Introduction
Over the past two decades, notable advancements in
the management of non-small cell lung cancer (NSCLC) have included
the identification of oncogenic-driven subtypes and the development
of targeted therapies (1). Among
these subtypes, epidermal growth factor receptor (EGFR) is the most
common driver mutation, present in ~51.4% of patients with lung
adenocarcinoma (LUAD) in the Asian population. The predominant EGFR
mutations, comprising exon 19 deletion (del) and exon 21 L858R
mutation, account for ≤90% of cases (2,3).
Furthermore, the introduction of EGFR-tyrosine kinase inhibitors
(TKIs) has substantially improved clinical outcomes, with overall
survival (OS) extending to 38.6 months in previously untreated
patients with EGFR-mutant advanced NSCLC (4). The standard first-line therapy for
patients with LUAD harboring EGFR mutations remains EGFR-TKIs
(5). However, the emergence of
resistance to EGFR-TKIs is inevitable, posing a marked therapeutic
challenge across all generations of these inhibitors (6). Existing guidelines recommended a
limited number of subsequent treatment options, such as
chemotherapy and angiogenesis inhibitors, which are associated with
modest limited clinical efficacy (7).
Furmonertinib is a newly developed irreversible
third-generation EGFR-TKI with a trifluoroethoxypyridine-based
molecular structure, designed to enhance target specificity and
therapeutic efficacy. Emerging studies and case reports indicate
that furmonertinib exerts favorable effects on patients previously
treated with other EGFR-TKIs or those harboring uncommon EGFR
mutations (8–10). Based on these findings, clinicians
are increasingly considering furmonertinib as a potential
therapeutic option in later-line therapy. Moreover, an increasing
number of patients with EGFR-mutated LUAD, particularly those who
have undergone multiple lines of therapy, are receiving
furmonertinib as a novel treatment strategy (9,11).
Therefore, the present study aimed to
retrospectively analyzed real-world data on furmonertinib use in
patients with EGFR-mutant advanced NSCLC, to assess its efficacy,
safety profile and potential predictors of treatment response after
multiple lines of therapy.
Materials and methods
Participants and study design
Data from patients with EGFR-mutant advanced LUAD
who received furmonertinib after the failure of multiple lines of
therapy at the China-Japan Friendship Hospital (Beijing, China)
from December 2021 to April 2024 were included. The final follow-up
was performed in July 2024. Inclusion criteria were as follows: i)
Histologically or cytologically confirmed unresectable locally
advanced or metastatic LUAD; ii) presence of EGFR mutations
confirmed through molecular testing; iii) failure of ≥2 prior lines
of treatment; iv) treatment with furmonertinib either as a
monotherapy or in combination with other anti-tumor treatments; and
v) presence of ≥1 measurable target lesion as defined by the
Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1
criteria (12). Exclusion criteria
were as follows: i) Duration of furmonertinib treatment of <1
month; ii) concurrent malignancies requiring active treatment; and
iii) unavailable or incomplete medical records. The present
retrospective study was approved by the Institutional Ethics Review
Committee of the China-Japan Friendship Hospital (approval no.
2024-KY-104) and was performed according to the principles of the
Declaration of Helsinki. Given the retrospective design of the
study, the requirement for written informed consent was waived.
Data collection
Data were extracted for each patient, including
clinical characteristics and treatment-related information. The
clinical characteristics included age, sex, surgical history,
histological subtype, EGFR mutation status at initial diagnosis,
clinical stage, presence of organ metastases (including pleura,
bone, lung, brain, leptomeninges, adrenal gland and liver), gene
mutation status before furmonertinib treatment and sites of disease
progression before initiating furmonertinib treatment.
Treatment-related information included previous administration of
EGFR-TKIs, time to initiation of furmonertinib, duration of
furmonertinib intake, use of combination therapies and occurrence
of adverse events (AEs).
Efficacy and safety assessments
Efficacy was assessed according to the RECIST v1.1
criteria. The primary efficacy was progression-free survival (PFS),
defined as the duration from initiation of furmonertinib to either
documented disease progression or death from any cause. Safety
assessments were performed using the National Cancer
Institute-Common Terminology Criteria for Adverse Events version
5.0 (13).
Statistical analysis
Statistical methods were selected according to the
data type. The Kolmogorov-Smirnov test was used to analyze the
normality of data distribution. Quantitative variables were
described as mean ± standard deviation or median (interquartile
range) according to their distribution (normal or non-normal,
respectively). Categorical variables are presented as n (%).
Kaplan-Meier survival curves were used to estimate the PFS, with
differences in survival distributions assessed using the log-rank
test. The median PFS (in months) and the corresponding 95%
confidence interval (CI) were reported. All statistical analyses
were performed using R software (version 4.0.3; The R Foundation).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics
The present study was a retrospective study
involving 25 patients with EGFR-mutant advanced LUAD who received
furmonertinib following the failure of multiple lines of therapy.
The participants were treated at the China-Japan Friendship
Hospital, between December 2021 and April 2024. The median age was
62.92±12.56 years and 60.0% of the included patients were female.
Additionally, 28.0% had a history of surgery and 96.0% were
diagnosed with stage IV disease. The pathological subtype for all
patients was adenocarcinoma. The most common sites of metastasis
included the lung (52.0%), pleura (44.0%), brain (40.0%) and bone
(40.0%). Further clinical assessments revealed that between the
predominant EGFR mutations, 15 (60.0%) patients harbored exon
19del, whilst 10 (40.0%) patients harbored exon21 L858R mutations.
Furthermore, 14 patients (56.0%) underwent genetic testing before
receiving furmonertinib therapy, with the results revealing a
diverse range of co-mutations, such as FGFR3/RB1, KRAS, MET,
MET/18E709K, T790M and TP53.
The median number of prior lines of therapy before
initiating furmonertinib treatment, either as a monotherapy or in
combination with other therapies, was 3 (interquartile range, 2–4).
Notably, one patient had undergone up to seven lines of systemic
treatment before furmonertinib. Furmonertinib monotherapy was the
most common (76.0%) treatment option. Among patients who received
combination therapy, three patients (12.0%) underwent radiotherapy
directed at the brain, two patients (8.0%) underwent chemotherapy,
involving pemetrexed and carboplatin, and one patient (4.0%)
underwent a combination of targeted therapy and chemotherapy. In
terms of dosage, six patients were administered a double dose (160
mg), whilst 19 patients received a conventional dose (80 mg). The
baseline demographics of the study population are presented in
Table I.
 | Table I.Clinical characteristics of patients
with advanced lung adenocarcinoma who received furmonertinib after
multiline therapy failure (n=25). |
Table I.
