Introduction
Neoadjuvant chemotherapy has long been an
established treatment for resectable non-small cell lung cancer
(NSCLC). It has demonstrated enhanced efficacy compared with
surgery alone, with a 5% increase in 5-year survival rates
(1). NSCLC accounts for ~85% of all
lung cancer cases and remains the leading cause of cancer-related
mortality worldwide. The integration of immunotherapy with
traditional chemotherapy in the neoadjuvant setting represents a
paradigm shift in the management of resectable NSCLC. Preoperative
chemotherapy combined with immunotherapy has gained traction due to
its potential to enhance antitumor immune responses while targeting
tumor cells directly. Multiple clinical trials and meta-analyses
have shown that this combined approach not only improves
pathological responses but also boosts overall survival compared to
chemotherapy alone (2–4). For instance, programmed death-1 (PD-1)
inhibitors like nivolumab and pembrolizumab have been pivotal in
eliciting durable immune-mediated tumor regression, paving the way
for more favorable surgical conditions.
Combined neoadjuvant chemotherapy and immunotherapy
can transform initially unresectable tumors into resectable tumors,
potentially enhancing surgical outcomes and prognosis (5,6). For
patients with locally advanced NSCLC, particularly those initially
evaluated for pneumonectomy, the combination of neoadjuvant
chemotherapy and immunotherapy offers a strategy to potentially
minimize the scope of surgery. This approach allows more patients
to undergo lobectomy instead of pneumonectomy by shrinking the
tumor and downstaging the disease (7). Reducing the scope of surgery not only
decreases surgical trauma but may also improve prognosis and
quality of life. Furthermore, this downstaging effect is attributed
to the synergistic action of chemotherapy in debulking the tumor
and immunotherapy in activating T-cell responses against residual
disease. However, challenges such as treatment-related toxicities,
including immune-related adverse events, must be carefully managed
to optimize patient outcomes. Recent guidelines from organizations
like the National Comprehensive Cancer Network (NCCN) emphasize
multidisciplinary evaluation to balance the benefits and risks of
neoadjuvant therapies (8).
However, the impact of reducing the surgical scope
in patients receiving neoadjuvant chemoimmunotherapy on the final
efficacy remains a critical question. The safety, efficacy and
long-term survival associated with this treatment approach still
require validation through large-scale clinical studies. Whilst
existing trials (such as CheckMate 816) have demonstrated the
efficacy of neoadjuvant chemoimmunotherapy in enabling downstaging
(9–11), they lack detailed real-world
comparisons of surgical scope reduction after therapy.
Retrospective data uniquely capture intraoperative decision-making
and long-term outcomes in heterogeneous patient populations,
filling this gap. Thus, the present study aimed to retrospectively
analyze real-world data to compare the safety and long-term
efficacy of several surgical methods following neoadjuvant
treatment, thereby providing a foundation for clinical
decision-making. In addition, real-world evidence is crucial for
addressing gaps in randomized controlled trials, such as patient
diversity, comorbidities and long-term follow-up beyond trial
endpoints. By leveraging data from a cohort of 195 patients, this
study provides insights into perioperative complications,
pathological responses and survival metrics, which could inform
future prospective studies and refine clinical protocols for NSCLC
management.
Materials and methods
Patient selection
A retrospective analysis was performed, including
data from 195 patients with NSCLC who were hospitalized between
December 2018 and February 2022 at Hunan Cancer Hospital (Changsha,
China). All patients received neoadjuvant chemotherapy in
combination with immunotherapy, followed by curative-intent
surgery. The timing of surgery was determined by the surgeons after
2–4 cycles of neoadjuvant therapy. Patients were categorized into
three groups according to the type of surgery performed: Lobectomy
group (n=137), reduced surgical scope group (initially assessed as
requiring pneumonectomy but ultimately undergoing lobectomy or
bilobectomy; n=42), and pneumonectomy group (n=16). The reduced
surgical scope group consisted of patients initially assessed by a
multidisciplinary team (MDT) as requiring pneumonectomy based on
preoperative imaging showing extensive tumor involvement, but who
underwent lobectomy or bilobectomy following a neoadjuvant
response. This included patients downgraded specifically to
bilobectomy from pneumonectomy, distinct from bilobectomy patients
in the lobectomy group who were initially planned for bilobectomy
without reduction from pneumonectomy. Decisions were made
collaboratively by the MDT preoperatively and were confirmed
intraoperatively by at least two senior surgeons. The present study
received approval from the Ethics Review Committee of Hunan Cancer
Hospital, and written informed consent was obtained from all
patients. The study adhered to the Declaration of Helsinki (2013
revision).
Patients who met the following criteria were
included in the present study: i) Pathologically confirmed NSCLC
with clinical stage II–III disease (12) that was initially evaluated as
resectable based on enhanced chest and abdominal CT, bone scans,
MRI or PET-CT imaging; ii) age of 18–80 years; iii) no evidence of
distant organ metastasis; and iv) normal organ function. The
exclusion criteria were as follows: i) Pathological results
indicating small cell lung cancer; ii) history of lung or
mediastinal surgery; iii) severe, uncontrolled comorbidities or
active bacterial infections; iv) determination of lack of fitness
for intubation and general anesthesia by the anesthesiologist; v)
pregnant or breastfeeding status; and vi) Eastern Cooperative
Oncology Group performance status (13) score of >2.
Neoadjuvant chemotherapy and
immunotherapy
All patients received neoadjuvant chemotherapy in
combination with immunotherapy. The chemotherapy regimen was
customized according to the characteristics and tumor profile of
each patient, including the use of agents such as cisplatin,
carboplatin, paclitaxel and pemetrexed. The immunotherapy agents
primarily consisted of programmed cell death protein 1/programmed
death-ligand 1 inhibitors, including pembrolizumab and nivolumab.
