Introduction
Primary gastric squamous cell carcinoma (PGSCC) is a
rare gastric malignancy, accounting for only 0.04–0.07% of all
gastric cancers (1), with ~100
cases documented in the literature (2). The incidence ratio of men to women is
5:1 (1), and the disease
predominantly affects patients >60 years old. The exact etiology
remains unknown; however, previous studies have suggested an
association with long-term smoking (3,4).
Therefore, PGSCC may be more commonly found in male patients. There
is no standardized treatment protocol for PGSCC, and it often
carries a poor prognosis due to its typical late-stage diagnosis.
The median survival time for patients with PGSCC has been reported
to be as short as 7 months (5),
reflecting the aggressive nature of this tumor. This poor prognosis
is primarily attributed to the non-specific symptoms of PGSCC, such
as dysphagia, weight loss and epigastric pain, which are often
mistaken for other gastric conditions. To date, to the best of our
knowledge, no research has definitively clarified the pathogenesis
of PGSCC (2). The primary aim of
the present case report was to contribute to the limited knowledge
of PGSCC and explore the potential treatment options, including
surgery, in managing this rare disease.
Case report
The patient, a 57-year-old man with a 30-year
history of smoking and tobacco pipe use (~20 cigarettes per day),
presented with epigastric pain and 6 kg of weight loss over the
past 3 months. The patient was admitted to the Vietnam National
Cancer Hospital (Hanoi, Vietnam) in March 2023. A physical
examination revealed no notable abnormalities. Upper
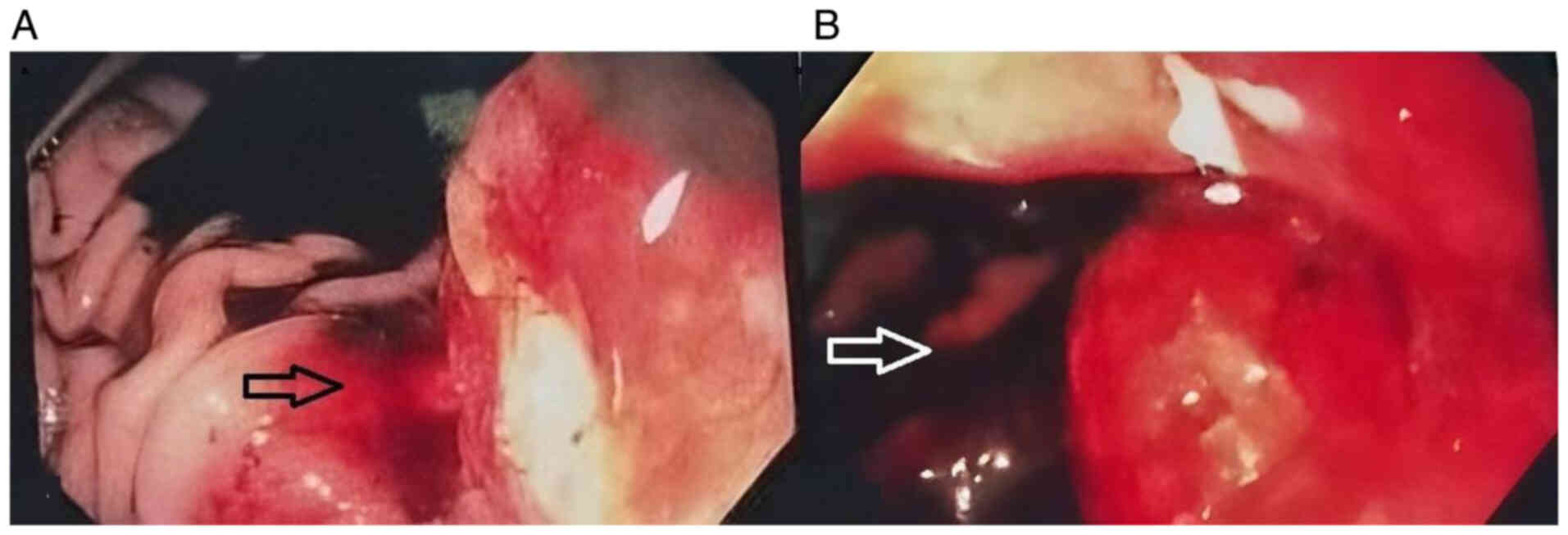
gastrointestinal endoscopy demonstrated a large, ulcerated lesion,
~5 cm in size, located at the posterior wall of the gastric body,
with gastrointestinal bleeding classified as Forrest class IIB
originating from the tumor (Fig.
