Introduction
Colorectal cancer (CRC) is one of the most common
malignancies of the digestive tract and is ranked third for
incidence and second for cancer-related mortality worldwide
(1). It has been reported that 20%
of patients with CRC have metastatic disease at the time of
presentation, and a further 25% who initially present with in
situ tumors develop metastases during follow-up (2). Notably, 5% of metastatic CRCs harbor
alterations in Erb-B2 receptor tyrosine kinase 2 (ERBB2), also
known as human epidermal growth factor receptor 2 (HER2). HER2 is a
known oncogene (3) that can trigger
carcinogenesis via the upregulation of HER2 protein expression,
gene amplification of ERBB2, or point mutations, which occur mainly
in patients with breast or gastric cancer (4).
HER2 positivity is considerably less common in CRC
than in breast or gastric cancer, affecting only ~5% cases overall,
but represents an important focus of research (5). First-line chemotherapeutic agents
currently approved for KRAS/NRAS wild-type (WT) metastatic CRC
include conventional cytotoxic agents such as fluorouracil,
oxaliplatin and irinotecan, vascular endothelial growth factor
inhibitors such as bevacizumab, and epidermal growth factor
receptor (EGFR) inhibitors, including cetuximab and panitumab
(2,6,7).
However, there is no standard treatment for HER2-positive
metastatic CRC. Although several recent studies have found
anti-HER2 therapy to be effective in HER2-positive CRC (8–10), the
evidence is not conclusive enough to warrant the inclusion of
anti-HER2 agents in the relevant diagnostic and treatment
guidelines. The present case report describes a patient who was
diagnosed with rectal adenocarcinoma and multiple metastases to the
liver in whom next-generation sequencing (NGS) results suggested
positive HER2 expression. As two cycles of an intensive folinic
acid (leucovorin) + fluorouracil + oxaliplatin (FOLFOX) exhibited
