Introduction
Sarcopenia is an age-related syndrome characterized
by a progressive and generalized loss of skeletal muscle mass,
strength and function. It is associated with various adverse
clinical outcomes, including fracture, falls and mortality
(1–3). Sarcopenia is particularly prevalent
among elderly patients with cancer (4,5) and
can occur across all types of cancer. Studies indicate that cancer
patients with low skeletal muscle mass during treatment face
increased risks of mortality, cancer recurrence and lower quality
of life (6–8). A systematic meta-analysis of 39
studies involving 8,966 cancer patients revealed an overall pooled
sarcopenia prevalence of 42% [95% confidence interval (CI)
0.36–0.48; P<0.001], with substantial heterogeneity across
cancer subtypes (8). Consistent
with these findings, a review revealed a particularly high
prevalence of sarcopenia (approaching 70%) in patients with
esophageal, gastrointestinal, lung, head and neck malignancies,
compared with a moderate prevalence (~50%) in those with breast,
renal cell and hematologic cancers (9). These tumor-specific prevalence
patterns exhibited correlations with disease stage, progression
kinetics and the severity of nutritional and absorptive
dysfunction. Although the pathogenesis of sarcopenia is not well
understood, early identification of at-risk patients and timely
intervention can markedly reduce the risk of adverse clinical
outcomes.
The diagnosis of sarcopenia requires assessing
muscle mass, muscle strength and physical performance (10). The assessment of muscle mass is a
critical component in the clinical diagnosis of sarcopenia
(1,11). Currently, the measurement of the
cross-sectional area (CSA) of the third lumbar vertebra (L3) using
computed tomography (CT) or magnetic resonance imaging (MRI) is
considered a reliable method for assessing muscle mass. However,
taking into account the expense, convenience, radiation exposure
and equipment availability, various alternative methods have been
developed, such as calf circumference (CC), mid-upper arm
circumference and surrogate vertebral measurements. The present
review summarized current methods for measuring skeletal muscle
mass, aiming to early identify potential sarcopenia cases and
facilitate timely intervention.
Methodology
Literature search strategy
A systematic literature search was performed across
three principal databases: PubMed/Medline (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(https://webofscience.clarivate.cn)
and China National Knowledge Infrastructure (CNKI, http://www.cnki.net), between January 2010 and
February 2024. The search strategy used Medical Subject Headings
(MeSH) terminology with Boolean operators, structured as
follows:
Pathology Concepts: sarcopenia OR ‘skeletal muscle’
OR ‘muscle wasting’ OR ‘muscle atrophy’ OR ‘body composition’ OR
‘muscle mass’ OR ‘cachexia’ OR ‘muscle weakness’
Measuring Methods: ‘calf circumference (CC)’ OR
‘mid-arm muscle circumference (MAMC)’ OR ‘Yubi-wakka test’ OR
‘bioimpedance analysis (BIA)’ OR ‘computed tomography (CT)’ OR
‘magnetic resonance imaging (MRI)’ OR ‘dual-energy X-ray
absorptiometry (DXA)’ OR ‘ultrasound (US)’
The search syntax employed Boolean AND operators
between these two conceptual groups. The study selection workflow
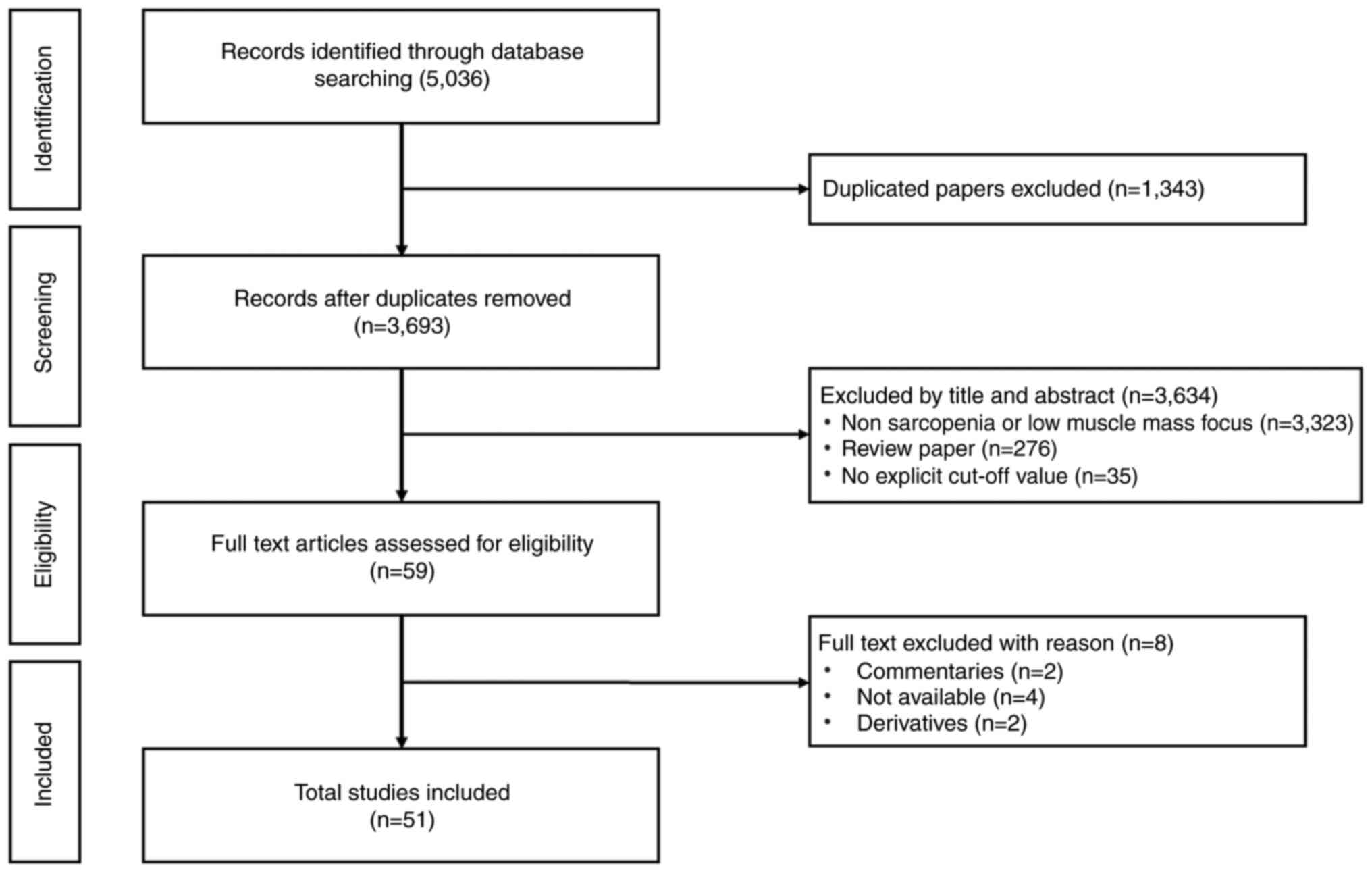
is schematically presented in Fig.
1.
Eligibility criteria
Inclusion criteria
Eligible studies met the following criteria:
Investigation of skeletal muscle mass assessment in sarcopenia
populations, with particular emphasis on cancer patients or
geriatric cohorts; employment of at least one validated measurement
modality for muscle mass evaluation, including, but not limited to,
anthropometric measurements, medical imaging techniques
(CT/MRI/DXA), or BIA; and peer-reviewed original research articles
published in English or Chinese.
Exclusion criteria
Studies were excluded based on the following
considerations: Case reports, conference abstracts, commentaries,
or non-peer-reviewed publications; articles lacking standardized
diagnostic thresholds for low muscle mass determination; duplicate
publications or studies with substantial cohort overlap.
Study selection and flowchart
The literature screening process followed the PRISMA
guidelines (Fig. 1). Initial
searches identified 5,036 records. After removing duplicates
(n=1,343), 3,693 titles/abstracts were screened. Of these, 3,323
were excluded for irrelevance (non-sarcopenia or low muscle mass
focus). A full-text review of 276 articles led to the exclusion of
142 studies (due to insufficient data or non-quantitative methods).
Ultimately, 51 studies were included for the present review.
Data extraction
A total of two independent reviewers extracted data
using a standardized form, including: Study characteristics
(author, year, design); population (sample size, age, cancer type);
muscle mass assessment methods and cut-off values.
Anthropometry
Calf circumference (CC)
CC serves as a straightforward and convenient
indicator for assessing muscle mass, playing a crucial role in
determining sarcopenia cut-off values (12–14).
