Introduction
Global epidemiological surveillance data have
indicated that ~20% of adults will receive a diagnosis of cancer
during their lifespan, with sex-specific mortality differentials
demonstrating that 11.1% of men and 8.3% of women ultimately
succumb to neoplastic diseases (1).
Contemporary epidemiological analyses have revealed that while
refinements in cancer prevention strategies and therapeutic
advancements have improved survival rates, the increasing incidence
of numerous types of cancer alongside the ageing population, is
leading to an increase in the number of cancer cases and
cancer-associated mortalities (2–4).
Therefore, cancer remains a global problem that requires an urgent
solution.
Nuclear receptor subfamily 2, group F, member 2
(NR2F2), also known as COUP-TF II or ARP1, was discovered in 1986
(5). It belongs to the
steroid/thyroid hormone receptor superfamily (6) and serves a role in the transcriptional
regulation of various genes (7). At
present, its natural ligand has not been found, thus it is called
an ‘orphan nuclear receptor’. NR2F2 has an important role in early
embryonic development in humans and mice, and its expression
decreases shortly after birth and is expressed at a very low basal
level in mature individuals (8,9). NR2F2
has emerged as a molecule of translational significance in
contemporary oncology; however, its oncogenic duality
(context-dependent tumor promotion/suppression) remains
mechanistically ambiguous.
The references for the present review were
systematically retrieved from the following databases: PubMed
(https://pubmed.ncbi.nlm.nih.gov/) and
Web of Science (https://www.webofscience.com/wos/woscc/basic-search).
The core search terms were: ‘NR2F2’ or ‘COUP-TFII’ or ‘ARP1’ and
(‘cancer’ or ‘tumor’ or ‘tumor lesion’) and (‘proliferation’ or
‘cell apoptosis’ or ‘extracellular matrix remodeling’ or ‘microRNA’
or ‘long non-coding RNA’). The current study reviewed the study
results regarding the role and mechanisms of NR2F2 in various
tumors (Fig. 1), with the aim of
identifying the clinical application of NR2F2 in targeted cancer
therapy, which could provide a novel strategy to overcome
cancer.
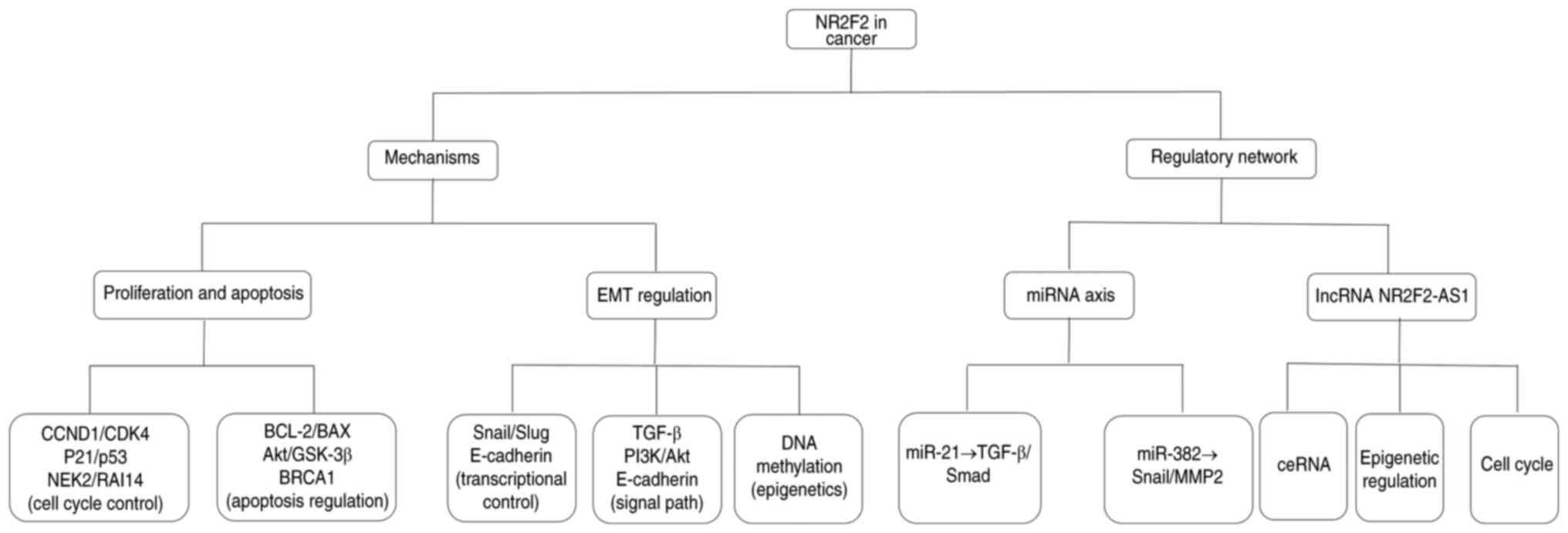 | Figure 1.The mechanism and regulatory network
through which NR2F2 exerts its functions in cancer development and
progression. NR2F2, nuclear receptor subfamily 2, group F, member
2; CCND1, cyclin D1; NEK2, nucleolar and spindle-associated protein
2; RAI14, retinoic acid induced 14; EMT, epithelial-mesenchymal
transition; miRNA/miR, microRNA; ceRNA, competing endogenous RNA;
lncRNA, long non-coding RNA; AS1, antisense 1. |
Mechanism of NR2F2 affecting proliferation
and apoptosis
In most types of cancer, the pathways limiting the
normal cellular proliferative response are disturbed, and mutations
required for tumor development act by shortening the normal
external mitogenic signals of somatic cells (10). Cyclin-dependent kinases (CDKs) are
closely associated with the regulation of cell cycle progression
and have been identified as kinases that can promote cell division
(11). Tumors are diverse and
heterogeneous, but they all have the ability to break through
normal tissue growth restrictions and to initiate abnormal
proliferation. Uncontrolled cell proliferation and the mandatory
compensatory mechanism of inhibiting cell apoptosis work together
to provide the necessary microenvironment for tumor progression
(12). Previous studies (13–19)
have demonstrated that NR2F2 can affect the proliferation,
programmed death and cell cycle changes of various tumor cells
(Table I).
 | Table I.Regulatory axis of NR2F2 in
proliferation and apoptosis. |
Table I.
Regulatory axis of NR2F2 in
proliferation and apoptosis.
| Cancer | Role | Axis | (Refs.) |
|---|
| Liver cancer | Unknown | NR2F2↓ → p53↑,
BCL-2↓ → apoptosis↑ | (13) |
| Ovarian cancer | Unknown | NR2F2↑ → NEK2,
RAI14↑, proliferation↑, apoptosis↑ | (13,14) |
| Renal cell
carcinoma | Oncogene | NR2F2 → BRCA1↓ | (15) |
| Uterine
fibroids | Oncogene | NR2F2 and CTNNB1
high expression co-localization | (16) |
| Colorectal
cancer | Oncogene
suppressor | NR2F2↓ →
Akt/GSK-3β/β-catenin↑ → FOXC1↑ → proliferation↑ | (17) |
|
|
| NR2F2↑ → p53↑,
PTEN↑ → Akt phosphorylation↓ → proliferation↓ | (18) |
| Breast cancer | Oncogene
suppressor | NR2F2 → cyclin D1↑,
p21↑, CDK2↓ → G2/M arrest | (19) |
NR2F2 serves an important role in maintaining venous
properties (6,20). In immortalized human lung
microvascular endothelial cells, NR2F2 regulates cell proliferation
and migration, thereby promoting the regeneration and repair of
vascular endothelial cells. NR2F2 promotes vascular endothelial
proliferation by directly binding to cyclin D1, and it directly
activates the VEGFA/neuropilin 1/VEGFR2 signaling pathway and
promotes cell migration (21).
Cyclin D1 is a cell cycle protein, which belongs to the D-type cell
cycle protein family; this protein serves a crucial role in the
G1 phase of the cell cycle. By forming complexes with
CDK4 and CDK6, it promotes the transition of cells from the
G1 phase to the S phase. Mesenchymal stem cells (MSCs)
isolated from Wharton's jelly (WJ) are highly proliferative and
have a wide range of differentiation potentials (22). Notably, knockdown of NR2F2 in
WJ-MSCs can decrease the expression of proliferation-related
factors cyclin D1 and CDK4, increase the expression of IL-6 and
IL-8 and slow cell proliferation (13). When WJ-MSCs with NR2F2 knockdown are
co-cultured with other cells, the expression levels of cyclin D1,
CDK4 and inflammatory factors are upregulated in the human
fibroblast-like synovial cell line MH7A, whereas in HepG2 hepatoma
cells, the levels of p53, a key regulator of apoptosis, are
elevated, BCL-expression is decreased and the apoptosis rate
increases (13). Therefore, NR2F2
could be considered a key regulator of the cell cycle.
