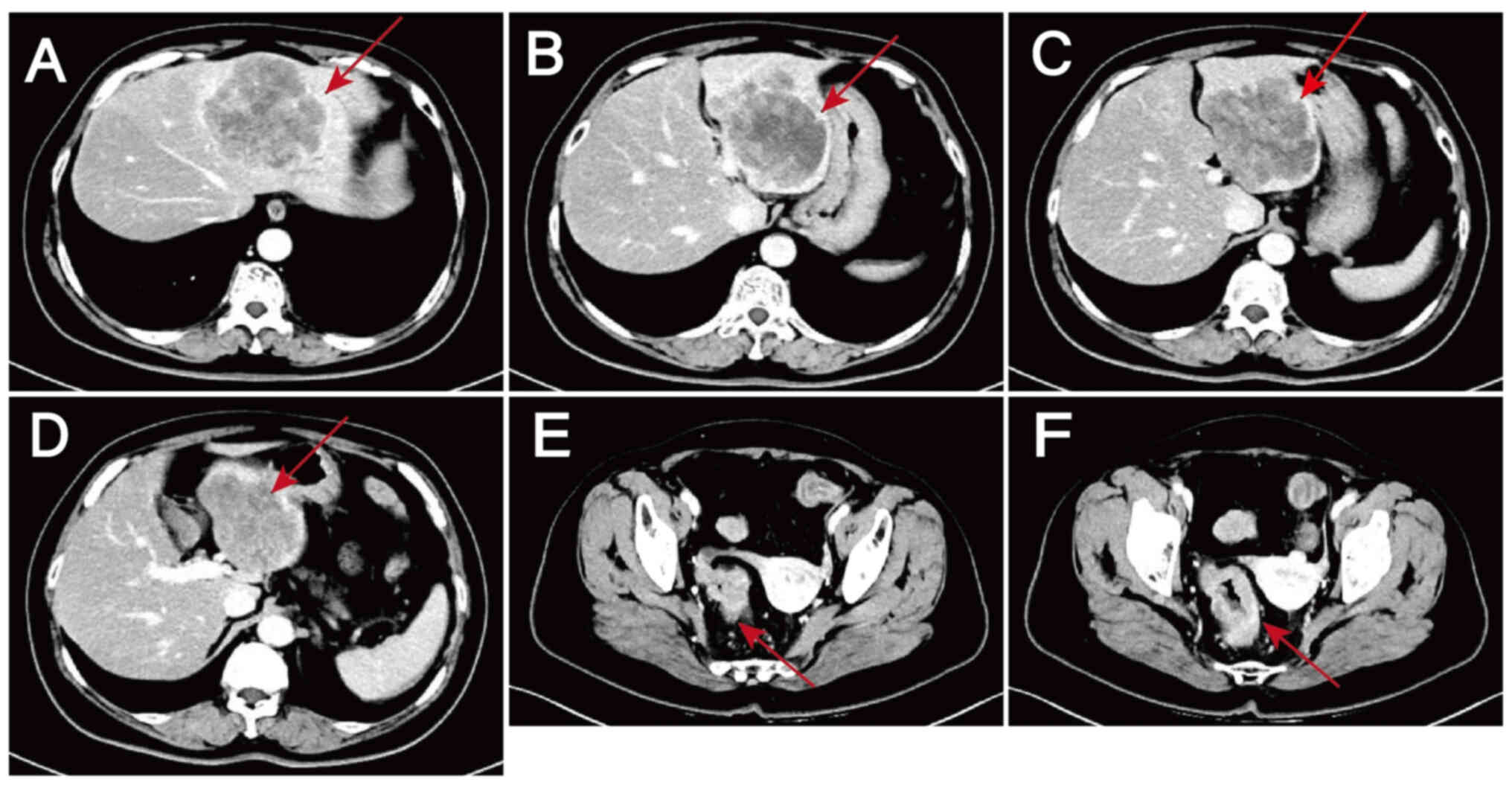
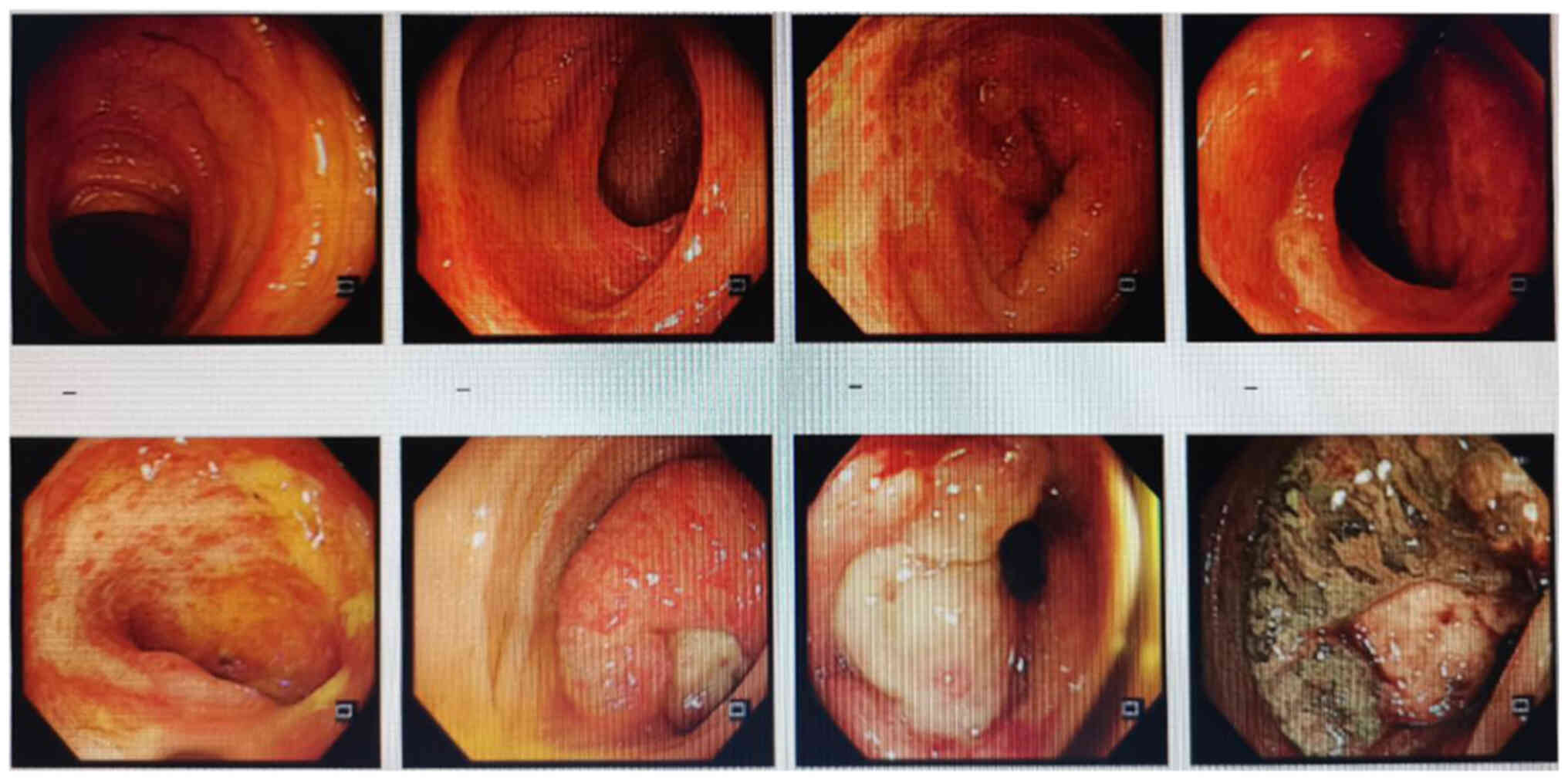
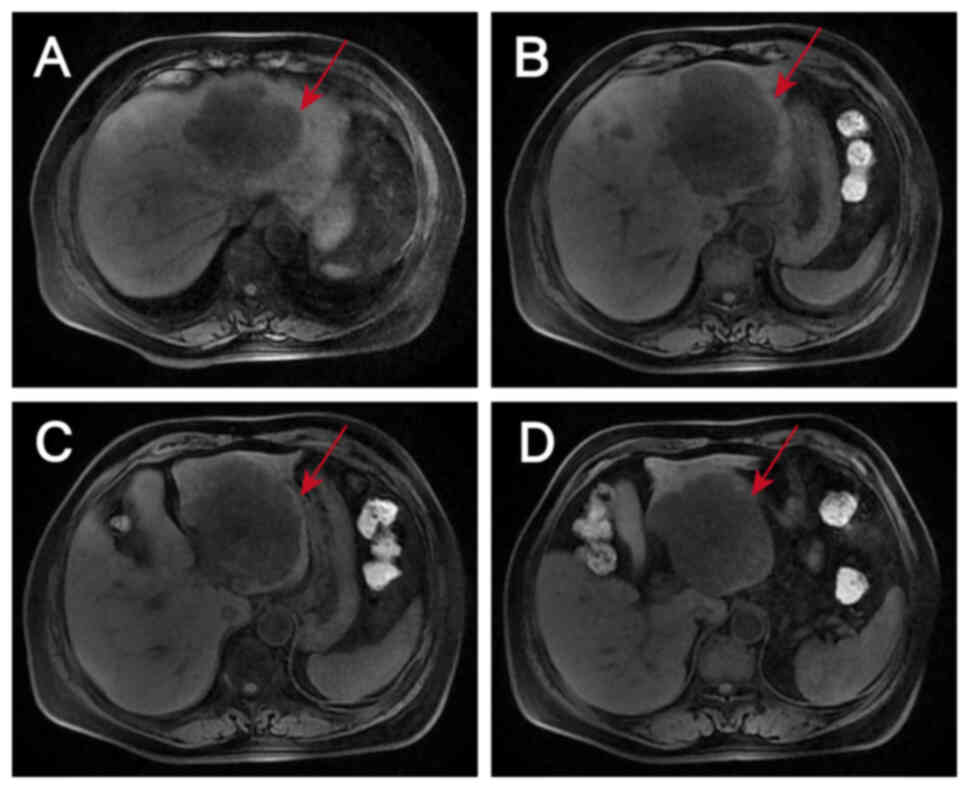
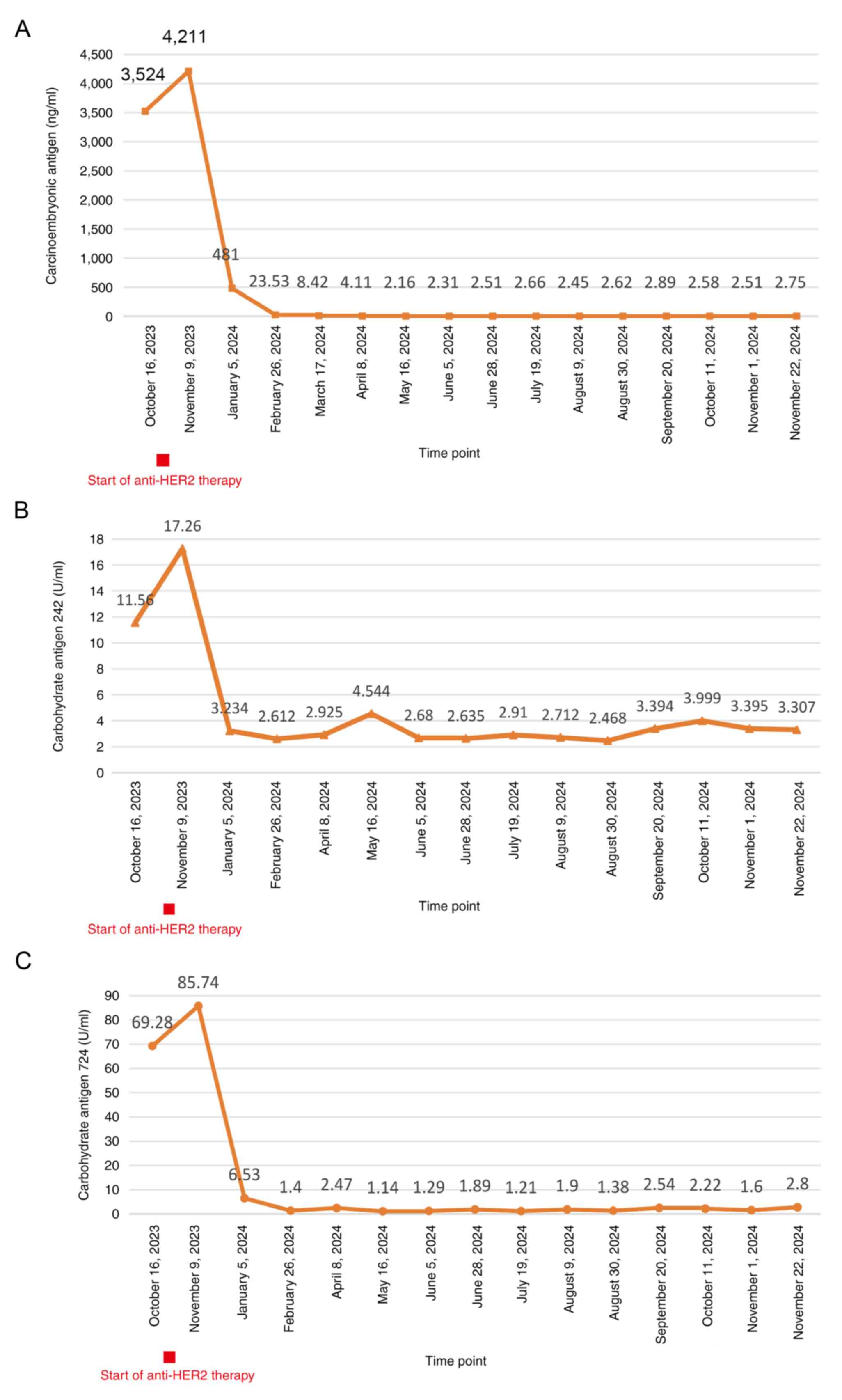
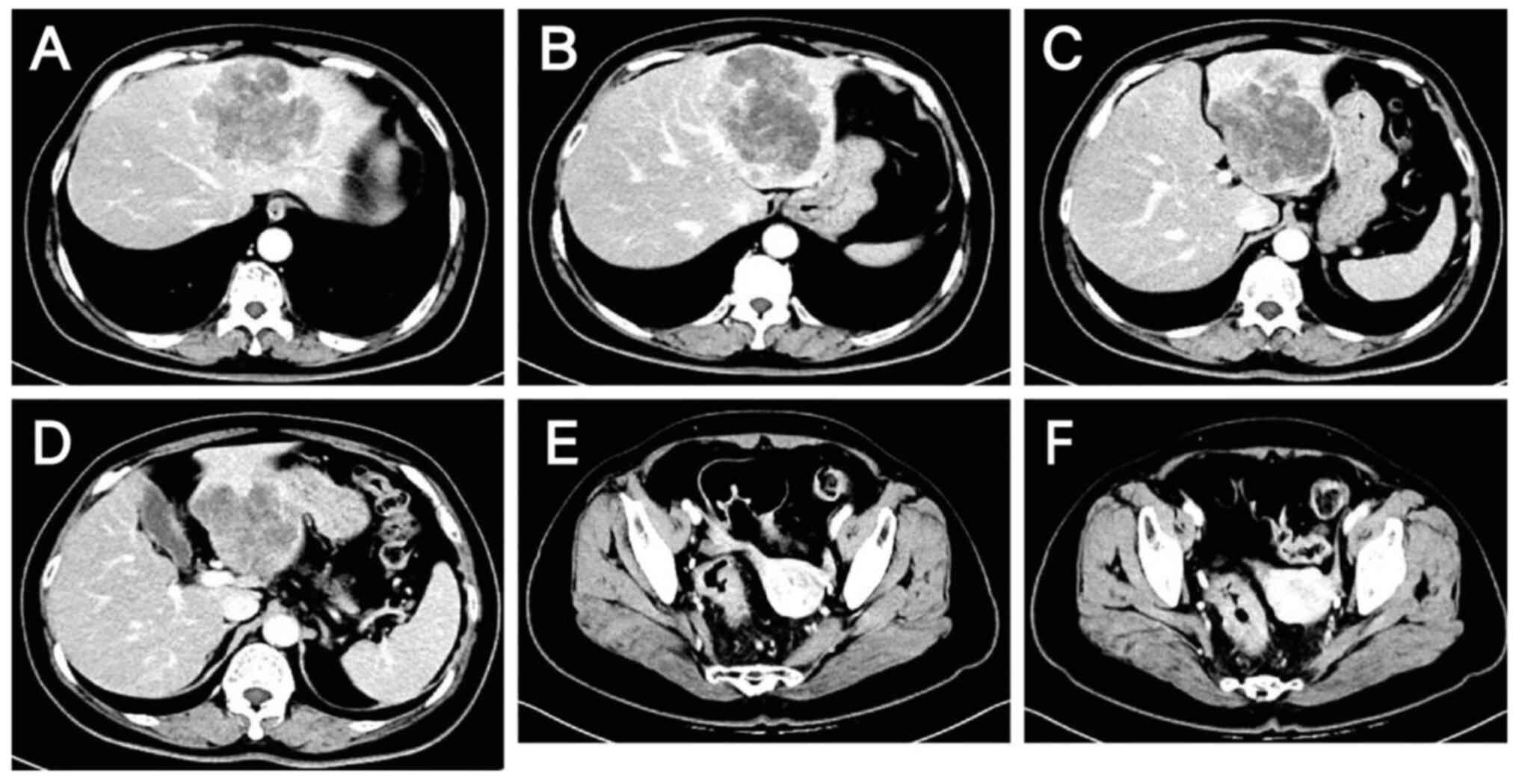
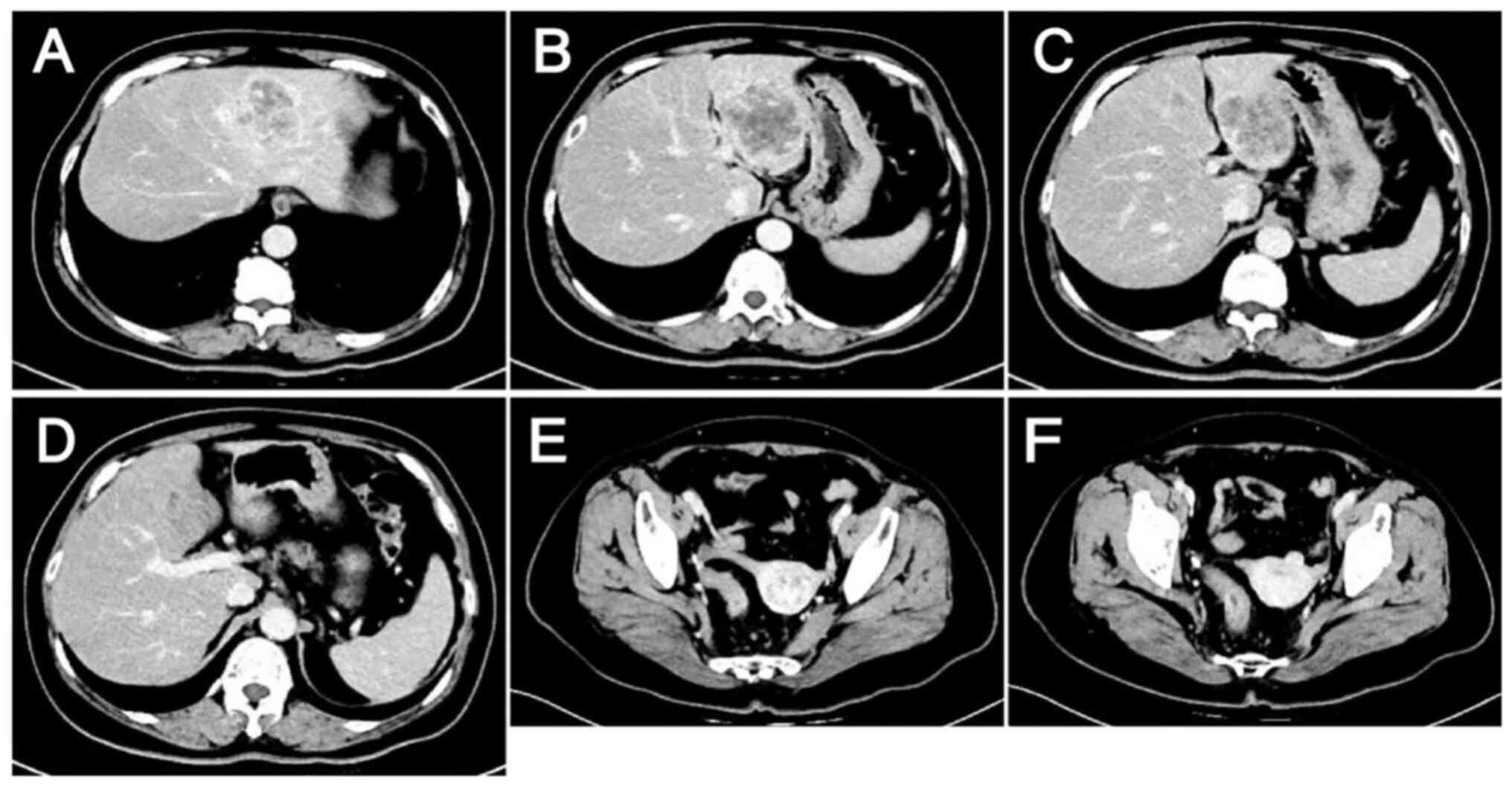
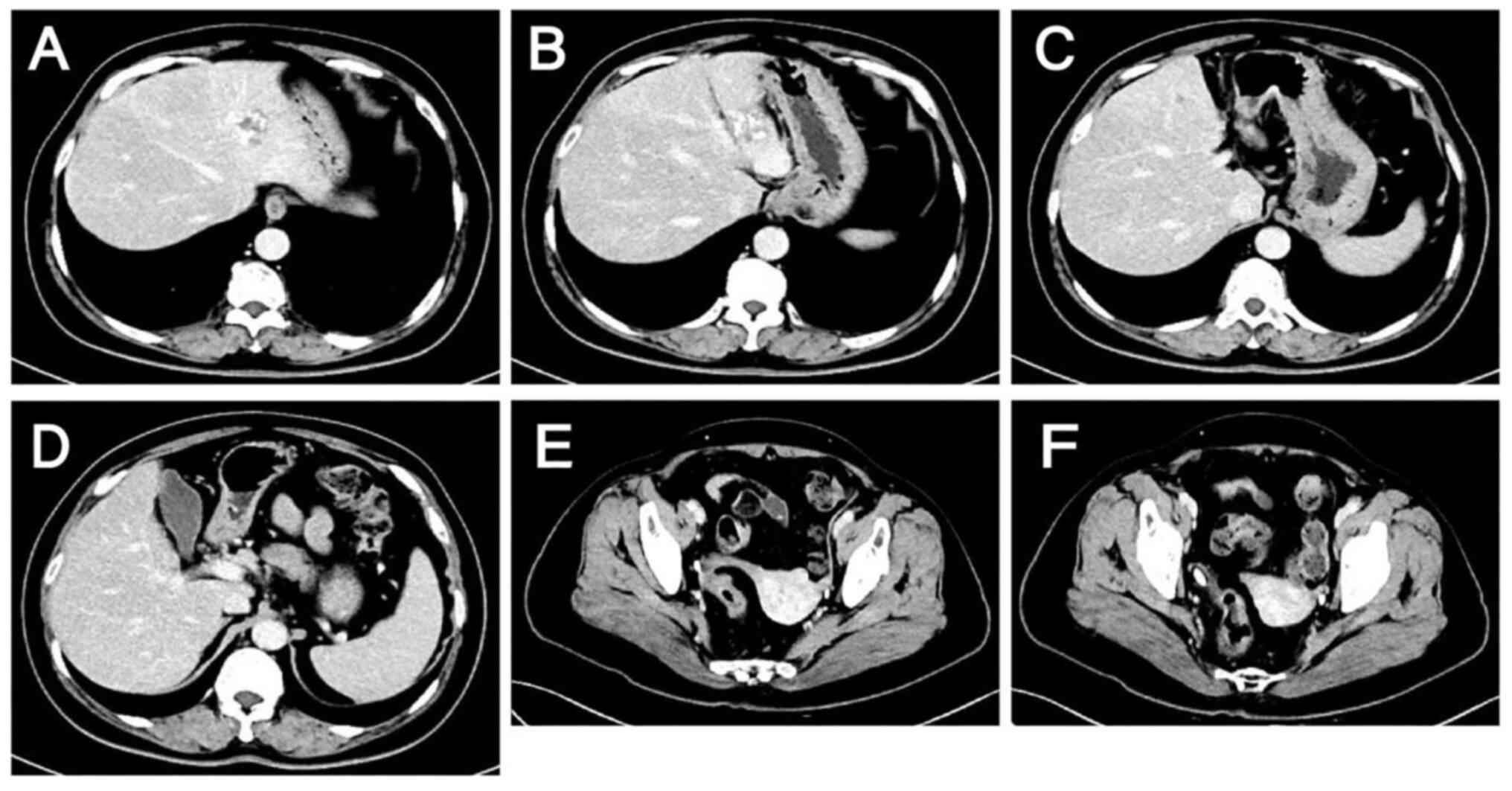
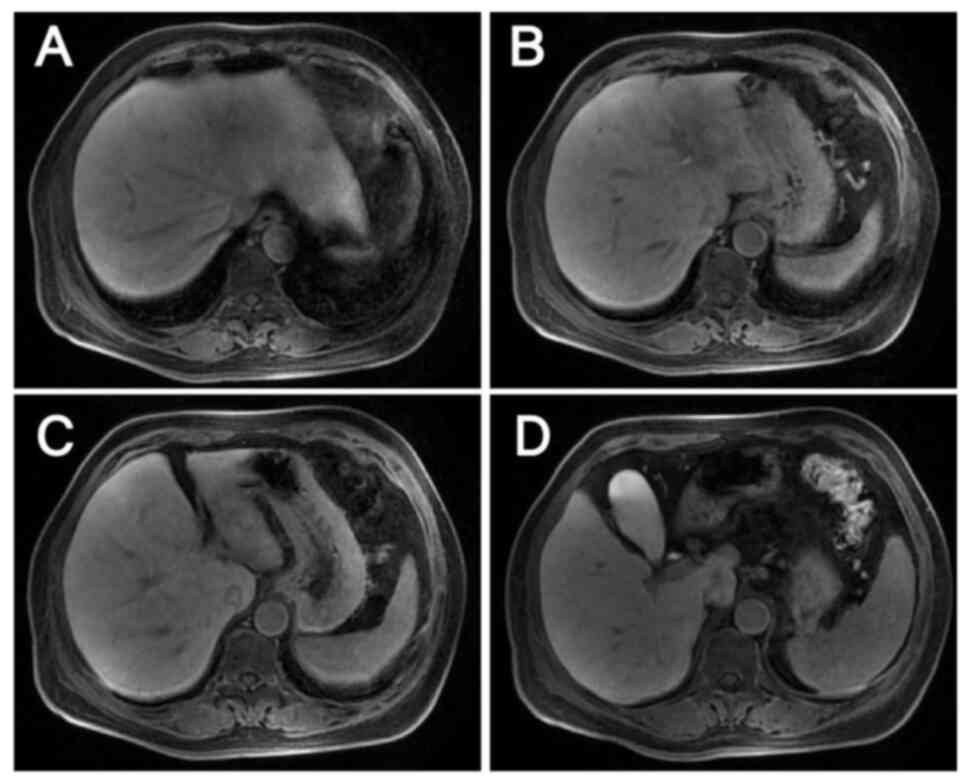
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross JS, Fakih M, Ali SM, Elvin JA,
Schrock AB, Suh J, Vergilio JA, Ramkissoon S, Severson E, Daniel S,
