Introduction
Anal cancer is a rare but serious malignancy
accounting for ~3% of all gastrointestinal types of cancer
(1). Squamous cell carcinoma (SCC)
is the most common type of anal cancer and is suspected to be
linked to inflammatory processes secondary to human papilloma virus
(HPV), particularly subtypes 16 and 18 (2). A third of anal cancers are
asymptomatic or present with non-specific symptoms, while the
remaining cases will present with anal bleeding or pain (3). Anal cancer treatment is
multi-disciplinary and stage dependent, consisting of combined
chemoradiotherapy (CRT) and/or surgical resection (2). The 5-year overall survival of
localized disease is ~78%, and 18% in metastatic disease, with the
median survival of metastatic disease being 12 months (4). In anal SCC, distant metastasis is
relatively uncommon, with the liver being the most common area for
metastatic spread (4). Distant
metastasis of anal SCC has been reported in 5–8% of cases at
diagnosis, with 10–20% after curative local treatment (5). An even more uncommon occurrence of
anal SCC is metastatic spread to the brain; to the best of our
knowledge, only 9 previous cases have reported cerebral metastatic
disease after an initial anal SCC diagnosis (6–13). The
current study presents the case of a 56-year-old female with anal
canal SCC who later developed cerebral metastasis, adding to the
limited literature of this rare phenomenon.
Case report
The current study presents the case of a now
56-year-old right-handed Caucasian female who initially presented
with anal SCC (T3 N1c Mx) and later went on to develop an isolated
cerebral metastasis. In September 2017, the patient presented to a
rural hospital in South Australia with a non-tender lump in the
right pubic area with no reported systemic symptoms. A fine-needle
aspiration under ultrasound guidance demonstrated malignant cells,
in keeping with poorly differentiated SCC that was reactive for
p16, a tumour marker highly associated with HPV (14). A computed tomography (CT) scan of
the abdomen and pelvis demonstrated no primary tumour. The patient
underwent pubic lumpectomy in February of 2017 and was subsequently
offered combination CRT but was lost to follow-up due to scheduling
difficulties.
In September 2022, the patient presented to the
Emergency Department of a local rural hospital with sharp anal pain
and obstipation, in which a CT scan demonstrated a perforated
rectal tumour above the anal verge. The perforation was repaired,
and the tumour was removed laparoscopically via loop colostomy in
November 2022 following a multi-disciplinary team meeting. The
subsequent pathology report confirmed the presence of invasive
basaloid SCC with focal necrosis, in keeping with the initial
primary diagnosis in 2017. The tumour expressed p40 positivity,
which indicated SCC origin (15).
Concurrent CRT with mitomycin-C with oral capecitabine was
commenced, and the patient also underwent 54 Gy of targeted
radiation therapy in 27 fractions. The patient was declared in
remission in December of 2023 and shortly thereafter underwent a
reversal of the colostomy the following month.
The patient presented to the Emergency Department in
March 2024 with several weeks of lethargy, disorientation, labile
mood, personality changes and headaches. Physical examination was
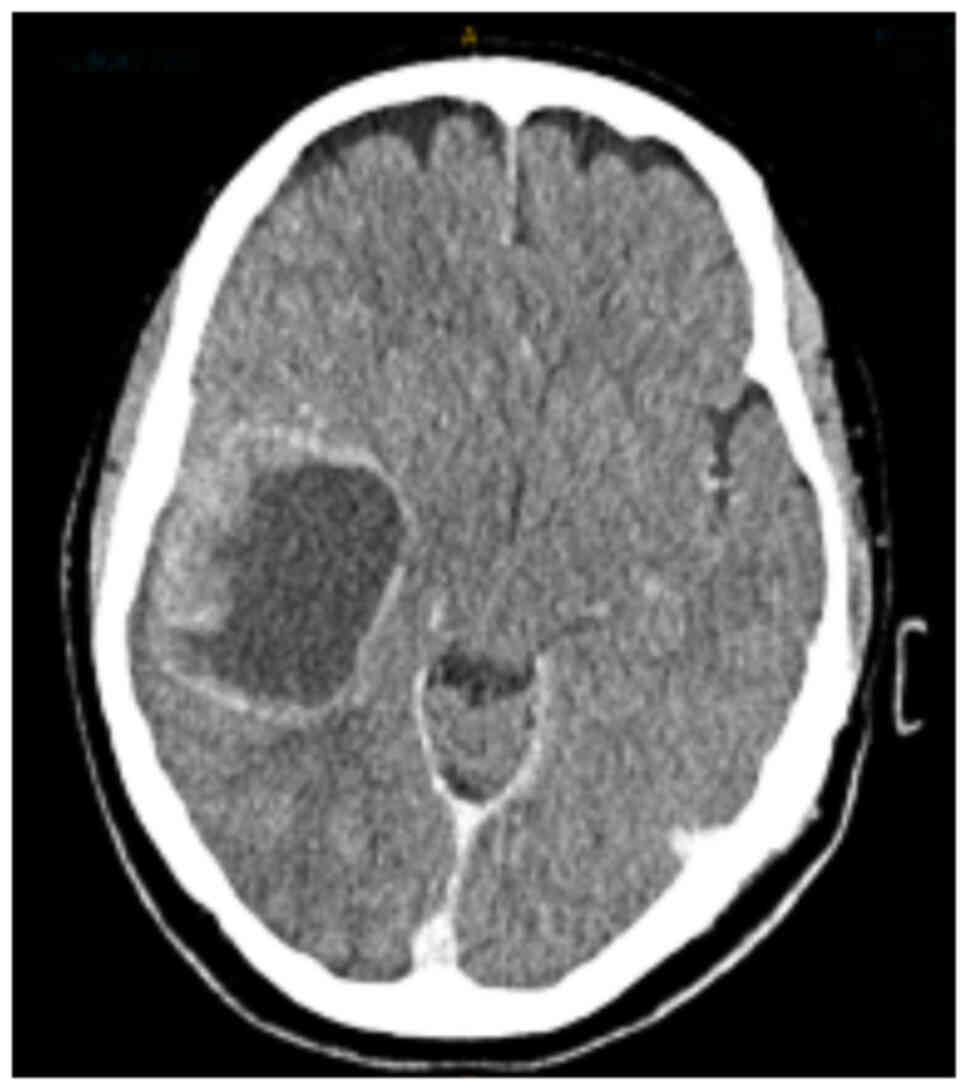
unremarkable on admission. A CT scan demonstrated a 5.2×4.2×4.9 cm
rounded solid and cystic, irregular enhancing mass in the right
frontotemporal region of the brain, in keeping with metastasis
(Fig. 1). There was midline shift
to the left, effacement of the right lateral ventricle and mild
perilesional oedema. The patient was subsequently transferred to
Flinders Medical Centre (Adelaide, Australia), and admitted for
review and treatment under the Neurosurgical Unit.
