Introduction
Pneumocystis jirovecii infection, classified
as Pneumocystis jirovecii pneumonia (PJP), is a leading
cause of pneumonia in immunocompromised populations, including
individuals with human immunodeficiency virus (HIV), solid organ
transplant recipients on immunosuppressive regimens, and patients
with cancer undergoing dose-dense chemotherapy (1). Notably, PJP carries a substantial
mortality risk, particularly in non-HIV immunocompromised
populations where 90-day mortality is significantly higher than in
HIV-infected patients (adjusted OR=5.47; 95% CI:2.16–14.1 for solid
tumor patients) (2). Owing to their
immunocompromised status, these patients exhibit heightened
susceptibility to Pneumocystis jirovecii colonization and
subsequent progression to clinically significant PJP (3), with critical risk factors including
prolonged high-dose corticosteroid use (≥10 mg/day prednisone
equivalent, increasing mortality by 80%) and lymphopenia
(<0.6×109/l). Mortality rates escalate to 43.3% for
non-HIV PJP patients requiring ICU admission, and reach 61.6% at 3
months in lung cancer population (4).
Advanced HR+/HER2- breast cancer survival has
significantly improved with CDK4/6 inhibitors (median OS now
exceeding 5 years in 1L), particularly in visceral metastasis
(5). Abemaciclib, a selective
CDK4/6 inhibitor, is approved for the treatment of hormone receptor
(HR)-positive/human epidermal growth factor receptor 2
(HER2)-negative breast cancer. As a CDK4/6 inhibitor, abemaciclib
functions by inhibiting CDK4 and CDK6 proteins, which are critical
regulators of cell division and growth. This inhibition directly
suppresses cancer cell proliferation (6). Clinically, abemaciclib is utilized in
combination with endocrine therapy (ET), demonstrating established
efficacy in both early-stage and advanced
HR+/HER2− breast cancer settings (7). It constitutes a first-line
standard-of-care option for HR+/HER2−
advanced disease, with key clinical trials (MONARCH-2 and
MONARCH-3) reporting therapeutic benefits when combined with
aromatase inhibitors or fulvestran (8,9). The
most common adverse event (AE) associated with abemaciclib is
diarrhea, with other frequently reported toxicities including
nausea, fatigue, elevated serum creatinine and myelosuppression
(neutropenia and lymphopenia) (10,11).
The present report details a case of PJP occurring
in a patient with HR+/HER2− advanced breast
cancer receiving abemaciclib in combination with ET. There have
been no prior documented cases of PJP in patients treated with
abemaciclib, to the best of our knowledge.
Case report
In February 2023, a 52-year-old treatment-naïve
woman diagnosed with stage IV HR+/HER2−
advanced breast cancer, confirmed via core needle biopsy of the
primary breast lesion and metastatic lung deposits at Longhua
Hospital affiliated to Shanghai University of Traditional Chinese
Medicine (Shanghai, China), had been receiving combination therapy
with abemaciclib (400 mg/day orally), letrozole (2.5 mg/day orally)
and goserelin (3.6 mg administered subcutaneously once monthly) in
28-day cycles for 6 cycles. Histopathology and immunohistochemistry
images of the primary breast lesion and metastatic lung deposits
via core needle biopsy are presented in Fig. S1. Tissue sections were
formalin-fixed and paraffin-embedded, and immunohistochemistry was
performed using antibodies following antigen retrieval. HER2
expression was scored using the 2023 American Society of Clinical
Oncology/College of American Pathologists guidelines (12).
Tissue specimens were initially fixed in 10% neutral
buffered formalin, prepared by mixing 100 ml of 37–40% formaldehyde
with 900 ml PBS, at 4°C for 24 h (cold fixation). Paraffin-embedded
tissues were prepared and sectioned to a thickness of 4 µm. For
antigen retrieval, heat-induced epitope retrieval was performed
using citrate buffer (pH 6.0) at 95°C for 20 min, followed by a
30-min cooling period at room temperature (RT). Sections were then
rehydrated through a descending alcohol series: Two washes in
xylene (5 min each), two washes in 100% ethanol (2 min each), one
wash in 95% ethanol (2 min), one wash in 70% ethanol (2 min) and
finally a rinse in dH2O. For intracellular or membrane
epitopes, permeabilization was carried out using 0.1% Triton X-100
in PBS for 10 min at RT. Non-specific binding sites were blocked by
incubating sections with 5% normal goat serum (cat. no. 16210064;
Thermo Fisher Scientific, Inc.) for 1 h at RT. Sections were
subsequently incubated overnight at 4°C with the primary antibody,
rabbit anti-CDK4 monoclonal antibody (cat. no. 12790; Cell
Signaling Technology, Inc.), diluted 1:200 in antibody diluent.
