Introduction
Lung cancer is a critical malignancy with both high
incidence and mortality. A 2020 world cancer statistical report
estimated that 2.21 million new cases and 1.80 million new deaths
occur due to lung cancer every year globally (1). Furthermore, according to an up-to-date
Chinese cancer statistical report, 1.06 million new cases and 0.73
million new deaths due to lung cancer occur every year in China
(2). As the major type of lung
cancer, non-small cell lung cancer (NSCLC) comprises 80–85% of the
total lung cancer cases (3). The
exploration of the mechanism of NSCLC has enabled the discovery of
various potential targets, such as EGFR, anaplastic lymphoma
kinase, ROS proto-oncogene 1, ret proto-oncogene and BRAF; tyrosine
kinase inhibitors that inhibit some of these targets have already
been applied in clinical therapy and improve the prognosis of
patients with NSCLC (4,5). This emphasizes the importance of
identifying the mechanism of progression of NSCLC.
Cancer-associated mesenchymal stem cells (CA-MSCs)
play a crucial role in modulating the tumor microenvironment and
promoting tumor development (6,7). MSCs
are abundant in the bone marrow, adipose tissue, placenta,
umbilical cord and other tissues, and are pluripotent stem cells
with multilineage differentiation potential, with the capability of
differentiating into osteoblasts, chondrocytes and adipocytes
(8). During tumor progression, MSCs
are recruited into the tumor and are transformed into CA-MSCs,
undergoing substantial changes in their phenotypes and functions
(9–11). Recently, certain studies have
demonstrated that CA-MSCs can also promote tumor progression by
secreting exosomes and transferring small molecules, such as
proteins and RNAs (12,13). For example, CA-MSCs are able to
transfer transmembrane BAX inhibitor motif containing 6 (TMBIM6)
via exosomes to modify hepatocellular carcinoma viability,
invasiveness and epithelial-to-mesenchymal transition (EMT),
promoting its malignant progression (12). However, the involvement of CA-MSCs
or CA-MSC exosomes in NSCLC pathogenesis has not been clearly
defined. Moreover, microRNA (miR)-182 has been reported to be an
oncogene in a number of cancer types, including NSCLC (14–19).
Our previous study also uncovered a tumor-promoting role of miR-182
in NSCLC (20).
Therefore, the present study aimed to investigate
the effect of CA-MSCs, CA-MSC exosomes and CA-MSC exosome-derived
miR-182 on the viability and invasiveness of NSCLC cells.
Materials and methods
Cell culture
The NSCLC cell lines (A549 and H1299) were provided
by Beyotime Institute of Biotechnology. The CA-MSCs were
established by treating MSCs (Procell Life Science & Technology
Co., Ltd.) with supernatant from A549 or H1299 cells for 14 days
(21). The cells were cultured in
Dulbecco's Modified Eagle's Medium (Procell Life Science &
Technology Co., Ltd.) supplemented with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2.
Isolation and identification of CA-MSC
exosomes
CA-MSC exosomes were separated via an Exosome
Isolation Kit (Shanghai Yeasen Biotechnology Co., Ltd.) in
accordance with the standard procedure. The exosomes were
identified with nanoparticle tracking analysis by Wuhan Servicebio
Technology Co., Ltd., as shown in Fig.
S1A. Western blotting was used to detect the marker proteins of
the exosomes Fig. S1B.
Co-culture experiments
For the inhibition of exosome generation, CA-MSCs
were treated with GW4869 for 24 h (10 µM; MedChemExpress) at 37°C.
The NSCLC cell and CA-MSC co-culture experiments were performed in
a Transwell insert (Corning, Inc.). In brief, A549
(5×104) or H1299 cells (5×104) were added to
the lower chamber and the CA-MSCs (5×104) or
GW4869-treated CA-MSCs (CA-MSC-GW; 5×104) were added to
the upper chamber. The mock group was A549 or H1299 cells cultured
alone, and the CA-MSC Exo group was A549 or H1299 cells cultured
with exosomes isolated from CA-MSCs (106
particles/cell). Following culture for 24 h at 37°C, the cells were
collected to assess cell viability, invasion and apoptosis as well
as to perform reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) assays.
Co-culture of NSCLC cells with
exosomes from miR-182-knockdown CA-MSCs
The CA-MSCs were transfected with miR-182 inhibitor
(miR-inh; 5′-AGUGUGAGUUCUACCAUUGCCAAA-3′; 0.5 pmol) or negative
control inhibitor (NC-inh; 5′-CAGUACUUUUGUGUAGUACAA-3′; 0.5 pmol)
(Genepharm, Inc.) using Lipofectamine™ 2000 (Thermo
Fisher Scientific., Inc.) at 37°C for 6 h. The exosomes from the
transfected or non-transfected CA-MSCs were subsequently isolated
(48 h after transfection). The non-transfected CA-MSCs were set as
a control. The miR-182 expression levels in CA-MSCs and their
exosomes were detected via RT-qPCR assay (48 h after transfection).
Subsequently, A549 and H1299 cells were treated with the exosomes
(106 particles/cell) from non-transfected CA-MSCs
(Control exo) or transfected CA-MSCs (NC-inh exo and miR-inh exo).
The mock group was prepared alone without exosome treatment.
Following culture for 24 h at 37°C, A549 and H1299 cells were
harvested for cell viability, invasion, apoptosis, RT-qPCR and
western blotting assays.
miR-182 target gene validation
The target gene of miR-182 was analyzed using
miRWalk (mirwalk.umm.uni-heidelberg.de). The wild type (WT) or
mutant type (MT) plasmid was constructed by cloning FBXW7 3′
untranslated region (UTR) wild sequence or mutant sequences into
pGL6 vector (Beyotime Institute of Biotechnology). The 0.8 µg WT
plasmid, 0.8 µg MT plasmid, 50 pmol NC mimics and 50 pmol miR-182
mimics were co-transfected into 293T cells (Beyotime Institute of
Biotechnology) in the presence of Lipofectamine™ 2000
(Thermo Fisher Scientific., Inc.). The cells were harvested 48 h
after transfection. Following the manufacturer's procedure, the
transcriptional regulation between miR-182 and FBXW7 was analyzed
using a Dual-Luciferase Reporter Gene Assay Kit (Beyotime Institute
of Biotechnology) and normalized to Renilla activity.
miR-182 and FBXW7 transfection
experiments in NSCLC cells
miR-182 mimic (sense,
5′-UUUGGCAAUGGUAGAACUCACACU-3′; and antisense,
5′-UGUGAGUUCUACCAUUGCCAAAUU-3′), negative control miR mimic (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′; and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′), the FBXW7 overexpression vector
(OE-FBXW7) and the negative control vector were constructed by
Shanghai GenePharma Co., Ltd. The pcDNA3.1 vectors were used for
FBXW7 overexpression and the negative control. A549 and H1299 cells
were transfected with the aforementioned mimics and vectors in the
following grouping: i) Mock group, without transfection; ii)
scramble group, transfected with 50 pmol negative control miR mimic
and 50 pmol negative control vector; iii) miR-mimic group,
transfected with 50 pmol miR-182 mimic and 0.8 µg negative control
vector; iv) OE-FBXW7 group, transfected with 0.8 µg negative
control 50 pmol miR mimic and 0.8 µg FBXW7 overexpression vector;
and v) miR-mimic + OE-FBXW7 group, transfected with 50 pmol miR-182
mimic and 0.8 µg FBXW7 overexpression vector. Following
transfection for 48 h according to the aforementioned protocol,
RT-qPCR, western blotting, cell viability, cell invasion and cell
apoptosis assays were performed. The transfection efficiency was
assessed by RT-qPCR assays after transfection with miR-mimic,
OE-FBXW7 and their respective negative controls.
Cell viability
The cell viability of A549 and H1299 cells was
assessed using Super-Enhanced Cell Counting Kit-8 (CCK-8; Beyotime
Institute of Biotechnology). Briefly, 10 µl CCK-8 reagent was added
to a 200 µl medium containing treated cells in a 96-well plate for
1 h. The optical density was detected using a microplate reader
(Shanghai Flash Biotechnology Co., Ltd.) and the relative cell
viability was measured (normalized to Mock group).
