Introduction
Primary central nervous system lymphoma (PCNSL) is
recognized as a distinct category of lymphoma, accounting for ~4%
of all brain tumors in the United States (1). In comparison with different lymphoma
types, the prognosis for PCNSL is considerably worse, with the 5-
and 10-year relative survival rates are 35.2 and 27.5%,
respectively (2–4). Furthermore, recent epidemiological
data showed a significant rise in PCNSL incidence, reaching 0.4
cases per 100,000 population in 2022 (4), partly due to the aging global
population (2,5). Therefore, several deep learning
methodologies, enhanced by computer technology, have been utilized
in the diagnosis and treatment of numerous cancers, including brain
tumors, to transform cancer care (6–8).
Despite considerable progress derived from a deeper comprehension
of the disease regarding treatment, challenges remain in the
management of PCNSL. Only a limited number of studies have used
advanced methodologies in their investigation, including our
previous research contributions (9–12).
This shortage largely stems from the relatively infrequent
occurrence of PCNSL, a constrained patient demographic and the high
mortality rate associated with PCNSL when compared with other
lymphoma types (4,5). Therefore, the present study aimed to
contribute meaningfully to tackling the aforementioned
challenges.
Magnetic resonance imaging (MRI) using
gadolinium-based contrast agents has been used for the detection of
PCNSL, serving as the most sensitive imaging modality within
radiographic evaluations. Accurate assessment of lesions using
gadolinium-enhanced MRI is essential for planning therapy,
monitoring and determining prognosis in PCNSL (13–15),
similar to glioma and meningioma. Studies concerning brain tumors,
such as glioma and meningioma, have reported that deep learning
techniques leveraging MRI can cohesively integrate processes such
as medical image fusion, segmentation, feature extraction and
classification to effectively classify and segment tumors with
marked reproducibility (16,17).
Advancements in computer-aided medicine have notably
improved the detection and segmentation of brain tumors using deep
learning techniques, facilitating their transition into broader
clinical practice (18–20). The implementation of deep learning
models for automated tumor detection and segmentation has emerged
as a transformative approach, presenting opportunities for
integration into long-term treatment monitoring. Nonetheless,
achieving precise segmentation of brain lymphoma remains a clinical
challenge due to the intricate anatomical features of the tumor and
the overlap between the tumor margins and normal brain tissue
(21). Moreover, previous findings
indicate that standard 2-dimentional (2D) measurements that have
traditionally been used to assess tumor size and progression, are
not as dependable or accurate as volumetric assessments. Whilst 2D
measurements rely on simplified metrics, such as maximum diameter
or cross-sectional area, they often fail to capture the complex
3-dimensional (3D) morphology of brain tumors, leading to potential
inaccuracies in clinical evaluation and treatment planning
(22). Manual segmentation for
evaluating brain tumor volumes is labor-intensive and highly
susceptible to variability among different raters, resulting in
inconsistent interpretations among imaging professionals (22,23).
Such inconsistencies can severely affect subsequent evaluations of
the disease, treatment choices and monitoring efforts, ultimately
impacting the survival and prognosis of patients with PCNSL. Thus,
there is a growing need for a segmentation approach that can
effectively address the challenges associated with 2D measurements
and manual volume assessments. The application of computer-assisted
automatic segmentation is regarded as a promising solution, and
deep learning enables automated lesion segmentation, reduces
variability, enhances throughput and improves the detection of
complex lesions.
Currently, deep learning-based approaches dominate
the field of medical image segmentation, with U-Net and its
variants (including U-Net++, Attention U-Net and 3D U-Net) serving
as landmark technologies. These architectures have demonstrated a
notable performance in several segmentation tasks, particularly in
brain tumors, lung cancer and breast cancer imaging (24). However, despite their clinical
potential, these advanced techniques have not been systematically
applied to PCNSL segmentation, to the best of our knowledge. Our
previous research did not explore the potential of nnU-Net. The
limited number of PCNSL cases and the available imaging data have
resulted in only a few studies focusing on deep learning models for
PCNSL, largely concentrating on differentiating it from other brain
tumors, such as glioblastoma (9,10).
Moreover, there has been a scarcity of recent investigations
applying deep learning for the automatic segmentation of
multi-parameter MRI in PCNSL (11).
As several studies have reported the effectiveness of automatic
segmentation models based on deep learning for specific brain
tumors, such as glioma and meningioma, with notable rates of
automated detection and precision across several tumor compartments
(22,25,26),
we hypothesize that these deep learning models could also attain a
certain level of accuracy in recognizing and segmenting PCNSL.
Consequently, the aim of the present study was to create automated
deep learning segmentation models that leverage multi-sequence MRI
images as input to reliably detect PCNSL tumors. This method would
enable the quantitative calculation of volumes and assess the
accuracy and consistency of the outcomes. To address the
challenges, the present study used the nnU-Net deep learning
framework (27), known for its
versatility and effectiveness with limited sample sizes, without
requiring manual parameter tuning (28,29).
Materials and methods
Study population
The present retrospective study enrolled patients
from the Fifth Medical Center of Chinese PLA General Hospital
(Beijing, China), Shengli Oilfield Central Hospital (Dongying,
China), Affiliated Hospital of Qingdao University (Qingdao, China),
Yantai Yuhuangding Hospital (Yantai, China) and Weifang People's
Hospital (Weifang, China). The study was performed according to the
principles of the Helsinki Declaration. To ensure consistent
ethical standards across all participating institutions, the five
hospitals adopted an ethical collaborative review mechanism (lead
institution review with participating institution endorsement). The
relevant approval documents were provided by Shengli Oilfield
Central Hospital and endorsed by the ethics committees of the other
four participating institutions (approval no. YX11202401101; March
12, 2024). The ethics committees of the participating hospitals
waived the requirement for patient informed consent.
