Introduction
Bladder cancer (BC) is a significant global health
burden, ranking as the ninth most common cancer worldwide, with
nearly half a million new cases reported annually (1). BC encompasses two primary categories:
Muscle-invasive BC (MIBC) and non-MIBC (NMIBC). Notably, NMIBC
constitutes ~75% of BC cases and encompasses various pathological
stages, including non-invasive bladder carcinoma confined to the
epithelium or mucosa, tumor invading the subepithelial connective
tissue and carcinoma in situ (2). Despite its generally favorable
prognosis, NMIBC presents distinct challenges, particularly in
high-risk subsets, such as those with high-grade tumors, large
tumor size, multifocality and those with a history of prior
recurrence (3). These subsets
demonstrate significant rates of recurrence and progression. For
example, patients with carcinoma in situ or those with a
high number of early recurrences within the first year after
initial treatment are considered at higher risk for progression to
MIBC (4). BC has a multifactorial
etiology, with smoking being the primary risk factor for its
development. While the majority of BC tumors originate from
urothelial cells, histological variations, such as squamous,
neuroendocrine, micropapillary and sarcomatoid subtypes are less
common. Notably, the classification of BC into NMIBC and MIBC
guides therapeutic approaches (5,6).
Moreover, the prognosis and treatment decisions for NMIBC are
heavily influenced by tumor grading, depth of invasion and risk
stratification systems, such as those developed by the European
Organization for Research and Treatment of Cancer and the European
Association of Urology (7,8).
The cornerstone of initial treatment for
intermediate- and high-risk NMIBC involves transurethral resection
of the bladder tumor followed by adjuvant therapy with intravesical
Bacillus Calmette-Guérin (BCG) (9).
This approach is considered the standard of care for these tumors.
BCG therapy, pioneered by Morales (10), has demonstrated efficacy in reducing
the risk of disease progression and recurrence. However, its use is
limited by the associated adverse events and recent shortages.
Despite its benefits, a subset of patients with high-risk factors,
such as those with carcinoma in situ, multiple recurrent
tumors or BCG failure, fail to achieve an adequate therapeutic
response, necessitating alternative treatment approaches (11,12).
BC, particularly NMIBC, is predominantly treated
with intravesical BCG therapy (13). However, resistance to BCG therapy
remains a significant clinical challenge. This resistance is often
associated with the expression of immunosuppressive molecules, such
as programmed death ligands [programmed death ligand 1 (PD-L1) and
programmed cell death 1 ligand 2], which can inhibit the immune
response and hinder the effectiveness of treatment (14). Experimental studies have
demonstrated that the upregulation of these ligands in the tumor
microenvironment is linked to poor BCG response, as they interfere
with T cell activation and tumor immunosurveillance (15,16).
Furthermore, the global shortage of BCG has created
notable challenges in managing NMIBC. Production issues, increased
demand and regulatory challenges have contributed to the scarcity
of BCG, thereby impacting treatment protocols worldwide (17). These shortcomings necessitate
alternative treatment strategies, including the use of intravesical
chemotherapy or other immunotherapeutic agents, such as
pembrolizumab (18). Mitomycin C,
gemcitabine and docetaxel have been explored as substitutes.
However, their efficacy and long-term outcomes compared with BCG
are still under evaluation (19).
Consequently, healthcare systems have had to adapt by prioritizing
BCG allocation for high-risk patients and utilizing alternative
treatment regimens for those with lower-risk profiles. Ongoing
research and development efforts aim to address these shortcomings
by improving the production processes and developing new
immunotherapeutic options (13,20).
Food and Drug Administration-approved injectable
immune checkpoint inhibitors (ICIs) for metastatic urothelial
carcinoma represent a new era of treatment (21). These ICIs show promise as
second-line treatments for BCG-unresponsive NMIBC, either alone or
in combination with other agents (22). However, systemic administration
leads to more adverse events, prompting the exploration of
alternative delivery routes. Notably, intravesical ICIs offer a
strategy for enhancing the therapeutic index and reducing systemic
toxicity (23). Preliminary
studies, such as the combination of intravesical pembrolizumab with
BCG induction therapy for patients with BCG-unresponsive NMIBC,
showed encouraging outcomes, improving recurrence-free survival and
progression-free survival (PFS) (24–26).
Other ICIs, such as atezolizumab, avelumab and nivolumab, have also
demonstrated potential in this setting, revolutionizing the
treatment landscape for NMIBC, particularly in cases of BCG
failure. Ongoing research continues to explore novel agents and
combination approaches to further optimize outcomes and reduce
adverse events (27,28).
The present study aimed to evaluate the efficacy and
safety of immunotherapy in BCG-refractory NMIBC by comparing
intravesical BCG with novel ICIs. Through a meta-analysis of
clinical trials and randomized controlled trials (RCTs), the impact
of ICIs on overall survival (OS) and progression-free survival
(PFS), along with their safety profiles were assessed.
Additionally, response predictors were identified and future
directions for optimizing treatment and biomarker development were
explored. The findings provide insights to guide clinicians in
selecting the most effective therapy based on individual patient
characteristics.
Materials and methods
Following the recommendations of the Preferred
Reporting Standards for Systematic Reviews and Meta-Analyses 2020
guidelines (29) and the protocol
registered in PROSPERO (https://www.crd.york.ac.uk/prospero/; no.
CRD42024544722. The meta-analysis primarily compared BCG with ICIs
(pembrolizumab/atezolizumab) in BCG-refractory NMIBC. Ethical
approval was considered unnecessary as the study did not involve
human or animal experiments.
Population, intervention, comparison,
outcomes and study design (PICOS) question
The present study aimed to evaluate the efficacy and
safety of immunotherapy in patients with BCG-refractory NMIBC by
comparing ICIs (pembrolizumab, atezolizumab) with standard
treatments. The study population included patients with
BCG-refractory NMIBC, with ICIs as the intervention and BCG as the
comparison. Primary outcomes assessed included OS, PFS and safety
based on adverse events.
Eligibility criteria
The inclusion criteria for the primary analysis in
the present study were RCTs and clinical trials published between
January 2015 and April 2024 that focused on patients diagnosed with
BCG-refractory NMIBC. Studies evaluating ICIs, such as
pembrolizumab or atezolizumab, and BCG therapy were included. Only
trials assessing immunotherapy in BCG-refractory NMIBC patients,
with or without prior platinum-based chemotherapy, were considered
for analysis. The primary outcomes evaluated in these studies were
OS, PFS and safety. Immunotherapy agents were considered ‘similar’
if they belonged to the same class of ICIs targeting the programmed
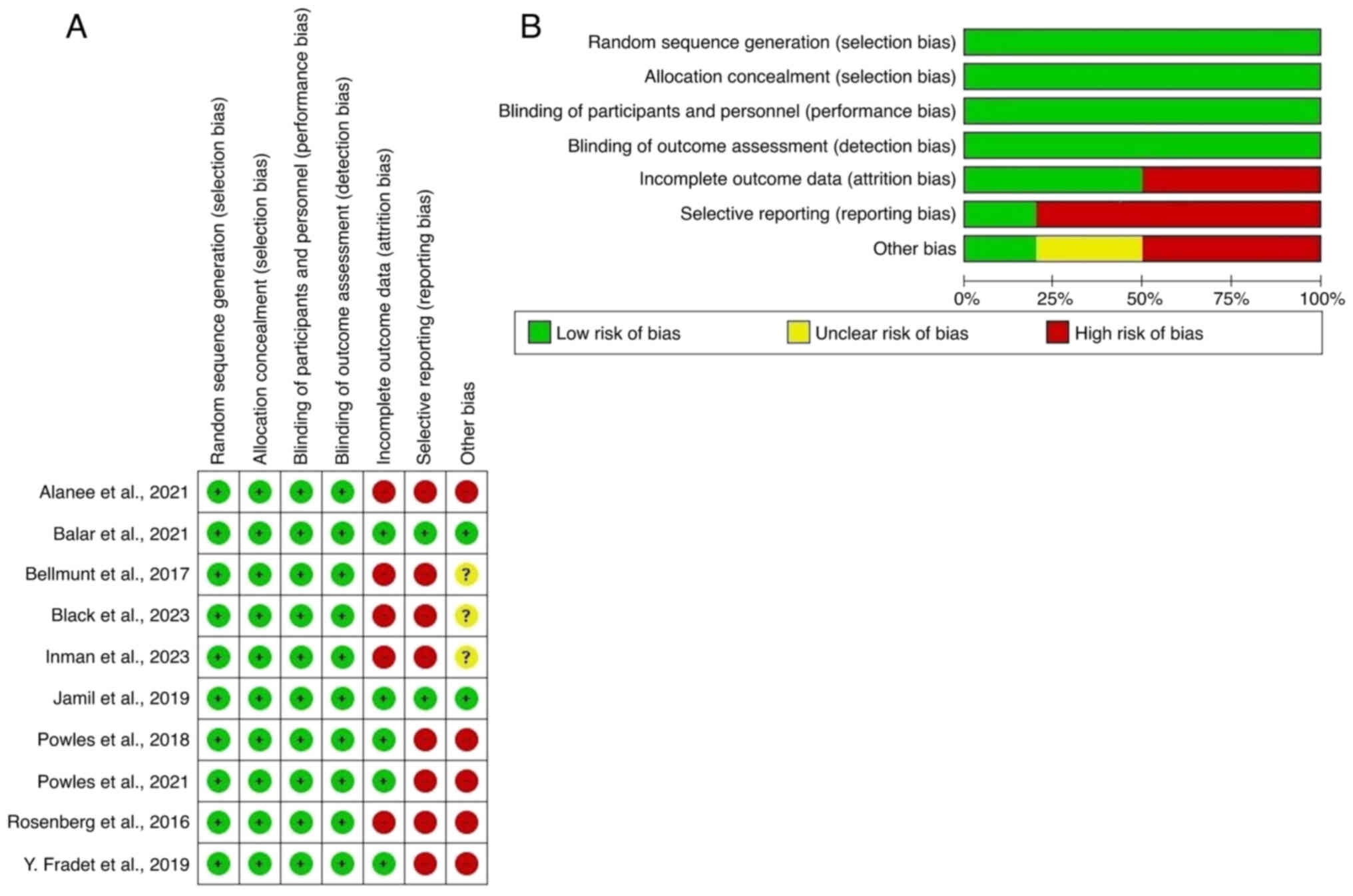
cell death protein 1/PD-L1 pathway. A risk of bias assessment was
conducted following the Cochrane Collaboration's Risk of Bias 2.0
(RoB 2.0) guidelines (30),
evaluating factors such as randomization methods, blinding and data
reporting. The exclusion criteria were duplicate studies, case
reports, retrospective analyses and non-English publications. For
the primary analysis (BCG vs. ICIs), trials comparing ICIs with
chemotherapy or those including non-NMIBC populations (such as
locally advanced/metastatic urothelial carcinoma) were excluded.
For the secondary analysis (ICIs vs. chemotherapy), trials
involving BCG-refractory NMIBC, BCG-naïve NMIBC or
platinum-refractory advanced/metastatic urothelial carcinoma were
included. Studies focusing on non-urothelial cancer or
non-refractory populations were excluded. Only prospective
multicenter pharmaceutical trials with a single treatment arm were
considered eligible, excluding studies involving animals, patients
with diseases other than BC, reanalyzed RCTs, non-randomized
allocation and publications in abstracts, reviews, editorials or
letters.
