Introduction
Rectal cancer is a common malignant tumor of the
digestive system with an increasing incidence rate in China.
According to the National Cancer Center's 2024 statistics,
colorectal cancer incidence and mortality rates have been rising in
recent years. In 2022, there were 517,100 new cases of colorectal
cancer in China, with an incidence rate of 20.1 per 100,000
individuals. Males accounted for 307,700 cases, while females for
209,400 cases, with a higher incidence observed in males.
Additionally, there were 240,000 deaths, corresponding to a
mortality rate of 8.56 per 100,000. The incidence of colorectal
cancer increases with age, particularly among those aged 40 and
above. Urban areas show higher incidence rates compared to rural
areas, with the South of China exhibiting the highest rates.
Notably, the proportion of low rectal cancer is relatively high in
China, accounting for ~60–75% of all rectal cancer cases (1). Low rectal cancer accounts for 60–70%
of all rectal cancer cases (2). Due
to the insidious onset of rectal cancer, patients are often
diagnosed at a locally advanced stage and/or with distant
metastasis. Currently, neoadjuvant chemoradiotherapy (nCRT),
surgical treatment and postoperative adjuvant therapy are standard
modalities for treating mid-low locally advanced rectal cancer
(3,4). nCRT works to reduce tumor volume and
achieve downstaging, thereby increasing the resection rate,
sphincter preservation rate, reducing local recurrence rate, as
well as prolonging disease-free survival (DFS) and overall survival
(OS) (5). A total of 10–30% of
patients achieve a pathological complete response (pCR) after nCRT
(6,7). Compared with patients with non-pCR,
patients with pCR have notably lower local recurrence and distant
metastasis rates, and prolonged DFS (8,9).
However, the impact of lymph node status after neoadjuvant therapy
(ypN) on the prognosis of patients with ‘tumor stage 0 after
neoadjuvant therapy’ (ypT0) still remains unclear. For instance,
the incidence of lymph node metastasis has been reported to be
6–20% in patients with pCR after nCRT, which is an important factor
affecting postoperative local recurrence and distant metastasis
(10,11). In recent years, the treatment effect
of rectal cancer has markedly improved with the continuous
development of neoadjuvant treatment strategies. For instance, in
the EORTC 22921 study, 13.7% of patients receiving neoadjuvant
chemoradiotherapy (nCRT) achieved a pathological complete response
(pT0), compared to 5.3% in the radiotherapy-only group
(P<0.0001). Additionally, nCRT significantly reduced the tumor
size and the number of involved lymph nodes, and the local
recurrence rate was 7.6% in the nCRT group, compared to 17.1% in
the radiotherapy-only group (12).
Furthermore, the study by Braendengen et al (13) showed a 5-year overall survival rate
of 70% in the nCRT group, significantly higher than the 60% in the
radiotherapy-only group, with a corresponding decrease in local
recurrence. However, despite regression of the primary tumor,
certain patients still have lymph node metastasis, which adversely
affects their long-term survival (14).
With the advancement of molecular biology
techniques, increasingly more studies have begun to assess the
molecular characteristics of rectal cancer and their impact on
treatment response (15). Specific
gene mutations, epigenetic modifications and microenvironmental
factors may all affect the efficacy of nCRT and the pCR rate
(16–18). These studies provide new ideas for
individualized treatment and suggest that multiple factors need to
be considered comprehensively during treatment to achieve the best
therapeutic effect. Accordingly, to assess the relationship between
different ypN statuses and prognosis, the present study
retrospectively analyzed the clinical data of 203 patients with
mid-low rectal cancer who achieved ypCR after nCRT. Through
in-depth analysis of these clinical data, the results may provide
valuable references for clinical practice and further reveal the
key factors affecting the prognosis of rectal cancer.
Materials and methods
Study recruitment and design
The present study had a retrospective case-control
design. Based on the inclusion and exclusion criteria, the clinical
data of 203 patients who received preoperative nCRT and were
postoperatively pathologically classified as ypT0N0 and ypT0N+ at
Hebei Cangzhou Hospital of Integrated Traditional Chinese and
Western Medicine (Cangzhou, China) from January 2010 to December
2020, were analyzed. The electronic medical records and clinical
databases of the two participating institutions were accessed and
reviewed from June 2021 to December 2023 to extract and verify the
relevant patient data. Final follow-up was completed on January 1,
2024. The second participating institution is Hengshui People's
Hospital (Hengshui, China).
The inclusion criteria before nCRT were as follows:
i) Tumor lower edge within 10 cm from the anal verge, with
adenocarcinoma confirmed by biopsy; ii) clinical staging as
clinical tumor stage (cT)3-4 or clinical lymph node stage (cN)+;
iii) no distant metastasis; and iv) no previous systemic treatment
(chemotherapy, immunotherapy or radiotherapy). The inclusion
criteria after nCRT were as follows: i) Treatment via radical
surgery; ii) pathologically classified as ypT0 regardless of ypN
classification; and iii) >3 years of follow-up after nCRT. The
exclusion criteria were as follows: i) Diagnosis of inflammatory
bowel disease or hereditary colorectal cancer, including familial
adenomatous polyposis or Lynch syndrome; ii) diagnosis of
synchronous unresectable multiple primary cancers; and iii) R1
resection performed.
The present study complied with the relevant
provisions of the Helsinki Declaration and was approved by the
Ethics Committee of Hebei Cangzhou Hospital of Integrated
Traditional Chinese and Western Medicine (approval no.
2021-KY-062.1). All patients and/or their families provided written
informed consent to participate in the study.
nCRT and postoperative adjuvant
chemotherapy
All patients received intensity-modulated
radiotherapy at 45.0–50.4 Gy, 1.8–2.0 Gy per session, for a total
of 25–28 sessions. The neoadjuvant chemotherapy regimen was
composed of concurrent single-agent capecitabine (1,000
mg/m2 orally, twice daily and 5 days a week for 5 weeks)
during radiotherapy. Surgery was performed 8–13 weeks after nCRT
following the principles of total mesorectal excision.
Postoperative adjuvant chemotherapy regimens included the XELOX
regimen (oxaliplatin, 130 mg/m2 intravenously on day 1;
and capecitabine, 1,000 mg/m2 orally twice daily on days
1–14, every 3 weeks) and the FOLFOX regimen (oxaliplatin, 85
mg/m2 intravenously on day 1; leucovorin, 400
mg/m2 intravenously on day 1; and fluorouracil, 400
mg/m2 intravenously on day 1, then 1,200
mg/m2/day for 2 days as a continuous intravenous
infusion). The decision-making of postoperative adjuvant
chemotherapy was performed jointly by surgeons and oncologists
based on the pathological results of the patients.
Preoperative assessment and evaluation
of nCRT efficacy
Pre- and post-nCRT cT and cN stages were evaluated
using pelvic MRI. The circumferential proportion of the tumor
occupying the intestinal lumen was comprehensively determined by
colonoscopy, pelvic MRI and digital rectal examination. All
postoperative pathological specimens from gastrointestinal tumors
were reviewed by two senior pathologists to determine the final
pathological results. The tumor regression grading system was used
as an indicator to evaluate nCRT efficacy (19). In addition, ypT0 was defined as the
complete disappearance of tumor cells under the microscope in the
tumor specimen resected after nCRT (20).
Follow-up
Data related to the follow-up of the enrolled
patients were collected from inpatient medical records, as well as
through outpatient reviews and regular telephone visits. Starting
from the end of surgery, outpatient follow-up was performed every 3
months for the first 3 years, every 6 months until 5 years
postoperatively, and annually after 5 years. The follow-up ended on
January 1, 2024. Follow-up included routine digital rectal
examination, complete blood count, liver and kidney function tests,
tumor markers, chest CT, liver ultrasound or enhanced MRI, as well
as colonoscopy. OS was defined as the time from surgery to death or
the last follow-up. DFS was calculated as the time to the last
follow-up without recurrence or metastasis.
Statistical analysis
Data analysis was performed using SPSS 27.0 (IBM
Corp.). Survival analysis was performed using the Kaplan-Meier
method and the log-rank test. Postoperative serum carcinoembryonic
antigen (CEA) levels were assessed at the first two routine
follow-up visits after surgery. Patients were stratified into
‘persistently elevated’ and ‘normalized/decreased’ CEA groups based
on whether CEA was >5.0 ng/ml on both measurements. The
χ2 test was used to compare categorical variables
between groups. Cox regression was used for univariate and
multivariate analysis of factors affecting OS and DFS. A two-sided
P<0.05 was considered to indicate a statistically significant
difference. To minimize time truncation bias due to variable
follow-up durations, all survival analyses were right-censored at a
fixed cutoff date of January 1, 2024. Additionally, ‘surgery year’
was extracted from the electronic medical record based on the date
of radical resection following nCRT, and modeled as a continuous
covariate (ranging from 2010–2020) in the univariate Cox regression
analysis. This variable was used to evaluate whether patients
treated in earlier years, (who had longer potential follow-up
periods) exhibited different survival outcomes, and to assess
whether time-related bias may have affected the observed results.
The resulting hazard ratio (HR) values reflect the change in risk
per 1-year increase in the surgery year (HR/year), without
standardization or transformation of the variable. This covariate
was included solely for the purpose of assessing time truncation
bias and was not interpreted as a clinically meaningful prognostic
factor due to the lack of statistical significance.
Results
Clinical characteristics analysis
Of the 203 patients included in the present study,
78 were from Hengshui People's Hospital and 125 from Hebei Cangzhou
Hospital of Integrated Traditional Chinese and Western Medicine. In
the Hengshui cohort, there were 42 men and 36 women, with a mean
age of 60.4±9.1 years. In the Cangzhou cohort, there were 60 men
and 65 women, with a mean age of 61.2±10.3 years. Furthermore,
among 203 patients included in the study, there were 127 cases in
the ypT0N0 group and 76 cases in the ypT0N+ group. However, there
were no significant inter-group differences for any of the general
clinical characteristics analyzed, such as age, sex and distance
from the anal verge (all P>0.05; Table I).
 | Table I.Comparison of general clinical data
between the ypT0N0 and ypT0N+ groups. |
Table I.