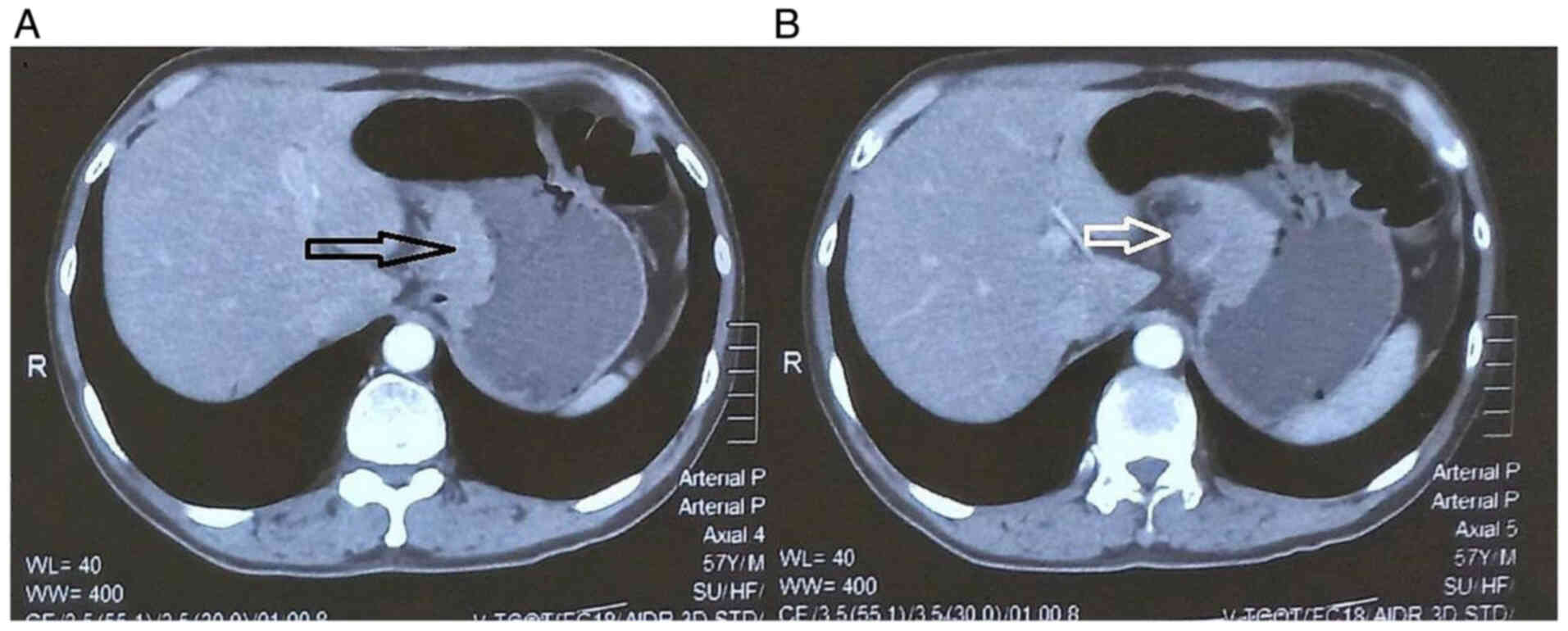
1). Computed tomography identified a tumor at the lesser
curvature of the stomach, measuring ~40 mm in length and 20 mm in
maximal thickness. Strong contrast enhancement following injection,
surrounding infiltration and an enlarged lymph node near the lesser
curvature measuring 14×15 mm were observed (Fig. 2). The tumor was classified as
cT4N1M0 (American Joint Committee on Cancer/International Union
Against Cancer, 8th edition) (6). A
preoperative biopsy previously performed at an external hospital
confirmed the diagnosis of PGSCC.