CT scans of the chest, abdomen and pelvis did not
demonstrate recurrent colorectal malignancy or new nodal or
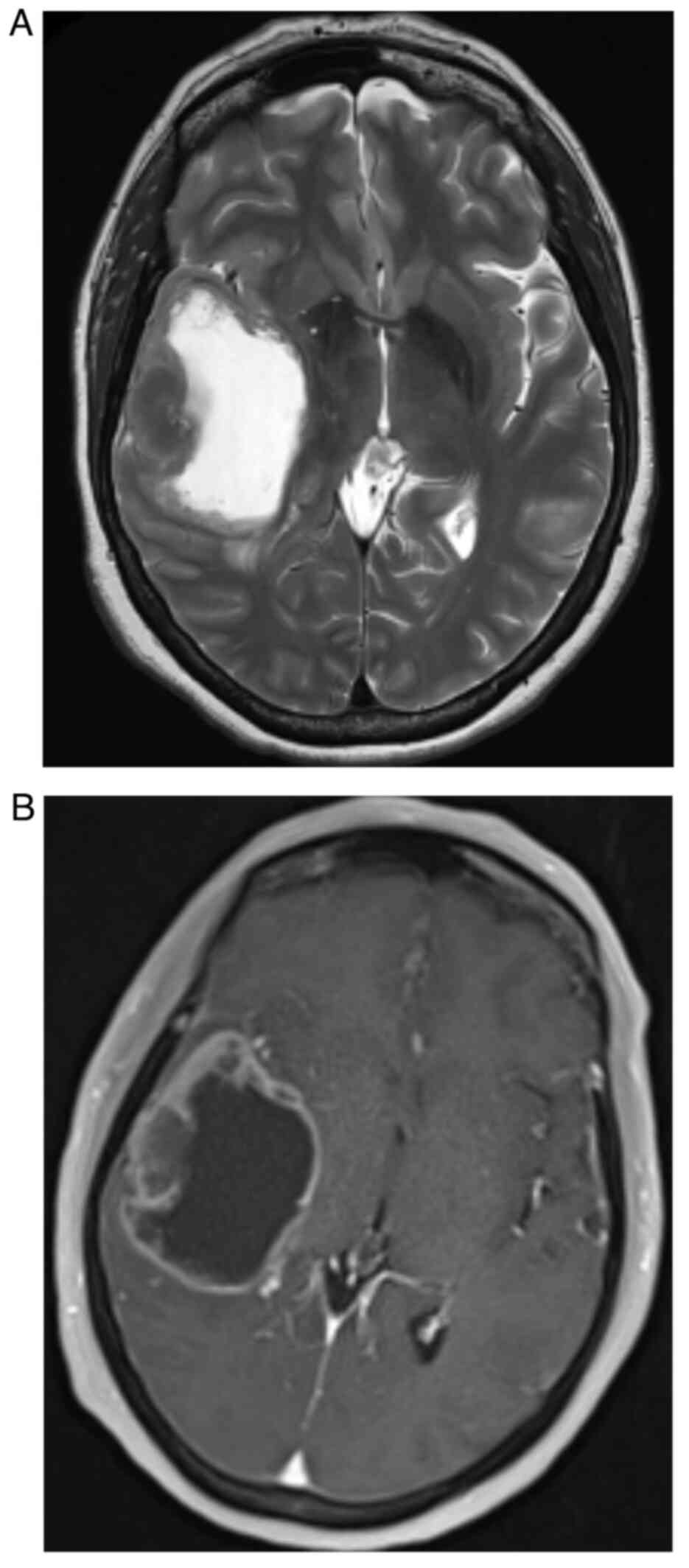
metastatic disease. Magnetic resonance imaging (MRI) of the brain
with gadolinium confirmed a large cystic and necrotic solitary
metastatic lesion within the temporal lobe (Fig. 2).
