Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, with non-small-cell lung cancer (NSCLC)
accounting for 80–85% of all cases (1). Somatic activating mutations in the
EGFR gene are the most frequent oncogenic driver mutations in Asian
patients with NSCLC, with a prevalence of ~47% % (2). Mutations in EGFR exons 18–21 notably
predict responses to targeted therapies therapies (3).
Among these mutations, the L858R substitution in
exon 21 is one of the most common, alongside exon 19 deletions,
together representing ~85–90% of all EGFR mutations in NSCLC. These
mutations are typically sensitive to first–generation tyrosine
kinase inhibitors (TKIs), such as gefitinib and erlotinib erlotinib
(4). By contrast, mutations in exon
18, including G719X, E709X and exon 18 deletions, are found in 3–4%
of all EGFR mutations in NSCLC NSCLC (5). The most frequent point mutations in
exon 18 are G719X and E709X, which together account for 84% of
mutations in this exon. DelE709_T710insX, the most frequent exon 18
deletion, accounts for ~2.4%. For patients with these rare
mutations, afatinib is considered a first–line treatment option
option (6), which demonstrates
moderate efficacy even against mutations that confer resistance to
osimertinib.
E709X mutations, although rare, are notable for
their sensitivity to targeted therapies. This mutation represents
~1.5% of all EGFR mutations and includes several variants, such as
E709K, E709A, E709G, E709V, E709H, E709D and E709Q. Among these,
E709K, E709A and E709G are the most common common (7,8).
However, due to the low incidence of E709X mutations and their
occurrence in combination with other EGFR mutations, systematic
studies on the efficacy of TKIs for E709X mutations remain limited.
The co-occurrence of E709K and L858R mutations, in particular, has
garnered notable attention due to their impact on disease
progression and treatment response.
The present study reported a patient with both E709K
and L858R mutations, treated sequentially with osimertinib,
afatinib and ametinib. A literature review was also conducted on
the rare E709X mutation in exon 18 and its implications for
treatment strategies.
Case report
A 64-year-old Chinese woman, a non-smoker with no
family history of cancer, was admitted to The People's Hospital of
Yingcheng City (Yincheng, China) in March 2022. The patient
experienced swelling, numbness and limited movement in the right
hip and knee for >10 days. Pelvic MRI indicated multiple
abnormal lesions in the right iliac crest, pubic bone, ischium,
upper right femur, bilateral femoral heads, L5 vertebra and
bilateral sacrum. CT scans of the neck revealed enlarged lymph
nodes in the left neck and left supraclavicular region. Chest and
upper abdomen CT revealed a tumor in the left lower lobe (3.1×1.7
cm) with enlarged left hilar and mediastinal lymph nodes, bilateral
lung nodules and some irregular ground-glass opacities. Cranial MRI
indicated multiple brain metastases in both the frontal and
temporal lobes.
A percutaneous lung biopsy was performed under CT
guidance, which revealed invasive adenocarcinoma (solid + acinar
type) (Fig. 1A and B), Hematoxylin
and eosin staining was performed according to standard
histopathological protocols routinely used in our pathology
department. Programmed cell death-ligand 1 (PD-L1) expression was
positive with 30% tumor cell interpretation, the test was performed
on a biopsy specimen using Ventana SP263 reagent and the Ventana
BenchMark Ultra platform (Fig. 1C).
Immunohistochemistry revealed that tumor cells were positive for
thyroid transcription factor 1 [TTF-1 (+)] (Fig. 1D), Napsin A (+) (Fig. 1E), cytokeratin (CK)7 (+) (Fig. 1F), Ki-67 (LI 10%) (Fig. 1G) and negative for P40 (Fig. 1H). Marker selection followed the
International Association for the Study of Lung Cancer 2021
consensus guidelines (9).
Formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples were
prepared using 10% neutral-buffered formalin at room temperature
for 24 h. Tissue sections were cut to a thickness of 4 µm using a
microtome. For antigen retrieval and intracellular epitope
exposure, slides were permeabilized with 0.2% Triton X-100 (Merck
KGaA; Sigma-Aldrich) in PBS for 10 min at room temperature. To
reduce nonspecific binding, tissue sections were blocked with 5%
normal goat serum (Abcam) in PBS for 30 min at room temperature.
Immunohistochemical staining was then performed using the following
primary antibodies: Anti-TTF-1 (1:200 dilution; cat. no. M3575;
Dako; Agilent Technologies, Inc.), anti-Napsin A (1:100 dilution;
cat. no. 760-4867; Roche Diagnostics), anti-CK7 (1:300 dilution;
cat. no. M7018; Dako; Agilent Technologies, Inc.), anti-Ki-67
(1:100 dilution; cat. no. M7240; Dako; Agilent Technologies, Inc.)
and anti-P40 (1:200 dilution; cat. no. 760-4863; Roche
Diagnostics). Primary antibodies were incubated at 4°C overnight.
Secondary antibody incubation was performed using the EnVision+
System-HRP Labeled Polymer Anti-Mouse/Rabbit (cat. no. K4007; Dako;
Agilent Technologies, Inc.) at room temperature for 30 min.
Diaminobenzidine was used as the chromogen, followed by
counterstaining with hematoxylin. All slides were examined using a
Nikon Eclipse E200 light microscope at ×200 and ×400
magnifications.
![The percutaneous lung biopsy tissue
in the lower lobe of the left lung was observed by H&E staining
and magnifications at (A) ×50 and (B) ×400. (C) Programmed cell
death ligand 1 test result: Positive, with tumor cell
interpretation indicating 30%. Quality control demonstrated an
assessable tumor cell count of 50%. Both positive and negative
controls were successful. Combined with the results of
immunohistochemistry [(D) thyroid transcription factor-1 (+)
(nuclear staining). (E) Napsin A (+) (cytoplasmic granular
staining). (F) Cytokeratin 7 (+) (diffuse cytoplasmic staining).
(G) Ki-67:10% (labeling index). (H) P40 (−) (no nuclear staining)
(magnification, ×200)], and the pathological diagnosis was
non-small cell lung cancer, which was consistent with the type of
pulmonary adenocarcinoma. (I) Emission CT bone scintigraphy. Four
panels show whole-body bone scans from different views. Panel I
(far left, anterior view): Increased radioactivity in the parietal
bone, right shoulder joint and left humerus. Panel II (left middle,
posterior view): Increased radioactivity in the left clavicle,
multiple ribs, and T2-T4 vertebrae. Panel III (right middle,
anterior view): Increased radioactivity in the sacrum, right
sacroiliac joint, and right ilium. Panel IV (far right, posterior
view): Increased radioactivity in the bilateral hip joints and
right femur. Remaining bones and joints show normal physiological
distribution. (J) NGS results: The EGFR p.L858R mutation, with a
frequency of 15.5% in the transthoracic lung biopsy tissue. (K) NGS
results: The EGFR p.E709K mutation, with a frequency of 21.2% in
the transthoracic lung biopsy tissue. (L) Serum tumor marker
levels: A line chart depicting changes in serum CEA levels during
the course of treatment (reference range, 0–5 ng/ml). Endobronchial
ultrasound-guided transbronchial needle aspiration biopsy was
performed to obtain tissue in the lower lobe of the left lung was
observed by H&E staining at magnifications of (M) ×50 and (N)
×400. PCK, phosphoenolpyruvate carboxykinase; INSM1,
insulinoma-associated protein 1; NGS, next-generation
sequencing.](/article_images/ol/30/5/ol-30-05-15255-g00.jpg) | Figure 1.The percutaneous lung biopsy tissue
in the lower lobe of the left lung was observed by H&E staining
and magnifications at (A) ×50 and (B) ×400. (C) Programmed cell
death ligand 1 test result: Positive, with tumor cell
interpretation indicating 30%. Quality control demonstrated an
assessable tumor cell count of 50%. Both positive and negative
controls were successful. Combined with the results of
immunohistochemistry [(D) thyroid transcription factor-1 (+)
(nuclear staining). (E) Napsin A (+) (cytoplasmic granular
staining). (F) Cytokeratin 7 (+) (diffuse cytoplasmic staining).
