Introduction
Small cell lung cancer (SCLC) is a highly aggressive
cancer that accounts for ~15% of lung cancer cases (1). SCLC cells originate from
neuroendocrine precursor cells (2),
and SCLC cells tend to grow rapidly and metastasize in the early
stage. Therefore, in most patients, late-stage SCLC has already
developed by the time of diagnosis (3), and the prognosis of SCLC is poor, with
a median overall survival (OS) of ~10 months (4–6). In
recent decades, the first-line treatment for SCLC has been the
combination of etoposide and platinum compounds (7,8).
Although approximately two-thirds of patients respond to this
regimen, most patients experience relapse or progression soon after
first-line treatment; hence, the prognosis remains poor (9). More recently, the novel immune
checkpoint inhibitors atezolizumab and durvalumab were approved for
first-line treatment in patients with late-stage SCLC. For
instance, in a randomised, controlled, open-label, phase 3 trial,
34% of the 268 patients administrated Durvalumab plus
platinum-etoposide as the first-line therapy remained alive at 18
months, whereas only 25% of the platinum-etoposide group (269
cases) survived, with an improvement in OS provided by addition of
Durvalumab [hazard ratio (HR): 0.73] (10). Similar results were also reported in
another double-blinded, placebo-controlled, phase 3 trial, where
201 patients were randomly assigned to the atezolizumab group and
202 patients to the placebo group. The median OS was 12.3 months in
the atezolizumab group and 10.3 months in the placebo group (HR:
0.70) (11). However, further
studies are needed to verify the new drugs and new combinations on
the outcomes for patients with SCLC.
SCLC is divided into sensitive and refractory
disease on the basis of the time elapsed between the first-line
treatment and relapse or resistance (within or beyond 90 days).
Currently, only topotecan is approved as a second-line treatment
for relapsed SCLC (12), and the
results of certain clinical trials have shown promise for
combination chemotherapy (13,14).
Temozolomide (TMZ) is an oral alkylating agent that is mainly
administered to patients with glioblastoma (GBM). In 2010, Zauderer
et al (15) reported for the
first time on two patients with SCLC with leptomeningeal metastases
who experienced a marked response to TMZ treatment. The results of
a phase II trial proved the activity of TMZ in patients with
relapsed SCLC, particularly those with brain metastases (16). A 5-day dosing regimen of TMZ was
subsequently reported to be effective for patients with relapsed
SCLC (17). In addition, synergy
between TMZ and novel poly(ADP-ribose) polymerase-1 inhibitors,
including veliparib, rucaparib and olaparib, has been demonstrated
in multiple clinical trials (18–20).
Currently, TMZ is incorporated into treatment guidelines for
relapsed SCLC, for which the level of evidence and strength of
recommendation is 2A (21). The
present study focused on the influence of clinical factors on TMZ
treatment, including a history of cigarette smoking and alcohol
consumption, cardiovascular complications and previous
radiotherapy, particularly prophylactic cerebral irradiation (PCI)
treatment, and provided insights into its effect on extracranial
lesions in patients with relapsed SCLC.
Patients and methods
Patients and data collection
This retrospective cohort study involved the
collection of data from patients with relapsed sensitive SCLC
according to their clinical records, who were treated at the 81st
Group Army Hospital of the People's Liberation Army (Zhangjiakou,
China) between June 2010 and December 2020; the dates refer to the
period from the first encounter at the hospital to the last
follow-up. The inclusion criteria were as follows: i) All patients
were pathologically diagnosed with SCLC and had received inpatient
radio- or chemotherapy; ii) sensitive patients were defined as
those who experienced progression or relapse ≥90 days after
completion of first-line chemotherapy; iii) patients with brain
metastasis were divided into TMZ and non-TMZ groups, where the
former group was administered 75 mg/m2/d TMZ for 21 days
of a 28-day cycle; iv) patients in the PCI group were prescribed
2.5 Gy irradiation, which was administered daily for 5 days per
week, and a total of 30 Gy through intensity-modulated radiation
therapy, no longer than one month after the first-line therapy. The
dose limit for both the hippocampus and the lens was 9.0 Gy; and v)
regular follow-up was carried out every month, mainly through
telephone or inpatient questionnaires.
The final study population comprised 164 patients
with SCLC (median age: 58.64 years, 74.4% male), with detailed
characteristics summarized in Table
SI. For patients in the limited stage, which refers to stages
I–III (any T, any N, M0), who could be safely treated with
definitive radiation doses according to the American Joint
Committee on Cancer definition from 2010 (22), all were prescribed PCI. However, for
those in the advanced stage, only patients evaluated as having
complete response or partial response (PR) were prescribed PCI. A
total of 20 patients were included in the TMZ treatment group, of
which 12 received PCI. For combination with vindesine (VDS), 8
patients in the TMZ group were simultaneously administered of VDS
at 3 mg/m2/week for a 28-day cycle. The clinical data
collected included age at diagnosis, sex, complications of diabetes
and hypertension, family history of cancer, habits of cigarette use
and alcohol consumption, OS, overall response rate (ORR), previous
radio/chemotherapy and the main types of side effects. The ORR was
evaluated according to the Response Evaluation Criteria in Solid
Tumors version 1.1 based on contrast-enhanced CT or MRI (23) and performed every two treatment
cycles. In light of limited cases receiving >4 courses of TMZ
treatment, patients were divided into three subgroups based on the
courses of TMZ treatment (≤2, 2–4 and >4).
Statistical analysis
GraphPad Prism 8.0 software (Dotmatics) was used to
analyse the data. Nonparametric data were presented as counts and
frequencies and comparisons were performed with the chi-square test
or Fisher's exact test. For continuous variables, expressed as the
mean ± standard deviation, a t-test or one-way ANOVA,
followed by Tukey's highly-significant differences post-hoc test
was used. OS was defined as the period between the date of first
primary malignancy diagnosis and the last known date of follow-up
or date of death. Cumulative survival was evaluated by Kaplan-Meier
analysis. Differences in survival curves between groups of patients
were assessed using the log-rank (Mantel-Cox) test. The risk ratio
(RR) of each variable with the corresponding 95% confidence
interval (CI) was calculated with the Koopman asymptotic score. A
two-tailed P<0.05 was considered to indicate statistical
significance.