Cell apoptosis
The Annexin V-Alexa Fluor 488/PI Apoptosis Detection
Kit (cat. no. 40305ES50; Shanghai Yeasen Biotechnology Co., Ltd.)
was used to assess the cell apoptotic rate. Briefly, A549 and H1299
cells were collected and washed with a binding solution.
Subsequently, 5 µl Annexin V-fluorescein isothiocyanate and 10 µl
propidium iodide were incubated with the sample for 20 min,
successively. The apoptotic cells were examined by flow cytometry
(FACSCalibur; BD Biosciences). The data was analyzed by FlowJo X
(BD Biosciences).
Cell invasion
A Transwell assay was carried out to assess cell
invasion. In brief, Transwell inserts were pre-coated with Matrigel
(Corning, Inc.) at 37°C for 1 h. A549 (2×104) and H1299
(2×104) cells were added to the upper chamber of a
24-well plate with Matrigel-coated inserts (Corning, Inc.), and the
lower chamber was filled with complete medium (Dulbecco's modified
Eagle's medium with 10% fetal bovine serum). Following treatment of
the cells for 24 h at 37°C, the invasive cells were stained with
crystal violet (Wuhan Servicebio Technology Co., Ltd.) at room
temperature for 10 min and subsequently counted manually using an
inverted optical microscope (Keyence Corporation).
RT-qPCR
Total RNA was isolated from cells or exosomes with
the TRIzol® reagent (Thermo Fisher Scientific, Inc.).
RT-qPCR was performed using the SweScript One-Step reverse
transcription-PCR Kit (Wuhan Servicebio Technology Co., Ltd.)
according to the manufacturer's instructions. The primer sequences
used were as follows: miR-182 [forward, GCGTTTGGCAATGGTAGAACT; and
reverse, AGTGCAGGGTCCGAGGTATT (universal primer)], U6 (forward,
CTCGCTTCGGCAGCACA; and reverse, AACGCTTCACGAATTTGCGT), FBXW7
(forward, TTCACCAACTCTCCTCCCCATT; and reverse,
GCTGAACATGGTACAAGCCCA) and GAPDH (forward, ACAACTTTGGTATCGTGGAAGG;
and reverse, GCCATCACGCCACAGTTTC). The result was analyzed with
2−ΔΔCq method (22). The
U6 and GAPDH were the reference genes for miR-182 and FBXW7,
respectively.
Western blotting
The proteins were separated from cells or exosomes
with RIPA buffer (Beyotime Institute of Biotechnology) and
quantified with a BCA kit (Wuhan Servicebio Technology Co., Ltd.).
Electrophoresis (4–20% precast gel) was performed and 20 µg
protein/lane was transferred to a nitrocellulose membrane (Wuhan
Servicebio Technology Co., Ltd.). Subsequently, the membranes were
blocked using 5% bovine serum albumin (Wuhan Servicebio Technology
Co., Ltd.) at 37°C for 1 h and incubated overnight at 4°C with the
following primary antibodies from Affinity Biosciences, Ltd.: Tumor
susceptibility gene 101 (cat. no. DF8427; 1:1,000), cluster of
differentiation (CD)9 (cat. no. AF5139; 1:500), CD81 (cat. no.
DF2306; 1:500), Calnexin (cat. no. AF5362; 1:500), FBXW7 (cat. no.
DF12400; 1:1,000), phosphorylated (p)-AKT (cat. no. AF0016; 1:500),
AKT (cat. no. AF6261; 1:1,000), p-ERK (cat. no. AF1015; 1:500), ERK
(cat. no. AF0155; 1:1,000) and GAPDH (cat. no. AF7021; 1:2,000).
The membrane was subsequently incubated with secondary antibodies
(cat. no. S0001; 1:5,000; Affinity Biosciences, Ltd.) at 37°C for 1
h. The bands were visualized using an electrochemiluminescence
(ECL) substrate (Affinity Biosciences, Ltd.). Density gray values
were measured by ImageJ V1.8 (https://imagej.net/ij/).
For p-AKT/p-ERK and AKT/ERK detection, the membrane
was first incubated with GAPDH antibody followed by visualization
via ECL. Then, the GAPDH antibody was eluted using stripping buffer
(Beyotime Institute of Biotechnology) and the membrane was
incubated with p-AKT/p-ERK antibody followed by visualization via
ECL. The p-AKT/p-ERK antibody was eluted using stripping buffer and
the membrane was incubated with AKT/ERK antibody followed by
visualization via ECL.
Statistical analysis
Repeats were in triplicates and data are presented
as the mean ± standard deviation. Unpaired students' t-test or
one-way ANOVA followed by Tukey's test were used to analyze
comparisons between two and multiple groups, respectively. Analyses
were performed using GraphPad Prism 9 (Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of CA-MSCs and CA-MSC exosomes
on NSCLC viability and invasiveness
CA-MSCs promoted the viability and invasiveness,
whereas they inhibited the apoptosis of A549 cells (CA-MSC vs. Mock
group; Fig. 1A and C). However,
following the elimination of exosome production by GW4869, the
effect of CA-MSCs was attenuated in A549 cells (CA-MSC-GW vs.
CA-MSC group). In addition, following exosome isolation, CA-MSC
exosomes facilitated the viability and invasiveness (Fig. 1B), whereas they repressed the
apoptosis of A549 cells (CA-MSC exo vs. mock group). Subsequently,
the aforementioned findings were further confirmed in H1299 cells
(Fig. 1D-F).
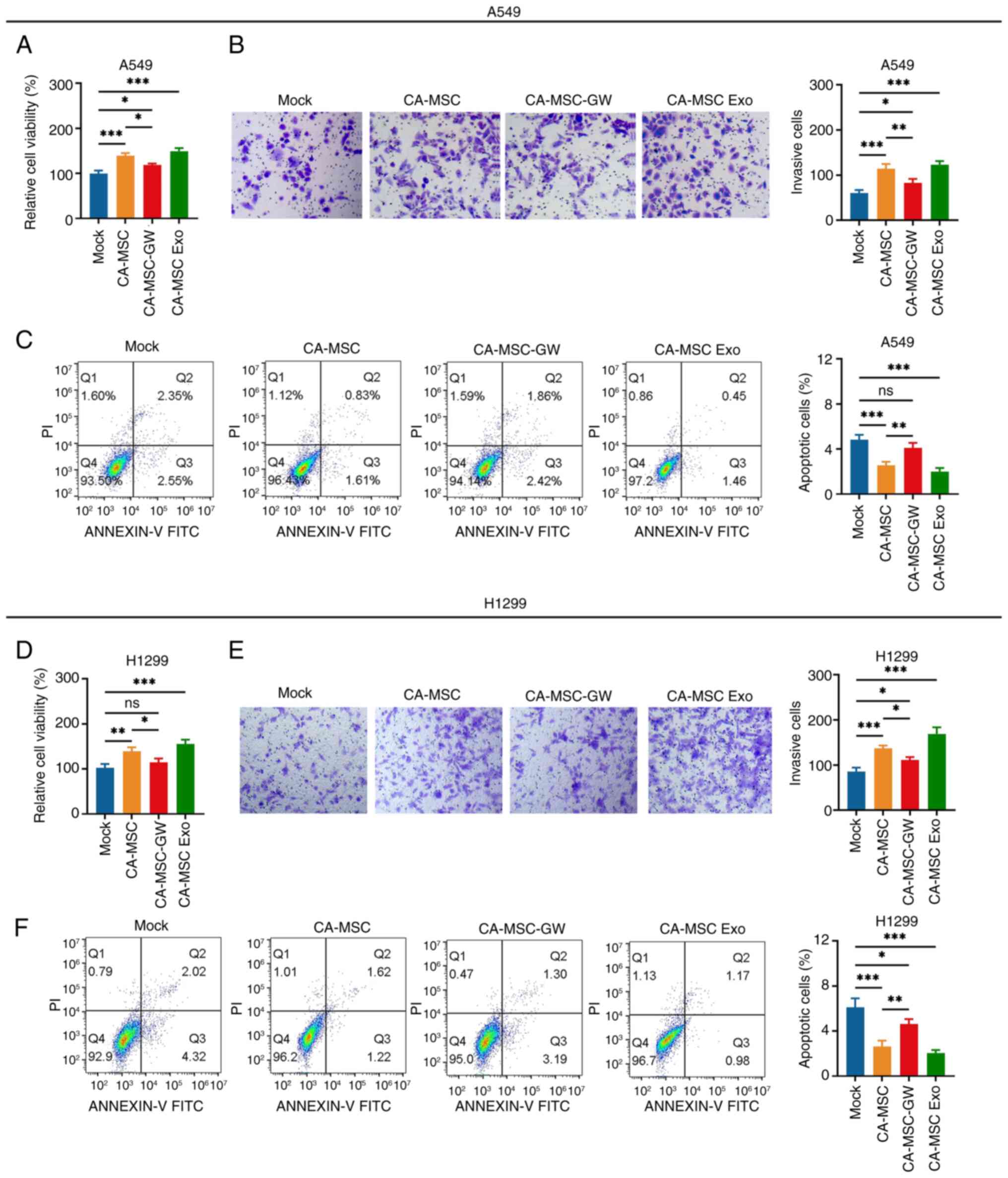 | Figure 1.CA-MSCs promote non-small cell lung
cancer cell viability and invasiveness via delivering exosomes.
