Introduction
Cancer has been a major threat to human health for
>5 decades and remains the primary cause of mortality in China
(1). Early detection, diagnosis and
treatment are vital for slowing tumor progression and improving
survival rates. Therefore, rapid detection of tumor cells serves a
notable role in the occurrence, treatment and prognosis of
malignant tumors (2). However, the
complexity of clinical samples presents challenges for several
methods in detecting low-abundance tumor cells, notably hindering
the specificity and sensitivity of multiple detection
techniques.
Currently, techniques such as histochemistry,
immunohistochemistry and flow cytometry are utilized to identify
tumor cells in clinical laboratories (3). However, histochemical staining often
lacks sensitivity for low-abundance tumor cells, while
immunohistochemistry is complex and time-consuming. Flow cytometry
offers sensitive quantification of several markers but requires
costly reagents and advanced equipment. Consequently, there is a
pressing need for a simple, cost-effective method with high
sensitivity and specificity for detecting low quantities of tumor
cells in specimens.
Previous years have seen a surge in interest in
nucleic acid aptamers, which are small molecules of RNA or
single-stranded DNA (ssDNA) molecules with unique three-dimensional
structures, typically comprising 20–80 bases and possessing a
relative molecular mass ranging from 6,000 to 30,000 Da. Similar to
antibodies, nucleic acid aptamers recognize targets specifically
through shape complementarity and are used in several applications,
including novel therapeutics, drug delivery, tumor cell detection
and biological imaging (4).
Aptamer-based tumor detection methods are flexible and
cost-effective, leading to the creation of numerous highly
sensitive detection techniques (5).
However, monovalent aptamers face challenges in selectivity and
targeting efficiency when identifying their targets, which can
result in suboptimal detection and therapeutic outcomes, thereby
hindering advancements in tumor detection and treatment.
In natural biological systems, multivalent
synergistic interactions are frequently employed, wherein multiple
ligands on a single biological entity simultaneously interact with
receptors on a different biological entity to achieve high affinity
and hyper selectivity. Currently, molecular biologists are
increasingly leveraging the mechanism of multivalent interactions
to develop novel molecular assemblies that can either introduce new
functionalities to aptamers or enhance the efficacy of existing
ones (6,7). Compared with monovalent aptamers,
multivalent aptamers offer several advantages. Firstly, they can
markedly enhance binding affinity. By engaging with the target
molecule through multiple binding sites, multivalent aptamers
establish multipoint interactions that strengthen overall binding,
a phenomenon known as the multivalent effect (8). When several aptamer molecules
simultaneously bind to the target, the resulting synergistic effect
further amplifies binding strength, akin to a zipper mechanism
(9). Secondly, multivalent aptamers
can enhance binding specificity. Their multiple binding sites
accurately match various epitopes of target molecules, thereby
minimizing interactions with non-target entities and improving
specificity (10). In addition, the
diversity of binding sites enables multivalent aptamers to
recognize different regions of the target molecule, further
reducing the cross-reaction with non-target molecules (11). Thirdly, multivalent aptamers exhibit
kinetic advantages. The presence of multiple binding sites
increases the likelihood of contact between the aptamer and the
target molecule, thereby enhancing the binding rate. The
probability of simultaneous dissociation from multiple binding
sites is low, resulting in a reduced overall dissociation rate and
prolonged binding duration. Finally, multivalent aptamers possess
functional advantages. In the context of biosensing, multivalent
aptamers can simultaneously bind multiple signaling molecules,
thereby amplifying the detection signal and enhancing sensitivity
(12). In targeted therapy,
multivalent aptamers can simultaneously bind multiple target
molecules, thereby enhancing the efficiency and accuracy of drug
delivery (13). Therefore, the
development of multivalent aptamers is of great importance.
Multivalent aptamers offer notable advantages over
conventional and bispecific antibodies in cancer diagnosis and
treatment, particularly in addressing the challenges associated
with antibody technology (14).
Firstly, multivalent aptamers can transcend structural limitations
and effectively target molecules often elusive to antibodies
(15). Due to their larger
molecular size, antibodies struggle to infiltrate dense tumor
tissues or cross the blood-brain barrier, while aptamers, being
notably smaller, can efficiently access the tumor microenvironment
to identify low-expression or concealed epitopes (16). Numerous tumor-associated targets
exhibit low immunogenicity or possess intricate structures,
complicating the generation of effective antibodies. By contrast,
aptamers can recognize small molecules, ions and complexly folded
transmembrane proteins through their ability to adapt
conformationally. They demonstrate a heightened sensitivity to
conformational alterations in the target. They can dynamically bind
to changing biomarkers on tumor cell surfaces in real time, a
capability that antibodies may lack due to fixed epitopes (17,18).
Secondly, bispecific aptamers can address the
shortcomings of bispecific antibodies (19). The development of bispecific
antibodies necessitates complex protein engineering, a process that
is both time-consuming and expensive, whereas aptamers can be
synthesized rapidly and cost-effectively through chemical methods,
achieving cost reductions of >90% (20). Furthermore, although bispecific
antibodies can induce the development of drug-resistant antibodies,
aptamers, which are based on nucleic acids, demonstrate low levels
of immunogenicity (21). Moreover,
aptamers can be chemically modified to enhance resistance to
nuclease degradation, extending their in vivo half-life.
Multivalent aptamers can integrate multiple targeting units and
non-protein targets, unlike bispecific antibodies, which are
limited by the compatibility of the two target proteins (22). Thirdly, multivalent aptamers can
modularly integrate diagnostic and therapeutic functionalities. For
instance, they can be linked with fluorescent moieties or
nanoparticles for tumor imaging or liquid biopsy (23). They can also serve as drug carriers
or be combined with immune checkpoint molecules for targeted
delivery and immunomodulation (24).
Previous advancements in aptamers are exemplified by
Macugen®, the first approved aptamer targeting vascular
endothelial growth factor (VEGF) for age-associated macular
degeneration (25). This has driven
research into antitumor aptamers. While studies show that
tumor-targeted aptamers can markedly inhibit tumor growth in animal
models, clinical trials have lagged, with most remaining in
preliminary phases and requiring further phase III trials to
validate safety and efficacy. Ferreira et al (26) screened Apt2, a nucleic acid aptamer
capable of specifically binding to triple-negative breast cancer
(TNBC) MDA-MB-231 cells, by cellular Systematic Evolution of
Ligands by Exponential Enrichment (SELEX) technology combined with
high-throughput sequencing. The target molecule of Apt2 has not yet
been identified; however, it exhibited high affinity, specificity
and low toxicity, and showed targeting ability in breast cancer
tissue sections, indicating it has potential to serve as a
therapeutically and diagnostically targeted ligand for TNBC. Hwang
et al (27) performed a
phase I/II clinical trial evaluating the safety and potential
efficacy of combined vitreous cavity injections of the VEGF
inhibitor ranibizumab and the platelet-derived growth factor
inhibitor E10030 for the treatment of severe ocular-type von
Hippel-Lindau disease-associated retinal hemangiomas. The results
demonstrated that the combination had a reasonable safety profile
but had limited results in terms of improvement in vision,
shrinkage of the tumor or reduced exudation.
To ensure a comprehensive collection of relevant
literature, the PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(https://webofscience.clarivate.cn/wos/alldb/basic-search)
and Scopus (http://www.scopus.com) databases were
searched for the present review. The timeframe of the search was
set from 2010 to 2025, which covers the various stages of this
research topic from initial exploration to gradual in-depth
development, providing a comprehensive and time-sensitive
literature base for the review. Based on the research topic, the
following search keyword combinations were used: ‘Multivalent
aptamers’, ‘tumor cells’, ‘capture’ and ‘detection’. A comparison
of key information in the literature was initially performed to
remove duplicates from the search results. Subsequently,
non-research literature was excluded, such as conference papers and
conference abstracts, and the present review focused on review
articles, clinical trials and other types of literature that could
provide substantive research data and in-depth analysis. The titles
and abstracts of the preliminarily screened literature were read
one by one to exclude those that were clearly inconsistent with the
topic, and those that were too broad or narrow in terms of research
content. Finally, the studies that passed the screening process
were read in full to further assess their quality and relevance and
to finalize the selection of literature to be included. The present
review examined the basic principles of aptamers, the obstacles and
potential solutions encountered in their development, the
methodologies for constructing multivalent aptamers, their
applications in oncology and the challenges and future prospects
associated with multivalent aptamers.
Basic principles of aptamers
Aptamers are oligonucleotide or peptide sequences
obtained by in vitro screening techniques. They bind target
molecules with a high affinity and specificity, functioning
similarly to antibodies in their ability to recognize and bind to
these targets. They are often called chemical antibodies (28,29)
and can be categorized into groups such as DNA, RNA, peptide,
xeno-nucleic acid (XNA), locked nucleic acid (LNA) and peptide
nucleic acid (PNA) aptamers, as well as complexes with
nanomaterials and fluorescence labeling.
DNA aptamers, made from ssDNA, are stable, easy to
synthesize and modifiable, making them valuable in biosensing and
diagnostics. Liu et al (30)
designed a non-G-Quadruplex DNA aptamer targeting nucleolin for
bladder cancer diagnosis and treatment. RNA aptamers, composed of
single-stranded RNAs, have complex secondary and tertiary
structures, making them suitable for intracellular application and
gene regulation. Yang et al (31) used RNA aptamers targeting the severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2) structural
protein for antiviral research. Peptide aptamers, consisting of
short peptide sequences typically derived from phage display
technology, can specifically bind to proteins or other
biomolecules. Shen et al (32) developed a peptide aptamer-paclitaxel
conjugate for tumor-targeted therapies to enhance drug delivery
precision while minimizing damage to normal cells. XNA aptamers are
composed of unnatural nucleic acids, such as 1′,5′-anhydrohexitol
nucleic acid and 2′-fluoroarabinonucleic acid, which offer greater
stability and chemical diversity. LNA aptamers, composed of locked
nucleic acids, whose ribose rings are locked into a rigid
structure, exhibit high stability, affinity and specificity. PNA
aptamers, consisting of peptide nucleic acid with a peptide chain
replacing its phosphate backbone, also demonstrate remarkable
stability and binding capabilities. The diversity of aptamers
allows for a broad spectrum of applications in disease diagnosis,
therapy, biosensing and basic research. With the advancement of
chemical modification and screening technologies, the design and
application of aptamers are expected to become increasingly precise
and diversified.