Patients who underwent MRI scans and were initially
pathologically diagnosed with PCNSL between September 2016 and
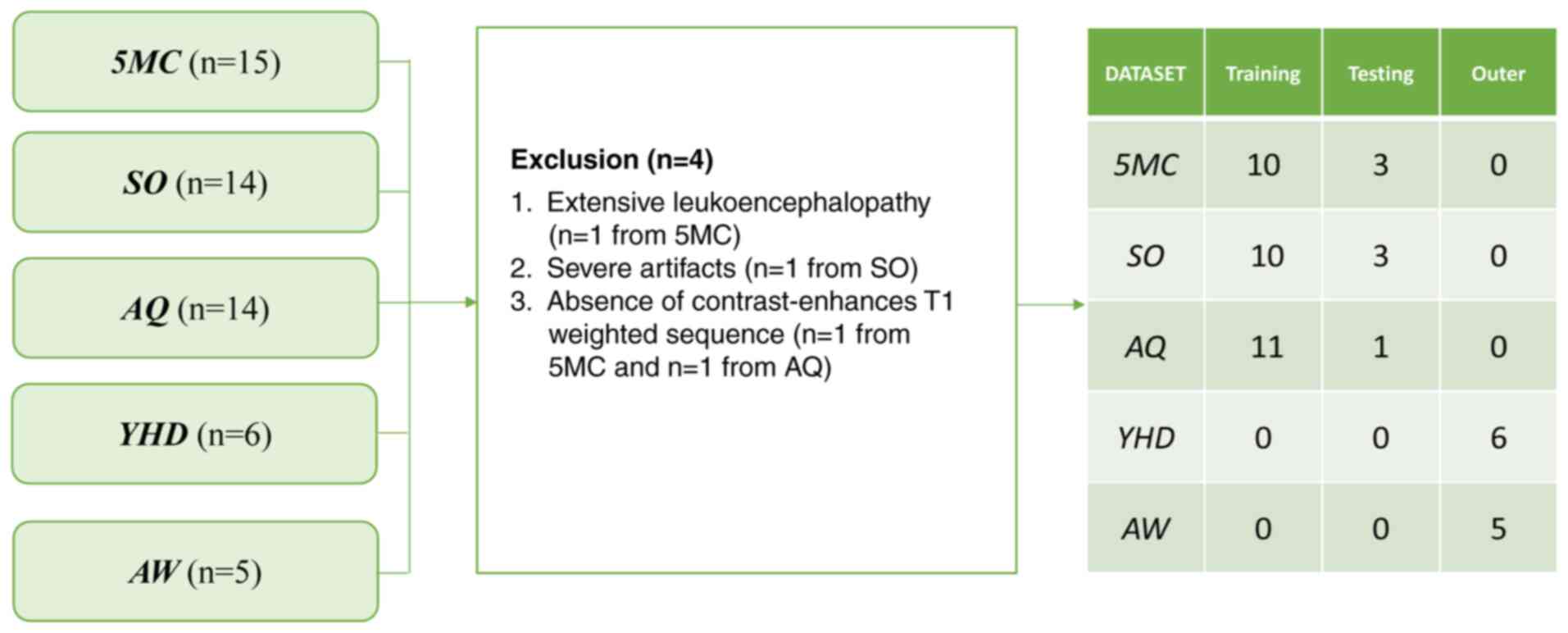
March 2022 were recruited. All 53 PCNSL cases identified for the
present study were evaluated for the predetermined exclusion and
inclusion criteria. These criteria were applied consistently to all
patients, regardless of initial or follow-up imaging. The inclusion
criteria were as follows: i) Confirmed PCNSL diagnosis by
pathology; ii) received treatment at the designated 5 hospitals
between September 2016 and March 2022; and iii) completed
in-hospital MRI examinations. The exclusion criteria were as
follows: i) Extensive leukoencephalopathy (Fazekas III; n=1)
(30); ii) severe artifacts (n=1);
and iii) absence of an available complete MRI dataset (n=2). The
exclusion of patients based on these confounding images was
essential for ensuring the accurate representation of tumor
morphology and minimizing feature extraction bias during model
training. Finally, the cohort consisted of 49 PCNSL who were
successfully assessed (Fig. 1).
Image acquisition
All examinations were performed using two MRI
scanners: 3T scanner (Siemens Healthineers) and 3T scanner Signa
(GE Healthcare), adhered to the standards outlined in the Expert
Consensus on MRI Examination Techniques. Contrast-enhanced
T1-weighted (T1W) and T2-weighted (T2W) images were collected.
Gadopentetate dimeglumine was administered intravenously at doses
based on the body weight (0.1 mmol/kg). The field of view was set
to 256 mm, the matrix was 512×512 and the slice thickness was 5 mm.
The images were reconstructed with a standard kernel and then were
transferred to an external workstation (syngo MMWP VE 36A; Siemens
Healthineers) for further postprocessing.
Image pre-processing
The images were reviewed by a radiologist from
Shengli Oilfield Central Hospital with >9 years of experience in
radiology, who manually delineated the tumors at the axial site
using the Medical Imaging Interaction Toolkit, version 2018.04.2
(www.mitk.org). The marked regions of interest
were confirmed as ground truth by another senior neuroradiologist
(with 11 years of experience in radiology) from the Fifth Medical
Center of Chinese PLA General Hospital, who was blinded to the
assessment.