Treatment generally involved 2–4 cycles, depending on patient
tolerance and response, with efficacy evaluated according to the
Response Evaluation Criteria in Solid Tumors (14).
Surgical procedures
Surgical procedures included lobectomy, bilobectomy
and pneumonectomy, with the surgical approach determined by the
tumor response and the overall condition of the patient following
neoadjuvant therapy. All surgeries aimed at radical resection and
emphasized the importance of achieving clear margins and adequate
lymph node dissection. Pathological complete response (pCR) and
major pathologic response (MPR) were defined as the presence of 0
and ≤10% residual viable tumor cells in the primary tumor and
resected lymph nodes, respectively.
Postoperative adjuvant therapy and
follow-up
Individualized postoperative adjuvant therapy plans,
which included immunotherapy maintenance, chemotherapy and
radiotherapy, were established by an MDT based on postoperative
pathology and patient recovery. Follow-up data were gathered
through outpatient visits and phone calls.
Data collection and statistical
analysis
Patient baseline characteristics, treatment
response, perioperative data, postoperative pathology results,
recurrence and survival data were collected. Categorical variables
were compared using χ2 or Fisher's exact tests, where
appropriate. Continuous variables were assessed for normality, with
nonnormally distributed data (such as hospital stay and operative
time) compared using the Kruskal-Wallis test, followed by pairwise
Mann-Whitney U-tests with Bonferroni correction for post-hoc
analysis where the Kruskal-Wallis result was significant, whereas
normally distributed data were compared using one-way ANOVA,
followed by Tukey's honestly significant difference post-hoc test
for significant results. Survival analyses were performed using the
Kaplan-Meier method with log-rank tests. Proportional hazards
assumptions for Cox models were tested using Schoenfeld residuals
(no violations found). Missing data were minimal (<5% for all
variables) and handled using listwise deletion; no imputation was
needed owing to low rates. Data analysis was performed using SPSS
Statistics, version 27 (IBM Corp.), including χ2 tests,
unpaired t-tests (used for preliminary pairwise comparisons between
specific subgroups or variables with only two groups, such as in
exploratory analyses of binary outcomes). Disease-free survival
(DFS) and overall survival (OS) curves were generated using R
software package (version 4.4; The R Foundation). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The present study included 195 patients diagnosed
with NSCLC. This cohort comprised 137 individuals in the lobectomy
group [129 men; mean age, 60.6±7.1 years; central tumors in 98
patients (71.5%)], 42 patients in the reduced surgical scope group
(39 men; mean age, 57.9±6.8 years) and 16 individuals in the
pneumonectomy group (all men; mean age, 54.4±7.1 years). The
distribution of lesions was as follows: 49 cases (25.1%) in the
left upper lobe, 39 cases (20.0%) in the left lower lobe, 53 cases
(27.2%) in the right upper lobe, 8 cases (4.1%) in the right middle
lobe and 46 cases (23.6%) in the right lower lobe. Initial clinical
staging revealed stage II disease in 37 patients (19.0%) and stage
III disease in 158 patients (81.0%). All patients were deemed
operable, with surgical plans that included lobectomy, bilobectomy,
sleeve resection and pneumonectomy. A total of 66 patients who were
initially evaluated for lobectomy underwent the procedure as
planned in the lobectomy group, whereas 28/29 patients assessed for
bilobectomy ultimately received that procedure in the lobectomy
group. Only one patient who was initially evaluated for bilobectomy
finally received a right pneumonectomy due to tumor invasion and
was assigned to the pneumonectomy group. In summary, the reduced
surgical scope group (n=42) specifically included patients
initially planned for pneumonectomy but downgraded to lobectomy
(n=36) or bilobectomy (n=6) after neoadjuvant therapy; these
bilobectomy patients were distinct from the 28 bilobectomy patients
in the lobectomy group who were initially planned for bilobectomy
(Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
| Final surgical
scope grouping |
|---|
|
|
|
|
|---|
| Variable | Total (n=195) | Lobectomy group
(n=137) | Reduced surgical
scope group (n=42) | Pneumonectomy group
(n=16) |
|---|
| Sex |
|
|
|
|
|
Male | 184 (94.4) | 129 (94.2) | 39 (92.9) | 16 (100.0) |
|
Female | 11 (5.6) | 8 (5.8) | 3 (7.1) | 0 (0.0) |
| Age, years | 59.5±7.2 | 60.6±7.1 | 57.9±6.8 | 54.4±7.1 |
| Smoking
history |
|
|
|
|
|
Non-smoker | 18 (9.2) | 13 (9.5) | 3 (7.1) | 2 (12.5) |
| Former
smoker | 177 (90.8) | 124 (90.5) | 39 (92.9) | 14 (87.5) |
| Location |
|
|
|
|
| LU | 49 (25.1) | 12 (8.8) | 24 (57.1) | 13 (81.2) |
| LL | 39 (20.0) | 28 (20.4) | 9 (21.4) | 2 (12.5) |
| RU | 53 (27.2) | 46 (33.6) | 7 (16.7) | 0 (0.0) |
| RM | 8 (4.1) | 8 (5.8) | 0 (0.0) | 0 (0.0) |
| RL | 46 (23.6) | 43 (31.4) | 2 (4.8) | 1 (6.3) |
| Clinical staging
(pretreatment) |
|
|
|
|
| II | 37 (19.0) | 31 (22.6) | 4 (9.5) | 2 (12.5) |
|
III | 158 (81.0) | 106 (77.4) | 38 (90.5) | 14 (87.5) |
| Planned operation
before treatment |
|
|
|
|
|
Lobectomy | 66 (33.9) | 66 (48.2) | 0 (0.0) | 0 (0.0) |
|
Bilobectomy | 29 (14.9) | 28 (20.4) | 0 (0.0) | 1 (6.2) |
| Sleeve
resection | 43 (22.1) | 43 (31.4) | 0 (0.0) | 0 (0.0) |
|
Pneumonectomy | 57 (29.1) | 0 (0.0) | 42 (100.0) | 15 (93.8) |
Surgical treatment and perioperative
data
A total of 52 patients (26.7%) underwent open
thoracotomy, 80 patients (41%) underwent video-assisted
thoracoscopic surgery (VATS), and 63 patients (32.3%) who were
initially assigned to VATS were reassigned to open surgery. The
primary reasons for conversion included local invasion of the
primary tumor and severe tissue adhesions. Ultimately, 145 patients
(74.4%) underwent lobectomy, 34 patients (17.4%) underwent
bilobectomy and 16 patients (8.2%) underwent pneumonectomy, which
included 15 left pneumonectomies and 1 right pneumonectomy. The
mean operative times for the lobectomy, reduced scope and
pneumonectomy groups were 165 min (range, 133–194), 180 min (range,
150–206) and 150.5 min (range, 140–180.8), respectively (P=0.083).
