Introduction
According to a global cancer study performed by the
International Agency for Research on Cancer in 2018, colorectal
cancer (CRC) ranks fourth and fifth in terms of new cases and
fatalities, respectively, among all malignant tumors (1). Furthermore, China has one of the
highest annual rates of new cases and deaths from CRC and there are
~376,000 new cases and ~191,000 deaths each year (2). The adenoma-adenocarcinoma pathway
serves a notable role in the pathophysiology of CRC, with adenoma
malignancy accounting for 50–80% of CRC cases (3). Although timely resection of early
colorectal lesions can improve survival rate, most patients are
diagnosed in the middle or late stages. Even after treatment, the
5-year survival rate remains very low at <50% (4,5). Early
detection and treatment are critical for colorectal adenoma, the
most common precancerous lesion in CRC.
The gold standard for diagnosing colorectal adenoma
is an electronic colonoscopy (e-colonoscopy) examination. However,
patients find this procedure less acceptable than others as it is
invasive and requires intestinal clearance beforehand. As a result,
e-colonoscopy is not part of the general medical examination
routine (6,7). In China, early CRC screening primarily
involves a fecal occult blood test, tumor marker detection and a
related symptom questionnaire. Following this, individuals
identified as high-risk are referred for a colonoscopy for further
examination (8). Several studies
have been performed in China and other counties to identify risk
factors for colorectal adenoma, and these studies reported that
several factors, including genetics, age, metabolic syndrome and
non-alcoholic fatty liver disease, influence the incidence of CRC
(9,10). However, the findings from these
several studies are incongruent. Therefore, the present study aimed
to gather relevant clinical data, create a prediction model through
statistical analyses and test the efficacy of the model to provide
data support and an evidence-based medical foundation for future
studies. The findings of the present could increase the diagnosis
rate of colorectal adenoma and achieve positive secondary
prevention of CRC by facilitating earlier screening of high-risk
populations for colorectal adenoma.
Materials and methods
Patients and study design
The present study was a retrospective case-control
study, approved by the Ethics Committee of the Affiliated Hospital
of Shandong University of Chinese Medicine [Jinan, China; approval
no. (2024) Ethics Review No. (017)-YJS]. The study only involved
the collection of case data using anonymized or de-identified
information that cannot be traced back to individuals and poses no
risk to patients. Therefore, the need for informed patient consent
was waived by the Ethics Committee. Data recorded from patients
receiving e-colonoscopy at the Department of Proctology of The
Affiliated Hospital of Shandong University of Traditional Chinese
Medicine from January 2013 to December 2023 were included. The
inclusion criteria were as follows: i) Age of >18 years old; ii)
presence of polyps identified using colonoscopy and precise
pathological report results; iii) detailed clinical and laboratory
data; and iv) no colorectal resection performed. The exclusion
criteria were as follows: i) Diagnosis of inflammatory bowel
disease, intestinal tuberculosis, CRC or schistosomiasis; ii) a
history of gastrointestinal surgery; iii) diagnosis of an
autoimmune disease or serious underlying disease, such as systemic
lupus erythematosus and severe heart disease; and iv) insufficient
basic information. A total of 730 patients were included in
analysis and randomly divided into training and validation cohorts
in a 7:3 ratio.
Data collection
The following data was collected: i) Background
information such sex, age, body mass index, the daily number of
bowel movements, stool texture, sleep status, as well as the
medical history of encephalopathy, lung disease, hypertension,
diabetes, liver disease, smoking and drinking and family history of
polyps or tumors of the digestive system; ii) results from blood
routine examinations, such as the level of red blood cells, white
blood cells and neutrophils, platelet count (PLT), hemoglobin
levels and thrombin time (TT); iii) biochemical indexes, such as
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
γ-glutamyl transpeptidase, AST/ALT ratio, albumin/globulin ratio,
total bilirubin, total bile acid, creatinine (Cr), uric acid (UA),
glucose (GLU), total cholesterol, triglycerides, high-density
lipoprotein (HDL), low density lipoprotein, amylase, series of
tumor carcinoembryonic antigen tests, α-fetoprotein, ferroprotein;
and iv) the number and pathological type of polyps.
Pathological diagnosis
A total of two experienced pathologists diagnosed
the patients according to the World Health Organization
classification of gastrointestinal neoplasms (diagnostic criteria
of the 2019 edition) (11,12). If agreement on the diagnosis could
not be achieved, a third pathologist assisted in making a final
decision.
Basis of grouping
The present study divided the patients into two
groups: i) The adenoma group, which included patients with
histological examinations identified as adenomatous polyps; and ii)
the control group, which included patients with non-adenomatous
polyps, such as inflammatory or hyperplastic polyps, or those with
no apparent abnormalities on endoscopic examination.
Statistical analyses
Establishment of the prediction model
A collinearity test was applied to the data. If
multicollinearity was confirmed, the Least Absolute Shrinkage and
Selection Operator cross-validation method was applied to filter
the variables (13). In the absence
of multicollinearity, data were screened using a single-factor
analysis. Before performing the single-factor analysis, the
Kolmogorov-Smirnov test was applied to continuous variables to
determine the normality of the data distribution. Continuous
variables with normal distributions are represented as mean ±
standard deviation and were analyzed using the unpaired Student's
t-test or a nonparametric Mann-Whitney U test. The χ2
test was used to analyze categorical variables. Finally, the
selected variables were incorporated in the multivariate logistic
regression analysis and the prediction model was built based on the
results by plotting a nomogram.
Validation of the model
The C-index, equivalent to the area under the
receiver operating characteristic (ROC) curve, estimated the
probability of uniformity between the predicted and actual results
(14). The model was considered to
have no discrimination ability when the C-index was 0.5, whereas it
possessed full discrimination ability when the C-index was 1. The
calibration diagram visually depicts the relationship between the
predicted and actual probabilities. The closer the prediction
probability is to the 45° diagonal, the higher the calibration
degree. The sensitivity, specificity and efficacy of the predictive
models were comprehensively evaluated by plotting calibration
curves, ROC curves and decision curve analysis (DCA) graphs. The
present analysis consisted of two parts: Internal and external
validations. For the internal validation, the Bootstrap method was
used to validate the model and draw calibration curves. For the
external validation, the logistic regression formula obtained in
the training cohort was applied to patients in the validation
cohort for logistic regression analysis. The C-index of the
validation set was then calculated and the calibration curve was
plotted. The calibration degree was then confirmed using the
Hosmer-Lemeshow test. ROC and DCA curves were employed for both
internal and external validation to assess the accuracy and
clinical utility of the model. R studio (version 4.1.2; http://www.r-project.org/) and IBM SPSS Statistics
(version 26.0: IBM Corp.) were used for statistical analysis. The
statistical significance levels reported are two-sided and
P<0.05 was considered to indicate a statistically significant
difference.
Reporting standard
The present study was reported in strict accordance
with the requirements in the STROBE Guidelines checklist (15).
Results
Essential characteristics of the
training and validation cohorts
A total of 730 cases were included based on the
inclusion and exclusion criteria, with 286 assigned to the case
group and 444 assigned to the control group. In a 7:3 ratio,
patients were randomly divided into the training set (n=511) and
the validation cohort (n=219). Table
I presents the 40 basic characteristics (39 independent
variables and 1 dependent variable) of the two sets.
 | Table I.Characteristics of patients in the
training and validation cohorts. |
Table I.
Characteristics of patients in the
training and validation cohorts.
