Introduction
Lung cancer was the leading cause of cancer-related
death worldwide in 2022, accounting for 18.7% of all cancer
fatalities (1). Small-cell lung
cancer (SCLC) accounts for 13–17% of all lung cancer cases. Despite
a high initial chemotherapy response rate (60–67%) (2), SCLC often recurs rapidly and develops
resistance to subsequent treatments, leading to a poor prognosis.
Patients with limited-stage SCLC (LS-SCLC) typically have a better
prognosis than those with extensive-stage SCLC (ES-SCLC) due to the
more localized nature of the disease. However, even with standard
concurrent chemoradiotherapy, the median overall survival (OS) for
patients with LS-SCLC remains 25–30 months, with a 5-year survival
rate of merely 31–34% (3).
Maintenance therapy has emerged as a critical strategy for
extending survival following first-line treatment. The ADRIATIC
study showed that durvalumab maintenance therapy significantly
improved OS in patients with LS-SCLC after concurrent
chemoradiotherapy [55.9 vs. 33.4 months, hazard ratio (HR)=0.73]
(4), underscoring its potential as
a new standard of care. The current study presented the case of a
patient who received oral temozolomide (TMZ) as maintenance therapy
after first-line treatment, achieving an exceptional survival of 6
years without disease recurrence, which highlights the potential of
TMZ as a maintenance therapy for LS-SCLC.
Case report
A 57-year-old woman, 165 cm tall and weighing 70 kg,
in February 2019 developed various symptoms, including cough,
expectoration, chest tightness and fatigue. The symptoms were
relieved after self-administered oral antibiotics but recurred in
March. The patient had no history of smoking and no family history
of cancer. The patient had been previously healthy, without any
chronic conditions such as hypertension, diabetes or any history of
cardiac, hepatic or renal insufficiency. A chest computed
tomography (CT) scan in March 2019 at the Affiliated Hospital of
