Introduction
Colorectal cancer (CRC) is one of the most common
and lethal malignancies worldwide (1). Despite advancements in early detection
and treatment modalities, including surgery, chemotherapy and
immunotherapy, the prognosis for patients with advanced-stage CRC
remains poor (2,3). Conventional prognostic factors, such
as tumor stage and histopathological features, often fail to fully
capture the complexity of the disease, underscoring the urgent need
for novel biomarkers that can enhance risk stratification and
inform therapeutic decisions (4,5). In
previous years, the integration of molecular profiling and
personalized treatment strategies has shown promise; however,
patient responses to immune checkpoint inhibitors and targeted
therapies remain highly variable (6), emphasizing the necessity for
biomarkers that predict therapeutic efficacy.
Palmitoylation, a dynamic post-translational
modification involving the reversible attachment of fatty acid
chains to cysteine residues of proteins, serves a critical role in
regulating protein function, membrane localization and cellular
signaling (7,8). This modification is essential for
several cellular processes, including signal transduction, protein
trafficking and cellular adhesion. Emerging evidence has suggested
that altered palmitoylation contributes to the pathogenesis of
several types of cancer, including CRC, by modulating key oncogenic
signaling pathways, enhancing cell survival and promoting immune
evasion (9,10). Palmitoylation of ERb can activate
17β-estradiol, thereby activating the p38/MAPK pathway to promote
apoptosis of human colon adenocarcinoma DLD-1 cells (11). However, it has also been observed
that the palmitoylation of ERb or inhibition of p38/MAPK signaling
promotes the proliferation of CRC cells (12). Therefore, palmitoylation serves a
notable biological role in CRC. In addition, several studies have
demonstrated that Wnt2B palmitoylation alters its cellular
localization, indirectly affecting Wnt signaling (13), and the level of Wnt2B palmitoylation
in mitochondria is negatively associated with the occurrence of
intestinal tumors (14). The
palmitoylation of YES in the SH4 domain regulates its localization
in the cholesterol-rich membrane microstructure domain, which
enhances the phosphorylation of upstream regulatory factors EGFR,
SHC and SHP2 in Ras/MAPK signaling, thereby inhibiting colon cancer
cell adhesion and promoting invasion (15). Therefore, palmitoylation can
regulate the activation of p38/MAPK, Wnt and Ras/MAPK signaling
pathways to control the proliferation and metastasis of CRC.
The ZDHHC family, a key enzyme involved in
regulating palmitoylation, also serves a notable role in the
progression of CRC. ZDHHC20 is one of the key ZDHHC enzymes located
on the plasma membrane. Draper and Smith (16) demonstrated that the mRNA levels of
ZDHHC20 in ovarian cancer, breast cancer, colon cancer, kidney
cancer and prostate cancer were markedly higher compared with those
in organ-matched normal tissues. In addition, Fas (also known as
CD95) is palmitoylated by ZDHHC7 at Cys199 to increase its
stability and lipid raft localization; notably, inhibition of Fas
palmitoylation by small interfering RNA knockout of ZDHHC7 can
promote CRC cell lines to escape FasL-induced cell death (17). However, the precise role of
palmitoylation in CRC progression and its potential as a prognostic
marker have yet to be comprehensively explored. With the advent of
bioinformatics approaches, the integration of multi-omics data has
facilitated the identification of molecular signatures that can
predict clinical outcomes and therapeutic responses, offering novel
options for personalized medicine (18).
The present study aimed to establish a
palmitoylation-related prognostic signature for CRC by performing a
systematic analysis of transcriptomics, clinical and genomics data.
Our findings offer valuable insights into the role of
palmitoylation-related genes in CRC and identify potential
biomarkers for prognosis and immunotherapy strategies.
Materials and methods
Data acquisition
Transcriptome data, survival information, somatic
mutation data and copy number variation (CNV) data for patients
with CRC were retrieved from The Cancer Genome Atlas (TCGA)
database (https://portal.gdc.cancer.gov/). After excluding
patients with a survival time of <30 days, a total of 418
patients were included in the subsequent analyses, and 41 healthy
individuals served as negative controls. The GSE17538 dataset, used
as an external validation cohort, was obtained from the Gene
Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/). Additionally, a
list of 3,532 palmitoylation-related genes was extracted from the
GeneCards database (https://www.genecards.org/).
Identification of a
palmitoylation-related risk signature
To identify prognostic genes, univariate Cox
regression analysis was first performed, with a significance
threshold of P<0.001. To enhance the selection process, Least
Absolute Shrinkage and Selection Operator (LASSO) regression was
performed using the glmnet package (https://cran.r-project.org/web/packages/glmnet/index.html)
in R studio 4.3.1 version software (https://cran.rstudio.com/). LASSO regression analysis
was performed employing 10-fold cross-validation to determine the
optimal regularization parameter (λ). The λ value selected was the
one that maximized model fit while simultaneously minimizing the
risk of overfitting. Samples with missing or insufficient clinical
data were excluded during univariate Cox analysis, ensuring the
accuracy of LASSO regression analysis, which was applied to select
the most relevant prognostic genes from the initial set of
identified candidates. Following this, a multivariate Cox
regression analysis was performed to establish a
palmitoylation-related prognostic model. In addition, the
differences in the expression profiles of the six model genes
between normal and CRC tissues were assessed and were depicted in a
heatmap, which was generated using the pheatmap package (https://cran.r-project.org/web/packages/pheatmap/index.html)
in R studio 4.3.1 version software (19). Patients in both the training and
validation cohorts were stratified into high- and low-risk groups
based on the median risk score derived from the model. The receiver
operating characteristic (ROC) curve was generated to evaluate the
prognostic performance of the model, with the area under the curve
(AUC) used to assess sensitivity and specificity. Kaplan-Meier (KM)
OS analysis was subsequently performed to assess the association
between the palmitoylation-related risk signature and OS. The OS of
patients was evaluated by KM analysis using the survival package
(https://cran.r-project.org/web/packages/survival/index.html)
of R studio 4.3.1 version software (19); log-rank test was used to obtain
P-values. Finally, the prognostic impact of the expression levels
of individual model genes was evaluated to understand their
contribution to patient survival outcomes.
Assessment of the prognostic
model
Firstly, the expression levels of the model genes
were compared between CRC tissues and adjacent normal tissues to
identify potential alterations associated with CRC. Subsequently,
the correlation between the expression levels of each model gene
and the risk score was assessed to understand their contributions
to the risk stratification and their prognostic relevance in
patients with CRC with Pearson correlation analysis. A nomogram was
constructed using the ‘rms’ package (https://cran.r-project.org/web/packages/rms/index.html)
in R studio 4.3.1 version software incorporating clinical features,
such as tumor (T) stage, lymph node (N) stage, clinical stage, age,
sex and risk score, to predict individualized prognosis.
Differences in clinical characteristics, including survival status
and tumor stage [T, N and metastasis (M) stages], were then
compared between the high- and low-risk groups to evaluate their
potential impact on prognosis. Finally, the prognostic accuracy of
the model was validated using the independent GSE17538 validation
dataset, and survival outcomes for patients in the high- and
low-risk groups were compared to assess the robustness of the risk
signature.
Functional enrichment and TMB
analyses
To explore the molecular mechanisms underlying the
palmitoylation-related risk signature, Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analyses were performed using the ClusterProfiler package
(https://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html)
(20). These analyses aimed to
identify key biological processes and signaling pathways associated
with the signature. Somatic mutation data for patients with CRC
were converted to Mutation Annotation Format using the maftools R
package for the subsequent TMB analysis (21). Mutational landscapes were
subsequently compared between the high- and low-risk groups to
assess potential differences in mutation profiles. Additionally,
the TMB was calculated for each sample, and the difference in
prognosis between the high-TMB and low-TMB groups was examined to
investigate the potential impact of TMB on the prognostic model
(22).
Identification of genomic alterations
through CNV analysis
CNV was assessed using the Genomic Identification of
Significant Targets in Cancer (GISTIC) algorithm (23). This tool identifies genomic regions
with recurrent amplifications or deletions across a set of samples,
assigning statistical significance to these alterations. The GISTIC
analysis aimed to uncover potential oncogenes or tumor suppressor
genes associated with CRC. The results of the CNV analysis were
visualized through copy number plots, highlighting the genomic
regions with significant gains or losses and their potential
relevance to cancer development.
Immune cell infiltration analysis
Immune cell infiltration and immune function scores
were assessed using the single-sample Gene Set Enrichment Analysis
algorithm (24), which calculates
rank scores for each gene based on gene expression profiles.
Differences in immune cell infiltration and immune function scores
between the high- and low-risk groups were then compared. A
comparative analysis of the differential expression of 50 immune
checkpoint genes between the two groups was also performed using
the CIBERSORT algorithm (https://github.com/Moonerss/CIBERSORT) (25).
Immunotherapy and drug sensitivity
analysis
Due to the critical role of immune checkpoint
inhibitors in CRC treatment, the correlation between the risk score
and the expression levels of programmed cell death protein 1
(PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T-lymphocyte
associated protein 4 (CTLA4) and T cell immunoreceptor with Ig and
ITIM domains (TIGIT) was examined. The Tumor Immune Dysfunction and
Exclusion (TIDE) algorithm (26)
was used to predict the response of high- and low-risk groups to
immune checkpoint inhibitors. TIDE analyzed gene expression data to
assess tumor immune evasion mechanisms, calculate immune evasion
scores and predict the likelihood of response to immune therapy for
each group (27). Subsequently,
SubMap analysis (http://cloud.genepattern.org/gp) was performed to
further evaluate the immune therapy responsiveness in the high- and
low-risk groups, identifying potential differences in treatment
outcomes (28). Finally, drug
sensitivity was assessed using the pRRophetic package (29), which calculated the half-maximal
inhibitory concentration values for various agents from the
Genomics of Drug Sensitivity in Cancer (GDSC) database (http://www.cancerrxgene.org), comparing sensitivity
between the high- and low-risk patient groups.
Cell culture and transduction
HCT116 (cat. no. CCL-247) and HT29 (cat. no. HTB-38)
cell lines were purchased from American Type Culture Collection.
