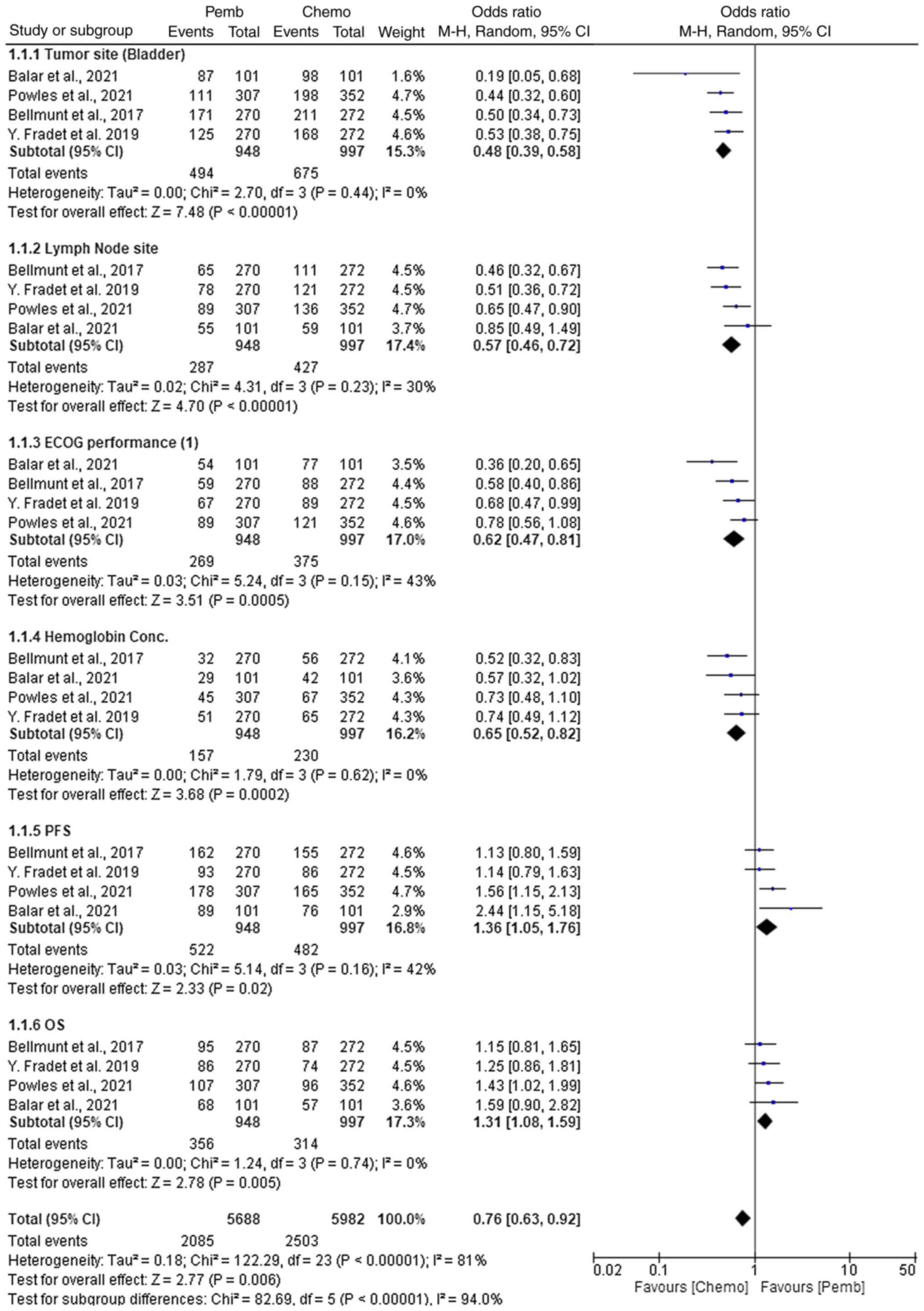
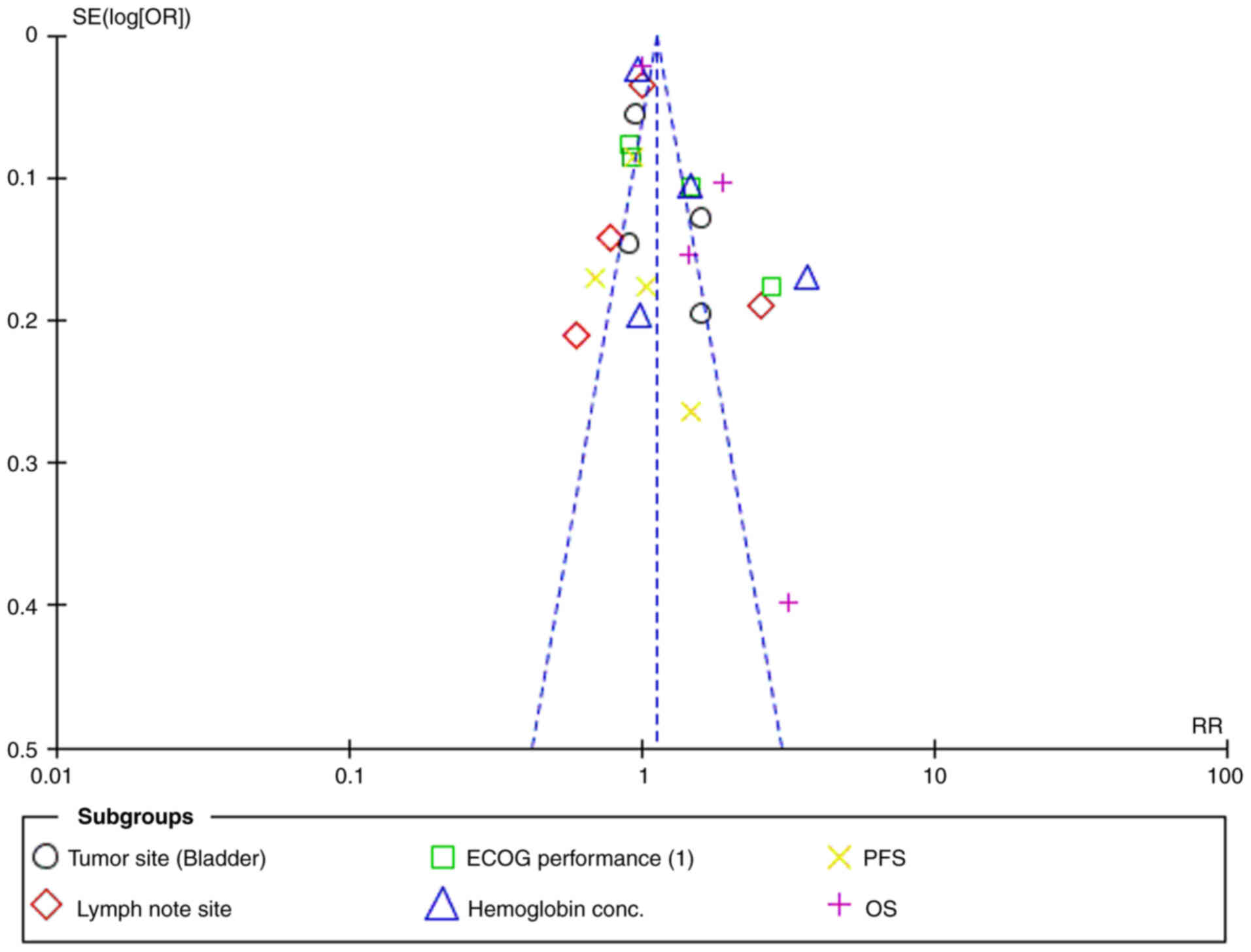
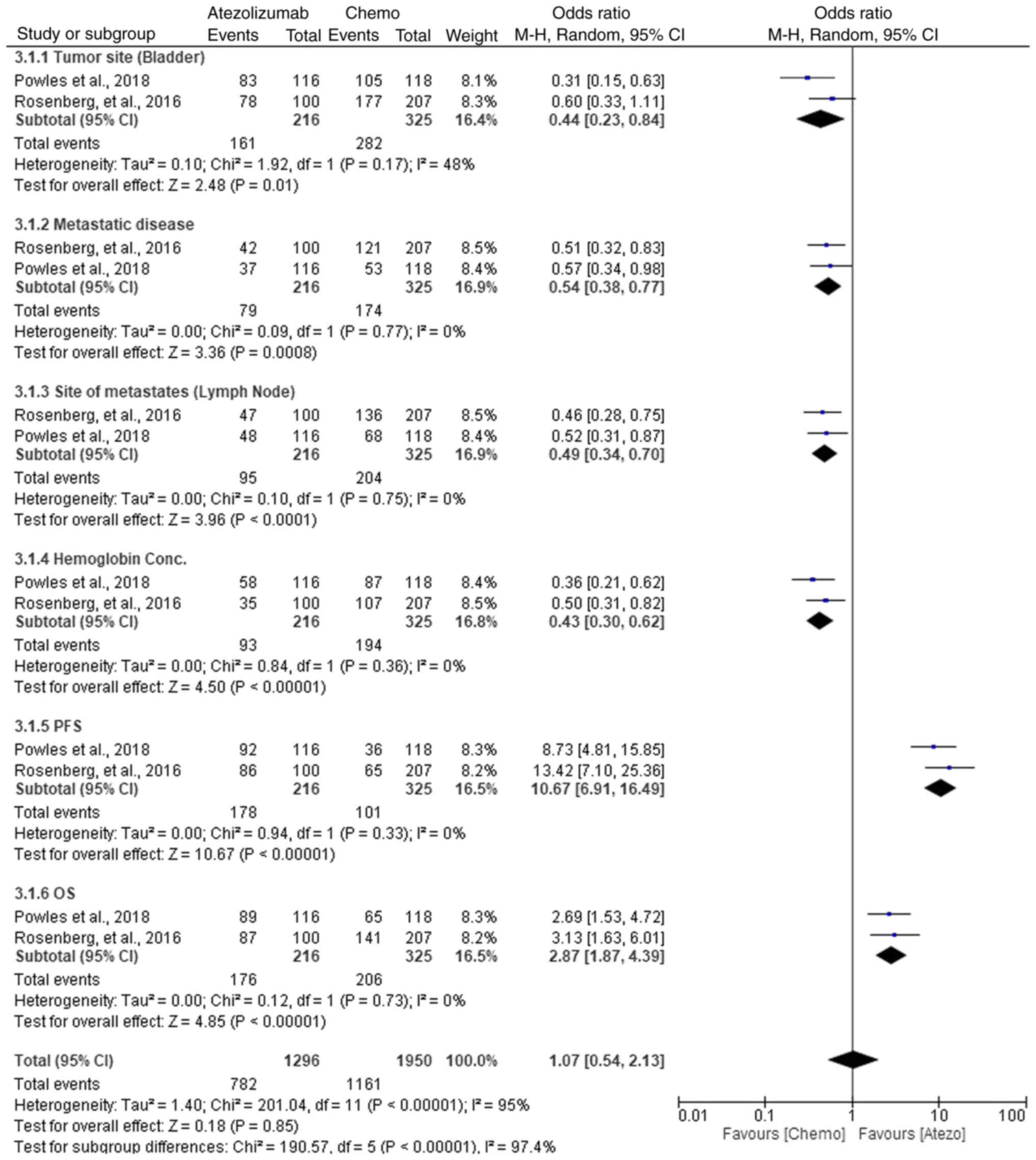
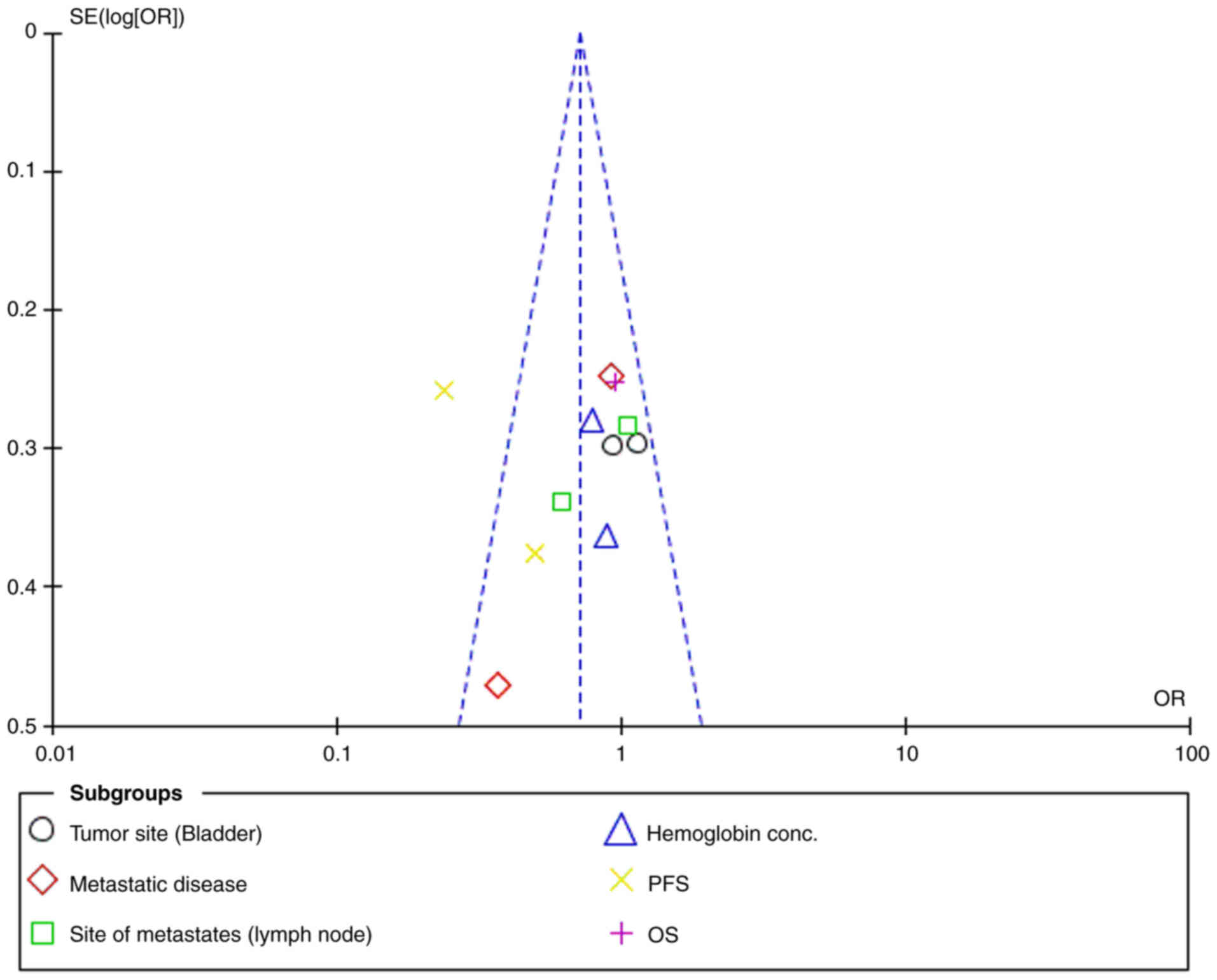
|
1
|
van Hoogstraten LM, Vrieling A, van der
Heijden AG, Kogevinas M, Richters A and Kiemeney LA: Global trends
in the epidemiology of bladder cancer: Challenges for public health
and clinical practice. Nat Rev Clin Oncol. 20:287–304. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sylvester RJ, Rodríguez O, Hernández V,
Turturica D, Bauerová L, Bruins HM, Bründl J, van der Kwast TH,
Brisuda A, Rubio-Briones J, et al: European Association of Urology
(EAU) prognostic factor risk groups for non-muscle-invasive bladder
cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973
classification systems for grade: An update from the EAU NMIBC
Guidelines Panel. Eur Urol. 79:480–488. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tse J, Singla N, Ghandour R, Lotan Y and
Margulis V: Current advances in BCG-unresponsive non-muscle
invasive bladder cancer. Expert Opin Investig Drugs. 28:757–770.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
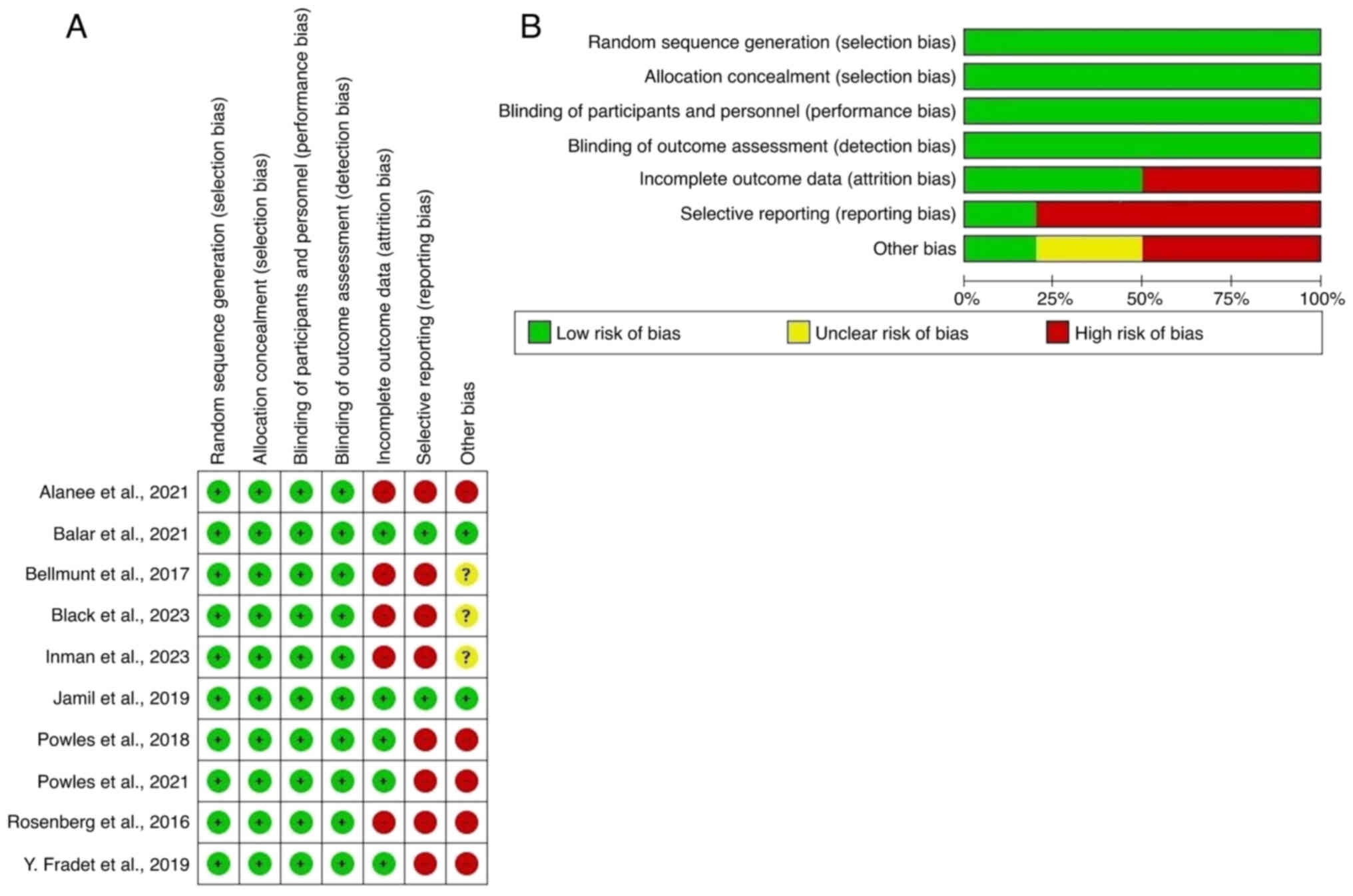
|
Ulamec M, Murgić J, Novosel L, Tomić M,
Terlević R, Tomašković I, Jazvić M, Froebe A and Krušlin B: New
insights into the diagnosis, molecular taxonomy, and treatment of