Hebei University of Engineering (Handan, China) revealed a
malignant mass in the left hilum (3.0×4.5 cm), along with several
enlarged lymph nodes in the regions of group 4L lower paratracheal,
group 10 left hilar and group 8 paraesophageal. The enlarged nodes
exhibited partial fusion, obscuring precise quantification (the
original CT films were inaccessible due to the passage of time.
Only the CT report and images captured by a mobile phone were
retained and submitted as supplementary material) (Fig. 1A; Fig.
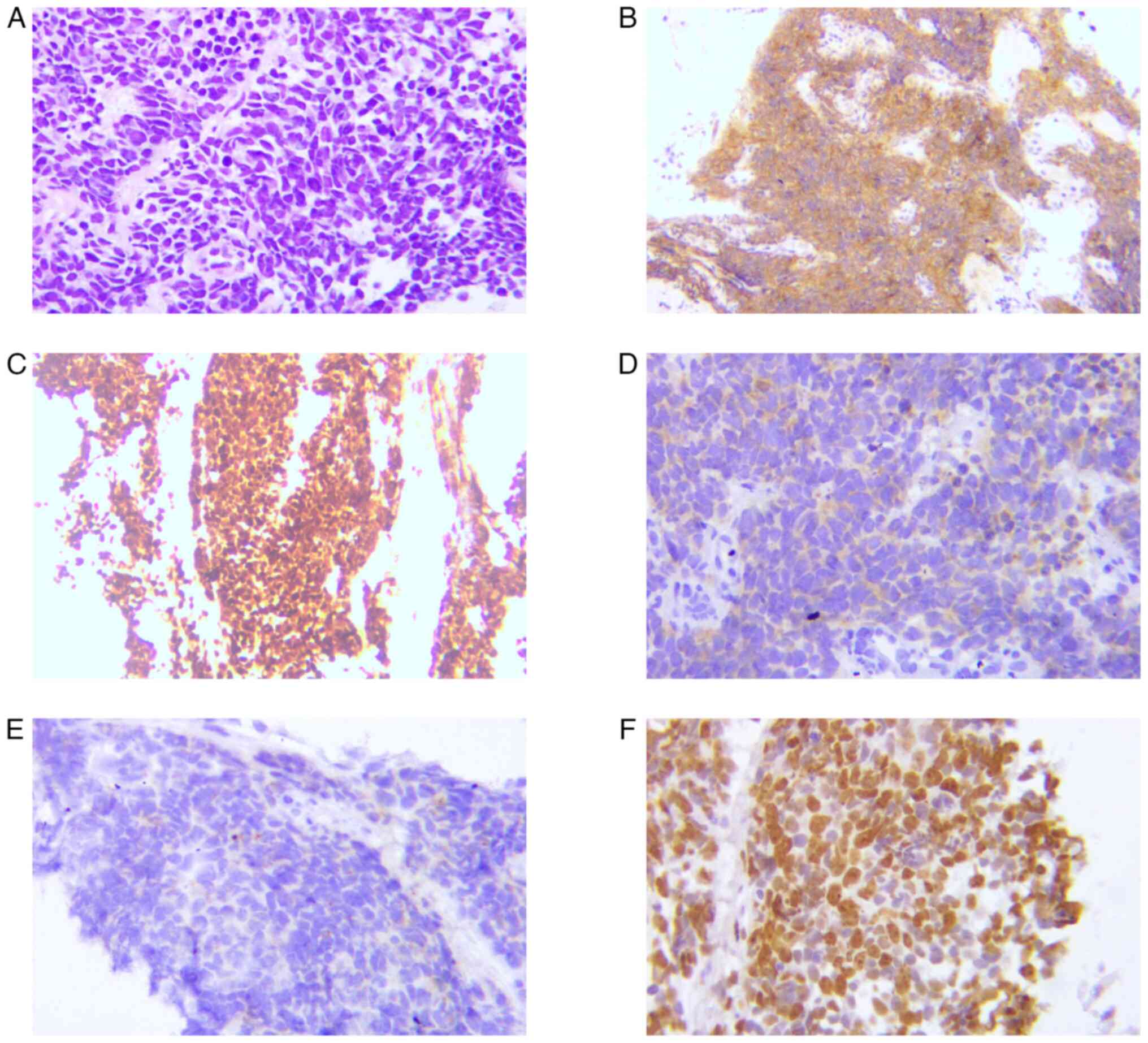
S1). On March 2019, a bronchoscopy biopsy confirmed SCLC with
aspergillus proliferation. Immunohistochemical analysis showed weak
cytokeratin positivity, thyroid transcription factor 1 (TTF-1) and
cluster of differentiation 56 (CD56) positivity and partial weak
positivity for chromogranin A and synaptophysin, while tumor
protein p63 (P63), P40 and lithodeoxycholic acid were negative,
with a Ki-67 index of 60% (Fig. 2).
The clinical staging was cT2N2M0,
stage IIIA.
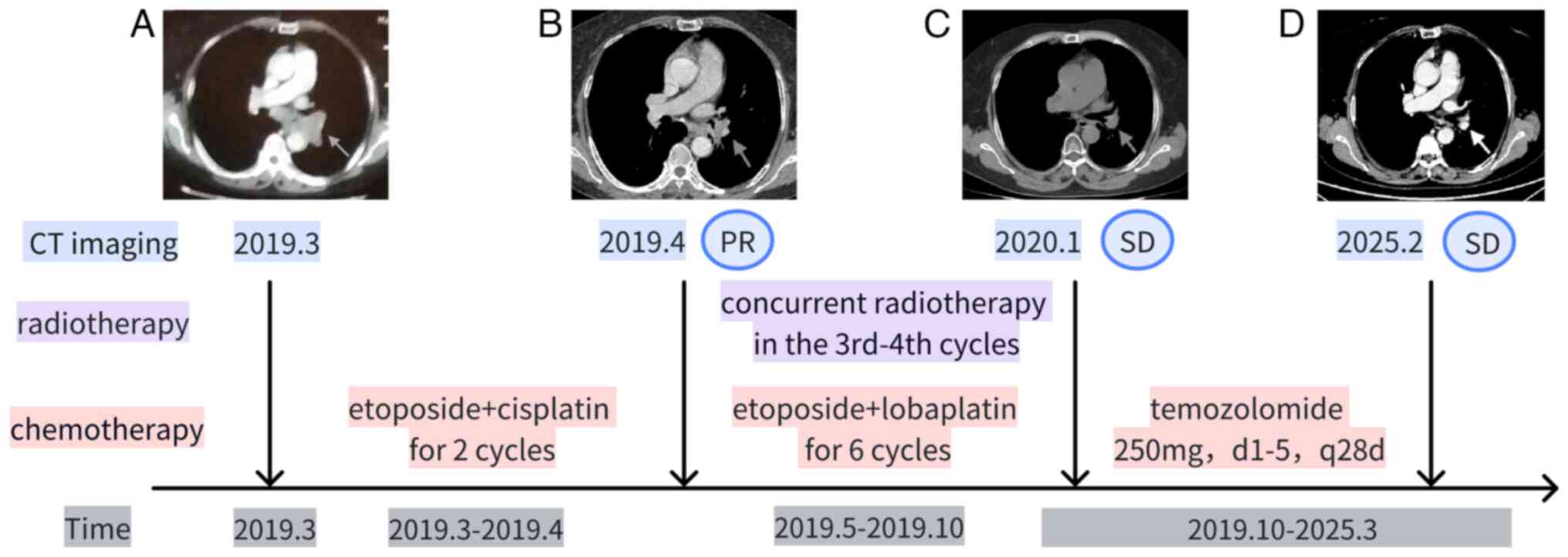 | Figure 1.Changes in the treatment and chest CT
of the patient. First-line treatment: Etoposide + platinum-based
drugs (cisplatin for 2 cycles, lobaplatin for 6 cycles), totaling 8
cycles. Synchronized radiotherapy commenced after the completion of
the 3rd chemotherapy cycle and was concluded before the initiation
of the 5th chemotherapy cycle. Maintenance therapy: Oral
temozolomide, 250 mg/day, days 1–5, every 28 days. Outcome: As of
March 2025, the OS of the patient was 72 months. Chest CT imaging:
(A) Pre-chemotherapy imaging in March 2019, with the arrow
indicating the lesion at the left hilum. (B) After completion of 2
cycles of chemotherapy in April 2019, the lesion at the left hilum
(indicated by the arrow) showed PR compared to pre-treatment. (C)
After completion of 3 cycles of maintenance therapy in January
2020, the lesion at the left hilum (indicated by the arrow) showed
