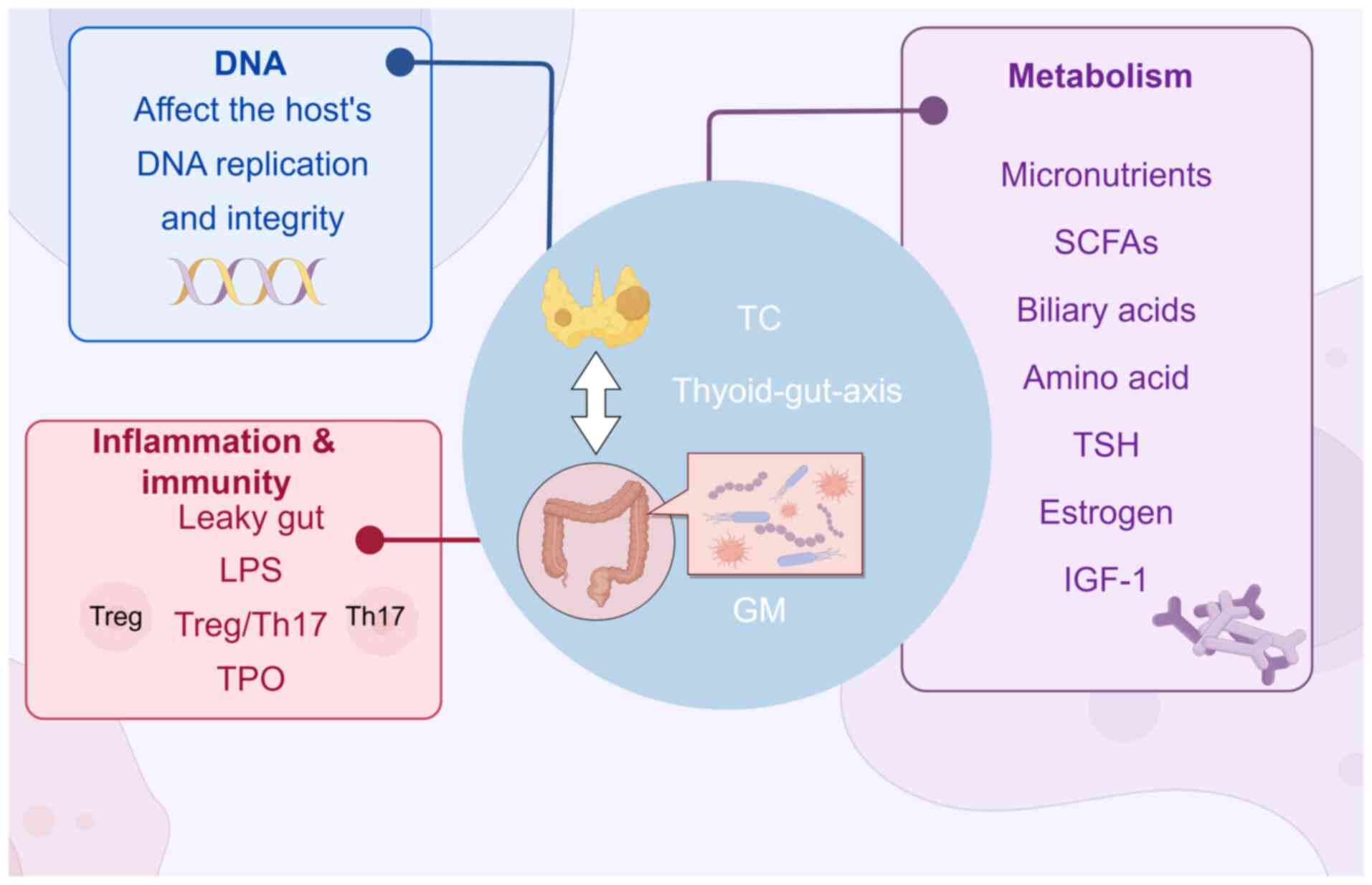
It has become established that the intricate
association between humans and their microbiota is vital for human
health (1). Various factors,
including diet, antibiotic use, genetics and the environment,
significantly shape the composition of the microbiota, which
matures in humans at an age of ~3 years, but continues to evolve
throughout life (1). The GM
comprises ~1013−1014 microorganisms, which
fulfill essential roles beyond digestive balance, including
nutrient assimilation, metabolic homeostasis, hormonal modulation
and immune regulation (2,3). The lymphocytes within the intestinal
mucosa orchestrate responses to microorganisms, making the
microbiota a key factor in determining an individual's health
status (4,5). Microbiota disturbances have also been
shown to contribute to numerous diseases (6), including thyroid cancer (TC). Previous
studies (7–11) investigating the relationship between
the microbiota and tumors (e.g., colorectal tumors) have reported
that the microbiota found in different parts of the body, including
the gut, mouth and within tumors (12–20),
can influence cancer growth and metastasis due to the common
embryonic lineage that thyroid follicular cells share with gastric
mucosal cells (21). Thyroid
disorders have been shown to be closely tied to thyroid hormone
levels and function, as well as the composition of the intestinal
flora (22). Furthermore, the
gut-brain axis allows intestinal microorganisms to modulate immune,
metabolic and endocrine interactions (23). Several studies have linked
intestinal microbiota with thyroid-associated conditions, including
Graves' disease, Hashimoto's thyroiditis and TC, highlighting the
importance of maintaining a healthy intestinal flora for thyroid
disease prevention (24–26).
TC, a common endocrine malignancy, has seen an
increased global incidence in recent years, particularly among
women, suggesting sex-associated factors (27–32).
Although various risk factors, such as smoking, obesity, hormone
exposure, family history and environmental factors, have been
implicated in the development of TC, the precise causes underlying
the disease remain largely unknown (33). Several studies have demonstrated a
significant association between the gut microbiome and risk factors
for TC, indicating its potential role in TC pathogenesis. There is
evidence to suggest a possible association between microbiome
diversity and composition with risk factors for thyroid diseases,
including hormonal imbalances and obesity (34–36).
TC is typically treated with thyroid surgery, radioactive iodine
(RAI) therapy and thyroid-stimulating hormone (TSH) suppression
(37). However, these treatments
have been shown to lead to various side effects (38–41),
potentially compromising patients' quality of life (41,42).
Recently, one randomized clinical trial demonstrated that
probiotics may help reduce postoperative reaction and
complications, possibly through modifying the gut and oral
microbiota (43). This suggests
that the GM may fulfill a crucial role in the development,
prevention, diagnosis, treatment and management of TC. Therefore, a
comprehensive understanding of the interaction between GM and TC is
crucial for improving clinical outcomes and patient care.
The present review aims to integrate and explore
this crucial interplay between GM and TC, offering novel avenues or
strategies for enhancing the understanding and management of
TC.
Previous studies have indicated a strong link
between the composition of the GM and the risk of TC, although the
exact causal association remains controversial (Table I). One study (44) employed 16S rRNA sequencing, which
showed that patients with TC had a higher richness and alpha
diversity of intestinal flora compared with healthy individuals. Of
note, the Firmicutes/Bacteroidetes ratio was found to
be markedly elevated, similarly to patterns observed in other
cancers, including breast cancer and colon cancer (45–47).