The Asian Working Group for Sarcopenia (AWGS) 2019 consensus
updated the diagnosis criteria for sarcopenia and recommended CC as
an effective screening tool in community (15–17).
Compared with commonly used methods for assessing muscle mass, CC
shows a positive association with muscle mass measured by BIA and
DXA, regardless of age or body fat percentage (18). Özcan et al (19) reported a positive association
between CC and low muscle mass in patients receiving maintenance
hemodialysis. Numerous studies have investigated the relationship
between CC and low muscle mass, establishing frequently utilized
cut-off values of <34 cm for males and <33 cm for females, as
detailed in Table I.
 | Table I.Summary of research on CC cut-off
values for predicting low skeletal muscle mass. |
Table I.
Summary of research on CC cut-off
values for predicting low skeletal muscle mass.
| First author/s,
year | Population | Country/Region | Position | Comparing | Criteria for
cut-off point | Cut-off for CC
(cm) | (Refs.) |
|---|
| Champaiboon et
al, 2023 | 6,404 older adults
(≥60 years) | Thailand | Sitting | BIA | AWGS | ♀<33, ♂<34
BMI ≥25:♀<34, ♂<35 older-old adults ≥75 years: ♀<33,
♂<34 | (13) |
| Fernandes et
al, 2022 | 796 older adults
(≥60 years) | Southeast region of
Brazil | Standing | GAMs | WHO (1995) Physical
Status: The Use and Interpretation of Anthropometry. WHO technical
report series; 854 Geneva, Switzerland | <34.5
(mortality) | (81) |
| Gonzalez et
al, 2021 | 3,104 participants
aged 18–39 years and with normal BMI (18.5–24.9) | US population | Siting | - | 1 and 2 SDs below
the mean | Moderately low
values ♂ ≤34, ♀ ≤33 severely low CC values: ♂≤32, ♀≤ 31 adjusted
BMI: adding 4 cm (BMI <18.5) or subtracting 3,7, or 12 cm (BMI
25–29, 30–39, and ≥40, respectively) | (12) |
| Xu et al,
2020 | 7,311 cases of
Chinese elderly hospitalized population aged >70 years | China | Supine | - | GLIM Criteria | ♂≤29.6, ♂≤27.5 | (20) |
| Kawakami et
al, 2020 | 1,239 adults ≥40
years | Japan | Standing | DEXA/BIA | AWGS | ♂<35 (BIA)/36
(DXA), ♀<33 (BIA)/34 (DXA) | (18) |
| Kim et al,
2018 | 657 older aged
(70–84 years) | Koreans | Standing | DXEA | AWGS | ♂<35, ♀<33 in
community-dwelling Korean elders | (64) |
| Pagotto et
al, 2018 | 132 elderly
people | Brazil | Standing | DEXA | LMM: ♂:7.26
kg/m2, ♀5.45 kg/m2 | ♂<34,
♀<33 | (82) |
| Maeda et al,
2017 | 1,164 hospitalized,
elderly adults | Japan | Supine | BIA | AWGS | ♀≤ 29, ♂≤30 | (83) |
| Barbosa-Silva et
al, 2016 | 1,291
community-dwelling elderly aged 60 y or over | Brazil | Standing | DEXA | EWGSOP2 | ♂≤34, ♀≤33 | (69) |
| Kawakami et
al, 2015 | 40~89 y adults | Japan | Standing | DEXA | AWGS | ♂<34,
♀<33 | (84) |
Xu et al conducted two consecutive studies
involving 7,311 hospitalized elderly patients aged >70 years in
China, using a prospective multi-center database established by the
authors. In the first study, the critical value of CC was
determined using the receiver operating characteristic curve
method, with in-hospital mortality as the primary outcome. This
value was further validated using clinical and financial metrics.
In the second study, three screening tools, NRS 2002, NA-SF and
MUST, were employed for risk assessment. Malnutrition was diagnosed
based on the GLIM criteria, incorporating the newly identified CC
values. The optimal cut-off for CC was determined to be 29.6 cm for
men and 27.5 cm for women (20).
The measurement of CC can be influenced by body type
and positioning. Studies have shown that CC tends to be markedly
higher in individuals with sedentary lifestyles (21). Furthermore, CC measurements vary
across regions, populations and levels of physical activity
intensity. These variations lead to differences in CC cut-off
values for predicting low muscle mass. Therefore, it is essential
to consider the effect of body type and positioning when measuring
CC. Additionally, the most suitable criteria for sarcopenia
screening using CC should be explored across diverse countries,
regions and populations.
Mid-arm muscle circumference
(MAMC)
A study assessed muscle mass in aging individuals
using MAMC within the community, suggesting MAMC as a valid tool
for evaluating nutrition and muscle mass in elderly adults
(22). Carnevale et al
(23) compared DXA and MAMC for
muscle mass assessment, showing that MAMC is a practical screening
tool for elderly patients and more effective in detecting low
muscle mass. Gort-van Dijk et al (24) conclude that MAMC is more specific
but less sensitive for assessing muscle mass. Despite these
considerations, the convenience of MAMC makes it a recommended tool
for screening decreased muscle mass in older populations (24). Notably, MAMC may be less suitable
for subjects with edema (lymphedema or generalized extreme edema)
or extreme obesity.
The cut-off values for determining low muscle mass
using MAMC vary across studies and populations. Carnevale et
al (23) established cut-off
values of <11.0 cm for males and <10.3 cm for females in
healthy young individuals. For older adults, the cut-offs were
<22.3 cm for males and <18.6 cm for females. Gort-van Dijk
et al (24) define low
muscle mass as falling below the 10th percentile of MAMC cut-off
values. However, there is no standardized cut-off value for MAMC to
define low muscle mass. Further research is needed to establish its
applicability across different age groups and to determine
appropriate cut-off values.
Yubi-wakka (finger-ring) test
The finger-ring measurement (Yubi-wakka) is a
self-testing tool developed by Tanaka et al (25) in 2018 to assess the risk of
sarcopenia among community-dwelling older adults. Participants
compared the circumference of their own hands with their CC. A
larger CC indicated no sarcopenia risk, while equal measurements
suggested potential early-stage sarcopenia. A smaller CC indicated
sarcopenia risk necessitating further evaluation and intervention.
Tanaka et al (25) verified
the Yubi-wakka test in a study of 1,904 older adults (≥65 years),
finding 53% in the larger CC group, 33% in the equal group and 14%
in the smaller CC group. Multiple-factor analysis revealed that,
compared with the larger CC group, the equal and smaller CC groups
were more likely to develop sarcopenia (OR=2.4, 95% CI 1.4–4.1;
OR=6.6, 95% CI 3.5–13, respectively). Lawongsa et al
(26) replicated these findings in
a study of Thai older adults, affirming the high sensitivity and
specificity of the Yubi-wakka test for sarcopenia risk
identification. At present, the Yubi-wakka test is primarily
validated in Asian populations (27–29).
Further research is needed to explore its applicability and
validity across diverse ethnic groups.
Computed tomography (CT) and magnetic
resonance imaging (MRI)
CT and MRI are widely used in clinical practice to
assess muscle mass (30–32). CT can be targeted to show different
body components by setting different thresholds, where the
threshold for muscle tissue is −29~150 Hounsfield units (33,34).
MRI relies on instrument-based marking or manual labeling of muscle
tissue for accurate measurement (30). CT or MRI assessment of muscle mass
usually involves measuring the skeletal muscle CSA from a single
image. This value is then adjusted for height to calculate the
skeletal muscle index (SMI, cm/m2) (35). Therefore, muscle mass measurements
using CT or MRI require both the selection of experienced staff to
circle the muscle area and, more importantly, the selection of
appropriate and typical vertebrae to measure the CSA of the muscle.
In addition to measuring skeletal muscle CSA for MRI, advanced MRI
sequences can also enable the estimation of body composition in
combination with the assessment of muscle mass abnormalities. This
combined approach enhances the diagnostic accuracy of sarcopenia in
aging patients (36). Xiao et
al (35) show that CT-based
diagnosis of sarcopenia can help to predict clinical outcomes and
prognosis in surgical patients and illustrate that CT is intuitive,
rapid and accurate in the diagnosis of sarcopenia. Beyond CSA
measurement, CT can assess muscle steatosis by measuring
intermuscular adipose tissue area or identifying muscle mass loss
(37). Despite their advantages in
sarcopenia diagnosis, CT and MRI are not widely used for screening
due to their high cost, time consumption and ionizing radiation (in
the case of CT) and the need for specialized technical expertise
(35,36).