There was no significant difference in intraoperative blood loss
among the three groups (P=0.406). R0 resection was achieved in 182
patients (93.3%), whereas 13 patients (6.7%) required palliative
resection due to factors such as tumor invasion of the pericardium.
The rates of palliative resection in the groups were 4 cases
(2.9%), 2 cases (4.8%) and 5 cases (31.3%), respectively
(P<0.001). Postoperative intensive care unit (ICU) admission was
necessary for 15 patients (11%), 6 patients (14.3%) and 5 patients
(31.3%), respectively (P=0.076). Severe postoperative complications
occurred in 29 patients (21.2%), 6 patients (14.3%) and 4 patients
(25%) in the lobectomy, reduced scope and pneumonectomy groups,
respectively (P=0.542). Only one patient in the pneumonectomy group
died within 30 days due to severe pulmonary infection. The median
postoperative hospital stay was 6 days (range, 5–8) for the
lobectomy group, 6 days (range, 6–7) for the reduced scope group
and 8 days (range, 7–10) for the pneumonectomy group (P=0.002)
(Table II).
 | Table II.Clinical staging. |
Table II.
Clinical staging.
|
|
| Final surgical
scope grouping |
|
|---|
|
|
|
|
|
|---|
| Variable | Total (n=195) | Lobectomy group
(n=137) | Reduced surgical
scope group (n=42) | Pneumonectomy group
(n=16) | P-value |
|---|
| Preoperative
clinical RECIST evaluation |
|
|
|
|
|
| CR | 16 (8.2) | 14 (10.2) | 2 (4.8) | 0 (0.0) |
|
| PR | 146 (74.9) | 96 (70.1) | 38 (90.5) | 12 (75.0) |
|
| SD | 31 (15.9) | 25 (18.3) | 2 (4.8) | 4 (25.0) |
|
| PD | 2 (1.0) | 2 (1.5) | 0 (0.0) | 0 (0.0) |
|
| Surgical
procedures |
|
|
|
|
|
|
VATS | 80 (41.0) | 72 (52.6) | 4 (9.5) | 4 (25.0) |
|
|
Thoracotomy | 52 (26.7) | 27 (19.7) | 15 (35.7) | 10 (62.5) |
|
|
Conversion to open
surgery | 63 (32.3) | 38 (27.7) | 23 (54.8) | 2 (12.5) |
|
| Reason for
conversion |
|
|
|
| 0.109 |
| Tumor
invasion | 16 (8.2) | 6 (4.4) | 8 (19.1) | 2 (12.5) |
|
|
Calcification of lymph
nodes | 6 (3.1) | 3 (2.2) | 3 (7.1) | 0 (0.0) |
|
|
Intraoperative bleeding | 4 (2.1) | 3 (2.2) | 1 (2.4) | 0 (0.0) |
|
| Tissue
fibrosis adhesions | 10 (5.1) | 8 (5.8) | 2 (4.8) | 0 (0.0) |
|
| Pleural
adhesion | 2 (1.0) | 2 (1.5) | 0 (0.0) | 0 (0.0) |
|
| Procedure |
|
|
|
| <0.001 |
|
Lobectomy | 145 (74.4) | 109 (79.6) | 36 (85.7) | 0 (0.0) |
|
|
Bilobectomy | 34 (17.4) | 28 (20.4) | 6 (14.3) | 0 (0.0) |
|
|
Pneumonectomy | 16 (8.2) | 0 (0.0) | 0 (0.0) | 16 (100.0) |
|
| Sleeve
resection |
|
|
|
| <0.001 |
|
Bronchial sleeve
lobectomy | 58 (29.7) | 41 (29.9) | 17 (40.5) | 0 (0.0) |
|
|
Lobectomy with pulmonary
artery angioplasty | 3 (1.5) | 2 (1.5) | 1 (2.4) | 0 (0.0) |
|
|
Lobectomy with bronchovascular
sleeve resection | 20 (10.3) | 6 (4.4) | 14(31.8) | 0 (0.0) |
|
| Operation time,
min | 167.0 | 165.0 | 180.0 | 150.5 | 0.083 |
|
| (139.0–195.0) | (133.0–194.0) | (150.0–206.0) | (140.0–180.8) |
|
| Blood loss, ml | 200.0 | 200.0 | 200.0 | 200.0 | 0.406 |
|
| (100.0–200.0) | (100.0–200.0) | (100.0–250.0) | (187.5–225.0) |
|
| Resection |
|
|
|
| 0.499 |
|
Complete (R0) | 182 (93.3) | 128 (93.4) | 40 (95.2) | 14 (87.5) |
|
|
Incomplete (R1/R2) | 13 (6.7) | 9 (6.6) | 2 (4.8) | 2 (12.5) |
|
| Postoperative
admission to ICU | 26 (13.3) | 15 (10.9) | 6 (14.3) | 5 (31.3) | 0.076 |
| Postoperative
hospital stay, days | 6 (6–8) | 6 (5–8) | 6 (6–7) | 8 (7–10) | 0.002 |
| Serious
postoperative complications | 39 (20.0) | 29 (21.2) | 6 (14.3) | 4 (25.0) | 0.542 |
Postoperative pathology and
follow-up
Postoperative pathological evaluation revealed that
75 patients (54.7%) in the lobectomy group, 28 patients (66.7%) in
the reduced scope group and 6 patients (37.5%) in the pneumonectomy
group achieved a pCR or MPR (P=0.114). The median postoperative
follow-up duration was 32.26 months (interquartile range
27.33–42.55), with 170 patients (87.2%) still alive and 149
patients (76.4%) exhibiting no signs of recurrence at that time
(Table III).
 | Table III.Surgical details. |
Table III.