Following counselling and consent, the patient
underwent neuronavigation-guided craniotomy and resection of the
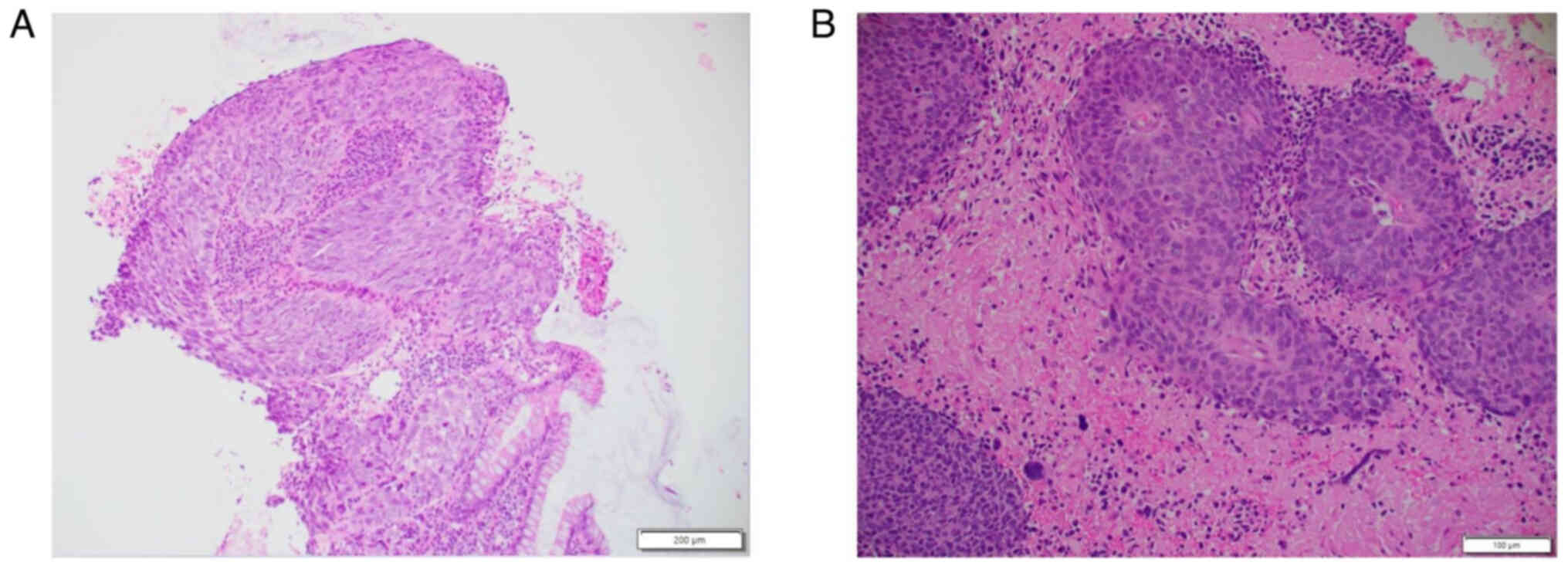
tumour in March of 2024. Histopathological analysis of haematoxylin
and eosin staining confirmed the presence of cells consistent with
sheets and nests of malignant basaloid epithelial cells
demonstrating nuclear enlargement, hyperchromasia and marked
pleomorphism, as well as brisk mitotic activity in addition to
focal keratinisation (Fig. 3). A
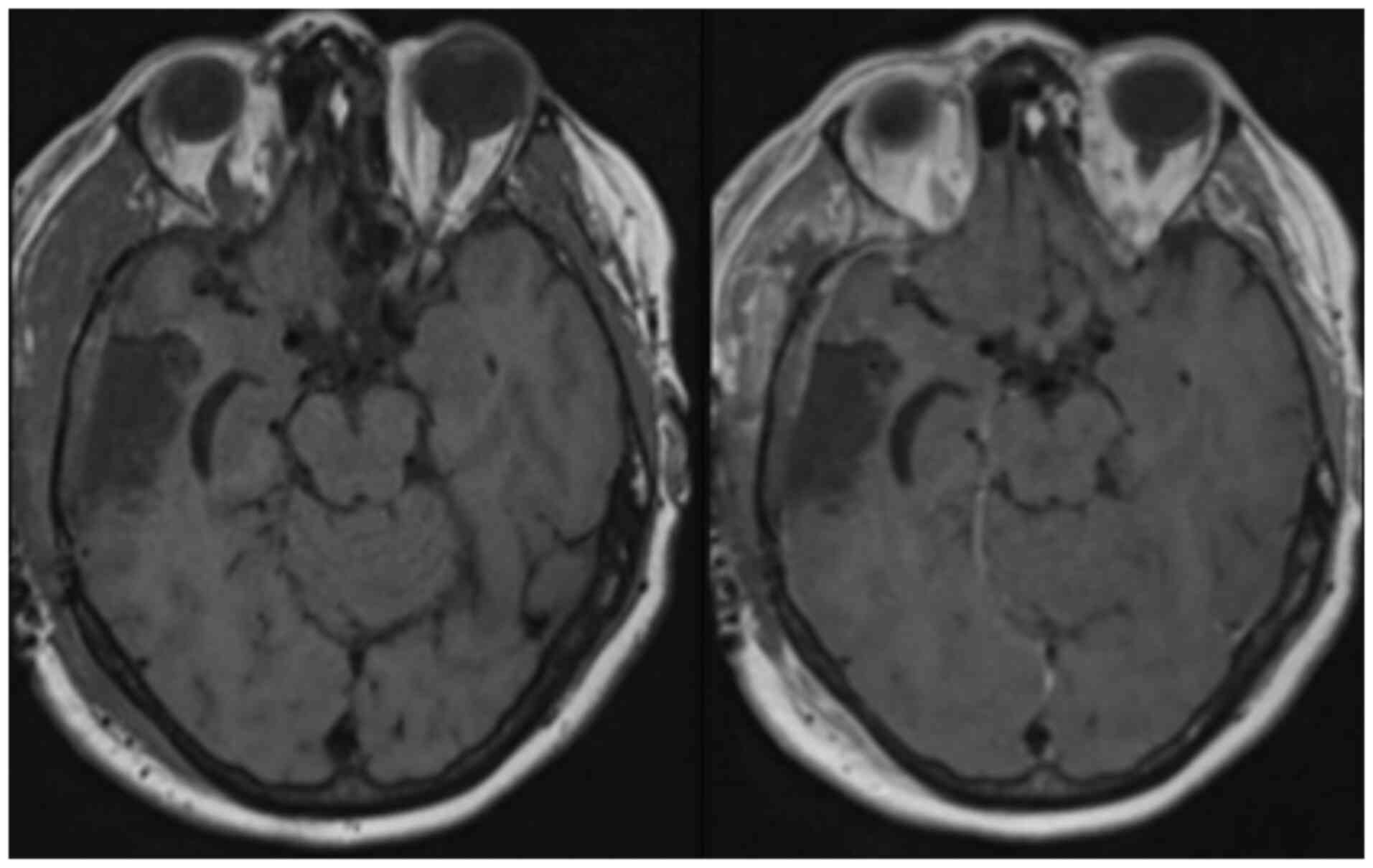
post-operative MRI did not demonstrate any residual tumour
(Fig. 4). The patient had recovered
well post-operatively without any complications and was discharged
home on day 9 post-operatively, with referrals to the original
oncologic team for consideration of further CRT.
