Introduction
Ovarian cancer (OC) was the eighth most commonly
diagnosed malignancy in the Global Cancer Statistics 2020 (1), with an estimated age-standardized
incidence rate of 6.6%. OC can be classified into three main types:
i) Epithelial OC (EOC); ii) sex cord stromal OC; and iii) germ cell
OC, the latter two accounting for ~5% of all OC cases (2). The main symptoms of OC are persistent
abdominal pain, abdominal distension or bloating, urinary system
symptoms, frequent urination and non-specific gastrointestinal
symptoms (3). After primary
debulking surgery, intravenous or intraperitoneal chemotherapy is
preferentially administrated to eliminate cancer cells and
maintenance drugs, such as olaparib and bevacizumab, are used to
slow recurrence (4). The
identification of complete molecular mechanisms underlying OC is
required to more efficiently prevent and treat OC.
Mesenchymal stem cells (MSCs) are pluripotent
stromal cells with multiple differentiation capabilities, and bone
marrow is a typical source of MSCs (5). In addition to MSCs, their nanoghosts
(nanovesicles reconstructed from the cytoplasmic membranes of MSCs)
also have promising therapeutic effects on certain types of cancer,
including gastrointestinal, lung and ovarian cancer (6). MSCs may secret large amounts of
exosomes (Exos) for cell-to-cell communication and keep a dynamic
and homeostatic microenvironment for tissue repair. The use of
MSCs-derived exosomes (MSCs-Exo) may have notable advantages over
their living cells and may reduce adverse side effects (7). MSCs-Exo possess numerous advantages,
consisting of non-immunogenicity, easy accessibility for isolation
and preparation, convenient storage at low temperature,
non-infusion toxicity and freedom from tumorigenic potential and
ethical issues (8). MSCs-Exo have
pluripotent functions through delivery of targeted microRNAs
(miRs/miRNAs) in certain types of human cancer (for example
ovarian, breast and prostate cancer), including increasing drug
sensitivity (9), promoting dormancy
(10) and restraining tumorigenesis
(11).
Recognition of miRNA signatures has identified
miR-125b-5p as a biomarker of endometrial cancer (12). In fact, miR-125b-5p downregulation
is indicative of poor prognosis in EOC (13) whereas miR-125b-5p upregulation
inhibits tumor growth in combination with cisplatin in a mouse
model with OC (14). DEAD-box
helicase 5 (DDX5) is an active RNA helicase that is involved in
cancer progression when it is upregulated by altering transcription
and signaling pathways (for example, the Wnt/β-catenin-ferroptosis
axis and the mTOR signaling pathway) (15–17).
Expression levels of DDX5 are associated with platinum resistance
in OC (18), but regulation of DDX5
can mediate the malignant phenotype of tumor cells (17,19).
In the present study, a targeting relationship between miR-125b-5p
and DDX5 was hypothesized using bioinformatical analysis via the
online ENCORI database, therefore, the aim of the study was to
investigate whether BMSCs-Exo delivery of miR-125b-5p could impede
the progression of OC through modulation of DDX5 expression
levels.
Materials and methods
Ethical approval
Experiments were performed following approval from
the Ethics Committee of Harbin Medical University Cancer Hospital
(approval no. 20200316; Harbin, China), and written informed
consent from each patient was obtained. Bone MSCs (BMSCs) were used
in accordance with the International Society for Stem Cells
Research guidelines for Stem Cell Research and Clinical Translation
and approved by the Ethics Committee of Harbin Medical University
Cancer Hospital (approval no. 20200518).
Clinical subjects
The present study included 100 patients with ovarian
cancer who underwent surgical treatment in the Department of
Gynecology of Harbin Medical University Cancer Hospital
(Heilongjiang, China) from May 2020 to January 2023, and these
patients were aged 39–67 years, with an average age of 55.31±7.64
years. Inclusion criteria: i) Patients were diagnosed with ovarian
cancer through pathological diagnosis; ii) no chemotherapy,
radiotherapy or other treatments were performed before surgery;
iii) the patients' ages were ≥18 years old; and iv) patients had
complete medical records. Exclusion criteria: i) Patients had with
other malignant tumors; and ii) patients with an expected survival
period of no more than 3 months. Ovarian cancer tissue and normal
tissue adjacent to the cancer (distance >3 cm from the cancer
tissue) were collected, and biological biopsy was performed on the
normal tissue adjacent to the cancer to confirm the absence of
cancer cells. The tissue samples (~1 mm3) were first
rapidly frozen in liquid nitrogen for 30–60 sec, then stored in a
−80°C freezer for later use. All samples were diagnosed with
ovarian cancer by pathologists who did not participate in this
study.
Bone marrow specimens were also collected from 3
patients (2 males and 1 female) who received inpatient treatment in
the Harbin Medical University Cancer Hospital for femoral head
necrosis during May 2020 to January 2023, aged 20–55 years old.
Inclusion criteria for patients: Aged >18 years old; without
high femoral head drop or trauma; no cardiovascular disease or
malignant tumors; and all underwent hip arthroplasty. Exclusion
criteria for patients: Presence of hematological diseases; severe
liver or kidney dysfunction; history of smoking; and a history of
alcohol consumption. During surgery, after the femoral head was
excised and the femoral medullary cavity was reamed, fresh bone
marrow (5–7 ml) was collected and placed into a 10 ml sterile
syringe preloaded with sodium heparin. Subsequently, the bone
marrow sample was transferred to a 15 ml sterile centrifuge tube
and immediately used for BMSCs isolation.
Cell culture
DMEM (Sigma-Aldrich; Merck KGaA) containing 10% FBS
(Biological Industries; Sartorius AG), 100 U/ml penicillin and 100
mg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) was used
to culture the OC cell line SKOV3 (American Type Culture
Collection), and incubated in a 95% humidified atmosphere at 37°C
with 5% CO2. The medium was replaced once every 2 days
(20).
Separation and identification of
BMSCs
BMSCs were isolated from bone marrow samples as
previously reported (21). In
brief, BMSCs were isolated by a density gradient centrifugation,
followed by suspension in α-MEM supplemented with 20% FBS, 1%
L-glutamine (Invitrogen (21);
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin. The
cells were then plated at a density of 1×106
cells/cm2. The culture medium was replaced after 4 days,
with the non-adherent cells removed by PBS washing and the adherent
cells were then cultured to reach 70–80% confluence. After that,
the cells were sub-cultured in low-glucose DMEM (DMEM-LG) with the
aforementioned supplements. BMSCs at passages 3 or 4 were chosen
for subsequent studies.
Following this, adipogenesis was induced for 21 days
in cultures by addition of DMEM-LG containing hydrocortisone (0.5
µM), 10% FBS, oboutyl methyl xanthine (0.5 µM), insulin (10 µg/ml)
and indomethacin (60 µM). The medium was replaced 3 times a week.
Lipid droplet formation was induced for 21 days in cultures and
detected using Oil Red Osteogenic (Oil Red O; Sigma-Aldrich; Merck
KGaA), followed by 1 h Oil Red O staining at room temperature.
Through the addition of DMEM-LG containing 10% FBS, ascorbic acid
(2.0×10−4 mol/l), dexamethasone (1.0×10−8
mol/l), β-glycero-phosphate (7×10−3 mol/l),
β-glycero-phosphate (10 µmol/l), dexamethasone (0.1 µmol/l) and
ascorbate (50 µmol/l). The cell cultures were stained with Alizarin
Red S (Sigma-Aldrich; Merck KGaA) for 5 min at room temperature to
assess mineralization.
BMSCs were subjected to dissociation using
trypsin/EDTA (Thermo Fisher Scientific, Inc.), cells were blocked
with 3% BSA (Gibco; Thermo Fisher Scientific, Inc.) for 30 min at
room temperature and the cell suspensions were dyed using multiple
antibodies against MSC markers at room temperature for 30 min,
consisting of CD29-FITC (1:100; cat. no. 561796), CD90-FITC (1:100;
cat. no. 561969), CD44-FITC (1:100; cat. no. 561859) and CD45-FITC
(1:100; cat. no. 561867) (BD Biosciences) and HRP-labeled secondary
goat anti-mouse IgG antibody (1:100; cat. no. 555988) (BD
Biosciences). In addition, the cells were marked with
isotype-matched antibodies, which served as background controls.
After the cells were treated with the appropriate secondary
antibodies, followed by sample analysis using the
FACSCalibur™ cytometer (BD Biosciences). Data were
analyzed using the CellQuest™ pro software (version 5.1;
BD Biosciences).