Following primary incubation, sections were incubated for 1 h at RT
with the horseradish peroxidase (HRP)-conjugated secondary
antibody, goat anti-rabbit IgG (H+L) (cat. no. ab6721; Abcam),
diluted 1:500 in PBS. Detection was performed using HRP/DAB with
3,3′-diaminobenzidine (DAB; cat. no. K3468; Dako; Agilent
Technologies, Inc.) as the chromogen.
For H&E staining, sections were stained with
Harris hematoxylin for 5 min at 25°C, followed by a 10-min rinse
under running tap water. Differentiation was then performed by
briefly treating sections with 1% acid alcohol (1 ml HCl in 99 ml
70% ethanol) for 5–10 sec at RT; this step was monitored
microscopically until the nuclei turned pale blue. Bluing was
achieved by immersing sections in 0.2% ammonium hydroxide for 30
sec at RT. Sections were counterstained with 0.5% aqueous eosin Y
solution for 2 min at RT. Immediately following eosin staining,
sections were dehydrated through a graded alcohol series. All
H&E and IHC-stained sections were routinely observed under a
light microscope during protocol optimization and quality control.
Finally, images were captured using a BX53 microscope (Olympus
Corp.) equipped with a DP74 camera at either ×200 or ×400
magnification (oil immersion for ×400).
Before starting antitumor therapy, the baseline
CD4+/CD8+ T-cell counts of the patient were
normal, and viral/bacterial infection screens were negative. Tumor
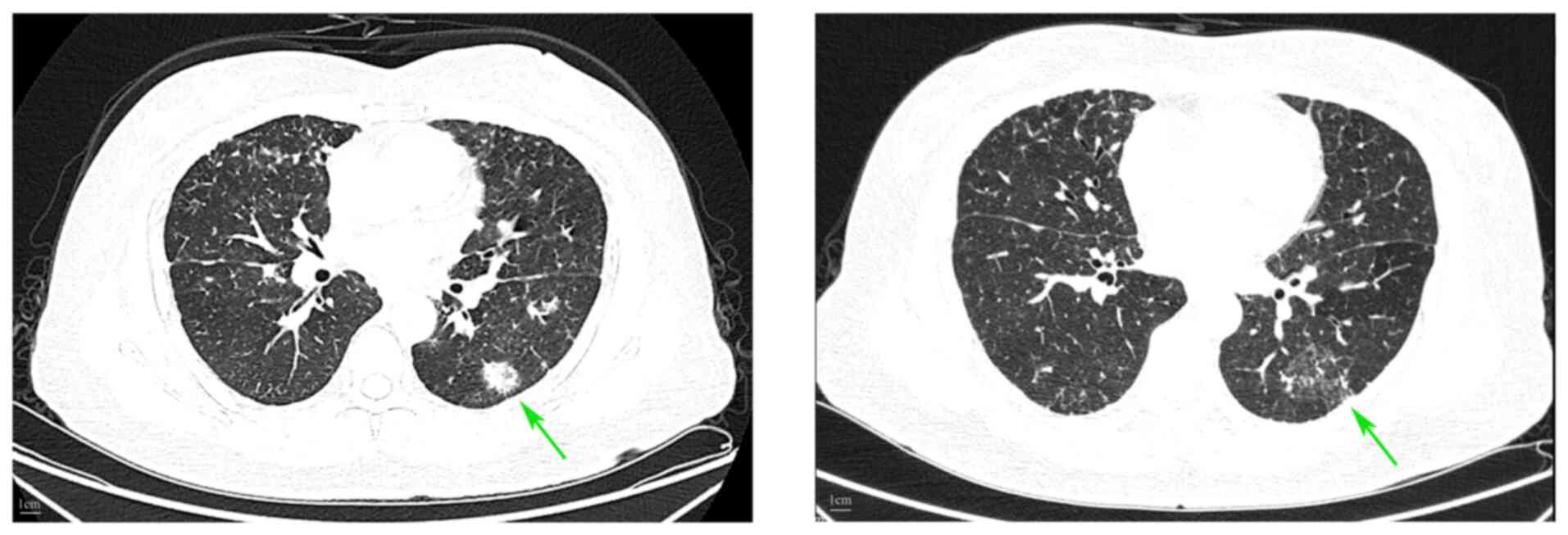
response assessed using CT demonstrated partial remission per the
Response Evaluation Criteria in Solid Tumors version 1.1 criteria
in June 2023 (13) (Fig. 1).