The third lumbar vertebra (L3)
The third lumbar vertebra (L3) is currently the most
commonly used for measuring the SMI to assess whole-body muscle
mass, given its strong correlation with whole-body musculature
(r=0.86 ~0.94; P<0.001) (38).
This method is considered the gold standard for diagnosis of low
muscle mass and sarcopenia (39).
Prado et al (40) measured
the L3 SMI in patients with respiratory and gastrointestinal tumors
to assess muscle mass. Martin et al (41) defined the cut-off value for
sarcopenia as SMI <52.4 cm2/m2 in men and
SMI <38.5 cm2/m2 in women. The L3 SMI
cut-off values for assessing whole-body muscle mass range from
40.2–55.0 cm2/m2 in men and 30.0–43.23
cm2/m2 in women, respectively, as shown in
Table II.
 | Table II.Summary of research on measuring
muscle mass at L3 using computerized tomography. |
Table II.
Summary of research on measuring
muscle mass at L3 using computerized tomography.
| First author/s,
year | Population | Software | Criteria for
cut-off point | Cutoff for Low SMI
(cm2/m2) | (Refs.) |
|---|
| Sahin et al,
2023 | 162 stomach cancer
patients, aged ≥18 years, Turkey | 3D Slicer (version
4.10.2) (Slicer) | - | ♂52.4; ♀38.5 | (85) |
| Sealy et al,
2020 | 213 head and neck
cancer patients, the Netherlands | Slice-O-Matic V5.0
(TomoVision) | Tertiles in the
cohort | ♂53.4; ♀43.23 | (86) |
| Van der Kroft et
al, 2020 | 180 patients with
Colorectal cancer, Germany | Slice-O-Matic V5.0
(TomoVision) | At the lowest
tertile | ♂46.6; ♀36.8 | (87) |
| Li et al,
2019 | 153 gastric cancer
patients, China | Slice-O-Matic V5.0
(TomoVision) | Optimum
stratification | ♂40.2; ♀30.0 | (88) |
|
Blauwhoff-Buskermolen et al,
2017 | 241 cancer
patients, aged ≥18 years, the Netherlands | Slice-O-Matic V5.0
(TomoVision) | - | ♂55.0; ♀39.0 | (89) |
By analyzing the relationship between SMI and
prognosis in ovarian cancer patients, Jin et al (42) found significant variability in the
cut-off values for low muscle mass among female patients. Commonly
used SMI cut-off values ranged from 38.5–41.0
cm2/m2, with 38.5 and 41.5
cm2/m2 being the most frequently applied
thresholds. The authors suggested that there was a large
heterogeneity in the cut-off values for low muscle mass,
potentially due to variations in methodology or population
characteristics. As a clinical reference standard for assessing low
SMI, the use of L3 needs further refinement to establish a robust
diagnostic criterion. Considering that most of the patients do not
routinely undergo CT scanning of the abdomen in clinical practice,
performing such examinations solely for sarcopenia diagnosis would
increase radiation exposure and assessment costs. In such case,
researchers have tried to measure the CSA of other non-L3 vertebrae
to evaluate muscle mass (43).
Thus, it may be a cost-effective alternative measurement of
skeletal muscle mass in screening of sarcopenia, improving patient
prognosis without increasing additional economic and radiation
burdens.
The third cervical vertebra (C3)
Patients with head and neck tumors have a higher
risk of developing sarcopenia (44), but abdominal CT scanning is not
routinely performed in this population. Thus, some researchers are
exploring whether the C3 could serve as an alternative for
screening low muscle mass in these patients. This approach could
facilitate timely treatment, such as nutritional support or
physical therapy and improve prognosis.
By measuring C3 and L3 muscle mass with CT in 51
trauma and 52 head and neck tumors patients, Swartz et al
(44) found a strong correlation
between L3 and C3 (r=0.891). The authors demonstrate that it is
feasible to use the head and neck CT to evaluate muscle mass in
head and neck tumors patients. This method also serves as a viable
alternative to abdominal CT for measuring L3 muscle mass. Jung
et al (45) also advocate
the use of C3 CSA measured by head and neck CT to estimate L3
muscle mass in patients with head and neck tumors and predict
overall survival. Meerkerk et al (30) used C3 muscle mass measurements to
predict frailty in patients with head and neck tumors. The authors'
findings showed that low SMI and sarcopenia are associated with
frailty in older patients. Additionally, a retrospective cohort
study has demonstrated a correlation between C3 muscle mass and
prognosis in oral cancer patients, suggesting its potential as an
imaging marker for predicting outcomes (46).
However, some researchers doubt whether the CSA
measured by C3 can accurately predict the CSA of L3. Yoon et
al (34) concluded that in
patients with head and neck tumors complicated with sarcopenia, the
muscle mass measured by C3 did not strongly correlate with that at
L3 (r=0.381). This discrepancy is probably due to the fact that the
sternocleidomastoid muscle at C3 was excluded from the analysis due
to tumor invasion, leading to variability in the results between
studies. Therefore, further research is needed to explore the
feasibility of using C3 as a substitute for L3 in evaluating
systemic muscle mass in patients' head and neck tumors. Key
questions include how to measure C3 muscle mass, which muscles
should be included and correlation between C3 and L3 muscle mass as
well as the determination of a cut-off value for low muscle mass
based on C3.
The fourth thoracic vertebra
(Th4)
The Th4 is commonly used to assess muscle mass in
patients undergoing chest CT scans for lung cancer and breast
cancer. Gronberg et al (47)
compared the concordance between Th4-measured SMI and L3-measured
SMI and showed that there was only moderate concordance between
them (r2=0.51 in men and r2=0.28 in women),
indicating that Th4 cannot yet replace L3 to assess low muscle
mass. Neefjes et al (48)
also explored the use of Th4 for assessing low muscle mass and the
findings suggested that further work is needed to validate the
reliability of Th4 for this purpose. The current studies on the
cut-off value of Th4 for assessing low muscle mass are summarized
in Table III.
 | Table III.Summary of research on measuring
muscle mass at Th4 using computerized tomography. |
Table III.
Summary of research on measuring
muscle mass at Th4 using computerized tomography.
| First author/s,
year | Population | Software | Criteria for
cut-off point/muscle area | Cut-off for low SMI
(cm2/m2) | (Refs.) |
|---|
| Tao et al,
2023 | 750 NSCLC patients,
China | Simens syngo via
VB20 (Siemens Healthineers) | X-tile
software/bilateral pectoralis major, pectoralis minor muscles | ♂ 18.1; ♀ 14.7 | (90) |
| Sealy et al,
2020 | 213 head and neck
cancer patients, the Netherlands | Slice-O-Matic V5.0
(TomoVision) | Tertiles in the
cohort/Total muscle | ♂69.45; ♀52.88 | (86) |
| Van der Kroft et
al, 2020 | 180 patients with
Colorectal cancer, Germany | Slice-O-Matic V5.0
(TomoVision) | The lowest
tertile/Total muscle | ♂65.2; ♀51.9 | (87) |
|
Blauwhoff-Buskermolen et al,
2017 | 241 cancer
patients, aged ≥18 years, the Netherlands | Slice-O-Matic V5.0
(TomoVision) | -/Total muscle | ♂66.0; ♀51.9 | (89) |
According to the current research results, the
cut-off values of Th4 in evaluating low muscle mass and the muscle
measurement area still are not standardized. Therefore, further
research is needed for the adoption of Th4 instead of L3 to
evaluate low muscle mass.
The twelfth thoracic vertebra
(Th12)
Chest CT scans do not cover the L3 region, as that
would increase both the financial burden and radiation exposure for
patients who only need a chest CT. It is worth exploring how to
diagnose sarcopenia without additional CT scans. Some researchers
have attempted to use Th10, Th12 and other thoracic vertebrae for
muscle mass assessment.
In a study by Matsuyama et al (49) Th12 muscle mass was measured using
chest CT in patients with progressive oral squamous cell carcinoma.
This value was compared with L3 muscle mass and the results showed
that SMI measured at Th12 using chest CT can be used to diagnose
sarcopenia and has predictive value for postoperative outcomes in
these patients. In conclusion, current evidence is insufficient to
support the use of Th10 and Th12 as alternatives to L3 for muscle
mass measurement and more studies are needed.
Masticatory muscle
Masticatory muscles reflect the chewing and
swallowing function of patients and are closely related to their
nutritional status. Studies have shown that the mass of masticatory
muscles is closely related to patients' grip strength, walking
speed and physical activity (50,51).