Comparison of general clinical data
between the ypT0N0 and ypT0N+ groups.
| Characteristic | ypT0N0 group
(n=127) | ypT0N+ group
(n=76) | χ2
value | P-value |
|---|
| Age, years |
|
| 0.016 | 0.900 |
|
<60 | 49 (38.6) | 30 (39.5) |
|
|
|
≥60 | 78 (61.4) | 46 (60.5) |
|
|
| Sex |
|
| 0.666 | 0.415 |
|
Female | 66 (52.0) | 35 (46.1) |
|
|
|
Male | 61 (48.0) | 41 (53.9) |
|
|
| Distance to anal
verge, cm |
|
| 0.040 | 0.842 |
| ≤5 | 67 (52.8) | 39 (51.3) |
|
|
|
>5 | 60 (47.2) | 37 (48.7) |
|
|
| Proportion of
circlea, % |
|
| 0.040 | 0.841 |
|
≤50 | 65 (51.2) | 40 (52.6) |
|
|
|
>50 | 62 (48.8) | 36 (47.4) |
|
|
| Degree of tumor
differentiation |
|
| 0.106 | 0.948 |
|
Low | 53 (41.7) | 30 (39.5) |
|
|
|
Intermediate | 64 (50.4) | 40 (52.6) |
|
|
|
High | 10 (7.9) | 6 (7.9) |
|
|
| cT stage |
|
| 0.031 | 0.960 |
|
cT1-2 | 32 (25.2) | 20 (26.3) |
|
|
|
cT3-4 | 95 (74.8) | 56 (73.7) |
|
|
| cN stage |
|
| 1.962 | 0.161 |
|
cN0 | 40 (31.5) | 17 (22.4) |
|
|
|
cN+ | 87 (68.5) | 59 (77.6) |
|
|
| CEA |
|
| 3.605 | 0.058 |
|
Normal | 59 (46.5) | 25 (32.9) |
|
|
|
Elevated | 68 (53.5) | 51 (67.1) |
|
|
| CA 19-9 |
|
| 0.179 | 0.672 |
|
Normal | 48 (37.8) | 31 (40.8) |
|
|
|
Elevated | 79 (62.2) | 45 (59.2) |
|
|
Comparison of OS and DFS
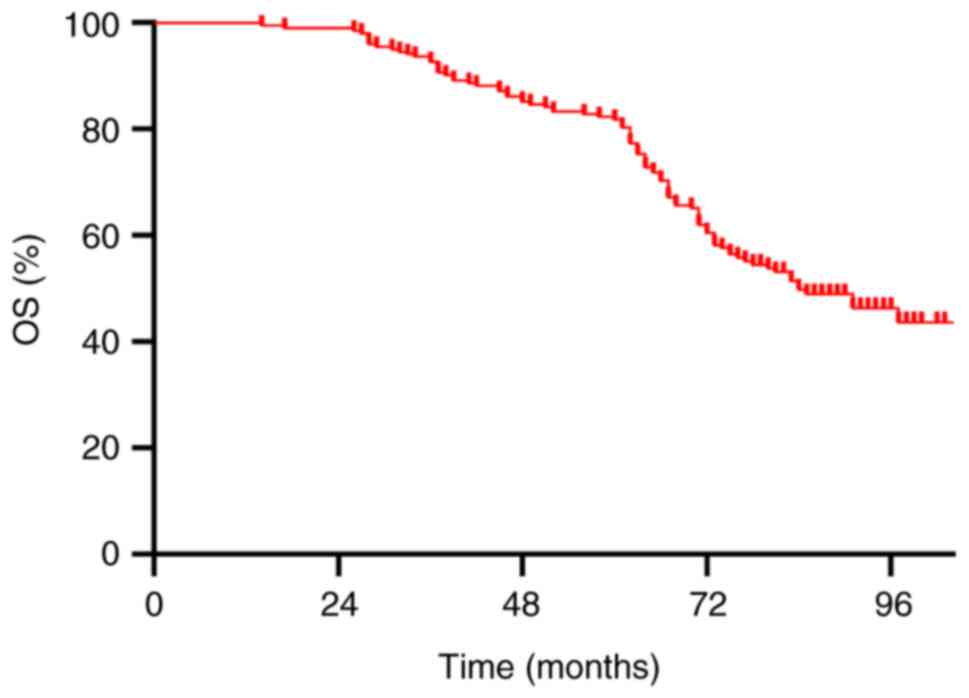
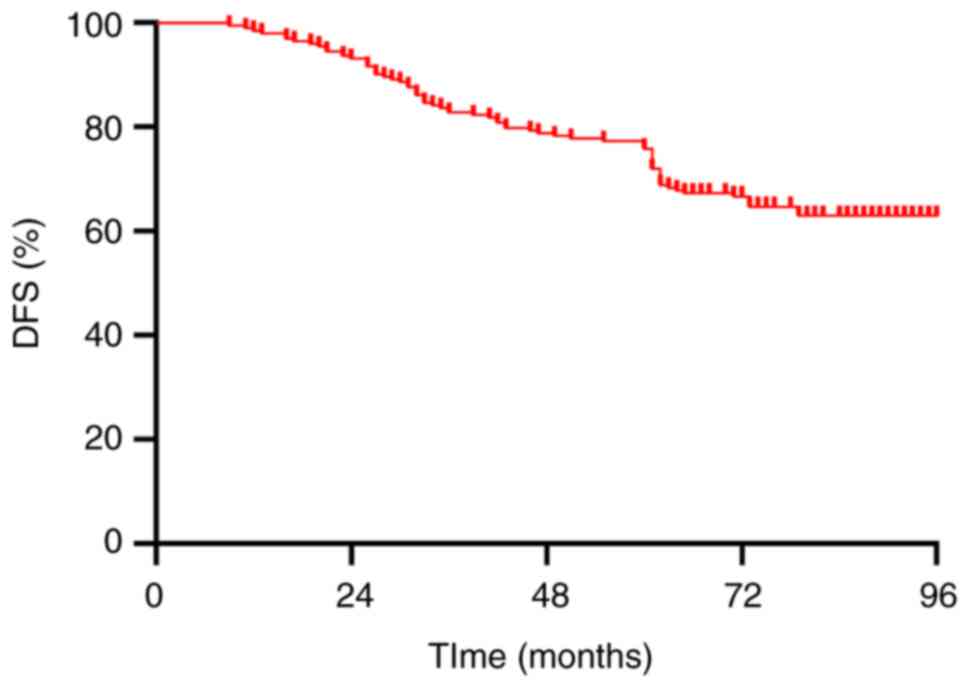
The 5-year OS and DFS rates for all patients were
82.3 and 77.3%, respectively (Figs.
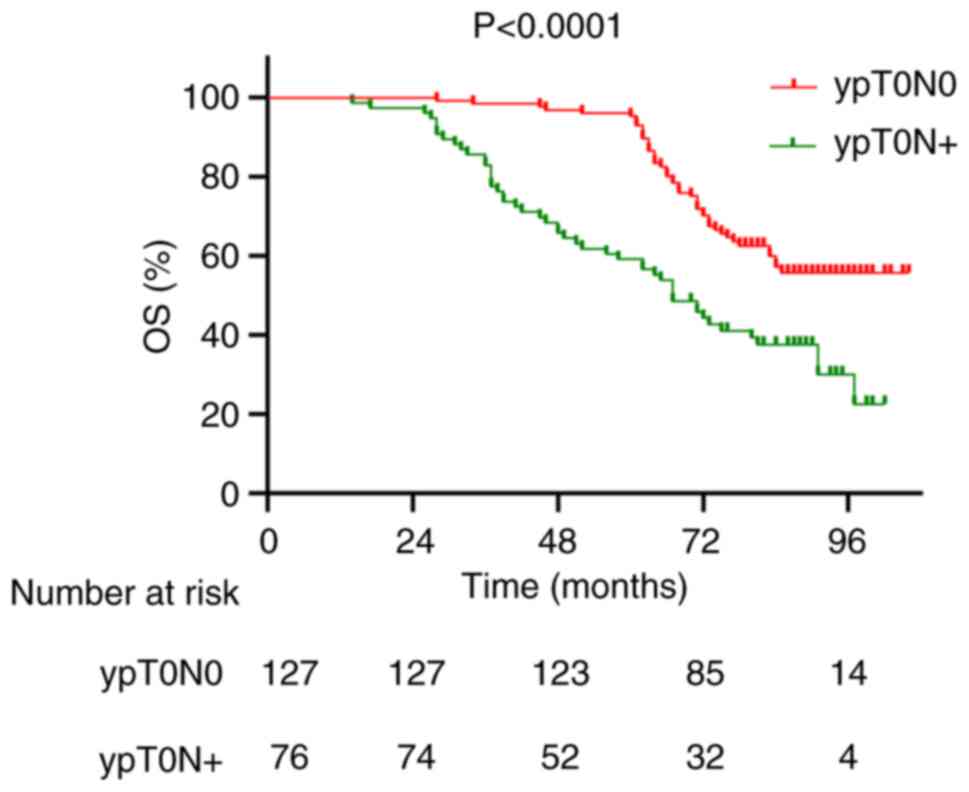
1 and 2). The 5-year OS rates
for patients in the ypT0N0 and ypT0N+ groups were 96.1 and 59.2%,
respectively (P<0.0001; Fig. 3),
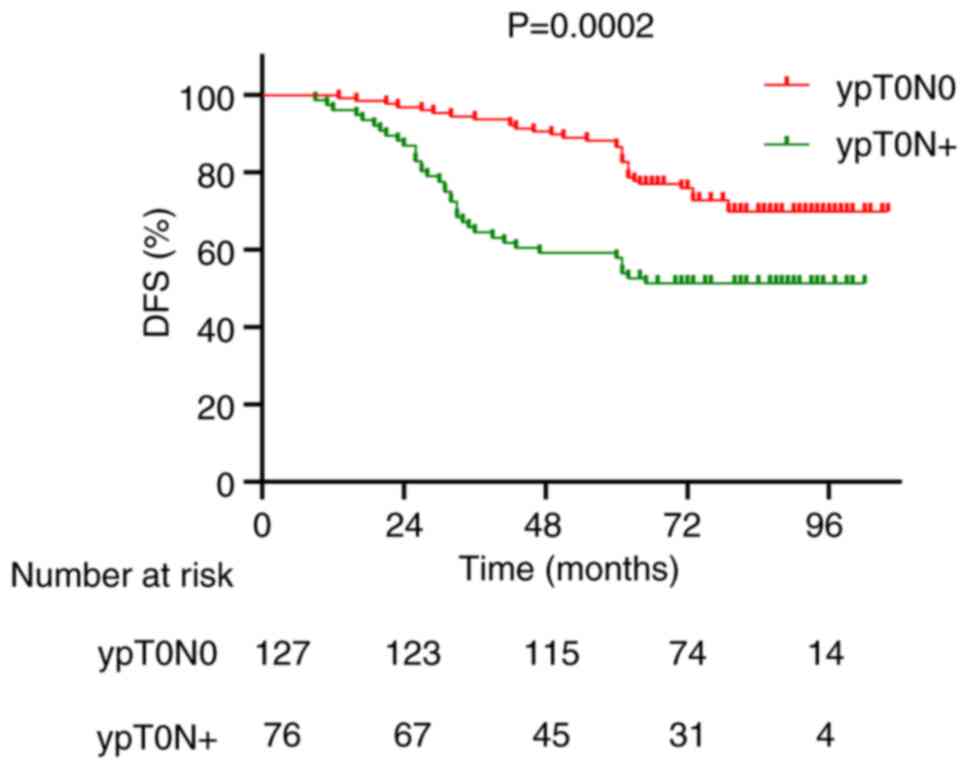
whilst the 5-year DFS rates were 88.2 and 59.2% for the ypT0N0 and
ypT0N+ groups, respectively (P=0.0002; Fig. 4). In addition, Kaplan-Meier survival
analyses were performed to assess the prognostic value of
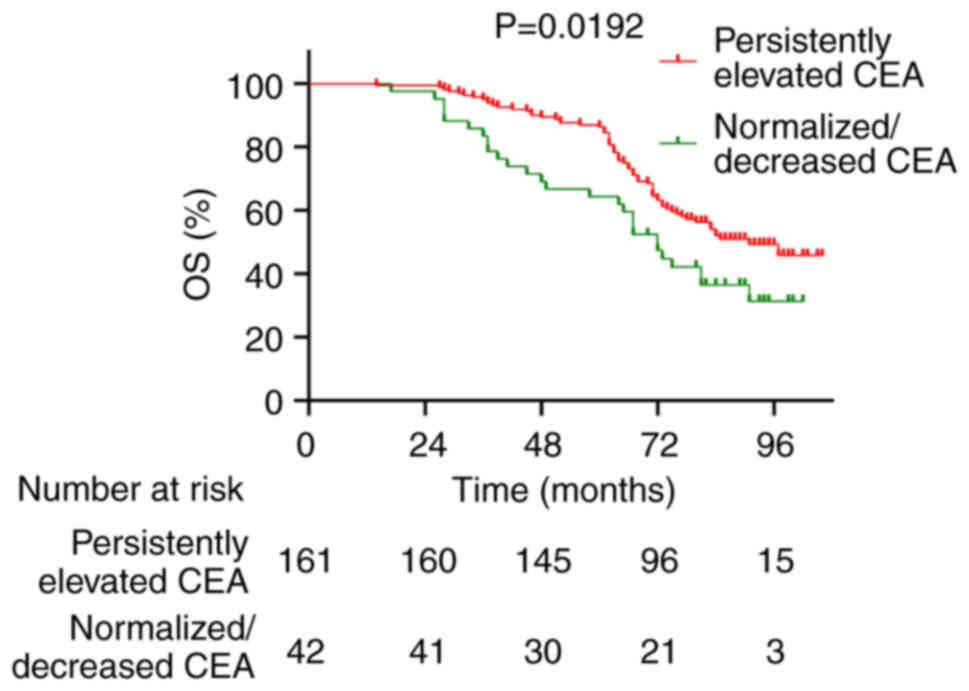
postoperative CEA levels. Patients in the ‘Persistently Elevated’
CEA group had significantly worse 5-year OS rates compared with
those in the ‘Normalized/Decreased’ CEA group (64.3 vs. 86.9%;
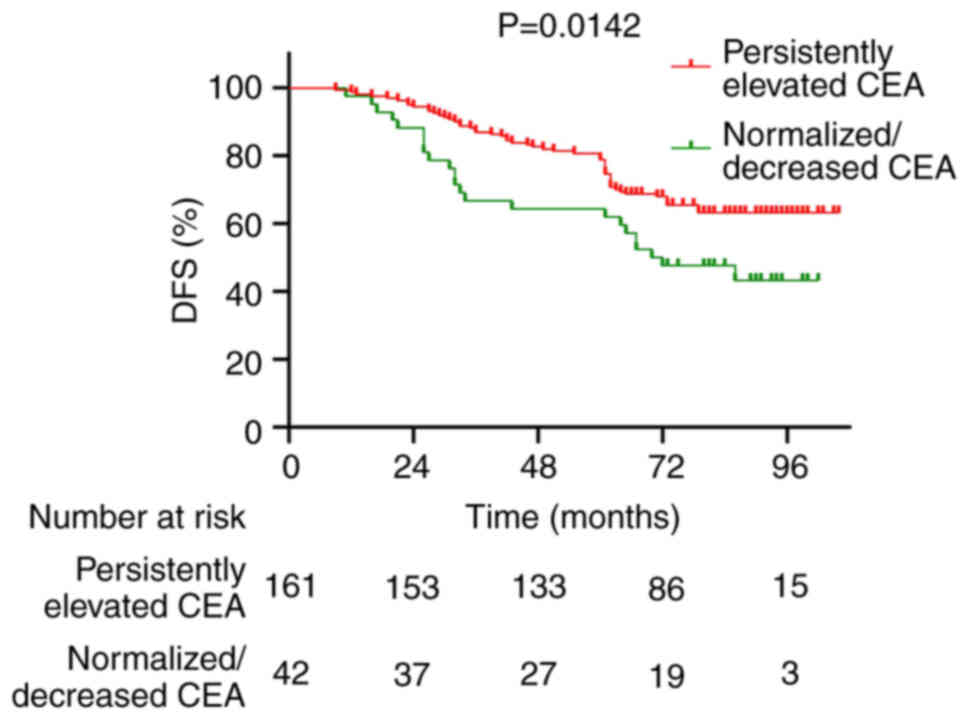