Comparison of the (A) viability, (B) number of invasive cells
(magnification, ×200) and (C) apoptosis rate of A549 cells in the
Mock, CA-MSC, CA-MSC-GW and CA-MSC Exo groups. Comparison of the
(D) viability, (E) number of invasive cells (magnification, ×200)
and (F) apoptosis rate of H1299 cells in the Mock, CA-MSC,
CA-MSC-GW and CA-MSC Exo groups. *P<0.05, **P<0.01 and
***P<0.001; ns, no significance. CA-MSC, cancer-associated
mesenchymal stem cell; CA-MSC-GW, GW4869-treated CA-MSCs; CA-MSC
Exo, exosomes derived from CA-MSCs. |
Effect of miR-182-knockdown CA-MSC
exosomes on NSCLC viability and invasiveness
CA-MSCs and CA-MSC exosomes increased the levels of
miR-182 in both A549 and H1299 cells (CA-MSC and CA-MSC exo vs.
Mock group; Fig. 2A and B).
However, following the elimination of exosome production by GW4869,
CA-MSCs had no effect on miR-182 levels (CA-MSC-GW vs. mock group),
indicating that CA-MSCs may deliver miR-182 to A549 and H1299
cells. Based on previous studies that examined the significant role
of miR-182 in the oncogenesis of lung cancer (14–16),
the miR-182 levels were knocked down by a miR-182 inhibitor in
CA-MSCs and the corresponding exosomes were collected to detect
whether its diminishment would weaken the effect of CA-MSC exosomes
on NSCLC viability and invasiveness. The miR-182 inhibitor
significantly decreased miR-182 levels in both CA-MSCs and CA-MSC
exosomes (miR-inh vs. NC-inh group; Fig. 2C-F).
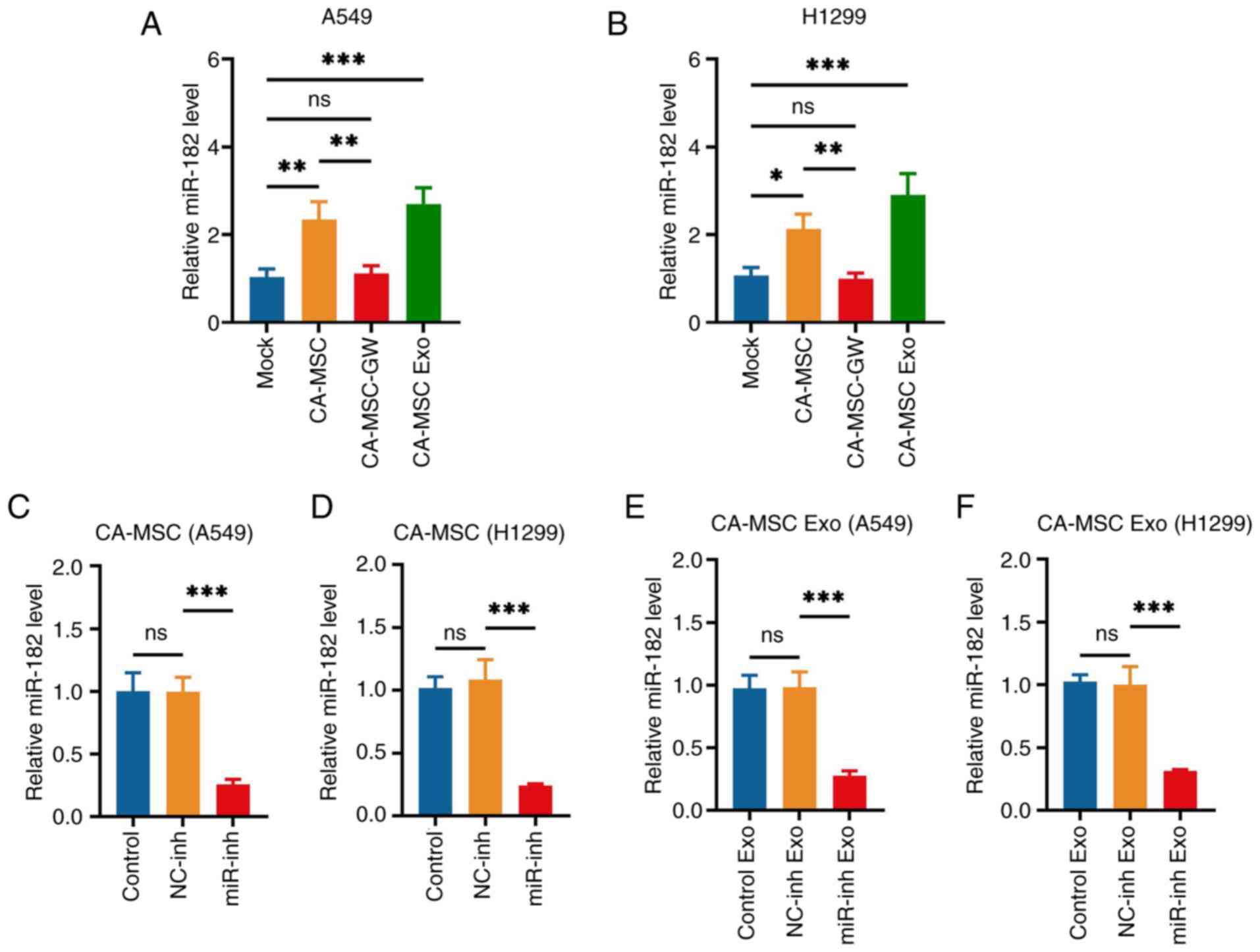 | Figure 2.miR-182 expression quantifications.
miR-182 expression in (A) A549 cells and (B) H1299 cells in the
Mock, CA-MSC, CA-MSC-GW and CA-MSC Exo groups. After transfection,
miR-182 expression in CA-MSCs cocultured with (C) A549 and (D)
H1299 cells and the corresponding (E,F) CA-MSC exosomes, in the
Control, NC-inh and miR-inh Exo groups. *P<0.05, **P<0.01 and
***P<0.001; ns, no significance. miR-182, microRNA-182; CA-MSC,
cancer-associated mesenchymal stem cell; CA-MSC-GW, GW4869-treated
CA-MSCs; CA-MSC Exo, exosomes derived from CA-MSCs; NC, negative
control; inh, inhibitor. |
CA-MSC exosomes increased the viability and
invasiveness while suppressing the apoptosis of A549 cells (Control
exo vs. Mock group); this effect was not altered following
transfection with the NC inhibitor (NC-inh exo vs. Control exo
group) (Fig. 3A-C). It is important
to note that following transfection with the miR-182 inhibitor, the
effect of CA-MSC exosomes on cell viability and apoptosis was
attenuated, whereas the invasiveness of A549 cells was not affected
(miR-inh exo vs. NC-inh exo group; Fig.
3A-C). Furthermore, similar results were observed in H1299
cells as those in A549 cells (Fig.
3D-F).