Results
Baseline characteristics of patients
with SCLC
The clinical data of the patients with SCLC are
provided in Table SI. There were
no significant differences in terms of sex, mean age at the time of
diagnosis, cigarette use or alcohol consumption, family history of
cancer or cardiovascular complications between patients treated
with or without TMZ. In addition, the proportions of patients who
received surgery, thoracic radiotherapy, prophylactic cerebral
irradiation and targeted therapy were similar between the two
groups. TMZ treatment did not increase OS, which may be explained
by the different proportions of patients with brain metastasis
among the patients with SCLC [18.89% (20/144) for those without TMZ
treatment and 100% for those in the TMZ group, P<0.001; Table SI]. Therefore, OS, risk factors and
the main cause of death were compared among patients with brain
metastasis who were or were not treated with TMZ (the TMZ or
non-TMZ group). Although there were no significant differences in
the potential risk factors (Table
I), the OS time of the TMZ group was significantly longer than
that of the non-TMZ group. Thus, the results of the present study
proved the benefits of TMZ for patients with SCLC with brain
metastasis.
 | Table I.Characteristics of the patients with
small-cell lung cancer with brain metastasis treated with TMZ or
not. |
Table I.
Characteristics of the patients with
small-cell lung cancer with brain metastasis treated with TMZ or
not.
| Characteristic | TMZ group
(n=20) | Non-TMZ group
(n=20) | P-value |
|---|
| Age, years | 56.33±1.308 | 57.50±2.036 | 0.6290 |
| Sex |
|
| >0.999 |
|
Female | 6 (30.0) | 5 (25.0) |
|
|
Male | 14 (70.0) | 15 (75.0) |
|
| Cigarette
consumption (longer than 30 years) | 8 (40.0) | 12 (60.0) | 0.3431 |
| Regular alcohol
consumption (longer than 20 years) | 6 (30.0) | 7 (35.0) | >0.999 |
| Diabetes
mellitus | 2 (10.0) | 1 (5.0) | >0.999 |
| Hypertension | 1 (5.0) | 1 (5.0) | >0.999 |
| Family history of
cancer | 4 (20.0) | 3 (15.0) | >0.999 |
| PCI | 12 (60.0) | 18 (90.0) | 0.3431 |
| Thoracic
radiotherapy | 16 (80.0) | 13 (65.0) | >0.999 |
| Non-TMZ
chemotherapy | 19 (95.0) | 19 (95.0) | >0.999 |
| Targeted
therapy | 4 (20) | 1 (5) | 0.3416 |
| Overall survival,
months | 25.80±2.769 | 16.70±2.413 | 0.0178 |
| Main cause of death
(brain metastasis/extracranial progress) | 7/13 | 9/11 | 0.7475 |
Factors influencing OS in patients
with SCLC treated with TMZ
The effect of TMZ on the survival of patients with
SCLC with brain metastasis was evaluated by Kaplan-Meier analysis,
and it was observed that the median survival time in the non-TMZ
group was only 12.0 months, whereas it was significantly increased
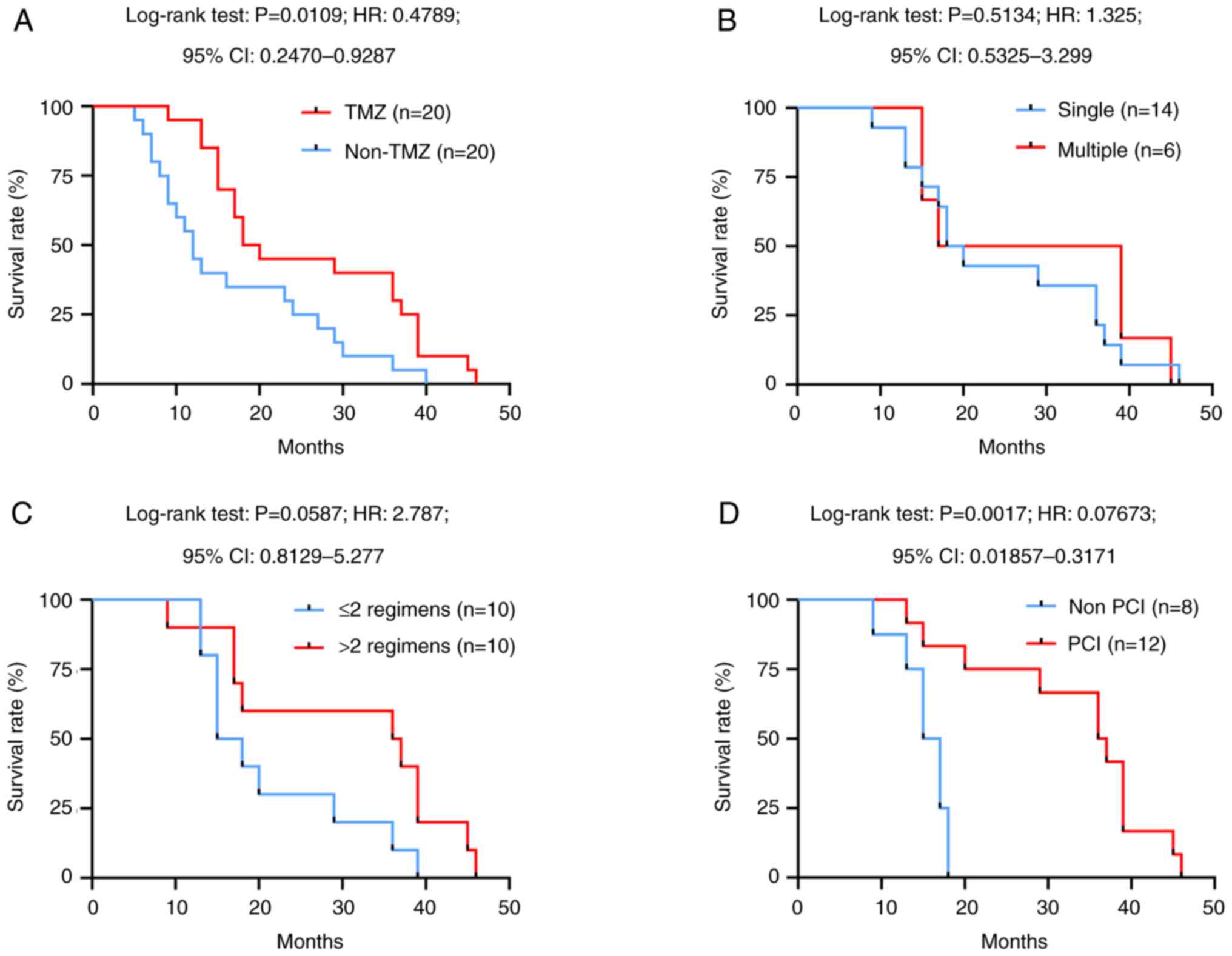
to 19.0 months in the TMZ group [P=0.0109, hazard ratio (HR):
0.4789, 95% CI: 0.2470–0.9287; Fig.