Image segmentation
A total of two segmentation models were established
using T1C and T2 scans, respectively. The obtained PCNSL dataset
was divided into a training dataset (n=30), a testing dataset (n=8)
and an outer validation dataset (n=11). Subsequently, the nnU-Net
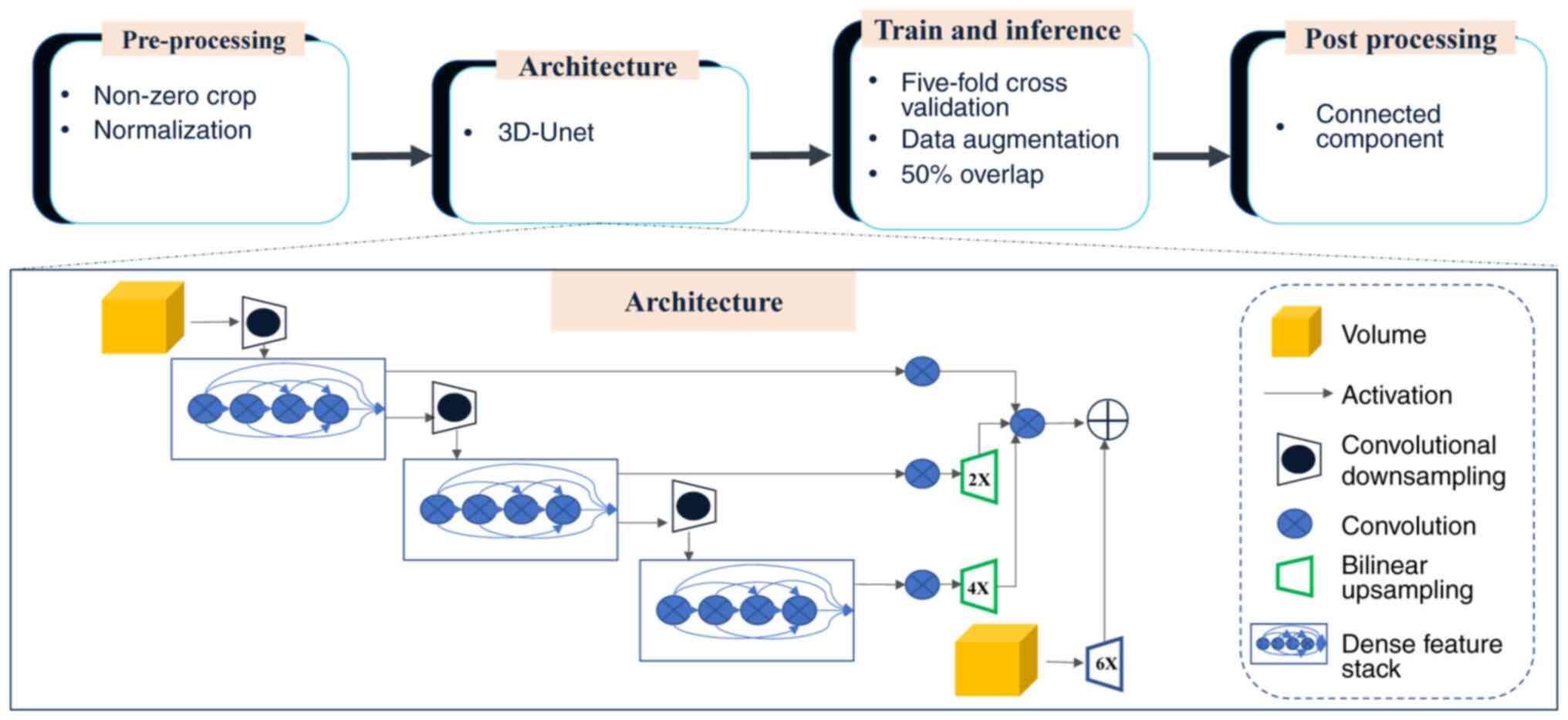
deep learning network was utilized to train an automated
segmentation model (27). nnU-Net
framework is an adaptive extension of the conventional U-Net
architecture. This innovative framework incorporates automated
adaptation mechanisms to accommodate diverse datasets and clinical
applications, thereby minimizing manual parameter optimization
requirements. The core design philosophy of the framework minimizes
unnecessary architectural complexity whilst optimizing essential
elements. This approach ensures reliable performance across diverse
medical imaging applications while preserving clinical relevance
(31). By leveraging nnU-Net, the
present study streamlined the key decisions involved in designing
an effective segmentation pipeline for the specific dataset. This
process is illustrated in Fig.
2.
The default framework of nnU-Net was used; however,
it was adapted for the characteristics of the data, choosing the 3D
fullres model and retaining the original resolution. Preprocessing
and data augmentation steps were used to improve classification
accuracy before training. To minimize the computational burden and
reduce the matrix size, the scans were cropped to the region with
non-zero values. The datasets were then resampled to median voxel
spacing by utilizing third-order spline interpolation for images
and neighbor interpolation for masks. The resampling approach
enabled the neural networks to improve the learning of the spatial
semantics of the scans. Finally, the entire dataset was normalized
by clipping to the (0.5, 99.5) percentile of these intensity
values, and the z-score was normalized according to the mean and
standard deviation of all collected intensity values in each
sample. In the training procedure, the 3D fullres model was used
and a combination of dice and cross-entropy loss was utilized
according to following formula: L=Lcrossentropy +
Ldice
The same data augmentation methods were performed to
prevent overfitting. The batch size was set adaptively according to
video memory. The number of training epochs was 500 at which epoch
the model converged. During the training process, 5-fold
cross-validation was utilized to optimize computational efficiency
and reduce deviation variance. Connected component analysis was
used as a postprocessing technique. After inference, the tumors
could be segmented automatically.
Statistical analysis
Categorical variables are presented as n (%), whilst
continuous variables are summarized as mean ± standard deviation
for normally distributed data, or as median (interquartile range)
for non-normally data. The Mann-Whitney U test was applied to
compare continuous clinical variables, whilst the χ2
test was used to assess the difference in categorical clinical
variables between groups. The Dice similarity coefficient (DSC),
which provides a measure of spatial overlap for each voxel of
segmented tissue, was used to evaluate the model performance.
Volumes of tumor components were calculated and volumetric
agreement was evaluated between the ground truth and automated
segmentations using the Wilcoxon rank-sum test. Pearson's
correlation coefficient (r) was calculated and a Bland-Altman
analysis was performed. P<0.05 was considered to indicate at
statistically significant difference. All statistical analyses were
performed using Python (version 3.5.6; Python Software
Foundation).
Results
Study population
In the present study, the PCNSL cohort comprised 49
patients (27 men and 22 women) with a mean age of 58.4±9.1 years.
The detailed patient demographics of the training and validation
sets are presented in Table I. The
diagnosis of PCNSL was confirmed through stereotactic biopsy in 40
of the cases and through surgical specimens in 9 of the cases. In
addition, systemic lymphatic disease was ruled out by additional
imaging and bone marrow biopsy in all cases. The majority of the
patients received chemotherapy (91.8%). A total of 93.8% of the
PCNSLs were located in the supratentorial region and showed
multifocal tumor spread (71.4%).
 | Table I.Demographic and clinical
characteristics of all patients with primary central nervous system
lymphoma in the present study (n=49). |
Table I.
