Introduction
Breast cancer is a widespread metastatic disease
among women, which is exceeded only by lung cancer, comprising ~29%
of all cases of female cancer in the USA in 2001 (1). A previous study revealed that in the
USA 1/8 women will develop invasive breast cancer during their
lifetime, compared with ~1/800 men. As of 2023 there were 4 million
cases of breast cancer among women in the USA, including all women
who were either still receiving treatment or had already finished
the treatment (2). A previous
study, including the study by Eccles et al (3), have focused on the epidemiology and
basic biology of breast cancer therapy, which have undergone
several revisions due to the detection of predictive biomarkers
that have enabled the use of a wider range of application therapies
for this disease. Memon et al (4) identified several primary risk factors
associated with breast cancer development, including family
history, hormone replacement therapy during menopause, and
nulliparity or delayed childbearing. Inherited genetic mutations,
including those in breast cancer susceptibility genes 1 and 2, as
well as other genes such as partner and localizer of BRCA2a, ataxia
telangiectasia mutated, checkpoint kinase 2 and tumor protein p53,
have also been shown to contribute to 5–10% of breast cancer cases
(5).
Wang et al (6) demonstrated that numerous signaling
pathways mediate epithelial-mesenchymal transition (EMT) and cancer
progression. KudoSaito et al (7) reported that Snail family
transcriptional repressor 1 induces EMT, leading to a promotion of
invasion and acceleration of cancer metastasis. Zinc finger
E-box-binding homeobox 1 (ZEB1) also acts as an inducer of EMT in
several types of human tumors, including breast, colorectal,
pancreatic and non-small cell lung cancers, where ZEB1 increases
the rates of invasion or metastasis of the cancer (8). In addition, a previous study by Tania
et al (9), have shown that
EMT may be induced by ZEB proteins using mouse models and primary
human carcinomas, which thereby increased tumor aggressiveness and
metastasis. Furthermore, the phosphoinositide 3-kinase (PI3K)/Akt
pathway is considered a critical signaling pathway, which serves
important roles in numerous cellular activities, including
development and proliferation, and is also frequently associated
with different types of human cancer (10), including breast cancer. The PI3K/Akt
pathway may be activated by Gprotein-coupled receptors through
receptor tyrosine kinases located on the cell surface, such as the
epidermal growth factor receptor, which is often found dysregulated
on the surface of breast cancer cells (11).
γ-aminobutyric acid (GABA) is an important
neurotransmitter that acts as an inhibitor in the central nervous
system. GABA acts on two different types of receptors, namely
ionotropic and metabotropic receptors, based on their
pharmacological and physiological properties, thereby eliciting
powerful inhibitory effects. Ionotropic GABA receptors (namely,
GABAA and GABAC receptors) are ligand-gated
chloride channels that mediate rapid inhibition responses, whereas
metabotropic GABA receptors (GABAB receptors) are
G-protein-coupled receptors that mediate slow inhibitory signals
(12). GABAB receptors
consist of two isomers, GABAB receptor 1 and
GABAB receptor 2. Previous studies have reported that
the GABAB receptors are involved in tumor progression
(13,14). A study by Tatsuta et al
(15) was the first to identify the
association between GABAB receptor activation and
cancer. It was demonstrated that prolonged administration of GABA
at high doses and the GABAB receptor agonist baclofen
significantly decreased the incidence and number of gastric cancers
in Wistar rats. This inhibitory effect on gastric carcinogenesis
occurred via GABAB receptors, not GABAA
receptors, as demonstrated by the lack of effect with muscimol (a
GABAA agonist). The aforementioned study also showed
that both GABA and baclofen decreased cell proliferation in antral
mucosa and increased serum gastrin levels, suggesting these
mechanisms may contribute to their anti-cancer effects.
GABAB receptor 1 is widely expressed in a range of
cancer tissues and cancer cells, and it is closely associated with
the occurrence and development of pancreatic, liver, colon and
ovarian cancer (16). Kanbara et
al (17) demonstrated that the
GABAB receptor antagonist CGP 35348 has antitumor
effects in the high-grade chondrosarcoma cell line OUMS27 and
regulates proliferation through apoptotic pathways in high-grade
chondrosarcoma cells. Another study reported that downregulation of
the mRNA expression level of GABAB receptor can inhibit
the proliferation of ovarian cancer cells (16). Furthermore, GABAB
receptor 1 has been shown to promote the secretion of the peptide
gastrin in neuroendocrine cells involved in prostate cancer
progression. This effect leads to enhanced invasive potential of
prostate cancer cells through GRP receptor activation, increased
cell migration and creation of a more aggressive cancer phenotype,
particularly in tumors with low levels of androgen receptor
expression (18). Previous studies
have also reported that GABAB receptors regulate the
proliferation of colorectal cancer cells via the GSK-3β/NF-κB
signaling pathway (18). In
addition, Zhang et al (19)
studied the role of GABA in breast cancer metastasis and reported
that GABAergic signaling facilitates breast cancer metastasis by
promoting ERK1/2-dependent phosphorylation and increasing matrix
metallopeptidase 2 (MMP-2) expression levels in 4T1 mouse mammary
carcinoma and MCF-7 human breast cancer cells. However, the effects
of GABAB receptor activation may be cell line-dependent,
as our preliminary observations with MDA-MB-231 cells suggested
potentially different outcomes compared to those reported in 4T1
and MCF-7 cell lines.
The hypothesis of the present study is that
GABAB receptor activation may inhibit metastatic
behavior in breast cancer cells through modulation of
EMT-associated pathways.
To test this hypothesis, the present study evaluated
the effects of baclofen-mediated GABAB receptor
activation on multiple phenotypes of MDA-MB-231 breast cancer
cells. Furthermore, computational approaches were used to identify
natural compounds with structural similarity to baclofen that could
potentially function as GABAB receptor agonists. Through
these experimental and computational approaches, the present study
investigated the potential role of GABAB receptor
modulation in breast cancer therapy.
Materials and methods
Cell culture and cell lines
Triple-negative MDA-MB-231 cells (Thermo Fisher
Scientific, Inc.) were cultured at 37°C for 48 h and subsequently
washed carefully with PBS to eliminate the dead cells and the
culture debris. Cells were then treated with 20% trypsin at 37°C
for several min. L-15 growth medium (5 ml; HyClone; Cytiva) was
subsequently added and thoroughly mixed using a pipette to separate
the cells. The cell suspension then underwent centrifugation for
1,200 × g for 5 min at 4°C. The supernatant was discarded and
resuspended in fresh L-15 medium (1 ml). Aliquots (200 µl) of the
cells were then placed in the culture plate with 10 ml L-15 medium
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100
U/ml penicillin-streptomycin. Cultures were incubated at 37°C under
standard conditions.
Treatment of the cells with
GABAB receptor agonist
The GABAB receptor agonist baclofen
(Tocris Bioscience) was dissolved in water to form a stock solution
(concentration, 10 mM) and stored at −20°C until use. Cells
(2×104) were seeded in 35-mm culture plates with L-15
medium containing 10% FBS. After 24 h, the medium was replaced with
L-15 medium containing 1% FBS to induce starvation. Cells were
subsequently treated with various concentrations of baclofen (0,
25, 50, 100 and 200 µM) for 24 h at 37°C. These concentration
ranges were selected based on previous studies investigating
GABAB receptor activation in breast cancer cells
(16,19).
Transwell migration and invasion
assay
MDA-MB-231 cells were cultured in the aforementioned
conditions in L-15 medium supplemented with 10% FBS. Migration and
invasion assays were performed using 24-well Transwell chambers
with 8 µm pore-size membranes. For the migration assay, 700 µl
serum-free L-15 medium was added to the lower chamber. The upper
chamber received 200 µl serum-free medium, followed by an
incubation at 37°C for 30 min to equilibrate the membrane and
establish optimal conditions for cell attachment prior to adding
the cell suspension. Subsequently, 2×104 MDA-MB-231
cells in serum-free medium containing different concentrations of
baclofen (0, 25, 50, 100 or 200 µM) were added to the upper
chamber. The lower chamber medium was then replaced with L-15
medium containing 10% FBS and 100 U/ml penicillin-streptomycin.