| Characteristic | Training cohort
(n=511) | Validation cohort
(n=219) | Normal reference
range |
|---|
| Sex |
|
| N/A |
|
Male | 327 (63.99) | 146 (66.67) | N/A |
|
Female | 184 (36.01) | 73 (33.33) | N/A |
| Daily number of
bowel movements |
|
| N/A |
|
Normal | 387 (75.73) | 167 (76.26) | N/A |
|
Abnormal | 124 (24.27) | 52 (23.74) | N/A |
| Stool texture |
|
| N/A |
|
Normal | 278 (54.40) | 107 (48.86) | N/A |
|
Abnormal | 233 (45.60) | 112 (51.14) | N/A |
| Sleep status |
|
| N/A |
|
Normal | 393 (76.91) | 191 (87.21) | N/A |
|
Abnormal | 118 (23.09) | 28 (12.79) | N/A |
| History of
encephalopathy |
|
| N/A |
| No | 480 (93.93) | 205 (94.98) | N/A |
|
Yes | 31 (6.07) | 14 (6.39) | N/A |
| History of lung
disease |
|
| N/A |
| No | 499 (97.65) | 211 (96.35) | N/A |
|
Yes | 12 (2.35) | 8 (3.65) | N/A |
| History of
hypertension |
|
| N/A |
| No | 358 (70.06) | 158 (72.15) | N/A |
|
Yes | 153 (29.94) | 61 (27.85) | N/A |
| History of
diabetes |
|
| N/A |
| No | 459 (89.82) | 194 (88.58) | N/A |
|
Yes | 52 (10.18) | 25 (11.42) | N/A |
| History of liver
disease |
|
| N/A |
| No | 494 (96.67) | 210 (95.89) | N/A |
|
Yes | 17 (3.33) | 9 (4.11) | N/A |
| Smoking
history |
|
| N/A |
| No | 389 (76.13) | 179 (81.74) | N/A |
|
Yes | 122 (23.87) | 40 (18.26) | N/A |
| Drinking
history |
|
| N/A |
| No | 382 (74.76) | 180 (82.19) | N/A |
|
Yes | 129 (25.24) | 39 (17.81) | N/A |
| Family history of
polyps or tumors of digestive system |
|
| N/A |
| No | 473 (92.56) | 210 (95.89) | N/A |
|
Yes | 38 (7.44) | 9 (4.11) | N/A |
| Classification |
|
| N/A |
| Adenoma
group | 203 (39.73) | 83 (37.90) | N/A |
| Control
group | 308 (60.27) | 136 (62.10) | N/A |
| Number of
polyps |
|
| N/A |
| 1 | 165 (32.29) | 78 (35.62) | N/A |
| 2 | 132 (25.83) | 53 (24.20) | N/A |
| ≥3 | 214 (41.88) | 88 (40.18) | N/A |
| Age, years | 57.00 (48.00,
65.00) | 60.00 (51.00,
66.00) | N/A |
| BMI,
kg/m2 | 25.00 (22.88,
27.39) | 24.91 (23.52,
26.97) | N/A |
| RBC,
×1012/l | 4.67 (4.30,
5.00) | 4.63 (4.30,
4.93) | Male, 4.3–5.8;
female, 3.8–5.2 |
| WBC,
×109/l | 5.77
(4.87,6.98) | 5.94 (4.94,
7.03) | 4.0–10.0 |
| NEU,
×109/l | 58.10 (51.65,
64.75) | 56.80 (51.50,
63.50) | 2.0–7.0 |
| PLT,
×109/l | 232.00 (199.00,
273.00) | 220.00 (185.00,
256.00) | 125-350 |
| HGB, g/l | 144.00 (132.00,
155.00) | 143.00 (132.50,
154.00) | Male, 130–175;
female, 120–150 |
| TT, sec | 16.60 (16.00,
17.40) | 16.30 (15.70,
17.10) | 14-21 |
| ALT, U/l | 18.00 (14.00,
26.00) | 18.00 (13.00,
25.50) | Male, 9–50; female,
7–40 |
| AST, U/l | 20.00 (16.10,
24.00) | 20.00 (17.05,
24.95) | Male, 15–40;
female, 13–35 |
| GGT, U/l | 21.00 (15.00,
32.00) | 22.00 (16.00,
31.35) | Male, 10–60;
female, 7–45 |
| AST/ALT | 1.10 (0.80,
1.30) | 1.10 (0.80,
1.40) | 0.8–1.4 |
| A/G | 1.50 (1.40,
1.70) | 1.50 (1.40,
1.70) | 1.2–2.4 |
| TBIL, µmol/l | 14.4 (11.30,
18.80) | 13.60 (11.00,
17.85) | 3.4–20.5 |
| TBA, µmol/l | 2.00 (1.10,
3.45) | 2.20 (1.30,
3.40) | 0.1–10.0 |
| Cr, µmol/l | 68.00 (59.00,
77.00) | 69.80 (60.50,
82.00) | Male, 53–106;
female, 44–97 |
| UA, µmol/l | 321.00 (267.00,
381, 00) | 319.00 (258.50,
374.00) | Male, 208–428;
female, 155–357 |
| GLU, mmol/l | 5.27 (4.89,
5.81) | 5.26 (4.85,
5.86) | 3.9–6.1 |
| CHOL, mmol/l | 5.10 (4.35,
5.84) | 5.06 (4.35,
5.80) | <5.2 |
| TG, mmol/l | 1.23 (0.89,
1.77) | 1.26 (0.90,
1.86) | ≤1.7 |
| HDL, mmol/l | 1.20 (1.00,
1.42) | 1.17 (0.98,
1.42) | Male, ≥1.0; female,
≥1.3 |
| LDL, mmol/l | 3.07 (2.48,
3.75) | 3.15 (2.66,
3.67) | <3.0 |
| AMY, U/l | 60.00 (48.00,
75.00) | 58.00 (45.00,
69.05) | 30-110 |
| CEA, ng/ml | 2.10 (1.45,
3.01) | 2.19 (1.44,
3.03) | <3.0 |
| AFP, ng/ml | 3.03 (2.28,
4.25) | 3.10 (2.12,
3.86) | <7 |
| Fer, ng/ml | 150.00 (87.00,
237.00) | 141.00 (85.80,
218.20) | Male, 30–400;
female, 15–150 |
Selection of variables and the
establishment of prediction model
Single-factor analysis
The Variance Inflation Factor (VIF) is a measure of
the severity of multicollinearity in a multiple linear regression
model. The absence of covariance is indicated when the VIF value is
<10. As presented in Table II,
the VIF values of all the variables included in the analysis were
<10, indicating that there was no multicollinearity in the data.
Therefore, a single-factor analysis was used to screen the
variables. Continuous variables did not conform to a normal
distribution, so the Mann-Whitney U test was applied, and
categorical variables were analyzed using the χ2 test.
The analysis revealed a significant difference (P<0.05) between
the adenoma and control groups for 12/39 variables, including for
sex, age, the daily number of bowel movements, history of
hypertension, history of diabetes, PLT, TT, Cr, UA, GLU, HDL and
the number of polyps (Table
III).
 | Table II.Results of the multicollinearity
test. |
Table II.
Results of the multicollinearity
test.
|
| Unstandardized
factor | Standardized
factor |
|
| Collinearity
statistics |
|---|
|
|
|
|
|
|
|
|---|
| Variable | B | Standard error | Beta | t | P-value | Tolerance | VIF |
|---|
| Sex | −0.094 | 0.075 | −0.092 | −1.255 | 0.210 | 0.311 | 3.218 |
| Age | 0.008 | 0.002 | 0.207 | 3.911 | <0.001 | 0.598 | 1.671 |
| BMI | −0.010 | 0.008 | −0.130 | −1.269 | 0.205 | 0.159 | 6.281 |
| Daily number of
bowel movements | 0.116 | 0.055 | 0.102 | 2.132 | 0.034 | 0.734 | 1.362 |
| Stool texture | −0.035 | 0.047 | −0.035 | −0.735 | 0.463 | 0.720 | 1.389 |
| Sleep status | 0.025 | 0.053 | 0.021 | 0.465 | 0.642 | 0.813 | 1.230 |
| History of
encephalopathy | 0.086 | 0.090 | 0.042 | 0.954 | 0.340 | 0.862 | 1.160 |
| History of lung
disease | 0.004 | 0.140 | 0.001 | 0.027 | 0.978 | 0.897 | 1.114 |
| History of
hypertension | −0.049 | 0.050 | −0.046 | −0.971 | 0.332 | 0.766 | 1.305 |
| History of
diabetes | 0.075 | 0.072 | 0.046 | 1.048 | 0.295 | 0.853 | 1.172 |
| History of liver
disease | 0.060 | 0.116 | 0.022 | 0.520 | 0.603 | 0.930 | 1.075 |
| Smoking
history | −0.031 | 0.063 | −0.027 | −0.499 | 0.618 | 0.562 | 1.780 |
| Drinking
history | 0.081 | 0.061 | 0.072 | 1.326 | 0.185 | 0.575 | 1.738 |
| Family history of
polyps | −0.105 | 0.080 | −0.056 | −1.323 | 0.186 | 0.922 | 1.085 |
| RBC | −0.038 | 0.042 | −0.046 | −0.902 | 0.367 | 0.650 | 1.537 |
| WBC | 0.013 | 0.013 | 0.046 | 0.958 | 0.339 | 0.729 | 1.371 |
| NEU | 0.001 | 0.001 | 0.076 | 1.810 | 0.071 | 0.947 | 1.056 |
| PLT | −0.001 | 0.000 | −0.090 | −1.812 | 0.071 | 0.688 | 1.453 |
| HGB | 0.000 | 0.000 | 0.052 | 1.166 | 0.244 | 0.858 | 1.166 |
| TT | 0.027 | 0.014 | 0.080 | 1.844 | 0.066 | 0.887 | 1.128 |
| ALT | −0.001 | 0.002 | −0.069 | −0.827 | 0.409 | 0.244 | 4.098 |
| AST | 0.002 | 0.003 | 0.075 | 0.669 | 0.504 | 0.132 | 7.579 |
| GGT | 0.000 | 0.000 | 0.026 | 0.360 | 0.719 | 0.317 | 3.151 |
| AST/ALT | 0.042 | 0.024 | 0.087 | 1.773 | 0.077 | 0.705 | 1.419 |
| A/G | −0.011 | 0.010 | −0.050 | −1.052 | 0.293 | 0.756 | 1.323 |
| TBIL | −0.002 | 0.003 | −0.029 | −0.637 | 0.525 | 0.788 | 1.269 |
| TBA | 0.005 | 0.008 | 0.027 | 0.588 | 0.557 | 0.813 | 1.230 |
| Cr | −0.000 | 0.002 | −0.001 | −0.025 | 0.98 | 0.591 | 1.691 |
| UA | 0.000 | 0.000 | 0.075 | 1.399 | 0.162 | 0.588 | 1.701 |
| GLU | −0.001 | 0.001 | −0.034 | −0.813 | 0.416 | 0.943 | 1.061 |
| CHOL | −0.001 | 0.001 | −0.045 | −1.067 | 0.287 | 0.962 | 1.040 |
| TG | −0.024 | 0.018 | −0.063 | −1.338 | 0.182 | 0.768 | 1.301 |
| HDL | −0.064 | 0.059 | −0.050 | −1.083 | 0.279 | 0.785 | 1.273 |
| LDL | −0.009 | 0.025 | −0.017 | −0.363 | 0.717 | 0.807 | 1.239 |
| AMY | 0.001 | 0.001 | 0.0490 | 1.111 | 0.267 | 0.858 | 1.166 |
| CEA | −0.018 | 0.008 | −0.099 | −2.217 | 0.027 | 0.839 | 1.192 |
| AFP | −0.002 | 0.011 | −0.008 | −0.194 | 0.847 | 0.889 | 1.125 |
| Fer | −0.000 | 0.000 | −0.028 | −0.596 | 0.551 | 0.754 | 1.326 |
| Number of
polyps | 0.109 | 0.025 | 0.190 | 4.322 | <0.001 | 0.867 | 1.153 |
 | Table III.P-values for comparisons between the
adenoma and the control groups. |
Table III.