Isolation and purification of
exosomes
BMSCs were rinsed with PBS and then moved to a
conditioned medium (DMEM) containing exosome-depleted FBS obtained
by centrifugation at 120,000 × g and 4°C for 18 h. FBS was
centrifuged at 120,000 × g and 4°C for 18 h to remove exosomes and
then used to culture BMSCs. After 48 h, cells or cellular debris
were removed by centrifugation at 800 × g for 10 min at 4°C, and
the supernatant was obtained and then centrifuged again (5,000 × g,
4°C, 10 min) to ensure that all cells or cellular debris were
removed to obtain the culture supernatant.
Ultracentrifugation was used for the extraction of
exosomes. In detail, the live cells were removed through
centrifugation at 300 × g for 10 min, the dead cells were removed
at 2,000 × g for 10 min, followed by removing cell debris at 10,000
× g for 30 min and lastly, at 110,000 × g for 70 min. The
aforementioned centrifugation steps were performed at 4°C.
Subsequently, the supernatant was removed to acquire exosome
pellets. The exosomes were filtered using a 0.22-µm filter after
PBS washing, which were then centrifuged at 110,000 × g 4°C for 70
min. Afterward, exosomes were resuspended in PBS and maintained at
−80°C for future use. BMSCs were transfected with either 10 nM
miR-125b-5p mimic or 10 nM scrambled miRNA (a negative control of
miR-125b-5p mimic; mimic NC) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C (the sequences
are presented in Table SI). Upon
48 h transfection, the culture medium was harvested to extract
exosomes, which were termed Exo-miR-125b-5p mimic and Exo-mimic NC.
The secreted number of exosomes was quantified using a BCA protein
assay kit (Thermo Fisher Scientific, Inc.) (22).
Characterization and quantification of
exosomes
The harvested exosomes were loaded on to 400 mesh
carbon grids and then fixed with 2.5% glutaraldehyde at 4°C for 5
min and dyed using 2.5% uranyl acetate at room temperature for 10
min (Electron Microscopy Sciences) and embedded with 1% methyl
cellulose on ice for 10 min (MilliporeSigma). Next, the grid was
dried completely at room temperature. Transmission electron
microscopy (TEM; JEM-2100F; JEOL, Ltd.) was performed to
characterize the exosome's morphology. The size distribution and
concentration of exosomes were analyzed by nanoparticle-tracking
analysis with a NanoSight NS300 instrument (Malvern Panalytical,
Malvern, UK) following the manufacturer's instructions. The
expression levels of exosome proteins CD9 and CD81 were evaluated
by western blotting.
Exosome uptake assay
Exosomes were fluorescently labeled with PKH26
(Sigma-Aldrich; Merck KGaA) following the manufacturer's
instructions. PKH26-labeled exosomes (50 µg) incubated for 24 h
with SKOV3 cells at 37°C. Aliquots of the cell suspension were
placed on microscope slides and subsequently mounted by a coverslip
with the Aqua-Poly/Mount (Polysciences, Inc.). After that, nuclei
were labelled blue using DAPI at 37°C for 10 min. Images of the
exosomes' cellular uptake was captured with a confocal laser
scanning microscope (Carl Zeiss AG) (23).
Cell grouping and treatment
To evaluate the influence of BMSCs-derived exosomes
(BMSCs-Exo) carrying miR-125b-5p targeting DDX5 on the biological
functions of SKOV3 cells, the following groups were evaluated: i)
Control (PBS co-cultured with SKOV3 cells); ii) Exo (BMSCs-Exo
co-cultured with SKOV3 cells); iii) Exo-mimic NC (Exo-mimic NC
co-cultured with SKOV3 cells); iv) Exo-miR-125b-5p mimic
(Exo-miR-125b-5p mimic co-cultured with SKOV3 cells); v) small
interfering RNA (siRNA)-NC (si-NC transfected into SKOV3 cells);
vi) si-DDX5 (DDX5 siRNA transfected into SKOV3 cells); vii)
Exo-miR-125b-5p mimic + pcDNA-NC (Exo-miR-125b-5p mimic was
co-cultured with SKOV3 cells and transfected using the pcDNA3.1
plasmid); and viii) Exo-miR-125b-5p mimic + pcDNA-DDX5
(Exo-miR-125b-5p mimic was co-cultured with SKOV3 cells and
transfected with pcDNA3.1 plasmid over-expressing DDX5) groups. The
corresponding exosomes (50 µg) were co-incubated with SKOV3 cells
(1×106 cells/well) for 24 h, respectively. The si-DDX5,
si-NC, pcDNA-DDX5 and pcDNA-NC were transfected into SKOV3 cells
using Lipofectamine® 3000. The aforementioned vectors
and plasmids used for transfection were purchased from Shanghai
GenePharma Co., Ltd., and the miR-125b-5p mimic (cat. no. MC10148)
and mimic NC (cat. no. 4464059) were purchased from Thermo Fisher
Scientific, Inc. (Table SI).
Cell counting kit (CCK-8) assay
SKOV3 cells were seeded at 2,000 cells/well in
96-well plates. After incubation with 10 µl CCK-8 reagent (Dojindo
Laboratories, Inc.) for 2 h at 37°C, absorbance at 450 nm was read
using a SpectraMax™ 190 spectrophotometer plate reader
(24).
Scratch assay
The 90% confluent, serum-starved SKOV3 cells were
seeded into 6-well plates (50,000 cells/well) for 24 h and then
scratched using a 200 µl pipette tip. At 0 and 48 h, images of the
cells were captured using an optical microscope and wound healing
rate was calculated as previously described (25).
Transwell assay
SKOV3 cells (5×105 cells/ml) were
resuspended in FBS-free medium. The Matrigel-coated (coat at 4°C,
then allow gelation for 2–3 h) upper chamber of 24-well inserts
Transwell plate (pore size, 8 µm; Corning, Inc.) was covered with
cell resuspension (100 µl) and the lower chamber with DMEM
containing 10% FBS (600 µl). After 48-h culture at 37°C, cells were
dyed with 0.5% crystal violet for 20 min at room temperature and
under the light microscope (Nikon Corporation), five fields
(magnification, ×200) were randomly selected, and the mean number
of cells was calculated and used for statistical analysis (26).
Annexin V/PI double staining
To assess apoptosis, SKOV3 cells were stained using
Annexin V and propidium iodide as part of the Annexin V-FITC/PI
Apoptosis Detection kit (BD Biosciences), according to the
manufacturer's protocol and early + late cell apoptosis were
analyzed using flow cytometry (Cytomics FC500 MPL; Beckman Coulter,
Inc.) and FACS DiVa 6.1.3 software (BD Biosciences) (27).
Reverse transcription-quantitative PCR
(RT-qPCR)
After RNA extraction from of OC tissue or cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
reverse transcription of RNA into cDNA was performed using the
PrimeScript RT Master Mix kit (Takara Biotechnology Co., Ltd.) and
Mir-X miRNA RT-qPCR SYBR kit (Takara Biotechnology Co., Ltd.) based
on manufacturer's instructions. qPCR was performed using SYBR
Premix Ex Taq II (Takara Biotechnology Co., Ltd.) in the ABI·7500
system. The reaction conditions were as follows, pre-denaturation
95°C for 5 min, 40 cycles: denaturation 95°C for 5 sec, annealing
60°C for 30 sec, and extension 74°C for 30 sec. The
2−ΔΔCq method was utilized to analyze the relative
expression levels of genes, which was normalized to U6 or GAPDH
(Table SII) (28).
Western blot assay
Total protein of OC tissue or cells was separated by
radio-immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) that contained protease inhibitors and
phenylmethanesulfonyl fluoride (Biocolor, Ltd.), and then
quantified using the BCA Protein Assay Kit (Beyotime Institute of
Biotechnology). Proteins (25 µg/lane) were separated by 10%
SDS-PAGE, transferred to a PVDF membrane (Bio-Rad), blocked in 5%
skim milk for 1 h at room temperature, mixed overnight at 4°C with
primary antibodies against DDX5 (1:1,000; cat. no. ab128928), CD9
(1:1,000; cat. no. ab236630), CD81 (1:2,000; cat. no. ab109201) and
GAPDH (1:2,500; cat. no. ab181602) and cultured for 1 h at room
temperature with goat anti-rabbit IgG (1:2,000; cat. no. ab6721)
(all from Abcam). Visualization of protein bands was performed in a
gel imaging system (G:BOXChemi XR5; Syngene Europe), and the data
analyzed using the ImageJ software (version 1.8.0; National
Institutes of Health) (29).
Bioinformatical analysis and dual
luciferase reporter gene assay
The targets of miR-125b-5p were predicted using
bioinformatical analysis via the online ENCORI database (version
3.0; http://rnasysu.com/encori/).