Cells were cultured in 90% Dulbecco's Modified Eagle Medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Shanghai Yeasen Biotechnology Co., Ltd.),
and were maintained in a humidified incubator at 37°C with 5%
CO2. Cells were passaged twice, resulting in the use of
third-generation cells for subsequent experiments. A
lentivirus-based short hairpin RNA (shRNA) approach was employed
using three distinct shRNA sequences. These shRNA sequences were
cloned into the pLKO.1 lentiviral vector with the aim of
downregulating the expression of KRT8P12 in HCT116 cells. In
addition, full-length KRT8P12 cDNA was synthesized and cloned into
the pLV-CMV-MCS-PGK-Puro lentiviral overexpression vector for the
purpose of inducing KRT8P12 overexpression in HT29 cells. Both
pLKO.1 and pLV-CMV-MCS-PGK-Puro vectors were procured from Beijing
Zhongyuan, Ltd. Lentiviral particles were produced by
co-transfecting 293T cells with a packaging plasmid mix
(REV:VSVG:PMDL, 2:3:5) in 400 µl serum-free DMEM, 1.5 µg core
plasmid and 1.5 µg viral packaging plasmid with 6 µl TurboFect
(TurboFect:plasmid, 2:1; cat. no. R0532; Fermentas; Thermo Fisher
Scientific, Inc.) at 37°C. The reagent was mixed thoroughly and let
sit for 20 min before being added to the cells. Then the cells were
harvested 48 h post-transfection. The resultant virus-containing
supernatant was collected and filtered. This supernatant was
subsequently utilized to infect HCT116 and HT29 cells. Briefly, the
HCT116 and HT29 cells were seeded into 6-well plates and cultured
until they reached 60–70% confluence, after which, lentiviral
particles (multiplicity of infection=10) along with poly-L-lysine
(8 µg/ml) were added to enhance viral transduction efficacy. The
cells were co-cultured with the virus for 12 h at 37°C, after which
they were replaced with fresh culture medium. A total of 48 h
post-infection, stable transductants were selected; puromycin was
the antibiotic used to confirm successful transduction. The
original concentration of puromycin used was 1 mg/ml and 2 µg/ml
puromycin was used for selection and maintenance. All culture
processes were conducted under conditions of 37°C and 5%
CO2. The sequences for KRT8P12 knockdown and
overexpression are shown in Table
SI. HCT116, HT29, sh-KRT8P12 and OE-KRT8P12 cells were cultured
in DMEM, supplemented with 10% FBS and 1% penicillin-streptomycin.
Cells without any treatment served as a blank control group,
whereas a pLKO.1 vector containing a nontargeting shRNA and an
empty pLV-CMV-MCS-PGK-Puro vector served as negative controls for
knockdown and overexpression, respectively. Cells were maintained
at 37°C in a 5% CO2 incubator, with medium changes every
2–3 days. For passaging, cells were detached using 0.25%
trypsin-EDTA, centrifuged at 80 × g for 3 min at room temperature
and reseeded at a 1:3 to 1:6 dilution. When freezing, cells were
suspended in a cryoprotectant medium (10% DMSO, 20% FBS and 70%
DMEM) and stored at −80°C or in liquid nitrogen for long-term
preservation.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from cell samples utilizing
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and was subsequently converted into cDNA employing a cDNA
synthesis kit (Vazyme Biotech Co., Ltd.) according to the
manufacturer's protocol. qPCR was conducted using SYBR Green
qRT-PCR Master Mix (Vazyme Biotech Co., Ltd.), with GAPDH serving
as the internal control for normalization. qPCR was performed as
follows: Pre-denaturation for 2 min at 95°C, followed by 40 cycles
of denaturation for 20 sec at 94°C, annealing for 20 sec at 60°C
and extension for 20 sec at 72°C. The 2−ΔΔCq method was
used for quantification (30). The
primer sequences used are listed in Table SII.
Cell Counting Kit-8 (CCK-8) cell
proliferation assay
To assess cell proliferation, the CCK-8 assay
(Beijing Solarbio Science & Technology Co., Ltd.) was performed
according to the manufacturer's instructions. CRC cells were seeded
in 96-well plates at a density of 1×104 cells/well and
allowed to adhere overnight. 2-Bromopalmitate (2-BP) is a
broad-spectrum inhibitor of protein palmitoylation and was
purchased from MilliporeSigma. Before CCK-8 assay, the cells
underwent different experimental conditions (sh-KRT8P12 or
OE-KRT8P12 transduction, and 2-BP treatment). For 2-BP treatment,
cells were treated with 50 µM 2-BP for 1 h at 37°C to inhibit
palmitoylation in CRC cells. After 24, 48 or 72 h incubation, 10 µl
CCK-8 solution was added to each well and the cells were incubated
for an additional 2 h at 37°C. The absorbance was measured at 450
nm using a microplate reader. The relative cell proliferation was
calculated by comparing the absorbance values of treated cells to
the control group. Each experiment was performed in triplicate and
repeated at least three times.
5-Ethynyl-2′-deoxyuridine (EdU) cell
proliferation assay
To evaluate cell proliferation, the EdU
incorporation assay (cat. no. E-CK-A376; Elabscience; Elabscience
Bionovation Inc.) was performed. CRC cells were seeded in 24-well
plates at a density of 2×104 cells/well and allowed to
adhere overnight. After treatment under different experimental
conditions (sh-KRT8P12 or OE-KRT8P12 transduction, and 2-BP
treatment), the cells were incubated with 10 µM EdU for 2 h at 37°C
in a 5% CO2 incubator. Following incubation, cells were
fixed with 4% paraformaldehyde for 15 min at room temperature, and
were then permeabilized with 0.3% Triton X-100 and stained with
Click-iT EdU reaction cocktail, according to the manufacturer's
protocol. The cell nuclei were counterstained with Hoechst to
visualize the total cells. Proliferating cells were observed by
fluorescence microscopy. Each experiment was performed in
triplicate and repeated at least three times.
Transwell cell migration assay
To assess cell migration, a Transwell assay was
performed using 24-well plates with a polycarbonate membrane insert
(pore size, 8 µm). CRC cells were seeded into the upper chamber of
the Transwell insert at a density of 1×104 cells/well in
200 µl serum-free medium. The lower chamber was filled with 600 µl
complete medium containing 10% FBS to act as a chemoattractant.
After incubating for 24 h at 37°C in a 5% CO2 incubator,
non-migrated cells on the upper surface of the membrane were gently
removed using a cotton swab. Migrated cells on the lower surface of
the membrane were fixed with 4% paraformaldehyde for 10 min and
stained with 0.1% crystal violet solution for 15 min at room
temperature. Images of the stained cells were then captured using
an inverted light microscope, and the number of migrated cells was
quantified by counting the cells in five random fields per well.
Each experiment was performed in triplicate and repeated at least
three times.
Flow cytometric analysis of
apoptosis
To assess apoptosis, flow cytometric analysis was
performed using an Annexin V-FITC/PI apoptosis detection kit (cat.
no. C1062S; Beyotime Institute of Biotechnology). CRC cells were
seeded in 6-well plates at a density of 1×105 cells/well
and underwent different experimental conditions: sh-KRT8P12 or
OE-KRT8P12 transduction, and 2-BP treatment (50 µM 2-BP for 1 h at
37°C in a 5% CO2 incubator). After treatment, the cells
were harvested by trypsinization, washed twice with
phosphate-buffered saline and resuspended in 1X binding buffer at a
concentration of 1×106 cells/ml. Subsequently, 5 µl
Annexin V-FITC and 5 µl PI were added to a 100-µl cell suspension,
and the mixture was incubated in the dark at room temperature for
15–20 min. After incubation, 400 µl 1X binding buffer was added to
each sample. The stained cells were analyzed using a FACSCalibur
flow cytometer (BD Biosciences). Annexin V-positive and PI-negative
cells were considered early apoptotic, whereas Annexin V-positive
and PI-positive cells were considered late apoptotic or necrotic.
The percentage of apoptotic cells was calculated by summing the
early and late apoptotic population using FlowJo version 10.8.1 (BD
Biosciences). Each experiment was performed in triplicate and
repeated at least three times.
Statistical analysis
All the bioinformatics analyses in this manuscript
were conducted using R studio 4.3.1 version software. Data were
analyzed using GraphPad Prism 9.0 software (Dotmatics). All
experiments were performed in triplicate and results are presented
as the mean ± standard deviation. Differences between two groups
were analyzed by unpaired Student's t-test. Differences between
more than two groups were analyzed using one-way ANOVA with the
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times to ensure reproducibility.
Results
Identification of a
palmitoylation-related risk signature
Univariate Cox regression analysis identified 10
palmitoylation-related genes significantly associated with OS
(Fig. 1A). Among these, ZDHHC3,
PARGC1A, PMM2, MMAA, LARS2, FDFT1 and CPT2 were protective factors
(HR <1), while TIGD1, MPP2 and KRT8P12 were risk factors (HR
>1). To refine the model, LASSO regression analysis was
performed, narrowing the candidate genes to a manageable subset for
multivariate analysis (Fig. 1B).
Multivariate Cox regression modeling ultimately identified six
critical genes, KRT8P12, ZDHHC3, PCOLCE2, MPP2, LARS2 and MMAA, to
construct a palmitoylation-related risk signature (Fig. 1C and D). The risk score formula was
defined as follows: RiskScore=(KRT8P12 × 0.105257166581237)-(ZDHHC3
× 0.0240068245440265) + (PCOLCE2 × 0.0952477383419697) + (MPP2 ×
0.177664830643707)-(LARS2 × 0.0426016477398653)-(MMAA ×
0.1200626992622).