(G) Ki-67:10% (labeling index). (H) P40 (−) (no nuclear staining)
(magnification, ×200)], and the pathological diagnosis was
non-small cell lung cancer, which was consistent with the type of
pulmonary adenocarcinoma. (I) Emission CT bone scintigraphy. Four
panels show whole-body bone scans from different views. Panel I
(far left, anterior view): Increased radioactivity in the parietal
bone, right shoulder joint and left humerus. Panel II (left middle,
posterior view): Increased radioactivity in the left clavicle,
multiple ribs, and T2-T4 vertebrae. Panel III (right middle,
anterior view): Increased radioactivity in the sacrum, right
sacroiliac joint, and right ilium. Panel IV (far right, posterior
view): Increased radioactivity in the bilateral hip joints and
right femur. Remaining bones and joints show normal physiological
distribution. (J) NGS results: The EGFR p.L858R mutation, with a
frequency of 15.5% in the transthoracic lung biopsy tissue. (K) NGS
results: The EGFR p.E709K mutation, with a frequency of 21.2% in
the transthoracic lung biopsy tissue. (L) Serum tumor marker
levels: A line chart depicting changes in serum CEA levels during
the course of treatment (reference range, 0–5 ng/ml). Endobronchial
ultrasound-guided transbronchial needle aspiration biopsy was
performed to obtain tissue in the lower lobe of the left lung was
observed by H&E staining at magnifications of (M) ×50 and (N)
×400. PCK, phosphoenolpyruvate carboxykinase; INSM1,
insulinoma-associated protein 1; NGS, next-generation
sequencing. |
Emission CT scan demonstrated clear body bone
imaging with strong distribution of radioactivity in the parietal,
right shoulder, left humerus, left clavicle, multiple ribs, T2-4
vertebrae, sacrum, right sacroiliac joint, right iliac, bilateral
hips and right femur, with physiological distribution elsewhere
(Fig. 1I). Next-generation
sequencing (NGS) of the transthoracic lung biopsy tissue identified
EGFR exon 21 p.L858R mutation (15.59%) and EGFR exon 18 p.E709K
mutation (21.2%) (Table I; Fig. 1J and K). Genomic DNA was extracted
from FFPE lung biopsy tissue using a commercial DNA extraction kit
(Auto-Pure 96; Hangzhou Allsheng Instruments Co., Ltd). The quality
and integrity of the extracted DNA were assessed using standardized
procedures, and the sample passed internal quality control metrics
(average sequencing depth: 1191.02×; Q30: 94.25%). NGS was
conducted using a hybrid capture-based 26-gene panel targeting lung
cancer-related genes (WuHan Kingmed Center for Clinical Laboratory
Co., Ltd). Paired-end sequencing was performed using the Illumina
NovaSeq platform, with a read length of 150 bp (2×150 bp). Library
preparation and enrichment were performed according to the
manufacturer's protocols (KM Miniseq-DX; Guangzhou Jinqi Rui
Biotechnology Co., Ltd). The final library was quantified using a
Qubit fluorometer and normalized to a loading concentration of 2.0
nM, calculated using standard molar concentration formulas. Variant
calling and visualization were performed using Integrative Genomics
Viewer software [analytical software 1: bcl2fastq (v2.19.0.316);
analytical software 2: fastp (v0.23.2); analytical software 3: BWA
(v0.7.17-r1188); analytical software 4: Mutect 2 (v4.2.3.0);
Analytical software 5: annovar (:v2020-06-08); Analytical software
6: msisensor-pro (v1.0.2); Analytical software 7: manta (v1.6.0);
Analytical software 8: Delly (v0.9.1); and Analytical software 9:
cnvkit (v0.9.10)]. For bioinformatic analysis, the laboratory used
proprietary pipelines and quality standards validated under
ISO15189 guidelines. The serum tumor biomarker CEA level was 17.82
ng/ml, above the normal range (<5.0 ng/ml) (Fig. 1L). Based on these findings, the
patient was diagnosed with stage IV left lower lung adenocarcinoma
with lymphatic, intracranial and extensive bone metastasis (TNM
staging, cT2N3M1; according to the guidelines from the
International Association for the Study of Lung Cancer, 9th
edition) (10). Osimertinib
targeted therapy was recommended in accordance with the National
Comprehensive Cancer Network guidelines (11).