Subsequently, DDX5 was selected, and the relationship was analyzed
using a dual luciferase reporter gene assay. The sequence
containing the miR-125b-5p binding site in the DDX5 3′UTR was
amplified and cloned into the pGL3-basic luciferase plasmid (Takara
Biotechnology Co., Ltd.) to construct a recombinant plasmid of
wild-type DDX5 (DDX5-WT; point mutation, 5′-UCAGGG-3′). Mutant DDX5
(DDX5-Mut; point mutation, 5′-ACCCCC-3′) recombinant plasmid was
constructed by mutating the miR-125b-5p binding site on DDX5-WT
using a point mutation kit (Takara Biotechnology Co., Ltd.).
Plasmid design and construction was performed by Takara
Biotechnology Co., Ltd.). After 48 h transfection with the
recombinant vector and miR-125b-5p mimic or mimic NC by using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), the luciferase activity of SKOV3 cells was
examined using the Dual Luciferase Reporter Assay System (Promega
Corporation) according to the manufacturer's instructions. Relative
firefly luciferase activity was normalized to Renilla
luciferase activity as a control for transfection efficiency
(30).
RNA immunoprecipitation (RIP)
assay
In accordance with the manufacturer's instructions,
the binding of miR-125b-5p to DDX5 was analyzed using the Magna RIP
RNA-Binding Protein Immunoprecipitation Kit (MilliporeSigma). Cells
in logarithmic growth period were harvested and lysed in 500 µl RIP
lysis buffer containing protease inhibitor cocktail and RNA
inhibitor (included in the kit). The supernatant was collected by
centrifugation at 5,000 × g for 10 min at 4°C. The supernatant was
incubated with 900 µl RIP buffer containing 5 µg of Ago2 antibody
(cat. no. ab186733; Abcam) or negative control anti-IgG (cat. no.
ab172730; Abcam) beads overnight at 4°C. After washing with 500 µl
washing buffer, the immunoprecipitated RNA was isolated with TRIzol
reagent. RT-qPCR was performed as aforementioned with the
immunoprecipitated RNA (31–33).
Statistical analysis
Data were statistically analyzed using the SPSS
software (version 21.0; IBM Corp.) and expressed as mean ± standard
deviation. Comparison between two groups was performed using an
unpaired independent samples t-test, while comparisons among
multiple groups were conducted using one-way ANOVA followed by
Tukey's post hoc test. Pearson's correlation test was utilized to
assess correlation. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
3 times.
Results
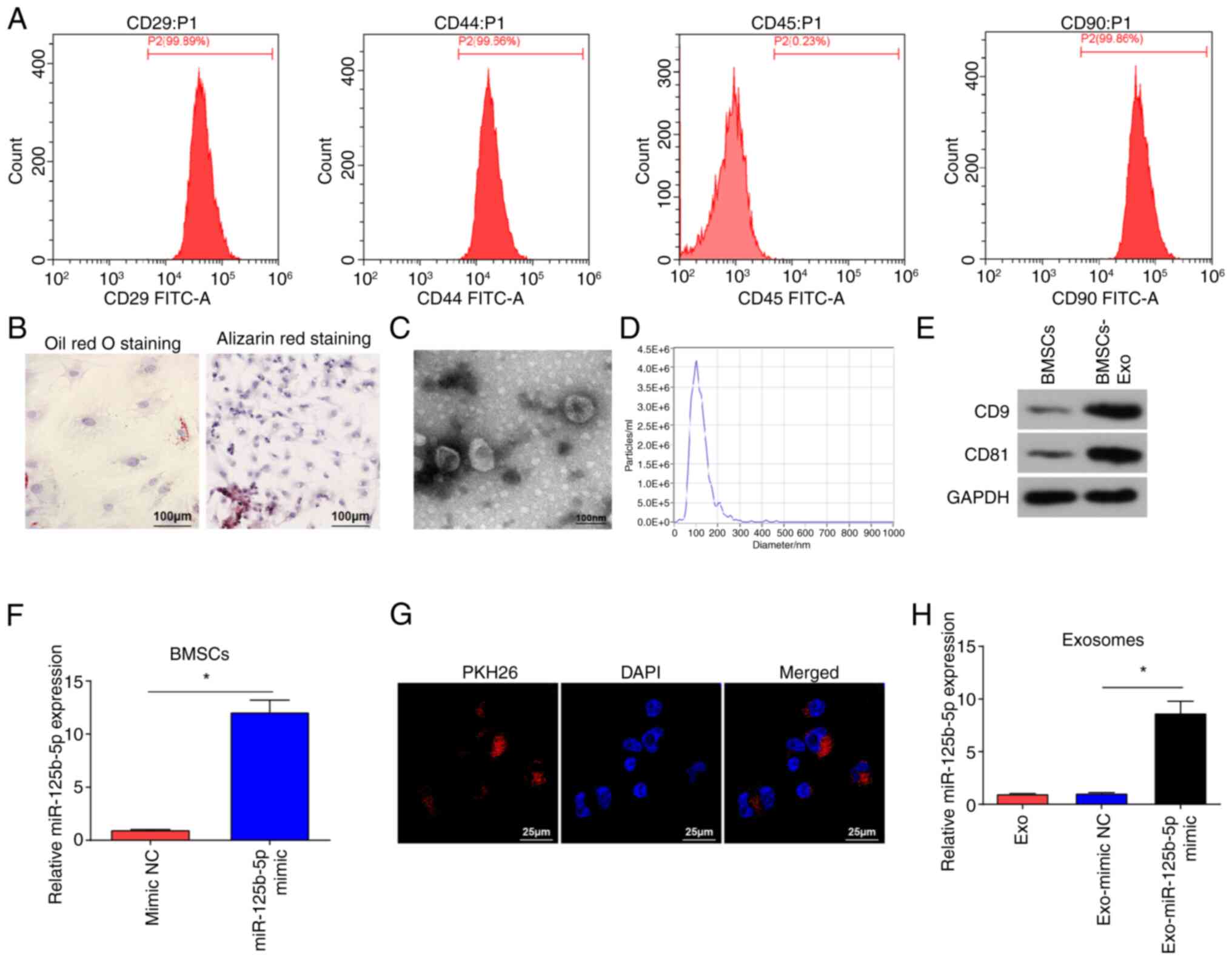
Identification of BMSCs and
exosomes
BMSCs were identified using flow cytometry, which
showed that BMSCs were positive for CD29, CD44 and CD90, and
negative for CD45 (Fig. 1A). BMSCs
were subjected to lipogenic and osteogenic differentiation
experiments (Fig. 1B). After Oil
Red O staining, red lipid droplets were observed in the cells.
Following Alizarin Red staining, BMSCs appeared cubic and had
aggregated to form mineralized nodules, further indicating that the
isolated cells were BMSCs.
TEM images demonstrated that isolated vesicles had
an elliptical shape (Fig. 1C). The
NTA results suggested that the vesicles diameter ranged from 30 to
200 nm (Fig. 1D). Western blotting
demonstrated that the vesicles expressed CD81 and CD9 (Fig. 1E), indicating that the isolated
vesicles were exosomes (34).
To evaluate if BMSCs-Exo could be used as effective
vehicles for miR-125b-5p delivery to suppress OC cells
proliferation and invasion, BMSCs were subject to miR-125b-5p mimic
transfection, and significantly increased miR-125b-5p expression
levels in BMSCs were demonstrated using RT-qPCR (Fig. 1F). PKH26-labeled exosomes were
incubated with SKOV3 cells, and fluorescence microscopy
demonstrated red fluorescence of PKH26 in SKOV3 cells after
co-culture (Fig. 1G). miR-125b-5p
mimic and mimic NC were transfected into BMSCs, post-transfected
cell culture medium was obtained, and Exos were extracted from the
supernatant of the medium. RT-qPCR was performed to evaluate
miR-125b-5p expression levels in the extracted exosomes, and it was
demonstrated (Fig. 1H) that
miR-125b-5p expression levels were significantly increased in
Exo-miR-125b-5p mimic group compared with that in the Exo-mimic NC
group.