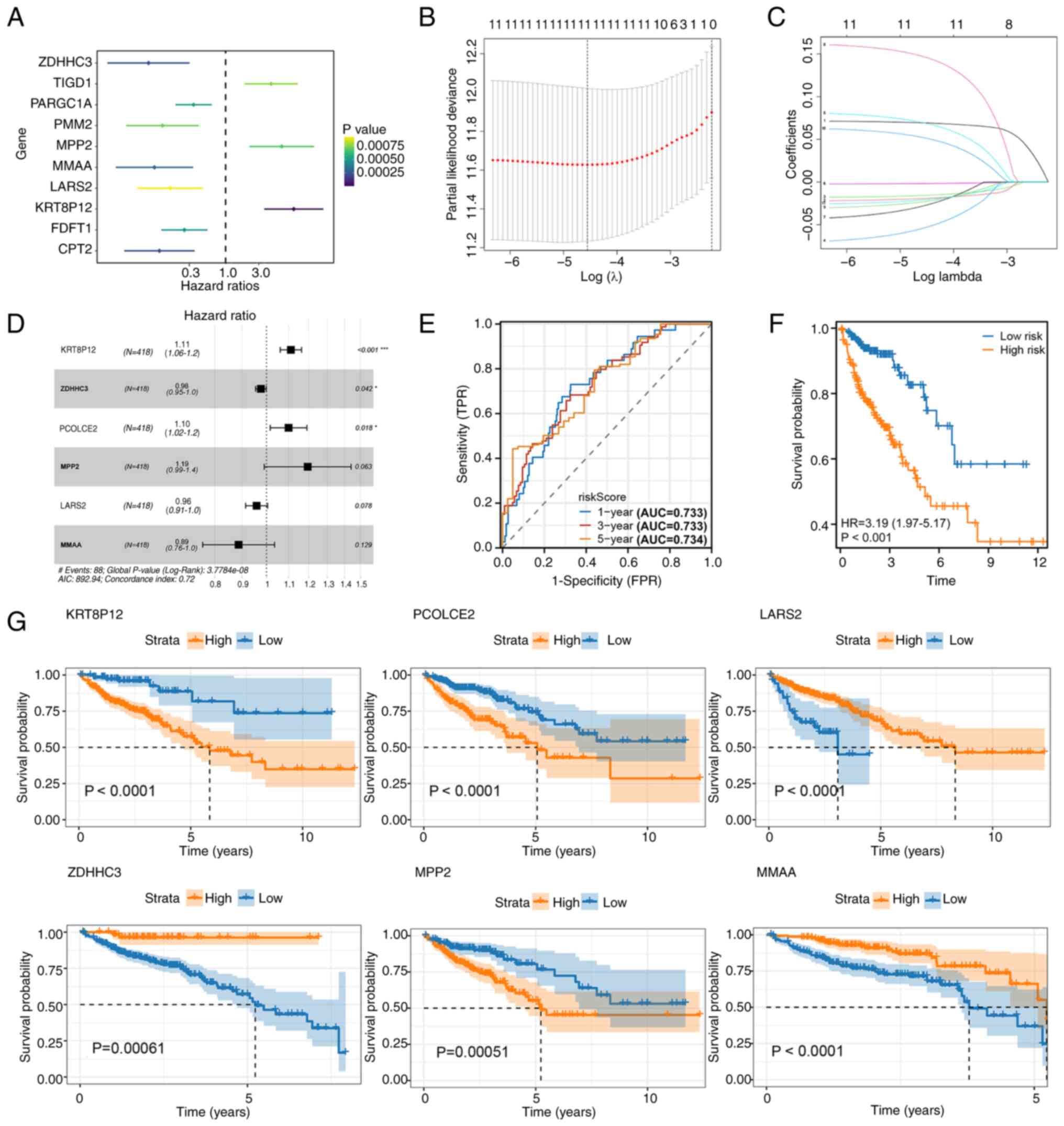 | Figure 1.Identification of a
palmitoylation-related risk signature. (A) Univariate Cox
regression analysis of palmitoylation-related genes identified 10
prognostic candidates (P<0.001). ZDHHC3, PARGC1A, PMM2, MMAA,
LARS2, FDFT1 and CPT2 were protective factors (HR <1), while
TIGD1, MPP2 and KRT8P12 were risk factors (HR >1). (B) LASSO
analysis for dimensionality reduction. (C) LASSO analysis for
selecting key prognostic genes. (D) Multivariate Cox regression
modeling incorporating six genes (KRT8P12, ZDHHC3, PCOLCE2, MPP2,
LARS2 and MMAA) to construct the risk signature. (E) Time-dependent
receiver operating characteristic curves for 1-, 3- and 5-year
survival predictions, with AUC values of 0.733, 0.733 and 0.734,
respectively. (F) KM survival analysis of high- and low-risk groups
based on the risk score, showing significantly worse survival in
the high-risk group (HR=3.19; P<0.001). (G) KM survival curves
of the six model genes (KRT8P12, ZDHHC3, PCOLCE2, MPP2, LARS2 and
MMAA), each demonstrating significant prognostic value. *P<0.05;
***P<0.001. LASSO, Least Absolute Shrinkage and Selection
Operator; KM, Kaplan-Meier; AUC, area under the curve; KRT8P12,
keratin 8 pseudogene 12. |
The prognostic efficacy of the model was evaluated
using time-dependent ROC curves, which yielded AUC values of 0.733,
0.733 and 0.734 for 1-, 3- and 5-year survival, respectively
(Fig. 1E). KM OS analysis
demonstrated that patients in the high-risk group exhibited
significantly worse survival compared with those in the low-risk
group (Fig. 1F). Furthermore,
individual KM analyses of the six model genes confirmed their
prognostic relevance, with all genes significantly associated with
survival outcomes (Fig. 1G). These
findings indicated the robust prognostic utility of the
palmitoylation-related risk signature in stratifying patients with
CRC.
Validation and clinical relevance of
the palmitoylation-related risk signature
The heatmap of six model genes, highlighted their
potential roles in tumor biology (Fig.
2A). Correlation analysis revealed a strong positive
association between KRT8P12 expression and the risk score, making
it the most influential contributor to the risk signature, as shown
in the lollipop plot (Fig. 2B). To
facilitate individualized prognostic prediction, a nomogram
integrating key clinical parameters, i.e. age, stage, T stage, N
stage, sex and the risk score, was constructed, providing a visual
tool for risk stratification (Fig.
2C). Analysis of clinical characteristics between high- and
low-risk groups demonstrated that patients in the high-risk group
had significantly worse prognoses and were more likely to present
with advanced disease, including higher stage, T stage, N stage and
M stage classifications (Fig. 2D).
The prognostic utility of the model was further validated in the
independent GSE17538 dataset, where Kaplan-Meier OS analysis
confirmed that patients in the high-risk group experienced
significantly worse outcomes (Fig.
2E). These findings underscore the robustness of the
palmitoylation-related risk signature and its strong association
with disease progression and survival outcomes.
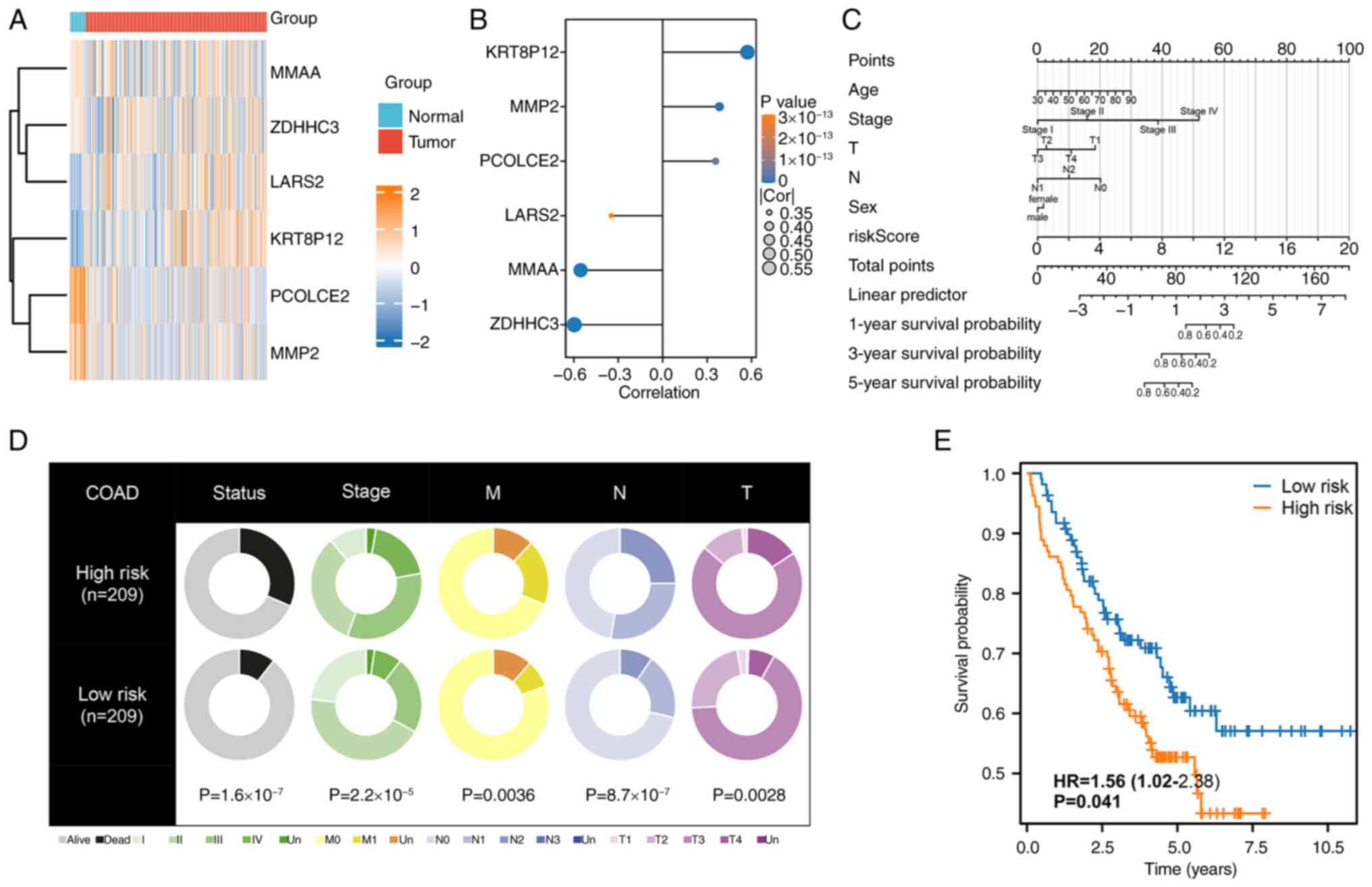 | Figure 2.Validation and clinical implications
of the palmitoylation-related risk signature. (A) Heatmap depicting
the differential expression of the six model genes between normal
and colorectal cancer samples, illustrating significant
alterations. (B) Lollipop plot showing the correlation between
model gene expression and the risk score, with KRT8P12 exhibiting
the strongest positive correlation. (C) Nomogram integrating
clinical parameters, including age, stage, T stage, N stage, sex
and risk score, to provide a comprehensive prognostic tool. (D)
Comparative analysis of clinical features between high- and
low-risk groups, demonstrating worse prognoses and more advanced
clinical stages (stage, T, N and M stages) in the high-risk group.
(E) Kaplan-Meier survival analysis validating the risk model in the
GSE17538 dataset, showing significantly poorer survival outcomes
for the high-risk group (P<0.05). KRT8P12, keratin 8 pseudogene
12; T, tumor; N, lymph node; M, metastasis; COAD, colon
adenocarcinoma. |
Functional enrichment and mutation
landscape analyses between risk groups
GO and KEGG analyses revealed both common and unique
pathways in the high- and low-risk groups. Shared pathways between
the groups included ‘olfactory transduction’ and ‘olfactory
receptor activity’, underscoring certain overlapping molecular
mechanisms (Fig. 3A and B).
However, distinct pathways were observed in each group: The
high-risk group was significantly enriched for ‘intermediate
filament’ and ‘sensory perception of smell’, whereas the low-risk
group exhibited unique enrichment in ‘odorant binding’ and
‘detection of chemical stimulus involved in sensory perception’.
These findings suggested distinct biological underpinnings
contributing to the differential prognosis between the two groups.
Somatic mutation landscape analyses demonstrated no significant
differences in mutation frequencies between the high- and low-risk
groups, with the five most frequently mutated genes being APC,
TP53, TTN, KRAS and PIK3CA in both groups (Fig. 3C and D). KM OS analysis stratified
by TMB revealed that patients with high TMB had significantly worse
survival outcomes compared with those with low TMB (Fig. 3E). Furthermore, combining TMB status
with risk score stratification provided a more refined prognostic
insight: Patients with high-risk/high-TMB demonstrated the worst
survival outcomes, highlighting a strong interplay between
mutational burden and risk group in determining prognosis (Fig. 3F). These results emphasized the
clinical relevance of integrating genetic and molecular signatures
for precise prognostic prediction in patients with CRC.