 | Table I.Results of NGS of tissue or
peripheral blood samples. |
Table I.
Results of NGS of tissue or
peripheral blood samples.
| Date (month and
year) | Sample | Gene | Cytoband | Chr | Ref | Variant | AAchange.refgene
add exons | Exons | Mutant frequency, %
(total reads) | Other
inspection |
|---|
| March 2022 | Transthoracic
lung | EGFR | 7p11.2 | 7 | G | A |
NM_005228:c.2125G>A | 18 | 15.5 (4536X) | / |
|
| biopsy tissue |
|
|
|
|
| (p.E709K)
exon18; |
|
|
|
|
|
|
| 7p11.2 | 7 | T | G |
NM_005228:c.2573T>G | 21 | 21.2 (4595X) |
|
|
|
|
|
|
|
|
| (p.L858R)
exon21; |
|
|
|
| December 2022 | EBUS-TBNA | EGFR | 7p11.2 | 7 | G | A |
NM_005228:c.2125G>A | 18 | 12.5 (4536X) | PD-L1 TC:30% |
|
| biopsy tissue |
|
|
|
|
| (p.E709K)
exon18; |
|
|
|
|
|
|
| 7p11.2 | 7 | T | G |
NM_005228:c.2573T>G | 21 | 12.4 (4595X) |
|
|
|
|
|
|
|
|
| (p.L858R)
exon21; |
|
|
|
| March 2023 | Cardiac
puncture | EGFR | 7p11.2 | 7 | G | A |
NM_005228:c.2125G>A | 18 | 25.2 (4536X) | i) ARID2
p.S1309*, |
|
| fluid |
|
|
|
|
| (p.E709K)
exon18; |
|
| ii) MAP2K4
c.634–2A>G; |
|
|
|
| 7p11.2 | 7 | T | G |
NM_005228:c.2573T>G | 21 | 23.6 (4595X) | and iii) FGFR4
chr5q35.1 |
|
|
|
|
|
|
|
| (p.L858R)
exon21; |
|
| duplication |
| March 2024 | Peripheral
blood | EGFR | 7p11.2 | 7 | G | A |
NM_005228:c.2125G>A | 18 | 1.18 (4536X) | BCL2L11 (BIM)
intron 2 |
|
|
|
|
|
|
|
| (p.E709K)
exon18; |
|
| deletion |
|
|
|
| 7p11.2 | 7 | T | G |
NM_005228:c.2573T>G | 21 | 0.92 (4595X) |
|
|
|
|
|
|
|
|
| (p.L858R)
exon21; |
|
|
|
In March 2022, the patient initially reported
improvement in right hip pain, the chest CT and head MRI of the
patient at that time are shown in Fig.
S1A. Oral osimertinib therapy was initiated in March 2022 (80
mg, once daily). After 10 days, the patient experienced notable
relief from lower back pain. A follow-up in April 2022, 1 month
after starting osimertinib, indicated partial response (PR)
(Fig. S1B). A 3-month follow-up in
July 2022 also indicated PR (Fig.
S1C).
However, by November 2022, after 8 months of
osimertinib treatment, progression of disease (PD) was observed
(Fig. S1D). Endobronchial
ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)
performed at Tongji Hospital (Wuhan, China) confirmed non-small
cell carcinoma with pathology consistent with lung adenocarcinoma
(Fig. 1M and N). NGS of the
EBUS-TBNA biopsy tissue revealed EGFR exon 21 p.L858R (12.4%) and
exon 18 p.E709K (12.5%) mutations and PD-L1 expression was again
30% (Fig. 1C).