Literature search approach
A comprehensive literature search was performed
across PubMed (https://pubmed.ncbi.nlm.nih.gov/), Medline (https://www.nlm.nih.gov/medline/), Embase
(https://www.embase.com/search/), Scopus
(www.scopus.com/search/) and the Cochrane
Research Register (https://www.cochranelibrary.com/) to identify relevant
studies for the present meta-analysis. The search strategy employed
Boolean operators (AND, OR) in combination with key words such as
‘non-muscle invasive bladder cancer’, ‘BCG therapy’, ‘immune
checkpoint inhibitors’, ‘pembrolizumab’, ‘atezolizumab’ and
‘clinical trials.’ Medical Subject Headings terms were used to
refine the search and enhance specificity, including terms such as
‘Bladder Neoplasms’, ‘Bacillus Calmette-Guerin’ and
‘Immunotherapy’. In addition, the reference lists of relevant
systematic reviews and literature reviews were manually searched to
ensure comprehensive coverage of the topic.
Study selection
In total, two independent reviewers rigorously
screened each article based on its title and abstract, resolving
discrepancies through discussion. Potentially eligible studies
underwent full-text screening and additional trials were identified
through a systematic review.
Data extraction
Data extraction involved meticulous collection of
relevant information, including the study approach, participant
characteristics, sample sizes, intervention specifics, control
groups, duration of observation, survival measures and adverse
events. These data were organized according to the PICOS structure
and independently retrieved by two assessors, with discrepancies
resolved through collaborative deliberation. Data extraction
prioritized BCG vs. ICI outcomes (such as OS, PFS safety).
Data analysis and risk assessment
The analysis was conducted by using Review Manager
version 5.4 (Cochrane Collaboration), employing a random-effects
model to address expected variability. Primary analysis compared
BCG vs. ICIs, while a secondary exploratory analysis included ICIs
vs. chemotherapy. Categorical outcomes such as OS, PFS and adverse
events were presented as odds ratios (ORs) with corresponding 95%
confidence intervals (CIs). Evaluation of statistical diversity was
performed using Cochran's Q test (χ2) and the
I2 index with significance set at P<0.05. Publication
bias was assessed using forest and funnel plots, with significance
set at P<0.05. Risk of bias assessment followed the Cochrane
Collaboration tool, considering factors such as random sequence
generation, allocation concealment, blinding and outcome data
completeness (Fig. 1).
Results
Characteristics of the selected
studies
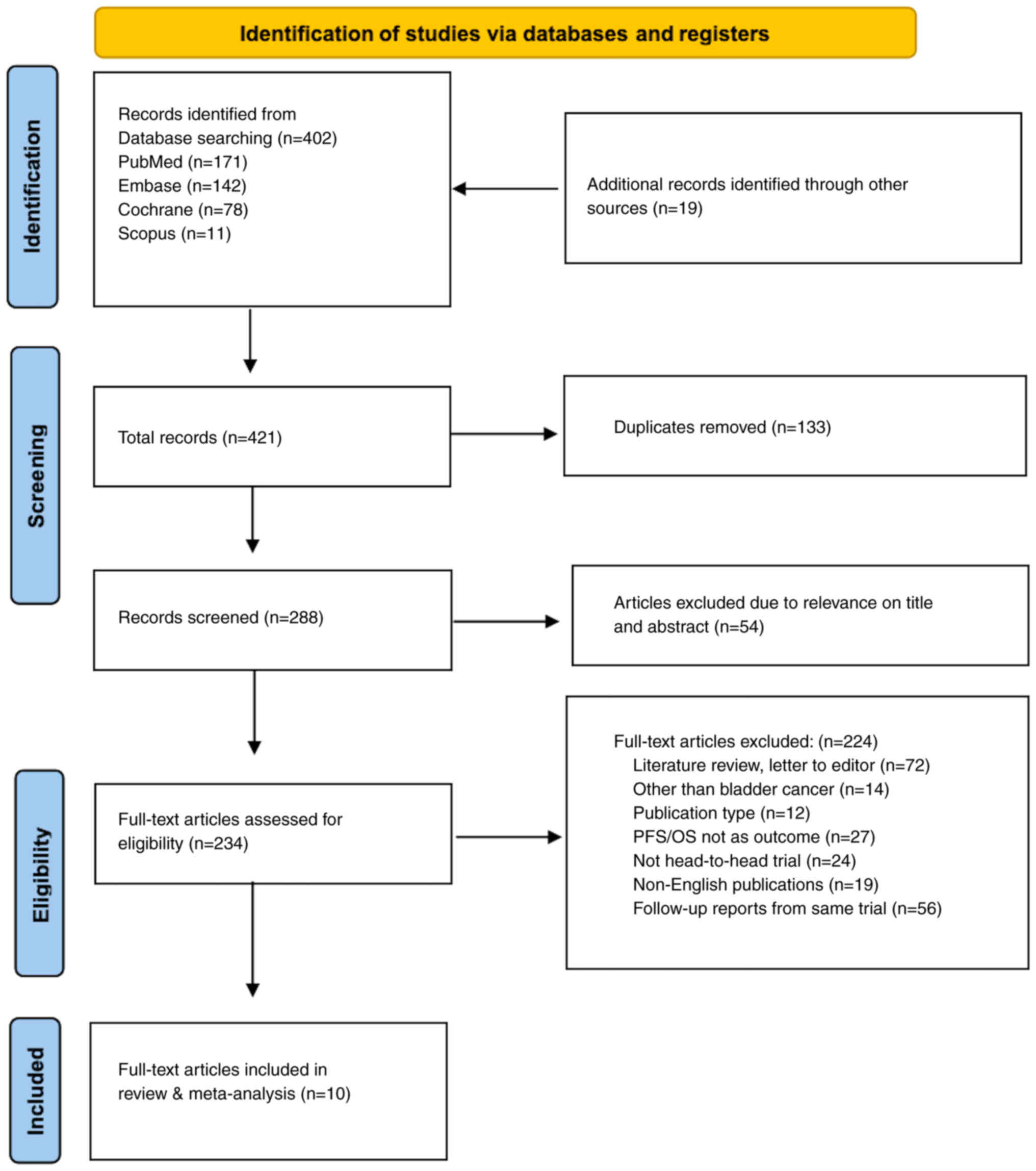
In the systematic review and meta-analysis, 402
records were initially identified through database searching, with
PubMed contributing 171, Embase 142, Cochrane 78 and Scopus 11
records. Additionally, 19 records were identified from other
sources. After removing duplicates (n=133), 288 records were
screened, of which 54 were excluded as their titles and abstracts
did not meet the inclusion criteria. Subsequently, 234 full-text
articles were assessed for eligibility, resulting in the exclusion
of 224 articles, primarily as they were literature reviews, letters
to editors, unrelated to BC or did not meet other inclusion
criteria. Finally, 10 full-text articles were included in the
present review and meta-analysis (Fig.
2).
Study participants and baseline
characteristics
This study included 2,154 participants from diverse
clinical trials exploring treatments for BC. The age of the
patients varied, with a median age of 63 years. A male predominance
was observed in some trials. The participants had high-risk NMIBC
including BCG-refractory NMIBC, defined as those with persistent or
recurrent high-grade disease despite ≥2 prior courses of
intravesical BCG therapy, or locally advanced or metastatic
urothelial carcinoma, specifically after failure of first-line
platinum-based chemotherapy. Patients with locally advanced or
metastatic urothelial carcinoma were included in secondary analyses
to assess broader efficacy, while the primary analysis focused on
BCG-refractory NMIBC populations. Chemotherapy treatments varied
across the trials, with most using cisplatin or carboplatin based
regimens. However, some trials followed different protocols,
including other platinum-based combinations. Trials spanned from
Phase 1 to Phase 3, employing treatments such as pembrolizumab,
atezolizumab, chemotherapy or intravesical BCG therapy. The
follow-up durations ranged from 11.7 to 31.7 months. The mean PFS
spanned 2.1 to 8.3 months and the mean OS ranged from 7.3 to 17.0
months. Adverse events, including fatigue, nausea, diarrhea,
asthenia, pyrexia, anemia and treatment-related effects, were
reported in all trials (Table
SI).
Primary analysis: BCG vs. ICIs
Comparison of pembrolizumab vs. BCG in the
treatment of NMIBC
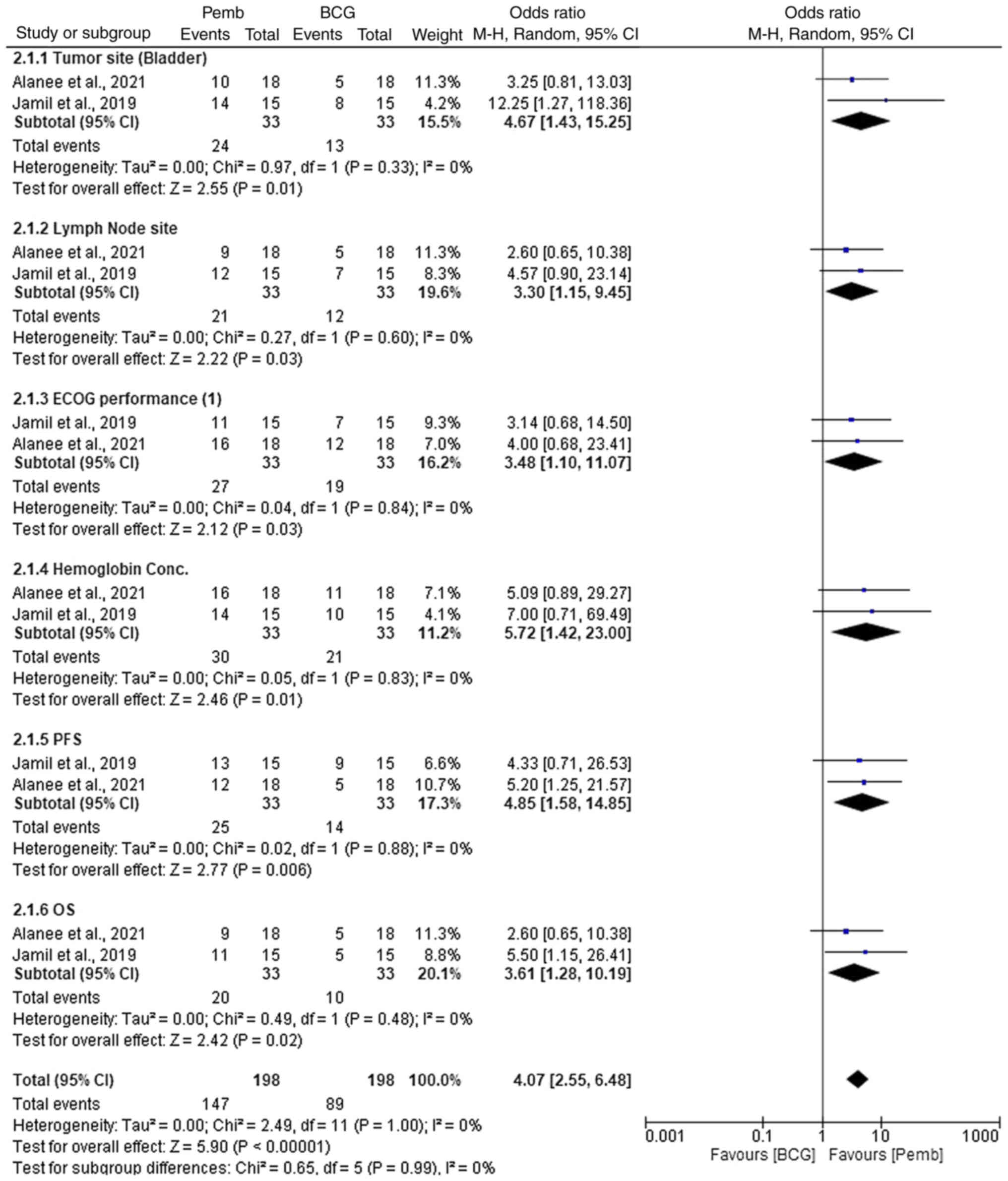
In the comparison of pembrolizumab versus BCG for
the treatment of NMIBC, pembrolizumab demonstrated superior
efficacy across various parameters (Fig. 3). For tumor site (bladder),
pembrolizumab showed a significantly high OR of 4.67 (95% CI,
1.43–15.25; P=0.01), indicating improved efficacy in tumor control
compared with BCG. Similarly, for lymph node site, pembrolizumab
again outperformed BCG with an OR of 3.30 (95% CI, 1.15–9.45;
P=0.03). In terms of ECOG performance (1), pembrolizumab showed a strong advantage
with an OR of 3.48 (95% CI, 1.10–11.07; P=0.03). Hemoglobin
concentration was also in favor of pembrolizumab, with an OR of
5.72 (95% CI, 1.42–23.00; P=0.01). This result suggests that
pembrolizumab was associated with a higher likelihood of improving
hemoglobin concentration compared with BCG. Regarding PFS and OS,
pembrolizumab demonstrated significant benefits, with ORs of 4.85
(95% CI, 1.58–14.85; P=0.006) and 3.61 (95% CI, 1.28–10.19;
P=0.02), respectively. Across all parameters, pembrolizumab
consistently showed higher ORs and significant P-values, suggesting
it may provide more effective outcomes compared with BCG. These
findings highlight pembrolizumab's potential for improved treatment
efficacy in NMIBC, possibly due to its targeted immune checkpoint
inhibition mechanism compared with BCG's non-specific immune
stimulation.
