Introduction
Malignant melanoma (MM) is primarily a highly
aggressive non-epithelial tumor affecting skin tissue. Patients
often have a history of skin melanocytic nevi and may present with
cutaneous ulcerations. Although MM is typically associated with the
skin, it can also occur in other sites, including the esophagus,
oral cavity, anus and vagina (1).
The clinical diagnosis of MM typically relies on histopathological
examination and immunohistochemical markers such as sry-box
transcription factor 10 (SOX10), melan-A and human melanoma
black-45 (HMB-45), which are essential for differentiating it from
other malignancies. Primary pulmonary malignant melanoma (pPMM) is
an extremely rare manifestation, accounting for ~0.001% of primary
malignant lung tumors and 0.4% of all malignant melanomas (1). Due to its rarity, pPMM poses
diagnostic challenges and is often misdiagnosed as other
malignancies, such as malignant peripheral nerve sheath tumors
(MPNSTs) or metastatic melanoma. The absence of a cutaneous or
mucosal primary site further complicates the diagnostic process.
H3K27Me3 is a post-translational modification associated with
transcriptional repression and tumor suppressor gene silencing
(2). Loss of H3K27Me3 has been
implicated in the progression of various aggressive tumors, such as
MPNSTs and glioblastoma (2,3). The present study discusses a unique
case of pPMM characterized by H3K27Me3 loss, examining its genomic
profile, pathogenesis and natural history, along with relevant
literature. Notably, this case was initially misdiagnosed as a
MPNST based on overlapping histopathological and
immunohistochemical features. This case underscores the challenges
in diagnosing rare malignancies, particularly when they mimic more