Surgical details.
|
|
| Final surgical
scope grouping |
|
|---|
|
|
|
|
|
|---|
| Variable | Total (n=195) | Lobectomy group
(n=137) | Reduced surgical
scope group (n=42) | Pneumonectomy group
(n=16) | P-value |
|---|
| Histology |
|
|
|
| <0.001 |
|
Squamous cell carcinoma | 151 (77.4) | 99 (72.3) | 39 (92.9) | 13 (81.3) |
|
|
Adenocarcinoma | 37 (19.0) | 35 (25.6) | 1 (2.4) | 1 (6.3) |
|
|
Adenosquamous carcinoma | 3 (1.5) | 0 (0.0) | 1 (2.4) | 2 (12.5) |
|
|
Sarcomatoid carcinoma | 3 (1.5) | 2 (1.5) | 1 (2.4) | 0 (0.0) |
|
|
Mucoepidermoid carcinoma | 1 (0.5) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
|
| ypTNM |
|
|
|
| 0.051 |
|
T0N0M0 | 67 (34.4) | 51 (37.2) | 12 (28.6) | 4 (25.0) |
|
|
T1N0 | 49 (25.1) | 34 (24.8) | 14 (33.3) | 1 (6.3) |
|
|
T2aN0 | 15 (7.7) | 9 (6.6) | 5 (11.9) | 1 (6.3) |
|
|
T2bN0 | 3 (1.5) | 2 (1.5) | 0 (0.0) | 1 (6.3) |
|
|
T3N0/T1-2N1 | 20 (10.3) | 10 (7.3) | 7 (16.7) | 3 (18.8) |
|
|
T3N1/T1-2N2/T4N0-1 | 36 (18.5) | 28 (20.4) | 3 (7.1) | 5 (31.3) |
|
|
T3-4N2/T1-2N3 | 5 (2.6) | 3 (2.2) | 1 (2.4) | 1 (6.3) |
|
| Pathological
response |
|
|
|
| 0.114 |
|
pCR | 67 (34.4) | 51 (37.2) | 12 (28.6) | 4 (25.0) |
|
|
MPR | 42 (21.5) | 24 (17.5) | 16 (38.1) | 2 (12.5) |
|
Survival analysis
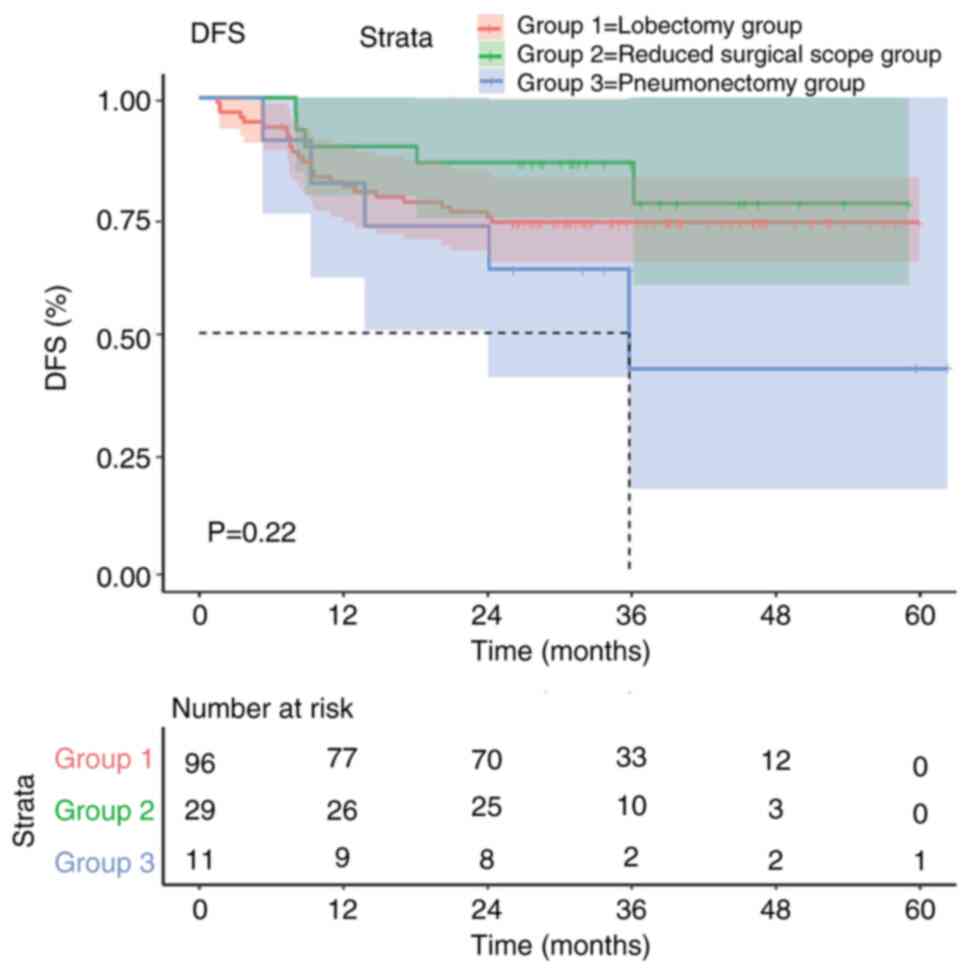
The DFS curve of the pneumonectomy group decreased
relatively quickly, and the median DFS was shorter, suggesting that
this surgical method had the worst effect. By contrast, the
patients in the reduced surgical scope group and the lobectomy
group had improved DFS curves in the first 36 months, and their
long-term survival was also longer. However, there was no
significant difference in DFS among the three groups (P=0.22;
Fig. 1).