The patient attended a follow-up appointment with a
medical oncologist 1 month after discharge, who discussed radiation
therapy. It was considered that systemic chemotherapy would not
have provided any benefit at the time due to the lack of
progression of systemic disease. The patient underwent 30 Gy over
10 fractions of hypofractionated radiotherapy to the cerebral
resection cavity. The patient had repeat abdomen CT and brain MRI
scans (Fig. 4) 4 months
post-operatively, which showed no recurrence of either anal primary
or cerebral metastasis. At a 2- and 5-month neurosurgical review,
no ongoing clinical issues were reported except mild fatigue, and
the patient remained neurologically intact. The patient was
subsequently discharged from the neurosurgical clinic with no
further follow ups.
Discussion
Anal cancer, a rare but deadly malignancy, is
increasingly prevalent in Western countries. SCC is the most common
form of anal cancer, accounting for 80% of cases (1). Primary anal adenocarcinoma comprises
10–20% of anal cancer cases, while anal melanoma and basal cell
carcinoma are exceedingly rare, seen at 1–4 and 0.2%, respectively
(1). Certain risk factors increase
the likelihood of developing anal cancer, such as smoking, HIV and
sexually transmitted diseases such as HPV subtypes 16 and 18. Once
anal cancer has metastasized, it carries a poor prognosis and is
often fatal (4). Other risk factors
include males who engage in sexual activity with males, receptive
anal intercourse, history of other sexually transmitted diseases,
use of immunosuppressive medication and women with a history of
cervical cancer (16). The
incidence of anal cancer has increased in Western countries over
the last few decades at ~2.2% per year, likely due to changes in
sexual behaviour leading to sexually transmitted infections.
Individuals who have undergone organ transplantation are also at an
increased risk for anal cancer due to the necessary
immunosuppressive medication regimen, allowing cancer cells to
proliferate without mounting a significant immune response
(16). Combined CRT, commonly
utilising 5-fluorouracil (5-FU) in addition to cisplatin or
mitomycin-C, is the standard treatment for anal SCC, but there is
no current consensus for treating metastatic disease (4).
To date, 9 previous cases of cerebral metastasis
from anal SCC have been documented in the literature, with ages
ranging from 44–69 years (see Table
I) (6–13). Among these cases, 5 patients were
female, 3 were male and the sex of 1 patient was not disclosed.
Treatment of metastatic brain lesions varied based on the location
and extent of the metastasis, the patient's preferences, and their
functional status at diagnosis. None of the reported cases
initially presented with known metastatic cerebral disease and 3
cases presented with no evidence of metastasis at all (6,11,12).
The most common locations for distant metastasis were the local
lymph nodes, the liver or both, as observed in 6 patients (7–10,13).
Only 2 cases initially presented with lung metastasis at diagnosis
(10,13). There was no discernible pattern when
comparing the natural history of these cases. The first documented
case of cerebral metastatic spread was secondary to anal
cloacogenic carcinoma in 1967. No further information was reported
regarding patient demographics, staging, treatment or outcome for
this patient (12).
 | Table I.Previously reported cases of anal
squamous cell carcinoma. |
Table I.
Previously reported cases of anal
squamous cell carcinoma.