common entities. The case highlights the importance of
comprehensive histological, immunohistochemical and molecular
evaluations in achieving a definitive diagnosis, especially in
cases with overlapping features.
Case report
Patient
The patient, a 54-year-old woman with no prior
history of skin, ear, or eye lesions, presented to Fujian
Provincial Hospital (Fuzhou, China) in November 2017 with symptoms
of chest tightness and 2.5 kg of weight loss, although no cough,
chest pain or hemoptysis were reported. At 8 months prior to
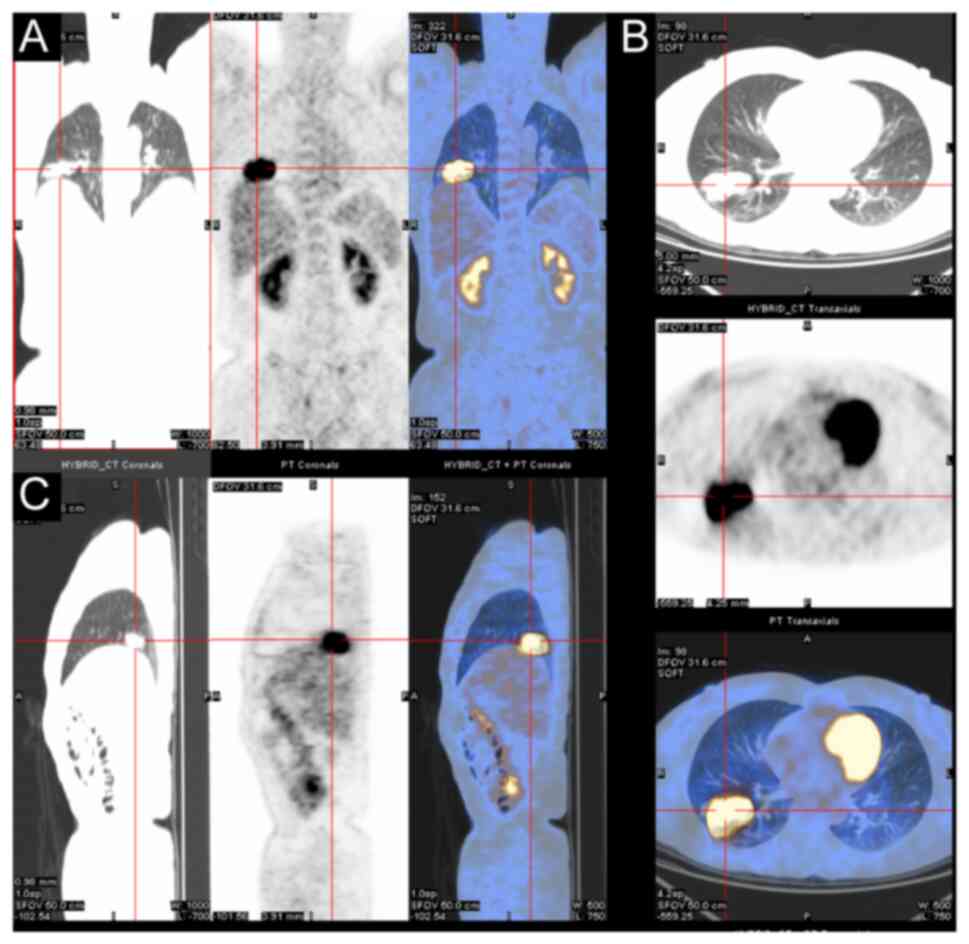
lobectomy, a computed tomography scan revealed a 4.1×2.3-cm mass in
the basal segment of the right lower lung lobe, suggesting a
possible lesion. No treatment was initiated at that time. After 5
months, a follow-up scan showed the mass had grown to 5.0×2.9 cm,
with irregular borders and high metabolic activity on positron
emission tomography-computed tomography, which raised suspicion of
a malignant mesenchymal tumor (Fig.
1). A lobectomy was subsequently performed, during which
laparoscopic exploration revealed no adhesions or effusions. The
mass involved the lung capsule but without any enlarged lymph
nodes.
Pathological examination. The resected lung lobe
specimen showed a nodular mass measuring 3×2.8×2.5 cm, located 1.5
cm from the lung capsule. The mass was unencapsulated, with a
well-defined border and a delicate grayish-white texture on the cut
surface. Histological analysis was performed on tissue sections
fixed in 10% neutral buffered formalin at room temperature for 24
h. The tissue was processed, embedded in paraffin and sectioned at
a thickness of 5 µm. Sections were stained with hematoxylin and