In healthy ovaries, NR2F2 is stably expressed in the
stroma but lowly expressed or not expressed in epithelial cells;
however, this expression pattern is disrupted in ovarian cancer,
where the expression levels in the stroma and ectopic epithelium
are decreased (14). Notably,
upregulating NR2F2 in ovarian cancer cell lines stimulates cell
death while also increasing cell proliferation. Nucleolar and
spindle-associated protein 2 (NEK2) is a serine/threonine kinase
that can phosphorylate centrosomal proteins, driving centrosome
separation and assembly of the mitotic spindle, thus accelerating
the cell cycle process. Specifically, NR2F2 influences ovarian
cancer progression by modulating cell cycle-related genes,
including NEK2 and retinoic acid induced 14; NR2F2 directly binds
to the promoter of NEK2, thereby promoting its transcriptional
expression. In OVCAR-3 cells, overexpression of NR2F2 can increase
the mRNA levels of NEK2, resulting in an increase in the proportion
of S-phase cells from 25 to 44%. Furthermore, overexpression of
NR2F2 in OVCAR-3 cells leads to an increase in Bim protein levels,
resulting in a 37% decrease in mitochondrial membrane potential and
activation of caspase-3, ultimately inducing cell apoptosis. This
contradictory effect is regulated by the PI3K/Akt pathway: When Akt
phosphorylates the Ser194 site of NR2F2, the binding ability of
NR2F2 to the Bim promoter is reduced; at this time, the
proliferative effect dominates. However, in a state of low Akt
activity, NR2F2 preferentially binds to the Bim promoter, and the
pro-apoptotic effect is notable (13,14,23).
In renal cell carcinoma (RCC), the mRNA expression
levels of NR2F2 are markedly higher compared with those in adjacent
non-cancerous tissues, and elevated NR2F2 levels are associated
with worse clinical outcomes in patients (24). When NR2F2 is knocked down in RCC
cells, it hinders cell proliferation, enhances programmed cell
death and causes cell cycle disruption. This mechanism also
triggers the mitochondrial-mediated apoptotic pathway, thereby
effectively inhibiting tumor growth in vivo (25). Knockdown of NR2F2 in RCC cells also
markedly upregulates breast cancer 1, early onset (BRCA1)
expression (2.3-fold increase in protein level), accompanied by
enhanced DNA damage repair and apoptosis. Conversely, co-knockdown
of BRCA1 and NR2F2 partially rescues the proliferation inhibition
caused by NR2F2 depletion, indicating that NR2F2 suppresses BRCA1
transcription via direct binding to its promoter region. This
mechanism aligns with the observation that high NR2F2 levels in RCC
tissues are associated with reduced BRCA1 expression and worse
patient survival (15).
In uterine fibroids, NR2F2 and the cell
proliferation-related factor β-catenin are abnormally highly
expressed, and both have strong vascular-specific localization
characteristics (16).
In colorectal cancer (CRC), knockout of NR2F2 can
activate the Akt/GSK-3β/β-catenin pathway and upregulate FOXC1,
thereby promoting cell proliferation and invasion (17). Overexpression of NR2F2 in SNU-C4, a
human CRC cell line, strongly impairs cell multiplication and
colony generation. Furthermore, it has been shown that p53 is
necessary for inhibiting cell proliferation; on the other hand, the
decreased expression of phosphorylated (p)-Akt enhances the
inhibitory effect of NR2F2 on cell proliferation and invasion
(18). These findings provide new
therapeutic ideas for metastatic CRC.
By exogenous overexpression of NR2F2 in the breast
cancer cell line MDA-MB-435, the cell growth rate can be decreased;
at the same time, NR2F2 can increase the expression of cyclin D1
and p21 in MDA-MB-435 cells (13).
Notably, NR2F2 transduction does not affect the speed of
G1-S phase progression but leads to a prolonged
G2/M phase compared with control cells. Compared with in
parental cells, the CDK2 activity necessary for G2/M
transition is reduced in NR2F2 overexpressing cells (19). Therefore, NR2F2 may influence the
cell cycle progression of breast cancer cells, thereby exerting
regulatory effects on cell proliferation and apoptosis.
Cancer stem cells (CSCs) are a small subset of cells
in tumors that possess the characteristics of self-renewal and
continuous proliferation. These cells can cause the occurrence and
metastasis of tumors, and maintain the heterogeneity of tumors
(26). Notably, CSCs may evade
immune recognition and attack by innate immune cells and adaptive
immune cells (27,28). CSCs have been demonstrated to be one
of the essential conditions for successful cancer metastasis
(29–31); therefore, targeting CSCs may be an
opportunity for treating metastatic diseases. Yes-associated
protein 1 (YAP1) positively regulates numerous genes related to
CSCs and lipid metabolism, and YAP1 is overexpressed in
enzalutamide-resistant (EnzaR) CSCs (28). NR2F2 functions in transcriptional
regulation and mediates the expression of YAP1 induced by
enzalutamide, and interacts with itself to form a transcriptional
complex. Therefore, YAP1 and NR2F2 can be detected in extracellular
vesicles isolated from EnzaR cells and prostate cancer patient
serum, indicating the therapeutic potential of NR2F2 in cancer
(32,33). Mauri et al (24) performed a comparative analysis of
the transcriptional profiles of CSCs in benign tumors and malignant
cutaneous squamous cell carcinoma (SCC) using microarray
technology. The results revealed that NR2F2 was highly expressed in
malignant SCC, and that NR2F2 promoted the malignant tumor state by
controlling the tumor stemness and maintenance of SCC in mice and
humans. Furthermore, NR2F2 is directly regulated by microRNA
(miRNA/miR)-302, which suppresses the promoter activity of OCT4,
weakening the mutual reinforcement between OCT4 and miR-302;
through this mechanism, miR-302 indirectly elevates OCT4 levels,
promoting pluripotency (34). This
indicates that NR2F2 is a key regulatory factor for the function of
malignant CSCs, which can promote tumor renewal and inhibit
differentiation, thereby maintaining the state of malignant
tumors.
NR2F2 and epithelial-mesenchymal transition
(EMT)
The most critical factor that accelerates the
deterioration of the prognosis of patients with cancer is
metastasis, which refers to the migration and invasion of cancer
cells and their settlement and continued growth in specific
locations outside the primary tumor site (35). Metastasis can occur through the EMT
pathway. During EMT, tumor cells undergo a phenotypic shift from
epithelial to mesenchymal characteristics. Key changes include the
downregulation of membrane-bound E-cadherin (leading to weakened
cell adhesion) and upregulation of N-cadherin (enhancing cell
migration) (36). The EMT pathway
mainly involves the remodeling of the cell surface proteome, which
is closely associated with EMT transcriptional regulation and
intermediate filament structural changes, to meet the migration and
invasion requirements of cancer cells during metastasis. When cells
exhibit a mesenchymal phenotype, this change (EMT and its reverse
process, mesenchymal-epithelial transition) is particularly
notable, and during the colonization process at distant metastatic
sites, cells regain an epithelial phenotype. These plastic events
are driven by metabolic conversion combined with direct
transcriptional regulation by TGF-β, but the ability of TGF-β to
induce cell apoptosis is inhibited (37). Studies have reported that NR2F2
participates in regulating the EMT process through multiple
signaling pathways; however, differences exist among the research
results (Table II).
 | Table II.Regulatory axis of NR2F2 in EMT. |
Table II.
Regulatory axis of NR2F2 in EMT.
| Cancer | Role | Axis | (Refs.) |
|---|
| Colorectal
cancer | Oncogene | NR2F2 → activation
of the transcription of miR-21 → TGF-β↑ → EMT↑ | (39) |
|
|
| NR2F2 → Snail↑ →
ZO-1↑, E-cadherin↑, β-catenin↑ | (40) |
| Metastatic
melanoma | Oncogene | DNA methylation →
increased activity of NR2F2 → melanocytes acquire the
characteristics of NCC and EMT | (41) |
| Intrahepatic
cholangiocarcinoma | Oncogene | PI3K/AKT↑ → NR2F2↑
→ Snail↑ → EMT↑ | (42) |
| Gastric cancer | Oncogene | Fbxo21 → NR2F2↓ →
EMT↓ | (43) |
| Prostate
cancer | Oncogene | NR2F2 → TGF-β↓ | (6) |
| Breast cancer | Oncogene
suppressor | NR2F2 → TGF-β↓ →
EMT↓ | (47) |
|
| Oncogene | Insulin → NR2F2↑ →
EMT↑ | (48) |
| Type A ductal
carcinoma of the breast | Oncogene
suppressor | NR2F2-ERα | (40) |
NR2F2 is increased in CRC cells, and is associated
with cancer metastasis and a shorter patient survival time
(38). Notably, NR2F2 serves a
crucial role in the onset and progression of CRC, and it drives the
EMT process in CRC through two mechanisms: i) The upregulation of
NR2F2 promotes the EMT of CRC cells, manifested as a decrease in
E-cadherin and an increase in N-cadherin and vimentin; and ii)
NR2F2 activates the expression of miR-21 through transcription and
inhibits the expression of Smad7, thereby promoting activation of
the TGF-β signaling pathway. The activated TGF-β signaling pathway
further promotes EMT of CRC cells, enhancing cell migration and
invasion (39). Furthermore, in CRC
tissues, the elevated expression of NR2F2 is associated with Snail
upregulation; NR2F2 directly targets the promoter of Snail1 to
regulate the transcription and expression of Snail1, and also
regulates the expression of adhesion molecules such as tight
junction protein 1, E-cadherin and β-catenin, thereby promoting the
metastasis of CRC (40). Therefore,
NR2F2 is considered a biomarker associated with the survival and
metastasis of patients with colon cancer, and may be a new
therapeutic target for CRC.