bladder cancer. Acta Med Acad. 50:143–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valenza C, Antonarelli G, Giugliano F,
Aurilio G, Verri E, Briganti A, Curigliano G and Necchi A: Emerging
treatment landscape of non-muscle invasive bladder cancer. Expert
Opin Biol Ther. 22:717–734. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanguedolce F, Calo B, Mancini V, Zanelli
M, Palicelli A, Zizzo M, Ascani S, Carrieri G and Cormio L:
Non-muscle invasive bladder cancer with variant histology:
Biological features and clinical implications. Oncology.
99:345–358. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babjuk M, Burger M, Compérat EM, Gontero
P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF,
Sylvester R, et al: European association of urology guidelines on
non-muscle-invasive bladder cancer (TaT1 and carcinoma in
situ)-2019 update. Eur Urol. 76:639–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Witjes JA, Bruins HM, Cathomas R, Compérat
EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A,
Neuzillet Y, et al: European association of urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2020
guidelines. Eur Urol. 79:82–104. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JH and Oh JJ: The emerging treatment
of BCG (Bacillus Calmette-Guérin)-unresponsive non-muscle-invasive
bladder cancer. J Urol Oncol. 22:246–255. 2024. View Article : Google Scholar
|
|
10
|
Morales A: BCG: A throwback from the stone
age of vaccines opened the path for bladder cancer immunotherapy.
Can J Urol. 24:8788–8793. 2017.PubMed/NCBI
|
|
11
|
Lerner SP, Tangen CM, Sucharew H, Wood D
and Crawford ED: Failure to achieve a complete response to
induction BCG therapy is associated with increased risk of disease
worsening and death in patients with high risk non-muscle invasive
bladder cancer. Urol Oncol. 27:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steinberg RL, Thomas LJ, Mott SL and
O'Donnell MA: Bacillus Calmette-Guérin (BCG) treatment failures
with non-muscle invasive bladder cancer: A data-driven definition
for BCG unresponsive disease. Bladder Cancer. 2:215–224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deininger S, Törzsök P, Mitterberger M,
Pallauf M, Oswald D, Deininger C and Lusuardi L: From interferon to
checkpoint inhibition therapy-A systematic review of new
immune-modulating agents in Bacillus Calmette-Guérin (BCG)
refractory non-muscle-invasive bladder cancer (NMIBC). Cancers.
14:6942022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Jong FC, Rutten VC, Zuiverloon TC and
Theodorescu D: Improving anti-PD-1/PD-L1 therapy for localized
bladder cancer. Int J Mol Sci. 22:28002021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del Giudice F, Asero V, Bologna E,