Clinical characteristics of patients
with advanced lung adenocarcinoma who received furmonertinib after
multiline therapy failure (n=25).
| Characteristic | Value |
|---|
| Age, years | 62.92±12.56 |
| Sex |
|
|
Female | 15 (60.0) |
|
Male | 10 (40.0) |
| Surgical
history |
|
|
Yes | 7 (28.0) |
| No | 18 (72.0) |
| Histological
type |
|
|
Adenocarcinoma | 25 (100.0) |
|
Other | 0 (0.0) |
| Clinical stage
before furmonertinib |
|
|
IIIB | 1 (4.0) |
|
IVA | 13 (52.0) |
|
IVB | 11 (44.0) |
| Organ
metastasis |
|
| Adrenal
glands | 2 (8.0) |
|
Bone | 10 (40.0) |
|
Brain | 10 (40.0) |
|
Leptomeninges | 3 (12.0) |
|
Lung | 13 (52.0) |
|
Liver | 2 (8.0) |
|
Pleura | 11 (44.0) |
| EGFR mutation
status at diagnosis |
|
| Exon
19del | 15 (60.0) |
| Exon21
L858R | 10 (40.0) |
| Combined mutations
at diagnosis |
|
|
Unknown | 23 (92.0) |
|
KRAS | 1 (4.0) |
|
T790M | 1 (4.0) |
| Genetic testing
before furmonertinib |
|
|
Yes | 14 (56.0) |
| No | 11 (44.0) |
| EGFR mutation
status before furmonertinib |
|
| Exon
19del | 8 (57.1) |
| Exon21
L858R | 4 (28.6) |
| No
mutation | 2 (14.3) |
| Combined
mutations |
|
|
FGFR3/RB1 | 1 (7.7) |
|
KRAS | 1 (7.7) |
|
MET | 1 (7.7) |
|
MET/18E709K | 1 (7.7) |
|
T790M | 3 (23.1) |
|
TP53 | 1 (7.7) |
| No | 5 (38.5) |
| First-generation
EGFR-TKIs |
|
|
Gefitinib | 10 (40.0) |
|
Icotinib | 7 (28.0) |
| No
treatment | 9 (32.0) |
| Second-generation
EGFR-TKIs |
|
|
Afatinib | 3 (12.0) |
|
Dacomitinib | 2 (8.0) |
| No
treatment | 20 (80.0) |
| Third-generation
EGFR-TKIs |
|
|
Almonertinib | 1 (4.0) |
|
Osimertinib | 17 (68.0) |
|
Rezivertinib | 1 (4.0) |
| No
treatment | 6 (24.0) |
| Combination
therapy |
|
|
Monotherapy | 19 (76.0) |
|
Chemotherapy | 2 (8.0) |
|
Radiotherapy | 3 (12.0) |
|
Targeted + chemotherapy | 1 (4.0) |
| Treatment line | 3.00
(2.00–4.00) |
| Medication
dosage |
|
| Normal
dose (80 mg) | 19 (76.0) |
| Double
dose (160 mg) | 6 (24.0) |
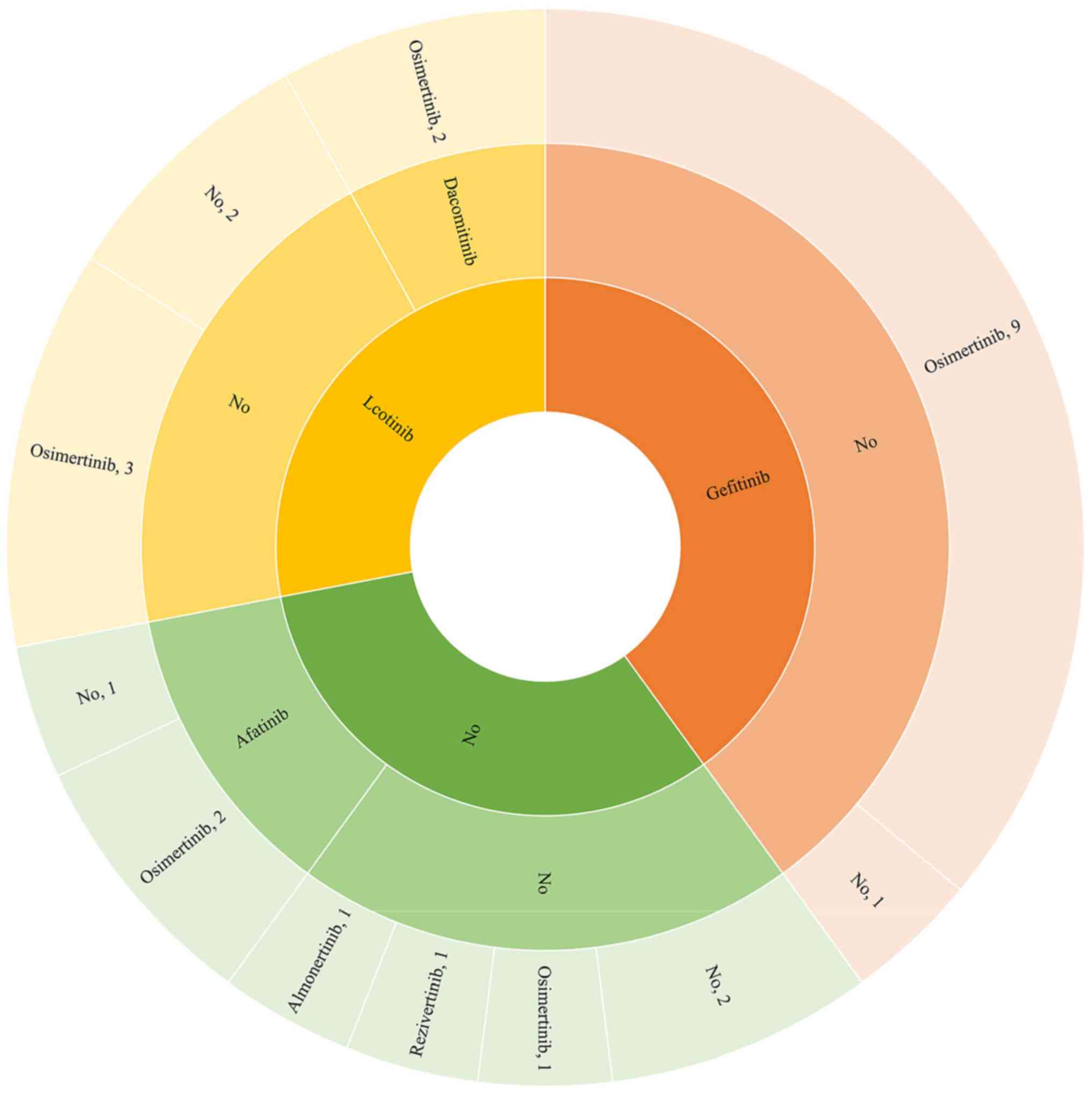
A summary of previous treatments with EGFR-TKIs for
patients receiving furmonertinib is presented in Table I and Fig. 1. Records indicated that 23 patients
(92.0%) had previously received EGFR-TKI treatment. Specifically,
17 patients had previously received first-generation EGFR-TKIs, 5
had received second-generation, and 19 had received
third-generation. Among these EGFR-TKI generations, gefitinib
(n=10; 40.0%), afatinib (n=3; 12.0%) and osimertinib (n=17; 68.0%)
were the most common treatments, respectively. Notably, the records
revealed that prior to furmonertinib therapy, a marked proportion
of patients (n=12) had received both first- and third-generation
EGFR-TKIs. First-generation EGFR-TKIs were administered to three
patients, second-generation EGFR-TKIs to one and third-generation
EGFR-TKIs to three. Additionally, two patients received sequential
treatment with EGFR-TKIs, spanning from the first to the third
generation. Furthermore, two patients had no history of prior
treatment involving EGFR-TKIs.
