Introduction
Breast cancer is among the most commonly diagnosed
types of cancer worldwide, and its burden has been growing over the
course of the last few decades. In 2020, >2.3 million new cases
and 685,000 associated deaths were reported globally (1). It is estimated that, owing to
population growth and aging, the numbers of annual new cases and
deaths from breast cancer will reach >3 million and >1
million, respectively, by the year 2040 (2). Clinical treatment of breast cancer has
remained challenging for clinicians.
Breast-conserving surgery (BCS) refers to a combined
treatment consisting of surgical tumor resection and postoperative
radiotherapy (PORT). A number of clinical trials have shown that
overall survival and local recurrence rates for BCS are comparable
with those associated with total mastectomy (3,4). As
such, BCS has been implemented as the first choice alternative to
radical mastectomy in patients, especially those with early breast
cancer (EBC). As an essential component of BCS, PORT is performed
using external whole-breast radiation and additional exposure to
the tumor bed for reducing local recurrence and mortality (5). PORT is typically initiated following
the surgical treatment and completed during a period of 5–7 weeks
(five irradiations per week, 1.8–2 Gy/time). The total dose of the
irradiation is generally 50–60 Gy for patients with a negative
tumor margin, although this may be increased to 66 Gy for those
with a positive margin (6). One
drawback of this therapy is that the high-dose irradiation received
may cause significant co-morbidities, including nausea, vomiting,
diarrhea and damage to adjacent tissues (7). Other aspects of PORT are a long
treatment time and high costs, which present a large burden for
patients. To obviate the need to attend long-term and daily RT, a
great number of patients are obliged to opt for a total mastectomy
instead, thereby precluding a significant proportion of patients
from receiving PORT. Moreover, in the case of patients receiving
chemotherapy, the initiation of PORT may be delayed, which is
likely to result in an increased risk of local relapse. Therefore,
there is an urgent need to develop novel irradiation strategies for
the purposes of eliminating such problems and improving clinical
outcomes in patients with EBC.
A previous study indicated that up to 90% of local
recurrences in patients with breast cancer occur near the primary
tumor site, which inspired the development of accelerated partial
breast irradiation (APBI), a targeted treatment approach (8). As a technique of APBI, intraoperative
RT (IORT) began to be utilized 20 years ago, and has since been
applied as an effective therapeutic strategy for irradiation
therapy in cases of breast cancer (9,10).
Unlike traditional PORT, IORT delivers a concentrated dose of
radiation directly to the tumor bed during surgery, thereby
minimizing radiation exposure to surrounding healthy tissue. This
technique aims to reduce the treatment time and to enhance patient
convenience, while maintaining efficacy (9,10).
Several retrospective and prospective clinical trials, and two
randomized clinical trials, have been conducted comparing IORT with
PORT (11–15). The data revealed that IORT had equal
local control and patient survival rate, and slightly improved
cosmetic outcomes compared with PORT. However, the role of IORT
combined with BCS remains controversial, largely because outcomes
could be influenced by multiple factors, such as patient selection
bias, surgical margin status, adjuvant systemic therapies or
surgeon expertise, making it challenging to isolate IORT's specific
impact. Based on the previous observations, we hypothesized that
IORT might be effective for the treatment of EBC and may produce
superior cosmetic results compared with PORT. The current
retrospective cohort study explores the role of IORT following BCS,
with focus on the oncological outcomes and cosmetic consequences
compared with PORT. The efficacy, safety, cosmetic outcome and
cost-effectiveness are compared between IORT and PORT, and the data
should contribute towards an improved understanding of the benefits
of IORT, which would aid clinical decision-making.
Patients and methods
Patient selection and data
collection
In the present retrospective cohort study, the data
of patients with early-stage breast cancer who underwent BCS
followed by IORT or PORT between January 2016 and October 2020 at
the Research Institute of General Surgery, Jinling Hospital,
Nanjing Medical University (Nanjing, China) were retrospectively
reviewed. All diagnoses were based on the 2012 World Health
Organization classification of breast tumors (16), with pathological type and
histological grade independently evaluated by two experienced
pathologists. Tumor staging followed the 7th edition of the
American Joint Committee on Cancer Tumor-Node-Metastasis guidelines
(17). Clinical data were extracted
from the hospital's electronic medical records and analyzed
retrospectively. Inclusion criteria were as follows: i) Female
patients, aged 18–75 years, with confirmed breast cancer; ii)
tumors located >3 cm from the nipple and being <2.5 cm in
size (verified by ultrasonography and magnetic resonance imaging);
iii) receipt of BCS with IORT or PORT; and iv) confirmed negative
tumor margins. Exclusion criteria included recurrent/metastatic
breast cancer, a history of upper limb surgery or trauma, systemic
diseases predisposing to swelling (e.g., myocardial infarction and
renal dysfunction), pregnancy/lactation and prior treatment for arm
lymphedema. After screening, 59 patients met the criteria and were
categorized into the IORT (n=21) or PORT (n=38) groups based on RT
records. Clinicopathological characteristics are summarized in
Table I. The study protocol
received ethical approval from the Ethics Committee of Jinling
Hospital, Nanjing Medical University (approval no. 2024DZKY-148)
and adhered to the Declaration of Helsinki (2013 revision).
 | Table I.Characteristics of all patients
(n=59) in the retrospective cohort, including the IORT (n=21) and
PORT (n=38) groups. |
Table I.
Characteristics of all patients
(n=59) in the retrospective cohort, including the IORT (n=21) and
PORT (n=38) groups.
| Parameters | All patients, %
(n) | IORT, % (n) | PORT, % (n) | P-value |
|---|
| Age, years |
|
|
| 0.028 |
|
<50 | 54.2 (32) | 33.3 (7) | 65.8 (25) |
|
|
≥50 | 45.8 (27) | 66.7 (14) | 34.2 (13) |
|
| Side |
|
|
| 0.786 |
|
Left | 44.1 (26) | 47.6 (10) | 42.1 (16) |
|
|
Right | 55.9 (33) | 52.4 (11) | 57.9 (22) |
|
| Tumor size, cm |
|
|
| 0.739 |
| ≤2 | 79.7 (47) | 76.2 (16) | 81.6 (31) |
|
|
>2 | 20.3 (12) | 23.8 (5) | 18.4 (7) |
|
| Number of positive
nodes |
|
|
| 0.699 |
| 0 | 88.1 (52) | 95.2 (20) | 84.2 (32) |
|
|
1-3 | 6.8 (4) | 4.8 (1) | 7.9 (3) |
|
| ≥4 | 5.1 (3) | 0.0 (0) | 7.9 (3) |
|
| Histology |
|
|
| 0.571 |
|
IDC | 64.4 (38) | 71.4 (15) | 60.5 (23) |
|
|
Mixeda | 20.3 (12) | 9.5 (2) | 26.3 (10) |
|
|
Other | 15.3 (9) | 19.0 (4) | 13.2 (5) |
|
| Grade |
|
|
| 0.766 |
| 1 | 72.9 (43) | 76.2 (16) | 71.1 (27) |
|
| 2 | 20.0 (13) | 23.8 (5) | 21.1 (8) |
|
| 3 | 5.1 (3) | 0.0 (0) | 7.9 (3) |
|
| Molecular
subtype |
|
|
| 0.337 |
| Luminal
A | 30.5 (18) | 42.9 (9) | 23.7 (9) |
|
| Luminal
B | 49.2 (29) | 47.6 (10) | 50.0 (19) |
|
|
HER2-positive
(non-luminal) | 8.5 (5) | 4.8 (1) | 10.5 (4) |
|
|
Triple-negative | 11.9 (7) | 4.8 (1) | 15.8 (6) |
|
| ER |
|
|
| 0.109 |
|
Positive | 78.0 (46) | 90.5 (19) | 71.1 (27) |
|
|
Negative | 22.0 (13) | 9.5 (2) | 28.9 (11) |
|
| PR |
|
|
| 0.370 |
|
Positive | 72.9 (43) | 81.0 (17) | 68.4 (26) |
|
|
Negative | 27.1 (16) | 19.0 (4) | 31.6 (12) |
|
| HER2 |
|
|
| 0.214 |
|
Positive | 74.6 (44) | 85.7 (18) | 68.4 (26) |
|
|
Negative | 25.4 (15) | 14.3 (3) | 31.6 (12) |
|
| Proliferative index
(Ki-67) |
|
|
| 0.034 |
|
≤20% | 47.5 (28) | 66.7 (14) | 36.8 (14) |
|
|
>20% | 52.5 (31) | 33.3 (7) | 63.2 (24) |
|
| Neoadjuvant
treatment |
|
|
| 0.286 |
|
Yes | 6.8 (4) | 0.0 (0) | 10.5 (4) |
|
| No | 93.2 (55) | 100.0 (21) | 89.5 (34) |
|
| Adjuvant
treatment |
|
|
|
|
|
Endocrine therapy | 81.4 (48) | 95.2 (20) | 73.7 (28) | 0.077 |
|
Chemotherapy | 86.4 (51) | 81.0 (17) | 89.5 (34) | 0.438 |
|
Anti-HER2 therapy | 20.3 (12) | 9.5 (2) | 26.3 (10) | 0.181 |
| Median follow-up
time, years [median (range)] | 6.1 (3.8–8.5) | 6.1 (3.8–8.0) | 6.0 (3.8–8.5) |
|
BCS assessment
All patients underwent an extended resection of a
breast tumor and a sentinel lymph node biopsy. Carbon nanoparticles
were used as the tracer in sentinel lymph node biopsy, and the
nodes underwent immediate pathological examination. If no lymph
node metastasis was identified, the extended resection of the tumor
was allowed to continue. In cases where sentinel lymph node
metastasis was identified, an axillary lymph node dissection was
also performed, in which a fusiform incision was made on the
surface of the tumor to remove the tumor. The distance from the
margin to the tumor was >1 cm. The resected specimens were
anatomically labeled as cephalic (up), caudal (down), lateral
(left/right), cutaneous (front), nipple (central) and deep/basal
(back) margins. Intraoperative pathological evaluation was
performed on all margins. Breast-conserving surgery (BCS) proceeded
only when clear margins were confirmed. In cases with positive
margins, additional tissue adjacent to the involved margin was
excised and re-evaluated by the pathologist until margin clearance
was achieved. For patients receiving IORT, RT was administered by
radiologists prior to the wound closure. All participants provided
written informed consent prior to undergoing surgical treatment and
subsequent adjuvant RT (either IORT or PORT), after being
comprehensively informed about the nature, risks, benefits and
alternatives of both therapeutic approaches, including detailed
explanations of the procedural differences, potential side effects
and expected outcomes associated with each modality.