Cells were subsequently incubated at 37°C for 24 h in an atmosphere
lacking CO2. Following this incubation, non-migrated
cells were removed from the upper surface of the membrane, whereas
migrated cells were fixed with 4% formaldehyde for 5 min at room
temperature, permeabilized with 100% methanol for 20 min and
stained with 1% crystal violet (prepared in a solution of 20%
methanol and 79% PBS) for 20 min at room temperature. The membranes
were subsequently air-dried and observed under an inverted light
microscope at ×20 magnification. Cell counts were obtained from at
least eight random fields. The invasion assay protocol was similar
to that of the migration assay, with the exception that a
Matrigel® layer (70–80 µl of a 1 mg/ml concentration;
Corning Life Sciences) was added to the upper chamber, which was
subsequently allowed to form a gel for 1 h at 37°C prior to cell
seeding. A total of 5×104 cells were seeded in the upper
chamber and the incubation period for the invasion assay was
extended to 48 h.
MTT assay
MDA-MB-231 cells at 75% confluence were starved in
serum-free L-15 medium for 24 h, followed by subsequent
trypsinization of 20% for 3 min. Cells were gently centrifuged at
125 × g for 6 min at room temperature, and the pellet was
subsequently resuspended in fresh L-15 medium. Cells (100 µl) were
then seeded into 24-well plates and treated with baclofen at
various concentrations (25, 50, 100 or 200 µM) for 3 days at 37°C.
DMSO (0.01%) served as a control. On day 3, the medium was removed,
cells were washed twice with PBS and 500 µl 0.5 mg/ml MTT solution
in Opti-MEM™ Reduced-Serum Medium (Thermo Fisher Scientific, Inc.)
was added to each well. After 30 min incubation at 37°C, the
solution was treated with 500 µl DMSO and shaken for 10 min at room
temperature. Finally, the optical density (OD) was measured at 570
nm, and cell viability was calculated using the following formula:
Cell viability (%)=[(OD (sample)-OD (blank)/(OD (control)-OD
(blank)] ×100.
Western blot analysis
MDA-MB-231 cells were treated with various
concentrations of baclofen (0, 25, 50, 100 and 200 µM) for 24 h at
37°C. Cells were lysed in 100–150 µl RIPA buffer (Beyotime
Institute of Biotechnology) supplemented with 10% protease
inhibitor cocktail and Na3VO4, and lysates
were centrifuged at 13,600 × g at 4°C for 10 min. Protein
concentrations were determined using a BCA kit (Pierce; Thermo
Fisher Scientific, Inc.) and subsequently diluted with 4X Laemmli
buffer containing dithiothreitol and heat-denatured and 30 µg of
protein per lane was loaded onto SDS-PAGE gels. Proteins were
separated by SDS-PAGE 10% and transferred to PVDF membranes.
Membranes were blocked with 5% non-fat milk in Tris-buffered saline
with 0.1% Tween-20 (TBST) for 1 h at room temperature, and
incubated with the following primary antibodies: Anti-β-catenin
(rabbit monoclonal; cat. no. #8480; 1:1,000; Cell Signaling
Technology, Inc.), anti-vimentin (rabbit monoclonal; cat. no.
#5741; 1:1,000; Cell Signaling Technology, Inc.), anti-phospho-Akt
(rabbit monoclonal; cat. no. #4060; 1:2,000; Cell Signaling
Technology, Inc.), anti-Akt (rabbit monoclonal; cat. no. #4691;
1:1,000; Cell Signaling Technology, Inc.), anti-phospho-ERK1/2
(rabbit monoclonal; cat. no. #4370; 1:2,000; Cell Signaling
Technology, Inc.), anti-ERK1/2 (rabbit monoclonal; cat. no. #4695;
1:1,000; Cell Signaling Technology. Inc.) and anti-GAPDH (mouse
monoclonal; cat. no. sc-32233; 1:2,000; Santa Cruz Biotechnology,
Inc.) overnight at 4°C. After washing three times with TBST (10 min
each), membranes were incubated with anti-rabbit IgG (cat. no.
#7074; 1:5,000; Cell Signaling Technology, Inc.) and anti-mouse IgG
(cat. no. #7076; 1:5,000; Cell Signaling Technology, Inc.)
HRP-conjugated secondary antibodies for 1 h at room temperature.
Following three additional TBST washes (10 min each), protein band
intensities were visualized using ECL detection reagent (Super
Signal West Pico PLUS; Thermo Fisher Scientific, Inc.) and were
quantified using Image Lab 5.2 software (Bio-Rad Laboratories,
Inc.)
Colony formation assay
A single-cell suspension of MDA-MB-231 cells was
prepared and diluted to a concentration of 200 cells/ml. Aliquots
(1 ml) of this suspension were seeded into each well of a 24-well
plate and incubated at 37°C for 24 h. The L-15 medium was
subsequently replaced with fresh medium containing 10% FBS and
baclofen at various concentrations (0, 25, 50, 100 and 200 µM).
Cells were then incubated at 37°C for 10 days in an atmosphere
lacking CO2. Subsequently, cells were fixed with 300 µl
methanol for 15 min at room temperature, washed with PBS and
stained with 100 µl 1% crystal violet (in a solution of 20%
methanol) for 20 min at room temperature. Plates were gently washed
with water and observed under an inverted light microscope at ×10
magnification. Colonies were defined as a cluster of ≥50 cells.
Colony-forming efficiency was quantified by counting the number of
colonies in each well using ImageJ software (version 1.53a;
National Institutes of Health) and was expressed as a percentage
relative to the control group.
Molecular docking studies
Molecular docking studies were performed using the
open-source program Auto Dock Vina version 1.1.2 (20). A crystal structure of the
GABAB receptor complexed with agonists (PDB ID 4MS4) was
downloaded from the PDB database (https://www.rcsb.org/) (21). All the small, crystallized molecules
were removed, and the structure was optimized by adding polar
hydrogens and computing the Gasteiger charges. A dataset of 1,635
natural lead-like compounds was subsequently retrieved from the
MPD3 database (https://www.bioinformation.info/) (22). A Tanimoto coefficient (TC)
similarity search was then performed against this dataset using
baclofen as a reference, with a cut-off of 0.5. The free chemical
informatics software Open Babel version 2.4.1 (23) was used for this similarity search.
Filtered compounds were optimized, again by adding polar hydrogens
and computing Gasteiger charges. A grid box of size 25×25×25 Å (25
Å3) was centered on the binding pocket. Finally, the
docking results were analyzed based on the interactions between the
docked molecules and the GABAB receptor.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software version 8.0 (GraphPad; Dotmatics). Data are
presented as mean ± standard error of the mean from at least three
independent experiments. One-way ANOVA analysis with Dunnett's post
hoc test was used to compare multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
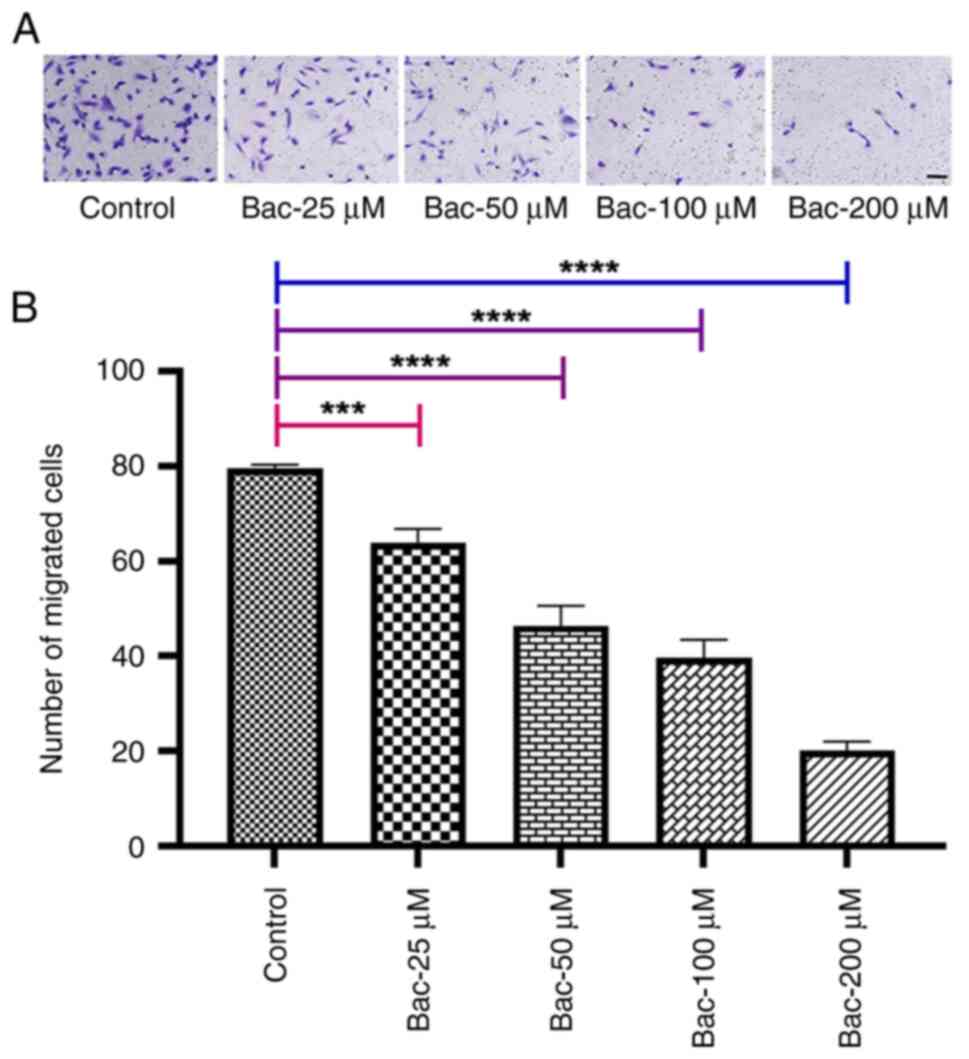
GABAB receptor activation
inhibits cell migration
To investigate the effects of GABAB
receptor activation on cell migration, migration assays were
performed using MDA-MB-231 breast cancer cells treated with various
concentrations of the GABAB receptor agonist, baclofen
(Fig. 1). The results demonstrated
that baclofen treatment significantly reduced cell migration
compared with the control group. This inhibition was
dose-dependent, with significant reductions observed at 25
(P<0.001), 50 (P<0.0001), 100 (P<0.0001) and 200
(P<0.0001) compared with the untreated control. The most
pronounced inhibition was observed at 200 µM. This observed
inhibition of cell migration in MDA-MB-231 cells following baclofen
treatment suggests a crucial role for GABAB signaling in
modulating metastatic potential.