Survival analysis
Using a data cut-off of July 2024, disease
progression or death was observed in 16 patients (64.0%), whilst 9
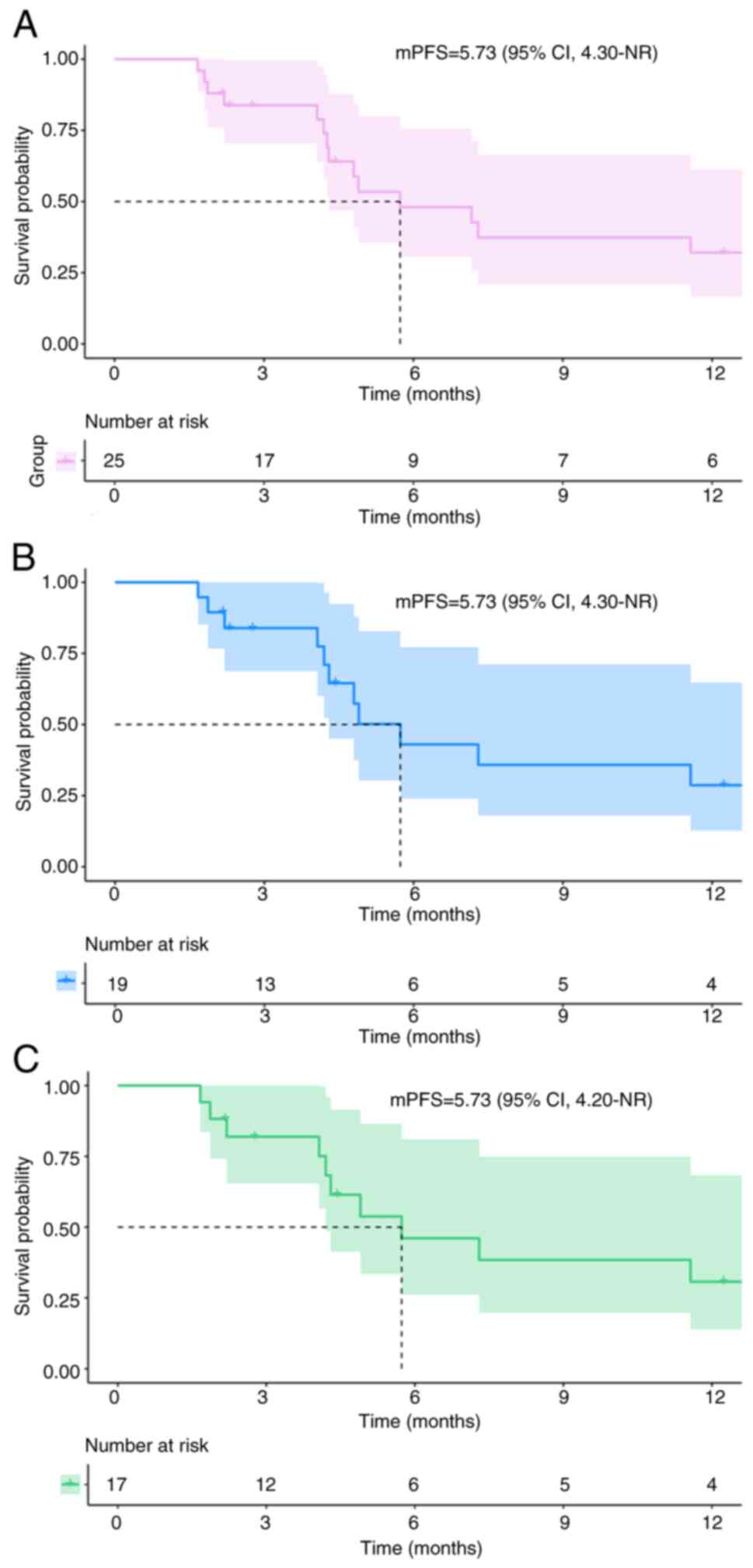
patients (36.0%) remained progression-free. The median (m)PFS was
5.73 months [95% CI, 4.30-not reached (NR)] for the 25 patients
with EGFR-mutant advanced NSCLC (Fig.
2A). Moreover, among the 25 patients included in the present
study, the best response was partial response (PR) in 4 patients
(16.0%), stable disease (SD) in 18 patients (72.0%) and progressive
disease in 3 patients (12.0%), with no patient achieving a complete
response. The objective response rate was 16.0% (n=4) and the
disease control rate was 88.0% (n=22). Notably, one patient who
received four cycles of furmonertinib combined with chemotherapy,
followed by furmonertinib monotherapy as maintenance, achieved
sustained SD for ~30.3 months. Furthermore, in the subgroup of 19
patients who had previously developed resistance to
third-generation EGFR-TKIs, the median PFS was also 5.73 months
(95% CI, 4.30-NR; Fig. 2B). Among
these patients, 17 had previously received treatment with
osimertinib. Similarly, the median PFS for this subgroup remained
at 5.73 months (95% CI, 4.20-NR; Fig.
2C). Only nine patients reached the mortality endpoint for OS,
and four were lost to follow-up by the cut-off date. Consequently,
the survival rate was 57.1%. However, the OS data are not yet
mature enough for analysis.