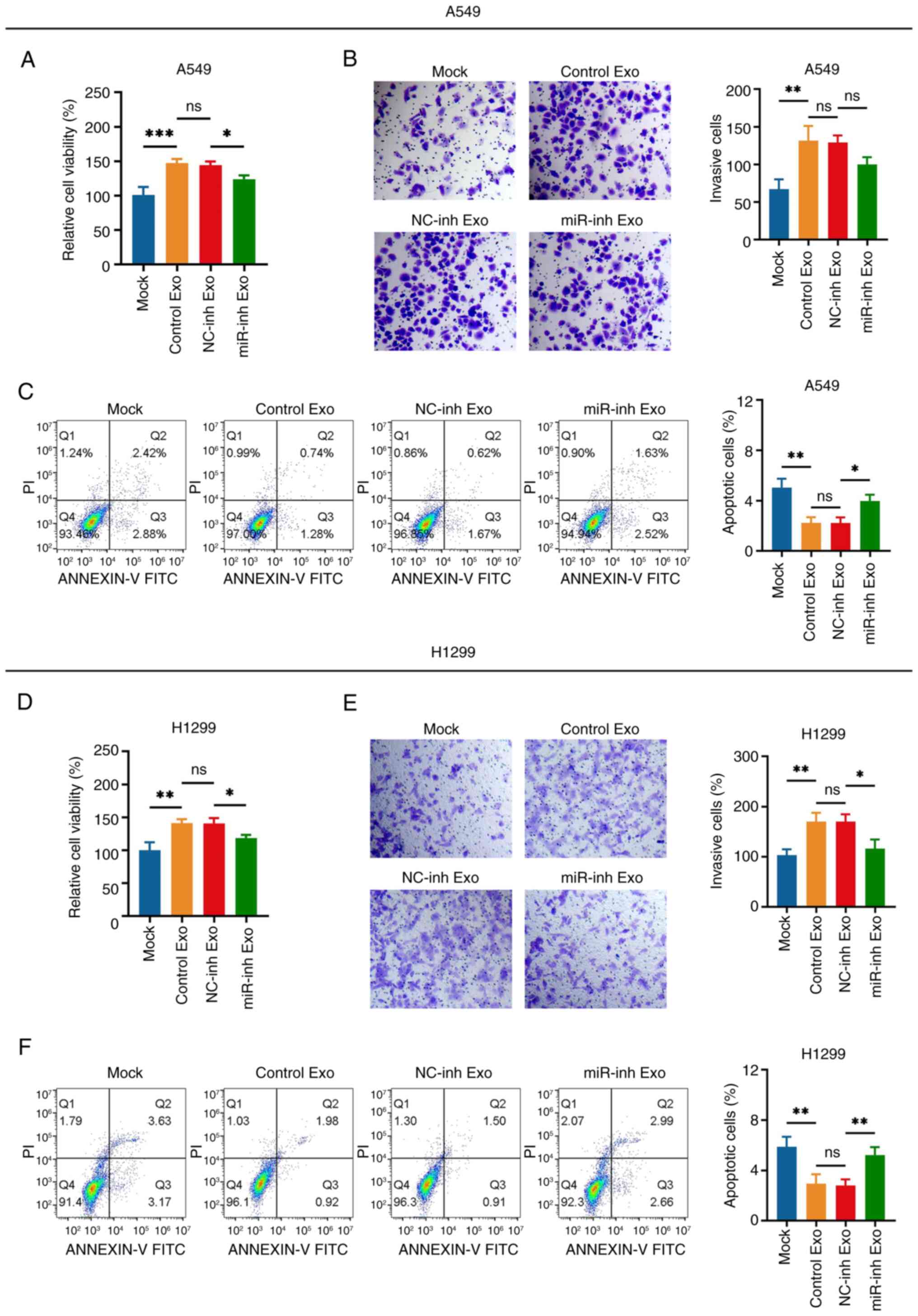 | Figure 3.miR-182 knockdown attenuates the
effect of CA-MSC exosomes on non-small cell lung cancer cell
viability and invasiveness. Comparison of the (A) viability, (B)
number of invasive cells (magnification, ×200) and (C) apoptosis
rate of A549 cells in the Mock, Control Exo, NC-inh Exo and miR-inh
Exo groups. Comparison of the (D) viability, (E) number of invasive
cells (magnification, ×200) and (F) apoptosis rate of H1299 cells
in the Mock, Control Exo, NC-inh Exo and miR-inh Exo groups.
*P<0.05, **P<0.01 and ***P<0.001; ns, no significance.
miR-182, microRNA-182; Exo, exosomes; NC, negative control; inh,
inhibitor. |
Effect of miR-182 knockdown in CA-MSC
exosomes on FBXW7 expression in NSCLC cells
FBXW7 was previously reported to be a direct target
of miR-182 in various cancer types (23–25);
therefore, it was further speculated that CA-MSC exosome-derived
miR-182 promoted NSCLC viability and invasiveness via sponging
FBXW7. Subsequently, it was observed that CA-MSC exosomes with
miR-182 knockdown increased the FBXW7 expression levels compared
with the CA-MSC exosomes with NC-knockdown (miR-inh exo vs. NC-inh
exo group) in A549 (Fig. 4A and B)
and H1299 cells (Fig. 4C and D). In
addition, miR-182 was confirmed to directly sponge FBXW7 via the
Dual-Luciferase Reporter Gene assay (Fig. S2).
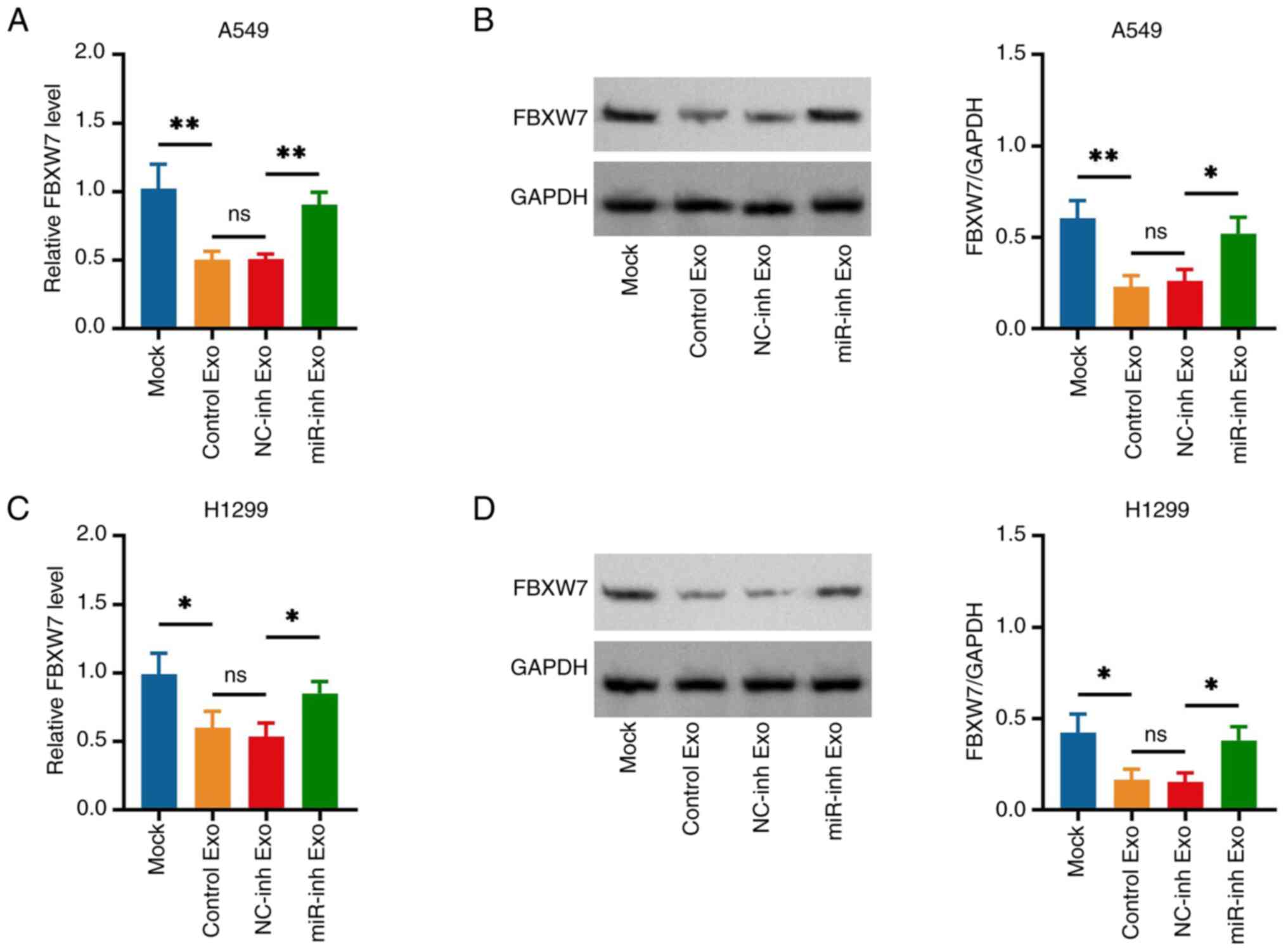 | Figure 4.miR-182-knockdown CA-MSC exosomes
increase FBXW7 expression in non-small cell lung cancer cells.