Demographic and clinical
characteristics of all patients with primary central nervous system
lymphoma in the present study (n=49).
| Characteristic | Value |
|---|
| Sex |
|
|
Male | 27 (55.1) |
|
Female | 22 (44.9) |
| Age, years | 58.4±9.1 |
| Tumor location |
|
|
Telencephalon | 37 (75.5) |
|
Thalamus | 1 (2.0) |
|
Brainstem | 5 (10.2) |
|
Cerebellum | 6 (12.2) |
| History of
malignancy |
|
| No | 47 (95.9) |
|
Yes | 2 (4.1) |
Evaluation of the segmentation
model
The performance results for the segmentation models
are outlined in Table II. Compared
with the ground truth, the T1WI sequences segmentation model
achieved the optimal performance with a training dice of 0.923, a
testing dice of 0.830 and an outer validation dice of 0.801. The
T1WI sequences model and the T2WI sequences model yielded a mean
tumor volume of 15.64±14.26 and 25.77 ± 24.20 cm3,
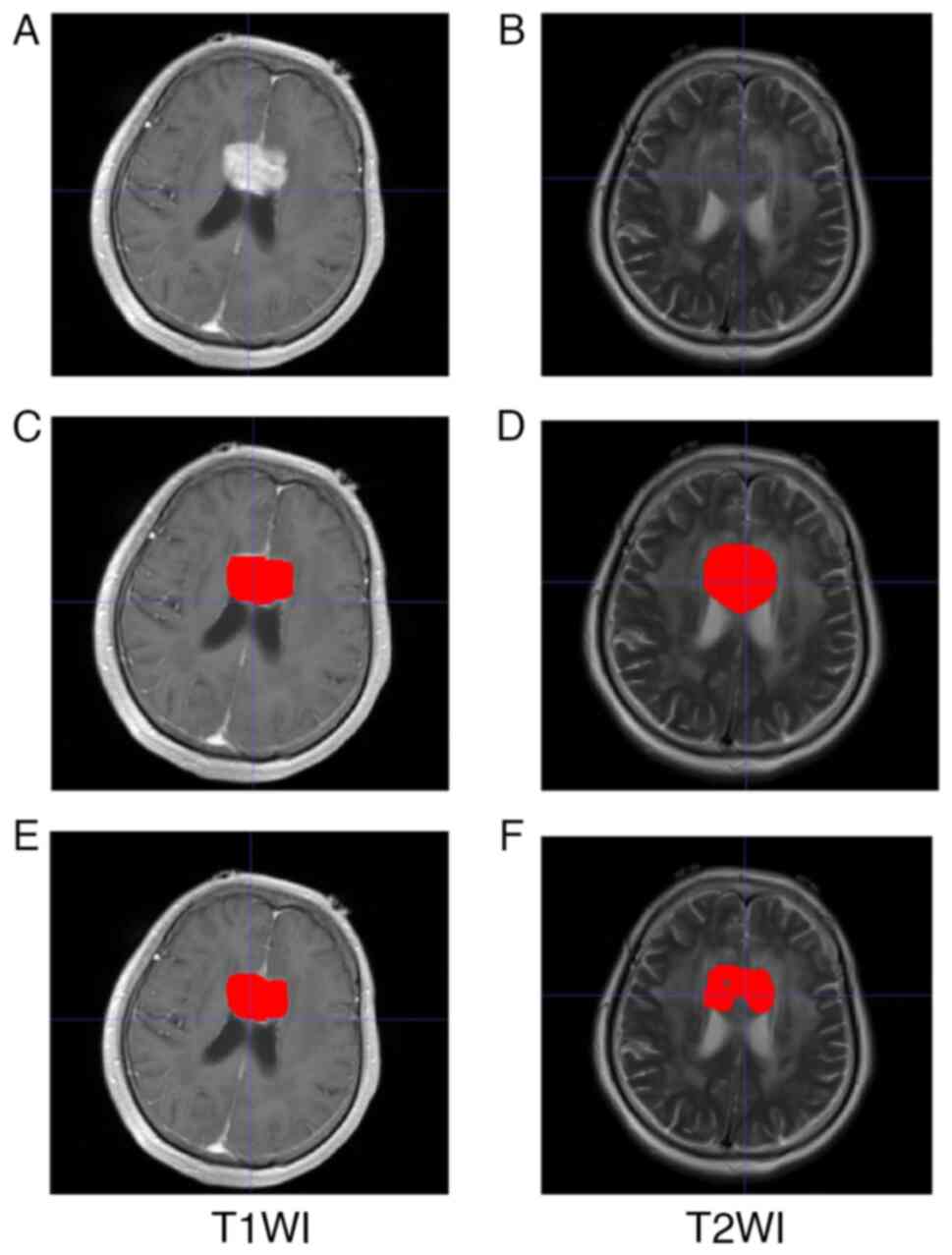
respectively. Representative images of two different automated
segmentation examples from a 57-year-old female patient are shown
in Fig. 3.
 | Table II.Correlation coefficients, dice
coefficients and the significant differences in tumor volume
between the tumor volumes of the training, testing and outer
datasets. |
Table II.
Correlation coefficients, dice
coefficients and the significant differences in tumor volume
between the tumor volumes of the training, testing and outer
datasets.