poor treatment efficacy, anti-HER2 therapy was introduced. The
clinical findings for this case are described to highlight the
important role of anti-HER2 therapy in HER2-positive CRC and
underscore the importance of further investigating personalized
treatment strategies for this disease.
Case report
A 60-year-old woman with complaints of abdominal
pain and passage of bloody stools was admitted to Hebei General
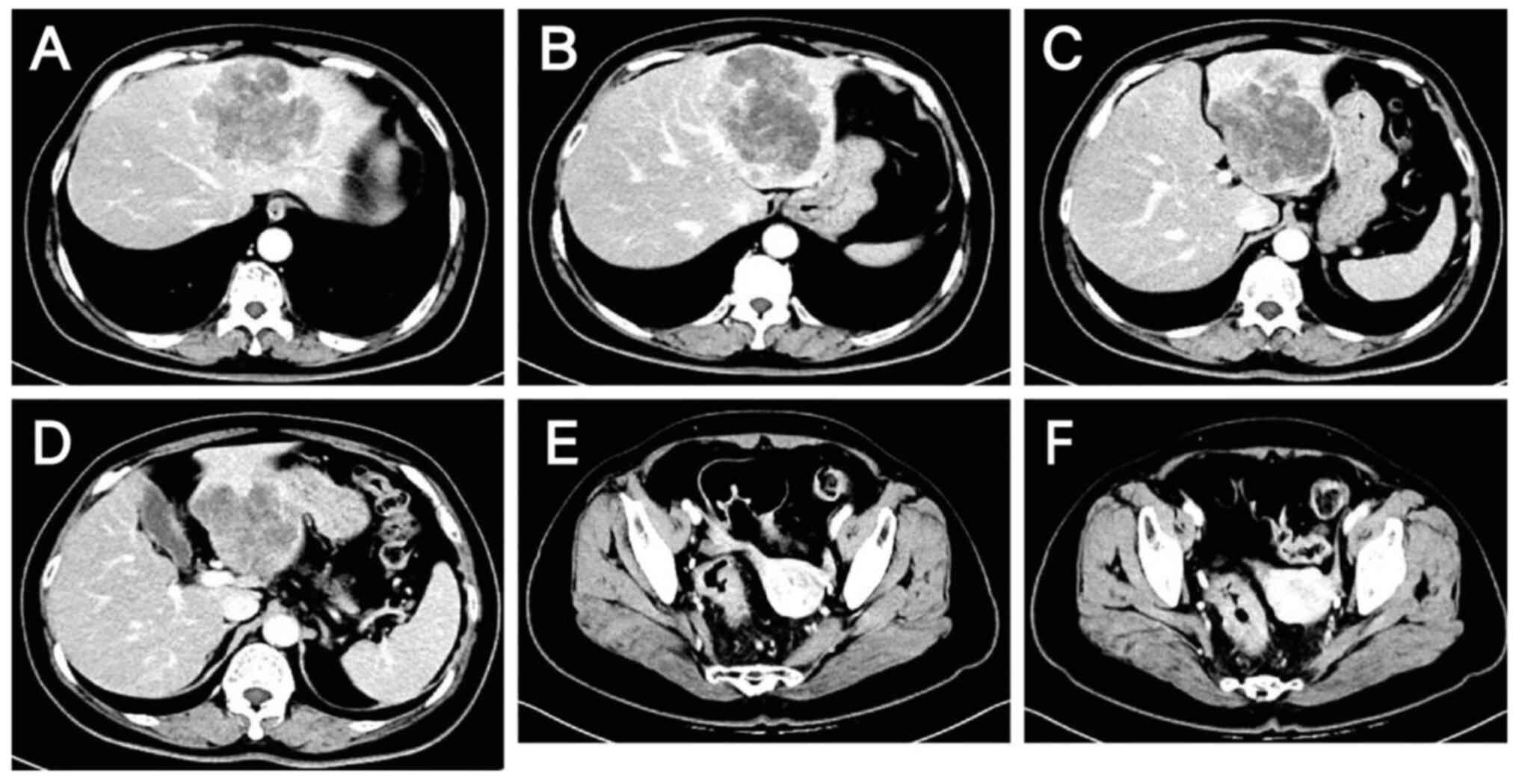
Hospital (Shijiazhuang, China) in October 2023. Contrast-enhanced
scans of the abdomen and pelvis revealed wall thickening in the
sigmoid colon and rectum, enlarged lymph nodes in the surrounding
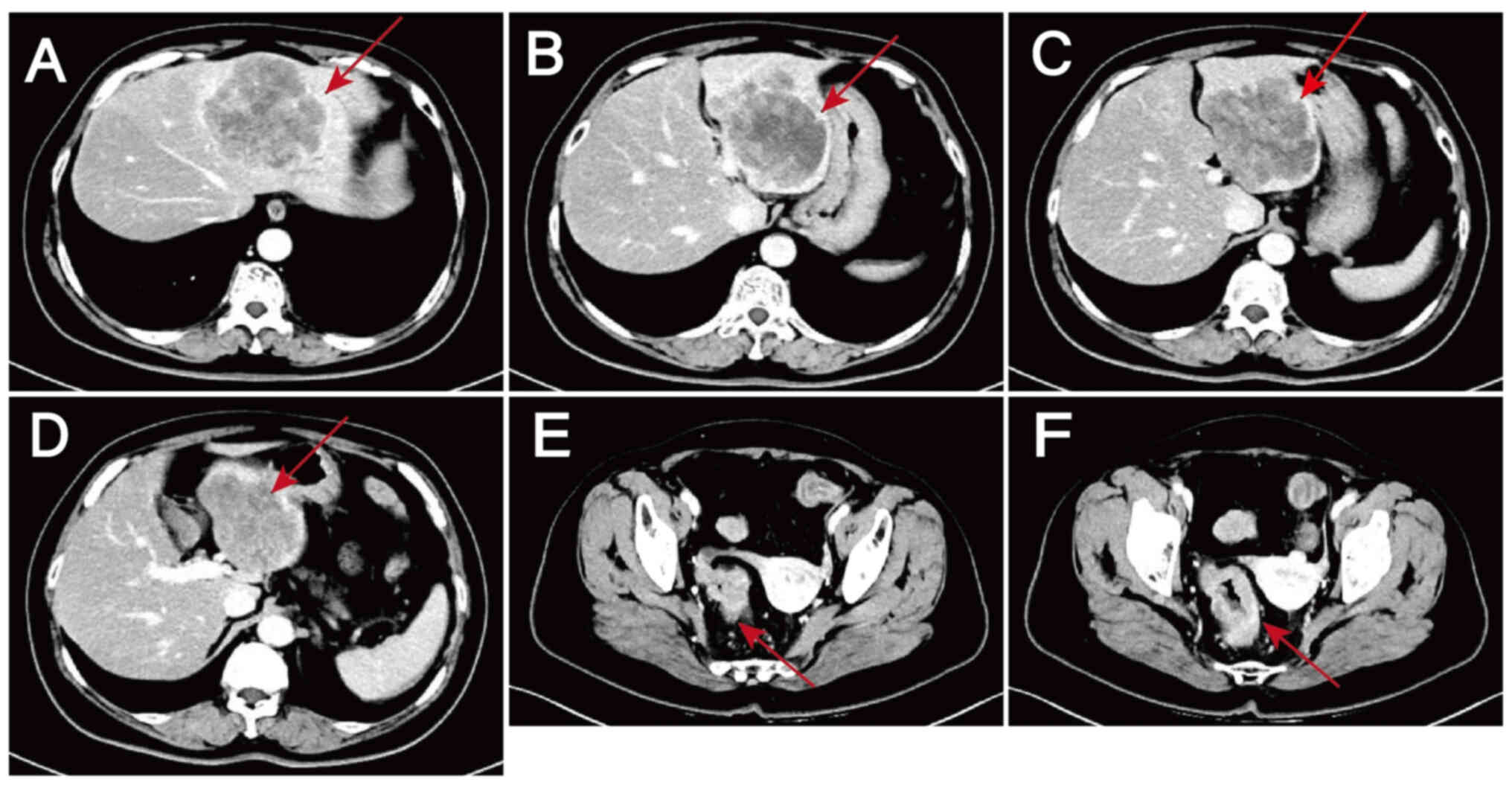
area suggestive of malignancy, and low-density shadows in the S2,
S3 and S4 segments of the liver that were thought to be metastases
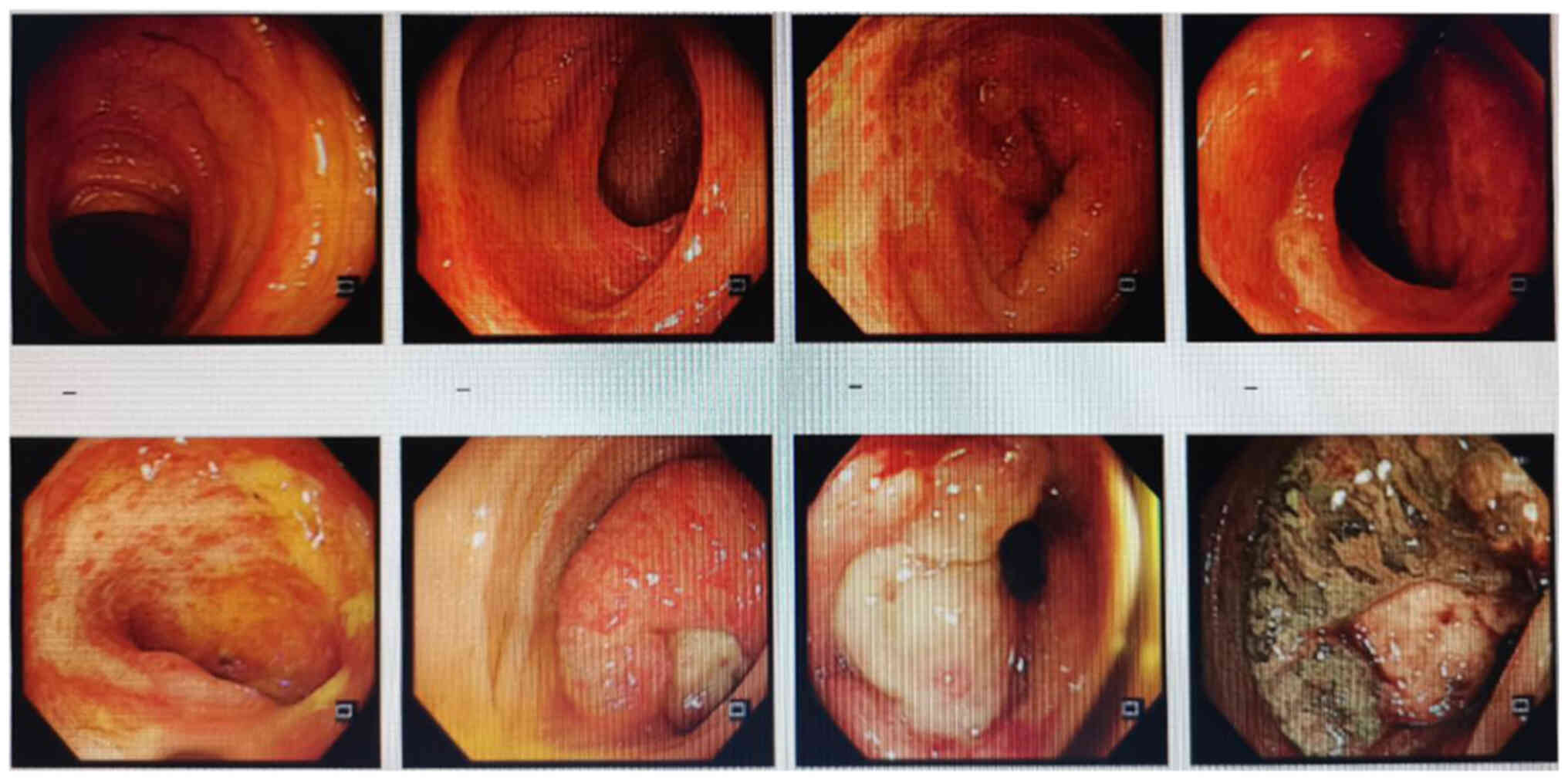
(Fig. 1). Colonoscopy suggested
rectal cancer, and a tissue biopsy revealed moderately and
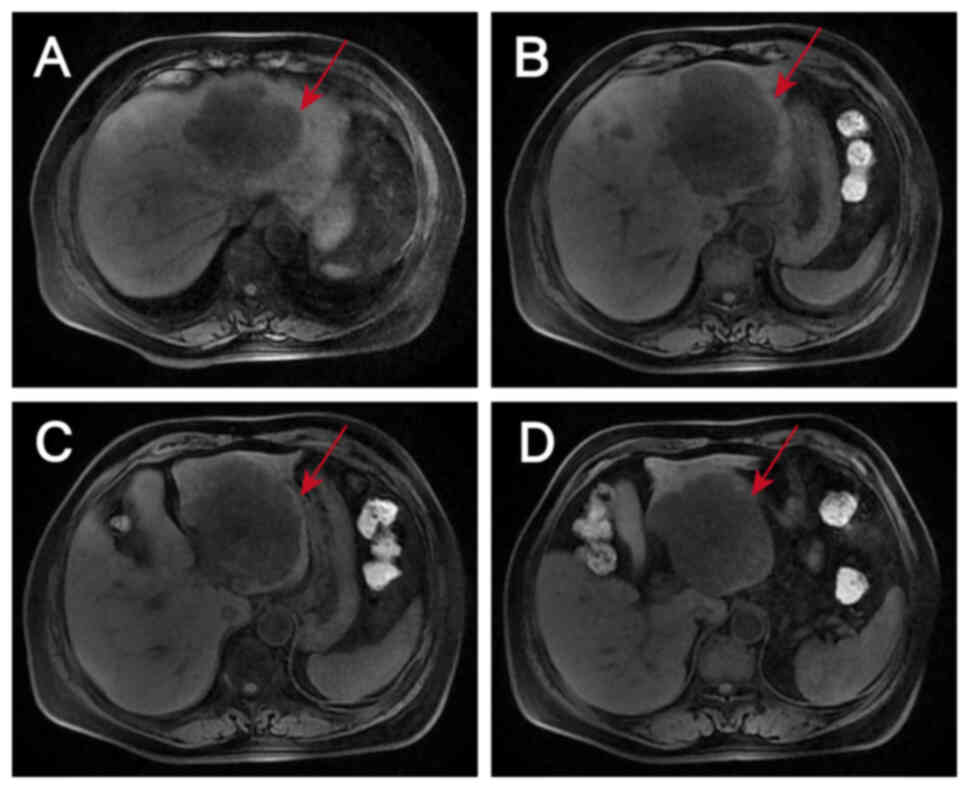
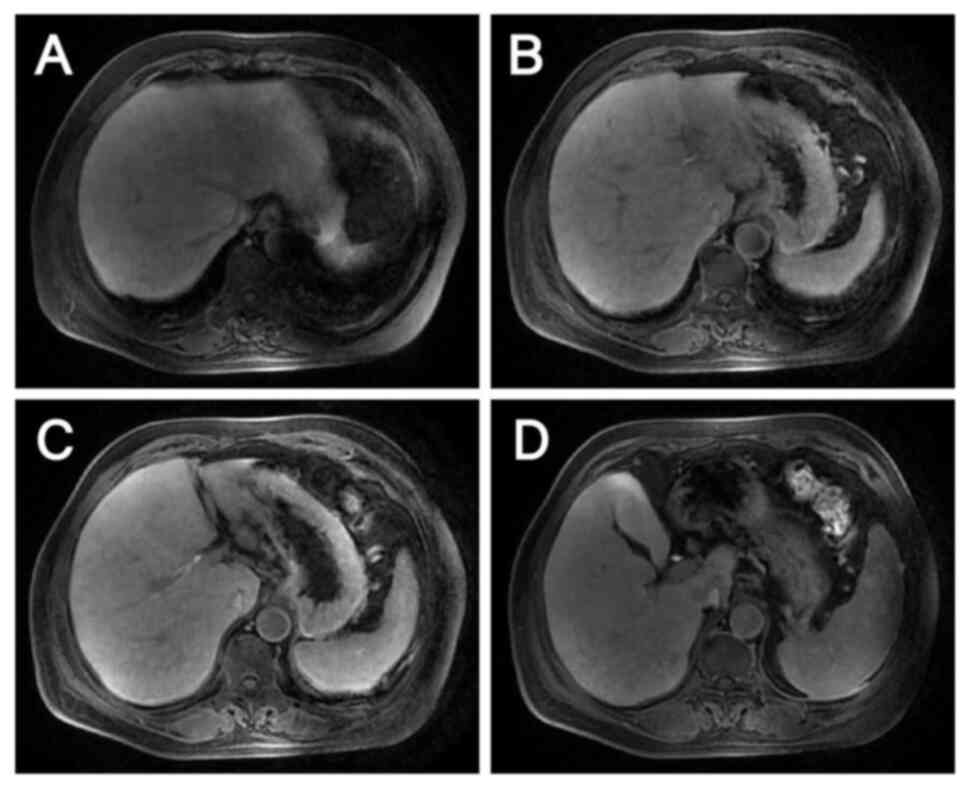
well-differentiated adenocarcinoma with necrosis (Fig. 2). Magnetic resonance images revealed
liver metastases in the S2, S3 and S4 segments and two enlarged
lymph nodes, one anterior and one posterior to the portal vein
(Fig. 3). Digestive tract tumor
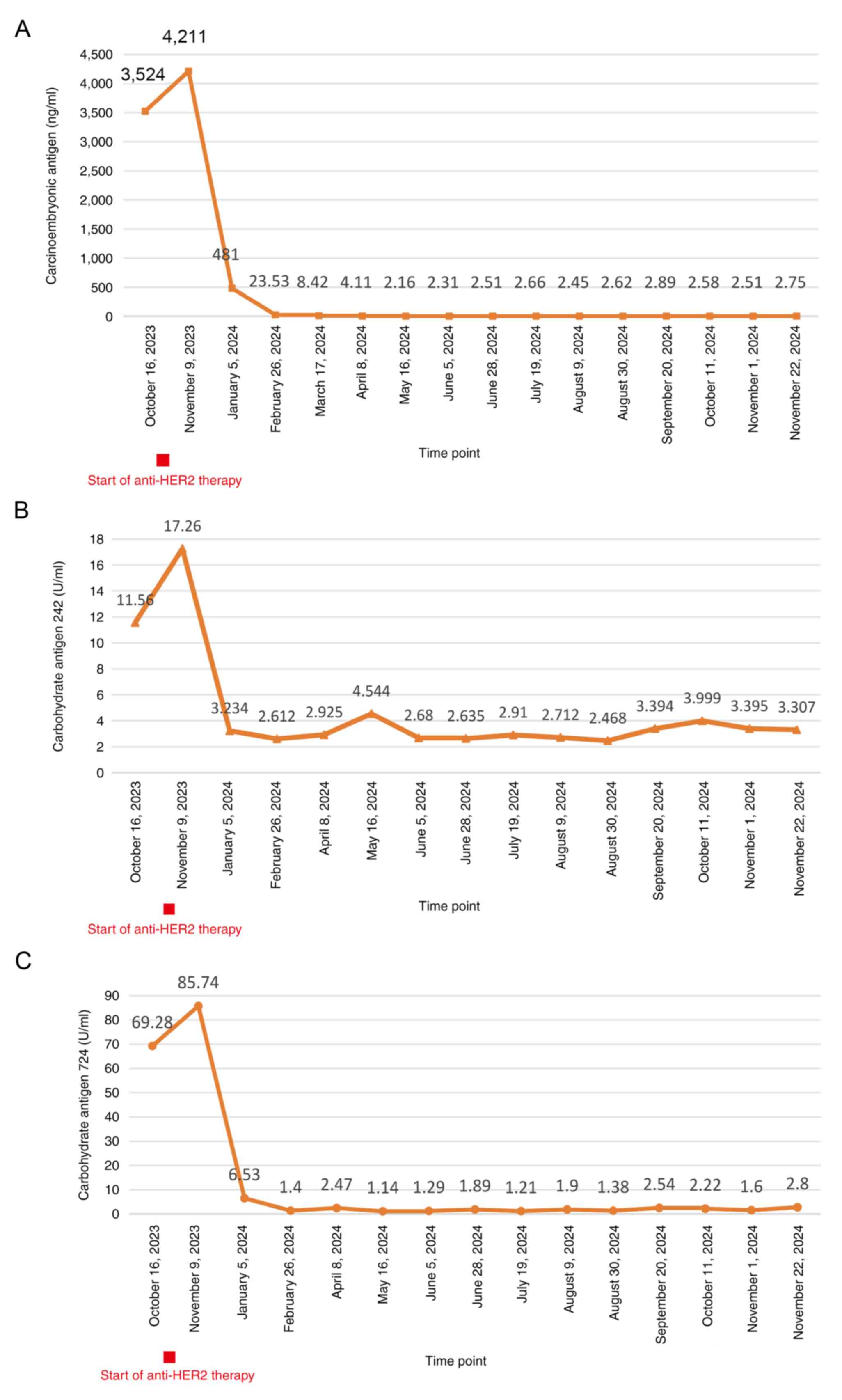
marker levels, including carcinoembryonic antigen, carbohydrate
antigen (CA)242 and CA724, were all higher than normal (Fig. 4). NGS of a biopsy specimen obtained
by colonoscopy suggested that KRAS, NRAS and BRAF were WT (Figs. S1 and S2). It also revealed that ERBB2 (HER2)
had a mutation abundance 8.3-fold higher than the reference level,
suggesting possible resistance to panitumumab and cetuximab. In
addition, TP53 exon5c.399del had a mutation abundance of 31.61%,
suggesting microsatellite stability. The NGS was performed by
Novogene Bioinformatics Technology Co., Ltd. DNA extraction and