In December 2022, the patient tested positive for
COVID-19 and was admitted to the First People's Hospital of Tianmen
City (Tianmen, China). The patient received 50 Gy/25 fractions of
radiotherapy to the lower left lung and 1 month of oral afatinib
therapy during the radiation (40 mg, once daily). However, afatinib
was discontinued due to poor tolerance and osimertinib was resumed.
In February 2023, following radiotherapy, afatinib was reintroduced
for 1 month and the patient was again reviewed, which indicated PR
(Fig. S1E).
In March 2023, the patient's condition progressed
again and the patient developed intermittent cough, dizziness,
nausea and vomiting. A CT scan indicated pericardial effusion and
cancerous lymphangitis (Fig. S1F),
with PD observed. In January 2023, 75 ml of bloody pericardial
effusion was drained and pathological examination confirmed
metastatic lung adenocarcinoma. Immunohistochemistry results were
positive for PCK, CK7, TTF-1 and Napsin A, and negative for P40,
CK5/6 and P40, with Ki-67 expression at ~40% (Anti-PCK: 1:150
dilution; cat. no. M3515; Dako; Agilent Technologies, Inc. Other
antibody information is the same as stated above). NGS of the
pericardial effusion revealed the same EGFR mutations (L858R and
E709K). Given disease progression and suspected resistance to
targeted therapy, cisplatin chemotherapy (cavum pericardii: 40 mg;
vein: 60 mg, once) was administered in March 2023. Systemic
chemotherapy with the paclitaxel-platinum (PP) (cisplatin: 100 mg;
pemetrexed: 750 mg, once a month) regimen was continued, which led
to PR after one cycle (Fig. S1G).
After one cycle, bevacizumab (400 mg, once a month) was added to
the PP regimen, which resulted in another PR (Fig. S1H).
In August 2023, after four cycles of the PP regimen,
the treatment was switched to a combination of pemetrexed (750 mg,
once a month) and bevacizumab (400 mg, once a month). After one
cycle, a SD was achieved (Fig.
S1I). However, after three cycles in November 2023, PD was
observed and compliance with chemotherapy in the patient was
suboptimal. Because the patient's self-perceived tolerance was
poor, treatment intervals were extended to 45 days (Fig. S1J).
In February 2024, after six cycles of pemetrexed and
bevacizumab, brain MRI revealed sporadic nodular enhancement and CT
scans demonstrated worsening left lower lobe tumor and metastasis
(Fig. S1K). The patient reported
occasional dizziness and headaches and PD was confirmed.
Whole-brain radiotherapy (WBRT) was initiated. NGS of peripheral
blood indicated EGFR exon 21 p.L858R (0.92%) and exon 18 p.E709K
(1.18%), with Bcl-2-like protein 11 interacting mediator of cell
death intron 2 deletion (Table I).
In May 2024, treatment with ameritinib (110 mg, once daily) and
bevacizumab (400 mg, once a month) was started and after 1 month of
WBRT, the patient demonstrated PR (Fig. S1L). The reexamination in August
2024 showed SD (Fig. S1M).
By November 2024, after 6 months of combined
treatment with ameritinib and bevacizumab, the patient developed
proteinuria (3+) and marked blood pressure fluctuations (≤170/120
mmHg), suspected to be side effects of bevacizumab. Consequently,
bevacizumab was discontinued and intravenous pemetrexed
chemotherapy (750 mg, once a month) was started. The patient
subsequently developed symptoms of dizziness, headache, vomiting,
somnolence, decreased vision, hearing loss, reduced appetite, lower
limb weakness and unsteady gait. Brain MRI revealed slight
enlargement of ventricles and cisterns, mild widening of sulci and
possible leptomeningeal metastasis (Fig. S1N).
