This
means that soluble E-cadherin cannot act as an oncogene like EGF.
Although spheroidogenesis might not be closely related to CDH1
expression in HCT116 cells, the soluble E-cadherin showed
significantly increases in a time-dependent manner. Therefore, it
could be a biological marker for monitoring the progression of
CRCs. However, this relationship of soluble E-cadherin with
inflammation should further be investigated in CRC.
However, the present study has certain limitations.
First of all, the possible role of soluble E-cadherin as on
oncogene was suggested in SNU-C5 CRC cells (19), but there remained the un-met issue
using exogenous E-cadherin or DECMA-1 treatment for
spheroidogenesis. To investigate the issue timely, the commercial
HCT116 cell line, well-known for 3D culture and already prepared
for CDH1 KO, were purchased from the company. In order to clarify
the feasible role of soluble E-cadherin, additional CRC cell lines
should be further included. Second, ERK activation should be more
precisely interpreted while spheroidogenesis. As ERK activation was
increased in spheroidogenesis regardless of E-cadherin level, an
ERK inhibitor PD98059 was adopted. PD98059 treatment inhibited ERK
activation and spheroidogenesis, but increased E-cadherin.
Therefore, the decreased ERK rather than ERK activation might be
more paid attention to interpret the present results. In addition,
PD98059 treatment did not affect EGFR expression compared to
spheroidogenesis, which might mean EGFR overexpression is an
essential to spheroidogenesis. As EGF-dependent ERK activation
(6–8) was suggested as a relevant pathway in
CRC, the related signaling pathways on E-cadherin, EGFR, ERK, or
β-catenin should be further investigated. Nevertheless, a candidate
signaling pathway could not be specified based on current results.
Finally, further experiments using animal models or human samples
including blood or urines are needed to ensure the hypothesis, a
biomarker for liquid biopsy for CRC.
In conclusion, E-cadherin did not affect
proliferation, viability, or spheroidogenesis of WT or CDH1 KO
HCT116 cells, although soluble E-cadherin was increased in a
time-dependent manner, especially being prominent in WT. These
results suggest that soluble E-cadherin could be a biomarker for
colorectal cancer although exogenous E-cadherin might not have a
further role like an oncogene in this experimental setting. Further
studies should be focused on multiple signaling pathways that can
be activated or inhibited by soluble E-cadherin.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science Research
Program through the National Research Foundation of Korea funded by
the Korea government (grant no. 2021R1F1A1063023) and by the
Ministry of Education (grant no. RS-2023-00270936).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
IYC and SPY conceived and designed the present
study. IYC, HJB and JWH performed the experiments for data
acquisition and analysis, and interpreted the experimental results.
IYC and SPY confirm the authenticity of all the raw data. IYC wrote
the original manuscript. SPY revised the manuscript. All authors
read and approved the final version of the manuscript, and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Conflict of interest statement
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hecht JR: Current and emerging therapies
for metastatic colorectal cancer: Applying research findings to
clinical practice. Am J Health Syst Pharm. 65 (11 Suppl 4):S15–S21;
quiz S22-4. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berx G, Becker KF, Höfler H and van Roy F:
Mutations of the human E-cadherin (CDH1) gene. Hum Mutat.
12:226–237. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bustos-Carpinteyro AR, Oliveira C, Sousa
A, Oliveira P, Pinheiro H, Carvalho J, Magaña-Torres MT,
Flores-Miramontes MG, Aguilar-Lemarroy A, Jave-Suárez LF, et al:
CDH1 somatic alterations in Mexican patients with diffuse and mixed
sporadic gastric cancer. BMC Cancer. 19:692019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bocca C, Bozzo F, Cannito S, Parola M and
Miglietta A: Celecoxib inactivates epithelial-mesenchymal
transition stimulated by hypoxia and/or epidermal growth factor in
colon cancer cells. Mol Carcinog. 51:783–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim K, Kim KH, Roh K, Yoo BC, Ku JL, Shin
YK, Cho JY, Kim M, Kwon MH, Goh SH, et al: Antitumor effects of
calgranulin B internalized in human colon cancer cells. Oncotarget.