Scornajenghi CM, Carino D, Dolci V, Viscuso P, Salciccia S, Sciarra
A, D'Andrea D, et al: Efficacy of different bacillus of
Calmette-Guérin (BCG) strains on recurrence rates among
intermediate/high-risk non-muscle invasive bladder cancers
(NMIBCs): Single-arm study systematic review, cumulative and
network meta-analysis. Cancers. 15:19372023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ibrahim OM: PGE2 Blockade Enhances the
Magnitude and Selectivity of the BCG-Induced Immune Response in the
Human Bladder Cancer Microenvironment. State University of New York
at Buffalo; 2020
|
|
17
|
Lobo N, Bree KK, Hensley PJ,
Nogueras-Gonzalez GM, Abraham P, Navai N, Dinney CP and Kamat AM:
Reduced-dose bacillus Calmette-Guérin (BCG) in an era of BCG
shortage: Real-world experience from a tertiary cancer centre. BJU
Int. 130:323–330. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamat AM, Flaig TW, Grossman HB, Konety B,
Lamm D, O'donnell MA, Uchio E, Efstathiou JA and Taylor JA III:
Expert consensus document: Consensus statement on best practice
management regarding the use of intravesical immunotherapy with BCG
for bladder cancer. Nat Rev Urol. 12:225–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Youssef SF and Buanz AB:
Intravesical combination therapies for non-muscle invasive bladder
cancer: Recent advances and future directions. Eur J Pharmacol.