1A]. In addition, the OS of the two groups remained identical
regardless of the number of brain metastases (Fig. 1B) and early chemotherapy regimens
(Fig. 1C). Furthermore, the median
survival time of patients who received PCI was 2.28-fold greater
than that of patients without PCI treatment (P=0.0017, HR: 0.07673,
95% CI: 0.01857–0.3171; Fig. 1D).
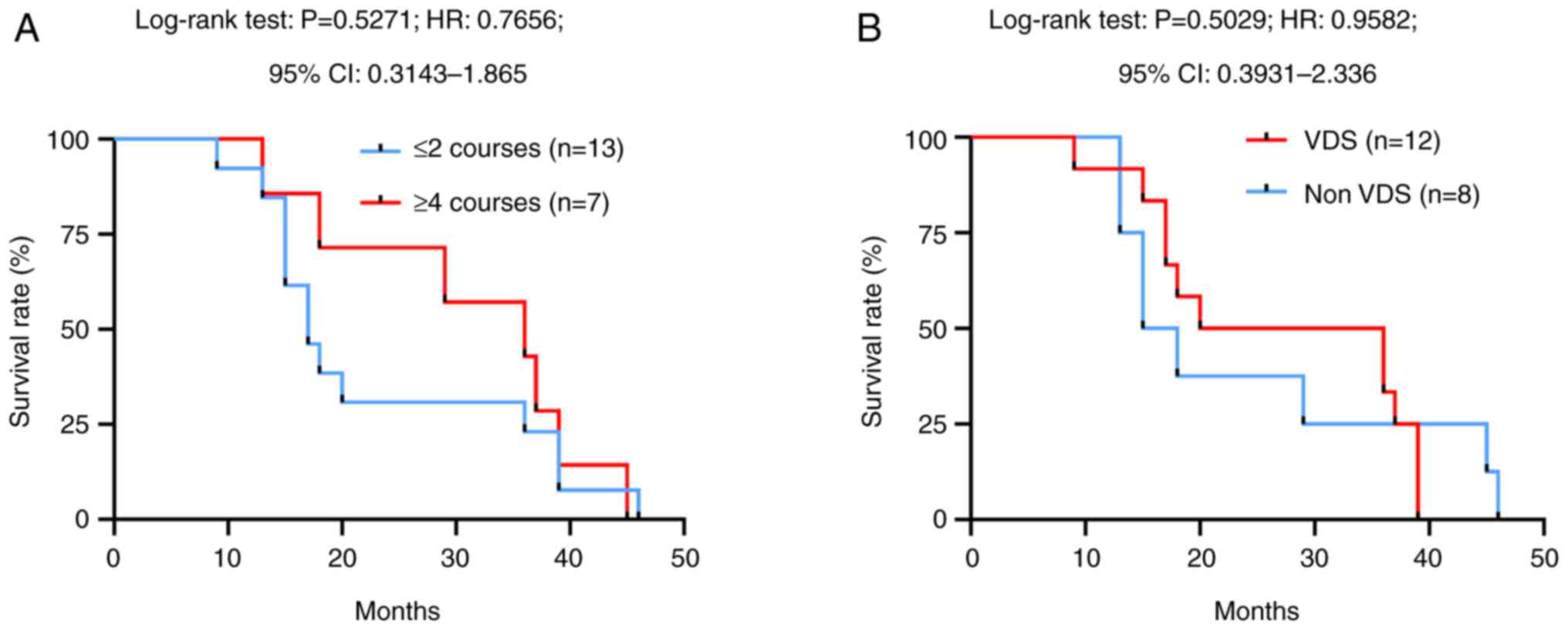
Next, the dose-dependent effects of TMZ on OS were analysed.
Although the difference was not significant, the median survival
time of those who underwent >4 cycles of TMZ treatment was 36.0
months, whereas patients who received 2 or 1 cycles of TMZ
treatment had a median survival time of only 17.0 months (P=0.5271,
HR: 1.306, 95% CI: 0.5363–3.181; Fig.
2A). In addition, during this study, 8 patients in the TMZ
group were simultaneously administered VDS (3 mg/m2/week
for a 28-day cycle), but there was no difference in OS between
those receiving TMZ plus VDS treatment and those receiving TMZ-only
treatment (P=0.5029, HR: 0.9582, 95% CI: 0.3931–2.336; Fig. 2B). Together, these results indicate
that the administration of PCI but not chemotherapy regimens or the
state of brain metastasis affects the prognosis of patients with
SCLC with brain metastasis after TMZ treatment and that there are
potential dose-dependent effects of TMZ treatment.