7:20368–20380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trinh NT, Nguyen TMN, Yook JI, Ahn SG and
Kim SA: Quercetin and quercitrin from Agrimonia pilosa ledeb
inhibit the migration and invasion of colon cancer cells through
the JNK signaling pathway. Pharmaceuticals (Basel). 15:3642022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu MN, Hu SH, Zhang XW, Xiong SM and Deng
H: Overview on new progress of hereditary diffuse gastric cancer
with CDH1 variants. Tumori. 106:346–355. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee Y, Ko D, Yoon J, Lee Y and Kim S:
TMEM52B suppression promotes cancer cell survival and invasion
through modulating E-cadherin stability and EGFR activity. J Exp
Clin Cancer Res. 40:582021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manuel Iglesias J, Beloqui I,
Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon
A, Menendez JA, Dopazo J and Martin AG: Mammosphere formation in
breast carcinoma cell lines depends upon expression of E-cadherin.
PLoS One. 8:e772812013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han SJ, Kwon S and Kim KS: Challenges of
applying multicellular tumor spheroids in preclinical phase. Cancer
Cell Int. 21:1522021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brouxhon SM, Kyrkanides S, Teng X, Athar
M, Ghazizadeh S, Simon M, O'Banion MK and Ma L: Soluble E-cadherin:
A critical oncogene modulating receptor tyrosine kinases, MAPK and
PI3K/Akt/mTOR signaling. Oncogene. 33:225–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu QP, Kuang JY, Yang QK, Bian XW and Yu
SC: Beyond a tumor suppressor: Soluble E-cadherin promotes the
progression of cancer. Int J Cancer. 138:2804–2812. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patil PU, D'Ambrosio J, Inge LJ, Mason RW
and Rajasekaran AK: Carcinoma cells induce lumen filling and EMT in
epithelial cells through soluble E-cadherin-mediated activation of
EGFR. J Cell Sci. 128:4366–4379. 2015.PubMed/NCBI
|
|
17
|
Ramírez Moreno M and Bulgakova NA: The
cross-talk between EGFR and E-cadherin. Front Cell Dev Biol.
9:8286732022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Min SO, Lee SW, Bak SY and Kim KS: Ideal
sphere-forming culture conditions to maintain pluripotency in a
hepatocellular carcinoma cell lines. Cancer Cell Int. 15:952015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang IY and Yoon SP: Increased soluble
E-cadherin of spheroid formation supplemented with fetal bovine
serum in colorectal cancer cells. Oncol Lett. 25:2072023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brouxhon SM, Kyrkanides S, Teng X, Raja V,
O'Banion MK, Clarke R, Byers S, Silberfeld A, Tornos C and Ma L:
Monoclonal antibody against the ectodomain of E-cadherin (DECMA-1)
suppresses breast carcinogenesis: Involvement of the
HER/PI3K/Akt/mTOR and IAP pathways. Clin Cancer Res. 19:3234–3246.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stanich PP, Elgindi D, Stoffel E, Koeppe
E, Bansal A, Stetson R, Collins DL, Clark DF, Karloski E, Dudley B,
et al: Colorectal neoplasia in CDH1 pathogenic variant carriers: A
multicenter analysis. Am J Gastroenterol. 117:1877–1879. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han XY, Wei B, Fang JF, Zhang S, Zhang FC,
Zhang HB, Lan TY, Lu HQ and Wei HB: Epithelial-mesenchymal
transition associates with maintenance of stemness in
spheroid-derived stem-like colon cancer cells. PLoS One.
8:e733412013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Bu X, Chen H, Wang Q and Sha W:
Bmi-1 promotes the invasion and migration of colon cancer stem
cells through the downregulation of E-cadherin. Int J Mol Med.