| First author,
year | Age, years | Sex | Histopathology | Location of distant
metastasis at time of diagnosis | Initial
treatment | Time from diagnosis
to cerebral metastasis | Location of cerebral
metastasis and subsequent treatment | Outcome | (Refs.) |
|---|
| Klotz et al,
1967 | Information not
provided. | Information not
provided. | Transitional
cloacogenic carcinoma (subtype of squamous cell carcinoma) | Nil | Information not
provided. | Information not
provided. | Information not
provided. | Information not
provided. | (12) |
| Davidson et
al, 1991 | 53 | F | Basaloid carcinoma
(subtype of SCC) | Nil | Abdominoperineal
surgery. | 8 years | Cerebellum. Surgical
excision and palliative radiotherapy. | Succumbed to disease
3 months after diagnosis of cerebral metastasis. | (11) |
| Rughani et al,
2011 | 63 | F | Poorly differentiated
SCC | Inguinal
lymphadenopathy and liver lesion. | 17 doses of pelvic
radiation with oral chemotherapy (capecitabine 1500 mg, twice
daily). | Unclear | Right parietal lobe
lesion. Elective craniotomy and whole brain radiation. | Neurological symptoms
unchanged at 1-month post-operative follow up. Patient succumbed to
disease 14 weeks post diagnosis of cerebral metastasis. | (10) |
| Gassman et al,
2012 | 67 | M | Invasive poorly
differentiated SCC. | Isolated hepatic
metastasis. | 5-FU, cisplatin +
50.4 Gy radiation, followed by further cisplatin therapy. | 6 months | Intracranial mass
with involvement of the sphenoid and temporal bones, in addition to
left orbit, CNII and rectus muscle. Stereotactic radiotherapy
planned for newly found brain metastasis. | Patient experienced
recurrence of primary disease, treated with abdominoperineal and
liver resection for recurrence. Patient succumbed to disease prior
to commencement of stereotactic radiotherapy for brain
metastasis. | (9) |
| Hernando-Hubero
et al, 2014 | 69 | M | Stage IV basaloid
undifferentiated carcinoma. | Mesorectal
lymphadenopathy, multiple pulmonary and liver metastases. | Cisplatin +
5-FU | 14 months | Right parietal,
left rolandic, cortical and subcortical, cerebellar and frontal
lobes, with tonsillar herniation. Steroid therapy + 30 Gy. | Patient succumbed
to disease 12 weeks after brain metastasis discovered. | (13) |
| Ogawa et al,
2017 | 60′s | F | Poorly
differentiated SCC, positive for CK5/6 and 34βE12, and negative for
CDX2. Negative for HPV and p16. | Liver and internal
iliac lymph node. | Two cycles of 5-FU
700 mg/m2 and cisplatin 70 mg/m2 + 54 Gy/27
fractions of radiotherapy. | 7 months | Metastatic lesions
in occipital lobe (19 Gy) and cerebellum (21 Gy). Patient then
underwent surgical excision of occipital tumour that had grown,
while cerebellar lesion showed full response to treatment. A
fluoropyrimidine anticancer agent was administered + 22 Gy for
progression of liver metastasis. Occipital tumour recurrence
detected, subsequently treated with 22 Gy of radiotherapy.
Recurrence of occipital lesion was treated surgically. | Patient experienced
adverse effects from treatment which included significant anorexia.
After 5 years, treatment was ceased. Out-patient follow up was
scheduled. Longest reported survival of a patient with a distant
cerebral metastasis. | (7) |
| Chihabeddine et
al, 2021 | 44 | F | Poorly
differentiated SCC | Extension into
gluteal soft tissue and involvement of mesorectal nodules,
cutaneous nodules of the sacral region, and bone lesions of greater
trochanter. | Chemotherapy with
capecitabine 825 mg/m2, cisplatin 80 mg/m2 +
radiotherapy total dose of 60 Gy. | A few months | Left parietal lobe
and frontal bone with involvement of soft tissue of scalp. Total
brain external radiotherapy, total dose of 20 Gy. | Patient succumbed
to disease during treatment. | (8) |
| Chihabeddine et
al, 2021 | 60 | M | Moderately
differentiated SCC with positive anti p40. Negative for programmed
death ligand 1 and microsatellite instability. | Secondary lung
localization, external iliac and inguinal lymphadenopathy. Lesions
seen in sacrum, pubis, and coccyx. | Palliative
chemotherapy docetaxel, cisplatin, FU + granulocyte
colony-stimulating factor, docetaxel, and 5-FU for 12 weeks.
Treatment ceased early due to persistent neutropenia. Second-line
treatment: 60 Gy, capecitabine and cisplatin. | A few months | Multiple metastatic
lesions above and below the tentorium Total brain radiotherapy
planned. | Patient succumbed
to disease prior to starting treatment. | (8) |
| Malla et al,
2022 | 54 | F | Poorly
differentiated SCC with ki-67 at 80%. Positive for p63 and
pancytokeratin. p16 also strongly positive. | Nil | 5-FU and
mitomycin | 3 months | Right frontal mass
with significant oedema. Surgical resection and g knife
radiosurgery. Whole brain radiotherapy with 3,000 Gy and intensity
modulated radiation therapy. | Patient experienced
recurrence 8 months later and had another round of g knife
radiosurgery. Following another recurrence of a brain lesion 7
months later, patient opted for hospice care. | (6) |
In the present study, the patient was diagnosed with
anal malignancy at the end of 2017. After being lost to follow-up
following their initial presentation, the patient underwent
appropriate treatment with CRT in 2022. However, despite undergoing
appropriate treatment for anal SCC, the patient was diagnosed with
a cerebral metastatic lesion ~6.5 years following initial diagnosis
and 6 months after achieving remission. The shortest reported
interval between primary and metastatic diagnosis was 3 months
(6) and the longest was 8 years
(11). Malla et al (6) reported a patient to have metastatic
spread to the frontal lobe who underwent surgical resection of the
brain tumour plus γ knife radiosurgery before experiencing a
recurrence of the brain lesion 8 months later and was subsequently
treated with whole-brain radiation therapy (WBRT). Despite
receiving WBRT, another cerebral recurrence was discovered 7 months
later. The patient then opted for hospice care instead of further
treatment.
The longest period between primary diagnosis and
metastatic discovery was reported by Davidson and Yong (11) in 1991. A 61-year-old female patient
with anal SCC presented with neurological symptoms 8 years after
undergoing surgical resection of the primary malignancy. The
patient subsequently underwent surgical resection and radiotherapy
for the brain lesion. This was the only reported case with surgical
resection of the primary malignancy, similar to the present
case.