This suggests that masticatory muscles could serve as a new site
for muscle mass measurement. Hwang et al (52) retrospectively analyzed 314 patients
in emergency department and confirmed a significant association
between masticatory muscle mass and systemic nutritional
biomarkers. These findings suggest that masticatory muscle mass is
a potential indicator of nutritional status, physical activity
levels and trauma-related prognosis (53).
Chang et al (53) attempted to measure the muscle mass
of the masticatory muscles in patients with head and neck cancers
to explore its relationship with L3 muscle mass, specifically at
the level of the mandibular notch. The authors found a strong
correlation between masticatory muscle mass and L3 muscle mass
(r=0.901). The study also suggested that C3 muscle mass
measurements may be unreliable in patients with head and neck
tumors involving lymphatic metastasis or severe muscle tissue
invasion. Thus, measuring masticatory muscle mass might be a useful
alternative. However, it should be noted that the number of studies
on masticatory muscle for assessing low muscle mass is insufficient
to support its validity as an alternative to L3.
Bilateral mid-thigh muscle area
(TMA)
TMA has also been used to assess muscle mass. It
provides more accurate evaluation of overall skeletal muscle mass
and is highly sensitive to changes. The 2019 European Working Group
on Sarcopenia in Older People (EWGSOP) guideline highlighted that
mid-thigh muscle mass correlates more strongly with whole-body
muscle mass than L1-L5 measurements (10). Tsai et al (54) found a strong correlation between TMA
and abdominal muscle area (AMA) while investigating the
relationship between lower extremity muscle mass and vascular
stenosis in patients with peripheral arterial disease. This
suggests that TMA could be another effective method for assessing
muscle mass and future research in this area is warranted.
Clinical ultrasound (US)
US enables evaluation of muscle echotexture by
detecting variations in intramuscular fat and connective tissue
composition. Recently, the Sarcopenia Group of the European
Geriatric Society published a consensus protocol for the detection
of sarcopenia using ultrasound, including the measurements of CSA,
muscle thickness, echo intensity, muscle wing angle and muscle
bundle length (55). While the
quadriceps remain the most studied muscle group, uncertainty
persists regarding optimal anatomical measurement sites for
assessing total skeletal muscle mass. Perkisas et al
(56) concluded that muscle mass
decreases before the number of muscle fibers is lost, which could
be a reliable indicator of early signs of sarcopenia in the
elderly. Although human errors in the interpretation of ultrasound
results, might affect the formulation of unified diagnostic
criteria, ultrasound still has the potential to become a tool for
early screening and clinical diagnosis of sarcopenia due to its
advantages of being non-invasive, painless, non-ionizing radiation
and low-cost.
Dual energy X-ray absorptiometry (DXA)
DXA is currently widely used in body composition
measurement due to its accuracy and repeatability. DXA enables
simultaneous quantification of fat mass, appendicular skeletal
muscle mass (ASM), fat-free mass (FFM) and bone mineral content.
Studies demonstrate strong concordance between DXA-derived
non-fat/adipose tissue measurements and corresponding values
obtained via CT or MRI (1,57). ASMI measured by DXA varied in
different study populations, with cut-off values ranging from 5.86
kg/m2−7.40 kg/m2 in males and from 4.42
kg/m2−5.67 kg/m2 in females (58). Based on available studies, EWGSOP2
has established the cut-off value for DXA to assess low muscle mass
as ASMI <7.0 kg/m2 for males and <5.5
kg/m2 for females (59).
The AWGS has established DXA cut-off values for assessing low
muscle mass as ASMI <7.0 kg/m2 for males and <5.7
kg/m2 for females (60).
Nevertheless, DXA is unable to assess intramuscular
fat infiltration (myosteatosis), which limits muscle quality
evaluation. Furthermore, DXA measurements can be inaccurate due to
variations in body thickness, fluid retention conditions (e.g.,
renal/hepatic dysfunction) and fluctuations in hydration
status.
Bioimpedance analysis (BIA)
BIA is widely used for body composition assessment,
indirectly calculating muscle mass using specific formulas. Cheng
et al (57) demonstrated
that BIA initially overestimates muscle mass but aligns closely
with DXA measurements after adjustment, making it a rapid and
reliable tool for sarcopenia screening. Compared with DXA, BIA is a
simple measurement method with the advantages of being
non-invasive, radiation-free and fast, making it an improved better
choice for muscle mass screening of sarcopenia.
Although the AWGS2019 diagnostic criteria for
sarcopenia define low muscle mass as muscle mass measured by BIA
<7.0 kg/m2 for males and <5.7 kg/m2 for
females (61), there is no
diagnostic criterion for low muscle mass determined by BIA in
EWGSOP2. This may be due to the heterogeneity of low muscle mass
cut-off values among different ethnicities. Therefore, the cut-off
values for low muscle mass range from 7.4 kg/m2−9.31
kg/m2 for males and from 5.14 kg/m2−7.4
kg/m2 for females, as shown in Table IV.
 | Table IV.Summary of research on BIA. |
Table IV.
Summary of research on BIA.
| First author/s,
year | Population | BIA instrument | Criteria for
cut-off point | Cut-off for low SMI
(kg/m2) | (Refs.) |
|---|
| Pineda-Zuluaga
et al, 2023 | 237 people, older
than 60 years, Colombia | Hydra 4200 Xitron
Technologies | −2 SD of the mean
value of Colombia older adults | ♂8.0; ♀6.1 | (91) |
| Bulut et al,
2020 | 1,150 participants,
>60 years old, Turkey | Tanita MC-780U | −2 SD of Turkish
young adults (18 ~40 years old) | ♂8.33; ♀5.7 | (92) |
| Björkman et
al, 2019 | 428 healthy people,
>75 years, Finland | ImpediMed SFB7 | Age-specific median
values | ♂9.31; ♀6.9 | (93) |
| Han et al,
2016 | 878 healthy
volunteers, >65 years, China | InBody 720 | −2 SD of Chinese
young adults (20~40 years old) | ♂7.40; ♀5.14 | (94) |
| Bahat et al,
2016 | 301 participants,
Turkey | Tanita BC 532 | −2 SD of Turkish
young adults (18–39 years old) | ♂9.2; ♀7.4 | (95) |
The use of different evaluation instruments results
in significant differences in cut-off values because different
formulas are used to measure muscle mass. In the future, more
studies should focus on establishing a unified and standardized
instrument to explore the cut-off values for low muscle mass in
different ethnicities, for the purpose of improving the sensitivity
and specificity of sarcopenia screening. In general, BIA is a
convenient and simple tool for measuring muscle mass and can be
used in muscle mass screening for sarcopenia. However, it should be
noted that muscle mass measurements by BIA are susceptible to the
hydration status of patients, leading to inaccurate muscle mass
values. Therefore, BIA is not recommended for patients with
edema.
Discussion
Sarcopenia, a syndrome characterized by progressive
and generalized loss of skeletal muscle mass and function, has
gained recognition as a critical determinant of health in older
adults (62). Accurate assessment
of muscle mass is crucial for the diagnosis, monitoring and
management of sarcopenia, markedly influencing clinical
decision-making and patient outcomes (63). Anthropometric measurements such as
CC, mid-upper arm circumference and the Yubi-wakka test, offer
non-invasive, rapid and cost-effective approaches for sarcopenia
screening (15). These methods are
particularly valuable in both in community and hospital settings,
enabling widespread screening initiatives (61). However, while the Yubi-wakka test
shows good applicability in Asian populations, further validation
in diverse ethnic groups is needed to ensure its global
applicability (64). BIA is a
widely used tool for assessing body composition, including muscle
mass, owing to its ease of use and non-invasive nature. However,
variability in BIA devices and methodologies can affect the
accuracy and reliability of measurements, highlighting the need for
standardization in sarcopenia diagnosis (65,66).
Cut-off values for BIA in sarcopenia screening exhibit variability,
with suggested thresholds being <7.0 kg/m2 for men
and <5.5 or 5.7 kg/m2 for women (61). The EWGSOP recommends DXA for muscle
mass assessment (39), due to its
precision and ability to evaluate regional lean mass. However, its
high cost and limited availability may restrict its use in routine
clinical practice (67). The
cut-off values for DXA in sarcopenia diagnosis are <7.0
kg/m2 for men and <5.4 kg/m2 for women.
However, these thresholds may not be universally applicable across
diverse populations (68). Imaging
techniques such as CT and MRI provide detailed visualization of
muscle mass and quality, making them valuable tools in sarcopenia
research and clinical practice (31). The use of skeletal muscle mass
measured at the L3 level serves as a common reference standard.
However, the lack of standardized cut-off values and variability in
the selection of vertebral levels and muscles for assessment pose
significant challenges (69).