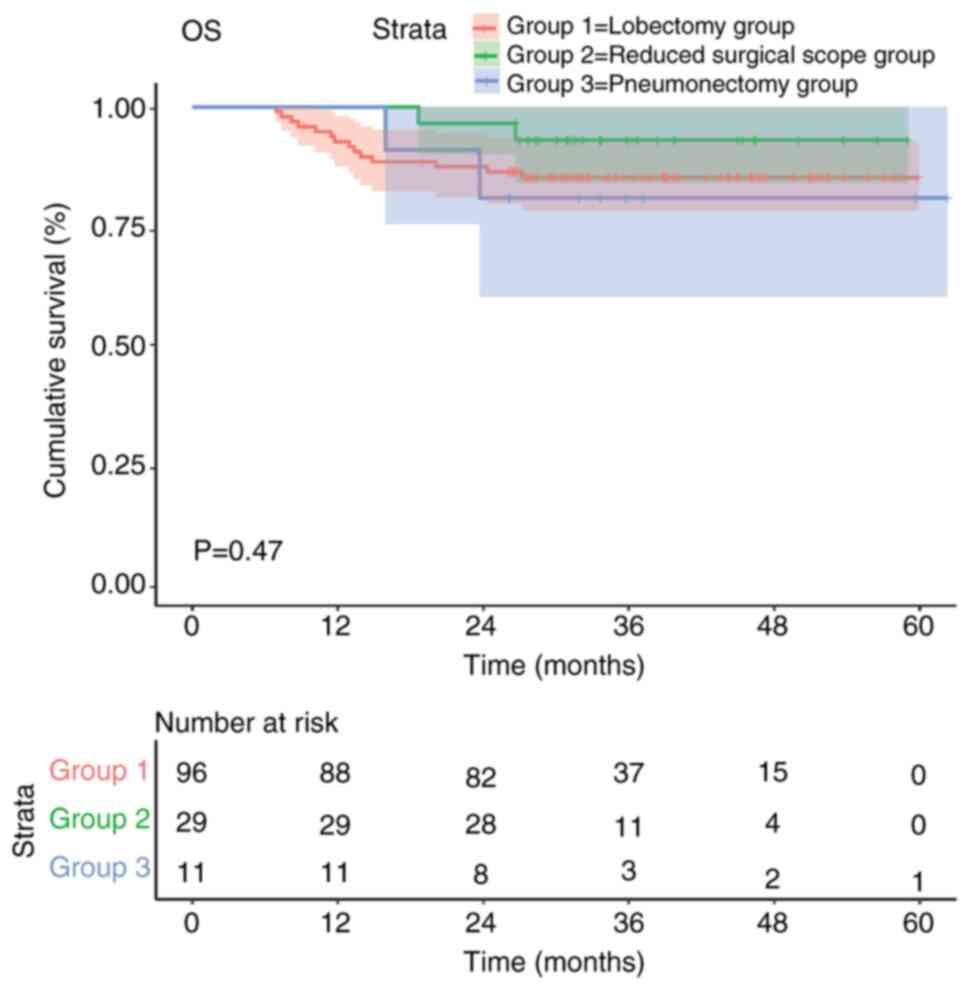
The Kaplan-Meier survival curves also indicated that
there was no statistically significant difference in OS among the
three surgical approaches evaluated for patients with NSCLC
(P=0.47; Fig. 2). Although the
reduced surgical scope group exhibited a greater trend in the
survival curve compared with the other groups, it was not possible
to clearly determine which surgical method was superior due to the
small sample size and lack of statistical significance.
Discussion
Perioperative treatment for NSCLC aims to improve
surgical outcomes and reduce recurrence, thereby increasing OS
rates. Neoadjuvant therapy focuses on shrinking tumors before
surgery, eliminating micrometastatic foci, increasing the
likelihood of complete resection and ultimately reducing
postoperative recurrence whilst extending DFS (15,16).
Recent advancements in perioperative treatment, particularly the
combination of neoadjuvant chemotherapy with immunotherapy, have
yielded promising results (17).
Neoadjuvant chemotherapy, particularly platinum-based regimens, has
been reported to markedly enhance surgical outcomes and decrease
recurrence rates (18). Previous
studies have indicated that neoadjuvant immunotherapy, such as
nivolumab, combined with chemotherapy can notably increase
downstaging rates, resulting in higher event-free and OS rates for
patients with NSCLC than chemotherapy alone (19,20).
In the present study, the rates of pCR across the three groups
ranged from 25–37.2%, which is consistent with other reports
(21–23).
Surgical resection remains the cornerstone of
treatment for stage I–III NSCLC (8). Neoadjuvant therapy does not notably
increase perioperative complication rates, and OS is markedly
improved with integrated treatment strategies, resulting in
substantial increases in 5-year survival rates. This multimodal
approach not only enhances disease control but also provides higher
success rates for surgery and DFS (3,24).
For centrally located NSCLC, determining the
necessity of pneumonectomy is a critical consideration. According
to data from the Checkmate 816, Rationale 315 and AEGEAN studies,
8–25% of patients with NSCLC receive pneumonectomy after
neoadjuvant therapy (11,25,26).