IORT and PORT
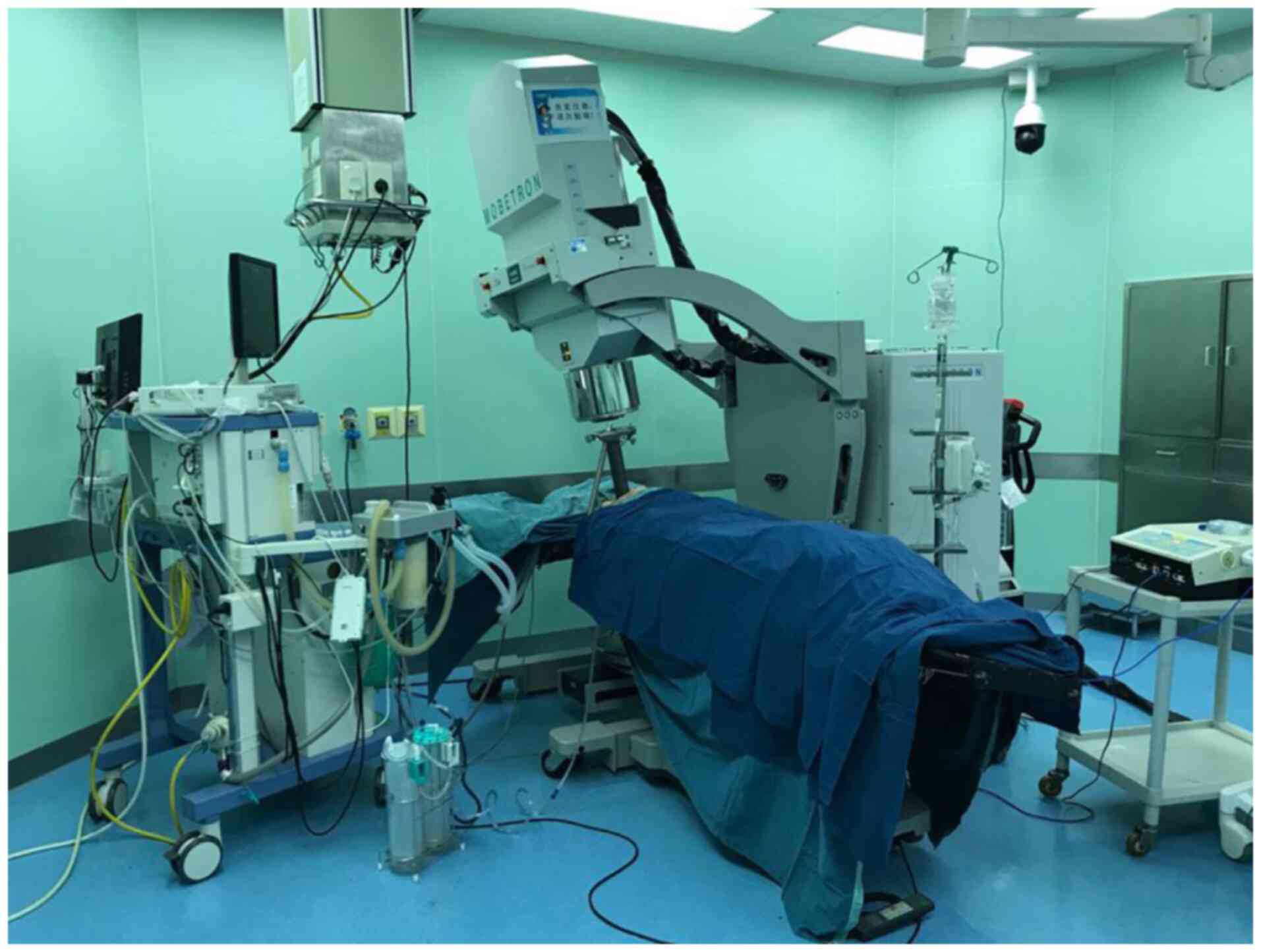
IORT was performed by the radiologists using a
mobile electron beam MOBETRON® system (cat. no.
Mobetron2000; Intraop Medical Corp.) placed in the operating room
(Fig. 1). The irradiation area
included the tumor bed and the tissues both surrounding it (~2 cm)
and below it (~1 cm). The patients received a radiation dose of
18–20 Gy, determined by tumor characteristics (size, pathological
type, molecular subtype and margin status) along with
patient-specific factors such as age and clinical condition. This
dosage was adjusted from a protocol described in a previous report
(18). Depending on the size of the
tumor bed, treatment cones and energy levels were adjusted to
ensure 90% coverage of the target irradiation area. The radiation
was delivered at a dose rate of 10 Gy/min, and the entire radiation
procedure, including pre-treatment alignment of the linear
accelerator with the collimator, was completed within 3–5 min.
Following IORT, the treatment cone was removed and the surgical
site was inspected. The surrounding glands near the tumor bed were
carefully sutured, with efforts made to close any residual lacunae.
Subcutaneous tissue and skin were then closed in layers.
Postoperatively, chemotherapy and endocrine therapy were initiated
based on the final pathological findings.
In the PORT group, patients were treated using
Elekta Precise RT equipment (cat. no. precise1070; Elekta
Instrument AB). Postoperative RT was performed by irradiation of
the entire breast and lymphatic area with a total dose of 50 Gy,
administered at doses of 1.8–2.0 Gy each time (5 times/week). The
patients with a negative margin were able to receive a total dosage
of up to 60 Gy, whereas those with a positive margin required
escalated dosing up to 70 Gy. Following postoperative RT,
chemotherapy and endocrine therapy were subsequently performed
according to the pathological results.
Follow-ups and outcome evaluation
The patients were followed up by visits to the
clinic or telephone calls to evaluate the outcomes of surgery and
RT, including cosmetic complications, recurrence, metastasis events
and survival. The acute and late radiation injuries were evaluated
according to the criteria of radiation injury constituted by the
Radiation Therapy Oncology Group (RTOG) and the European
Organization for Research and Treatment of Cancer (19,20).
Cosmetic outcomes were assessed according to the cosmetic scale of
the Radiation Therapy Oncology Group (21,22).
The scale included four different categories, designated as
follows: i) ‘Excellent’, signifying that the shape of the affected
breast was not different from that of the healthy breast, and there
was no visible distortion or skin change; ii) ‘good’, meaning that
a slight difference in the shape of the affected breast, such as
slight skin distortion, retraction or mild hyperpigmentation, was
identified; iii) ‘fair’, which meant that <25% of the affected
breast exhibited distortion of breast symmetry or moderate
hyperpigmentation; and iv) ‘poor’, which signified that >25% of
the affected breast exhibited a severe distortion of breast
symmetry or moderate hyperpigmentation. Cosmetic outcomes were also
assessed using a modified evaluation framework across five
dimensions: Breast shape, skin condition, nipple and areola,
texture of the breast and patient satisfaction (23,24).
Detailed criteria for this scoring framework are provided in
Table II.
 | Table II.Evaluation criteria for cosmetic
outcomes of standardized breast-conserving treatment in early
breast cancer. |
Table II.
