Introduction
Malignant mesothelioma (MM) is a rare cancer, which
poses a formidable challenge to clinicians. Individuals carrying
germline mutations in the tumor suppressor gene BRCA1-associated
protein 1 (BAP1) are predisposed to develop MM; however,
familial cases are seldom reported (1). The prognostic significance of
BAP1 mutations in predicting response to systemic therapies
remains elusive. In the systemic treatment of MM, conventional
chemotherapy offers only a moderate survival benefit and is
associated with poor outcomes (2).
Of note, the dual immune checkpoint inhibitors (ICIs) regimen of
nivolumab plus ipilimumab has become a standard first-line
treatment for MM, significantly extending overall survival compared
to chemotherapy in a phase 3 trial (median overall survival: 18.1
vs. 14.1 months; hazard ratio, 0.74; P=0.0020) (2). The current study presented a Chinese
patient and their family whose hereditary MMs were associated with
a novel BAP1 frameshift mutation, underscoring the
importance of genetic factors in MM pathogenesis, and reported on
the efficacy of dual ICIs treatment in this context.
Case presentation
A 48-year-old male patient with no history of
asbestos exposure was admitted to Peking Union Medical College
Hospital (Beijing, China) for recurrent pleural and peritoneal
effusion in December 2022. The patient had presented with bilateral
hydropneumothorax at the age of 30 years. The disease was resistant
to anti-tuberculosis (TB) treatment and lingered on into the
patient's forties. The patient suffered from another episode of
pleural and peritoneal effusion since January 2022. Symptoms
including abdominal swelling and pain persisted after 9 months of
the standard 4-drug anti-TB regimen. A positive blood T-SPOT.TB
assay but negative findings of the Xpert MIB/RIF test, acid-fast
staining and bacterial culture test using peritoneal effusion
suggested no active TB infection. An abdominopelvic MRI showed a
collapsed right rib cage, thickened right pleural membrane, omentum
and pelvic peritoneum, as well as large effusion. A diagnostic
laparoscopy was performed in late December 2022 and a biopsy to
nodules throughout the omentum uncovered an MM with
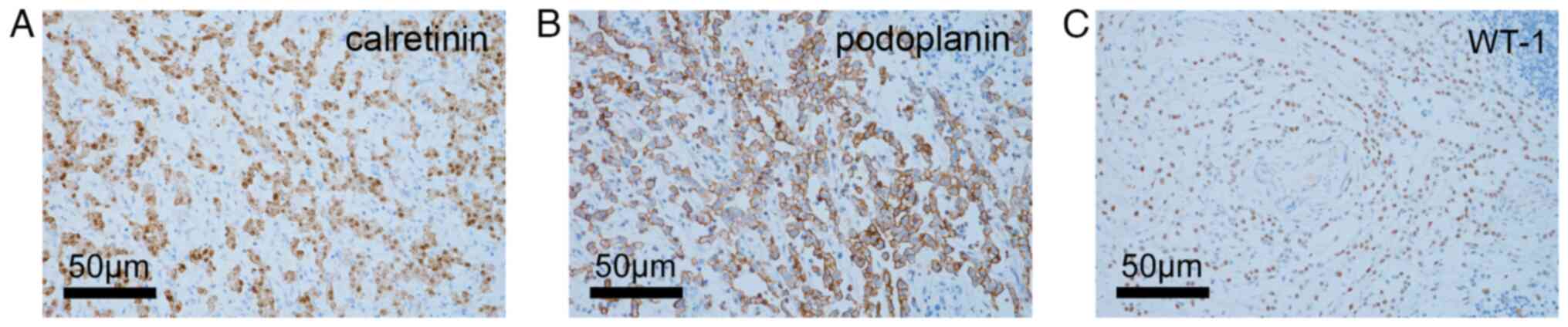
immunohistochemistry (IHC) positive for calretinin, podoplanin and
Wilms' tumor 1 (WT-1) (Fig. 1A-C).