The formation of metastatic melanoma begins with the
dedifferentiation of transformed melanocytes, resulting in the
formation of migratory and invasive melanoma cells with the
characteristics of neural crest cells (NCCs) and EMT. NR2F2 isoform
2 (NR2F2-Iso2) drives the progression of metastatic melanoma by
regulating the functional activity of full-length NR2F2 (isoform 1)
on EMT and NCC-related target genes (41). During this process, DNA methylation
has a crucial role; its regulation of NR2F2 activity enables
transformed melanocytes to exhibit characteristics similar to NCCs
and EMT. This epigenetic regulation-induced transcriptional
plasticity promotes the transformation and metastatic spread of
cells (41).
The research results of Lang et al (42) indicate that in intrahepatic
cholangiocarcinoma (ICC) cells, the expression of NR2F2 is
excessively expressed at the protein level, but not at the mRNA
level. This suggests that the factors and/or signaling pathways
that promote the development of ICC may have upregulated the
expression of NR2F2 in ICC cells at the post-transcriptional level.
They speculate that the PI3K/Akt signaling pathway may be the main
mechanism leading to the upregulation of NR2F2 protein expression
in ICC (42). Higher NR2F2
expression levels in ICC are associated with enhanced tumor growth,
lymph node spread and reduced survival. Moreover, the activation of
PI3K/Akt signaling can promote the upregulation of NR2F2 protein
expression in ICC, and NR2F2 promotes EMT in ICC cells through
Snail upregulation. These findings suggest that NR2F2 promotes EMT
and metastasis in ICC, indicating that NR2F2 could be a potential
target for adjuvant therapy in these patients (42).
In gastric cancer, F-box protein 21 (Fbxo21)
inhibits EMT by downregulating NR2F2. The expression levels of
Fbxo21 are negatively associated with those of NR2F2 in gastric
cancer tissues and cell lines (43).
Analysis of samples from patients with prostate
cancer has also demonstrated the biological importance of NR2F2 in
the development of prostate cancer. Notably, the expression levels
of NR2F2 have a notable association with tumor recurrence and
disease advancement, exhibiting a negative association with TGF-β
signaling (44). These findings
indicate that the disruption of TGF-β-dependent barriers by NR2F2
is crucial for the development of phosphatase and tensin homolog
(PTEN)-mutant prostate cancer into a life-threatening disease, and
support NR2F2 as a potential drug target for the intervention of
metastatic human prostate cancer (6).
Certain studies have predicted that NR2F2 is
negatively associated with genes related to EMT and TGF-β signaling
pathways (45,46). The expression of NR2F2 inhibits
TGF-β-induced EMT and suppresses breast cancer metastasis; this has
been supported by observing changes in EMT-like morphology,
alongside a decrease in E-cadherin and an increase in Slug
expression, which are known EMT markers (45). Table
III provides a detailed description of the functional
heterogeneity of NR2F2 in different breast cancer subtypes. After
being treated with insulin, the expression levels of NR2F2 are
increased in breast cancer cells (MCF-7 and MDA-MB-231), and the
high expression of NR2F2 promotes the invasion and migration of
breast cancer cells, accompanied by a decrease in E-cadherin
expression and an increase in N-cadherin and vimentin expression
(45). Some authors have also
proposed that it is inappropriate to generalize all subtypes of
breast cancer. In specific breast cancer subgroups, such as in
patients with type A ductal carcinoma of the breast, higher
expression of NR2F2 is associated with improved survival rates.
Chromatin immunoprecipitation and high-throughput sequencing
methods have been used to map the genomic NR2F2 and estrogen
receptor (ER)α binding sites in type A ductal breast carcinoma
cells, revealing that most NR2F2 overlaps with ERα. Transcriptome
analysis of breast cancer cells with low NR2F2 expression has shown
that NR2F2 is associated with endocrine therapy resistance and has
confirmed that NR2F2 is a target gene of ERα. This indicates that
NR2F2 may serve a key role in ERα-mediated transcription and could
provide a potential therapeutic target for patients with luminal
A-type breast cancer (7).
 | Table III.Functional differences and mechanisms
of NR2F2 in different subtypes of breast cancer. |
Table III.
Functional differences and mechanisms
of NR2F2 in different subtypes of breast cancer.
| Subtypes | Role | Axis | (Refs.) |
|---|
| Luminal A
(ER+/HER2−) | Suppressor | NR2F2 + ERα →
cyclin D1↓ p53-PTEN↑-p-Akt↓ | (7,45) |
| HER2+
(ER−/HER2+) | Oncogene | ERK1/2 → p-NR2F2 →
binding with β-catenin↑ → EMT↑ N-cadherin↑ | (47) |
| Triple-negative
breast cancer | Bidirectional
regulation | Mut-p53: NR2F2 →
YAP1↑ → stemness↑ | (7,47) |
| Luminal B
(ER+/HER2+) | Mixed-effects | Estrogen↓: NR2F2 +
ERα → proliferation↓ Insulin↑: p-NR2F2 → migration↑ | (7,47) |
As well as in cancer, pulmonary fibrosis is also
closely related to EMT of type II alveolar epithelial cells
(AECII). The long non-coding RNA (lncRNA)-ASLNC12002 is highly
expressed in the AECII of patients with sepsis-induced acute
respiratory distress syndrome, and the enhanced expression of
ASLNC12002 leads to inactivation of the NR2F2/miR-128-3p/Snail1
pathway, thereby enabling the EMT progression of AECII in patients
with sepsis-induced acute respiratory distress syndrome (40). This study suggests that NR2F2 may
have a universal role in EMT regulation across different diseases,
providing a cross-system reference basis for understanding the
universal function of NR2F2 in cell phenotypic transformation.
NR2F2 and miRNA
miRNAs are a type of non-coding RNA that are 18–22
nucleotides long. By interacting with the 3′UTR of mRNA, miRNAs
reduce the stability of mRNA or inhibit its translation efficiency,
thereby suppressing gene expression at the post-transcriptional
level (45). To date, a large
number of miRNAs have been recognized as either oncogenes or tumor
suppressor genes, and they have been extensively studied in the
field of cancer diagnosis and treatment (46–49).
NR2F2 also interacts with miRNAs (Table IV).
 | Table IV.Regulatory axis of NR2F2 and
miRNAs. |
Table IV.
Regulatory axis of NR2F2 and
miRNAs.
| Cancer | Role | Axis | (Refs.) |
|---|
| Colorectal
cancer | Oncogene | NR2F2 → miR-21↑ →
TGF-β↑ → EMT↑ | (39) |
|
|
| NR2F2 → miR-382↓ →
proliferation, migration, invasion↑ | (53) |
|
|
| NR2F2 → miR-34a↓ →
EMT↑ | (54) |
| Gastric cancer | Oncogene | NR2F2 → miR-27b↓ →
tumor metastasis↑ | (55) |
| Prostate
cancer | Oncogene | miR-382 → NR2F2↓ →
Snail↓, MMP2↓ → proliferation, migration, invasion↓ | (67) |
Increased NR2F2 expression is associated with
unfavorable survival rates in patients with CRC. Notably, in CRC
NR2F2 can promote TGF-β-induced EMT by transactivating miR-21
(39). Some researchers have also
discovered that when NR2F2 is upregulated, it reduces the
suppressive impact of miR-382 on the proliferation, migration and
invasiveness of CRC cells (50).
Furthermore, the tumor suppressor miR-34a is negatively associated
with NR2F2 expression, and NR2F2 promotes EMT by inhibiting the
expression of miR-34a in CRC (51).
The Oncomine (https://www.oncomine.org) and Kaplan-Meier plotter
(http://kmplot.com/analysis) databases
indicate that NR2F2 is upregulated in gastric cancer and is
associated with a lower survival rate. Furthermore, miR-27b can
bind to the 3′UTR region of NR2F2 mRNA and inhibit its expression;
knockdown of NR2F2 reduces the migration and invasion abilities of
gastric cancer cells. These results indicate that miR-27b
suppresses gastric cancer metastasis through targeting NR2F2
(52).
Upregulation of miR-382 expression can markedly
inhibit the proliferation, migration and invasion of prostate
cancer cells (53). The results of
dual luciferase reporter assay, reverse transcription-quantitative
polymerase chain reaction and western blot analysis have confirmed
that NR2F2 is a direct target of miR-382. Notably, the expression
of downstream genes of NR2F2 (such as Snail and matrix
metalloproteinase 2) is inhibited by miR-382. miR-382 can inhibit
the proliferation and metastasis of prostate cancer cells by
suppressing NR2F2, providing crucial insights into how prostate
cancer develops and suggesting a new molecular target for potential
treatments (53).
lncRNA NR2F2 antisense RNA 1
(NR2F2-AS1)
In previous years, lncRNAs have drawn considerable
interest in cancer research owing to their crucial functions in
this disease (54,55). NR2F2-AS1 is a lncRNA, and its gene
is located in the antisense direction of the NR2F2 gene. It is
located in the chromosomal region 15q26.2 and contains 12 exons.