|
| Correlation
coefficient, r | Dice
coefficient | P-value |
|---|
|
|
|
|
|
|---|
| Dataset | T1WI | T2WI | T1WI | T2WI | T1WI | T2WI |
|---|
| Training | 0.76a | 0.89a | 0.923 | 0.761 | 0.329 | 0.229 |
| Testing | 0.98a | 0.66a | 0.830 | 0.647 | 0.093 | 0.779 |
| Outer | 0.95a | 0.98a | 0.801 | 0.643 | 0.013 | 0.477 |
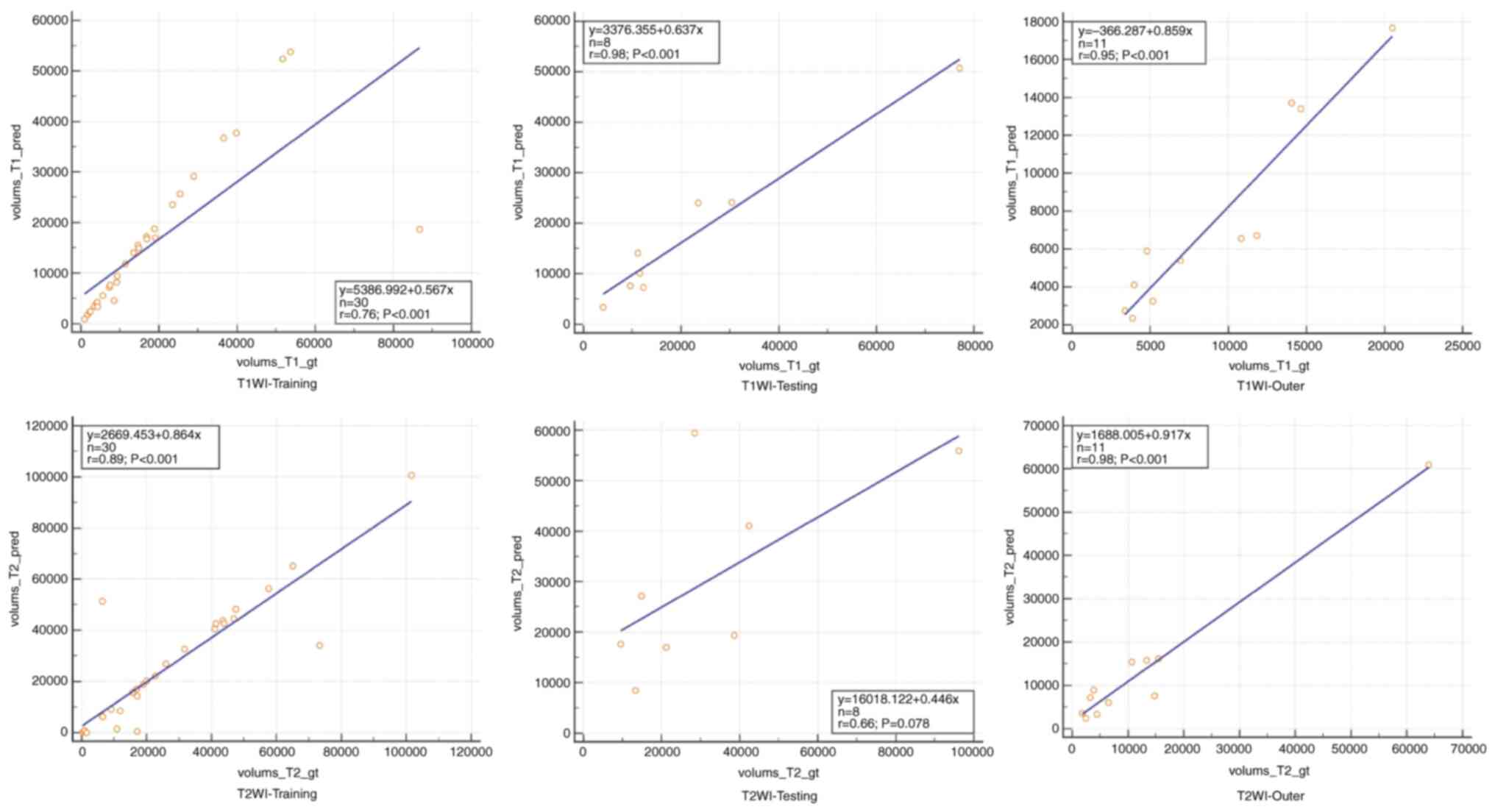
To compare volumetric assessment by automated
segmentations with the ground truth, regression lines for two
segmentation models were drawn (Fig.
4). The correlation coefficients for the three models are
presented in Table II, which also
demonstrates the significant differences in tumor volume between
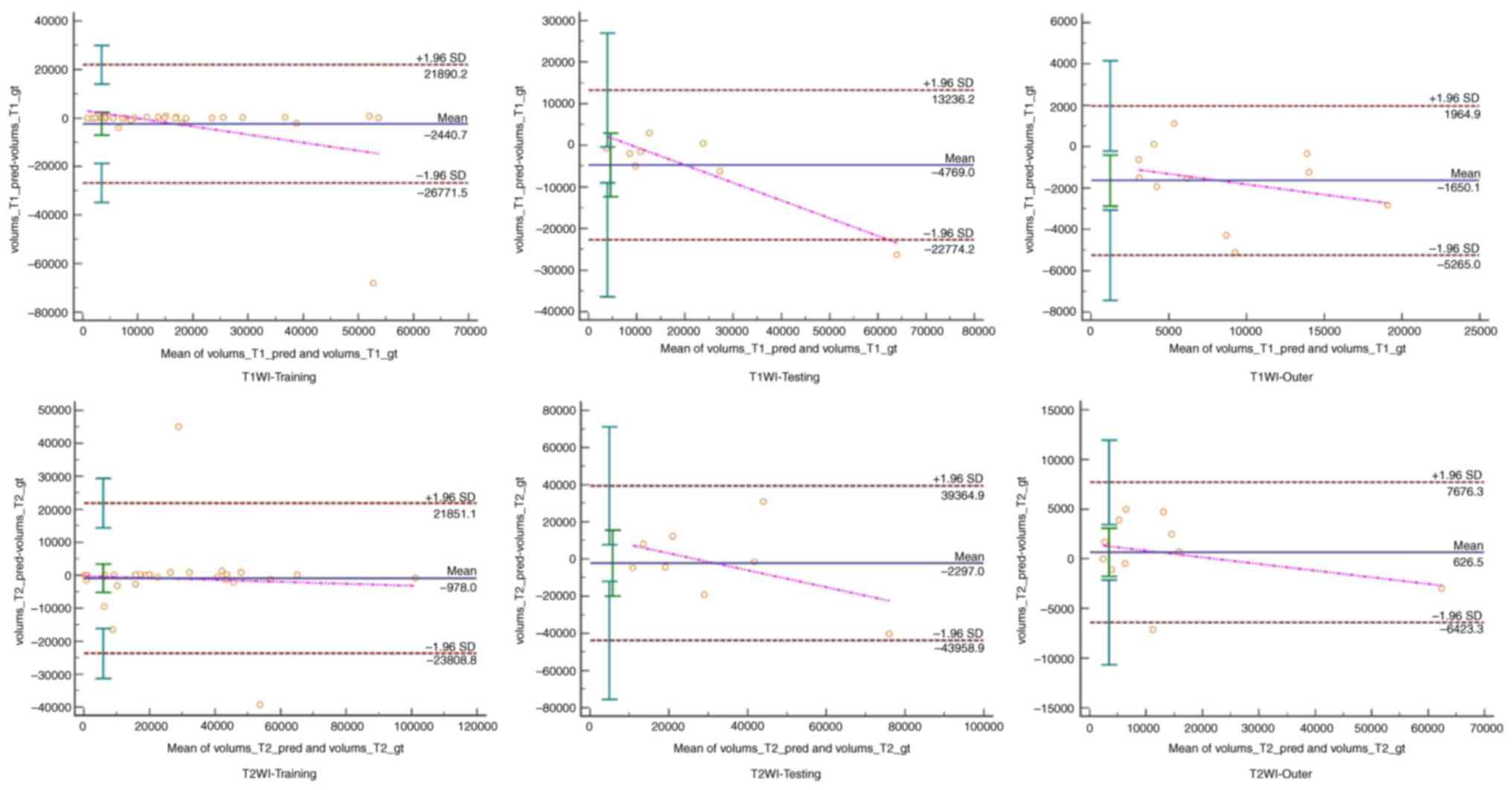
the training, testing and outer datasets. Furthermore, Bland-Altman
analysis revealed a good agreement between the manual and automated
segmentations (Fig. 5).