The patient underwent open surgery at 7 days
post-admission. Intraoperative findings included no peritoneal
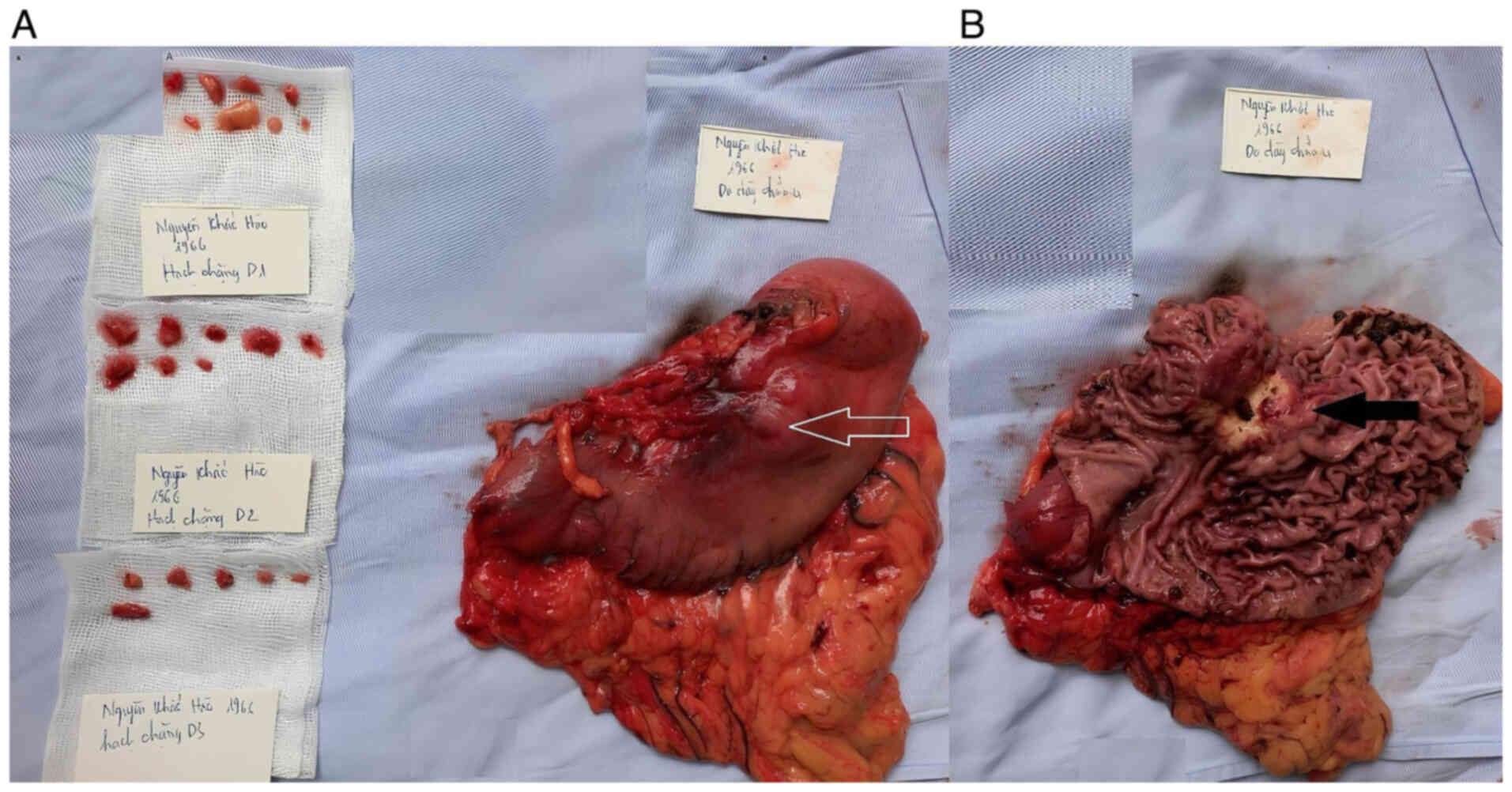
effusion or peritoneal metastasis. The tumor was located at the
lesser curvature, posterior to the gastric body, and measured 5×6
cm, with invasion into the peritoneum covering the pancreas
(Fig. 3). Lymph nodes in groups 7,
8a, 9, 10, 11p and 11d, ranging from 1 to 1.5 cm in size, were
suspected of metastasis.
