Introduction
Acute leukemia (AL), a highly heterogeneous
malignant clonal disease affecting hematopoietic stem cells
(1), is characterized by the
infiltration of leukemia cells from the bone marrow into various
organs and tissues, ultimately suppressing normal hematopoietic
function. Based on the French-American-British classification, AL
is divided into acute myelocytic leukemia (AML) and acute
lymphoblastic leukemia (ALL) (2).
While modern chemotherapy regimens have achieved high rates of
complete remission, both AML and ALL are associated with decreased
survival rates and poor prognosis due to recurrence within 5 years
(3). The significance of the
accurate identification of AML and ALL is paramount, as treatment
strategies for the two subtypes notably differ (4). However, several challenges hinder the
accurate diagnosis of leukemia worldwide, including limited medical
resources, a scarcity of experienced technicians and the
prohibitive cost of necessary equipment. These factors have led to
instances of leukemia classification errors (5,6).
Additionally, some patients with AL exhibit ambiguous expression of
myeloid or lymphoid immune markers (7), while others have leukemia cells that
simultaneously express both myeloid and lymphoid antigens (8). This complexity not only complicates
the diagnostic process but also poses challenges in selecting
appropriate chemotherapy regimens, ultimately impacting treatment
outcomes. Given these challenges, there is an urgent need to
identify a simple and reliable method for distinguishing AML from
ALL.
Circular RNAs (circRNAs) constitute a unique
subclass of non-coding RNAs (ncRNAs) that predominantly reside
within the cytoplasm. These molecules are not affected by RNA
exonuclease (9) and their
expression shows marked stability and has been observed in a wide
range of eukaryotic organisms (10). A key mechanism by which circRNAs
exert their biological influence is through the competitive
adsorption of microRNAs (miRNAs) (11). circRNAs, with their higher abundance
compared with mRNAs, have emerged as promising biomarkers for
cancer diagnosis. A growing body of evidence suggests a strong
association between circRNAs and the diagnosis as well as prognosis
of AL (12–16). Certain studies have shown that
circ-VIM (13) is significantly
upregulated and hsa_circ_0004277 (14) is significantly downregulated in AML.
In addition, the expression level of circPVT1 is increased in ALL
(15). Meanwhile, Guo et al
(16) utilized microarray analysis
to compare circRNA expression profiles across different groups.
Notably, hsa_circ_0012152 and hsa_circ_0001857 emerged as effective
discriminators between AML and ALL in bone marrow samples, further
underscoring their potential as diagnostic biomarkers. These
findings underscore the increasing recognition of circRNAs as
valuable players in the diagnosis and prognosis of AL.
However, current studies aiming to distinguish
between AML and ALL predominantly rely on bone marrow samples,
leaving peripheral blood as an underexplored alternative. The
process of extracting bone marrow specimens is fraught with
potential complications that can lead to unreliable test results,
Lin et al (17) found that
11.8% of patients with bone marrow necrosis were still misdiagnosed
after bone marrow aspiration. These factors include patient
psychological issues, technical errors by the extraction personnel,
the volume of extraction, age, puncture site and pathological
conditions (18). Peripheral blood
sampling offers a notably more convenient and less invasive
approach compared with bone marrow sampling, potentially reducing
patient discomfort (19). In
suspected cases of leukemia, testing peripheral blood can provide
earlier insights into AL subtypes, facilitating the timely
determination of appropriate chemotherapy regimens. Chinese
guideline for diagnosis and treatment of adult acute lymphoblastic
leukemia (2024) (20) suggest using
peripheral blood for essential tests when bone marrow aspiration
yields no sample. This method is especially beneficial in scenarios
where obtaining bone marrow samples poses challenges, such as in
the presence of bone marrow fibrosis.
The present study aimed to identify peripheral
blood-based circRNA biomarkers for minimally invasive distinction
between AML and ALL. Using experimental validation and
bioinformatic profiling, the diagnostic utility of hsa_circ_0012152
and hsa_circ_0020093 were assessed while exploring their potential
regulatory roles in AL.
Materials and methods
Patients and specimen collection
The present study recruited 110 patients newly
diagnosed with AL and 20 healthy individuals (10 males and 10
females; age range, 14–70 years) from January 2023 to September
2024 at the First Affiliated Hospital of Ningbo University (Ningbo,
China). Specifically, the 110 patients with AL comprised 86
patients with AML (53 males and 33 females; age range, 11–87 years)
and 24 patients with ALL (12 males and 12 females; age range, 2–74
years). The inclusion criteria required confirmation of diagnosis
according to the 2022 World Health Organization Classification of
Haematolymphoid Tumours (21,22)
and the availability of peripheral blood samples. The exclusion
criteria comprised patients with chronic myeloid leukemia,
therapy-related AML, those who had undergone prior chemotherapy,
individuals with concurrent malignancies and T cell ALL (T-ALL)
cases. For the patients with ALL included in the study, a phased
grouping strategy was applied. During the initial phase, all ALL
cases were analyzed as a single unified group (n=24) and compared
directly against the AML group. Subsequently, to investigate
biomarker performance differences among intrinsic ALL subtypes, the
ALL cohort was further stratified into two subgroups: ALL with
myeloid antigen expression (n=12) and B-ALL (n=12) for independent
comparative analysis. It should be noted that all specimens
utilized in this investigation were prospectively collected
residual peripheral blood samples obtained from the patients.
Screening target circRNAs
The circRNA microarray data analyzed in the present
study were archived in the OMIX database (National Genomics Data
Center; accession no: OMIX009143; https://ngdc.cncb.ac.cn/omix/release/OMIX009143) and
were from our previous study (16).
The sequencing data of hsa_circ_0012152 and hsa_circ_0020093
examined during the present study are also available in the
GSA-human repository (https://bigd.big.ac.cn/gsa-human/browse/HRA007384).
The R language (23) (version
4.1.2) limma package was utilized to standardize and analyze the
raw circRNA microarray data. Using a fold change (FC) threshold of
≥10 and P<0.05 as criteria, those circRNAs with the most
significant differential expression and higher expression abundance
were selected when comparing AML to ALL as the focus of the present
study.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Peripheral blood mononuclear cells (PBMCs) were
isolated by density gradient centrifugation: 2 ml Ficoll solution
(GE Healthcare) was vertically added to the bottom of a 15 ml
centrifuge tube, followed by gentle layering of 4 ml
EDTA-anticoagulated peripheral blood onto the Ficoll layer. After
centrifugation at 500 × g for 30 min at 20°C, the PBMC layer
(opaque white interphase) was carefully aspirated. The harvested
PBMCs were washed three times with 5 ml phosphate-buffered saline
(PBS) (200 × g, 10 min each). Residual red blood cells, if present,
were lysed using 5 ml Red Blood Cell Lysis Buffer (Beijing Solarbio
Science & Technology Co., Inc.) at 37°C for 10 min, followed by
centrifugation (200 × g, 10 min). The pellet was resuspended in 1
ml PBS and transferred to RNase-free EP tubes for subsequent
analysis. Total RNA was extracted from the PBMCs using RNAiso Plus
reagent (Takara Bio, Inc.), following the manufacturer's
instructions. The NanoDrop 2000 ultra microspectrophotometer
(Thermo Fisher Scientific, Inc.) was then utilized to determine the
concentration and purity of the RNA samples. RNA purity was deemed
satisfactory when the optical density 260/280 ratio fell within the
acceptable range of 1.8–2.1, with a concentration maintained at
~500 µg/ml. Following RNA extraction, cDNA synthesis was carried
out using the RevertAid First Strand cDNA Synthesis Kit (Thermo
Fisher Scientific, Inc.) as per the manufacturer's protocol.
Subsequently, qPCR was performed using the TB Green PCR reagent kit
(Takara Bio, Inc.) on the StepOnePlus system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Each 20 µl qPCR reaction mixture
comprised 1 µl cDNA, 1.6 µl primer, 0.4 µl ROX, 10 µl TB Green and
7 µl diethylpyrocarbonate-treated (DEPC) water. The qPCR reaction
program consisted of an initial pre-denaturation step at 95°C for
60 sec, followed by 40 cycles of amplification. Each cycle included
denaturation at 95°C for 15 sec, primer annealing at 64°C for 30
sec and extension at 72°C for 32 sec, with fluorescence recording.
Melting curve analysis was then performed, consisting of
denaturation at 95°C for 15 sec, annealing at 60°C for 60 sec,
re-denaturation at 95°C for 15 sec and a final annealing step at
60°C for 15 sec. For internal standardization, the MOLM-13 cell
line (from The First Affiliated Hospital, Zhejiang University
School of Medicine; RRID: CVCL_2119) was used as a reference for
hsa_circ_0012152 detection, while the NALM-6 cell line (from The
First Affiliated Hospital, Zhejiang University School of Medicine;
RRID: CVCL_0092) served as a benchmark for hsa_circ_0020093
detection. GAPDH was employed as the reference gene and DEPC water
was included as a negative control. Gene expression levels were
analyzed using the comparative cycle threshold (2−ΔΔCq)
method (24). Primer sequences are
provided in Table I. Post-PCR
analysis involved confirming the absence of any jumps in the
negative control, checking the corresponding Cq values of the
reference gene (GAPDH), hsa_circ_0012152 and hsa_circ_0020093 as
well as analyzing the melting curve. A single peak in the melting
curve, with a melting temperature ranging between 80–90°C,
indicated good primer specificity. This was further verified
through gel electrophoresis imaging, product sequencing and
amplification efficiency validation. Adjustments were made to the
baseline, amplification curve and threshold for each gene based on
the observed amplification patterns, and all experimental data were
recorded accordingly.
 | Table I.Sequence of primers used in reverse
transcription-quantitative PCR. |
Table I.
Sequence of primers used in reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5′ to 3′) |
|---|
| GAPDH | Forward:
ATGGGGAAGGTGAAGGTCG |
|
| Reverse:
GGGTCATTGATGGCAACAATATC |
|
hsa_circ_0012152 | Forward:
TCTCCCCACTTGCGCTTCTC |
|
| Reverse:
GCCAACCAGCACTTTGGGTC |
|
hsa_circ_0020093 | Forward:
AATTGCGGCAGTCCAGATCA |
|
| Reverse:
TGGATAGCCTTCAATGAGCCA |
Construction of competing endogenous
RNA (ceRNA) network
Using the CircInteractome database (https://circinteractome.irp.nia.nih.gov/) and
starBase3.0 database (https://starbase.sysu.edu.cn/), the downstream target
miRNA and miRNA-targeted genes of hsa_circ_0020093 were predicted.