Discussion
Precise assessment of size is clinically relevant
for therapy planning, prognosis and monitoring in PCNSL. Automated
segmentation of PCNSL may enable a precise and objective assessment
of tumor burden and response in longitudinal imaging, which is
often difficult to determine due to its multifaceted appearance of
multiple scattered lesions (22,23).
In the present study, two automated segmentation models of PCNSL
were established using T1C and T2 scans. The nature of PCNSL tumors
generally results in a complex and heterogeneous structure on
multiple MRI image sequences. However, the present study observed
that the automatic segmentation model demonstrated marked accuracy
in its segmentation performance.
The nnU-net-based automated segmentation model in
the present study demonstrated a notable performance. Compared with
the ground truth, the T1WI sequences segmentation model achieved
the optimal performance with a training dice of 0.923, a testing
dice of 0.830 and an outer validation dice of 0.801. Bland-Altman
analysis revealed high agreement between manual and automated
segmentations. Furthermore, both models had strong correlation
coefficient and the automated segmentation architecture was
successfully deployed across diverse input data types with ease.
Therefore, the deep learning model proposed in the present study is
highly clinically relevant as it allows for the automated detection
of cerebral masses. This can enable the preselection of lesions and
serve as a control mechanism for radiologists and treating
physicians.
In comparison with the previously suggested models,
the deep learning model in the present study demonstrated
superiority. In prior studies, several methods were proposed for
glioma, another type of central nervous system tumor, due to
similar imaging features and larger glioma datasets. Perkuhn et
al (32) reported DSCs ranging
from 0.62–0.86 for different tumor components of gliomas. This
trained segmentation model for gliomas could be successfully
applied to PCNSL without any decrease in segmentation performance,
despite the different appearance and overall complex structure of
PCNSL. Using a different convolutional neural network (CNN), Menze
et al (33) and Bousabarah
et al (34) calculated DSCs
of 0.74–0.85 and 0.84–0.90 for glioma segmentations, respectively.
Kickingereder et al (26)
reported DSCs of 0.89–0.93 for an artificial neural network trained
on a larger cohort of patients with glioblastoma. Pennig et
al (11) evaluated the efficacy
of a deep learning model initially trained on gliomas for fully
automated detection and segmentation of PCNSL on multiparametric
MRI including heterogeneous imaging data from several scanner
vendors and study centers. The median DSC was 0.76 for total tumor
volume (11).
CNNs require large annotated datasets for effective
training, as small datasets limit generalizability. This is
particularly challenging for lymphoma analysis, where variations in
lesion size, shape and metabolic activity complicate training
without reliable anatomical references. In terms of performance,
the present model achieved notable results using only a small
sample dataset compared with the previous study (35,36).
The T1WI model achieved an improved performance in comparison with
the T2WI, and this can be attributed to the improved visualization
of tumor morphology on contrast-enhanced T1WI, where tumors
typically exhibit distinct homogeneous enhancement patterns
(37). Moreover, peritumoral edema
appears hyperintense on T2WI, which reflects the infiltration and
dissemination of tumor cells along the perivascular space, and is
easily confused with the slightly hyperintense signal of the tumor
(37). The lower T2WI performance
was mainly related to imaging features, rather than model
architecture or training bias. Other studies have also reported a
similar phenomenon (10,38,39).
Therefore, T2WI performance may be improved in the following ways:
i) Data enhancement: Improve T2WI data quality through enhancement
technology; ii) multimodal fusion: Combine T1WI and T2WI data to
improve the segmentation effect; and iii) model optimization:
Incorporate a boundary sensing module into the segmentation network
to improve the extraction of target boundary features and refine
segmentation precision.
The main limitations of the present study include
the small sample size and the inherent constraints associated with
its retrospective design. Thus, further investigation is needed to
determine whether automatic segmentation for PCNSL is suitable for
specific clinical tasks and parameters. Despite evaluating the
automatic segmentation model using several MRI multi-sequence
images from multiple hospital treatment centers, it is essential to
acknowledge the potential bias associated with these images. It is
also acknowledged that the MRI techniques and learning methods used
in the present study could be further optimized. However, at
present, acquiring a substantial volume of MRI data from additional
patients with PCNSL for external validation remains challenging due
to the rarity of this condition. If more PCNSL imaging data become
accessible in the future, the performance of the model in the
present study could be further optimized. In subsequent studies,
the focus should be on compiling a more comprehensive and
higher-quality dataset of medical imaging data for PCNSL, refining
the methodology and validating the results in larger, multi-center
cohorts to improving the robustness of the model and enhance its
potential clinical applicability. The development of brain tumor
segmentation methods that can address the issue of missing
modalities should also be investigated. Furthermore, data on the
prognosis of PCNSL should be collected and the method to form a set
of full-process automated diagnosis and treatment solutions for
PCNSL should be improved. Although the present research is still in
the initial stage, the precise quantification of the lesions will
lay a solid foundation for this goal. Furthermore, future studies
should perform analyses to explore potential correlations between
the artificial intelligence-generated findings (such as tumor
segmentation results and predictive scores) and key clinical
outcomes, such as treatment response, progression-free survival or
overall survival, where data is available. These analyses should
aim to establish a stronger link between artificial intelligence
findings and clinical outcomes, thereby enhancing the translational
value and practical applicability of the model in the present
study.