In another study (48), it was
shown that patients with TC had lower numbers of Butyricum
and Lactobacillus, which was found to be connected with
trace elements such as selenium, which protect the thyroid and
fight against oxidative stress, whereas the numbers of
Clostridium, Neisseria and Streptococcus were
enhanced. Furthermore, TSH was positively correlated with
Porphyromonas (r=0.57; P<0.01), triiodothyronine was
correlated with Streptococcus (r=0.43; P<0.001) and
thyroglobulin was negatively correlated with Bacteroides and
Lactobacillaceae (r=−0.43; P<0.001), suggesting that
these genera could serve as biomarkers for TC (48). A subsequent study (49) reported changes in the GM of patients
with TC, marked by increased numbers of Bacteroidetes,
Clostridium and Lachnospiraceae, whereas the numbers of
Prevotella and Faecalibacterium were decreased. This
research group also identified a four-genus signature
(‘g_Hungatella’, ‘g_Alistipes’, ‘g_Bacterium’, and
‘g_Phascolarctobacterium’), which suggested that patients with TC
also had metastatic lymphadenopathy. However, their findings
contradicted those of other studies (48–50),
as they observed reduced richness and diversity of intestinal
microbiota in patients with TC. Additionally, a study by Lu et
al (50) noted a decrease in
lipid metabolism-associated genera and elevated levels of
27-hydroxycholesterol, whereas other research groups (44,51)
described shifts in microbiota composition, with increased numbers
of Escherichia coli and decreased numbers of Bacteroides
vulgatus in patients with TC. Furthermore, several Mendelian
randomization analyses have been published (51–58),
which suggested a potential bidirectional causal association
between GM composition and TC. For instance, Streptococcus
and bacteria of the class Betaproteobacteria were identified
as risk factors and protective factors for TC, respectively. Taken
together, these findings have highlighted the importance of
understanding the role of GM in the development and progression of
TC.
Overall, alterations of the GM in patients with TC
have been shown to include increases in the numbers of
Clostridium, Streptococcus, Proteus and
Lachnospiraceae bacteria, alongside decreases in the numbers
of Lactobacillus, Prevotella and Ruminococcaceae. The
populations of clinical trials mentioned in Table I (details of gut microbiota
composition in TC) are Asian (44,48–51),
whereas the populations of Mendelian randomization studies
(52–57) are from various ethnicities. The
conflicting findings of the above studies on microbiota diversity
may be attributed to small sample sizes, differences in the
demographics, tumor stage and treatment, or dietary considerations.
Furthermore, it should be noted that these studies only used 16S
rRNA sequencing, thereby necessitating the use of further, more
advanced methods.
Another significant mechanism involves inflammation.
Cancer-associated microbiota and pattern recognition receptors,
such as Toll-like receptors, have been linked to the activation of
nuclear factor κB (NF-κB) signaling in the tumor microenvironment
(63). This process sets off a
chain reaction of chronic inflammation, causing both the continuous
damage and repair of epithelial cells and the release of cytokines,
promoting malignancy (64). The
inflammatory response also stimulates immune cells to release
cytokines, thereby enhancing cell proliferation, inhibiting
apoptosis and deactivating tumor suppressor genes via the NF-κB and
STAT3 signaling pathways (65).
The adult body stores 15–20 mg iodine in the thyroid
gland, absorbed through the sodium/iodine symporter (NIS) present
in the stomach, duodenum and jejunum. Both the thyroid gland and
extra-glandular tissues express NIS, with iodine also being
absorbed via the cystic fibrosis and salt multivitamin transporters
(70–72). Previous studies have identified that
individuals with inflammatory bowel disease may have lower levels
of Firmicutes and Bacteroidetes, leading to iodine
malabsorption and decreased rates of thyroid hormone synthesis,
suggesting a potential association between iodine absorption and GM
(73,74). Furthermore, thyroid hormones
influence the motility of the small intestine, which, in turn,
affects the composition of the intestinal flora. Therefore, it may
be proposed that changes in GM due to the prevailing thyroid
conditions may affect iodine uptake, the synthesis of thyroid
hormones and RAI treatment efficacy, and these aspects warrant
further research.
SCFAs, such as butyric, acetic and propionic acids,
are essential compounds produced by GM, particularly
Flachnospiraceae and Butyricimonas, which are
potentially able to prevent cancer (78–80). A
study by Wang et al (81)
highlighted that Lactobacillus species produce pyruvate
through glycolysis, thereby promoting butyrate production, which
serves to support normal cell growth and inhibit tumor cell
proliferation. A different study (82) demonstrated how butyrate leads to a
decrease in the expression level of c-Myc and the resultant
inhibition of microRNA (miR)-92a transcription, thereby promoting
apoptosis in colon cancer cells. Furthermore, SCFAs fulfill a
crucial role in reducing chronic vascular inflammation by
regulating the levels of inflammatory cytokines, such as
interleukin (IL)-6 and IL-8, and modulating endothelial activation
(83). Butyrate was shown to
strengthen intestinal immune barriers, thereby decreasing
pro-inflammatory factors, and inhibiting inflammation-associated
pathways (84). Furthermore, SCFAs,
derived from the fermentation of dietary fibers, were shown to
induce apoptosis of TC cells and to promote cell cycle arrest (G1
and G2/M). They also inhibit histone deacetylases,
increasing the expression of the p21, p27 and Bax genes, as well as
that of Notch1 protein, while causing a decrease in the expression
of pro-survival genes, such as Bcl-2, Bcl-xL and cyclins A and B,
and reducing the activities of cyclin-dependent kinase 1 and 2.
Additionally, the NIS was found to be significantly upregulated,
and the level of thyroglobulin mRNA was increased, thereby
enhancing iodine uptake (85–90).
However, previous studies (44,48)
have also demonstrated a decrease in the numbers of SCFA-producing
bacteria in patients with TC, potentially increasing the TC cancer
risk due to lower butyrate levels. This reduction in the numbers of
SCFA-producing bacteria may affect Lactobacillus species,
thereby compromising butyrate production and leading to the
dysregulation of thyroid malignancies and inflammatory responses.
Therefore, modulating the levels of SCFAs may be a means of
improving tumor cell sensitivity to RAI by increasing the
expression of NIS, thereby providing valuable insights into future
therapeutic strategies.
The GM are also able to influence the metabolism of
amines and secondary bile acids. For instance, histamine, an amine
metabolism byproduct, has been shown to stimulate tumor cell growth
(44,91). In addition, cholesterol and
27-hydroxycholesterol have both been linked with increased
aggressiveness in TC, with 27-hydroxycholesterol being associated
with the Christensenellaceae R7 group, potentially promoting
estrogen receptor-driven TC growth (50,92).
Furthermore, a study by Wang (65)
using the Kyoto Encyclopedia of Genes and Genomes database data
revealed important roles for amino acid metabolites and specific
bacteria in PTC development, particularly regarding tryptophan
metabolism, as this affects intestinal permeability and immune
responses. Disruptions in bacterial amino acid metabolism may
therefore contribute to PTC by fostering inflammatory and
immunosuppressive conditions.
Changes in intestinal flora are a potential factor
in TC development. Changes in the numbers/levels of gut bacteria
may activate galactose and ketone body metabolic pathways, which
result in the fueling of TC progression (93,94).
Feng et al (44) found a
notable decrease in the number of Megamonas bacteria,
accompanied by elevated flavonoid levels in patients with TC;
therefore, these flavonoids were negatively correlated with the
abundance of Megamonas. Flavonoids affect the TPO enzyme,
disrupting thyroid hormone synthesis either by altering the
structure of TPO or by competitively inhibiting its activity
(95). This disruption may lead to
reduced hormone production and increased serum TSH levels, which
are recognized as a risk factor for TC development (69,96).
Taken together, these findings emphasize the interplay between gut
flora and TC progression, highlighting the necessity of exploring
further the association between GM metabolism and thyroid
tumorigenesis.
Having a history of breast cancer significantly
increases the likelihood of developing TC, particularly when there
is a positive family history (97).