Heterogeneity across the subgroups was low, with
I2 values of 0% for tumor site (bladder), lymph node
site, ECOG performance, hemoglobin concentration, PFS and OS,
indicating minimal variability between studies. χ2 tests
also showed no significant heterogeneity (P>0.05). The overall
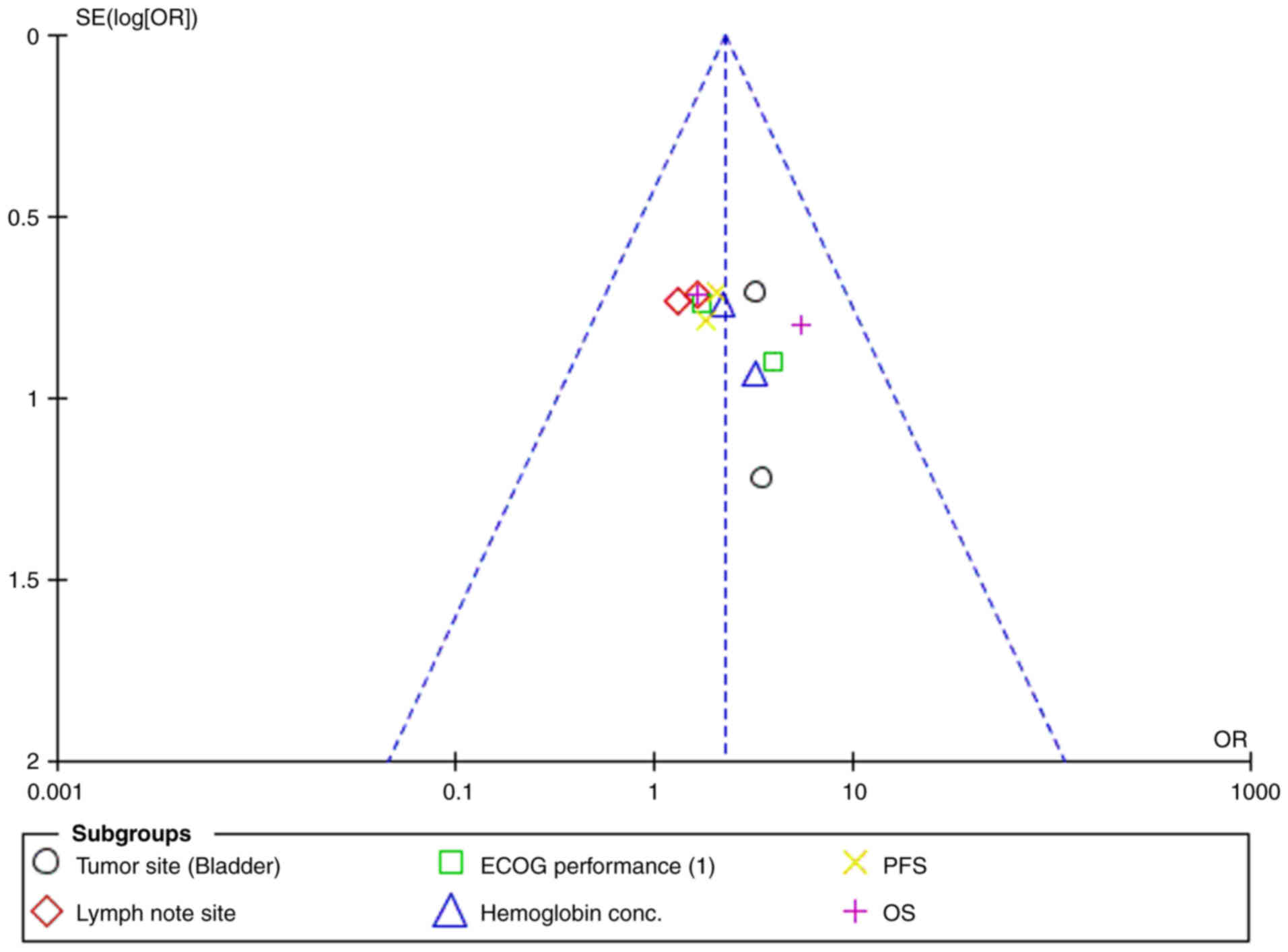
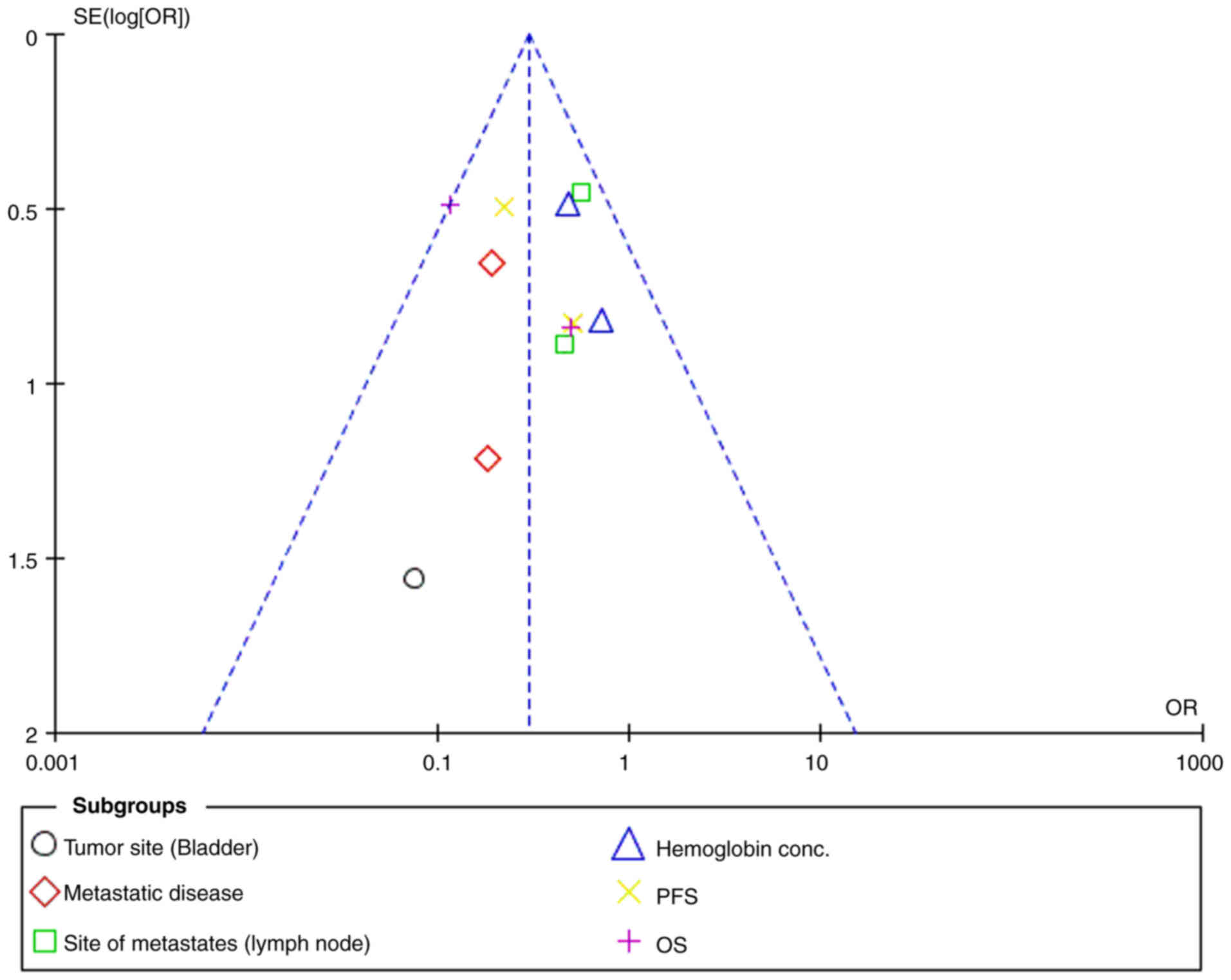
I2 value was 0%, and the funnel plot (Fig. 4) did not indicate publication bias,
suggesting consistent results across the trials analyzed.