In conclusion, the present study established two
automated segmentation models of PCNSL using T1C and T2. Compared
with the ground truth, the T1C sequences segmentation model
achieved optimal performance. The proposed automatic segmentation
model performed well despite the complex and multifaceted
appearance of PCNSL. This indicates its great potential for use in
the entire follow-up monitoring process for this lymphoma
subtype.
Acknowledgements
The authors would like to thank Dr Lixi Miao
(Department of Medical Imaging Center, Shengli Oilfield Central
Hospital) and Dr Ruyi Tian (Department of Radiology, Fourth Medical
Center Chinese PLA General Hospital, Beijing, P.R. China) for
assessing the images. Their evaluations provided the essential data
foundation for establishing the segmentation model. As doctors from
the hospitals that supplied the imaging data, they voluntarily
participated in the MRI evaluations but were not involved in any
other aspects of this study. In accordance with their wishes and
academic standards, they have not been listed as co-authors of this
manuscript.
Funding
The present project was funded by the Natural Science Foundation
of Dongying, China (grant no. 2023ZR028).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TW, XT, JD, and YJ participated in study design and
manuscript conceptualization. XT, JD, and YJ participated in study
design and manuscript conceptualization. TW developed the model and
performed the statistical analysis. GL and XT drafted the work. XT
revised the manuscript critically for important intellectual
content, provided a discussion of the results and an explanation of
the main nnU-Net techniques. JD and YJ submitted ethics
applications to five affiliated hospitals; recruited radiologists
for manual segmentation of imaging data and coordinated with
co-authors to distribute workloads. JD and YJ confirm the
authenticity of all the raw data. GL and WM contributed to the
conception of the work and oversaw project progress while ensuring
workflow execution. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present retrospective study was performed in
compliance with the Helsinki Declaration and was approved by the
Independent Ethics Committees of the Fifth Medical Center of
Chinese PLA General Hospital, the Affiliated Hospital of Qingdao
University, Yantai Yuhuangding Hospital, Weifang People's Hospital
and Shengli Oilfield Central Hospital (March 12, 2024; approval no.
YX11202401101). The ethics committees waived the requirement for
obtaining informed consent from patients.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villano JL, Shaikh H, Dolecek TA and
McCarthy BJ: Age, gender, and racial differences in incidence and
survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chukwueke U, Grommes C and Nayak L:
Primary central nervous system lymphomas. Hematol Oncol Clin North
Am. 36:147–159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morales-Martinez A, Nichelli L,
Hernandez-Verdin I, Houillier C, Alentorn A and Hoang-Xuan K:
Prognostic factors in primary central nervous system lymphoma. Curr
Opin Oncol. 34:676–684. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schaff LR and Grommes C: Primary central
nervous system lymphoma. Blood. 140:971–979. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lukas RV, Stupp R, Gondi V and Raizer JJ:
Primary central nervous system Lymphoma-PART 1: Epidemiology,
diagnosis, staging, and prognosis. Oncology (Williston Park).
32:17–22. 2018.PubMed/NCBI
|
|
6
|
Sangeetha SKB, Muthukumaran V, Deeba K,
Rajadurai H, Maheshwari V and Dalu GT: Multiconvolutional transfer
learning for 3D brain tumor magnetic resonance images. Comput
Intell Neurosci. 2022:87224762022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadad T, Rehman A, Munir A, Saba T, Tariq
U, Ayesha N and Abbasi R: Brain tumor detection and
multi-classification using advanced deep learning techniques.
Microsc Res Tech. 84:1296–1308. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abd-Ellah MK, Awad AI, Khalaf AAM and
Hamed HFA: A review on brain tumor diagnosis from MRI images:
Practical implications, key achievements, and lessons learned. Magn
Reson Imaging. 61:300–318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu G, Zhang Y, Wang W, Miao L and Mou W:
Machine learning and deep learning CT-based models for predicting
the primary central nervous system lymphoma and glioma types: A
multicenter retrospective study. Front Neurol. 13:9052272022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia W, Hu B, Li H, Shi W, Tang Y, Yu Y,
Geng C, Wu Q, Yang L, Yu Z, et al: Deep learning for automatic
differential diagnosis of primary central nervous system lymphoma
and glioblastoma: Multi-parametric magnetic resonance imaging based
convolutional neural network model. J Magn Reson Imaging.
54:880–887. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pennig L, Hoyer UCI, Goertz L, Shahzad R,
Persigehl T, Thiele F, Perkuhn M, Ruge MI, Kabbasch C, Borggrefe J,
et al: Primary central nervous system lymphoma: Clinical evaluation
of automated segmentation on multiparametric MRI using deep
learning. J Magn Reson Imaging. 53:259–268. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramadan S, Radice T, Ismail A, Fiori S and
Tarella C: Advances in therapeutic strategies for primary CNS
B-cell lymphomas. Expert Rev Hematol. 15:295–304. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Batchelor TT: Primary central nervous
system lymphoma. Hematology Am Soc Hematol Educ Program.
2016:379–385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonm AV, Ritterbusch R, Throckmorton P and
Graber JJ: Clinical imaging for diagnostic challenges in the
management of gliomas: A review. J Neuroimaging. 30:139–145. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang RY, Bi WL, Griffith B, Kaufmann TJ,
la Fougère C, Schmidt NO, Tonn JC, Vogelbaum MA, Wen PY, Aldape K,
et al: Imaging and diagnostic advances for intracranial
meningiomas. Neuro Oncol. 21:i44–i61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yadav AS, Kumar S, Karetla GR,
Cotrina-Aliaga JC, Arias-Gonzáles JL, Kumar V, Srivastava S, Gupta
R, Ibrahim S, Paul R, et al: A feature extraction using
probabilistic neural network and BTFSC-Net model with deep learning
for brain tumor classification. J Imaging. 9:102022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
ZainEldin H, Gamel SA, El-Kenawy EM,
Alharbi AH, Khafaga DS, Ibrahim A and Talaat FM: Brain tumor
detection and classification using deep learning and Sine-cosine
fitness grey wolf optimization. Bioengineering (Basel). 10:182022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taher F, Shoaib MR, Emara HM, Abdelwahab
KM, Abd El-Samie FE and Haweel MT: Efficient framework for brain
tumor detection using different deep learning techniques. Front
Public Health. 10:9596672022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang K, Park J, Kwon YJ, Cho SJ, Choi BS,
Kim J, Kim E, Jang J, Ahn KS, Kim S and Kim CY: Fully automated
segmentation models of supratentorial meningiomas assisted by
inclusion of normal brain images. J Imaging. 8:3272022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dang K, Vo T, Ngo L and Ha H: A deep
learning framework integrating MRI image preprocessing methods for
brain tumor segmentation and classification. IBRO Neurosci Rep.