Aptamers are mainly identified using an in
vitro evolutionary approach called phylogenetic exponential
enrichment of ligands. Current screening methods include the
conventional SELEX technique and its enhanced variants [such as
capillary electrophoresis-SELEX (CE-SELEX), Cell-SELEX,
Capture-SELEX], non-SELEX techniques and computer-assisted design,
each with unique advantages and disadvantages.
The conventional SELEX technique serves as the
standard approach for aptamer screening, encompassing a four-step
process: Construction of random oligonucleotide libraries,
isolation of target-bound and unbound sequences, enrichment of
high-affinity sequences by polymerase chain reaction (PCR)
amplification and the execution of several cycles of screening
until highly specific aptamers are identified. However, the
traditional SELEX technique has some limitations in screening small
molecule aptamers (33).
Numerous enhanced SELEX technologies have evolved
from the original methodology. Notably, the CE-SELEX technique
employs CE to differentiate between aptamers and unbound sequences
of target molecules, leveraging variations in electrophoretic
mobility for effective screening. This method necessitates a
smaller sample size, markedly reducing the number of screening
iterations required (1–4 rounds) (34). Researchers have developed advanced
CE-SELEX techniques to address the limitations of traditional SELEX
methods. For instance, low pH-CE-SELEX technology mitigates
electroosmotic flow and the adsorption of basic proteins, thereby
enhancing screening efficiency (35). Additionally, single-step CE-SELEX
technology facilitates a one-step, online reaction, making it
particularly suitable for valuable, rare and difficult-to-prepare
proteins. Furthermore, synchronized competition CE-SELEX technology
allows for simultaneous competition to screen two target proteins,
thereby improving both screening efficiency and aptamer affinity
(36). Cell-SELEX technology
permits the direct screening of aptamers on the surface of living
cells, targeting cell membrane proteins or surface markers, such as
cancer cell-specific receptors. This approach maintains the
target's natural conformation and post-translational modifications,
eliminating the need for protein purification (37). Capture-SELEX technology immobilizes
the target on a solid-phase carrier (such as magnetic beads or
microarrays) and performs aptamer screening in a capture-elution
format. This method effectively addresses the limitations of the
traditional SELEX method for small molecule target screening
(33,38) and is noted for its straightforward
operation.
In addition to SELEX technology, alternative methods
such as non-SELEX technology can be employed for aptamer screening.
For instance, microfluidic SELEX leverages a microfluidic chip to
consolidate the processes of binding, isolation and amplification,
thereby facilitating high-throughput and automated screening.
Currently, the main microfluidic chip SELEX technologies that have
been developed include magnetic bead and sol-gel methods. The
combination of microfluidic chip and SELEX technology enhances the
screening efficiency of aptamers while simultaneously lowering
associated costs (39).
Furthermore, in vitro display technologies, such as ribosome
or mRNA display, directly link aptamer sequences to functional
proteins (such as green fluorescent protein) using in vitro
translation systems. This approach negates the need for PCR and
mitigates the impact of amplification bias on library diversity
(40).
It is also possible to predict aptamer-target
interactions and optimize sequences using computer-aided design,
which integrates bioinformatics, molecular docking and machine
learning techniques (such as deep learning). This approach can
reduce the number of experimental screening phases and expedite the
development timeline. Jeddi and Saiz (41) developed a computational model to
explore the effect of different system designs on the efficacy of
aptamer-based biosensors, utilizing an anti-mucin 1 (MUC1) aptamer
alongside a silica biosensor substrate. The authors investigated
different aptamer attachment termini, surface densities,
orientations and solvent solutions. The results showed that the
5′end fixation was superior to the 3′end; high surface density
(8×1012/cm2) could reduce the fluctuation,
but spatial site-blocking needed to be avoided; and 0.8 M NaCl
solvent could optimize the electrostatic environment and stabilize
the aptamer conformation.
Challenges and solutions in aptamer
development
Challenges in optimizing aptamer
properties
Aptamers offer several advantages over antibodies,
including straightforward synthesis, enhanced stability and ease of
modification, making them suitable for diverse applications in
biosensors, drug development and diagnostics (42). However, the three-dimensional
structure of aptamers is susceptible to environmental influences,
resulting in issues such as inadequate affinity, low specificity,
limited functional activity and reduced detection sensitivity.
Consequently, individual optimization of each aptamer is
essential.
To address the issue of inadequate aptamer affinity,
particularly at low target concentrations, the binding strength of
the aptamer to the target often falls short of requirements
(43). Firstly, the development of
multivalent aptamers is proposed, as their apparent high affinity
allows for effective signal capture even at low target
concentrations while resisting interference from the sample matrix
(such as salt ions and pH fluctuations) (44). The detection limits of multivalent
aptamers can achieve levels as low as fg/ml, which can be
comparable with or even exceed those of antibodies. For instance, a
DNA hydrogel-based bivalent aptamer detects apolipoprotein A1, a
bladder cancer marker, at 0.01 nm without interference from urea
and creatinine (45). Secondly,
sequence truncation and reconstruction mitigate spatial site
resistance by removing non-critical nucleotides and retaining the
core binding sequence. Thirdly, chemical modifications or the
introduction of organic compounds are utilized to incorporate
phosphorothioate bonds or 2′-fluorine modifications, thereby
enhancing the rigidity of the binding site. Research indicates that
the binding capacity of aptamers can be adjusted by adding specific
organic compounds (46). For
instance, tumor microenvironment-activated drug conjugation
markedly reduces the binding of all aptamers, whereas polyethylene
glycol (PEG) 8000 markedly improves the binding signal of certain
aptamers (such as salicylic acid-aptamer) (46). Subsequently, structural optimization
is performed, wherein secondary structures such as hairpins and
G-quadruplexes are designed to enhance target binding efficiency
through pre-folding (47).
Furthermore, buffer conditions are optimized, revealing that an
increase in NaCl concentration leads to a reduction in the binding
of all aptamers, indicating that aptamer binding is primarily
mediated by electrostatic interactions, with enhanced stability
observed within a neutral pH range (48). The binding signals of certain
aptamers (such as pyruvate dehydrogenase/lactate
dehydrogenase-aptamer) show reduced binding signals without
divalent cations, whereas the addition of Ca2+ and
Mg2+ doubles these signals (48). This highlights the importance of
divalent cations in aptamer binding affinity. Lastly, higher
affinity variants can be identified using mismatch PCR or targeted
mutation libraries (49).
Aptamer specificity presents notable challenges.
Firstly, aptamers may exhibit cross-reactivity with non-target
molecules (such as structural analogs and serum proteins), leading
to false-positive results. Secondly, prolonged incubation can lead
to non-specific binding of the aptamer to proteins or lipids on
cell surfaces due to charge interactions (50). Numerous existing aptamers are
affected by these specificity issues, which may hinder the
advancement of aptamer-based diagnostics onto the market. The
development of multivalent aptamers is anticipated to address the
limitations of low specificity through synergistic effects and
structural enhancements. This approach can notably improve the
specificity of aptamers, particularly in complex biological samples
(such as blood and urine) by reducing non-specific binding
(43). The use of multivalent
aptamers in complex samples can effectively mitigate non-specific
adsorption, thereby fulfilling the requirements for clinical
testing. For instance, in cancer exosome detection, the
construction of heterologous bivalent aptamers targeting cluster of
differentiation 63 and epithelial cell adhesion molecule (EpCAM)
can eliminate the interference from normal cellular exosomes,
achieving a specificity of >95% (51). In serum epidermal growth factor
receptor (EGFR) assays, a trivalent aptamer sensor demonstrated the
ability to distinguish between EGFR and human EGFR 2 in 10% serum,
whereas the monovalent aptamer exhibited a false-positive rate of
>30% due to non-specific adsorption (52). In addition, Kelly et al
(53) found that the addition of
the adding non-specific competitors, such as ssDNA, inhibited the
non-specific binding of aptamers.
Specificity can also be improved through
counter-screening, where non-target molecules are added to
eliminate cross-binding sequences during the screening phase.
Computer-assisted methods can also predict binding sites via
molecular docking, allowing for the optimization of complementary
regions (54). Chemical
modifications, such as PEGylation, can be employed to introduce
charge or spatial resistance outside the binding region, further
reducing non-specific adsorption. Modifying buffer conditions has
shown that proteins such as interferon-γ and thrombin tend to
non-specifically bind to aptamers at low pH levels. At a pH level
<5, all aptamers exhibit this non-specific binding, which is
further enhanced by divalent cations (55).
Several strategies can be employed to address the
issues of inadequate functional activity, particularly for the
aptamer to efficiently modulate its function (such as inhibiting
enzyme activity or blocking receptor signaling) after binding to
the target. Firstly, by utilizing binding site-directed design, the
functional domain of the target can be identified using structural
biology to create aptamers that specifically target this region.
Secondly, developing multimeric aptamers or aptamer-nanocomplexes
can enhance target conformation through synergistic binding.
Finally, functionalized coupling can link aptamers with effector
molecules (such as anti-PD-1 antibody) to enhance downstream
effects (56).
To address the issue of inadequate detection
sensitivity, characterized by the weak signal response of aptamers
in sensing or diagnosis, two approaches can be employed. Firstly,
implementing signal amplification strategies, combined with
nanomaterials or enzyme-catalyzed reactions, can enhance
sensitivity. Secondly, the design of ‘molecular beacon’ aptamers,
which induce fluorescence or electrical signal changes upon binding
to the target, presents another viable solution (57).
Challenges at the application
level
Aptamers have a wide range of applications due to
their specific binding capabilities to target molecules,
particularly in drug development and diagnostic fields. However, a
notable challenge for the in vivo application of aptamers is
their susceptibility to degradation by nucleases (58). Conventional methods for aptamer
modification, such as the incorporation of a phosphorothioate
backbone or modifications to the sugar structure, can enhance their
resistance to nuclease activity. However, these modifications may
affect the binding affinity and specificity of the aptamer. To
address this issue, Tabuchi et al (59) introduced the sulfur-fluoride
exchange reaction, which facilitates the covalent attachment of the
aptamer to the target protein. This covalent binding not only
increased the binding strength of the aptamer to the target protein
but also conferred relative nuclease resistance of the aptamer.