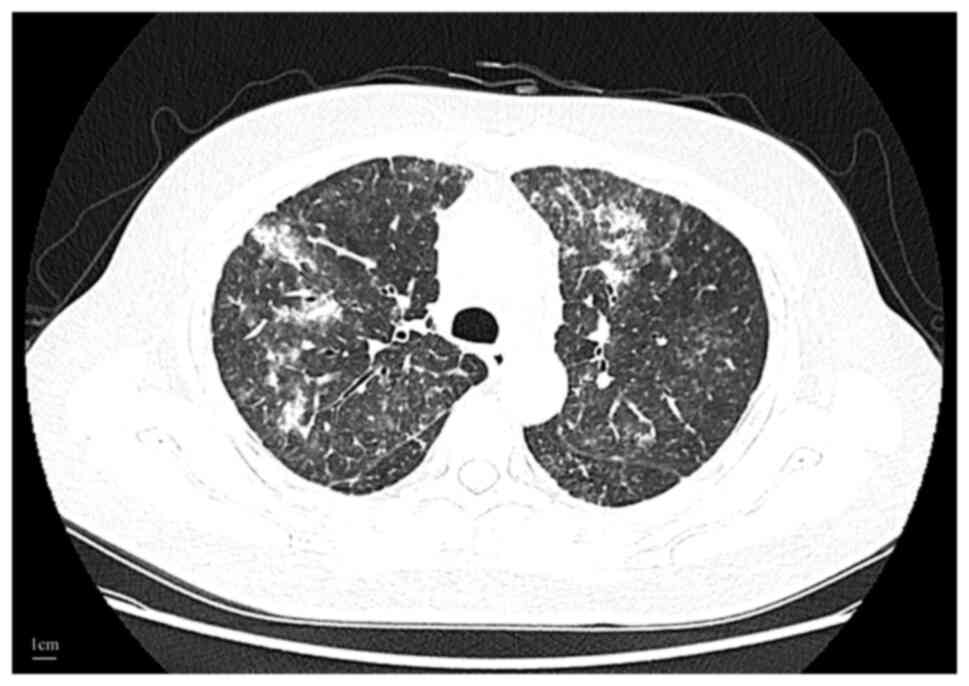
In August 2023, the patient presented with fever,
fatigue and a cough, requiring hospitalization. Chest CT revealed
minimal lung metastases alongside bilateral interstitial pneumonia
(Fig. 2). Laboratory investigations
revealed elevated serum high-sensitivity C-reactive protein (CRP)
and serum amyloid A (SAA), reduced circulating T-cell
(CD4+ and CD8+) and B-cell (CD19+)
counts, and negative serum (1,3)-β-D-glucan (BG). Nucleic acid
amplification tests for SARS-CoV-2 and common respiratory viruses
returned negative results. Detailed laboratory results are
presented in Table I. The
laboratory findings indicate that the patient developed grade 3
lymphopenia, grade 1 neutropenia and anemia during abemaciclib
therapy. Bacterial pneumonia was initially suspected based on
clinical presentation and elevated inflammatory markers. Anticancer
therapy was discontinued, and the patient received levofloxacin
(750mg/day intravenous) combined with piperacillin-tazobactam (4.5
grams every 6 h intravenously) as empirical antimicrobial therapy
for 3 days.
 | Table I.Laboratory data. |
Table I.
Laboratory data.
| Variable | Value (Pre-PJP
treatment) | Value (Post-PJP
treatment) | Reference
rangea |
|---|
| White-cell count,
/µl | 4,170 | 5,280 | 3,500-9,500 |
| Neutrophil count,
/µl | 2,790 | 5,370 | 1,800-6,300 |
| Lymphocyte count,
/µl | 590 | 1,260 | 1,100-3,200 |
| CD4+,
/Ul | 344 | 512 | 430-980 |
| CD8+,
/Ul | 199 | 287 | 380-670 |
| CD19+,
/Ul | 15 | 97 | 128-388 |
| C-reactive protein,
mg/l | 16.34 | 3.11 | 0.00–5.00 |
| Serum amyloid A,
mg/l | 90.97 | 9.09 | 0.00–10.00 |
| (1,3)-β-D-glucan | Negative | - | - |
|
Lipopolysaccharide | Negative | - | - |
| SARS-CoV-2 | Negative | - | - |
| Respiratory
syncytial virus | Negative | - | - |
| Adenovirus | Negative | - | - |
| Influenza
virus | Negative | - | - |
| Mycoplasma | Negative | - | - |
| Chlamydia | Negative | - | - |
| Legionella
pneumophila | Negative | - | - |
| Human
immunodeficiency virus | Negative | - | - |
After 3 days, the temperature of the patient
increased to 39.6°C with severe hypoxia (SpO2, 71% on
room air). High-flow nasal cannula oxygen therapy was initiated and
bronchoalveolar lavage (BAL) was performed. Metagenomic
next-generation sequencing (mNGS) of BAL fluid identified P.