The level of estrogen, a known risk factor for breast cancer, may
be increased due to its conversion from bound to free estrogen in
the gut (98,99). This rise in circulating estrogen has
been connected to TC development, particularly through estrogen
receptor (ER) activation, including activation of the ER subtype
ERα, which is highly expressed in PTC tissues (100–102). ERα activation may impede the
tumor-suppressive effects of miR-299-5p, thereby promoting TC
progression (103). Furthermore,
estrogen has been shown to induce proangiogenic changes in
endothelial cells, fostering tumor growth and metastasis.
Intestinal dysbiosis, coupled with elevated estrogen levels, may
significantly contribute to the development of TC in women. This
underscores the need to improve the understanding of the
association between hormones, gut health and cancer in order to
develop potential targeted prevention and treatment strategies for
TC.
Obesity and insulin resistance exert a crucial
impact on TC development. A previous study by He et al
(104) demonstrated a close
correlation between body mass index (BMI) and the incidence of TC,
where higher BMI values were associated with an increased risk of
TC. Previous studies (44,105) have also indicated that changes in
gut bacteria composition, specifically decreased numbers of
Bacteroidetes and increased numbers of Firmicutes
bacteria, are associated with TC in obese individuals. Individuals
with obesity consuming high-fat diets often have increased
Gram-negative bacteria levels, leading to the production of
lipopolysaccharides that trigger chronic intestinal inflammation
(106). This increase in
inflammation may disrupt the integrity of the intestinal barrier,
allowing bacteria to enter into the bloodstream, resulting in
chronic inflammation in adipose tissue. Chronic inflammation often
leads to insulin resistance (107), which is associated with an
increased risk of various malignancies, including TC. Insulin
resistance, in turn, elicits increases in the level of insulin-like
growth factor-1 (IGF-1), which is overexpressed in TC. High levels
of IGF-1 can fuel cancer growth by promoting cell malignancy and
inhibiting apoptosis. Insulin, acting as a growth factor, activates
pathways that further enhance the risk of developing TC (108,109). Additionally, the disruption of the
IGF axis by high insulin levels may contribute to the progression
of TC (110). Previous studies
(111,112) identified high expression levels of
IGF-1 and IGF-1 receptor in patients with TC, suggesting that IGF-1
enhances tumor growth through TSH stimulation, thereby activating
the AKT and Raf-1/MEK/ERK signaling pathways and promoting tumor
proliferation. Considered altogether, the future treatment of TC
should focus on weight control as an important factor acting
against this malignancy, where obesity and insulin resistance need
to be strategically avoided or overcome.
Studies have revealed the changes that occur in the
GM of patients with TC when compared with healthy individuals
(44,48,49).
Despite the small sample sizes used in sequencing studies, these
findings have raised important questions regarding post-operative
alterations in the GM of patients with TC. One study (113), which utilized 16S RNA sequencing,
demonstrated that patients with TC had a lower fecal microbial
community richness compared with healthy individuals, with six
bacterial species, including Bacteroidetes, Blautia, Eubacterium
rectum, Bifidobacterium, Eubacterium hallii and
Fusobacterium, exhibiting notable differences.
Interestingly, no significant disparities were observed between the
thyroid peroxidase antibody positive and thyroid peroxidase
antibody negative groups. The impact of GM on the prognosis and
complications of patients with TC following thyroidectomy cannot be
overstated. For instance, one study noted a negative correlation
between the abundance of Bifidobacteriales and the occurrence and
severity of post-operative nausea and vomiting in female patients,
thereby suggesting that regulating GM may alleviate these symptoms
(114). In addition, patients with
PTC often need to have the dosage level of levothyroxine hormone
adjusted post-surgery. Probiotics have also been shown to affect
the absorption of levothyroxine, necessitating lower dosage
adjustments (115). A randomized
controlled trial involving thyroid hormone withdrawal (THW)
combined with probiotics demonstrated that patients who received
probiotics experienced improvements in microbial dysbiosis and
reduced withdrawal side effects compared with those who received a
placebo. These findings emphasized the importance of GM management
in post-operative care (43,116).
A growing number of studies have supported the
potential of fecal microbiota transplantation (FMT) as a promising
treatment for different types of TC and associated complications
(117). A previous study by Routy
et al (118) showed that
modulating the microbiome via the application of FMT may lead to
enhancements in the effectiveness of cancer immunotherapy,
particularly when combined with immune checkpoint inhibitors (ICIs)
that target the cytotoxic T-lymphocyte associated protein 4 and
programmed cell death protein 1 (PD-1) pathways. Personalized GM
modulation, including FMT during PTC treatment, may also promote
positive responses to 131I therapy (119). FMT is also being studied for its
applicability in various other thyroid-associated conditions,
including primary hypothyroidism (120–122). Probiotics have also demonstrated
promising results. A previous study revealed that administering one
specific probiotic led to notable decreases in the levels of
Firmicutes and circulating autoantibodies in patients with
Graves' disease, leading to lower recurrence rates 6 months after
antithyroid treatment (123).
Probiotics such as Bacillus subtilis, Bifidobacterium and
Lactobacillus, derived from Firmicutes and
Actinobacteria, positively impact gut flora composition and
metabolic pathways in patients with PTC. Furthermore, this study
revealed reduced levels of specific amino acids that are closely
associated with gut flora and metabolic processes. Therefore,
patients with PTC may benefit from amino acid supplementation to
restore microbial balance and metabolic functions. However, further
studies are required to fully understand the association between
changes in GM and prognosis, in order to address current gaps in
knowledge within this field.
The microbiota is able to significantly influence
the effectiveness and toxicity of various anticancer therapies,
including chemotherapy and immunotherapy (85). RAI therapy, a key adjuvant treatment
for TC, is often used following thyroidectomy (124). 131I treatment,
particularly multiple high doses of RAI therapy, in patients with
TC may disrupt the balance of GM and the radiation-sensitive
pathways of linoleic acid, arachidonic acid and tryptophan
metabolites (125). However, RAI
may cause complications such as salivary gland inflammation,
leading to xerostomia (also known as dry mouth), with dysfunction
rates reported as high as 72.73% (126). Dry mouth negatively diminishes
patients' long-term quality of life through disrupting normal
salivary secretion. Furthermore, THW following RAI treatment may
cause fatigue, constipation, weight gain, edema and
hypercholesterolemia, thereby reducing the patients' quality of
life (38–42). Probiotics have emerged as a strategy
to manipulate the microbiota in order to improve outcomes during
anticancer treatment. Several randomized clinical trials have
demonstrated that probiotics may reduce the incidence of
complications in patients with THW postoperatively by restoring
microbiota diversity (43,127,128). One study found that patients with
dry mouth had a higher Firmicutes-to-Bacteroidetes
ratio, and an increased abundance of Streptococcus (128). In addition, the abundance of
inflammation-associated bacteria, such as Neisseria,
Veillonella, Porphyromonas, Corynebacterium and
Capnocytophaga, was found to be higher in patients with dry
mouth (129,130). For instance, Prevotella may
promote inflammation via Toll-like receptor 2 activation and Th17
cell-mediated immune responses (131); however, probiotics were able to
decrease the abundance of bacteria associated with dry mouth, such
as Prevotella_9, Haemophilus, Fusobacterium and
Lautropia, and their use is anticipated to lead to
improvements regarding a series of side effects caused by RAI
treatment and THW.
GM may also serve as a predictor of the responses of
patients with PTC to RAI therapy or 131I treatment.
Researchers have found that butyric acid-producing Dorea
serve as an independent predictor of the response to
131I treatment, suggesting that increasing the abundance
of Dorea and Bifidobacterium in the GM may lead to
improvements in the response rates of postoperative patients with
PTC (119). In addition,
macrogenomic sequencing revealed markedly lower Faecalibacterium
prausnitzii levels in patients post-RAI treatment compared with
healthy controls (132). This
species produces anti-inflammatory butyrate, potentially mitigating
radiation-induced damage (132).