P-values for comparisons between the
adenoma and the control groups.
| Independent
variable | Adenoma group
(n=203) | Control group
(n=308) | P-value |
|---|
| Sex |
|
| 0.002 |
|
Male | 147 (72.4) | 180 (58.4) |
|
|
Female | 56 (27.6) | 128 (41.6) |
|
| Daily number of
bowel movements |
|
| 0.018 |
|
Normal | 142 (70) | 245 (79.5) |
|
|
Abnormal | 61 (30.0) | 63 (20.5) |
|
| Stool texture |
|
| 0.281 |
|
Normal | 104 (51.2) | 174 (56.5) |
|
|
Abnormal | 99 (48.8) | 134 (43.5) |
|
| Sleep status |
|
| 0.321 |
|
Normal | 151 (74.4) | 242 (78.6) |
|
|
Abnormal | 52 (25.6) | 66 (21.4) |
|
| History of
encephalopathy |
|
| 0.113 |
| No | 186 (91.6) | 294 (95.5) |
|
|
Yes | 17 (8.4) | 14 (4.5) |
|
| History of lung
disease |
|
| 0.662 |
| No | 197 (97.0) | 302 (98.1) |
|
|
Yes | 6 (3.0) | 6 (1.9) |
|
| History of
hypertension |
|
| 0.034 |
| No | 131 (64.5) | 227 (73.7) |
|
|
Yes | 72 (35.5) | 81 (26.3) |
|
| History of
diabetes |
|
|
|
| No | 173 (85.2) | 286 (92.9) | 0.008 |
|
Yes | 30 (14.8) | 22 (7.1) |
|
| History of liver
disease |
|
|
|
| No | 194 (95.6) | 300 (97.4) | 0.379 |
|
Yes | 9 (4.4) | 8 (2.6) |
|
| Smoking
history |
|
|
|
| No | 154 (75.9) | 235 (76.3) | 0.994 |
|
Yes | 49 (24.1) | 73 (23.7) |
|
| Drinking
history |
|
|
|
| No | 147 (72.4) | 235 (76.3) | 0.376 |
|
Yes | 56 (27.6) | 73 (23.7) |
|
| Family history of
polyps or tumors of the digestive system |
|
| 0.371 |
| No | 191 (94.1) | 282 (91.6) |
|
|
Yes | 12 (5.9) | 26 (8.4) |
|
| Number of
polyps |
|
| <0.001 |
| 1 | 44 (21.7) | 121 (39.3) |
|
| 2 | 47 (23.2) | 85 (27.6) |
|
| ≥3 | 112 (55.2) | 102 (33.1) |
|
| Age, years | 61.00 (52.50,
67.00) | 55.00 (45.00,
62.25) | <0.001 |
| BMI,
kg/m2 | 25.51 (23.23,
27.42) | 24.80 (22.80,
27.35) | 0.150 |
| RBC,
×1012/l | 4.64 (4.24,
4.98) | 4.68 (4.32,
5.02) | 0.268 |
| WBC,
×109/l | 5.92 (5.00,
7.04) | 5.72 (4.79,
6.85) | 0.190 |
| NEU,
×109/l | 59.10 (51.95,
65.95) | 57.70 (51.55,
63.90) | 0.060 |
| PLT,
×109/l | 220.00 (191.50,
254.50) | 237.00 (202.75,
280.25) | 0.001 |
| HGB, g/l | 144.00 (134.50,
155.00) | 143.50 (130.00,
155.00) | 0.538 |
| TT, sec | 16.90 (16.10,
17.50) | 16.50 (16.00,
17.30) | 0.014 |
| ALT, U/l | 19.00 (14.00,
25.00) | 18.00 (13.00,
27.00) | 0.442 |
| AST, U/l | 21.00 (17.00,
24.00) | 20.00 (16.00,
25.00) | 0.127 |
| GGT, U/l | 21.00 (15.00,
32.00) | 21.00 (15.00,
31.00) | 0.634 |
| AST/ALT | 1.10 (0.90,
1.30) | 1.10 (0.80,
1.30) | 0.573 |
| A/G | 1.50 (1.40,
1.70) | 1.50 (1.40,
1.70) | 0.532 |
| TBIL, µmol/l | 14.90 (11.70,
19.20) | 14.00 (11.20,
18.20) | 0.122 |
| TBA, µmol/l | 2.10 (1.20,
3.50) | 1.80 (1.10,
3.23) | 0.058 |
| Cr, µmol/l | 71.00 (62.00,
78.00) | 66.50 (56.00,
77.00) | 0.014 |
| UA, µmol/l | 331.00 (287.00,
388.00) | 305.50 (259.00,
378.00) | 0.009 |
| GLU, mmol/l | 5.38 (4.97,
6.04) | 5.22 (4.84,
5.67) | 0.009 |
| CHOL, mmol/l | 5.07 (4.20,
5.81) | 5.11 (4.44,
5.85) | 0.187 |
| TG, mmol/l | 1.16 (0.91,
1.78) | 1.27 (0.88,
1.72) | 0.612 |
| HDL, mmol/l | 1.19 (0.98,
1.37) | 1.24 (1.01,
1.44) | 0.020 |
| LDL, mmol/l | 3.08 (2.42,
3.72) | 3.07 (2.53,
3.81) | 0.578 |
| AMY, U/l | 61.00 (48.00,
79.50) | 60.00 (47.75,
71.00) | 0.200 |
| CEA, ng/ml | 2.13 (1.47,
3.09) | 2.06 (1.42,
2.96) | 0.365 |
| AFP, ng/ml | 3.12 (2.21,
4.32) | 2.91 (2.31,
4.21) | 0.549 |
| Fer, ng/ml | 155.20 (89.30,
248.00) | 144.00 (85.98,
226.50) | 0.304 |
Single- and multi-factor logistic
regression analyses
The univariate logistic regression analysis
demonstrated that sex, age, the daily number of bowel movements,
history of hypertension, history of diabetes, PLT, TT, Cr, UA, HDL
and the number of polyps were significantly associated with the
likelihood of colorectal adenomas (P<0.05). Colorectal adenomas
were more common in men than in women and in those who had an
abnormal daily number of bowel movements, a history of hypertension
and diabetes and in those with ≥3 polyps. Furthermore, Cr, UA, TT
and age were positively associated with the likelihood of
colorectal adenomas, whereas PLT and HDL were negatively associated
(Table IV).
 | Table IV.Results of the univariate and
multifactor logistic regression models for the probability of
colorectal adenoma. |
Table IV.
Results of the univariate and
multifactor logistic regression models for the probability of
colorectal adenoma.
|
| Single-factor
logistic regression analysis | Multi-factor
logistic regression analysis |
|---|
|
|
|
|
|---|
| Characteristic | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex (female vs.
male) | 0.536 | 0.364–0.782 | 0.001 | 0.630 | 0.378–1.045 | 0.073 |
| Age (adenoma vs.
control) | 1.048 | 1.031–1.065 | <0.001 | 1.044 | 1.025–1.065 | <0.001 |
| Daily number of
bowel movements (abnormal vs. normal) | 1.671 | 1.110–2.515 | 0.014 | 1.616 | 1.026–2.547 | 0.038 |
| History of
hypertension (yes vs. no) | 1.540 | 1.049–2.261 | 0.027 | 0.897 | 0.569–1.404 | 0.635 |
| History of diabetes
(yes vs. no) | 2.254 | 1.265–4.074 | 0.006 | 1.505 | 0.789–2.897 | 0.216 |
| PLT (adenoma vs.
control) | 0.994 | 0.991–0.997 | <0.001 | 0.997 | 0.993–1.000 | 0.066 |
| TT (adenoma vs.
control) | 1.185 | 1.037–1.378 | 0.020 | 1.181 | 1.021–1.390 | 0.036 |
| Cr (adenoma vs.
control) | 1.013 | 1.001–1.026 | 0.033 | 1.002 | 0.987–1.018 | 0.785 |
| UA (adenoma vs.
control) | 1.002 | 1.000–1.004 | 0.030 | 1.002 | 0.999–1.004 | 0.142 |
| GLU (adenoma vs.
control) | 0.996 | 0.555–1.008 | 0.624 | 0.995 | 0.888–1.007 | 0.615 |
| HDL (adenoma vs.
control) | 0.498 | 0.284–0.836 | 0.012 | 0.705 | 0.364–1.288 | 0.280 |
| Number of polyps
(≥3 vs. 1 or 2) | 3.020 | 1.961–4.706 | <0.001 | 2.442 | 1.526–3.944 | <0.001 |
The results of the multifactorial logistic
regression analysis demonstrated a significant association between
age, the daily number of bowel movements, TT and the number of
polyps, with the likelihood of colorectal adenomas (P<0.05;
Table IV). This suggests that the
four aforementioned factors are independent risk factors for
colorectal adenoma when >1 factor is evaluated at the same
time.
After several rounds of cascading statistical
methods, the range of variables initially included was
progressively narrowed. Eventually only age, the daily number of
bowel movements, TT and the number of polyps were included in the
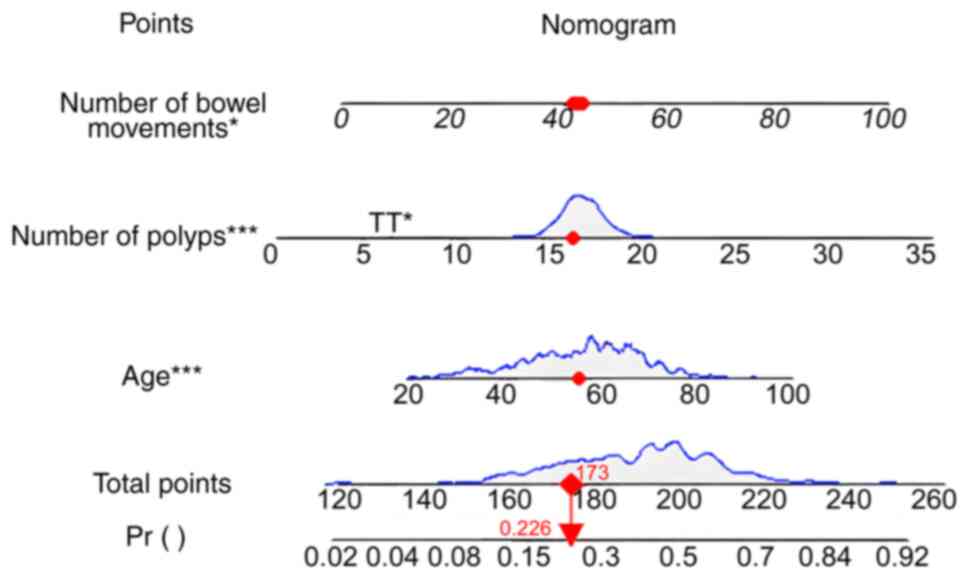
nomogram to build the prediction model (Fig. 1). Different positions on each line
segment represent specific ranges of values, whilst the length
reflects the extent of their influence on the results. The nomogram
consists of the variable name, score and probability. Depending on
the location of a variable, the score for that criterion is
determined by the value at the top of the chart. The scores of all
metrics are summed to give the total score, and finally, the
probability of occurrence of each outcome event is further derived
through the function transformation relationship (16).