38:1199–1207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian Y, Wu X, Yokoyama Y, Okuzaki D,
Taguchi M, Hirose H, Wang J, Hata T, Inoue A, Hiraki M, et al:
E-cadherin-Fc chimera protein matrix enhances cancer stem-like
properties and induces mesenchymal features in colon cancer cells.
Cancer Sci. 110:3520–3532. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tay PN, Tan P, Lan Y, Leung CH, Laban M,
Tan TC, Ni H, Manikandan J, Rashid SB, Yan B, et al: Palladin, an
actin-associated protein, is required for adherens junction
formation and intercellular adhesion in HCT116 colorectal cancer
cells. Int J Oncol. 37:909–926. 2010.PubMed/NCBI
|
|
26
|
Rao C, Frodyma DE, Southekal S, Svoboda
RA, Black AR, Guda C, Mizutani T, Clevers H, Johnson KR, Fisher KW
and Lewis RE: KSR1- and ERK-dependent translational regulation of
the epithelial-to-mesenchymal transition. Elife. 10:e666082021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Díaz VM, Hurtado M, Kort EJ, Resnati M,
Blasi F, Thomson T and Paciucci R: Requirement of the enzymatic and
signaling activities of plasmin for phorbol-ester-induced
scattering of colon cancer cells. Exp Cell Res. 312:2203–2213.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao S, Hu J, Wu X and Liang Z: PMA treated
THP-1-derived-IL-6 promotes EMT of SW48 through STAT3/ERK-dependent
activation of Wnt/β-catenin signaling pathway. Biomed Pharmacother.
108:618–624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park YR, Seo SY, Kim SL, Zhu SM, Chun S,
Oh JM, Lee MR, Kim SH, Kim IH, Lee SO, et al: MiRNA-206 suppresses
PGE2-induced colorectal cancer cell proliferation, migration, and
invasion by targetting TM4SF1. Biosci Rep. 38:BSR201806642018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong L, Ding C, Zheng T, Pu Y, Liu J,
Zhang W, Xue F, Kang P, Ma Y, Wang X and Mao C: Extracellular
vesicles from human umbilical cord mesenchymal stem cells treated
with siRNA against ELFN1-AS1 suppress colon adenocarcinoma
proliferation and migration. Am J Transl Res. 11:6989–6999.
2019.PubMed/NCBI
|
|
31
|
Yang X, Zheng YT and Rong W: Sevoflurane
induces apoptosis and inhibits the growth and motility of colon
cancer in vitro and in vivo via inactivating Ras/Raf/MEK/ERK
signaling. Life Sci. 239:1169162019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng B, Rong A, Zhou Q and Li W: LncRNA
LINC00662 promotes colon cancer tumor growth and metastasis by
competitively binding with miR-340-5p to regulate CLDN8/IL22
co-expression and activating ERK signaling pathway. J Exp Clin
Cancer Res. 39:52020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jun JH, Oh JE, Shim JK, Kwak YL and Cho
JS: Effects of bisphenol A on the proliferation, migration, and
tumor growth of colon cancer cells: In vitro and in vivo evaluation
with mechanistic insights related to ERK and 5-HT3. Food Chem
Toxicol. 158:1126622021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Minelli R, Serpe L, Pettazzoni P, Minero
V, Barrera G, Gigliotti C, Mesturini R, Rosa AC, Gasco P, Vivenza
N, et al: Cholesteryl butyrate solid lipid nanoparticles inhibit
the adhesion and migration of colon cancer cells. Br J Pharmacol.
166:587–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Satow R, Hirano T, Batori R, Nakamura T,
Murayama Y and Fukami K: Phospholipase Cδ1 induces E-cadherin
expression and suppresses malignancy in colorectal cancer cells.