P=0.0192; Fig. 5). Similarly, the
5-year DFS rate was significantly lower in the ‘Persistently
Elevated’ group compared with in the ‘Normalized/Decreased’ group
(54.7 vs. 80.7%; P=0.0142; Fig.
6).
Analysis of factors influencing OS and
DFS
The results of multivariate analysis demonstrated
that cT3-4 before nCRT [HR=5.941; 95% confidence interval (CI),
3.036–11.625; P<0.001], cN+ (HR=2.636; 95% CI, 1.576–4.411;
P<0.001), elevated serum CEA level (HR=2.735; 95% CI,
1.678–4.459; P<0.001) and postoperative pathology ypT0N+
(HR=2.394; 95% CI, 1.604–3.573; P<0.001) were independent risk
factors affecting OS (Table II).
Moreover, the independent risk factors influencing DFS were cT3-4
(HR=4.818; 95% CI, 2.270–10.226; P<0.001), cN+ (HR=2.641; 95%
CI, 1.426–4.893; P=0.002), elevated serum CEA level (HR=1.820; 95%
CI, 1.079–3.071; P=0.025) and postoperative pathology ypT0N+
(HR=2.280; 95% CI, 1.426–3.644; P<0.001) (Table III).
 | Table II.Influencing factors of overall
survival of the 203 patients in the present study. |
Table II.
Influencing factors of overall
survival of the 203 patients in the present study.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥60 vs. <60
years) | 1.248 | 0.825–1.888 | 0.293 |
|
|
|
| Sex (female vs.
male) | 1.139 | 0.768–1.689 | 0.518 |
|
|
|
| Distance to anal
verge (>5 vs. ≤5 cm) | 1.095 | 0.737–1.625 | 0.654 |
|
|
|
| Proportion of
circlea (≤50 vs.
>50%) | 1.005 | 0.677–1.491 | 0.980 |
|
|
|
| cT stage (cT3-4 vs.
cT1-2) | 4.024 | 2.091–7.745 | <0.001 | 5.941 | 3.036–11.625 | <0.001 |
| cN stage (cN+ vs.
cN0) | 1.903 | 1.153–3.141 | 0.012 | 2.636 | 1.576–4.411 | <0.001 |
| CEA (elevated vs.
normal) | 3.502 | 2.161–5.673 | <0.001 | 2.735 | 1.678–4.459 | <0.001 |
| CA 19-9 (elevated
vs. normal) | 1.142 | 0.763–1.709 | 0.520 |
|
|
|
| ypT0N stage (ypT0N+
vs. ypT0N0) | 2.349 | 1.582–3.486 | <0.001 | 2.394 | 1.604–3.573 | <0.001 |
| Lymph node
involvement (≥3 vs. 1–2) | 1.504 | 1.027–3.459 | 0.097 |
|
|
|
| Surgery year | 1.387 | 0.787–2.445 | 0.258 |
|
|
|
 | Table III.Influencing factors of disease-free
survival of the 203 patients in the present study. |
Table III.