Given that the predictive analysis of hsa_circ_0012152 has been
systematically established in a previous study (16), the current investigation
specifically explored the prediction of hsa_circ_0020093 to advance
novel findings. With the assistance of the STRING 11.5 database
(https://string-db.org/), a protein-protein
interaction (PPI) network was constructed, which was then visually
represented using Cytoscape (25)
(version 3.8.0). The essential hub genes were pinpointed in this
PPI network. Furthermore, leveraging the capabilities of Cytoscape,
the hsa_circ_0020093-miRNA-mRNA crosstalk network and the ceRNA
network were crafted.
Gene ontology (GO) and kyoto
encyclopedia of genes and genomes (KEGG) pathway analyses for
target genes
Using the interaction relationship within the ceRNA
network as a foundation, GO and KEGG enrichment analyses on the
miRNA target genes were performed employing R software. To
visualize these enrichment results, the enrich plot package was
utilized, generating graphical representations that provided
insights into the functional and pathway associations of the target
genes.
Statistical analysis
In the present study, SPSS 26.0 software (IBM Corp.)
was harnessed to analyze the experimental data, while GraphPad
Prism 9.0 (Dotmatics) was used to visualize the outcomes. To assess
the diagnostic efficacy of hsa_circ_0012152 and hsa_circ_0020093 as
auxiliary markers for AML and ALL in peripheral blood, as well as
their utility in subtype classification for AL, the receiver
operating characteristic (ROC) curve was employed to compute the
area under the curve (AUC) value. The diagnostic cut-off values for
both hsa_circ_0012152 and hsa_circ_0020093 were established using
Youden's index, enabling the calculation of sensitivity,
specificity, positive predictive value and negative predictive
value. A comprehensive analysis of the diagnostic efficacy for AL
subtypes was conducted using a range of experiments, including
series and parallel tests, along with a multi-factor logistic
regression model. AML cases were stratified into high- and
low-expression groups based on the median expression level of
hsa_circ_0012152, while ALL cases were similarly categorized
according to hsa_circ_0020093 expression. Differences in laboratory
indicators and clinical data between these groups were
statistically analyzed. Qualitative data were subjected to the
χ2 test, while quantitative data with three or more
groups were analyzed using the non-parametric Kruskal-Wallis
H-test, followed by Dunn's multiple comparison test for post hoc
pairwise comparisons. For datasets with 2 groups, the Wilcoxon rank
sum test was directly employed. P<0.05 was considered to
indicate a statistically significant difference.
Results
circRNA screening results
In our previous investigation utilizing circRNA
microarray analysis (16), 10,171
circRNAs were identified. By applying stringent criteria of a FC
≥10 and a P<0.05, the selection was narrowed down to 101
differentially expressed circRNAs between ALL and AML. These
included 67 circRNAs that were upregulated and 34 that were
downregulated in ALL compared with AML. Notably, among this subset,
hsa_circ_0012152 stood out as the most notably downregulated
circRNA, exhibiting a 77-fold decrease and being the sole circRNA
to exhibit a downregulation exceeding 50-fold. Conversely,
hsa_circ_0020093 emerged as the most significantly upregulated
circRNA, achieving a 228-fold increase and being the only one to
surpass a 200-fold upregulation (Fig.
1A). When comparing the AML sample to the normal control, of
the 2 differentially expressed circRNAs that were upregulated,
hsa_circ_0012152 demonstrated the most significant upregulation,
reaching a 22-fold increase (Fig.
1B). By contrast, when ALL was compared with the normal
control, 201 circRNAs exhibited differential expression, comprising
88 upregulated and 113 downregulated circRNAs. Once again,
hsa_circ_0020093 was the most significantly upregulated circRNA,
achieving a 133-fold increase and being the only circRNA to exceed
a 100-fold up-regulation (Fig. 1C).
Notably, hsa_circ_0012152 and hsa_circ_0020093 exhibited the
highest expression levels in AML and ALL, respectively, making them
ideal candidates for further investigation. Consequently, these two
circRNAs were selected as the focal targets of the present
study.
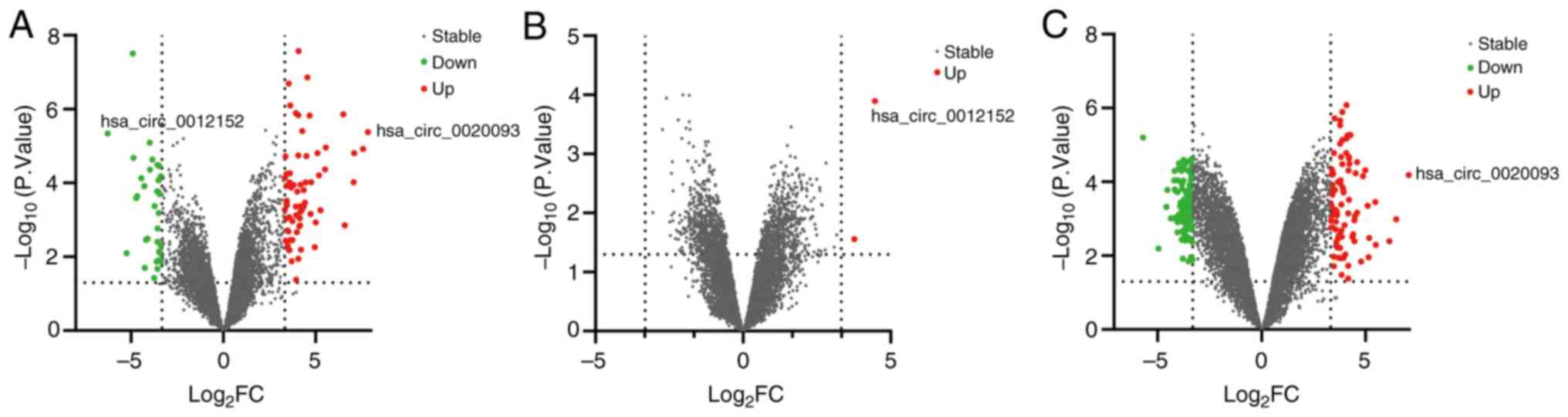 | Figure 1.Volcano plot of differentially
expressed circRNAs between ALL, AML and NC. ‘log2FC’ is
plotted on the horizontal axis and ‘-log10 (P-value)’ is
plotted on the vertical axis; that is, the larger the
‘log2FC’ the greater the difference, and the larger the
‘-log10 (P-value)’ the more significant the statistical
difference. Red and green represent the upregulated and
downregulated circRNAs, respectively, with |log2FC| ≥10
and P<0.05, while gray represents the circRNAs with
|log2FC|<10 or P≥0.05. (A) ALL vs. AML, (B) AML vs.
NC and (C) ALL vs. NC. circRNA, circular RNA; ALL, acute
lymphoblastic leukemia; AML, acute myeloid leukemia; NC, normal
control; FC, fold change. |
Expression levels of hsa_circ_0012152
and hsa_circ_0020093 in the peripheral blood of patients with
AL
To gain deeper insights into the expression profiles
of hsa_circ_0012152 and hsa_circ_0020093 in AL, a sample validation
study for these two circRNAs was undertaken. The results presented
in Fig. 2A highlight that the
expression levels of hsa_circ_0012152 were significantly elevated
in the peripheral blood samples from patients with AML compared
with ALL (median value, 8.40 vs. 0.51; P<0.0001). Furthermore,
the expression of hsa_circ_0012152 was also significantly higher in
patients with AML compared with the normal controls (median value,
8.40 vs. 0.29; P<0.0001). By contrast, the expression levels of
hsa_circ_0020093were significantly increased in patients with ALL
compared with AML (median value, 0.1601 vs. 0.0017; P<0.0001;
Fig. 2B) and were also
significantly higher in patients with ALL than in the normal
controls (median value, 0.1601 vs. 0.0027; P=0.0003; Fig. 2B). These findings suggest that the
expression levels of both hsa_circ_0012152 and hsa_circ_0020093 in
peripheral blood could potentially serve as diagnostic and
classification biomarkers for AML and ALL.