Another IHC test indicated a programmed death 1 (PD-1) ligand 1
(PD-L1) (22C3) combined positive score (CPS) of 10. IHC staining
was performed using an automated immunostainer (Ventana BenchMark
ULTRA; Roche Diagnostics), following the manufacturer's recommended
standard protocol. The antibodies utilized were anti-calretinin
(cat. no. PA0346; Leica Biosystems), anti-podoplanin (cat. no.
IR0720), anti-WT-1 (cat. no. IR055) and anti-PD-L1 (cat. no. M3653;
all from DAKO; Agilent Technologies, Inc.). PD-L1 expression was
assessed using the CPS, calculated as the number of PD-L1-stained
cells (tumor cells, lymphocytes, macrophages) divided by the total
number of viable tumor cells, multiplied by 100 (3). In line with clinical practice and
prior investigations, the predefined cutoff was set at 1 (3).
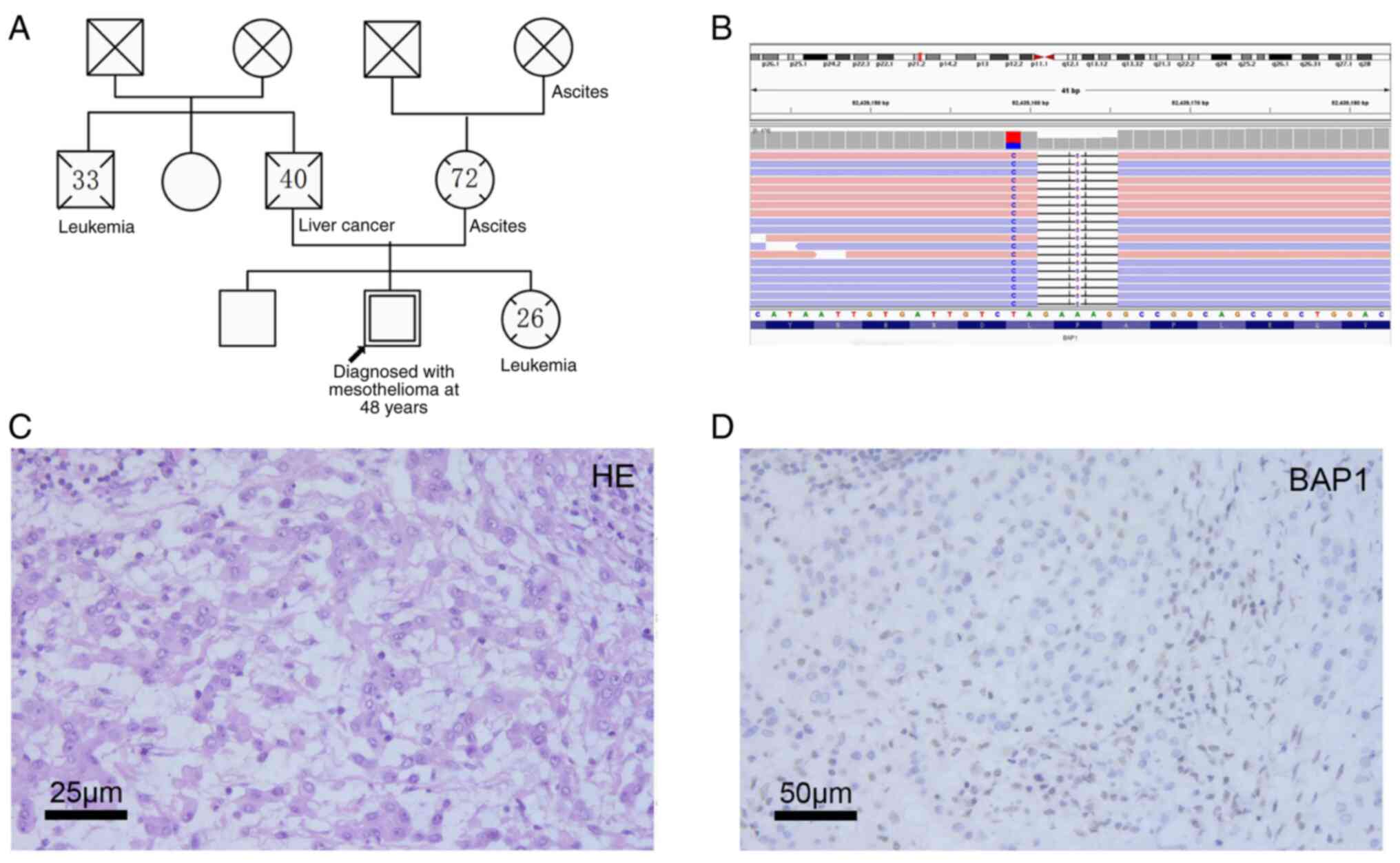
The patient has a maternal history of suspected
peritoneal mesotheliomas. The patient's mother and grandmother both
died in their seventies due to tenacious peritoneal effusions after
rounds of unsuccessful anti-TB treatment. Furthermore, the
patient's younger sister had died of acute leukemia at age 26. The
patient's family cancer history is shown in a pedigree chart in
Fig. 2A. Next-generation sequencing
(NGS) was performed using a 520-gene panel for multiple solid
tumors. Libraries were prepared using a target enrichment method,
followed by sequencing on the NextSeq 550 Dx platform (Illumina,
Inc.). NGS analysis of the tumor tissue and the patient's control
blood sample revealed a novel, heterozygous, germline frameshift
mutation of the BAP1 gene (c.1077_1083delinsTG, pPhe360fs)
(Fig. 2B), which was interpreted as
likely pathogenic, since it may lead to protein loss through
nonsense-mediated mRNA decay by introducing a termination codon
(4). The Combined Annotation
Dependent Depletion score of this mutation was 34 and the minor
allele frequency was 51.88% (5).
Copy number variation gain of the Ataxia-Telangiectasia mutated
(ATM) gene was also observed, which was a 3.9-fold gain. The tumor
mutation burden was 0 mutations/Mb. Tissue samples were fixed in