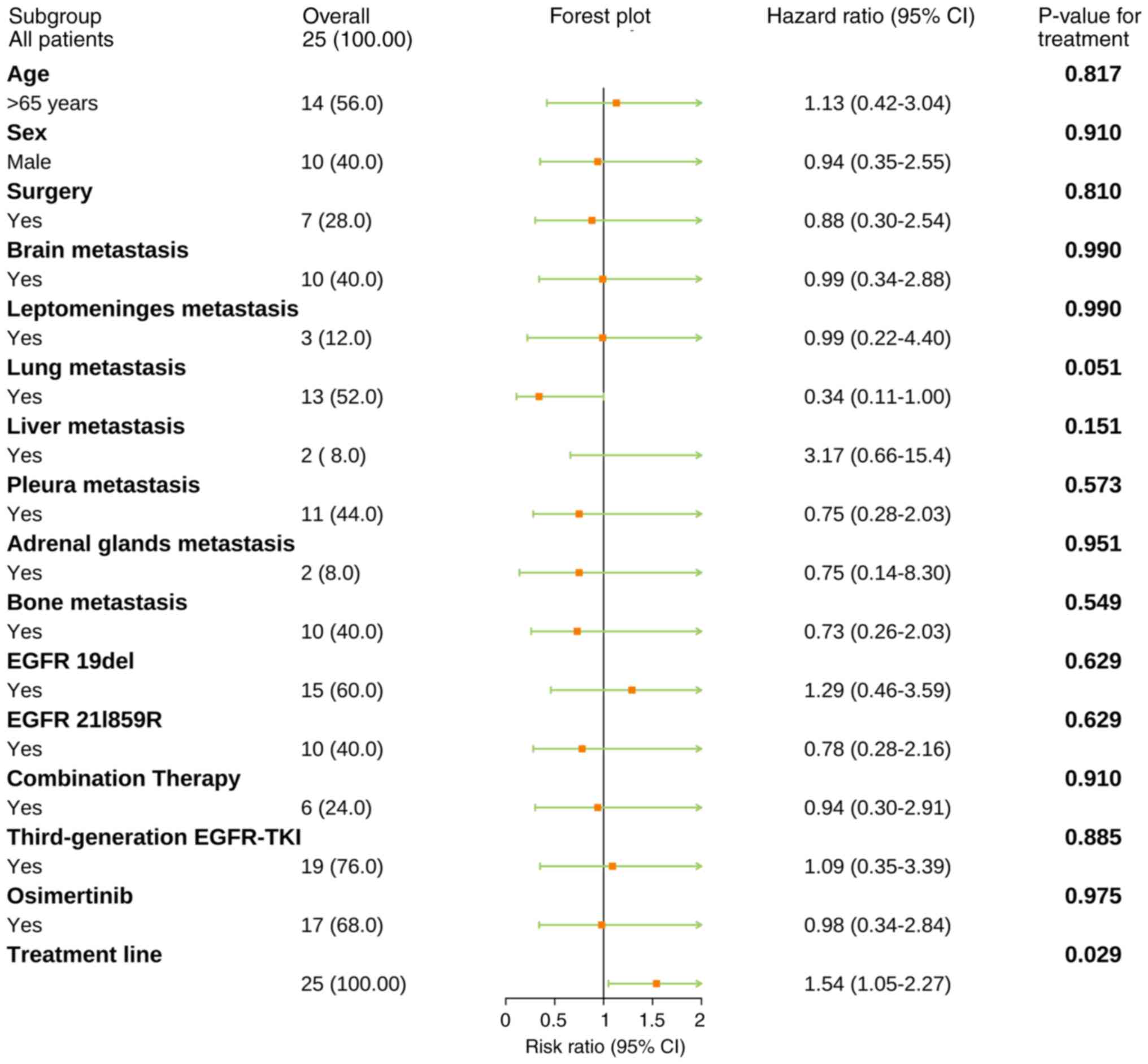
Subgroup analysis
The present study aimed to assess whether certain
clinical characteristics influence the efficacy of furmonertinib as
a subsequent treatment. To achieve this, subgroup analyses were
performed based on clinical characteristics, EGFR mutation status
at initial diagnosis and previous treatment agents. The results
indicated that the number of treatment lines may be a significant
negative prognostic factor [hazard ratio (HR)=1.54; P=0.029].
However, the reliability of these findings is limited by the
relatively small sample size. Beyond this observation, the results
did not reveal any other significant differences in the subgroup
analyses (Fig. 3).
To validate the aforementioned findings, a
sensitivity analysis was performed, excluding patients who received
combination therapy (Table SI).
Additionally, Bonferroni's correction was applied to the
multiplicity test. However, the association between the number of
treatment line and survival outcome did not remain significant
after adjustment for multiple comparisons.
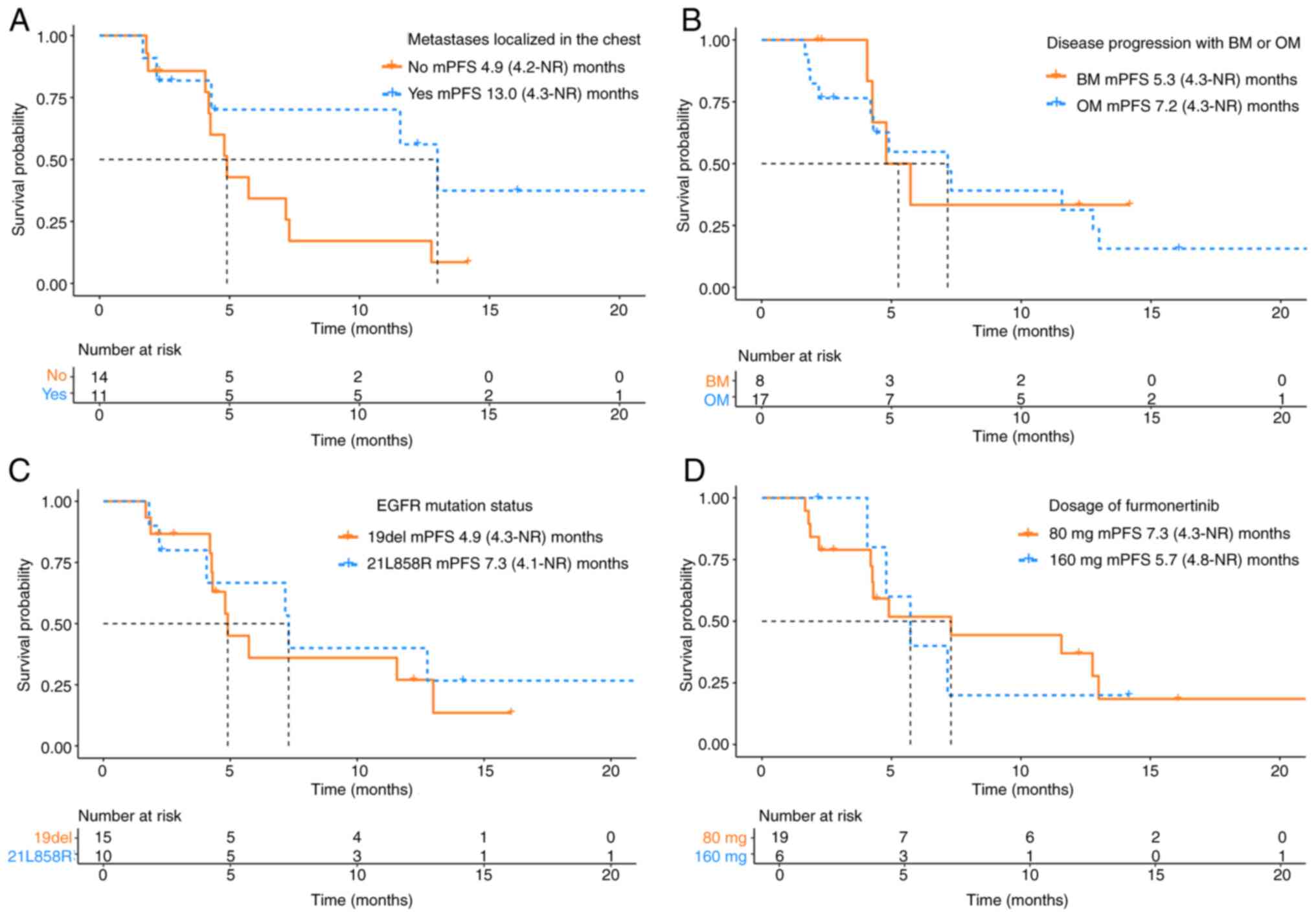
Furthermore, to evaluate potential prognostic
factors influencing the efficacy of furmonertinib, subgroup
analyses were performed considering the influence of distant organ
metastases and other clinical factors. The patients were classified
into four groups based on the existing published literature
(9,14) and clinical experience: i) Based on
the metastatic site, patients were divided into two groups based on
whether the metastatic lesions were localized in the chest region.