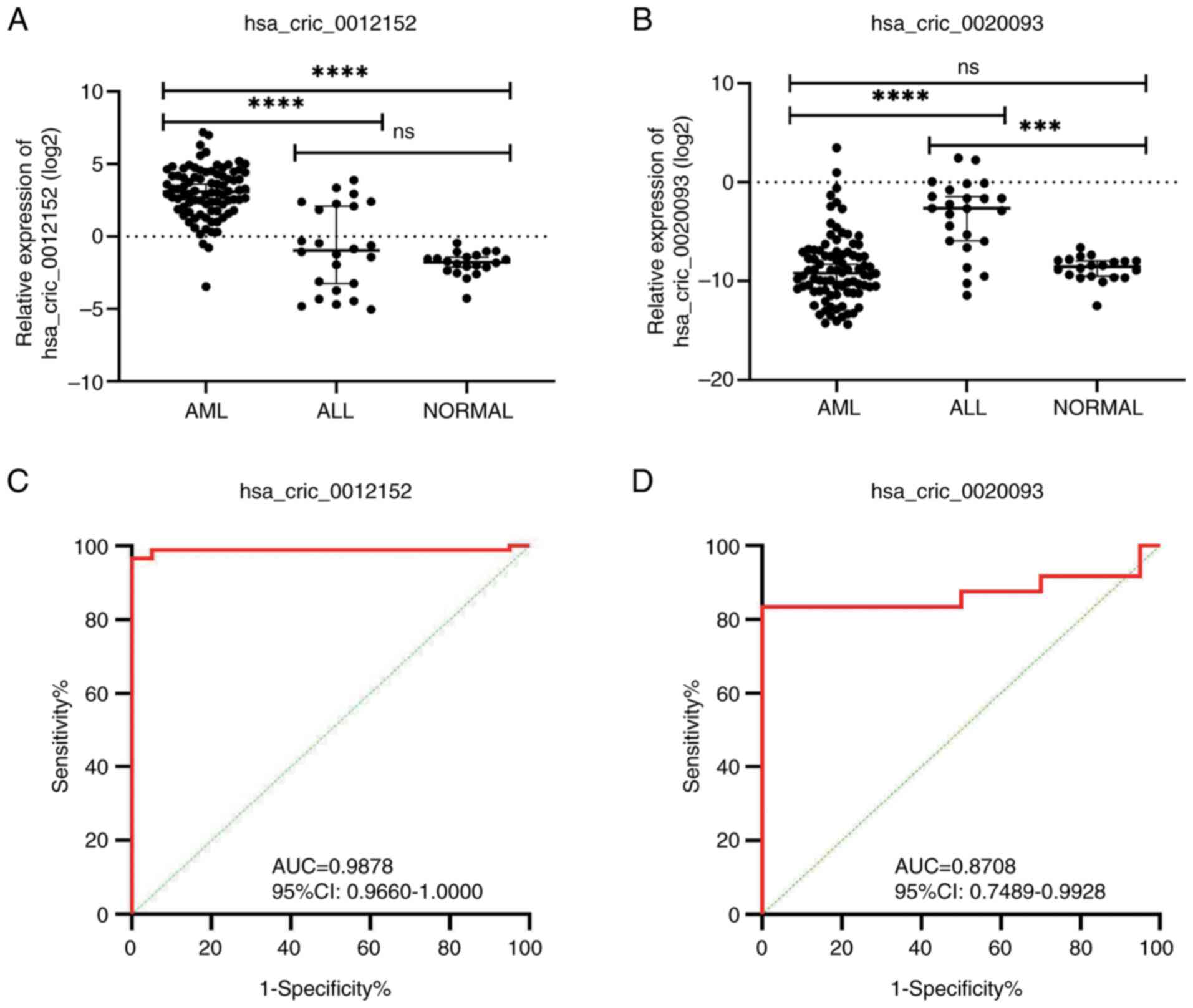 | Figure 2.Relative expression levels of
hsa_circ_0012152 and hsa_circ_0020093 in peripheral blood samples
of patients with AL. (A) The relative expression levels of
hsa_circ_0012152 in patients with AL, exhibiting significant
differences. (B) The relative expression levels of hsa_circ_0020093
in patients with AL, highlighting distinct patterns. (C) ROC curve
of hsa_circ_0012152, demonstrating its potential as a diagnostic
biomarker for distinguishing AML from healthy individuals. (D) ROC
curve of hsa_circ_0020093, emphasizing its diagnostic value in
differentiating ALL from healthy controls. ****P<0.0001,
***P<0.001. ns, no significant difference; circ, circular (RNA);
AL, acute leukemia; ALL, acute lymphoblastic leukemia; AML, acute
myeloid leukemia; AUC, area under the curve; 95% CI, 95% confidence
interval; ROC, receiver operating characteristic. |
Upon further evaluation of their diagnostic efficacy
using ROC curve analysis, it was found that hsa_circ_0012152
exhibited high sensitivity (0.9651), perfect specificity (1.0000)
and an excellent AUC of 0.9878 [95% confidence interval (CI),
0.9660–1.0000; P<0.0001; Fig. 2C
and Table II] as an adjunctive
diagnostic marker for AML in peripheral blood. Similarly,
hsa_circ_0020093 demonstrated good sensitivity (0.8333), perfect
specificity (1.0000) and a promising AUC of 0.8708 (95% CI,
0.7489–0.9928; P<0.0001; Fig. 2D
and Table II) as an adjunctive
diagnostic marker for ALL in peripheral blood. Thus, both circRNAs
showed promising potential as adjunctive diagnostic markers for AML
and ALL in peripheral blood samples. Notably, the expression levels
of hsa_circ_0012152 and hsa_circ_0020093 remained consistent across
different patient demographics, including sex, age, laboratory
parameters and survival outcomes (Table III). However, in patients with AML
exhibiting high expression levels of hsa_circ_0012152, there was a
higher proportion of primitive cells in the peripheral blood
(P=0.008; Table III). This
finding hints at a possible association between hsa_circ_0012152
expression and disease progression in patients with AML, which
warrants further investigation.
 | Table II.Diagnostic efficacy of
hsa_circ_0012152 and hsa_circ_0020093 in acute leukemia. |
Table II.
Diagnostic efficacy of
hsa_circ_0012152 and hsa_circ_0020093 in acute leukemia.
| circRNA | Disease | Sensitivity | Specificity | PPV | NPV | Youden | AUC | 95% CI | P-value |
|---|
|
hsa_circ_0012152 | AML | 0.9651 | 1.0000 | 1.0000 | 0.8695 | 0.9651 | 0.9878 | 0.9660–1.0000 | <0.0001 |
|
hsa_circ_0020093 | ALL | 0.8333 | 1.0000 | 1.0000 | 0.8333 | 0.8333 | 0.8708 | 0.7498–0.9928 | <0.0001 |
 | Table III.Relationship between high and low
expression of hsa_circ_0012152 or hsa_circ_0020093 in peripheral
blood with the clinical characteristics of patients with acute
leukemia. |
Table III.
Relationship between high and low
expression of hsa_circ_0012152 or hsa_circ_0020093 in peripheral
blood with the clinical characteristics of patients with acute
leukemia.
|
| hsa_circ_0012152
expression | hsa_circ_0020093
expression |
|---|
|
|
|
|
|---|
| Characteristic | High | Low | P-value | High | Low | P-value |
|---|
| Sex, n (%) |
|
| 0.121 |
|
| 0.414 |
|
Male | 23 (43.40) | 30 (56.60) |
| 7 (58.33) | 5 (41.67) |
|
|
Female | 20 (60.61) | 13 (39.39) |
| 5 (41.67) | 7 (58.33) |
|
| Median age, years
(IQR) | 53 (11,78) | 63 (14,87) | 0.269 | 49.5 (3,66) | 44.5 (2,74) | 0.817 |
| Median BMPC, %
(IQR) | 59.5 (25.5,93) | 60.0
(23.0,89.5) | 0.276 | 84 (59,92) | 80.5 (46,96.5) | 0.743 |
| Median PBIC, %
(IQR) | 45 (4,90) | 20 (1,90) | 0.008a | 36 (3,77) | 50 (8,86) | 0.744 |
| Median WBC,
×109/l (IQR) | 15.3
(1.1,259.1) | 8.2
(0.4,186.7) | 0.141 | 11.9
(1.1,84.0) | 16.5
(1.1,86.3) | 0.954 |
| Median RBC,
×109/l (IQR) | 2.2 (0.7,5.4) | 2.32 (1.3,4.8) | 0.638 | 3.91
(1.53,5.00) | 2.67
(1.48,5.06) | 0.386 |
| Median Hb, g/l
(IQR) | 76 (26,131) | 74 (46,142) | 0.917 | 115.5 (54,136) | 86.5 (56,153) | 0.525 |
| Median PLT,
×109/l (IQR) | 42 (9,507) | 35 (4,316) | 0.273 | 37 (4,173) | 89 (8,294) | 0.273 |
| Median LDH, IU/l
(IQR) | 431 (102,1835) | 324 (115,2540) | 0.182 | 570 (199,7138) | 525
(152,13207) | 0.644 |
| Median β2-MG, mg/l
(IQR) | 2.1 (1.2,8.5) | 2.3 (1.0,8.6) | 0.365 | 2.4 (1.2,3.4) | 2.1 (1.1,5.2) | 0.795 |
| Median OS, days
(IQR) | 191 (1,632) | 225 (5,747) | 0.904 | 157 (105, 362) | 136 (9,780) | 0.670 |
| Median EFS, days
(IQR) | 181 (1,632) | 215.5 (5,747) | 0.959 | 153 (105,362) | 136 (9,780) | 0.250 |
hsa_circ_001252 and hsa_circ_0020093
can accurately discriminate AML from ALL in peripheral blood
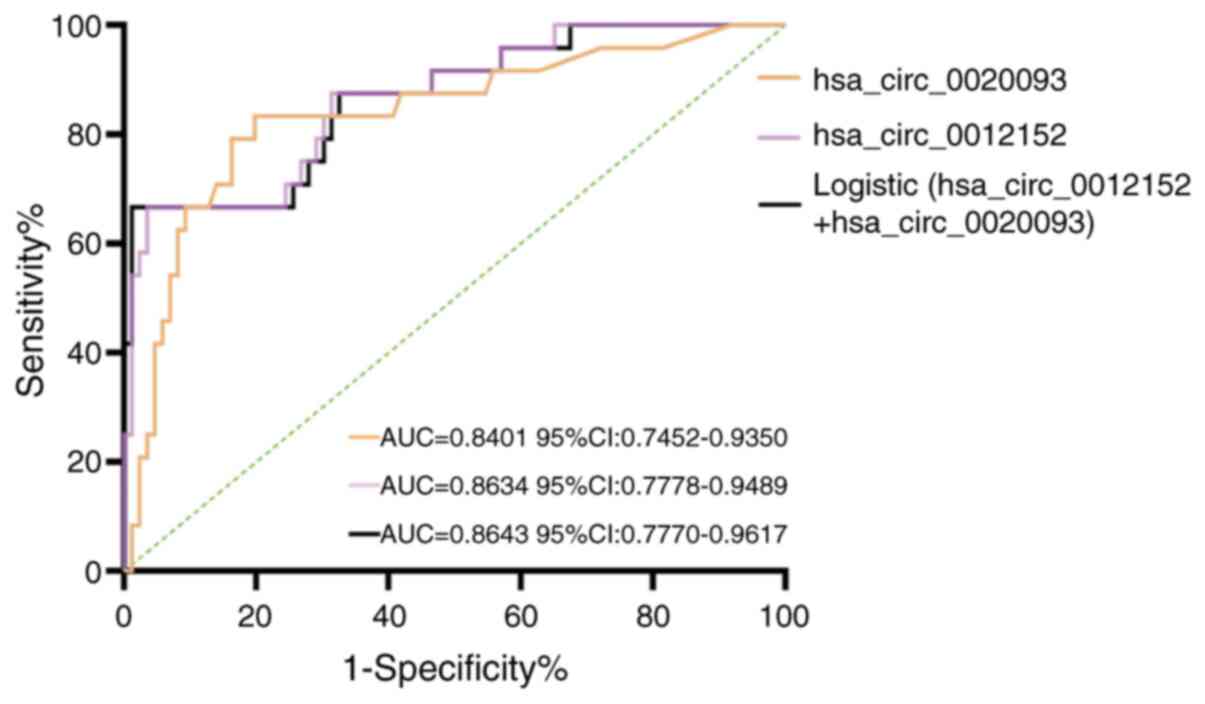
Subsequently, ROC curves were constructed to
evaluate the subtype-distinguishing capacity of hsa_circ_0012152
and hsa_circ_0020093 in differentiating AML from ALL (Fig. 3 and Table IV), extending their utility beyond
initial diagnostic applications in leukemia detection. The findings
revealed that in peripheral blood samples, hsa_circ_0012152
exhibited a sensitivity of 0.9651, a specificity of 0.6667 and an
AUC of 0.8634 (95% CI, 0.7778–0.9489; P<0.0001) in
discriminating AML from ALL. Additionally, hsa_circ_0020093
demonstrated a sensitivity of 0.8333, a specificity of 0.8023 and
an AUC of 0.8401 (95% CI, 0.7452–0.9350; P<0.0001) for the same
purpose. To further enhance the diagnostic accuracy of these two
circRNAs, series, parallel and logistic regression experiments were
conducted for each. In the series experiments, the specificity and
positive predictive value increased to 0.9444 and 0.9804,
respectively, albeit with a slight decrease in sensitivity and
negative predictive value (Table
IV). Conversely, in the parallel experiments, the sensitivity
and negative predictive value rose to 0.9931 and 0.9574,
respectively, but there was a decrease in specificity and positive
predictive value (Table IV). Using
both hsa_circ_0012152 and hsa_circ_0020093 as independent
variables, a logistic regression model was established. The
predictive probability derived from this model was then utilized as
the diagnostic discriminator to construct an ROC curve. The results
indicated that the sensitivity and AUC increased to 0.9884 and
0.8643 (95% CI, 0.7770–0.9617; P<0.0001; Table IV), respectively, with the logistic
regression model offering a slight improvement in diagnostic
discrimination compared with using these markers individually. Each
type of testing (series, parallel and logistic regression) has
distinct advantages and disadvantages. Series testing inherently
reduces sensitivity as an intrinsic trade-off for specificity
enhancement, while parallel testing inherently reduces specificity
as an intrinsic characteristic to improve sensitivity (26). Logistic regression testing provides
a notable enhancement in diagnostic accuracy (27). Therefore, it is recommended that
these three tests be combined when distinguishing AML from ALL
using a diagnostic marker, as this approach leverages the strengths
of each method while mitigating their respective weaknesses.