Comparison of (A) gene and (B) protein expression of FBXW7 in A549
cells in the Mock, Control Exo, NC-inh Exo and miR-inh Exo groups.
Comparison of (C) gene and (D) protein expression of FBXW7 in H1299
cells in the Mock, Control Exo, NC-inh Exo and miR-inh Exo groups.
*P<0.05 and **P<0.01; ns, no significance. miR-182,
microRNA-182; Exo, exosomes; NC, negative control; inh, inhibitor;
FBXW7, F-box and WD repeat domain containing 7. |
Effect of miR-182 and FBXW7 on NSCLC
viability and invasiveness
miR-182 expression was increased after miR-182 mimic
transfection (miR-mimic vs. NC-mimic group; Fig. S3A and B), and FBXW7 expression was
elevated after OE-FBXW7 vector transfection (OE-FBXW7 vs. Control
vector group; Fig. S3C and D),
indicating transfection success. Following transfection in A549
cells, the miR-182 mimic decreased FBXW7 expression (miR-mimic vs.
Scramble group), while FBXW7 overexpression increased the
expression of this protein (OE-FBXW7 vs. Scramble group) and
attenuated the impact of miR-182 mimic (miR-mimic + OE-FBXW7 vs.
miR-mimic group) (Fig. 5A and B).
The aforementioned observations were further confirmed in H1299
cells (Fig. 5C and D).
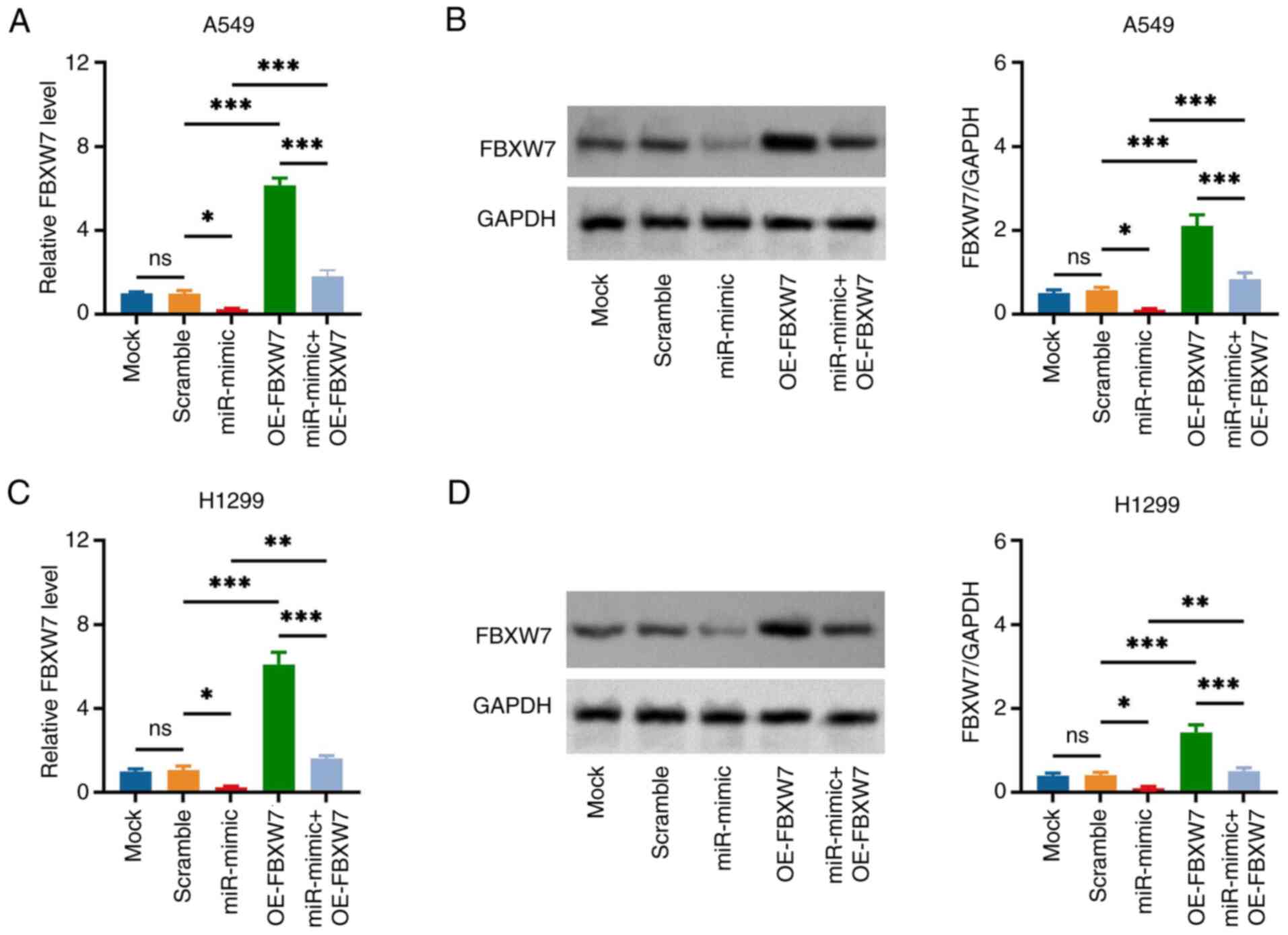 | Figure 5.miR-182 mimic and FBXW7 OE vector
modifies FBXW7 expression in non-small cell lung cancer cells.
Comparison of (A) gene and (B) protein expression of FBXW7 in A549
cells in the Mock, Scramble, miR-mimic, OE-FBXW7 and miR-mimic +
OE-FBXW7 groups. Comparison of (C) gene and (D) protein expression
of FBXW7 in H1299 cells in the Mock, Scramble, miR-mimic, OE-FBXW7
and miR-mimic + OE-FBXW7 groups. *P<0.05, **P<0.01 and
***P<0.001; ns, no significance. miR-182, microRNA-182; FBXW7,
F-box and WD repeat domain containing 7; OE, overexpression
(vector). |
The miR-182 mimic elevated cell viability while
reducing apoptosis, whereas it impacted the invasiveness of A549
cells to a lesser extent (miR-mimic vs. scramble group; Fig. 6A-C). FBXW7 overexpression decreased
cell viability and invasiveness, whereas it enhanced the apoptosis
of A549 cells (OE-FBXW7 vs. scramble group). In addition, FBXW7
overexpression weakened the effect of the miR-182 mimic on the
aforementioned functions of A549 cells (miR-mimic + OE-FBXW7 vs.
miR-mimic group). Furthermore, similar results were observed in
H1299 cells as those in A549 cells (Fig. 6D-F).