Whilst pneumonectomy may offer benefits for certain patients
(27–29), it is not necessary for all patients.
The goal of lung cancer surgery is to remove tumors effectively
whilst preserving as much lung tissue and function as possible.
Particularly after neoadjuvant therapy, patients who were initially
assessed as requiring pneumonectomy may instead be candidates for
lobectomy, which can preserve more lung function without
compromising treatment efficacy. With advancements in surgical
techniques, alternatives such as bronchoplasty, angioplasty, sleeve
lobectomy and bronchovascular sleeve resection can help avoid
pneumonectomy whilst still achieving radical resection of locally
advanced tumors (30–32).
The high pCR rate of 25% observed in the
pneumonectomy group in the present study suggests that
pneumonectomy may not be necessary for all patients. The potential
to achieve similar outcomes with lobectomy in locally advanced
cases warrants further investigation. Additionally, segmentectomy
or wedge resection in early-stage NSCLC can provide survival rates
comparable with those of lobectomy whilst reducing surgical risk
(33,34). Therefore, for locally advanced
cases, lobectomy as a substitute for pneumonectomy may also be a
feasible strategy.
The present study performed comparisons of baseline
characteristics, perioperative data, postoperative pathology,
recurrence rates and survival outcomes among the lobectomy, reduced
scope and pneumonectomy groups. The results indicated that reducing
the extent of surgery after neoadjuvant therapy, specifically
switching from pneumonectomy to lobectomy, does not lead to
increased local recurrence rates or adversely affect DFS or OS.
Patients in the pneumonectomy group experienced higher rates of ICU
admission, longer hospital stays and increased perioperative
mortality, with only 37.5% achieving pCR or MPR. Therefore,
pneumonectomy should be avoided whenever possible. Based on the
present findings, an individualized multidisciplinary evaluation
after neoadjuvant chemotherapy and immunotherapy-to determine the
feasibility of downstaging and reducing surgical extent (e.g., from
pneumonectomy to lobectomy or bilobectomy)-appears safe and
effective in appropriately selected patients, though this requires
further prospective validation.
The cohort in the present study was predominantly
male, which may reflect the higher incidence of NSCLC in male
smokers in China (35), but limits
generalizability to female patients. In the present study, the
underrepresentation of women may have masked potential sex-specific
benefits or risks of reduced surgical scope post-neoadjuvant
chemoimmunotherapy, such as differences in recurrence rates or
survival trends. Future research should aim for more balanced
cohorts to explore these interactions and enhance applicability
across the sexes.
Additionally, the uneven group sizes [70% of
patients in the lobectomy group (n=137)] stem from real-world
neoadjuvant chemoimmunotherapy dynamics, which enable downstaging
to less extensive resections such as lobectomy in certain cases,
reserving pneumonectomy for a minority. However, this may reflect
therapeutic efficacy, not design flaws. Moreover, it may introduce
bias and reduce statistical power for detecting differences in
survival or recurrence, elevating type II error risks in smaller
subgroups. This common retrospective challenge calls for larger,
balanced prospective studies with propensity matching to improve
generalizability.
Furthermore, although no statistically significant
differences were observed in terms of DFS and OS, the observed
trends suggest improved outcomes in the reduced-scope group (such
as lower recurrence at 14.3 compared with 31.3% in the
pneumonectomy group), potentially due to preserved lung function,
reduced surgical trauma and improved postoperative recovery. These
trends may arise from the selection of patients with favorable
neoadjuvant responses, allowing for less invasive surgery without
compromising oncologic outcomes. Supporting evidence from previous
studies reinforces this, as neoadjuvant chemoimmunotherapy has been
reported to enable tumor downstaging and facilitate reduced
surgical extent, such as shifting from pneumonectomy to lobectomy,
which is associated with improved long-term survival metrics
(36). For example, meta-analyses
and clinical trials report that neoadjuvant immunotherapy combined
with chemotherapy not only enhances pCR but also supports safer
resections with higher event-free survival rates, particularly when
surgical scope is minimized to preserve pulmonary function
(9). Additionally, real-world data
highlights that post-neoadjuvant approaches such as
robotic-assisted thoracic surgery in downstaged patients reduce ICU
stays and complications, contributing to trends in improved DFS by
mitigating perioperative risks (37).
Future studies should concentrate on minimizing the
need for pneumonectomy following neoadjuvant therapy, increasing
the rates of minimally invasive surgeries and reducing
perioperative risks. Research, such as the RATIONALE-315 and
CheckMate 816 studies, suggests that higher rates of minimally
invasive procedures and lobectomies may enhance postoperative
recovery and improve quality of life (25,38,39).
Avoiding pneumonectomy whilst achieving a high rate of pCR or MPR
remains a key focus of future research. The integration of new
adjuvant and neoadjuvant therapies has the potential to improve
outcomes for patients with resectable NSCLC (40). Identifying patients who can safely
avoid pneumonectomy and customizing interventions to reduce
invasiveness whilst enhancing prognosis are priorities for ongoing
research.
However, the present study had several limitations.
The single-center, retrospective design may have introduced
selection bias. Additionally, the relatively small sample size
could have led to statistical bias. As a retrospective,
single-center study without propensity score matching, the results
may be confounded by selection bias (such as reduced-scope patients
may have had improved neoadjuvant responses or fewer
comorbidities). Moreover, comorbidity data were not systematically
collected in the present study, which is a limitation as it could
influence surgical decisions and outcomes; future studies should
include standardized comorbidity assessments (such as the Charlson
Comorbidity Index). However, baseline characteristics were
comparable across groups (Table I)
and multivariate adjustments in survival analyses mitigated bias to
a certain extent, supporting the hypothesis-generating nature of
the findings without invalidating the observed safety trends.