 | Table IV.Differential diagnostic efficacy of
acute myeloid leukemia and acute lymphoblastic leukemia by
hsa_circ_0012152 and hsa_circ_0020093. |
Table IV.
Differential diagnostic efficacy of
acute myeloid leukemia and acute lymphoblastic leukemia by
hsa_circ_0012152 and hsa_circ_0020093.
| Components and
models | Sensitivity | Specificity | PPV | NPV | Youden | AUC | 95% CI | P-value |
|---|
|
hsa_circ_0012152 | 0.9651 | 0.6667 | 0.9121 | 0.8420 | 0.6318 | 0.8634 | 0.7778–0.9489 | <0.0001 |
|
hsa_circ_0020093 | 0.8333 | 0.8023 | 0.5405 | 0.9452 | 0.6357 | 0.8401 | 0.7452–0.9350 | <0.0001 |
| Logistic
(hsa_circ_0012152 + hsa_circ_0020093) | 0.9884 | 0.6667 | 0.9140 | 0.9413 | 0.6550 | 0.8643 | 0.7770–0.9617 | <0.0001 |
| Parallel test | 0.9931 | 0.5556 | 0.8890 | 0.9574 | 0.5487 | - | - | - |
| Serial test | 0.7742 | 0.9444 | 0.9804 | 0.5386 | 0.7186 | - | - | - |
Diagnostic efficacy of the
hsa_circ_0012152 levels in peripheral blood in distinguishing the
molecular subtypes of AML and the presence or absence of myeloid
antigen expression in patients with ALL
Utilizing the 5th edition of the World Health
Organization Classification of Haematolymphoid Tumours: Myeloid and
Histiocytic/Dendritic Neoplasms (21), which relies on cellular morphology,
genetics and immunophenotype characteristics, enables more precise
differentiation and consequently accurate guidance on the origin
and prognostic assessment of AML. Against this backdrop, it was
next determined whether the expression of hsa_circ_0012152 in
peripheral blood could inform this WHO classification. Adhering to
the WHO classification standards, 86 patients with AML were
stratified into distinct groups. The expression profiles of
hsa_circ_0012152 across these groups are presented in Fig. 4E. Notably, a statistically
significant difference in hsa_circ_0012152 expression was observed
across most groups, indicating the presence of a subset of patients
with distinct expression patterns. Upon closer examination of the
inter-group differences, it was found that the ‘APL with PML-RARA
or t (15;17)’ group exhibited lower expression levels of
hsa_circ_0012152 compared with the other groups (median value, 3.40
vs. 12.24; P<0.0001; Fig. 4A).
This observation suggests that hsa_circ_0012152 may serve as a
diagnostic marker, differentiating this specific subgroup from
others when examining peripheral blood samples. With a sensitivity
of 0.8947, a specificity of 0.7463 and an AUC of 0.8366 (95% CI,
0.7532–0.9201; P<0.0001; Fig. 4C
and Table V), its diagnostic
potential is further highlighted.
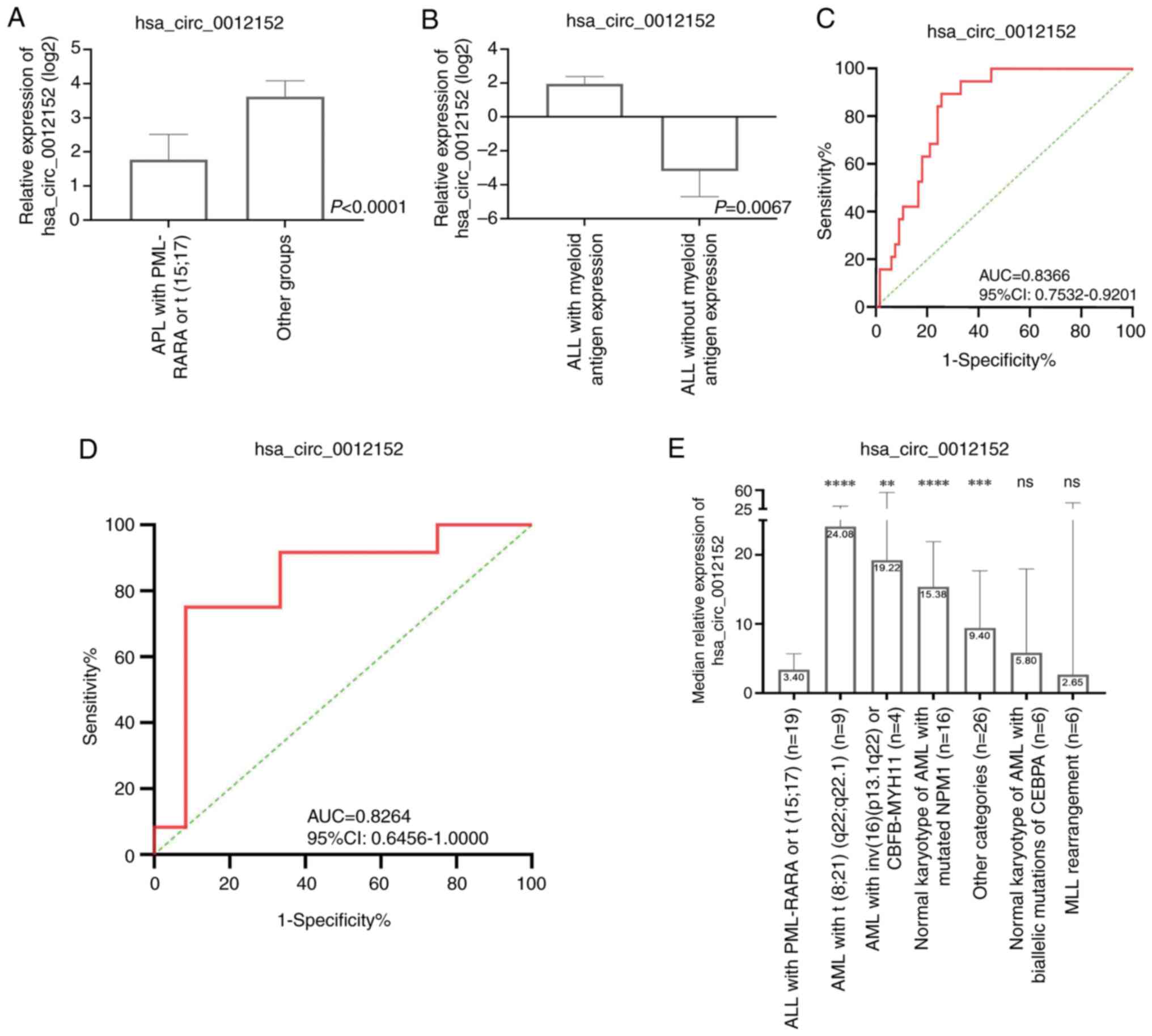 | Figure 4.Diagnostic Efficacy of
hsa_circ_0012152 levels in peripheral Blood for distinguishing
molecular subtypes of AML and assessing myeloid antigen expression
in patients with ALL. (A) The expression levels of hsa_circ_0012152
specifically in ‘APL with PML-RARA or t (15;17)’ compared with the
other AML subtypes, highlighting significant differences. (B) The
expression patterns of hsa_circ_0012152 in patients with ALL with
and without myeloid antigen expression, indicating potential
diagnostic utility. (C) ROC curve analysis for identifying ‘APL
with PML-RARA or t (15;17)’ within the AML population based on the
hsa_circ_0012152 expression levels. (D) ROC curve analysis for
differentiating myeloid antigen expression in patients with ALL
using hsa_circ_0012152 as a diagnostic biomarker. (E)
Classification of hsa_circ_0012152 expression in patients with AML
according to the World Health Organization criteria.