library preparation was performed using the Qiagen QIAamp DNA FFPE
Kit (Qiagen, GmbH) and Agilent SureSelect XT HS2 (Agilent
Technologies, Inc.), respectively. Sample quality was assessed by
pathological analysis of tumor cell content, nucleic acid quality
assessment (total DNA amount, DNA degradation degree and total
pre-library amount) and sequencing quality assessment (average
sequencing depth, coverage uniformity, genome alignment rate and
base quality Q30 proportion). The hybridization capture method was
used, and the read length and sequencing direction were double ends
of 150 bp and double-end sequencing, respectively. The sequencing
platform and sequencing kit were the Illumina NextSeq 550 and
Illumina NextSeq 550 High Output kits (Illumina, Inc.),
respectively. Final library loading concentration was 1.2–1.8 pM.
The software used for the analysis included CNVkit (version 0.9.9;
University of California), GATK Mutect2 (version 4.1.8.1; Broad
Institute of MIT and Havard) and PierianDx (version 7.3; Velsera,
Inc.).
Following discussions among the multidisciplinary
team and considering the patient's preference for surgery,
neoadjuvant therapy was initiated, with plans to proceed to surgery
for the primary CRC lesions and liver metastases if the treatment
was effective. After obtaining informed consent, the patient was
treated with two cycles of a modified FOLFOX6 regimen based on a
body surface area of 1.66 m2, comprising oxaliplatin 140
mg + leucovorin calcium 600 mg + fluorouracil 0.625 g by
intravenous injection + fluorouracil 3.75 g. This regimen was
administered by continuous intravenous drip on 13 days post
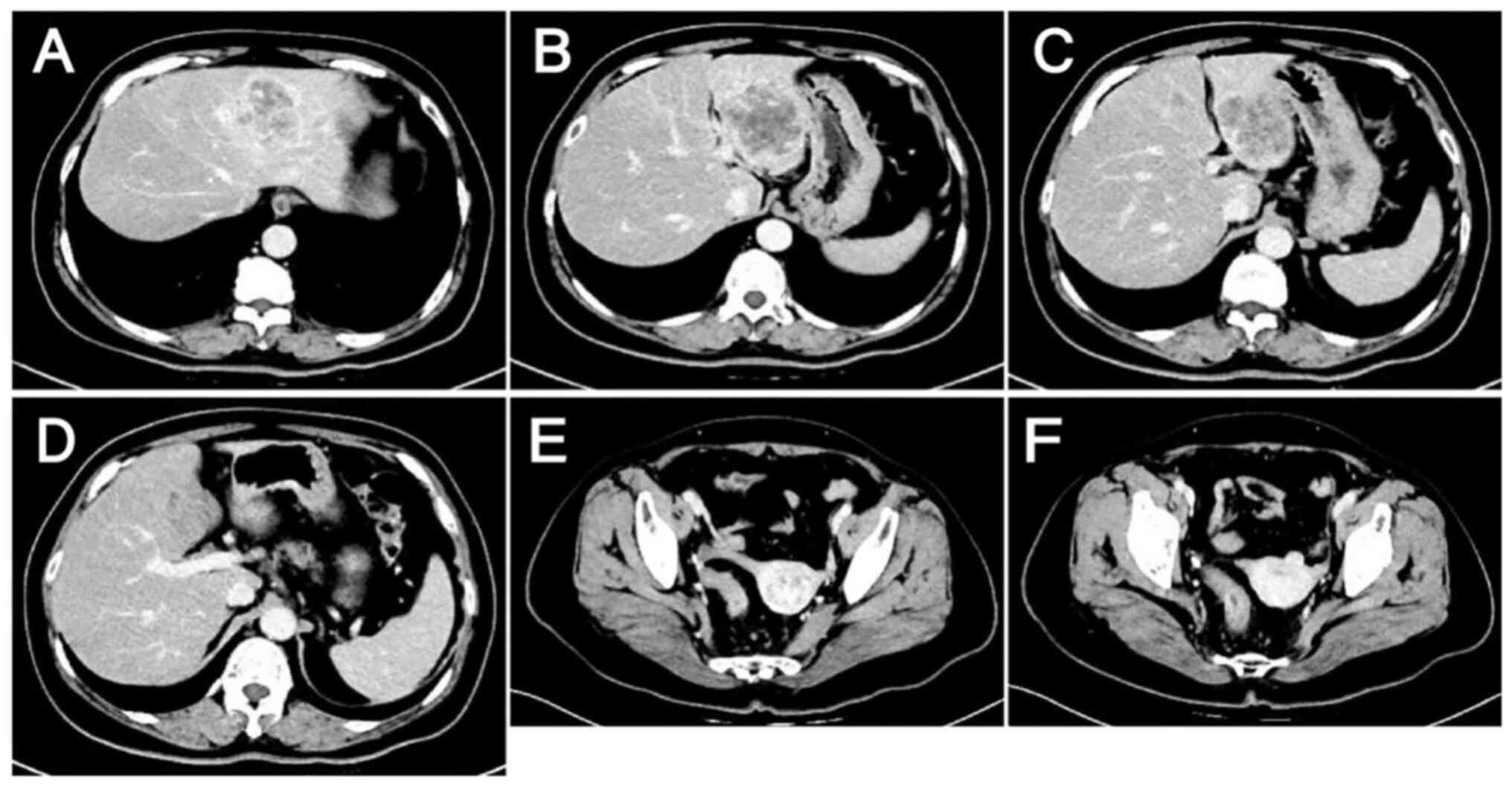
admission and 28 days post admission. However, after the two
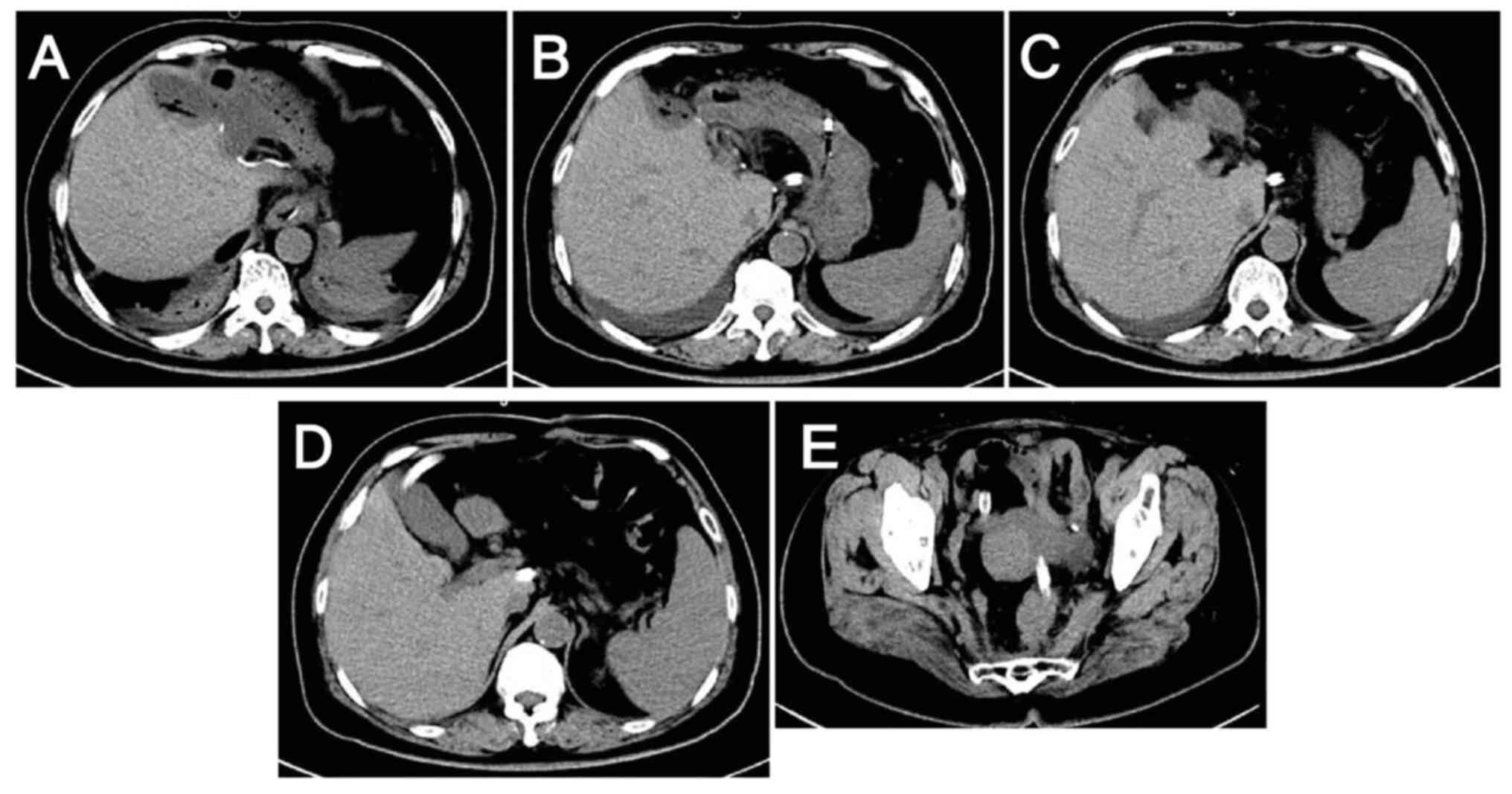
cycles, no reduction in the size of the tumor lesions was evident
(Fig. 5) and the tumor marker
levels remained elevated (Fig. 4),
suggesting poor treatment efficacy.