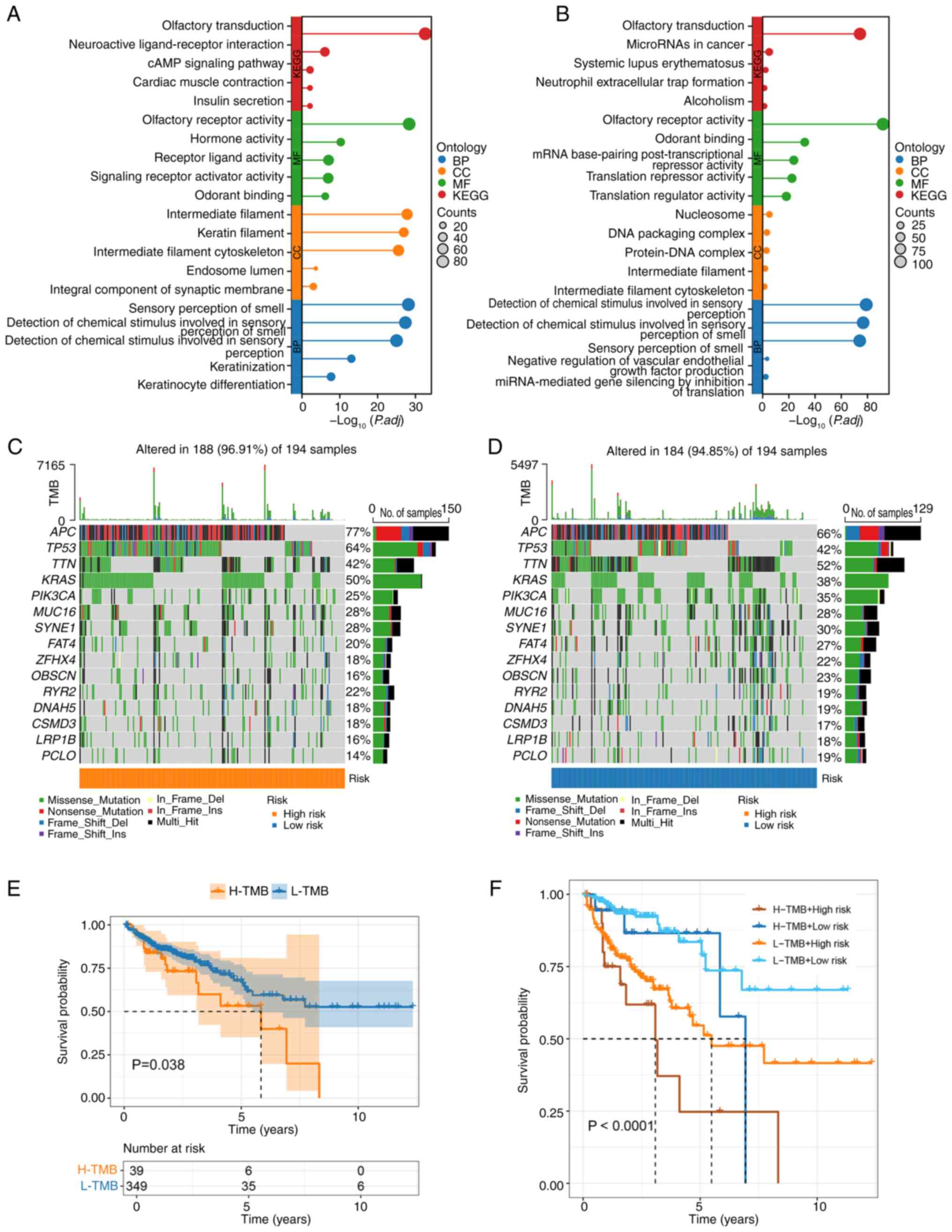 | Figure 3.Functional enrichment and mutational
landscape analysis between risk groups. (A) GO and KEGG enrichment
analyses of the high-risk group showing significant enrichment in
pathways such as ‘olfactory transduction’, ‘olfactory receptor
activity’, ‘intermediate filament’ and ‘sensory perception of
smell’. (B) GO and KEGG enrichment analyses of the low-risk group
highlighting overlapping pathways (‘olfactory transduction’ and
‘olfactory receptor activity’) as well as unique pathways,
including ‘odorant binding’ and ‘detection of chemical stimulus
involved in sensory perception’. (C) Waterfall plot illustrating
the mutation profile of the high-risk group, with the top five most
frequently mutated genes being APC, TP53, TTN, KRAS and PIK3CA. (D)
Waterfall plot of the mutation profile in the low-risk group,
showing similar mutation frequencies to the high-risk group. (E) KM
survival curve comparing patients with high-TMB and low-TMB,
indicating significantly worse survival in the high-TMB group
(P=0.038). (F) KM survival curve combining TMB status and risk
group stratification, revealing the worst survival outcomes in the
high-risk/high-TMB subgroup (P<0.0001). KM, Kaplan-Meier; GO,
Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; TMB,
tumor mutational burden; BP, biological process; CC, cellular
component; MF, molecular function. |
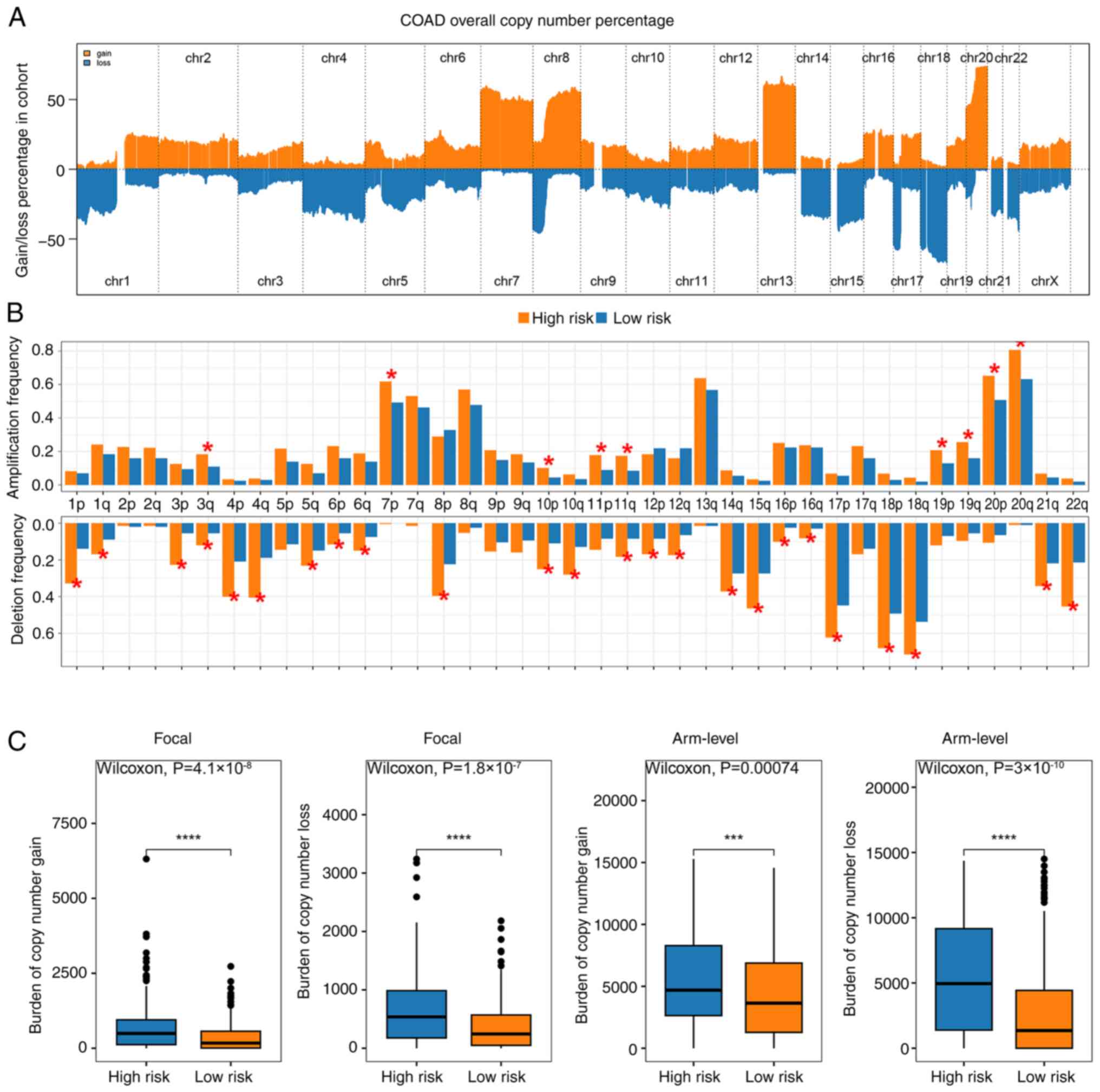
CNV analysis in high- and low-risk
groups
CNV analysis uncovered distinct genomic alterations
between high- and low-risk groups. The percentages of copy number
gains and losses displayed significant differences, with the
high-risk group showing a notably different distribution compared
with the low-risk group (Fig. 4A).
Additionally, the high-risk group exhibited a higher frequency of
amplification and deletion events, reflecting greater genomic
instability (Fig. 4B). Further
analysis of CNV burden at both focal and arm levels revealed that
the high-risk group consistently demonstrated significantly
elevated levels of copy number gains and losses compared with the
low-risk group (Fig. 4C). These
findings suggested that the increased CNV burden in the high-risk
group may contribute to the worse prognosis observed in these
patients, highlighting the potential role of genomic instability in
risk stratification.