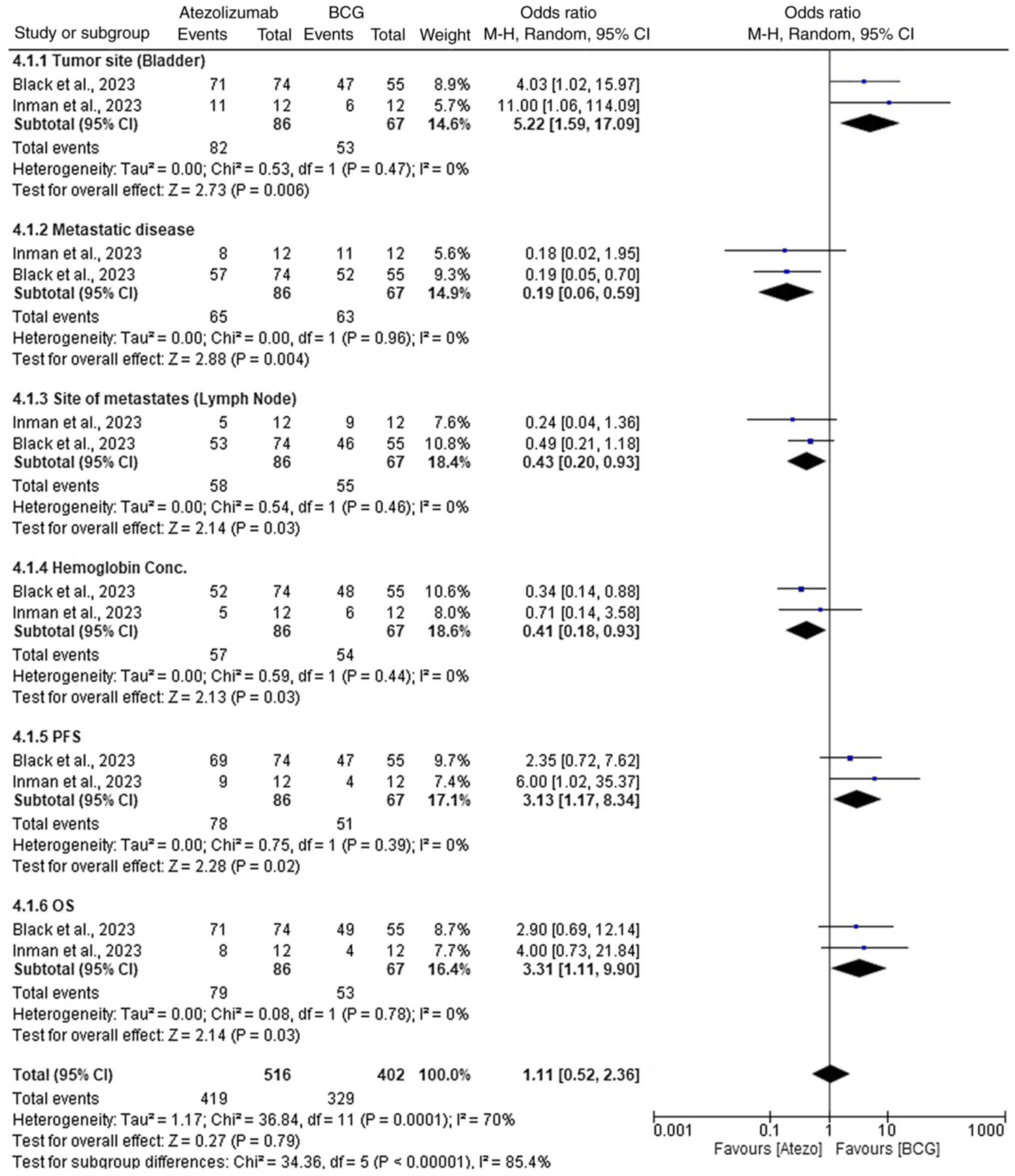
Comparative efficacy of atezolizumab
vs. BCG in NMIBC
In the comparison of atezolizumab versus BCG for the
treatment of NMIBC, both treatments demonstrated varying levels of
efficacy across different parameters (Fig. 5). For tumor site (bladder),
atezolizumab showed improved efficacy with an OR of 5.22 (95% CI,
1.59–17.09; P=0.006). Similarly, for metastatic disease,
atezolizumab performed better with an OR of 0.19 (95% CI,
0.06–0.59; P=0.004). Atezolizumab also showed superior outcomes in
terms of site of metastases (lymph nodes) with an OR of 0.43 (95%
CI, 0.20–0.93; P=0.03), and hemoglobin concentration with an OR of
0.41 (95% CI, 0.18–0.93; P=0.03). Atezolizumab again proved more
effective in terms of PFS with an OR of 3.13 (95% CI, 1.17–8.34;
P=0.02), and OS with an OR of 3.31 (95% CI, 1.11–9.90; P=0.03). The
overall effect for all parameters favored atezolizumab with an OR
of 1.11 (95% CI, 0.52–2.36; P=0.79); however, this was not
statistically significant. These results highlight that
atezolizumab generally provided more consistent and superior
efficacy across multiple clinical outcomes, likely due to its
immune-modulating properties compared with BCG's direct
immunotherapy mechanism.
Throughout the results, low heterogeneity was
observed for most individual parameters, with I2 values
of 0% for tumor site (bladder), metastatic disease, site of
metastases (lymph node), hemoglobin concentration, PFS and OS.
However, when examining the overall effect, moderate heterogeneity
was observed, with I2=70%, and significant subgroup
differences were noted (P<0.00001) (Fig. 6).
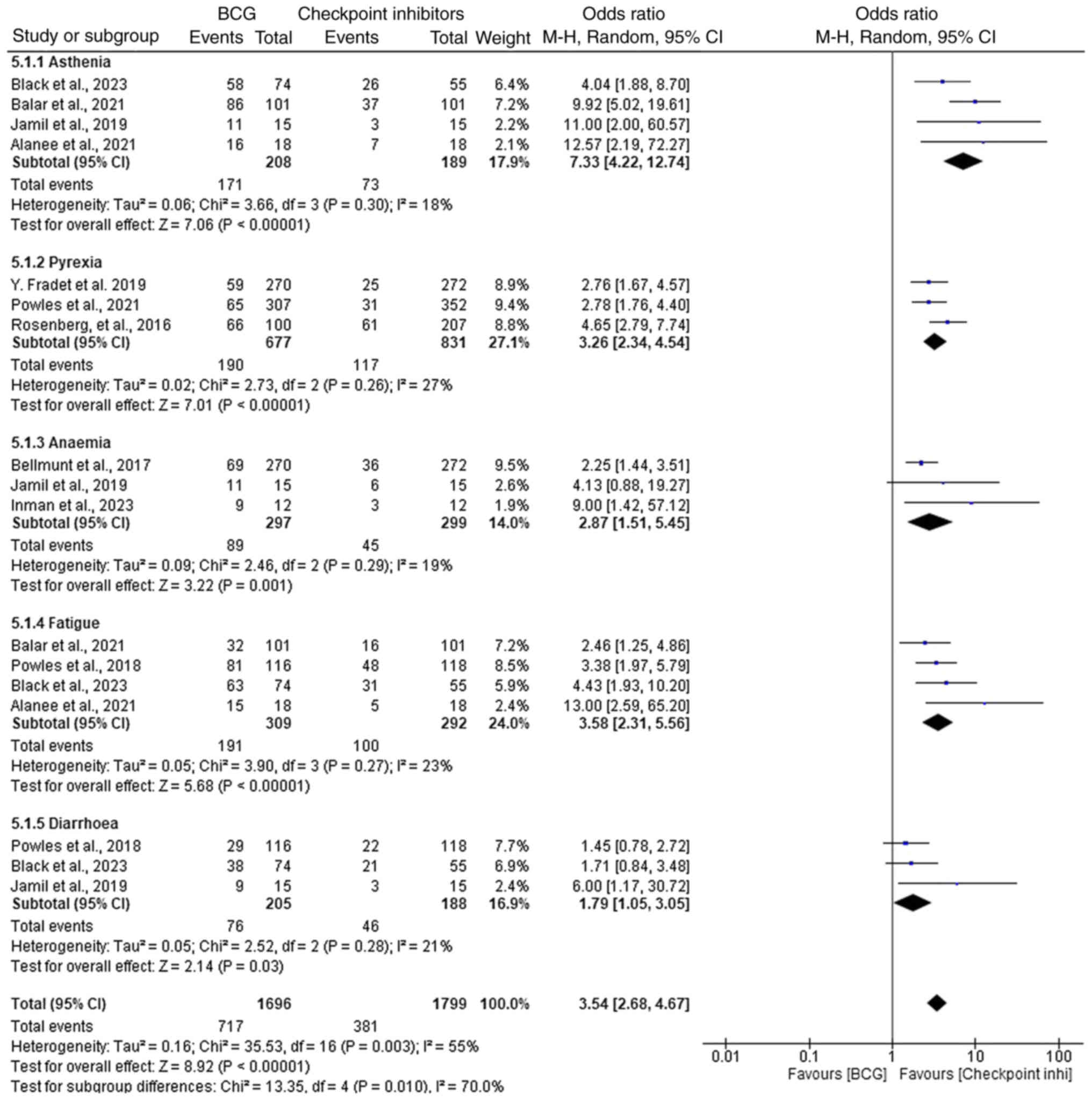
Adverse events: ICIs vs. BCG therapy
in NMIBC
In the comparison of adverse events between ICIs and
BCG therapy for NMIBC, ICIs demonstrated a higher incidence of
several adverse events (Fig. 7).
Asthenia was significantly more common in the ICI group, with an OR
of 7.33 (95% CI, 4.22–12.74; P<0.00001), indicating a higher
incidence compared with BCG therapy. Similarly, pyrexia (fever) was
more frequently observed in the ICI group, with an OR of 3.26 (95%
CI, 2.34–4.54; P<0.00001), suggesting that fever management may
be more challenging in patients receiving ICIs. Despite higher
rates of certain adverse events, ICIs were associated with a lower
incidence of anemia (OR, 2.87; 95% CI, 1.51–5.45; P=0.001), fatigue
(OR, 3.58, 95% CI, 2.31–5.56; P<0.00001) and diarrhea (OR, 1.79;
95% CI, 1.05–3.05; P=0.03), suggesting improved tolerability and
fewer serious complications compared with BCG therapy. Although the
ORs are all >1, indicating a higher likelihood of adverse events
in the ICI group, the results show that ICIs demonstrate improved
outcomes in terms of anemia management, fatigue and diarrhea,
indicating a more favorable safety profile in these aspects.
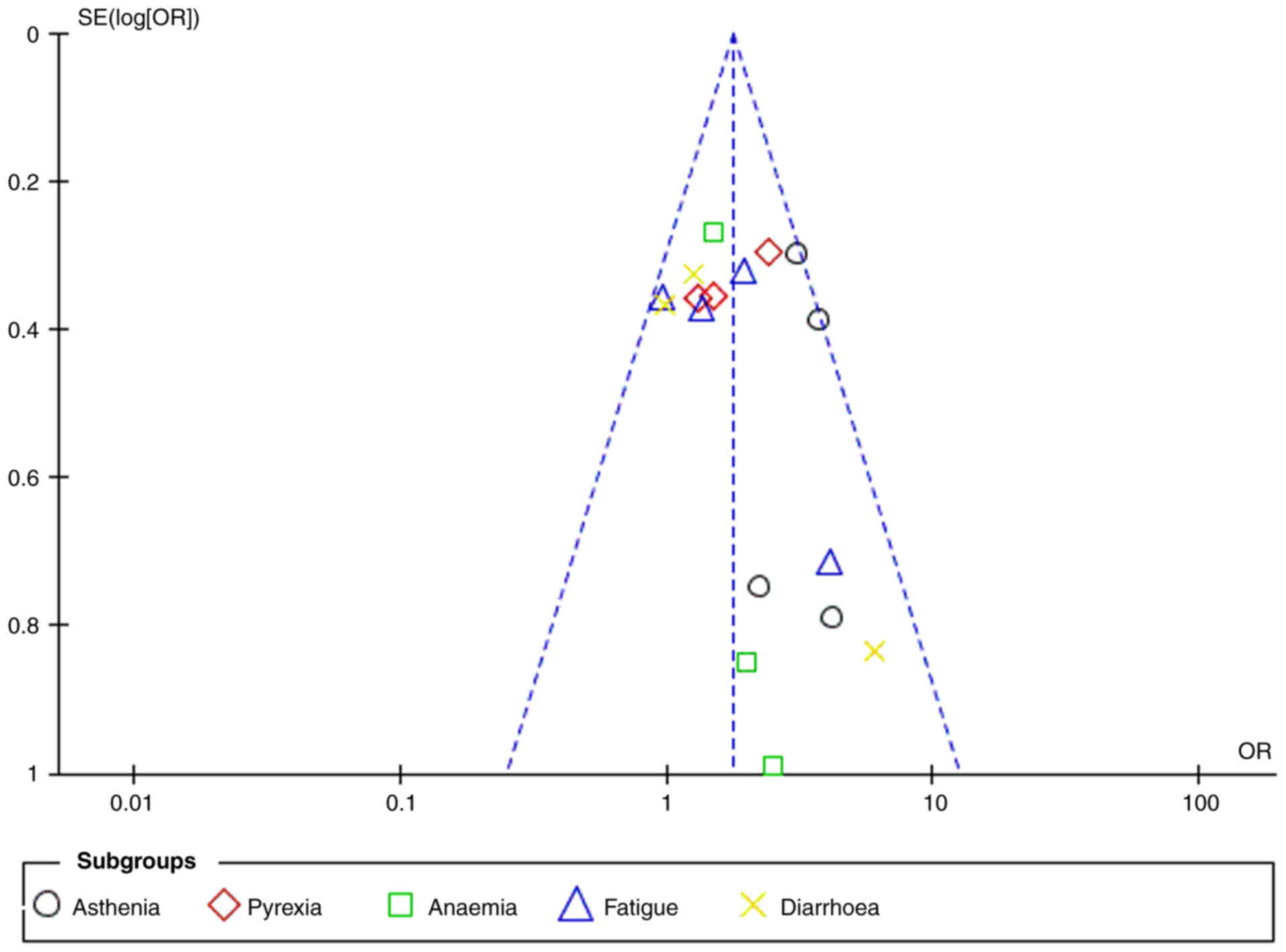
Overall, low heterogeneity was observed throughout
the results, with the I2 values generally indicating
minimal variability across studies. The heterogeneity for asthenia,
pyrexia, anemia, fatigue and diarrhea was 18, 27, 19, 23 and 21%,
respectively. The overall I2 value for all adverse
events was 55%, indicating moderate heterogeneity. The funnel plot
analysis further confirmed the absence of significant publication
bias (Fig. 8). Statistical tests
for heterogeneity showed acceptable values, with the
χ2=12.45 (P=0.09) and Tau2=0.02, indicating
low to moderate variability in the data.