Influencing factors of disease-free
survival of the 203 patients in the present study.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95%CI | P-value |
|---|
| Age (≥60 vs. <60
years) | 1.487 | 0.900–2.456 | 0.121 |
|
|
|
| Sex (female vs.
male) | 1.193 | 0.751–1.895 | 0.455 |
|
|
|
| Distance to anal
verge (>5 vs. ≤5 cm) | 1.053 | 0.663–1.673 | 0.826 |
|
|
|
| Proportion of
circlea (≤50 vs.
>50%) | 1.162 | 0.732–1.845 | 0.525 |
|
|
|
| cT stage (cT3-4 vs.
cT1-2) | 3.367 | 1.613–7.027 | 0.001 | 4.818 | 2.270–10.226 | <0.001 |
| cN stage (cN+ vs.
cN0) | 2.017 | 1.106–3.679 | 0.022 | 2.641 | 1.426–4.893 | 0.002 |
| CEA (elevated vs.
normal) | 2.301 | 1.373–3.856 | 0.002 | 1.820 | 1.079–3.071 | 0.025 |
| CA 19-9 (elevated
vs. normal) | 1.087 | 0.672–1.758 | 0.735 |
|
|
|
| ypT0N stage (ypT0N+
vs. ypT0N0) | 2.322 | 1.461–3.690 | <0.001 | 2.280 | 1.426–3.644 | <0.001 |
| Lymph node
involvement (≥3 vs. 1–2) | 1.391 | 0.957–3.018 | 0.219 |
|
|
|
| Surgery year | 1.006 | 0.563–1.795 | 0.895 |
|
|
|
Follow-up duration and time truncation
adjustment
The follow-up duration for the entire cohort ranged
from 13–107 months, with a median follow-up time of 56 months. To
minimize the potential impact of time truncation bias due to
variation in follow-up duration, all survival data were uniformly
right-censored at a fixed cutoff date of January 1, 2024, which
represented the final follow-up point for the present study. In
addition, a univariate Cox regression analysis was performed
including surgery year as a continuous variable to evaluate whether
differences in the timing of treatment affected survival outcomes.
The results demonstrated that surgery year was not significantly
associated with either OS (HR=1.387; 95% CI, 0.787–2.445; P=0.258;
Table II) or DFS (HR=1.006; 95%
CI, 0.563–1.795; P=0.895). This suggests that time-related bias did
not significantly influence survival analyses in this cohort.
Comparison of recurrence
Of the 203 patients enrolled in the present study,
72 cases (35.5%) experienced recurrence during the follow-up
period, with 35 cases (27.6%) in the ypT0N0 group and 37 cases
(48.7%) in the ypT0N+ group (χ2=9.271; P=0.002).
Regarding the pattern of recurrence, 53 patients (26.1%) developed
distant metastasis, with 26 cases (20.5%) in the ypT0N0 group and
27 cases (35.5%) in the ypT0N+ group. There were 10 cases (26.1%)
of local recurrence, with 5 cases (3.9%) in the ypT0N0 group and 5
cases (6.6%) in the ypT0N+ group. In addition, 9 patients (4.4%)
experienced both distant metastasis and local recurrence, including
4 cases (3.1%) in the ypT0N0 group and 5 cases (6.6%) in the ypT0N+
group. Statistical analysis revealed no significant difference in
the comparison of the pattern of recurrence between the two groups
(χ2=0.074; P=0.963) (Table
IV).
 | Table IV.Recurrence pattern comparison between
ypT0N0 and ypT0N+ groups. |
Table IV.
Recurrence pattern comparison between
ypT0N0 and ypT0N+ groups.
| Parameter | ypT0N0 group
(n=127) | ypT0N+ group
(n=76) | χ2
value | P-value |
|---|
| Recurrence
cases | 35 (27.6) | 37 (48.7) | 9.271 | 0.002 |
| Distant
metastasis | 26 (20.5) | 27 (35.5) | 0.016 | 0.899 |
| Local
recurrence | 5 (3.9) | 5 (6.6) | 0.009 | 0.925 |
| Both distant and
local recurrence | 4 (3.1) | 5 (6.6) | 0.074 | 0.963 |
Discussion
In recent years, marked progress has been made in
the evaluation, staging and treatment of rectal cancer, leading to
the improvement of the oncological prognosis of patients. The
application of nCRT contributes further to improving local tumor
control and reducing adverse drug reactions (21), particularly for patients with
postoperative pCR, resulting in markedly extended DFS (22). Sell et al (23) performed a study on 370 patients with
rectal cancer who underwent preoperative chemoradiotherapy and
reported that 50 patients (13.5%) achieved pCR, with 5-year OS and
DFS rates of 95 and 92%, respectively; this is similar to the
results of the present study. Moreover, through the follow-up of 58
patients with mid-low locally advanced rectal cancer who achieved
pCR after nCRT with a median time of >4 years, Campos-Lobato
et al (24) reported that
the distant metastasis rate in pCR patients was notably lower
compared with non-pCR patients, with a 5-year OS rate of 93%. A
multicenter study including 566 ypT0N0 patients from 36 centers
also reported that the local recurrence rate, distant metastasis
rate, 5-year DFS and OS were 1.6, 8.9, 85.0 and 90.0%,
respectively, in patients after a median follow-up time of 46.4
months (25). In the present study,
the 5-year DFS and OS of 127 ypT0N0 patients were 88.2 and 96.1%,
respectively, similar to the aforementioned reports.
The ypN status is an important factor for the
prognosis of patients with locally advanced rectal cancer after
nCRT (26). Among the 203 ypT0
patients in the present study, 76 had positive lymph node remnants.
Moreover, the present study demonstrated that residual mesorectal
lymph node metastasis after nCRT, even after the complete
regression of the primary tumor in the rectal wall, adversely
affected OS and DFS. In light of these findings, the results of the
present study were further compared with those of several previous
publications addressing lymph node status, pCR and prognostic
evaluation after nCRT. Li Destri et al (27) assessed the impact of nCRT on lymph
node retrieval and prognosis in 142 patients with rectal cancer.
Their findings highlighted the prognostic value of the lymph node
ratio (LNR) and the potential staging inaccuracy due to reduced
lymph node yield post-nCRT. By contrast, the present study did not
focus on lymph node count or LNR, but instead identified ypT0N+
patients as a distinct high-risk subgroup based on pathological
nodal involvement despite complete tumor regression. This offers
more direct implications for postoperative treatment planning.
Furthermore, Zhang et al (28) evaluated 432 patients with rectal
cancer and identified predictors for achieving pCR, such as low
baseline CEA and extended interval between nCRT and surgery. Their
results support a ‘watch-and-wait’ strategy for ypT0N0 patients.
However, the present study specifically excluded ypT0N0 patients
and focused on ypT0N+ individuals who exhibited inferior survival
outcomes, thereby underscoring the necessity of intensified
adjuvant therapy in this subgroup. Zhu et al (29) analyzed 482 patients and reported
that the total number of examined lymph nodes markedly affected
staging accuracy and long-term survival. Whilst this emphasizes the
importance of adequate lymph node dissection, the results of the
present study indicate that even patients with complete tumor
regression can harbor nodal metastasis, which independently
predicts poor prognosis. Thus, the findings of the present study
shift the focus from lymph node quantity to the biological impact
of ypT0N+ status. Moreover, Ozturk et al (30) proposed a lymph node regression
grading (LNRG) system based on histopathological response in 469
patients. Although they reported that LNRG may provide additional
prognostic information, the present study adopted a clinically
translational approach by directly identifying ypT0N+ patients as
requiring different postoperative management. Nodal regression
grade was analyzed; however, survival data and risk stratification
specific to the ypT0N+ population were provided. Collectively,
these comparisons underscore the novelty of the present study in
defining ypT0N+ as a distinct prognostic entity. Unlike previous
studies that emphasize lymph node count, regression grade or pCR
prediction, the present work focused on a specific, understudied
subgroup and proposes actionable treatment strategies to improve
their outcomes. Lu et al (31) analyzed 59 ypT0N0 patients after nCRT
and reported that 8 patients (13.6%) had mesorectal lymph node
metastasis, and the 5-year DFS and OS rates of these patients were
markedly longer than those of ypT0N+ patients. Zhang et al
(32) analyzed 76 ypT0 patients,
with 9 (11.8%) classed as ypN+. They reported that the 5-year DFS
and OS of ypT0N+ patients were 62.5 and 72.9%, respectively.
Furthermore, multivariate analysis in the present study revealed
that ypT0N+ was an independent risk factor for DFS.
In addition, the findings of the present study
demonstrated that there was a lower distant metastasis rate of
patients with pCR after nCRT. This may be due to the fact that
during the period from the end of radiotherapy to surgery, patients
who achieve pCR had no residual tumor cells, whilst patients who
did not achieve pCR may have had a continuous interaction between
tumor cells and the surrounding environment, markedly increasing
the possibility of distant metastasis. Therefore, a good response
of the primary tumor to nCRT can be regarded as a sign of systemic
treatment effectiveness. However, unlike the tumor in the rectal
wall, lymph node metastasis did not completely disappear with the
achievement of pCR in the rectal wall tumor. This may be attributed
to the continuous evolution of tumor cells in primary and
metastatic sites, further enhancing their invasive, metastatic and
chemoradiotherapy-resistant malignant potentials (33,34).
Therefore, even if the tumor in the rectal wall disappears
completely, metastatic cells in the lymph nodes can survive and
lead to distant metastasis that produces an adverse impact on
patient prognosis.
In the present study, independent risk factors
affecting OS and DFS included cT stage, cN stage and serum CEA
levels. A study performed in the United States, which included
23,747 patients with rectal cancer, reported that the rate of pCR
gradually decreased as the cT stage (cT1-cT4) and cN stage
(cN0-cN2) changed, with a lower rate of pCR in patients with a
higher stage and cN+ compared to those with patients a lower stage
and cN0 (P<0.001), indicating a worse prognosis (35). Peng et al (36) also reported that cT stage (P=0.043)
and N stage (P=0.003) could predict pCR rates. Patients with a
larger tumor burden often experience resistance to radiotherapy.