Future research should prioritize the establishment of standardized
criteria to enable more consistent diagnosis and monitoring of
sarcopenia (70–72). US is emerging as a promising tool
for sarcopenia assessment, offering a non-invasive, portable and
repeatable method to evaluate muscle mass and quality (25). Its potential for real-time imaging
and the absence of radiation exposure represent significant
advantages. However, US results are operator-dependent and further
research is required to establish its reliability and validity in
sarcopenia assessment (55).
Despite the variety of available methods, several challenges
persist, such as the lack of standardized cut-off values across
methodologies, the reliance on equipment that may not be
universally accessible and the dependence on techniques requiring
specialized training (63). The
comparative merits and drawbacks of these measurement approaches
are detailed in Table V.
Additionally, the heterogeneity in sarcopenia presentation across
diverse populations underscores the need for more inclusive
research to ensure diagnostic accuracy (73). Given the limitations of individual
methods, a combined approach may offer a more comprehensive
assessment of sarcopenia. Integrating anthropometric measures with
BIA or imaging techniques can yield a more accurate representation
of muscle mass and function (58,74).
Future research should explore the integration of these methods to
improve diagnostic accuracy and enhance clinical applicability. The
field of sarcopenia assessment is rapidly evolving, necessitating
innovative approaches that balance accuracy with practicality for
widespread implementation. The development of emerging
technologies, such as advanced imaging software and machine
learning algorithms, holds promise for delivering more precise and
accessible methods for sarcopenia diagnosis (75). Furthermore, incorporation of
biomarkers and genetic factors into the assessment framework could
enable a more comprehensive understanding of sarcopenia (76).
 | Table V.Comparative analysis of body
composition assessment modalities for sarcopenia evaluation. |
Table V.
Comparative analysis of body
composition assessment modalities for sarcopenia evaluation.
| Methods | Strengths | Limitations |
|---|
| CT | • Quantitative gold
standard | • Radiation
burden |
|
| • Widely
available | • Threshold
variability |
|
| • Validated
prognostic value | • Cost prohibitive
for serial monitoring |
| MRI | • Superior soft
tissue resolution | • Limited
availability |
|
| • Multi-parametric
tissue characterization | • Lengthy
acquisition |
|
| • No ionizing
radiation | • Motion
artifacts |
| DXA | • Established
reference ranges | • Hydration status
confounding |
|
| • Low radiation
dose | • Regional
variability |
|
| • Rapid
acquisition | • Limited quality
assessment |
|
| • Widely
available |
|
| US | • Point-of-care
application | • Operator
expertise required |
|
| • Dynamic muscle
evaluation | • Limited
reproducibility |
|
| •
Cost-effective | • Depth
constraints |
| Anthropometry | • Universally
accessible | • Insensitive to
early changes |
|
| • Minimal training
required | • Body habitus
confounding |
|
| • Low-cost
screening | • Qualitative
only |
Sarcopenia is a prevalent condition among cancer
patients, with significant implications for clinical outcomes.
Current evidence establishes CT as the most widely adopted and
clinically validated modality for body composition assessment in
this population. The systematic review by McGovern et al
(77) highlights that low SMI and
skeletal muscle density, as assessed by CT, are prevalent across
malignancies, independent of disease stage. However, the review
underscores the lack of standardized thresholds for defining
sarcopenia in oncology, with studies employing heterogeneous
cut-offs. By contrast, Ubachs et al (78) provide compelling evidence for the
prognostic value of CT-based assessments, demonstrating significant
associations between both SMI [P=0.02; hazard ratio (HR): 1.17; 95%
CI: 1.03–1.33] and skeletal muscle radiation attenuation
(P<0.001; HR: 1.14; 95% CI: 1.08–1.20) with overall survival.
The study highlights the particular clinical utility of CT in
ovarian cancer, where ascites-related weight masking renders
anthropometric measurements unreliable for nutritional assessment.
The applicability of sarcopenia assessment varies markedly across
cancer types, with methodology selection often dictated by
anatomical considerations. Notably, studies in head and neck
cancers predominantly employ cervical skeletal muscle index (C3
SMI) measurements, whereas investigations of gastrointestinal
malignancies typically utilize lumbar skeletal muscle index (L3
SMI), reflecting site-specific clinical relevance (8). Other tools such as DXA (limited by
hydration status), MRI (costly) and US (operator-dependent) are
less robust for cancer-specific prognostication. Anthropometrics
(such as CC) are accessible but lack precision. A meta-analysis by
Zhang et al (8)
systematically compared prognostic capabilities across skeletal
muscle quantification methods, revealing that while L3 SMI remains
the preferred modality for cross-cancer applications due to its
standardized implementation, C3 SMI emerges as particularly
predictive of mortality outcomes in head and neck malignancies; a
finding that aligns with the anatomical and pathophysiological
characteristics of these tumors. However, C3 SMI limited
application in non-head/neck cancers restricts broader
generalizability. Future research should prioritize tumor-specific
diagnostic thresholds and multimodal assessment frameworks to
optimize SMI assessment in cancer patients.
Normative values and cut-off criteria for muscle
mass exhibit variations across ethnicities, sexes and age groups.
However, a number of countries, including China, currently lack
clinically validated cut-off standards and population-specific
reference values derived from rigorous methodologies. To address
this gap, future research is needed to determine benchmarks across
diverse populations using advanced technologies, large-scale
studies and high-quality methodologies. Future research should
define clinically supported indices, such as FFM index in different
ethnic groups, to determine sex- and age-specific normal ranges and
clinically supported indices for diagnosing reduced muscle
mass.
The present review presented a systematic evaluation
framework that innovatively combined both conventional and emerging
assessment modalities, thereby offering distinct methodological
advantages compared with previous works by Muraki (methodological
analysis) (79) and Li et al
(technical imaging review) (80).
The present review broadened the assessment paradigm by
incorporating low-cost clinical tools including anthropometric
measurements (such as CC) and validated self-assessment methods
(such as the Yubi-wakka test), implemented through standardized
diagnostic criteria (AWGS 2019) to enhance clinical utility across
diverse settings without compromising diagnostic accuracy.
Furthermore, the present review assessed multi-modal strategies,
such as combining BIA with anthropometry, to improve accuracy while
addressing limitations such as hydration effects or positioning
errors, often overlooked in prior studies, with particular emphasis
on population-specific validation for Asian cohorts to address
diagnostic heterogeneity and technical rigor. Notably, the present
review bridged research and practice by advocating harmonized
protocols that balance gold-standard imaging (such as L3-CT) with
scalable tools (such as US and CC), while also exploring emerging
technologies such as machine learning for muscle segmentation. By
comprehensively integrating multiple assessment approaches, our
study extends the current evaluative paradigm to improve clinical
utility.
Conclusion
While current methods provide valuable tools for
both clinical practice and research, there remains a critical need
for further standardization, validation and innovation. Future
research should focus on the development and validation of
assessment methods that are accessible, reliable and applicable
across diverse populations, improving the care and outcomes for
individuals affected by sarcopenia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Hunan Province (grant no. 2023JJ40907), the Natural
Science Foundation of Changsha (grant no. kq2208355) and the Hunan
Cancer Hospital Climb Plan (grant no. IIT2021005).
Availability of data and materials
Not applicable.
Authors' contributions
JH and CT conceived the study and contributed to the
critical revision of the manuscript. WW and ML designed the study,
collected data, prepared tables, drafted the original draft and
conducted the review and editing. QZ contributed to manuscript
drafting, prepared the tables, and reviewed the manuscript. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cruz-Jentoft AJ and Sayer AA: Sarcopenia.
Lancet. 393:2636–2646. 2019. View Article : Google Scholar
|
|
2
|
Damluji AA, Alfaraidhy M, AlHajri N,
Rohant NN, Kumar M, Al Malouf C, Bahrainy S, Ji Kwak M, Batchelor
WB, Forman DE, et al: Sarcopenia and cardiovascular diseases.
Circulation. 147:1534–1553. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sayer AA and Cruz-Jentoft A: Sarcopenia
definition, diagnosis and treatment: Consensus is growing. Age and
ageing. 51:afac2202022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang FM, Wu HF, Shi HP, Yu Z and Zhuang
CL: Sarcopenia and malignancies: Epidemiology, clinical
classification and implications. Ageing Res Rev. 91:1020572023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang P, Zhou J, Xiao X, Yang Y, Luan S,
Liang Z, Li X, Zhang H, Shang Q, Zeng X and Yuan Y: The prognostic
value of sarcopenia in oesophageal cancer: A systematic review and
meta-analysis. J Cachexia Sarcopenia Muscle. 14:3–16. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT
and Han DS: Association between loss of skeletal muscle mass and
mortality and tumor recurrence in hepatocellular carcinoma: A
systematic review and Meta-analysis. Liver Cancer. 7:90–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nipp RD, Fuchs G, El-Jawahri A, Mario J,
Troschel FM, Greer JA, Gallagher ER, Jackson VA, Kambadakone A,
Hong TS, et al: Sarcopenia is associated with quality of life and
depression in patients with advanced cancer. Oncologist. 23:97–104.
