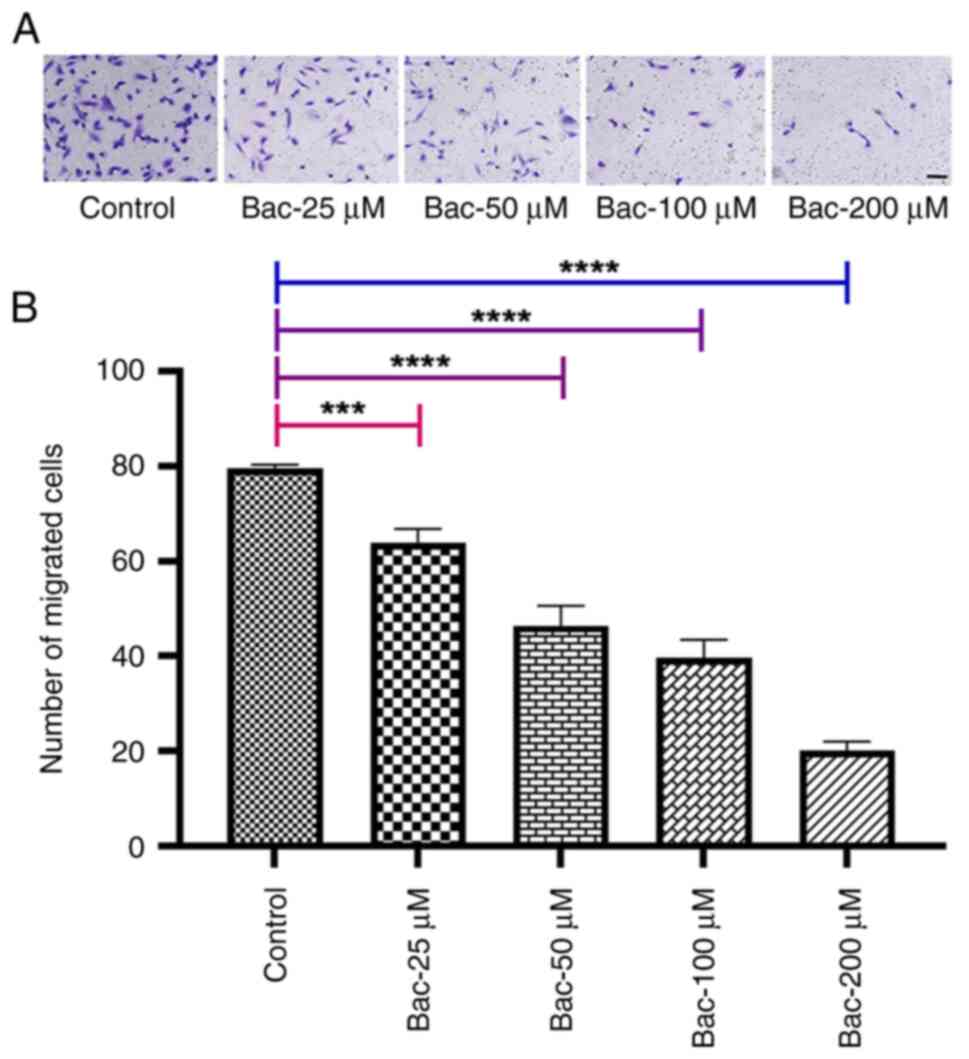
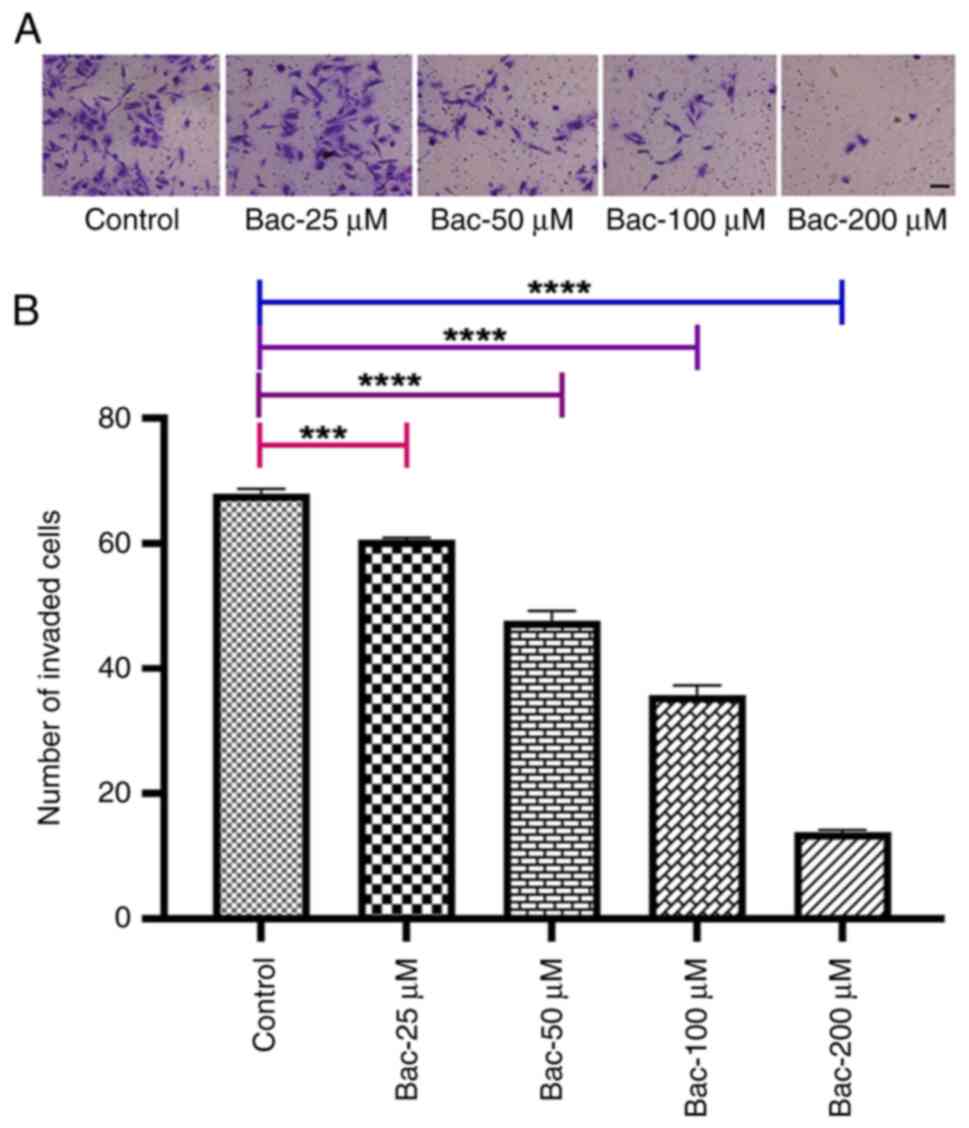
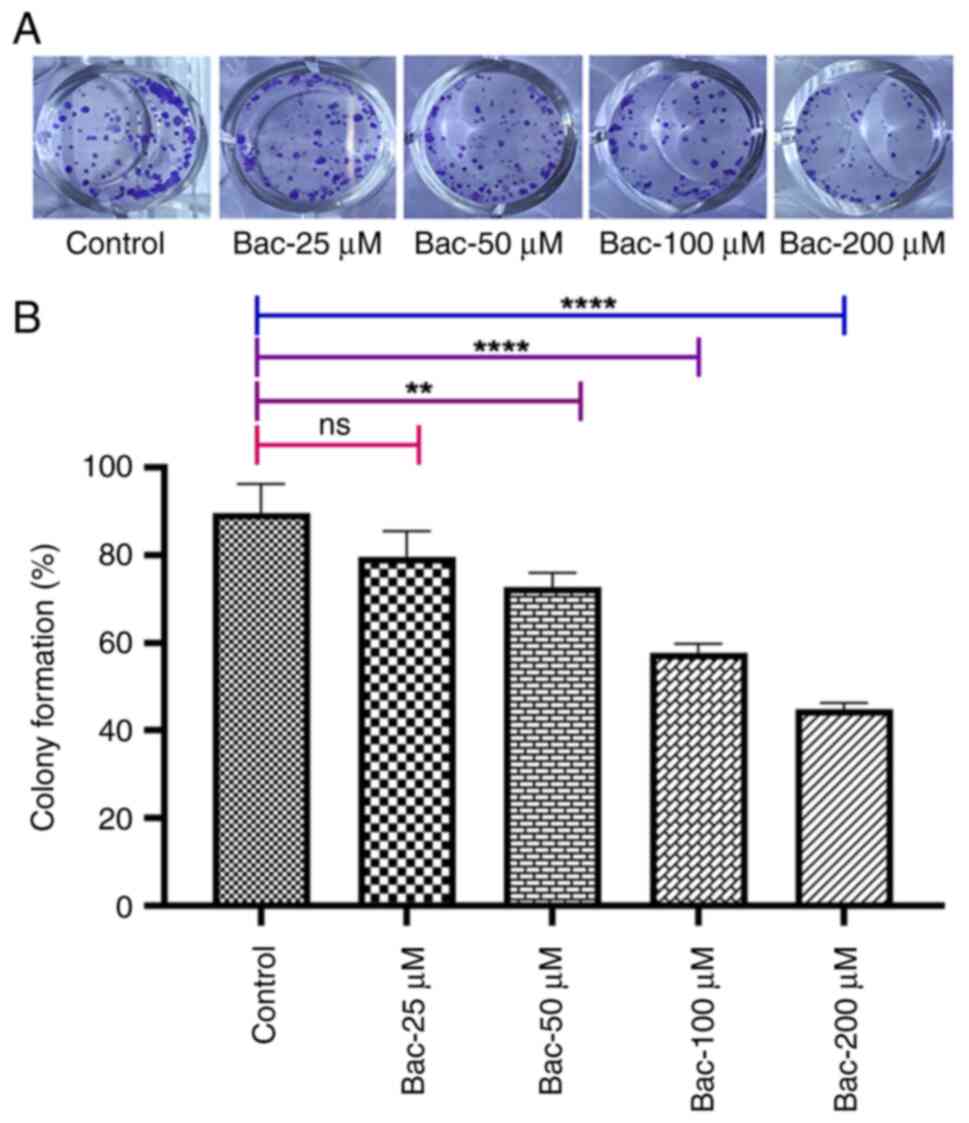
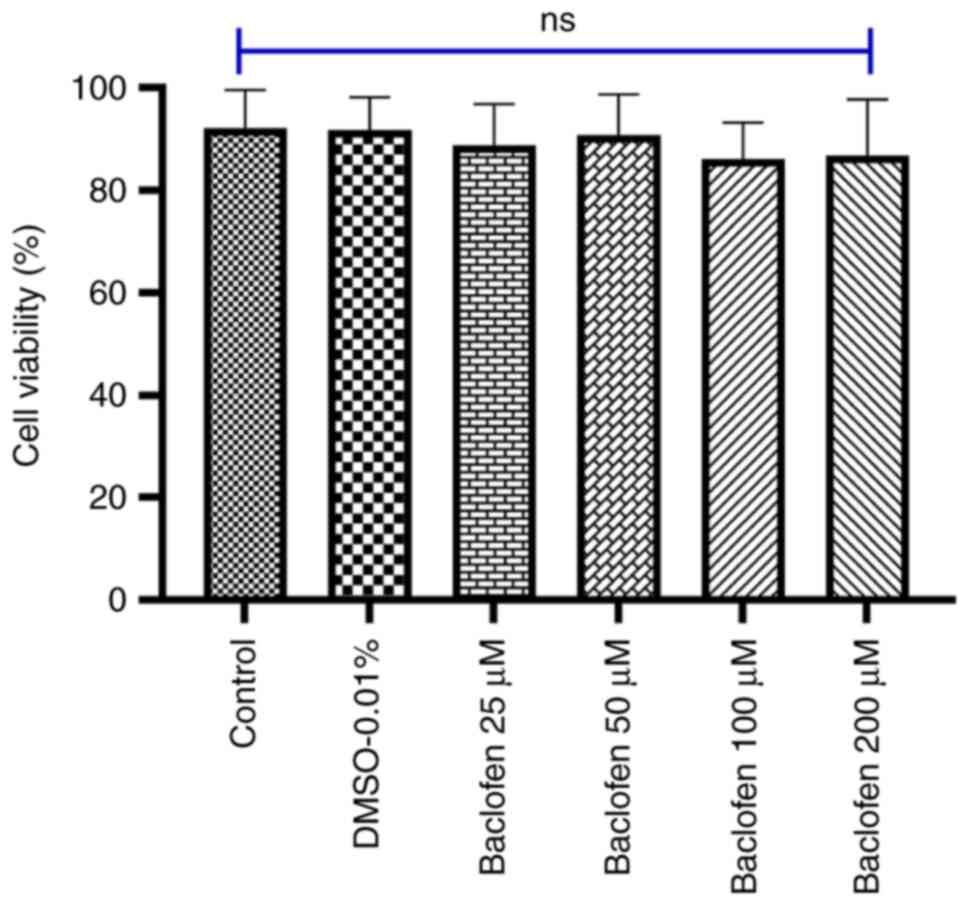
|
1
|
Smith RA, von Eschenbach AC, Wender R,
Levin B, Byers T, Rothenberger D, Brooks D, Creasman W, Cohen C,
Runowicz C, et al: American Cancer Society guidelines for the early
detection of cancer: Update of early detection guidelines for
prostate, colorectal, and endometrial cancers: Also: Update
2001-testing for early lung cancer detection. CA Cancer J Clin.