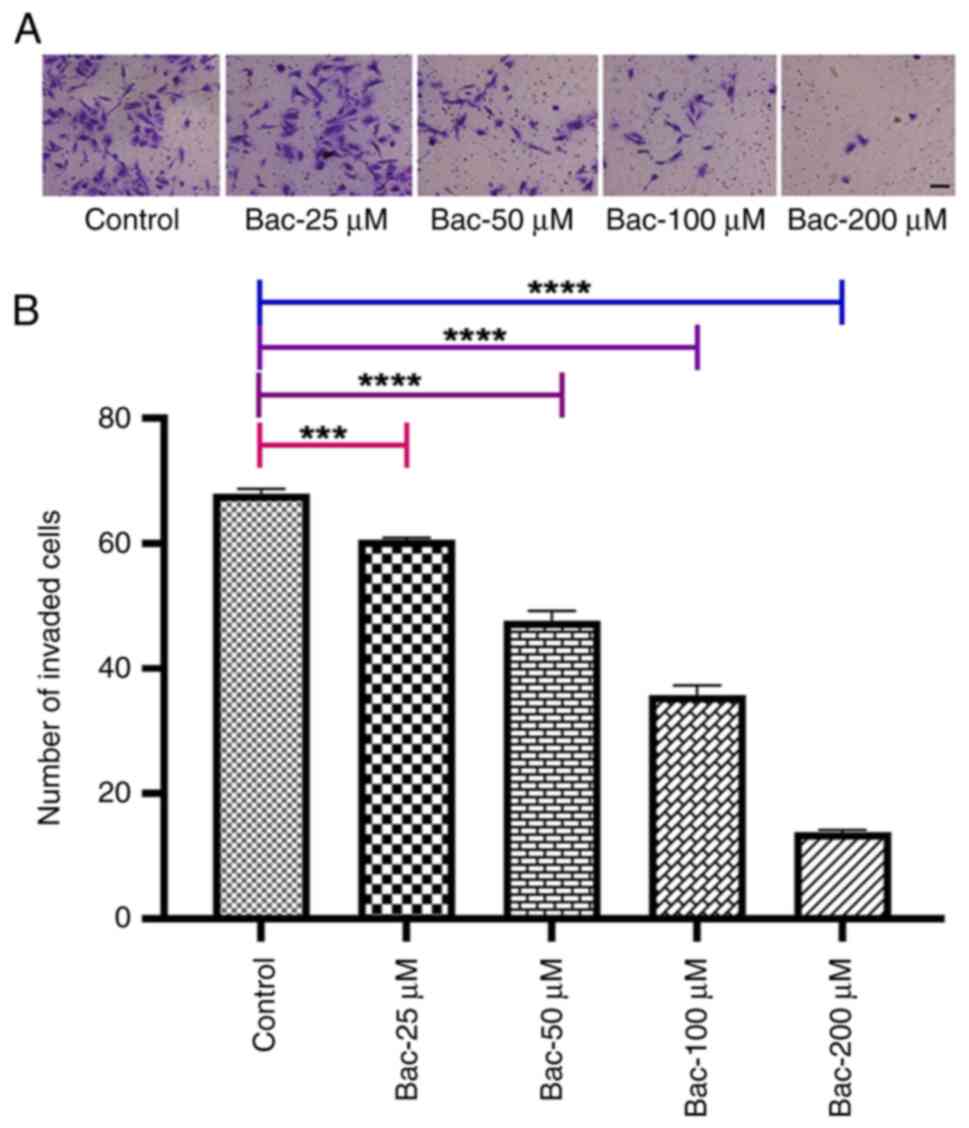
GABAB receptor activation
inhibits cell invasion
To investigate the effect of GABAB
receptor activation on cell invasion, Transwell invasion assays
using MDA-MB-231 cells treated with baclofen were performed
(Fig. 2). The results revealed
dose-dependent inhibition of cell invasion in response to baclofen
treatment. A significant reduction in the number of invaded cells
was observed at the lowest concentration of baclofen tested (25 µM;
P<0.001). This inhibitory effect became more pronounced with
increasing baclofen concentrations, showing enhanced significance
at 50, 100 and 200 µM (all P<0.0001), indicating that a robust,
dose-dependent association existed between GABAB
receptor activation and a decreased invasive capability of the
MDA-MB-231 cells. The observed inhibition of cell invasion in
MDA-MB-231 cells following baclofen treatment also suggests a
crucial role for GABAB signaling in regulating breast
cancer cell metastasis.
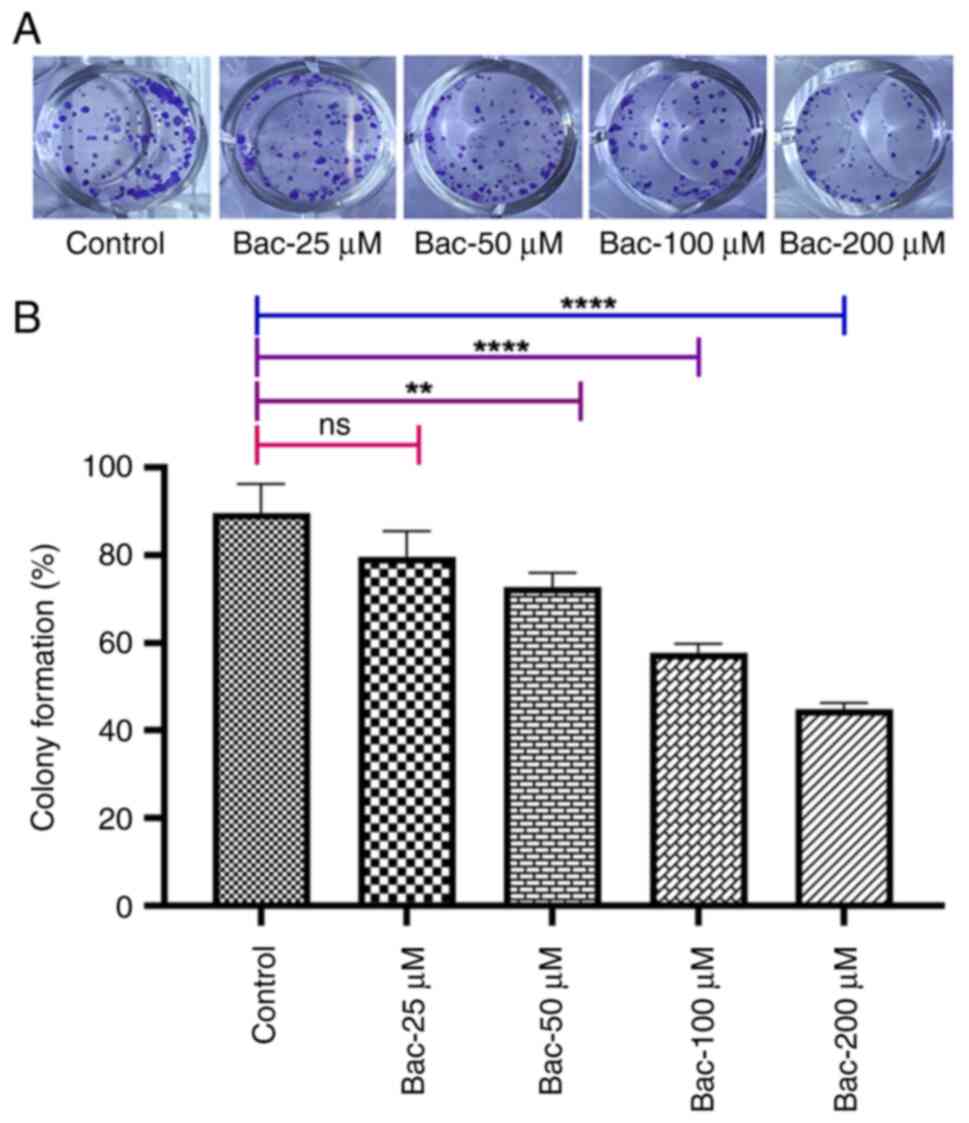
GABAB receptor activation
inhibits cell clonogenicity
To investigate the long-term effects of
GABAB receptor activation on breast cancer cell survival
and proliferation, colony formation assays were performed.