Previous studies have shown that NR2F2-AS1 serves a notable role in
the occurrence and progression of various types of cancer (Table V) (41,46,56–65).
 | Table V.Regulatory axis of the long
non-coding RNA NR2F2-AS1 in cancer. |
Table V.
Regulatory axis of the long
non-coding RNA NR2F2-AS1 in cancer.
| Cancer | Role | Axis | (Refs.) |
|---|
| Non-small cell
lung | Oncogene | NR2F2-AS1↓ →
miR-320b/BMI1 → apoptosis↑ | (56) |
| cancer |
| NR2F2-AS1↑ →
miR-545-5p↓ → c-Met/BVR/ATF-2↑ → EMT↑ | (57) |
| Prostate
carcinoma | Oncogene | NR2F2-AS1↑ → CDK4↑
→ proliferation↑ | (58) |
| Osteosarcoma | Oncogene | NR2F2-AS1 →
miR-425-5p↓ → HMGB2↑ → proliferation, growth, invasion and
inhibition of apoptosis↑ | (59) |
| Colorectal
cancer | Oncogene | NR2F2-AS1↓ → cyclin
D1↓ → G0/G1 arrest | (60) |
|
|
|
miR-106b/NR2F2-AS1/PLEKHO2/MAPK | (61) |
| Cervical
cancer | Oncogene | NR2F2-AS1↑ →
miR-4429/MBD1↑ → proliferation, growth, invasion and inhibition of
apoptosis↑ | (62) |
| Clear cell Renal
cell carcinoma | Oncogene | NR2F2-AS1↑ → Rac1↑
→ stem cell characteristics↑ | (63) |
| Nasopharyngeal
carcinoma | Oncogene | NR2F2-AS1↑ → PTEN↓
→ proliferation↑ apoptosis↓ | (64) |
| Gastric cancer | Oncogene
suppressor | NR2F2-AS1↓ →
miR-320b↓ → PDCD4↓ | (65) |
| Oral squamous
cell | Oncogene | NR2F2-AS1↑ →
miR-494↑ → miR-494 methylation↓ → proliferation↓ | (41) |
| carcinoma
cells | suppressor | NR2F2-AS1↑ →
miR-32-5p/SEMA3A → HUVECs↓, tumor metastasis↓ | (45) |
lncRNA NR2F2-AS1 was initially identified as being
anomalously high expressed in non-small cell lung cancer (NSCLC).
Downregulation of lncRNA NR2F2-AS1 can regulate the miR-320b/BMI1
proto-oncogene, polycomb ring finger mechanism, promote apoptosis
of A549 and SPC-A-1 cells, and inhibit cell proliferation and
invasion (66). Furthermore, in
NSCLC tissues and cells, in addition to high expression of lncRNA
NR2F2-AS1, c-Met, biliverdin reductase (BVR) and activating
transcription factor 2 (ATF-2) are also highly expressed, whereas
miR-545-5p is downregulated. The results of a previous study have
shown that knocking down NR2F2-AS1 or upregulating the expression
levels of miR-545-5p can notably inhibit the proliferation,
migration, invasion and EMT process of NSCLC cells. After lncRNA
NR2F2-AS1 inhibits miR-545-5p, it activates the EMT process through
the c-Met/BVR/ATF-2 axis, thereby promoting the development of
NSCLC. Studies have shown that regulating NR2F2-AS1 and miR-545-5p
may be an effective method for improving the treatment of NSCLC
(66,67).
lncRNA NR2F2-AS1 is highly expressed in prostate
carcinoma and is positively associated with CDK4 expression. NR2F2
promotes the proliferation of prostate carcinoma cells and serves a
positive role in the cell cycle (68).
In human osteosarcoma (OS), the lncRNA NR2F2-AS1
exhibits high expression levels and is associated with an
unfavorable prognosis. Furthermore, elevated levels of NR2F2-AS1
can enhance the proliferation and invasive capabilities of OS
cells, while also suppressing their apoptosis (56). By acting as a sponge for miR-425-5p,
NR2F2-AS1 increases high mobility group box 2 (HMGB2) expression,
facilitating OS development. Therefore, the lncRNA
NR2F2-AS1/miR-425-5p/HMGB2 regulatory axis is considered to be a
promising target for the treatment of human OS (56).
The expression levels of NR2F2-AS1 are increased in
CRC and its upregulation is associated with a reduced overall
survival rate among patients with CRC. Cyclin D1 is also
upregulated, and there is a positive association between cyclin D1
and NR2F2-AS1 (57). Small
interfering RNA (siRNA)-mediated silencing of NR2F2-AS1 leads to
decreased cyclin D1 levels and the occurrence of
G0/G1 phase arrest, whereas the
overexpression of cyclin D1 can reverse the
G0/G1 phase arrest caused by the
siRNA-induced silencing of NR2F2-AS1 (57). Compared with in normal tissues in
The Cancer Genome Atlas (https://portal.gdc.cancer.gov), CRC tissues exhibit a
marked increase in miR-106b expression levels. Notably, the direct
interaction between miR-106b and NR2F2-AS1/pleckstrin homology
domain containing O2 (PLEKHO2) has been confirmed using dual
luciferase reporter experiments. Additionally, as a competing
endogenous RNA (ceRNA), NR2F2-AS1 can absorb miR-106b through the
‘sponge’ effect and thereby regulate the expression levels of
PLEKHO2. By affecting the MAPK signaling axis, NR2F2-AS1 and
PLEKHO2 serve a key role in driving CRC progression. Therefore, the
miR-106b/lncRNA NR2F2-AS1/PLEKHO2/MAPK signaling axis may have
potential therapeutic effects in CRC (58).
Elevated expression levels of lncRNA NR2F2-AS1 have
been detected in both cervical cancer cells and tissues. NR2F2-AS1
induces cell proliferation, migration, invasion and the EMT process
by regulating the miR-4429/methyl-CpG binding domain protein 1
axis, and induces cell apoptosis to accelerate the progression of
cervical cancer (59).
The expression levels of NR2F2-AS1 are also
increased in clear cell RCC (ccRCC), and its high expression in
ccRCC tissues is associated with an unfavorable prognosis. Notably,
NR2F2-AS1 may enhance the CSC characteristics of ccRCC by
upregulating Rac1 (60).
In nasopharyngeal carcinoma (NPC), the expression of
NR2F2-AS1 is negatively associated with that of PTEN. NR2F2-AS1
inhibits the proliferation of nasopharyngeal cancer cells and
induces apoptosis by regulating the expression of PTEN. Therefore,
the upregulation of NR2F2-AS1 expression and the downregulation of
PTEN expression indicate a worse survival prognosis for patients
with NPC (61).
Unlike in other types of cancer, NR2F2-AS1 is lowly
expressed in gastric cancer cells. As a ceRNA, NR2F2-AS1 absorbs
miR-320b to downregulate the expression of programmed cell death 4
(PDCD4), thereby inhibiting the development of gastric cancer. The
NR2F2-AS1/miR-320b/PDCD4 pathway suggests a new therapeutic route
for gastric cancer treatment (62).
In oral SCC (OSCC) cells, NR2F2-AS1 may weaken the
metastatic ability of OSCC cells through the miR-32-5p/semaphorin
3A axis, inhibit the angiogenesis of human umbilical vein
endothelial cells, suppress tumor growth and metastasis in mice
(63) and regulate the
proliferation of OSCC cells by inhibiting miR-494 methylation
(64).
Expression, regulatory mechanisms and
prospects of targeting NR2F2 in tumors
The expression characteristics and
functional heterogeneity of NR2F2 in tumors
NR2F2 is broadly expressed across various tissues in
the human body; however, its expression in different tumors
exhibits species-specific and tissue-specific characteristics
(65). Drawing from the
aforementioned existing research, NR2F2 has been extensively
investigated in the context of prostate cancer and seems to exert a
carcinogenic effect (6,53,68).
In CRC, although NR2F2 inhibits the proliferation of tumor cells
(17,18), it promotes the EMT process (39,40)
and also interacts with miRNAs (39,50,51).
In gastric cancer, NR2F2 promotes the EMT process (43) and interacts with miRNAs (52), but the lncRNA NR2F2-AS1 inhibits the
development of gastric cancer (62). The function of NR2F2 in breast
cancer remains highly debated. On the one hand, the expression of
NR2F2 inhibits breast cancer proliferation and metastasis (19,45);
however, on the other hand, NR2F2 expression levels are increased
in insulin-treated breast cancer cells, and the high expression of
NR2F2 promotes breast cancer cell invasion and migration (45). Currently, the research on NR2F2 in
NSCLC, OS, cervical cancer, RCC, uterine leiomyoma, ccRCC, NPC,
metastatic melanoma and ICC remains limited. The limited studies
available generally indicate that NR2F2 may serve a role in
promoting tumor progression (15,16,41,42,56,59–61,66).