In conclusion, the results of the present study
indicate that reducing the scope of surgery in select patients with
NSCLC after neoadjuvant chemotherapy combined with immunotherapy is
safe and effective. Future research should focus on identifying
patients who are most likely to benefit from this approach and
optimizing neoadjuvant therapy to further enhance treatment
outcomes and quality of life.
Acknowledgements
The abstract was presented at the 33rd Annual
Meeting of the Asian Society for Cardiovascular and Thoracic
Surgery 14–17 May, 2025 in Singapore as an oral presentation.
Funding
The present study was supported by the Sanming Project of
Medicine in Shenzhen (grant no. SZSM202211011).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WL contributed to conceptualization, formal
analysis, investigation and software, and led the writing of the
original draft. JT contributed to formal analysis, and project
administration. XL contributed to project administration. HY
contributed to funding acquisition, formal analysis, investigation,
project administration, and writing-review and editing. WW
contributed to funding acquisition, formal analysis, investigation,
project administration, and writing-review and editing. BZ
contributed to funding acquisition, formal analysis, investigation,
project administration, and writing-review and editing. WL, JT, XL,
LG, DY, HY WW and BZ contributed to data curation, which involved
the systematic collection, organization, validation, and management
of retrospective clinical data from the 195 patients included in
the analysis. BZ and WL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study conformed to the provisions of the
Declaration of Helsinki (as revised in 2013) and was approved by
the Ethics Review Committee of Hunan Cancer Hospital (approval no.
2025-30). All patients signed informed consent forms, and the
consent process ensured that patients were fully informed about the
use of their anonymized data for research and publication purposes,
in accordance with ethical guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee JM, Tsuboi M and Brunelli A: Surgical
perspective on neoadjuvant immunotherapy in non-small cell lung
cancer. Ann Thorac Surg. 114:1505–1515. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang J, Zhang C and Zhong WZ: Neoadjuvant
immunotherapy for non-small cell lung cancer: State of the art.
Cancer Commun (Lond). 41:287–302. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia XH, Xu H, Geng LY, Jiao M, Wang WJ,
Jiang LL and Guo H: Efficacy and safety of neoadjuvant
immunotherapy in resectable nonsmall cell lung cancer: A
meta-analysis. Lung Cancer. 147:143–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng H, Liu J, Cai X, Chen J, Rocco G,
Petersen RH, Brunelli A, Ng CSH, D'Amico TA, Liang W and He J:
Radical minimally invasive surgery after immuno-chemotherapy in
initially-unresectable stage IIIB non-small cell lung cancer. Ann
Surg. 275:e600–e602. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong P, Yan Y, Yang L, Wu D, Wang H, Lv Y,
Zhang J and Yu X: Neoadjuvant immunotherapy improves treatment for
early resectable non-small-cell lung cancer: A systematic review
and meta-analysis. J Oncol. 2022:20852672022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalvapudi S, Vedire Y, Yendamuri S and
Barbi J: Neoadjuvant therapy in non-small cell lung cancer: Basis,
promise, and challenges. Front Oncol. 13:12861042023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen LN, Wei AZ and Shu CA: Neoadjuvant
immunotherapy in resectable non-small-cell lung cancer. Ther Adv
Med Oncol. 15:175883592311637982023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deutsch JS, Cimino-Mathews A, Thompson E,
Provencio M, Forde PM, Spicer J, Girard N, Wang D, Anders RA,
Gabrielson E, et al: Association between pathologic response and
survival after neoadjuvant therapy in lung cancer. Nat Med.
30:218–228. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verma S, Breadner D, Mittal A, Palma DA,
Nayak R, Raphael J and Vincent M: An updated review of management
of resectable stage III NSCLC in the era of neoadjuvant
immunotherapy. Cancers (Basel). 16:13022024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matilla JM, Zabaleta M, Martínez-Téllez E,
Abal J, Rodríguez-Fuster A and Hernández-Hernández J: New TNM
staging in lung cancer (8th edition) and future perspectives. J
Clin Transl Res. 6:145–154. 2020.PubMed/NCBI
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sorin M, Prosty C, Ghaleb L, Nie K,
Katergi K, Shahzad MH, Dubé LR, Atallah A, Swaby A, Dankner M, et
al: Neoadjuvant chemoimmunotherapy for NSCLC: A systematic review
and meta-analysis. JAMA Oncol. 10:621–633. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aguado C, Chara L, Antoñanzas M, Matilla
Gonzalez JM, Jiménez U, Hernanz R, Mielgo-Rubio X, Trujillo-Reyes
JC and Couñago F: Neoadjuvant treatment in non-small cell lung
cancer: New perspectives with the incorporation of immunotherapy.
World J Clin Oncol. 13:314–322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferrari V and Helissey C: Revolutionizing
localized lung cancer treatment: Neoadjuvant chemotherapy plus
immunotherapy for all? J Clin Med. 13:27152024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bunn PA Jr, Mault J and Kelly K: Adjuvant
and neoadjuvant chemotherapy for non-small cell lung cancer: A time
for reassessment? Chest. 117 (4 Suppl 1):119S–122S. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aguado C, Jiménez Maestre UJ and
Mielgo-Rubio X: Neoadjuvant immunotherapy in non-small-cell lung
cancer: Times are changing-and fast. World J Clin Oncol.