jirovecii and an ultra-high pathogen load (read counts of
>500 with 72.87% coverage). All other pathogens, including
bacteria, Mycoplasma, Chlamydia, Mycobacterium, parasites
and DNA viruses, were excluded using mNGS. No malignant cells were
detected in the BAL specimen. The laboratory findings indicate that
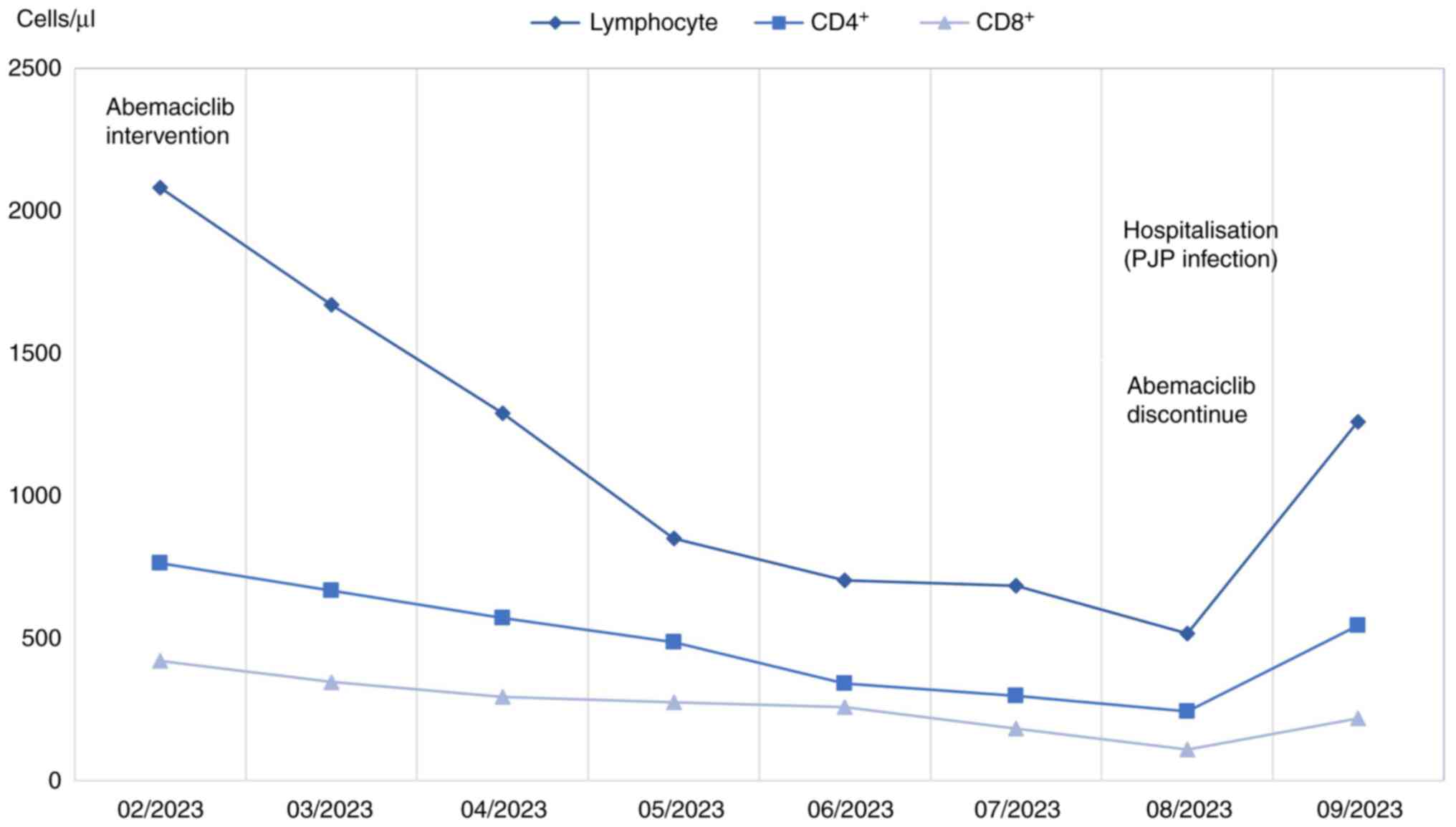
the patient's lymphocyte count significantly decreased from
1.5×109/l pre-treatment to 0.5×109/l during
therapy, accompanied by a decline in CD4+ T-cells as
well (Fig. 3). Based on these
findings, a diagnosis of PJP was established and the therapeutic
regimen was switched to trimethoprim-sulfamethoxazole (TMP/SMX;
160/800 mg orally every 6 h). Concurrently, methylprednisolone was
administered at 500 mg daily. The fever resolved to 36.8°C after 4
days of targeted therapy. Clinical symptoms (cough, dyspnoea and
fatigue) and laboratory parameters (CRP, SAA and lymphocyte counts)
demonstrated gradual improvement during the first week (Table I). The patient was discharged for
outpatient management after 14 days of hospitalization. TMP/SMX was
continued for 21 days, whilst methylprednisolone was tapered and
discontinued over 4 weeks. The patient remained afebrile without
recurrence of fatigue, chest pain, dyspnoea or cough.