Another study (133) also reported
that the gut microecology was disrupted by post-high-dose
131I therapy, with arachidonic acid acting as a key
metabolite in radioprotection. In addition, GM and RAI-refractory
papillary TC may be associated via different mechanisms that are
connected with NIS regulation, although the exact role of GM in
this context has yet to be fully elucidated (134). Additional studies in this regard
may have important clinical implications and lead to the discovery
of probiotics that facilitate the treatment of RAI-refractory
TC.
In 2005, the European Medicines Agency approved the
use of recombinant human TSH (rhTSH) for TSH stimulation prior to
RAI in patients with TC subjected to thyroidectomy. This involves
two intramuscular injections of 0.9 mg rhTSH, followed by RAI
administration on the third day, allowing patients to continue
thyroid hormone supplementation and avoid profound hypothyroidism.
Although treatment with rhTSH may cause side effects such as nausea
and fatigue, it has been shown to reduce the long-term salivary
gland dysfunction that is associated with RAI (135). Another study, by Horvath et
al (136), revealed that
administering lower RAI doses in low-to-intermediate-risk patients
resulted in comparable 5-year survival rates, yet with fewer
adverse effects, when rhTSH was included as a part of the regimen
compared with THW. However, further research is needed to confirm
these findings, and to investigate the potential of probiotics or
fecal microbiota transplantation to alleviate rhTSH side effects
and to reduce salivary gland dysfunction following RAI (137). In conclusion, numerous additional
studies are required to properly investigate the best use of RAI
therapy (whether using THW or rTSH) combined with intestinal flora
stabilization therapy.
The intestinal flora exerts a critical role in
modulating the PD-1/programmed death ligand 1 (PD-L1) pathway and
regulating the efficacy of ICIs. Given that >70% of immune cells
reside in the intestine, the GM enhances the host's mucosal immune
response, thereby strengthening epithelial tight junctions and
mitigating pathogen invasion. A study by Sivan et al
(138) reported that
bifidobacteria enhance anti-tumor activity when combined with ICIs,
thereby preventing tumor progression and significantly boosting the
efficacy of ICIs by activating dendritic cells and enhancing
CD8+ T-cell activation through resistance to the
negative regulation mediated by PD-1/PD-L1. PD-L1 expression is
significantly higher in thyroid tumors, with positive rates ranging
from 6.1–82.5% in patients with PTC, and from 22.2–81.2% in
patients with anaplastic TC. In spite of the fact that ICIs show
promise in terms of treating invasive and iodine-refractory TC
(139), the 2020 ASCO Phase II
trial of spartalizumab (PDR001) revealed a 35% response rate,
although some of the patients exhibited drug resistance (140). Controlling the gut flora may
mitigate primary resistance, with E. muciniphila having been
shown to be associated with improved ICI responses via IL-12
(118). The intestinal flora has
also been shown to enhance the responses of patients with melanoma
to ICIs, particularly through Faecalibacterium, which boosts
effector T-cell functions (141).
Additionally, the intestinal flora impacts Th17 and Treg cell
differentiation, with Firmicutes and Lachnospiraceae
being found to be enriched in patients with TC, further implicating
the GM in regulating immune functions and tumor immunotherapy
outcomes via mechanisms such as SCFA-mediated production (141). Taken together, these findings have
demonstrated that controlling the intestinal flora may represent a
significant breakthrough in improving tumor immunotherapy.
The GM has been shown to perform a range of crucial
roles in immune regulation, hormone control, metabolic equilibrium
and nutritional absorption (2,3). As a
result, the microbiota has been associated with the proliferation
of cancer cells (4,5), which may be useful in the diagnosis
and prognosis of cancer. In particular, the GM has been shown to
influence TC proliferation directly and indirectly via various
mechanisms, including chronic inflammation, regulation of trace
elements, metabolism of a range of compounds (e.g., SCFAs and amino
acids), hormones (e.g., TSH, FT3 and estrogen) and insulin
resistance. However, the precise details of these mechanisms remain
unclear and further studies are required. TC causes GM dysbiosis
and changes in the gut microbiome may correlate with the prognosis
of patients with TC. For the majority of patients with TC, the
cancer typically grows slowly and these patients have a better
prognosis. However, ~1% of TC cases are anaplastic TC, which has a
poor prognosis and is associated with rapid progression and high
mortality rates, with a one-year survival rate of only 20%.
Although checkpoint immunotherapy is commonly used for anaplastic
TC, few patients survive beyond 2 years following diagnosis
(142). Currently, surgical
removal and adjuvant therapies are effective for TC, although
patients must be treated with dosages of levothyroxine (via THW or
rTSH) post-surgery, and this is associated with a number of
complications that lower patients' quality of life (38–42,124–126). Changes in GM have been associated
with thyroid surgery, RAI and checkpoint inhibitors, suggesting
that GM may serve as biomarkers for TC diagnosis and prognosis. In
addition, the combined use of probiotics and FMT may enhance the
quality of life for patients with TC, and improve the prognosis for
patients with anaplastic TC. However, further studies, particularly
randomized controlled trials and high-quality observational
studies, are required to confirm these hypotheses. Ultimately,
exploring the specific mechanisms that link GM with TC may provide
novel insights into new therapies for TC.
Not applicable.
This study was funded by the Kunshan First People's Hospital
Innovation Team Development Program (grant no. Y24-071-101366), a
horizontal project supported by Shanghai United Imaging Healthcare
Co., Ltd. and Kunshan First People's Hospital (grant no.
H23-126-101180), and the 2022 National Key Laboratory of Radiation
Medicine and Radiation Protection Open Topics (grant no.
GZK1202219).
MW designed the study, wrote the manuscript and
performed a literature search. YZ critically reviewed, edited and
approved the manuscript. All authors read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Virili C, Fallahi P, Antonelli A, Benvenga
S and Centanni M: Gut microbiota and Hashimoto's thyroiditis. Rev
Endocr Metab Disord. 19:293–300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shanahan F: The gut microbiota in 2011:
Translating the microbiota to medicine. Nat Rev Gastroenterol
Hepatol. 9:72–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walsh CJ, Guinane CM, OToole PW and Cotter
PD: Beneficial modulation of the gut microbiota. FEBS Letters.
588:4120–4130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Power SE, OToole PW, Stanton C, Ross RP
and Fitzgerald GF: Intestinal microbiota, diet and health. Br J
Nutr. 111:387–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao L, Wang JY, Ma SF and Li SJ: Research
progress on the relationship between intestinal flora and
thyroid-related diseases. J Shanxi Med Univ. 5:707–710. 2023.
|
|
6
|
Kamada N, Chen GY, Inohara N and Núñez G:
Control of pathogens and pathobionts by the gut microbiota. Nat
Immunol. 14:685–690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sears CL and Garrett WS: Microbes,
microbiota, and colon cancer. Cell Host Microbe. 15:317–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwabe RF and Jobin C: The microbiome and
cancer. Nat Rev Cancer. 13:800–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Louis P, Hold GL and Flint HJ: The gut
microbiota, bacterial metabolites and colorectal cancer. Nat Rev
Microbiol. 12:661–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, Immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irrazábal T, Belcheva A, Girardin SE,
Martin A and Philpott DJ: The multifaceted role of the intestinal
microbiota in colon cancer. Mol Cell. 54:309–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Luo M, Zhang Y, Cao G and Wang S:
Association of high-risk human papillomavirus infection duration
and cervical lesions with vaginal microbiota composition. Ann
Transl Med. 8:11612020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitra A, MacIntyre DA, Ntritsos G, Smith
A, Tsilidis KK, Marchesi JR, Bennett PR, Moscicki AB and Kyrgiou M:
The vaginal microbiota associates with the regression of untreated
cervical intraepithelial neoplasia 2 lesions. Nat Commun.