Validation of predictive models
Internal validation
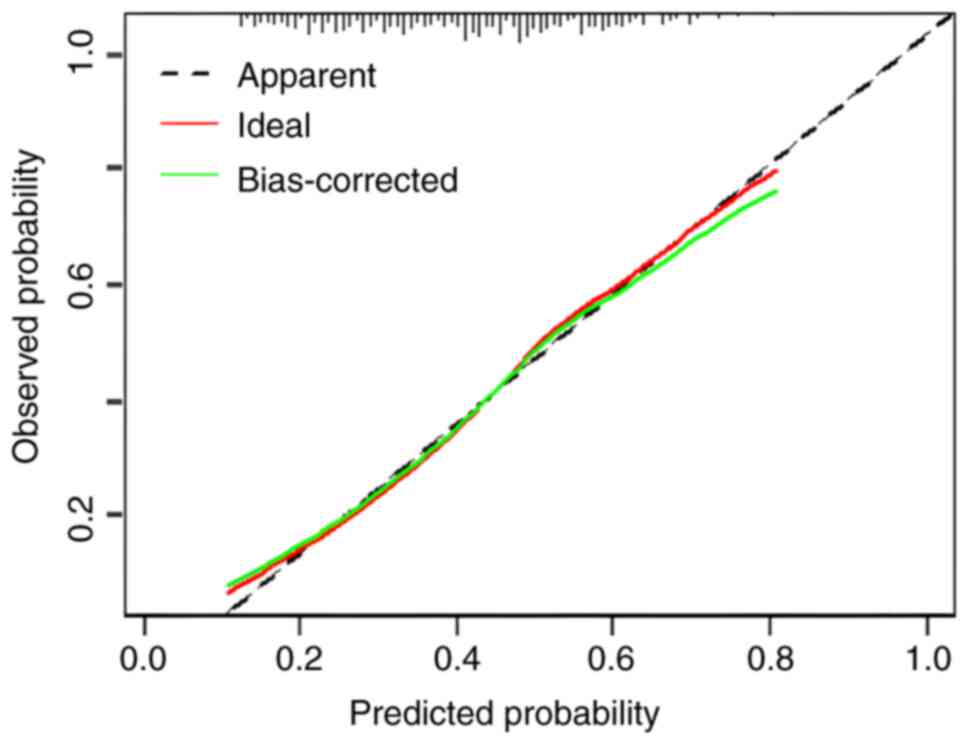
The C-index in the training cohort was 0.7054 [95%
confidence interval (CI), 0.6596–0.7512]. The calibration curve was
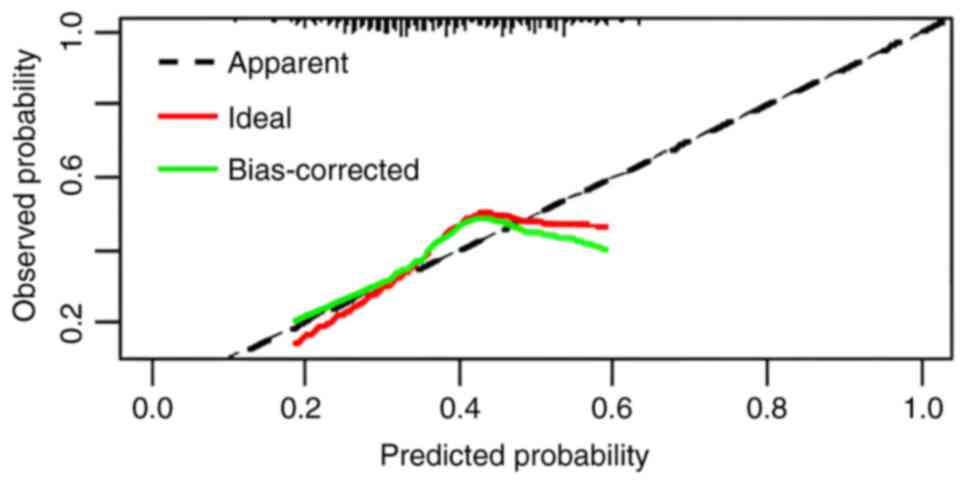
plotted using the Bootstrap method with n=1,000 (Fig. 2). The prediction probability curve
was very close to the 45° diagonal, indicating that the model had a
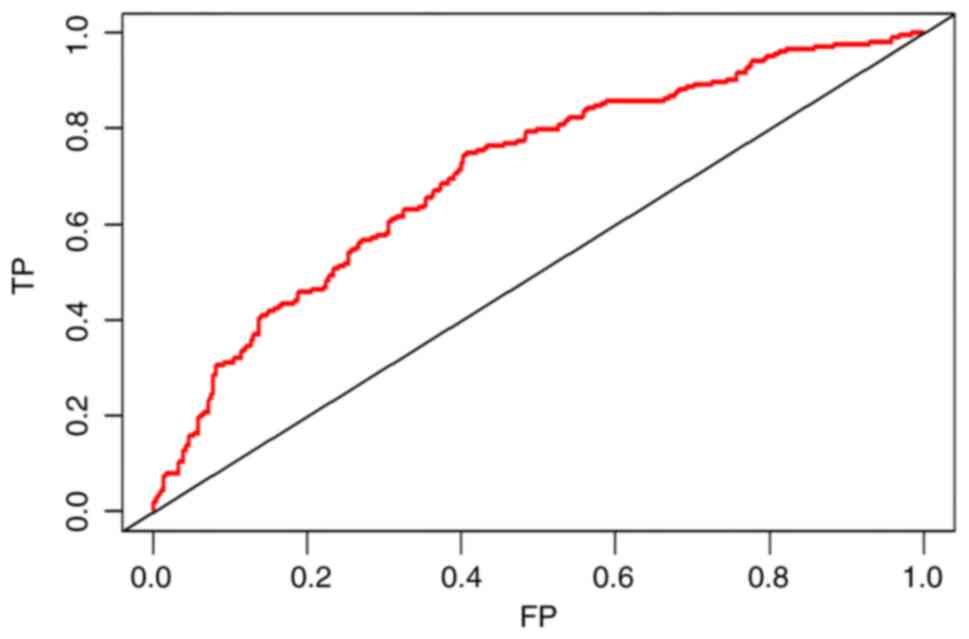
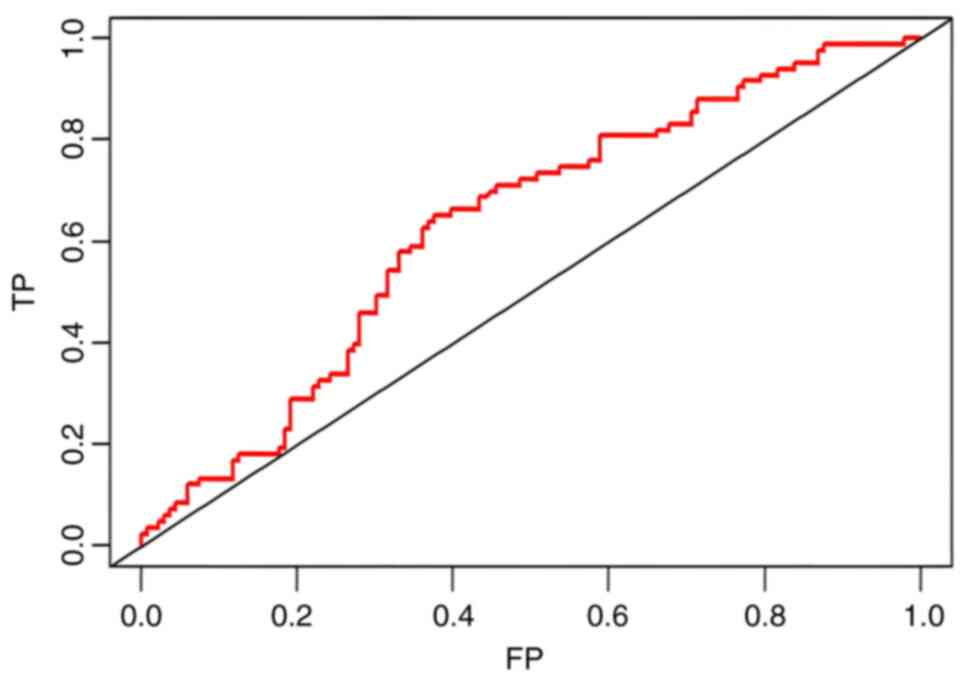
high level of predictability. The ROC curve is a tool used to
evaluate the performance of a binary classification model by
plotting the relationship between True Positive Rate and False
Positive Rate, to reflect the performance of the classifier. The
closer the ROC curve is to the point (1,0), the more effective the
generalization performance of the model is. Fig. 3 displays the ROC curve for the
training set, indicating that the model demonstrated a strong
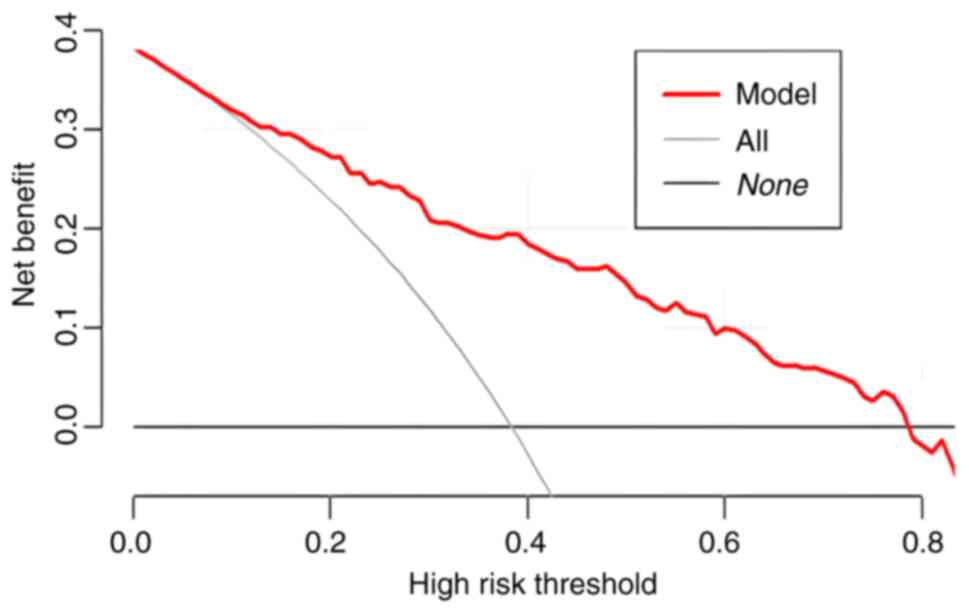
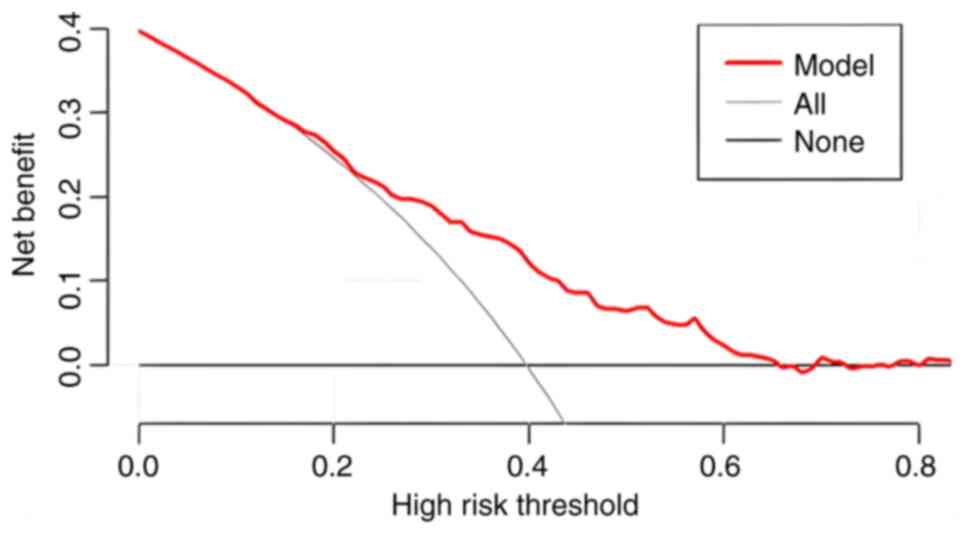
performance. By contrast, DCA is more focused on the practical
application of clinical decision-making, integrating patient or
decision-maker preferences into the analysis and considering the
clinical utility of a particular model. It quantifies the balance
between the benefits of using the model and the potential
drawbacks. As such, the core significance of the DCA curve is to
translate the statistical performance of a predictive model into
the actual value of clinical decision-making, and it is an
important tool for assessing the diagnostic accuracy and clinical
utility of predictive models. Traditional metrics (such as area
under the curve, sensitivity and specificity) do not directly
reflect the clinical value of the model, and DCA fills this gap by
providing a more intuitive decision support tool (17–19).
In DCA, the horizontal coordinate is the threshold probability and
the vertical coordinate is the net benefit (NB). The greater the NB
value of the DCA curve is above the extreme curve in the range of
the horizontal coordinates, the more improved the clinical
application. The DCA curve of the training cohort in the present
study is presented in Fig. 4, which
reveals that the model had a good clinical application value.
External validation
The logistic regression formula derived from the
training cohort was applied to the validation cohort, yielding a
C-index of 0.6306 (95% CI, 0.5560–0.7053). Furthermore, the
calibration curves also demonstrated good predictive power
(Fig. 5). The Hosmer-Lemeshow test
demonstrated that P=0.28 and χ2=9.7893 with P<0.05,
indicating that the model fit was good. The ROC and DCA curves of
the validation cohort are presented in Figs. 6 and 7, demonstrating that this model also
showed a good performance and clinical application value in the
validation cohort.
Discussion
The present study applied appropriate statistical
methods to analyze relevant clinical data and develop a predictive
model of risk factors for colorectal adenoma. The line graph, also
called nomogram, is widely used in clinical practice to assess the
risk of disease occurrence and prognosis (20,21).
The core principle is to integrate multiple predictors based on the
results of regression analysis, use the regression model to measure
the degree of influence of different variables on the outcome
events and transform this information into intuitive and
easy-to-understand lines with scale markings. Nomograms transform
complex regression equations into visual graphs, making regression
analysis results and predictive model profiles more readable to
facilitate fast and efficient assessment of clinical data. It is
due to this intuitive and efficient advantage that it has gradually
gained more attention and application in medical research and
clinical practice as well (21). To
date, several clinical researchers have used the method of
predictive modeling with nomograms to present their data analysis
results, including certain high-level studies published in
high-impact factor journals, including studies by Gafita et
al (22), Liu et al
(23) and Pietrantonio et al
(24). These high-level nomogram
studies similarly construct a model through multifactorial logistic
regression analysis and then assess model performance through ROC
curves, calibration curves, as well as clinical applicability
through DCA curves. However, they tend to use large samples or
multi-center clinical data to enhance the accuracy of the results.
By contrast to the aforementioned studies, a broader range of
variables was included in the present study. In addition to the
conventional variables reported in previous research, additional
factors that may be related to colorectal adenomas were