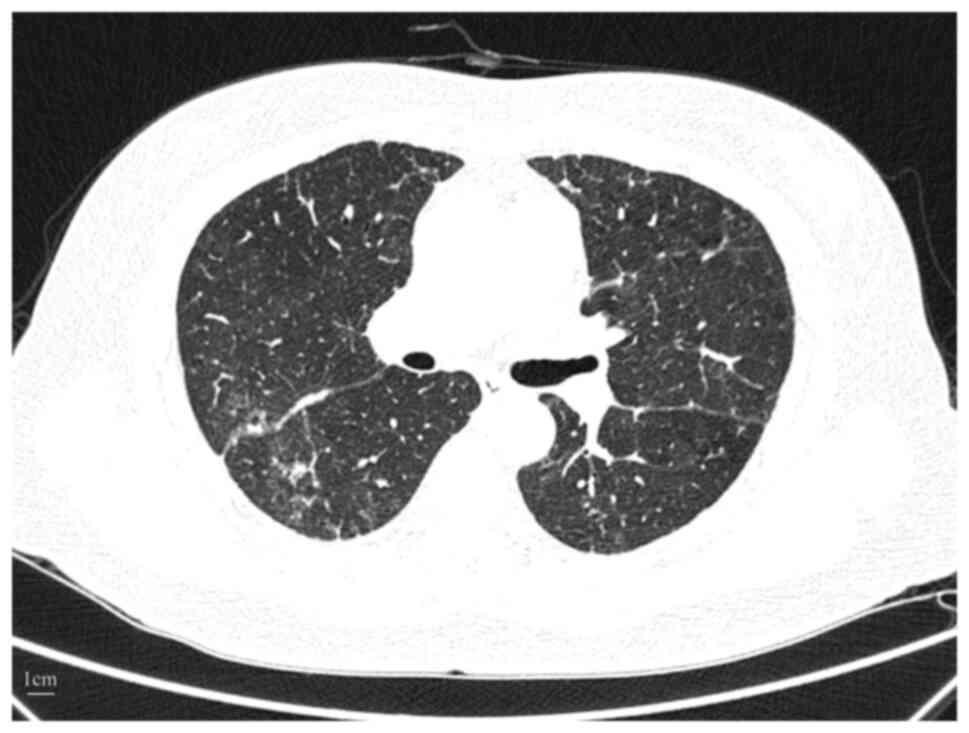
Follow-up chest CT after 1 month revealed partial
resolution of interstitial infiltrates (Fig. 4). Antitumor therapy was resumed 2
months later, with abemaciclib permanently discontinued in favor of
fulvestrant (500 mg intramuscular subcutaneously once monthly) +
goserelin (3.6 mg administered subcutaneously once monthly).
Discussion
The present report describes the first documented
case of PJP occurring in a patient with advanced breast cancer
receiving abemaciclib therapy, to the best of our knowledge.
Lymphopenia is established as an adverse effect of CDK4/6
inhibitors, including abemaciclib, palbociclib and ribociclib.
These agents operate through a shared mechanism of action targeting
CDK4/6, which regulates cell cycle progression in both malignant
cells and normal lymphocytes. CDK4/6 inhibitors serve a pivotal
role in regulating the G1-to-S phase transition during the cell
cycle, a process critical for T-cell clonal expansion following
activation (14). Abemaciclib
exerts potent inhibitory activity against CDK4/6, leading to
hypophosphorylation of the retinoblastoma protein, sustained E2F
transcription factor suppression and resultant G1-phase cell cycle
arrest in lymphocytes (15). This
mechanism directly impairs T-cell proliferation and reduces
circulating lymphocyte counts. Notably, abemaciclib has been
associated with markedly higher incidences of severe lymphopenia
compared with palbociclib and ribociclib. This difference may
relate to the greater selectivity of abemaciclib for CDK4 over
CDK6, which could preferentially affect lymphocyte subsets
dependent on CDK4 signaling (16).
To date, there are no documented cases of PJP linked to palbociclib
or ribociclib use in the published literature, to the best of our
knowledge.
PJP is associated with elevated overall mortality in
hospitalized populations, and the present case underscores the
necessity of considering Pneumocystis as a differential
diagnosis in patients receiving abemaciclib who develop pulmonary
infections. PJP remains a predominant cause of pneumonia in
immunocompromised hosts, particularly among HIV-infected
individuals (17).