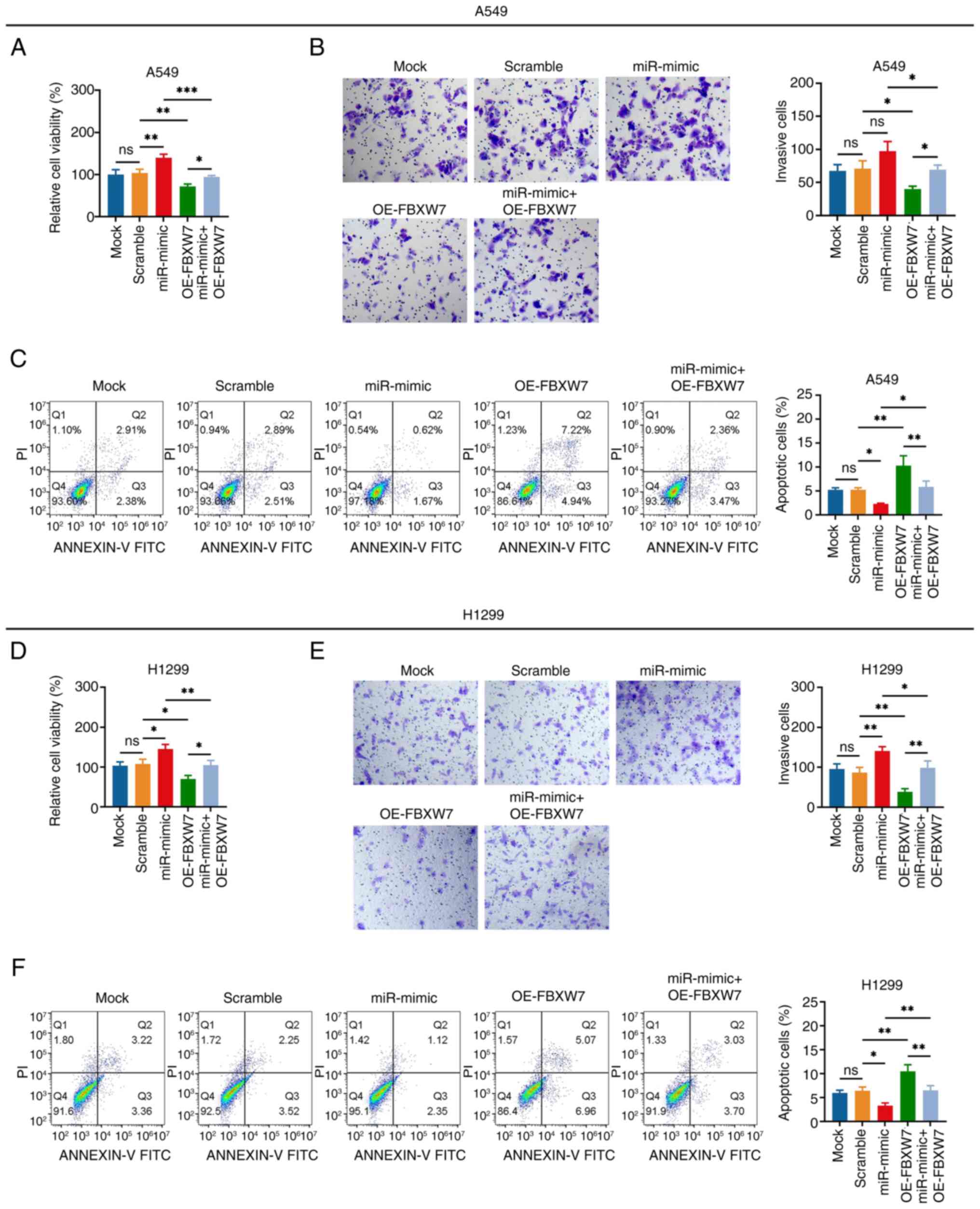 | Figure 6.miR-182 mimic promotes non-small cell
lung cancer cell viability and invasiveness via targeting FBXW7.
Comparison of the (A) viability, (B) number of invasive cells
(magnification, ×200) and (C) apoptosis rate of A549 cells in the
Mock, Scramble, miR-mimic, OE-FBXW7 and miR-mimic + OE-FBXW7
groups. Comparison of the (D) viability, (E) number of invasive
cells (magnification, ×200) and (F) apoptosis rate of H1299 cells
in the Mock, Scramble, miR-mimic, OE-FBXW7 and miR-mimic + OE-FBXW7
groups. *P<0.05, **P<0.01 and ***P<0.001; ns, no
significance. miR-182, microRNA-182; FBXW7, F-box and WD repeat
domain containing 7; OE, overexpression (vector). |
Effect of miR-182 and FBXW7 on AKT and
ERK expression in NSCLC cells
The miR-182 mimic elevated the p-AKT and p-ERK1/2
expression levels in A549 cells (miR-mimic vs. Scramble group;
Fig. 7A). FBXW7 overexpression
reduced the p-AKT and p-ERK1/2 expression levels in A549 cells
(OE-FBXW7 vs. scramble group) and attenuated the effect of miR-182
mimic (miR-mimic + OE-FBXW7 vs. miR-mimic group). Subsequently, the
aforementioned observations were also verified in H1299 cells
(Fig. 7B).
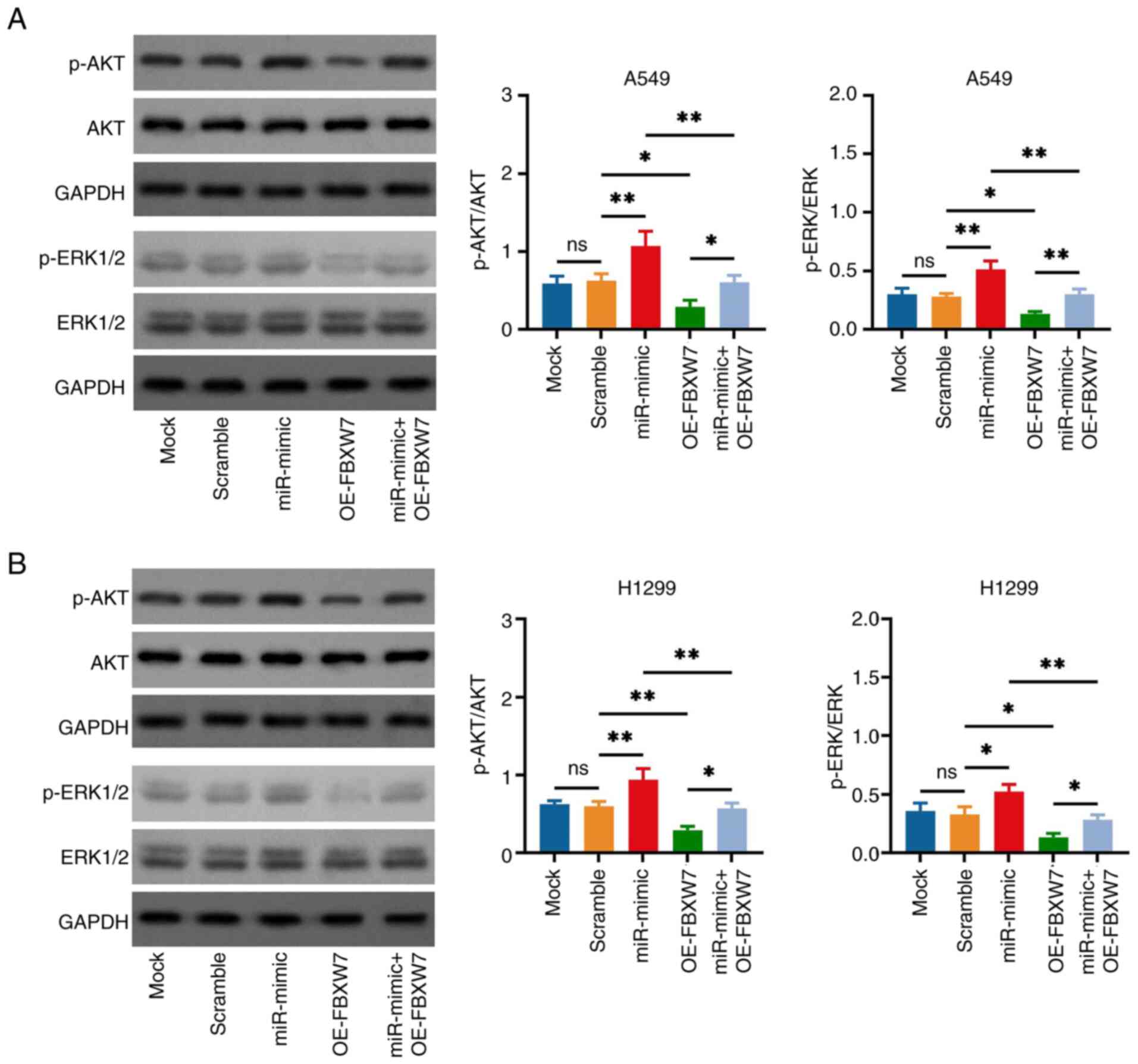 | Figure 7.miR-182 mimic and FBXW7 OE vector
modifies AKT and ERK signaling in non-small cell lung cancer cells.