Due to the aggressive nature of PGSCC, a total
gastrectomy was performed along with an omentectomy, a bursectomy
of the peritoneum covering the pancreas and an extended D2 lymph
node dissection (D2 + 10, 12a, 12p, 13 and 16). A Roux-en-Y
anastomosis was completed and a gastric tube was placed to
facilitate early postoperative feeding. The patient recovered well
with no complications reported. A postoperative barium swallow
study demonstrated normal findings 1 week after surgery, allowing
for nasogastric tube removal. The patient was discharged 3 days
later.
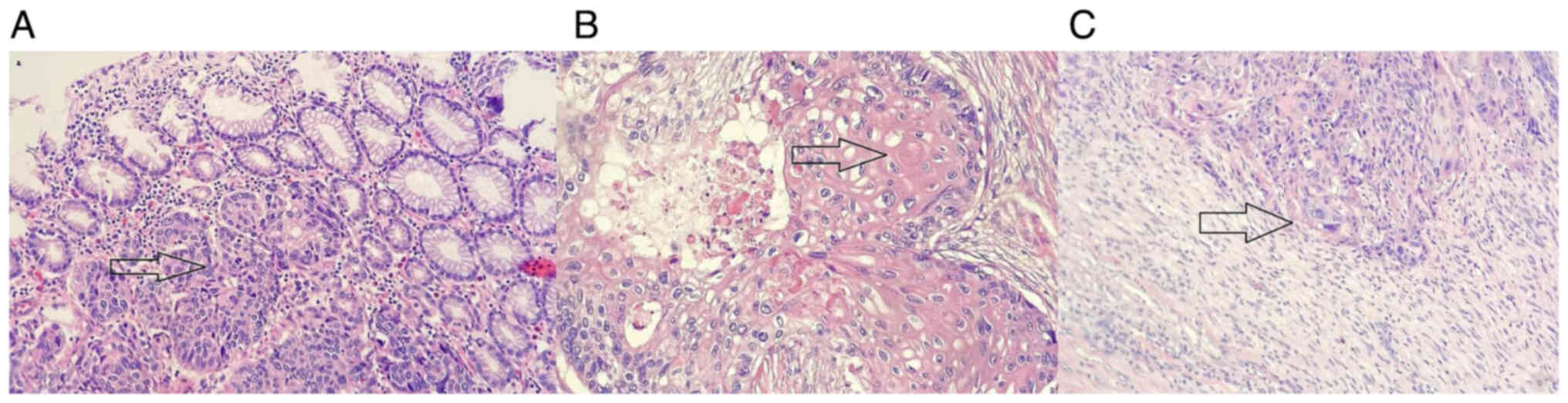
Pathological examination (10% neutral-buffered
formalin at 20–25°C for 24 h; 4-µm thick sections; hematoxylin and
eosin staining processed using automatic HE Staining on Dako
Systems; Olympus BX53 microscopy) reported a moderately
differentiated primary SCC (Fig.