11:19992020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tango CN, Seo SS, Kwon M, Lee DO, Chang HK
and Kim MK: Taxonomic and functional differences in cervical
microbiome associated with cervical cancer development. Sci Rep.
10:97202020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu S, Rhee KJ, Albesiano E, Rabizadeh S,
Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al: A
human colonic commensal promotes colon tumorigenesis via activation
of T helper type 17 T cell responses. Nat Med. 15:1016–1022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Housseau F and Sears CL: Enterotoxigenic
Bacteroides fragilis (ETBF)-mediated colitis in Min (Apc+/-) mice:
A human commensal-based murine model of colon carcinogenesis. Cell
Cycle. 9:3–5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q, Sun W and Zhang H: Interaction of
gut microbiota with endocrine homeostasis and thyroid cancer.
Cancers. 14:26562022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li JZ, Ma DS and Ma ZJ: Research progress
on the correlation between intestinal flora and thyroid cancer.
Chin J General Surg. 6:482–484. 2021.
|
|
20
|
Xie Z, Zhou J, Zhang X and Li Z: Clinical
potential of microbiota in thyroid cancer therapy. Biochimica Et
Biochim Biophys Acta Mol Basis Dis. 1870:1669712024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cellini M, Santaguida MG, Virili C,
Capriello S, Brusca N, Gargano L and Centanni M: Hashimotos
thyroiditis and autoimmune gastritis. Front Endocrinol (Lausanne).
8:922017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Virili C and Centanni M: ‘With a little
help from my friends’-The role of microbiota in thyroid hormone
metabolism and enterohepatic recycling. Mol Cell Endocrinol.
458:39–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jašarević E, Morrison KE and Bale TL: Sex
differences in the gut microbiome-brain axis across the lifespan.
Philos Trans R Soc Lond B Biol Sci. 371:201501222016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Covelli D and Ludgate M: The thyroid, the
eyes and the gut: A possible connection. J Endocrinol Invest.
40:567–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishaq HM, Mohammad IS, Guo H, Shahzad M,
Hou YJ, Ma C, Naseem Z, Wu X, Shi P and Xu J: Molecular estimation
of alteration in intestinal microbial composition in Hashimotos
thyroiditis patients. Biomed Pharmacother. 95:865–874. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao F, Feng J, Li J, Zhao L, Liu Y, Chen
H, Jin Y, Zhu B and Wei Y: Alterations of the gut microbiota in
hashimotos thyroiditis patients. Thyroid. 28:175–186. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du L, Li R, Ge M, Wang Y, Li H, Chen W and
He J: Incidence and mortality of thyroid cancer in China,
2008–2012. Chin J Cancer Res. 31:144–151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cabanillas ME, McFadden DG and Durante C:
Thyroid cancer. Lancet. 388:2783–2795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lortet-Tieulent J, Franceschi S, Dal Maso
L and Vaccarella S: Thyroid cancer ‘epidemic’ also occurs in low-
and middle-income countries. Int J Cancer. 144:2082–2087. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim J, Gosnell JE and Roman SA: Geographic
influences in the global rise of thyroid cancer. Nat Rev
Endocrinol. 16:17–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maruvada P, Leone V, Kaplan LM and Chang
EB: The human microbiome and obesity: Moving beyond associations.
Cell Host Microbe. 22:589–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stefura T, Zapała B, Gosiewski T,
Skomarovska O, Dudek A, Pędziwiatr M and Major P: Differences in
compositions of oral and fecal microbiota between patients with
obesity and controls. Medicina (Kaunas). 57:6782021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong T, Zhao F, Yuan K, Zhu X, Wang N, Xia
F, Lu Y and Huang Z: Association between serum Thyroid-stimulating
hormone levels and salivary microbiome shifts. Front Cell Infect
Microbiol. 11:6032912021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nabhan F, Dedhia PH and Ringel MD: Thyroid
cancer, recent advances in diagnosis and therapy. Int J Cancer.
149:984–992. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee J, Yun MJ, Nam KH, Chung WY, Soh EY
and Park CS: Quality of life and effectiveness comparisons of
thyroxine withdrawal, triiodothyronine withdrawal, and recombinant
thyroid-stimulating hormone administration for low-dose radioiodine
remnant ablation of differentiated thyroid carcinoma. Thyroid.
20:173–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schroeder PR, Haugen BR, Pacini F, Reiners
C, Schlumberger M, Sherman SI, Cooper DS, SchuffK G, Braverman LE,
Skarulis MC, et al: A comparison of short-term changes in
health-related quality of life in thyroid carcinoma patients
undergoing diagnostic evaluation with recombinant human thyrotropin
compared with thyroid hormone withdrawal. J Clin Endocrinol Metab.
91:878–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rubic M, Kuna SK, Tesic V, Samardzic T,
Despot M and Huic D: The most common factors influencing on quality
of life of thyroid cancer patients after thyroid hormone
withdrawal. Psychiatr Danub. 26:520–527. 2014.PubMed/NCBI
|
|
41
|
Sigal GA, Tavoni TM, Silva BMO, Kalil
Filho R, Brandão LG and Maranhão RC: Effects of Short-term
hypothyroidism on the lipid transfer to High-density lipoprotein
and other parameters related to lipoprotein metabolism in patients
submitted to thyroidectomy for thyroid cancer. Thyroid. 29:53–58.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh R, Tandon A, Gupta SK and Saroja K:
Optimal levothyroxine replacement adequately improves symptoms of
hypothyroidism; residual symptoms need further evaluation for other
than hypothyroidism causation. Indian J Endocrinol Metab.
21:830–835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin B, Zhao F, Liu Y, Wu X, Feng J, Jin X,
Yan W, Guo X, Shi S, Li Z, et al: Randomized clinical trial:
Probiotics alleviated Oral-Gut microbiota dysbiosis and thyroid
hormone Withdrawal-related complications in thyroid cancer patients
before radioiodine therapy following thyroidectomy. Front
Endocrinol (Lausanne). 13:8346742022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng J, Zhao F, Sun J, Lin B, Zhao L, Liu
Y, Jin Y, Li S, Li A and Wei Y: Alterations in the gut microbiota
and metabolite profiles of thyroid carcinoma patients. Int J
Cancer. 144:2728–2745. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Feng Q, Liang S, Jia H, Stadlmayr A, Tang
L, Lan Z, Zhang D, Xia H, Xu X, Jie Z, et al: Gut microbiome
development along the colorectal adenoma-carcinoma sequence. Nat
Commun. 6:65282015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fuhrman BJ, Feigelson HS, Flores R, Gail
MH, Xu X, Ravel J and Goedert JJ: Associations of the fecal
microbiome with urinary estrogens and estrogen metabolites in
postmenopausal women. J Clin Endocrinol Metab. 99:4632–4640. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeffery IB, OToole PW, Öhman L, Claesson
MJ, Deane J, Quigley EM and Simrén M: An irritable bowel syndrome
subtype defined by species-specific alterations in faecal
microbiota. Gut. 61:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang J, Zhang F, Zhao C, Xu Q, Liang C,
Yang Y, Wang H, Shang Y, Wang Y, Mu X, et al: Dysbiosis of the gut
microbiome is associated with thyroid cancer and thyroid nodules
and correlated with clinical index of thyroid function. Endocrine.