Of the 9 reported cases, 2 patients had succumbed to
their disease before commencing treatment (9,10).
Both patients were found to have multiple cerebral metastases in
different areas of the brain several months after their initial
diagnosis of anal cancer. Both patients passed away before
beginning the planned radiotherapy. In total, 3 patients succumbed
to the disease either during the treatment of the brain metastasis
or shortly after completing secondary treatment (8,10,13).
All cerebral metastases were treated or planned for treatment with
surgical resection, radiation therapy or a combination of the two.
No case report documented the use of chemotherapy in treating
cerebral metastasis. In a review by Sclafani et al (17) in 2019, treatment protocols in 8
reports of advanced anal SCC with liver and lung metastasis were
compared and it was found that although there is a lack of
high-level evidence to guide the treatment of advanced anal SCC, a
multi-disciplinary approach to treating metastatic disease was
considered a superior approach. It was concluded that aggressive
management with various treatment modalities, including surgery,
radiotherapy and radiofrequency ablation, can offer more extended
disease control compared with systemic chemotherapy alone. However,
given the rarity of the disease and lack of supportive evidence,
further randomised control trials are urgently necessary to develop
a substantial treatment regime tailored to individualised
treatments.
A case reported in 2017 by Ogawa et al
(7) documented the longest survival
period from diagnosis of primary malignancy to death, which was 5
years. The case involved a female patient in her 60s who was
initially diagnosed with poorly differentiated SCC, which was
positive for cytokeratin (CK) 5, 6 and 34bE12, and negative for
CDX2. CK5 and 6 are understood to be indicative of squamous cell
origin, ruling out adenocarcinoma and neuroendocrine origins
(18). At the initial presentation,
the patient also had liver and internal iliac lymph node
involvement. Initial treatment included CRT with 5-FU and cisplatin
with 54 Gy over 27 fractions of radiotherapy. However, 7 months
after initial diagnosis, metastatic brain lesions were found in the
occipital lobe and cerebellum. The patient received 19 Gy of
radiation to the occipital lesion and 21 Gy to the cerebellar
lesion. Over the next 4 years, the patient experienced multiple
recurrences of the primary cancer, as well as further cerebral
metastasis. Despite receiving various treatments, including
radiation, surgical resection and chemotherapeutics, the patient
experienced significant adverse events, including significant
anorexia and ceased further treatment after 5 years (7).
The present patient's primary SCC displayed strong,
diffuse cytoplasmic and nuclear labelling for p16. The only other
documented case of p16 positivity was reported by Malla et
al (6). p16 upregulation was
previously considered an indicator of HPV-induced transformation to
anal carcinoma; however, it has since been discovered to occur
independent of HPV infection. HPV-negative tumours more frequently
correlate with TP53 mutations compared with p16 upregulation
(19), while p16 upregulation can
occur independent of HPV via the p16/ retinoblastoma protein (Rb)
pathway. Specifically, if p16 expression is lost, unregulated
CDK4/6 expression leads to hyperphosphorylated Rb, ultimately
leading to uncontrolled cell proliferation (20). p16 has also been used as a marker of
malignancy in cervical SCC. It was found that the intensity with
which p16 stained immunohistochemically has a direct relationship
to the severity of cervical lesion or disease (21).
HPV and p16 should only be considered in tandem; p16
independently is not considered to be a reliable marker of
HPV-induced transformation as p16 can be positive for malignancies
unrelated to HPV (22). Although
not an exhaustive list, p16 overexpression is seen in endometrial
carcinoma (23) and mesothelioma
(24). The presence of p16
positivity without HPV in anal SCC tends to lead to a poorer
prognosis due to a reduced response to treatment (14). The presence of both HPV and p16 is
linked to an increased response to treatment, whereas HPV and p16
negative status records the lowest rate of response to treatment
and the worst prognosis overall (14). A meta-analysis in 2017 concluded
that patients with HPV+/p16+ status anal SCC had improved outcomes
than those with HPV-/p16+ or HPV-/p16-status. Additionally, it was
suggested that an individual's HPV and p16 status can be used for
therapeutic guidance and of prognostic utility in anal SCC
(25). The American Society of
Colon and Rectal Cancer Surgeons guidelines suggests that there may
be benefit in vaccination against HPV to prevent anal cancer
altogether (26). The present
patient did not have any risk factors for anal cancer other than
smoking. At the time of initial diagnosis of anal SCC, the patient
had been a smoker for a couple of decades, although had since
ceased smoking.
Patients who complete CRT may benefit from
assessment of clinical response to the primary malignancy at 6
months with a digital rectal exam, palpation of inguinal lymph
nodes, and a yearly CT/positron emission tomography scan for up to
3 years (27,28). However, no significant data supports
investigating distant metastasis during the surveillance period
(27). The benefits of full-body
scanning in patients who present with evidence of liver and/or lung
metastasis at the initial diagnosis of an anal primary should be
considered. This does, however, carry implications. For example,
the cost-effectiveness of a full-body scan, as well as the
availability of such scans. Furthermore, a false positive can
inflict unnecessary distress on a patient and their family members.
Other logistical barriers also exist, including insurance
limitations and transport to such facilities. Further research
aiming to optimise surveillance may potentially benefit
gastrointestinal malignancies altogether.