EGFR inhibitor therapy was not considered due to the
NGS result and the evidence suggesting that patients with
HER2-positive CRC are highly resistant to EGFR inhibitors (11,12).
Furthermore, targeted bevacizumab therapy was not started because
hemorrhage is one of the most serious adverse effects of
bevacizumab and the patient had persistent hematochezia. In light
of the recent developments in CRC research and the financial
circumstances of the patient, single-agent anti-HER2 therapy
combined with a modified FOLFOX6 regimen was selected as the
subsequent treatment, in which the doses of chemotherapeutic agents
were adjusted and administered on a 3-week cycle. The patient
received six cycles of trastuzumab 450 mg + FOLFOX, with the latter
comprising oxaliplatin 150 mg + leucovorin calcium 600 mg +
fluorouracil 0.625 g by injection + fluorouracil 4 g by continuous
intravenous drip. These agents were administered on 41 days post
admission, 62 days post admission, 83 days post admission, 108 days
post admission, 134 days post admission, and 157 days post
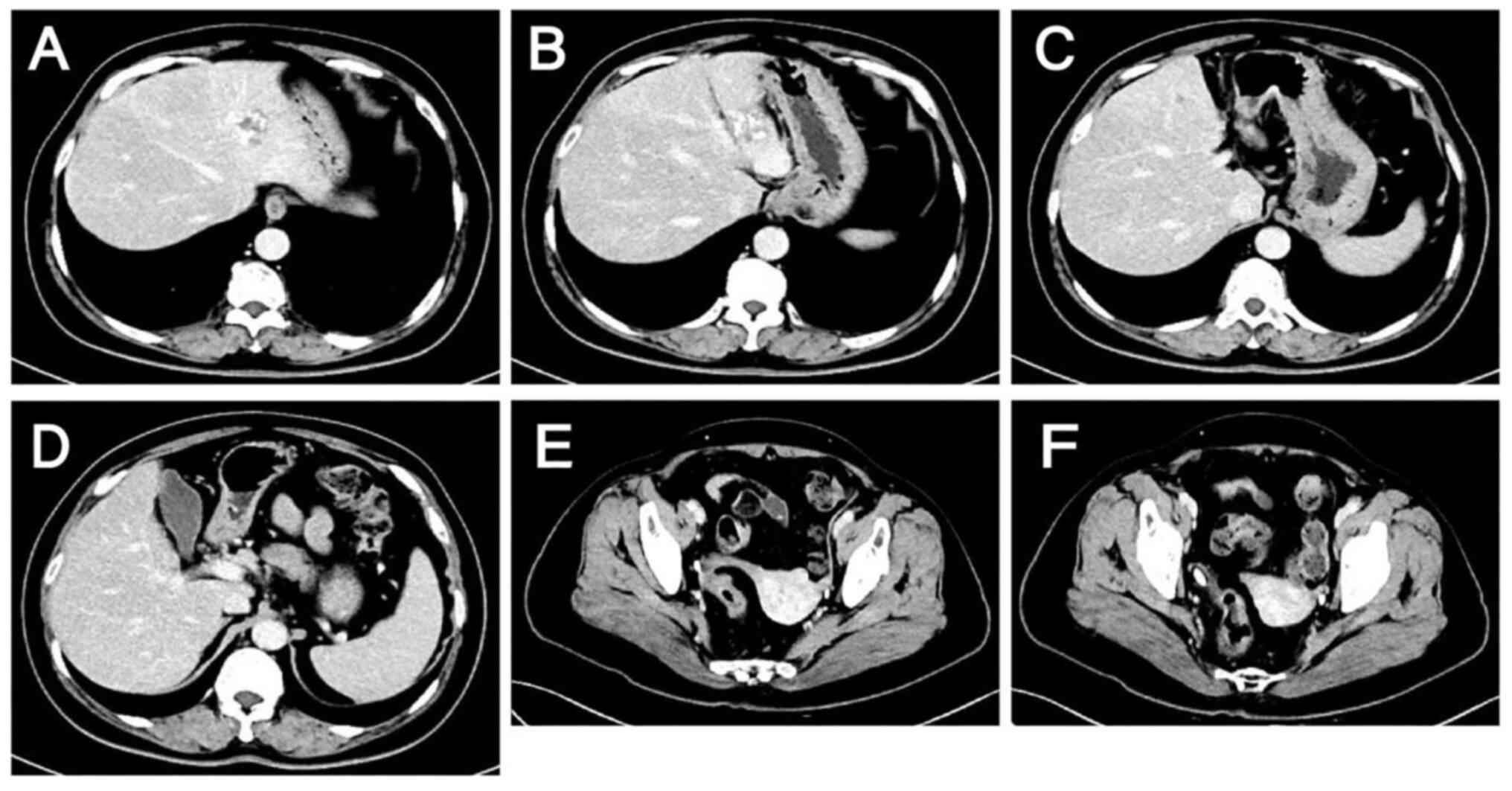
admission. During treatment, the tumor marker levels gradually
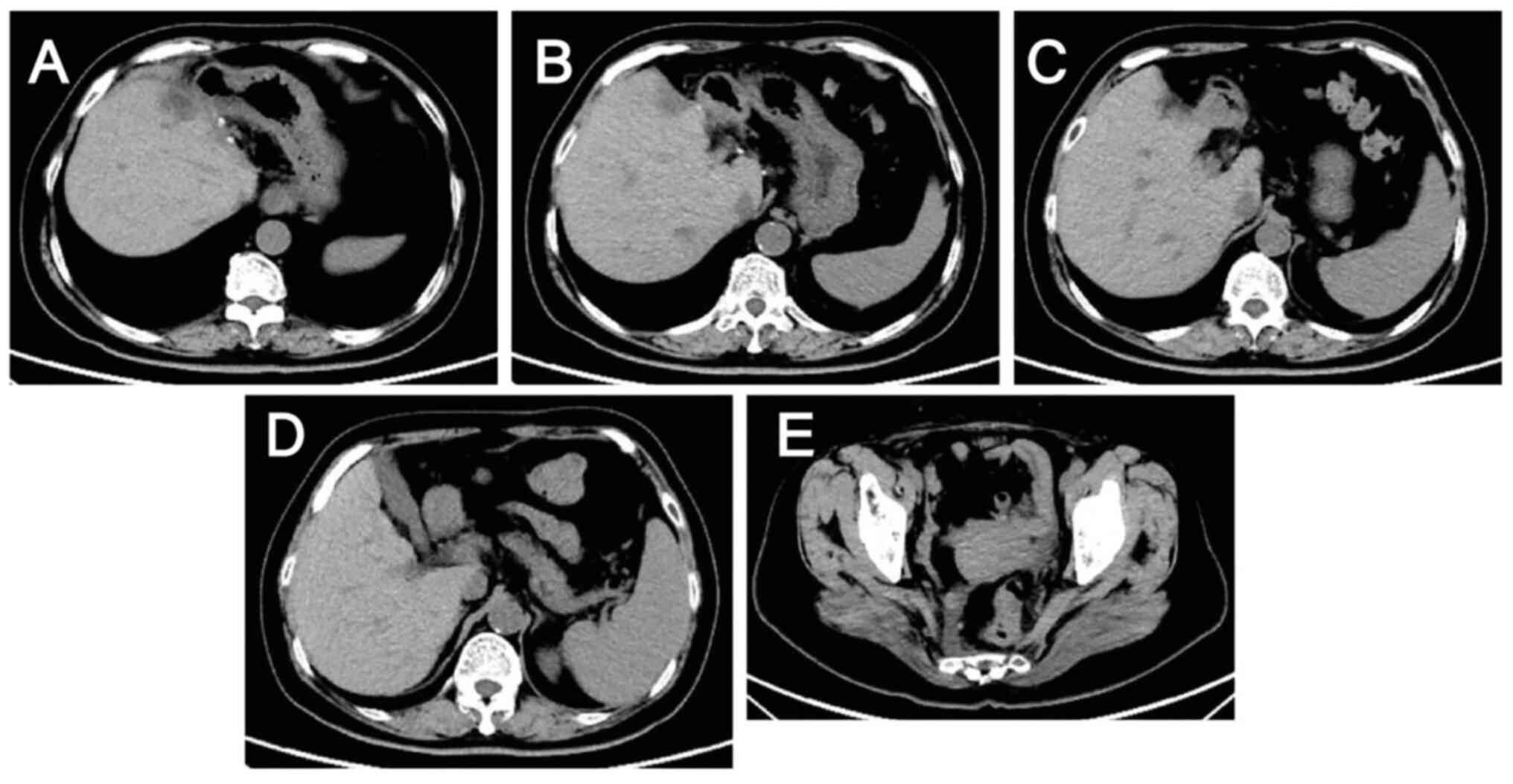
decreased (Fig. 4), and computed
tomography (CT) and magnetic resonance scans of the liver indicated
a marked reduction in the size of the tumor lesions (Fig. 6, Fig.