926:1750242022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wołącewicz M, Hrynkiewicz R, Grywalska E,
Suchojad T, Leksowski T, Roliński J and Niedźwiedzka-Rystwej P:
Immunotherapy in bladder cancer: Current methods and future
perspectives. Cancers. 12:11812020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abd El-Salam MA, Smith CE and Pan CX:
Insights on recent innovations in bladder cancer immunotherapy.
Cancer Cytopathol. 130:667–683. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mancini M, Righetto M and Noessner E:
Checkpoint inhibition in bladder cancer: Clinical expectations,
current evidence, and proposal of future strategies based on a
tumor-specific immunobiological approach. Cancers. 13:60162021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tyagi P, Hafron J, Kaufman J and
Chancellor M: Enhancing therapeutic efficacy and safety of immune
checkpoint inhibition for bladder cancer: A comparative analysis of
injectable vs. Intravesical administration. Int J Mol Sci.
25:49452024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roviello G, Catalano M, Santi R, Palmieri
VE, Vannini G, Galli IC, Buttitta E, Villari D, Rossi V and Nesi G:
Immune checkpoint inhibitors in urothelial bladder cancer: State of
the art and future perspectives. Cancers. 13:44112021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Álvarez-Maestro M, Guerrero-Ramos F,
Rodríguez-Faba O, Domínguez-Escrig J and Fernández-Gómez J: Current
treatments for BCG failure in non-muscle invasive bladder cancer
(NMIBC). Actas Urol Esp (Engl Ed). 45:93–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee CU, Song W, Kang M, Sung HH, Jeon HG,
Seo SI, Jeon SS, Park SH and Jeong BC: Early experience with
pembrolizumab in bacillus Calmette-Guérin unresponsive
non-muscle-invasive bladder cancer. Korean J Urol Oncol.
21:241–248. 2023. View Article : Google Scholar
|
|
27
|
Ruiz-Lorente I, Gimeno L, López-Abad A,
López Cubillana P, Fernández Aparicio T, Asensio Egea LJ, Moreno
Avilés J, Doñate Iñiguez G, Guzmán Martínez-Valls PL, Server G, et
al: Exploring the immunoresponse in bladder cancer immunotherapy.
Cells. 13:19372024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song SH and Oh JJ: The evolving role of
checkpoint inhibitors in the treatment of urothelial carcinoma: A
literature review of practice-changing trials. J Urol Oncol.
21:154–164. 2023. View Article : Google Scholar
|
|
29
|
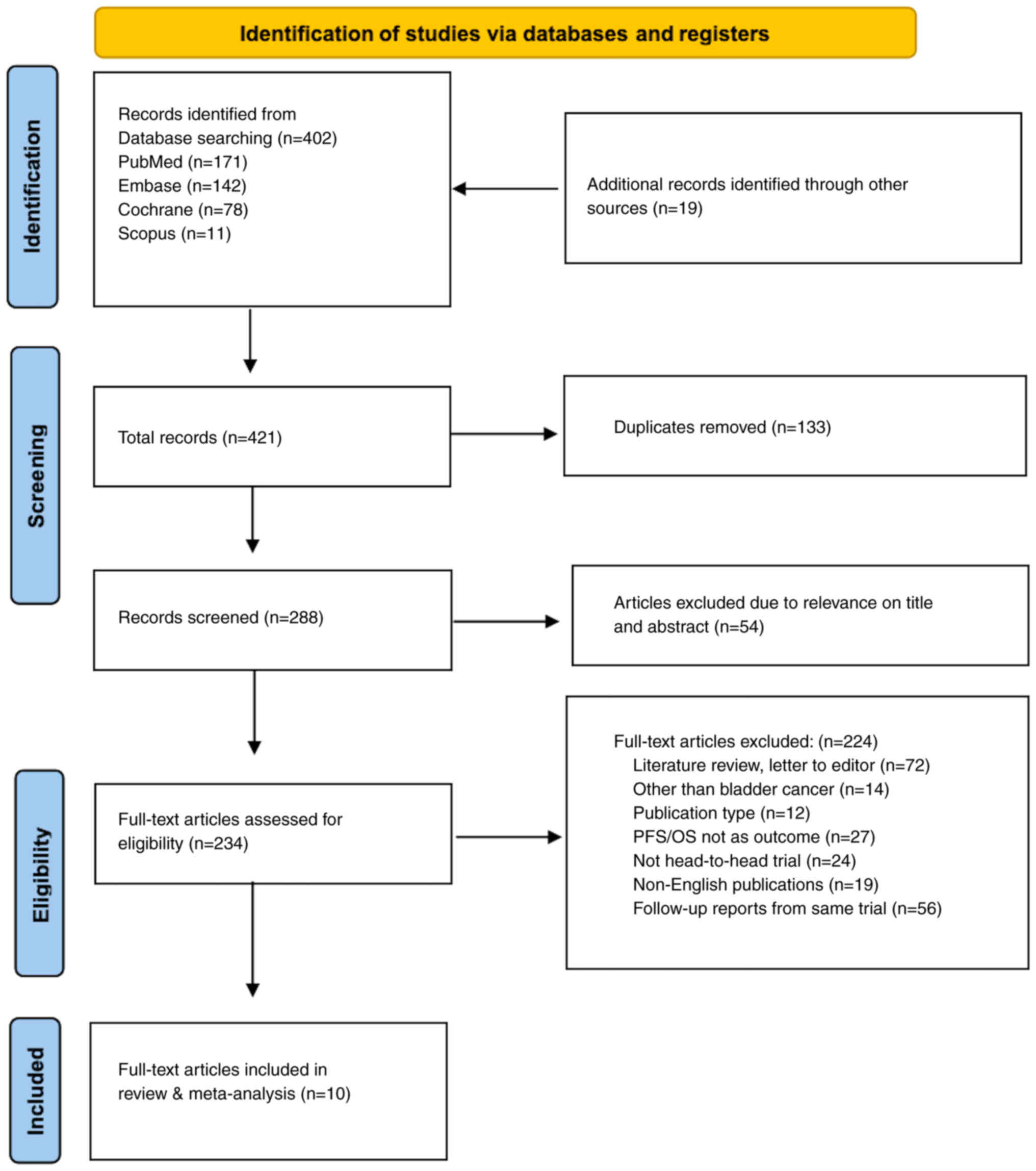
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366:l48982019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schulz KF, Altman DG and Moher D: CONSORT
2010 Statement: Updated guidelines for reporting parallel group
randomised trials. BMJ. 340:c3322010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hernán MA and Robins JM: Per-protocol
analyses of pragmatic trials. N Engl J Med. 377:1391–1398. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bell ML, Fiero M, Horton NJ and Hsu CH:
Handling missing data in RCTs; a review of the top medical
journals. BMC Med Res Methodol. 14:1182014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dwan K, Williamson PR, Gamble C, Higgins
J, Sterne J, Altman DG, Clarke M and Kirkham JJ: Guidance to
detect, evaluate and prevent the problem of selective reporting in
trial publications. Trials. 14 (Suppl 1):O912013. View Article : Google Scholar
|
|
35
|
He Y, Ren T, Ji C, Zhao L and Wang X: The
baseline hemoglobin level is a positive biomarker for immunotherapy
response and can improve the predictability of tumor mutation
burden for immunotherapy response in cancer. Front Pharmacol.
15:14568332024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galsky MD, Mortazavi A, Milowsky MI,
George S, Gupta S, Fleming MT, Dang LH, Geynisman DM, Walling R,