comprehensively considered. For the first time to the best our
knowledge, three new variables, sleep status, history of
encephalopathy and lung disease, were introduced.
The link between sleep status and colorectal
adenomas has been previously investigated. In a clinical
case-control study, a marked association was reported between
patients with colorectal adenomas and their corresponding sleep
duration, with an average sleep duration of <6 h per night
increasing the risk of adenomas by ~50% (25). Sleep deprivation increases the
expression of TNF-α, triggering a localized inflammatory response.
Melatonin, which is closely related to sleep, it increases the
expression of oncogene P53 and promotes the repair of DNA. At the
same time, melatonin can inhibit the cell cycle and reduce cell
proliferation, contributing to tumor suppression. Poor sleep
conditions will lead to a large reduction in the secretion of
melatonin, which may increase the risk of developing intestinal
tumors. In addition, circadian rhythm disorders can also trigger
mutations in circadian rhythm protein 2, which can lead to a series
of diseases (26,27). Moreover, the concept of the
brain-gut axis was proposed as early as the 1960s. Further research
on microorganisms has gradually recognized that the gut microbiota
serves a key regulatory role in the interaction between the brain
and the gastrointestinal tract; therefore, the term
‘microbial-brain-gut axis’ was coined (28). The microbial-brain-gut axis is
involved in the development of several intestinal diseases. It also
influences tumor cell proliferation, apoptosis, invasion and other
processes in intestinal tumors. Meanwhile the brain can regulate
gastrointestinal tumors through anatomical neural and
neuroendocrine pathways (29).
Several studies have reported that CRC and precancerous lesions are
associated with cognitive function and mental health. The
microbiota-gut-brain axis may serve a notable role in this
relationship (30–32). Additionally, dysregulation of the
microbiota composition and abnormalities in intestinal barrier
function may be key factors in the underlying pathological
mechanisms (33). Dysregulation of
the intestinal flora has been reported in a number of patients with
colorectal adenomas, inflammatory bowel disease and cancer.
Intestinal flora can form a cancer-promoting intestinal
microenvironment through chronic inflammation thus affecting the
development of the disease (34).
Therefore, in the prevention and treatment of colorectal tumors and
precancerous lesions, the concept of ‘brain and intestines
together’ could represent a breakthrough.
Furthermore, the present study incorporated lung
disease history into the model as Chinese medical theory posits
that the lung and large intestine are interconnected and influence
each other (35). In previous
years, several researchers have assessed this theory by performing
experimental studies, reporting that there is a molecular biology
basis for the link between the lung and the large intestine
(36–38). First, histoembryological studies
have reported that the differentiation and development of the
lungs, trachea and intestines originate from the endoderm and share
a common embryonic origin (39–42).
The mucosal immunity has a crucial role in connecting the lungs and
the large intestine through both anatomical and physiological
activities. The mucous membranes found in the gastrointestinal and
respiratory tracts are integral parts of the overall mucosal immune
system, which allows for interactions and communication between
these two systems. The molecular basis of this mucosal immunity is
the secretory IgA (SIgA), which can be secreted in large amounts by
both the respiratory and gastrointestinal tract, the latter being
the primary site of the SIgA immune response. Through the ‘homing’
and the common immune system, the mucosal immunity of the
intestinal tract activates the lymphocytes and reaches the
respiratory tract and other mucosal lymphoid tissues, where it
exerts the immune response against the same antigen, forming the
common mucosal immune system (43).
Intestinal trefoil factor 3 (TFF3) is secreted by the cup cells of
the intestinal mucosa, where it exerts an important protective
effect on the mucosa of the digestive tract. Notably, TFF3 was
reported to have a higher level of expression in the respiratory
tract than in the colonic tissues and is closely associated with
lung function, which further suggests that an inextricable link
exists between the lungs and the intestines (44,45).
In addition, it was reported that the increase in inflammatory
cells (neutrophils, eosinophils and B-lymphocytes) in the lungs,
stimulated by lung pathologies such as pneumonia, was accompanied
by marked changes in the microbial community in the gut (38,46).