Effects of TMZ on the ORR of patients
with SCLC
To evaluate the ORR of TMZ treatment, both brain and
extracranial metastatic lesions were evaluated through CT or MRI
images. As shown in Table II, 7
patients received four courses of TMZ and 4 of these patients had
the opportunity to receive more courses. On the basis of the
Response Evaluation Criteria in Solid Tumors, the state of stable
disease (SD) and partial response (PR) was considered responsive to
TMZ treatment (with no complete response observed in this study).
The ORRs for brain metastasis were 75.0, 30.0 and 15% in the
2-cycle, 4-cycle and >4-cycle evaluations, respectively. The
ORRs for lesions with extracranial metastasis were 75.0, 25.0 and
20%, respectively (Table II). In
addition, the RR of the combination of VDS with 2-cycle TMZ
treatment was 1.500 (95% CI: 0.6803–3.6893) and 1.125 (95% CI:
0.4039–3.108) for the brain and extracranial metastatic lesions,
respectively. Administration of PCI before TMZ showed a limited
influence on the ORRs, with a RR of 1.061 (95% CI: 0.4961–2.949)
and 1.250 (95% CI: 0.4516–4.627) for brain and extracranial
metastatic lesions, respectively. Similar results were observed for
different chemotherapy regimens before TMZ. Although there were no
significant differences in the ORRs, >15% patients retained
non-progressive disease after two courses of TMZ treatment.
Furthermore, the initial treatment regimens and medication duration
did not affect the effectiveness of TMZ.
 | Table II.Overall response rate of brain and
extracranial lesions of metastasis for TMZ. |
Table II.
Overall response rate of brain and
extracranial lesions of metastasis for TMZ.
| A, Brain
metastasis |
|---|
|
|---|
|
Treatment/response | TMZ courses, n
(patients) |
|---|
| Total (n=20) | 2 (n=20) | 4 (n=7) | >4 (n=4) |
| SD | 10 | 5 | 2 |
| PR | 5 | 1 | 1 |
| PD | 1 | 2 | 1 |
| ORR,
% | 75.0 | 30.0 | 15.0 |
| Adjuvant |
|
|
|
| No
(n=8) | 2 (n=8) | 4 (n=4) | >4 (n=1) |
|
SD | 6 | 2 | 0 |
|
PR | 2 | 0 | 0 |
|
PD | 0 | 2 | 1 |
|
ORR, % | 100 | 25.0 | 0 |
| Yes
(n=12) | 2 (n=12) | 4 (n=4) | >4 (n=3) |
|
SD | 4 | 3 | 2 |
|
PR | 3 | 1 | 1 |
|
PD | 1 | 0 | 0 |
|
ORR, % | 58.3 | 33.3 | 25.0 |
| RR (95%
CI); P-value | 1.500
(0.6803–3.6893); NS | 0.6467
(0.1844–2.084); NS | / |
| PCI |
|
|
|
| Yes
(n=12) | 2 (n=12) | 4 (n=5) | >4 (n=4) |
|
SD | 7 | 4 | 2 |
|
PR | 3 | 0 | 1 |
|
PD | 1 | 1 | 1 |
|
ORR, % | 83.3 | 33.3 | 25.0 |
| No
(n=8) | 2 (n=5) | 4 (n=3) | >4 (n=0) |
|
SD | 3 | 1 | 0 |
|
PR | 2 | 1 | 0 |
|
PD | 0 | 1 | 0 |
|
ORR, % | 62.5 | 25.0 | 0 |
| RR (95%
CI); P-value | 1.0610
(0.4961–2.949); NS | 2.4000
(0.6897–13.41); NS | / |
| Number of
regimens |
|
|
|
| ≤2
(n=10) | 2 (n=10) | 4 (n=3) | >4 (n=1) |
|
SD | 6 | 2 | 0 |
|
PR | 2 | 0 | 1 |
|
PD | 1 | 2 | 0 |
|
ORR, % | 80.0 | 20.0 | 0 |
| ≥3
(n=10) | 2 (n=10) | 4 (n=4) | >4 (n=3) |
|
SD | 4 | 3 | 2 |
|
PR | 3 | 1 | 0 |
|
PD | 0 | 0 | 1 |
|
ORR, % | 70.0 | 40.0 | 20.0 |
| RR (95%
CI); P-value | 1.670
(0.5312–2.877); NS | 0.6667
(1.866–2.084); NS | / |
|
| B, Extracranial
metastasis |
|
|
Treatment/response | TMZ courses, n
(patients) |
|
| Total (n=20) | 2 (n=20) | 4 (n=7) | >4 (n=4) |
|
SD | 8 | 4 | 1 |
|
PR | 7 | 1 | 3 |
|
PD | 2 | 2 | 0 |
|
ORR, % | 75.0 | 25.0 | 20.0 |
| Adjuvant |
|
|
|
| No
(n=8) | 2 (n=8) | 4 (n=3) | >4 (n=1) |
|
SD | 4 | 1 | 1 |
|
PR | 4 | 1 | 0 |
|
PD | 0 | 1 | 0 |
|
ORR, % | 100 | 25.0 | 12.5 |
| Yes
(n=12) | 2 (n=12) | 4 (n=4) | >4 (n=3) |
|
SD | 4 | 3 | 0 |
|
PR | 3 | 0 | 2 |
|
PD | 2 | 1 | 0 |
|
ORR, % | 58.3 | 25.0 | 16.7 |
|
RR (95% CI);
P-value | 1.125
(0.4039–3.108); NS | 0.6467
(0.1844–2.084); NS | / |
| PCI |
|
|
|
| Yes
(n=12) | 2 (n=12) | 4 (n=11) | >4 (n=4) |
|
SD | 6 | 4 | 1 |
|
PR | 4 | 6 | 3 |
|
PD | 2 | 1 | 0 |
|
ORR, % | 83.3 | 83.3 | 33.3 |
| No
(n=8) | 2 (n=5) | 4 (n=3) | >4 (n=0) |
|
SD | 2 | 0 | 0 |
|
PR | 3 | 1 | 0 |
|
PD | 0 | 1 | 0 |
|
ORR, % | 62.5 | 12.5 | 0 |
| RR (95%
CI); P-value | 1.250
(0.4516–4.627); NS | (0.9803–2.684);
NS | / |
| Number of
regimens |
|
|
|
| ≤2
(n=10) | 2 (n=10) | 4 (n=3) | >4 (n=1) |
|
SD | 5 | 1 | 0 |
|
PR | 3 | 1 | 1 |
|
PD | 1 | 1 | 0 |
|
ORR, % | 80.0 | 20.0 | 10.0 |
| ≥3
(n=10) | 2 (n=10) | 4 (n=4) | >4 (n=3) |
|
SD | 3 | 3 | 1 |
|
PR | 4 | 0 | 2 |
|
PD | 1 | 1 | 0 |
|
ORR, % | 70.0 | 30.0 | 30.0 |
| RR (95%
CI); P-value | 1.4810
(0.5407–4.511); NS | 0.4444
(0.07810–1.770); NS | / |
Adverse effects of TMZ treatment in
patients with SCLC
In the present study, 13 patients failed to receive