Proc Natl Acad Sci USA. 111:13505–13510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng C, Li Z, Niu Z, Niu W, Xu Z, Gao H,
Niu W, Wang J, He Z, Gao C, et al: Norcantharidin suppresses colon
cancer cell epithelial-mesenchymal transition by inhibiting the
αvβ6-ERK-Ets1 signaling pathway. Sci Rep. 6:205002016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Zhang G, Wang Y, Elgehama A, Sun Y,
Li L, Gu Y, Guo W and Xu Q: Loss of periplakin expression is
associated with the tumorigenesis of colorectal carcinoma. Biomed
Pharmacother. 87:366–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang YF, Wang HH, Shu CW, Tsai WL, Lee
CH, Chen CL and Liu PF: TMEM211 promotes tumor progression and
metastasis in colon cancer. Curr Issues Mol Biol. 45:4529–4543.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Zhu G, Zhai H, Jia J, Yang W, Li X
and Liu L: Simultaneous stimulation with tumor necrosis factor-α
and transforming growth factor-β1 induces epithelial-mesenchymal
transition in colon cancer cells via the NF-κB pathway. Oncol Lett.
15:6873–6880. 2018.PubMed/NCBI
|
|
40
|
Lin MC, Wang FY, Kuo YH and Tang FY:
Cancer chemopreventive effects of lycopene: Suppression of MMP-7
expression and cell invasion in human colon cancer cells. J Agric
Food Chem. 59:11304–11318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bartucci M, Svensson S, Ricci-Vitiani L,
Dattilo R, Biffoni M, Signore M, Ferla R, De Maria R and Surmacz E:
Obesity hormone leptin induces growth and interferes with the
cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells.
Endocr Relat Cancer. 17:823–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T and Akagi M: Characterizing inflammatory markers in highly
aggressive soft tissue sarcomas. Medicine (Baltimore).
101:e306882022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kleer CG, van Golen KL, Braun T and
Merajver SD: Persistent E-cadherin expression in inflammatory
breast cancer. Mod Pathol. 14:458–464. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cohen EN, Gao H, Anfossi S, Mego M, Reddy
NG, Debeb B, Giordano A, Tin S, Wu Q, Garza RJ, et al: Inflammation
mediated metastasis: Immune induced epithelial-to-mesenchymal
transition in inflammatory breast cancer cells. PLoS One.
10:e01327102015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shirahama S, Furukawa F, Wakita H and
Takigawa M: E- and P-cadherin expression in tumor tissues and
soluble E-cadherin levels in sera of patients with skin cancer. J
Dermatol Sci. 13:30–36. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Devaux CA, Mezouar S and Mege JL: The
E-cadherin cleavage associated to pathogenic bacteria infections
can favor bacterial invasion and transmigration, dysregulation of
the immune response and cancer induction in humans. Front
Microbiol. 10:25982019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katayama M, Hirai S, Yasumoto M, Nishikawa
K, Nagata S, Otsuka M, Kamihagi K and Kato I: Soluble fragments of
e-cadherin cell-adhesion molecule increase in urinary-excretion of
cancer-patients, potentially indicating its shedding from
epithelial tumor-cells. Int J Oncol. 5:1049–1057. 1994.PubMed/NCBI
|
|
48
|
Repetto O, De Paoli P, De Re V, Canzonieri
V and Cannizzaro R: Levels of soluble E-cadherin in breast,
gastric, and colorectal cancers. Biomed Res Int. 2014:4080472014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weiss JV, Klein-Scory S, Kübler S,
Reinacher-Schick A, Stricker I, Schmiegel W and Schwarte-Waldhoff
I: Soluble E-cadherin as a serum biomarker candidate: Elevated
levels in patients with late-stage colorectal carcinoma and FAP.
Int J Cancer. 128:1384–1392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Okugawa Y, Toiyama Y, Inoue Y, Iwata T,
Fujikawa H, Saigusa S, Konishi N, Tanaka K, Uchida K and Kusunoki
M: Clinical significance of serum soluble E-cadherin in colorectal
carcinoma. J Surg Res. 175:e67–e73. 2012. View Article : Google Scholar : PubMed/NCBI
|