et al: Targeting HER2 in colorectal cancer: The landscape of
amplification and short variant mutations in ERBB2 and ERBB3.
Cancer. 124:1358–1373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siena S, Sartore-Bianchi A, Marsoni S,
Hurwitz HI, McCall SJ, Penault-Llorca F, Srock S, Bardelli A and
Trusolino L: Targeting the human epidermal growth factor receptor 2
(HER2) oncogene in colorectal cancer. Ann Oncol. 29:1108–1119.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Margonis GA, Buettner S, Andreatos N, Kim
Y, Wagner D, Sasaki K, Beer A, Schwarz C, Løes IM, Smolle M, et al:
Association of BRAF mutations with survival and recurrence in
surgically treated patients with metastatic colorectal liver
cancer. JAMA Surg. 153:e1809962018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suwaidan AA, Lau DK and Chau I: HER2
targeted therapy in colorectal cancer: New horizons. Cancer Treat
Rev. 105:1023632022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosati G, Aprile G, Colombo A, Cordio S,
Giampaglia M, Cappetta A, Porretto CM, De Stefano A, Bilancia D and
Avallone A: Colorectal cancer heterogeneity and the impact on
precision medicine and therapy efficacy. Biomedicines. 10:10352022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Li W, Lu Z, Lu M, Zhou J, Peng Z,
Zhang X, Wang X, Shen L and Li J: Clinicopathological features of
HER2 positive metastatic colorectal cancer and survival analysis of
anti-HER2 treatment. BMC Cancer. 22:3552022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chitkara A, Bakhtiar M, Sahin IH, Hsu D,
Zhang J, Anamika F, Mahnoor M, Ahmed R, Gholami S and Saeed A: A
meta-analysis to assess the efficacy of HER2-targeted treatment
regimens in HER2-positive metastatic colorectal cancer (mCRC). Curr
Oncol. 30:8266–8277. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin
R, He WL, Cai SR, He YL and Ye JN: Anti-EGFR therapy in metastatic
colorectal cancer: Mechanisms and potential regimens of drug
resistance. Gastroenterol Rep (Oxf). 8:179–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sartore-Bianchi A, Amatu A, Porcu L,
Ghezzi S, Lonardi S, Leone F, Bergamo F, Fenocchio E, Martinelli E,
Borelli B, et al: HER2 positivity predicts unresponsiveness to
EGFR-targeted treatment in metastatic colorectal cancer.