SD. (D) The most recent contrast-enhanced CT imaging in February
2025 shows that the lesion at the left hilum (indicated by the
arrow) is in a state of SD. PR, partial response; SD, stable
disease; OS, overall survival. |
The specimens were fixed in 10% neutral buffered
formalin at room temperature for 24 to 48 h and embedded in
paraffin. The tissue blocks were sectioned into slices of 4 or 5 µm
in thickness. H&E staining was performed by staining with
hematoxylin for 10 min and eosin for 5 min at room temperature.
Elastic fiber staining was conducted using the iron hematoxylin
method, also at room temperature (5). A mixture of 5% ethanol hematoxylin,
10% ferric chloride and Verhoeff's iodine solution in a ratio of
20:8:8 drops was prepared and applied to the tissue sections.
Counterstaining with eosin was performed for 2 min.
Immunohistochemistry was carried out by EnVision system. Antigen
retrieval was performed by high pressure treatment at 120°C for 5
min, followed by blocking endogenous enzyme activity with 3%
H2O2 for 10 min. The primary antibodies
included ALK (clone D5F3; cat. no. K18082; Roche Diagnostics) and
TTF-1 (clone SPT24; cat. no. 18092706; OriGene Technologies, Inc.),
both diluted at 1:200 and incubated at room temperature for 1 h.
The secondary antibody was a horseradish peroxidase labeled polymer
(1:2,000 dilution; cat. no. M00855-M01010; Roche Diagnostics),
incubated at 37°C for 30 min. Next, DAB was used for color
development at room temperature for 10 min, and hematoxylin was
used for counterstaining at room temperature for 10 min. All
sections were examined under a light microscope.
The patient was diagnosed with LS-SCLC with
mediastinal lymph node metastasis and a concomitant fungal
infection. Treatment commenced in March 2019, utilizing a regimen
of etoposide and a platinum-based drug for eight cycles (Fig. 1). After two cycles of etoposide (120
mg on days 1–3) and cisplatin (85 mg on day 1), the patient
exhibited a partial response (Fig.