These suggest that there is a notable interaction between the
functions of the lungs and the intestines, and this inspired the
present study.
According to the prediction model in the present
study, abnormal bowel frequency is an independent risk factor for
developing colorectal adenoma. Changes in bowel frequency, habits
and stool characteristics are strongly associated with the
emergence of colorectal adenoma (47). These were also early symptoms of
CRC, which may be caused by long-term diarrhea and constipation,
resulting in intestinal dysfunction and intestinal microecological
changes, resulting in chronic inflammation (48). Inflammation is inextricably linked
to tumorigenesis. Inflammation can cause DNA changes in intestinal
cells and abnormal microbial metabolism, resulting in a series of
lesions and, eventually, tumorigenesis (49). This is in line with findings that
indicate that constipation could cause the accumulation of harmful
substances in the intestinal lumen and damage the intestinal
mucosa. This results in repeated repair and healing, leading to DNA
changes in the epithelial cells of the mucosal surface, which could
lead to adenomas (50,51). The aforementioned findings are
consistent with the results of the present study, indicating that
an abnormal number of bowel movements was a significant risk factor
for colorectal adenomas.
The prediction model revealed that the TT was
positively associated with a risk of colorectal adenoma. The TT is
the time it takes for blood to clot after standardized thrombin is
added to the plasma. TT is considered prolonged only when it is
>3 sec from the standard control, suggesting hyperfibrinolysis,
increased heparin and heparin-like substances (52). Only 3/730 patients in the present
study had abnormal TT values, with the rest within the normal
range, implying no association between abnormal TT values and the
risk of colorectal adenoma. However, this could indicate that even
within the normal range, the greater the TT value, the more likely
a colorectal adenoma is to occur. TT is one of the indicators
commonly used in clinical practice to reflect the coagulation
function and its relationship with malignant tumors has received
increasing attention in recent years (53,54).
Several researchers have assessed the concept of
malignancy-associated coagulation abnormality. Most studies
reported that alterations in coagulation and fibrinolytic processes
are associated with the occurrence and progression of malignant
tumors and 95% of patients are accompanied by abnormalities in
coagulation function, such as pancreatic, nasopharyngeal, lung and
gastric cancers (55–62). In addition, it is generally
considered that activation of the coagulation system is closely
associated with the angiogenesis process occurring in human
malignant tumors (63). Moreover,
the relationship between CRC and coagulation function has been
previously assessed, and certain studies have reported that the
risk of coagulation abnormality in CRC is higher than that in other
tumors (64). Certain studies have
also reported that there is an association between the development
of CRC and the hypercoagulable state of the blood in several
malignant tumors (65,66). Abnormal coagulation can affect the
proliferation and migration of tumor cells and even promote tumor
angiogenesis, but its specific mechanism needs to be further
elucidated (67). The significance
of one of the coagulation function indicators, TT, has also been
increasingly recognized. Several researchers have included TT in
similar diagnostic or prognostic modeling studies. For instance, a
predictive modeling study by Junsheng et al (68) reported that TT is an independent
risk factor for the progression of colorectal adenoma to CRC and
this finding was integrated into the predictive modeling. The
clinical study by Fu et al (69) also reported that the prothrombin
time, prothrombin time activity, activated partial thromboplastin
time, TT and fasting blood glucose levels were higher in patients
with CRC than in control patients, suggesting that patients with
CRC have notable coagulation abnormalities. Zihong et al
(70) reported that there was a
marked difference in TT between patients with early-stage CRC and
the controls. These findings suggest that coagulation indicators
such as TT should be considered in the early diagnosis, treatment
and prevention of colorectal adenoma and CRC.
The number of polyps was associated with the risk of
colorectal adenoma in the present study. Individuals with ≥3 polyps
were more likely to develop colorectal adenoma than those with one
to two polyps. According to one study, >50% of colorectal
adenomas were multiple polyps (71). Another study reported that most
patients with adenomas had ≥3 polyps (72). These findings are consistent with
the results of the present study.
The relationship between age and the risk of
colorectal adenoma has been assessed by several studies, resulting
in generally consistent conclusions. Both colorectal adenoma and
CRC have been reported to be were strongly associated with age, and
the prevalence increases with advanced age (73–77).
Karsenti et al (78)
analyzed 6,027 colonoscopy results and reported that the detection
rate of adenomas and the rate of advanced tumor formation in
individuals aged ≥45 years increased 2-fold compared with those
<45 years old. Lin et al (79), in a CRC screening of 2 million
elderly individuals, reported that the positive predictive value of
CRC and advanced adenomas increased with age. The results of an
epidemiologic survey of adenomas based on the Chinese population
reported that the prevalence of adenomas increased from 4.6% at age
39 years to a peak of 27.3% at age ≥65 years (80). The detection rate of adenomas and
the risk of cancerous transformation in individuals >60 years of
age increased dramatically (81,82).
The reason for this may be that with age, the function of all
organs of the human body gradually declines and the ability of the
intestinal mucosa to repair damage is greatly weakened, making it
prone to lesions. The older the age, the greater the chance of
developing metabolic syndromes such as abnormalities in blood
glucose, lipids and blood, pressure which are closely related to
the risk of early-onset CRC (83).
Certain studies have also suggested that the main reason for this
phenomenon is the decreasing stability of colonic pluripotent stem
cells with age. This leads to the overproliferation of cells when
exposed to adverse factors, ultimately resulting in the formation
of adenomas (84). Multiple reasons
make age one of the non-negligible risk factors associated with
colorectal precancerous lesions.
Numerous previous studies have reported that smoking
and drinking history, hypertension, diabetes, uric acid, lipids and
triglycerides levels, non-alcoholic fatty liver disease and a
family history of polyps are associated with an increased risk of
colorectal adenoma (85–90). However, the aforementioned variables
were not among the independent risk factors identified in the
present study. This discrepancy may be caused by variations in the
patient population of different studies, including differences in
regions, ethnic groups and sample sizes.
By contrast to conventional epidemiologic studies,
the present study introduces the method of nomogram and provides a
comprehensive assessment of their performance and clinical utility.
The statistical methods used in the present study are more complex
and rigorous, and the research process is completely different. In
addition to incorporating potential association variables
identified in previous studies, the current study included further
variables, such as history of encephalopathy and lung disease for
the first time, to the best of our knowledge. The results also
identified new potential risk factors, such as TT, daily number of
bowel movements, increased age and the number of polyps. Using this
new research method, further possible influences on diseases could
be identified with greater confidence. The clinical prediction
model enables the identification of high-risk individuals for
colorectal adenomas, thereby facilitating timely e-colonoscopic
surveillance, thus increasing the detection rate of adenoma whilst
reducing medical costs, preventing the occurrence of colorectal
precancerous lesions and interrupting the progression of colorectal
adenoma to CRC. This could effectively lower the incidence and
mortality rates from CRC.
However, there are several limitations to the
present research. First, the C-index suggested that the constructed
model had only a moderate predictive performance and there may be a
certain degree of selection bias as the study was retrospective.
Second, the data for both the training and validation cohorts came
from the same source, which may lead to model overfitting. Third,
most of the patients included in the study were from Shandong
Province in China and the surrounding areas; therefore, it is
unclear whether the model applies to individuals from other
regions. Subsequently, multicenter studies employing larger samples
are needed to validate the reliability and applicability of the
present model. Moreover, the aim of the study was to derive the
overall risk factors for developing adenomas by comparing the data
of adenomatous and non-adenomatous populations, so the study did
not take adenoma grading into consideration. However, the grade of
colorectal adenoma is an important indicator; therefore, assessing
the association between risk factors and certain grades of
colorectal cancer is a future research direction.
In conclusion, the present study analyzed a large
amount of clinical data and constructed a predictive model of
colorectal adenoma risk factors with good efficacy and stability,
which provide new ideas for timely screening of high-risk groups
and improve the early detection rate of colorectal adenoma. It is
expected that the model in the present study will contribute to the
development of clinical medicine.
Acknowledgements
Not applicable.
Funding
The present research was supported by the Qilu Traditional
Chinese Medicine Superiority Specialty Cluster Project (grant no.
YWC2022KJQ0003).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DLL and ZAG confirm the authenticity of all the raw
data. DLL designed the study framework, acquired the research data,
conducted the statistical analyses for the validation of the model
and drafted the initial manuscript. LLM finalized the study design
details, performed data cleaning and organization, compiled the
datasets and conducted data analysis for the establishment of the
prediction model. ZAG evaluated and optimized the final study
design, applied for and obtained ethical approvals to ensure that
the research data could be accessed, and agreed to be accountable
for the work in ensuring that questions related to the integrity of
any part of the work are appropriately investigated and resolved.
YXZ and CJ assisted with data acquisition, conducted data
cross-checking and verification, checked all statistical methods
and medical terminology in the manuscript and revised it critically
for important intellectual content. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Shandong University of
Chinese Medicine [approval no. (2024) Ethics Review No. (017)-YJS].
The study only involved the collection of case data using
anonymized or de-identified information that cannot be traced back
to individuals and poses no risk to patients; therefore, the need
for informed patient consent was waived by the Ethics
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The Chinese standard of care for
colorectal cancer (2020 edition). Chin J Surg. 58:561–585.
2020.PubMed/NCBI
|
|
3
|
Hui YF, Li C and Guo TH: Colorectal
adenomas in TCM syndrome distribution laws and its clinical
intervention study. China's Basic Med J Traditional Chin Med.
29:1866–1870. 2023.(In Chinese).
|
|
4
|
Yu M, Liu XB, Zhou M, Zhu ZD, Pan JG and
WQ: Change trend of colorectal malignant tumors detected by
electronic colonoscopy from 2015 to 2020. J Clin Gastroenterol.