Evaluation criteria for cosmetic
outcomes of standardized breast-conserving treatment in early
breast cancer.
| Evaluation
Dimension | Specific
description | Score |
|---|
| Breast shape | Bilateral breasts
are largely symmetrical, with minor differences visible only upon
close inspection. Contour is near-normal, with minimal impact on
overall appearance. | 2 points |
|
| Moderate asymmetry
or contour irregularity in bilateralbreasts, visibly affecting
overall appearance. | 1 point |
|
| Severe asymmetry or
deformity, with loss of normal breast shape and significant
aesthetic detriment. | 0 point |
| Skin condition | Mild skin
irregularity, faint scarring or minimal pigmentation/wrinkling; not
readily noticeable. | 2 points |
|
| Moderate skin
irregularity, visible scarring or noticeable
pigmentation/wrinkling, but without severe aesthetic impact. | 1 point |
|
| Severe skin
irregularity, thick/obvious scarring or significant
pigmentation/wrinkling, markedly affecting appearance. | 0 point |
| Nipple and
areola | Vertical
discrepancy between bilateral nipple-areola complexes ≤2 cm. Minor
variations in size, shape or color (subtle or absent compared with
contralateral side). | 2 points |
|
| Vertical
discrepancy >2 and ≤3 cm. Significant differences in size, shape
or color, causing noticeable aesthetic impact. | 1 point |
|
| Vertical
discrepancy >3 cm or severe abnormalities (e.g., discoloration),
substantially compromising breast appearance. | 0 point |
| Texture of the
breast | Soft texture,
consistent with contralateral breast; no indurations, lumps or
abnormal sensations. | 2 points |
|
| Slight firmness or
mild induration present, without affecting overall tactile
quality. | 1 point |
|
| Marked firmness,
obvious indurations or palpable lumps, significantly impairing
tactile quality. | 0 point |
| Patient
satisfaction | Patient expresses
high satisfaction, reporting results meet or exceed expectations;
fully accepts post-operative breast appearance and function. | 2 points |
|
| Patient reports
general satisfaction, with minor unresolved concerns about
outcomes. | 1 point |
|
| Patient
dissatisfied; results fail to meet expectations, with significant
concerns about breast appearance or function. | 0 point |
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation for normally distributed data or the median with
interquartile range (IQR) for non-normally distributed data.
Categorical variables are reported as counts and percentages.
Normality of continuous variables was assessed using the
Shapiro-Wilk test. Unpaired Student's t-test was applied for
normally distributed continuous variables, and the Wilcoxon
rank-sum test was used for non-normal or ordinal data (e.g.,
cosmetic scores). Categorical variables were analyzed using the
Fisher's exact test. All tests were two-sided, with P<0.05
considered to indicate a statistically significant difference.
Analyses and graph generation were performed using GraphPad Prism
9.0 (Dotmatics).
Results
Clinical characteristics of the
patients
A total of 59 patients diagnosed with breast cancer
were included in the present study and categorized into the IORT
(n=21 patients) and PORT (n=38 patients) groups. The clinical data
of the patients were collected retrospectively and analyzed. When
comparing the two groups, no significant differences were
identified in terms of the tumor side, tumor size, number of
positive nodes, histology, grade, molecular subtype, estrogen
receptor (ER), progesterone receptor (PR), human epidermal growth
factor receptor 2 (HER2), neoadjuvant treatment or adjuvant
treatment (Table I). The IORT
cohort demonstrated a significantly younger mean age compared with
the PORT group (P<0.05), while exhibiting a significantly
decreased prevalence of high tumor proliferative activity
(Ki-67>20%) than the PORT cohort (P<0.05).
Follow-up and prognosis
Long-term follow-ups of the patients were conducted
to assess the efficiency and safety of IORT in patients with EBC
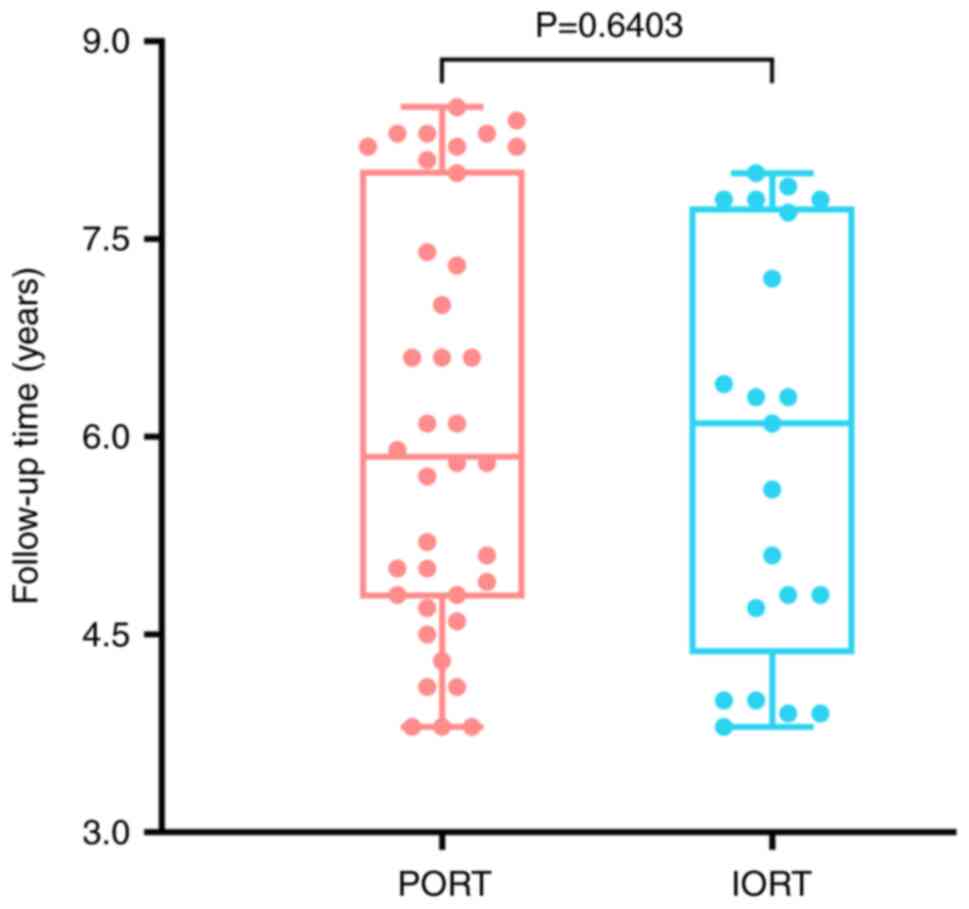
who were receiving BCS. No significant difference in the follow-up
time was observed in the IORT (5.89±1.57 years) and PORT (6.09±1.60
years) groups (P>0.05) (Fig. 2).
The median follow-up time in the IORT group was 6.1 years, ranging
from 3.8–8.0 years (Table I). For
the patients receiving PORT, the median follow-up time was 6.0
years (range: 3.8–8.5 years).
Local control and recurrence
rates
In the IORT group, none of the patients developed
local relapse or distant metastasis in the period of follow-up.
Similarly, none of the patients developed local relapse in the PORT
group, although one patient did develop lung metastasis and was
treated with thorascopic surgery. The overall survival rate for
both groups was 100% in the follow-up period. These data indicated
that IORT provided effective local control of the tumor, and the
local recurrence rates were comparable with those achieved using
PORT. Furthermore, the use of IORT did not result in any compromise
of the overall survival rates when compared with PORT.
Comparative analysis of
treatment-related morbidity
The analysis of treatment-related complications
revealed distinct patterns between the IORT and PORT groups. In the
IORT cohort, no radiation-associated complications were documented
(Table III). Surgical
complications occurred in 2 IORT patients (9.5%, 2/21), manifesting
as fat liquefaction that resolved within ~10 days through dressing
changes. One of these cases developed abscesses requiring drainage
and antibiotics, achieving full wound healing within 2 weeks. Fat
liquefaction presented in 3 cases (7.9%) of 38 patients in the PORT
group, with no significant intergroup difference compared with the
IORT group (P>0.05). Conversely, the PORT group demonstrated
substantial radiation-induced morbidity: 24 patients (63.2%, 24/38)
developed grade 1–4 acute skin toxicity per RTOG criteria (19), including 5 grade 3–4 cases (13.2%,
5/38). Hematological toxicity affected 28 patients in the PORT
group (28.6% grade 3–4), accompanied by 2 cases of grade 1–2
pneumonitis. Additionally, PORT exhibited significantly higher
rates of hyperpigmentation (P<0.001), radiodermatitis
(P<0.001), radiation-related pain (P<0.05) and acute
hematological toxicity (P<0.01) compared with IORT. Notably,
neither acute nor late radiation injuries were observed in the IORT
group (Table III).
 | Table III.Complications of surgery and
radiotherapy. |
Table III.