Exploratory analyses: ICIs vs.
Chemotherapy
Comparative efficacy of pembrolizumab vs.
chemotherapy in NMIBC
In the comparative analysis of pembrolizumab and
chemotherapy (gemcitabine, docetaxel, everolimus or valrubicin) for
NMIBC, the treatment outcomes across various parameters were
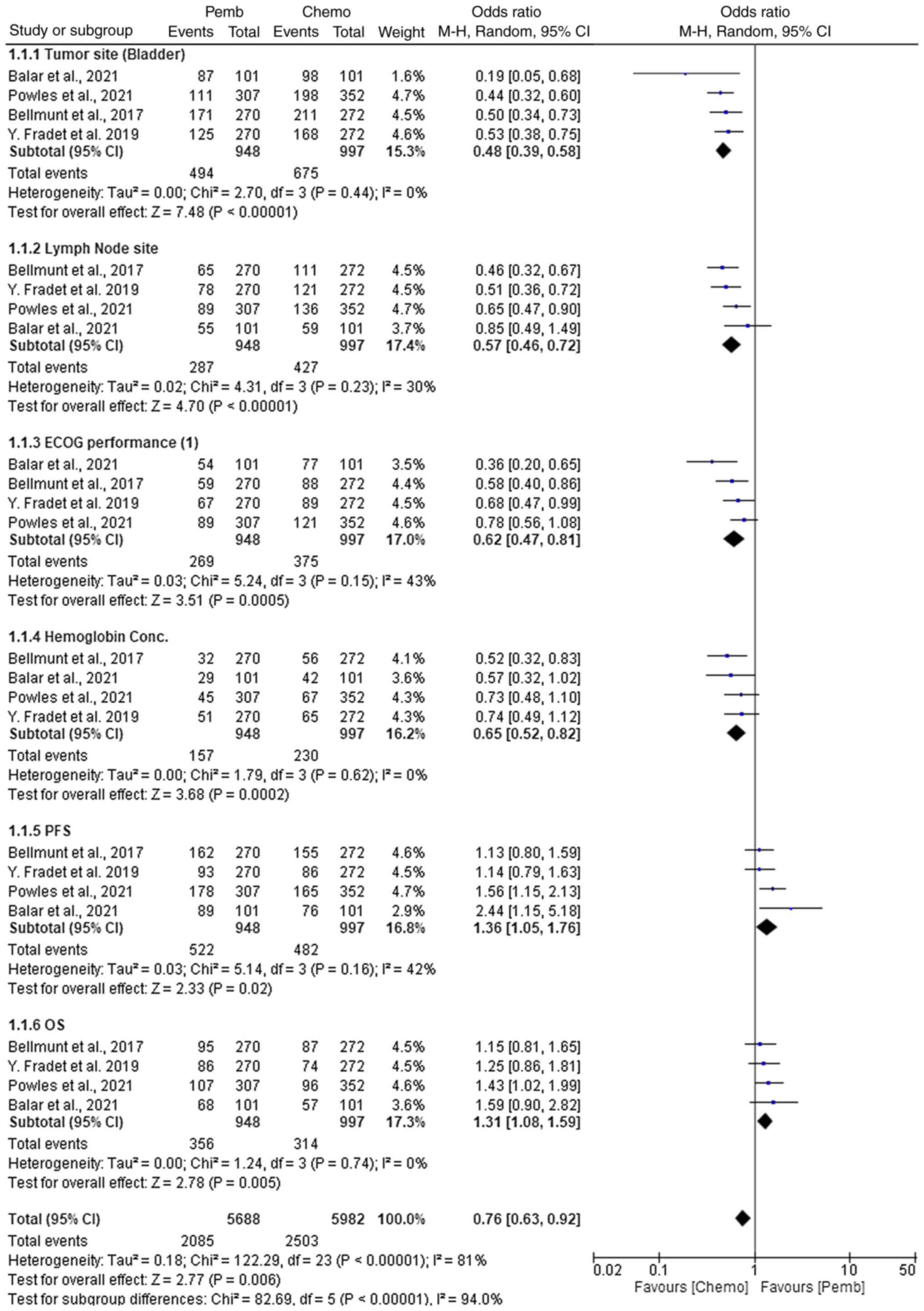
assessed (Fig. 9). For tumor site
(bladder), pembrolizumab demonstrated a statistically significant
advantage in terms of efficacy compared with chemotherapy, with an
OR of 0.48 (95% CI, 0.39–0.58; P<0.00001). This indicated that
pembrolizumab had a higher probability of achieving tumor control
compared with chemotherapy, regardless of the specific chemotherapy
agent used. Similarly, pembrolizumab showed a significant benefit
in lymph node involvement with an OR of 0.57 (95% CI, 0.46–0.72;
P<0.00001), suggesting that pembrolizumab may be more effective
in managing lymph node metastasis. In terms of the Eastern
Cooperative Oncology Group (ECOG) performance status (1), which measures a patient's level of
functioning in terms of their ability to perform daily activities
and their overall physical status, pembrolizumab showed superior
efficacy with an OR of 0.62 (95% CI, 0.47–0.81; P=0.0005), compared
with chemotherapy. Hemoglobin concentration outcomes favored
pembrolizumab with an OR of 0.65 (95% CI, 0.52–0.82; P=0.0002),
indicating a more favorable hematological profile. In terms of PFS,
pembrolizumab also demonstrated a notable advantage with an OR of
1.36 (95% CI, 1.05–1.76; P=0.02), suggesting prolonged disease
control compared with chemotherapy. For OS, pembrolizumab had a
statistically significant OR of 1.31 (95% CI, 1.08–1.59; P=0.005)
compared with chemotherapy. These findings indicate that
pembrolizumab, by enhancing immune-mediated tumor clearance,
offered superior efficacy in terms of survival and disease control,
when compared with chemotherapy, which primarily exerts cytotoxic
effects.
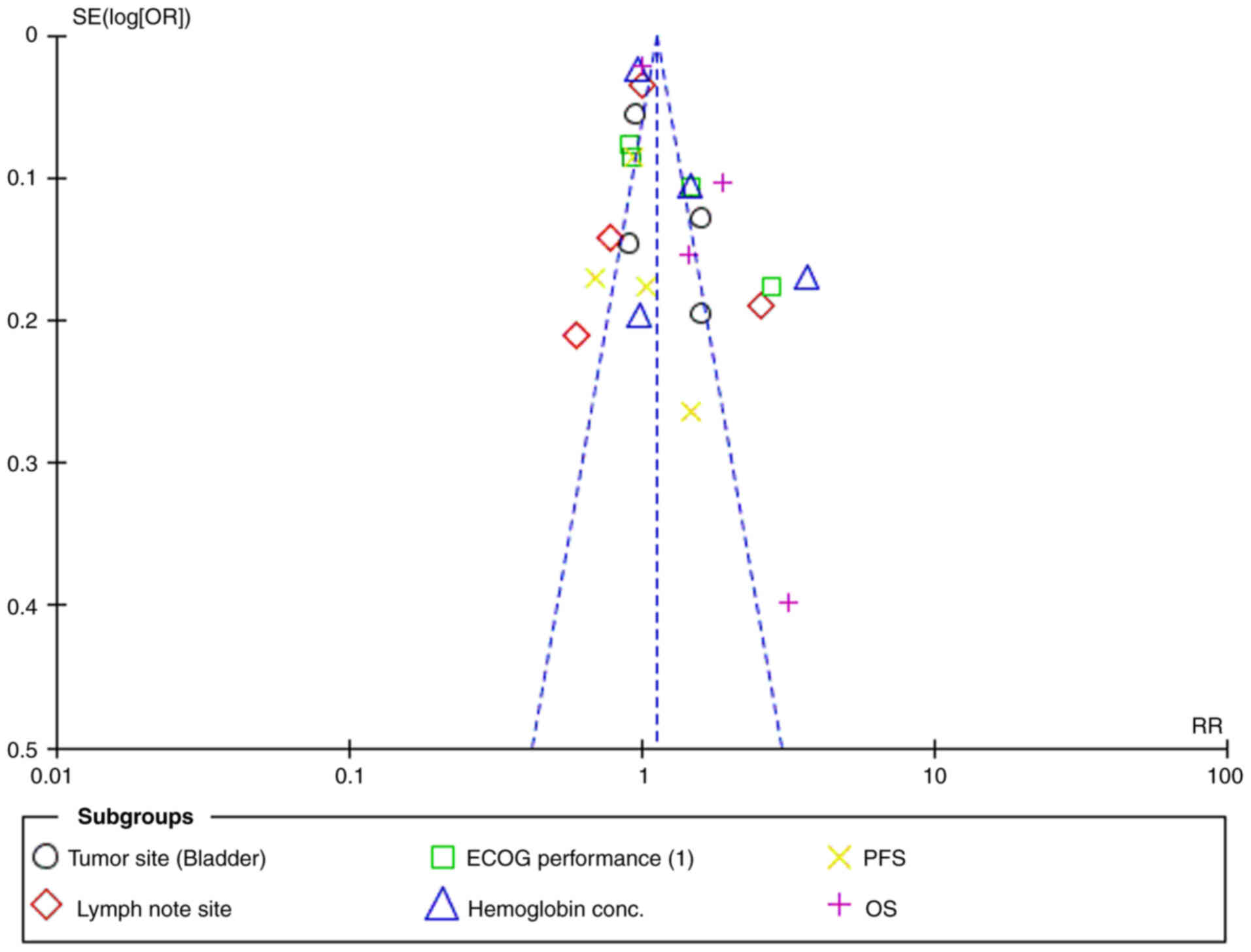
Heterogeneity across the results was assessed using
I2 and Tau2 values. Most subgroups, including
tumor site (bladder), lymph node site, hemoglobin concentration and
ECOG performance, showed low to moderate heterogeneity
(I2 values ranging from 0 to 43%). However, PFS and OS
showed moderate heterogeneity (I2=42–43%). The overall
heterogeneity was high (I2=81%), reflecting variability
across the studies. The funnel plot indicated no substantial
publication bias, suggesting the heterogeneity was due to true
differences between the study populations (Fig. 10).