Zhang et al (60) developed
a nanocarrier based on polyvalent aptamer-protein (pAPNC) utilizing
a bovine serum albumin (BSA) core with a multivalent XQ-2d aptamer
shell. By binding multiple XQ-2d aptamers to the surface of BSA,
pAPNC not only bolstered the stability of the aptamers but also
enhanced the drug loading capacity through a synergistic charge
effect. This increased resistance to nucleases allows pAPNC to
maintain a prolonged circulation time in vivo, thereby
augmenting its potential for targeted therapeutic and imaging
applications.
Aptamers face limitations in pharmacokinetics,
including a short half-life or poor tissue penetration in
vivo (61). PEGylation can
increase their molecular weight, effectively prolonging kidney
clearance (62). Designing
bispecific aptamers that simultaneously target cell surface
receptors and targets can prolong the local duration of aptamer
action (63). Furthermore, the
accumulation of aptamers in tissues can be improved through
aptamer-mediated targeting of delivery vehicles, such as exosomes
and liposomes (64). Xiao et
al (65) developed a novel
method to covalently attach aptamer-drug conjugates (ApDC) to the
surface of attenuated Salmonella using click chemistry. This
approach markedly enhanced the stability of the ApDC in
vivo, prolonged its half-life in serum and reduced premature
degradation and clearance of the drug.
The application of aptamers also faces the risk of
immunogenicity. In vivo, aptamers may be identified by the
immune system, leading to the activation of an antibody response
(66). To mitigate this issue,
aptamers can be humanized to eliminate the use of sequences that
are readily detected by the immune system, such as CpG sequences
(67). Chemical modifications can
also be used to conceal immunogenic properties, such as modifying
PEG or sugar chains on the surface of the aptamer (68).
Challenges at the screening phase
Screening phase issues arise from the limited
diversity of the initial aptamer library, resulting in a narrow
evaluation range. This can be addressed by using longer random
sequence regions or chemically modified nucleotides to increase
structural diversity. Additionally, next-generation sequencing can
monitor library diversity, allowing for adaptive refinement of the
screening process (69).
Method for constructing multivalent
aptamers
Nucleic acid nanostructure
self-assembly
The self-assembly of nucleic acid nanostructures
relies on the principle of base complementary pairing among DNA
molecules, facilitating the construction of multivalent aptamers by
designing specific DNA sequences and structures. This process
results in the formation of precise DNA templates and auxiliary
strands that contribute to the development of nanostructures. Due
to the highly specific interactions of Watson-Crick base pairing,
DNA molecules can self-assemble into well-defined DNA
nanostructures, such as dimer, trimer, tetrahedron, hexamer and
origami in a highly precise and predictable manner. Peng et
al (70) designed a
dual-recognition controlled electrochemical biosensor containing
two aptamer hairpin probes that form DNA dimers that can bind to
MUC1 and EpCAM proteins located on the cell membrane. When both
probes simultaneously bind to the target cell, a roll-over
amplification reaction is initiated, resulting in the production of
numerous DNA products. These products include G-quadruplex forming
sequences and complementary sequences of tetrahedral DNA
structures. The tetrahedral DNA structure is affixed to the surface
of the electrode and can capture the rolling circle amplification
(RCA) products, thereby notably amplifying the electrochemical
signal. Li et al (71)
designed a trimeric aptamer with triple rotational symmetry that
aligns perfectly with the trimeric structure of the SARS-CoV-2
spiny protein for coronavirus disease 2019 detection. This
symmetrical design enables the aptamer to bind simultaneously to
all three subunits of the spiny protein, leading to a substantial
increase in binding affinity. This symmetrical trimeric aptamer
demonstrates a two-order-of-magnitude enhancement in binding
affinity compared with traditional monomeric or linear multimeric
aptamers. Ge et al (72)
constructed a novel drug delivery system targeting
prostate-specific membrane antigen (PSMA)-positive prostate cancer
cells utilizing multivalent aptamers through the DNA origami
technique. This system exhibits a high loading capacity, precise
targeting capabilities and a slow drug release profile compared
with conventional antibody-drug conjugates.
Bio-coupling
Bio-coupling is a chemical and biological method
that employs non-covalent bonding between biological macromolecules
(such as proteins and nucleic acids) or covalent bonding to link
multiple aptamers, thereby creating multivalent aptamers. This
process enhances the affinity and specificity of multivalent
aptamer-target binding. Wang et al (73) developed multivalent aptamer nanodrug
couplers with a nucleosome-like structure, based on copper
alkalosis in the form of cell death, to improve tumor cuproptosis
treatment by leveraging mitochondrial copper overload and
glutathione depletion. The resulting multivalent aptamer, featuring
molecular aptamers for tumor targeting and repetitive polyT
sequences for copper chelation, facilitates efficient loading and
targeted delivery of copper peroxide-elesclomol nanodots and
improves the affinity and specificity of the multivalent aptamers
to the target. However, its diversity in loading remains
constrained due to the limited range of drugs suitable for
coupling, and an excess of multivalent aptamers may potentially
diminish the targeting efficacy.
Nanomaterial loading
Nanomaterial loading methods leverage the surface
properties and internal structure of nanomaterials to bind to
aptamer molecules, resulting in the formation of multivalent
aptamers. Commonly used nanomaterials include metallic [such as
gold nanoparticles (AuNPs)], carbon-based, semiconductor and
polymer nanomaterials. Due to their distinctive attributes,
including diminutive size, unique optical characteristics and ease
of biofunctionalization, nanomaterials have found applications in
biosensing, imaging, cancer diagnostics and therapeutic
interventions (74). Nanomaterials
measuring <100 nm can circulate in the bloodstream for long
periods and accumulate non-specifically in tumors with low
selectivity through enhanced permeability and retention effects
(75). The high surface area to
volume ratio of these nanomaterials allows for the loading of
numerous aptamers, facilitating the construction of multivalent
aptamers. These multivalent aptamers enhance the specific
recognition capabilities of nanomaterials, thereby improving the
efficiency of cancer cell identification and targeted delivery. In
addition, the incorporation of nanomaterials increases the density
and molecular weight of the aptamers, which improves the binding
affinity, nuclease resistance and aptamer circulation time within
the bloodstream. Zhang et al (76) developed a biofunctionalized AuNP
probe featuring ~3000 6-FAM-Sgc8 aptamers per AuNP, specifically
designed to bind to the protein tyrosine kinase 7 on target cells.
The binding constants of this probe to MCF-7 cells were 170-fold
higher compared with those of the monovalent Sgc8 aptamers,
ensuring stable binding to the target cells. Multivalent aptamers
developed using nanomaterial-loading methods can interact with
targets through multiple binding sites, markedly improving their
affinity and specificity. However, these approaches can be
expensive and certain nanomaterials (such as nano sliver and nano
titanium dioxide) may exhibit bio-toxic properties.
Chemical cross-linking
Chemical cross-linking is the process of chemically
linking multiple aptamer molecules together to form a complex with
multiple binding sites, which notably improves its binding ability
and specificity toward the target. A critical consideration in
designing effective multivalent aptamers using chemical
cross-linking methods is determining the optimal distance for
linking monovalent aptamers to maximize the affinity of the
multivalent aptamer. The effectiveness of this aptamer attachment
method is primarily influenced by the specificity of the
aptamer-target interaction and the desired functionality of the
multivalent aptamer. Generally, the inclusion of flexible junctions
of varying lengths and materials is the effective operation for
multivalent constructs. These junctions help minimize the formation
of unintended secondary structures within individual aptamers and
can enhance the affinity of multivalent aptamers by mitigating
spatial site barriers posed by adjacent aptamers. Commonly used
junctions include oligonucleotide-based junctions, such as those
for ssDNA and double-stranded DNA, non-nucleotide linkers,
PEG-derived linkers and polyacrylamide (10).
Aptamer-protein fusion
The aptamer-protein fusion represents a method that
leverages the inherent stability and functionality of proteins to
integrate nucleic acid aptamers with proteins to form multivalent
aptamers (9). Amini et al
(77) established an
aptamer-protein fusion strategy to identify a bivalent aptamer with
ultra-high affinity for the SARS-CoV-2 spike protein. This was
achieved using structured libraries of double random structural
domains, highlighting the potential of aptamer-protein fusion in
enhancing binding affinity. While this technique enhances the
stability and targeting capabilities of the aptamer, the resulting
fusion protein may exhibit immunogenic properties, potentially
triggering an immune response and raising safety concerns.
Application of multivalent aptamers in tumor
diagnostic analysis
Application in the efficient detection
of tumor cells
The monitoring of circulating tumor cells (CTCs) in
human bloodstreams offers notable insights for diagnosing
metastasis, assessing prognosis and managing cancer therapies.
However, their low abundance in blood complicates detection.
Multivalent aptamers have demonstrated promise in addressing this
challenge.
Application in the aptamer
biosensors
Yang et al (78) developed an electrode interface
modified with an in situ-produced multivalent aptamer
network for effective capture and sensitive detection of CTCs in
whole blood using electrochemical methods. The authors synthesized
long ssDNA strands with repetitive aptamer fragments, immobilized
on the electrode using RCA, to efficiently isolate rare CTCs.
Functional AuNPs, modified with antibodies and horseradish
peroxidase, specifically bind to CTCs, amplifying the
electrocatalytic signal and increasing current output. This
approach markedly enhances the sensitivity of CTC detection,
achieving a detection limit as low as five cells. The interface
with a multivalent aptamer network effectively distinguished target
cells from control cells, enabling precise detection of CTCs
(Fig. 1A).