7, Fig. 8, Fig. 9). This was evaluated as a partial
response.
On April, 2024, the patient underwent
laparoscopy-assisted resection of rectal cancer and abdominal lymph
node dissection, followed by surgery for the liver metastases. This
included dissection of the group 8a lymph nodes, complete resection
of the left outer liver lobe with intrahepatic metastases, and S4
resection, including metastases. This radical resection of the
rectal cancer and liver metastases provided a foundation for
subsequent treatment and potential improvements in survival and
quality of life. Postoperative pathological examination of the
rectal specimen suggested moderately and well-differentiated
adenocarcinoma penetrating the muscularis propria to the subserosa,
with no intravascular cancer thrombus or nerve invasion, and
negative resection margins. One positive regional lymph node was
detected. Immunohistochemistry (IHC) of the rectal specimen
suggested positivity for mismatch repair endonuclease PMS2 (PMS2),
DNA mismatch repair protein Msh2 (MSH2), DNA mismatch repair
protein Msh6 (MSH6) and DNA mismatch repair protein Mlh1 (MLH1),
which indicated microsatellite stability (Fig. S3). Postoperative pathological
examination of the excised liver tissue suggested moderately and
well-differentiated adenocarcinoma with a negative resection margin
and no lymph node involvement. For IHC, the tissues were
paraffin-embedded and fixed in 10% neutral-buffered formalin for 24
h at room temperature. The tissues were section to a 3-µm
thickness. 3% Hydrogen peroxide was used for blocking for 10 min at
room temperature. The following ready-to-use primary antibodies
supplied by Roche Diagnostics, were added at 37°C for 32 min:
VENTANA anti-MLH1 (M1) Mouse Monoclonal Primary Antibody (cat. no.
08033668001), VENTANA anti-MSH2 (G219-1129) Mouse Monoclonal
Primary Antibody (cat. no. 07862253001), VENTANA anti-MSH6 (SP93)
Rabbit Monoclonal Primary Antibody (cat. no. 07862245001) and
VENTANA anti-PMS2 (A16-4) Mouse Monoclonal Primary Antibody (cat.
no. 08033692001). These primary antibodies and OptiView DAB IHC
Detection Kit (cat. no. 06396500001) supplied by Roche Diagnostics
were used together to detect mismatch repair protein. The OptiView
DAB IHC Detection Kit includes HRP-conjugated secondary antibodies
optimized for automated staining on Ventana BenchMark instruments
and was added at 37°C for 30 min. Results were assessed under a
light microscope.
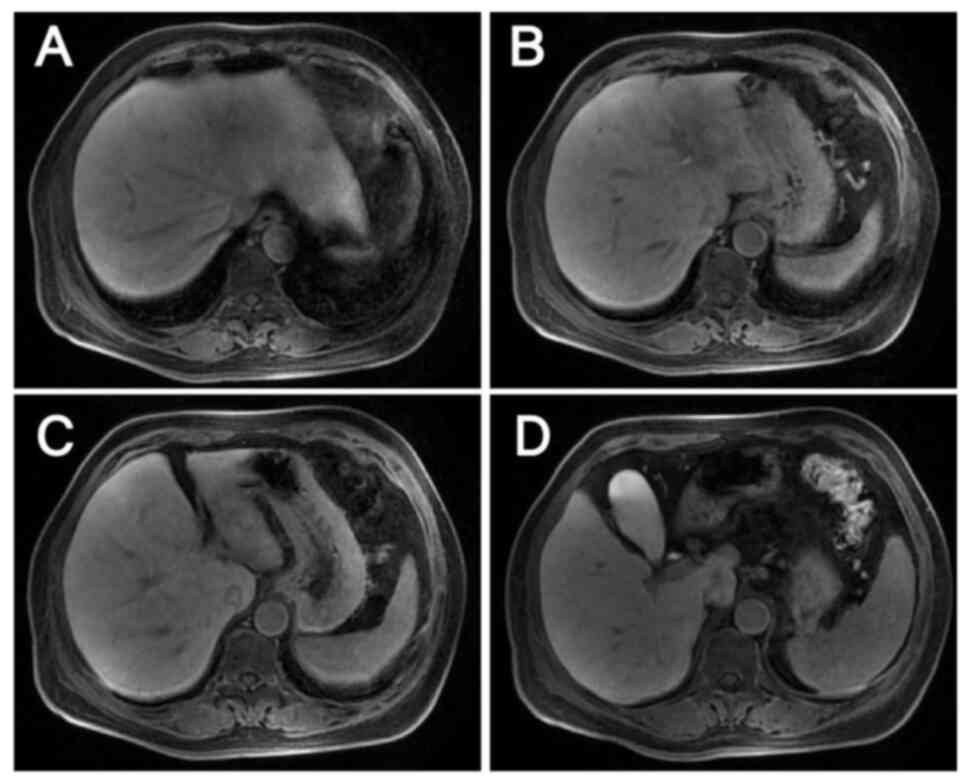
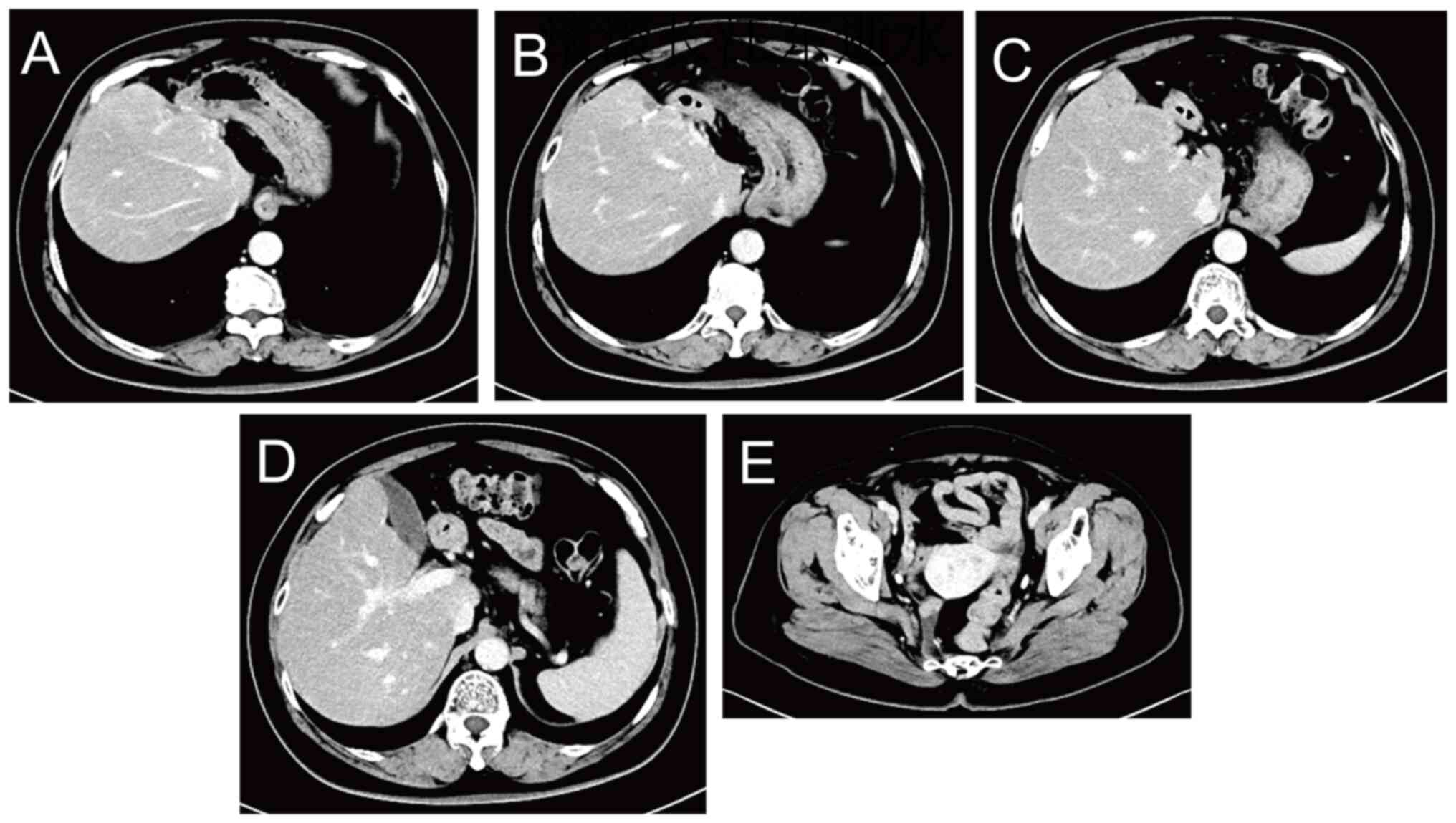
On postoperative day 6, abdominal-pelvic CT revealed
postoperative changes in the rectum and liver, along with blurring
of the left abdominal fat space, and small local nodules and small
lymph nodes in the hilar area and omental capsule (Fig. 10). Repeat CT examination on 27 days
post surgery showed less shadowing from the small nodules (Fig. 11).
The CT results indicated that a small lymph node in
the hilum and omental capsule had a short diameter of ~8 mm, could
be malignant, and would be difficult to remove surgically.
Therefore, seven 3-week cycles of anti-HER2 therapy (300 mg
trastuzumab on day 1 of the cycle) combined with oral chemotherapy
(1 g capecitabine twice daily on days 1–14 of the cycle) were
administered on 28, 39, 60, 81, 102, 123 and 145 days post-surgery.