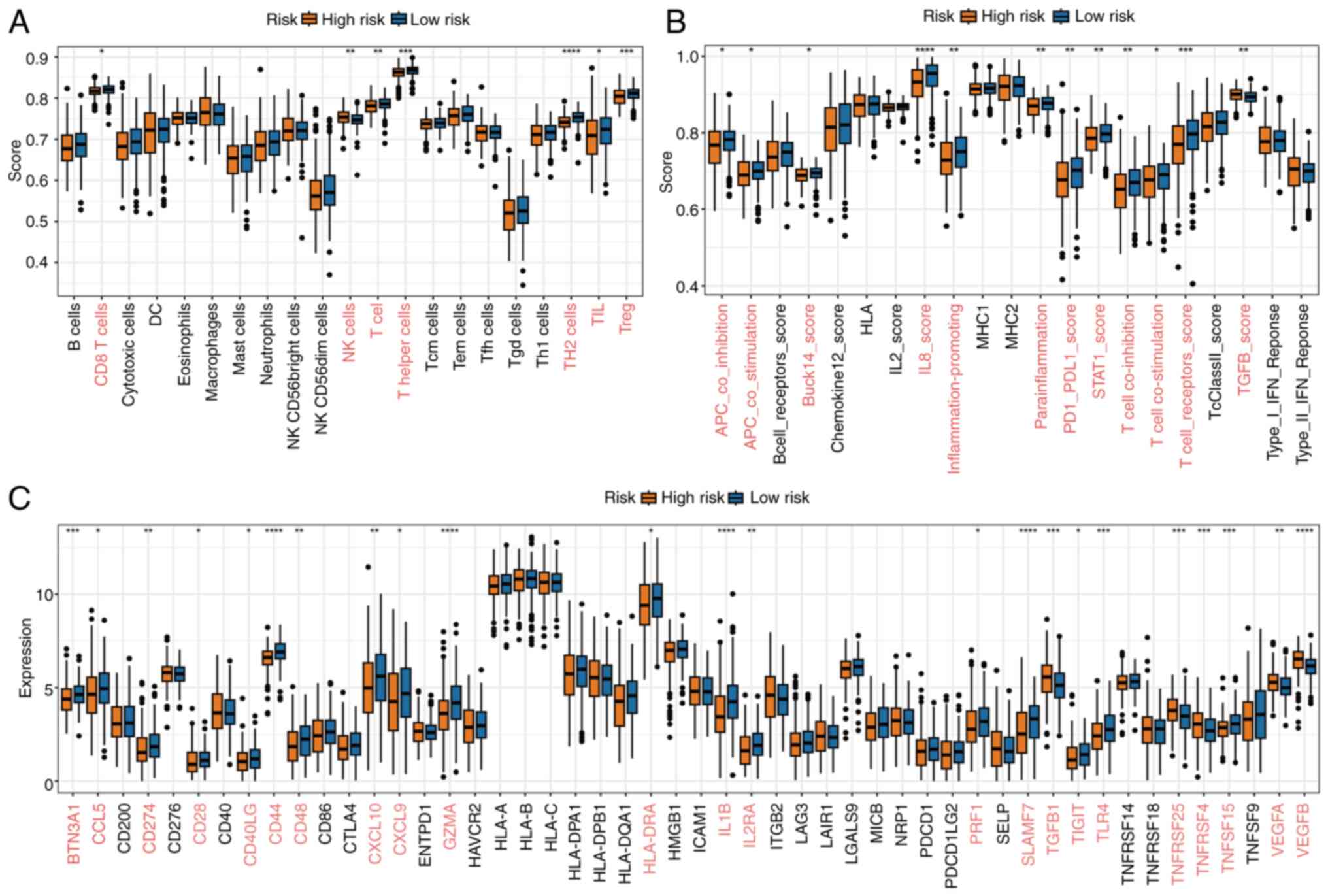
Infiltration and treatment prediction
analysis
Immune cell infiltration analysis demonstrated
notable differences in immune cell abundance between the high- and
low-risk groups. Differential expression analysis revealed that
most immune cell types were significantly more abundant in the
low-risk group, suggesting enhanced immune activity (Fig. 5A). Similarly, functional pathway
analysis indicated that key immune-related processes were more
active in the low-risk group, further highlighting the distinct
immune landscape between the two groups (Fig. 5B). Additionally, the expression
levels of common immune checkpoint genes exhibited significant
differences between the groups, with an overall trend of higher
expression in the low-risk group, reflecting its more robust immune
environment (Fig. 5C).
Treatment prediction analysis further elucidated the
clinical implications of the risk signature. Correlation analysis
of the risk score with the expression of immune checkpoint genes,
including PD-1, CD274 (PD-L1), CTLA4 and TIGIT, revealed a strong
negative association, with higher expression of these genes in the
low-risk group; however, although the association was observed, it
was not statistically significant (Fig.
6A). TIDE analysis supported these findings, showing that
low-risk patients had lower TIDE scores, indicating a greater
likelihood of responding to immune checkpoint inhibitors (Fig. 6B). SubMap analysis further
demonstrated that low-risk patients might benefit more from
immunotherapy (Fig. 6C).
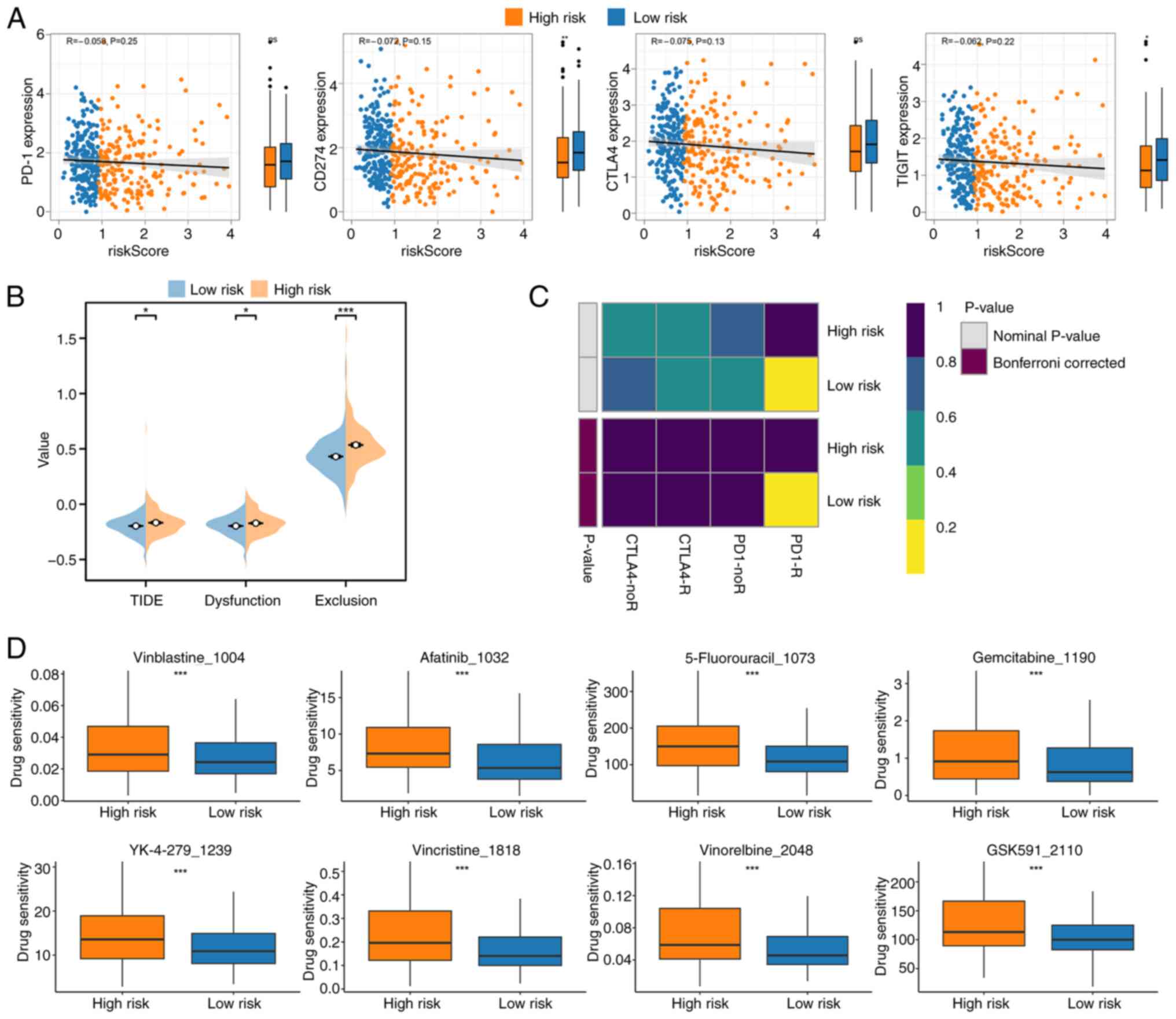 | Figure 6.Treatment prediction analysis based
on risk score. (A) Correlation analysis of risk score with the
expression of immune checkpoint genes [PD-1, CD274 (PD-L1), CTLA4,
TIGIT] and differential expression analysis, showing significantly
higher expression in the low-risk group. (B) TIDE analysis
demonstrating lower TIDE scores in the low-risk group, suggesting a
greater likelihood of response to immune checkpoint inhibitors. (C)
SubMap analysis further indicating enhanced sensitivity to
immunotherapy in the low-risk group. (D) Drug sensitivity analysis
from the GDSC database, highlighting the top eight drugs with the
most significant differences in sensitivity between high- and
low-risk groups. *P<0.05; ***P<0.001. PD-1, programmed cell
death protein 1; PD-L1, programmed death-ligand 1; CTLA4, cytotoxic
T-lymphocyte associated protein 4; TIGIT, T cell immunoreceptor
with Ig and ITIM domains; TIDE, Tumor Immune Dysfunction and
Exclusion. |
In addition to immunotherapy, drug sensitivity
analysis based on the GDSC database identified significant
differences in response to several therapeutic agents. The analysis
highlighted the top eight drugs with the most pronounced
differences in sensitivity between the high- and low-risk groups,
with the drug sensitivity being lower in the low risk group,
underscoring distinct drug response profiles associated with the
risk score (Fig. 6D). Vinblastine
is a microtubule inhibitor that prevents cell mitosis by inhibiting
microtubule polymerization, which is primarily used for treating
Hodgkin's lymphoma, non-Hodgkin's lymphoma and certain solid tumors
(31). Afatinib is an irreversible
tyrosine kinase inhibitor that targets EGFR mutations, mainly used
in non-small cell lung cancer (32). 5-Fluorouracil is a pyrimidine analog
that inhibits thymidylate synthase, thus disrupting DNA synthesis,
which is widely used in CRC, gastric and breast cancer (33). Gemcitabine is a nucleoside analog
that inhibits DNA replication, commonly used in pancreatic, lung
and breast cancer (34). YK-4-279
is an EWS-FLI1 inhibitor, primarily targeting EWS-FLI1 fusion
gene-associated tumors such as Ewing sarcoma (35). Vincristine is a microtubule
inhibitor that blocks mitosis, and is mainly used for leukemia,
lymphoma and certain solid tumors (36). Vinorelbine, a vinca alkaloid,
inhibits microtubule function and is mainly used for non-small cell
lung cancer and breast cancer (37). GSK591 is a protein arginine
methyltransferase 5 inhibitor that affects epigenetic regulation
and has potential applications in several types of cancer (38). These findings provide a strong
foundation for leveraging the risk signature to guide personalized
treatment strategies, including both immunotherapy and targeted
drug therapies.