1B) but suffered severe nausea and vomiting, necessitating a
switch from cisplatin to lobaplatin. The third cycle was completed
with etoposide (120 mg on days 1–3) and lobaplatin (40 mg on day
1), alongside 28 sessions of radiotherapy (56 Gy; 2.0 Gy daily,
from May to July 2019). The reason for initiating concurrent
radiotherapy after the third cycle was that the patient had a
combined aspergillus infection. Administering radiotherapy during
the first two cycles carried the risk of further compromising the
immune system and exacerbating the pulmonary infection. The patient
developed grade II myelosuppression, with a white blood cell count
of 2.82×109/l (normal ranges, 3.5–9.5×109/l),
prompting a dosage reduction of etoposide to 100 mg in cycles 4–6
to reduce the risk further of myelosuppression. In cycle 4, during
radiotherapy, the patient developed grade III myelosuppression,
with a white blood cell count of 1.28×109/l and a
neutrophil count of 0.53×109/l (normal range,
1.8–6.3×109/l). The National Comprehensive Cancer
Network guidelines recommend 4–6 cycles of chemotherapy for SCLC
(6). However, clinical practice
should be adjusted based on the patient's personal preferences and
physical tolerance: Dose reduction or reduced cycles may be
considered when chemotherapy is intolerable, while extended cycles
could be an option for patients demonstrating adequate tolerance
who desire enhanced therapeutic efficacy. Considering the patient's
desire for better therapeutic outcomes and physical tolerance, an
additional two cycles of chemotherapy were initiated, with
treatment intervals extended to 28 days to minimize side effects.
Cycles 7 and 8 were completed with etoposide (100 mg on days 1–4)
and lobaplatin (75 mg on day 2), resulting in grade III
myelosuppression, with a white blood cell count of
1.4×109/l, neutrophil count of 0.37×109/l and
platelet count of 27×109/l (normal range,
125–350×109/l). After first-line treatment, the patient
did not choose prophylactic cranial irradiation due to the risk of
cognitive decline and instead opted for regular brain MRI checks to
detect potential brain metastases early. In October 2019, following
the completion of first-line therapy, the patient commenced oral
TMZ maintenance therapy at a dosage of 250 mg, administered on days
1–5 of each 28-day cycle. After 3 cycles of maintenance therapy, a
chest CT scan revealed stable disease (Fig. 1C). The patient then continued with
regular follow-up assessments, including brain MRI, chest and
abdomen CT, and whole-body bone scintigraphy, with the frequency of
follow-ups gradually extended from 3 to 6 months, and eventually to
once a year. The last follow-up was conducted in February 2025,
with the chest CT scan showing a stable lesion (Fig. 1D). The patient has now survived for
6 years since the definitive diagnosis, maintaining a stable
condition without significant adverse reactions.
Discussion
This case report highlights the long-term survival
of a patient with LS-SCLC receiving oral TMZ as maintenance therapy
following first-line treatment. Remarkably, the patient has
survived for 6 years without disease recurrence, a rare achievement
in SCLC cases. This prolonged survival prompts critical inquiries
about the benefits of maintenance therapy and the potential
underlying mechanisms that may contribute to this unique
outcome.
A 2005 meta-analysis found that maintenance or
consolidation therapy increased the 1-year survival rate by 9%
(from 30 to 39%), the 2-year survival rate by 4% (from 10 to 14%),
the 1-year progression-free survival (PFS) rate by 10% (from 13 to
23%) and the 2-year PFS rate by 3% (from 10 to 13%) (7). In addition, a 2013 meta-analysis
indicated that maintenance chemotherapy improved PFS [HR=0.72, 95%
confidence interval (CI) 0.58–0.89, P=0.003] in ES-SCLC but did not
significantly affect OS (8).
Regarding maintenance strategies, conversion strategies, which
utilize a regimen different from the initial treatment, showed a
trend toward better PFS and OS but without statistical
significance. By contrast, continuous strategies that use the same
regimen as the initial treatment had no significant impact on OS
and even worsened PFS (HR=1.27, 95% CI 1.04–1.54). These findings
underscore the importance of maintenance therapy in improving
survival outcomes for patients with ES-SCLC, although the optimal
strategy is under investigation.