4 cycles of TMZ treatment, of whom only one experienced progression
of brain metastasis, and the other 12 patients discontinued TMZ
treatment because of metastasis to the liver, lymph nodes or
splanchnocoel. However, the risk of severe anaemia or fatigue, the
two most common adverse effects, was not elevated in this study.
Additionally, only slight side effects associated with the
digestive system were observed (Table
III). These results revealed that TMZ treatment was a safe
second-line strategy against SCLC.
 | Table III.Adverse effects of TMZ treatment. |
Table III.
Adverse effects of TMZ treatment.
|
| TMZ group
(n=20) | Non-TMZ group
(n=20) |
|
|---|
|
|
|
|
|
|---|
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P-value |
|---|
| Anemia | 2 (10) | 4 (20) | 3 (15) | 1 (5) | 4 (20) | 4 (20) | 3 (15) | 0 (0) | >0.999 |
| Fatigue | 8 (40) | 6 (30) | 4 (25) | 0 (0) | 6 (30) | 7 (35) | 5 (25) | 0 (0) | >0.999 |
| Vomiting | 6 (30) | 6 (30) | 0 (0) | 0 (0) | 8 (40) | 5 (25) | 0 (0) | 0 (0) | NA |
| Anorexia | 2 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | NA |
Discussion
In recent decades, the combination of TMZ or
whole-brain irradiation with or without stereotactic radiotherapy
has been considered a beneficial option for patients with GBM and
non-SCLC (24,25). However, the effect of PCI in
adjuvant TMZ treatment for patients with SCLC has remained
underexplored. Recently, a case study reported that permanent
radioactive iodine-125 seed implantation combined with TMZ
metronomic chemotherapy triggered an abscopal effect and provided
good local control of liver metastases in patients with SCLC
(26). Thus, radiotherapy may
sensitize patients with SCLC to TMZ. In addition, TMZ, as a
third-line therapy, has been reported to result in a partial
response in patients with pulmonary large-cell neuroendocrine
carcinoma, with no severe side effects (27), which suggests TMZ is effective for
treating pulmonary neuroendocrine lesions, including SCLC.
To investigate the effect of TMZ in patients with
sensitive relapsed SCLC and the related risk factors, the present
retrospective cohort study was performed. In accordance with a
previous study (16), a significant
improvement in OS was observed in the TMZ group. The present
results are the first to show an underlying relationship between
PCI and the effects of TMZ treatment in patients with sensitive
relapsed SCLC with brain metastasis, to the best of our knowledge.
In addition, although vinorelbine alone (28) or in combination with other agents
(29,30) has been reported to exert toxic
effects on patients with SCLC in clinical trials, no synergy
between vindesine and TMZ was found in the cohort of the present
study. Interestingly, in addition to a dramatic response of brain
metastases to TMZ, a relatively high response rate was observed in
extracranial lesions even in the four-cycle assessment. Therefore,
the results of the present study support the beneficial effects of
TMZ in patients with SCLC.
TMZ is an oral DNA alkylating agent that is known to
cause cell cycle arrest (31),
apoptosis (32) and autophagy
(33,34). Because of its ability to cross the
blood-brain barrier, TMZ is highly recommended as a first-line
chemotherapy for astrocytoma and GBM (35,36).
Through the methylation of DNA adenine and guanine residues, TMZ
treatment leads to the formation of O6-methylguanine,
N3-methyladenine and N7-methylguanine, which
exert cytotoxic effects (37). In
addition, methylated DNA can be repaired by base excision or DNA
mismatch repair pathways, and mutation of related genes results in
TMZ resistance (38). To date,
mutations in TP53 (39), MGMT
(40,41), APEX1 (42), STAT3 (43), BCRP1 (44) and other genes have been reported to
result in TMZ resistance in patients with GBM. However, the state
of these genes in populations of patients with SCLC has been poorly
investigated. In the present study, the gene mutation data of 249
patients with SCLC and 520 patients with GBM available from the
cBioPortal website (http://www.cbioportal.org/) were compared. In
accordance with a previous study (45), TP53 and RB1 are the most commonly
mutated genes in patients with SCLC, and of note, TP53 and TTN are
among the top five commonly mutated genes in both patients with
SCLC and GBM. However, the resistance-related genes discussed above
all exhibited relatively low mutation rates in patients with these
two kinds of cancer (Table SII).