Complications of surgery and
radiotherapy.
| A, Wound-associated
complication |
|---|
|
|---|
|
Complication/injury | IORT (n=21) | PORT (n=38) | P-value |
|---|
| Fat
liquefaction | 2/21 | 3/38 | 0.999 |
| Wound
infection | 2/21 | 1/38 | 0.999 |
| Pain | 0/21 | 0/38 | >0.999 |
| Breast oedema | 0/21 | 0/38 | >0.999 |
| Seroma | 0/21 | 0/38 | >0.999 |
|
| B, RTOG acute
radiation injury |
|
|
Complication/injury | IORT
(n=21) | PORT
(n=38) | P-value |
|
| Leukocyte |
|
|
|
| Grade
1–2 | 0/21 | 22/38 | <0.001 |
| Grade
3–4 | 0/21 | 6/38 | 0.207 |
| Skin |
|
|
|
| Grade
1–2 | 0/21 | 19/38 | <0.001 |
| Grade
3–4 | 0/21 | 5/38 | 0.293 |
| Lung |
|
|
|
| Grade
1–2 | 0/21 | 2/38 | 0.344 |
| Grade
3–4 | 0/21 | 0/38 | - |
| Heart |
|
|
|
| Grade
1–4 | 0/21 | 0/38 | - |
|
| C, RTOG/EORTC
late radiation injury |
|
|
Complication/injury | IORT
(n=21) | PORT
(n=38) | P-value |
|
| Leukocyte |
|
|
|
| Grade
1–3 | 0/21 | 9/38 | 0.071 |
| Grade
4–5 | 0/21 | 0/38 | - |
| Skin |
|
|
|
| Grade
1–3 | 0/21 | 3/38 | 0.241 |
| Grade
4–5 | 0/21 | 0/38 | - |
| Lung |
|
|
|
| Grade
1–5 | 0/21 | 0/38 | - |
| Heart |
|
|
|
| Grade
1–5 | 0/21 | 0/38 | - |
Late radiation injury
According to the definition of RTOG late radiation
injury (18), late skin toxicity
(grades 1–3) was observed in 3 patients of the PORT group. A total
of 9 patients had late radiation injury of the leukocytes. Of these
patients, 5 (55.6%) still had different levels of leukopenia at 1
year after RT. Patients with RT-induced lung injury did recover 1
year after RT, as confirmed by CT. None of the patients in the IORT
group experienced late radiation injury (Table III).
Dosimetric profile of organs at risk
(OARs)
The dosimetric profile of OARs represents a crucial
parameter for assessing treatment safety and efficacy of
irradiation therapy. The V5 and V20 dose-volume parameters, defined
as the percentage of organ volume exposed to ≥5 Gy and ≥20 Gy
radiation respectively, were employed to quantify post-treatment
radiation burden on OARs. In the PORT group, the dosimetric
analysis revealed the following: The V5 and V20 values for the
ipsilateral lung were 46.62±16.11 and 21.65±8.29%, respectively
(Table IV). For the total lung,
the V5 and V20 values were 24.46±8.84 and 11.05±4.39%,
respectively, with a mean lung dose (MLD) of 8.06±2.98 Gy. For the
heart, the V5 and V20 values were 13.74±11.56 and 1.86±2.40,
respectively, with a mean heart dose of 3.32±2.00 Gy. For IORT,
electron beams exhibited a rapid dose fall-off, which significantly
limits radiation exposure to surrounding normal tissues. Combined
with the precise energy control and the use of shielding, IORT
could ensure effective sparing of the OARs, and as a result, no
significant injuries in adjacent organs and tissues were seen in
the IORT group. Although the PORT technique could achieve an
optimal dose distribution to reduce the risk of
irradiation-associated complications, including pneumonitis and
cardiotoxicity, it was evident that IORT might be a much safer
treatment alternative for selected patients with breast cancer.
 | Table IV.Dosimetric profile of OARs. |
Table IV.
Dosimetric profile of OARs.
|
| PORT |
|
|---|
|
|
|
|
|---|
| OAR | V5 value, % | V20 value, % | Mean dose, Gy | IORT |
|---|
| Total lung | 24.46±8.84 | 11.05±4.39 | 8.06±2.98 | No significant
injury |
| Ipsilateral
lung | 46.62±16.11 | 21.65±8.29 |
|
|
| Heart | 13.74±11.56 | 1.86±2.40 | 3.32±2.00 | No significant
injury |
Breast cosmetic outcomes
Postoperative cosmetic outcomes were assessed using
standardized grading and scoring criteria as aforementioned.
High-quality outcomes (excellent/good grades) were achieved in
95.2% (20/21) of the IORT group, including 16 patients (76.2%) with
excellent ratings and 4 with good ratings (Table V). By contrast, only 42.1% (16/38)
of the PORT group achieved these grades, with 4 excellent ratings
and 12 good ratings. The IORT cohort demonstrated a significantly
higher rate of excellent cosmetic outcomes compared with the PORT
group (P<0.001; Table V).
Longitudinal cosmetic scores further demonstrated the advantage of
IORT, with a stable median score of 10 (IQR, 10–10; ranges, 7–10 at
1 week, 8–10 at 1 month, and 9–10 at 6 months and 1 year). In the
PORT group, the cosmetic score was significantly lower, as revealed
by a median score of 8 (IQR, 6–9; range, 4–10), 7 (IQR, 6–8; range,
3–10), 8 (IQR, 7–9; range, 5–10) and 9 (IQR, 8–10; range, 6–10) at
1 week, 1 month, 6 months and 1 year, respectively (all P<0.01;
Fig. 3).
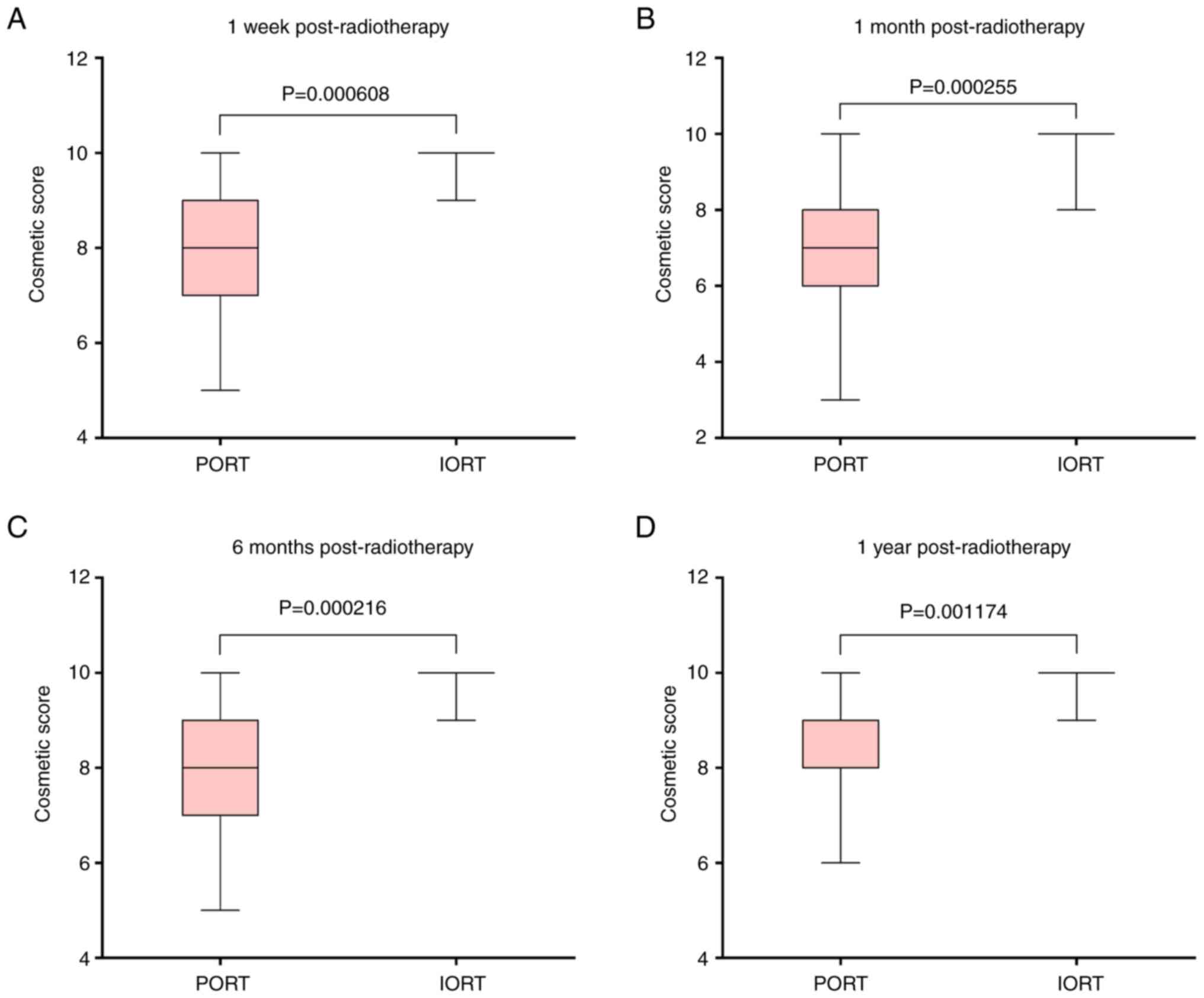 | Figure 3.Breast cosmetic score comparisons
between the IORT and PORT groups at four postoperative time points.
(A) At 1 week: PORT=median, 8 (IQR, 6–9; range 4–10) vs.