Anal SCC with metastatic spread to the brain
presents a poor prognosis as survival from the time of discovery of
cerebral lesions to death is a short few months to years (5). Distant spread to the brain follows no
understood pattern concerning either location, quantity or
timeline. Additionally, those who present without metastasis
initially do not necessarily carry an improved prognosis when
compared with those who present with metastasis. The case report by
Davidson and Young was the first to report that their patient had
been in remission of their primary anal SCC at the time of
presentation with cerebral disease (11). This implies that at the time of
declaring remission, there is a likelihood that microscopic
malignant cells will linger undetected at the time of a follow-up
scan. Colorectal cancer may spread to the liver via the portal
circulation, which can lead to hepatic metastasis while increasing
the risk of spread to the lungs as well. From here, a highway is
created for dissemination of metastases to the central nervous
system (29).
Additionally, a brain lesion may then be missed in
the workup and surveillance for anal cancer as the brain is not
routinely imaged unless otherwise clinically indicated (30). It may be beneficial to consider a CT
of the brain with contrast in patients with initial metastasis in
the liver and/or lungs to rule out cerebral metastasis at
presentation confidently.
Furthermore, utilisation of a chemotherapeutic agent
that can effectively cross the blood-brain barrier (BBB) could be
considered, to ensure that spread to the brain is minimised or
prevented in patients with known existing metastatic disease. For
example, an agent such as temozolomide, which has historically been
used in treating gliomas and can penetrate the BBB, may be
considered in preventing cerebral metastasis (31). A review of 21 clinical trials in
2014 investigated the efficacy of temozolomide in treating cerebral
metastases from various solid tumours which included non-small cell
lung cancer and malignant melanoma. It was noted that trials with
temozolomide-monotherapy had modest response, while metastases
treated with combination chemotherapy with temozolomide and
radiation therapy saw an even greater response to treatment
compared with monotherapy alone (32).
Of the previous cases reported, no two primary anal
malignancies nor cerebral metastasis were treated identically.
Primary treatment consisted of either surgery, chemotherapy,
radiation or a combination of the three. This area of
gastrointestinal malignancies would benefit from further research
in standardising primary treatment to decrease the risk of
recurrence and metastatic spread and develop treatment protocols
for advanced disease and surveillance protocols. Whether
investigating cerebral lesions before declaring remission would be
of benefit warrants further study. Finally, testing for tumour
markers such as p16 may be considered when planning treatment and
to further understand the disease course. In conclusion, patients
at risk for anal cancer should take steps to protect their health
by mitigating risks and undergoing regular screening to detect any
potential problems early.
Acknowledgements
The authors would like to thank Ms. Eri Sakata for
her assistance with the translation of Japanese to English for
reference 7.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to patient confidentiality.
Author's contributions
EAP, VMT and ARM made substantial contributions to
the conception/design of the work. PSM, EQ and CT were responsible
for the acquisition, analysis and interpretation of medical
information for the work, advised on patient treatment, obtained
CT/MRI images and pathology images. All authors contributed to
drafting the work or revising it critically for important
intellectual content. All authors read and approved the final
version of the manuscript. EAP, VMT, ARM, PSM, EQ and CT confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Southern
Adelaide Clinical Human Research Ethics (SAC HREC) Committee
(November 12 2024; Adelaide, Australia). Ethics and Governance
compliance within the network is regulated by the Southern Adelaide
Local Health Network Research Hub through the SAC HREC. Flinders
Medical Centre is part of the same network.
Patient consent for publication
The patient provided oral (recorded) informed
consent for the publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Young AN, Jacob E, Willauer P, Smucker L,
Monzon R and Oceguera L: Anal Cancer. Surg Clin North Am.
100:629–634. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babiker HM, Kashyap S, Mehta SR, Lekkala
MR and Cagir B: Anal Cancer. 2024. View Article : Google Scholar
|
|
3
|
Morton M, Melnitchouk N and Bleday R:
Squamous cell carcinoma of the anal canal. Curr Probl Cancer.
42:486–492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gnanajothy R, Warren GW, Okun S and
Peterson LL: A combined modality therapeutic approach to metastatic
anal squamous cell carcinoma with systemic chemotherapy and local
therapy to sites of disease: Case report and review of literature.
J Gastrointest Oncol. 7:E58–E63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sawai K, Goi T, Tagai N, Kurebayashi H,
Morikawa M, Koneri K, Tamaki M, Murakami M, Hirono Y and Maeda H:
Stage IV anal canal squamous cell carcinoma with Long-term
survival: A case report. Surg Case Rep. 8:1192022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malla M, Hatoum H and George S: Brain
metastases in anal squamous cell carcinoma: Case report and review
of the literature. J Clin Images Med Case Rep. 32022.PubMed/NCBI
|
|
7
|
Ogawa S, Fujishiro H, Fujiwara A, Tsukano
K, Kotani S, Yamanouchi S, Kusunoki R, Aimi M, Miyaoka Y, Kohge N
and Onuma H: A case of Long-term survival in stage IV squamous cell
carcinoma of the anal canal with multidisciplinary treatment. Nihon
Shokakibyo Gakkai Zasshi. 114:1987–1995, (In Japanese). PubMed/NCBI
|
|
8
|
Chihabeddine M, Naim A, Habi J, Kassimi M,
Mahi M and Kouhen F: Anal cancer with atypical brain and cranial
bones metastasis: About 2 cases and literature review. Case Rep
Oncol. 14:778–783. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gassman AA, Fernando E, Holmes CJ, Kapur U
and Eberhardt JM: Development of cerebral metastasis after medical
and surgical treatment of anal squamous cell carcinoma. Case Rep
Oncol Med. 2012:9121782012.PubMed/NCBI
|
|
10
|
Rughani AI, Lin C, Tranmer BI and Wilson
JT: Anal cancer with cerebral metastasis: A case report. J
Neurooncol. 101:141–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davidson NG and Yong PP: Brain metastasis
from basaloid carcinoma of the anal canal 8 years after
abdominoperineal resection. Eur J Surg Oncol. 17:227–230.