Alter RS, et al: Randomized double-blind phase II study of
maintenance pembrolizumab versus placebo after first-line
chemotherapy in patients with metastatic urothelial cancer. J Clin
Oncol. 38:1797–1806. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rose TL, Harrison MR, Deal AM, Ramalingam
S, Whang YE, Brower B, Dunn M, Osterman CK, Heiling HM, Bjurlin MA,
et al: Phase II study of gemcitabine and split-dose cisplatin plus
pembrolizumab as neoadjuvant therapy before radical cystectomy in
patients with muscle-invasive bladder cancer. J Clin Oncol.
39:3140–3148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang T, Tan A, Shah AY, Iyer G, Morris V,
Michaud S and Sridhar SS: Reevaluating the role of platinum-based
chemotherapy in the evolving treatment landscape for patients with
advanced urothelial carcinoma. Oncologist. 29:1003–1013. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martini A, Raggi D, Fallara G, Nocera L,
Schultz JG, Belladelli F, Marandino L, Salonia A, Briganti A,
Montorsi F, et al: Immunotherapy versus chemotherapy as first-line
treatment for advanced urothelial cancer: A systematic review and
meta-analysis. Cancer Treat Rev. 104:1023602022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szabados B, Kockx M, Assaf ZJ, van Dam PJ,
Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, Pous AF,
Gravis G, et al: Final results of neoadjuvant atezolizumab in
cisplatin-ineligible patients with muscle-invasive urothelial
cancer of the bladder. Eur Urol. 82:212–222. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holmsten K: Improving Chemotherapy in
Advanced Urothelial Cancer: Real-world Data Studies and Prospective
Clinical Trials. Karolinska Institutet; Stockholm: 2020, PubMed/NCBI
|
|
42
|
Huang Y, Liao C, Shen Z, Zou Y, Xie W, Gan
Q, Yao Y, Zheng J and Kong J: A bibliometric insight into
neoadjuvant chemotherapy in bladder cancer: Trends, collaborations,
and future avenues. Front Immunol. 15:12975422024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lidagoster S, Ben-David R, De Leon B and
Sfakianos JP: BCG and alternative therapies to BCG therapy for
non-muscle-invasive bladder cancer. Curr Oncol. 31:1063–1078. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Michaud E, Mansure JJ and Kassouf W:
Integrating novel immunotherapeutic approaches in organ-preserving
therapies for bladder cancer. Br J Pharmacol. Dec 13–2023.(Epub
ahead of print). doi: 10.1111/bph.16300. PubMed/NCBI
|
|
45
|
Peng M, Xiao D, Bu Y, Long J and Yang X,
Lv S and Yang X: Novel combination therapies for the treatment of
bladder cancer. Front Oncol. 10:5395272021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Steinberg RL, Thomas LJ, O'Donnell MA and
Nepple KG: Sequential intravesical gemcitabine and docetaxel for
the salvage treatment of non-muscle invasive bladder cancer.
Bladder Cancer. 1:65–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Milbar N, Kates M, Chappidi MR, Pederzoli
F, Yoshida T, Sankin A, Pierorazio PM, Schoenberg MP and Bivalacqua
TJ: Oncological outcomes of sequential intravesical gemcitabine and
docetaxel in patients with non-muscle invasive bladder cancer.
Bladder Cancer. 3:293–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hannouneh ZA, Hijazi A, Alsaleem AA, Hami
S, Kheyrbek N, Tanous F, Khaddour K, Abbas A and Alshehabi Z: Novel
immunotherapeutic options for BCG-unresponsive high-risk
non-muscle-invasive bladder cancer. Cancer Med. 12:21944–21968.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Claps F, Pavan N, Ongaro L, Tierno D,
Grassi G, Trombetta C, Tulone G, Simonato A, Bartoletti R, Mertens
LS, et al: BCG-unresponsive non-muscle-invasive bladder cancer:
Current treatment landscape and novel emerging molecular targets.