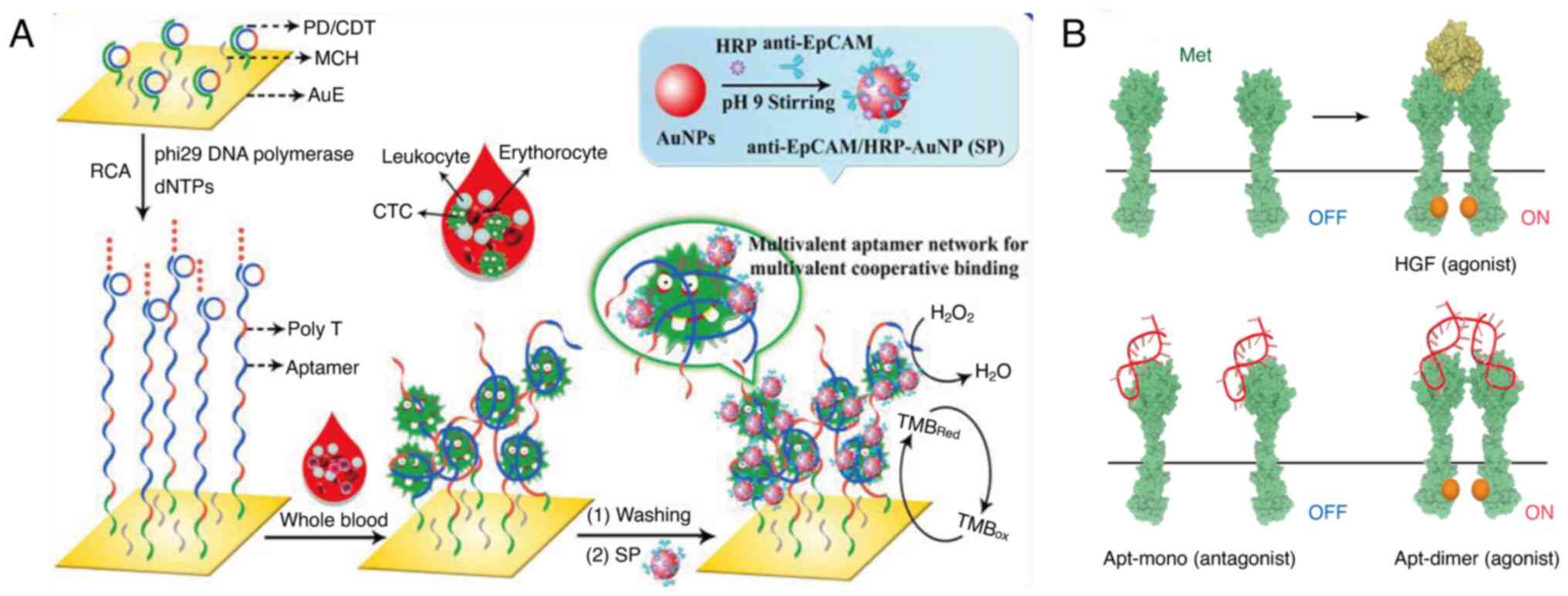 | Figure 1.Examples of multivalent aptamers in
tumor diagnostic analysis. (A) An electrode interface modified with
an in situ produced multivalent aptamer network to
efficiently capture and sensitively detect CTCs in whole blood.
Reprinted with permission from (77). Copyright © 2020, American Chemical
Society. (B) Schematic representation of HGF-induced Met activation
and the Met activation potential of the Apt-mono and the Apt-dimer.
Reprinted with permission from (83). Copyright © 2020 The Authors, some
rights reserved; exclusive licensee American Association for the
Advancement of Science. CTC, circulating tumor cell; HGF,
hepatocyte growth factor; AuNP, gold nanoparticle; RCA, rolling
circle amplification; EpCAM, epithelial cell adhesion molecule;
Met, MET proto-oncogene, receptor tyrosine kinase; Apt, aptamer;
HRP, horseradish peroxidase; TMB, 3,3′5,5′-tetramethylbenzidine;
SP, anti-EpCAM/HRP-AuNP, anti-EpCAM antibodies and horseradish
peroxidase co-conjugated gold nanoparticle; dNTPs,
deoxyribonucleotides mixture; PD, primer DNA; CDT; circular DNA
template; MCH, 6-mercapto-1-hexanol; AuE, gold electrode. |
Application in the molecular capture
probes
Su et al (79) developed a novel immunomagnetic
substrate, Fe3O4@polyamidoamine dendrimer
(PAMAM)@amphiphilic
trimester amine N-oxide (TMAO)@Aptamer, for efficient exosome
detection and capture. This was achieved through the sequential
modification of the fifth-generation PAMAM, TMAO and EpCAM aptamer
onto a magnetic core. The substrate leverages the strong magnetic
properties of magnetite (Fe3O4), the abundant
affinity sites of PAMAM, the strong hydrophilicity of TMAO and the
enhanced affinity of EpCAM aptamer. It demonstrates an impressive
capture efficiency of up to 90.5% for tumor-derived exosomes (TEXs)
within 30 min, while the non-specific adsorption of non-TEXs
remains low at only 8.2%. Song et al (80) introduced a novel approach for the
detection and in situ imaging of MUC1, utilizing aptamer
conformational changes and hybridization chain reaction (HCR). By
creating a specialized aptamer-trigger probe, the aptamer
selectively binds to MUC1, initiating the HCR reaction and
resulting in signal amplification. This approach not only detects
MUC1 in solution but also allows for in situ imaging at the
cellular level, making it suitable for the localization and
quantitative analysis of tumor cells.
Application in efficient capture and
non-invasive release of tumor cells
Detection and capture of tumor cells in blood or
other bodily fluids has been proposed to be an important approach
for improving early diagnosis of tumors and helping to determine
tumor metastasis. Currently, target cells are isolated based on
their size, surface adhesion, cell surface or distinctive marker
proteins using affinity ligands that selectively recognize these
surface markers. Such ligands include antibodies, aptamer molecules
or peptide compounds. Conventional immunomagnetic-based cell
capture techniques demonstrate poor sensitivity and specificity,
with suboptimal overall efficiency and economic feasibility
(81). By contrast, multivalent
aptamers have demonstrated high potential to efficiently capture
and release tumor cells. Liu et al (82) designed novel dual aptamer-modified
nitrogen-doped carbon quantum dot probes to improve the capture of
CTCs. The probes contained EpCAM and vimentin dual aptamers,
exhibiting potential to capture CTCs with different programmed
death-ligand 1 (PD-L1) expression levels (such as H1299 and A549
cells). It was observed that the dual aptamer modification markedly
improved the capture efficiency and purity of CTCs surpassing the
traditional single EpCAM capture method, particularly for CTCs
undergoing epithelial-mesenchymal transition.
Application in tumor cells' behavior
characterization
Besides their use in detecting and enriching tumor
cells, multivalent aptamers on cell surface can be used to promote
the receptor dimerization, activation and inhibition
mechanisms.
Ueki et al (83) designed a DNA aptamer that mimics the
function of hepatocyte growth factor (HGF) through dimerization
(Apt-dimer). Previous studies have demonstrated that HGF stimulates
the downstream signaling pathways by binding to and inducing
dimerization of the receptor MET proto-oncogene, receptor tyrosine
kinase (Met). It was observed that the Apt-dimer, designed by
dimerization, successfully activated the Met receptor and triggered
cell signaling similar to that of HGF (Fig. 1B). Nuclease stability is considered
a major limitation to the in vivo application of DNA
aptamers. Through serum stability experiments, i.e., Apt-dimer and
monomeric aptamer were dissolved in serum-containing solution
respectively, while serum-free buffer was set up as a negative
control; samples were taken at different time points and changes in
the integrity of the nucleic acid bands were observed by
electrophoresis, it was observed that the Apt-dimer showed high
nuclease stability in serum, whereas the monomeric form of the DNA
aptamer was rapidly degraded (84).
Therefore, it was postulated that the 3′ end of the Apt-dimer forms
a stem-loop structure that protects the DNA aptamer from 3′
exonuclease degradation. This finding provides new ideas for
designing highly stable DNA aptamers.
Using the SELEX technology, Menon et al
(85) reported RNA aptamers that
can specifically bind CD3 to improve the efficacy of overt T-cell
therapy. Unlike conventional CD3 agonistic antibodies, the designed
aptamers expanded T cells in an antigen-specific manner and avoided
non-specific T-cell expansion. These aptamers markedly enhanced
T-cell activation and the proliferation of low-affinity T-cell
receptors, improving the treatment of solid tumors, which have low
affinity. In addition, the aptamers promoted T-cell activation and
proliferation, but increased T-cell persistence in vivo,
achieving antitumor effects.
Zlinska et al (86) designed a DNA aptamer named VZ23,
which could specifically inhibit fibroblast growth factor receptor
1 (FGFR1) signaling. The aptamer demonstrated good efficacy in
treating human diseases, including growth disorders, degenerative
diseases and cancer. Currently, the treatment of FGFR is mainly
based on small-molecule tyrosine kinase inhibitors, but these
inhibitors are not sufficiently specific and inhibit multiple FGFR
isoforms and other receptor tyrosine kinases simultaneously,
inducing side effects and toxicity (87). Therefore, the VZ23 aptamer, which
specifically inhibits FGFR1 signaling without interfering with
other FGFR subtypes or non-FGFR receptors, may be an effective
treatment for cancer.
Application of multivalent aptamers in tumor
therapy
Currently, the lack of efficient and accurate
delivery systems for targeting tumor cells is a major limitation to
the effective treatment of tumors. Researchers have proposed the
tumor-immune bispecific antibody to improve cancer treatment
strategy by enhancing the immune system's ability to attack tumor
cells (88). This approach
leverages bispecific antibodies to target two different molecules
simultaneously (88). A major
advantage of tumor-immune bispecific antibodies is their high
specificity and few side effects. However, bispecific antibodies
are associated with certain limitations (89). For instance, their production
requires complex genetic engineering and purification processes,
which are costly. It has been shown that they may induce
immunogenic effects such as cytokine release syndrome (CRS). In
addition, the macromolecular structure of bispecific antibodies
restricts their penetration into the microenvironment of solid
tumors, resulting in inadequate penetration into solid tumors.