Thereafter, the chemotherapy was discontinued and the patient
received three 3-week cycles of anti-HER2 therapy (trastuzumab 300
mg) on 165, 186 and 207 days post-surgery. The tumor marker levels
were within the normal range during the postoperative period, and
the patient remained on anti-HER2 therapy for 1 year. The latest CT
result, which was recorded on 210 days post-surgery, showed
postoperative changes in the rectum and liver, along with no signs
of recurrence (Fig. 12). The
patient was found to be in good condition and discontinued
anti-HER2 therapy with instructions to attend for review every 3
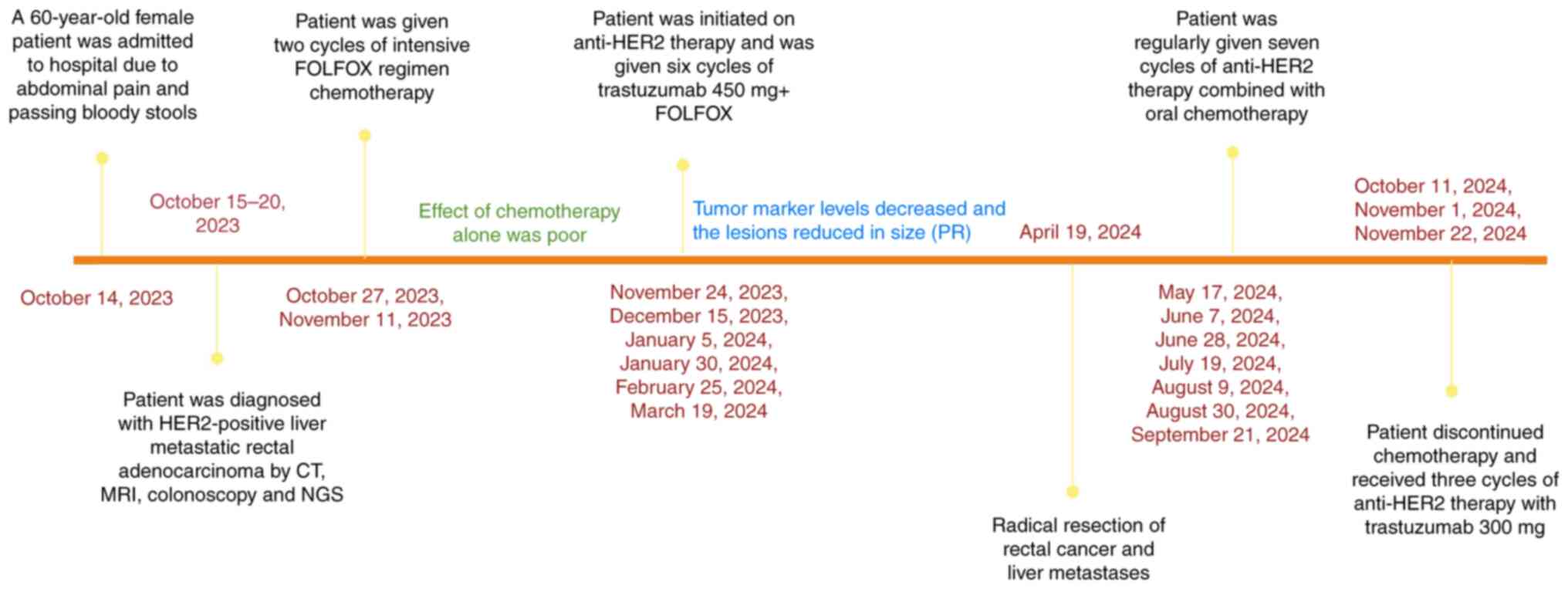
months. The timeline of the treatment sequences, imaging
evaluations and key clinical events are shown in Fig. 13.
Discussion
The incidence of HER2 positivity in CRC is
relatively low, at only ~5% overall (5). HER2 is a member of the ERBB family of
transmembrane receptor tyrosine kinases (RTKs), which also includes
ERBB1 (EGFR), ERBB3 and ERBB4 (13,14).
It is widely accepted that the overexpression, amplification or
mutation of HER2 results in resistance to EGFR inhibitors and can
be used as a biomarker for the prediction of treatment efficacy
(11,12,15).
The EGFR inhibitor cetuximab exerts an antitumor effect by
inhibiting EGFR and downstream ERK activation, thereby hindering
cancer cell proliferation (16).
The mechanism underlying resistance to EGFR inhibitors is thought
to involve HER2 amplification, leading to aberrant activation of
HER2-ERK signal transduction and the bypass of EGFR-ERK signaling
(16). Accordingly, ERK activation
is maintained in HER2-amplified cells, conferring resistance to
EGFR inhibitors in patients with HER2-positive tumors (16). The specific targeting of HER2 with
small interfering RNA or HER2 inhibitors has been shown to block
HER2 and ERK activation in HER2-amplified cells, thereby restoring
sensitivity to cetuximab (16).
These findings suggest that the HER2-mediated activation of
alternative signaling pathways promotes resistance to EGFR
inhibitors and that anti-HER2 therapy could potentially restore
sensitivity to EGFR inhibitors and exert an anticancer effect in
HER2-positive tumors. Preclinical and clinical studies have shown
that the progression-free and overall survival rates achieved by
EGFR inhibitors in patients with HER2-amplified CRC are
significantly lower than those of their WT counterparts and
comparable with those in patients with RAS or BRAF mutations
(17,18). This suggests that HER2 predicts a
poor response to EGFR inhibitor therapy. Therefore, we hypothesize
that CRC with HER2 positivity, whether amplified, overexpressed or
mutated, can lead to resistance to EGFR inhibitors and that the
early initiation of anti-HER2 therapy should be considered.
In the present case, the NGS results indicated that
KRAS, NRAS and BRAF were all WT, while ERBB2 (HER2) was mutated and
amplified with a mutation abundance elevated 8.3-fold. These
findings suggest possible resistance to drugs such as panitumumab
and cetuximab, based on the aforementioned findings. Accordingly,
first-line chemotherapy alone was initiated for metastatic CRC;
however, after two cycles, an increase in tumor marker levels was
observed and no clear change in the size of the lesions was shown
by imaging, indicating that chemotherapy alone was having a poor
effect. Given advances in clinical trials on CRC diagnosis and
treatment, as well as the patient's circumstances, the patient was
subsequently treated with anti-HER2 therapy combined with
chemotherapy. Notably, tumor markers gradually decreased during the
treatment, and imaging revealed a reduction in the size of the
lesions. After six cycles of treatment, the tumor marker levels
were within the normal range and the lesions were markedly smaller.
Simultaneous surgical resection of the rectal cancer and liver
metastases was subsequently achieved, demonstrating that anti-HER2
therapy can be beneficial in the treatment of metastatic CRC.
Although HER2 has a well-established prognostic significance in
breast cancer, its prognostic role in metastatic CRC remains
unclear (19). However, HER2
positivity appears to have been beneficial in the present case,
converting unresectable lesions into resectable ones after
anti-HER2 therapy, potentially improving the prognosis and quality
of life of the patient.
HER2 positivity is more common in patients with
RAS/BRAF WT disease (20), with one
study reporting HER2 upregulation in only 1.0% of patients with
KRAS/BRAF mutations compared with 5.2% in those with WT disease
(21). In the MyPathway trial, 23%
of patients had KRAS mutations, with an objective response rate
(ORR) of only 8%, much lower than the ORR of 40% observed for
patients with KRAS WT disease (22). An exploratory analysis of the Phase
II TRIUMPH study also showed the treatment responses in patients
with alterations in RTK/RAS/PI3K-related genes detected by
circulating tumor DNA were poorer compared with those of patients
without these alterations (23).
These studies suggest that patients with KRAS WT disease may have a
stronger response to anti-HER2 therapy. The microsatellite
instability (MSI) status of CRC can be classified as MSI-high,
MSI-low or microsatellite-stable (MS-stable) (24). Immunotherapy offers significant
benefits to patients with MSI-high CRC and has been approved in
this indication by the US Food and Drug Administration. However, it
does not provide a significant survival advantage in patients with
MS-stable CRC (25). In the present
case, NGS results showed that KRAS, NRAS and BRAF were WT, the
tumor microsatellite status was MS-stable, and HER2 expression was
positive; therefore, anti-HER2 therapy was recommended.
HER2 overexpression or amplification is
traditionally assessed by IHC to evaluate protein expression and by
in situ hybridization (ISH) to evaluate gene amplification.
The diagnostic criteria developed by Valtorta et al
(26) for the HERACLES clinical
trial established a standardized procedure for defining HER2
positivity in CRC by IHC and ISH. According to these criteria, HER2
status is considered positive if there is intense (3+) IHC staining
in ≥50% of cells, or either intense (3+) IHC staining in >10 to
<50% of cells or moderate (2+) IHC staining in ≥50% of cells,
combined with amplification on ISH testing, defined as an ERBB2 to
centromere of chromosome 17 ratio of ≥2 in ≥50% of cells (26). Studies (27,28)
have shown that NGS results are closely consistent with IHC/ISH
testing results, and can also be used to evaluate the amplification
of HER2 in CRC. Furthermore, the assessment of circulating tumor
DNA by liquid biopsy is a simple method for the detection of HER2
status that requires only a blood sample rather than a tissue
biopsy, with some studies demonstrating its success in metastatic
CRC (23,29,30).
Therefore, the circulating tumor DNA assay can be used to
complement tissue-based methods or serve as an alternative when
tissue testing is not feasible. Sartore-Bianchi et al
(12) proposed that HER2 status
assessment should be included in the molecular diagnostic workup of
all patients with metastatic CRC to allow prompt referral to
clinical trials involving HER2-targeted double blockade, regardless
of previous anti-EGFR treatment. Another study emphasized the
importance of detecting HER2 status in patients with advanced or
metastatic CRC, particularly those with RAS WT and MS-stable
disease (31). The current National
Comprehensive Cancer Network guidelines recommend the assessment of
HER2 status in all patients with metastatic CRC using either
IHC/ISH or NGS (32). This
indicates that greater emphasis should be placed on the detection
of HER2 expression in patients with CRC, particularly those with
metastatic disease. Timely treatment based to these results may
lead to enhanced clinical outcomes and an improved prognosis.