Furthermore, the function of NR2F2 in liver cancer and ovarian
cancer is still largely unclear (13,69).
Fig. 2 visually presents the
multi-level regulatory network of NR2F2 in tumors, covering the
interactions among non-coding RNA regulation, cell cycle
regulation, EMT regulation and epigenetic regulation pathways.
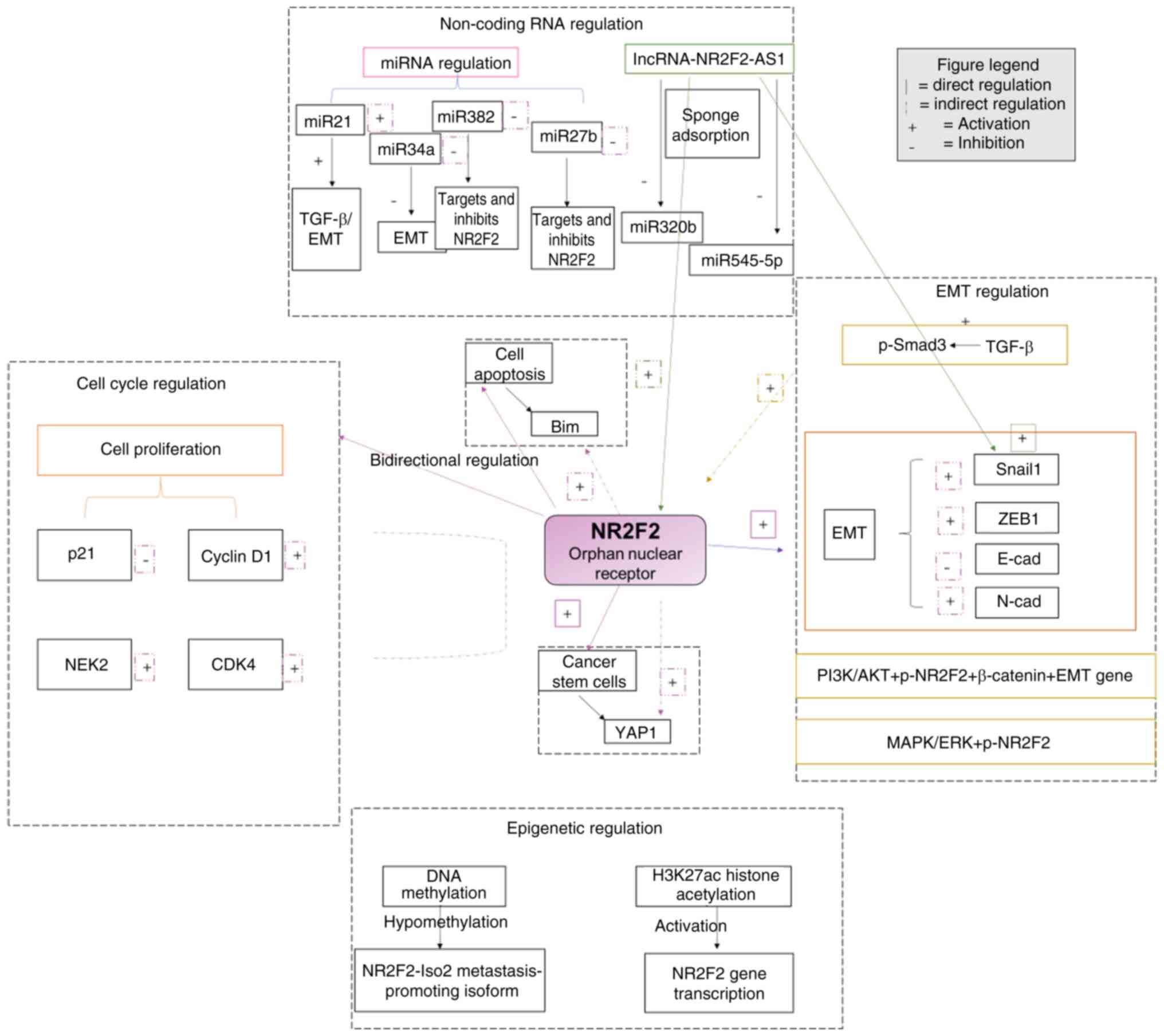 | Figure 2.Panoramic model of the core
regulatory network of NR2F2. NR2F2, nuclear receptor subfamily 2,
group F, member 2; EMT, epithelial-mesenchymal transition;
miRNA/miR, microRNA; lncRNA, long non-coding RNA; NEK2, nucleolar
and spindle-associated protein 2; ZEB1, zinc finger E-box-binding
homeobox 1; cad, cadherin; p, phosphorylated; CDK4, cyclin
dependent kinase; AS1, antisense 1; Iso, isoform. |
NR2F2 exhibits both tumor suppressor and oncogenic
functions in different types of cancer and at different stages of
the same cancer, and there are complex molecular mechanisms
underlying this. The influence of the tissue microenvironment on
the function of NR2F2 is crucial. In breast cancer, the synergistic
effect of ERα and NR2F2 is the core of functional differentiation.
In luminal A-type breast cancer, NR2F2 binds to ERα to form a
transcriptional complex, which activates p53 and PTEN to inhibit
cell proliferation, demonstrating a tumor suppressive effect
(7). In insulin-stimulated breast
cancer cells, the activation of the insulin-PI3K/Akt pathway leads
to phosphorylation of NR2F2 (at Ser294 site), causing it to bind to
β-catenin and promote EMT. At this time, NR2F2 acts as a
carcinogenic factor to promote metastasis (51). In the gastric cancer
microenvironment, the absence of Fbxo21 ubiquitin ligase leads to a
reduction in ubiquitination and degradation of NR2F2, and the
abnormal accumulation of NR2F2 activates Snail to promote EMT,
exerting a carcinogenic effect (43).
The regulatory mechanisms of NR2F2
(including transcriptional isoforms, upstream and downstream
regulation, and epigenetic modifications)
At the same time, NR2F2 has multiple transcriptional
isoforms, and these distinct isoforms may have differences in
structure and function. The expression levels of these isoforms
vary in different tissues or cellular environments, which may lead
to the diversity of the functions of NR2F2. For example, in
metastatic melanoma, NR2F2-Iso2 competitively binds to the target
genes of Iso1 (such as the EMT-related gene Snail1), thereby
relieving the inhibitory effect of Iso1 on metastasis and driving
tumor progression (41).
Post-translational modifications are also important factors in
regulating the function of NR2F2. Studies have shown that
post-translational modifications of other nuclear receptors, such
as phosphorylation and acetylation, can notably affect their
activity, stability and interactions with other molecules (70). In CRC, low methylation of the NR2F2
promoter is notably associated with its high expression, and the
increased levels of H3K27 acetylation in the promoter region
further enhances its transcriptional activity (39). Given the limited scope of tumor
types and the small number of samples examined, the conclusions
regarding the role of NR2F2 in the development of various tumors
remain inconsistent. Thus, the complete role of NR2F2 in cancer
still requires further investigation.
The upstream regulatory factors of NR2F2 are
complex. In breast cancer, an NRAS proto-oncogene, GTPase mutation
activates ERK1/2, phosphorylates the Ser294 site of NR2F2 and
enhances its binding with β-catenin to promote EMT (45). In CRC, TGF-β activates NR2F2 through
Smad3 phosphorylation, and thereby promote the transcription of
miR-21. Meanwhile, miR-21, by targeting and binding to the 3′UTR of
Smad7 mRNA, inhibits its translation process, ultimately leading to
a decrease in the level of Smad7 protein (34,71).
Regarding downstream effectors, in ER-positive breast cancer, NR2F2
regulates ER-dependent transcription programs through multiple
mechanisms, influencing the expression of a series of downstream
genes. For example, NR2F2 inhibits the transcriptional program of
ER by redistributing the binding sites of ER, altering the balance
of transcriptional co-regulators, and modifying chromatin
accessibility (72). In lung cancer
cell lines, NR2F2 can regulate immune-related pathways and cell
cycle-related proteins (such as cyclin D3, cyclin A2, CDK inhibitor
1A and TP53), and affect the immune function and cell cycle
progression of the cells (13).
Epigenetic modifications also serve a role in the
functional regulation of NR2F2. Epigenetic modifications, such as
DNA methylation and histone modifications, can affect gene
expression. In the endometrium, NR2F2 mainly occupies genomic
regions with H3K27ac and H3K4me1 histone modifications, which are
closely associated with the activation state of the genes (73). In melanoma, the expression of the
NR2F2 isoform NR2F2-Iso2 is regulated by DNA methylation. When the
promoter region of NR2F2-Iso2 undergoes low methylation, its
expression is upregulated, thereby promoting the metastasis of
melanoma (41). In terms of
co-transcription factors, NR2F2 interacts with a series of
transcriptional co-regulatory factors in breast cancer, such as
histone deacetylase 1/2 (HDAC1/2), nuclear receptor co-repressor ½
(NCOR1/2), CREB-binding protein (CBP) and FOXA1. These factors work
in synergy with NR2F2 to jointly regulate the transcriptional
activity of ER (7). In other cancer
types, there may also be different co-transcription factors that
cooperate with NR2F2 to participate in the occurrence and
development process of tumors; however, further in-depth research
in this area is still needed.