13:523–532. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin YY, Guo WY, Lu CF, Peng SJ, Wu YT and
Lee CC: Application of artificial intelligence to stereotactic
radiosurgery for intracranial lesions: Detection, segmentation, and
outcome prediction. J Neurooncol. 161:441–450. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laukamp KR, Pennig L, Thiele F, Reimer R,
Görtz L, Shakirin G, Zopfs D, Timmer M, Perkuhn M and Borggrefe J:
Automated meningioma segmentation in multiparametric MRI:
comparable effectiveness of a deep learning model and manual
segmentation. Clin Neuroradiol. 31:357–366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang K, Beers AL, Bai HX, Brown JM, Ly
KI, Li X, Senders JT, Kavouridis VK, Boaro A, Su C, et al:
Automatic assessment of glioma burden: A deep learning algorithm
for fully automated volumetric and bidimensional measurement. Neuro
Oncol. 21:1412–1422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng J, Luo H, Zhao G, Lin C, Yi X and
Chen S: A Review of medical image segmentation algorithms based on
deep learning. Computer Engineering Appl. 57:44–57. 2021.(In
Chinese).
|
|
25
|
Latif G: DeepTumor: Framework for brain MR
image classification, segmentation and tumor detection. Diagnostics
(Basel). 12:28882022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kickingereder P, Isensee F, Tursunova I,
Petersen J, Neuberger U, Bonekamp D, Brugnara G, Schell M, Kessler
T, Foltyn M, et al: Automated quantitative tumour response
assessment of MRI in neuro-oncology with artificial neural
networks: A multicentre, retrospective study. Lancet Oncol.
20:728–740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Isensee F, Jaeger PF, Kohl SAA, Petersen J
and Maier-Hein KH: nnU-Net: A self-configuring method for deep
learning-based biomedical image segmentation. Nat Methods.
18:203–211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pemberton HG, Wu J, Kommers I, Müller DMJ,
Hu Y, Goodkin O, Vos SB, Bisdas S, Robe PA, Ardon H, et al:
Multi-class glioma segmentation on real-world data with missing MRI
sequences: Comparison of three deep learning algorithms. Sci Rep.
13:189112023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ganesan P, Feng R, Deb B, Tjong FVY,
Rogers AJ, Ruipérez-Campillo S, Somani S, Clopton P, Baykaner T,
Rodrigo M, et al: Novel domain Knowledge-encoding algorithm enables
Label-efficient deep learning for cardiac CT segmentation to guide
atrial fibrillation treatment in a pilot dataset. Diagnostics
(Basel). 14:15382024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fazekas F, Chawluk JB, Alavi A, Hurtig HI
and Zimmerman RA: MR signal abnormalities at 1.5 T in Alzheimer's
dementia and normal aging. AJR Am J Roentgenol. 149:351–356. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Isensee F, Petersen J, Klein A, Zimmerer
D, Jaeger PF, Kohl S, Wasserthal J, Koehler G, Norajitra T, Wirkert
S and Maier-Hein KH: nnU-Net: Self-adapting framework for
U-Net-based medical image segmentation. ArXiv. abs/1809.10486.
2018.
|
|
32
|
Perkuhn M, Stavrinou P, Thiele F, Shakirin
G, Mohan M, Garmpis D, Kabbasch C and Borggrefe J: Clinical
evaluation of a multiparametric deep learning model for
glioblastoma segmentation using heterogeneous magnetic resonance
imaging data from clinical routine. Invest Radiol. 53:647–654.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Menze BH, Jakab A, Bauer S,
Kalpathy-Cramer J, Farahani K, Kirby J, Burren Y, Porz N, Slotboom
J, Wiest R, et al: The multimodal brain tumor image segmentation
benchmark (BRATS). IEEE Trans Med Imaging. 34:1993–2024. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bousabarah K, Letzen B, Tefera J, Savic L,
Schobert I, Schlachter T, Staib LH, Kocher M, Chapiro J and Lin M:
Automated detection and delineation of hepatocellular carcinoma on
multiphasic contrast-enhanced MRI using deep learning. Abdom Radiol
(NY). 46:216–225. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Naser PV, Maurer MC, Fischer M,
Karimian-Jazi K, Ben-Salah C, Bajwa AA, Jakobs M, Jungk C, Jesser
J, Bendszus M, et al: Deep learning aided preoperative diagnosis of
primary central nervous system lymphoma. iScience. 27:1090232024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cassinelli Petersen GI, Shatalov J, Verma
T, Brim WR, Subramanian H, Brackett A, Bahar RC, Merkaj S, Zeevi T,
Staib LH, et al: Machine learning in differentiating gliomas from
primary CNS lymphomas: A systematic review, reporting quality, and
risk of bias assessment. AJNR Am J Neuroradiol. 43:526–533. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferreri AJM, Calimeri T, Cwynarski K,
Dietrich J, Grommes C, Hoang-Xuan K, Hu LS, Illerhaus G, Nayak L,
Ponzoni M and Batchelor TT: Primary central nervous system
lymphoma. Nat Rev Dis Primers. 9:292023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gill CM, Loewenstern J, Rutland JW, Arib
H, Pain M, Umphlett M, Kinoshita Y, McBride RB, Bederson J, Donovan
M, et al: Peritumoral edema correlates with mutational burden in
meningiomas. Neuroradiology. 63:73–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reszec J, Hermanowicz A, Rutkowski R,
Turek G, Mariak Z and Chyczewski L: Expression of MMP-9 and VEGF in
meningiomas and their correlation with peritumoral brain edema.
Biomed Res Int. 2015:6468532015. View Article : Google Scholar : PubMed/NCBI
|