Although mutation of TP53 has been proven to affect the sensitivity
of GBM cells to TMZ in certain studies (37,46),
the results remain controversial. Hence, mutation of the TP53 gene
is not considered the primary indicator of resistance to TMZ.
Therefore, in terms of gene mutations, patients with SCLC may also
benefit from TMZ treatment.
TMZ has a favourable side effect profile; the most
common adverse reactions are mild, e.g., myelosuppression (anaemia
and fatigue) and gastrointestinal side effects (vomiting and
anorexia). However, TMZ has also been reported to induce severe
skin reactions (47), aplastic
anaemia (48) and organizing
pneumonitis (49). The occurrence
and severity of side effects limit the use of TMZ in clinical
applications. The dose and treatment course of TMZ, or its
combination with other medications, are key factors in the
occurrence of side effects. For instance, high-dose or long-term
use may lead to serious side effects such as neutropenia or
thrombocytopenia. The combined use of TMZ with radiotherapy may
exacerbate myelosuppression or skin reactions (50). However, no significant difference in
the incidence of side effects was observed between the PCI and
non-PCI groups of the present study, which may be a result of
separate administration of PCI and TMZ. In addition, a patient's
genetic background and health status determine the severity of the
side effects. Patients with an unmethylated MGMT promoter may be
more resistant to TMZ and require a higher dose (51). Elderly patients or patients with
impaired liver and kidney function have decreased drug metabolism
ability, which may lead to drug accumulation and increased risk of
toxicity (52). In the present
study, no difference was observed in age, sex, smoking, alcohol
consumption, diabetes mellitus, hypertension or medications between
the TMZ and non-TMZ groups. Therefore, the similar incidence of
side effects confirmed the safety of TMZ.
Since the present study was a single-centre study
with a limited number of patients, adjusting for the influence of
confounding factors through stratified analysis was impossible. For
instance, it was not possible to divide the TMZ group into patients
who received chemotherapy alone, radiotherapy alone or both
chemotherapy and radiotherapy. For the same reason, only the role
of adjuvant VDS treatment in combination with TMZ was investigated.
In addition, as it was a retrospective study, selection bias was
inevitable. The included patients were mostly low- and
middle-income patients who underwent mainly primary examinations
and chemotherapy and/or radiotherapy. Therefore, the roles of
genetic testing and immunotherapy in SCLC were not investigated. A
larger sample size including patients from multiple centres is
necessary to determine the effect of PCI on the sensitivity of
patients with SCLC to TMZ and other related risk factors.
In conclusion, because of its convenience and
safety, TMZ may be an alternative treatment for patients with SCLC
who have no access to hospitals (53), such as when hospital access was
limited due to the COVID-19 pandemic, to control lesions within or
outside of the brain.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 82203973 and 82073477), Sichuan
University-Luzhou Joint Scientific Innovation Project (grant no.
2021CDLZ-9), Sichuan Provincial Health Commission (grant nos.
20PJ226 and 21PJ159) and the Science & Technology Department of
Sichuan Province (grant nos. 2021YJ0204 and 2022JDJQ0051).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FG, SL, SZ and DH conceived and designed the study,
developed the protocol, defined inclusion criteria and obtained
ethical approval. SW, YG, ZH and FL recruited patients,
administered TMZ/VDS treatments and monitored adverse events. YG,
ZH and FJ coordinated TMZ acquisition, validated dosing protocols
and supervised data integrity. WL, RL, LL, FJ, ZH and CM collected
clinical records and imaging data and performed statistical
analyses. FG and FJ performed the literature review. FG, LL, SW, FJ
and FL drafted the initial manuscript. SZ and FJ revised the
manuscript. All authors have read and approved the final
manuscript. FG and SZ independently verified the authenticity of
the raw data.
Ethics approval and consent to
participate
This study was approved by the Human Research Ethics
Committee of PLA 81st Group Army Hospital (Zhangjiakou, China;
approval no. JTJYY-202501). Written informed consent was obtained
from all individual participants involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rudin CM, Ismaila N, Hann CL, Malhotra N,
Movsas B, Norris K, Pietanza MC, Ramalingam SS, Turrisi AT III and
Giaccone G: Treatment of Small-cell lung cancer: American society
of clinical oncology endorsement of the american college of chest
physicians guideline. J Clin Oncol. 33:4106–4111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Denninghoff V, Russo A, de Miguel-Pérez D,
Malapelle U, Benyounes A, Gittens A, Cardona AF and Rolfo C: Small
cell lung cancer: State of the art of the molecular and genetic
landscape and novel perspective. Cancers (Basel). 13:17232021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oronsky B, Reid TR, Oronsky A and Carter
CA: What's new in SCLC? A Review. Neoplasia. 19:842–884. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farago AF and Keane FK: Current standards
for clinical management of small cell lung cancer. Transl Lung
Cancer Res. 7:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tiseo M, Boni L, Ambrosio F, Camerini A,
Baldini E, Cinieri S, Brighenti M, Zanelli F, Defraia E,
Passalacqua R, et al: Italian, multicenter, phase III, randomized
study of cisplatin plus etoposide with or without bevacizumab as
First-line treatment in Extensive-Disease Small-Cell lung cancer:
The GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol. 35:1281–1287. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jalal SI, Lavin P, Lo G, Lebel F and
Einhorn L: Carboplatin and etoposide with or without palifosfamide
in untreated Extensive-stage Small-Cell lung cancer: A multicenter,
adaptive, randomized phase III study (MATISSE). J Clin Oncol.
35:2619–2623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans WK, Shepherd FA, Feld R, Osoba D,
Dang P and Deboer G: VP-16 and cisplatin as first-line therapy for
small-cell lung cancer. J Clin Oncol. 3:1471–147. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roth BJ, Johnson DH, Einhorn LH, Schacter
LP, Cherng NC, Cohen HJ, Crawford J, Randolph JA, Goodlow JL, Broun
GO, et al: Randomized study of cyclophosphamide, doxorubicin, and
vincristine versus etoposide and cisplatin versus alternation of
these two regimens in extensive small-cell lung cancer: A phase III
trial of the southeastern cancer study group. J Clin Oncol.