34:361–364. 2022.
|
|
5
|
Courtney RJ, Paul CL, Carey ML,
Sanson-Fisher RW, Macrae FA, D'Este C, Hill D, Barker D and Simmons
J: A population-based cross-sectional study of colorectal cancer
screening practices of first-degree relatives of colorectal cancer
patients. BMC Cancer. 13:132013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rex DK, Boland CR, Dominitz JA, Giardiello
FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D and Robertson
DJ: Colorectal cancer screening: Recommendations for physicians and
patients from the U.S. Multi-society task force on colorectal
cancer. Gastroenterology. 153:307–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bretthauer M, Kaminski MF, Løberg M,
Zauber AG, Regula J, Kuipers EJ, Hernán MA, McFadden E, Sunde A,
Kalager M, et al: Population-based colonoscopy screening for
colorectal cancer: A randomized clinical trial. JAMA Intern Med.
176:894–902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li P, Wang CJ and Chen GY: Consensus on
screening and diagnosis of early colorectal cancer and precancerous
lesions in China. Chin J Practical Internal Med. 35:211–227.
2015.(In Chinese). View Article : Google Scholar
|
|
9
|
Wu XL, Wang LK and Huang XT: A
retrospective study on the epidemiological characteristics of
colorectal cancer. China Med Herald. 16:60–63. 2019.(In
Chinese).
|
|
10
|
Li QQ, Wang J and Zhao Y: Current status
of research on risk factors associated with the development of
colorectal polyps. Med Rev. 26:3196–3200. 2020.
|
|
11
|
Huang D, Zhu XZ and Sheng WQ:
Interpretation of the gastrointestinal epithelial tumors of 2019
edition of <WHO classification of tumors of the digestive
system>. Chin J Pathol. 49:209–213. 2020.
|
|
12
|
Fang SG, Wei JG and Chen ZW: WHO
classification of tumours of the digestive system (2019). J Diagn
Pathol. 26:865–870. 2019.(In Chinese).
|
|
13
|
Tibshirani R: Regression shrinkage and
selection via the lasso: A ret-rospective. J Royal Statistical Soc.
58:273–282. 2011. View Article : Google Scholar
|
|
14
|
Sninsky JA, Shore BM, Lupu GV and Crockett
SD: Risk factors for colorectal polyps and cancer. Gastrointest
Endosc Clin N Am. 32:195–213. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP; STROBE Initiative, : The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. Lancet. 370:1453–1457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang R: Construction of a mult-icenter
sleep database and a risk prediction modeling of moderate to severe
obstructive sleep apnea in the population with sleep disorders
(unpublished PhD thesis). Southern Medical University; 2024, (In
Chinese).
|
|
17
|
Zhao L, Leng Y, Hu Y, Xiao J, Li Q, Liu C
and Mao Y: Understanding decision curve analysis in clinical
prediction model research. Postgrad Med J. 100:512–515. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vickers AJ and Holland F: Decision curve
analysis to evaluate the clinical benefit of prediction models.
Spine J. 21:1643–1648. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kerr KF, Brown MD, Zhu K and Janes H:
Assessing the clinical impact of risk prediction models with
decision curves: Guidance for correct interpretation and
appropriate use. J Clin Oncol. 34:2534–2540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iasonos A, Schrag D, Raj GV and Panageas
KS: How to build and interpret a nomogram for cancer prognosis. J
Clin Oncol. 26:1364–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balachandran VP, Gonen M, Smith JJ and
DeMatteo RP: Nomograms in oncology: More than meets the eye. Lancet
Oncol. 16:e173–e180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gafita A, Calais J, Grogan TR, Hadaschik
B, Wang H, Weber M, Sandhu S, Kratochwil C, Esfandiari R, Tauber R,
et al: Nomograms to predict outcomes after 177Lu-PSMA therapy in
men with metastatic castration-resistant prostate cancer: An
international, multicentre, retrospective study. Lancet Oncol.
22:1115–1125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Li J, Guo J, Shi Y and Wang L: A
prediction nomogram for neonatal acute respiratory distress
syndrome in late-preterm infants and full-term infants: A
retrospective study. EClinicalMedicine. 50:1015232022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pietrantonio F, Lonardi S, Corti F,
Infante G, Elez ME, Fakih M, Jayachandran P, Shah AT, Salati M,
Fenocchio E, et al: Nomogram to predict the outcomes of patients
with microsatellite instability-high metastatic colorectal cancer
receiving immune checkpoint inhibitors. J Immunother Cancer.
9:e0033702021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Viswanathan AN, Hankinson SE and
Schernhammer ES: Night shift work and the risk of endometrial
cancer. Cancer Res. 67:10618–10622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thompson CL, Larkin EK, Patel S, Berger
NA, Redline S and Li L: Short duration of sleep increases risk of
colorectal adenoma. Cancer. 117:841–8747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Um K, Park CS, Yoo C, Ahn YS, Kim M and
Jeong KS: Risk factors including night shift work of colorectal
polyp. Ann Occup Environ Med. 32:e262020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alpert O, Begun L, Issac T and Solhkhah R:
The brain-gut axis in gastrointestinal cancers. J Gastrointest
Oncol. 12 (Suppl 2):S301–S331. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Wang S and Cao K: Prevention and
treatment of colorectal cancer by ‘brain-intestinal axis’ of
traditional Chinese medicine. World TCM. 18:3085–3089. 2023.
|
|
30
|
Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu
KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling
C, Golubeva AV, et al: The microbiota-gut-brain axis. Physiol Rev.
99:1877–2013. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao W, Su J, Gao X, Yang H, Weng R, Ni W
and Gu Y: The microbiota-gut-brain axis participates in chronic
cerebral hypoperfusion by disrupting the metabolism of short-chain
fatty acids. Microbiome. 10:622022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu S, Liu X, Jiang R, Yan X and Ling Z:
Roles and mechanisms of gut microbiota in patients With Alzheimer's
disease. Front Aging Neurosci. 13:6500472021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma C, Li Y, Mei Z, Yuan C, Kang JH,
Grodstein F, Ascherio A, Willett WC, Chan AT and Huttenhower C:
Association between bowel movement pattern and cognitive function:
Prospective cohort study and a metagenomic analysis of the gut
microbiome. Neurology. 101:e2014–e2025. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Polimeno L, Barone M, Mosca A, Viggiani
MT, Joukar F, Mansour-Ghanaei F, Mavaddati S, Daniele A, Debellis
L, Bilancia M, et al: Soy Metabolism by gut microbiota from
patients with precancerous intestinal lesions. Microorganisms.
8:4692020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei H: Theoretical connotation and
biological mechanism of ‘The Lung is Connected with the Large
Intestine, and the Large Intestine Corresponds to the Skin’. J
Beijing Univ Traditional Chin Med. 46:1350–1356. 2023.
|
|
36
|
Liu B, Yu Y, Zhao M, Xiao K, Yan P, Duan
Z, Wang K, Zhao N, Cao J, Wang J and Xie L: Correlation analysis of
the microbiome and immune function in the lung-gut axis of
critically Ill patients in the ICU. Front Med (Lausanne).
9:8083022022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao M, Shao F, Yu D, Zhang J, Liu Z, Ma
J, Xia P and Wang S: Maturation and specialization of group 2
innate lymphoid cells through the lung-gut axis. Nat Commun.
13:76002022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mazumder MHH, Gandhi J, Majumder N, Wang
L, Cumming RI, Stradtman S, Velayutham M, Hathaway QA, Shannahan J,
Hu G, et al: Lung-gut axis of microbiome alterations following
co-exposure to ultrafine carbon black and ozone. Part Fibre
Toxicol. 20:152023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zorn AM and Wells JM: Vertebrate endoderm
development and organ formation. Annu Rev Cell Dev Biol.
25:221–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rankin SA, Han L, McCracken KW, Kenny AP,
Anglin CT, Grigg EA, Crawford CM, Wells JM, Shannon JM and Zorn AM:
A retinoic acid-hedgehog cascade coordinates mesoderm-inducing
signals and endoderm competence during lung specification. Cell
Rep. 16:66–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu S, Liu XY, Li LH, Wang X, Cui YF, Sun
GZ and Guo XZ: Histology and cytology basic research on'Lung and
Large Intestine being interior-exteriorly Related'. Chin J Trad
Chin Med Pharm. 27:1167–1170. 2012.(In Chinese).
|
|
42
|
Li LH: ‘Biological mechanism of the
relationship between the lung and the large intestine: A study on
the physiological mechanism of the correlation between lung and
intestinal tissues in a rat]. Beijing: Beijing University of
Chinese Medicine; pp. 1–4. 2012
|
|
43
|
Tulic MK, Piche T and Verhasselt V:
Lung-gut cross-talk: Evidence, mechanisms and implications for the
mucosal inflammatory diseases. Clin Exp Allergy. 46:519–528. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan XD, Chen YH, Liu QP, Gonzalez-Crussi F
and Liu XL: Prostanoids mediate the protective effect of trefoil
factor 3 in oxidant-induced intestinal epithelial cell injury: Role
of cyclooxygenase-2. J Cell Sci. 113:2149–2155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ganguly K and Schulz H: Association
studies of lung function in mice. Dtsch Tierarztl Wochenschr.
115:276–284. 2008.PubMed/NCBI
|
|
46
|
Pietrantonio F, Lonardi S, Corti F,
Infante G, Elez ME, Fakih M, Jayachandran P, Shah AT, Salati M,
Fenocchio E, et al: Nomogram to predict the outcomes of patients
with microsatellite instability-high metastatic colorectal cancer
receiving immune checkpoint inhibitors. J Immunother Cancer.
9:e0033702021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mao WX, Zhong ZS, Huang SP, Zhang W and
Wang J: Analysis of risk factors and TCM mechanism of adenomatous
polyps. Chin J Integr Tradit West Med Dig. 27:726–729,734.
2019.
|
|
48
|
Hao Y, Wang Y, Qi M, He X, Zhu Y and Hong
J: Risk factors for recurrent colorectal polyps. Gut Liver.
14:399–411. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lucas C, Barnich N and Nguyen HTT:
Microbiota, inflammation and colorectal cancer. Int J Mol Sci.