Lymphopenia is documented as a frequent AE in prior
clinical trials of abemaciclib. In the MONARCH 2 and 3 trials,
lymphopenia occurred in 52.7–62.9% of patients treated with
abemaciclib (compared with 25.6–31.7% with the placebo). Moreover,
7.9–12.2% developed grade ≥3 lymphopenia compared with 1.8% in the
control arm. Furthermore, MONARCH 2 reported a higher incidence of
any-grade infections in the abemaciclib group (42.6%) compared with
in the placebo group (24.7%), although infection types were not
characterized but predominantly low-grade. In MONARCH 3, pulmonary
infections represented the most common severe AE (2.8%), resulting
in three fatalities in the abemaciclib cohort. However, these
trials did not establish a statistically significant association
between infection risk and lymphopenia severity (7,8). In
the present case, the patient developed grade 3 lymphopenia, grade
1 neutropenia and anemia during abemaciclib therapy, suggestive of
myelosuppression. Neutropenia was mild and not considered the
primary etiology for PJP, and the endocrine agents letrozole and
goserelin administered concurrently have no documented association
with lymphopenia in prior studies (18,19).
Consequently, abemaciclib-induced lymphopenia was postulated as the
critical factor predisposing to PJP. Furthermore, despite the
negative HIV serology and absence of pre-existing immunosuppressive
comorbidities, the patient exhibited a marked reduction in
lymphocyte counts from 1.5 G/l pre-treatment to 0.5 G/l (August
2023), with CD4+ T-cell counts of 244 cells/µl during
abemaciclib therapy (Fig. 3). Such
profound lymphopenia likely induced immunosuppression, heightening
susceptibility to Pneumocystis infection.
Drug-induced interstitial lung disease (ILD) is
indeed a recognized AE of abemaciclib, and this possibility was
thoroughly considered in the present case. However, there are
distinct differences between ILD and PJP. Drug-induced ILD
typically manifests with low-grade fever (20), whereas the patient in the present
case exhibited a high fever of 39.6°C. Moreover, chest CT revealed
focal consolidation, whereas ILD typically presents with bilateral
symmetrical ground-glass opacities on imaging (21). The patient also showed rapid
clinical improvement following administration of TMP/SMX, whereas
ILD-related changes usually resolve more gradually, often requiring
corticosteroids (22). Based on
these findings, particularly the focal consolidation on CT, high
fever and dramatic response to anti-PJP therapy, a diagnosis of PJP
infection was strongly favored over drug-induced ILD in the present
case.
Diagnostic modalities for PJP include serum BG, PCR,
Gomori methenamine silver (GMS) staining and mNGS. In the present
case, serum BG was negative, and a negative serum BG result may
reflect insufficient β-glucan release during early-stage infection
(<48 h) (23). Whilst repeat
testing during the symptomatic peak is feasible, even a positive
result cannot specify the causative pathogen. Thus, mNGS was
performed for pathogen identification. mNGS is an untargeted,
broad-spectrum pathogen detection platform wherein total DNA/RNA
from clinical specimens undergoes high-throughput sequencing,
generating pathogen taxonomic data through alignment with reference
databases. This enables rapid, unbiased pathogen identification
without prior targeting. A single sequencing run can identify
diverse pathogens, including viruses, bacteria, fungi and parasites
(24). Recent studies have reported
the diagnostic performance of mNGS for PJP, with sensitivity and
specificity of 92.3 and 87.4%, respectively (25,26).
Whilst mNGS exhibits comparable sensitivity with PCR, it
demonstrates superiority in detecting co-infections. Moreover,
although combined GMS staining and mNGS enhances diagnostic
accuracy to 96.2% (27), empiric
TMP/SMX therapy was promptly initiated in the present case, given
the risk of clinical deterioration during the diagnostic interval,
leading to rapid symptomatic resolution. Moreover, to avoid
incurring additional costs, GMS and PCR were not performed.
Lymphopenia, akin to neutropenia, compromises immune
function, rendering the host susceptible to bacterial, viral and
other pathogenic invasions. This elevates the risk of infections,
potentially culminating in fatal outcomes. Whilst standardized
approaches exist for detecting and managing abemaciclib-induced
neutropenia (28), evidence-based
protocols for identifying and mitigating abemaciclib-associated
lymphopenia remain deficient. Currently, there are no established
protocols for discontinuing treatment, adjusting doses or offering
symptomatic care after lymphopenia, thereby notably elevating the
risk of opportunistic infections such as PJP.
In conclusion, the present case demonstrates that
lymphopenia is a common and clinically significant AE associated
with abemaciclib therapy. Therefore, routine surveillance of
absolute lymphocyte counts (ALC) and CD4+ T-cell subsets
is warranted during abemaciclib treatment. PJP prophylaxis should
be considered in patients exhibiting persistent lymphopenia (ALC,
<0.5×109/l) or CD4+ T-cell depletion
(<300 cells/µl), particularly when concomitant risk factors
exist, including concomitant immunosuppressive agents or
pre-existing immunodeficiencies. The preferred regimen is oral
TMP/SMX (160/800 mg) once daily; for patients intolerant to
TMP/SMX, oral dapsone (100 mg once daily or 50 mg twice daily) may
be administered as an alternative. Clinicians should monitor for
hematologic toxicities associated with TMP/SMX and dapsone, both of
which are associated with a risk of neutropenia (29,30).