Oncologist. 24:1395–1402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popescu NC, King CR and Kraus MH:
Localization of the human erbB-2 gene on normal and rearranged
chromosomes 17 to bands q12-21.32. Genomics. 4:362–366. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, He Y, Sun Y, Wang W, Qian X, Yu X
and Pan Y: Prevalence, prognosis and predictive status of HER2
amplification in anti-EGFR-resistant metastatic colorectal cancer.
Clin Transl Oncol. 22:813–822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yonesaka K, Zejnullahu K, Okamoto I, Satoh
T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda
M, et al: Activation of ERBB2 signaling causes resistance to the
EGFR-directed therapeutic antibody cetuximab. Sci Transl Med.
3:99ra862011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sawada K, Nakamura Y, Yamanaka T, Kuboki
Y, Yamaguchi D, Yuki S, Yoshino T, Komatsu Y, Sakamoto N, Okamoto W
and Fujii S: Prognostic and predictive value of HER2 amplification
in patients with metastatic colorectal cancer. Clin Colorectal
Cancer. 17:198–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong JH, Kim J, Hong YS, Kim D, Kim JE,
Kim SY, Kim KP, Yoon YK, Kim D, Chun SM, et al: HER2 amplification
and cetuximab efficacy in patients with metastatic colorectal
cancer harboring wild-type RAS and BRAF. Clin Colorectal Cancer.
16:e147–e152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang JY, Jeon YK, Jeong SY, Lim SH, Park
YS, Lim HY, Lee JY and Kim ST: Effect of human epidermal growth
factor receptor 2 overexpression in metastatic colorectal cancer on
standard chemotherapy outcomes. J Gastrointest Oncol. 14:2097–1110.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nam SK, Yun S, Koh J, Kwak Y, Seo AN, Park
KU, Kim DW, Kang SB, Kim WH and Lee HS: BRAF, PIK3CA, and HER2
oncogenic alterations according to KRAS mutation status in advanced
colorectal cancers with distant metastasis. PLoS One.
11:e01518652016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richman SD, Southward K, Chambers P, Cross
D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM,
et al: HER2 overexpression and amplification as a potential
therapeutic target in colorectal cancer: Analysis of 3256 patients
enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials.
J Pathol. 238:562–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meric-Bernstam F, Hurwitz H, Raghav KPS,
McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R,
Burris H, Sweeney C, et al: Pertuzumab plus trastuzumab for
HER2-amplified metastatic colorectal cancer (MyPathway): An updated
report from a multicentre, open-label, phase 2a, multiple basket
study. Lancet Oncol. 20:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura Y, Okamoto W, Kato T, Esaki T,
Kato K, Komatsu Y, Yuki S, Masuishi T, Nishina T, Ebi H, et al:
Circulating tumor DNA-guided treatment with pertuzumab plus
trastuzumab for HER2-amplified metastatic colorectal cancer: A
phase 2 trial. Nat Med. 27:1899–1903. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zumwalt TJ and Goel A: Immunotherapy of
metastatic colorectal cancer: Prevailing challenges and new
perspectives. Curr Colorectal Cancer Rep. 11:125–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valtorta E, Martino C, Sartore-Bianchi A,
Penaullt-Llorca F, Viale G, Risio M, Rugge M, Grigioni W,
Bencardino K, Lonardi S, et al: Assessment of a HER2 scoring system
for colorectal cancer: Results from a validation study. Mod Pathol.
28:1481–1491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujii S, Magliocco AM, Kim J, Okamoto W,
Kim JE, Sawada K, Nakamura Y, Kopetz S, Park WY, Tsuchihara K, et
al: International harmonization of provisional diagnostic criteria
for ERBB2-amplified metastatic colorectal cancer allowing for
screening by next-generation sequencing panel. JCO Precis Oncol.
4:6–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada Y, Yagi R, Kameyama H, Nagahashi
M, Ichikawa H, Tajima Y, Okamura T, Nakano M, Nakano M, Sato Y, et
al: Utility of comprehensive genomic sequencing for detecting
HER2-positive colorectal cancer. Hum Pathol. 66:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siravegna G, Sartore-Bianchi A, Nagy RJ,
Raghav K, Odegaard JI, Lanman RB, Trusolino L, Marsoni S, Siena S
and Bardelli A: Plasma HER2 (ERBB2) copy number predicts response
to HER2-targeted therapy in metastatic colorectal cancer. Clin
Cancer Res. 25:3046–3053. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takegawa N, Yonesaka K, Sakai K, Ueda H,
Watanabe S, Nonagase Y, Okuno T, Takeda M, Maenishi O, Tsurutani J,
et al: HER2 genomic amplification in circulating tumor DNA from
patients with cetuximab-resistant colorectal cancer. Oncotarget.