1991.PubMed/NCBI
|
|
12
|
Klotz RG, Pamukcoglu T and Souilliard DH:
Transitional cloacogenic carcinoma of the anal canal.
Clinicopathologic study of three hundred Seventy-three cases.
Cancer. 20:1727–1745. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernando-Cubero J, Alonso-Orduña V,
Hernandez-Garcia A, De Miguel AC, Alvarez-Garcia N and Anton-Torres
A: Brain metastasis in basaloid undifferentiated anal carcinoma: A
case report. Oncol Lett. 7:1276–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mai S, Welzel G, Ottstadt M, Lohr F,
Severa S, Prigge ES, Wentzensen N, Trunk MJ, Wenz F, von
Knebel-Doeberitz M and Reuschenbach M: Prognostic relevance of HPV
infection and p16 overexpression in squamous cell anal cancer. Int
J Radiat Oncol Biol Phys. 93:819–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rami S, Han YD, Jang M, Cho MS, Hur H, Min
BS, Lee KY and Kim NK: Efficacy of Immunohistochemical staining in
differentiating a squamous cell carcinoma in poorly differentiated
rectal cancer: Two case reports. Ann Coloproctol. 32:1502016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Zee RP, Richel O, de Vries HJC and
Prins JM: The increasing incidence of anal cancer: Can it be
explained by trends in risk groups? Neth J Med. 71:401–411.
2013.PubMed/NCBI
|
|
17
|
Sclafani F, Hesselberg G, Thompson SR,
Truskett P, Haghighi K, Rao S and Goldstein D: Multimodality
treatment of oligometastatic anal squamous cell carcinoma: A case
series and literature review. J Surg Oncol. 119:489–496. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gondal TA, Chaudhary N, Bajwa H, Rauf A,
Le D and Ahmed S: Anal cancer: The past, present and future.
Current Oncol. 30:3232–3250. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meulendijks D, Tomasoa NB, Dewit L, Smits
PHM, Bakker R, van Velthuysen MLF, Rosenberg EH, Beijnen JH,
Schellens JH and Cats A: HPV-negative squamous cell carcinoma of
the anal canal is unresponsive to standard treatment and frequently
carries disruptive mutations in TP53. Br J Cancer. 112:1358–1366.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rayess H, Wang MB and Srivatsan ES:
Cellular senescence and tumor suppressor gene p16. Int J Cancer.
130:1715–1725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izadi-Mood N, Asadi K, Shojaei H, Sarmadi
S, Ahmadi SA, Sani S and Chelavi LH: Potential diagnostic value of
P16 expression in premalignant and malignant cervical lesions. J
Res Med Sci. 17:428–433. 2012.PubMed/NCBI
|
|
22
|
Mai S, Welzel G, Ottstadt M, Lohr F,
Severa S, Prigge ES, Wentzensen N, Trunk MJ, Wenz F, von
Knebel-Doeberitz M and Reuschenbach M: Prognostic relevance of HPV
infection and p16 overexpression in squamous cell anal cancer. Int
J Radiat Oncol Biol Phys. 93:819–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon G, Koh CW, Yoon N, Kim JY and Kim HS:
Stromal p16 expression is significantly increased in endometrial
carcinoma. Oncotarget. 8:4826–4836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kettunen E, Savukoski S, Salmenkivi K,
Böhling T, Vanhala E, Kuosma E, Anttila S and Wolff H: CDKN2A copy
number and p16 expression in malignant pleural mesothelioma in
relation to asbestos exposure. BMC Cancer. 19:5072019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun G, Dong X, Tang X, Qu H, Zhang H and
Zhao E: The prognostic value of HPV combined p16 status in patients
with anal squamous cell carcinoma: A meta-analysis. Oncotarget.
9:8081–8088. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stewart DB, Gaertner WB, Glasgow SC,
Herzig DO, Feingold D and Steele SR: The American society of colon
and rectal surgeons clinical practice guidelines for anal squamous
cell cancers (revised 2018). Dis Colon Rectum. 61:755–774. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adams R: Surveillance of anal canal
cancers. Surg Oncol Clin N Am. 26:127–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glynne-Jones R, Sebag-Montefiore D,
Meadows HM, Cunningham D, Begum R, Adab F, Benstead K, Harte RJ,
Stewart J, Beare S, et al: Best time to assess complete clinical
response after chemoradiotherapy in squamous cell carcinoma of the
anus (ACT II): A post-hoc analysis of randomised controlled phase 3
trial. Lancet Oncol. 18:347–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhong X, He X, Hu Z, Huang H, Chen
J, Chen K, Zhao S, Wei P and Li D: Liver metastasis from colorectal
cancer: Pathogenetic development, immune landscape of the tumour
microenvironment and therapeutic approaches. J Exp Clin Cancer Res.
42:1772023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riihimäki M, Hemminki A, Sundquist J and
Hemminki K: Patterns of metastasis in colon and rectal cancer. Sci
Rep. 6:297652016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tashima T: Brain cancer chemotherapy
through a delivery system across the Blood-brain barrier into the
brain based on Receptor-mediated transcytosis using monoclonal
antibody conjugates. Biomedicines. 10:15972022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu W: Temozolomide for treatment of brain
metastases: A review of 21 clinical trials. World J Clin Oncol.
5:192013. View Article : Google Scholar
|