****P<0.0001, ***P<0.001, **P<0.01, all groups were
compared with APL with PML-RARA or t (15;17). ns, no significant
difference circ, circular (RNA); AUC, area under curve; 95% CI, 95%
confidence interval; ALL, acute lymphoblastic leukemia; AML, acute
myeloid leukemia; ROC, receiver operating characteristic; PML,
promyelocytic leukemia; RARA, retinoic acid receptor α; APL, acute
promyelocytic leukemia; CBFB, core-binding factor subunit β; MYH11,
myosin heavy chain 11; NPM1, nucleophosmin 1; MLL, mixed lineage
leukemia gene; CEBPA, CCAAT/enhancer binding protein α. |
 | Table V.Diagnostic efficacy of the levels of
hsa_circ_0012152 in peripheral blood in distinguishing the
molecular subtypes of AML and the presence or absence of myeloid
antigen expression in patients with ALL. |
Table V.
Diagnostic efficacy of the levels of
hsa_circ_0012152 in peripheral blood in distinguishing the
molecular subtypes of AML and the presence or absence of myeloid
antigen expression in patients with ALL.
| Identity | Sensitivity | Specificity | PPV | NPV | Youden | AUC | 95% CI | P-value |
|---|
| APL with PML-RARA
and other groups in AML | 0.8947 | 0.7463 | 0.5000 | 0.9615 | 0.6410 | 0.8366 | 0.7532–0.9201 | <0.0001 |
| Presence or absence
of myeloid antigen expression in ALL | 0.7500 | 0.9167 | 0.9000 | 0.7857 | 0.6667 | 0.8264 | 0.6456–1.0000 | 0.0067 |
In addition, when comparing the ‘APL with PML-RARA
or t (15;17)’ group to other specific AML subgroups, such as ‘AML
with t (8;21) (q22; q22.1)’ and ‘AML with inv (16) (p13.1q22) or CBFB-MYH11’, the
expression levels of hsa_circ_0012152 were significantly reduced
(P<0.0001 and P=0.0021, respectively; Table VI). Similarly, the ‘APL with
PML-RARA or t (15;17)’ group showed significantly lower expression
compared with the ‘Normal karyotype of AML with mutated NPM1’ and
‘Other categories’ groups (P<0.0001 and P=0.0006, respectively;
Table VI). However, no significant
differences were observed when compared with the ‘MLL
rearrangement’ and ‘Normal karyotype of AML with biallelic
mutations of CEBPA’ groups, potentially due to the limited sample
size within these subgroups (Table
VI).
 | Table VI.Comparison of the expression levels
of hsa_circ_0012152 in APL with PML-RARA and other groups of
AML. |
Table VI.
Comparison of the expression levels
of hsa_circ_0012152 in APL with PML-RARA and other groups of
AML.
| Molecular subtype
of AML | Expression level of
hsa_circ_0012152 | P-value |
|---|
| APL with PML-RARA
vs. |
|
|
| AML
with t (8;21) (q22; q22.1) | 3.40 vs. 24.08 |
<0.0001a |
| AML
with inv (16) (p13.1q22) or
CBFB-MYH11 | 3.40 vs. 19.22 | 0.0021a |
| Normal
karyotype of AML with mutated NPM1 | 3.40 vs. 15.38 |
<0.0001a |
| Other
categories | 3.40 vs. 9.40 | 0.0006a |
| MLL
rearrangement | 3.40 vs. 2.65 | 0.3302 |
| Normal
karyotype of AML with biallelic mutations of CEBPA | 3.40 vs. 5.8 | 0.3094 |
Furthermore, ALL encompasses various subtypes,
including B-ALL, T-ALL and mixed-phenotype ALL expressing myeloid
antigens. The present study aimed to investigate whether the
expression of myeloid antigens in ALL influences the expression of
hsa_circ_0012152. To this end, patients with ALL were categorized
based on the presence or absence of myeloid antigen expression.
Notably, it was found that hsa_circ_0012152 expression was
significantly elevated in ALL samples expressing myeloid antigens
compared with those without (median value, 3.88 vs. 0.11; P=0.0067;
Fig. 4B). With a sensitivity of
0.75, a specificity of 0.9167 and an AUC of 0.8264 (95% CI,
0.6456–1.0000; P=0.0067; Fig. 4D
and Table V), this finding suggests
that hsa_circ_0012152 may aid in distinguishing between ALL
subtypes based on myeloid antigen expression. However, it should be
noted that no significant difference in hsa_circ_0020093 expression
was observed between these two ALL groups (0.03 vs. 0.27,
P=0.149).
Construction of the ceRNA network for
hsa_circ_0020093
hsa_circ_0020093 and hsa_circ_0012152 have been
identified as potential markers for distinguishing patients with
ALL from patients with AML and healthy individuals, respectively.
Based on this observation, we hypothesize that these circRNAs may
be involved in the underlying mechanisms of ALL and AML
pathogenesis. While the preliminary research team has conducted
comprehensive bioinformatics analyses on hsa_circ_0012152 (16), hsa_circ_0020093 was chosen as the
focus of further investigations on. Using the CircInteractome and
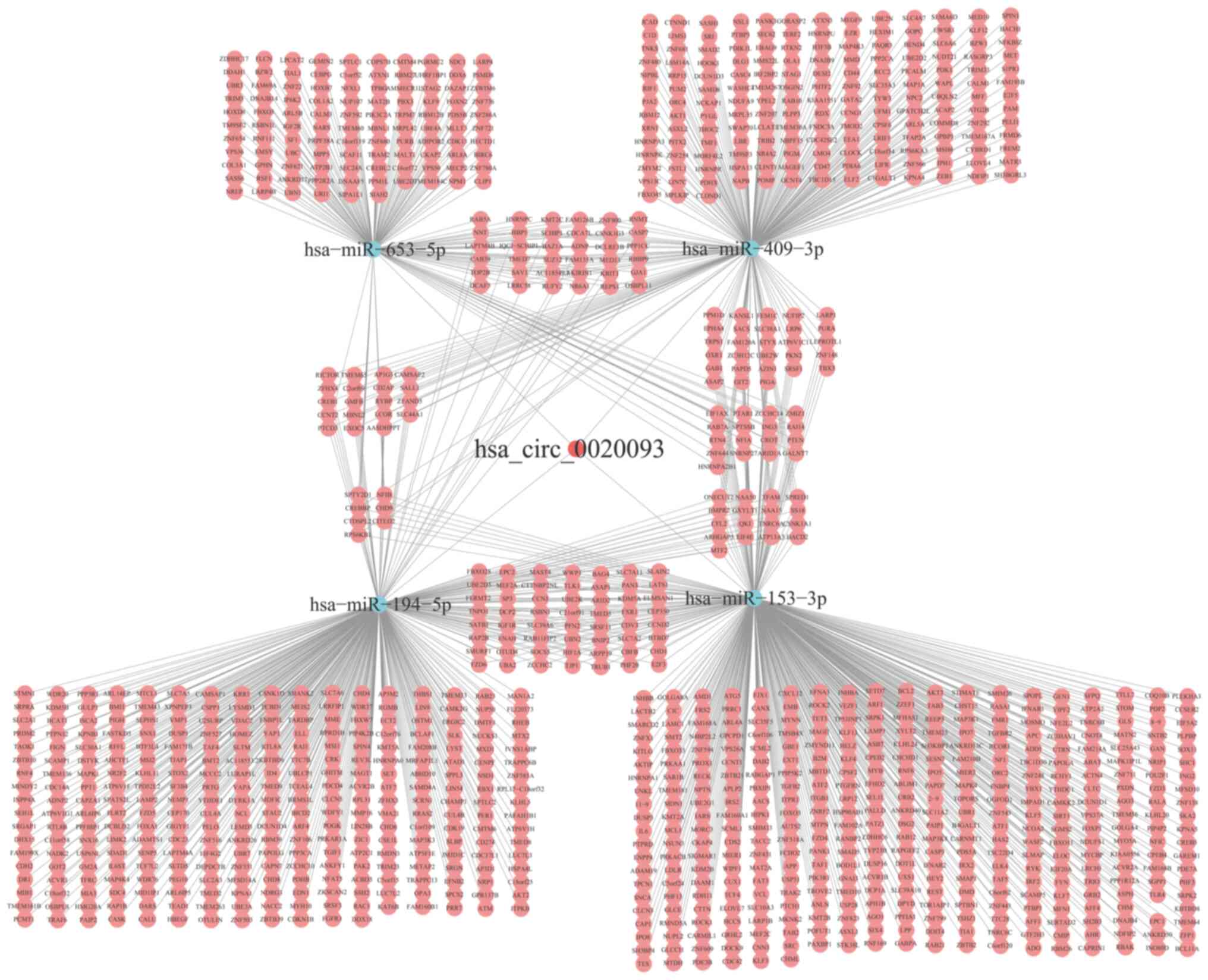
StarBase 3.0 databases, it was predicted that hsa_circ_0020093 can
bind to four miRNAs: hsa-miR-153-3p, hsa-miR-194-5p, hsa-miR-409-3p
and hsa-miR-653-5p. Subsequently, the starBase3.0 database was
utilized to identify a total of 1,146 downstream target genes for
these miRNAs. The intricate interactions between hsa_circ_0020093,
the miRNAs and their target genes are visualized in Fig. 5. To gain a deeper understanding of
these target genes, a PPI network was constructed using the STRING
11.5 database (Fig. 6). Within this
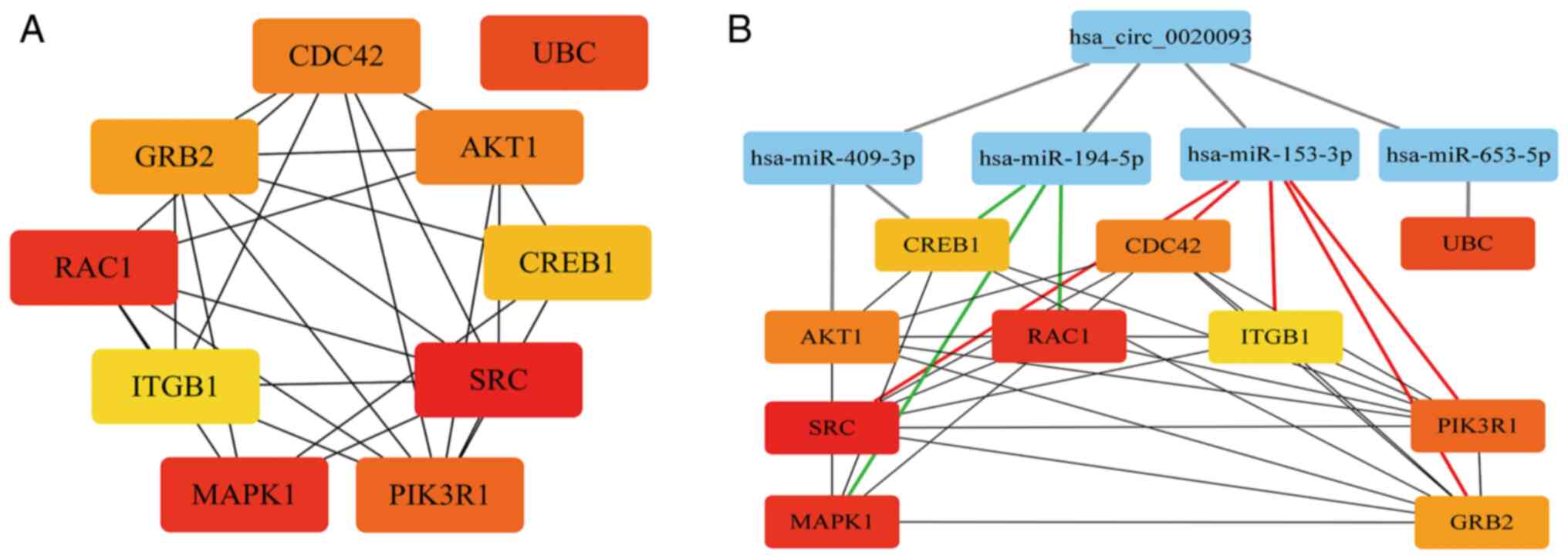
network, the top 10 hub genes were identified using the Degree
algorithm in Cytoscape (v3.8.0): SRC, RAC1, MAPK1, UBC, PIK3R1,
AKT1, CDC42, GRB2, CREB1 and ITGB1 (Fig. 7A). Furthermore, a ceRNA network was
constructed based on the interactions between hsa_circ_0020093, the
miRNAs and their target genes (Fig.