The results revealed that patients with metastasis localized in the
chest region had notably longer mPFS compared with those with extra
chest metastases (4.9 vs. 13.0 months; HR=0.43; 95% CI, 0.16–1.15;
P=0.102; Fig. 4A); ii) based on the
mode of disease progression before furmonertinib initiation,
patients were categorized into brain metastases (BM) and other
metastases (OM) groups. Individuals in the OM group exhibited
markedly longer mPFS compared with those in the BM group (5.3 vs.
7.2 months; HR=1.26; 95% CI, 0.43–3.69; P=0.687; Fig. 4B); iii) based on the EGFR mutation
subtype, patients with EGFR exon21 L858R mutation exhibited notably
longer mPFS compared with those with EGFR 19del (4.9 vs. 7.3
months; HR=0.78; 95% CI, 0.29–2.10; P=0.628; Fig. 4C); and iv) based on furmonertinib
dosage, patients who received 80 mg had markedly longer mPFS
compared with those receiving 160 mg (7.3 vs. 5.7 months; HR=1.02;
95% CI, 0.33–3.19; P=0.968; Fig.
4D). However, there was no statistically significant difference
in survival for these subgroups.
Safety
A total of 18 patients (72.0%) experienced at least
one AE over the treatment course. Among these patients, four
received combination therapy, while 14 received monotherapy with
furmonertinib. A total of 25 AEs were reported across 16 distinct
categories, with an 80.0% rate (n=20) in grade 1–2 and 20.0% (n=5)
in grade 3–4. The incidence of AEs was 72.0% (n=18) in the
monotherapy group and 28.0% (n=7) in the combination therapy group.
The incidence of suspected drug-related AEs was 48% (n=12). The
majority of AEs (17/25; 68.0%) were managed with supportive care.
All grade 3–4 AEs were treated accordingly. Notably, no AEs
required dose reductions or treatment discontinuation, which may be
due to the conventional application of supportive care measures.
The distribution of AEs is presented in Table II.
 | Table II.Adverse events in patients who
received furmonertinib monotherapy or combination therapy as
subsequent treatment (n=25). |
Table II.
Adverse events in patients who
received furmonertinib monotherapy or combination therapy as
subsequent treatment (n=25).
|
| Monotherapy | Combination
therapy |
|---|
|
|
|
|
|---|
| Adverse event | Grade 1–2
(n=16) | Grade 3–4
(n=2) | Grade 1–2
(n=4) | Grade 3–4
(n=3) |
|---|
| Cough | 2 (8.0) | - | - | - |
| Diarrhea | 1
(4.0)a | - | - | 1
(4.0)a |
| Dizziness | 1 (4.0) | - | - | - |
| Dry eye | 2
(8.0)a | - | - | - |
| Dry skin | - | - | 1
(4.0)a | - |
| Fatigue | 2 (8.0) | - | - | - |
| Headache | 1 (4.0) | - | - | - |
| Hemoptysis | 1 (4.0) | - | - | - |
| Mucositis oral | 1
(4.0)a | - | - | - |
| Nail changes | - | - | 1
(4.0)a | - |
| Neutrophil count
decreased | - | - | 1
(4.0)a | - |
| Pain | 1 (4.0) | 1 (4.0) | 1 (4.0) | - |
| Palpitations | 1
(4.0)a | - | - | - |
| Paronychia | 1
(4.0)a | - | - | 1 (4.0) |
| Shingles | 1 (4.0) | 1 (4.0) | - | - |
| Decreased white
blood cell count | 1
(4.0)a | - | - | 1
(4.0)a |
Discussion
The present retrospective study summarized the
efficacy and safety profiles of furmonertinib therapy in patients
with EGFR-mutant advanced LUAD following the failure of multiple
lines of therapy. The majority of patients harboring the
EGFR-sensitizing mutations are inevitably faced with the challenge
of receiving effective subsequent treatment options after the
failure of multiple-line therapies. Notably, furmonertinib therapy
was initiated in most patients as a subsequent treatment option
after multiple progression of the disease. Furmonertinib is a
third-generation EGFR-TKI designed to target both the
EGFR-sensitizing mutations and the T790M resistance mutation. It
was independently developed in China (15). Additionally, furmonertinib has
exhibited promising efficacy and an acceptable safety profile in
patients with central nervous system (CNS) metastases (16). It has been approved as the
first-line treatment for patients with LUAD harboring
EGFR-sensitizing mutations. Clinical data indicate that
furmonertinib can prolong PFS to 20.8 months, exceeding the PFS
observed with other EGFR-TKIs (17). Additionally, a real-world
retrospective study reported an mPFS of 19.5 months in patients
with NSCLC with EGFR mutations who received furmonertinib as the
initial therapy (18).
Furmonertinib has also demonstrated a survival
advantage as a salvage therapy. A retrospective study involving 39
patients with advanced NSCLC who had developed resistance to
third-generation EGFR-TKIs reported that 160 mg furmonertinib was a
viable treatment option, with an mPFS of 4.70 months and OS of 7.73
months (9). In the present study,
the median PFS was 5.73 months in patients with EGFR-mutant
advanced LUAD, with one patient achieving sustained SD for ~30.3
months. Notably, among the 19 patients previously treated with
third-generation EGFR-TKIs, the mPFS remained at 5.73 months.
Whilst patients in the present study received several previous
lines of systemic therapy, only nine patients reached the mortality
endpoint and four were lost to follow-up at the cut-off date,
rendering the analysis of the OS data immature. Future studies with
longer follow-up periods are warranted to confirm these initial
observations and address potential attrition bias.
Treatment options are limited for patients with LUAD
harboring EGFR-sensitizing mutations after failure of
multiple-lines of therapy. In clinical practice, patients usually
receive platinum-based doublet chemotherapy, either as a
monotherapy or in combination with anti-angiogenic therapy, with
the observed mPFS being ~5 months after treatment (19,20).
The advancement of immune checkpoint inhibitors has led to a
paradigm shift in the management of NSCLC. However, previous
studies reported that patients harboring the EGFR mutation who
received immunotherapy exhibited suboptimal clinical outcomes, with
an mPFS ranging between 1.3–2.1 months (21,22);
however, improvement in the outcomes was observed in those patients
who received immunotherapy in combination with other treatment
options. Specifically, this combination treatment resulted in an
mPFS ranging from 3.6–7.6 months (23,24).
Moreover, treatment involving sunvozertinib yielded an mPFS of 5.9
months in patients with NSCLC with EGFR-sensitizing mutations
following the failure of EGFR-TKIs treatment (25). A meta-analysis assessing the
efficacy of immunotherapy in combination with chemotherapy along
with antiangiogenic therapy suggested that this approach conferred
notably superior survival benefits on PFS in patients with NSCLC
with EGFR mutations, especially those who had previously undergone
EGFR-TKI therapy (26). However,
these combination therapies also exhibit an elevated risk of
toxicity, thereby exacerbating the treatment burden (23,26).