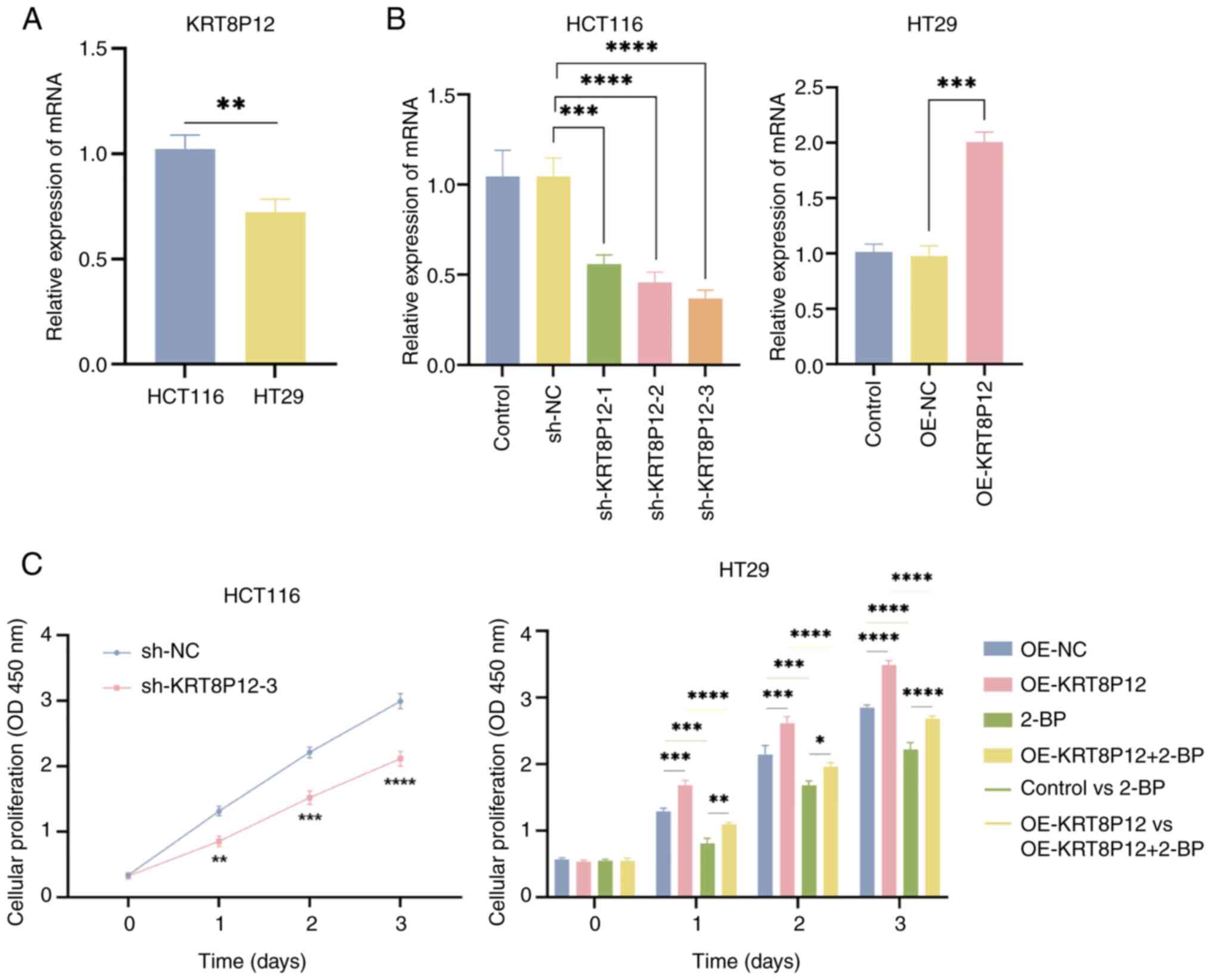
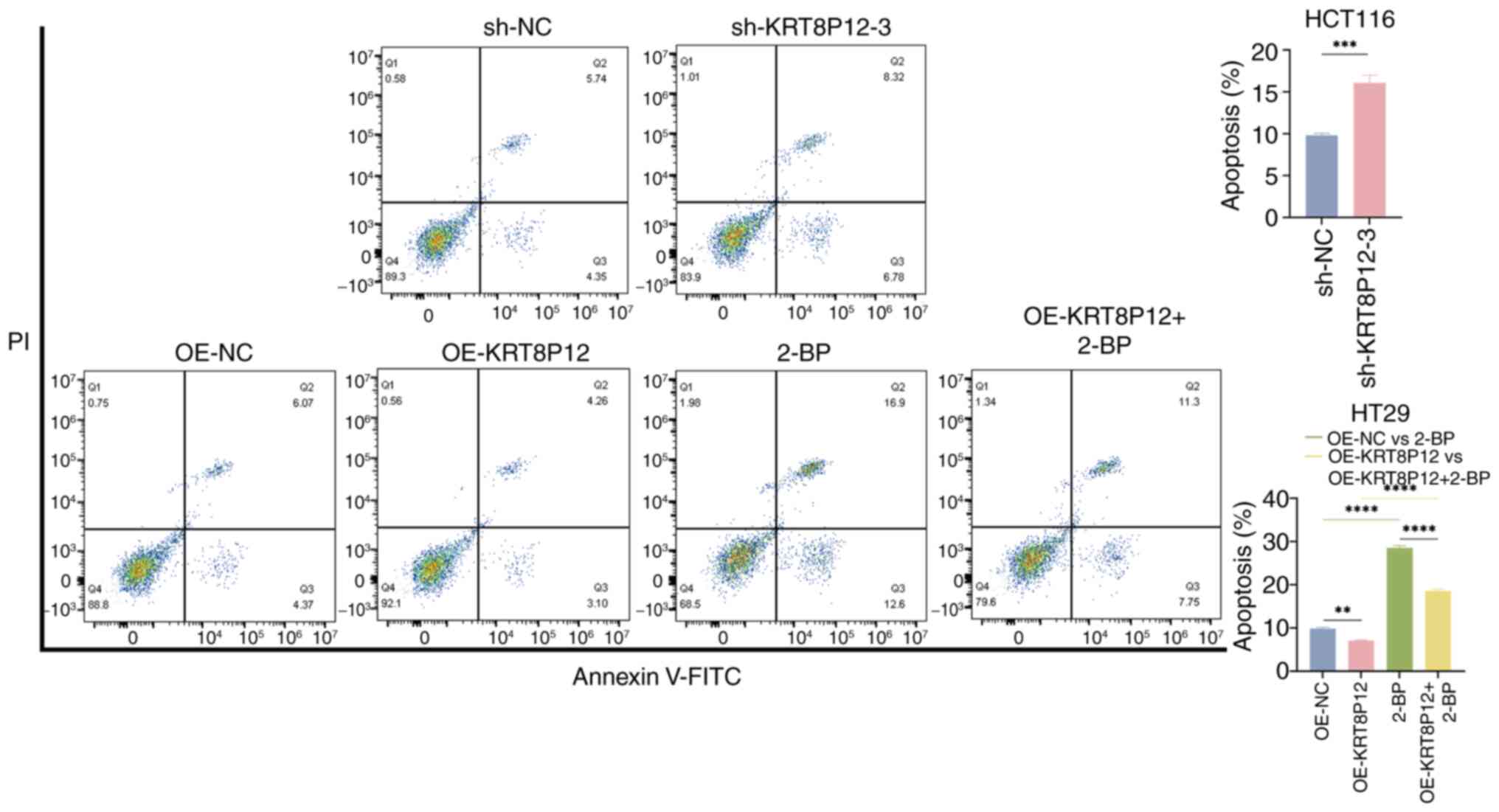
Effects of KRT8P12 on CRC cells
KRT8P12 is a gene associated with palmitoylation. To
verify the regulatory effect of KRT8P12 on CRC cells, the
expression of the KRT8P12 gene was assessed in CRC cell lines
HCT116 and HT29 using qPCR. Higher expression was demonstrated in
HCT116 cells (Fig. 7A). The
expression of KRT8P12 was subsequently knocked down in the HCT116
cell line using shRNA lentiviruses (sh-KRT8P12-3) and
overexpression was performed in the HT29 cell line (OE-KRT8P12)
(Fig. 7B). Since the target
sequence of sh-KRT8P12-2 was absolutely matched with KRT8, it might
also knock down KRT8, so it was not chosen for subsequent
experiments. Subsequently, the proliferation of the constructed
cell lines was assessed and it was demonstrated that sh-KRT8P12
decreased the proliferation of CRC cells, whereas overexpression of
KRT8P12 promoted the proliferation of CRC cells. By contrast, 2-BP
inhibited the proliferation induced by KRT8P12 overexpression
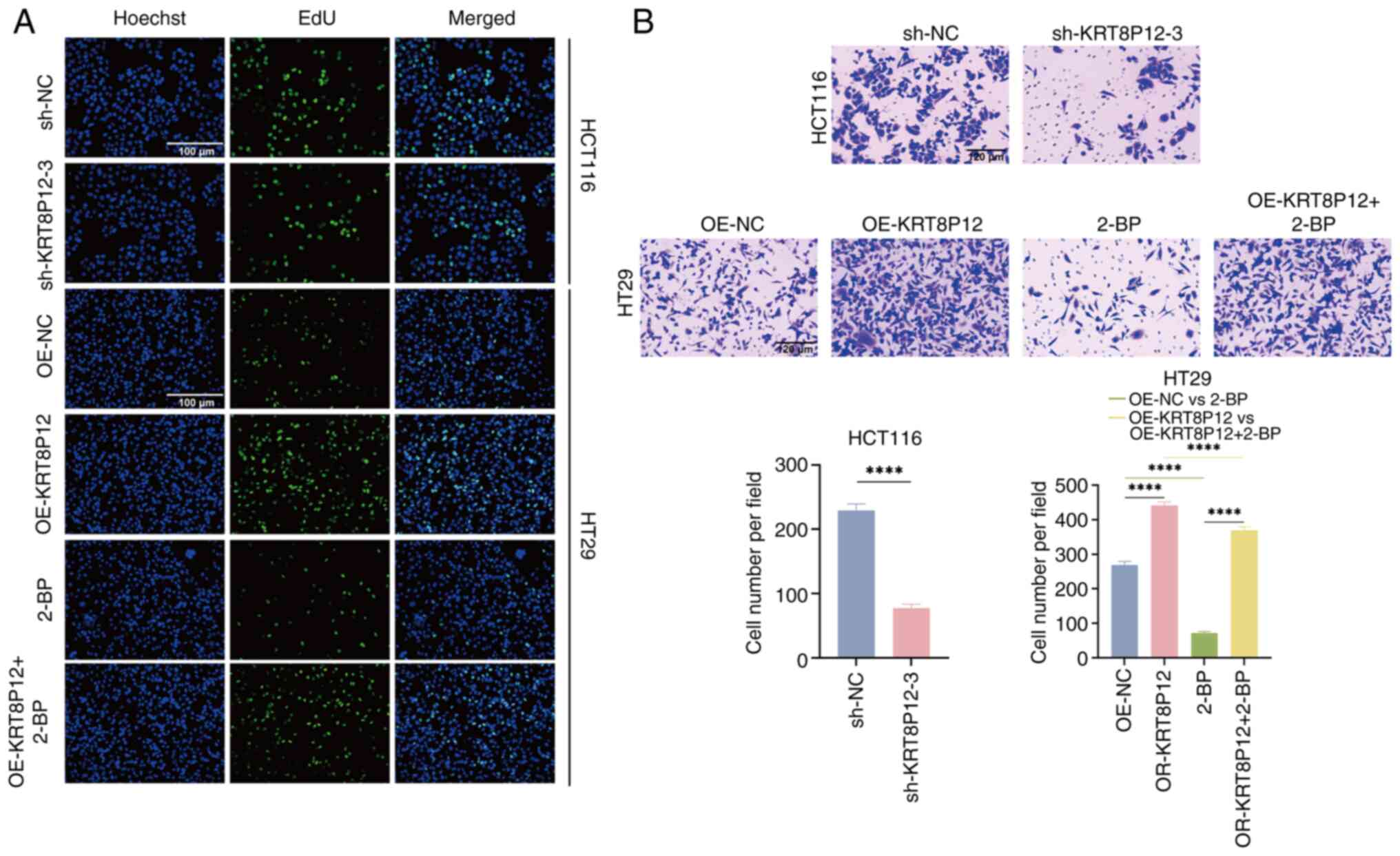
(Figs. 7C and 8A). The effect of KRT8P12 on cell
migration was evaluated using Transwell chambers, and it was
demonstrated that silencing of KRT8P12 reduced the migration of CRC
cells, whereas KRT8P12 overexpression significantly promoted tumor
cell migration. Treatment with 2-BP inhibited the migration induced
by KRT8P12 overexpression (Fig.