eosin at room temperature, following standard protocols. The slides
were examined using a light microscope (Leica DM3000) at
magnifications of ×100, ×200 and ×400, and scale bars were included
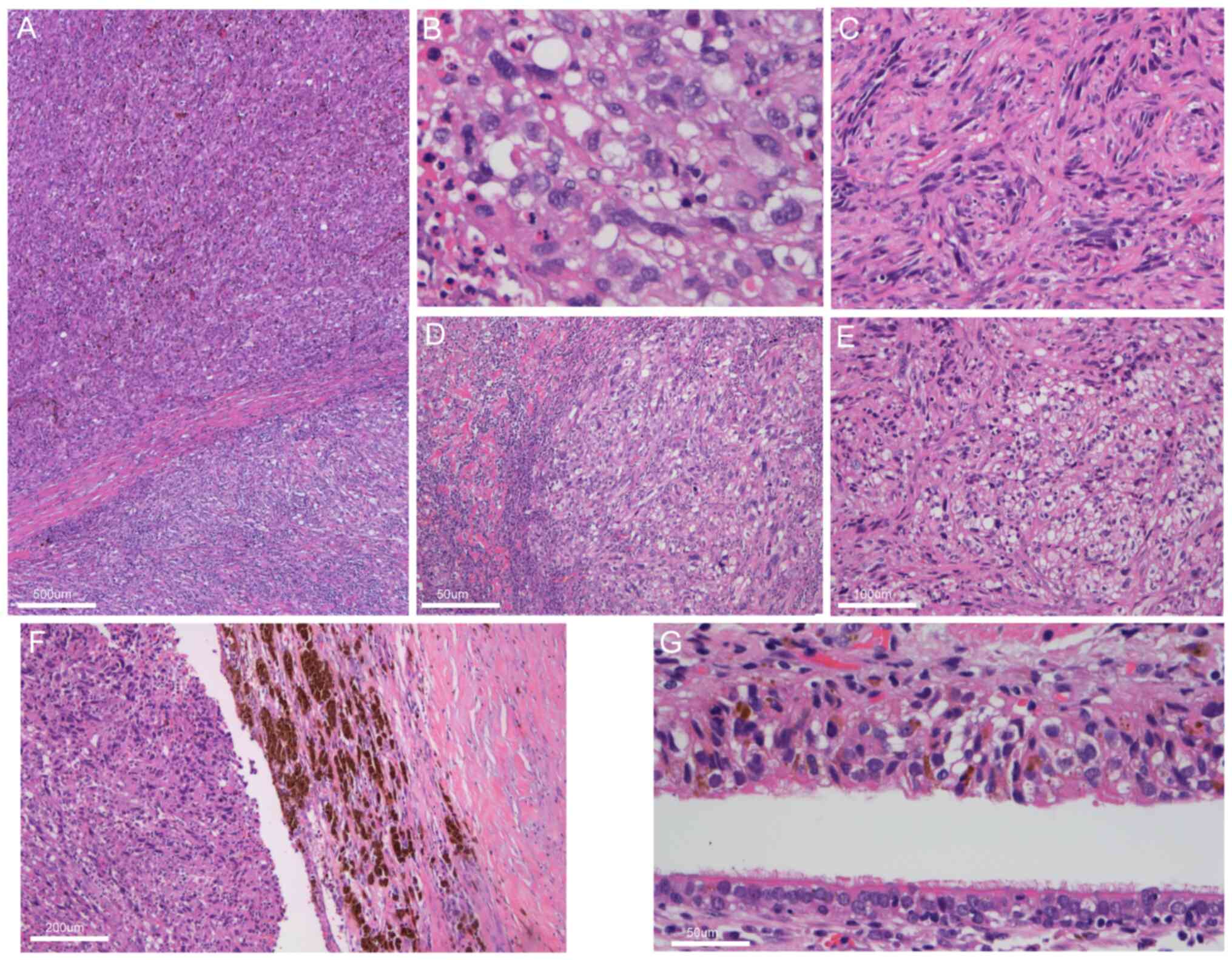
in the figure legends for reference. The analysis revealed a
nodular tumor with infiltrative growth and biphasic
differentiation, consisting of epithelioid and spindle-shaped cells
(Fig. 2A). The epithelioid cell
region displayed marked cellular atypia with pleomorphic nuclei and
eosinophilic cytoplasm (Fig. 2B),
while the spindle cell region showed whorl-like structures and
hyaline degeneration of the interstitium (Fig. 2C). Tumor cells exhibited bizarre
appearances (Fig. 2D) and
transparent cytoplasm (Fig. 2E).
The mitotic rate was 12 mitoses per 10 high-power fields, with
frequent necrosis and melanin accumulation (Fig. 2F). Extensive fibrous stroma was
present, with infiltrating lymphocytes, plasma cells and
eosinophils. The residual bronchial mucosa showed no significant
atypia, but scattered or nest-like melanoma cells were observed
infiltrating beneath the bronchial mucosal epithelium without
ulceration present (Fig. 2G).
Immunophenotype and genomic assessment.
Immunohistochemistry (IHC) staining was performed on 4-µm sections
obtained from the formalinfixed paraffinembedded tissues using
automated immunostainers. The IHC automated staining protocol was
performed as described previously (4). The detailed information on the
immunohistochemical experiments has been provided in Table SI, including the concentrations of
reagents, suppliers, product catalog numbers, incubation conditions
(temperature and duration). PBS was used as the negative control.