Comparison of p-AKT and p-ERK1/2 expression levels in (A) A549 and
(B) H1299 cells in the Mock, Scramble, miR-mimic, OE-FBXW7 and
miR-mimic + OE-FBXW7 groups. p-ERK1/2 was analyzed using ERK1/2 as
a reference control, p-AKT was analyzed using AKT as a reference
control, and the loading control (GAPDH) was set to ensure that the
loading amount of all samples was consistent. *P<0.05 and
**P<0.01; ns, no significance. miR-182, microRNA-182; FBXW7,
F-box and WD repeat domain containing 7; OE, overexpression
(vector); p-, phosphorylated. |
Discussion
CA-MSCs are a group of cells derived from MSCs that
interact with cancer cells; they present essential roles in the
development of the tumor microenvironment and are involved in
oncogenesis (6). Since this concept
was proposed in recent years and the related research studies are
insufficient, the detailed mechanism of CA-MSCs remains unclear.
However, several reviews have preliminarily summarized the
potential effects of CA-MSCs on regulating the microenvironment or
modulating progression in various types of cancer, such as gastric,
breast, ovarian and lung cancer (11,26,27).
The possible effects of CA-MSCs can be categorized as follows: i)
Differentiation into other stromal components that promote tumor
development; ii) suppression of the immune responses; iii)
facilitating angiogenesis; iv) promoting EMT in tumor cells; v)
enhancing tumor cell stemness; and vi) inhibiting tumor cell
death.
The present study was based on previous studies that
examined the function of MSCs in cancer or other diseases via
delivering exosomes and related molecules (28,29);
moreover, a recent report indicated that CA-MSCs enhance
hepatocellular carcinoma via transmitting exosomal TMBIM6 (12). It was therefore hypothesized that
CA-MSCs may facilitate NSCLC progression via delivering exosomes.
The present study observed that CA-MSCs promoted NSCLC cell
viability and invasiveness; however, following repression of
exosome secretion, this effect was weakened. Furthermore, direct
addition of CA-MSC exosomes similarly enhanced NSCLC cell viability
and invasiveness. These observations verified that CA-MSCs may
enhance NSCLC cell viability and invasiveness by delivering
exosomes. Although some studies have revealed that CA-MSCs promote
lung cancer tumorigenesis and metastasis (10,30),
to the best of our knowledge, the implication of exosomes in the
function of CA-MSCs in lung cancer progression has not yet been
reported. The possible explanations of the aforementioned findings
are the following: MSCs present with tumor-promoting and antitumor
functions simultaneously, which is determined by the source of MSC,
tumor type and tumor features (31,32).
Then, following the induction of NSCLC cells by co-culture with
MSCs, the transformed CA-MSCs demonstrate sufficient/elevated
levels of markers related to NSCLC progression (for instance,
miR-182 in the present study). Subsequently, CA-MSCs transmit
proteins, lipids and RNAs that demonstrate an oncogenic role in
NSCLC cells (for instance, miR-182 in the present study), which
results in promoting the viability and invasiveness of NSCLC
cells.
miR-182 is a well-known oncogene identified in
various cancer types and specifically in NSCLC (14–19).
Notably, the tumor-promoting role of miR-182 in NSCLC was observed
in our previous study (20).
Besides, miR-182 was found to be enriched in CA-MSC exosomes in the
present study. Therefore, miR-182 was selected for verification.
The present study demonstrated that CA-MSCs and CA-MSC exosomes
transmitted sufficient levels of miR-182 to NSCLC cells. miR-182
expression was knocked down in CA-MSCs to detect whether it was
important for the carcinogenetic effect of CA-MSCs in NSCLC. The
present study assessed whether knockdown of miR-182 expression
attenuated the effect of CA-MSC exosomes on NSCLC viability and
invasiveness, and the results indicated that CA-MSC exosomes may
promote NSCLC progression by delivering miR-182. The explanations
for this finding may be as follows: i) The co-culture of NSCLC
cells and MSCs increased the expression levels of the oncogene,
miR-182, in CA-MSCs, which was subsequently transmitted via
exosomes; and ii) miR-182 promoted NSCLC viability and invasiveness
via multiple mechanisms of action, such as the regulation of
IL-8/STAT3, RNA binding motif protein 5, endothelial PAS
domain-containing protein 1, FOXO3 and domain protein 13 (14–16,20,33).
Besides, the findings regarding the effect of miR-182 on NSCLC
progression in the present study were in-line with previous studies
(16,20,24,34).
Specifically, previous studies revealed that miR-182 induced
metastasis and EMT in NSCLC (16),
and it increased cell proliferation and colony formation of NSCLC
(16); furthermore, miR-182
promotes the radioresistance of NSCLC (20), and its inhibition could repress
NSCLC progression (34).
In the present study, to further identify the
associated mechanism of action, the target of miR-182 was
investigated. Using miRWalk database prediction, FBXW7 was
identified as a candidate target of miR-182. Subsequently, by
searching the related articles, it was discovered that several
studies have reported that FBXW7 is a direct target of miR-182 in
kidney, lung and breast cancer (23–25).
In the present study, it was further confirmed that miR-182
negatively regulated FBXW7 in NSCLC and sponged FBXW7. This finding
was in line with a previous study reporting that miR-182 represses
FBXW7 in NSCLC cells (24).
Moreover, the present study demonstrated that FBXW7 suppressed
NSCLC viability and invasiveness and attenuated the impact of
miR-182 on these two processes, indicating that miR-182 promoted
NSCLC progression via targeting FBXW7. This finding was in
accordance with a previous study that reported that miR-182
enhances NSCLC proliferation via suppressing FBXW7 (24). The explanation of the finding that
FBXW7 suppressed NSCLC viability and invasiveness and attenuated
the impact of miR-182 on these two processes may be as follows: i)
FBXW7 suppresses NSCLC malignant growth and invasiveness via
multiple mechanisms of action, such as coiled-coil domain
containing 6-induced DNA damage, zinc finger protein SNAI1-mediated
ubiquitin-dependent degradation and ERK3 degeneration (35–37);
and ii) miR-182 directly binds to FBXW7 at the nucleotide sequence
level to silence FBXW7 expression (23–25);
therefore, FBXW7 attenuates the impact of miR-182 on NSCLC cell
function. Furthermore, previous studies uncovered that FBXW7
negatively regulates the AKT and ERK pathways, which are important
in NSCLC progression (38–40). The present study also found that
miR-182 positively while FBXW7 negatively regulated the AKT and ERK
pathways, which was in-line with previous findings in cancer
(23–25,38–40).
Specifically, previous studies revealed that miR-182 targets FBXW7
to promote kidney cancer growth and metastasis (23), NSCLC proliferation and colony
formation ability (24) and breast
cancer proliferation and invasion (25). Furthermore, previous studies also
disclosed that FBXW7 inactivates the AKT and ERK pathways to
regulate the progression of oral squamous cell carcinoma and
esophageal squamous cell carcinoma, respectively (38,39),
and that the AKT and ERK pathways are important in cancer
oncogenesis (40,41).
The findings of the present study uncover the
potency of CA-MSC-derived miR-182 as a treatment target for NSCLC
treatment. However, some limitations of this study still exist.
Firstly, the findings need further exploration in animal
experiments. Secondly, further studies are needed to explore
alternative pathways in addition to the FBXW7-mediated AKT and ERK
pathway.
In conclusion, the present study demonstrated that
CA-MSCs promote NSCLC viability and invasiveness by delivering
exosomal miR-182 in a FBXW7-mediated AKT and ERK-dependent
pathway.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Health Science and
Technology Project (grant no. 2022KY394).
Availability of data and materials
The datasets generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS, XZ and ZL contributed to the study conception
and design. Material preparation, data collection and analysis were
performed by YS, XZ, LY, HD and ZL. The first draft of the
manuscript was written by YS, XZ, LY, HD and ZL. YS and ZL confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024.PubMed/NCBI
|
|
3
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu J and Lin Z: Non-small cell lung cancer
targeted therapy: Drugs and mechanisms of drug resistance. Int J
Mol Sci. 23:150562022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang KH and Ding DC: Role of
cancer-associated mesenchymal stem cells in the tumor
microenvironment: A review. Tzu Chi Med J. 35:24–30. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frisbie L, Buckanovich RJ and Coffman L:
Carcinoma-associated mesenchymal stem/stromal cells: Architects of
the pro-tumorigenic tumor microenvironment. Stem Cells. 40:705–715.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adelipour M, Lubman DM and Kim J:
Potential applications of mesenchymal stem cells and their derived
exosomes in regenerative medicine. Expert Opin Biol Ther.