By contrast, furmonertinib therapy exhibits a superior efficacy and
safety profile, and an optimal treatment burden with an mPFS of
5.73 months in this study. Meanwhile, Ding et al (11) reportedthat furmonertinib may be a
promising prospect to treat the vast majority activating
EGFR-mutant NSCLC. However, existing clinical studies have failed
to perform comparative analyses of furmonertinib with chemotherapy
or immunotherapy in patients with EGFR-mutant advanced NSCLC who
progressed on EGFR-TKI treatment, to the best of our knowledge.
Furmonertinib exhibits favorable clinical activity
in patients with BM due to its ability to penetrate the blood-brain
barrier. Notably, a previous study reported that furmonertinib
levels in the brain were higher compared with those in the plasma
(27). The FURLONG Study reported
that CNS PFS was 20.8 months in the furmonertinib group and 9.8
months in the gefitinib group (HR=0.40; P=0.001) (16). Another study reported that high-dose
furmonertinib in combination with intraventricular chemotherapy as
salvage treatment for patients with leptomeningeal metastases (LM)
harboring EGFR ex20ins mutations had promising clinical benefits
and a modest safety profile (8).
Additionally, Hu et al (28)
performed a pooled analysis of two Phase II studies and reported
that furmonertinib exhibited promising CNS efficacy in patients
with EGFR T790M-mutated NSCLC when administered orally at a dose of
≥80 mg once daily. These findings indicate that furmonertinib
exhibits promising potential in treating CNS metastases. However,
its clinical advantages in the BM population have not been
validated in this study.
Patients with tumors localized to the lung may
represent a population that could potentially benefit from
furmonertinib treatment as the clinical efficacy of furmonertinib
may be associated with its pharmacological properties. In a study
where a single oral dose of [14C]-furmonertinib was administered to
rats, lung tissue had the highest levels of radioactivity, with the
total radioactivity concentration ~100-fold greater than that in
the plasma (29). In the present
study, patients with metastasis localized in the chest exhibited a
prolonged mPFS. Moreover, these findings indicate that patients
harboring the EGFR exon 21 L858R mutations had longer mPFS, which
was a favorable outcome for this subgroup. Notably, previous
studies have suggested that patients with the EGFR L858R mutation
typically have a worse PFS compared with those with exon 19del
(4,30,31).
Additionally, in the present study, subgroup analysis suggested
that a higher number of prior treatment lines may be a negative
prognostic factor. However, the sample size in the subgroup
analysis was too small, thereby limiting the reliability of these
findings. Consequently, large-scale studies are needed to validate
these findings these trends.
Furmonertinib exhibits a superior clinical safety
profile and a wider dosage range compared with other EGFR-TKIs
(11). According to previously
reported data, treatment-related AEs of grade ≥3 occurred in 11% of
178 patients with NSCLC who received 80 mg furmonertinib once daily
as first-line therapy (17).
Regarding the toxicity of high-dose furmonertinib (dosages of ≥160
mg), existing studies suggest that it exhibits a favorable
tolerability profile. Xu et al (14) enrolled 28 patients with advanced
NSCLC with BM/LM and administered 160 mg furmonertinib, either as a
monotherapy or in combination with anti-angiogenic agents. The
results indicated that 14.3% of these patients (n=4) who had grade
≥3 AEs experienced controlled outcomes with no dose reductions or
therapeutic suspension (14).
Another prospective real-world study involving 48 patients with
EGFR-mutated NSCLC and LM, who were administered a high-dose
furmonertinib (240 mg once daily), either as a monotherapy or in
combination with other treatment agents, reported that 22 (45.8%)
had AEs possibly related to furmonertinib, and 3 (6.3%) had a grade
3 AEs leading to a dose reduction to 160 mg daily (32). In summary, a double dose of
furmonertinib (160 mg) exhibits a favorable tolerability profile,
and this observation extends to a daily dose of up to 240 mg, where
the response is both safe and tolerable for patients with NSCLC
(27). The aforementioned studies
demonstrate that a higher dose of furmonertinib may have a greater
efficacy with a favorable safety profile compared with the
conventional dose. In the present study, 76% of patients received a
conventional dose of furmonertinib, with no AEs observed in
association with dose reductions or discontinuation, which may be
attributed to the implementation of conventional supportive care
measures. Additionally, the present study assessed the
dose-response relationship and demonstrated that the group
administered 80 mg furmonertinib had longer mPFS; however, this
observation was not statistically significant.
The findings regarding genetic testing must be
interpreted with caution. First, the proportion of patients who
underwent repeat genetic testing was relatively small, which may be
attributed to limiting factors such as the high cost and
availability of biopsy specimens. Specifically, only 14 patients
(56.0%) underwent genetic testing prior to furmonertinib,
potentially introducing selection bias. Untested patients may
harbor undetected driver mutations. They were highly likely to
exhibit drug-resistant mutations, such as MET amplification, EGFR
C797S and TP53 mutations (33,34).
These could confound the observed association between genetic
profiles and treatment outcomes, leading to an underestimation of
the efficacy of furmonertinib in the present study. Secondly, the
majority of specimens for genetic testing were peripheral blood
samples rather than tissue specimens, which inherently exhibit a
high false-negative rate (35,36).
These findings present a common clinical obstacle in the subsequent
treatment of patients with cancer. With an extended treatment
period, the proportion of genetic mutations also increases
(37), which may lead to
underestimation of targetable mutations and misclassification of
true responders. Ultimately, as patients progress to the terminal
phase, they may opt for essential genetic testing rather than
comprehensive genomic profiling. Consequently, future studies
should require tissue-based next-generation sequencing (NGS) to
mitigate the influence of potential confounding factors associated
with co-mutations and to improve the detection of more potential
therapeutic targets to guide clinical decisions.
Currently, studies focusing on furmonertinib
involving specific gene mutations in LUAD can be divided into two
categories. First, furmonertinib has been explored in patients with
EGFR ex20ins with an encouraging antitumor activity (38). In multiple retrospective studies,
furmonertinib demonstrated promising antitumor and CNS activity in
patients with advanced NSCLC with EGFR ex20ins (8,39,40). A
phase-III trial of furmonertinib vs. chemotherapy as first-line
treatment for advanced NSCLC with EGFR ex20ins mutations
(FURMO-004) is ongoing (41).
Additionally, furmonertinib has demonstrated clinical efficacy in
patients with uncommon EGFR mutations, such as L861Q/G719X
(42), exon 18 (G719X) and 20
(S768I) (10), EGFR kinase domain
duplication (EGFR-KDD) (43), exon
20 R776S, C797S, exon 21 L858R compound EGFR mutations (44) and HER2 T8962A and L869R (45). These suggest that furmonertinib may
have suppressive effects on EGFR resistance mutations; however, the
clinical evidence primarily stems from case reports.
The present study has certain limitations. Firstly,
the sample size included is relatively small to ensure the
generalizability of the study findings. The findings from the
subgroup analyses should serve as exploratory foundations rather
than definitive due to the inherent limitations of the sample size.