10:282–291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johal S, Hettle R, Carroll J, Maguire P
and Wynne T: Real-world treatment patterns and outcomes in
small-cell lung cancer: A systematic literature review. J Thorac
Dis. 13:3692–3707. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldman JW, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab, with or without tremelimumab, plus
Platinum-etoposide versus Platinum-etoposide alone in First-line
treatment of Extensive-stage Small-cell lung cancer (CASPIAN):
Updated results from a randomised, controlled, Open-label, phase 3
trial. Lancet Oncol. 22:51–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
Extensive-Stage Small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk
Y, Cuceviá B, Juhasz G, Thatcher N, Ross GA, Dane GC and Crofts T:
Phase III trial comparing supportive care alone with supportive
care with oral topotecan in patients with relapsed small-cell lung
cancer. J Clin Oncol. 24:5441–5447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baize N, Monnet I, Greillier L, Geier M,
Lena H, Janicot H, Vergnenegre A, Crequit J, Lamy R, Auliac JB, et
al: Carboplatin plus etoposide versus topotecan as Second-line
treatment for patients with sensitive relapsed small-cell lung
cancer: An Open-label, multicentre, randomised, phase 3 trial.
Lancet Oncol. 21:1224–1233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goto K, Ohe Y, Shibata T, Seto T,
Takahashi T, Nakagawa K, Tanaka H, Takeda K, Nishio M, Mori K, et
al: Combined chemotherapy with cisplatin, etoposide, and irinotecan
versus topotecan alone as second-line treatment for patients with
sensitive relapsed Small-cell lung cancer (JCOG0605): A
multicentre, Open-label, randomised phase 3 trial. Lancet Oncol.
17:1147–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zauderer M, Krug LM, Pietanza MC and
O'Rourke D: Leptomeningeal metastases from small cell lung cancer
responsive to temozolomide therapy. J Thorac Oncol. 5:1716–1717.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pietanza MC, Kadota K, Huberman K, Sima
CS, Fiore JJ, Sumner DK, Travis WD, Heguy A, Ginsberg MS, Holodny
AI, et al: Phase II trial of temozolomide in patients with relapsed
sensitive or refractory small cell lung cancer, with assessment of
methylguanine-DNA methyltransferase as a potential biomarker. Clin
Cancer Res. 18:1138–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zauderer MG, Drilon A, Kadota K, Huberman
K, Sima CS, Bergagnini I, Sumner DK, Travis WD, Heguy A, Ginsberg
MS, et al: Trial of a 5-day dosing regimen of temozolomide in
patients with relapsed small cell lung cancers with assessment of
methylguanine-DNA methyltransferase. Lung Cancer. 86:237–240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pietanza MC, Waqar SN, Krug LM, Dowlati A,
Hann CL, Chiappori A, Owonikoko TK, Woo KM, Cardnell RJ, Fujimoto
J, et al: Randomized, Double-blind, phase II study of temozolomide
in combination with either veliparib or placebo in patients with
Relapsed-sensitive or refractory Small-cell lung cancer. J Clin
Oncol. 36:2386–2394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lok BH, Gardner EE, Schneeberger VE, Ni A,
Desmeules P, Rekhtman N, de Stanchina E, Teicher BA, Riaz N, Powell
SN, et al: PARP inhibitor activity correlates with SLFN11
expression and demonstrates synergy with temozolomide in small cell
lung cancer. Clin Cancer Res. 23:523–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farago AF, Yeap BY, Stanzione M, Hung YP,
Heist RS, Marcoux JP, Zhong J, Rangachari D, Barbie DA, Phat S, et
al: Combination olaparib and temozolomide in relapsed Small-cell
lung cancer. Cancer Discov. 9:1372–1387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalemkerian GP, Akerley W, Bogner P,
Borghaei H, Chow LQ, Downey RJ, Gandhi L, Ganti AK, Govindan R,
Grecula JC, et al: Small cell lung cancer. J Natl Compr Canc Netw.
11:78–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmad I, Chufal KS, Gupta S and Bhatt CP:
Defining limited stage small cell lung cancer: A radiation
oncologist's perspective. BMJ Case Rep. 2018:bcr20172237082018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han J, Qiu M, Su L, Wu C, Cheng S, Zhao Z,
Li D, Wang M, Tao W and Du S: Response and safety of whole-brain
radiotherapy plus temozolomide for patients with brain metastases
of non-small-cell lung cancer: A meta-analysis. Thorac Cancer.
12:3177–3183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan H, Zheng SY, Zhou T, Cui HJ and Hu
KW: Temozolomide plus whole brain radiotherapy for the treatment of
non-small-cell lung cancer patients with brain metastases: A
protocol of an updated systematic review and meta-analysis.
Medicine (Baltimore). 99:e184552020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu L, Wang Y, Li L, Yu L, Liu L, Qu B and
Zhang X: 125I Radiotherapy combined with metronomic chemotherapy
may boost the abscopal effect, leading to complete regression of
liver metastasis in an SCLC patient with a 58.5-month OS: A case
report. Front Oncol. 13:9651662023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei J, Dong XF, Hu ZL, Tang S and Lu YF:
Successful treatment with temozolomide in an elderly woman with
advanced pulmonary Large-cell neuroendocrine carcinoma: A case
report. Medicine (Baltimore). 97:e133182018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Furuse K, Kubota K, Kawahara M, Takada M,
Kimura I, Fujii M, Ohta M, Hasegawa K, Yoshida K, Nakajima S, et
al: Phase II study of vinorelbine in heavily previously treated
small cell lung cancer. Japan Lung Cancer Vinorelbine Study Group.