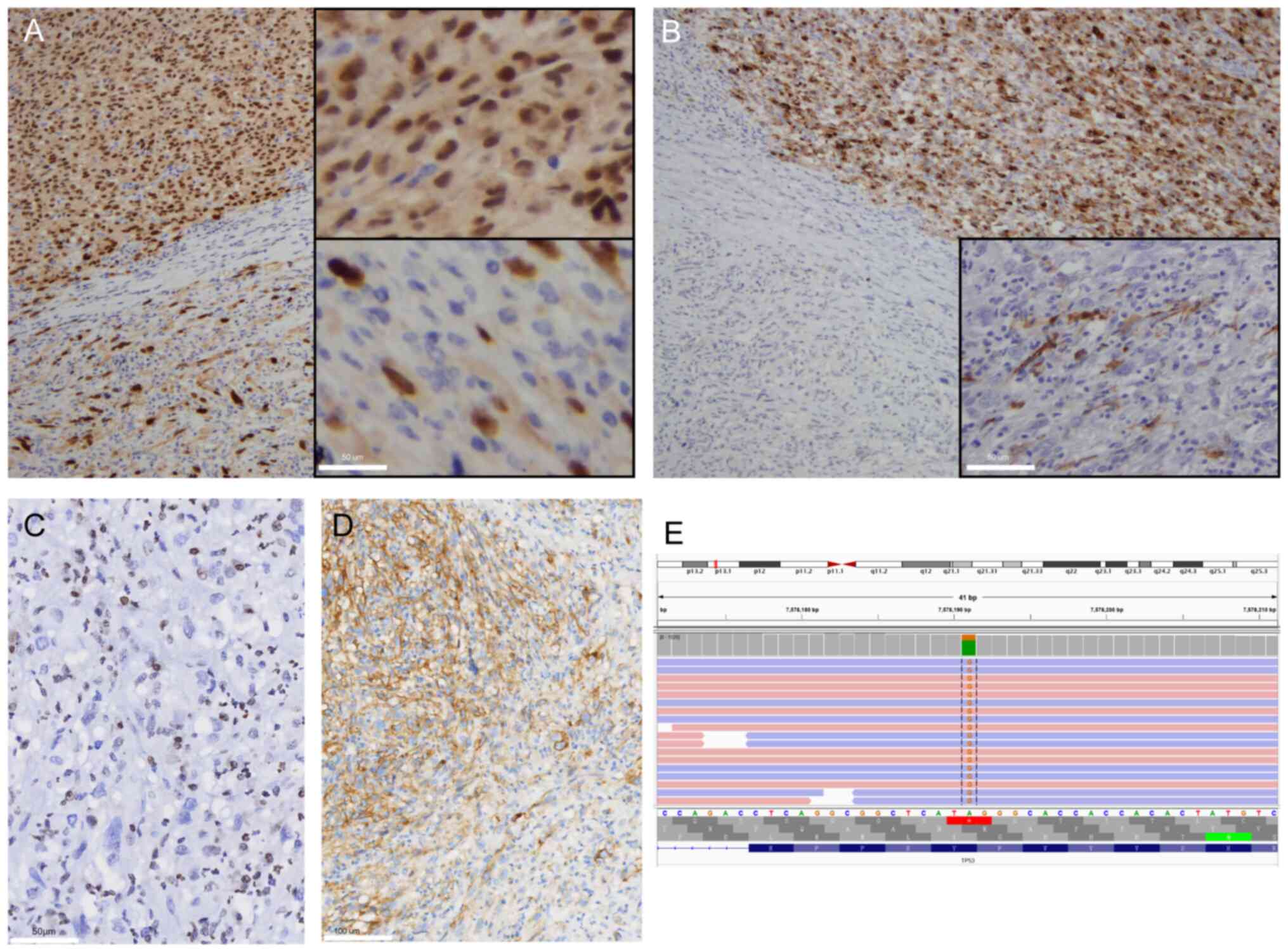
The tumor cells expressed melanoma markers, including S-100 (data
not shown), SOX10 (Fig. 3A),
melan-A (Fig. 3B), HMB-45 and
microphthalmia-associated transcription factor (data not shown),
with stronger expression in the epithelioid regions compared with
that in the spindle cell regions. Immunohistochemical staining for
H3K27Me3 was negative in the tumor cells (Fig. 3C). The Ki-67 proliferation index was
~70%, and the programmed death-ligand 1 (PD-L1) (SP263) positivity
rate was 60% (Fig. 3D). Other
markers, including BRAF, neurotrophic tyrosine receptor kinase
(NTRK), transcription factor e3, AE1/AE3, epithelial membrane
antigen (EMA), anaplastic lymphoma kinase (ALK), CD34, smooth
muscle actin (SMA), desmin, thyroid transcription factor-1 (TTF1)
and Napsin-A, were negative.