MDA-MB-231 cells were treated with various concentrations of
baclofen (25–200 µM) and the ability of the cells to form colonies
was evaluated over 10 days. The results demonstrated that
GABAB receptor activation by baclofen significantly
inhibited the clonogenicity potential of MDA-MB-231 cells in a
dose-dependent manner (Fig. 3).
Moreover, the lowest concentration of baclofen tested (25 µM) did
not show a significant effect, whereas concentrations of 50 µM
(P<0.01), 100 µM (P<0.001) and 200 µM (P<0.001)
significantly progressively reduced colony formation capabilities.
This inhibitory effect on colony formation, combined with the
observation that baclofen did not significantly affect short-term
cell viability in the MTT assay (Fig.
4), suggested that GABAB receptor activation
preferentially impaired the cells' capacity for sustained
proliferation rather than inducing immediate cytotoxicity.
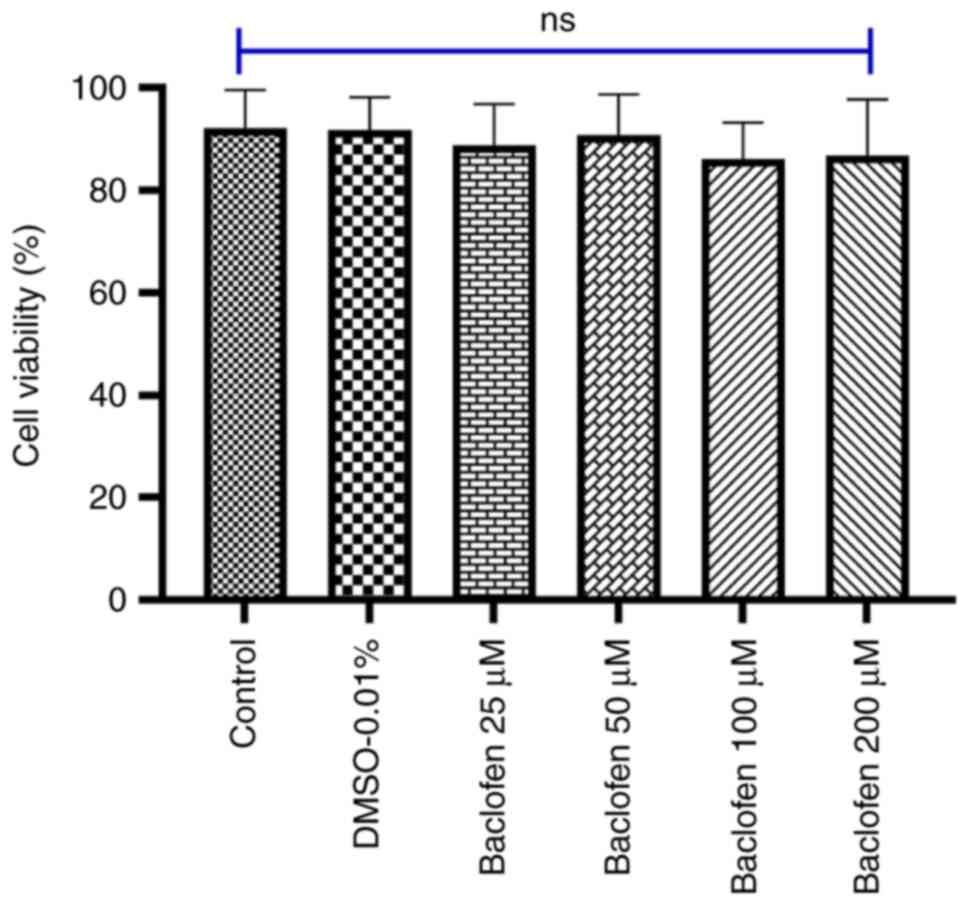
Effect of GABAB receptor
activation on MDA-MB-231 cell viability
The viability of the cells incubated with the
specified concentrations of baclofen is shown in Fig. 4. In the present study, an MTT assay
was performed to examine whether GABAB receptor
activation could inhibit the viability of MDA-MB-231 cells. The MTT
assay results demonstrated that GABAB receptor
activation by baclofen did not significantly affect the viability
of MDA-MB-231 cells across all tested concentrations. Even at the
highest concentration of baclofen tested (200 µM), baclofen
treatment exhibited no significant inhibitory effects on cell
survival.
GABAB receptor activation
modulates EMT markers in breast cancer cells
Subsequently, the effects of GABAB
receptor activation on EMT markers in MDA-MB-231 breast cancer
cells were investigated by examining the protein expression levels
of β-catenin (a mesenchymal marker) and vimentin (an epithelial
marker), with GAPDH serving as a loading control. Western blot
analysis revealed concentration-dependent modulation of EMT markers
following baclofen treatment. The protein expression levels of the
EMT-associated genes βcatenin and vimentin were evaluated as shown
in Fig. 5. While the lowest
concentration of baclofen tested (25 µM) exhibited minimal effects
(P>0.05), administering higher concentrations led to significant
changes in EMT marker expression. β-catenin levels were
significantly increased at 100 µM (P<0.01) and 200 µM
(P<0.001), while vimentin expression was markedly decreased at
these same concentrations (P<0.01 and P<0.01, respectively).
Taken together, these results provide a molecular basis for
understanding how GABAB receptor activation suppresses
metastatic behavior in breast cancer cells.