8B). The effect of KRT8P12 on CRC cell apoptosis was assessed
using Annexin-V/PI staining, and it was demonstrated that
sh-KRT8P12 enhanced apoptosis in CRC cells. Furthermore, 2-BP
reversed the effect of KRT8P12 overexpression on inhibiting
apoptosis (Fig. 9). In conclusion,
the palmitoylation-associated gene KRT8P12 may promote tumor cell
proliferation and migration, while inhibiting tumor cell
apoptosis.
Discussion
CRC remains a leading cause of cancer-associated
mortality, with a poor prognosis in advanced stages (39). Despite notable advances in
treatment, patient outcomes are often hindered by late-stage
diagnosis and a lack of effective prognostic biomarkers.
Palmitoylation, a reversible post-translational modification,
serves a critical role in regulating protein function, membrane
localization and cellular signaling (40). Previous studies have suggested that
alterations in palmitoylation may contribute to tumorigenesis and
metastasis in CRC, underscoring its potential as a therapeutic
target and prognostic marker (41,42).
In the present study, a palmitoylation-related
prognostic signature was developed for CRC, which was validated
using both TCGA and GSE17538 datasets. The findings demonstrated
that this signature, comprising six key genes (KRT8P12, ZDHHC3,
PCOLCE2, MPP2, LARS2 and MMAA), was significantly associated with
OS in patients with CRC. Patients stratified into high- and
low-risk groups based on this signature exhibited distinct clinical
outcomes, with high-risk patients showing significantly worse
survival. Notably, the signature was associated with immune cell
infiltration, immune checkpoint gene expression and TMB, suggesting
it has potential in predicting therapeutic responses, particularly
to immunotherapy.
PD-1/PD-L1 has emerged as a prominent strategy in
immunotherapy, serving a vital role in the treatment of various
tumors (43). Concurrently,
palmitoylation, as a post-translational modification, is
increasingly being explored in the context of tumor immunotherapy
(44). The application of 2-BP to
inhibit the palmitoylation of PD-L1 or silencing of ZDHHC3 has
demonstrated the ability to activate antitumor immunity both in
vitro and in mice bearing MC38 tumor cells (45). Furthermore, Benzosceptrin C can
prevent the palmitoylation of PD-L1 by inhibiting ZDHHC3 enzyme
activity. This leads to the translocation of PD-L1 from the plasma
membrane to the cytoplasm, preventing its recycling back to the
membrane via endosomes, thereby triggering lysosomal degradation of
PD-L1. Additionally, the combination of Benzosceptrin C with
anti-CTLA4 antibodies effectively enhances antitumor T cell
immunity (46). However, the
therapeutic mechanisms of palmitoylation in CRC warrant further
investigation.
The six key genes included in the
palmitoylation-related prognostic signature, KRT8P12, ZDHHC3,
PCOLCE2, MPP2, LARS2 and MMAA, are integral to the molecular
mechanisms underlying the development and progression of CRC. To
the best of our knowledge, KRT8P12, a key molecule in the present
study, has not yet been reported to serve a role in CRC. However,
members of the same family KRT8 and KRT19, which are associated
with keratin production, may serve a role in tumor biology,
particularly in CRC, where their expression levels can influence
tumor behavior and patient prognosis (47). A previous study reported that ZDHHC3
expression is markedly downregulated in CRC, and higher expression
levels of ZDHHC3 are associated with improved patient prognosis
(48). Moreover,
immunohistochemical analysis of CRC clinical samples has revealed
that PCOLCE2 expression is markedly higher in tumor tissue compared
with that in normal tissue. Knockdown experiments further
demonstrated that silencing PCOLCE2 expression can notably reduce
the proliferative capacity and epithelial-to-mesenchymal transition
process in CRC cells (49). A
previous study revealed that MPP2 is associated with the prognosis
of several types of cancer, particularly CRC, and its expression
level may notably influence patient survival rates (50). The LATS2 gene is regarded as a
potential tumor suppressor, with its dysregulation implicated in
tumorigenesis and cancer progression. A previous study reported a
strong association between LATS2 expression levels, EGFR mutations
and patient survival (51). In CRC,
altered LATS2 expression may influence the regulation of cell
proliferation and apoptosis, thereby impacting tumor progression.
Together, these findings highlight the crucial roles of KRT8P12,
ZDHHC3, PCOLCE2, MPP2, LATS2 and MMAA in the pathophysiology of
CRC, suggesting that these genes may serve as potential biomarkers
for prognosis and therapeutic targets in CRC management.
Functional enrichment analysis revealed that the
palmitoylation-related risk signature was associated with critical
pathways involved in tumorigenesis, including protein localization,
signal transduction and intermediate filament dynamics. Notably,
the high-risk group exhibited stronger enrichment in pathways
associated with cell structure and metastasis such as ‘cAMP
signaling pathway’ and ‘intermediate filament’, suggesting a
potential role in CRC progression. These findings underscore the
molecular mechanisms underlying the signature, highlighting its
potential as a therapeutic target in CRC.
Immune cell infiltration analysis revealed
significant differences between the high- and low-risk groups, with
the low-risk group exhibiting higher levels of immune cell
abundance, suggesting a more active antitumor immune response.
Moreover, the low-risk group demonstrated increased expression of
immune checkpoint genes, including PD-1, PD-L1 and CTLA4,
indicating a greater potential for response to immune checkpoint
inhibitors. TIDE analysis further supported these findings,
revealing that the low-risk group had lower immune evasion scores,
suggesting a higher likelihood of benefiting from PD-1/PD-L1
blockade. Additionally, SubMap analysis, which predicts responses
to immunotherapy based on gene expression profiles, indicated that
patients in the low-risk group might experience improved
therapeutic outcomes with immune checkpoint inhibitors.
Collectively, these results indicated the prognostic value of the
palmitoylation-related signature, not only in predicting survival
but also in guiding personalized immunotherapy strategies for
patients with CRC, particularly those likely to benefit from immune
checkpoint blockade.
In the present study, KRT8P12 was found to
significantly promote the proliferation and migration of HCT116 and
HT29 CRC cells, while inhibiting apoptosis. This result is
consistent with the role of keratins in other types of cancer,
where they enhance tumor invasiveness by promoting cell
proliferation and migration. For example, co-expression of K20 and
K7 was said to be indicative of advanced CRC. In CRC, increased
expression of K7 and K20 is associated with epithelial to
mesenchymal transition, which usually indicates higher tumor
invasiveness and reduced patient survival (52). Additionally, the use of 2-BP to
inhibit palmitoylation effectively blocked these effects, further
confirming the crucial role of KRT8P12 and palmitoylation in CRC
cells. Notably, the expression levels of KRT8P12 varied across
different cell lines, which may influence its specific role in
different tumor environments. As evidenced in the present study,
the expression levels of KRT8P12 were shown to vary in HCT116 and
HT29 cell lines. In addition, it was demonstrated that KRT8P12
serves an important role in tumor progression by promoting the
proliferation and migration of CRC cells and inhibiting apoptosis.
This finding not only provides new insights into the role of
keratins in CRC but also offers potential targets for future
therapeutic strategies. Future research could further explore the
specific molecular mechanisms of KRT8P12 and its role in different
tumor microenvironments, to develop more effective treatments.
Despite the promising findings of the
palmitoylation-related prognostic signature, there are several
limitations to the present study. Firstly, the present research is
mainly based on the retrieval of public databases, which contain
relatively small sample sizes and are not updated in a timely
manner. The main research object was palmitoylation, and the
specific molecular mechanism of its regulation of CRC progression
still requires extensive research. Therefore, the interpretation of
the results needs further validation and supplementation.
Meanwhile, the signature was primarily validated using public
datasets (TCGA and GSE17538), which may have inherent biases and
lack clinical validation in independent, real-world patient
cohorts. Secondly, while the immune cell infiltration and
immunotherapy prediction analyses provide valuable insights, the
precise mechanisms underlying the relationship between
palmitoylation and immune responses in CRC remain unclear and
require further experimental investigation. Additionally, the
present research suggested that KRT8P12 may modulate immune
responses and promote the progression of CRC through palmitoylation
pathways. A comprehensive review of the literature on KRT8P12 was
performed and found that it represents a novel therapeutic target.
However, the molecular mechanisms of KRT8P12 in regulating CRC
remain underexplored and warrant further investigation. The present
study also did not assess the potential interactions between the
six key genes and other molecular pathways that could contribute to
CRC progression, which warrants further exploration. Finally,
although the signature demonstrated potential for guiding
immunotherapy strategies, the lack of prospective clinical trials
to validate its predictive power limits its clinical applicability.
Future studies should aim to validate the prognostic signature in
larger, more diverse patient populations and explore the
mechanistic roles of palmitoylation in CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Study conception and design was performed by XW, JL
and QW. XW, JL and ZW conducted the literature research, and
performed the experimental studies, data acquisition, data analysis
and statistical analysis. The manuscript was prepared by XW and JL.
Manuscript review was performed by XW, JL, ZW and QW. XW, JL and QW
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
TCGA
|
The Cancer Genome Atlas
|
|
CNV
|
copy number variation
|
|
LASSO
|
Least Absolute Shrinkage and Selection
Operator
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
|
KM
|
Kaplan-Meier
|
|
OS
|
overall survival
|
|
TMB
|
tumor mutational burden
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
GISTIC
|
Genomic Identification of Significant
Targets in Cancer
|
|
TIDE
|
Tumor Immune Dysfunction and
Exclusion
|
|
GDSC
|
Genomics of Drug Sensitivity in
Cancer
|
|
qPCR
|
quantitative PCR
|
|
FBS
|
fetal bovine serum
|
|
2-BP
|
2-bromopalmitate
|
|
CCK-8
|
Cell Counting Kit-8
|
|
PI
|
propidium iodide
|
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltz L: Systemic therapy for metastatic
colorectal cancer. J Natl Compr Canc Netw. 11 (Suppl 5):S649–S652.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang D, Zhao K, Han T, Zhang X, Xu X, Liu
Z, Ren X, Zhang X, Lu Z and Qin C: Bisphenol A promote the cell
proliferation and invasion ability of prostate cancer cells via
regulating the androgen receptor. Ecotoxicol Environ Saf.