64:564–574. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu X, Jiang W, Kosik RO, Song Y, Luo Q,
Qiao T, Tong J, Liu S, Deng C, Qin S, et al: Gut microbiota changes
and its potential relations with thyroid carcinoma. J Adv Res.
35:61–70. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu G, Yu X, Jiang W, Luo Q, Tong J, Fan S,
Chai L, Gao D, Qiao T, Wang R, et al: Alterations of gut microbiome
and metabolite profiles associated with anabatic lipid
dysmetabolism in thyroid cancer. Front Endocrinol (Lausanne).
13:8931642022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ishaq HM, Mohammad IS, Hussain R, Parveen
R, Shirazi JH, Fan Y, Shahzad M, Hayat K, Li H, Ihsan A, et al:
Gut-Thyroid axis: How gut microbial dysbiosis associated with
euthyroid thyroid cancer. J Cancer. 13:2014–2028. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Quan Z, Zhang X, Wang S and Meng Y: Causal
analysis of the gut microbiota in differentiated thyroid carcinoma:
A two-sample Mendelian randomization study. Front Genetics.
14:12999302023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun X, Chen S, Zhao S, Wang J and Cheng H:
Causal relationship of genetically predicted gut microbiota with
thyroid cancer: A bidirectional two-sample mendelian randomization
study. Front Endocrinol (Lausanne). 15:12844722024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hou T, Wang Q, Dai H, Hou Y, Zheng J, Wang
T, Lin H, Wang S, Li M, Zhao Z, et al: Interactive association
between gut microbiota and thyroid cancer. Endocrinology.
165:bqad1842023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu F, Zhang P, Liu Y, Bao C, Qian D, Ma
C, Li H and Yu T: Mendelian randomization suggests a causal
relationship between gut dysbiosis and thyroid cancer. Front Cell
Infect Microbiol. 13:12984432023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou J, Zhang X, Xie Z and Li Z: Exploring
reciprocal causation: Bidirectional mendelian randomization study
of gut microbiota composition and thyroid cancer. J Cancer Res Clin
Oncol. 150:752024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hu S, Tang C, Wang L, Feng F, Li X, Sun M
and Yao L: Causal relationship between gut microbiota and
differentiated thyroid cancer: A two-sample Mendelian randomization
study. Front Oncol. 14:13755252024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yan S, He J, Yu X, Shang J, Zhang Y, Bai
H, Zhu X, Xie X and Lee L: Causal relationship between gut
microbiota and thyroid nodules: A bidirectional two-sample
Mendelian randomization study. Front Endocrinol (Lausanne).
15:14170092024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Knezevic J, Starchl C, Tmava Berisha A and
Amrein K: Thyroid-gut-axis: How does the microbiota influence
thyroid function? Nutrients. 12:17692020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao Y, Oh J, Xue M, Huh WJ, Wang J,
Gonzalez-Hernandez JA, Rice TA, Martin AL, Song D, Crawford JM, et
al: Commensal microbiota from patients with inflammatory bowel
disease produce genotoxic metabolites. Science. 378:eabm32332022.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Trapani KM, Boghossian LJ and Caskey E:
Clostridium subterminale septicemia in a patient with metastatic
gastrointestinal adenocarcinoma. Case Rep Infect Dis.
2018:60315102018.PubMed/NCBI
|
|
62
|
Dahmus JD, Kotler DL, Kastenberg DM and
Kistler CA: The gut microbiome and colorectal cancer: A review of
bacterial pathogenesis. J Gastrointest Oncol. 9:769–777. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kostic AD, Chun E, Robertson L, Glickman
JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold
GL, et al: Fusobacterium nucleatum potentiates intestinal
tumorigenesis and modulates the tumor-immune microenvironment. Cell
Host Microbe. 14:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fan Y, Mao R and Yang J: NF-κB and STAT3
signaling pathways collaboratively link inflammation to cancer.
Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang HP: Multiomics study on screening
biomarkers for papillary thyroid cancer (Doctors thesis). China
Medical University; Liaoning: 2024
|
|
66
|
Zhang L, Chen J, Xu C, Qi L and Ren Y:
Effects of iodine-131 radiotherapy on Th17/Tc17 and Treg/Th17 cells
of patients with differentiated thyroid carcinoma. Exp Ther Med.
15:2661–2666. 2018.PubMed/NCBI
|
|
67
|
Wang AY, Li CY, Xue G and Wang JF: The
relationship between intestinal flora and thyroid disease. J
Otorhinolaryngol Ophthalmol Shandong Univ. 1:132–139. 2023.
|
|
68
|
McBrearty N, Arzumanyan A, Bichenkov E,
Merali S, Merali C and Feitelson M: Short chain fatty acids delay
the development of hepatocellular carcinoma in HBx transgenic mice.
Neoplasia. 23:529–538. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao H, Li H and Huang T: High urinary
iodine, thyroid autoantibodies, and Thyroid-stimulating hormone for
papillary thyroid cancer risk. Biol Trace Elem Res. 184:317–324.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Guerrero-Preston R, Godoy-Vitorino F,
Jedlicka A, Rodríguez-Hilario A, González H, Bondy J, Lawson F,
Folawiyo O, Michailidi C, Dziedzic A, et al: 16S rRNA amplicon
sequencing identifies microbiota associated with oral cancer, human
papilloma virus infection and surgical treatment. Oncotarget.
7:51320–51334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhao J, Nian L, Kwok LY, Sun T and Zhao J:
Reduction in fecal microbiota diversity and short-chain fatty acid
producers in Methicillin-resistant Staphylococcus aureus infected
individuals as revealed by PacBio single molecule, real-time
sequencing technology. Eur J Clin Microbiol Infect Dis.
36:1463–1472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang YY, Zhang JY, Jin XR and Wang NP:
Research progress on the relationship between intestinal flora and
thyroid diseases. Prog Modern Gen Surg China. 26:793–796. 2023.
|
|
73
|
Fröhlich E and Wahl R: Microbiota and
thyroid interaction in health and disease. Trends Endocrinol Metab.
30:479–490. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Frank DN, St Amand AL, Feldman RA,
Boedeker EC, Harpaz N and Pace NR: Molecular-phylogenetic
characterization of microbial community imbalances in human
inflammatory bowel diseases. Proc Natl Acad Sci USA.
104:13780–13785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lamberti C, Mangiapane E, Pessione A,
Mazzoli R, Giunta C and Pessione E: Proteomic characterization of a
selenium-metabolizing probiotic Lactobacillus reuteri Lb26 BM for
nutraceutical applications. Proteomics. 11:2212–2221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tam AA, Ozdemir D, Aydın C, Bestepe N,
Ulusoy S, Sungu N, Ersoy R and Cakir B: Association between
preoperative thyrotrophin and clinicopathological and aggressive
features of papillary thyroid cancer. Endocrine. 59:565–572. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pushalkar S, Hundeyin M, Daley D,
Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres
LE, et al: The pancreatic cancer microbiome promotes oncogenesis by
induction of innate and adaptive immune suppression. Cancer Discov.
8:403–416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Virili C, Stramazzo I, Bagaglini MF,
Carretti AL, Capriello S, Romanelli F, Trimboli P and Centanni M:
The relationship between thyroid and human-associated microbiota: A
systematic review of reviews. Rev Endocr Metab Disord. 25:215–237.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kiss B, Mikó E, Sebő É, Toth J, Ujlaki G,
Szabó J, Uray K, Bai P and Árkosy P: Oncobiosis and microbial
metabolite signaling in pancreatic adenocarcinoma. Cancers (Basel).
12:10682020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pitt JM, Vétizou M, Gomperts Boneca I,
Lepage P, Chamaillard M and Zitvogel L: Enhancing the clinical
coverage and anticancer efficacy of immune checkpoint blockade
through manipulation of the gut microbiota. Oncoimmunology.