Co-administration with abemaciclib may amplify the risk and
severity of neutropenia, necessitating vigilant hematologic
surveillance. Additionally, abemaciclib is predominantly
metabolized by CYP3A4, whereas TMP/SMX is primarily metabolized via
CYP2C9/CYP2C19, and dapsone via CYP2E1/CYP2C. Consequently, no dose
adjustment of abemaciclib is required when used concomitantly with
these medications (31–33).
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Commission of Shanghai Municipality (grant no. 20Z21900300).
Availability of data and materials
The mNGS data generated in the present study may be
found in the figshare database under accession number 29591006 or
at the following URL: https://figshare.com/s/88124d28658523f8f3f9. The
personal information of the patient is anonymized for privacy
protection.
Authors' contributions
WH and SL was involved in the study's
conceptualization, methodology, validation, data curation and
writing - original draft preparation. YQ participated in the
study's conceptualization and contributed by performing patient
follow-up, writing-reviewing and editing and supervision. CS was
involved in data acquisition. CW and JB were involved in patient
follow-up. All authors read and approved the final version of the
manuscript. WH, SL, YQ, CS, CW and JB confirm the authenticity of
all raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written and signed consent was obtained from the
patient to publish their history, images, clinical data and other
data included in the present manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou S and Aitken SL: Prophylaxis against
pneumocystis jirovecii pneumonia in adults. JAMA. 330:182–183.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masur H, Brooks JT, Benson CA, Holmes KK,
Pau AK and Kaplan JE; National Institutes of Health, Centers for
Disease Control and Prevention HIV Medicine Association of the
Infectious Diseases Society of America, : Prevention and treatment
of opportunistic infections in HIV-infected adults and adolescents:
Updated guidelines from the centers for disease control and
prevention, national institutes of health, and HIV medicine
association of the infectious diseases society of America. Clin
Infect Dis. 58:1308–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilroy SA and Bennett NJ: Pneumocystis
pneumonia. Semin Respir Crit Care Med. 32:775–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Zhou X, Saimi M, Huang X, Sun T,
Fan G and Zhan Q: Risk factors of mortality from pneumocystis
pneumonia in non-HIV patients: A meta-analysis. Front Public
Health. 9:6801082021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jerzak KJ, Bouganim N, Brezden-Masley C,
Edwards S, Gelmon K, Henning JW, Hilton JF and Sehdev S: HR+/HER2-
Advanced breast cancer treatment in the first-line setting: Expert
review. Curr Oncol. 30:5425–5447. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cooley L, Dendle C, Wolf J, Teh BW, Chen
SC, Boutlis C and Thursky KA: Consensus guidelines for diagnosis,
prophylaxis and management of pneumocystis jirovecii pneumonia in
patients with haematological and solid malignancies, 2014. Intern
Med J. 44:1350–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnston SRD, Harbeck N, Hegg R, Toi M,
Martin M, Shao ZM, Zhang QY, Martinez Rodriguez JL, Campone M,
Hamilton E, et al: Abemaciclib combined with endocrine therapy for
the adjuvant treatment of HR+, HER2-, node-positive, high-risk,
early breast cancer (monarchE). J Clin Oncol. 38:3987–3998. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2- advanced breast cancer who had progressed while
receiving endocrine therapy. J Clin Oncol. 35:2875–2884. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patnaik A, Rosen LS, Tolaney SM, Tolcher
AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW,
Hilton JF, et al: Efficacy and safety of abemaciclib, an inhibitor
of CDK4 and CDK6, for patients with breast cancer, non-small cell
lung cancer, and other solid tumors. Cancer Discov. 6:740–753.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Wu J, Ji Z, Zhao Y, Li S, Li J,
Chen L, Zou J, Zheng J, Lin W, et al: Comparative efficacy and
safety of different combinations of three CDK4/6 inhibitors with
endocrine therapies in HR+/HER-2- metastatic or advanced breast
cancer patients: A network meta-analysis. BMC Cancer. 23:8162023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lombardo JF: American society of clinical
oncology/college of American pathologists guidelines should be
scientifically validated. Arch Pathol Lab Med. 131:1510–1511. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guillaume Z, Medioni J, Lillo-Lelouet A,
Marret G, Oudard S and Simonaggio A: Severe cellular
immunodeficiency triggered by the CDK4/6 inhibitor palbociclib.