In the future, multicenter and prospective studies should be
performed, expanding the sample size to acquire higher-level
evidence, thereby clearly identifying the patient population that
derives clinical benefit from furmonertinib therapy. Secondly, the
immature OS data, involving only 9 mortality events, limits the
robustness of the survival analysis and the ability to establish
definitive conclusions on the long-term outcomes. The low event
rate notably reduces the statistical power to detect clinically
meaningful survival differences. Furthermore, the loss of four
patients to follow-up introduces the potential for attrition bias.
These limitations underscore the necessity of future studies with
extended follow-up periods to validate the preliminary findings of
the present study. Ultimately, not all patients included underwent
genetic testing before treatment with furmonertinib. Most patients
had only a single gene mutation, and EGFR mutational status varied.
Therefore, establishing an association between available genetic
data and treatment outcomes was challenging. This highlights the
fact that the current level of genetic testing availability is
insufficient for clinicians. To identify actionable targets and
address the effects of potential co-mutation confounders, future
studies should incorporate tissue-based NGS for more comprehensive
molecular profiling.
In conclusion, furmonertinib is a viable treatment
option for patients with EGFR-mutant advanced LUAD following the
failure of multiple lines of therapy, even after resistance to
third-generation EGFR-TKIs targeted agents. The clinical
application of furmonertinib is continuously advancing from
first-line to subsequent treatment. In the future, clinical
research with a large sample size is needed to enhance the
robustness and generalizability of the findings of the present
study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was funded by the National High-Level Hospital
Clinical Research Funding (grant no. 2022-NHLHCRF-LX-02-0111).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ and HC designed the study. YZ and XL were
responsible for the writing of the original draft of the manuscript
and data analyses. JL, HD, YS and ZL contributed to data
collection, data analyses and manuscript preparation. YZ and HC
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Institutional Ethics Review Committee of the China-Japan Friendship
Hospital (approval no. 2024-KY-104). The requirement for informed
consent for participation was waived due to the retrospective
nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ding PN, Becker TM, Bray VJ, Chua W, Ma
YF, Lynch D, Po J, Luk AWS, Caixeiro N, de Souza P and Roberts TL:
The predictive and prognostic significance of liquid biopsy in
advanced epidermal growth factor receptor-mutated non-small cell
lung cancer: A prospective study. Lung Cancer. 134:187–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi Y, Au JSK, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gou LY and Wu YL: Prevalence of driver
mutations in non-small-cell lung cancers in the People's Republic
of China. Lung Cancer (Auckl). 5:1–9. 2014.PubMed/NCBI
|
|
4
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reckamp KL: Targeted therapy for patients
with metastatic non-small cell lung cancer. J Natl Compr Canc Netw.
16((5S)): S601–S604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Wang H and He C: Drug resistance
of targeted therapy for advanced non-small cell lung cancer
harbored EGFR mutation: From mechanism analysis to clinical
strategy. J Cancer Res Clin Oncol. 147:3653–3664. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu C, Xu T, Fang H, Wang X, Liu N, Yang L
and Fang S: High-dose furmonertinib combined with intraventricular
chemotherapy as salvage therapy for leptomeningeal metastasis from
EGFR exon 20 insertion-mutated lung cancer. J Neurooncol.
169:203–213. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi R, Fu X, Yu Y, Xu H, Shen M, He S and
Lv D: Efficacy and safety of re-challenging 160 mg furmonertinib
for advanced NSCLC after resistance to third-generation EGFR-TKIs
targeted agents: A real-world study. Lung Cancer. 184:1073462023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan X and Shi M: Successful therapy of a
critically ill non-small cell lung cancer patient with compound
mutations in EGFR G719X and S768I genes using furmonertinib: A case
report. Heliyon. 10:e271062024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding J, Ding X, Zeng J and Liu X:
Furmonertinib for EGFR-mutant advanced non-small cell lung cancer:
A glittering diamond in the rough of EGFR-TKI. Front Pharmacol.
15:13579132024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuchida Y and Therasse P: Response
evaluation criteria in solid tumors (RECIST): New guidelines. Med
Pediatr Oncol. 37:1–3. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events (CTCAE). Version 5.0.
2017.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf
|
|
14
|
Xu Z, Hao X, Wang Q, Yang K, Li J and Xing
P: Intracranial efficacy and safety of furmonertinib 160 mg with or
without anti-angiogenic agent in advanced NSCLC patients with BM/LM
as salvage therapy. BMC Cancer. 23:2062023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Y, Hu X, Zhang S, Lv D, Wu L, Yu Q,
Zhang Y, Liu L, Wang X, Cheng Y, et al: Efficacy, safety, and
genetic analysis of furmonertinib (AST2818) in patients with EGFR
T790M mutated non-small-cell lung cancer: A phase 2b, multicentre,
single-arm, open-label study. Lancet Respir Med. 9:829–839. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y,
Liu C, Zhu S, Zhang X, Li Y, et al: Central nervous system efficacy
of furmonertinib (AST2818) versus gefitinib as first-line treatment
for egfr-mutated NSCLC: Results from the FURLONG study. J Thorac
Oncol. 17:1297–1305. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y,
Liu C, Zhu S, Zhang X, Li Y, et al: Furmonertinib (AST2818) versus
gefitinib as first-line therapy for Chinese patients with locally
advanced or metastatic EGFR mutation-positive non-small-cell lung
cancer (FURLONG): A multicentre, double-blind, randomised phase 3
study. Lancet Respir Med. 10:1019–1028. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan N, Guo S, Huang S, Zhang H and Li X:
The efficacy of furmonertinib in untreated advanced NSCLC patients
with sensitive EGFR mutations in a real-world setting: A single
institutional experience. Front Oncol. 14:13311282024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida T, Kuroda H, Oya Y, Shimizu J,
Horio Y, Sakao Y, Hida T and Yatabe Y: Clinical outcomes of
platinum-based chemotherapy according to T790M mutation status in
EGFR-positive non-small cell lung cancer patients after initial
EGFR-TKI failure. Lung Cancer. 109:89–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park S, Keam B, Kim SH, Kim KH, Kim YJ,
Kim JS, Kim TM, Lee SH, Kim DW, Lee JS and Heo DS: Pemetrexed
singlet versus nonpemetrexed-based platinum doublet as second-line
chemotherapy after first-line epidermal growth factor receptor
(EGFR) tyrosine kinase inhibitor failure in non-small cell lung
cancer patients with EGFR mutations. Cancer Res Treat. 47:630–637.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Yang L, Yang Y, Yang G, Xu H, Niu
X and Wang U: The efficacy and safety of chemo-free therapy in
epidermal growth factor receptor tyrosine kinase
inhibitor-resistant advanced non-small cell lung cancer: A
single-arm, phase II study. Cancer Med. 12:19438–19448. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haratani K, Hayashi H, Tanaka T, Kaneda H,
Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et
al: Tumor immune microenvironment and nivolumab efficacy in EGFR
mutation-positive non-small-cell lung cancer based on T790M status
after disease progression during EGFR-TKI treatment. Ann Oncol.