2018. View Article : Google Scholar
|
|
8
|
Zhang Y, Zhang J, Zhan Y, Pan Z, Liu Q and
Yuan W: Sarcopenia is a prognostic factor of adverse effects and
mortality in patients with tumour: A systematic review and
Meta-analysis. J Cachexia Sarcopenia Muscle. 15:2295–2310. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan S and Larsson SC: Epidemiology of
sarcopenia: Prevalence, risk factors, and consequences. Metabolism.
144:1555332023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie
Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA,
et al: Sarcopenia: Revised European consensus on definition and
diagnosis. Age Ageing. 48:6012019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dent E, Woo J, Scott D and Hoogendijk EO:
Sarcopenia measurement in research and clinical practice. Eur J
Intern Med. 90:1–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonzalez MC, Mehrnezhad A, Razaviarab N,
Barbosa-Silva TG and Heymsfield SB: Calf circumference: Cutoff
values from the NHANES 1999–2006. Am J Clin Nutr. 113:1679–1687.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Champaiboon J, Petchlorlian A, Manasvanich
BA, Ubonsutvanich N, Jitpugdee W, Kittiskulnam P, Wongwatthananart
S, Menorngwa Y, Pornsalnuwat S and Praditpornsilpa K: Calf
circumference as a screening tool for low skeletal muscle mass:
Cut-off values in independent Thai older adults. BMC Geriatr.
23:8262023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiss CM, Bertschi D, Beerli N, Berres M,
Kressig RW and Fischer AM: Calf circumference as a surrogate
indicator for detecting low muscle mass in hospitalized geriatric
patients. Aging Clin Exp Res. 36:252024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen LK, Woo J, Assantachai P, Auyeung TW,
Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al: Asian
working group for sarcopenia: 2019 consensus update on sarcopenia
diagnosis and treatment. J Am Med Dir Assoc. 21:300–307.e2. 2020.
View Article : Google Scholar
|
|
16
|
Oh MH, Shin HE, Kim KS, Won CW and Kim M:
Combinations of sarcopenia diagnostic criteria by asian working
group of sarcopenia (AWGS) 2019 guideline and incident adverse
health outcomes in community-dwelling older adults. J Am Med Dir
Assoc. 24:1185–1192. 2023. View Article : Google Scholar
|
|
17
|
Sri-On J, Fusakul Y, Kredarunsooksree T,
Paksopis T and Ruangsiri R: The prevalence and risk factors of
sarcopenia among Thai community-dwelling older adults as defined by
the Asian Working Group for Sarcopenia (AWGS-2019) criteria: A
cross-sectional study. BMC Geriatr. 22:7862022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawakami R, Miyachi M, Sawada SS, Torii S,
Midorikawa T, Tanisawa K, Ito T, Usui C, Ishii K, Suzuki K, et al:
Cut-offs for calf circumference as a screening tool for low muscle
mass: WASEDA's Health study. Geriatr Gerontol Int. 20:943–950.
2020. View Article : Google Scholar
|
|
19
|
Özcan B, Güner M, Ceylan S, Öztürk Y,
Girgin S, Okyar Baş A, Koca M, Balcı C, Doğu BB, Cankurtaran M, et
al: Calf circumference predicts sarcopenia in maintenance
hemodialysis. Nutr Clin Pract. 39:193–201. 2024.
|
|
20
|
Xu JY, Zhu MW, Zhang H, Li L, Tang PX,
Chen W and Wei JM: A Cross-Sectional study of GLIM-defined
malnutrition based on new validated calf circumference Cut-off
values and different screening tools in hospitalised patients over
70 years old. J Nutr Health Aging. 24:832–838. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rigby L, Frey M, Alexander KL and De
Carvalho D: Monitoring calf circumference: Changes during prolonged
constrained sitting. Ergonomics. 65:631–641. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Landi F, Russo A, Liperoti R, Pahor M,
Tosato M, Capoluongo E, Bernabei R and Onder G: Midarm muscle
circumference, physical performance and mortality: Results from the
aging and longevity study in the Sirente geographic area (ilSIRENTE
study). Clin Nutr. 29:441–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carnevale V, Castriotta V, Piscitelli PA,
Nieddu L, Mattera M, Guglielmi G and Scillitani A: Assessment of
skeletal muscle mass in older people: Comparison between 2
Anthropometry-based methods and Dual-energy X-ray absorptiometry. J
Am Med Dir Assoc. 19:793–796. 2018. View Article : Google Scholar
|
|
24
|
Gort-van Dijk D, Weerink LBM, Milovanovic
M, Haveman JW, Hemmer PHJ, Dijkstra G, Lindeboom R and
Campmans-Kuijpers MJE: Bioelectrical impedance analysis and
Mid-upper arm muscle circumference can be used to detect low muscle
mass in clinical practice. Nutrients. 13:23502021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka T, Takahashi K, Akishita M, Tsuji T
and Iijima K: ‘Yubi-wakka’ (finger-ring) test: A practical
self-screening method for sarcopenia, and a predictor of disability
and mortality among Japanese community-dwelling older adults.
Geriatr Gerontol Int. 18:224–232. 2018. View Article : Google Scholar
|
|
26
|
Lawongsa K, Srisuwan P, Tejavanija S and
Gesakomol K: Sensitivity and specificity of Yubi-wakka
(finger-ring) screening method for sarcopenia among older Thai
adults. Geriatr Gerontol Int. 24:263–268. 2024. View Article : Google Scholar
|
|
27
|
Hiraoka A, Izumoto H, Ueki H, Yoshino T,
Aibiki T, Okudaira T, Yamago H, Suga Y, Iwasaki R, Tomida H, et al:
Easy surveillance of muscle volume decline in chronic liver disease
patients using finger-circle (yubi-wakka) test. J Cachexia
Sarcopenia Muscle. 10:347–354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hiraoka A, Nagamatsu K, Izumoto H, Yoshino
T, Adachi T, Tsuruta M, Aibiki T, Okudaira T, Yamago H, Suga Y, et
al: SARC-F combined with a simple tool for assessment of muscle
abnormalities in outpatients with chronic liver disease. Hepatol
Res. 50:502–511. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishikawa H, Yoh K, Enomoto H, Nishimura
T, Nishiguchi S and Iijima H: Clinical impact of the finger-circle
test in patients with liver diseases. Hepatol Res. 51:603–613.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meerkerk CDA, Chargi N, de Jong PA, van
den Bos F and de Bree R: Low skeletal muscle mass predicts frailty
in elderly head and neck cancer patients. Eur Arch
Otorhinolaryngol. 279:967–977. 2022. View Article : Google Scholar
|
|
31
|
Albano D, Messina C, Vitale J and
Sconfienza LM: Imaging of sarcopenia: Old evidence and new
insights. Eur Radiol. 30:2199–2208. 2020. View Article : Google Scholar
|
|
32
|
O'Brien ME, Zou RH, Hyre N, Leader JK,
Fuhrman CR, Sciurba FC, Nouraie M and Bon J: CT pectoralis muscle
area is associated with DXA lean mass and correlates with emphysema
progression in a Tobacco-exposed cohort. Thorax. 78:394–401. 2023.
View Article : Google Scholar
|
|
33
|
Huang W, Tan P, Zhang H, Li Z, Lin H, Wu
Y, Du Q, Wu Q, Cheng J, Liang Y and Pan Y: Skeletal muscle mass
measurement using Cone-beam computed tomography in patients with
head and neck cancer. Front Oncol. 12:9029662022. View Article : Google Scholar
|
|
34
|
Yoon JK, Jang JY, An YS and Lee SJ:
Skeletal muscle mass at C3 may not be a strong predictor for
skeletal muscle mass at L3 in sarcopenic patients with head and
neck cancer. PLoS One. 16:e02548442021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Y, Xiao-Yue Z, Yue W, Ruo-Tao L,
Xiang-Jie L, Xing-Yuan W, Qian W, Xiao-Hua Q and Zhen-Yi J: Use of
computed tomography for the diagnosis of surgical sarcopenia:
Review of recent research advances. Nutr Clin Pract. 37:583–593.