7B). Within this network, it was found that hsa-miR-153-3p
targets 5 genes, while hsa-miR-194-5p targets 3 genes. This leads
us to consider that hsa_circ_0020093 may play a critical role in
ALL by regulating downstream target genes through its interaction
with hsa-miR-153-3p or hsa-miR-194-5p.
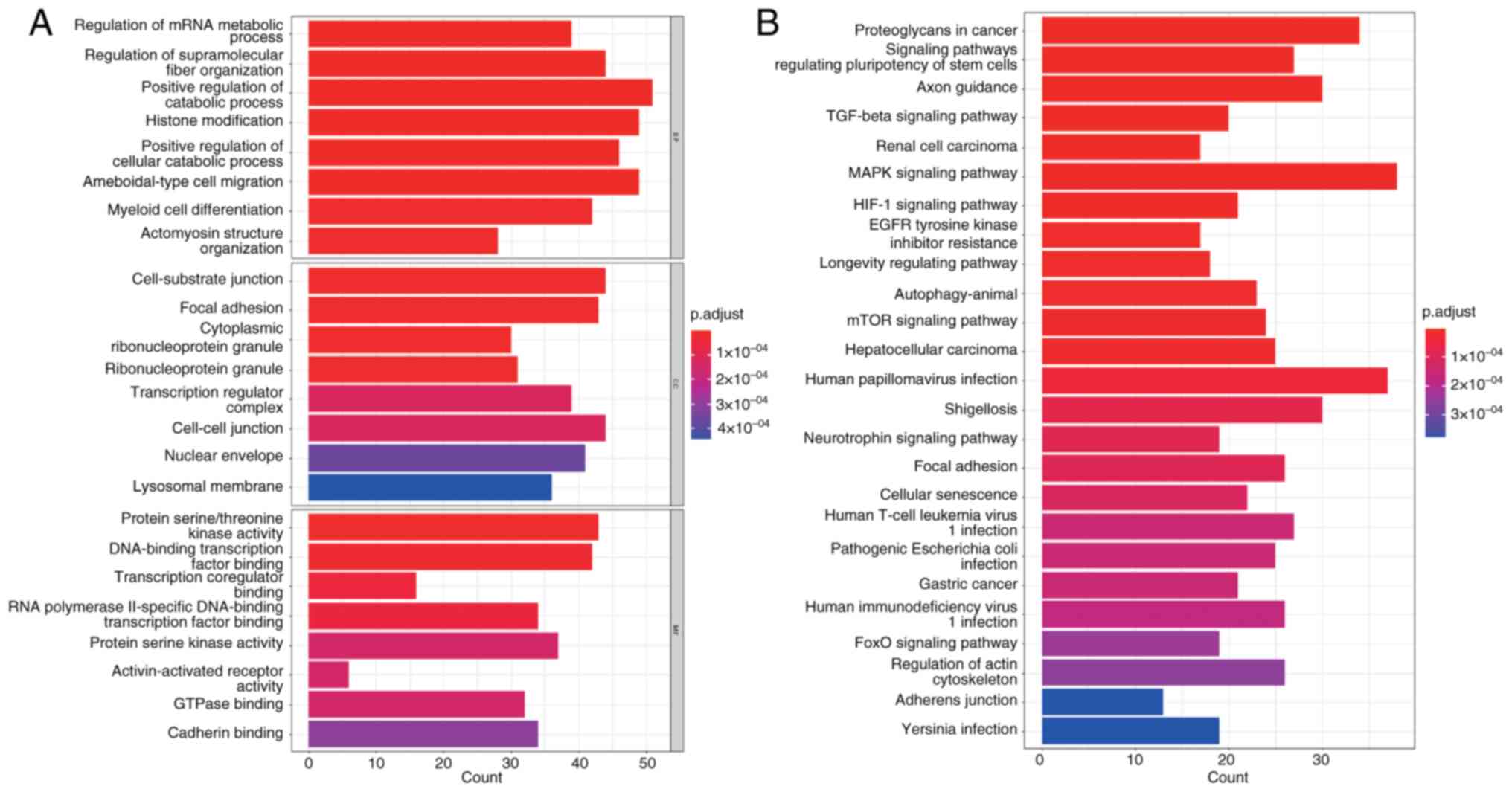
GO and KEGG analysis for
hsa_circ_0020093
Using the enrich plot package in R, GO and KEGG
enrichment analyses were conducted for the 843 target genes of
hsa-miR-153-3p and hsa-miR-194-5p (Fig.
8). Through GO analysis, it was observed that these target
genes play crucial roles in several biological processes, including
‘positive regulation of cellular catabolic processes’ and
‘amoeboid-type cell migration’. In terms of cellular components,
they are involved in ‘cell-substrate junction’ and ‘cytoplasmic
ribonucleoprotein granule’. Within the molecular functions
category, they exhibit activities such as ‘DNA-binding
transcription factor binding’, ‘protein serine kinase activity’ and
‘cadherin binding’, among others. KEGG analysis further suggested
that these target genes might be implicated in key pathways such as
the ‘Neurotrophin signaling pathway’, ‘Axon guidance’, ‘Human
T-cell leukemia virus 1 infection’, ‘HIF-1 signaling pathway’,
‘MAPK signaling pathway’, ‘mTOR signaling pathway’ and ‘Focal
adhesion’. These findings provided valuable insights into the
potential functions and regulatory mechanisms of these target genes
in various biological processes and pathways.
Discussion
AL represents a highly diverse group of blood
malignancies, necessitating precise classification. While the
conventional Morphology, Immunology, Cytogenetics and Molecular
Biology examination can differentiate most AL cases (28–30),
it requires considerable expertise from diagnosticians and costly
equipment, posing significant challenges to leukemia diagnoses
globally (4,5). Consequently, researchers have been
actively pursuing innovative and more efficient methods to improve
AL diagnosis and subtype identification. Studies have demonstrated
the potential of long ncRNAs (31,32),
miRNAs (33–35) and circRNAs (16) in the diagnosis and prognosis of AL.
However, the exploration in AL diagnostic typing is primarily
limited to bone marrow samples. Challenges arise when bone marrow
fibrosis results in dry pumping, rendering samples unavailable.
Therefore, there is an unmet need to investigate the expression
levels of circRNAs specifically in peripheral blood samples and
their role in AL. Peripheral blood samples provide convenient
access to the circRNAs, which can greatly reduce the patient's
suffering. Addressing this gap could notably contribute to
advancing the field and improving patient outcomes.
The present study aimed to develop a streamlined
approach for diagnosing AL by focusing exclusively on peripheral
blood samples. The present study delves into the role of circRNAs
in AL classification and preliminarily explores their underlying
mechanisms. Extensive sample analysis revealed that the expression
levels of hsa_circ_0012152 in peripheral blood were higher in
patients with AML compared with patients with ALL and healthy
individuals. This finding allows for the differentiation of
patients with AML from healthy subjects with high accuracy (AUC,
0.9878; P<0.0001) and serves as a potential marker for
distinguishing AML from ALL (AUC, 0.8634; P<0.0001). Similarly,
hsa_circ_0020093 expression is elevated in patients with ALL,
enabling differentiation from patients with AML and healthy
individuals (AUC, 0.8708; P<0.0001) and serving as another
potential marker for AML-ALL classification (AUC, 0.8401;
P<0.0001). Previous research has identified circ-PVT1 (15) as a diagnostic marker for ALL, while
circ-VIM (13), circAF4 (36) and circ-FOXO3 (37) can diagnose AML; however, these
studies were primarily based on bone marrow samples. The results of
the present study suggest that hsa_circ_0012152 and
hsa_circ_0020093 can serve as auxiliary and differential diagnostic
markers for AML and ALL, respectively, enriching the circRNA
expression profile for AL diagnosis and classification.