The Concurrent ONce-daily VErsus twice-daily
RadioTherapy (CONVERT) trial found that ~30% of patients with
LS-SCLC developed brain metastasis after concurrent
chemoradiotherapy, significantly impacting mortality rates in this
population (3). By contrast, the
patient of the present study survived for 6 years without
developing brain metastasis, likely due to TMZ's ability to cross
the blood-brain barrier. While there is no direct evidence
supporting TMZ as a preventive therapy for brain metastases in
SCLC, several studies have explored its efficacy in treating
existing brain metastases. One case study reported on a patient
with ES-SCLC who achieved complete remission (CR) following
whole-brain radiotherapy (WBRT) but developed multiple new brain
metastases after 15 months. After treatment with TMZ, the patient
attained CR after 6 months with good tolerance (9). Another study involving two patients
with SCLC receiving a combination of TMZ and etoposide showed
stabilization of central nervous system lesions, both
radiologically and clinically, for 12 and 29 weeks, respectively
(10). A Phase II study found that
TMZ alone controlled the brain disease in 41% of patients with
recurrent brain metastasis (11).
The prolonged use of oral TMZ raises concerns about
potential adverse effects. Nausea and vomiting, the most common
non-hematologic toxicities, affect ~50% of patients, although they
are typically mild to moderate in severity (12). Thrombocytopenia and neutropenia are
considered dose-limiting toxicities. While TMZ at 150
mg/m2/day is generally well tolerated in patients with
solid tumors, higher doses can lead to severe hematologic toxicity
(13). In the present case, the
patient took 250 mg/day, which was calculated based on the standard
dose of 150 mg/m2/day (calculation: 165 cm, 70 kg, body
surface area of 1.75 m2, resulting in 1.75 m2
× 150 mg/m2/day=262.5 mg/day). The patient's complete
blood count, liver and kidney function, as well as symptom changes
are being regularly monitored to detect potential adverse
reactions. Supportive treatment is provided based on any discomfort
the patient experiences. After 65 months of TMZ therapy (as of
March 2025), the patient did not experience any grade II or higher
myelosuppression. It is noteworthy that challenges arose in
obtaining all relevant test results due to the patient consulting
at external hospitals. The findings are based on follow-up
examinations from the China-Japan Friendship Hospital. In addition,
the patient did not experience significant vomiting after the
administration of ondansetron to manage nausea, suggesting that
long-term oral TMZ is safe and well-tolerated. However, rare
toxicities such as aplastic anemia, cholestatic hepatitis,
lymphopenia-induced opportunistic infections, myelodysplastic
syndromes and leukemia have been reported during TMZ treatment
(14). Although these adverse
effects are uncommon, their severity and specificity require
careful clinical monitoring. Regular assessments of blood counts,
as well as liver and kidney function, are crucial during the
long-term use of TMZ.
Immunotherapy shows potential as a maintenance
treatment for SCLC. Studies suggest that certain medications may
improve the effectiveness of immunotherapy, indicating that
combination therapies could be a valuable avenue for future
research in SCLC maintenance therapies.
A retrospective study found that combining TMZ with
programmed cell death protein-1 (PD-1)/programmed cell death-ligand
1 (PD-L1) inhibitors resulted in an ORR of 26.19% and a DCR of
64.29% in patients with NSCLC brain metastasis (15). Furthermore, a Phase II trial showed
that TMZ combined with nivolumab for treating recurrent or
refractory SCLC and advanced neuroendocrine tumors achieved an ORR
of 30%, a median PFS of 2.4 months and a median OS of 6.3 months;
the median OS was 9 months for patients with brain metastasis
(16). The NCT0491938 trial is
currently exploring the combination of TMZ with atezolizumab as
maintenance therapy for relapsed or refractory ES-SCLC (17).