28:1532–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu L, Hu Y, Xu J, Qiao R, Zhong H, Han B,
Xia J and Zhong R: Multi-target angiogenesis inhibitor combined
with PD-1 inhibitors may benefit advanced non-small cell lung
cancer patients in late line after failure of EGFR-TKI therapy. Int
J Cancer. 153:635–643. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu X, Li J, Ye L, Zhao J, Xie M, Zhou J,
Shen Y, Zhou F, Wu Y, Han C, et al: Real-world outcomes of
chemo-antiangiogenesis versus chemo-immunotherapy combinations in
EGFR-mutant advanced non-small cell lung cancer patients after
failure of EGFR-TKI therapy. Transl Lung Cancer Res. 10:3782–3792.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang JCH, Xu Y, Huang WT, Su WC, Gao B,
Lee CK, Fang J, Yu W, Wang M and Janne PA: Anti-tumor activity of
sunvozertinib in NSCLC with EGFR sensitizing mutations after
failure of EGFR TKI treatment. J Clin Oncol. 41 (16
Suppl):S91032023. View Article : Google Scholar
|
|
26
|
Zhao Y, He Y, Wang W, Cai Q, Ge F, Chen Z,
Zheng J, Zhang Y, Deng H, Chen Y, et al: Efficacy and safety of
immune checkpoint inhibitors for individuals with advanced
EGFR-mutated non-small-cell lung cancer who progressed on EGFR
tyrosine-kinase inhibitors: A systematic review, meta-analysis, and
network meta-analysis. Lancet Oncol. 25:1347–1356. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y, Zhang S, Hu X, Feng J, Ma Z, Zhou
J, Yang N, Wu L, Liao W, Zhong D, et al: Safety, clinical activity,
and pharmacokinetics of alflutinib (AST2818) in patients with
advanced NSCLC with EGFR T790M mutation. J Thorac Oncol.
15:1015–1026. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu X, Zhang S, Ma Z, Feng J, Wu L, Lv D,
Zhou J, Zhang X, Liu L, Yu Q, et al: Central nervous system
efficacy of furmonertinib (AST2818) in patients with EGFR T790M
mutated non-small cell lung cancer: A pooled analysis from two
phase 2 studies. BMC Med. 21:1642023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meng J, Zhang H, Bao JJ, Chen ZD, Liu XY,
Zhang YF, Jiang Y, Miao LY and Zhong DF: Metabolic disposition of
the EGFR covalent inhibitor furmonertinib in humans. Acta Pharmacol
Sin. 43:494–503. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jackman DM, Yeap BY, Sequist LV, Lindeman
N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS,
et al: Exon 19 deletion mutations of epidermal growth factor
receptor are associated with prolonged survival in non-small cell
lung cancer patients treated with gefitinib or erlotinib. Clin
Cancer Res. 12:3908–3914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen H, Yang S, Wang L, Wu Y, Wu Y, Ma S,
He Z, Zhang C, Liu Y, Tang H, et al: High-dose furmonertinib in
patients with EGFR-mutated NSCLC and leptomeningeal metastases: A
prospective real-world study. J Thorac Oncol. 20:65–75. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ang YLE, Zhao X, Reungwetwattana T, Cho
BC, Liao BC, Yeung R, Loong HH, Kim DW, Yang JC, Lim SM, et al: A
phase II study of osimertinib in patients with advanced-stage
non-small cell lung cancer following prior epidermal growth factor
receptor tyrosine kinase inhibitor (EGFR TKI) therapy with EGFR and
T790M mutations detected in plasma circulating tumour DNA (PLASMA
study). Cancers (Basel). 15:49992023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leonetti A, Verzè M, Minari R, Perrone F,
Gnetti L, Bordi P, Pluchino M, Nizzoli R, Azzoni C, Bottarelli L,
et al: Resistance to osimertinib in advanced EGFR-mutated NSCLC: A
prospective study of molecular genotyping on tissue and liquid
biopsies. Br J Cancer. 130:135–142. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Esagian SM, Grigoriadou GI, Nikas IP,
Boikou V, Sadow PM, Won JK and Economopoulos KP: Comparison of
liquid-based to tissue-based biopsy analysis by targeted next
generation sequencing in advanced non-small cell lung cancer: A
comprehensive systematic review. J Cancer Res Clin Oncol.
146:2051–2066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin LH, Allison DHR, Feng Y, Jour G, Park
K, Zhou F, Moreira AL, Shen G, Feng X, Sabari J, et al: Comparison
of solid tissue sequencing and liquid biopsy accuracy in
identification of clinically relevant gene mutations and
rearrangements in lung adenocarcinomas. Mod Pathol. 34:2168–2174.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vatrano S, Righi L, Vavalá T, Rapa I,
Busso M, Izzo S, Cappia S, Veltri A, Papotti M, Scagliotti GV and
Novello S: Molecular and histological changes in post-treatment
biopsies of non-squamous non-small cell lung cancer: A
retrospective study. Target Oncol. 11:157–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han B, Zhou C, Wu L, Yu X, Li Q, Liu F and
Shen C: 1210P Preclinical and preliminary clinical investigations
of furmonertinib in NSCLC with EGFR exon 20 insertions (20ins). Ann
Oncol. 32 (Suppl 5):S9642021. View Article : Google Scholar
|
|
39
|
Sa H, Shi Y, Ding C and Ma K: A real-world
study of the efficacy and safety of furmonertinib for patients with
non-small cell lung cancer with EGFR exon 20 insertion mutations. J
Cancer Res Clin Oncol. 149:7729–7742. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu S, Ming H, He Q, Ding M, Ding H and Li
C: A study of high dose furmonertinib in EGFR exon 20 insertion
mutation-positive advanced non-small cell lung cancer. Front Oncol.
14:13143012024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Spira A, Cho BC, Felip E, Garon EB, Goto
K, Johnson M, Leighl N, Passaro A, Planchard D, Popat S, et al:
FURVENT: Phase 3 trial of firmonertinib vs chemotherapy as
first-line treatment for advanced NSCLC with EGFR exon 20 insertion
mutations (FURMO-004). Lung Cancer. 199:1080662025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu G, Chen Q, Lv D, Lin L and Huang J:
Pulmonary adenocarcinoma patient with complex mutations on EGFR
benefits from furmonertinib after acquiring gefitinib resistance: A
case report. Recent Pat Anticancer Drug Discov. 19:247–252. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin H, Yang Z, Li Z, Chen J, Wang H and
Lin Y: EGFR kinase domain duplication in lung adenocarcinoma with
systemic and intracranial response to a double-dose of
furmonertinib: A case report and literature review. Front Oncol.
14:13215872024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jia G, Bashir S, Ye M, Li Y, Lai M, Cai L
and Xu: Furmonertinib and intrathecal pemetrexed chemotherapy
rechallenges osimertinib-refractory leptomeningeal metastasis in a
non-small cell lung cancer patient harboring EGFR20 R776S, C797S,
and EGFR21 L858R compound EGFR mutations: A case report. Anticancer
Drugs. 35:542–547. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ni C, Zhang L, Yu X, Pang Y and Xu J:
Response to furmonertinib in a patient with non-small cell lung
cancer harboring HER2 exon 21 insertion mutation: A case report.
Front Oncol. 14:14403792024. View Article : Google Scholar : PubMed/NCBI
|