Multivalent aptamers, on the other hand, exhibit high chemical
stability, low immunogenicity and their synthesis is highly
flexible. Thus, they are expected to be an alternative to
bispecific antibodies for cancer treatment. Multivalent aptamers
can mimic the ‘bridging’ function of bispecific antibodies by
binding to multiple targets (such as tumor antigens and immune cell
receptors) owing to their modular design, which allows greater
freedom of design. Aptamers have a low molecular weight and can
efficiently penetrate solid tumors. Based on the solid-phase
synthesis approach, large-scale production of aptamers can be
achieved at a notably lower cost compared with antibody-based
drugs. Specifically, being nucleic acid molecules, multivalent
aptamers are less likely to trigger antibody-dependent immune
responses and may avoid side effects such as CRS. Their stability
and half-life can be chemically modified (such as phosphorothioate
backbone or PEGylation). The potential application scenarios of
multivalent aptamers in tumor therapy are discussed in the present
review.
Alternative T-cell splicing dual
antibodies
Bispecific aptamers can bind two different target
molecules simultaneously, usually an antigen on the surface of
cancer cells and a receptor on the surface of immune cells
(90). Based on this mechanism,
bispecific aptamers can form an artificial immune synapse between
cancer cells and immune cells, stimulating T-cell activation and
cancer cell lysis, while overcoming the penetration limitations of
large molecule antibodies, making them ideal bispecific antibodies.
For instance, Sun et al (91) designed a bispecific aptamer, Ap3-7c,
that can target both programmed cell death protein 1 (PD-1) and
PD-L1. Mechanistically, it not only inhibits the PD-1/PD-L1
interaction, but also promotes the physical contact between the T
cells and the tumor cells, thereby potentiating the antitumor
activity of the T cells. The bispecific aptamer binds to
dibenzocyclooctyne through a novel ‘recognize-then-conjugate’
mechanism, in which it covalently binds to the target cells
(Fig. 2A). This therapeutic
maneuver extends the retention time of the aptamer at the tumor
site and exerts an optimal therapeutic effect.
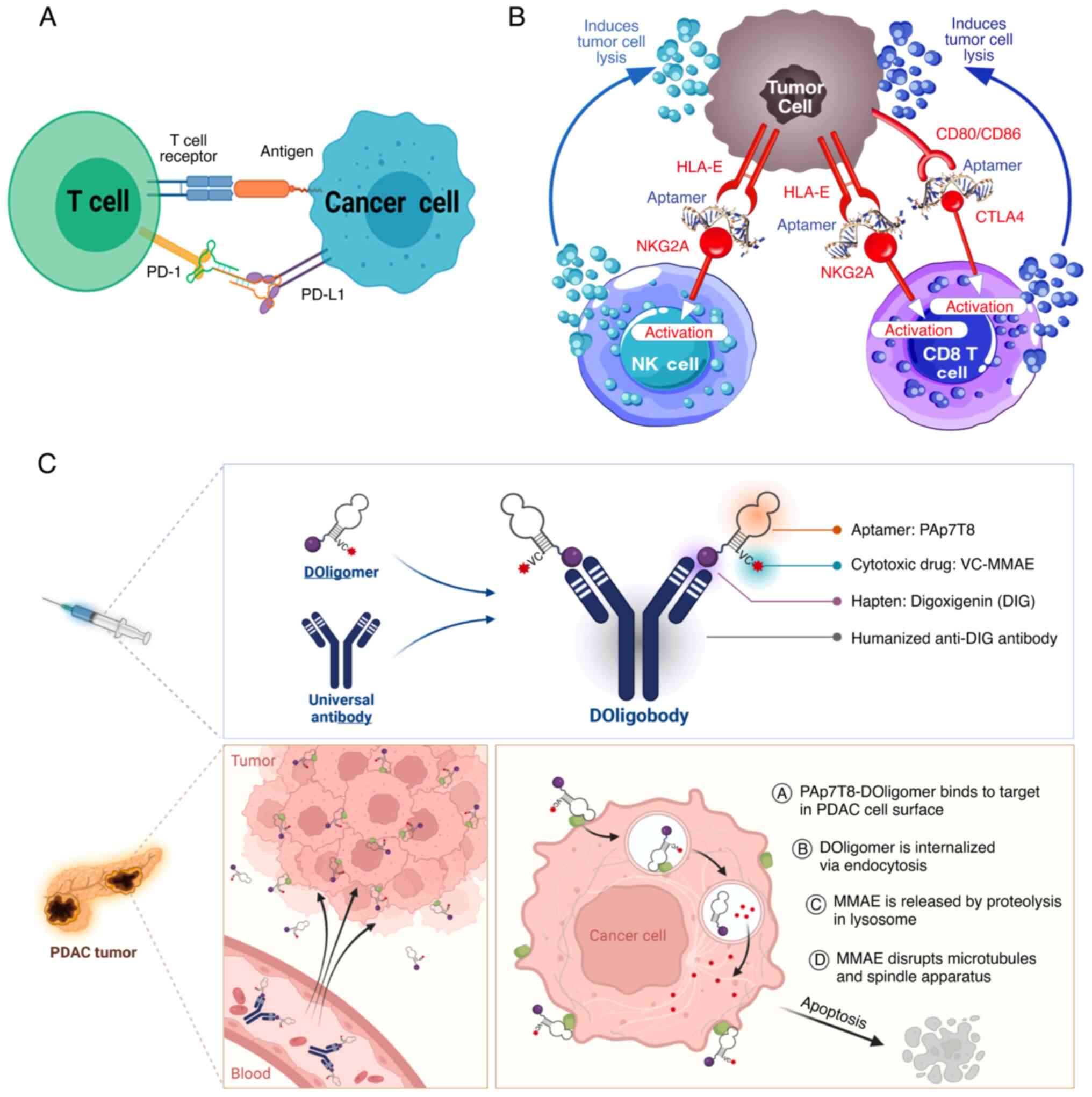 | Figure 2.Examples of multivalent aptamers in
tumor therapy. (A) Schematic representation of the mechanism by
which Ap3-7c blocks the PD1/PD-L1 axis. Reprinted with permission
from (91). Copyright © 2022,
American Chemical Society. (B) A comprehensive mechanistic
representation of the dual CTLA4/NKG2A aptamer (AYA22T) targeting
immunotherapy. Reprinted with permission from (94). Copyright © 2024 by the authors. (C)
Proposed mechanism of anticancer effect by PAp7T8-DOligobody in
PDAC. Reprinted with permission from (96). Copyright © 2023 The Authors.
Published by Elsevier B.V. PD-1, programmed cell death protein 1;
PD-L1, programmed death-ligand 1; CTLA4, cytotoxic T-lymphocyte
associated protein 4; NKG2A, natural killer group protein 2; HLA,
human leukocyte antigen; PDAC, pancreatic ductal adenocarcinoma;
MMAE, monomethyl auristatin E; CD80/CD86, cooperative B7-1/2;
Doligomer, drug-conjugated oligomer; DOligobody, drug-conjugated
oligobody. |
Dual signaling pathway inhibition
Cancer immunotherapy is increasingly transforming
the treatment of tumors. Furthermore, the emergence of immune
checkpoint inhibitors [such as anti-cytotoxic T-lymphocyte
associated protein 4 (CTLA-4) and anti-PD-1/PD-L1 antibodies] has
been a major breakthrough (92).
However, existing immune checkpoint inhibitors exhibit limited
response rates and immune-related side effects (92,93).
Therefore, it is crucial to formulate novel immunotherapeutic
strategies with high specificity and lower toxicity. Ayass et
al (94) designed a
bifunctional aptamer that could target CTLA-4 and natural killer
group protein 2 (NKG2A) to mimic dual immune checkpoint inhibitors.
Designed using computational biology approaches, this aptamer
selectively targets CTLA-4 and NKG2A, relieving T and natural
killer cell inhibition and thereby enhancing their antitumor
activity (Fig. 2B).
Multifunctional carriers
ApDCs have been extensively investigated with
respect to their potential to treat cancer, with previous studies
showing that aptamers can specifically deliver drugs to cancer
cells without affecting normal cells. In the study by Chen et
al (95), a multivalent
hydrophobic polymer was conjugated with an sgc8 aptamer to
construct an ultra-stable nano-micellar system that can maintain
the structural integrity in physiological environments. The system
exhibited good targeting and drug delivery efficiency. The
nano-micelle system was loaded with photosensitizer Ce6 for
photodynamic therapy and other hydrophobic drugs (such as
Adriamycin and paclitaxel), demonstrating its potential as a
multifunctional platform for drug delivery. In addition, Choi et
al (96) constructed a novel
pancreatic ductal adenocarcinoma-targeted therapeutic platform,
which could efficiently combine cytotoxic drug monomethyl
auristatin E with an aptamer and was delivered through a generic
semi-antigenic antibody (anti-digoxin antibody) to extend the
half-life of the aptamer in vivo. The aptamer specifically
recognizes pancreatic cancer cells, achieving specific tumor
delivery of drugs, which reduces toxicity to normal cells (Fig. 2C). The use of a universal antibody
allows easier replacement of different aptamers and improves
simultaneous targeting of multiple targets.
Challenges and prospects
Challenges
Studies have demonstrated that multivalent aptamers
have unique advantages, but they also have several limitations
(9,56,97,98).
Firstly, the use of multivalent aptamers requires balancing between
target specificity and affinity. Notably, aptamers bind to targets
through three-dimensional structures, but they are likely to
introduce risks due to non-specific binding. For instance, for
highly heterogeneous targets in the tumor microenvironment,
multivalent aptamers may trigger off-target effects as a function
of excessive cross-linking, decreasing their therapeutic effects.
Secondly, although they have a low molecular weight, aptamers have
a multivalent structure, which increases the size and limits its
penetration ability into solid tumors. In addition, nucleic acid
molecules may be degraded by serum nuclease, have a short half-life
and rely on nanocarriers or liposome encapsulation for effective
delivery. For instance, in glioma treatment, aptamers must
effectively penetrate the blood-brain barrier; however, most
research remains at the in vitro or animal model stage, with
limited success in clinical translation (99). Thirdly, the efficacy of aptamers is
limited by the interference of the complex tumor microenvironment.