10% neutral-buffered formalin at room temperature for 24 h.
Sections (4 µm) were stained with hematoxylin for 5 min and eosin
for 2 min, then examined under a light microscope. The hematoxylin
and eosin (H&E) staining showed heterotypical cells
infiltration in omentum (Fig. 2C),
and IHC showed nuclear total loss of the BAP-1 protein in tumor
cells but not in immune cells (Fig.
2D), indicating loss of function in the tumor. The IHC
protocols have been described previously. The anti-BAP1 antibody
(used at 1:50 dilution) was obtained from Abcam (cat. no.
EPR22826-65). Regrettably, the patient did not consent to any
genetic analysis and consultation provided to their child and
samples of the patient's living maternal relatives were
unavailable.
According to positive PD-L1 expression and the
patient's wishes, nivolumab (Opdivo; Bristol Myers Squibb) 3 mg/kg
every 2 weeks and ipilimumab (Yervoy; Bristol Myers Squibb) 1 mg/kg
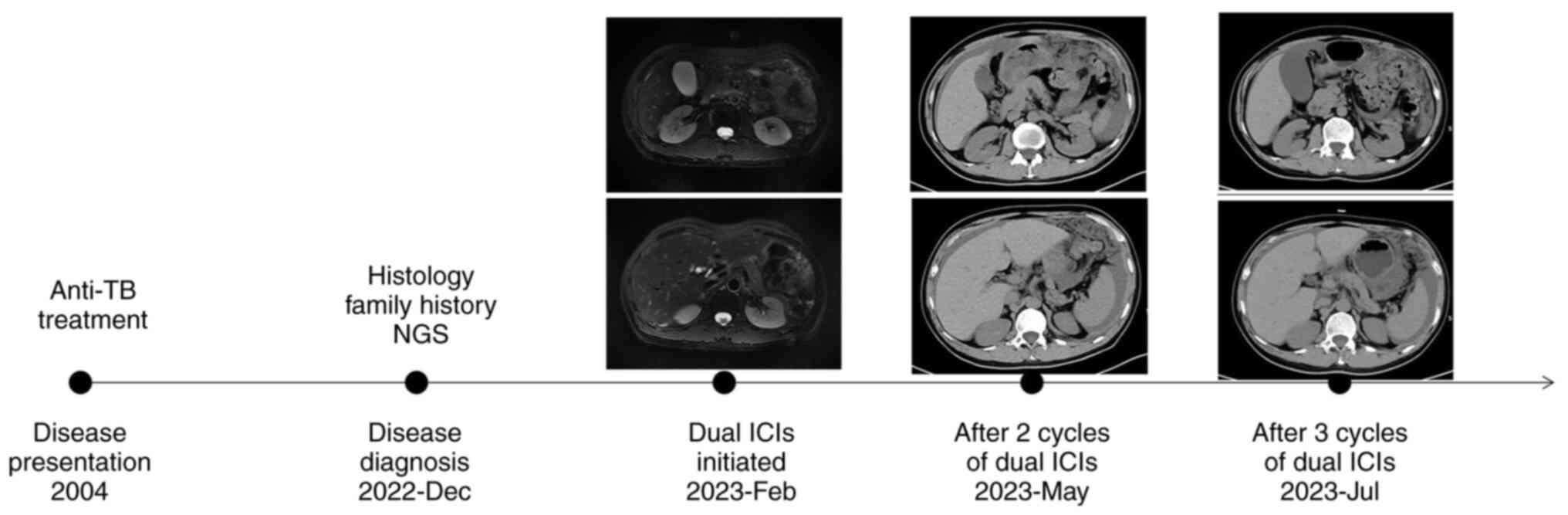
every 6 weeks were prescribed since February 2023. After 2 cycles,
the patient's symptoms were slightly alleviated but computed
tomography (CT) imaging showed stable disease (Fig. 3). However, the patient developed
Steven-Johnson syndrome (SJS) after the 3rd cycle of dual ICIs,
resulting in a disruption of ICI treatment. SJS symptoms were
relieved after 80 mg/day (1.5 mg/kg) methyprednisolone (Pfizer) for
7 days, followed by 60 mg/day (1.0 mg/kg) prednisone (Tianjin
Lisheng Pharmaceutical) for 5 days, then tapered by 10 mg every 3
days until 10 mg/day, followed by a further taper of 5 mg every 7
days until completion. At two months after the cessation of
steroids, the patient received 3 cycles of combination therapy with
pemetrexed (Huiyu Pharmaceutical) 500 mg/m2, cisplatin
(Qilu Pharmaceutical) 75 mg/m2 and bevacizumab (Avastin;
Roche) 15 mg/kg, every 3 weeks. CT following the 3rd cycle of
therapy showed a reduction of pleural and peritoneal effusion but
unchanged thickening of pleura and omentum. Considering the
favorable prognosis of mesothelioma carrying a germline BAP1
mutation (6), as well as the rather
torpid clinical course, the patient decided not to continue