Oncology. 53:169–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rapti A, Agelidou A, Stergiou I, Agelidou
M, Nikolakopoulos I, Varthalitis J, Kalykaki A, Chainis K, Tzanakis
N and Georgoulias V; Lung Cancer Committee of the Hellenic Oncology
Research Group (HORG), : Combination of vinorelbine plus
gemcitabine in previously treated patients with small cell lung
cancer: A multicentre phase II study. Lung Cancer. 49:241–244.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dudek AZ, Leśniewski-Kmak K, Bliss RL,
Brunstein C, Condon DL and Kratzke RA: Pilot phase II study of
gemcitabine and vinorelbine in patients with recurrent or
refractory small cell lung cancer. Lung. 183:43–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirose Y, Berger MS and Pieper RO:
Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates
temozolomide-induced toxicity in a p53-independent manner in human
glioblastoma cells. Cancer Res. 61:5843–5849. 2001.PubMed/NCBI
|
|
32
|
Günther W, Pawlak E, Damasceno R, Arnold H
and Terzis AJ: Temozolomide induces apoptosis and senescence in
glioma cells cultured as multicellular spheroids. Br J Cancer.
88:463–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Würstle S, Schneider F, Ringel F, Gempt J,
Lämmer F, Delbridge C, Wu W and Schlegel J: Temozolomide induces
autophagy in primary and established glioblastoma cells in an EGFR
independent manner. Oncol Lett. 14:322–328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou Y, Wang Q, Li B, Xie B and Wang W:
Temozolomide induces autophagy via ATM-AMPK-ULK1 pathways in
glioma. Mol Med Rep. 10:411–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld
SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O'Neill
AM, et al: Multicenter phase II trial of temozolomide in patients
with anaplastic astrocytoma or anaplastic oligoastrocytoma at first
relapse. Temodal Brain Tumor Group. J Clin Oncol. 17:2762–2771.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baumert BG, Hegi ME, van den Bent MJ, von
Deimling A, Gorlia T, Hoang-Xuan K, Brandes AA, Kantor G, Taphoorn
MJB, Hassel MB, et al: Temozolomide chemotherapy versus
radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): A
randomised, open-label, phase 3 intergroup study. Lancet Oncol.
17:1521–1532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SY: Temozolomide resistance in
glioblastoma multiforme. Genes Dis. 3:198–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu W, Klockow JL, Zhang M, Lafortune F,
Chang E, Jin L, Wu Y and Daldrup-Link HE: Glioblastoma Multiforme
(GBM): An overview of current therapies and mechanisms of
resistance. Pharmacol Res. 171:1057802021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Chen JX, Liu JP, You C, Liu YH and
Mao Q: Gain of function of mutant TP53 in glioblastoma: Prognosis
and response to temozolomide. Ann Surg Oncol. 21:1337–1344. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Norden AD, Lesser GJ, Drappatz J, Ligon
KL, Hammond SN, Lee EQ, Reardon DR, Fadul CE, Plotkin SR, Batchelor
TT, et al: Phase 2 study of Dose-intense temozolomide in recurrent
glioblastoma. Neuro Oncol. 15:930–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Binabaj MM, Bahrami A, ShahidSales S,
Joodi M, Joudi Mashhad M, Hassanian SM, Anvari K and Avan A: The
prognostic value of MGMT promoter methylation in glioblastoma: A
meta-analysis of clinical trials. J Cell Physiol. 233:378–386.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hudson AL, Parker NR, Khong P, Parkinson
JF, Dwight T, Ikin RJ, Zhu Y, Chen J, Wheeler HR and Howell VM:
Glioblastoma recurrence correlates with increased APE1 and
polarization toward an Immuno-suppressive microenvironment. Front
Oncol. 8:3142018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan MSY, Sandanaraj E, Chong YK, Lim SW,
Koh LWH, Ng WH, Tan NS, Tan P, Ang BT and Tang C: A STAT3-based
gene signature stratifies glioma patients for targeted therapy. Nat
Commun. 10:36012019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
George J, Lim JS, Jang SJ, Cun Y, Ozretić
L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al:
Comprehensive genomic profiles of small cell lung cancer. Nature.
524:47–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Egaña L, Auzmendi-Iriarte J, Andermatten
J, Villanua J, Ruiz I, Elua-Pinin A, Aldaz P, Querejeta A,
Sarasqueta C, Zubia F, et al: Methylation of MGMT promoter does not
predict response to temozolomide in patients with glioblastoma in
Donostia Hospital. Sci Rep. 10:184452020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Madan R and Goyal S: Temozolomide induced
cutaneous reaction. Neurol India. 70:435–443. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gilbar PJ, Pokharel K and Mangos HM:
Temozolomide-induced aplastic anaemia: Case report and review of
the literature. J Oncol Pharm Pract. 27:1275–1280. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maldonado F, Limper AH, Lim KG and Aubrey
MC: Temozolomide-associated organizing pneumonitis. Mayo Clin Proc.
82:771–773. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma W, Li N, An Y, Zhou C, Bo C and Zhang
G: Effects of temozolomide and radiotherapy on brain metastatic
tumor: A systematic review and Meta-analysis. World Neurosurg.
92:197–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gong L, Yin Y, Chen C, Wan Q, Xia D, Wang
M, Pu Z, Zhang B and Zou J: Characterization of EGFR-reprogrammable
Temozolomide-resistant cells in a model of glioblastoma. Cell Death
Discov. 8:4382022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Klotz U: Pharmacokinetics and drug
metabolism in the elderly. Drug Metab Rev. 41:67–76. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chow MC, Chambers P, Singleton G, Patel J,
Cooper S, Mythen C, Bautista-González E, Chisnall G, Djellouli N,
Thwaites B, et al: Global changes to the chemotherapy service
during the covid-19 pandemic. J Oncol Pharm Pract. 27:1073–1079.
2021. View Article : Google Scholar : PubMed/NCBI
|