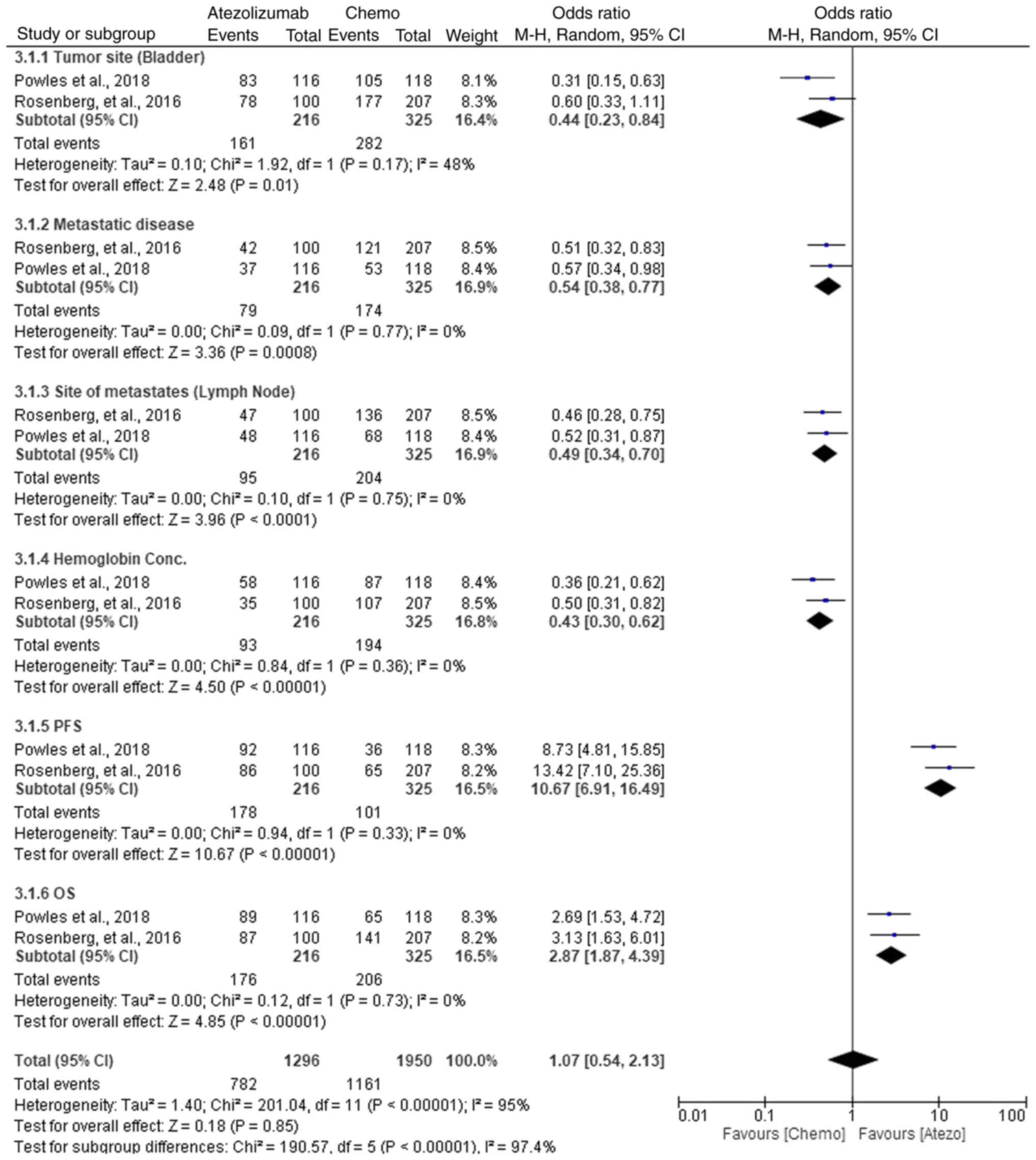
Comparative efficacy of atezolizumab
vs. chemotherapy in NMIBC treatment
In the comparative analysis of atezolizumab versus
chemotherapy for NMIBC treatment, multiple parameters were
evaluated (Fig. 11). For tumor
site (bladder), chemotherapy showed a slight advantage with an OR
of 0.44 (95% CI, 0.23–0.84; P=0.01). In metastatic disease,
atezolizumab was more effective, with an OR of 0.54 (95% CI,
0.38–0.77; P=0.0008), indicating improved control of the disease.
Lymph node metastasis also favored atezolizumab with an OR of 0.49
(95% CI, 0.34–0.70; P<0.0001), demonstrating its superior
efficacy in managing lymph node involvement. For hemoglobin
concentration, chemotherapy was more effective, showing an OR of
0.43 (95% CI, 0.30–0.62; P<0.00001). In terms of PFS,
atezolizumab showed a clear benefit (OR, 10.67; 95% CI, 6.91–16.49;
P<0.00001), while for OS, chemotherapy demonstrated improved
outcomes with an OR of 2.87 (95% CI, 1.87–4.39; P<0.00001).
Despite the high heterogeneity observed across the studies, the
subgroup analyses revealed that atezolizumab had a superior
response in metastatic and lymph node metastasis, while
chemotherapy performed better in bladder tumor control and
hemoglobin concentration. The overall effect, however, showed no
significant difference between the two treatments (OR, 1.07; 95%
CI, 0.54–2.13; P=0.85). These findings suggest that while
chemotherapy may be more effective in specific areas, atezolizumab
offers a better option for metastatic disease and PFS, thus
supporting its use in these subgroups.
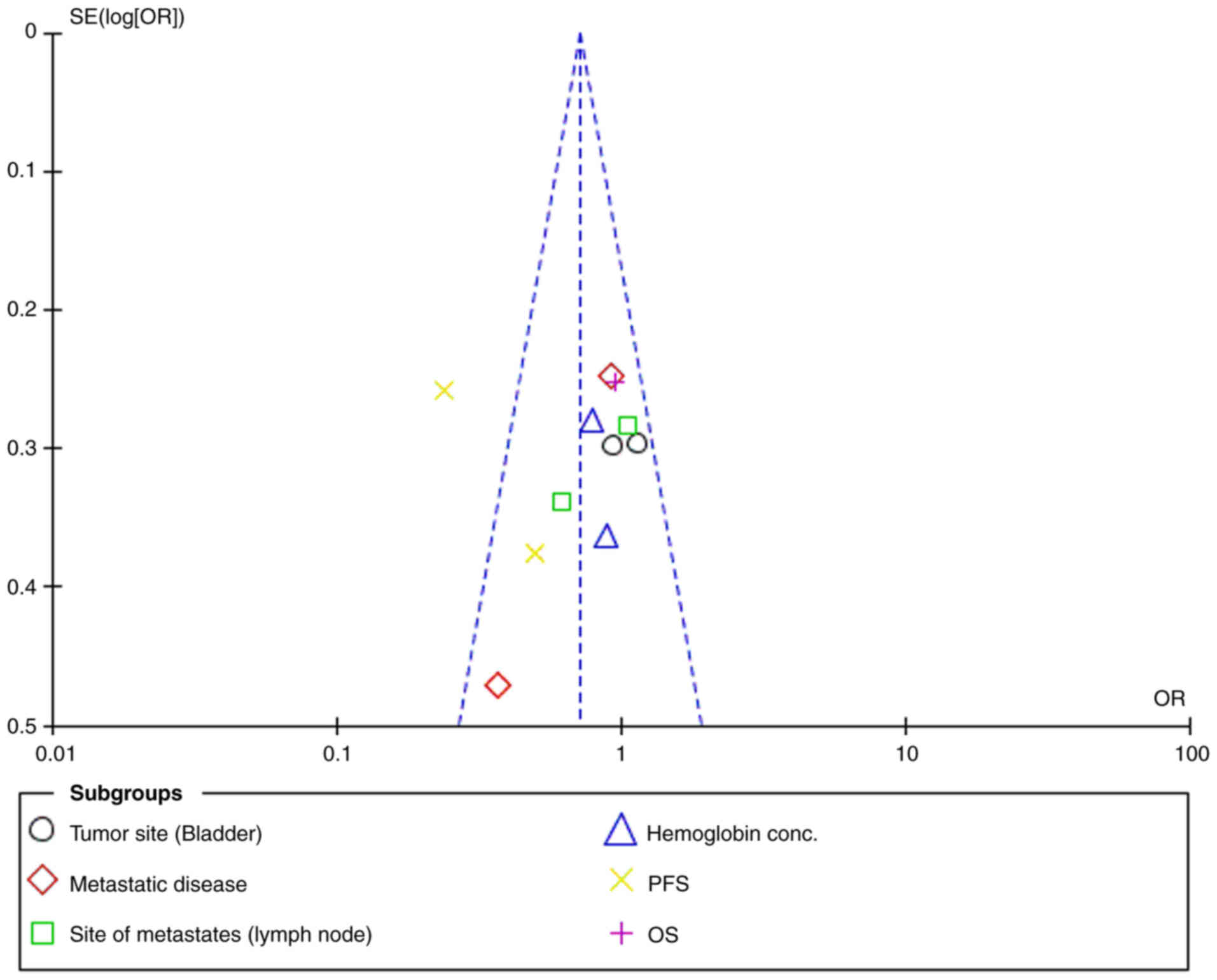
Overall, low heterogeneity was observed across
individual subgroups, with I2 values consistently
<50%, indicating minimal variation between the studies. The
χ2 tests further supported the absence of significant
heterogeneity, with P>0.05 in all comparisons. The funnel plot
showed no significant asymmetry (Fig.
12), reinforcing that the low heterogeneity was not due to
publication bias. The overall heterogeneity across all studies was
I2=95%, indicating high variability when combining all
parameters.
Assessment of publication bias and
risk of bias
In the present meta-analysis, publication bias was
assessed using the Cochrane Risk of Bias 2 tool. Specifically, the
risk of bias was evaluated across several domains: Randomization,
where most studies demonstrated adequate methods (31); deviation from intended
interventions, with some trials showing discrepancies in adherence
to treatment protocols (32);
missing outcome data, where only a small number of studies had
incomplete data, which did not significantly affect the results
(33); measurement of outcomes,
with most trials adequately blinding outcome assessors; and
selection of the reported result (34), which was generally well-reported
across studies (Fig. 1). Despite
the low to moderate heterogeneity, the robustness of the findings
suggests that the results are reliable, with minimal risk of
publication bias affecting the conclusions of the present
meta-analysis.