Considering the different growth rates of blood vessels and tumors,
patients with a larger tumor burden tend to have a larger
proportion of hypoxic tumor cells, and the hypoxic microenvironment
may promote tumor progression, increase tumor heterogeneity, and
enhance tumor tolerance to radiotherapy and chemotherapy (37). Zhou et al (38) analyzed 124 patients with locally
advanced rectal cancer and reported that pre-treatment elevation of
CEA was an independent high-risk factor affecting OS in ypT0N
patients. Colloca et al (39) performed a meta-analysis on 20
studies and demonstrated that patients in the elevated CEA group
had markedly prolonged 3- and 5-year DFS rates compared with the
normal pre-treatment CEA group. CEA has been reported to affect the
biological behavior of tumor cells through autocrine secretion,
increasing tumor cell survival, inhibiting tumor cell
differentiation, as well as promoting endothelial cell activation
and tumor angiogenesis through paracrine secretion (40). The present study further
demonstrated the prognostic value of pre-treatment CEA for patients
with locally advanced rectal cancer. In addition to pre-treatment
levels, the present study revealed that persistently elevated
postoperative CEA was significantly associated with worse OS and
DFS. This highlights the importance of serial postoperative CEA
monitoring, not only for recurrence detection, but also for
postoperative risk stratification and treatment planning. These
findings support the integration of dynamic CEA assessment into
follow-up strategies, especially for identifying patients who may
benefit from intensified adjuvant therapy or closer
surveillance.
For patients with elevated CEA levels, previous
studies have suggested several potential improvements in treatment
strategies. Firstly, personalized treatment plans are crucial for
these patients. Specifically, more aggressive preoperative and
postoperative treatment regimens can be considered for patients
with elevated CEA. For example, enhancing the nCRT regimen (such as
introducing more potent chemotherapy agents like FOLFOX or adding
irinotecan) can increase the pCR rate and improve long-term
survival (41). Secondly, dynamic
monitoring of CEA levels can serve as an important indicator of
treatment response. Post-nCRT changes in CEA after nCRT may suggest
the sensitivity to treatment in patients with elevated preoperative
CEA levels. If postoperative CEA remains elevated, particularly
when the local tumor has completely disappeared, it may suggest the
presence of micro-residual tumors or micrometastases, requiring
closer follow-up and further treatment for such patients.
Therefore, regular monitoring of CEA levels is essential for
predicting postoperative recurrence or metastasis in the management
of patients with elevated CEA (42).
Moreover, molecular mechanisms serve a critical role
in treatment response after nCRT in patients with rectal cancer. In
particular, certain gene mutations and epigenetic changes can
enhance tumor cell resistance to chemotherapy and radiotherapy,
thereby worsening prognosis through multiple molecular pathways
(43). KRAS mutations have been
reported to be common in rectal cancer, especially in locally
advanced cases. Although nCRT can notably reduce tumor size, the
effectiveness of chemotherapy and radiotherapy is often suboptimal
in KRAS-mutant patients (44). This
may be attributed to the continuous activation of cell
proliferation and growth signals caused by the mutations, leading
to treatment resistance. NRAS and BRAF mutations are also
considered important factors affecting the efficacy of nCRT, with
BRAF-mutant patients generally exhibiting worse survival and a
higher recurrence risk compared to BRAF wild-type patients
(45). In addition, epigenetic
changes such as DNA methylation and histone modifications can also
have an impact on the treatment response by affecting the
proliferation, migration and invasiveness of tumor cells. For
instance, dysregulated DNA methylation of tumor suppressor genes is
considered a mechanism contributing to tumor cell resistance to
treatment. Specifically, methylation of tumor suppressor genes
(such as MutL protein homolog 1 and adenomatous polyposis coli) can
induce microsatellite instability (MSI), an epigenetic alteration
that is associated with variable sensitivity to nCRT, with MSI-high
(MSI-H) tumors often demonstrating enhanced responsiveness to
immunotherapy but inconsistent responses to chemoradiotherapy.
Epigenetic modifications can also alter the immune escape
mechanisms and angiogenesis responses in the tumor microenvironment
(TME), thus affecting the sensitivity to radiotherapy and
chemotherapy (46).
Furthermore, the role of the TME in cancer
progression, immune evasion and drug resistance has received
increasing attention in recent decades. The TME consists of several
cell types (including immune cells, fibroblasts, and endothelial
cells), as well as their secreted cytokines and metabolic products.
These factors can modulate the treatment response of patients and
long-term survival by altering tumor progression and therapeutic
resistance (47). Future research
should further investigate the specific mechanisms by which the TME
impacts prognosis and develop personalized treatment strategies
tailored for ypT0N+ patients.
The results of the present study indicated that
ypT0N+ patients had a worse prognosis compared to ypT0N0 patients,
with the requirement for more aggressive treatment strategies.
Therefore, based on domestic and international guidelines, and the
latest research (48,49), the following specific treatment
strategies are proposed: First, intensified postoperative adjuvant
chemotherapy should be considered for ypT0N+ patients. Standard
oxaliplatin-based regimens such as XELOX (capecitabine plus
oxaliplatin) and FOLFOX (fluorouracil plus oxaliplatin) remain the
recommended postoperative therapies (50). In the present study, ypT0N+ patients
exhibited significantly higher recurrence rates and worse long-term
survival compared with ypT0N0 patients, suggesting that current
standard treatments may be insufficient in this high-risk subgroup.
Although irinotecan-containing regimens such as FOLFIRI have
demonstrated efficacy in the management of metastatic colorectal
cancer (51), their role in the
postoperative treatment of ypT0N+ patients remains unproven. These
approaches are therefore considered hypothesis-generating and
warrant further prospective evaluation. Second, although anti-EGFR
agents (such as cetuximab) and anti-VEGF agents (such as
bevacizumab) have demonstrated efficacy in metastatic colorectal
cancer (52), their role in the
adjuvant treatment of ypT0N+ rectal cancer remains to be
established. Current clinical guidelines do not recommend the
routine use of these agents in the postoperative setting for
non-metastatic disease (53).
However, further research should explore whether selected high-risk
patients with specific genetic mutations (such as KRAS, NRAS and
BRAF) could benefit from such targeted strategies. Similarly,
immune checkpoint inhibitors [such as programmed cell death 1
(PD-1)/programmed cell death-ligand 1 (PD-L1) inhibitors] have
shown promising efficacy in MSI-H colorectal cancers, particularly
in advanced or recurrent settings (54). Whilst their role in the adjuvant
treatment of ypT0N+ patients remains investigational, the presence
of MSI-H or high tumor mutation burden may serve as a biomarker for
future clinical trials assessing the value of immunotherapy in this
subgroup. In the present study, none of the 203 patients received
targeted therapy or immunotherapy as part of their adjuvant
treatment. All patients were treated according to institutional
protocols at the time, which were based on standard chemotherapy
regimens such as XELOX or FOLFOX. Therefore, the discussion on
targeted therapy and immunotherapy in ypT0N+ patients is provided
to reflect current advances in the literature and highlight
potential directions for future individualized treatment
strategies. Lastly, ypT0N+ patients should undergo enhanced
follow-ups. Regular monitoring of CEA levels, imaging (such as MRI
and CT) and colonoscopy should be performed to detect potential
recurrence or metastasis at an early stage. For high-risk patients
(such as those with elevated CEA or KRAS mutations), there is a
need to increase the follow-up frequency, with imaging assessments
potentially required every 3–6 months. Collectively, treatment
strategies for ypT0N+ patients should include individualized
chemotherapy regimens, potential targeted therapy and
immunotherapy. Further prospective studies should facilitate the
determination of the optimal combination of these treatment
approaches to improve therapeutic outcomes and reduce recurrence
risks. Moreover, notably in the present study, ypT0N+ patients were
classified as having complete primary tumor regression (ypT0) but
with concurrent lymph node metastasis. Due to potential limitations
in pathological specimen sampling, the identification of certain
pCR cases may not be entirely accurate, indicating the presence of
false-positive pCR cases. This is particularly relevant when
microscopic residual disease is not fully reflected in pathological
evaluation, potentially underestimating recurrence risks in certain
patients. To minimize the impact of false-positive pCR, future
research should focus on standardizing pathological evaluations,
expanding the scope of tissue sampling and integrating liquid
biopsy techniques to enhance the accuracy of early recurrence
detection. Previous studies have reported that persistent
postoperative detection of circulating tumor (ct)DNA is associated
with an increased risk of recurrence in patients with colorectal
cancer, including those with rectal cancer (55,56).
Integrating ctDNA analysis into postoperative surveillance may help
identify ypT0N+ patients who require intensified adjuvant therapy
and more rigorous follow-up protocols.
The present study demonstrated that elevated
preoperative CEA levels are an independent risk factor for poor
prognosis in patients with rectal cancer, consistent with previous
findings. Zhou et al (38)
reported that pretreatment CEA elevation was markedly associated
with reduced OS in patients with ypT0N+ rectal cancer. Similarly,
Peng et al (36) reported
that cT stage, cN stage and CEA levels were notable predictors of
pCR following nCRT. However, inconsistencies still exist. For
example, Zhang et al (32)
reported no significant survival differences based on preoperative
CEA levels. These discrepancies may be attributed to heterogeneity
in patient populations, differences in tumor location, radiotherapy
regimens (such as inclusion of short-course radiotherapy) and
varying definitions of CEA thresholds. Biologically, CEA promotes
tumor progression through autocrine and paracrine mechanisms,
enhancing tumor cell survival, inhibiting differentiation and
stimulating angiogenesis. These effects may contribute to
chemoradiotherapy resistance in CEA-elevated tumors. Clinically,
dynamic CEA monitoring is valuable in assessing treatment response.