Factors such as acidic pH, hypoxia and high interstitial pressure
in the tumor microenvironment may affect the binding efficiency of
aptamers and targets. In addition, the dynamic expression of
antigens on the surface of tumor cells (such as drug-resistant
mutations after treatment) may decrease the efficacy of the
aptamer. Therefore, dynamically responsive aptamers need to be
developed to cope with the hostile microenvironment. Fourthly,
large-scale production and quality control may be important
challenges. Although chemical synthesis of aptamers is relatively
low-cost, the precise assembly of multivalent structures (such as
dimer or trimer design) may be a complex production process. The
length and flexibility of the linker arm can influence aptamer
binding efficiency, necessitating optimization through
high-throughput screening. Finally, clinical studies have primarily
tested the efficacy of single-target applications (26,100,101), but few systematic investigations
have explored combination therapy strategies for the use of
multivalent aptamers (such as with immune checkpoint inhibitors).
In addition, little attention has been paid to the identification
of biomarkers that can be used to accurately predict patient
response.
Prospects
In the future, research into multivalent aptamers
should aim to explore the following aspects. Firstly, there is a
need to adopt multivalent design and dynamic response technologies
to establish efficient environment-responsive aptamers (such as pH
or enzyme-sensitive) to achieve selective activation of the tumor
microenvironment. Multimodal co-design of aptamers with other
therapeutic modules (such as photo-thermolysis, chemotherapeutic
agents or gene editing tools) are needed to build an ‘all-in-one’
platform. For instance, aptamers should be integrated with
proteolysis-targeting chimeras technology to degrade
difficult-to-adhere targets (such as transcription factors) via the
ubiquitin-proteasome system. Secondly, researchers should optimize
nanocarriers by leveraging liposomes, exosomes or metal-organic
frameworks to encapsulate aptamers, which will potentially enhance
their in vivo stability and tumor accumulation. Penetration
enhancement strategies are carried out to enhance solid tumor
penetration by aptamer-modified penetrating peptides or using
physical means such as ultrasound/magnetic fields. Thirdly,
artificial intelligence-driven aptamer development should be
investigated in future to integrate machine learning to predict
aptamer-target binding conformations, thereby accelerating the
SELEX screening process. For instance, leveraging AlphaFold-based
protein structure prediction could facilitate the reverse design of
aptamer sequences. Furthermore, it is imperative to integrate
multi-omics using spatial transcriptome technology to resolve tumor
heterogeneity and enhance the precise selection of aptamer targets.
Fourthly, investigators should aim to expand the target range of
aptamers and develop combination therapies. For membrane proteins,
which cannot be targeted efficiently using traditional small
molecules (such as delta-like ligand 3 or PSMA), multivalent
aptamer-ADC or bispecific chimeras may be used to address the drug
resistance limitation. Coupling aptamers with immune agonists (such
as IL-15) to remodel the tumor immunosuppressive microenvironment
and enhance T cell infiltration. Future research should prioritize
clinical translation and personalized medicine by integrating
aptamer therapy with liquid biopsy techniques, such as circulating
tumor DNA testing, to identify and stratify potential beneficiary
populations for more precise and effective treatment. Based on the
tumor-specific antigen profiles of patients, multivalent aptamer
combinations could be customized to achieve precision therapy.
Future research should focus on advancing multivalent aptamers for
oncology, exploring their potential from diagnosis to therapy,
targeted drug delivery and dynamic regulation. This will require
interdisciplinary collaboration and further technological
innovations to overcome existing challenges and enhance their
clinical applicability. Furthermore, efforts to integrate aptamers
and tumor biology are warranted to develop strategies to improve
the clinical application of aptamers.
The present review has the following limitations in
terms of content and scope of research: The study primarily focuses
on literature from the past decade and fails to cover research
achievements from earlier periods. This may lead to the neglect of
the historical background and foundational research of multivalent
nucleic acid aptamers in the early development of tumor diagnosis
and treatment. Although a comprehensive search in the PubMed, Web
of Science and Scopus databases was performed, some relevant
literature may still have been overlooked. For instance, conference
papers or preprints not indexed in these databases may contain
valuable cutting-edge research findings. Although the review
mentions the potential applications of multivalent aptamers in
tumor diagnosis and treatment, most studies are still at the in
vitro or animal model stage, with limited clinical trial
evidence, making it difficult to accurately assess their efficacy
and safety in real-world medical practice. The present review
includes only a limited discussion on the differences in the
application of multivalent aptamers in various types of tumors
(such as solid tumors and hematological malignancies). The
biological characteristics and microenvironmental differences among
different tumors are substantial, which may necessitate different
aptamer design strategies. The present review also has limited
discussion on the range of biomolecules that multivalent aptamers
can target, particularly in terms of certain membrane proteins or
intracellular targets that are difficult to target. This may result
in an inaccurate assessment of the potential and challenges of
multivalent aptamers in expanding their application scope.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HYZ conceived the study, wrote the original
manuscript and made subsequent revisions to the article. WJZ and
YYL searched the literature and participated in writing the
manuscript. All authors have read and approved the final version of
the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CTCs
|
circulating tumor cells
|
|
VEGF
|
vascular endothelial growth factor
|
|
XNA
|
xeno-nucleic acid
|
|
LNA
|
locked nucleic acid
|
|
PNA
|
peptide nucleic acids
|
|
SARS-CoV-2
|
severe acute respiratory syndrome
coronavirus 2
|
|
HNA
|
anhydrohexitol nucleic acid
|
|
SELEX
|
Systematic Evolution of Ligands by
Exponential Enrichment
|
|
CE-SELEX
|
capillary electrophoresis-SELEX
|
|
MUC1
|
mucin 1
|
|
PCR
|
polymerase chain reaction
|
|
PEG
|
polyethylene glycol
|
|
EpCAM
|
epithelial cell adhesion molecule
|
|
EGFR
|
epidermal growth factor receptor
|
|
ssDNA
|
single-stranded DNA
|
|
pAPNC
|
nanocarrier based on polyvalent
aptamer-protein
|
|
BSA
|
bovine serum albumin
|
|
ApDC
|
aptamer-drug conjugate
|
|
RCA
|
rolling circle amplification
|
|
PSMA
|
prostate-specific membrane
antigen
|
|
AuNP
|
gold nanoparticle
|
|
PAMAM
|
polyamidoamine dendrimer
|
|
TMAO
|
amphiphilic trimester amine
N-oxide
|
|
TEXs
|
tumor-derived exosomes
|
|
HCR
|
hybridization chain reaction
|
|
HGF
|
hepatocyte growth factor
|
|
FGFR1
|
fibroblast growth factor receptor
1
|
|
CRS
|
cytokine release syndrome
|
References
|
1
|
Wei W, Zeng H, Zheng R, Zhang S, An L,
Chen R, Wang S, Sun K, Matsuda T, Bray F and He J: Cancer
registration in China and its role in cancer prevention and
control. Lancet Oncol. 21:e342–e349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu J and Gao D: Recent advances in
aptamer-based microfluidic biosensors for the isolation, signal
amplification and detection of exosomes. Sensors (Basel).
25:8482025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Jiang H and Wang X: Advances in
cancer research: Current and future diagnostic and therapeutic
strategies. Biosensors (Basel). 14:1002024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu R, Li J, Salena BJ and Li Y: Aptamer
and DNAzyme based colorimetric biosensors for pathogen detection.
Angew Chem Int Ed Engl. 64:e2024187252025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He Y, Zeng X, Xiong Y, Shen C, Huang K and
Chen P: Portable aptasensor based on parallel rolling circle
amplification for tumor-derived exosomes liquid biopsy. Adv Sci
(Weinh). 11:24033712024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin M, Zhang J, Wan H, Yan C and Xia F:
Rationally designed multivalent aptamers targeting cell surface for
biomedical applications. ACS Appl Mater Interfaces. 13:9369–9389.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aiassa LV, Battaglia G and Rizzello L: The
multivalency game ruling the biology of immunity. Biophys Rev
(Melville). 4:0413062023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeldell SB and Seitz O: Nucleic acid
constructs for the interrogation of multivalent protein
interactions. Chem Soc Rev. 49:6848–6865. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Yang X, Lee NZ and Cao X:
Multivalent aptamer approach: Designs, strategies, and
applications. Micromachines (Basel). 13:4362022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moradi Z, Abnous K, Taghdisi SM, Zamanian
J, Moshiri M, Etemad D, Etemad L, Kesharwani P and Sahebkar A:
Designing multivalent aptamers: Recent advancements in diagnostic
and therapeutic approaches for cancer treatment. J Drug Delivery
Sci Technol. 105:1066142025. View Article : Google Scholar
|
|
11
|
Duan Q, Jia H, Chen W, Qin C, Zhang K, Jia
F, Fu T, Wei Y, Fan M, Wu Q and Tan W: Multivalent aptamer-based
lysosome-targeting chimeras (LYTACs) platform for mono- or
dual-targeted proteins degradation on cell surface. Adv Sci
(Weinh). 11:23089242024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Peng Y, Yao L, Shang H, Zheng Z,
Chen W and Xu J: Self-assembly of multivalent aptamer-tethered DNA
monolayers dedicated to a fluorescence polarization-responsive
circular isothermal strand displacement amplification for
salmonella assay. Anal Chem. 95:2570–2578. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang GQ, Zhong LP, Yang N and Zhao YX:
Screening of aptamers and their potential application in targeted
diagnosis and therapy of liver cancer. World J Gastroenterol.
25:3359–3369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Omer M, Andersen VL, Nielsen JS, Wengel J
and Kjems J: Improved cancer targeting by multimerizing aptamers on
nanoscaffolds. Mol Ther Nucleic Acids. 22:994–1003. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Sheng W and Fan ZH: An ensemble
of aptamers and antibodies for multivalent capture of cancer cells.
Chem Commun. 50:67222014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu S, Li X, Gao H, Chen J and Jiang H:
Progress in aptamer research and future applications.
ChemistryOpen. e2024004632025.doi: 10.1002/open.202400463 (Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanjanwala D and Patravale V: Aptamers and
nanobodies as alternatives to antibodies for ligand-targeted drug
delivery in cancer. Drug Discovery Today. 28:1035502023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kovacevic KD, Gilbert JC and Jilma B:
Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv Drug
Deliv Rev. 134:36–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vandghanooni S, Eskandani M, Barar J and
Omidi Y: Bispecific therapeutic aptamers for targeted therapy of
cancer: A review on cellular perspective. J Mol Med (Berl).