Discussion
The present systematic review and meta-analysis
aimed to evaluate the efficacy and safety of ICIs compared with BCG
therapy for BCG-refractory NMIBC, alongside exploratory comparisons
with chemotherapy. The findings revealed that pembrolizumab and
atezolizumab, two commonly used ICIs, demonstrated superior
efficacy over chemotherapy and BCG across multiple clinical
parameters, including tumor control, PFS and OS. Specifically,
pembrolizumab showed significant benefits in managing tumor site,
lymph node involvement, ECOG performance status and hematological
outcomes, consistently outperforming BCG in tumor control,
survival, and lymph node involvement. An increase in hemoglobin
levels can be indicative of improved overall health and better
systemic response to treatment (35), suggesting that pembrolizumab may
contribute to enhanced treatment outcomes beyond tumor control,
possibly through its immune-modulating effects. Despite higher
incidences of certain adverse events, such as asthenia and pyrexia,
ICIs were associated with a more favorable safety profile in
managing anemia, fatigue and diarrhea. The overall heterogeneity in
the analysis was moderate (I2=55%), with minimal
variation across most subgroups, indicating consistent findings.
These results suggest that ICIs, particularly pembrolizumab and
atezolizumab, offer superior efficacy and a potentially more
favorable safety profile compared with chemotherapy and BCG,
marking them as promising treatment options for advanced NMIBC.
Pembrolizumab and atezolizumab have been extensively
studied in patients with advanced urothelial carcinoma, with
notable improvements in OS and durable responses observed in
previous trials (36,37). For instance, a randomized phase 3
trial comparing pembrolizumab with standard chemotherapy regimens
demonstrated a superior OS outcome with pembrolizumab, alongside
favorable safety profiles (38).
These findings align closely with the outcomes of the present
meta-analysis, which also revealed significant improvements in OS
with pembrolizumab and atezolizumab compared with chemotherapy in
patients with advanced urothelial carcinoma.
Chemotherapy remains a cornerstone of BC treatment,
albeit with varying efficacy and tolerability profiles among
different agents (39,40). The present meta-analysis identified
modest clinical benefits associated with various chemotherapy
regimens, such as vinflunine, gemcitabine and taxanes, while
highlighting significant toxicity concerns (41). Notably, while the present
meta-analysis did not specifically analyze chemotherapy type and
patient selection, the findings align with existing research
emphasizing the role of these factors in optimizing treatment
outcomes, as observed in a comparative study evaluating different
chemotherapy regimens (42).
BCG therapy has long been established as the
mainstay in the management of NMIBC, although its efficacy may be
limited in patients who are unresponsive or intolerant to treatment
(43). Emerging evidence suggests
that combination therapies, such as pembrolizumab + BCG or
atezolizumab + BCG, hold promise in improving treatment outcomes in
these patients (44). The present
meta-analysis contributes to this growing body of evidence by
demonstrating the safety and potential efficacy of immunotherapy in
advanced BC, particularly in the BCG-unresponsive NMIBC setting.
While effective, intravesical BCG therapy is associated with
several adverse events. Common side effects include cystitis,
hematuria and flu-like symptoms, with severe complications such as
sepsis being rare but notably concerning (45). In comparison, intravesical
chemotherapy agents such as gemcitabine and docetaxel generally
exhibit severe adverse events. For instance, gemcitabine has been
associated with local side effects, such as irritative voiding
symptoms and chemical cystitis, but systemic side effects are
minimal (46,47). Moreover, the toxicity profile of
docetaxel is also manageable, with less severe local toxicity
compared with BCG (47).
Pembrolizumab, an ICI, represents a novel approach for treating
NMIBC, particularly in BCG-unresponsive cases (48). However, its side effects include
immune-related adverse events, such as colitis, hepatitis and
pneumonitis, which can be serious but are typically manageable with
appropriate interventions (49).
Although, pembrolizumab has a different side effect profile, its
use in combination with BCG has been investigated to enhance
therapeutic outcomes while effectively managing adverse effects
(49,50). Furthermore, the findings of the
present study resonate with those of comparative studies exploring
similar combination therapies and treatment modalities. For
instance, studies comparing pembrolizumab + BCG or atezolizumab +
BCG with standard BCG therapy alone have consistently shown
improved outcomes in terms of response rates and adverse event
profiles (51,52). Hence, the comparative analyses in
the present study provide additional support for the efficacy and
safety of combination immunotherapy in BC management, reinforcing
the relevance of the findings of the meta-analysis in the context
of existing literature (53).
The strength of the present meta-analysis lies in
the comprehensive inclusion of relevant studies evaluating the
efficacy and safety of ICIs, BCG therapy and chemotherapy in BC
treatment. By synthesizing data from multiple trials, a robust
overview of the current evidence landscape has been provided,
offering valuable insights into the comparative effectiveness of
these therapeutic modalities. The combination of ICIs with BCG
therapy was not explored in the present study as the analysis
specifically aimed to compare the effectiveness of ICIs against
standard therapies in patients with BCG-refractory NMIBC. The
present study provides critical evidence to guide treatment
decisions, particularly for BCG-unresponsive patients.
Consequently, the findings suggest that ICIs, such as pembrolizumab
and atezolizumab, offer improved efficacy and safety profiles
compared with BCG, marking a promising therapeutic option for
high-risk cases. These insights could influence clinical
guidelines, promote personalized treatment strategies and improve
patient outcomes. Nevertheless, further clinical trials are needed
to optimize these combinations and establish the best treatment
protocols. However, the findings of the present study suggest a
promising shift toward more personalized and potent therapies in
managing BCG-refractory NMIBC.
The limitations of the present study include
potential publication bias, heterogeneity among the included
studies, variations in study design and methodology and the
inability to access individual patient data for more detailed
analyses. Therefore, these factors may have influenced the overall
interpretation of the results and should be considered when
interpreting the findings of the present meta-analysis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 82072809 and 82173221) and the
Joint Funds of the Zhejiang Provincial Natural Science Foundation
of China (grant no. LHDMZ23H160004).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MMA, XM and KMA conceptualized the study and
contributed to the original draft, including the literature search,
data collection and analysis. LD, GL and XM were responsible for
data acquisition and analysis. KMA, LD and MMA interpreted the
results. MA and GL confirm the authenticity of all the raw data. GL
and XM revised and edited the manuscript, while MMA, GL and KMA
provided critical analysis and thorough revisions of the results.
GL supervised the study. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Hoogstraten LM, Vrieling A, van der
Heijden AG, Kogevinas M, Richters A and Kiemeney LA: Global trends
in the epidemiology of bladder cancer: Challenges for public health
and clinical practice. Nat Rev Clin Oncol. 20:287–304. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sylvester RJ, Rodríguez O, Hernández V,
Turturica D, Bauerová L, Bruins HM, Bründl J, van der Kwast TH,
Brisuda A, Rubio-Briones J, et al: European Association of Urology
(EAU) prognostic factor risk groups for non-muscle-invasive bladder
cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973
classification systems for grade: An update from the EAU NMIBC
Guidelines Panel. Eur Urol. 79:480–488. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tse J, Singla N, Ghandour R, Lotan Y and
Margulis V: Current advances in BCG-unresponsive non-muscle
invasive bladder cancer. Expert Opin Investig Drugs. 28:757–770.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ulamec M, Murgić J, Novosel L, Tomić M,
Terlević R, Tomašković I, Jazvić M, Froebe A and Krušlin B: New
insights into the diagnosis, molecular taxonomy, and treatment of
bladder cancer. Acta Med Acad. 50:143–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valenza C, Antonarelli G, Giugliano F,
Aurilio G, Verri E, Briganti A, Curigliano G and Necchi A: Emerging
treatment landscape of non-muscle invasive bladder cancer. Expert
Opin Biol Ther. 22:717–734. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanguedolce F, Calo B, Mancini V, Zanelli
M, Palicelli A, Zizzo M, Ascani S, Carrieri G and Cormio L:
Non-muscle invasive bladder cancer with variant histology:
Biological features and clinical implications. Oncology.
99:345–358. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babjuk M, Burger M, Compérat EM, Gontero
P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF,
Sylvester R, et al: European association of urology guidelines on
non-muscle-invasive bladder cancer (TaT1 and carcinoma in
situ)-2019 update. Eur Urol. 76:639–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Witjes JA, Bruins HM, Cathomas R, Compérat
EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A,
Neuzillet Y, et al: European association of urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2020
guidelines. Eur Urol. 79:82–104. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JH and Oh JJ: The emerging treatment
of BCG (Bacillus Calmette-Guérin)-unresponsive non-muscle-invasive
bladder cancer. J Urol Oncol. 22:246–255. 2024. View Article : Google Scholar
|
|
10
|
Morales A: BCG: A throwback from the stone
age of vaccines opened the path for bladder cancer immunotherapy.
Can J Urol. 24:8788–8793. 2017.PubMed/NCBI
|
|
11
|
Lerner SP, Tangen CM, Sucharew H, Wood D
and Crawford ED: Failure to achieve a complete response to
induction BCG therapy is associated with increased risk of disease
worsening and death in patients with high risk non-muscle invasive
bladder cancer. Urol Oncol. 27:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steinberg RL, Thomas LJ, Mott SL and
O'Donnell MA: Bacillus Calmette-Guérin (BCG) treatment failures
with non-muscle invasive bladder cancer: A data-driven definition
for BCG unresponsive disease. Bladder Cancer. 2:215–224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deininger S, Törzsök P, Mitterberger M,
Pallauf M, Oswald D, Deininger C and Lusuardi L: From interferon to
checkpoint inhibition therapy-A systematic review of new
immune-modulating agents in Bacillus Calmette-Guérin (BCG)
refractory non-muscle-invasive bladder cancer (NMIBC). Cancers.
14:6942022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Jong FC, Rutten VC, Zuiverloon TC and
Theodorescu D: Improving anti-PD-1/PD-L1 therapy for localized
bladder cancer. Int J Mol Sci. 22:28002021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del Giudice F, Asero V, Bologna E,
Scornajenghi CM, Carino D, Dolci V, Viscuso P, Salciccia S, Sciarra
A, D'Andrea D, et al: Efficacy of different bacillus of
Calmette-Guérin (BCG) strains on recurrence rates among
intermediate/high-risk non-muscle invasive bladder cancers
(NMIBCs): Single-arm study systematic review, cumulative and
network meta-analysis. Cancers. 15:19372023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ibrahim OM: PGE2 Blockade Enhances the
Magnitude and Selectivity of the BCG-Induced Immune Response in the
Human Bladder Cancer Microenvironment. State University of New York
at Buffalo; 2020
|
|
17
|
Lobo N, Bree KK, Hensley PJ,
Nogueras-Gonzalez GM, Abraham P, Navai N, Dinney CP and Kamat AM:
Reduced-dose bacillus Calmette-Guérin (BCG) in an era of BCG
shortage: Real-world experience from a tertiary cancer centre. BJU
Int. 130:323–330. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamat AM, Flaig TW, Grossman HB, Konety B,
Lamm D, O'donnell MA, Uchio E, Efstathiou JA and Taylor JA III:
Expert consensus document: Consensus statement on best practice
management regarding the use of intravesical immunotherapy with BCG
for bladder cancer. Nat Rev Urol. 12:225–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Youssef SF and Buanz AB:
Intravesical combination therapies for non-muscle invasive bladder
cancer: Recent advances and future directions. Eur J Pharmacol.
926:1750242022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wołącewicz M, Hrynkiewicz R, Grywalska E,
Suchojad T, Leksowski T, Roliński J and Niedźwiedzka-Rystwej P:
Immunotherapy in bladder cancer: Current methods and future
perspectives. Cancers. 12:11812020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abd El-Salam MA, Smith CE and Pan CX:
Insights on recent innovations in bladder cancer immunotherapy.