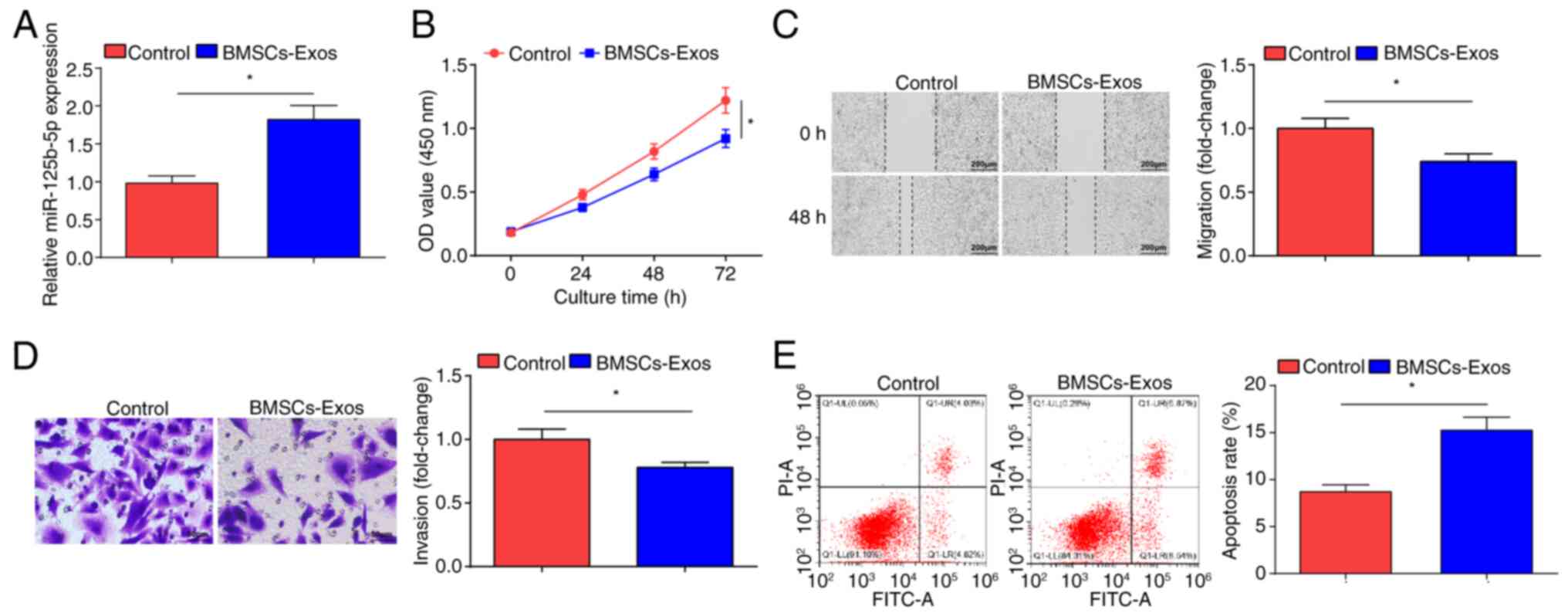
BMSCs-Exo limits OC cell
malignancy
MSCs can inhibit the development of SKOV3 cells
(35). miR-125b-5p expression
levels in SKOV3 cells after co-culture with BMSCs-Exo were
examined. BMSCs-Exo treatment resulted in significantly increased
miR-125b-5p expression levels in SKOV3 cells (Fig. 2A). The CCK-8 (Fig. 2B), scratch (Fig. 2C) and Transwell (Fig. 2D) assays demonstrated that treatment
with BMSCs-Exo significantly impaired the proliferation, migration
and invasion of SKOV3 cells compared with the control cells;
however, flow cytometry demonstrated that the apoptotic rate of the
BMSCs-Exo treated cells significantly increased (Fig. 2E). Overall, BMSCs-Exo inhibited the
development of OC cells in vitro.
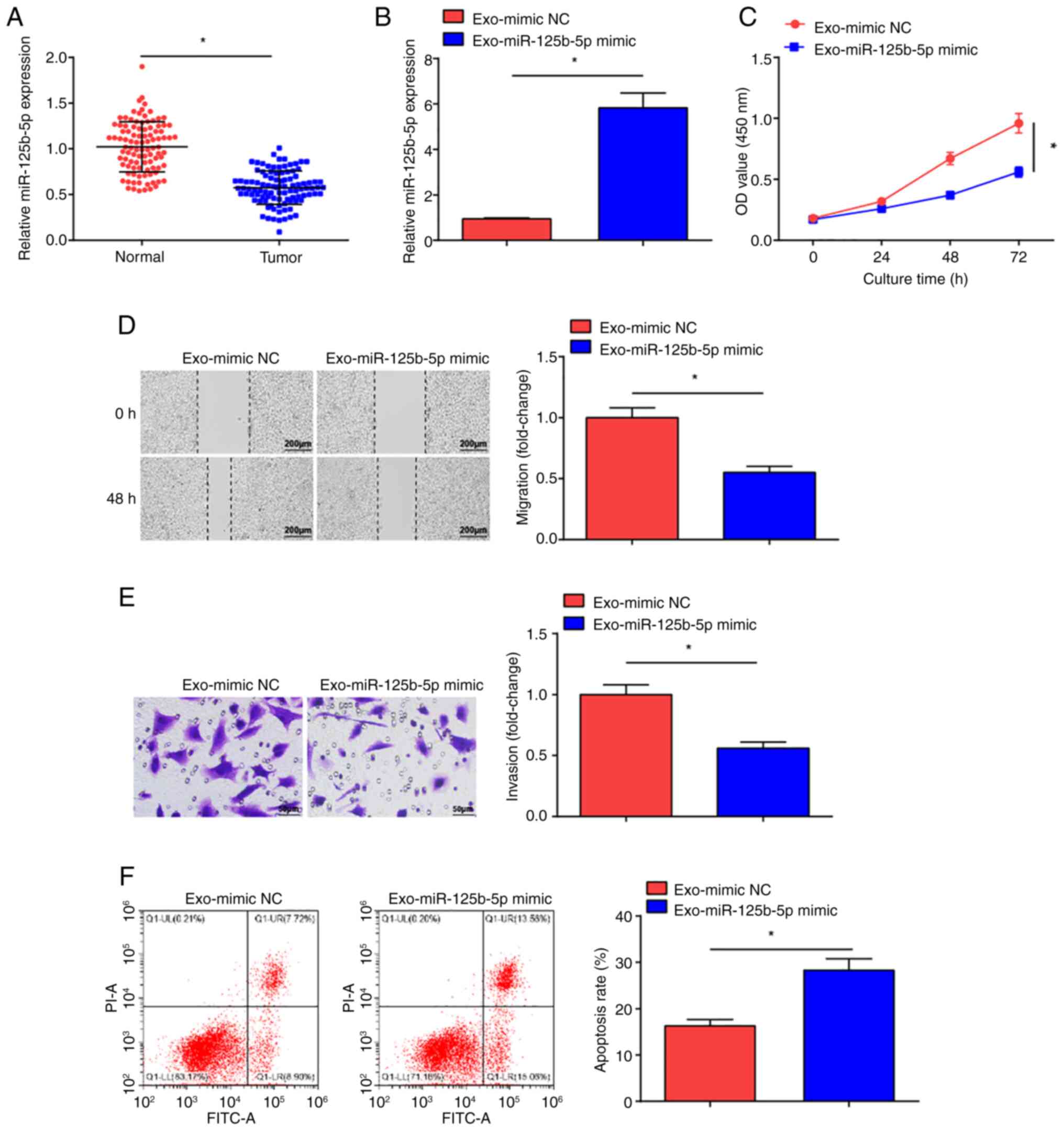
Exosome mediated delivery of
miR-125b-5p represses malignant progression of OC cells
miR-125b-5p can inhibit cancer cell malignancy
(36). Significantly decreased
miR-125b-5p expression levels were measured in the tumor tissue
samples from patients with OC compared with normal tissue samples
(Fig. 3A). To determine whether
BMSCs-Exo carrying miR-125b-5p could impact OC cell development,
the exosomes Exo-miR-125b-5p mimic and Exo-mimic NC were
co-cultured with SKOV3 cells, and miR-125b-5p expression levels in
SKOV3 cells were evaluated (Fig.
3B). Significantly increased expression levels of miR-125b-5p
were observed in the Exo-miR-125b-5p mimic-treated SKOV3 cells
compared with the Exo-mimic NC treatment. The CCK-8 assay (Fig. 3C), scratch (Fig. 3D) and Transwell (Fig. 3E) assays, and flow cytometry
(Fig. 3F) demonstrated that the
proliferative, migratory and invasive properties of Exo-miR-125b-5p
mimic-treated SKOV3 cells were significantly reduced and apoptosis
was significantly increased in comparison with Exo-mimic NC
treatment. These results suggest that miR-125b-5p delivered by
BMSCs-Exo impeded OC development.