IORT=median, 10 (IQR, 10–10; range, 7–10), P=0.000608. (B) At 1
month: PORT=median, 7 (IQR, 6–8; range, 3–10) vs. IORT=median, 10
(IQR, 10–10; range, 8–10), P=0.000265. (C) At 6 months:
PORT=median, 8 (IQR, 7–9; range, 5–10) vs. IORT=median, 10 (IQR,
10–10; range, 9–10), P=0.000216. (D) At 1 year: PORT=median, 9
(IQR, 8–10; range, 6–10) vs. IORT=median, 10 (IQR, 10–10; range,
9–10), P=0.001174. IORT group boxes are absent due to identical Q1,
median and Q3 values (all scores=10). IORT, intraoperative
radiation therapy; PORT, postoperative radiation therapy; IQR,
interquartile range. |
 | Table V.Percentages of breast cosmetic
grading in the patients treated with PORT (n=38) and IORT
(n=21). |
Table V.
Percentages of breast cosmetic
grading in the patients treated with PORT (n=38) and IORT
(n=21).
| Treatment | Excellent | Good | Fair | Poor |
|---|
| PORT, % (n) | 10.53 (4) | 31.58 (12) | 39.47 (15) | 18.42 (7) |
| IORT, % (n) | 76.19 (16) | 19.04 (4) | 4.76 (1) | 0.00 (0) |
| P-value | <0.001 | 0.300 | 0.004 | 0.094 |
Length of stay
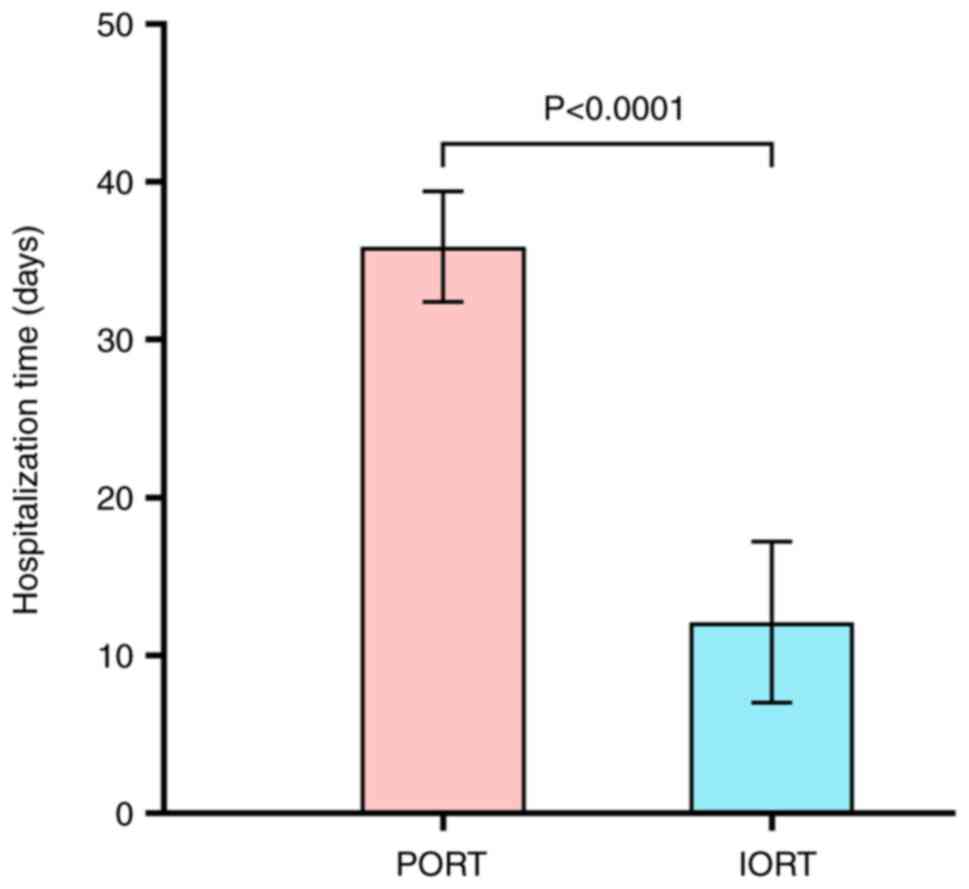
In the IORT group, the total duration of
hospitalization was 12.1±5.1 days. By contrast, in the PORT group,
the total length of stay (including RT) was 35.9±3.5 days (Fig. 4), representing a significant
difference in the treatment time between the two groups
(P<0.001). In addition, the patients treated with PORT also
spent more time on trips to the hospital (data not shown).
Health care costs
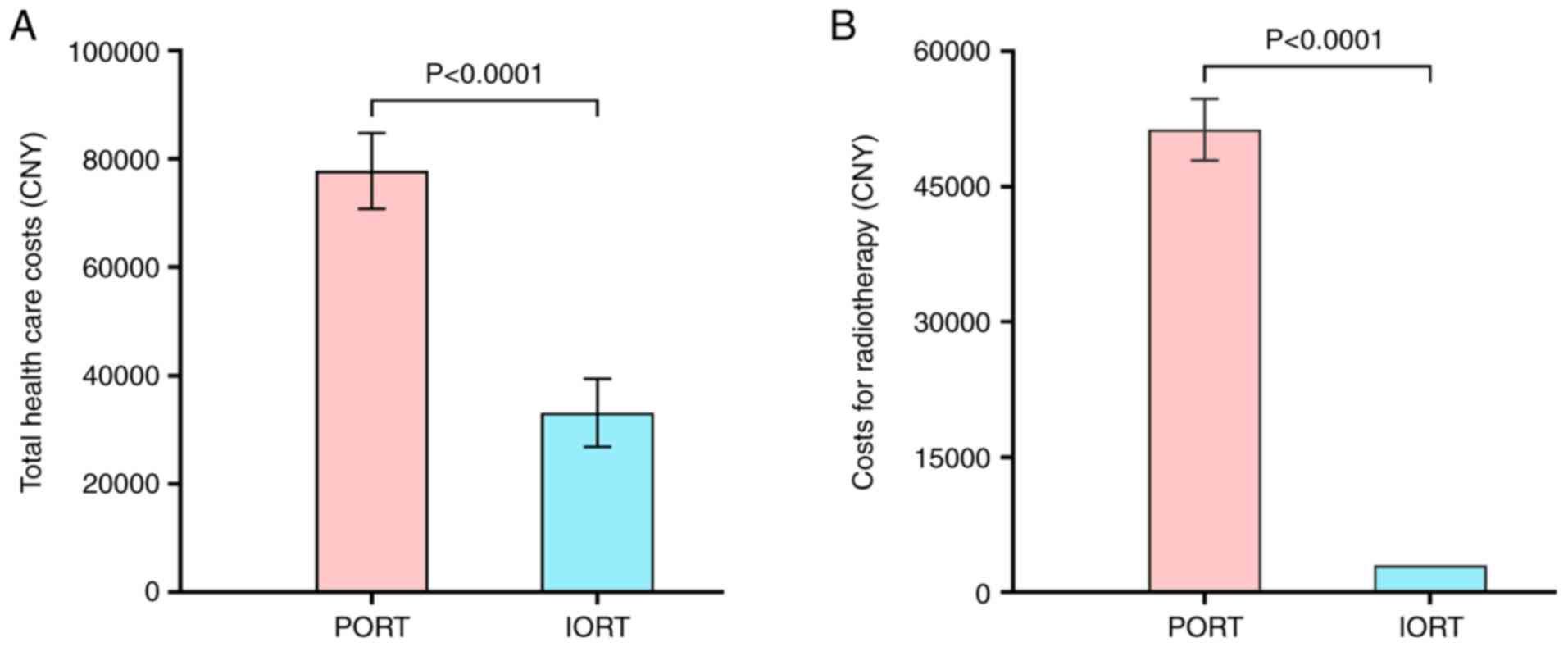
A comparative cost analysis was conducted between
the two groups, quantifying healthcare expenditures specific to
radiation therapy, including treatment delivery, hospitalization,
nursing care and associated pharmaceuticals/medical supplies. In
the IORT group, the mean health care costs were 33,117.98±6,281.17
Chinese Yuan (CNY), which was significantly lower compared with
that of the PORT group (77,789.55±7,000.90 CNY) (P<0.001)
(Fig. 5A). Also, the mean cost of
IORT was significantly lower than that of PORT (P<0.001)
(Fig. 5B). The patients receiving
PORT also tended to spend more on clinical visits and
transportation, although these costs were not included in the
overall health care costs. These data suggested that the use of
IORT could alleviate some of the economic burden for patients with
EBC who receive BCS.
Discussion
BCS combined with RT is a standard treatment for
EBC. Traditionally, PORT has been the norm; however, IORT has
emerged as an innovative alternative (9). The current study was a retrospective
cohort study exploring the efficacy, safety and cosmetics of IORT
in patients with early-stage breast cancer undergoing BCS. The data
demonstrated that IORT offered similar effectiveness to PORT while
at the same time causing fewer common complications and less
financial burdens than PORT. More importantly, IORT exhibited
better cosmetic outcomes for such patients, suggesting it as a new
treatment option for patients with breast cancer undergoing
BCS.