7:3453–3460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh H, Kang A, Bloudek L, Hsu LI,
Corinna Palanca-Wessels M, Stecher M, Siadak M and Ng K: Systematic
literature review and meta-analysis of HER2 amplification,
overexpression, and positivity in colorectal cancer. JNCI Cancer
Spectr. 8:pkad0822024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benson AB, Venook AP, Adam M, Chang G,
Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna
I, et al: Colon cancer, version 3.2024, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 22:e2400292024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang FH, Zhang XT, Tang L, Wu Q, Cai MY,
Li YF, Qu XJ, Qiu H, Zhang YJ, Ying JE, et al: The Chinese society
of clinical oncology (CSCO): Clinical guidelines for the diagnosis
and treatment of gastric cancer, 2023. Cancer Commun (Lond).
44:127–172. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakamura Y, Sawada K, Fujii S and Yoshino
T: HER2-targeted therapy should be shifted towards an earlier line
for patients with anti-EGFR-therapy naïve, HER2-amplified
metastatic colorectal cancer. ESMO Open. 4:e0005302019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sartore-Bianchi A, Trusolino L, Martino C,
Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I,
Martinelli E, et al: Dual-targeted therapy with trastuzumab and
lapatinib in treatment-refractory, KRAS codon 12/13 wild-type,
HER2-positive metastatic colorectal cancer (HERACLES): A
proof-of-concept, multicentre, open-label, phase 2 trial. Lancet
Oncol. 17:738–746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sartore-Bianchi A, Lonardi S, Martino C,
Fenocchio E, Tosi F, Ghezzi S, Leone F, Bergamo F, Zagonel V,
Ciardiello F, et al: Pertuzumab and trastuzumab emtansine in
patients with HER2-amplified metastatic colorectal cancer: The
phase II HERACLES-B trial. ESMO Open. 5:e0009112020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siena S, Di Bartolomeo M, Raghav K,
Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Fakih
M, Elez E, et al: Trastuzumab deruxtecan (DS-8201) in patients with
HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A
multicentre, open-label, phase 2 trial. Lancet Oncol. 22:779–789.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Raghav K, Siena S, Takashima A, Kato T,
Van den Eynde M, Pietrantonio F, Komatsu Y, Kawakami H, Peeters M,
Andre T, et al: Trastuzumab deruxtecan in patients with
HER2-positive advanced colorectal cancer (DESTINY-CRC02): Primary
results from a multicentre, randomised, phase 2 trial. Lancet
Oncol. 25:1147–1162. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Long HD, Lin YE, Zhang JJ, Zhong WZ and
Zheng RN: Risk of congestive heart failure in early breast cancer
patients undergoing adjuvant treatment with trastuzumab: A
meta-analysis. Oncologist. 21:547–554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suter TM and Ewer MS: Cancer drugs and the
heart: Importance and management. Eur Heart J. 34:1102–1111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Swain SM, Nishino M, Lancaster LH, Li BT,
Nicholson AG, Bartholmai BJ, Naidoo J, Schumacher-Wulf E, Shitara
K, Tsurutani J, et al: Multidisciplinary clinical guidance on
trastuzumab deruxtecan (T-DXd)-related interstitial lung
disease/pneumonitis-Focus on proactive monitoring, diagnosis, and
management. Cancer Treat Rev. 106:1023782022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Armenian SH, Lacchetti C, Barac A, Carver
J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M,
et al: Prevention and monitoring of cardiac dysfunction in
survivors of adult cancers: American society of clinical oncology
clinical practice guideline. J Clin Oncol. 35:893–911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Bailey J,
Burstein HJ, et al: Breast cancer, version 3.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:331–357. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Edoardo C and Giuseppe C:
Trastuzumab-deruxtecan in solid tumors with HER2 alterations: From
early phase development to the first agnostic approval of an
antibody-drug conjugate. Expert Opin Investig Drugs. 33:851–865.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao M, Jiang T, Li P, Zhang J, Xu K and
Ren T: Efficacy and safety of HER2-targeted inhibitors for
metastatic colorectal cancer with HER2-amplified: A meta-analysis.
Pharmacol Res. 182:1063302022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Siravegna G, Lazzari L, Crisafulli G,
Sartore-Bianchi A, Mussolin B, Cassingena A, Martino C, Lanman RB,
Nagy RJ, Fairclough S, et al: Radiologic and genomic evolution of
individual metastases during HER2 blockade in colorectal cancer.
Cancer Cell. 34:148–162.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang N, Cao Y, Si C, Shao P, Su G, Wang K,
Bao J and Yang L: Emerging role of ERBB2 in targeted therapy for
metastatic colorectal cancer: Signaling pathways to therapeutic
strategies. Cancers (Basel). 14:51602022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lazzari L, Corti G, Picco G, Isella C,
Montone M, Arcella P, Durinikova E, Zanella ER, Novara L, Barbosa
F, et al: Patient-derived xenografts and matched cell lines
identify pharmacogenomic vulnerabilities in colorectal cancer. Clin
Cancer Res. 25:6243–6259. 2019. View Article : Google Scholar : PubMed/NCBI
|