chemotherapy and still had a good performance status at the last
follow-up in October 2024. The patient was lost to follow-up
thereafter. Regarding the patient's maternal history of suspected
peritoneal mesothelioma, attempts to obtain accurate information
about the patient's cousins were unsuccessful.
Discussion
The present study was the very first report of this
original germline BAP1 frameshift mutation
(c.1077_1083delinsTG, pPhe360fs) in a newly-found Chinese family
with hereditary MMs, to the best of our knowledge. BAP1, a
deubiquitinase, exerts its functions of orchestrating
transcription, DNA replication and repair in the nucleus, as well
as promoting cell death and maintaining metabolism homeostasis in
the cytoplasm, through deubiquitination of its miscellaneous
substrates in these cellular activities (7). While various rare germline BAP1
mutations have been detected in breast cancer and renal cancer in
the Chinese population (8–10), there have been no specific reports
about any Chinese families affected by BAP1 hereditary
mesothelioma.
Several questions need to be answered. Firstly,
BAP1 germline mutation usually involves one allele and a
second hit is required to trigger total loss of the protein
function and the development of mesotheliomas. It may be speculated
that exposure to carcinogens other than asbestos or
post-translational modification may be the second hit, although DNA
methylation-mediated BAP1 inactivation has not been detected
(11). Besides, the development of
mesotheliomas in Bap1+/− mice or in familial carriers of
BAP1 mutations independently of carcinogens has also been
reported (7). Another notable
molecular event was the co-occurring ATM gain in the number of
copies. The upregulation of the ATM gene and other genetic
alterations in the DNA damage repair pathway in BAP1
haploinsufficient mesotheliomas were also observed by a previous
study (12). BAP1 is known
as a substrate of ATM phosphorylation in DNA replication stress
(13), but the role of the
BAP1 germline mutation in carcinogenesis and mechanisms of
secondary changes needs to be investigated in the future.