RT is the critical component of breast-conserving
therapy received by patients with EBC. The traditional routine of
PORT is to irradiate the entire breast and lymphatic area 5 days a
week, with the administration of 1.8–2.0 Gy/fraction up to a total
dose of 50 Gy (9). Certain
patients, such as those with breast cancer presenting with positive
margins, multifocal disease, young age (<40 years) and
high-grade tumors, have to receive an additional irradiation dose
of 10–15 Gy to the tumor bed, leading to an extended RT duration
(5–6 weeks, or even much longer). Although PORT has been
demonstrated to reduce local recurrence by 70% and to improve
survival by 5–7% (25), certain
patients may reject BCS due to the lengthy period of PORT. In
addition, PORT needs to be performed after chemotherapy and wound
healing. This delay may cause a higher risk of local relapse
(26). The goal of BCS is to remove
the tumor and to minimize the effects of changes in breast
appearance and function, thereby improving the quality of life for
patients after the operation (27).
Moreover, the cosmetic side effects of PORT, including skin
fibrosis and hyperpigmentation, may lead to a considerable number
of patients giving up BCS. A previous study showed that 21% of
women did not receive RT following BCS on account of the
aforementioned reasons (28).
Given the fact that local recurrence mainly occurs
near to the tumor bed following BCS, APBI has gradually gained in
popularity. Current technologies of APBI include brachytherapy,
three-dimensional conformal RT (3D-CRT), intensity-modulated
radiation therapy (IMRT) and IORT. Brachytherapy is mainly
performed according to the MammoSite® RT system
(29). The major advantage of this
technology is that the device can be placed in the operation room
and guided by ultrasound following the operation. 3D-CRT and IMRT
are non-invasive techniques, although they have the disadvantages
of being susceptible to the breathing and positioning of the
patients (30). IORT is the latest
APBI technique to have been developed. Compared with the other APBI
techniques, IORT can be performed at the same time as surgery, and
is not affected by the patient's breathing and posture, which
ensures the accuracy of irradiation of the tumor bed (31). The radiation dosage of IORT on the
tumor bed is usually 20 Gy, which is less than that of fractionated
irradiation, but is equivalent to the dose of fractionated
irradiation (70 Gy) (32). At
present, several studies have shown the non-inferiority of IORT for
EBC in terms of overall survival rates when comparing between
patients receiving IORT and those receiving whole-breast PORT
(33,34). Representative prospective clinical
trials include the TARGIT-A trial reported by Vaidya et al
(13) and the ELIOT trial reported
by Veronesi et al (14).
Both trials showed that IORT and PORT achieve similar overall
survival rates for EBC, supporting the effectiveness of IORT in its
clinical application (35).
However, the TARGIT-A trial showed that the 5-year overall
recurrence rate in the IORT group was higher compared with that of
the whole-breast irradiation group (hazard ratio, 1.44; P=0.053),
whereas the 5-year ipsilateral breast tumor recurrence rate was
higher than the IORT target value (3.3% vs. 1.3%; P=0.042). This
may have been due to the fact that some of the patients in the
TARGIT-A trial did not meet the criteria recommended by the
American Society of Radiological Oncology for APBI treatment
(36). In terms of skin
side-effects, the incidence of grade 3–4 skin toxicity in the
TARGIT-A group was found to be lower compared with that in the
whole-breast irradiation group (0.2% vs. 0.8%; P=0.029), which
contributed to the difference in cosmetic outcomes of BCS.
Cosmetic outcome is an important determinant in
treatment choice following BCS. Previous studies have shown that
the cosmetic effects of IORT are neither inferior nor better
compared with those achieved by external irradiation (37–39).
In the present study, the cosmetic outcomes in the IORT group were
found to be satisfactory. Only 1 patient was graded as fair due to
the presence of an abscess, whereas the remaining 20 patients
(95.2%) were graded as excellent (76.2%) or good (19.0%) (Table V). By contrast, only 42.1% (16/38)
of the PORT group achieved excellent or good grades, with 4
excellent ratings (10.5%) and 12 good ratings (31.6%). This result
demonstrated that IORT could achieve improved cosmetic outcomes in
patients, which was in agreement with the recent findings reported
by Shandiz et al (12).
Furthermore, additional complications of RT occurred in the PORT
group in the present study, including hyperpigmentation,
radiodermatitis, radiation-induced pain and radiation-induced acute
hematological toxicity. Fat liquefaction was more frequent in the
IORT group compared with the PORT group, although this difference
was not found to be significant. Age appears to be an important
factor associated with fat liquefaction and infection of the
incision following IORT, since the patients with fat liquefaction
or local infection were all >60 years old. This may be due to
the fact that the ratio of the breast gland to adipose tissue is
higher in older patients, and older patients were more common in
the IORT group.
Recent studies have established the long-term
oncological efficacy of IORT (40–42).
Chi et al (40) conducted a
retrospective analysis of 164 patients with breast cancer and 83
ductal carcinoma in situ (DCIS) cases treated with IORT (20
Gy single fraction) at a single institution in Taiwan. With a mean
follow-up time of 64.3 months, a higher locoregional recurrence
rate was observed using IORT (9.8%) compared with using
whole-breast irradiation (3.0%), although metastasis and mortality
rates were comparable between groups. Hanna et al (41) prospectively validated the
feasibility of non-dedicated linear accelerators for IORT (21 Gy
single fraction), reporting a 7.2% local recurrence rate over a
median follow-up time of 145 months (range, 12.8–187.1 months).
These findings underscored the importance of an extended follow-up,
as late recurrences may occur beyond 10 years. Sarria et al
(42) further supported the utility
of IORT as an upfront boost in an international cohort of 653
patients from Peru, Spain and Germany, demonstrating local control
outcomes comparable to external beam RT. Short-term outcomes were
explored by Fadavi et al (11) and Shandiz et al (12). Fadavi et al (11) compared intraoperative electron RT
followed by hypofractionated whole breast irradiation to
conventional whole-breast irradiation, finding comparable acute and
late toxicity profiles over a median follow-up time of 12 months.
Shandiz et al (12) reported
an 8.3% recurrence rate and manageable toxicity (e.g., grade III
fibrosis in 8.3% of cases) with IORT as a tumor bed boost
post-neoadjuvant chemotherapy (NAC) over a median follow-up time of
29.5 months. Both studies concluded that IORT achieved acceptable
short-term outcomes in local control, toxicity and cosmesis,
aligning with the findings of Keramati et al (9) in their 2020 review of post-NAC
settings. This consistency further supports the applicability of
IORT beyond NAC-specific cohorts. Collectively, these findings
affirm the viability of IORT as a simplified, effective alternative
to whole-breast irradiation in appropriately selected patients,
which is consistent with the present findings on long-term
oncological safety. In contrast to prior works, the present study
advances the clinical understanding of IORT by addressing
underexplored dimensions. First, while earlier research prioritized
oncological outcomes, the present study highlights cosmetic results
as a critical metric, demonstrating significantly higher rates of
‘excellent/good’ outcomes with IORT (95.2%) vs. PORT (44.7%). This
distinction emphasizes the potential of IORT to enhance quality of
life, a priority for patient-centered care. Second, the present
study uniquely quantified socioeconomic benefits, revealing that
IORT could markedly reduce healthcare costs and shorten
hospitalization compared with PORT, offering actionable insights
for cost-sensitive healthcare systems. Third, whilst Chi et
al (40) demonstrated the
long-term efficacy of IORT in a retrospective single-institution
cohort, the present study built on these findings by confirming its
safety in another Chinese population and offering new insights into
cost-effectiveness. Finally, by focusing on a distinct cohort of
Chinese patients, the present work addressed regional disparities
in treatment response and healthcare-resource allocation, factors
often overlooked in global IORT research. Collectively, the present
study bridged a critical gap in the literature and provided a
comprehensive framework to optimize IORT implementation across
diverse clinical and socioeconomic settings.
The primary advantage of IORT lies in its ability to
deliver adjuvant radiation concurrently with surgery, significantly
shortening treatment duration compared with PORT. Additionally,
IORT minimizes radiation exposure to healthy tissues, reducing
toxicity risks. However, this approach has inherent limitations.
For instance, final pathological details, such as surgical margin
status and lymph node involvement, remain unavailable during IORT
delivery, creating potential discrepancies between intraoperative
frozen-section and final histopathological results. Thus, rigorous
patient selection is critical in clinical practice. While the
present findings offer therapeutic insights for EBC, several
limitations warrant acknowledgment. First, this preliminary
retrospective study included a small, heterogeneous cohort,
encompassing node-positive patients and those receiving neoadjuvant
therapies, reflecting real-world practice but introducing
variability. Treatment allocation (IORT vs. PORT) was influenced by
clinician judgment and patient preference, risking selection bias
and baseline imbalances in tumor biology or comorbidities. Reliance
on retrospective data restricted access to detailed information,
such as HER2 status and adjuvant therapy adherence, limiting robust
confounder adjustment. The modest sample size further precluded
subgroup analyses or propensity score matching.