51:38–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eccles SA, Aboagye EO, Ali S, Anderson AS,
Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant
HE, et al: Critical research gaps and translational priorities for
the successful prevention and treatment of breast cancer. Breast
Cancer Res. 15:R922013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Memon MA, Abbasi F, Abbasi IHR, Mughal GA,
Soomro RN and Memon AS: Surgical approaches to cat breast cancer
(Mammary tumor), their treatment and management at Richmond
Crawford Veterinary Hospital Karachi (RCVH), Sindh, Pakistan. ARC J
Animal and Veterinary Sciences (AJAVS). 2:23–28. 2016.
|
|
5
|
Chang JW, Ding Y, Ul Qamar MT, Shen Y, Gao
J and Chen LL: A deep learning model based on sparse autoencoder
for prioritizing cancerrelated genes and drug target combinations.
Carcinogenesis. 40:624–632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
KudoSaito C, Shirako H, Ohike M, Tsukamoto
N and Kawakami Y: CCL2 is critical for immunosuppression to promote
cancer metastasis. Clin Exp Metastasis. 30:393–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang H, Yu T, Han Y, Jiang H, Wang C, You
T, Zhao X, Shan H, Yang R, Yang L, et al: LncRNA PTAR promotes EMT
and invasionmetastasis in serous ovarian cancer by competitively
binding miR1013p to regulate ZEB1 expression. Mol Cancer.
17:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumor Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH,
Chen PJ and Cheng AL: Activation of phosphatidylinositol
3-kinase/Akt signaling pathway mediates acquired resistance to
sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther.
337:155–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahajan K and Mahajan NP: PI3K-independent
AKT activation in cancers: A treasure trove for novel therapeutics.
J Cell Physiol. 227:3178–3184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vithlani M, Terunuma M and Moss SJ: The
dynamic modulation of GABAA receptor trafficking and its role in
regulating the plasticity of inhibitory synapses. Physiol Rev.
91:1009–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
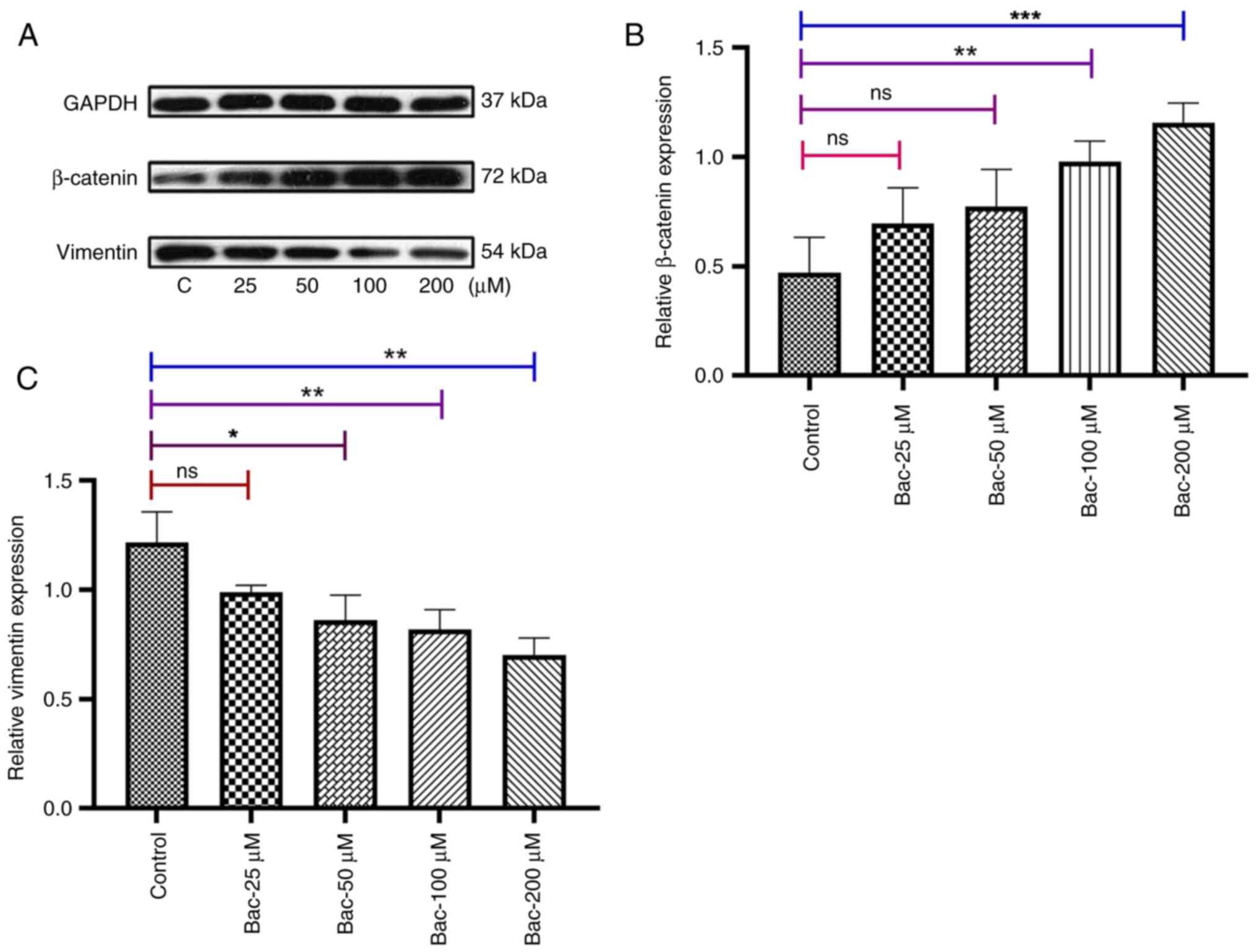
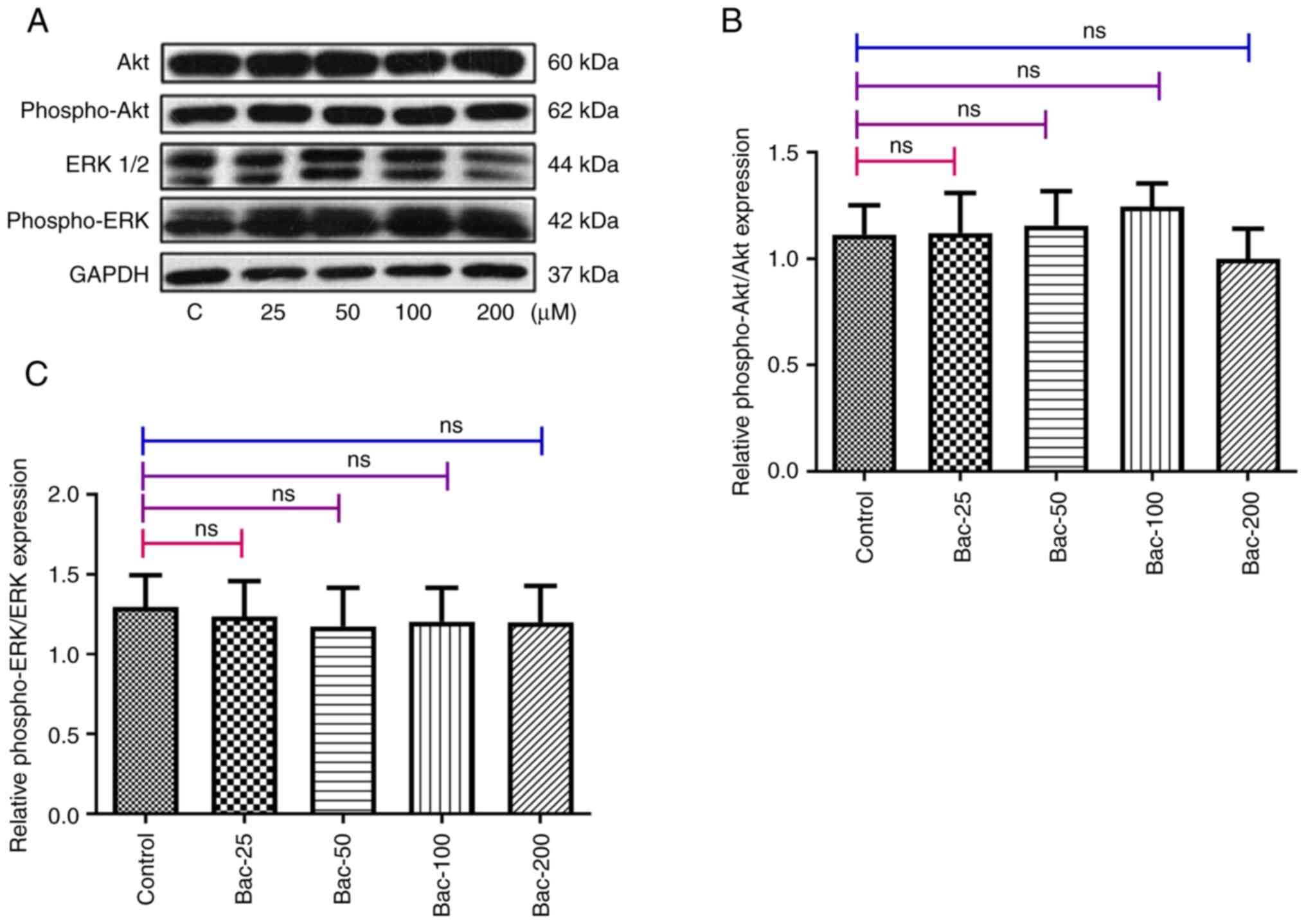
Tian H, Wu JX, Shan FX, Zhang SN, Cheng Q,
Zheng JN and Pei DS: Gamma aminobutyric acid induces tumor cells
apoptosis via GABABR1·β-arrestins·JNKs signaling module. Cell
Biochem Biophys. 71:679–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tatsuta M, Ishi H, Baba M, Nakaizumi A,