4), with peritoneal and perineural invasion. Metastatic nodes
were found in groups D1 (2/8) and D2 (2/9), with no metastatic
nodes found in group D2+ (0/7). This result was thoroughly
discussed with the pathologist, who affirmed that additional
immunohistochemical staining was not required to establish the
diagnosis. Accordingly, the postoperative stage was classified as
pT4bN2M0.
The patient began an adjuvant chemotherapy regimen
at 1-month post-surgery with capecitabine and oxaliplatin (XELOX)
for a total of eight cycles. The XELOX regimen consisted of
oxaliplatin at a dose of 130 mg/m2 administered
intravenously on day 1, and capecitabine at a dose of 1,000-1,250
mg/m2 taken orally twice daily on days 1–14 of a 3-week
cycle. Follow-up was conducted every 3 months, with no recurrence
or metastasis detected after 1 year of follow-up.
Discussion
PGSCC comprises 0.04–0.07% of all gastric cancers
(1), with only ~100 cases recorded
in the literature to date (2). The
incidence ratio of male to female patients is ~5:1 (1), with most cases of disease occurring in
patients >60 years old. Despite its rarity, PGSCC is often
diagnosed at an advanced stage due to the lack of specific symptoms
and reliable biomarkers for early detection. The aggressive nature
of this tumor is reflected in its poor prognosis, with a median
survival time of only 7 months reported (5). The present case shares several
characteristics commonly described in other reports on PGSCC. For
instance, in a study of 21 PGSCC cases by Chen et al
(3), 85.7% were male patients, with
two-thirds of tumors located in the upper third of the stomach, and
62% of patients had a history of smoking. Long-term tobacco use can
lead to chronic inflammation and oxidative stress in the gastric
mucosa, contributing to genetic mutations and carcinogenesis
(7). This finding suggests a
potential association between prolonged tobacco use and the
occurrence of PGSCC, similar to lung or esophageal cancers. This
observation aligns with the present case, as the patient was a
69-year-old male with a marked history of smoking, further
supporting the potential association between prolonged tobacco use
and the development of PGSCC.
The etiology of PGSCC remains uncertain despite
several hypotheses. Mori et al (8) identified an adenocarcinoma component
in the histology of three PGSCC cases, leading to the hypothesis
that PGSCC might originate from adenocarcinoma. Takita et al
(9) utilized polymerase chain
reaction to detect Epstein-Barr virus (EBV) infection in surgical
specimens of the tumor. The findings proposed that EBV infection
could play a role in the pathogenesis of PGSCC in some cases.
Typically, PGSCC is diagnosed as SCC based on biopsy
samples from an upper gastrointestinal endoscopy. However,
postoperative pathological findings often reveal gastric
adenosquamous carcinoma or SCC extending from the esophagus.
Therefore, clear diagnostic criteria for PGSCC are essential to
differentiate it from other cases. Parks (10) proposed the following diagnostic
criteria: i) The tumor should not be located at the cardia; ii) it
should not be esophageal cancer spreading to the stomach; and iii)
there should be no SCC in other organs (such as the uterus, lung,
bronchus or pancreas). Additionally, the Japanese Gastric Cancer
Association (JGCA) suggested the following criteria (8): i) All tumor cells must be squamous
cells without glandular elements; and ii) there must be evidence
that the tumor originated from the gastric mucosa. The present case
met both Parks' and JGCA criteria.
Currently, there are no standardized treatment
protocols for PGSCC due to limited data on surgical,
chemotherapeutic and radiotherapeutic approaches for this rare
disease (1). Gastrectomy and
lymphadenectomy remain the primary treatments. Postoperative
adjuvant chemotherapy, including regimens based on 5-fluorouracil,
is administered similarly to gastric adenocarcinoma treatment.