Furthermore, the present study demonstrated that the circRNA
expression profiles in the peripheral blood of newly diagnosed
patients with AL can be used for auxiliary diagnosis, offering a
more convenient alternative to bone marrow extraction. To improve
the diagnostic efficacy of hsa_circ_0012152 and hsa_circ_0020093 in
identifying AL, serial and parallel testing approaches were
evaluated. Both methods respectively increased specificity and
sensitivity, but with trade-offs. To enhance diagnostic efficiency
comprehensively, logistic regression models were explored (27), which integrate multiple indicators
into a single evaluation index. Combining hsa_circ_0012152 and
hsa_circ_0020093 using logistic regression models improved the
sensitivity in discriminating AL to 0.9884, resulting in a slightly
improved AUC of 0.8643. While the increase is modest, it may be due
to the limited number of ALL cases in the present study,
necessitating further sample collection for validation.
Additionally, it was found in the present study that
hsa_circ_0012152 can distinguish ‘APL with PML-RARA or t (15;17)’
from other AML groups using peripheral blood (AUC, 0.8366;
P<0.0001), and differentiate whether ALL expresses myeloid
antigens in peripheral blood (AUC, 0.8264; P=0.0067), further
enriching the circRNA expression profiles for AML subtype
differentiation, and providing valuable diagnostic assistance for
identifying patients with AL harboring chaotic immunological marker
expression. Overall, the findings of the present study suggest that
circRNAs hold promise as diagnostic and classification markers for
AL, offering a more convenient and less invasive alternative to
bone marrow-based methods.
The involvement of circRNAs in the onset and
progression of diseases is significantly influenced by their
function as miRNA sponges. Through the ceRNA network, circRNAs bind
to miRNAs, effectively modulating the expression of downstream
target genes that are crucial for disease development. For
instance, Han et al (38)
demonstrated that hsa_circ_0001947 inhibits AML cell proliferation
via the hsa_miR-329-5p/CREBRF axis. Similarly, Jamal et al
(39) found that the
circRNA-100290/miRNA-293/Rab10 axis can upregulate the RAS
signaling pathway, accelerating AML progression. In pediatric AML,
circRNA-0004136 adsorbs miRNA-142, promoting tumor cell
proliferation (40). These findings
underscore the ability of circRNAs to regulate target genes through
miRNA sponging, thereby influencing disease progression and
prognosis. In the present study, the expression levels of
hsa_circ_0012152 and hsa_circ_0020093 were significantly elevated
in AML and ALL, respectively, suggesting their involvement in the
pathogenesis of these diseases and potential diagnostic utility.
Guo et al (16) conducted
bioinformatics analyses on hsa_circ_0012152, speculating that it
may contribute to AML development through the activation of the
MAPK pathway via the
hsa_circ_0012152/miR-491-5p/miR-512-3p/EGFR/mitogen-activated
protein kinase 1 (MAPK1) axis. Meanwhile, bioinformatics analysis
of hsa_circ_0020093 in the present study, revealed its potential
involvement in ALL pathogenesis through miRNA sponging effects
involving hsa-miR-153-3p or hsa-miR-194-5p and subsequent
regulation of downstream target genes. A previous study has
established a negative correlation between growth factor
receptor-bound protein 2 (GRB2) expression and overall survival in
patients with ALL, highlighting its prognostic significance
(41). GRB2 plays a crucial
role in tyrosine kinase signaling, leading to the activation of
MAPK1 and MAPK3 (42). Inhibition of GRB2 has been
shown to suppress ALL progression, making it a promising
therapeutic target (42). Another
study implicated reduced miR335 expression in elevated MAPK1
levels and poor prognosis in pediatric patients with ALL,
emphasizing the prognostic significance of MAPK1 expression
levels (43). Signaling pathway
analysis has revealed an association between the ATP binding
cassette subfamily B member 1 gene and poor diagnosis and prognosis
in ALL, mediated through MAPK pathway activation (44). Furthermore, a study has shown that
deferoxamine may inactivate hypoxia-inducible factor 1 (HIF1) and
inhibit ALL progression via MAPK signaling pathways (45), further highlighting the contribution
of MAPK and HIF1 signaling in ALL development and progression.
Regarding the mammalian target of rapamycin (mTOR) signaling
pathway, numerous studies have linked mTOR dysregulation to
malignant cell proliferation (46–48).
Activation of the mTOR signaling pathway is often associated with
poor prognosis and chemoresistance in ALL. Taken together, these
findings underscore the complexity and interconnectedness of
molecular mechanisms underlying ALL pathogenesis and highlight the
potential diagnostic and therapeutic implications of
circRNA-mediated miRNA sponging in these diseases. In the expansion
of KEGG pathway prediction analysis for hsa_circ_0020093 downstream
target mRNA genes in the present study, it was determined that
PIK3R1, MAPK1 and GRB2 are intricately linked to the
‘mTOR signaling pathway’, whereas MAPK1, GRB2 and
CDC42 play a part in the ‘MAPK signaling pathway’. Based on
these findings, we hypothesize that hsa_circ_0020093 might regulate
the expression of downstream genes, namely CDC42, GRB2 and
MAPK1, by competitively binding to either hsa-miR-153-3p or
hsa-miR-194-5p. This, in turn, could activate crucial signaling
cascades such as MAPK and mTOR, ultimately driving the onset and
progression of ALL. Notably, this implicates not just CDC42,
GRB2 and MAPK1 as potential therapeutic targets for ALL,
but also highlights hsa_circ_0020093, hsa-miR-153-3p and
hsa-miR-194-5p as precise therapeutic candidates for the
disease.
The present study prospectively investigated the
expression patterns of hsa_circ_0012152 and hsa_circ_0020093 in
diagnosing AL and distinguishing its subtypes, thereby offering
valuable tools to enhance clinical management. These findings hold
notable significance in mitigating patient suffering and refining
the diagnostic categorization of AL. However, owing to the limited
availability of ALL samples, the study did not include T-ALL,
necessitating larger sample sizes and extended follow-up durations
in future research to validate the findings. Additionally, further
exploration is warranted to elucidate the association of
hsa_circ_0012152 or hsa_circ_0020093 with gene mutations,
chemotherapy resistance and their mechanistic role in AL.
In conclusion, the present study demonstrated that
hsa_circ_0020093 may regulate downstream target genes via
hsa-miR-153-3p or hsa-miR-194-5p, thereby contributing to the
initiation and progression of ALL. The combination of
hsa_circ_0012152 with hsa_circ_0020093 in peripheral blood can
facilitate the differentiation between AML and ALL, highlighting
their potential utility as diagnostic biomarkers for the disease
and providing a less invasive alternative to bone marrow
aspiration.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of
Zhejiang Province (grant no. LY20H080001), the Natural Science
Foundation of Ningbo (grant no. 202003N4228) and the Medical and
Health Science and Technology Projects of Zhejiang Province (grant
no. 2021KY283/2023KY263/2024KY1495).
Availability of data and materials
The sequencing data of hsa_circ_0012152 and
hsa_circ_0020093 examined in the present study may be found in the
GSA-human repository under the accession number HRA007384 or at the
following URL: https://bigd.big.ac.cn/gsa-human/browse/HRA007384. The
circRNA microarray data analyzed in the present study may be found
in the OMIX database (National Genomics Data Center, China) under
the accession number OMIX009143 or at the following URL: https://ngdc.cncb.ac.cn/omix/release/OMIX009143. All
other data generated in the present study may be requested from the
corresponding author.
Authors' contributions
QM was responsible for the study design and
framework development; QY, DL, YC and QM secured funding; DL
conducted the data collection and developed the statistical
analysis methods; YC performed chart and diagram creation. QM
supervised the overall project coordination and provided critical
oversight. QY led the writing of the original manuscript and QY and
YC contributed to data analysis and interpretation. QM reviewed and
edited the manuscript. QY, DL, YC and QM confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Ningbo University (Ningbo, China;
approval no. 159A for Research in 2023). All patients and
volunteers involved in the study provided written informed consent,
which includes permission for the use of remaining peripheral blood
specimens in this research. For those patients who were minors,
written consent was obtained from their parents or legal guardians,
and these minors also provided assent where applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AL
|
acute leukemia
|
|
AML
|
acute myeloid leukemia
|
|
ALL
|
acute lymphoblastic leukemia
|
|
circRNA
|
circular RNA
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
|
ROC
|
receiver operating characteristic
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
ncRNAs
|
non-coding RNAs
|
|
miRNAs
|
microRNAs
|
|
AUC
|
area under the curve
|
|
FC
|
Fold Change
|
|
PPI
|
protein-protein interaction
|
|
ceRNA
|
competing endogenous RNA
|
|
95% CI
|
95% confidence interval
|
References
|
1
|
Devine SM and Larson RA: Acute leukemia in
adults: Recent developments in diagnosis and treatment. CA Cancer J
Clin. 44:326–352. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng H, Huang CM, Wang Y, Hu XX, Xu XQ,
Song XM, Tang GS, Chen L and Yang JM: Microarray profiling and
co-expression network analysis of the lncRNAs and mRNAs associated
with acute leukemia in adults. Mol Biosyst. 13:1102–1108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pui CH, Schrappe M, Ribeiro RC and
Niemeyer CM: Childhood and adolescent lymphoid and myeloid
leukemia. Hematology Am Soc Hematol Educ Program. 118–145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miranda-Filho A, Piñeros M, Ferlay J,
Soerjomataram I, Monnereau A and Bray F: Epidemiological patterns
of leukaemia in 184 countries: A population-based study. Lancet
Haematol. 5:e14–e24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oliveira PD: Leukaemia prevalence
worldwide: Raising aetiology questions. Lancet Haematol. 5:e2–e3.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porwit A and Béné MC: Acute leukemias of
ambiguous origin. Am J Clin Pathol. 144:361–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu XQ, Wang JM, Lu SQ, Chen L, Yang JM,
Zhang WP, Song XM, Hou J, Ni X and Qiu HY: Clinical and biological
characteristics of adult biphenotypic acute leukemia in comparison
with that of acute myeloid leukemia and acute lymphoblastic
leukemia: A case series of a Chinese population. Haematologica.