6:e11321372017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang SP, Rubio LA, Duncan SH, Donachie GE,
Holtrop G, Lo G, Farquharson FM, Wagner J, Parkhill J, Louis P, et
al: Pivotal roles for pH, Lactate, and Lactate-utilizing bacteria
in the stability of a human colonic microbial ecosystem. mSystems.
5:e00645–e00620. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hu S, Liu L, Chang EB, Wang JY and Raufman
JP: Butyrate inhibits pro-proliferative miR-92a by diminishing
c-Myc-induced miR-17-92a cluster transcription in human colon
cancer cells. Mol Cancer. 14:1802015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li M, van Esch BCAM, Henricks PAJ, Garssen
J and Folkerts G: Time and concentration dependent effects of short
chain fatty acids on lipopolysaccharide- or tumor necrosis factor
α-Induced endothelial activation. Front Pharmacol. 9:2332018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang JT, Yi M, Li ZJ and Sun SX: Research
progress on the mechanism of butyrate in inflammatory response. J
Immunology. 12:1101–1104. 2015.
|
|
85
|
Zhou L, Zhang M, Wang Y, Dorfman RG, Liu
H, Yu T, Chen X, Tang D, Xu L, Yin Y, et al: Faecalibacterium
prausnitzii produces butyrate to maintain Th17/Treg balance and to
ameliorate colorectal colitis by inhibiting histone deacetylase 1.
Inflamm Bowel Dis. 24:1926–1940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rathod M, Kelkar M, Valvi S, Salve G and
De A: FOXA1 Regulation turns Benzamide HDACi treatment
effect-specific in BC, Promoting NIS gene-mediated targeted
radioiodine therapy. Mol Ther Oncolytics. 19:93–104. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Greenberg VL, Williams JM, Cogswell JP,
Mendenhall M and Zimmer SG: Histone deacetylase inhibitors promote
apoptosis and differential cell cycle arrest in anaplastic thyroid
cancer cells. Thyroid. 11:315–325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xiao X, Ning L and Chen H: Notch1 mediates
growth suppression of papillary and follicular thyroid cancer cells
by histone deacetylase inhibitors. Mol Cancer Ther. 8:350–356.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Duan H, Wang L, Huangfu M and Li H: The
impact of microbiota-derived short-chain fatty acids on macrophage
activities in disease: Mechanisms and therapeutic potentials.
Biomed Pharmacother. 165:1152762023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shen WT, Wong TS, Chung WY, Wong MG,
Kebebew E, Duh QY and Clark OH: Valproic acid inhibits growth,
induces apoptosis, and modulates apoptosis-regulatory and
differentiation gene expression in human thyroid cancer cells.
Surgery. 138:979–985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Aponte-López A, Fuentes-Pananá EM,
Cortes-Muñoz D and Muñoz-Cruz S: Mast cell, the neglected member of
the tumor microenvironment: Role in breast cancer. J Immunol Res.
2018:25842432018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Revilla G, Pons MP, Baila-Rueda L,
García-León A, Santos D, Cenarro A, Magalhaes M, Blanco RM, Moral
A, Ignacio Pérez J, et al: Cholesterol and 27-hydroxycholesterol
promote thyroid carcinoma aggressiveness. Sci Rep. 9:102602019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shang X, Zhong X and Tian X: Metabolomics
of papillary thyroid carcinoma tissues: Potential biomarkers for
diagnosis and promising targets for therapy. Tumour Biol.
37:11163–11175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li JZ: Research on the correlation between
papillary thyroid carcinoma and intestinal flora (Masters thesis).
QingHai University; Xining: 2023
|
|
95
|
Mahfoudi R, Djeridane A, Benarous K,
Gaydou EM and Yousfi M: Structure-activity relationships and
molecular docking of thirteen synthesized flavonoids as horseradish
peroxidase inhibitors. Bioorg Chem. 74:201–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Danilovic DLS, Ferraz-de-Souza B, Fabri
AW, Santana NO, Kulcsar MA, Cernea CR, Marui S and Hoff AO:
25-Hydroxyvitamin D and TSH as risk factors or prognostic markers
in thyroid carcinoma. PLoS One. 11:e01645502016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Huang NS, Chen XX, Wei WJ, Mo M, Chen JY,
Ma B, Yang SW, Xu WB, Wu J, Ji QH, et al: Association between
breast cancer and TC: A study based on 13 978 patients with breast
cancer. Cancer Med. 7:6393–6400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ervin SM, Li H, Lim L, Roberts LR, Liang
X, Mani S and Redinbo MR: Gut microbial β-glucuronidases reactivate
estrogens as components of the estrobolome that reactivate
estrogens. J Biol Chem. 294:18586–18599. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pollet RM, DAgostino EH, Walton WG, Xu Y,
Little MS, Biernat KA, Pellock SJ, Patterson LM, Creekmore BC,
Isenberg HN, et al: An atlas of β-Glucuronidases in the human
intestinal microbiome. Structure. 25:967–977.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rubio GA, Catanuto P, Glassberg MK, Lew JI
and Elliot SJ: Estrogen receptor subtype expression and regulation
is altered in papillary thyroid cancer after menopause. Surgery.
163:143–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Qiu YB, Liao LY, Jiang R, Xu M, Xu LW,
Chen GG and Liu ZM: PES1 promotes the occurrence and development of
papillary thyroid cancer by upregulating the ERα/ERβ protein ratio.
Sci Rep. 9:10322019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Z, He L, Sun W, Qin Y, Dong W, Zhang
T, Zhang P and Zhang H: miRNA-299-5p regulates estrogen receptor
alpha and inhibits migration and invasion of papillary thyroid
cancer cell. Cancer Manag Res. 10:6181–6193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Vannucchi G, De Leo S, Perrino M, Rossi S,
Tosi D, Cirello V, Colombo C, Bulfamante G, Vicentini L and
Fugazzola L: Impact of estrogen and progesterone receptor
expression on the clinical and molecular features of papillary
thyroid cancer. Eur J Endocrinol. 173:29–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
He Q, Sun H, Li F and Liang N: Obesity and
risk of differentiated thyroid cancer: A large-scale case-control
study. Clin Endocrinol (Oxf). 91:869–878. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Riva A, Borgo F, Lassandro C, Verduci E,
Morace G, Borghi E and Berry D: Pediatric obesity is associated
with an altered gut microbiota and discordant shifts in Firmicutes
populations. Environ Microbiol. 19:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Watanabe H, Katsura T, Takahara M,
Miyashita K, Katakami N, Matsuoka TA, Kawamori D and Shimomura I:
Plasma lipopolysaccharide binding protein level statistically
mediates between body mass index and chronic microinflammation in
Japanese patients with type 1 diabetes. Diabetol Int. 11:293–297.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
De Melo TG, Souza AL, Ficher E, Fernandes
AM, Montali Da Assumpção LV, Monte Alegre S and Zantut-Wittmann DE:
Reduced insulin sensitivity in differentiated thyroid cancer
patients with suppressed TSH. Endo Res. 43:73–79. 2018. View Article : Google Scholar
|
|
108
|
Heidari Z, Abdani M and Mansournia MA:
Insulin resistance associated with differentiated thyroid
carcinoma: Penalized conditional logistic regression analysis of a
matched case-control study data. Int J Endocrinol Metab.