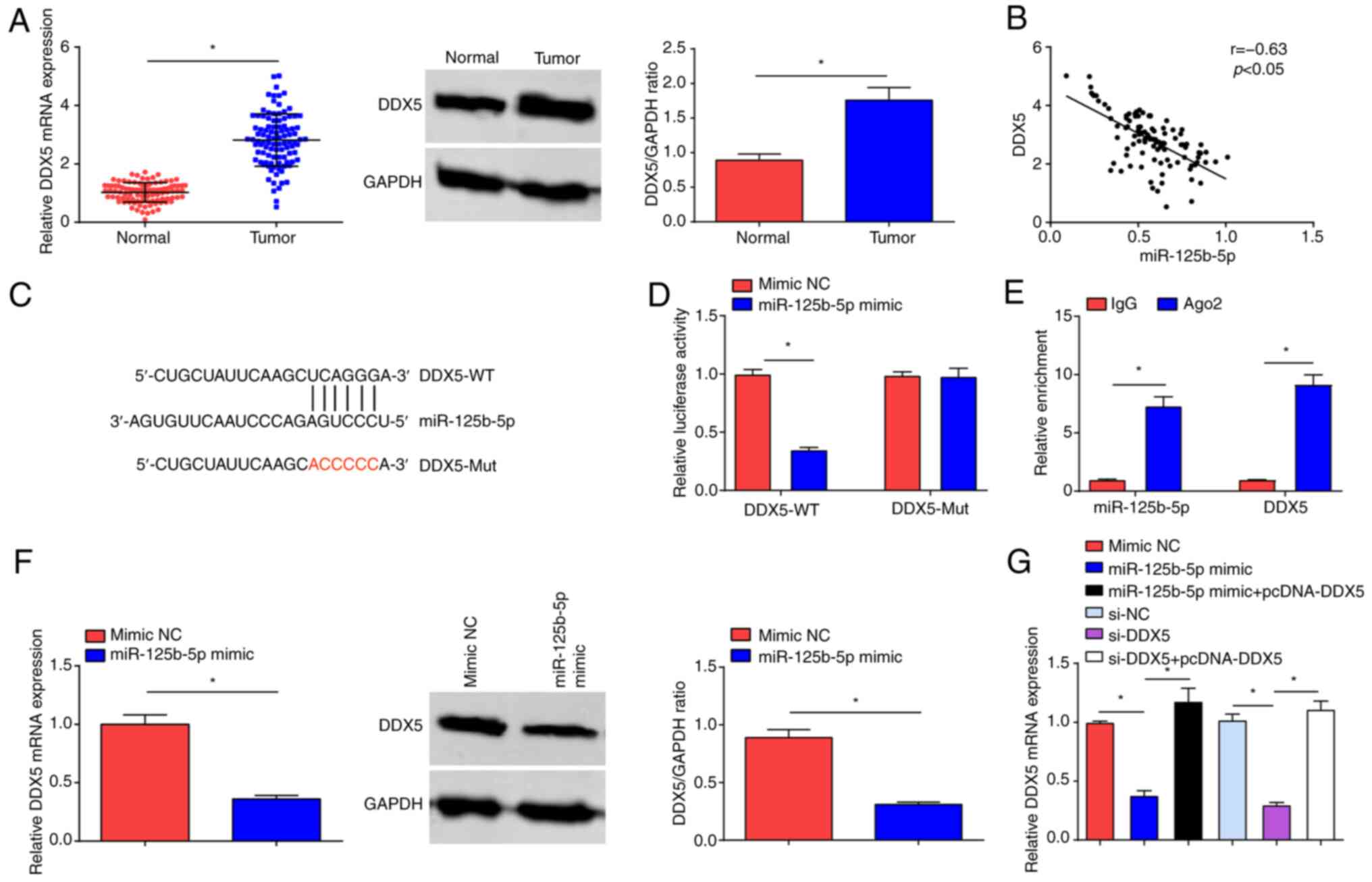
Targeting relationship between
miR-125b-5p and DDX5
RT-qPCR and western blot assays demonstrated that
DDX5 expression was significantly increased in OC tumor tissues
compared with normal tissue (Fig.
4A). To confirm the regulatory relationship between miR-125b-5p
and DDX5, bioinformatics analysis software predicted the binding
sites between them (Fig. 4B), and
Pearson's correlation test demonstrated significant negative
correlation between the mRNA expression levels of miR-125b-5p and
DDX5 (Fig. 4C). Furthermore,
miR-125b-5p mimic inhibited the luciferase activity of DDX5-WT,
while had no impact on the luciferase activity of DDX5-Mut
(Fig. 4D). The RIP experiment
demonstrated that miR-125b-5p and DDX5 expression levels were
significantly increased with Ago2 treatment (Fig. 4E). RT-qPCR and western blot analysis
indicated that DDX5 expression levels were significantly reduced
upon miR-125b-5p overexpression (Fig.
4F). RT-qPCR also demonstrated that overexpression of DDX5
reversed the suppressive effect of either miR-125b-5p mimic or
si-DDK5 on DDK5 expression levels (Fig.
4G).
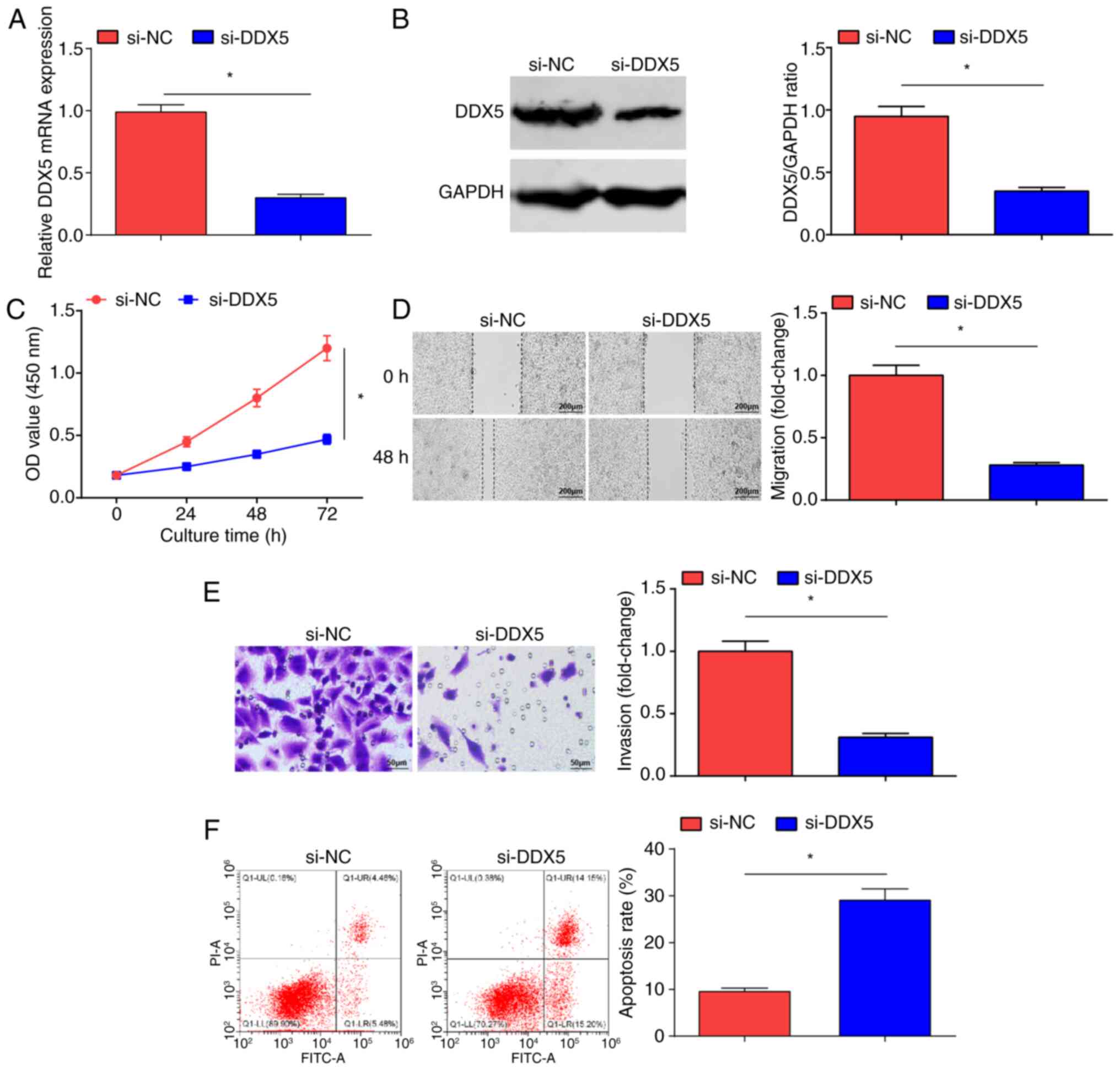
DDX5 knockdown inhibits the malignant
progression of OC cells
si-NC and si-DDX5 were introduced into SKOV3 cells;
si-DDX5 transfection inhibited DDX5 expression levels in SKOV3
cells (Fig. 5A and B). The CCK-8
(Fig. 5C), scratch (Fig. 5D) and Transwell (Fig. 5E) assays, and flow cytometry
(Fig. 5F) demonstrated that
following the inhibition of DDX5 expression, the proliferative,
migratory and invasive properties of OC cells were significantly
reduced, and cell apoptosis was significantly increased in the
si-DDX5 group compared with that in the si-NC group. In brief, DDX5
suppression inhibited OC cell progression.