According to the role of BAP1 in DNA
double-strand break repair and the generally lower aggressiveness
of mesothelioma with germline BAP1 mutations (7), one may wonder what is the optimal
initial systemic strategy for such patients with advanced diseases:
Platinum-doublet chemotherapy, dual ICIs or the combination of
chemotherapy with anti-PD-1 antibodies (2,14).
Loss of BAP1 was suggested to be associated with improved
survival in patients with pleural mesothelioma but also with
resistance to cisplatin-based chemotherapy through apoptosis
inhibition (15,16). These contradictory phenomena were
the reflections of its multiple biological activities and the
uncertainty of the value of BAP1 alterations as a predictive
marker for current systemic therapies. The patient of the present
study received dual ICIs according to their own will. However, the
efficacy was modest, although findings have shown that BAP1
deletion correlates with an inflammatory tumor microenvironment and
is a potential target for checkpoint blockade (12). Additionally, the patient had
positive PD-L1 expression (CPS=10). As confirmed by the CheckMate
743 (2), PD-L1 positivity did not
correlate with the degree of benefit from nivolumab plus ipilimumab
in this patient. The instigation of SJS associated with this
regimen suggests an off-target effect by activated cytotoxic T
cells. The optimal treatment modality for MM associated with
germline BAP1 mutation should be explored in future
comparative studies.
In conclusion, a novel BAP1 germline
frameshift mutation (c. 1077_1083delinsTG, pPhe360fs) was found to
be associated with a Chinese patient with MM. Dual ICIs achieved a
modest effect in this patient and treatment was disrupted by a
serious skin immune-related adverse event.
Acknowledgements
Not applicable.
Funding
This work was supported by the National High Level Hospital
Clinical Research Funding (grant nos. 2022-PUMCH-A-215 and
2022-PUMCH-A-086) and the Chinese Academy of Medical Sciences
Innovation Fund for Medical Sciences (grant no.
2021-I2M-C&T-B-023).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The raw sequence data
reported in this paper have been deposited in the Genome Sequence
Archive (Genomics, Proteomics & Bioinformatics 2021) in the
National Genomics Data Center (Nucleic Acids Res 2022), China
National Center for Bioinformation/Beijing Institute of Genomics,
Chinese Academy of Sciences (GSA-Human: HRA009720) and are publicly
accessible at https://ngdc.cncb.ac.cn/gsa-human/browse/HRA009720.
Authors' contributions
NZ, SY and LZ was involved in the conception and
design of the study. SY and LZ provided administrative support. HW,
XG, SY, LZ and YC provided study materials or patients and were
involved in the acquisition of data. NZ, CW, MW and MY performed
data analysis and interpretation. All authors wrote the manuscript.
NZ and MW edited the manuscript. All authors have read and approved
the final manuscript. SY and LZ confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable. Written informed consent was
obtained from the patient for genetic testing.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report, including
accompanying images and genetic test results.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BAP1
|
BRCA1-associated protein 1
|
|
TB
|
tuberculosis
|
|
NGS
|
next generation sequencing
|
|
MM
|
malignant mesothelioma
|
|
IHC
|
immunohistochemistry
|
|
ATM
|
Ataxia-Telangiectasia mutated
|
|
TMB
|
tumor mutation burden
|
|
SJS
|
Steven-Johnson syndrome
|
|
ICIs
|
immune checkpoint inhibitors
|
References
|
1
|
Testa JR, Cheung M, Pei J, Below JE, Tan
Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, et al: Germline
BAP1 mutations predispose to malignant mesothelioma. Nat Genet.
43:1022–1025. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulangara K, Zhang N, Corigliano E,
Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J,
et al: Clinical utility of the combined positive score for
programmed death ligand-1 expression and the approval of
pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med.