SCLC cells can repair DNA damage, particularly
through the poly(ADP-ribose) polymerase (PARP) pathway. PARP
inhibitors can block this repair process, thereby enhancing the
cytotoxic effects of TMZ. Research has shown that combining TMZ
with talazoparib is more effective than monotherapy in
patient-derived xenograft models with high Schlafen family member
11 expression (18). A randomized,
double-blinded Phase II trial found that the TMZ and veliparib
combination significantly increased the ORR (39 vs. 14%) in
patients with relapsed or refractory ES-SCLC; however, no
significant differences were found in PFS and OS (19).
PARP inhibitors may enhance the tumor
microenvironment, boosting the effectiveness of immunotherapy.
Studies suggest that PARP inhibitors may exert immune-modulatory
effects by activating the cyclic GMP-AMP synthase/stimulator of
interferon genes (STING) pathway, thereby transforming ‘cold’
tumors into ‘hot’ tumors and enhancing the anti-tumor activity of
immunotherapy in Excision repair cross-complementation group
1-deficient NSCLC (20). In
addition, DNA damage response inhibitors such as prexasertib and
olaparib can increase PD-L1 expression in SCLC cell lines,
promoting CD8+ T-cell infiltration into tumors and anti-tumor
immunity through the activation of the STING/TANK-binding kinase
1/interferon regulatory factor 3 pathway, which leads to the
production of type I interferons (e.g., interferon-β) and
chemokines (e.g., C-X-C motif chemokine ligand 10 and C-C motif
chemokine ligand 5) (21).
Lurbinectedin has been shown to reduce tumor-associated macrophages
and modulate the inflammatory tumor microenvironment (22). Ongoing clinical trials, such as
NCT03830918, are investigating the combination of TMZ, niraparib
and atezolizumab as maintenance therapy for SCLC, with PFS as the
primary endpoint.
In conclusion, this case report details a patient
with LS-SCLC who survived for 6 years without disease recurrence
after receiving oral TMZ as maintenance therapy. Immunotherapy has
emerged as a standard maintenance treatment for SCLC. Future
research should explore the combination of immunotherapy with TMZ
or PARP inhibitors to enhance treatment outcomes for patients with
SCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the horizontal research project from
China-Japan Friendship Hospital (grant no. 2023-HX-130, funded by
Beijing Bethune Charitable Foundation, grant no. STLKY2-077).
Availability of data and materials
All data generated in the present study are included
in the figures/tables of this article.
Authors' contributions
DW and HC designed the study. AW, XZ and CW were
responsible for patient management and interpreted the patient
data. DW, TX, YG and YX acquired and analyzed the data. DW and YX
drafted the manuscript. HC and CW revised the manuscript and
checked and confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ganti AKP, Loo BW, Bassetti M, Blakely C,
Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M,
et al: Small cell lung cancer, version 2.2022, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:1441–1464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faivre-Finn C, Snee M, Ashcroft L, Appel
W, Barlesi F, Bhatnagar A, Bezjak A, Cardenal F, Fournel P, Harden
S, et al: Concurrent once-daily versus twice-daily
chemoradiotherapy in patients with limited-stage small-cell lung
cancer (CONVERT): An open-label, phase 3, randomised, superiority
trial. Lancet Oncol. 18:1116–1125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng Y, Spigel DR, Cho BC, Laktionov KK,
Fang J, Chen Y, Zenke Y, Lee KH, Wang Q, Navarro A, et al:
Durvalumab after chemoradiotherapy in limited-stage small-cell lung
cancer. N Engl J Med. 391:1313–1327. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Musto L: Improved iron-hematoxylin stain
for elastic fibers. Stain Technol. 56:185–187. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ganti AKP, Loo BW, Bassetti M, Blakely C,
Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M,
et al: Small cell lung cancer, version 2.2022, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:1441–1464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bozcuk H, Artac M, Ozdogan M and Savas B:
Does maintenance/consolidation chemotherapy have a role in the
management of small cell lung cancer (SCLC)? A metaanalysis of the