DDX5 overexpression abrogates
miR-125b-5p-induced suppression of OC development
To verify that BMSCs-Exo carrying miR-125b-5p
targeting DDX5 can regulate OC development, pcDNA-NC and pcDNA-DDX5
were transfected into SKOV3 cells. RT-qPCR experiments showed that
the expression levels of DDX5 mRNA were significantly elevated in
the pcDNA-DDX5 group compared with the pcDNA-NC group (Fig. 6A). Next, the Exo-miR-125b-5p mimic +
pcDNA-NC group and the Exo-miR-125b-5p mimic + pcDNA-DDX5 group
were examined. Significantly increased DDX5 expression levels were
observed in the Exo-miR-125b-5p mimic + pcDNA-DDX5 group compared
with the Exo-miR-125b-5p mimic + pcDNA-NC group (Fig. 6B and C). The CCK-8, (Fig. 6D) scratch (Fig. 6E) and Transwell (Fig. 6F) assays, and flow cytometry
(Fig. 6G) demonstrated that the
proliferative, migratory and invasive properties of OC cells were
significantly increased, and that cell apoptosis was significantly
reduced in the Exo-miR-125b-5p mimic + pcDNA-DDX5 group compared
with the Exo-miR-125b-5p mimic + pcDNA-NC group. In summary,
miR-125b-5p, derived from BMSCs-Exo, served a key role in
inhibiting OC cell growth by targeting DDX5.
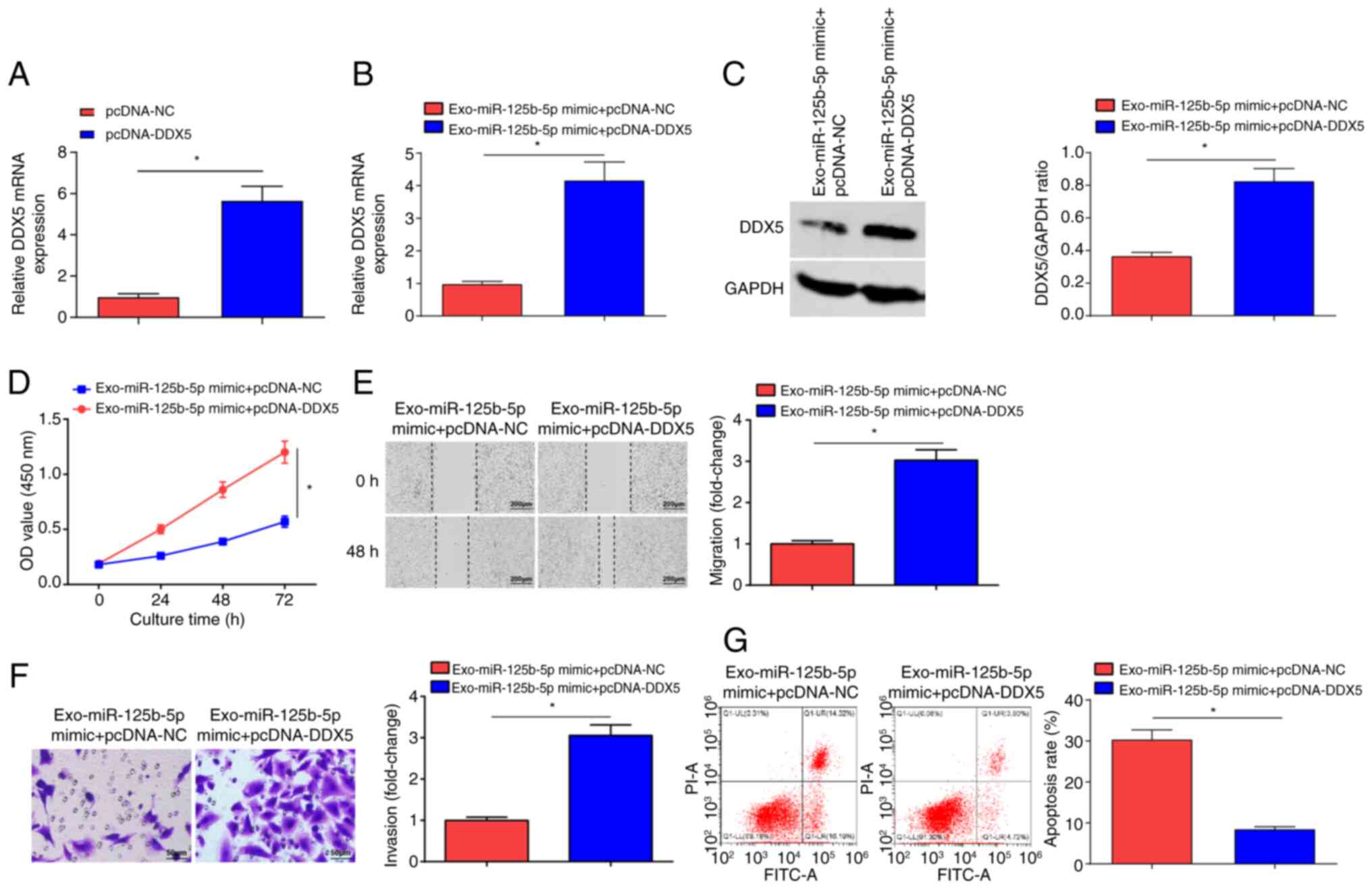 | Figure 6.DDX5 overexpression inhibits
miR-125b-5p-induced suppression of OC development. (A) DDX5 mRNA
expression in the pcDNA-NC group and pcDNA-DDX5 group. DDX5 (B)
mRNA and (C) protein expression levels in the Exo-miR-125b-5p mimic
+ pcDNA-NC and Exo-miR-125b-5p mimic + pcDNA-DDX5 groups. (D)
Proliferation of OC cells examined using the CCK-8 assay in the
Exo-miR-125b-5p mimic + pcDNA-NC and Exo-miR-125b-5p mimic +
pcDNA-DDX5 groups. (E) Migration of OC cells evaluated using a
scratch test in the Exo-miR-125b-5p mimic + pcDNA-NC and
Exo-miR-125b-5p mimic + pcDNA-DDX5 groups (scale bar, 200 µm;
magnification, ×100). (F) Invasion of OC cells assessed using a
Transwell assay in the Exo-miR-125b-5p mimic + pcDNA-NC and
Exo-miR-125b-5p mimic + pcDNA-DDX5 groups (scale bar, 50 µm;
magnification, ×200). (G) Apoptosis of OC cells examined using flow
cytometry in the Exo-miR-125b-5p mimic + pcDNA-NC and
Exo-miR-125b-5p mimic + pcDNA-DDX5 groups. Data were represented by
the mean ± standard deviation. *P<0.05. OC, ovarian cancer; OD,
optical density; miR-125b-5p, microRNA-125b-5p; NC, negative
control; Exo-, exosome encapsulated; WT, wild-type; Mut, mutant;
DDX, DEAD-box helicase 5; transfected using the pcDNA3.1
plasmid. |
Discussion
OC is the deadliest gynecological malignancy
worldwide that is often diagnosed at advanced stages with poor
outcomes (37). Focusing on the
biological functions of OC cells, the present study demonstrated
that BMSCs-Exo delivery of miR-125b-5p had anti-tumorigenic effects
in OC cells via direct targeting of DDX5.
BMSCs-Exo co-culture reduced proliferation,
invasion, migration and elevated apoptosis of OC cells in
vitro. A previous study by Qiu et al (9) reported MSC-Exo-mediated effects on
reducing cell growth in OC. The combination of MSC-Exo and
radiotherapy has a regulatory role in the control of enhanced
radiation effects on metastasis and the spread of melanoma cells
(38). Furthermore, an observation
by Xu et al (39)
demonstrated that glioma cells treated with MSCs-Exo show reduced
proliferation, invasion and migration. Shang et al (40) reported the tumor-suppressor effects
of MSCs-Exo delivering high levels of miR-1231 on the
proliferation, migration and invasion of pancreatic cancer cells.