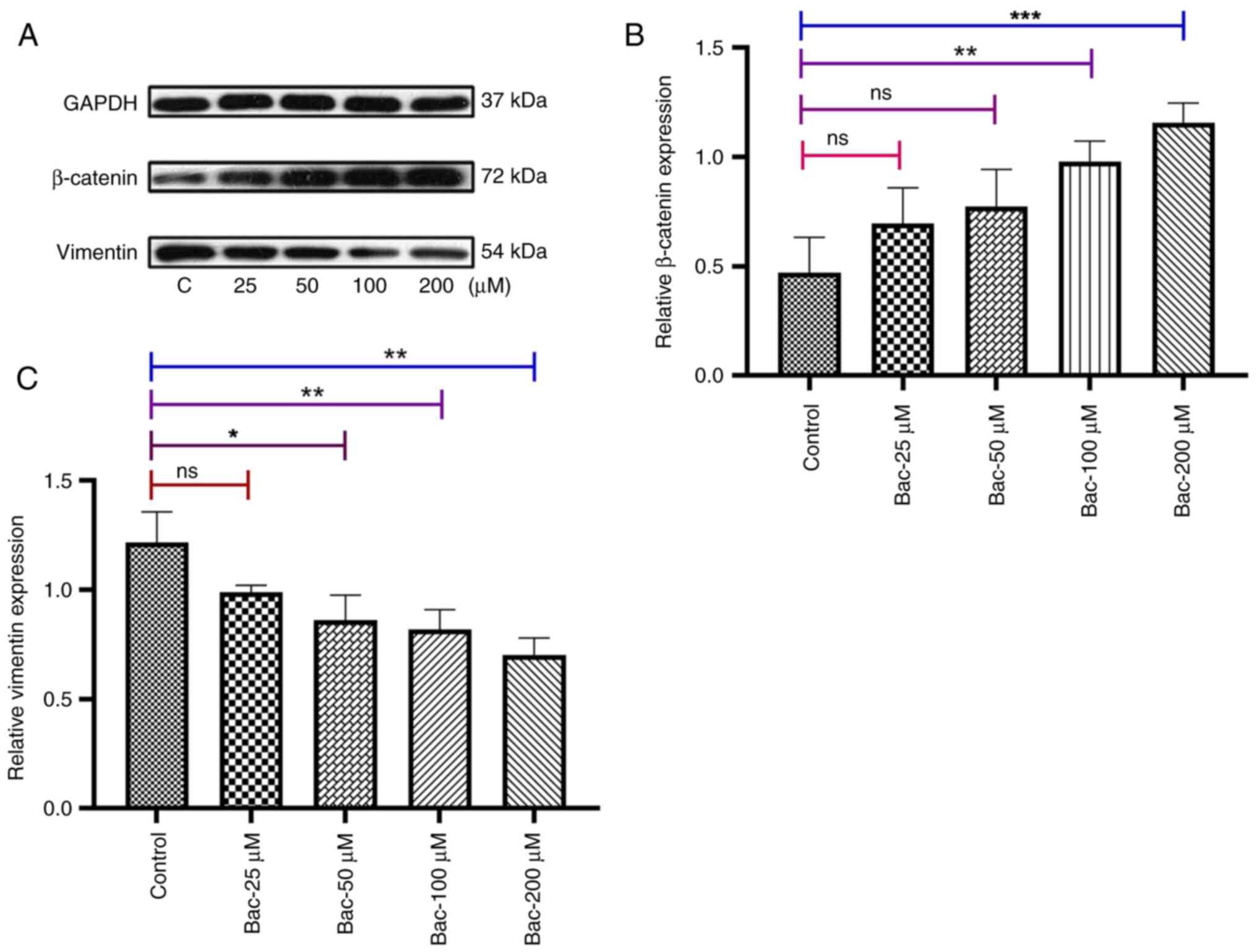 | Figure 5.γ-aminobutyric acid B receptor
activation modulates epithelial-mesenchymal transition markers in
MDA-MB-231 breast cancer cells. (A) Representative immunoblots
showing the expression of β-catenin and vimentin in MDA-MB-231
cells treated with various concentrations of baclofen. Following 24
h of serum starvation, cells were exposed to baclofen (0, 25, 50,
100, 200 µM) for 24 h. GAPDH served as a loading control. (B)
Quantification of the expression of β-catenin relative to GAPDH is
shown. Baclofen treatment induced dose-dependent increases in
β-catenin levels, with significant upregulation observed at
concentrations of 50, 100 and 200 µM. (C) Quantification of the
expression of vimentin relative to GAPDH is shown. A significant
decrease in vimentin expression was observed at a baclofen
concentration of 100 and 200 µM. Data represent the mean ± standard
error of the mean. from at least three independent experiments.
Protein band intensities were quantified using ImageJ software.
Statistical analysis was performed using one-way ANOVA with
Dunnett's post hoc test for comparisons with the control group.
*P<0.05, **P<0.01 and ***P<0.001. ns, not significant; C,
control, Bac, baclofen. |
Effects of activated GABAB
receptors on the P13K/Akt and Ras/Raf/MAPK signaling pathways
Subsequently, the potential involvement of the
PI3K/Akt and Ras/Raf/MAPK signaling pathways in mediating the
effects of the GABAB receptor in breast cancer cells
were investigated. Using western blot analysis with
phosphorylation-specific antibodies, the activation status of both
Akt and ERK1/2 following baclofen treatment was examined. The
results demonstrated that GABAB receptor activation by
baclofen did not significantly alter the phosphorylation levels of
either Akt or ERK1/2 in MDA-MB-231 cells (Fig. 6). These findings suggested that the
anti-metastatic effects of GABAB receptor activation in
MDA-MB-231 cells occured through mechanisms independent of the
PI3K/Akt and Ras/Raf/MAPK signaling pathways, pointing to the
involvement of alternative signaling cascades in mediating these
effects.
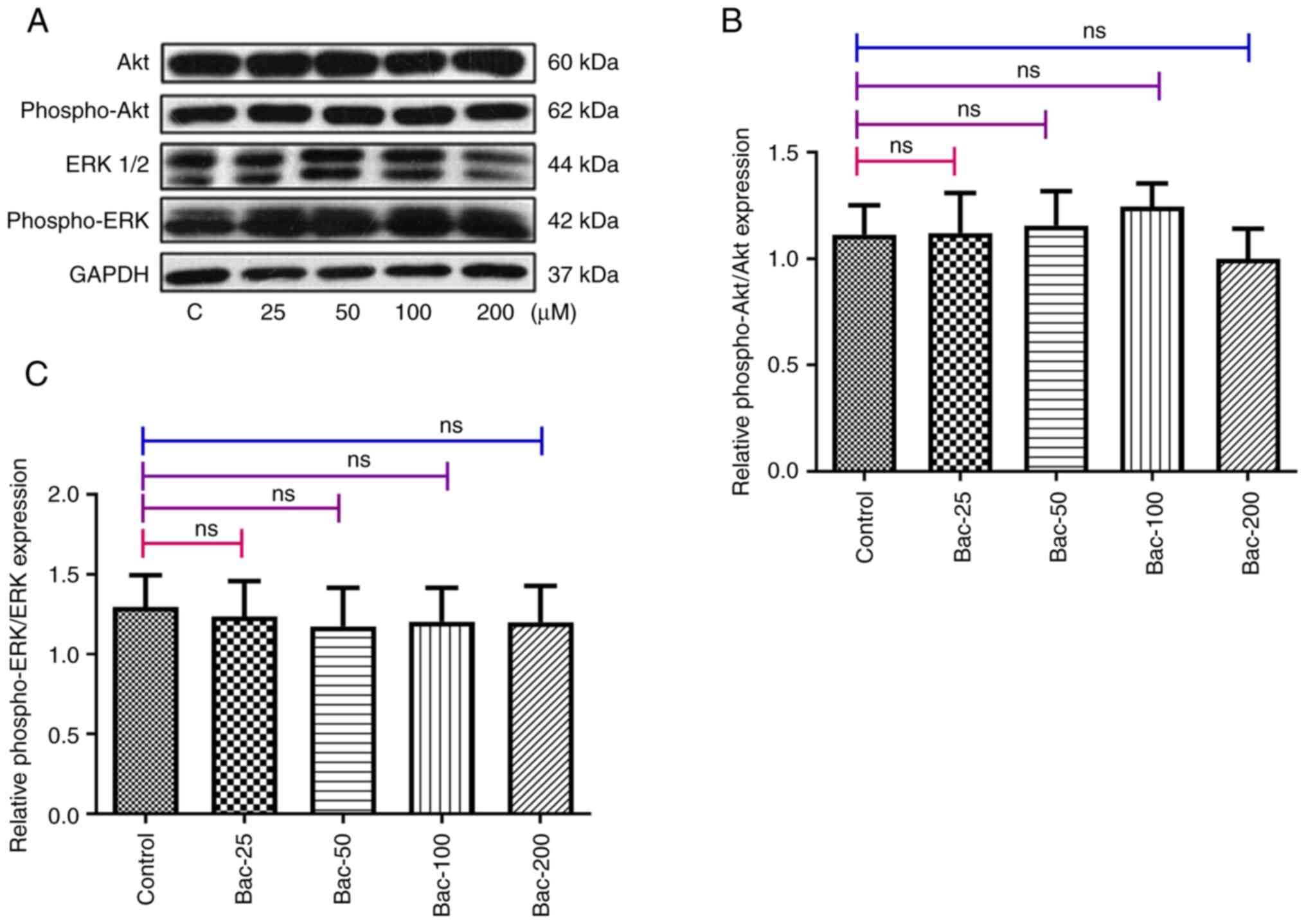 | Figure 6.Effects of γ-aminobutyric acid B
receptor activation on the P13K/Akt and Ras/Raf/MAPK signaling
pathways. (A) After 24 h of treatment with different concentrations
of baclofen (25, 50, 100 and 200 µM), the phosphorylation levels of
Akt and ERK1/2 were found not to have been significantly altered.