Research progress on targeted
inhibitors of NR2F2
Currently, while the specific ligand for NR2F2 has
yet to be identified, small-molecule compounds that can suppress
its activity have been discovered (74). Moreover, reducing NR2F2 expression
in the normal tissues of adults does not result in notable damage
to the organism (75). Therefore,
implementing targeted therapy focused on NR2F2 is likely to offer a
promising option for the treatment of malignant tumors.
At present, research on small molecule inhibitors
targeting NR2F2 has made some progress. Researchers have developed
inhibitors, such as Z021, for the ligand-binding domain (LBD) of
NR2F2. Through thermal shift experiments, it has been confirmed
that Z021 can directly bind to NR2F2 (ΔTm=2°C). Moreover, in cell
lines and patient-derived xenograft models, Z021 combined with
fulvestrant can completely eliminate neurofibromin 1-knockout
tumors and markedly inhibit tumor growth in three different
drug-resistant patient-derived xenograft models (72,76).
Challenges and practical obstacles in
the treatment based on targeting NR2F2
However, there are still numerous challenges in
targeting NR2F2 for treatment in cancer therapy. Firstly, NR2F2 is
also expressed at a certain level in normal tissues. How to achieve
specific inhibition of NR2F2 in tumor cells while minimizing the
side effects on normal tissues has become an urgent issue to be
addressed. Secondly, the functions of NR2F2 vary in different
cancer types and at different stages of the same cancer. For
example, in breast cancer, NR2F2 can mediate endocrine therapy
resistance through mechanisms such as interaction with ER in the
ER+ tumor microenvironment and exert carcinogenic
effects (72). In some lung cancer
cell lines, upregulation of NR2F2 can inhibit cell cycle-related
signaling pathways and exert tumor suppressor functions (77). Moreover, the current understanding
of the interaction networks between NR2F2 and other molecules is
not comprehensive. NR2F2 not only interacts with a series of
transcriptional co-regulators (such as HDAC1/2, NCOR1/2, CBP and
FOXA1) to regulate gene transcription (7), but may also affect cell functions
through unknown molecular pathways. This may affect the full
effectiveness of small molecule inhibitors. Despite the challenges,
targeted NR2F2 therapy also holds great potential. NR2F2 serves a
crucial role in the occurrence and development of various types of
cancer, especially in some drug-resistant cancer subtypes. For
example, in the case of endocrine therapy resistance in
ER+ breast cancer, NR2F2 is a key regulatory factor
(72). Developing effective NR2F2
inhibitors is expected to provide novel treatment options for these
refractory cancers. Moreover, as the research on NR2F2 continues,
the understanding of its structure, function and mechanism of
action will be more comprehensive, providing a solid theoretical
basis for the design of more efficient and specific small molecule
inhibitors.
The practical obstacles to targeting NR2F2, such as
the absence of specific ligands and potential off-target effects,
include: i) As an ‘orphan nuclear receptor’, the natural ligand of
NR2F2 has not yet been identified. This has led to the lack of a
clear target for traditional small molecule inhibitors based on
ligand design. Currently developed inhibitors (such as Z021) mainly
target the hydrophobic pocket of the LBD, but this region has a
homology of up to 68% with other nuclear receptors (such as
COUP-TF1 and retinoid X receptor α), which may cause off-target
effects. For example, in vitro experiments have shown that
the IC50 value of Z021 on COUP-TF1 was only 2.3× higher
compared with that on NR2F2, suggesting a potential risk of
hepatotoxicity (72,73). ii) Single-cell sequencing has
revealed that the proportion of NR2F2-expressing cells within the
same tumor could range from 12 to 79% (for example, primary tumor
vs. metastatic tumor), resulting in the ‘targeting gap’ phenomenon.
Residual highly-expressing cells may trigger recurrence (7). iii) The oral bioavailability of the
reported NR2F2 inhibitors is generally low, and their distribution
in tumor tissues is uneven. Although nanocarrier delivery systems
(such as poly lactic-co-glycolic acid nanoparticles encapsulating
Z021) can increase tumor accumulation, they may cause
immunogenicity issues (72).
Future research directions of NR2F2
based on cutting-edge technologies
Focusing on cutting-edge technologies, such as
single-cell analysis and organoid models, the present review
discusses the future research directions of NR2F2. Using
single-cell RNA sequencing (scRNA-seq) technology to analyze the
heterogeneous expression of NR2F2 in tumor cell subpopulations
(such as stem cells and drug-resistant cells) and the
microenvironment (immune cells and fibroblasts), a cell interaction
map could be constructed to reveal the mechanism of functional
differences (78). For example, in
the field of tumor research, scRNA-seq can be utilized to finely
classify NR2F2-positive cells in tumor tissues. Analyzing the
co-expression gene networks of NR2F2 in different subpopulations,
the specific functions of NR2F2 in key tumor phenotypes, such as
proliferation, migration and immune escape, can be clarified. In
CRC, a study has investigated how NR2F2-positive tumor cells
interact with tumor-associated macrophages to influence the
polarization state of macrophages and thereby alter the
immunosuppressive characteristics of the tumor microenvironment
(79). Using tumor organoids
derived from patients to simulate the physiological environment,
the regulatory effect of NR2F2 on tumor growth and invasion can be
verified, which may be used for high-throughput screening of
targeted inhibitors. Combined with gene expression profiles,
personalized treatment markers can be developed. For example, in
the research on liver cancer, tumor tissues from patients have been
used to construct organoids. Through gene editing technology, the
expression of NR2F2 was regulated to observe its effects on the
growth, differentiation and invasion of the organoids (13). In the study of endocrine therapy
resistance in breast cancer, organoids with high expression of
NR2F2 and endocrine therapy resistance were constructed. Small
molecule inhibitors that could reverse resistance were screened,
and at the same time, gene expression profiling analysis was
combined to search for biomarkers that predict drug sensitivity
(72). This may help provide more
precise personalized treatment plans for patients and improve the
clinical efficacy of NR2F2-targeted therapy.
Conclusion
NR2F2 exhibits complex dual functions in various
cancers, functioning as both a tumor suppressor gene and a tumor
promoter gene. Its function is regulated by various factors such as
the tissue microenvironment, differences in transcriptional
isoforms and post-translational modifications. Although there have
been certain studies on the role of NR2F2 in some cancers, its
function remains unclear in numerous cancer types, and the existing
research conclusions show inconsistencies across different tumors.
Moreover, the upstream regulatory factors and downstream effector
molecules of NR2F2 are diverse, and their interactions with
epigenetic modifications and co-transcription factors also affect
its function.
Currently, targeted therapy targeting NR2F2 has
shown certain potential, such as the positive role of the small
molecule inhibitor Z021 in reversing the resistance of breast
cancer to endocrine therapy. However, it still faces practical
challenges such as the lack of specific ligands, potential
off-target effects, tumor internal expression heterogeneity and
drug delivery efficiency. In the future, combining cutting-edge
technologies such as scRNA-seq and organoid models to deeply
analyze the heterogeneous functional mechanisms of NR2F2 in
different tumor subtypes and microenvironments and to develop more
efficient and more specific targeted drugs and delivery systems,
will provide new ideas and strategies for the precise treatment of
NR2F2-related tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZNL performed the integration and analysis of
references and their interpretation, and wrote the manuscript. XY
revised the content of the manuscript. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Pienta KJ, Goodin PL and Amend SR:
Defeating lethal cancer: Interrupting the ecologic and evolutionary
basis of death from malignancy. CA Cancer J Clin. 75:183–202.
2025.PubMed/NCBI
|
|
3
|
Pilleron S, Soto-Perez-de-Celis E, Vignat
J, Ferlay J, Soerjomataram I, Bray F and Sarfati D: Estimated
global cancer incidence in the oldest adults in 2018 and
projections to 2050. Int J Cancer. 148:601–608. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung
H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45.
2025.PubMed/NCBI
|
|
5
|
Yang J, Sun W and Cui G: Roles of the NR2F
family in the development, disease, and cancer of the lung. J Dev
Biol. 12:242024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sajinovic T and Baier G: New Insights Into
The Diverse Functions of the NR2F nuclear orphan receptor family.
Front Biosci (Landmark Ed). 28:132023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erdős E and Bálint BL: NR2F2 orphan
nuclear receptor is involved in estrogen receptor alpha-mediated
transcriptional regulation in luminal a breast cancer cells. Int J
Mol Sci. 21:19102020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumel-Alterzon S, Katz LS, Lambertini L,
Tse I, Heidery F, Garcia-Ocaña A and Scott DK: NRF2 is required for
neonatal mouse beta cell growth by maintaining redox balance and
promoting mitochondrial biogenesis and function. Diabetologia.
67:547–560. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Estermann MA, Grimm SA, Kitakule AS,
Rodriguez KF, Brown PR, McClelland K, Amato CM and Yao HH: NR2F2
regulation of interstitial cell fate in the embryonic mouse testis
and its impact on differences of sex development. Nat Commun.