While the heterogeneity of the cohort enhances
real-world generalizability, several confounding factors may
influence the analysis of IORT and PORT outcomes in the present
study. Patient selection bias could arise from imbalances in tumor
characteristics (e.g., size and HER2 status), age or comorbidities
between groups (43,44). For instance, younger patients or
those with aggressive tumor biology in the IORT group might skew
recurrence rates, independent of treatment efficacy. Surgical
variability, such as differences in margin status or surgeon
proficiency with IORT techniques, could disproportionately affect
local control outcomes if one group had suboptimal tumor excision
or inconsistent radiation delivery (45). Adjuvant therapies further complicate
comparisons: Disparities in chemotherapy, endocrine therapy or PORT
protocols (e.g., whole-breast vs. partial irradiation) might mask
or amplify treatment-related effects on survival or toxicity
(46). Baseline cosmetic
differences, including breast size or tumor location, could
confound aesthetic outcomes, as smaller breasts or peripherally
located tumors may inherently favor better cosmesis regardless of
RT type (47). Socioeconomic and
geographic factors might introduce systemic bias, as patients
opting for IORT (often requiring specialized resources) may have
better healthcare access or higher health literacy, improving
compliance and artificially enhancing outcomes (46). Follow-up heterogeneity, such as
variations in surveillance intensity (e.g., imaging frequency),
could lead to underdetection of recurrence in less-monitored groups
(48,49). Finally, psychosocial factors, such
as patient preferences for shorter treatment duration, might select
for populations prioritizing convenience over risk, influencing
perceived satisfaction or compliance (50,51).
Collectively, these confounders risk distorting comparisons by
attributing outcomes to treatment modality rather than underlying
imbalances. For example, if IORT cohorts included more low-risk
tumors or received more adjuvant therapies, its apparent
superiority in local control could reflect selection bias rather
than inherent efficacy. Conversely, socioeconomic disparities might
exaggerate the cost burden of PORT if marginalized populations
disproportionately received it. To mitigate these issues,
multivariable adjustments, propensity score matching or randomized
trials are critical to isolate the true effects of IORT vs. PORT.
These factors collectively weaken causal inferences regarding the
non-inferior safety and superior cosmetic efficacy of IORT,
underscoring the need for prospective trials with standardized data
collection to validate these findings. Future studies with
prospective and randomized design, which will feature larger
patient cohorts with selected patients, are warranted to validate
the current findings.
In conclusion, in patients with EBC undergoing BCS,
IORT demonstrates non-inferior oncological safety and tumor control
compared with PORT, while excelling in cosmetic outcomes,
cost-effectiveness and treatment efficiency. For
low-to-intermediate-risk patients prioritizing quality of life and
convenience, IORT offers a patient-centric alternative without
compromising oncological rigor. PORT remains critical for high-risk
cases with the need for comprehensive radiation. This paradigm
aligns with personalized oncology, balancing clinical efficacy with
practical benefits to enhance therapeutic value. Further studies,
including large-scale trials and investigations into multimodal
strategies, are warranted to refine patient selection and validate
long-term outcomes, ensuring broader applicability of IORT in
modern EBC management.
Acknowledgements
Not applicable.
Funding
This study was funded by the Project of Social Development
Foundation of Jiangsu Province, China (grant no. BE2017726) and the
Medical Research Project of Jinling Hospital, Nanjing Medical
University (grant no. 22LCZLXJS33).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QL, JG and XX conceptualized the study and designed
the experimental methods. XX, LW, YC, JY and WD collected and
analyzed the data. LH and YS performed the follow-up of the
patients. JW and XX analyzed data. QL, XX and LW were responsible
for interpretation of the data. XX was responsible for writing the
original draft and editing the figures. QL, JG and XX reviewed and
edited the manuscript. QL supervised the study and contributed to
the revision of the manuscript. All authors read and approved the
final version of the manuscript. XX, LW, YC, JW, JY, LH, YS, WD, JG
and QL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jinling Hospital, Nanjing Medical University (Nanjing, China;
approval no. 2024DZKY-148) and was conducted in accordance with The
Declaration of Helsinki. The participants provided written informed
consent prior to taking part in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajan KK, Fairhurst K, Birkbeck B,
Novintan S, Wilson R, Savović J, Holcombe C and Potter S: Overall
survival after mastectomy versus breast-conserving surgery with
adjuvant radiotherapy for early-stage breast cancer: Meta-analysis.
BJS Open. 8:zrae0402024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Christiansen P, Carstensen SL, Ejlertsen
B, Kroman N, Offersen B, Bodilsen A and Jensen MB: Breast
conserving surgery versus mastectomy: Overall and relative
survival-a population based study by the Danish Breast Cancer
Cooperative Group (DBCG). Acta Oncol. 57:19–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Recht A: Whole-breast irradiation is the
preferred standard of care for the majority of patients with
early-stage breast cancer. J Clin Oncol. 38:2263–2267. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polgár C, Kahán Z, Ivanov O, Chorváth M,
Ligačová A, Csejtei A, Gábor G, Landherr L, Mangel L, Mayer Á and
Fodor J: Radiotherapy of breast cancer-professional guideline 1st
central-eastern european professional consensus statement on breast
cancer. Pathol Oncol Res. 28:16103782022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang K and Tepper JE: Radiation
therapy-associated toxicity: Etiology, management, and prevention.
CA Cancer J Clin. 71:437–454. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaidya JS, Baum M, Tobias JS, D'Souza DP,
Naidu SV, Morgan S, Metaxas M, Harte KJ, Sliski AP and Thomson E:
Targeted intra-operative radiotherapy (Targit): An innovative
method of treatment for early breast cancer. Ann Oncol.
12:1075–1080. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keramati A, Javadinia SA, Gholamhosseinian
H, Fanipakdel A, Shandiz FH and Taghizadeh–Hesary F: A review of
intraoperative radiotherapy after neoadjuvant chemotherapy in
patients with locally advanced breast cancer: From bench to
bedside. Indian J Gynecol Oncolog. 8:1102020. View Article : Google Scholar
|
|
10
|
Hepel JT and Wazer DE: Partial breast
irradiation is the preferred standard of care for a majority of
women with early-stage breast cancer. J Clin Oncol. 38:2268–2272.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fadavi P, Nafissi N, Mahdavi SR,
Jafarnejadi B and Javadinia SA: Outcome of hypofractionated breast
irradiation and intraoperative electron boost in early breast
cancer: A randomized non-inferiority clinical trial. Cancer Rep
(Hoboken). 4:e13762021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shandiz FH, Fanipakdel A, Forghani MN,
Javadinia SA, Shahi EM, Keramati A, Fazilat–Panah D and Moein
Babaei M: Clinical efficacy and side effects of IORT as tumor bed
boost during breast-conserving surgery in breast cancer patients
following neoadjuvant chemotherapy. Indian J Gynecol Oncolog.
18:462020. View Article : Google Scholar
|
|
13
|
Vaidya JS, Wenz F, Bulsara M, Tobias JS,
Joseph DJ, Saunders C, Brew-Graves C, Potyka I, Morris S, Vaidya
HJ, et al: An international randomised controlled trial to compare
TARGeted intraoperative radiotherapy (TARGIT) with conventional
postoperative radiotherapy after breast conserving surgery for
women with early-stage breast cancer (the TARGIT-A trial). Health
Technol Assess. 20:1–188. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Veronesi U, Orecchia R, Maisonneuve P,
Viale G, Rotmensz N, Sangalli C, Luini A, Veronesi P, Galimberti V,
Zurrida S, et al: Intraoperative radiotherapy versus external
radiotherapy for early breast cancer (ELIOT): A randomised
controlled equivalence trial. Lancet Oncol. 14:1269–1277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Zhou Z, Mei X, Yang Z, Ma J, Chen
X, Wang J, Liu G, Yu X and Guo X: Intraoperative radiotherapy
versus whole-breast external beam radiotherapy in early-stage
breast cancer: A systematic review and meta-analysis. Medicine
(Baltimore). 94:e11432015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO classification of tumours of the breast.
4th edition. 4. Lyon; IARC Press: 2012
|
|
17
|
Singletary SE, Connolly JL and Hortobagyi
GN: Breast. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL and
Trotti A: AJCC Cancer Staging Manual. 7th edition. Springer; NY,
USA: pp. 347–376. 2010
|
|
18
|
Vaidya JS, Wenz F, Bulsara M, Tobias JS,
Joseph DJ, Keshtgar M, Flyger HL, Massarut S, Alvarado M, Saunders
C, et al: Risk-adapted targeted intraoperative radiotherapy versus
whole-breast radiotherapy for breast cancer: 5-year results for
local control and overall survival from the TARGIT-A randomised
trial. Lancet. 383:603–613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toxicity criteria of the Radiation Therapy
Oncology Group (RTOG) and the European Organization for Research
and Treatment of Cancer (EORTC), . Int J Radiat Oncol Biol Phys.