Patients with normalized postoperative CEA levels generally have a
favorable prognosis, whereas persistently elevated postoperative
CEA (even in the absence of detectable residual tumor) may indicate
micro-metastatic disease or occult recurrence risk (57). In the cohort in the present study,
patients with persistently elevated postoperative CEA had
significantly worse 5-year OS (64.3 vs. 86.9%; P=0.0192) and DFS
rates (54.7 vs. 80.7%; P=0.0142) compared with those whose CEA
normalized after surgery. Based on this, CEA-based postoperative
risk stratification is proposed: Patients with normalized
postoperative CEA may follow standard surveillance intervals, and
patients with persistently elevated CEA should undergo intensified
follow-up and be considered for escalated adjuvant therapy. The
findings of the present study align with much of the existing
literature (58,59) and emphasize the importance of
individualized follow-up strategies based on dynamic tumor markers
(42). However, future studies are
warranted to further validate the integration of CEA kinetics into
treatment algorithms for patients with rectal cancer.
Micro (mi)RNAs serve a direct role in rectal cancer
progression and resistance to nCRT, as demonstrated in studies
assessing their impact on chemoradiotherapy outcomes. For example,
De Palma et al (60)
reported that miR-21 enhances resistance to nCRT by inhibiting
apoptosis-related pathways and upregulating tumor cell survival
signals. Conversely, Li et al (47) reported that lower expression of
miR-223 was associated with an improved response to
chemoradiotherapy. These findings suggest that miRNA profiling
could serve as a biomarker for predicting nCRT efficacy in patients
with rectal cancer.
KRAS mutations are common in rectal cancer and are
closely associated with the development of lymph node metastasis
(61). Studies have reported that
KRAS mutations not only affect the biological behavior of the
primary tumor, but are also associated with an increased risk of
lymph node metastasis (43). In
ypT0N+ patients, KRAS mutations could contribute to the development
of lymph node metastasis. Treatment strategies targeting KRAS
mutations, such as anti-EGFR therapy (for example, cetuximab), may
improve therapeutic outcomes, particularly in patients with lymph
node metastasis after neoadjuvant therapy. Prognostically, KRAS
mutations are associated with a worse outcome, warranting more
aggressive postoperative treatment and close follow-up (62).
BRAF mutations, particularly the V600E mutation,
drive tumor progression in rectal cancer through constitutive
activation of the MAPK/ERK signaling pathway, promoting cell
proliferation, angiogenesis and resistance to apoptosis (63). These mutations are associated with
poor prognosis, higher recurrence rates and increased lymph node
involvement (43). In patients with
rectal cancer, BRAF mutations have been associated with lower pCR
rates after nCRT and inferior survival outcomes (64). Although BRAF-targeted therapies have
shown efficacy in metastatic colorectal cancer, particularly when
combined with MEK inhibitors and anti-EGFR agents (65), there is currently limited evidence
supporting their use in patients with locally advanced rectal
cancer with residual nodal disease after nCRT. Further studies are
warranted to investigate whether such strategies may be beneficial
for the ypT0N+ subgroup.
MSI is caused by mutations or deletions in DNA
mismatch repair genes, resulting in genetic instability (66). MSI-H is commonly found in rectal
cancer and is associated with lower rates of lymph node metastasis
and improved responses to chemotherapy (67). However, certain MSI-H patients may
still exhibit ypT0N+ status, possibly due to immune evasion
mechanisms within the tumor (68).
For these patients, immune checkpoint inhibitors (such as PD-1
inhibitors) could be considered as part of their treatment plan.
Moreover, immunotherapy has been reported to be highly effective in
MSI-H colorectal cancers, particularly in cases of recurrence or
metastasis (69).
Furthermore, the present study evaluated the
prognostic value of the number of positive lymph nodes. Although
the univariate Cox regression analysis did not show a statistically
significant association between lymph node count (1–2 vs. ≥3) and
OS or DFS (both P>0.05), this may be due to the limited sample
size in the ≥3 positive node subgroup (n=21), which may have
reduced the power to detect a difference. Nonetheless, lymph node
burden remains a clinically relevant consideration. Prior studies
have reported that patients with higher numbers of positive nodes
are at greater risk for recurrence and worse long-term outcomes
(27,70–72).
Therefore, lymph node count should still be incorporated into
postoperative risk stratification, especially in ypT0N+ patients,
to guide treatment intensity and follow-up frequency.
However, the present study has several limitations.
Whilst no confirmed false-positive pCR cases were identified in the
present study, the potential for sampling bias and microscopic
residual disease must be acknowledged. The classification of ypT0N+
patients as having achieved pCR in the primary tumor was based on
standard histopathological examination, which may not always
capture residual tumor cells hidden within fibrotic tissue.
Additionally, the absence of liquid biopsy or molecular residual
disease assessment limited the ability to verify complete tumor
eradication. Potential false-positive pCR cases could have led to
an underestimation of recurrence risk and an overestimation of nCRT
effectiveness, reinforcing the need for intensified postoperative
surveillance in ypT0N+ patients. Future studies should incorporate
advanced molecular and imaging techniques, including ctDNA
analysis, to improve the accuracy of pCR assessment and guide
treatment decisions. Furthermore, in the present study, treatment
response categories were defined as follows: Complete response was
defined as ypT0N0, indicating no residual tumor at both the primary
and nodal sites; partial response included ypT1-2N0 or ypT0N+
cases, where tumor regression was observed; stable disease included
ypT3-4N0 or persistent positive nodes without significant
regression; and progressive disease was characterized by evidence
of disease progression or distant metastasis. These classifications
align with standard rectal cancer response criteria and were used
to assess treatment outcomes in our cohort. However, due to the
retrospective design of the present study and the lack of routine
molecular testing protocols at the study institutions during the
study period (2010–2020), genetic and immunological data such as
KRAS, NRAS, BRAF mutations, MSI and PD-1/PD-L1 expression were not
available for analysis. These biomarkers are critical determinants
of prognosis and treatment efficacy in patients with rectal cancer,
particularly for identifying patients who might benefit from
targeted therapies or immunotherapy. The absence of these molecular
data prevented the performance of a more precise risk
stratification and tailored therapeutic recommendations for ypT0N+
patients. Future studies, ideally prospective in design, should
incorporate comprehensive genetic and molecular profiling to
further elucidate their prognostic significance and improve
individualized treatment strategies. Additionally, due to the
retrospective design, the present study relied on existing medical
records, which limited the availability of certain prognostic
variables, including lifestyle factors such as diet, body mass
index, smoking, alcohol consumption and physical activity. These
factors have been associated with rectal cancer outcomes in
previous studies (73–75), but their impact could not be
assessed in the present study due to a lack of systematic
documentation. Additionally, whilst patients were enrolled from two
institutions, the study remains regionally specific and
single-center in nature, which may limit the generalizability of
the findings. Variations in chemotherapy regimens and radiotherapy
doses were also present, although their effect on survival was not
statistically significant. Furthermore, the relatively short
follow-up period for certain patients could influence survival
estimates. Therefore, future research should include prospective
lifestyle data collection, longer follow-up periods and
multi-center validation to further refine prognostic models and
treatment strategies for patients with rectal cancer. Lastly,
standard Cox proportional hazards models were employed to assess
survival outcomes without incorporating time-dependent covariates.
Although the proportional hazards assumption was satisfied,
time-dependent covariates were not incorporated into the final
multivariate analysis, which represents a methodological
limitation. To minimize the potential impact of time truncation
bias due to variable follow-up durations, a uniform right-censoring
strategy was applied by setting January 1, 2024 as the fixed cutoff
date for all survival analyses. Additionally, surgery year was
included as a continuous variable in the univariate Cox regression
model, with no significant association with OS or DFS, suggesting
minimal confounding from differences in enrollment time. Future
studies should consider using extended Cox models or longitudinal
statistical methods to better account for dynamic variables such as
postoperative biomarker changes.
In summary, patients with locally advanced rectal
cancer who achieve ypT0N0 after preoperative nCRT have a good
prognosis, whereas those with ypT0N+ have a worse prognosis.
Therefore, in future studies, the definition of pCR should be
limited to ypT0N0, and postoperative chemotherapy strategies for
patients with ypT0N+ may need to be adjusted accordingly.
Meanwhile, ypN+, pre-nCRT elevated CEA levels, cT3-4 and cN+ are
independent risk factors for OS and DFS in ypT0 patients,
emphasizing the need for enhanced postoperative treatment and
follow-up for these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ performed the data analysis and paper writing.
YNZ was responsible for the research design and guided the revision
of the paper. YS provided clinical cases, participated in data
analysis and interpretation, and revised the manuscript critically
for important intellectual content. LX contributed to the research
design, provided data collection support and assisted in drafting
the manuscript. HL participated in data analysis and contributed to
the revision of the manuscript. XY assisted in data analysis and
provided clinical case data. YK assisted in data collection and
analysis, and contributed to manuscript writing. YYZ made
substantial contributions to the acquisition and analysis of
clinical data. WG assisted in data collection and initial analysis.
JZ and YNZ confirm the authenticity of all the raw data. All
authors have read and approved the final version and agreed to be
accountable for the integrity of the work.
Ethics approval and consent to
participate
The current study was performed in accordance with
the Declaration of Helsinki and approved by the local Ethics
Committee of Cangzhou Hospital of Integrated Traditional Chinese
Medicine and Western Medicine (Cangzhou, China; approval no.