The findings from the Trastuzumab for Gastric Cancer
trial, published in 2010, revealed that the mean overall survival
of patients with HER2-positive (IHC 3+ or overexpression on
fluorescence ISH) recurrent or metastatic gastric adenocarcinoma or
gastric-esophageal junction cancer was prolonged when trastuzumab
was added to first-line chemotherapy. In addition, the combination
of trastuzumab and chemotherapy exhibited a safety profile
comparable with that of first-line chemotherapy alone (33). This discovery marked a shift from
traditional therapy to a new era of molecular targeted therapy for
gastric cancer. Trastuzumab combined with chemotherapy is now
considered the standard first-line treatment for HER2-positive
gastric cancer (34,35). Furthermore, the 2023 Chinese Society
of Clinical Oncology gastric cancer guidelines emphasize that HER2
status testing is necessary in all patients with a confirmed
pathological diagnosis of gastric adenocarcinoma (36).
Patients with gastric cancer are tested early for
HER2 status, and those who are positive receive first-line
anti-HER2 treatment. However, in CRC, the testing of anti-HER2
treatment typically occurs later. Insights from the present case
and a review of the literature on anti-HER2 therapy for gastric
cancer suggest some notable similarities between the diagnosis and
treatment principles of gastric cancer and those of CRC (33). This leads to the suggestion that the
early detection of the HER2 expression status of patients with CRC,
particularly those with metastatic CRC, could allow anti-HER2
therapy to be brought forward into the first-line setting,
potentially improving patient outcomes and quality of life.
Similarly, another study proposed that anti-HER2 therapy for
HER2-amplified CRC should be initiated as soon as possible rather
than reserved for last-line application (37). However, further research is
necessary to determine whether the earlier application of anti-HER2
therapy should be included in clinical guidance on the diagnosis
and treatment of CRC.
At present, anti-HER2 therapy mainly includes
monoclonal antibodies such as trastuzumab and pertuzumab, tyrosine
kinase inhibitors such as lapatinib and tucatinib, and
antibody-drug conjugates, including trastuzumab deruxtecan and
trastuzumab emtansine. Advances in molecular pathology and
detection methods have led to a series of studies in patients with
HER2-positive CRC, including MyPathway, TRIUMPH, MOUNTAINEER,
DESTINY-CRC and HERACLES (22,38–40).
Most treatments in these studies involved a dual-targeted approach,
such as a combination of two anti-HER2 antibodies or a monoclonal
antibody combined with a tyrosine kinase inhibitor. Significant
improvements have been reported in the overall prognosis for these
treatments compared with standard treatment or best supportive care
in the aforementioned trials, particularly with regard to the ORR,
suggesting that patients with HER2-positive CRC can benefit from
anti-HER2 therapy. Based on these promising outcomes, the National
Comprehensive Cancer Network guidelines recommend four
HER2-targeted regimens, namely, trastuzumab combined with
pertuzumab, lapatinib or tucatinib, or trastuzumab deruxtecan
(DS-8201) alone for patients with CRC, HER2 amplification and WT
RAS/BRAF (32). In the present
case, a dual-targeted regimen was not feasible due to financial
constraints. Therefore, single-agent trastuzumab was used as a
targeted therapy and achieved favorable results. Nevertheless, a
dual-targeted approach is recommended when financial resources
allow.
Trastuzumab deruxtecan is an antibody-drug conjugate
consisting of trastuzumab linked to a topoisomerase I inhibitor.
The Phase II DESTINY-CRC01 trial assessed the activity of this
agent in patients with HER2-positive, WT RAS/BRAF and previously
treated metastatic CRC at a dosage of 6.4 mg/kg every 3 weeks
(40). In contrast with the
aforementioned trials (22,38,39),
the DESTINY-CRC01 trial allowed the inclusion of patients who had
previously received anti-HER2 therapy (40). This trial found no significant
reduction in the response rate in patients who had previously
received anti-HER2 therapy, suggesting that trastuzumab deruxtecan
is effective in anti-HER2-pretreated patients (40). The subsequent DESTINY-CRC02 trial
explored the efficacy of two dose levels of trastuzumab deruxtecan
(6.4 and 5.4 mg/kg) administered every 3 weeks (41). Unlike DESTINY-CRC01, DESTINY-CRC02
enrolled patients with both RAS-WT and RAS-mutant CRC (41). No significant difference in
treatment response was observed between the two dose levels
(41). However, due to toxicity
concerns, the 5.4 mg/kg dosage was preferred, supporting its use in
future treatments. This trial demonstrated a promising treatment
effect in patients with RAS-mutated disease (n=20; ORR 20.0%) and
those who had previously received anti-HER2 therapy (n=27; ORR
40.1%), suggesting that trastuzumab deruxtecan could be considered
for the subsequent treatment of HER2-positive and RAS-mutated
metastatic CRC (41). In addition,
both trials showed that the response of patients with IHC 3+
disease was improved compared with that of patients with IHC
2+/ISH+ disease, indicating that high HER2 expression may be
important for an effective response to anti-HER2 therapy (40,41).
Most anti-HER2 therapy regimens used in clinical
trials for HER2-positive CRC have demonstrated good tolerability.
However, cardiotoxicity associated with anti-HER2 agents, and
interstitial lung disease or pneumonia associated with trastuzumab
deruxtecan warrant careful monitoring (42–44).
In the present case, the patient tolerated anti-HER2 therapy well,
with no adverse effects observed. The results of regular
electrocardiograms and measurements of left ventricular ejection
fraction were within the normal ranges. Cardiac function should be
assessed before, during and after the completion of HER2-targeted
therapy, and treatment should be withheld or discontinued if a
significant reduction in ejection fraction is observed (45,46).
Grade ≥3 treatment-related adverse events have been reported in
clinical trials of trastuzumab deruxtecan for breast cancer,
gastric cancer and CRC (47–49).
Therefore, patients treated with this agent should be promptly
evaluated for any signs or symptoms of pulmonary toxicity. The
aforementioned studies (40,41)
have investigated a variety of anti-HER2 agents, including some
that remain under investigation. Further clinical research is
necessary to determine the optimal anti-HER2 regimen and the
appropriate sequencing of subsequent anti-HER2 therapies.
Currently available anti-HER2 therapies have
provided clinical benefits to certain patients with HER2-positive
CRC. However, more than half of patients do not experience any
benefit, suggesting that anti-HER2 therapy has some limitations. A
meta-analysis found a median progression-free survival of only 4.89
months in patients with advanced CRC receiving anti-HER2 therapy
(50). The relatively lower
efficacy of anti-HER2 therapy in patients with HER2-positive CRC in
comparison with that in patients with other types of cancer may be
associated with HER2 resistance (51). While a number of studies have shown
that the overexpression or mutation of genes involved in the
RTK/RAS and PI3K pathways may contribute to resistance to anti-HER2
therapy (51–53), additional studies are necessary to
explore the mechanisms of this resistance. This may facilitate the
identification of strategies that can overcome drug resistance and
enhance the clinical benefits of this therapy for patients with
HER2-positive CRC.
In conclusion, anti-HER2 therapy has not been
thoroughly investigated in CRC, and the relationship between HER2
positivity and the prognosis of patients with CRC remains
inconclusive. The present case provides evidence that patients with
HER2-positive CRC could significantly benefit from anti-HER2
therapy, and such therapy may confer a good prognosis for patients.
Moreover, given that HER2 positivity is associated with resistance
to EGFR inhibitors, early anti-HER2-containing combination therapy
is warranted. Therefore, it is important to assess HER2 status
promptly and accurately in patients with CRC, particularly those
with metastatic disease. Further studies are required to determine
whether anti-HER2 therapy can be moved into the frontline setting
for patients with HER2-positive CRC and to identify the optimal
anti-HER2 regimen.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
Hebei Province in 2021 and 2024 (grant nos. H2021307016 and
H2024206176) and Projects from Health and Family Planning
Commission of Hebei Province (grant no. 20220829).
Availability of data and materials
The NGS data generated in the present study may be
found in the BioProject database under accession number
PRJNA1247027 or at the following URL: https://www.ncbi.nlm.nih.gov/sra/PRJNA1247027. The
remaining data generated in the present study are included in the
figures and/or tables of this article.
Authors' contributions
JH, ZL and XF collected clinical data, reviewed the
literature and drafted the manuscript. DH, XL, YW, QL and DW
collected clinical data and contributed to drafting the manuscript.
XF and JH confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CT
|
computed tomography
|
|
EGFR
|
epidermal growth factor receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
NGS
|
next-generation sequencing
|
|
ORR
|
objective response rate
|
|
WT
|
wild-type
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross JS, Fakih M, Ali SM, Elvin JA,
Schrock AB, Suh J, Vergilio JA, Ramkissoon S, Severson E, Daniel S,
et al: Targeting HER2 in colorectal cancer: The landscape of
amplification and short variant mutations in ERBB2 and ERBB3.
Cancer. 124:1358–1373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siena S, Sartore-Bianchi A, Marsoni S,
Hurwitz HI, McCall SJ, Penault-Llorca F, Srock S, Bardelli A and
Trusolino L: Targeting the human epidermal growth factor receptor 2
(HER2) oncogene in colorectal cancer. Ann Oncol. 29:1108–1119.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Margonis GA, Buettner S, Andreatos N, Kim
Y, Wagner D, Sasaki K, Beer A, Schwarz C, Løes IM, Smolle M, et al:
Association of BRAF mutations with survival and recurrence in
surgically treated patients with metastatic colorectal liver
cancer. JAMA Surg. 153:e1809962018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suwaidan AA, Lau DK and Chau I: HER2
targeted therapy in colorectal cancer: New horizons. Cancer Treat
Rev. 105:1023632022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosati G, Aprile G, Colombo A, Cordio S,
Giampaglia M, Cappetta A, Porretto CM, De Stefano A, Bilancia D and
Avallone A: Colorectal cancer heterogeneity and the impact on
precision medicine and therapy efficacy. Biomedicines. 10:10352022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Li W, Lu Z, Lu M, Zhou J, Peng Z,
Zhang X, Wang X, Shen L and Li J: Clinicopathological features of
HER2 positive metastatic colorectal cancer and survival analysis of
anti-HER2 treatment. BMC Cancer. 22:3552022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chitkara A, Bakhtiar M, Sahin IH, Hsu D,
Zhang J, Anamika F, Mahnoor M, Ahmed R, Gholami S and Saeed A: A
meta-analysis to assess the efficacy of HER2-targeted treatment
regimens in HER2-positive metastatic colorectal cancer (mCRC). Curr
Oncol. 30:8266–8277. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin
R, He WL, Cai SR, He YL and Ye JN: Anti-EGFR therapy in metastatic
colorectal cancer: Mechanisms and potential regimens of drug
resistance. Gastroenterol Rep (Oxf). 8:179–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sartore-Bianchi A, Amatu A, Porcu L,
Ghezzi S, Lonardi S, Leone F, Bergamo F, Fenocchio E, Martinelli E,
Borelli B, et al: HER2 positivity predicts unresponsiveness to
EGFR-targeted treatment in metastatic colorectal cancer.