(B) Quantification of phospho-Akt/Akt. (C) Quantification of
phospho-ERK/ERK. The experiments were repeated three times with
similar results. Protein quantification was performed using ImageJ
software. Statistical analysis was performed using one-way ANOVA
with Dunnett's post hoc test for comparisons with the control
group. The data are shown as the mean ± standard error of the mean.
ERK, extracellular signal-regulated kinase; PI3K,
phosphatidylinositol 3-kinase; Akt, protein kinase B; MAPK,
mitogen-activated protein kinase; ns, not significant; Bac,
baclofen; phospho, phosphorylated. |
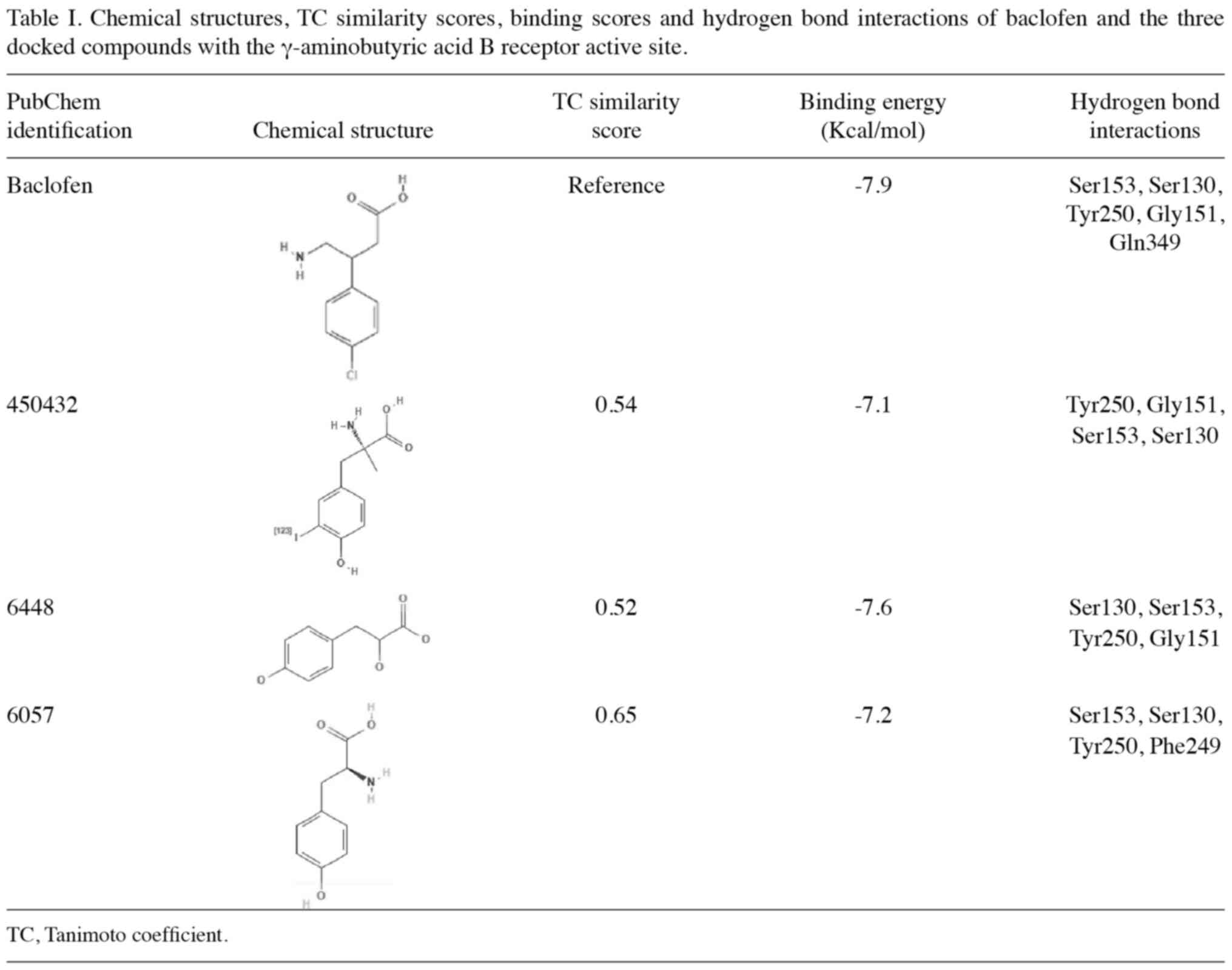
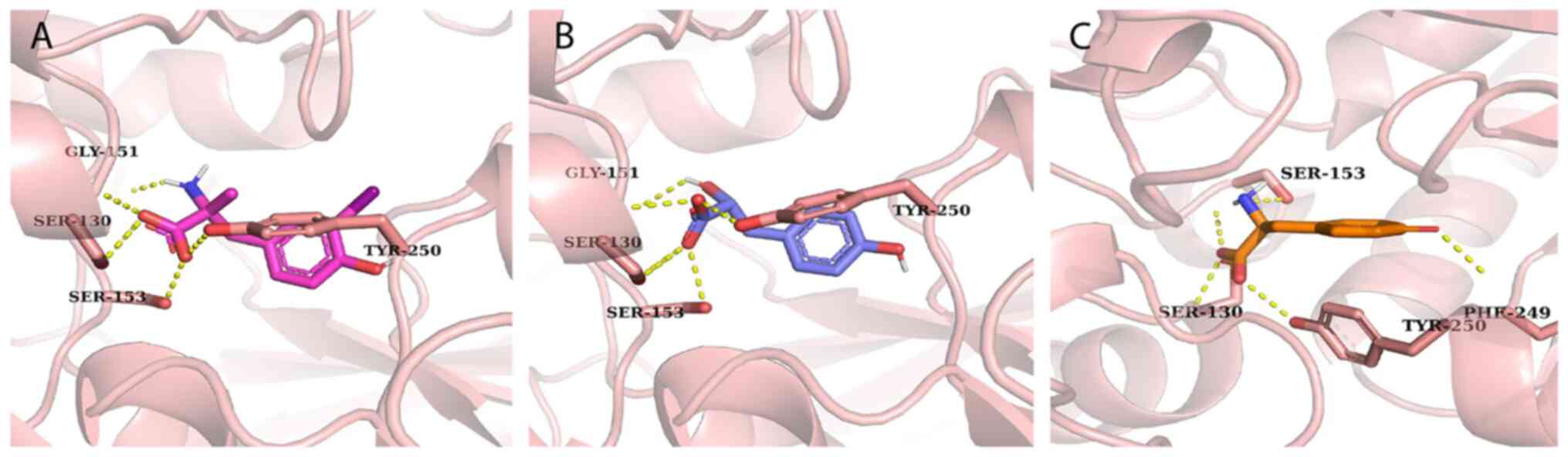
Molecular docking and interaction
analysis with GABAB receptor
Analysis of GABAB receptor binding
identified five key residues (Ser-130, Ser-153, Tyr-250, Gly-151
and Glu-249) that form hydrogen bond interactions with baclofen, as
shown in Fig. S1. Using a TC-based
similarity search against 1,635 natural lead-like compounds from
the MPD3 database, 17 compounds were identified with coefficients
>0.4. Molecular docking analysis using Autodock Vina revealed
that six compounds (PubChem identification, 450432, 31226, 441457,
6448, 10394 and 6057) formed three common hydrogen bond
interactions with critical residues (including Ser-130, Ser-153 and
Tyr-250) of the GABAB receptor (Table SI).
Further detailed binding interaction analysis,
comparing both structural similarity scores (TC) and binding
energies, identified three compounds with particularly favorable
profiles (Table I). These compounds
showed high structural similarity to baclofen (TC, 0.54–0.65) and
comparable binding energies (−7.1 to −7.6 kcal/mol vs. −7.9
kcal/mol for baclofen). Specifically, compound 450432 (TC=0.54;
binding energy=−7.1 kcal/mol) formed hydrogen bonds with Tyr-250,
Gly-151, Ser-153 and Ser-130; compound 6448 (TC=0.52; binding
energy=−7.6 kcal/mol) interacted with Ser-130, Ser-153, Tyr-250 and
Gly-151; and compound 6057 (TC=0.65; binding energy=−7.2 kcal/mol)
formed hydrogen bonds with Ser-130, Ser-153, Tyr-250 and Phe-249.
The docked conformations and hydrogen bond interactions are
illustrated in Figs. 7 and S2.
Discussion
The present study aimed to evaluate the role of
GABAB receptor activation in breast cancer progression
through complementary experimental and computational approaches.