2021-KY-062.1). All patients and/or their families signed informed
consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
pCR
|
pathological complete response
|
|
nCRT
|
neoadjuvant chemoradiotherapy
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
CEA
|
carcinoembryonic antigen
|
References
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 1:47–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu X, Qi R, Xu Y, Wang X, Cai Y and Wang
C: Tumor regression grade in locally advanced rectal cancer after
neoadjuvant chemoradiotherapy: Influencing factors and prognostic
significance. Int J Clin Exp Pathol. 16:124–132. 2023.PubMed/NCBI
|
|
3
|
Glynne-Jones R, Wyrwicz L, Tiret E, Brown
G, Rödel C, Cervantes A and Arnold D; ESMO Guidelines Committee, :
Rectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 29 (Suppl 4):iv2632018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: Rectal cancer, version 2.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:874–901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung M, Shin SJ, Koom WS, Jung I, Keum KC,
Hur H, Min BS, Baik SH, Kim NK, Kim H, et al: A randomized phase 2
study of neoadjuvant chemoradiaton therapy with
5-fluorouracil/leucovorin or irinotecan/S-1 in patients with
locally advanced rectal cancer. Int J Radiat Oncol Biol Phys.
93:1015–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung KU, Kim HO, Kim H, Lee D and Cheong
C; on the behalf of Korean Society of Korean Society of
Coloproctology, : Unveiling the profound advantages of total
neoadjuvant therapy in rectal cancer: A trailblazing exploration.
Ann Surg Treat Res. 105:341–352. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu Z, Hao Y, Huang Y, Ling L, Hu X and
Qiao S: Radiotherapy in the preoperative neoadjuvant treatment of
locally advanced rectal cancer. Front Oncol. 13:13005352023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Sluis FJ, van Westreenen HL, van
Etten B, van Leeuwen BL and de Bock GH: Pretreatment identification
of patients likely to have pathologic complete response after
neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal
Dis. 33:149–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Latif A, Shirkhoda M, Rouhollahi MR,
Nemati S, Yahyazadeh SH, Zendehdel K, Soroush AR and Yaghoobi
Notash A: Predicting factors of complete pathological response in
locally advanced rectal cancer. Middle East J Dig Dis. 14:443–451.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith FM and Winter D: Pathologic complete
response of primary tumor following preoperative chemoradiotherapy
for locally advanced rectal cancer: long-term outcomes and
prognostic significance of pathologic nodal status (KROG 09-01).
Ann Surg. 265:e27–e28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin JK, Huh JW, Lee WY, Yun SH, Kim HC,
Cho YB and Park YA: Clinical prediction model of pathological
response following neoadjuvant chemoradiotherapy for rectal cancer.
Sci Rep. 12:71452022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bosset JF, Calais G, Mineur L, Maingon P,
Radosevic-Jelic L, Daban A, Bardet E, Beny A, Briffaux A and
Collette L: Enhanced tumorocidal effect of chemotherapy with
preoperative radiotherapy for rectal cancer: Preliminary
results-EORTC 22921. J Clin Oncol. 23:5620–5627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Braendengen M, Tveit KM, Berglund A,
Birkemeyer E, Frykholm G, Påhlman L, Wiig JN, Byström P, Bujko K
and Glimelius B: Randomized phase III study comparing preoperative
radiotherapy with chemoradiotherapy in nonresectable rectal cancer.
J Clin Oncol. 26:3687–3694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collard MK, Rullier E, Panis Y, Manceau G,
Benoist S, Tuech JJ, Alves A, Laforest A, Mege D, Cazelles A, et
al: Nonmetastatic ypt0 rectal cancer after neoadjuvant treatment
and total mesorectal excision: Lessons from a retrospective
multicentric cohort of 383 patients. Surgery. 171:1193–1199. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JS, Yoon G, Kim HJ, Park SY, Choi GS,
Kang MK, Kim JG, Jang JS and Seo AN: HER2 status in patients with
residual rectal cancer after preoperative chemoradiotherapy: The
relationship with molecular results and clinicopathologic features.
Virchows Arch. 473:413–423. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jo P, Jung K, Grade M, Conradi LC, Wolff
HA, Kitz J, Becker H, Rüschoff J, Hartmann A, Beissbarth T, et al:
CpG island methylator phenotype infers a poor disease-free survival
in locally advanced rectal cancer. Surgery. 151:564–570. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Li N, Wang F, Liu H, Zhang Y,
Xiao J, Qiu W, Zhang C, Fan X, Qiu M, et al: Characterization of
the tumor microenvironment and identification of spatially
predictive biomarkers associated with beneficial neoadjuvant
chemoradiotherapy in locally advanced rectal cancer. Pharmacol Res.
197:1069742023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mandard AM, Dalibard F, Mandard JC, Marnay
J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G,
et al: Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:2680–2686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iskander O, Courtot L, Tabchouri N, Artus
A, Michot N, Muller O, Pabst-Giger U, Bourlier P, Kraemer-Bucur A,
Lecomte T, et al: Complete pathological response following
radiochemotherapy for locally advanced rectal cancer: Short and
long-term outcome. Anticancer Res. 39:5105–5113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Capirei C, Valentini V, Cionini L, De
Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G,
Palazzi S, et al: Prognostic value of pathologic complete response
after neoadjuvant therapy in locally advanced rectal cancer:
Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol
Phys. 72:99–107. 2008. View Article : Google Scholar
|
|
22
|
Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C,
Hui B, Liu R, Ma H and Ren J: A Review of neoadjuvant
chemoradiotherapy for locally advanced rectal cancer. Int J Biol
Sci. 12:1022–1031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sell NM, Qwaider YZ, Goldstone RN, Cauley
CE, Cusack JC, Ricciardi R, Bordeianou LG, Berger DL and Kunitake
H: Ten-year survival after pathologic complete response in rectal
adenocarcinoma. J Surg Oncol. 123:293–298. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Campos-Lobato LF, Stocchi L, da Luz
Moreira A, Geisler D, Dietz DW, Lavery IC, Fazio VW and Kalady MF:
Pathologic complete response after neoadjuvant treatment for rectal
cancer decreases distant recurrence and could eradicate local
recurrence. Ann Surg Oneol. 18:1590–1598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Capirci C, Valentini V, Cionini L, De
Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G,
Palazzi S, et al: Prognostic value of pathologic complete response
after neoadjuvant therapy in locally advanced rectal cancer:
Long-term analysis of 566 ypCR patients. Int J Radiat Oneol Biol
Phys. 72:99–107. 2008. View Article : Google Scholar
|
|
26
|
Zeman M, Skałba W, Szymański P, Hadasik G,
Żaworonkow D, Walczak DA and Czarniecka A: Risk factors for
long-term survival in patients with ypN+ M0 rectal cancer after
radical anterior resection. BMC Gastroenterol. 22:1412022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Destri G, Maugeri A, Ramistella A, La
Greca G, Conti P, Trombatore G, Vecchio GM, Magro GG, Barchitta M
and Agodi A: The prognostic impact of neoadjuvant chemoradiotherapy
on lymph node sampling in patients with locally advanced rectal
cancer. Updates Surg. 72:793–800. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Q, Liang J, Chen J, Mei S and Wang
Z: Predictive factors for pathologic complete response following
neoadjuvant chemoradiotherapy for rectal cancer. Asian Pac J Cancer
Prev. 22:1607–1611. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu L, Wang L, Gao Z, Zeng Y, Tao K, Wang
Q, Li X, Zhang H, Shen Z, Zhou J, et al: Examined lymph node
numbers influence prognosis in rectal cancer treated with
neoadjuvant therapy. Cancer Pathog Ther. 1:168–176. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozturk SK, Martinez CG, Mens D, Verhoef C,
Tosetto M, Sheahan K, de Wilt JHW, Hospers GAP, van de Velde CJH,
Marijnen CAM, et al: Lymph node regression after neoadjuvant
chemoradiotherapy in rectal cancer. Histopathology. 84:935–946.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu Z, Cheng P, Yang F, Zheng Z and Wang X:
Long-term outcomes in patients with ypT0 rectal cancer after
neoadjuvant chemoradiotherapy and curative resection. Chin J Cancer
Res. 30:272–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Sun G, Zheng K, Lou Z, Gao XH,
Meng RG, Furnée EJB and Zhang W: Prognostic factors in patients
with complete response of the tumour (ypT0) after neoadjuvant
chemoradiotherapy and radical resection of rectal cancer. ANZ J
Surg. 91:E190–E195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jamal-Hanjani M, Wilson GA, MeGranahan N,
Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R,
Rosenthal R, et al: Trackingthe evolution of non-small-cell lung
cancer. N Engl J Med. 376:2109–2121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abbosh C, Birkbak NJ, Wilson GA,
Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA,
Veeriah S, Rosenthal R, et al: Corrigendum: Phylogenetic ctDNA
analysis depicts early-stage lung cancer evolution. Nature.
554:2642018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Sukhni E, Attwood K, Mattson DM,
Gabriel E and Nurkin SJ: Predictors of pathologic complete response
following neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg
Oncol. 23:1177–1186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng J, Lin J, Qiu M, Wu X, Lu Z, Chen G,
Li L, Ding P, Gao Y, Zeng Z, et al: Clinical factors of
post-chemoradiotherapy as valuable indicators for pathological
complete response in locally advanced rectal cancer. Clinics (Sao
Paulo). 71:449–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Petrova V, Annicchiarico-Petruzzelli M,
Melino G and Amelio I: The hypoxic tumour microenvironment.
Oncogenesis. 7:102018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou C, Wang K, Zhang X, Xiao Y, Yang C,
Wang J, Qu F, Wang X, Liu M, Gao C, et al: Assessing the predictive
value of clinical factors to pathological complete response for
locally advanced rectal cancer: An analysis of 124 patients. Front
Oncol. 13:11254702023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Colloca G, Venturino A and Vitucci P:
Pre-treatment carcinoembryonic antigen and outcome of patients with
rectal cancer receiving neo-adjuvant chemo-radiation and surgical
resection: A systematic review and meta-analysis. Med Oncol.