94:919–927. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rong D, Sun H, Li Z, Liu S, Dong C, Fu K,
Tang W and Cao H: An emerging function of circRNA-miRNAs-mRNA axis
in human diseases. Oncotarget. 8:73271–73281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lei K, Bai H, Wei Z, Xie C, Wang J, Li J
and Chen Q: The mechanism and function of circular RNAs in human
diseases. Exp Cell Res. 368:147–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo SS, Li BX, Zou DB, Yang SJ, Sheng LX,
Ouyang GF, Mu QT and Huang H: Tip of the iceberg: Roles of circRNAs
in hematological malignancies. Am J Cancer Res. 10:367–382.
2020.PubMed/NCBI
|
|
13
|
Yi YY, Yi J, Zhu X, Zhang J, Zhou J, Tang
X, Lin J, Wang P and Deng ZQ: Circular RNA of vimentin expression
as a valuable predictor for acute myeloid leukemia development and
prognosis. J Cell Physiol. 234:3711–3719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Zhong C, Jiao J, Li P, Cui B, Ji C
and Ma D: Characterization of hsa_circ_0004277 as a new biomarker
for acute myeloid leukemia via circular RNA profile and
bioinformatics analysis. Int J Mol Sci. 18:5972017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu J, Han Q, Gu Y, McGrath M, Qiao F, Chen
B, Song C and Ge Z: Circular RNA PVT1 expression and its roles in
acute lymphoblastic leukemia. Epigenomics. 10:723–732. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo S, Li B, Chen Y, Zou D, Yang S, Zhang
Y, Wu N, Sheng L, Huang H, Ouyang G and Mu Q: Hsa_circ_0012152 and
Hsa_circ_0001857 accurately discriminate acute lymphoblastic
leukemia from acute myeloid leukemia. Front Oncol. 10:16552020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CQ, Yang Y, Mao H, Zhao Q, Wu JQ, Li
DP and Huang YH: Clinical characteristics, prognostic factors and
misdiagnosis of bone marrow necrosis patients. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 29:1637–1644. 2021.(In Chinese). PubMed/NCBI
|
|
18
|
Ridgeway JA, Tinsley S and Kurtin SE:
Practical guide to bone marrow sampling for suspected
myelodysplastic syndromes. J Adv Pract Oncol. 8:29–39.
2017.PubMed/NCBI
|
|
19
|
Butler JT, Yashar WM and Swords R:
Breaking the bone marrow barrier: Peripheral blood as a gateway to
measurable residual disease detection in acute myelogenous
leukemia. Am J Hematol. 100:638–651. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hematology Oncology Committee, Chinese
Anti-Cancer Association, Leukemia & Lymphoma Group, Chinese
Society of Hematology and Chinese Medical Association, . Chinese
guideline for diagnosis and treatment of adult acute lymphoblastic
leukemia (2024). Zhonghua Xue Ye Xue Za Zhi. 45:417–429. 2024.(In
Chinese). PubMed/NCBI
|
|
21
|
Khoury JD, Solary E, Abla O, Akkari Y,
Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, et
al: The 5th edition of the World Health Organization classification
of haematolymphoid tumours: myeloid and histiocytic/dendritic
neoplasms. Leukemia. 36:1703–1719. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the World Health
Organization classification of haematolymphoid tumours: Lymphoid
neoplasms. Leukemia. 36:1720–1748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Staples TL: Expansion and evolution of the
R programming language. R Soc Open Sci. 10:2215502023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doncheva NT, Morris JH, Gorodkin J and
Jensen LJ: Cytoscape StringApp: Network analysis and visualization
of proteomics data. J Proteome Res. 18:623–632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan Y, Tan Y, Li J, Hu P, Guan P, Kuang H,
Liang Q, Yu Y, Chen Z, Wang Q, et al: Combined IFN-γ and IL-2
release assay for detect active pulmonary tuberculosis: A
prospective multicentre diagnostic study in China. J Transl Med.
19:2892021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi K, Uchiyama H, Yanagisawa S and
Kamae I: The logistic regression and ROC analysis of group-based
screening for predicting diabetes incidence in four years. Kobe J
Med Sci. 52:171–180. 2006.PubMed/NCBI
|
|
28
|
Löwenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weir EG and Borowitz MJ: Flow cytometry in
the diagnosis of acute leukemia. Semin Hematol. 38:124–138. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McKenna RW: Multifaceted approach to the
diagnosis and classification of acute leukemias. Clin Chem.
46:1252–1259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang WT, Chen TQ, Zeng ZC, Pan Q, Huang W,
Han C, Fang K, Sun LY, Yang QQ, Wang D, et al: The lncRNA LAMP5-AS1
drives leukemia cell stemness by directly modulating DOT1L
methyltransferase activity in MLL leukemia. J Hematol Oncol.
13:782020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bárcenas-López DA, Núñez-Enríquez JC,
Hidalgo-Miranda A, Beltrán-Anaya FO, May-Hau DI, Jiménez-Hernández
E, Bekker-Méndez VC, Flores-Lujano J, Medina-Sansón A, Tamez-Gómez
EL, et al: Transcriptome analysis identifies LINC00152 as a
biomarker of early relapse and mortality in acute lymphoblastic
leukemia. Genes (Basel). 11:3022020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gentner B, Pochert N, Rouhi A, Boccalatte
F, Plati T, Berg T, Sun SM, Mah SM, Mirkovic-Hösle M, Ruschmann J,
et al: MicroRNA-223 dose levels fine tune proliferation and
differentiation in human cord blood progenitors and acute myeloid
leukemia. Exp Hematol. 43:858–868.e7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hornick NI, Huan J, Doron B, Goloviznina
NA, Lapidus J, Chang BH and Kurre P: Serum exosome MicroRNA as a
minimally-invasive early biomarker of AML. Sci Rep. 5:112952015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Avigad S, Verly IRN, Lebel A, Kordi O,
Shichrur K, Ohali A, Hameiri-Grossman M, Kaspers GJL, Cloos J,
Fronkova E, et al: miR expression profiling at diagnosis predicts
relapse in pediatric precursor B-cell acute lymphoblastic leukemia.
Genes Chromosomes Cancer. 55:328–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang W, Fang K, Chen TQ, Zeng ZC, Sun YM,
Han C, Sun LY, Chen ZH, Yang QQ, Pan Q, et al: circRNA circAF4
functions as an oncogene to regulate MLL-AF4 fusion protein
expression and inhibit MLL leukemia progression. J Hematol Oncol.
12:1032019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou J, Zhou LY, Tang X, Zhang J, Zhai LL,
Yi YY, Yi J, Lin J, Qian J and Deng ZQ: Circ-Foxo3 is positively
associated with the Foxo3 gene and leads to better prognosis of
acute myeloid leukemia patients. BMC Cancer. 19:9302019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han F, Zhong C, Li W, Wang R, Zhang C,
Yang X, Ji C and Ma D: hsa_circ_0001947 suppresses acute myeloid
leukemia progression via targeting hsa-miR-329-5p/CREBRF axis.
Epigenomics. 12:935–953. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jamal M, Song T, Chen B, Faisal M, Hong Z,
Xie T, Wu Y, Pan S, Yin Q, Shao L and Zhang Q: Recent progress on
circular RNA research in acute myeloid leukemia. Front Oncol.
9:11082019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan DM, Ma J and Fang WB: Identification
of non-coding RNA regulatory networks in pediatric acute myeloid
leukemia reveals circ-0004136 could promote cell proliferation by
sponging miR-142. Eur Rev Med Pharmacol Sci. 23:9251–9258.
2019.PubMed/NCBI
|
|
41
|
Puil L, Liu J, Gish G, Mbamalu G, Bowtell
D, Pelicci PG, Arlinghaus R and Pawson T: Bcr-Abl oncoproteins bind
directly to activators of the Ras signalling pathway. EMBO J.
13:764–773. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohanian M, Tari Ashizawa A, Garcia-Manero
G, Pemmaraju N, Kadia T, Jabbour E, Ravandi F, Borthakur G,
Andreeff M, Konopleva M, et al: Liposomal Grb2 antisense
oligodeoxynucleotide (BP1001) in patients with refractory or
relapsed haematological malignancies: A single-centre, open-label,
dose-escalation, phase 1/1b trial. Lancet Haematol. 5:e136–e146.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan J, Jiang N, Huang G, Tay JL, Lin B, Bi
C, Koh GS, Li Z, Tan J, Chung TH, et al: Deregulated MIR335 that
targets MAPK1 is implicated in poor outcome of paediatric acute
lymphoblastic leukaemia. Br J Haematol. 163:93–103. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tomiyasu H, Watanabe M, Sugita K,
Goto-Koshino Y, Fujino Y, Ohno K, Sugano S and Tsujimoto H:
Regulations of ABCB1 and ABCG2 expression through MAPK pathways in
acute lymphoblastic leukemia cell lines. Anticancer Res.
33:5317–5323. 2013.PubMed/NCBI
|
|
45
|
You H, Wang D, Wei L, Chen J, Li H and Liu
Y: Deferoxamine inhibits acute lymphoblastic leukemia progression
through repression of ROS/HIF-1 α, Wnt/β-catenin, and p38MAPK/ERK
pathways. J Oncol. 2022:82812672022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Simioni C, Martelli AM, Zauli G, Melloni E
and Neri LM: Targeting mTOR in acute lymphoblastic leukemia. Cells.
8:1902019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Evangelisti C, Chiarini F, Cappellini A,
Paganelli F, Fini M, Santi S, Martelli AM, Neri LM and Evangelisti
C: Targeting Wnt/β-catenin and PI3K/Akt/mTOR pathways in T-cell
acute lymphoblastic leukemia. J Cell Physiol. 235:5413–5428. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ge Z, Song C, Ding Y, Tan BH, Desai D,
Sharma A, Gowda R, Yue F, Huang S, Spiegelman V, et al: Dual
targeting of MTOR as a novel therapeutic approach for high-risk
B-cell acute lymphoblastic leukemia. Leukemia. 35:1267–1278. 2021.
View Article : Google Scholar : PubMed/NCBI
|