Ichii M and Taniguchi H: Inhibition by γ-amino-n-butyric acid and
baclofen of gastric carcinogenesis induced by
N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res.
50:4931–4934. 1990.PubMed/NCBI
|
|
16
|
Gao J, Gao Y, Lin S, Zou X, Zhu Y, Chen X,
Wan H and Zhu H: Effects of activating GABAB1 receptor on
proliferation, migration, invasion and epithelialmesenchymal
transition of ovarian cancer cells. J Ovarian Res. 13:1262020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanbara K, Otsuki Y, Watanabe M, Yokoe S,
Mori Y, Asahi M and Neo M: GABA B receptor regulates proliferation
in the highgrade chondrosarcoma cell line OUMS27 via apoptotic
pathways. BMC Cancer. 18:2632018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Solorzano SR, ImazRosshandler I,
CamachoArroyo I, García-Tobilla P, Morales-Montor G, Salazar P,
Arena-Ortiz ML and Rodríguez-Dorantes M: GABA promotes
gastrinreleasing peptide secretion in NE/NElike cells: Contribution
to prostate cancer progression. Sci Rep. 8:102722018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Li X, Yao Z, Wei C, Ning N and Li
J: GABAergic signaling facilitates breast cancer metastasis by
promoting ERK1/2dependent phosphorylation. Cancer Lett.