16:e145452018.PubMed/NCBI
|
|
109
|
Knuppel A, Fensom GK, Watts EL, Gunter MJ,
Murphy N, Papier K, Perez-Cornago A, Schmidt JA, Smith Byrne K,
Travis RC and Key TJ: Circulating Insulin-like Growth Factor-I
concentrations and risk of 30 cancers: Prospective analyses in UK
Biobank. Cancer Res. 80:4014–4021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Vella V and Malaguarnera R: The emerging
role of insulin receptor isoforms in TC: Clinical implications and
new perspectives. Int J Mol Sci. 19:38142018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Manzella L, Massimino M, Stella S, Tirrò
E, Pennisi MS, Martorana F, Motta G, Vitale SR, Puma A, Romano C,
et al: Activation of the IGF axis in thyroid cancer: Implications
for tumorigenesis and treatment. Int J Mol Sci. 20:32582019.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li YH, Gao XF, Guo TT, Zhang J, Zhang CZ
and Li J: Research progress on the mechanism of obesity combined
with thyroid cancer. Chin J Gen Surg. 4:311–315. 2023.
|
|
113
|
Song YC, Yu XQ and Li D: Structural
changes of gut microbiota in papillary thyroid carcinoma patients
with postoperative hypothyroidism. J Tongji Univ. 2:144–151.
2019.
|
|
114
|
Tang YJ: Relationships of Preoperative Gut
Microbiota and Postoperative Nausea and Vomiting in Female Patients
Undergoing Thyroid Cancer Surgery: A Prospective Observational
Study (Masters thesis). Fujian Medicine University; Fuzhou, China:
2023
|
|
115
|
Spaggiari G, Brigante G, De Vincentis S,
Cattini U, Roli L, De Santis MC, Baraldi E, Tagliavini S, Varani M,
Trenti T, et al: Probiotics ingestion does not directly affect
thyroid hormonal parameters in hypothyroid patients on
levothyroxine treatment. Front Endocrinol (Lausanne). 8:3162017.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ludgate ME, Masetti G and Soares P: The
relationship between the gut microbiota and thyroid disorders. Nat
Rev Endocrinol. 20:511–525. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen D, Wu J, Jin D, Wang B and Cao H:
Fecal microbiota transplantation in cancer management: Current
status and perspectives. Int J Cancer. 145:2021–2031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zheng L, Zhang L, Tang L, Huang D, Pan D,
Guo W, He S, Huang Y, Chen Y, Xiao X, et al: Gut microbiota is
associated with response to 131I therapy in patients
with papillary thyroid carcinoma. Eur J Nucl Med Mol Imaging.
50:1453–1465. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Moshkelgosha S, Verhasselt HL, Masetti G,
Covelli D, Biscarini F, Horstmann M, Daser A, Westendorf AM,
Jesenek C, Philipp S, et al: Modulating gut microbiota in a mouse
model of Graves orbitopathy and its impact on induced disease.
Microbiome. 9:452021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Su X, Yin X, Liu Y, Yan X, Zhang S, Wang
X, Lin Z, Zhou X, Gao J, Wang Z and Zhang Q: Gut dysbiosis
contributes to the imbalance of Treg and Th17 cells in graves
disease patients by propionic acid. J Clin Endocrinol Metab.
105:dgaa5112020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Su X, Zhao Y, Li Y, Ma S and Wang Z: Gut
dysbiosis is associated with primary hypothyroidism with
interaction on gut-thyroid axis. Clin Sci (Lond). 134:1521–1535.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Docimo G, Cangiano A, Romano RM,
Pignatelli MF, Offi C, Paglionico VA, Galdiero M, Donnarumma G,
Nigro V, Esposito D, et al: The Human Microbiota in endocrinology:
Implications for pathophysiology, treatment, and prognosis in
thyroid diseases. Front Endocrinol (Lausanne). 11:5865292020.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lin JD, Chao TC, Huang MJ, Weng HF and
Tzen KY: Use of RAI for thyroid remnant ablation in
well-differentiated thyroid carcinoma to replace thyroid
reoperation. Am J Clin Oncol. 21:77–81. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lu G, Gao D, Liu Y, Yu X, Jiang W and Lv
Z: Early and long-term responses of intestinal microbiota and
metabolites to 131I treatment in differentiated thyroid cancer
patients. BMC Medicine. 22:3002024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Luster M, Clarke SE, Dietlein M, Lassmann
M, Lind P, Oyen WJG, Tennvall J and Bombardieri E; European
Association of Nuclear Medicine (EANM), : Guidelines for
radioiodine therapy of differentiated thyroid cancer. Eur J Nucl
Med Mol Imaging. 35:1941–1959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Panebianco C, Andriulli A and Pazienza V:
Pharmacomicrobiomics: Exploiting the drug-microbiota interactions
in anticancer therapies. Microbiome. 6:922018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lin B, Zhao F, Liu Y, Sun J, Feng J, Zhao
L, Wang H, Chen H, Yan W, Guo X, et al: Alterations in oral
microbiota of differentiated thyroid carcinoma patients with
xerostomia after radioiodine therapy. Front Endocrinol (Lausanne).
13:8959702022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Feller L, Altini M and Lemmer J:
Inflammation in the context of oral cancer. Oral Oncol. 49:887–892.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Atarashi K, Suda W, Luo C, Kawaguchi T,
Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, et
al: Ectopic colonization of oral bacteria in the intestine drives
TH1 cell induction and inflammation. Science. 358:359–365. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Larsen JM: The immune response to
Prevotella bacteria in chronic inflammatory disease. Immunology.
151:363–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Mahowald MA, Rey FE, Seedorf H, Turnbaugh
PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, et
al: Characterizing a model human gut microbiota composed of members
of its two dominant bacterial phyla. Proc Natl Acad Sci USA.
106:5859–5864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lu G, Gao D, Jiang W, Yu X, Tong J, Liu X,
Qiao T, Wang R, Zhang M, Wang S, et al: Disrupted gut microecology
after high-dose 131I therapy and radioprotective effects
of arachidonic acid supplementation. Eur J Nucl Med Mol Imaging.
51:2395–2408. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Samimi H and Haghpanah V: Gut microbiome
and radioiodine-refractory papillary thyroid carcinoma
pathophysiology. Trends Endocrinol Metab. 31:627–630. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Coerts HI, de Keizer B, Marlowe RJ and
Verburg FA: Recombinant or endogenous TSH for RAI therapy in
thyroid cancer: State of knowledge and current controversies. Eur J
Endocrinol. 188:lvad0062023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Horvath E, Skoknic V, Majlis S, Tala H,
Silva C, Castillo E, Whittle C, Niedmann JP and González P:
Radioiodine-induced salivary gland damage detected by
ultrasonography in patients treated for papillary TC: Radioactive
iodine activity and risk. Thyroid. 30:1646–1655. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Sunavala-Dossabhoy G and Petti S: Effect
of recombinant human thyroid stimulating hormone on long-term
salivary gland dysfunction in thyroid cancer patients treated with
RAI. A systematic review. Oral Oncol. 136:1062802023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Shi X, Yu PC, Lei BW, Li CW, Zhang Y, Tan
LC, Shi RL, Wang J, Ma B, Xu WB, et al: Association between
programmed death-ligand 1 expression and clinicopathological
characteristics, structural recurrence, and biochemical
recurrence/persistent disease in medullary thyroid carcinoma.
Thyroid. 29:1269–1278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Capdevila J, Wirth LJ, Ernst T, Ponce Aix
S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto
PA, et al: PD-1 blockade in anaplastic thyroid carcinoma. J Clin
Oncol. 38:2620–2627. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Xu JW and Gu J: Research progress on the
relationship between intestinal flora and thyroid cancer. J Clin
Oncol. 2:176–180. 2021.
|
|
142
|
Schlumberger M and Leboulleux S: Current
practice in patients with differentiated TC. Nat Rev Endocrinol.
17:176–188. 2021. View Article : Google Scholar : PubMed/NCBI
|