269:1158182024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marks KM, West NP, Morris E and Quirke P:
Clinicopathological, genomic and immunological factors in
colorectal cancer prognosis. Br J Surg. 105:e99–e109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anaya YA, Bracho RP, Chauhan SC, Tripathi
MK and Bandyopadhyay D: Small Molecule B-RAF inhibitors as
anti-cancer therapeutics: Advances in discovery, development, and
mechanistic insights. Int J Mol Sci. 26:26762025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linder ME and Deschenes RJ:
Palmitoylation: Policing protein stability and traffic. Nat Rev Mol
Cell Biol. 8:74–84. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salaun C, Greaves J and Chamberlain LH:
The intracellular dynamic of protein palmitoylation. J Cell Biol.
191:1229–1238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tape CJ, Ling S, Dimitriadi M, McMahon KM,
Worboys JD, Leong HS, Norrie IC, Miller CJ, Poulogiannis G,
Lauffenburger DA and Jørgensen C: Oncogenic KRAS regulates tumor
cell signaling via stromal reciprocation. Cell. 165:18182016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bivona TG: Dampening oncogenic RAS
signaling. Science. 363:1280–1281. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galluzzo P, Caiazza F, Moreno S and Marino
M: Role of ERbeta palmitoylation in the inhibition of human colon
cancer cell proliferation. Endocr Relat Cancer. 14:153–167. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caiazza F, Galluzzo P, Lorenzetti S and
Marino M: 17Beta-estradiol induces ERbeta up-regulation via
p38/MAPK activation in colon cancer cells. Biochem Biophys Res
Commun. 359:102–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwab RHM, Amin N, Flanagan DJ, Johanson
TM, Phesse TJ and Vincan E: Wnt is necessary for mesenchymal to
epithelial transition in colorectal cancer cells. Dev Dyn.
247:521–530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klaus C, Schneider U, Hedberg C, Schütz
AK, Bernhagen J, Waldmann H, Gassler N and Kaemmerer E: Modulating
effects of acyl-CoA synthetase 5-derived mitochondrial Wnt2B
palmitoylation on intestinal Wnt activity. World J Gastroenterol.
20:14855–14864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dubois F, Leroy C, Simon V, Benistant C
and Roche S: YES oncogenic activity is specified by its SH4 domain
and regulates RAS/MAPK signaling in colon carcinoma cells. Am J
Cancer Res. 5:1972–1987. 2015.PubMed/NCBI
|
|
16
|
Draper JM and Smith CD: DHHC20: A human
palmitoyl acyltransferase that causes cellular transformation. Mol
Membr Biol. 27:123–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rossin A, Durivault J, Chakhtoura-Feghali
T, Lounnas N, Gagnoux-Palacios L and Hueber AO: Fas palmitoylation
by the palmitoyl acyltransferase DHHC7 regulates Fas stability.
Cell Death Differ. 22:643–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kristensen VN, Lingjærde OC, Russnes HG,
Vollan HK, Frigessi A and Børresen-Dale AL: Principles and methods
of integrative genomic analyses in cancer. Nat Rev Cancer.
14:299–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rich JT, Neely JG, Paniello RC, Voelker
CC, Nussenbaum B and Wang EW: A practical guide to understanding
Kaplan-Meier curves. Otolaryngol Head Neck Surg. 143:331–336. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mayakonda A, Lin DC, Assenov Y, Plass C
and Koeffler HP: Maftools: Efficient and comprehensive analysis of
somatic variants in cancer. Genome Res. 28:1747–1756. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chalmers ZR, Connelly CF, Fabrizio D, Gay
L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J,
et al: Analysis of 100,000 human cancer genomes reveals the
landscape of tumor mutational burden. Genome Med. 9:342017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mermel CH, Schumacher SE, Hill B, Meyerson
ML, Beroukhim R and Getz G: GISTIC2.0 facilitates sensitive and
confident localization of the targets of focal somatic copy-number
alteration in human cancers. Genome Biol. 12:R412011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Powers RK, Goodspeed A, Pielke-Lombardo H,
Tan AC and Costello JC: GSEA-InContext: Identifying novel and
common patterns in expression experiments. Bioinformatics.
34:i555–i564. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Newman AM, Steen CB, Liu CL, Gentles AJ,
Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA,
Steiner D, et al: Determining cell type abundance and expression
from bulk tissues with digital cytometry. Nat Biotechnol.
37:773–782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoshida Y, Brunet JP, Tamayo P, Golub TR
and Mesirov JP: Subclass mapping: Identifying common subtypes in
independent disease data sets. PLoS One. 2:e11952007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geeleher P, Cox N and Huang RS:
pRRophetic: An R package for prediction of clinical
chemotherapeutic response from tumor gene expression levels. PLoS
One. 9:e1074682014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sebastian J and Rathinasamy K:
Microtubules and cell division: Potential pharmacological targets
in cancer therapy. Curr Drug Targets. 24:889–918. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Chen WD, Xu YX, Ren YY, Zheng C,
Lin YY and Zhou JL: Strategies for enhancing non-small cell lung
cancer treatment: Integrating Chinese herbal medicines with
epidermal growth factor receptor-tyrosine kinase inhibitors
therapy. Eur J Pharmacol. 980:1768712024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JC, Oludare A and Jung H: Connecting
dots between nucleotide biosynthesis and DNA lesion repair/bypass
in cancer. Biosci Rep. 44:BSR202313822024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jordheim LP: Clinical use of biomarkers in
the field of cytotoxic nucleoside analogues. Nucleosides
Nucleotides Nucleic Acids. 43:822–830. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conn E, Hour S, Allegakoen D, Graham G,
Petro J, Kouassi-Brou M, Hong SH, Selvanathan S, Çelik H, Toretsky
J and Üren A: Development of an Ewing sarcoma cell line with
resistance to EWS-FLI1 inhibitor YK-4-279. Mol Med Rep.
21:1667–1675. 2020.PubMed/NCBI
|
|
36
|
Banyal A, Tiwari S, Sharma A, Chanana I,
Patel SKS, Kulshrestha S and Kumar P: Vinca alkaloids as a
potential cancer therapeutics: Recent update and future challenges.
3 Biotech. 13:2112023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun K, Sun Z, Zhao F, Shan G and Meng Q:
Recent advances in research of colchicine binding site inhibitors
and their interaction modes with tubulin. Future Med Chem.
13:839–858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun J, Yuan H, Sun L, Zhao L, Wang Y, Hou
C, Zhang H, Lv P, Yang G, Zhang N, et al: Tumor-intrinsic PRMT5
upregulates FGL1 via methylating TCF12 to inhibit CD8+
T-cell-mediated antitumor immunity in liver cancer. Acta Pharm Sin
B. 15:188–204. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eng C, Yoshino T, Ruíz-García E, Mostafa
N, Cann CG, O'Brian B, Benny A, Perez RO and Cremolini C:
Colorectal cancer. Lancet. 404:294–310. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Z, Xiao M, Mo Y, Wang H, Han Y, Zhao
X, Yang X, Liu Z and Xu B: Emerging roles of protein palmitoylation
and its modifying enzymes in cancer cell signal transduction and
cancer therapy. Int J Biol Sci. 18:3447–3457. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu J, Cao X, Chen Z, Lai B, Xi L, Zhang
J, Zhu S, Qi S, Liang Y, Cao F, et al: Inhibiting S-palmitoylation
arrests metastasis by relocating Rap2b from plasma membrane in
colorectal cancer. Cell Death Dis. 15:6752024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kong Y, Liu Y, Li X, Rao M, Li D, Ruan X,
Li S, Jiang Z and Zhang Q: Palmitoylation landscapes across human
cancers reveal a role of palmitoylation in tumorigenesis. J Transl
Med. 21:8262023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsai MY, Lu CK, Shu LH, Liu HT, Wu YH, Lin
YS, Yang YH, Shih WT, Lee IY, Wu YH and Wu CY: Antrodia
cinnamomea formula suppresses prostate cancer progression via
immune modulation and PD-1/PD-L1 pathway inhibition. Int J Mol Sci.
26:26842025. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee TA, Tsai EY, Liu SH, Hsu Hung SD,
Chang SJ, Chao CH, Lai YJ, Yamaguchi H and Li CW:
Post-translational modification of PD-1: Potential targets for
cancer immunotherapy. Cancer Res. 84:800–807. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao H, Lan J, Li C, Shi H, Brosseau JP,
Wang H, Lu H, Fang C, Zhang Y, Liang L, et al: Inhibiting PD-L1
palmitoylation enhances T-cell immune responses against tumours.
Nat Biomed Eng. 3:306–317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Q, Wang J, Yu D, Zhang Q, Hu H, Xu M,
Zhang H, Tian S, Zheng G, Lu D, et al: Benzosceptrin C induces
lysosomal degradation of PD-L1 and promotes antitumor immunity by
targeting DHHC3. Cell Rep Med. 5:1013572024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang W, He J, Lu H, Kong Q and Lin S: KRT8
and KRT19, associated with EMT, are hypomethylated and
overexpressed in lung adenocarcinoma and link to unfavorable
prognosis. Biosci Rep. 40:BSR201934682020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang X, Hou J, Zhou G, Wang H and Wu Z:
zDHHC3-mediated S-palmitoylation of SLC9A2 regulates apoptosis in
kidney clear cell carcinoma. J Cancer Res Clin Oncol. 150:1942024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yin Y, Yang X, Cheng Z, Wang H, Lei J,
Wang D, Wang P, Li B, Mi J and Yuan Q: Identification of
extracellular matrix-related biomarkers in colon adenocarcinoma by
bioinformatics and experimental validation. Front Immunol.
15:13715842024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang L, Yu S, Zhang Q, Cai Y, Li W, Yao S
and Cheng H: Identification of hub genes related to CD4(+) memory T
cell infiltration with gene co-expression network predicts
prognosis and immunotherapy effect in colon adenocarcinoma. Front
Genet. 13:9152822022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Luo SY, Sit KY, Sihoe AD, Suen WS, Au WK,
Tang X, Ma ES, Chan WK, Wistuba II, Minna JD, et al: Aberrant large
tumor suppressor 2 (LATS2) gene expression correlates with EGFR
mutation and survival in lung adenocarcinomas. Lung Cancer.
85:282–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Karantza V: Keratins in health and cancer:
More than mere epithelial cell markers. Oncogene. 30:127–138. 2011.
View Article : Google Scholar : PubMed/NCBI
|