2022. View Article : Google Scholar
|
|
36
|
Codari M, Zanardo M, di Sabato ME,
Nocerino E, Messina C, Sconfienza LM and Sardanelli F: MRI-derived
biomarkers related to sarcopenia: A systematic review. J Magn Reson
Imaging. 51:1117–1127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pishgar F, Shabani M, Quinaglia ACST,
Bluemke DA, Budoff M, Barr RG, Allison MA, Post WS, Lima JAC and
Demehri S: Quantitative analysis of adipose depots by using chest
CT and associations with All-cause mortality in chronic obstructive
pulmonary disease: Longitudinal analysis from MESArthritis
ancillary study. Radiology. 299:703–711. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mourtzakis M, Prado CM, Lieffers JR,
Reiman T, McCargar LJ and Baracos VE: A practical and precise
approach to quantification of body composition in cancer patients
using computed tomography images acquired during routine care. Appl
Physiol Nutr Metab. 33:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tagliafico AS, Bignotti B, Torri L and
Rossi F: Sarcopenia: How to measure, when and why. Radiol Med.
127:228–237. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Prado CM, Lieffers JR, McCargar LJ, Reiman
T, Sawyer MB, Martin L and Baracos VE: Prevalence and clinical
implications of sarcopenic obesity in patients with solid tumours
of the respiratory and gastrointestinal tracts: A population-based
study. Lancet Oncol. 9:629–635. 2008. View Article : Google Scholar
|
|
41
|
Martin L, Birdsell L, Macdonald N, Reiman
T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB and
Baracos VE: Cancer cachexia in the age of obesity: Skeletal muscle
depletion is a powerful prognostic factor, independent of body mass
index. J Clin Oncol. 31:1539–1547. 2013. View Article : Google Scholar
|
|
42
|
Jin Y, Ma X, Yang Z and Zhang N: Low L3
skeletal muscle index associated with the clinicopathological
characteristics and prognosis of ovarian cancer: A meta-analysis. J
Cachexia Sarcopenia Muscle. 14:697–705. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ufuk F, Herek D and Yuksel D: Diagnosis of
sarcopenia in head and neck computed tomography: Cervical muscle
mass as a strong indicator of sarcopenia. Clin Exp
Otorhinolaryngol. 12:317–324. 2019. View Article : Google Scholar
|
|
44
|
Swartz JE, Pothen AJ, Wegner I, Smid EJ,
Swart KM, de Bree R, Leenen LP and Grolman W: Feasibility of using
head and neck CT imaging to assess skeletal muscle mass in head and
neck cancer patients. Oral Oncol. 62:28–33. 2016. View Article : Google Scholar
|
|
45
|
Jung AR, Roh JL, Kim JS, Choi SH, Nam SY
and Kim SY: Efficacy of head and neck computed tomography for
skeletal muscle mass estimation in patients with head and neck
cancer. Oral Oncol. 95:95–99. 2019. View Article : Google Scholar
|
|
46
|
Lin SC, Lin YS, Kang BH, Yin CH, Chang KP,
Chi CC, Lin MY, Su HH, Chang TS, She YY, et al: Sarcopenia results
in poor survival rates in oral cavity cancer patients. Clin
Otolaryngol. 45:327–333. 2020. View Article : Google Scholar
|
|
47
|
Gronberg BH, Sjoblom B, Wentzel-Larsen T,
Baracos VE, Hjermstad MJ, Aass N, Bremnes RM, Fløtten Ø, Bye A and
Jordhøy M: A comparison of CT based measures of skeletal muscle
mass and density from the Th4 and L3 levels in patients with
advanced non-small-cell lung cancer. Eur J Clin Nutr. 73:1069–1076.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neefjes ECW, van den Hurk RM,
Blauwhoff-Buskermolen S, van der Vorst M, Becker-Commissaris A, de
van der Schueren MAE, Buffart LM and Verheul HMW: Muscle mass as a
target to reduce fatigue in patients with advanced cancer. J
Cachexia Sarcopenia Muscle. 8:623–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matsuyama R, Maeda K, Yamanaka Y, Ishida
Y, Kato R, Nonogaki T, Shimizu A, Ueshima J, Kazaoka Y, Hayashi T,
et al: Assessing skeletal muscle mass based on the cross-sectional
area of muscles at the 12th thoracic vertebra level on computed
tomography in patients with oral squamous cell carcinoma. Oral
Oncol. 113:1051262021. View Article : Google Scholar
|
|
50
|
Gaszynska E, Godala M, Szatko F and
Gaszynski T: Masseter muscle tension, chewing ability, and selected
parameters of physical fitness in elderly care home residents in
Lodz, Poland. Clin Interv Aging. 9:1197–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamaguchi K, Tohara H, Hara K, Nakane A,
Yoshimi K, Nakagawa K and Minakuchi S: Factors associated with
masseter muscle quality assessed from ultrasonography in
community-dwelling elderly individuals: A cross-sectional study.
Arch Gerontol Geriatr. 82:128–1232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hwang Y, Lee YH, Cho DH, Kim M, Lee DS and
Cho HJ: Applicability of the masseter muscle as a nutritional
biomarker. Medicine. 99:e190692020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang SW, Tsai YH, Hsu CM, Huang EI, Chang
GH, Tsai MS and Tsai YT: Masticatory muscle index for indicating
skeletal muscle mass in patients with head and neck cancer. PLoS
One. 16:e02514552021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tsai PS, Lin DC, Jan YT, Liu YP, Wu TH and
Huang SC: Lower-extremity muscle wasting in patients with
peripheral arterial disease: Quantitative measurement and
evaluation with CT. Eur Radiol. 33:4063–4072. 2023. View Article : Google Scholar
|
|
55
|
Perkisas S, Bastijns S, Baudry S, Bauer J,
Beaudart C, Beckwee D, Cruz-Jentoft A, Gasowski J, Hobbelen H and
Jager-Wittenaar H: Application of ultrasound for muscle assessment
in sarcopenia: 2020 SARCUS update. Eur Geriatr Med. 12:45–59. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Perkisas S, Baudry S, Bauer J, Beckwee D,
De Cock AM, Hobbelen H, Jager-Wittenaar H, Kasiukiewicz A, Landi F,
Marco E, et al: Application of ultrasound for muscle assessment in
sarcopenia: Towards standardized measurements. Eur Geriatr Med.
9:739–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cheng KY, Chow SK, Hung VW, Wong CH, Wong
RM, Tsang CS, Kwok T and Cheung WH: Diagnosis of sarcopenia by
evaluating skeletal muscle mass by adjusted bioimpedance analysis
validated with dual-energy X-ray absorptiometry. J Cachexia
Sarcopenia Muscle. 12:2163–2173. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Walowski CO, Braun W, Maisch MJ, Jensen B,
Peine S, Norman K, Müller MJ and Bosy-Westphal A: Reference values
for skeletal muscle mass-current concepts and methodological
considerations. Nutrients. 12:7552020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marini ACB, Perez DRS, Fleuri JA and
Pimentel GD: SARC-F is better correlated with muscle function
indicators than muscle mass in older hemodialysis patients. J Nutr
Health Aging. 24:999–1002. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee YA, Kim HN and Song SW: Associations
between hair mineral concentrations and skeletal muscle mass in
korean adults. J Nutr Health Aging. 26:515–520. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim M and Won CW: Sarcopenia in Korean
Community-dwelling adults aged 70 years and older: Application of
screening and diagnostic tools from the asian working group for
sarcopenia 2019 update. J Am Med Dir Assoc. 21:752–758. 2020.
View Article : Google Scholar
|
|
62
|
Janssen I, Baumgartner RN, Ross R,
Rosenberg IH and Roubenoff R: Skeletal muscle cutpoints associated
with elevated physical disability risk in older men and women. Am J
Epidemiol. 159:413–421. 2004. View Article : Google Scholar
|
|
63
|
Fielding RA, Vellas B, Evans WJ, Bhasin S,
Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J,
Breuille D, et al: Sarcopenia: An undiagnosed condition in older
adults. Current consensus definition: Prevalence, etiology, and
consequences. International working group on sarcopenia. J Am Med
Dir Assoc. 12:249–256. 2011. View Article : Google Scholar
|
|
64
|
Kim S, Kim M, Lee Y, Kim B, Yoon TY and
Won CW: Calf circumference as a simple screening marker for
diagnosing sarcopenia in older korean adults: The Korean frailty
and aging cohort study (KFACS). J Korean Med Sci. 33:e1512018.