The Next-generation sequencing (NGS) assay was
conducted using a laboratory developed test kit from Amoy
Diagnostics, Co., Ltd., which was designed to sequence the entire
coding regions of 116 cancer-relate genes. Raw sequencing reads
were converted into FastQ files using bcl2fastq (Illumina),
enabling reliable downstream analysis. The platform enables the
detection of various classes of genomic alterations, including
point mutations, indel, gene fusions and copy number variations.
The NGS protocol was implemented as described previously (5). The NGS identified four somatic
mutations, namely TP53, BCL2L11, NF1 and NTRK3. Among
them, TP53 is significant for targeted therapeutics
(Fig. 3E; Table I). BRAF and KIT
mutations, typically associated with skin melanoma, were not
identified.
 | Table I.Somatic mutation identified by the
next-generation sequencing. |
Table I.
Somatic mutation identified by the
next-generation sequencing.
| Gene | Detection result
[accession number] | Abundance/copy
number, % |
|---|
| TP53 | exon6 c.658T>C p.
(Y220H) [NM_000546.5] | 30.03 |
| NTRK3 | exon3 c.148G>A p.
(D50N) [NM_002530.4] | 60.73 |
| BCL2L11 |
intron2c.394+1479_394+4381del
[NM_138621.5] | 28.98 |
| NF1 | exon3c.266C>G p.
(T89R) [NM_001042492.3] | 8.40 |
Follow-up
After several days of postoperative recovery, the
patient was scheduled for follow-up visits every 3 months for the
first 3 years. Due to financial constraints, the patient opted out
of further therapy. Currently, at 72 months post-surgery, the
patient is on a favorable recovery trajectory, showing no evidence
of disease.
Discussion
MM, typically a highly aggressive tumor, is
predominantly found in the skin. Patients often present with a
history of melanocytic nevi and may have skin ulcerations. Although
PMM is usually metastatic from skin lesions (6), pPMM is exceptionally rare. The median
age of diagnosis is ~59.1 years, with no significant sex
differences reported. Unlike for numerous lung cancers, smoking is
not considered a risk factor for pPMM. Most cases are incidentally
discovered during routine physical examinations, often appearing as
solitary lung nodules on imaging (1).
Histologically, pPMM shares feature with melanomas
from skin or mucosal sites, including epithelioid and
spindle-shaped cells with significant nuclear atypia and melanin
deposits. Distinguishing between pPMM and metastatic melanoma can
be challenging due to the tendency of skin melanomas to degenerate
after metastasis (7). The accurate
diagnosis of pPMM requires a thorough evaluation of clinical,
imaging and pathological findings. Previous studies (8,9) have
proposed specific criteria for diagnosing pPMM. Jensen and Egedorf
(8) suggested the following six
criteria: i) No prior skin tumors removed; ii) no prior ocular
tumors removed; iii) a solitary lung tumor; iv) morphology
consistent with a primary tumor; v) no involvement of other organs;
and vi) absence of primary MM demonstrated in other organs,
particularly in the skin or eyes, confirmed by biopsy. According to
the pathological diagnostic criteria for pPMM by Allen and Drash
(9), the diagnosis of MM is based
on bronchial epithelium invasion by melanoma cells, junctional
changes such as ‘dropping off’ or ‘nesting’ just beneath the
bronchial epithelium, and confirmation of melanoma cells through
immunohistochemical staining (S-100, HMB-45 and Melan-A) or
electron microscopy. The present case met all these clinical and
pathological criteria, leading to a diagnosis of pPMM.
The key differential diagnosis in the present case
was MPNST, due to the rare presentation of MM in the pulmonary
region, and as both MPNST and MM can exhibit melanin deposits and
similar morphological features, making differentiation difficult
(10,11). The biphasic differentiation in the
present case, with both epithelioid and spindle cell
characteristics, added complexities to the diagnosis. H3K27Me3 is a
crucial epigenetic marker of gene silencing, intricately associated
with heterochromatic regions; it plays a significant role in
inflammation, DNA damage repair, cell proliferation, metastasis,
regulatory cell death, ferroptosis and angiogenesis, thereby
influencing various epigenetic mechanisms in the pathogenesis of
multiple cancer types, such as glioblastoma (2). H3K27me3 is frequently observed to be
lost in MPNST (3) and has also been
documented in MM (12), indicating
that this marker alone is insufficient to distinguish between the
two. The correct identification of pPMM had significant clinical
implications, as the treatment strategies for MM and MPNSTs differ
substantially. Accurate diagnosis enabled the initiation of
appropriate therapy, including targeted and immune-based treatments
tailored to MM, which contributed to an improved clinical outcome
for the patient. Differentiation from other malignancies, such as
pulmonary sarcomatoid carcinoma, epithelioid inflammatory
myofibroblastic sarcoma, angiosarcoma and leiomyosarcoma, is also
crucial. Negative immunohistochemical markers for AE1/AE3, EMA,
SMA, ALK and others helped rule out these diagnoses in the present
study.