96:885–902. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu G and Chen X: Aptamer-based targeted
therapy. Adv Drug Deliv Rev. 134:65–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herrera M, Pretelli G, Desai J, Garralda
E, Siu LL, Steiner TM and Au L: Bispecific antibodies: Advancing
precision oncology. Trends Cancer. 10:893–919. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adachi T and Nakamura Y: Aptamers: A
review of their chemical properties and modifications for
therapeutic application. Molecules. 24:42292019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar Kulabhusan P, Hussain B and Yüce M:
Current perspectives on aptamers as diagnostic tools and
therapeutic agents. Pharmaceutics. 12:6462020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathavan S, Tam YJ, Mustaffa KMF and Tye
GJ: Aptamer based immunotherapy: A potential solid tumor
therapeutic. Front Immunol. 16:15365692025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lauridsen LH, Shamaileh HA, Edwards SL,
Taran E and Veedu RN: Rapid one-step selection method for
generating nucleic acid aptamers: Development of a DNA aptamer
against α-bungarotoxin. PLoS One. 7:e417022012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferreira D, Barbosa J, Sousa DA, Silva C,
Melo LDR, Avci-Adali M, Wendel HP and Rodrigues LR: Selection of
aptamers against triple negative breast cancer cells using high
throughput sequencing. Sci Rep. 11:86142021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang CK, Chew EY, Cukras CA, Keenan TDL,
Wong WT, Linehan WM, Chittiboina P, Pacak K and Wiley HE:
Intravitreous treatment of severe ocular von Hippel-Lindau disease
using a combination of the VEGF inhibitor, ranibizumab and PDGF
inhibitor, E10030: Results from a phase 1/2 clinical trial. Clin
Exp Ophthalmol. 49:1048–1059. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Du Y, Zhuo Y and Qiu L:
Functional nucleic acid-based live-cell fluorescence imaging. Front
Chem. 8:5980132020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He J, Duan Q, Ran C, Fu T, Liu Y and Tan
W: Recent progress of aptamer-drug conjugates in cancer therapy.
Acta Pharm Sin B. 13:1358–1370. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Hu B, Pei X, Li J, Qi D, Xu Y, Ou
H, Wu Y, Xue L, Huang JH, et al: A non-G-quadruplex DNA aptamer
targeting NCL for diagnosis and therapy in bladder cancer. Adv
Healthc Mater. 12:e23007912023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang LF, Ling M, Kacherovsky N and Pun SH:
Aptamers 101: Aptamer discovery and in vitro applications in
biosensors and separations. Chem Sci. 14:4961–4978. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen X, Ma Y, Luo H, Abdullah R, Pan Y,
Zhang Y, Zhong C, Zhang B and Zhang G: Peptide aptamer-paclitaxel
conjugates for tumor targeted therapy. Pharmaceutics. 17:402024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lyu C, Khan IM and Wang Z: Capture-SELEX
for aptamer selection: A short review. Talanta. 229:1222742021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cossu J, Ravelet C, Martel-Frachet V,
Peyrin E and Boturyn D: Peptide-based CE-SELEX enables convenient
isolation of aptamers specifically recognizing CD20-expressing
cells. Bioorg Med Chem. 110:1178312024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Q, Zhao X, Liu H and Qu F: Low pH
capillary electrophoresis application to improve capillary
electrophoresis-systematic evolution of ligands by exponential
enrichment. J Chromatogr A. 1364:289–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu C, Zhao XY, Yang G and Qu F: Capillary
electrophoresis involving in high efficiency screening for
aptamers. Chin J Analytical Chemistry. 48:583–589. 2020. View Article : Google Scholar
|
|
37
|
Li Y, Tam WW, Yu Y, Zhuo Z, Xue Z, Tsang
C, Qiao X, Wang X, Wang W, Li Y, et al: The application of aptamer
in biomarker discovery. Biomark Res. 11:702023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lam SY, Lau HL and Kwok CK: Capture-SELEX:
Selection strategy, aptamer identification, and biosensing
application. Biosensors (Basel). 12:11422022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meng X, Wen K, Citartan M and Lin Q: A
comparative study of aptamer isolation by conventional and
microfluidic strategies. Analyst. 148:787–798. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kubo T, Koike T, Ouchi T, Khaliq N, Sasaki
E, Kuroda K, Ueda M, Hanaoka K and Nemoto N: In vitro selection of
dye-fluorescence-enhancing peptide aptamer by cDNA display. Anal
Biochem. 698:1157222025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeddi I and Saiz L: Computational design
of single-stranded DNA hairpin aptamers immobilized on a biosensor
substrate. Sci Rep. 11:109842021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan R, Tao X, Zhai X, Zhu Y, Li Y, Chen Y,
Dong D, Yang S and Lv L: Application of aptamer-drug delivery
system in the therapy of breast cancer. Biomed Pharmacother.
161:1144442023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Lai BS and Juhas M: Recent
advances in aptamer discovery and applications. Molecules.
24:9412019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vorobyeva M, Vorobjev P and Venyaminova A:
Multivalent aptamers: Versatile tools for diagnostic and
therapeutic applications. Molecules. 21:16132016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Zhang Y, Li PC, Guo J, Huo F, Yang
J, Jia R, Wang J, Huang Q, Theodorescu D, et al: Development of
novel aptamer-based targeted chemotherapy for bladder cancer.
Cancer Res. 82:1128–1139. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lakshmipriya T, Fujimaki M, Gopinath SCB,
Awazu K, Horiguchi Y and Nagasaki Y: A high-performance
waveguide-mode biosensor for detection of factor IX using PEG-based
blocking agents to suppress non-specific binding and improve
sensitivity. Analyst. 138:2863–2870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moreira D, Leitão D, Lopes-Nunes J, Santos
T, Figueiredo J, Miranda A, Alexandre D, Tomaz C, Mergny JL and
Cruz C: G-quadruplex aptamer-ligand characterization. Molecules.
27:67812022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schmidt C, Kammel A, Tanner JA, Kinghorn
AB, Khan MM, Lehmann W, Menger M, Schedler U, Schierack P and
Rödiger S: A multiparametric fluorescence assay for screening
aptamer-protein interactions based on microbeads. Sci Rep.
12:29612022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Connell GC and Smothers CG: Optimized
methodology for product recovery following emulsion PCR:
Applications for amplification of aptamer libraries and other
complex templates. J Biol Methods. 7:e1282020.PubMed/NCBI
|
|
50
|
Zheng X, Gao S, Wu J and Hu X: Recent
advances in aptamer-based biosensors for detection of pseudomonas
aeruginosa. Front Microbiol. 11:6052292020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salunkhe S, Dheeraj, Basak M, Chitkara D
and Mittal A: Surface functionalization of exosomes for
target-specific delivery and in vivo imaging & tracking:
Strategies and significance. J Control Release. 326:599–614. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Park NJ, Wang X, Diaz A, Goos-Root DM,
Bock C, Vaught JD, Sun W and Strom CM: Measurement of cetuximab and
panitumumab-unbound serum EGFR extracellular domain using an assay
based on slow off-rate modified aptamer (SOMAmer) reagents. PLoS
One. 8:e717032013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kelly L, Maier KE, Yan A and Levy M: A
comparative analysis of cell surface targeting aptamers. Nat
Commun. 12:62752021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen Z, Hu L, Zhang BT, Lu A, Wang Y, Yu Y
and Zhang G: Artificial intelligence in aptamer-target binding
prediction. Int J Mol Sci. 22:36052021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Stuber A and Nakatsuka N: Aptamer
renaissance for neurochemical biosensing. ACS Nano. 18:2552–2563.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gao S, Zheng X, Jiao B and Wang L:
Post-SELEX optimization of aptamers. Anal Bioanal Chem.
408:4567–4573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu L, Wang Y, Xu X, Liu Y, Lin B, Zhang M,
Zhang J, Wan S, Yang C and Tan W: Aptamer-based detection of
circulating targets for precision medicine. Chem Rev.
121:12035–12105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Miao Y, Fu C, Yu Z, Yu L, Tang Y and Wei
M: Current status and trends in small nucleic acid drug
development: Leading the future. Acta Pharm Sin B. 14:3802–3817.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tabuchi Y, Yang J and Taki M: Relative
nuclease resistance of a DNA aptamer covalently conjugated to a
target protein. Int J Mol Sci. 23:77782022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, Dong Q, Xiao J, Fang X, Huang W,
Li Q, Chen Z, Liu H and Tan W: In-vivo polyvalent simpleaptamer@protein-based
nanocarrier with synergistic charge effect for high drug loading,
high nuclease resistance, and high receptor accessibility. CCS
Chem. 1–13. 2024. View Article : Google Scholar
|
|
61
|
Zhang Y, Zhang H, Chan DWH, Ma Y, Lu A, Yu
S, Zhang B and Zhang G: Strategies for developing long-lasting
therapeutic nucleic acid aptamer targeting circulating protein: The
present and the future. Front Cell Dev Biol. 10:10481482022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yu Y, Wang L, Ni S, Li D, Liu J, Chu HY,
Zhang N, Sun M, Li N, Ren Q, et al: Targeting loop3 of sclerostin
preserves its cardiovascular protective action and promotes bone
formation. Nat Commun. 13:42412022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Camorani S, Caliendo A, Morrone E, Agnello
L, Martini M, Cantile M, Cerrone M, Zannetti A, La Deda M, Fedele
M, et al: Bispecific aptamer-decorated and light-triggered
nanoparticles targeting tumor and stromal cells in breast cancer
derived organoids: Implications for precision phototherapies. J Exp
Clin Cancer Res. 43:922024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang N, Wang J, Bing T, Liu X and
Shangguan D: Transferrin receptor-mediated internalization and
intracellular fate of conjugates of a DNA aptamer. Mol Ther Nucleic
Acids. 27:1249–1259. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiao Y, Pan T, Da W, Liu Y, Chen S, Chen
D, Liu K, Zheng Y, Xie D, Gao Y, et al: Aptamer-drug
conjugates-loaded bacteria for pancreatic cancer synergistic
therapy. Sig Transduct Target Ther. 9:2722024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Safarkhani M, Ahmadi S, Ipakchi H, Saeb
MR, Makvandi P, Ebrahimi Warkiani M, Rabiee N and Huh Y:
Advancements in aptamer-driven DNA nanostructures for precision
drug delivery. Adv Sci (Weinh). 11:e24016172024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Papaefthymiou A, Doukatas A and
Galanopoulos M: Pancreatic cancer and oligonucleotide therapy:
Exploring novel therapeutic options and targeting chemoresistance.