published controlled trials. Cancer. 104:2650–2657. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou H, Zeng C, Wei Y, Zhou J and Yao W:
Duration of chemotherapy for small cell lung cancer: A
meta-analysis. PLoS One. 8:e738052013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gay CM, de Groot PM, Pietanza MC and Byers
LA: Durable, exceptional response to temozolomide in a patient with
extensive-stage small cell lung cancer (ES-SCLC) metastatic to
brain. Cancer Treatment and Research Communications. 10:17–20.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lukas RV, Vigneswaran J and Salgia R:
Etoposide and temozolomide in combination for the treatment of
progressive small-cell lung cancer central nervous system
metastases: Two cases. Tumori. 99:e73–e76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abrey LE, Olson JD, Raizer JJ, Mack M,
Rodavitch A, Boutros DY and Malkin MG: A phase II trial of
temozolomide for patients with recurrent or progressive brain
metastases. J Neurooncol. 53:259–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trinh VA, Patel SP and Hwu WJ: The safety
of temozolomide in the treatment of malignancies. Expert Opin Drug
Saf. 8:493–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hammond LA, Eckardt JR, Baker SD, Eckhardt
SG, Dugan M, Forral K, Reidenberg P, Statkevich P, Weiss GR,
Rinaldi DA, et al: Phase I and pharmacokinetic study of
temozolomide on a daily-for-5-days schedule in patients with
advanced solid malignancies. J Clin Oncol. 17:2604–2613. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dixit S, Baker L, Walmsley V and Hingorani
M: Temozolomide-related idiosyncratic and other uncommon
toxicities: A systematic review. Anticancer Drugs. 23:1099–1106.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wu D, Tang J and Wu Y: The
efficiency and safety of temozolomide and PD-1/L1 inhibitors in
pretreated NSCLC with brain metastasis: A retrospective cohort. J
Cancer Res Clin Oncol. 150:2712024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Owen DH, Wei L, Benner B, Pilcher C,
Christenson G, Ferguson S, Jukich M, Sukrithan V, Konda B, Shah M,
et al: OA12.04 efficacy of nivolumab and temozolomide in extensive
stage small cell lung cancer after chemo-immunotherapy: A phase 2
trial. J Thorac Oncol. 17:S32–S33. 2022. View Article : Google Scholar
|
|
17
|
Owen DH, Durm GA, Wei L, Pilcher C,
Ferguson S, Benner B, Jukich M, Sukrithan V, Konda B, Savardekar H,
et al: EP14.05–004 temozolomide and atezolizumab as second line
treatment for extensive stage small cell lung cancer: A randomized,
multi-cohort phase 2 trial. J Thorac Oncol. 17:S544–S545. 2022.
View Article : Google Scholar
|
|
18
|
Lok BH, Gardner EE, Schneeberger VE, Ni A,
Desmeules P, Rekhtman N, de Stanchina E, Teicher BA, Riaz N, Powell
SN, et al: PARP inhibitor activity correlates with SLFN11
expression and demonstrates synergy with temozolomide in small cell
lung cancer. Clin Cancer Res. 23:523–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pietanza MC, Waqar SN, Krug LM, Dowlati A,
Hann CL, Chiappori A, Owonikoko TK, Woo KM, Cardnell RJ, Fujimoto
J, et al: Randomized, double-blind, phase II study of temozolomide
in combination with either veliparib or placebo in patients with
relapsed-sensitive or refractory small-cell lung cancer. J Clin
Oncol. 36:2386–2394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chabanon RM, Muirhead G, Krastev DB, Adam
J, Morel D, Garrido M, Lamb A, Hénon C, Dorvault N, Rouanne M, et
al: PARP inhibition enhances tumor cell-intrinsic immunity in
ERCC1-deficient non-small cell lung cancer. J Clin Invest.
129:1211–1228. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sen T, Rodriguez BL, Chen L, Corte CMD,
Morikawa N, Fujimoto J, Cristea S, Nguyen T, Diao L, Li L, et al:
Targeting DNA damage response promotes antitumor immunity through
STING-mediated T-cell activation in small cell lung cancer. Cancer
Discov. 9:646–661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Belgiovine C, Bello E, Liguori M,
Craparotta I, Mannarino L, Paracchini L, Beltrame L, Marchini S,
Galmarini CM, Mantovani A, et al: Lurbinectedin reduces
tumour-associated macrophages and the inflammatory tumour
microenvironment in preclinical models. Br J Cancer. 117:628–638.
2017. View Article : Google Scholar : PubMed/NCBI
|