Genomic analysis of this pPMM revealed mutations
similar to those found in mucosal and esophageal melanomas,
contrasting with the common mutations seen in skin melanoma
(13–15). In the present patient, NGS
identified mutations in TP53, NTRK3, NF1 and BCL2L11,
while BRAF and KIT mutations, typically associated
with skin melanoma, were absent. Research indicates that NF1
mutations are prevalent in primary esophageal melanomas (13), supporting the idea that different
anatomical locations may have distinct mutational profiles.
The prognosis for patients with pPMM is generally
poor (4). However, the present
patient has achieved long-term disease-free survival for 72 months,
possibly indicating a potential cure following surgical resection.
This favorable outcome may be attributed to the incidental nature
of the diagnosis, absence of clinical symptoms, early detection and
H3K27Me3 loss, which has been suggested in previous studies to
correlate with a more favorable prognosis in melanoma (16,17).
High H3K27Me3 expression is associated with more aggressive
melanoma cell phenotype and poorer outcomes (18), emphasizing the significance of this
biomarker in assessing melanoma prognosis and possible therapeutic
approaches based on the gennomic findings.
There are some limitations to the present study. The
report is based on a single case, which limits the generalizability
of the findings and the exploration of potential treatment
strategies for pPMM. Given the rarity of the condition, discussing
possible treatment options based on the genomic findings would have
been beneficial. Therefore, more cases are needed to validate the
observations and conclusions in future studies.
In conclusion, the current study presents a rare
case of pPMM, initially misdiagnosed as MPNST, characterized by
H3K27me3 loss and a histological profile displaying both epithelial
and spindle cell features. The case findings highlight the
complexities involved in diagnosing pPMM and the critical
importance of comprehensive clinical and pathological
evaluation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Fujian Provincial Natural
Science Foundation (grant no. 2024J011006) and Fujian Provincial
Science and Technology Innovation Joint Funds (grant no.
2024Y96010076).
Ethics approval and consent to
participate
The Ethics Committee of Fujian Provincial Hospital
(Fuzhou, China) approved the present study (approval no.
K2024-09-070) and waived the requirement for informed consent. The
present study was performed in accordance with the principles of
the Declaration of Helsinki.
Availability of data and materials
The data generated in the present study may be found
in the SRA database under accession number PRJNA1245001 or at the
following URL: https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1245001.
All other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
HL and LZ conceived and designed the experiments.
HL, LZ, XY, LC and SL performed the experiments. HL, XY, LC, SL and
XC analyzed the data. HL, LZ and XC provided the reagents,
materials and/or the analysis tools. HL and LZ confirm the
authenticity of all the raw data. HL and LZ wrote the paper. All
authors have read and approved the final manuscript.
Patient consent for publication
The patient provided written informed consent for
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kyriakopoulos C, Zarkavelis G,
Andrianopoulou A, Papoudou-Bai A, Stefanou D, Boussios S and
Pentheroudakis G: Primary pulmonary malignant melanoma: Report of
an important entity and literature review. Case Rep Oncol Med.
2017:86543262017.PubMed/NCBI
|
|
2
|
Di L and Zhu WG: The role of H3K27me3
methylation in cancer development. Genome Instab Dis. 5:17–34.