31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sawaki M, Sato S, Noda S, Idota A, Uchida
H, Tsunoda N, Kikumori T, Aoyama Y, Ishihara S, Itoh Y and Imai T:
Phase I/II study of intraoperative radiotherapy for early breast
cancer in Japan. Breast Cancer. 19:353–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimple RJ, Klauber-DeMore N, Kuzmiak CM,
Pavic D, Lian J, Livasy CA, Esler L, Moore DT, Sartor CI and Ollila
DW: Cosmetic outcomes for accelerated partial breast irradiation
before surgical excision of early-stage breast cancer using
single-dose intraoperative radiotherapy. Int J Radiat Oncol Biol
Phys. 79:400–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Li XR, Ma L, Zhang YJ, Wang JD,
Kong QL, Zheng YQ, Yu W and Li R: Observation on short-term effect
of breast-conserving operation plus intraoperative radiotherapy in
64 breast cancer patients. Chin J Breast Dis (Electronic Ed).
4:683–691. 2010.(In Chinese).
|
|
23
|
Harris JR, Levene MB, Svensson G and
Hellman S: Analysis of cosmetic results following primary radiation
therapy for stages I and II carcinoma of the breast. Int J Radiat
Oncol Biol Phys. 5:257–261. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang BN, Shao ZM, Qiao XM, Li B, Jiang J,
Yang MT, Wang S, Song ST, Zhang B and Yang HJ: A prospective
multicenter clinical trial of breast conserving therapy for early
breast cancer in China. Zhonghua Zhong Liu Za Zhi. 27:680–684.
2005.(In Chinese). PubMed/NCBI
|
|
25
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Punglia RS, Saito AM, Neville BA, Earle CC
and Weeks JC: Impact of interval from breast conserving surgery to
radiotherapy on local recurrence in older women with breast cancer:
Retrospective cohort analysis. BMJ. 340:c8452010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chai F, Zhao QL, Zhang JF and Huang YQ:
Short-term effect of breast conserving surgery combined with
intraoperative radiotherapy in 60 patients with breast cancer. Chin
J Breast Dis (Electronic Ed). 11:13–18. 2017.(In Chinese).
|
|
28
|
Tuttle TM, Jarosek S, Habermann EB, Yee D,
Yuan J and Virnig BA: Omission of radiation therapy after
breast-conserving surgery in the United States: A population-based
analysis of clinicopathologic factors. Cancer. 118:2004–2013. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niehoff P, Ballardini B, Polgár C, Major
T, Hammer J, Richetti A and Kovács G: Early European experience
with the MammoSite radiation therapy system for partial breast
brachytherapy following breast conservation operation in low-risk
breast cancer. Breast. 15:319–325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banglan KL, Sharpe MB, Jaffray D, Frazier
RC, Fayad J, Kestin LL, Remouchamps V, Martinez AA, Wong J and
Vicini FA: Accelerated partial breast irradiation using 3D
conformal radiation therapy (3D-CRT). Int J Radiat Oncol Biol Phys.
55:302–311. 2003. View Article : Google Scholar
|
|
31
|
Liu L and Li XR: Research progress of
breast conserving surgery combined with intraoperative radiotherapy
for breast cancer. Chin J Health Care Med. 13:166–169. 2011.
|
|
32
|
Herskind C, Griebel J, Kraus-Tiefenbacher
U and Wenz F: Sphere of equivalence-a novel target volume concept
for intraoperative radiotherapy using low energy X rays. Int J
Radiat Oncol Biol Phys. 72:1575–1581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Melnik I, Yerushalmi R, Sobol Y, Magen A,
Givon-Madhala O, Birinbaum Y, Fenig E and Sharon E: Intraoperative
radiation therapy for breast cancer-Immediate and 30-month
oncological outcomes. Breast J. 26:946–951. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gondim GRM, Makdissi FBA, Fogaroli RC,
Collins JBD, Iyeyasu H, de Castro DG, Silva MLG, Chen MJ, Coelho
TM, Ramos H and Pellizzon ACA: Intraoperative breast radiotherapy:
Survival, local control and risk factors for recurrence. Rep Pract
Oncol Radiother. 24:551–555. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng YQ, Xu XF, Gu J, Wu B, Zhu XX and
Guan XX: Application of intraoperative radiotherapy in breast
conserving surgery for early breast cancer. J Med Postgra.
30:534–536. 2017.(In Chinese).
|
|
36
|
Shaitelman SF, Lin HY, Smith BD, Shen Y,
Bedrosian I, Marsh GD, Bloom ES, Vicini FA, Buchholz TA and Babiera
GV: Practical implications of the publication of consensus
guidelines by the American society for radiation oncology:
Accelerated partial breast irradiation and the national cancer data
base. Int J Radiat Oncol Biol Phys. 94:338–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Keshtgar MR, Williams NR, Bulsara M,
Saunders C, Flyger H, Cardoso JS, Corica T, Bentzon N,
Michalopoulos NV and Joseph DJ: Objective assessment of cosmetic
outcome after targeted intraoperative radiotherapy in breast
cancer: Results from a randomized controlled trial. Breast Cancer
Res Treat. 140:519–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cracco S, Semprini G, Cattin F, Gregoraci
G, Zeppieri M, Isola M, Ceschia T, Cedolini C and Parodi PC: Impact
of intraoperative radiotherapy on cosmetic outcome and
complications after oncoplastic breast surgery. Breast J.
21:285–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Corica T, Nowak AK, Saunders CM, Bulsara
MK, Taylor M, Williams NR, Keshtgar M, Joseph DJ and Vaidya JS:
Cosmetic outcome as rated by patients, doctors, nurses and
BCCT.core software assessed over 5 years in a subset of patients in
the TARGIT-A Trial. Radiat Oncol. 13:682018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chi MS, Ko HL, Yang TL, Liu YF, Chi KH and
Cheng FT: Comparative long-term oncological outcomes of
intraoperative radiotherapy vs. whole-breast irradiation in early
breast cancer: A single institute study. Front Oncol.
14:14115982024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hanna SA, Bevilacqua JLB, de Barros ACSD,
de Andrade FEM, Piato JRM, Pelosi EL, Martella E, da Silva JLF,
Carvalho HA and Jacomo AL: Long-term results of intraoperative
radiation therapy for early breast cancer using a nondedicated
linear accelerator. Adv Radiat Oncol. 8:1012332023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sarria GR, Ramos ML, Palacios A, Del
Castillo R, Castro F, Calvo A, Cotrina JM, Heredia A, Galarreta JA,
Fuentes-Rivera P, et al: Long-term outcomes of an international
cooperative study of intraoperative radiotherapy upfront boost with
low energy x-rays in breast cancer. Front Oncol. 12:8503512022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lv Y, He L, Wang C, Zhang L, Zhang B and
Song Y: A systematic review of clinical outcomes and
radiotherapy-associated toxicity in multicatheter accelerated
partial breast irradiation. Medicine (Baltimore). 98:e144072019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao XR, Tang Y, Wu HF, Guo QS, Zhang YJ,
Shi M, Cheng J, Wang HM, Liu M, Ma CY, et al: Influence of age as a
continuous variable on the prognosis of patients with pT1-2N1
breast cancer. Breast. 66:136–144. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bundred JR, Michael S, Stuart B, Cutress
RI, Beckmann K, Holleczek B, Dahlstrom JE, Gath J, Dodwell D and
Bundred NJ: Margin status and survival outcomes after breast cancer
conservation surgery: Prospectively registered systematic review
and meta-analysis. BMJ. 378:e0703462022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Curigliano G, Burstein HJ, Gnant M, Loibl
S, Cameron D, Regan MM, Denkert C, Poortmans P, Weber WP and
Thürlimann B; St Gallen Consensus Conference Panelists 2023, :
Understanding breast cancer complexity to improve patient outcomes:
The St Gallen International Consensus Conference for the Primary
Therapy of Individuals with Early Breast Cancer 2023. Ann Oncol.
34:970–986. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jansen LA, Backstein RM and Brown MH:
Breast size and breast cancer: A systematic review. J Plast
Reconstr Aesthet Surg. 67:1615–1623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lafranconi A, Pylkkänen L, Deandrea S,
Bramesfeld A, Lerda D, Neamțiu L, Saz-Parkinson Z, Posso M, Rigau
D, Sola I, et al: Intensive follow-up for women with breast cancer:
Review of clinical, economic and patient's preference domains
through evidence to decision framework. Health Qual Life Outcomes.
15:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Browall M, Forsberg C and Wengström Y:
Assessing patient outcomes and cost-effectiveness of nurse-led
follow-up for women with breast cancer-have relevant and sensitive
evaluation measures been used? J Clin Nurs. 26:1770–1786. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jensen AB: Psychosocial factors in breast
cancer and their possible impact upon prognosis. Cancer Treat Rev.
18:191–210. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Saita E, Acquati C, Molgora S, Vagnini D,
Piccolo EM, Valenti F, Stratta G and Grassi MM: Locally advanced
breast cancer (LABC) and delayed care: A qualitative analysis of
psychosocial factors. Psychol Health Med. 28:408–418. 2023.
View Article : Google Scholar : PubMed/NCBI
|