143:330–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carbone M, Yang H, Pass HI, Krausz T,
Testa JR and Gaudino G: BAP1 and Cancer. Nat Rev Cancer.
13:153–159. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Novelli F, Yoshikawa Y, Vitto VAM, Modesti
L, Minaai M, Pastorino S, Emi M, Kim JH, Kricek F, Bai F, et al:
Germline BARD1 variants predispose to mesothelioma by impairing DNA
repair and calcium signaling. Proc Natl Acad Sci USA.
121:e24052311212024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carbone M, Pass HI, Ak G, Alexander HR Jr,
Baas P, Baumann F, Blakely AM, Bueno R, Bzura A, Cardillo G, et al:
Medical and surgical care of patients with mesothelioma and their
relatives carrying germline BAP1 mutations. J Thorac Oncol.
17:873–889. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carbone M, Harbour JW, Brugarolas J,
Bononi A, Pagano I, Dey A, Krausz T, Pass HI, Yang H and Gaudino G:
Biological mechanisms and clinical significance of BAP1 mutations
in human cancer. Cancer Discov. 10:1103–1120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Wang H, Ricketts CJ, Yang Y, Merino
MJ, Zhang H, Shi G, Gan H, Linehan WM, Zhu Y and Ye D: Germline
mutations of renal cancer predisposition genes and clinical
relevance in Chinese patients with sporadic, early-onset disease.
Cancer. 125:1060–1069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong L, Zhang H, Zhang H, Ye Y, Cheng Y,
Li L, Wei L, Han L, Cao Y, Li S, et al: The mutation landscape of
multiple cancer predisposition genes in Chinese familial/hereditary
breast cancer families. Cancer Biol Med. 19:850–870.
2021.PubMed/NCBI
|
|
10
|
Kong W, Yang T, Wen X, Mu Z, Zhao C, Han
S, Tian J, Zhang X, Zhou T, Zhang Y, et al: Germline mutation
landscape and associated clinical characteristics in Chinese
patients with renal cell carcinoma. Front Oncol. 11:7375472021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nasu M, Emi M, Pastorino S, Tanji M,
Powers A, Luk H, Baumann F, Zhang YA, Gazdar A, Kanodia S, et al:
High Incidence of Somatic BAP1 alterations in sporadic malignant
mesothelioma. J Thorac Oncol. 10:565–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shrestha R, Nabavi N, Lin YY, Mo F,
Anderson S, Volik S, Adomat HH, Lin D, Xue H, Dong X, et al: BAP1
haploinsufficiency predicts a distinct immunogenic class of
malignant peritoneal mesothelioma. Genome Med. 11:82019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ismail IH, Davidson R, Gagné JP, Xu ZZ,
Poirier GG and Hendzel MJ: Germline mutations in BAP1 impair its
function in DNA double-strand break repair. Cancer Res.
74:4282–4294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chu Q, Perrone F, Greillier L, Tu W,
Piccirillo MC, Grosso F, Lo Russo G, Florescu M, Mencoboni M,
Morabito A, et al: Pembrolizumab plus chemotherapy versus
chemotherapy in untreated advanced pleural mesothelioma in Canada,
Italy, and France: A phase 3, open-label, randomised controlled
trial. Lancet. 402:2295–2306. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Louw A, Panou V, Szejniuk WM, Meristoudis
C, Chai SM, van Vliet C, Lee YCG, Dick IM, Firth T, Lynggaard LA,
et al: BAP1 loss by immunohistochemistry predicts improved survival
to first-line platinum and pemetrexed chemotherapy for patients
with pleural mesothelioma: A validation study. J Thorac Oncol.
17:921–930. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oehl K, Vrugt B, Wagner U, Kirschner MB,
Meerang M, Weder W, Felley-Bosco E, Wollscheid B, Bankov K, Demes
MC, et al: Alterations in BAP1 are associated with cisplatin
resistance through inhibition of apoptosis in malignant pleural
mesothelioma. Clin Cancer Res. 27:2277–2291. 2021. View Article : Google Scholar : PubMed/NCBI
|