The results have provided insights into how GABAB
receptor activation regulates breast cancer metastasis by
selectively modulating cellular behavior and EMT programming. The
results demonstrate that GABAB receptor activation
significantly inhibits metastatic phenotypes in MDA-MB-231 breast
cancer cells, as evidenced by dose-dependent reductions in both
cell migration and invasion following baclofen treatment.
Furthermore, the rapid onset of these inhibitory effects, observed
within 24 h baclofen administration, indicates direct modulation of
cellular machinery controlling movement, including cytoskeletal
reorganization pathways, cell-matrix adhesion molecules and actin
polymerization regulators that are essential for cellular motility
and invasiveness. These findings extend observations previously
made by Gao et al (16) in
ovarian cancer cells, who demonstrated that baclofen inhibited
migration, invasion and EMT in SKOV3 cells. Lodewyks et al
(24) reported that in Huh-7
hepatocellular carcinoma cells, baclofen inhibited cell migration
without affecting proliferation and Zhang et al (19) in breast cancer cell lines (4T1 and
MCF-7), found that GABAergic signaling influenced metastasis
through ERK1/2 phosphorylation, suggesting that a conserved
mechanism exists across diverse cancer types.
The analysis of clonogenicity and cell survival in
the present study revealed an effect of GABAB receptor
activation on cancer cell behavior. Treating the cells with
baclofen, a GABAB receptor agonist, caused significant
inhibition of colony formation at concentrations as low as 50 µM,
while preserving short-term cell viability even at a concentration
of 200 µM. These results align with the observations made in the
study by Zhang et al (19)
regarding cell viability in vitro. Furthermore, the observed
differential effect between acute survival and long-term
proliferation implied a specific targeting of sustained growth
pathways by baclofen rather than any immediate triggering of cell
death mechanisms. This selective inhibition of metastatic behavior
without direct cytotoxicity suggests baclofen could potentially
serve as an anti-metastatic agent that specifically prevents cancer
spread while minimizing adverse effects on healthy tissues. Such
targeted anti-metastatic therapies represent an important clinical
need, as metastasis is responsible for ~90% of cancer-related
deaths (2). By inhibiting invasion
pathways and EMT programming without inducing widespread cell
death, GABA-B receptor agonists like baclofen might be particularly
valuable in combination with conventional cytotoxic therapies or as
maintenance treatment to prevent disease progression and metastatic
dissemination in high-risk patients with minimal additional
systemic toxicity.
At the molecular level, the modulation of EMT
markers provides crucial mechanistic insights into how
GABAB receptor activation mediates metastasis
suppression (25). The plasticity
of EMT is evident in its classification into three distinct types
(I, II and III), each serving diverse roles in biological processes
ranging from embryogenesis and organ development to wound healing
and tumor metastasis (26). The
increase in β-catenin expression that was observed in the present
study, which occurred concurrently with a decrease in the levels of
vimentin, indicated a transition toward a less metastatic
phenotype. This molecular reprogramming was aligned with the
functional observations made in the Transwell assay experiments
showing reduced cell migration and invasion. Taken together, these
findings corroborate those of the study by Gao et al
(16), who reported that baclofen
inhibits EMT in ovarian cancer cells, suggesting a direct
regulation of EMT programming through GABAB receptor
activation. The mechanism likely involves GABA-B receptor-mediated
modulation of key transcription factors that govern EMT. Upon
GABA-B receptor activation, the resulting G protein-coupled
signaling appears to stabilize epithelial phenotype markers like
β-catenin while suppressing mesenchymal markers such as vimentin.
This effect may be mediated through inhibition of transcriptional
repressors of E-cadherin such as snail family transcriptional
repressor 1, ZEB1 and twist related protein 1, which are known
master regulators of the EMT process (7). As the present results showed that this
occured independently of PI3K/Akt and MAPK pathways, this suggested
that GABA-B receptors may influence EMT transcription factors
through alternative signaling intermediates, possibly involving
regulation of intracellular calcium levels or modifications to
chromatin remodeling complexes that control EMT gene
expression.
The PI3K/Akt signaling pathway is a notable
intracellular signaling pathway that serves important roles in cell
growth and cell proliferation. Abnormal activation of this pathway
has been observed in numerous human cancers, including breast,
lung, ovarian and prostate cancer (27). The present study revealed that the
GABAB receptor-mediated anti-metastatic effects occurred
independently of PI3K/Akt signaling in breast cancer cells, as
evidenced by the unchanged phosphorylation levels. This finding
stands in contrast with previous observations made in neuronal
systems, where, in a previous study, baclofen was found to activate
Akt through transactivation of the insulin-like growth factor 1
receptor (28). That the
GABAB receptor-mediated anti-metastatic effects were
found to occur independently of the PI3K/Akt pathway suggests the
operation of tissue-specific signaling mechanisms, and that
alternative pathways may exist to mediate the anti-metastatic
effects. Alternative mechanisms could potentially be involved,
including modulation of Rho GTPases controlling cytoskeletal
dynamics, JAK/STAT signaling, calcium channel inhibition affecting
adhesion, cAMP/PKA pathway influencing gene expression or
Wnt/β-catenin signaling through GSK3β regulation.
The molecular docking analysis provided notable
insights into potential natural GABAB receptor agonists.
At present, baclofen [β-(4-chlorophenyl)] represents the only
marketed GABAB receptor agonist (29), and it is primarily used as a muscle
relaxant and antispastic agent (30). The identification of natural
compounds with similar binding profiles to baclofen is particularly
important, given the therapeutic potential of phytochemicals, which
historically have been shown to exhibit reduced side effects
compared with synthetic compounds (31). The three compounds that were
identified in the present study to have enhanced hydrogen bonding
profiles compared with baclofen warrant attention. Their
interaction patterns with key receptor residues suggest potential
agonist activity, which may provide new scaffolds for drug
development. Both the favorable docking scores and specific
hydrogen bonding interactions suggest that these compounds may
serve as promising leads for developing novel GABAB
receptor-targeted therapies.
Several key questions emerge from these findings. A
primary limitation of the present study is the use of a single cell
line (MDA-MB-231). However, the findings align with and extend
previous research across multiple cancer models, and future
validation in additional breast cancer cell lines such as SK-BR-3
(HER2-positive) and T-47D (hormone receptor-positive) would further
strengthen these findings. Interestingly, the present findings
appear to contrast with those reported by Gao et al
(16), who demonstrated that
baclofen, a GABAB receptor agonist, inhibited
proliferation, migration, invasion and EMT in ovarian cancer cells
by activating the GABAB1 receptor. By contrast, the
present results demonstrated that baclofen treatment promoted these
processes in MDA-MB-231 breast cancer cells, while the antagonist
CGP inhibited them. This apparent discrepancy with the present
results may reflect tissue-specific differences in GABAB
receptor signaling between ovarian and breast cancer cells.
Similarly, Kanbara et al (17) reported that the GABAB
receptor antagonist CGP had antitumor effects in chondrosarcoma
cells, which aligns with the present observations in breast cancer.
These conflicting findings underscore the complexity of GABAergic
signaling in different cancer types and suggest that
GABAB receptor modulation may elicit distinct, and
sometimes opposing, effects depending on the specific cancer tissue
context. This tissue-specificity in GABAB receptor
signaling might be explained by differences in downstream signaling
pathways or receptor coupling to various effector systems across
different cell types. Future studies comparing GABAB
receptor signaling mechanisms in these different cancer types would
help elucidate the molecular basis for these differential
responses. Additionally, the lack of in vivo experiments
represents another notable limitation, as these findings need to be
validated in a physiological context. Another important limitation
was the use of single time points for each assay, which restricts
the ability to fully distinguish between immediate and delayed
effects of GABA-B receptor activation. This limitation particularly
affects our understanding of the temporal dynamics of the observed
anti-metastatic effects and whether they represent transient or
sustained responses. Elucidating the complete signaling cascade
that links GABAB receptor activation with EMT modulation
also requires further study, as does identifying the potential
impact on the tumor microenvironment. Furthermore, experimental
validation of the computationally identified compounds represents a
crucial next step in drug development.
In conclusion, baclofen, an agonist of the
GABAB receptor, has been widely used for treating
various diseases associated with GABA in previous years. Previous
studies have demonstrated that baclofen exhibits marked regulatory
effects in carcinoma (32,33). The present study has demonstrated
that GABAB receptor activation functions as a selective
regulator of breast cancer metastasis through multiple mechanisms.