348:100–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
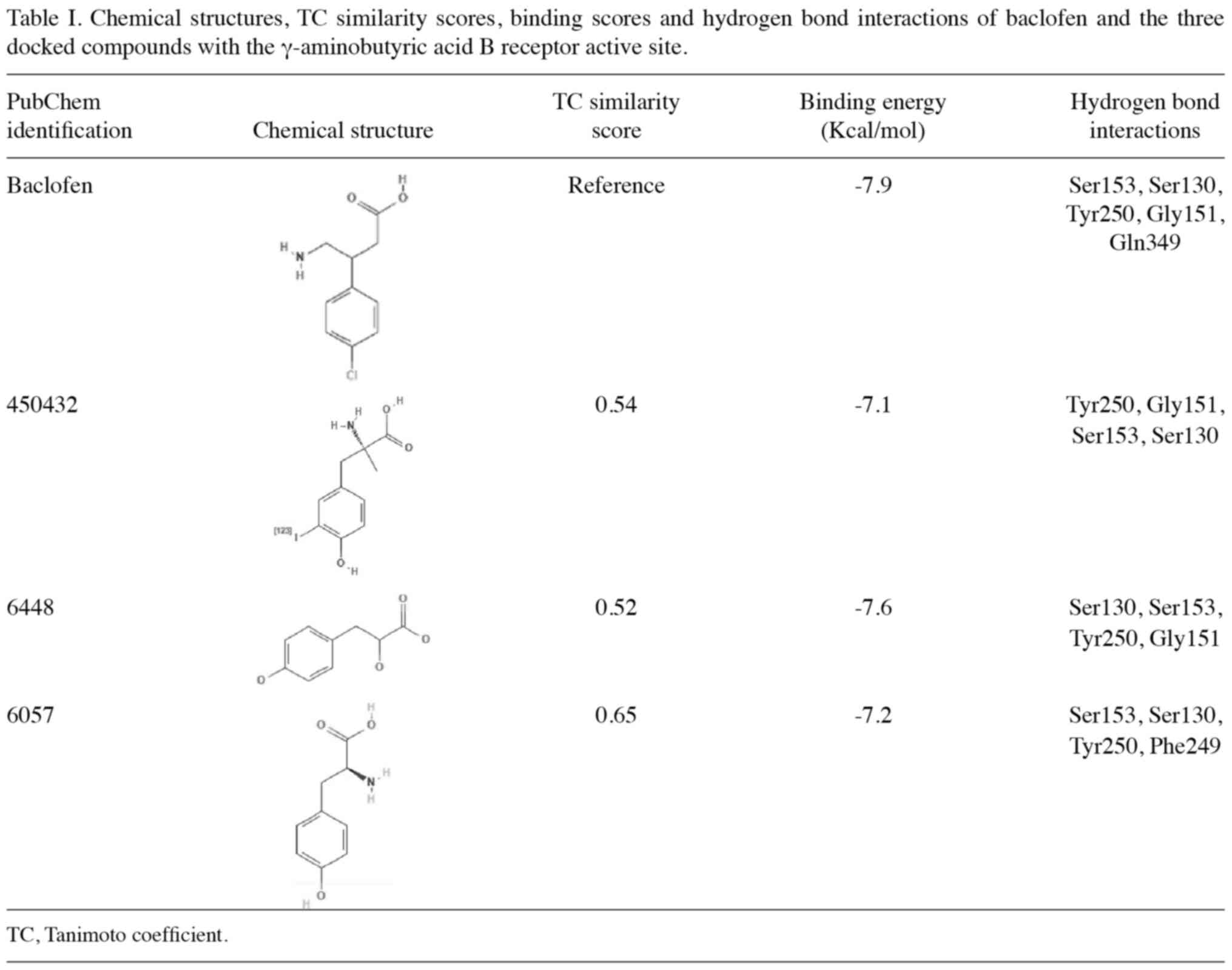
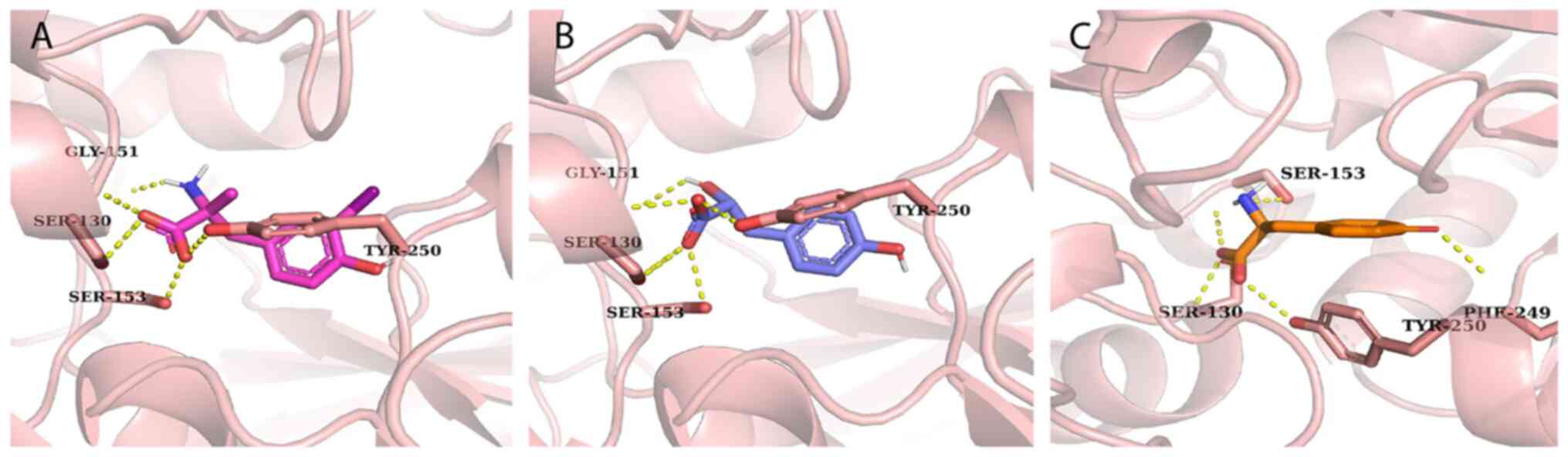
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sussman JL, Lin D, Jiang J, Manning NO,
Prilusky J, Ritter O and Abola E: Protein data bank (PDB): Database
of threedimensional structural information of biological
macromolecules. Acta Crystallogr D Biol Crystallogr. 54:1078–1084.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mumtaz A, Ashfaq U, Qamar M, Anwar F,
Gulzar F, Ali MA, Saari N and Pervez MT: MPD3: A useful medicinal
plants database for drug designing. Nat Prod Res. 31:1228–1236.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Boyle NM, Banck M, James CA, Morley C,
Vandermeersch T and Hutchison GR: Open babel: An open chemical
toolbox. J Cheminform. 3:332011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lodewyks C, Rodriguez J, Yan J, Lerner B,
Lipschitz J, Nfon C, Rempel JD, Uhanova J and Minuk GY: GABAB
receptor activation inhibits the in vitro migration of malignant
hepatocytes. Can J Physiol Pharmacol. 89:393–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jolly MK and Celià-Terrassa T: Dynamics of
phenotypic heterogeneity associated with EMT and stemness during
cancer progression. J Clin Med. 8:15422019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu
Y, Yao Y and Li D: The epithelial to mesenchymal transition (EMT)
and cancer stem cells: Implication for treatment resistance in
pancreatic cancer. Mol Cancer. 16:522017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Myers AP and Cantley LC: Targeting a