16:39872025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deshmukh S and Saini S: Phenotypic
heterogeneity in tumor progression, and its possible role in the
onset of cancer. Front Genet. 11:6045282020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pellarin I, Dall'Acqua A, Favero A,
Segatto I, Rossi V, Crestan N, Karimbayli J, Belletti B and
Baldassarre G: Cyclin-dependent protein kinases and cell cycle
regulation in biology and disease. Signal Transduct Target Ther.
10:112025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song G, Liu J, Tang X, Zhong J, Zeng Y,
Zhang X, Zhou J, Zhou J, Cao L, Zhang Q and Li Y: Cell cycle
checkpoint revolution: targeted therapies in the fight against
malignant tumors. Front Pharmacol. 15:14590572024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L, Huang M, Liao X, Cai X and Wu Q:
NR2F2 regulates cell proliferation and immunomodulation in
whartons' jelly stem cells. Genes (Basel). 13:14582022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hawkins SM, Loomans HA, Wan YW,
Ghosh-Choudhury T, Coffey D, Xiao W, Liu Z, Sangi-Haghpeykar H and
Anderson ML: Expression and functional pathway analysis of nuclear
receptor NR2F2 in ovarian cancer. J Clin Endocrinol Metab.
98:E1152–E1162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng J, Qin W, Jiao D, Ren J, Wei M, Shi
S, Xi W, Wang H, Yang AG, Huan Y and Wen W: Knockdown of COUP-TFII
inhibits cell proliferation and induces apoptosis through
upregulating BRCA1 in renal cell carcinoma cells. Int J Cancer.
139:1574–1585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue L, Yang E, Gou J, Nie D, Yi T, Min W
and Li Z: MiR-142-3p may be involved in the development of solitary
and multiple uterine leiomyomasby interacting with CTNNB1 and
AXIN-2 through Wnt signaling pathway. Res Sq. 2020.
|
|
17
|
Yun SH, Han SH and Park JI: COUP-TFII
knock-down promotes proliferation and invasion in colorectal cancer
cells via activation of Akt pathway and up-regulation of FOXC1.
Anticancer Res. 40:177–190. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yun SH and Park JI: COUP-TFII
overexpression inhibits cell proliferation and invasion via
increased expression of p53 and PTEN and decreased Akt
phosphorylation in human colorectal cancer SNU-C4 cells. Anticancer
Res. 40:767–777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lastra D, Escoll M and Cuadrado A:
Transcription factor NRF2 participates in cell cycle progression at
the level of G1/S and mitotic checkpoints. Antioxidants (Basel)
Antioxidants (Basel). 11:9462022.PubMed/NCBI
|
|
20
|
Ferreira LGA, Kizys MML, Gama GAC,
Pachernegg S, Robevska G, Sinclair AH, Ayers KL and Dias-da-Silva
MR: COUP-TFII regulates early bipotential gonad signaling and
commitment to ovarian progenitors. Cell Biosci. 14:32024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao G, Weiner AI, Neupauer KM, de Mello
Costa MF, Palashikar G, Adams-Tzivelekidis S, Mangalmurti NS and
Vaughan AE: Regeneration of the pulmonary vascular endothelium
after viral pneumonia requires COUP-TF2. Sci Adv. 6:eabc44932020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abbaszadeh H, Ghorbani F, Derakhshani M,
Movassaghpour AA, Yousefi M, Talebi M and Shamsasenjan K:
Regenerative potential of Wharton's jelly-derived mesenchymal stem
cells: A new horizon of stem cell therapy. J Cell Physiol.
235:9230–9240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baghal-Sadriforoush S, Bagheri M, Abdi Rad
I and Sotoodehnejadnematalahi F: Melatonin sensitizes OVCAR-3 cells
to cisplatin through suppression of PI3K/Akt pathway. Cell Mol Biol
(Noisy-le-grand). 68:158–169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mauri F, Schepkens C, Lapouge G, Drogat B,
Song Y, Pastushenko I, Rorive S, Blondeau J, Golstein S, Bareche Y,
et al: NR2F2 controls malignant squamous cell carcinoma state by
promoting stemness and invasion and repressing differentiation. Nat
Cancer. 2:1152–1169. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang X, Liu CX, Zeng XR, Huang XM, Chen
WL, Wang Y and Ai F: Orphan nuclear receptor COUP-TFII is an
oncogenic gene in renal cell carcinoma. Clin Transl Oncol.
22:772–781. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu X, Tian W, Ning J, Xiao G, Zhou Y,
Wang Z, Zhai Z, Tanzhu G, Yang J and Zhou R: Cancer stem cells:
Advances in knowledge and implications for cancer therapy. Signal
Transduct Target Ther. 9:1702024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bayik D and Lathia JD: Cancer stem
cell-immune cell crosstalk in tumour progression. Nat Rev Cancer.
21:526–536. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang B, Yan X and Li Y: Cancer stem cell
for tumor therapy. Cancers (Basel). 13:48142021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Sousa e Melo F, Kurtova AV, Harnoss JM,
Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z,
Koeppen H, et al: A distinct role for Lgr5+ stem cells
in primary and metastatic colon cancer. Nature. 543:676–680. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Taftaf R, Kawaguchi M, Chang YF,
Chen W, Entenberg D, Zhang Y, Gerratana L, Huang S, Patel DB, et
al: Homophilic CD44 interactions mediate tumor cell aggregation and
polyclonal metastasis in patient-derived breast cancer models.
Cancer Discov. 9:96–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nallasamy P, Nimmakayala RK, Parte S, Are
AC, Batra SK and Ponnusamy MP: Tumor microenvironment enriches the
stemness features: The architectural event of therapy resistance
and metastasis. Mol Cancer. 21:2252022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HC, Ou CH, Huang YC, Hou PC, Creighton
CJ, Lin YS, Hu CY and Lin SC: Correction: YAP1 overexpression
contributes to the development of enzalutamide resistance by
induction of cancer stemness and lipid metabolism in prostate
cancer. Oncogene. 40:40602021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohammadipoor A, Hershfield MR,
Linsenbardt HR, Smith J, Mack J, Natesan S, Averitt DL, Stark TR
and Sosanya NM: Biological function of extracellular vesicles
(EVs): A review of the field. Mol Biol Rep. 50:8639–8651. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li HL, Wei JF, Fan LY, Wang SH, Zhu L, Li
TP, Lin G, Sun Y, Sun ZJ, Ding J, et al: miR-302 regulates
pluripotency, teratoma formation and differentiation in stem cells
via an AKT1/OCT4-dependent manner. Cell Death Dis. 7:e20782016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging biological principles of metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bakir B, Chiarella AM, Pitarresi JR and
Rustgi AK: EMT, MET, plasticity, and tumor metastasis. Trends Cell
Biol. 30:764–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jinesh GG and Brohl AS: Classical
epithelial-mesenchymal transition (EMT) and alternative cell death
process-driven blebbishield metastatic-witch (BMW) pathways to
cancer metastasis. Signal Transduct Target Ther. 7:2962022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Torrente L, Maan G, Oumkaltoum Rezig A,
Quinn J, Jackson A, Grilli A, Casares L, Zhang Y, Kulesskiy E,
Saarela J, et al: High NRF2 levels correlate with poor prognosis in
colorectal cancer patients and with sensitivity to the kinase
inhibitor AT9283 in vitro. Biomolecules. 10:13652020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Nie L, Wu L, Liu Q and Guo X:
NR2F2 inhibits Smad7 expression and promotes TGF-β-dependent
epithelial-mesenchymal transition of CRC via transactivation of
miR-21. Biochem Biophys Res Commun. 485:181–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng K, Huang W, Shang J, Ping F, Tan Q,
Wang W, Li Y and Cao Y: Knockdown of lncRNA-ASLNC12002 alleviates
epithelial-mesenchymal transition of type II alveolar epithelial
cells in sepsis-induced acute respiratory distress syndrome. Hum
Cell. 36:568–582. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Davalos V, Lovell CD, Von Itter R,
Dolgalev I, Agrawal P, Baptiste G, Kahler DJ, Sokolova E, Moran S,
Piqué L, et al: An epigenetic switch controls an alternative NR2F2
isoform that unleashes a metastatic program in melanoma. Nat
Commun. 14:18672023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lang Q, Xiao P, Zhao M, Liang D, Meng Q
and Pei T: COUP-TFII promotes metastasis and
epithelial-to-mesenchymal transition through upregulating Snail in
human intrahepatic cholangiocarcinoma. Acta Biochim Biophys Sin
(Shanghai). 52:1247–1256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang Y, Liu X, Shen R, Gu X and Qian W:
Fbxo21 regulates the epithelial-to-mesenchymal transition through
ubiquitination of Nr2f2 in gastric cancer. J Cancer. 12:1421–1430.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shao Y, Chan Y, Zhang C, Zhao R and Zu Y:
Dihydroartemisinin modulates prostate cancer progression by
regulating multiple genes via the transcription factor NR2F2. Curr
Pharm Biotechnol. 26:935–955. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xia B, Hou L, Kang H, Chang W, Liu Y,
Zhang Y and Ding Y: NR2F2 plays a major role in insulin-induced
epithelial-mesenchymal transition in breast cancer cells. BMC
Cancer. 20:6262020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liang Q, Xu Z, Liu Y, Peng B, Cai Y, Liu W
and Yan Y: NR2F1 regulates TGF-β1-mediated epithelial-mesenchymal
transition affecting platinum sensitivity and immune response in
ovarian cancer. Cancers (Basel). 14:46392022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dienstmann R, Vermeulen L, Guinney J,
Kopetz S, Tejpar S and Tabernero J: Consensus molecular subtypes
and the evolution of precision medicine in colorectal cancer. Nat
Rev Cancer. 17:79–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang XJ, Wang W and Hann SS: Interactions
among lncRNAs, miRNAs and mRNA in colorectal cancer. Biochimie.