Alternative regimens include fluorouracil + oxaliplatin + calcium
folinate (FOLFOX) or capecitabine + oxaliplatin (XELOX). As with
chemotherapy, the role of radiotherapy in PGSCC has not been
established; however, some cases have utilized combined
chemoradiotherapy. Schmidt et al (11) reported a PGSCC case with a 5-year
disease-free survival following surgery, radiotherapy and
chemotherapy. However, Wakabayashi et al (1) reported a case treated solely with
surgery and chemotherapy. Postoperative follow-up for this patient
for the first 18 months was uneventful. However, the patient
subsequently developed multiple liver metastases and para-aortic
lymph node metastases. Neoadjuvant chemotherapy may be effective
for some patients with PGSCC (12),
although its indications and role remain uncertain (3). Therefore, further studies with a
larger patient population are needed to thoroughly evaluate
appropriate treatment strategies for PGSCC.
In the present case, a total gastrectomy,
omentectomy and lymphadenectomy were performed as recommended by
the JGCA for advanced gastric cancer. A bursectomy was conducted
due to the location of the tumor at the posterior gastric body with
invasion into the anterior pancreatic peritoneum. Typically, the
JGCA recommends a D2 lymphadenectomy in cases of locally advanced
disease with suspected lymph node metastasis (13). In the present case, an extended D2
lymphadenectomy (D2+) was chosen, including hepatic pedicle nodes
(12a, 12p and 12b), groups 10, 13 and 16. Given the poor prognosis
associated with PGSCC and its high malignancy, along with the
advanced stage of the tumor, including perineural and adjacent
organs invasion, it was reasonable for extensive surgery combined
with more extensive lymphadenectomy to be performed.
PGSCC is often diagnosed at an advanced stage,
frequently presenting with lymph node or liver metastases. This may
be due to its rarity and non-specific symptoms, which overlap with
other more common gastric conditions. Additionally, the lack of
specific biomarkers for early detection contributes to the
difficulty in diagnosing PGSCC at an earlier stage. Therefore,
endoscopic screening programs, especially in high-risk populations,
such as those with a history of smoking, should also be considered.
Compared with adenocarcinoma, PGSCC is more aggressive and has a
higher risk of lymph node metastasis (the present case was
classified as pT3N2M0 with four metastatic lymph nodes and
perineural invasion) (2).
Therefore, PGSCC often has a poor prognosis. Meng et al
(5) conducted a retrospective study
showing that PGSCC has a worse prognosis than gastric
adenocarcinoma, with a median survival time of only 7 months.
Meanwhile, advanced gastric adenocarcinoma typically has a median
survival time of 11–17 months with standard treatment (14). This difference further underscores
the aggressive nature and poor prognosis of PGSCC. Each case
represents a valuable opportunity to explore adjuvant or
alternative treatment strategies. The decision to proceed with
upfront surgery, followed by postoperative adjuvant therapy, is
also a strategy that could be proposed. However, further studies
with larger sample sizes are needed to clarify this approach.
To conclude, PGSCC is a rare form of gastric
malignancy that is often diagnosed at an advanced stage.
Gastrectomy combined with D2 lymphadenectomy remains the standard
treatment approach, with the use of chemotherapy and radiotherapy
remaining contentious. Additional research with larger sample sizes
is essential to accurately evaluate the optimal therapeutic regimen
for PGSCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conception and design was performed by BVP, DDN,
MDT, TDN, ADT, BTN and KVL. Administrative support was provided by
BVP, TDN, ADT, BTN, KVL and HTTN. Provision of study materials of
patients was performed by BVP, DDN, MDT, ADT, KVL and HTTN.