Oncologist. 24:1395–1402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popescu NC, King CR and Kraus MH:
Localization of the human erbB-2 gene on normal and rearranged
chromosomes 17 to bands q12-21.32. Genomics. 4:362–366. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, He Y, Sun Y, Wang W, Qian X, Yu X
and Pan Y: Prevalence, prognosis and predictive status of HER2
amplification in anti-EGFR-resistant metastatic colorectal cancer.
Clin Transl Oncol. 22:813–822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yonesaka K, Zejnullahu K, Okamoto I, Satoh
T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda
M, et al: Activation of ERBB2 signaling causes resistance to the
EGFR-directed therapeutic antibody cetuximab. Sci Transl Med.
3:99ra862011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sawada K, Nakamura Y, Yamanaka T, Kuboki
Y, Yamaguchi D, Yuki S, Yoshino T, Komatsu Y, Sakamoto N, Okamoto W
and Fujii S: Prognostic and predictive value of HER2 amplification
in patients with metastatic colorectal cancer. Clin Colorectal
Cancer. 17:198–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong JH, Kim J, Hong YS, Kim D, Kim JE,
Kim SY, Kim KP, Yoon YK, Kim D, Chun SM, et al: HER2 amplification
and cetuximab efficacy in patients with metastatic colorectal
cancer harboring wild-type RAS and BRAF. Clin Colorectal Cancer.
16:e147–e152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang JY, Jeon YK, Jeong SY, Lim SH, Park
YS, Lim HY, Lee JY and Kim ST: Effect of human epidermal growth
factor receptor 2 overexpression in metastatic colorectal cancer on
standard chemotherapy outcomes. J Gastrointest Oncol. 14:2097–1110.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nam SK, Yun S, Koh J, Kwak Y, Seo AN, Park
KU, Kim DW, Kang SB, Kim WH and Lee HS: BRAF, PIK3CA, and HER2
oncogenic alterations according to KRAS mutation status in advanced
colorectal cancers with distant metastasis. PLoS One.
11:e01518652016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richman SD, Southward K, Chambers P, Cross
D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM,
et al: HER2 overexpression and amplification as a potential
therapeutic target in colorectal cancer: Analysis of 3256 patients
enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials.
J Pathol. 238:562–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meric-Bernstam F, Hurwitz H, Raghav KPS,
McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R,
Burris H, Sweeney C, et al: Pertuzumab plus trastuzumab for
HER2-amplified metastatic colorectal cancer (MyPathway): An updated
report from a multicentre, open-label, phase 2a, multiple basket
study. Lancet Oncol. 20:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura Y, Okamoto W, Kato T, Esaki T,
Kato K, Komatsu Y, Yuki S, Masuishi T, Nishina T, Ebi H, et al:
Circulating tumor DNA-guided treatment with pertuzumab plus
trastuzumab for HER2-amplified metastatic colorectal cancer: A
phase 2 trial. Nat Med. 27:1899–1903. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zumwalt TJ and Goel A: Immunotherapy of
metastatic colorectal cancer: Prevailing challenges and new
perspectives. Curr Colorectal Cancer Rep. 11:125–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valtorta E, Martino C, Sartore-Bianchi A,
Penaullt-Llorca F, Viale G, Risio M, Rugge M, Grigioni W,
Bencardino K, Lonardi S, et al: Assessment of a HER2 scoring system
for colorectal cancer: Results from a validation study. Mod Pathol.
28:1481–1491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujii S, Magliocco AM, Kim J, Okamoto W,
Kim JE, Sawada K, Nakamura Y, Kopetz S, Park WY, Tsuchihara K, et
al: International harmonization of provisional diagnostic criteria
for ERBB2-amplified metastatic colorectal cancer allowing for
screening by next-generation sequencing panel. JCO Precis Oncol.
4:6–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada Y, Yagi R, Kameyama H, Nagahashi
M, Ichikawa H, Tajima Y, Okamura T, Nakano M, Nakano M, Sato Y, et
al: Utility of comprehensive genomic sequencing for detecting
HER2-positive colorectal cancer. Hum Pathol. 66:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siravegna G, Sartore-Bianchi A, Nagy RJ,
Raghav K, Odegaard JI, Lanman RB, Trusolino L, Marsoni S, Siena S
and Bardelli A: Plasma HER2 (ERBB2) copy number predicts response
to HER2-targeted therapy in metastatic colorectal cancer. Clin
Cancer Res. 25:3046–3053. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takegawa N, Yonesaka K, Sakai K, Ueda H,
Watanabe S, Nonagase Y, Okuno T, Takeda M, Maenishi O, Tsurutani J,
et al: HER2 genomic amplification in circulating tumor DNA from
patients with cetuximab-resistant colorectal cancer. Oncotarget.
7:3453–3460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh H, Kang A, Bloudek L, Hsu LI,
Corinna Palanca-Wessels M, Stecher M, Siadak M and Ng K: Systematic
literature review and meta-analysis of HER2 amplification,
overexpression, and positivity in colorectal cancer. JNCI Cancer
Spectr. 8:pkad0822024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benson AB, Venook AP, Adam M, Chang G,
Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna
I, et al: Colon cancer, version 3.2024, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 22:e2400292024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang FH, Zhang XT, Tang L, Wu Q, Cai MY,
Li YF, Qu XJ, Qiu H, Zhang YJ, Ying JE, et al: The Chinese society
of clinical oncology (CSCO): Clinical guidelines for the diagnosis
and treatment of gastric cancer, 2023. Cancer Commun (Lond).
44:127–172. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakamura Y, Sawada K, Fujii S and Yoshino
T: HER2-targeted therapy should be shifted towards an earlier line
for patients with anti-EGFR-therapy naïve, HER2-amplified
metastatic colorectal cancer. ESMO Open. 4:e0005302019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sartore-Bianchi A, Trusolino L, Martino C,
Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I,
Martinelli E, et al: Dual-targeted therapy with trastuzumab and
lapatinib in treatment-refractory, KRAS codon 12/13 wild-type,
HER2-positive metastatic colorectal cancer (HERACLES): A
proof-of-concept, multicentre, open-label, phase 2 trial. Lancet
Oncol. 17:738–746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sartore-Bianchi A, Lonardi S, Martino C,
Fenocchio E, Tosi F, Ghezzi S, Leone F, Bergamo F, Zagonel V,
Ciardiello F, et al: Pertuzumab and trastuzumab emtansine in
patients with HER2-amplified metastatic colorectal cancer: The
phase II HERACLES-B trial. ESMO Open. 5:e0009112020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siena S, Di Bartolomeo M, Raghav K,
Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Fakih
M, Elez E, et al: Trastuzumab deruxtecan (DS-8201) in patients with
HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A
multicentre, open-label, phase 2 trial. Lancet Oncol. 22:779–789.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Raghav K, Siena S, Takashima A, Kato T,
Van den Eynde M, Pietrantonio F, Komatsu Y, Kawakami H, Peeters M,
Andre T, et al: Trastuzumab deruxtecan in patients with
HER2-positive advanced colorectal cancer (DESTINY-CRC02): Primary
results from a multicentre, randomised, phase 2 trial. Lancet
Oncol. 25:1147–1162. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Long HD, Lin YE, Zhang JJ, Zhong WZ and
Zheng RN: Risk of congestive heart failure in early breast cancer
patients undergoing adjuvant treatment with trastuzumab: A
meta-analysis. Oncologist. 21:547–554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suter TM and Ewer MS: Cancer drugs and the
heart: Importance and management. Eur Heart J. 34:1102–1111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Swain SM, Nishino M, Lancaster LH, Li BT,
Nicholson AG, Bartholmai BJ, Naidoo J, Schumacher-Wulf E, Shitara
K, Tsurutani J, et al: Multidisciplinary clinical guidance on
trastuzumab deruxtecan (T-DXd)-related interstitial lung
disease/pneumonitis-Focus on proactive monitoring, diagnosis, and
management. Cancer Treat Rev. 106:1023782022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Armenian SH, Lacchetti C, Barac A, Carver
J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M,
et al: Prevention and monitoring of cardiac dysfunction in
survivors of adult cancers: American society of clinical oncology
clinical practice guideline. J Clin Oncol. 35:893–911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Bailey J,
Burstein HJ, et al: Breast cancer, version 3.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:331–357. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Edoardo C and Giuseppe C:
Trastuzumab-deruxtecan in solid tumors with HER2 alterations: From
early phase development to the first agnostic approval of an
antibody-drug conjugate. Expert Opin Investig Drugs. 33:851–865.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao M, Jiang T, Li P, Zhang J, Xu K and
Ren T: Efficacy and safety of HER2-targeted inhibitors for
metastatic colorectal cancer with HER2-amplified: A meta-analysis.
Pharmacol Res. 182:1063302022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Siravegna G, Lazzari L, Crisafulli G,
Sartore-Bianchi A, Mussolin B, Cassingena A, Martino C, Lanman RB,
Nagy RJ, Fairclough S, et al: Radiologic and genomic evolution of
individual metastases during HER2 blockade in colorectal cancer.
Cancer Cell. 34:148–162.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang N, Cao Y, Si C, Shao P, Su G, Wang K,
Bao J and Yang L: Emerging role of ERBB2 in targeted therapy for
metastatic colorectal cancer: Signaling pathways to therapeutic
strategies. Cancers (Basel). 14:51602022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lazzari L, Corti G, Picco G, Isella C,
Montone M, Arcella P, Durinikova E, Zanella ER, Novara L, Barbosa
F, et al: Patient-derived xenografts and matched cell lines
identify pharmacogenomic vulnerabilities in colorectal cancer. Clin
Cancer Res. 25:6243–6259. 2019. View Article : Google Scholar : PubMed/NCBI
|