Clin Res Hepatol Gastroenterol. 46:1019112022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang J, Tan M, Wang Y, Liu X and Lin A:
Advances in modification and delivery of nucleic acid drugs.
Zhejiang Da Xue Xue Bao Yi Xue Ban. 52:417–428. 2023.(In English,
Chinese). PubMed/NCBI
|
|
69
|
Pfeiffer F, Rosenthal M, Siegl J, Ewers J
and Mayer G: Customised nucleic acid libraries for enhanced aptamer
selection and performance. Curr Opin Biotechnol. 48:111–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Peng Y, Lu B, Deng Y, Yang N and Li G: A
dual-recognition-controlled electrochemical biosensor for accurate
and sensitive detection of specific circulating tumor cells.
Biosens Bioelectron. 201:1139732022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li J, Zhang Z, Gu J, Amini R, Mansfield
AG, Xia J, White D, Stacey HD, Ang JC, Panesar G, et al: Three on
three: Universal and high-affinity molecular recognition of the
symmetric homotrimeric spike protein of SARS-CoV-2 with a symmetric
homotrimeric aptamer. J Am Chem Soc. 144:23465–23473. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ge Z, Guo L, Wu G, Li J, Sun Y, Hou Y, Shi
J, Song S, Wang L, Fan C, et al: DNA origami-enabled engineering of
ligand-drug conjugates for targeted drug delivery. Small.
16:e19048572020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang S, Liu X, Wei D, Zhou H, Zhu J, Yu Q,
Luo L, Dai X, Jiang Y, Yu L, et al: Polyvalent aptamer nanodrug
conjugates enable efficient tumor cuproptosis therapy through
copper overload and glutathione depletion. J Am Chem Soc.
146:30033–30045. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Eilers A, Witt S and Walter J:
Aptamer-modified nanoparticles in medical applications. Aptamers in
Biotechnology. vol. 174. Urmann K and Walter JG: Springer
International Publishing; Cham: pp. 161–193. 2020, View Article : Google Scholar
|
|
75
|
Diao W, Yang B, Sun S, Wang A, Kou R, Ge
Q, Shi M, Lian B, Sun T, Wu J, et al: PNA-modified liposomes
improve the delivery efficacy of CAPIRI for the synergistic
treatment of colorectal cancer. Front Pharmacol. 13:8931512022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang X, Wei X, Wu CX, Men X, Wang J, Bai
JJ, Sun XY, Wang Y, Yang T, Lim CT, et al: Multiplex profiling of
biomarker and drug uptake in single cells using microfluidic flow
cytometry and mass spectrometry. ACS Nano. 18:6612–6622. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Amini R, Ma J, Zhang Z, Wang Q, Gu J,
Soleymani L and Li Y: Dimeric DNA aptamers for the spike protein of
SARS-CoV-2 derived from a structured library with dual random
domains. Small Methods. Dec 20;e24016002024.doi:
10.1002/smtd.202401600 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yang J, Li X, Jiang B, Yuan R and Xiang Y:
In situ-generated multivalent aptamer network for efficient capture
and sensitive electrochemical detection of circulating tumor cells
in whole blood. Anal Chem. 92:7893–7899. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Su N, Zhang J, Liu W, Zheng H, Li M, Zhao
J, Gao M and Zhang X: Specific isolation and quantification of
PD-L1 positive tumor derived exosomes for accurate breast cancer
discrimination via aptamer-functionalized magnetic composites and
SERS immunoassay. Talanta. 281:1269562025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Song Z, Zhou Y, Shen M, Zhao D, Hu H, Zeng
S, Sun L and Cai S: MUC1 detection and in situ imaging method based
on aptamer conformational switch and hybridization chain reaction.
Talanta. 239:1231292022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen Y, Tyagi D, Lyu M, Carrier AJ, Nganou
C, Youden B, Wang W, Cui S, Servos M, Oakes K, et al: Regenerative
NanoOctopus based on multivalent-aptamer-functionalized magnetic
microparticles for effective cell capture in whole blood. Anal
Chem. 91:4017–4022. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu Y, Zhang B, Wu X, Wang F, Yang Z, Li
M, Sheng K, Yan Y, Zhu L, Jing H, et al: A facile liquid biopsy
assay for highly efficient CTCs capture and reagent-less monitoring
of immune checkpoint PD-L1 expression on CTCs with non-small cell
lung cancer patients. Biosens Bioelectron. 275:1172362025.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ueki R, Uchida S, Kanda N, Yamada N, Ueki
A, Akiyama M, Toh K, Cabral H and Sando S: A chemically unmodified
agonistic DNA with growth factor functionality for in vivo
therapeutic application. Sci Adv. 6:eaay28012020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lei Y, Qiao Z, Tang J, He X, Shi H, Ye X,
Yan L, He D and Wang K: DNA nanotriangle-scaffolded activatable
aptamer probe with ultralow background and robust stability for
cancer theranostics. Theranostics. 8:4062–4071. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Menon AP, Villanueva H,
Meraviglia-Crivelli D, van Santen HM, Hellmeier J, Zheleva A,
Nonateli F, Peters T, Wachsmann TLA, Hernandez-Rueda M, et al: CD3
aptamers promote expansion and persistence of tumor-reactive T
cells for adoptive T cell therapy in cancer. Mol Ther Nucleic
Acids. 35:1021982024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zlinska V, Feketova Z, Czyrek A, Chudzian
J, Zivkovic ML, Ursachi VC, Dudeja P, Fafilek B, Rynes J,
Rico-Llanos G, et al: Specific inhibition of fibroblast growth
factor receptor 1 signaling by a DNA aptamer. Mol Ther Nucleic
Acids. 36:1024052025. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zschäbitz S and Grüllich C: Lenvantinib: A
tyrosine kinase inhibitor of VEGFR 1–3, FGFR 1–4, PDGFRα, KIT and
RET. Small Molecules in Oncology. vol. 211. Martens UM: Springer
International Publishing; Cham: pp. 187–198. 2018, View Article : Google Scholar
|
|
88
|
Shapir Itai Y, Barboy O, Salomon R,
Bercovich A, Xie K, Winter E, Shami T, Porat Z, Erez N, Tanay A, et
al: Bispecific dendritic-T cell engager potentiates anti-tumor
immunity. Cell. 187:375–389.e18. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
De Assis LH, Fassi DE and Hutchings M:
Bispecific antibody therapies. Hematology Am Soc Hematol Educ
Program. 2023:216–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Thomas BJ, Porciani D and Burke DH: Cancer
immunomodulation using bispecific aptamers. Mol Ther Nucleic Acids.
27:894–915. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sun Y, Mo L, Hu X, Yu D, Xie S, Li J, Zhao
Z, Fang X, Ye M, Qiu L, et al: Bispecific aptamer-based
recognition-then-conjugation strategy for PD1/PDL1 axis blockade
and enhanced immunotherapy. ACS Nano. 16:21129–21138. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shalata W, Weissmann S, Itzhaki Gabay S,
Sheva K, Abu Saleh O, Jama AA, Yakobson A and Rouvinov K: A
retrospective, single-institution experience of bullous pemphigoid
as an adverse effect of immune checkpoint inhibitors. Cancers
(Basel). 14:54512022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ji P, Gong Y, Jin ML, Wu HL, Guo LW, Pei
YC, Chai WJ, Jiang YZ, Liu Y, Ma XY, et al: In vivo
multidimensional CRISPR screens identify Lgals2 as an immunotherapy
target in triple-negative breast cancer. Sci Adv. 8:eabl82472022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ayass MA, Tripathi T, Griko N, Okyay T,
Ramankutty Nair R, Zhang J, Zhu K, Melendez K, Pashkov V and
Abi-Mosleh L: Dual checkpoint aptamer immunotherapy: Unveiling
tailored cancer treatment targeting CTLA-4 and NKG2A. Cancers
(Basel). 16:10412024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen G, Mao D, Wang X, Chen J, Gu C, Huang
S, Yang Y, Zhang F and Tan W: Aptamer-based self-assembled
nanomicelle enables efficient and targeted drug delivery. J
Nanobiotechnol. 21:4152023. View Article : Google Scholar
|
|
96
|
Choi SI, Lee YS, Lee YM, Kim HJ, Kim WJ,
Jung S, Im JE, Lee MR, Kim JK, Jeon AR, et al: Complexation of drug
and hapten-conjugated aptamer with universal hapten antibody for
pancreatic cancer treatment. J Control Release. 360:940–952. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Szymanowski W, Szymanowska A, Bielawska A,
Lopez-Berestein G, Rodriguez-Aguayo C and Amero P: Aptamers as
potential therapeutic tools for ovarian cancer: Advancements and
challenges. Cancers (Basel). 15:53002023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xie S, Wang Z, Fu T, Zheng L, Wu H, He L,
Huang H, Yang C, Wang R, Qian X, et al: Engineering aptamers with
selectively enhanced biostability in the tumor microenvironment.
Angew Chem Int Ed Engl. 61:e2022012202022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mojarad-Jabali S, Farshbaf M, Walker PR,
Hemmati S, Fatahi Y, Zakeri-Milani P, Sarfraz M and Valizadeh H: An
update on actively targeted liposomes in advanced drug delivery to
glioma. Int J Pharm. 602:1206452021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rosenberg JE, Bambury RM, Van Allen EM,
Drabkin HA, Lara PN Jr, Harzstark AL, Wagle N, Figlin RA, Smith GW,
Garraway LA, et al: A phase II trial of AS1411 (a novel
nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma.
Invest New Drugs. 32:178–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Giordano FA, Layer JP, Leonardelli S,
Friker LL, Turiello R, Corvino D, Zeyen T, Schaub C, Müller W,
Sperk E, et al: L-RNA aptamer-based CXCL12 inhibition combined with
radiotherapy in newly-diagnosed glioblastoma: Dose escalation of
the phase I/II GLORIA trial. Nat Commun. 15:42102024. View Article : Google Scholar : PubMed/NCBI
|
