Int J Mol Sci. 24:125962023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shore ND, Redorta JP, Robert G, Hutson TE,
Cesari R, Hariharan S, Faba OR, Briganti A and Steinberg GD:
Non-muscle-invasive bladder cancer: An overview of potential new
treatment options. Urol Oncol. 39:642–663. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li R, Shah PH, Stewart TF, Nam JK,
Bivalacqua TJ, Lamm DL, Uchio EM, Geynisman DM, Jacob JM, Meeks JJ,
et al: Oncolytic adenoviral therapy plus pembrolizumab in
BCG-unresponsive non-muscle-invasive bladder cancer: The phase 2
CORE-001 trial. Nat Med. 30:2216–2223. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Necchi A, Roumiguié M, Kamat AM, Shore ND,
Boormans JL, Esen AA, Lebret T, Kandori S, Bajorin DF, Krieger LEM,
et al: Pembrolizumab monotherapy for high-risk non-muscle-invasive
bladder cancer without carcinoma in situ and unresponsive to BCG
(KEYNOTE-057): A single-arm, multicentre, phase 2 trial. Lancet
Oncol. 25:720–730. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aurilio G, Cimadamore A, Lopez-Beltran A,
Scarpelli M, Massari F, Verri E, Cheng L, Santoni M and Montironi
R: Narrative review: Update on immunotherapy and pathological
features in patients with bladder cancer. Transl Androl Urol.
10:15212021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Powles T, Durán I, Van der Heijden MS,
Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano
D, Bamias A, et al: Atezolizumab versus chemotherapy in patients
with platinum-treated locally advanced or metastatic urothelial
carcinoma (IMvigor211): A multicentre, open-label, phase 3
randomised controlled trial. Lancet. 391:748–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rosenberg JE, Hoffman-Censits J, Powles T,
Van Der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bellmunt J, De Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fradet Y, Bellmunt J, Vaughn D, Lee J,
Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi
A, et al: Randomized phase III KEYNOTE-045 trial of pembrolizumab
versus paclitaxel, docetaxel, or vinflunine in recurrent advanced
urothelial cancer: Results of> 2 years of follow-up. Ann Oncol.