Furthermore, a previous study suggested human umbilical cord
MSCs-Exo as a feasible therapeutic strategy in the control of
outgrowth of esophageal squamous cell carcinoma cells in
vitro and in vivo through transfer of miR-375 (41). Briefly, MSCs-Exo themselves have
anti-tumorigenic effects and their delivery of targeted nucleic
acids also contributes to cancer control.
Expression levels of miR-125b-5p were significantly
reduced in tumor tissue samples of patients with OC. miR-125b-5p
downregulation has been demonstrated in several types of cancer,
including EOC (13) and colon
cancer (42). In regards to the
delivery capacity of BMSCs-Exo, miR-125b-5p was transported into
SKOV3 cells, so as to lower the malignant abilities of cells in
vitro. Liu et al (43)
have reported that in bladder cancer cells, upon overexpressing
miR-125b-5p, cell viability and migration are inhibited, whereas
apoptosis is induced. By contrast, overexpression of miR-125b-5p
has been reported to have a role in impairing the proliferative,
migratory and invasive properties of breast cancer cells (44). In the presence of cisplatin,
overexpression of miR-125b-5p in gallbladder cancer cells increases
apoptosis levels and decreases tumor formation, but inhibition of
miR-125b-5p decreases apoptosis levels and increases tumor
formation (45). It was reported
that in hepatocellular carcinoma, miR-125b-5p overexpression limits
the malignant phenotype of tumor cells (36). In the development of esophageal
squamous cell carcinoma cells, overexpression of miR-125b-5p can
induce cell senescence and also slow down the process of epithelial
to mesenchymal transition (46).
Overall, in numerous types of cancer, including OC, miR-125b-5p
exerts protective effects to slow down malignant progression.
DDX5 was upregulated in OC, and DDX5 silencing led
to the blockade of OC cell outgrowth in vitro. Similar to
the present findings, Zhang et al (47) also reported high expression levels
of DDX5 in colorectal cancer. DDX5 is upregulated in small cell
lung cancer and its inhibition results in reduced growth of tumor
cells resistant to chemotherapy (48). DDX5 is upregulated in gastric
cancer, and inhibition of DDX5 inhibits growth of cells and
xenografts, whereas overexpression of DDX5 promotes cell
proliferation, migration and invasion (17). Also, DDX5 was identified as a target
involved in miR-125b-5p-mediated OC cell growth, as DDX5
overexpression caused the reversal of upregulated
miR-125b-5p-induced repression of OC cell development.
Mechanistically, high expression levels of DDX5 can re-activate
carcinogenesis of endometrial cancer cells mediated by silenced
hepatoma-derived growth factor (49). Furthermore, Wang et al
(50) demonstrated that DDX5
overexpression increases the proliferation of non-small-cell lung
cancer cells in vitro and in vivo. Collectively,
modification of DDX5 expression represents a switch in the
progression of tumors.
In the present study, a binding site between
miR-125b-5p and DDX5 was identified by confirming the regulatory
relationship between miR-125b-5p and DDX5 and demonstrating that
miR-125b-5p was negatively correlated with DDX5. Following this, a
binding site between miR-125b-5p and DDX5 was experimentally
demonstrated by online database, indicating that miR-125b-5p had a
role in regulating DDX5 expression. In addition, downregulation of
DDX5 expression levels could inhibit the proliferation and
malignant progression of OC cells. It was hypothesized that there
was a targeting relationship between miR-125b-5p and DDX5
expression in OC cells derived from exosomes of bone marrow MSCs.
The experimental results were as hypothesized, as the Exo +
miR-125b-5p + pcDNA-DDX5 group demonstrated increased cell
viability, migratory and invasive ability, and decreased apoptosis
rate in OC cells. DDX5 overexpression reversed the effects of
MSC-derived Exo miR-125b-5p on OC cell proliferation and invasion,
while bone marrow MSC-derived Exo miR-125b-5p inhibited OC cell
proliferation and tumor progression by targeting DDX5. In the
future, we will conduct further validation of the related
downstream mechanisms.
In summary, miR-125b-5p delivered by BMSCs-Exo could
inhibit OC progression, which was associated with DDX5
downregulation. The present study advances the understanding of
existing molecular mechanisms in OC, and explores the feasibility
of drug delivery using BMSCs-Exo.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW and WW contributed to conception and design of
manuscript and manuscript editing. DZ contributed to revising the
manuscript critically for important intellectual content and
experimental studies. YG contributed to analysis and interpretation
of data. WW and YW confirmed the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Experiments were performed after approval by the
Ethics Committee of Harbin Medical University Cancer Hospital
(approval no. 20200316; Harbin, China), and written informed
consent was provided by each patient. BMSCs were used in accordance
with the International Society for Stem Cells Research guidelines
for Stem Cell Research and Clinical Translation and approved by the
Ethics Committee of Harbin Medical University Cancer Hospital
(approval no. 20200518).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rooth C: Ovarian cancer: Risk factors,
treatment and management. Br J Nurs. 22:S23–S30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Narod S: Can advanced-stage ovarian cancer
be cured? Nat Rev Clin Oncol. 13:255–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crapnell K, Blaesius R, Hastings A, Lennon
DP, Caplan AI and Bruder SP: Growth, differentiation capacity, and
function of mesenchymal stem cells expanded in serum-free medium
developed via combinatorial screening. Exp Cell Res. 319:1409–1418.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohr A and Zwacka R: The future of
mesenchymal stem cell-based therapeutic approaches for cancer-from
cells to ghosts. Cancer Lett. 414:239–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendt M, Rezvani K and Shpall E:
Mesenchymal stem cell-derived exosomes for clinical use. Bone
Marrow Transplant. 54 (Suppl 2):S789–S792. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan F, Li X, Wang Z, Li J, Shahzad K and
Zheng J: Clinical applications of stem cell-derived exosomes.
Signal Transduct Target Ther. 9:172024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu L, Wang J, Chen M, Chen F and Tu W:
Exosomal microRNA-146a derived from mesenchymal stem cells
increases the sensitivity of ovarian cancer cells to docetaxel and
taxane via a LAMC2-mediated PI3K/Akt axis. Int J Mol Med.
46:609–620. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ono M, Kosaka N, Tominaga N, Yoshioka Y,
Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K and Ochiya
T: Exosomes from bone marrow mesenchymal stem cells contain a
microRNA that promotes dormancy in metastatic breast cancer cells.
Sci Signal. 7:ra632014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang S, Mo C, Guo S, Zhuang J, Huang B
and Mao X: Human bone marrow mesenchymal stem cells-derived
microRNA-205-containing exosomes impede the progression of prostate
cancer through suppression of RHPN2. J Exp Clin Cancer Res.
38:4952019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalinkova L, Kajo K, Karhanek M,
Wachsmannova L, Suran P, Zmetakova I and Fridrichova I:
Discriminating miRNA profiles between endometrioid well- and
poorly-differentiated tumours and endometrioid and serous subtypes
of endometrial cancers. Int J Mol Sci. 21:60712020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Lima AB, Silva LM, Gonçales NG,
Carvalho MRS, da Silva Filho AL and da Conceição Braga L:
Three-dimensional cellular arrangement in epithelial ovarian cancer
cell lines TOV-21G and SKOV-3 is associated with apoptosis-related
miRNA expression modulation. Cancer Microenviron. 11:85–92. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Zhang X, Huang Y, Zhang Q, Zhou J,
Zhang X and Wang X: miR-200b and miR-200c co-contribute to the
cisplatin sensitivity of ovarian cancer cells by targeting DNA
methyltransferases. Oncol Lett. 17:1453–1460. 2019.PubMed/NCBI
|
|
15
|
Xing Z, Ma WK and Tran EJ: The DDX5/Dbp2
subfamily of DEAD-box RNA helicases. Wiley Interdiscip Rev RNA.
10:e15192019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Caron de Fromentel C, Kim W, Wang
WH, Sun J, Yan B, Utturkar S, Lanman NA, Elzey BD, Yeo Y, et al:
RNA helicase DDX5 modulates sorafenib sensitivity in hepatocellular
carcinoma via the Wnt/β-catenin-ferroptosis axis. Cell Death Dis.
14:7862023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du C, Li DQ, Li N, Chen L, Li SS, Yang Y,
Hou MX, Xie MJ and Zheng ZD: DDX5 promotes gastric cancer cell
proliferation in vitro and in vivo through mTOR signaling pathway.