13:758–761. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guven DC, Sahin TK and Kilickap S: The
efficacy and safety of neoadjuvant immunotherapy in patients with
non-small cell lung cancer. Cancers (Basel). 16:1562023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Provencio M, Nadal E, González-Larriba JL,
Martínez-Martí A, Bernabé R, Bosch-Barrera J, Casal-Rubio J, Calvo
V, Insa A, Ponce S, et al: Perioperative nivolumab and chemotherapy
in stage III non-small-cell lung cancer. N Engl J Med. 389:504–513.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Provencio M, Nadal E, Insa A, García
Campelo R, Casal J, Dómine M, Massuti B, Majem M, Rodríguez-Abreu
D, Martínez-Martí A, et al: Perioperative chemotherapy and
nivolumab in non-small-cell lung cancer (NADIM): 5-Year clinical
outcomes from a multicentre, single-arm, phase 2 trial. Lancet
Oncol. 25:1453–1464. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Chen KN, Chen Q, Wu L, Wang Q, Li
X, Ying K, Wang W, Zhao J, Liu L, et al: Neoadjuvant nivolumab plus
chemotherapy versus chemotherapy for resectable NSCLC:
Subpopulation analysis of Chinese patients in CheckMate 816. ESMO
Open. 8:1020402023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Ma X, Ma K, Chen X, He H, Zhao X,
Fan M and Xu Y: Efficacy and safety of neoadjuvant
chemoimmunotherapy and chemotherapy in patients with potentially
resectable stage IIIA/IIIB NSCLC: A retrospective study. Front
Immunol. 15:14792632025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yue D, Wang W, Liu H, Chen Q, Chen C, Liu
L, Zhang P, Zhao G, Yang F, Han G, et al: Perioperative
tislelizumab plus neoadjuvant chemotherapy for patients with
resectable non-small-cell lung cancer (RATIONALE-315): An interim
analysis of a randomised clinical trial. Lancet Respir Med.
13:119–129. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heymach JV, Harpole D, Mitsudomi T, Taube
JM, Galffy G, Hochmair M, Winder T, Zukov R, Garbaos G, Gao S, et
al: Perioperative durvalumab for resectable non-small-cell lung
cancer. N Engl J Med. 389:1672–1684. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weder W, Collaud S, Eberhardt WEE,
Hillinger S, Welter S, Stahel R and Stamatis G: Pneumonectomy is a
valuable treatment option after neoadjuvant therapy for stage III
non-small-cell lung cancer. J Thorac Cardiovasc Surg.
139:1424–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
White A, Kucukak S, Bueno R, Servais E,
Lee DN, Colson Y, Jaklitsch M, McNamee C, Mentzer S, Wee J and
Swanson SJ: Pneumonectomy is safe and effective for non-small cell
lung cancer following induction therapy. J Thorac Dis. 9:4447–4453.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang G, Liu L, Zhang J and Li S: The
analysis of prognosis factor in patients with non-small cell lung
cancer receiving pneumonectomy. J Thorac Dis. 12:1366–1373. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schirren J, Bölükbas S, Bergmann T,
Fisseler-Eckhoff A, Trainer S and Beqiri S: Prospective study on
perioperative risks and functional results in bronchial and
bronchovascular sleeve resections. Thorac Cardiovasc Surg.
57:35–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maurizi G, D'Andrilli A, Venuta F and
Rendina EA: Bronchial and arterial sleeve resection for
centrally-located lung cancers. J Thorac Dis. 8 (Suppl
11):S872–S881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nitsche LJ, Jordan S, Demmy T, Dexter E,
Hennon M, Nwogu C, Yendamuri S and Picone A: Analyzing the impact
of minimally invasive surgical approaches on post-operative
outcomes of pneumonectomy and sleeve lobectomy patients. J Thorac
Dis. 15:2497–2504. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kates M, Swanson S and Wisnivesky JP:
Survival following lobectomy and limited resection for the
treatment of stage I non-small cell lung cancer<=1 cm in size: A
review of SEER data. Chest. 139:491–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi Y, Wu S, Ma S, Lyu Y, Xu H, Deng L and
Chen X: Comparison between wedge resection and
lobectomy/segmentectomy for early-stage non-small cell lung cancer:
A bayesian meta-analysis and systematic review. Ann Surg Oncol.
29:1868–1879. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parascandola M and Xiao L: Tobacco and the
lung cancer epidemic in China. Transl Lung Cancer Res. 8 (Suppl
1):S21–S30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu Y, Ren SY, Wang RY, Zeng C, Li JN, Xiao
P, Wu F, Yu FL and Liu WL: Surgical outcomes after neoadjuvant
chemoimmunotherapy for resectable non-small cell lung cancer. Front
Oncol. 11:6840702021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Romero Román A, Campo-Cañaveral de la Cruz
JL, Macía I, Escobar Campuzano I, Figueroa Almánzar S, Delgado Roel
M, Gálvez Muñoz C, García Fontán EM, Muguruza Trueba I, Romero
Vielva L, et al: Outcomes of surgical resection after neoadjuvant
chemoimmunotherapy in locally advanced stage IIIA non-small-cell
lung cancer. Eur J Cardiothorac Surg. 60:81–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gu L, Wang X, Sun Y, Xu Y, Niu X, Zhao R,
Yao Y, Jian H, Han Y, Wei J, et al: An open, observational,
three-arm clinical study of 2–3 cycles of treatment as neoadjuvant
therapy in operable locally advanced non-small cell lung cancer: An
interim analysis. Front Immunol. 13:9382692022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei J, Zhao J, Gong L, Ni Y, Zhou Y, Tian
F, Liu H, Gu Z, Huang L, Lu Q, et al: Neoadjuvant camrelizumab plus
platinum-based chemotherapy vs chemotherapy alone for chinese
patients with resectable stage IIIA or IIIB (T3N2) non-small cell
lung cancer: The TD-FOREKNOW randomized clinical trial. JAMA Oncol.
9:1348–1355. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heymach JV, Mitsudomi T, Harpole D,
Aperghis M, Jones S, Mann H, Fouad TM and Reck M: Design and
rationale for a phase III, double-blind, placebo-controlled study
of neoadjuvant durvalumab + chemotherapy followed by adjuvant
durvalumab for the treatment of patients with resectable stages II
and III non-small-cell lung cancer: The AEGEAN trial. Clin Lung
Cancer. 23:e247–e251. 2022. View Article : Google Scholar : PubMed/NCBI
|