2024. View Article : Google Scholar
|
|
3
|
Cortes-Ciriano I, Steele CD, Piculell K,
Al-Ibraheemi A, Eulo V, Bui MM, Chatzipli A, Dickson BC,
Borcherding DC, Feber A, et al: Genomic patterns of malignant
peripheral nerve sheath tumor (MPNST) evolution correlate with
clinical outcome and are detectable in cell-free DNA. Cancer
Discov. 13:654–671. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Zheng L, Zhang X, Yu X, Zhong G,
Chen X, Chen X and Chen L: SH3 domainbinding glutamic acidrich
proteinlike 3 is associated with hyperglycemia and a poor outcome
in EpsteinBarr virusnegative gastric carcinoma. Oncol Lett.
29:82024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Z, Dong H, Wu J, Dong W, Guo X, Yu H,
Fang J, Gao S, Chen X, Lu H, et al: Targeted genomic profiling
revealed a unique clinical phenotype in intrahepatic
cholangiocarcinoma with fibroblast growth factor receptor
rearrangement. Transl Oncol. 14:1011682021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Bing Z, Xu X and Cui Y: Primary
pulmonary malignant melanoma: Case report and literature review.
Thorac Cancer. 9:1185–1189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamposioras K, Pentheroudakis G,
Pectasides D and Pavlidis N: Malignant melanoma of unknown primary
site. To make the long story short. A systematic review of the
literature. Crit Rev Oncol Hematol. 78:112–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jensen OA and Egedorf J: Primary malignant
melanoma of the lung. Scand J Respir Dis. 48:127–135.
1967.PubMed/NCBI
|
|
9
|
Allen MS Jr and Drash EC: Primary melanoma
of the lung. Cancer. 21:154–159. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaspard M, Lamant L, Tournier E, Valentin
T, Rochaix P, Terrier P, Ranchere-Vince D, Coindre JM, Filleron T
and Le Guellec S: Evaluation of eight melanocytic and neural
crest-associated markers in a well-characterised series of 124
malignant peripheral nerve sheath tumours (MPNST): Useful to
distinguish MPNST from melanoma? Histopathology. 73:969–982. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hrycaj SM, Szczepanski JM, Zhao L,
Siddiqui J, Thomas DG, Lucas DR, Patel RM, Harms PW, Bresler SC and
Chan MP: PRAME expression in spindle cell melanoma, malignant
peripheral nerve sheath tumour, and other cutaneous sarcomatoid
neoplasms: A comparative analysis. Histopathology. 81:818–825.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Guellec S, Macagno N, Velasco V, Lamant
L, Lae M, Filleron T, Malissen N, Cassagnau E, Terrier P, Chevreau
C, et al: Loss of h3k27 trimethylation is not suitable for
distinguishing malignant peripheral nerve sheath tumor from
melanoma: A study of 387 cases including mimicking lesions. Mod
Pathol. 30:1677–1687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsuyama S, Kohsaka S, Hayashi T, Suehara
Y, Hashimoto T, Kajiyama Y, Tsurumaru M, Ueno T, Mano H, Yao T and
Saito T: Comprehensive clinicopathological and molecular analysis
of primary malignant melanoma of the oesophagus. Histopathology.
78:240–251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Guan W, Ren W, Liu Z, Wu H, Chen Y,
Liu S, Quan X, Yang Z, Jiang C, et al: Longitudinal genomic
alternations and clonal dynamics analysis of primary malignant
melanoma of the esophagus. Neoplasia. 30:1008112022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tímár J and Ladányi A: Molecular pathology
of skin melanoma: Epidemiology, differential diagnostics, prognosis
and therapy prediction. Int J Mol Sci. 23:53842022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou C, Xiao L, Ren X, Cheng L, Guo B,
Zhang M and Yan N: EZH2-mediated H3K27me3 is a predictive biomarker
and therapeutic target in uveal melanoma. Front Genet.
13:10134752022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo C, Balsa E, Perry EA, Liang J, Tavares
CD, Vazquez F, Widlund HR and Puigserver P: H3K27me3-mediated PGC1α
gene silencing promotes melanoma invasion through WNT5A and YAP. J
Clin Invest. 130:853–862. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoffmann F, Niebel D, Aymans P,
Ferring-Schmitt S, Dietrich D and Landsberg J: H3K27me3 and EZH2
expression in melanoma: Relevance for melanoma progression and
response to immune checkpoint blockade. Clin Epigenetics.
12:242020. View Article : Google Scholar : PubMed/NCBI
|