34:1772017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Machado Carvalho JV, Dutoit V, Corrò C and
Koessler T: Promises and challenges of predictive blood biomarkers
for locally advanced rectal cancer treated with neoadjuvant
chemoradiotherapy. Cells. 12:4132023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Huang Y, Xu M, Zhuang J, Zhou Z,
Zheng S, Zhu B, Guan G, Chen H and Liu X: Pathomics-based machine
learning models for predicting pathological complete response and
prognosis in locally advanced rectal cancer patients
post-neoadjuvant chemoradiotherapy: Insights from two independent
institutional studies. BMC Cancer. 24:15802024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu Y, Wu H, Hong L, Qiu J, Wu S, Shao L,
Lin C, Wang Z and Wu J: A large population-based and validated
study on the follow-up management and supportive strategy of
locally advanced rectal cancer patients. Support Care Cancer.
32:6522024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bengala C, Bettelli S, Bertolini F,
Sartori G, Fontana A, Malavasi N, Depenni R, Zironi S, Del Giovane
C, Luppi G and Conte PF: Correction to: Prognostic role of EGFR
gene copy number and KRAS mutation in patients with locally
advanced rectal cancer treated with preoperative chemoradiotherapy.
Br J Cancer. 131:9542024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng S, You Z, Guo G, Lin Z, Wang S and
Yang G: Effect of KRAS mutation status on clinicopathological
characteristics and overall survival in patients with rectal
cancer. BMC Gastroenterol. 25:372025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
De Mattia E, Polesel J, Mezzalira S,
Palazzari E, Pollesel S, Toffoli G and Cecchin E: Predictive and
prognostic value of oncogene mutations and microsatellite
instability in locally-advanced rectal cancer treated with
neoadjuvant radiation-based therapy: A systematic review and
meta-analysis. Cancers (Basel). 15:14692023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Swets M, Graham Martinez C, van Vliet S,
van Tilburg A, Gelderblom H, Marijnen CAM, van de Velde CJH and
Nagtegaal ID: Microsatellite instability in rectal cancer: What
does it mean? A study of two randomized trials and a systematic
review of the literature. Histopathology. 81:352–362. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li C, Liu T, Liu Y, Zhang J and Zuo D:
Prognostic value of tumour microenvironment-related genes by TCGA
database in rectal cancer. J Cell Mol Med. 25:5811–5822. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schrag D, Shi Q, Weiser MR, Gollub MJ,
Saltz LB, Musher BL, Goldberg J, Al Baghdadi T, Goodman KA,
McWilliams RR, et al: Preoperative treatment of locally advanced
rectal cancer. N Engl J Med. 389:322–334. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Scott AJ, Kennedy EB, Berlin J, Brown G,
Chalabi M, Cho MT, Cusnir M, Dorth J, George M, Kachnic LA, et al:
Management of locally advanced rectal cancer: ASCO guideline. J
Clin Oncol. 42:3355–3375. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sobrero A, Lonardi S, Rosati G, Di
Bartolomeo M, Ronzoni M, Pella N, Scartozzi M, Banzi M, Zampino MG,
Pasini F, et al: FOLFOX or CAPOX in stage II to III colon cancer:
Efficacy results of the Italian three or six colon adjuvant trial.
J Clin Oncol. 36:1478–1485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bond MJG, Bolhuis K, Loosveld OJL, de
Groot JWB, Droogendijk H, Helgason HH, Hendriks MP, Klaase JM,
Kazemier G, Liem MSL, et al: First-line systemic treatment
strategies in patients with initially unresectable colorectal
cancer liver metastases (CAIRO5): An open-label, multicentre,
randomised, controlled, phase 3 study from the Dutch colorectal
cancer group. Lancet Oncol. 24:757–771. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ciardiello F, Ciardiello D, Martini G,
Napolitano S, Tabernero J and Cervantes A: Clinical management of
metastatic colorectal cancer in the era of precision medicine. CA
Cancer J Clin. 72:372–401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kajiwara Y and Ueno H: Essential updates
2022–2023: Surgical and adjuvant therapies for locally advanced
colorectal cancer. Ann Gastroenterol Surg. 8:977–986. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Grothey A: Pembrolizumab in MSI-H-dMMR
advanced colorectal cancer-A new standard of care. N Engl J Med.
383:2283–2285. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tie J, Wang Y, Tomasetti C, Li L, Springer
S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, et al:
Circulating tumor DNA analysis detects minimal residual disease and
predicts recurrence in patients with stage II colon cancer. Sci
Transl Med. 8:346ra922016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Reinert T, Schøler LV, Thomsen R, Tobiasen
H, Vang S, Nordentoft I, Lamy P, Kannerup AS, Mortensen FV,
Stribolt K, et al: Analysis of circulating tumour DNA to monitor
disease burden following colorectal cancer surgery. Gut.
65:625–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Linders D, Deken M, van der Valk M,
Tummers W, Bhairosingh S, Schaap D, van Lijnschoten G, Zonoobi E,
Kuppen P, van de Velde C, et al: CEA, EpCAM, αvβ6 and uPAR
expression in rectal cancer patients with a pathological complete
response after neoadjuvant therapy. Diagnostics (Basel).
11:5162021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Boogerd LS, van der Valk MJ, Boonstra MC,
Prevoo HA, Hilling DE, van de Velde CJ, Sier CF, Fariña Sarasqueta
A and Vahrmeijer AL: Biomarker expression in rectal cancer tissue
before and after neoadjuvant therapy. Onco Targets Ther.
11:1655–1664. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu L, Zhu JJ, Liang XH, Tong H and Song Y:
Predictive value of magnetic resonance imaging parameters combined
with tumor markers for rectal cancer recurrence risk after surgery.
World J Gastrointest Surg. 17:1018972025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
De Palma FDE, Luglio G, Tropeano FP,
Pagano G, D'Armiento M, Kroemer G, Maiuri MC and De Palma GD: The
role of Micro-RNAs and circulating tumor markers as predictors of
response to neoadjuvant therapy in locally advanced rectal cancer.
Int J Mol Sci. 21:70402020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chatila WK, Kim JK, Walch H, Marco MR,
Chen CT, Wu F, Omer DM, Khalil DN, Ganesh K, Qu X, et al: Genomic
and transcriptomic determinants of response to neoadjuvant therapy
in rectal cancer. Nat Med. 28:1646–1655. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang J, Lin Y, Huang Y, Jin J, Zou S,
Zhang X, Li H, Feng T, Chen J, Zuo Z, et al: Genome landscapes of
rectal cancer before and after preoperative chemoradiotherapy.
Theranostics. 9:6856–6866. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hammarström K, Nunes L, Mathot L,
Mezheyeuski A, Lundin E, Korsavidou Hult N, Imam I, Osterlund E,
Sjöblom T and Glimelius B: Clinical and genetic factors associated
with tumor response to neoadjuvant (chemo)radiotherapy, survival
and recurrence risk in rectal cancer. Int J Cancer. 155:40–53.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Corcoran RB, André T, Atreya CE, Schellens
JHM, Yoshino T, Bendell JC, Hollebecque A, McRee AJ, Siena S,
Middleton G, et al: Combined BRAF, EGFR, and MEK inhibition in
patients with BRAFV600E-mutant colorectal cancer. Cancer
Discov. 4:428–443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hasan S, Renz P, Wegner RE, Finley G, Raj
M, Monga D, McCormick J and Kirichenko A: Microsatellite
instability (MSI) as an independent predictor of pathologic
complete response (PCR) in locally advanced rectal cancer: A
national cancer database (NCDB) analysis. Ann Surg. 271:716–723.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Koopman M, Kortman GAM, Mekenkamp L,
Ligtenberg MJL, Hoogerbrugge N, Antonini NF, Punt CJA and van
Krieken JHJM: Deficient mismatch repair system in patients with
sporadic advanced colorectal cancer. Br J Cancer. 100:266–273.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Acar T, Acar N, Kamer E, Tekindal MA,
Cengiz F, Kar H, Atahan K and Haciyanli M: Do microsatellite
instability (MSI) and deficient mismatch repair (dMMR) affect the
pathologic complete response (pCR) in patients with rectal cancer
who received neoadjuvant treatment? Updates Surg. 72:73–82. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yu JH, Liao LE, Xiao BY, Zhang X, Wu AW,
Cheng Y, Tang JH, Jiang W, Kong LH, Han K, et al: Long-term
outcomes of dMMR/MSI-H rectal cancer treated with anti-PD-1-based
immunotherapy as curative-intent treatment. J Natl Compr Canc Netw.
22:e2370962024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Langman G, Patel A and Bowley DM: Size and
distribution of lymph nodes in rectal cancer resection specimens.
Dis Colon Rectum. 4:406–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Achilli P, Ferrari D, Calini G, Bertoglio
CL, Magistro C, Origi M, Carnevali P, Alampi BD, Giusti I, Ferrari
G, et al: Preoperative lateral lymph node features and impact on
local recurrence after neoadjuvant chemoradiotherapy and total
mesorectal excision for locally advanced rectal cancer: Results
from a multicentre international cohort study. Colorectal Dis.
26:466–475. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Beppu N, Matsubara N, Noda M, Yamano T,
Kakuno A, Doi H, Kamikonya N, Yamanaka N, Yanagi H and Tomita N:
Pathologic evaluation of the response of mesorectal positive nodes
to preoperative chemoradiotherapy in patients with rectal cancer.
Surgery. 157:743–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kokaine L, Gardovskis A and Gardovskis J:
Evaluation and predictive factors of complete response in rectal
cancer after neoadjuvant chemoradiation therapy. Medicina (Kaunas).
57:10442021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yuhara H, Steinmaus C, Cohen SE, Corley
DA, Tei Y and Buffler PA: Is diabetes mellitus an independent risk
factor for colon cancer and rectal cancer? Am J Gastroenterol.
106:1911–1922. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cai Z, Xie X, Chen Y, Chen Z, Cao W, Saad
KSS, Zou Y, Lan P and Wu X: Risk factor analysis for inaccurate
pre-operative MRI staging in rectal cancer. BMC Cancer. 20:2532020.
View Article : Google Scholar : PubMed/NCBI
|