18:13102017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He X, Wu K, Ogino S, Giovannucci EL, Chan
AT and Song M: Association between risk factors for colorectal
cancer and risk of serrated polyps and conventional adenomas.
Gastroenterology. 155:355–373.e18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yun GY, Moon HS, Kwon IS, Kim JS, Kang SH,
Lee ES, Kim SH, Sung JK, Lee BS and Jeong HY: Left-sided colectomy:
One of the important risk factors of metachronous colorectal
adenoma after colectomy for colon cancer. Dig Dis Sci.
63:1052–1061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
International Society on Thrombosis and
Haemostasis (ISTH), . ISTH guidelines for coagulation testing.
2021.https://www.isth.org
|
|
53
|
Ren Y, Liang H, Huang Y, Miao Y, Li R,
Qiang J, Wu L, Qi J, Li Y, Xia Y, et al: Key candidate genes and
pathways in T lymphoblastic leukemia/lymphoma identified by
bioinformatics and serological analyses. Front Immunol.
15:13412552024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu LL, Lin WK, Qian JY, Ma SS, Li MJ, Li
K, Li ZX, Lan G and Xie D: Prognostic assessment of lung
adenocarcinoma patients with early-staging diseases: A nomogram
based on coagulation-related factors. Eur J Cardiothorac Surg.
64:ezad3132023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang HY, Xiu DR, Li ZF and Wang G:
Coagulation function in patients with pancreatic carcinoma. Chin
Med J (Engl). 122:697–700. 2009.PubMed/NCBI
|
|
56
|
Ward MP, E Kane L, Norris L, Mohamed BM,
Kelly T, Bates M, Clarke A, Brady N, Martin CM, Brooks RD, et al:
Platelets, immune cells and the coagulation cascade; friend or foe
of the circulating tumour cell? Mol Cancer. 20:592021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yin J and Zhu SS: Routine coagulation
molecules predict nasopharyngeal carcinoma and associated
metastases. Br J Biomed Sci. 76:178–183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Peng PH, Wu CC, Liu SC, Chang KP, Chen CD,
Chang YT, Hsu CW, Chang YS and Yu JS: Quantitative plasma proteome
analysis reveals aberrant level of blood Coagulation-related
proteins in nasopharyngeal carcinoma. J Proteomics. 74:744–757.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marinho FC and Takagaki TY:
Hypercoagulability and lung cancer. J Bras Pneumol. 34:312–322.
2008.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hammouda A, Souilah S, Ferhat-Hamida MY,
Amir ZC, Aouichat-Bouguerra S and Hariti G: Activation of
coagulation in patients with lung cancer. Ann Biol Clin (Paris).
77:272–280. 2019.(In French). PubMed/NCBI
|
|
61
|
Repetto O and De Re V: Coagulation and
fibrinolysis in gastric cancer. Ann N Y Acad Sci. 1404:27–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang B, Zou D, Wang N, Wang H, Zhang T,
Gao L, Ma C, Zheng P, Gu B, Li X, et al: Construction and
validation of a novel coagulation-related 7-gene prognostic
signature for gastric cancer. Front Genet. 13:9576552022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chuang IW, Cho YH, Ahn MJ, Lee MJ, Kim GM,
Chung CS and Bang OY: Association of cancer cell type and
extracellular vesicles with coagulopathy in patients with lung
cancer and stroke. Stroke. 49:1282–1285. 2018. View Article : Google Scholar
|
|
64
|
Kessler CM: The link between cancer and
venous thromboembolism: A review. Am J Clin Oncol. 32 (4
Suppl):S2–S7. 2009. View Article : Google Scholar
|
|
65
|
Guo Q, Zhang B, Dong X, Xie Q, Guo E,
Huang H and Wu Y: Elevated levels of plasma fibrinogen in patients
with pancreatic cancer: Possible role of a distant metastasis
predictor. Pancreas. 38:e75–e79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tang L, Liu K, Wang J, Wang C, Zhao P and
Liu J: High preoperative plasma fibrinogen levels are associated
with distant metastases and impaired prognosis after curative
resection in patients with colorectal cancer. J Surg Oncol.
102:428–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sahni A, Simpson-Haidaris PJ, Sahni SK,
Vaday GG and Francis CW: Fibrinogen synthesized by cancer cells
augments the prolifrative effect of fibroblast growth factor-2. J
Thromb Haemost. 6:176–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Junsheng L, Ziling Y and Zhengyuan H:
Construction of a prediction model to distinguish colorectal
adenoma from colorectal cancer based on conventional test
indicators. Tumor Control Res. 51:353–360. 2024.(In Chinese).
|
|
69
|
Liu ZH, Zhang N, Li FX, Li SX and Liu JT:
Correlation between homocysteine, coagulation function indicators
and the risk of colorectal cancer incidence: a case-control study.
Chin J Clin Oncol. 50:654–660. 2023.(In Chinese).
|
|
70
|
Chen ZH, Sun SB, Wang XM, Yu SS, Wu XF,
Chen XS, Geng T, Liu H, Liu XM and Nan Q: Abnormal Coagulation
Parameters in Patients with Early Colorectal Cancer and Its
Clinical Significance. Journal of Kunming Medical University.
42:96–100. 2021.(In Chinese).
|
|
71
|
Negro S, Bao QR, Scarpa M, Scognamiglio F,
Pucciarelli S, Remo A, Agostini M, D'Angelo E, Mammi I, Schiavi F,
et al: Multiple colorectal adenomas syndrome: The role of MUTYH
mutation and the polyps' number in clinical management and
colorectal cancer risk. Dig Liver Dis. 56:1087–1094. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang W and Wang XR: Exploring Risk Factors
for Colorectal Polyp Malignant Transformation in Middle-Aged and
Elderly Patients Based on Multivariate Logistic Regression
Analysis. Modern Medicine and Health Research Electronic Journal.
9:122–124. 2025.(In Chinese).
|
|
73
|
Kim SE, Shim KN, Jung SA, Yoo K and Moon
IH: An association be-tween obesity and the prevalence of colonic
adenoma according to age and gender. J Gastroenterol. 42:616–623.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Meester RGS, Mannalithara A,
Lansdorp-Vogelaar I and Ladabaum U: Trends in incidence and stage
at diagnosis of colorectal cancer in adults aged 40 through 49
years, 1975–2015. JAMA. 321:1933–1934. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen XY and Kong LB: Progress of research
on factors and mechanisms associated with carcinogenesis of
colorectal adenomatous polyps. Chin J Cancer Prevention Treatment.
26:354–358. 2019.
|
|
76
|
Wu H, Zhang J and Zhou B: Metabolic
syndrome and colorectal adenoma risk: A systematic review and
metaanalysis. Clin Res Hepatol Gastroenterol. 45:1017492021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Han X, Qian W, Liu Y, Zheng T, Su XJ,
Zhang PP, Chen Y, Hu LH and Li ZS: Effects of age, sex and
pathological type on the risk of multiple polyps: A Chinese
teaching hospital study. J Dig Dis. 21:505–511. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Karsenti D, Tharsis G, Burtin P, Venezia
F, Tordjman G, Gillet A, Samama J, Nahon-Uzan K, Cattan P and
Cavicchi M: Adenoma and advanced neoplasia detection rates increase
from 45 years of age. World J Gastroen. 25:447–456. 2019.
View Article : Google Scholar
|
|
79
|
Lin G, Feng Z, Liu H, Li Y, Nie Y, Liang Y
and Li K: Mass screening for colorectal cancer in a population of
two million older adults in Guangzhou, China. Sci Rep. 9:104242019.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hong W, Dong L, Stock S, Basharat Z, Zippi
M and Zhou M: Prevalence and characteristics of colonic adenoma in
mainland China. Cancer Manag Res. 10:2743–2755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sun DH: Clinical characteristics of 543
cases of tubular adenomatous polyps and their carcinogenesis of the
large intestine. Gansu Med. 36:705–707. 2017.
|
|
82
|
McCashland TM, Brand R, Lyden E and de
Garmo P; CORI Research Project, : Gender differences in colorectal
polyps and tumors. Am J Gastroenterol. 96:882–886. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jin EH, Han K, Lee DH, Shin CM, Lim JH,
Choi YJ and Yoon K: Association between metabolic syndrome and the
risk of colorectal cancer diagnosed before age 50 years according
to tumor location. Gastroenterology. 163:637–648.e2. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L,
Li X, Guo L, Zheng Z, Zou S, et al: Participation and yield of a
population-based colorectal cancer screening programme in China.
Gut. 68:1450–1457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Soltani G, Poursheikhani A, Yassi M,
Hayatbakhsh A, Kerachian M and Kerachian MA: Obesity, diabetes and
the risk of colorectal adenoma and cancer. BMC Endocr Disord.
19:1132019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lin CC, Huang KW, Luo JC, Wang YW, Hou MC,
Lin HC, Lee FY and Chan WL: Hypertension is an important predictor
of recurrent colorectal adenoma after screening colonoscopy with
adenoma polypectomy. J Chin Med Assoc. 77:508–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xing J, Ren J and Zhang Q: Analysis of
risk factors for the development of colorectal adenomatous polyps.
J Capital Med Univ. 42:601–608. 2021.
|
|
88
|
Liu ZH, Zhang GX, Zhang H, Jiang L, Deng
Y, Chan FSY and Fan JKM: Association of body fat distribution and
metabolic syndrome with the occurrence of colorectal adenoma: A
Case-control study. J Dig Dis. 22:222–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Blackett JW, Verna EC and Lebwohl B:
Increased prevalence of colorectal adenomas in patients with
nonalcoholic fatty liver disease: A Cross-sectional study. Dig Dis.
38:222–230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Han X, Qian W, Liu Y, Zheng T, Su XJ,
Zhang PP, Chen Y, Hu LH and Li ZS: Effects of age, sex and
pathological type on the risk of multiple polyps: A Chinese
teaching hospital study. J Dig Dis. 21:505–511. 2020. View Article : Google Scholar : PubMed/NCBI
|