Sci Rep. 7:428762017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye X: Confluence analysis of multiple
omics on platinum resistance of ovarian cancer. Eur J Gynaecol
Oncol. 36:514–519. 2015.PubMed/NCBI
|
|
19
|
Xu CM, Chen LX, Gao F, Zhu MF, Dai Y, Xu Y
and Qian WX: MiR-431 suppresses proliferation and metastasis of
lung cancer via down-regulating DDX5. Eur Rev Med Pharmacol Sci.
23:699–707. 2019.PubMed/NCBI
|
|
20
|
Mallmann-Gottschalk N, Sax Y, Kimmig R,
Lang S and Brandau S: EGFR-specific tyrosine kinase inhibitor
modifies NK cell-mediated antitumoral activity against ovarian
cancer cells. Int J Mol Sci. 20:46932019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahmoudian-Sani MR, Forouzanfar F,
Asgharzade S and Ghorbani N: Overexpression of MiR-183/96/182
triggers retina-like fate in human bone marrow-derived mesenchymal
stem cells (hBMSCs) in culture. J Ophthalmol. 2019:24543622019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li B, Luan S, Chen J, Zhou Y, Wang T, Li
Z, Fu Y, Zhai A and Bi C: The MSC-derived exosomal lncRNA H19
promotes wound healing in diabetic foot ulcers by upregulating PTEN
via MicroRNA-152-3p. Mol Ther Nucleic Acids. 19:814–826. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Han Y, Zhao Z, Ji X, Wang X, Jin J,
Wang Q, Guo X, Cheng Z, Lu M, et al: Oral mucosal mesenchymal stem
cell-derived exosomes: A potential therapeutic target in oral
premalignant lesions. Int J Oncol. 54:1567–1578. 2019.PubMed/NCBI
|
|
24
|
Sun P, Fan X, Hu X, Fu X, Wei Q and Zang
Y: circPCNX and pecanex promote hepatocellular carcinoma cell
viability by inhibiting miR-506. Cancer Manag Res. 11:10957–10967.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang F, Chen Y, Ren S, Li Z, Sun K, Xing
Y, Zhu Y and Piao D: Cyclovirobuxine D inhibits colorectal cancer
tumorigenesis via the CTHRC1-AKT/ERK-Snail signaling pathway. Int J
Oncol. 57:183–196. 2020.PubMed/NCBI
|
|
26
|
Wang S, Su X, Xu M, Xiao X, Li X, Li H,
Keating A and Zhao RC: Exosomes secreted by mesenchymal
stromal/stem cell-derived adipocytes promote breast cancer cell
growth via activation of Hippo signaling pathway. Stem Cell Res
Ther. 10:1172019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun H, Wang H and Wang X, Aoki Y and Wang
X, Yang Y, Cheng X, Wang Z and Wang X: Aurora-A/SOX8/FOXK1
signaling axis promotes chemoresistance via suppression of cell
senescence and induction of glucose metabolism in ovarian cancer
organoids and cells. Theranostics. 10:6928–6945. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Zhao J, Ruan Y, Sun L, Xu C and
Jiang H: Sialyltransferase ST3GAL1 promotes cell migration,
invasion, and TGF-β1-induced EMT and confers paclitaxel resistance
in ovarian cancer. Cell Death Dis. 9:11022018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Wang P, Cao L, Li F, Duan S, Yuan
G, Xiao L, Guo L, Yin H, Xie D, et al: Long intergenic non-coding
RNA 01121 promotes breast cancer cell proliferation, migration, and
invasion via the miR-150-5p/HMGA2 axis. Cancer Manag Res.
11:10859–10870. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li N, Cui T, Guo W, Wang D and Mao L:
MiR-155-5p accelerates the metastasis of cervical cancer cell via
targeting TP53INP1. Onco Targets Ther. 12:3181–3196. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu SC, Cao YH, Chen LB, Kang R, Huang ZX
and Lu XS: BMSC-derived exosomal lncRNA PTENP1 suppresses the
malignant phenotypes of bladder cancer by upregulating SCARA5
expression. Cancer Biol Ther. 23:1–13. 2022. View Article : Google Scholar
|
|
32
|
Xiu C, Zheng H, Jiang M, Li J, Zhou Y, Mu
L and Liu W: MSCs-derived miR-150-5p-expressing exosomes promote
skin wound healing by activating PI3K/AKT pathway through PTEN. Int
J Stem Cells. 15:359–371. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Furuta T, Miyaki S, Ishitobi H, Ogura T,
Kato Y, Kamei N, Miyado K, Higashi Y and Ochi M: Mesenchymal stem
cell-derived exosomes promote fracture healing in a mouse model.
Stem Cells Transl Med. 5:1620–1630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Ma Y, Hu X, Zheng Y and Chen X:
Targeting PRMT5/Akt signalling axis prevents human lung cancer cell
growth. J Cell Mol Med. 23:1333–1342. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reza AMMT, Choi YJ, Yasuda H and Kim JH:
Human adipose mesenchymal stem cell-derived exosomal-miRNAs are
critical factors for inducing anti-proliferation signalling to
A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 6:384982016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hua S, Quan Y, Zhan M, Liao H, Li Y and Lu
L: miR-125b-5p inhibits cell proliferation, migration, and invasion
in hepatocellular carcinoma via targeting TXNRD1. Cancer Cell Int.
19:2032019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wieser V, Tsibulak I, Reimer DU, Zeimet
AG, Fiegl H, Hackl H and Marth C: An angiogenic tumor phenotype
predicts poor prognosis in ovarian cancer. Gynecol Oncol.
170:290–299. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Araujo Farias V, O'Valle F,
Serrano-Saenz S, Anderson P, Andrés E, López-Peñalver J, Tovar I,
Nieto A, Santos A, Martín F, et al: Exosomes derived from
mesenchymal stem cells enhance radiotherapy-induced cell death in
tumor and metastatic tumor foci. Mol Cancer. 17:1222018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu H, Zhao G, Zhang Y, Jiang H, Wang W,
Zhao D, Hong J, Yu H and Qi L: Mesenchymal stem cell-derived
exosomal microRNA-133b suppresses glioma progression via
Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res
Ther. 10:3812019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shang S, Wang J, Chen S, Tian R, Zeng H,
Wang L, Xia M, Zhu H and Zuo C: Exosomal miRNA-1231 derived from
bone marrow mesenchymal stem cells inhibits the activity of
pancreatic cancer. Cancer Med. 8:7728–7740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He Z, Li W, Zheng T, Liu D and Zhao S:
Human umbilical cord mesenchymal stem cells-derived exosomes
deliver microRNA-375 to downregulate ENAH and thus retard
esophageal squamous cell carcinoma progression. J Exp Clin Cancer
Res. 39:1402020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi H, Li K, Feng J, Liu G, Feng Y and
Zhang X: LncRNA-DANCR interferes with miR-125b-5p/HK2 axis to
desensitize colon cancer cells to cisplatin vis activating
anaerobic glycolysis. Front Oncol. 10:10342020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu S, Chen Q and Wang Y: MiR-125b-5p
suppresses the bladder cancer progression via targeting HK2 and
suppressing PI3K/AKT pathway. Hum Cell. 33:185–194. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Wang Y, Fan H, Zhang Z and Li N:
miR-125b-5p inhibits breast cancer cell proliferation, migration
and invasion by targeting KIAA1522. Biochem Biophys Res Commun.
504:277–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang D, Zhan M, Chen T, Chen W, Zhang Y,
Xu S, Yan J, Huang Q and Wang J: miR-125b-5p enhances chemotherapy
sensitivity to cisplatin by down-regulating Bcl2 in gallbladder
cancer. Sci Rep. 7:431092017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and
Shi ZZ: miR-125b-5p functions as a tumor suppressor gene partially
by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS
One. 12:e01856362017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan
C, Xu M, Sun H, Liu C, Wei P and Du X: The lncRNA NEAT1 activates
Wnt/β-catenin signaling and promotes colorectal cancer progression
via interacting with DDX5. J Hematol Oncol. 11:1132018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xing Z, Russon MP, Utturkar SM and Tran
EJ: The RNA helicase DDX5 supports mitochondrial function in small
cell lung cancer. J Biol Chem. 295:8988–8998. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu C, Wang L, Jiang Q, Zhang J, Zhu L,
Lin L, Jiang H, Lin D, Xiao Y, Fang W and Guo S: Hepatoma-derived
growth factor and DDX5 promote carcinogenesis and progression of
endometrial cancer by activating β-catenin. Front Oncol. 9:2112019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Z, Luo Z, Zhou L, Li X, Jiang T and
Fu E: DDX5 promotes proliferation and tumorigenesis of
non-small-cell lung cancer cells by activating β-catenin signaling
pathway. Cancer Sci. 106:1303–1312. 2015. View Article : Google Scholar : PubMed/NCBI
|