163:58–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma L, Guo H, Zhao Y, Liu Z, Wang C, Bu J,
Sun T and Wei J: Liquid biopsy in cancer current: Status,
challenges and future prospects. Signal Transduct Target Ther.
9:3362024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou B, Song J, Han T, Huang M, Jiang H,
Qiao H, Shi J and Wang Y: MiR-382 inhibits cell growth and invasion
by targeting NR2F2 in colorectal cancer. Mol Carcinog.
55:2260–2267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bao Y, Lu Y, Feng W, Yu H, Guo H, Tao Y,
Shi Q, Chen W and Wang X: COUP-TFII promotes epithelial-mesenchymal
transition by inhibiting miR-34a expression in colorectal cancer.
Int J Oncol. 54:1337–1344. 2019.PubMed/NCBI
|
|
52
|
Feng Q, Wu X, Li F, Ning B, Lu X, Zhang Y,
Pan Y and Guan W: miR-27b inhibits gastric cancer metastasis by
targeting NR2F2. Protein Cell. 8:114–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang W, Liu J, Qiu J, Fu X, Tang Q, Yang
F, Zhao Z and Wang H: MicroRNA-382 inhibits prostate cancer cell
proliferation and metastasis through targeting COUP-TFII. Oncol
Rep. 36:3707–3715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Coan M, Haefliger S, Ounzain S and Johnson
R: Targeting and engineering long non-coding RNAs for cancer
therapy. Nat Rev Genet. 25:578–595. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sanchez Calle A, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ye J, He H, Chen S, Ren Y, Guo W and Jin
Z: Long non-coding RNA NR2F2-AS1 regulates human osteosarcoma
growth and metastasis through miR-425-5p-mediated HMGB2. Int J Clin
Oncol. 27:1891–1903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu J, Qian J, Mo Q, Tang L and Xu Q:
LncRNA NR2F2-AS1 silencing induces cell cycle arrest in G0/G1 phase
via downregulating cyclin D1 in colorectal cancer. Cancer Manag
Res. 12:1835–1843. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu S, An G, Cao Q, Li T, Jia X and Lei L:
The miR-106b/NR2F2-AS1/PLEKHO2 axis regulates migration and
invasion of colorectal cancer through the MAPK pathway. Int J Mol
Sci. 22:58772021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu D, Huang K, Wang T, Zhang X, Liu W,
Yue X and Wu J: NR2F2-AS1 accelerates cell proliferation through
regulating miR-4429/MBD1 axis in cervical cancer. Biosci Rep.
40:BSR201942822020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen L, Zhang D, Ding T, Liu F, Xu X, Tian
Y, Xiao J and Shen H: LncRNA NR2F2-AS1 upregulates rac1 to increase
cancer stemness in clear cell renal cell carcinoma. Cancer Biother
Radiopharm. 35:301–306. 2020.PubMed/NCBI
|
|
61
|
Qin H and Qin C: Downregulation of long
non-coding RNA NR2F2-AS1 inhibits proliferation and induces
apoptosis of nasopharyngeal carcinoma cells by upregulating the
expression of PTEN. Oncol Lett. 19:1145–1150. 2020.PubMed/NCBI
|
|
62
|
Luo M, Deng S, Han T, Ou Y and Hu Y:
LncRNA NR2F2-AS1 functions as a tumor suppressor in gastric cancer
through targeting miR-320b/PDCD4 pathway. Histol Histopathol.
37:575–585. 2022.PubMed/NCBI
|
|
63
|
Qin SY, Li B, Liu JM, Lv QL and Zeng XL:
LncRNA NR2F2-AS1 inhibits the progression of oral squamous cell
carcinoma by mediating the miR-32-5p/SEMA3A axis. Kaohsiung J Med
Sci. 40:877–889. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liang Y, Wu X, Lee J, Yu D, Su J, Guo M,
Meng N, Qin J and Fan X: lncRNA NR2F2-AS1 inhibits the methylation
of miR-494 to regulate oral squamous cell carcinoma cell
proliferation. Arch Oral Biol. 134:1053162022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Polvani S, Pepe S, Milani S and Galli A:
COUP-TFII in health and disease. Cells. 9:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang S, Zhang X, Sun Q, Zhuang C, Li G,
Sun L and Wang H: LncRNA NR2F2-AS1 promotes tumourigenesis through
modulating BMI1 expression by targeting miR-320b in non-small cell
lung cancer. J Cell Mol Med. 23:2001–2011. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu C, Li QG, Zhou Y, Cao YY, Wei ZX, Jin
YH, Wang X, Chen YY, Qi L, Geng JX and Liu F: LncRNA NR2F2-AS1
induces epithelial-mesenchymal transition of non-small cell lung
cancer by modulating BVR/ATF-2 pathway via regulating
miR-545-5p/c-Met axis. Am J Cancer Res. 11:4844–4865.
2021.PubMed/NCBI
|
|
68
|
Fu X, Wang D, Shu T, Cui D and Fu Q:
LncRNA NR2F2-AS1 positively regulates CDK4 to promote cancer cell
proliferation in prostate carcinoma. The Aging Male. 23:1073–1079.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xiao J, Liu H, Yao J, Yang S, Shen F, Bu
KP, Wang X, Liu F, Xia N, Yuan Q, et al: The characterization of
serum proteomics and metabolomics across the cancer trajectory in
chronic hepatitis B-related liver diseases. View. 5:202400312024.
View Article : Google Scholar
|
|
70
|
Yang X, Liu Y, Cao J, Wu C, Tang L, Bian
W, Chen Y, Yu L, Wu Y, Li S, et al: Targeting epigenetic and
post-translational modifications of NRF2: Key regulatory factors in
disease treatment. Cell Death Discov. 11:1892025. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang R, Yang S, Wang M, Zhou Y, Li X, Chen
W, Liu W, Huang Y, Wu J, Jing C, et al: A sustainable approach to
universal metabolic cancer diagnosis. Nat Sustain. 7:602–615. 2024.
View Article : Google Scholar
|
|
72
|
Cai Y, Zhao P, Wu F, Zhao H, Shao H, Marra
A, Patel P, O'Connell E, Fink E, Miele MM, et al: Inhibition of
NR2F2 restores hormone therapy response to endocrine refractory
breast cancers. Sci Transl Med. 17:eadk77862025. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Oh Y, Quiroz E, Wang T, Medina-Laver Y,
Redecke SM, Dominguez F, Lydon JP, DeMayo FJ and Wu SP: The
NR2F2-HAND2 signaling axis regulates progesterone actions in the
uterus at early pregnancy. Front Endocrinol (Lausanne).
14:12290332023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Le Guével R, Oger F, Martinez-Jimenez CP,
Bizot M, Gheeraert C, Firmin F, Ploton M, Kretova M, Palierne G,
Staels B, et al: Inactivation of the nuclear orphan receptor
COUP-TFII by small chemicals. ACS Chem Biol. 12:654–663. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang X, Feng S and Tang K: COUP-TF genes,
human diseases, and the development of the central nervous system
in murine models. Nuclear Receptors in Development and Disease.
Current Topics in Developmental Biology. Elsevier; pp. 275–301.
2017, PubMed/NCBI
|
|
76
|
Wang J, Abhinav P, Xu YJ, Li RG, Zhang M,
Qiu XB, Di RM, Qiao Q, Li XM, Huang RT, et al: NR2F2
loss-of-function mutation is responsible for congenital bicuspid
aortic valve. Int J Mol Med. 43:1839–1846. 2019.PubMed/NCBI
|
|
77
|
Wan R, Long S, Ma S, Yan P, Li Z, Xu K,
Lian H, Li W, Duan Y, Zhu M, et al: NR2F2 alleviates pulmonary
fibrosis by inhibition of epithelial cell senescence. Respir Res.
25:1542024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tostivint V, Racaud-Sultan C, Roumiguié M,
Soulié M, Gamé X and Beauval JB: Progress in prostate cancer study:
3D cell culture enables the ex vivo reproduction of tumor
characteristics. Presse Med. 46:954–965. 2017.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Feng R, Morine Y, Ikemoto T, Imura S,
Iwahashi S, Saito Y and Shimada M: Nrf2 activation drive
macrophages polarization and cancer cell epithelial-mesenchymal
transition during interaction. Cell Commun Signal. 16:542018.
View Article : Google Scholar : PubMed/NCBI
|
