23:491–507. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papaccio F, Paino F, Regad T, Papaccio G,
Desiderio V and Tirino V: Concise review: Cancer cells, cancer stem
cells, and mesenchymal stem cells: Influence in cancer development.
Stem Cells Transl Med. 6:2115–2125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arena S, Salati M, Sorgentoni G, Barbisan
F and Orciani M: Characterization of tumor-derived mesenchymal stem
cells potentially differentiating into cancer-associated
fibroblasts in lung cancer. Clin Transl Oncol. 20:1582–1591. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hazrati A, Malekpour K, Mirsanei Z,
Khosrojerdi A, Rahmani-Kukia N, Heidari N, Abbasi A and Soudi S:
Cancer-associated mesenchymal stem/stromal cells: Role in
progression and potential targets for therapeutic approaches. Front
Immunol. 14:12806012023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shang C, Ke M, Liu L, Wang C, Liu Y and
Zheng X: Exosomes from cancer-associated mesenchymal stem cells
transmit TMBIM6 to promote the malignant behavior of hepatocellular
carcinoma via activating PI3K/AKT pathway. Front Oncol.
12:8687262022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garnier D, Ratcliffe E, Briand J, Cartron
PF, Oliver L and Vallette FM: The activation of mesenchymal stem
cells by glioblastoma microvesicles alters their exosomal secretion
of miR-100-5p, miR-9-5p and let-7d-5p. Biomedicines. 10:1122022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao MN, Zhang LF, Sun Z, Qiao LH, Yang T,
Ren YZ, Zhang XZ, Wu L, Qian WL, Guo QM, et al: A novel
microRNA-182/interleukin-8 regulatory axis controls osteolytic bone
metastasis of lung cancer. Cell Death Dis. 14:2982023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang F, Pei Y, Xu W and Rong L:
hsa_circ_0003176 suppresses the progression of non-small-cell lung
cancer via regulating miR-182-5p/RBM5 axis. Dis Markers.
2022:84021162022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang W, Yin Y, Bi L, Wang Y, Yao J, Xu L
and Jiao L: MiR-182-5p promotes the metastasis and
epithelial-mesenchymal transition in non-small cell lung cancer by
targeting EPAS1. J Cancer. 12:7120–7129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stafford MYC and McKenna DJ: MiR-182 is
upregulated in prostate cancer and contributes to tumor progression
by targeting MITF. Int J Mol Sci. 24:18242023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Yuan H, Xu H, Zhao H and Xiong N:
Hypoxic cancer-secreted exosomal miR-182-5p promotes glioblastoma
angiogenesis by targeting kruppel-like factor 2 and 4. Mol Cancer
Res. 18:1218–1231. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao F, Yin J, Wang Y, Li H and Wang D:
miR-182 promotes cervical cancer progression via activating the
Wnt/β-catenin axis. Am J Cancer Res. 13:3591–3598. 2023.PubMed/NCBI
|
|
20
|
Chen G, Yu L, Dong H, Liu Z and Sun Y:
MiR-182 enhances radioresistance in non-small cell lung cancer
cells by regulating FOXO3. Clin Exp Pharmacol Physiol. 46:137–143.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cascio S, Chandler C, Zhang L, Sinno S,
Gao B, Onkar S, Bruno TC, Vignali DAA, Mahdi H, Osmanbeyoglu HU, et
al: Cancer-associated MSC drive tumor immune exclusion and
resistance to immunotherapy, which can be overcome by Hedgehog
inhibition. Sci Adv. 7:eabi57902021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao J, Yu U, Li L, Yuan X, Chen S, Xu H,
Yi M and Liu S: circKL inhibits the growth and metastasis of kidney
cancer by sponging miR-182-5p and upregulating FBXW7. Oncol Rep.
47:752022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang H, Liu YH, Wang LL, Wang J, Zhao ZH,
Qu JF and Wang SF: MiR-182 promotes cell proliferation by
suppressing FBXW7 and FBXW11 in non-small cell lung cancer. Am J
Transl Res. 10:1131–1142. 2018.PubMed/NCBI
|
|
25
|
Chiang CH, Chu PY, Hou MF and Hung WC:
MiR-182 promotes proliferation and invasion and elevates the
HIF-1α-VEGF-A axis in breast cancer cells by targeting FBXW7. Am J
Cancer Res. 6:1785–1798. 2016.PubMed/NCBI
|
|
26
|
Razmkhah M, Abtahi S and Ghaderi A:
Mesenchymal stem cells, immune cells and tumor cells crosstalk: A
sinister triangle in the tumor microenvironment. Curr Stem Cell Res
Ther. 14:43–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bussard KM, Mutkus L, Stumpf K,
Gomez-Manzano C and Marini FC: Tumor-associated stromal cells as
key contributors to the tumor microenvironment. Breast Cancer Res.
18:842016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Deng S, Han L, Ren Y, Gu J, He L,
Liu T and Yuan ZX: Mesenchymal stem cells, exosomes and
exosome-mimics as smart drug carriers for targeted cancer therapy.
Colloids Surf B Biointerfaces. 209:1121632022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gemayel J, Chaker D, El Hachem G, Mhanna
M, Salemeh R, Hanna C, Harb F, Ibrahim A, Chebly A and Khalil C:
Mesenchymal stem cells-derived secretome and extracellular
vesicles: Perspective and challenges in cancer therapy and clinical
applications. Clin Transl Oncol. 25:2056–2068. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan C, Chang J, Song X, Qi Y, Ji Z, Liu T,
Yu W, Wei F, Yang L and Ren X: Lung cancer-associated mesenchymal
stem cells promote tumor metastasis and tumorigenesis by induction
of epithelial-mesenchymal transition and stem-like reprogram. Aging
(Albany NY). 13:9780–9800. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yassine S and Alaaeddine N: Mesenchymal
stem cell exosomes and cancer: Controversies and prospects. Adv
Biol (Weinh). 6:e21010502022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Z, Wu Y, Xu Y, Li G, Li Z and Liu T:
Mesenchymal stem cell-derived exosomes in cancer therapy
resistance: Recent advances and therapeutic potential. Mol Cancer.
21:1792022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu X, Wang W, Wu G, Peng C and Liu J:
miR-182-5p serves as an oncogene in lung adenocarcinoma through
binding to STARD13. Comput Math Methods Med. 2021:70743432021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang T, Goel A, Xu X, Wu Y, Tang E, Zhang
F, Li Y, Li H, Cai Y and Weng W: N-mytistoyltransferase 1 and 2 are
potential tumor suppressors and novel targets of miR-182 in human
non-small cell lung carcinomas. Lung Cancer. 171:70–81. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao J, Tang J, Men W and Ren K:
FBXW7-mediated degradation of CCDC6 is impaired by ATM during DNA
damage response in lung cancer cells. FEBS Lett. 586:4257–4263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao G, Li Y, Wang M, Li X, Qin S, Sun X,
Liang R, Zhang B, Du N, Xu C, et al: FBXW7 suppresses
epithelial-mesenchymal transition and chemo-resistance of
non-small-cell lung cancer cells by targeting snai1 for
ubiquitin-dependent degradation. Cell Prolif. 51:e124732018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
An HJ, Lee CJ, Lee GE, Choi Y, Jeung D,
Chen W, Lee HS, Kang HC, Lee JY, Kim DJ, et al: FBXW7-mediated ERK3
degradation regulates the proliferation of lung cancer cells. Exp
Mol Med. 54:35–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, Lin XF, Wang JN and Ren XS: FBXW7
inhibited cell proliferation and invasion regulated by miR-27a
through PI3K/AKT signaling pathway and epithelial-to-mesenchymal
transition in oral squamous cell carcinoma. Eur Rev Med Pharmacol
Sci. 24:3701–3709. 2020.PubMed/NCBI
|
|
39
|
Pan Y, Liu J, Gao Y, Guo Y, Wang C, Liang
Z, Wu M, Qian Y, Li Y, Shen J, et al: FBXW7 loss of function
promotes esophageal squamous cell carcinoma progression via
elevating MAP4 and ERK phosphorylation. J Exp Clin Cancer Res.
42:752023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ullah R, Yin Q, Snell AH and Wan L:
RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer
Biol. 85:123–154. 2022. View Article : Google Scholar : PubMed/NCBI
|





