Clin Breast Cancer. 20:e192–e195. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng J, Wang ES, Jenkins RW, Li S, Dries
R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, et al: CDK4/6
inhibition augments antitumor immunity by enhancing T-cell
activation. Cancer Discov. 8:216–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gebbia V, Valerio MR, Firenze A and
Vigneri P: Abemaciclib: Safety and effectiveness of a unique
cyclin-dependent kinase inhibitor. Expert Opin Drug Saf.
19:945–954. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weyant RB, Kabbani D, Doucette K, Lau C
and Cervera C: Pneumocystis jirovecii: A review with a focus on
prevention and treatment. Expert Opin Pharmacother. 22:1579–1592.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruhstaller T, Giobbie-Hurder A, Colleoni
M, Jensen MB, Ejlertsen B, de Azambuja E, Neven P, Láng I, Jakobsen
EH, Gladieff L, et al: Adjuvant letrozole and tamoxifen alone or
sequentially for postmenopausal women with hormone
receptor-positive breast cancer: Long-term follow-up of the BIG
1–98 trial. J Clin Oncol. 37:105–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pagani O, Francis PA, Fleming GF, Walley
BA, Viale G, Colleoni M, Láng I, Gómez HL, Tondini C, Pinotti G, et
al: Absolute improvements in freedom from distant recurrence to
tailor adjuvant endocrine therapies for premenopausal women:
Results from TEXT and SOFT. J Clin Oncol. 38:1293–1303. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spagnolo P, Bonniaud P, Rossi G,
Sverzellati N and Cottin V: Drug-induced interstitial lung disease.
Eur Respir J. 60:21027762022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pipavath S and Godwin JD: Imaging of
interstitial lung disease. Clin Chest Med. 25:455–465. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Conte P, Ascierto PA, Patelli G, Danesi R,
Vanzulli A, Sandomenico F, Tarsia P, Cattelan A, Comes A, De
Laurentiis M, et al: Drug-induced interstitial lung disease during
cancer therapies: Expert opinion on diagnosis and treatment. ESMO
Open. 7:1004042022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Damiani C, Demey B, Pauc C, Le Govic Y and
Totet A: A negative (1,3)-β-D-glucan result alone is not sufficient
to rule out a diagnosis of pneumocystis pneumonia in patients with
hematological malignancies. Front Microbiol. 12:7132652021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Wang X, Xu J, Yang Q, Zhu H, Liu Y
and Yang J: Diagnostic value of metagenomic next-generation
sequencing of lower respiratory tract specimen for the diagnosis of
suspected pneumocystis jirovecii pneumonia. Ann Med.
55:22323582023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JZ, Wang JB, Yuan D, Sun CH, Hou LL,
Zhang Y, Yang XH, Xie HX and Gao YX: Metagenomic next-generation
sequencing-based diagnosis of pneumocystis jirovecii pneumonia in
patients without human immunodeficiency virus infection: A
dual-center retrospective propensity matched study. J Infect Public
Health. 18:1028312025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Li Z, Ye J and Ye W: Diagnostic
performance of metagenomic next-generation sequencing for
pneumocystis jirovecii pneumonia. BMC Infect Dis. 23:4552023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pang Y, Qiu J, Yang H, Zhang J, Mo J,
Huang W, Zeng C and Xu P: Application value of metagenomic
next-generation sequencing based on protective bronchoalveolar
lavage in nonresponding pneumonia. Microbiol Spectr.
13:e03138242025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spring LM, Zangardi ML, Moy B and Bardia
A: Clinical management of potential toxicities and drug
interactions related to cyclin-dependent kinase 4/6 inhibitors in
breast cancer: Practical considerations and recommendations.
Oncologist. 22:1039–1048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen JK, Guerci J, Corbo H, Richmond M and
Martinez M: Low-dose TMP-SMX for pneumocystis jirovecii pneumonia
prophylaxis in pediatric solid organ transplant recipients. J
Pediatr Pharmacol Ther. 28:123–128. 2023.PubMed/NCBI
|
|
30
|
Hsieh CY and Tsai TF: Drug-induced
neutropenia during treatment of non-neoplastic dermatologic
diseases: A review. Clin Drug Investig. 40:915–926. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wolf R, Matz H, Orion E, Tuzun B and Tuzun
Y: Dapsone. Dermatol Online J. 8:22002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang YS, Tseng SY, Chang TE, Perng CL and
Huang YH: Sulfamethoxazole-trimethoprim-induced liver injury and
genetic polymorphisms of NAT2 and CYP2C9 in Taiwan. Pharmacogenet
Genomics. 31:200–206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Posada MM, Morse BL, Turner PK,
Kulanthaivel P, Hall SD and Dickinson GL: Predicting clinical
effects of CYP3A4 modulators on abemaciclib and active metabolites
exposure using physiologically based pharmacokinetic modeling. J
Clin Pharmacol. 60:915–930. 2020. View Article : Google Scholar : PubMed/NCBI
|