common collaborator in cancer development. Sci Transl Med.
2:48ps452010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu H, Xu C, Zhang W, Liu Q, Rondard P, Pin
JP and Liu J: GABAB receptor activation protects neurons from
apoptosis via IGF-1 receptor transactivation. J Neurosci.
30:749–759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bowery NG, Doble A, Hill DR, Hudson AL,
Shaw JS, Turnbull MJ and Warrington R: Bicucullineinsensitive GABA
receptors on peripheral autonomic nerve terminals. Eur J Pharmacol.
71:53–70. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Herman RM, D'Luzansky SC and Ippolito R:
Intrathecal baclofen suppresses central pain in patients with
spinal lesions. A pilot study. Clin J Pain. 8:338–345. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choudhari AS, Mandave PC, Deshpande M,
Ranjekar P and Prakash O: Phytochemicals in cancer treatment: From
preclinical studies to clinical practice. Front Pharmacol.
10:16142020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kirui JK, Xie Y, Wolff DW, Jiang H, Abel
PW and Tu Y: Gbetagamma signaling promotes breast cancer cell
migration and invasion. J Pharmacol Exp Ther. 333:393–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Calver A, Medhurst A, Robbins M, Charles
KJ, Evans ML, Harrison DC, Stammers M, Hughes SA, Hervieu G, Couve
A, et al: The expression of GABAB1 and GABAB2 receptor subunits in
the CNS differs from that in peripheral tissues. Neuroscience.
100:155–170. 2000. View Article : Google Scholar : PubMed/NCBI
|