Collection and assembly of data was performed by BVP, DDN, MDT,
TDN, ADT and HTTN. Data analysis and interpretation was performed
by BVP, DDN, ADT, BTN and HTTN. The manuscript was written by all
authors. All authors read and approved the final version of the
manuscript. BVP and HTTN confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was conducted with informed
patient consent to participate and received the requisite ethical
approval from the Scientific Council of Vietnam National Cancer
Hospital (Hanoi, Vietnam; approval no. 3366/BVK-HDDD; dated
February 3, 2023). The council comprises expert representatives
from relevant specialties, including gastrointestinal surgeons,
radiologists, oncologists, gastroenterologists and pathologists.
Their comprehensive review and endorsement ensured adherence to the
highest ethical standards throughout the research process. The
procedures adhered to the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wakabayashi H, Matsutani T, Fujita I,
Kanazawa Y, Nomura T, Hagiwara N, Hosone M, Katayama H and Uchida
E: A rare case of primary squamous cell carcinoma of the stomach
and a review of the 56 cases reported in Japan. J Gastric Cancer.
14:58–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gülçiçek OB, Solmaz A, Özdoğan K, Erçetin
C, Yavuz E, Yiğitbaş H, Çelebi F and Altınay S: Primary squamous
cell carcinoma of the stomach. Ulus Cerrahi Derg. 32:221–223.
2015.PubMed/NCBI
|
|
3
|
Chen Y, Zhu H, Xu F, Cao Y, Gu X, Wan Y
and Gou H: Clinicopathological characteristics, treatment, and
prognosis of 21 patients with primary gastric squamous cell
carcinoma. Gastroenterol Res Pract. 2016:30625472016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Lange G, Bouroumeau A, Coron E and
Koessler T: Gastric squamous cell carcinoma: A rare malignancy,
literature review and management recommendations (Review). Mol Clin
Oncol. 19:1–6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng Y, Zhang J, Wang H, Zhang Y, Sun R,
Zhang Z, Gao F, Huang C and Zhang S: Poorer prognosis in patients
with advanced gastric squamous cell carcinoma compared with
adenocarcinoma of the stomach: Case report. Medicine (Baltimore).
96:e92242017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. 8th Edition.
John Wiley and Sons; Hoboken, NJ: pp. 63–66. 2017
|
|
7
|
Beattie M, Mansour R, Thigpin D and Haus
C: Metastatic primary gastric squamous cell carcinoma: An uncommon
presentation of a rare malignancy. Case Rep Gastrointest Med.
2019:e53050232019.PubMed/NCBI
|
|
8
|
Mori M, Iwashita A and Enjoji M: Squamous
cell carcinoma of the stomach: Report of three cases. Am J
Gastroenterol. 81:339–342. 1986.PubMed/NCBI
|
|
9
|
Takita J, Kato H, Miyazaki T, Nakajima M,
Fukai Y, Masuda N, Manda R, Fukuchi M and Kuwano H: Primary
squamous cell carcinoma of the stomach: A case report with
immunohistochemical and molecular biologic studies.
Hepatogastroenterology. 52:969–974. 2005.PubMed/NCBI
|
|
10
|
Parks RE: Squamous neoplasms of the
stomach. Am J Roentgenol Radium Ther Nucl Med. 101:447–449. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt C, Schmid A, Lüttges JE, Kremer B
and Henne-Bruns D: Primary squamous cell carcinoma of the stomach.
Report of a case and review of literature. Hepatogastroenterology.
48:1033–1036. 2001.PubMed/NCBI
|
|
12
|
Chang YS, Kim MS, Kim DH, Park S, You JY,
Han JK, Kim SH and Lee HJ: Primary squamous cell carcinoma of the
remnant stomach after subtotal gastrectomy. J Gastric Cancer.
16:120–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd english edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogata T, Narita Y, Oze I, Kumanishi R,
Nakazawa T, Matsubara Y, Kodama H, Nakata A, Honda K, Masuishi T,
et al: Chronological improvement of survival in patients with
advanced gastric cancer over 15 years. Ther Adv Med Oncol.
16:175883592412294282024. View Article : Google Scholar : PubMed/NCBI
|