View Article : Google Scholar
|
|
65
|
Sergi G, De Rui M, Veronese N, Bolzetta F,
Berton L, Carraro S, Bano G, Coin A, Manzato E and Perissinotto E:
Assessing appendicular skeletal muscle mass with bioelectrical
impedance analysis in free-living Caucasian older adults. Clin
Nutr. 34:667–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lee MM, Jebb SA, Oke J and Piernas C:
Reference values for skeletal muscle mass and fat mass measured by
bioelectrical impedance in 390 565 UK adults. J Cachexia Sarcopenia
Muscle. 11:487–496. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Harvey NC, Kanis JA, Liu E, Johansson H,
Lorentzon M and McCloskey E: Appendicular lean mass and fracture
risk assessment: Implications for FRAX® and sarcopenia.
Osteoporos Int. 30:537–539. 2019. View Article : Google Scholar
|
|
68
|
Yamada Y, Yamada M, Yoshida T, Miyachi M
and Arai H: Validating muscle mass cutoffs of four international
sarcopenia-working groups in Japanese people using DXA and BIA. J
Cachexia Sarcopenia Muscle. 12:1000–1010. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Barbosa-Silva TG, Bielemann RM, Gonzalez
MC and Menezes AM: Prevalence of sarcopenia among
community-dwelling elderly of a medium-sized South American city:
Results of the COMO VAI? study. J Cachexia Sarcopenia Muscle.
7:136–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang L, Zhu B, Xue C, Lin H, Zhou F and
Luo Q: A prospective cohort study evaluating impact of sarcopenia
on hospitalization in patients on continuous ambulatory peritoneal
dialysis. Sci Rep. 14:169262024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Benz E, Pinel A, Guillet C, Capel F,
Pereira B, De Antonio M, Pouget M, Cruz-Jentoft AJ, Eglseer D,
Topinkova E, et al: Sarcopenia and sarcopenic obesity and mortality
among older people. JAMA Netw Open. 7:e2436042024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kirk B, Cawthon PM, Arai H, Avila-Funes
JA, Barazzoni R, Bhasin S, Binder EF, Bruyere O, Cederholm T, Chen
LK, et al: The conceptual definition of sarcopenia: Delphi
consensus from the global leadership initiative in sarcopenia
(GLIS). Age Ageing. 53:afae0522024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Morley JE, Abbatecola AM, Argiles JM,
Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR,
Evans WJ, et al: Sarcopenia with limited mobility: An international
consensus. J Am Med Dir Assoc. 12:403–449. 2011. View Article : Google Scholar
|
|
74
|
Mizuno T, Matsui Y, Tomida M, Suzuki Y,
Ishizuka S, Watanabe T, Takemura M, Nishita Y, Tange C, Shimokata
H, et al: Relationship between quadriceps muscle computed
tomography measurement and motor function, muscle mass, and
sarcopenia diagnosis. Front Endocrinol (Lausanne). 14:12593502023.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cruz-Jentoft AJ, Landi F, Schneider SM,
Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel
JP, et al: Prevalence of and interventions for sarcopenia in ageing
adults: A systematic review. Report of the International Sarcopenia
Initiative (EWGSOP and IWGS). Age Ageing. 43:748–759. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cawthon PM, Peters KW, Shardell MD, McLean
RR, Dam TT, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM,
et al: Cutpoints for low appendicular lean mass that identify older
adults with clinically significant weakness. J Gerontol A Biol Sci
Med Sci. 69:567–575. 2014. View Article : Google Scholar
|
|
77
|
McGovern J, Dolan RD, Horgan PG, Laird BJ
and McMillan DC: Computed tomography-defined low skeletal muscle
index and density in cancer patients: Observations from a
systematic review. J Cachexia Sarcopenia Muscle. 12:1408–1417.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ubachs J, Ziemons J, Minis-Rutten IJG,
Kruitwagen R, Kleijnen J, Lambrechts S, Olde Damink SWM, Rensen SS
and Van Gorp T: Sarcopenia and ovarian cancer survival: A
systematic review and meta-analysis. J Cachexia Sarcopenia Muscle.
10:1165–1174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Muraki I: Muscle mass assessment in
sarcopenia: A narrative review. JMA J. 6:381–386. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li C, Huang Y, Wang H, Lu J and He B:
Application of imaging methods and the latest progress in
sarcopenia. Chin J Academic Radiol. 7:15–27. 2024. View Article : Google Scholar
|
|
81
|
Fernandes DPS, Juvanhol LL, Lozano M and
Ribeiro AQ: Calf circumference is an independent predictor of
mortality in older adults: An approach with generalized additive
models. Nutr Clin Pract. 37:1190–1198. 2022. View Article : Google Scholar
|
|
82
|
Pagotto V, Santos KFD, Malaquias SG,
Bachion MM and Silveira EA: Calf circumference: Clinical validation
for evaluation of muscle mass in the elderly. Rev Bras Enferm.
71:322–328. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Maeda K, Koga T, Nasu T, Takaki M and
Akagi J: Predictive accuracy of calf circumference measurements to
detect decreased skeletal muscle mass and European society for
clinical nutrition and metabolism-defined malnutrition in
hospitalized older patients. Ann Nutr Metab. 71:10–15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kawakami R, Murakami H, Sanada K, Tanaka
N, Sawada SS, Tabata I, Higuchi M and Miyachi M: Calf circumference
as a surrogate marker of muscle mass for diagnosing sarcopenia in
Japanese men and women. Geriatr Gerontol Int. 15:969–976. 2015.
View Article : Google Scholar
|
|
85
|
Sahin MEH, Akbas F, Yardimci AH and Sahin
E: The effect of sarcopenia and sarcopenic obesity on survival in
gastric cancer. BMC Cancer. 23:9112023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sealy MJ, Dechaphunkul T, van der Schans
CP, Krijnen WP, Roodenburg JLN, Walker J, Jager-Wittenaar H and
Baracos VE: Low muscle mass is associated with early termination of
chemotherapy related to toxicity in patients with head and neck
cancer. Clin Nutr. 39:501–509. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
van der Kroft G, van Dijk DPJ, Rensen SS,
Van Tiel FH, de Greef B, West M, Ostridge K, Dejong CHC, Neumann UP
and Olde Damink SWM: Low thoracic muscle radiation attenuation is
associated with postoperative pneumonia following partial
hepatectomy for colorectal metastasis. HPB (Oxford). 22:1011–1019.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li Y, Wang WB, Jiang HG, Dai J, Xia L,
Chen J, Xie CH, Peng J, Liao ZK, Gao Y, et al: Predictive value of
pancreatic dose-volume metrics on sarcopenia rate in gastric cancer
patients treated with adjuvant chemoradiotherapy. Clin Nutr.
38:1713–1720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Blauwhoff-Buskermolen S, Langius JAE,
Becker A, Verheul HMW and de van der Schueren MAE: The influence of
different muscle mass measurements on the diagnosis of cancer
cachexia. J Cachexia Sarcopenia Muscle. 8:615–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tao J, Fang J, Chen L, Liang C, Chen B,
Wang Z, Wu Y and Zhang J: Increased adipose tissue is associated
with improved overall survival, independent of skeletal muscle mass
in non-small cell lung cancer. J Cachexia Sarcopenia Muscle.
14:2591–2601. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pineda-Zuluaga MC, Gonzalez-Correa CH and
Sepulveda-Gallego LE: Cut-off points for low skeletal muscle mass
in older adults: Colombia versus other populations. F1000Res.
11:3042022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bulut EA, Soysal P, Dokuzlar O, Kocyigit
SE, Aydin AE, Yavuz I and Isik AT: Validation of population-based
cutoffs for low muscle mass and strength in a population of Turkish
elderly adults. Aging Clin Exp Res. 32:1749–1755. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bjorkman MP, Pitkala KH, Jyvakorpi S,
Strandberg TE and Tilvis RS: Bioimpedance analysis and physical
functioning as mortality indicators among older sarcopenic people.
Exp Gerontol. 122:42–46. 2019. View Article : Google Scholar
|
|
94
|
Han DS, Chang KV, Li CM, Lin YH, Kao TW,
Tsai KS, Wang TG and Yang WS: Skeletal muscle mass adjusted by
height correlated better with muscular functions than that adjusted
by body weight in defining sarcopenia. Sci Rep. 6:194572016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bahat G, Tufan A, Tufan F, Kilic C,
Akpinar TS, Kose M, Erten N, Karan MA and Cruz-Jentoft AJ: Cut-off
points to identify sarcopenia according to European Working Group
on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr.
35:1557–1563. 2016. View Article : Google Scholar : PubMed/NCBI
|