The present study has also demonstrated that baclofen treatment
significantly inhibited the migratory, invasive and clonogenic
properties of MDA-MB-231 cells through the modulation of EMT
markers, as evidenced by the downregulation of vimentin and
upregulation of β-catenin. These effects were found to occur
independently of the PI3K/Akt and ERK1/2 signaling pathways,
suggesting the existence of alternative signaling mechanisms. The
present results may provide a future direction for the clinical
treatment of breast cancer with GABAB receptor agonists
and help to elucidate the role and viable mechanism of the
GABAB receptor in breast cancer. In addition to these
biological findings, the computational analysis identified three
promising natural compounds with potential GABAB
receptor agonist activity. Collectively, these findings have
extended current understanding of GABAB receptor
function in breast cancer and offered additional mechanistic
insights for targeted breast cancer treatments. These findings
further validated and extended previous observations regarding
GABAB receptor activation in breast cancer,
demonstrating specific effects on metastatic potential in
MDA-MB-231 cells.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present research was funded by the Researchers Supporting
Project number (grant no. RSP2025R491), King Saud University,
Riyadh, Saudi Arabia.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization, methodology, formal analysis and
visualization were performed by MHA. Validation of experimental
results was performed by ZYA, HHA, AA, NAA and ZTM through
independent data verification, experiment replication, and critical
assessment for reliability. Data collection was performed by MHA,
ZYA, HHA, AA, NAA and ZTM. MHA wrote the original draft and ZYA,
HHA, AA, NAA and ZTM revised and edited the manuscript. Project
administration and funding acquisition were performed by MHA, NAA
and ZTM. MHA and ZTM confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
ZEB1
|
zinc finger E-box binding homeobox
1
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
PBS
|
phosphate-buffered saline
|
|
GABA
|
γ-aminobutyric acid
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
protein kinase B
|
|
MAPK
|
mitogen-activated protein kinase
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
References
|
1
|
Smith RA, von Eschenbach AC, Wender R,
Levin B, Byers T, Rothenberger D, Brooks D, Creasman W, Cohen C,
Runowicz C, et al: American Cancer Society guidelines for the early
detection of cancer: Update of early detection guidelines for
prostate, colorectal, and endometrial cancers: Also: Update
2001-testing for early lung cancer detection. CA Cancer J Clin.
51:38–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eccles SA, Aboagye EO, Ali S, Anderson AS,
Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant
HE, et al: Critical research gaps and translational priorities for
the successful prevention and treatment of breast cancer. Breast
Cancer Res. 15:R922013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Memon MA, Abbasi F, Abbasi IHR, Mughal GA,
Soomro RN and Memon AS: Surgical approaches to cat breast cancer
(Mammary tumor), their treatment and management at Richmond
Crawford Veterinary Hospital Karachi (RCVH), Sindh, Pakistan. ARC J
Animal and Veterinary Sciences (AJAVS). 2:23–28. 2016.
|
|
5
|
Chang JW, Ding Y, Ul Qamar MT, Shen Y, Gao
J and Chen LL: A deep learning model based on sparse autoencoder
for prioritizing cancerrelated genes and drug target combinations.
Carcinogenesis. 40:624–632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
KudoSaito C, Shirako H, Ohike M, Tsukamoto
N and Kawakami Y: CCL2 is critical for immunosuppression to promote
cancer metastasis. Clin Exp Metastasis. 30:393–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang H, Yu T, Han Y, Jiang H, Wang C, You
T, Zhao X, Shan H, Yang R, Yang L, et al: LncRNA PTAR promotes EMT
and invasionmetastasis in serous ovarian cancer by competitively
binding miR1013p to regulate ZEB1 expression. Mol Cancer.
17:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumor Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH,
Chen PJ and Cheng AL: Activation of phosphatidylinositol
3-kinase/Akt signaling pathway mediates acquired resistance to
sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther.
337:155–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahajan K and Mahajan NP: PI3K-independent
AKT activation in cancers: A treasure trove for novel therapeutics.
J Cell Physiol. 227:3178–3184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vithlani M, Terunuma M and Moss SJ: The
dynamic modulation of GABAA receptor trafficking and its role in
regulating the plasticity of inhibitory synapses. Physiol Rev.
91:1009–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian H, Wu JX, Shan FX, Zhang SN, Cheng Q,
Zheng JN and Pei DS: Gamma aminobutyric acid induces tumor cells
apoptosis via GABABR1·β-arrestins·JNKs signaling module. Cell
Biochem Biophys. 71:679–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tatsuta M, Ishi H, Baba M, Nakaizumi A,
Ichii M and Taniguchi H: Inhibition by γ-amino-n-butyric acid and
baclofen of gastric carcinogenesis induced by
N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res.
50:4931–4934. 1990.PubMed/NCBI
|
|
16
|
Gao J, Gao Y, Lin S, Zou X, Zhu Y, Chen X,
Wan H and Zhu H: Effects of activating GABAB1 receptor on
proliferation, migration, invasion and epithelialmesenchymal
transition of ovarian cancer cells. J Ovarian Res. 13:1262020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanbara K, Otsuki Y, Watanabe M, Yokoe S,
Mori Y, Asahi M and Neo M: GABA B receptor regulates proliferation
in the highgrade chondrosarcoma cell line OUMS27 via apoptotic
pathways. BMC Cancer. 18:2632018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Solorzano SR, ImazRosshandler I,
CamachoArroyo I, García-Tobilla P, Morales-Montor G, Salazar P,
Arena-Ortiz ML and Rodríguez-Dorantes M: GABA promotes
gastrinreleasing peptide secretion in NE/NElike cells: Contribution
to prostate cancer progression. Sci Rep. 8:102722018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Li X, Yao Z, Wei C, Ning N and Li
J: GABAergic signaling facilitates breast cancer metastasis by
promoting ERK1/2dependent phosphorylation. Cancer Lett.
348:100–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sussman JL, Lin D, Jiang J, Manning NO,
Prilusky J, Ritter O and Abola E: Protein data bank (PDB): Database
of threedimensional structural information of biological
macromolecules. Acta Crystallogr D Biol Crystallogr. 54:1078–1084.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mumtaz A, Ashfaq U, Qamar M, Anwar F,
Gulzar F, Ali MA, Saari N and Pervez MT: MPD3: A useful medicinal
plants database for drug designing. Nat Prod Res. 31:1228–1236.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Boyle NM, Banck M, James CA, Morley C,
Vandermeersch T and Hutchison GR: Open babel: An open chemical
toolbox. J Cheminform. 3:332011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lodewyks C, Rodriguez J, Yan J, Lerner B,
Lipschitz J, Nfon C, Rempel JD, Uhanova J and Minuk GY: GABAB
receptor activation inhibits the in vitro migration of malignant
hepatocytes. Can J Physiol Pharmacol. 89:393–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jolly MK and Celià-Terrassa T: Dynamics of
phenotypic heterogeneity associated with EMT and stemness during
cancer progression. J Clin Med. 8:15422019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu
Y, Yao Y and Li D: The epithelial to mesenchymal transition (EMT)
and cancer stem cells: Implication for treatment resistance in
pancreatic cancer. Mol Cancer. 16:522017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Myers AP and Cantley LC: Targeting a
common collaborator in cancer development. Sci Transl Med.
2:48ps452010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu H, Xu C, Zhang W, Liu Q, Rondard P, Pin
JP and Liu J: GABAB receptor activation protects neurons from
apoptosis via IGF-1 receptor transactivation. J Neurosci.
30:749–759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bowery NG, Doble A, Hill DR, Hudson AL,
Shaw JS, Turnbull MJ and Warrington R: Bicucullineinsensitive GABA
receptors on peripheral autonomic nerve terminals. Eur J Pharmacol.
71:53–70. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Herman RM, D'Luzansky SC and Ippolito R:
Intrathecal baclofen suppresses central pain in patients with
spinal lesions. A pilot study. Clin J Pain. 8:338–345. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choudhari AS, Mandave PC, Deshpande M,
Ranjekar P and Prakash O: Phytochemicals in cancer treatment: From
preclinical studies to clinical practice. Front Pharmacol.
10:16142020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kirui JK, Xie Y, Wolff DW, Jiang H, Abel
PW and Tu Y: Gbetagamma signaling promotes breast cancer cell
migration and invasion. J Pharmacol Exp Ther. 333:393–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Calver A, Medhurst A, Robbins M, Charles
KJ, Evans ML, Harrison DC, Stammers M, Hughes SA, Hervieu G, Couve
A, et al: The expression of GABAB1 and GABAB2 receptor subunits in
the CNS differs from that in peripheral tissues. Neuroscience.
100:155–170. 2000. View Article : Google Scholar : PubMed/NCBI
|