Cancer Cytopathol. 130:667–683. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mancini M, Righetto M and Noessner E:
Checkpoint inhibition in bladder cancer: Clinical expectations,
current evidence, and proposal of future strategies based on a
tumor-specific immunobiological approach. Cancers. 13:60162021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tyagi P, Hafron J, Kaufman J and
Chancellor M: Enhancing therapeutic efficacy and safety of immune
checkpoint inhibition for bladder cancer: A comparative analysis of
injectable vs. Intravesical administration. Int J Mol Sci.
25:49452024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roviello G, Catalano M, Santi R, Palmieri
VE, Vannini G, Galli IC, Buttitta E, Villari D, Rossi V and Nesi G:
Immune checkpoint inhibitors in urothelial bladder cancer: State of
the art and future perspectives. Cancers. 13:44112021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Álvarez-Maestro M, Guerrero-Ramos F,
Rodríguez-Faba O, Domínguez-Escrig J and Fernández-Gómez J: Current
treatments for BCG failure in non-muscle invasive bladder cancer
(NMIBC). Actas Urol Esp (Engl Ed). 45:93–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee CU, Song W, Kang M, Sung HH, Jeon HG,
Seo SI, Jeon SS, Park SH and Jeong BC: Early experience with
pembrolizumab in bacillus Calmette-Guérin unresponsive
non-muscle-invasive bladder cancer. Korean J Urol Oncol.
21:241–248. 2023. View Article : Google Scholar
|
|
27
|
Ruiz-Lorente I, Gimeno L, López-Abad A,
López Cubillana P, Fernández Aparicio T, Asensio Egea LJ, Moreno
Avilés J, Doñate Iñiguez G, Guzmán Martínez-Valls PL, Server G, et
al: Exploring the immunoresponse in bladder cancer immunotherapy.
Cells. 13:19372024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song SH and Oh JJ: The evolving role of
checkpoint inhibitors in the treatment of urothelial carcinoma: A
literature review of practice-changing trials. J Urol Oncol.
21:154–164. 2023. View Article : Google Scholar
|
|
29
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366:l48982019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schulz KF, Altman DG and Moher D: CONSORT
2010 Statement: Updated guidelines for reporting parallel group
randomised trials. BMJ. 340:c3322010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hernán MA and Robins JM: Per-protocol
analyses of pragmatic trials. N Engl J Med. 377:1391–1398. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bell ML, Fiero M, Horton NJ and Hsu CH:
Handling missing data in RCTs; a review of the top medical
journals. BMC Med Res Methodol. 14:1182014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dwan K, Williamson PR, Gamble C, Higgins
J, Sterne J, Altman DG, Clarke M and Kirkham JJ: Guidance to
detect, evaluate and prevent the problem of selective reporting in
trial publications. Trials. 14 (Suppl 1):O912013. View Article : Google Scholar
|
|
35
|
He Y, Ren T, Ji C, Zhao L and Wang X: The
baseline hemoglobin level is a positive biomarker for immunotherapy
response and can improve the predictability of tumor mutation
burden for immunotherapy response in cancer. Front Pharmacol.
15:14568332024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galsky MD, Mortazavi A, Milowsky MI,
George S, Gupta S, Fleming MT, Dang LH, Geynisman DM, Walling R,
Alter RS, et al: Randomized double-blind phase II study of
maintenance pembrolizumab versus placebo after first-line
chemotherapy in patients with metastatic urothelial cancer. J Clin
Oncol. 38:1797–1806. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rose TL, Harrison MR, Deal AM, Ramalingam
S, Whang YE, Brower B, Dunn M, Osterman CK, Heiling HM, Bjurlin MA,
et al: Phase II study of gemcitabine and split-dose cisplatin plus
pembrolizumab as neoadjuvant therapy before radical cystectomy in
patients with muscle-invasive bladder cancer. J Clin Oncol.
39:3140–3148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang T, Tan A, Shah AY, Iyer G, Morris V,
Michaud S and Sridhar SS: Reevaluating the role of platinum-based
chemotherapy in the evolving treatment landscape for patients with
advanced urothelial carcinoma. Oncologist. 29:1003–1013. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martini A, Raggi D, Fallara G, Nocera L,
Schultz JG, Belladelli F, Marandino L, Salonia A, Briganti A,
Montorsi F, et al: Immunotherapy versus chemotherapy as first-line
treatment for advanced urothelial cancer: A systematic review and
meta-analysis. Cancer Treat Rev. 104:1023602022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szabados B, Kockx M, Assaf ZJ, van Dam PJ,
Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, Pous AF,
Gravis G, et al: Final results of neoadjuvant atezolizumab in
cisplatin-ineligible patients with muscle-invasive urothelial
cancer of the bladder. Eur Urol. 82:212–222. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holmsten K: Improving Chemotherapy in
Advanced Urothelial Cancer: Real-world Data Studies and Prospective
Clinical Trials. Karolinska Institutet; Stockholm: 2020, PubMed/NCBI
|
|
42
|
Huang Y, Liao C, Shen Z, Zou Y, Xie W, Gan
Q, Yao Y, Zheng J and Kong J: A bibliometric insight into
neoadjuvant chemotherapy in bladder cancer: Trends, collaborations,
and future avenues. Front Immunol. 15:12975422024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lidagoster S, Ben-David R, De Leon B and
Sfakianos JP: BCG and alternative therapies to BCG therapy for
non-muscle-invasive bladder cancer. Curr Oncol. 31:1063–1078. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Michaud E, Mansure JJ and Kassouf W:
Integrating novel immunotherapeutic approaches in organ-preserving
therapies for bladder cancer. Br J Pharmacol. Dec 13–2023.(Epub
ahead of print). doi: 10.1111/bph.16300. PubMed/NCBI
|
|
45
|
Peng M, Xiao D, Bu Y, Long J and Yang X,
Lv S and Yang X: Novel combination therapies for the treatment of
bladder cancer. Front Oncol. 10:5395272021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Steinberg RL, Thomas LJ, O'Donnell MA and
Nepple KG: Sequential intravesical gemcitabine and docetaxel for
the salvage treatment of non-muscle invasive bladder cancer.
Bladder Cancer. 1:65–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Milbar N, Kates M, Chappidi MR, Pederzoli
F, Yoshida T, Sankin A, Pierorazio PM, Schoenberg MP and Bivalacqua
TJ: Oncological outcomes of sequential intravesical gemcitabine and
docetaxel in patients with non-muscle invasive bladder cancer.
Bladder Cancer. 3:293–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hannouneh ZA, Hijazi A, Alsaleem AA, Hami
S, Kheyrbek N, Tanous F, Khaddour K, Abbas A and Alshehabi Z: Novel
immunotherapeutic options for BCG-unresponsive high-risk
non-muscle-invasive bladder cancer. Cancer Med. 12:21944–21968.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Claps F, Pavan N, Ongaro L, Tierno D,
Grassi G, Trombetta C, Tulone G, Simonato A, Bartoletti R, Mertens
LS, et al: BCG-unresponsive non-muscle-invasive bladder cancer:
Current treatment landscape and novel emerging molecular targets.
Int J Mol Sci. 24:125962023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shore ND, Redorta JP, Robert G, Hutson TE,
Cesari R, Hariharan S, Faba OR, Briganti A and Steinberg GD:
Non-muscle-invasive bladder cancer: An overview of potential new
treatment options. Urol Oncol. 39:642–663. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li R, Shah PH, Stewart TF, Nam JK,
Bivalacqua TJ, Lamm DL, Uchio EM, Geynisman DM, Jacob JM, Meeks JJ,
et al: Oncolytic adenoviral therapy plus pembrolizumab in
BCG-unresponsive non-muscle-invasive bladder cancer: The phase 2
CORE-001 trial. Nat Med. 30:2216–2223. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Necchi A, Roumiguié M, Kamat AM, Shore ND,
Boormans JL, Esen AA, Lebret T, Kandori S, Bajorin DF, Krieger LEM,
et al: Pembrolizumab monotherapy for high-risk non-muscle-invasive
bladder cancer without carcinoma in situ and unresponsive to BCG
(KEYNOTE-057): A single-arm, multicentre, phase 2 trial. Lancet
Oncol. 25:720–730. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aurilio G, Cimadamore A, Lopez-Beltran A,
Scarpelli M, Massari F, Verri E, Cheng L, Santoni M and Montironi
R: Narrative review: Update on immunotherapy and pathological
features in patients with bladder cancer. Transl Androl Urol.
10:15212021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Powles T, Durán I, Van der Heijden MS,
Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano
D, Bamias A, et al: Atezolizumab versus chemotherapy in patients
with platinum-treated locally advanced or metastatic urothelial
carcinoma (IMvigor211): A multicentre, open-label, phase 3
randomised controlled trial. Lancet. 391:748–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rosenberg JE, Hoffman-Censits J, Powles T,
Van Der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bellmunt J, De Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fradet Y, Bellmunt J, Vaughn D, Lee J,
Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi
A, et al: Randomized phase III KEYNOTE-045 trial of pembrolizumab
versus paclitaxel, docetaxel, or vinflunine in recurrent advanced
urothelial cancer: Results of> 2 years of follow-up. Ann Oncol.
30:970–976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Balar AV, Kamat AM, Kulkarni GS, Uchio EM,
Boormans JL, Roumiguié M, Krieger LEM, Singer EA, Bajorin DF,
Grivas P, et al: Pembrolizumab monotherapy for the treatment of
high-risk non-muscle-invasive bladder cancer unresponsive to BCG
(KEYNOTE-057): An open-label, single-arm, multicentre, phase 2
study. Lancet Oncol. 22:919–930. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Powles T, Csőszi T, Özgüroğlu M, Matsubara
N, Géczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales
Barrera R, et al: Pembrolizumab alone or combined with chemotherapy
versus chemotherapy as first-line therapy for advanced urothelial
carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial.
Lancet Oncol. 22:931–945. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jamil ML, Deebajah M, Sood A, Robinson K,
Rao K, Sana S and Alanee S: Protocol for phase I study of
pembrolizumab in combination with Bacillus Calmette-Guérin for
patients with high-risk non-muscle invasive bladder cancer. BMJ
Open. 9:e0282872019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Alanee S, Sana S, El-Zawahry A, Peabody J,
Pearce T, Adams N, Deebajah M, Crabtree J, Delfino K, McVary K, et
al: Phase I trial of intravesical Bacillus Calmette-Guérin combined
with intravenous pembrolizumab in recurrent or persistent
high-grade non-muscle-invasive bladder cancer after previous
Bacillus Calmette-Guérin treatment. World J Urol. 39:3807–3813.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Inman BA, Hahn NM, Stratton K, Kopp R,
Sankin A, Skinner E, Pohar K, Gartrell BA, Pham S, Rishipathak D,
et al: A phase 1b/2 study of atezolizumab with or without bacille
Calmette-Guérin in patients with high-risk non-muscle-invasive
bladder cancer. Eur Urol Oncol. 6:313–320. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Black PC, Tangen CM, Singh P, McConkey DJ,
Lucia MS, Lowrance WT, Koshkin VS, Stratton KL, Bivalacqua TJ,
Kassouf W, et al: Phase 2 trial of atezolizumab in bacillus
Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder
cancer: SWOG S1605. Eur Urol. 84:536–544. 2023. View Article : Google Scholar : PubMed/NCBI
|