30:970–976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Balar AV, Kamat AM, Kulkarni GS, Uchio EM,
Boormans JL, Roumiguié M, Krieger LEM, Singer EA, Bajorin DF,
Grivas P, et al: Pembrolizumab monotherapy for the treatment of
high-risk non-muscle-invasive bladder cancer unresponsive to BCG
(KEYNOTE-057): An open-label, single-arm, multicentre, phase 2
study. Lancet Oncol. 22:919–930. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Powles T, Csőszi T, Özgüroğlu M, Matsubara
N, Géczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales
Barrera R, et al: Pembrolizumab alone or combined with chemotherapy
versus chemotherapy as first-line therapy for advanced urothelial
carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial.
Lancet Oncol. 22:931–945. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
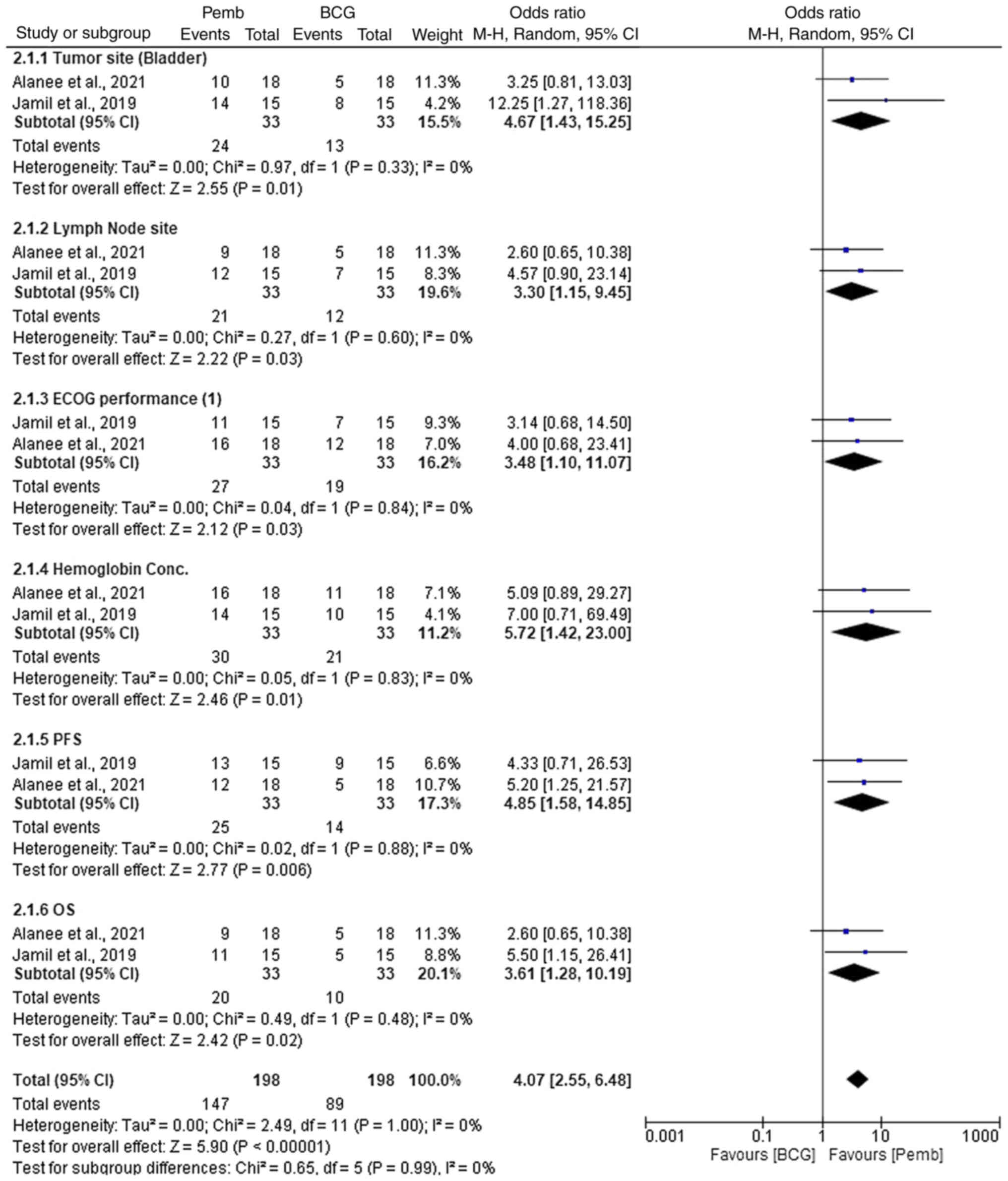
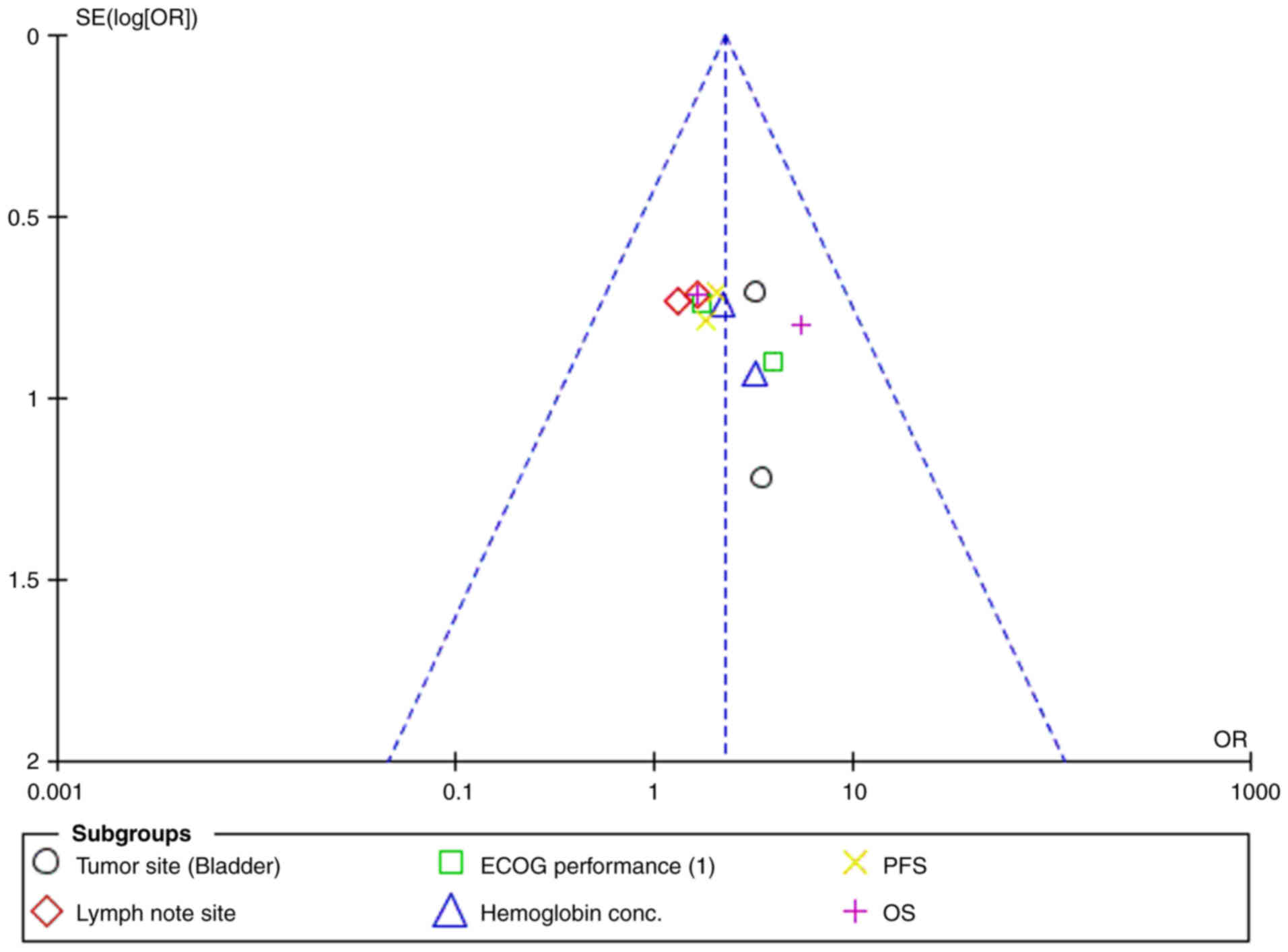
Jamil ML, Deebajah M, Sood A, Robinson K,
Rao K, Sana S and Alanee S: Protocol for phase I study of
pembrolizumab in combination with Bacillus Calmette-Guérin for
patients with high-risk non-muscle invasive bladder cancer. BMJ
Open. 9:e0282872019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Alanee S, Sana S, El-Zawahry A, Peabody J,
Pearce T, Adams N, Deebajah M, Crabtree J, Delfino K, McVary K, et
al: Phase I trial of intravesical Bacillus Calmette-Guérin combined
with intravenous pembrolizumab in recurrent or persistent
high-grade non-muscle-invasive bladder cancer after previous
Bacillus Calmette-Guérin treatment. World J Urol. 39:3807–3813.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Inman BA, Hahn NM, Stratton K, Kopp R,
Sankin A, Skinner E, Pohar K, Gartrell BA, Pham S, Rishipathak D,
et al: A phase 1b/2 study of atezolizumab with or without bacille
Calmette-Guérin in patients with high-risk non-muscle-invasive
bladder cancer. Eur Urol Oncol. 6:313–320. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Black PC, Tangen CM, Singh P, McConkey DJ,
Lucia MS, Lowrance WT, Koshkin VS, Stratton KL, Bivalacqua TJ,
Kassouf W, et al: Phase 2 trial of atezolizumab in bacillus
Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder
cancer: SWOG S1605. Eur Urol. 84:536–544. 2023. View Article : Google Scholar : PubMed/NCBI
|