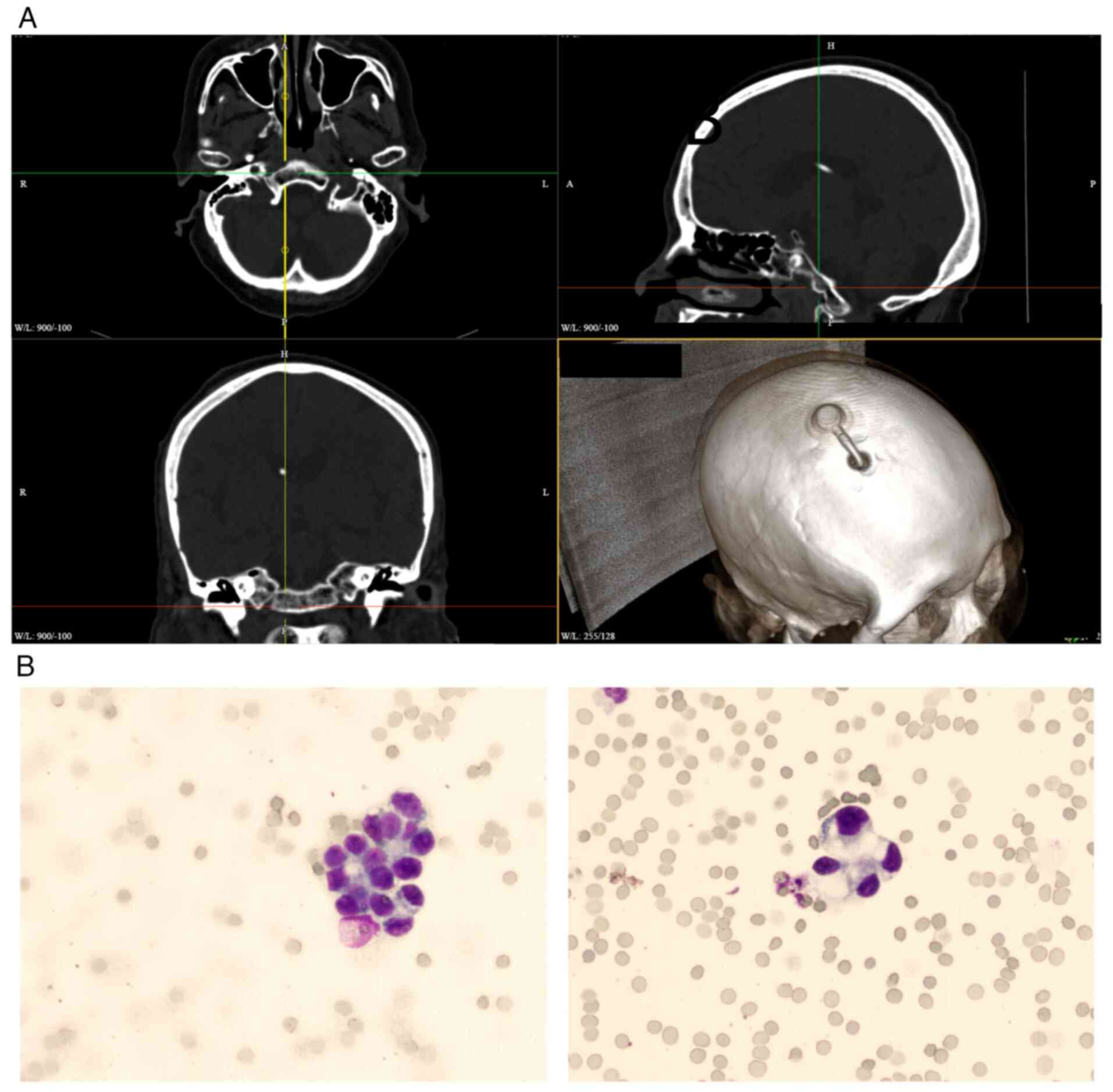
Following the confirmation of leptomeningeal
metastasis, an Ommaya reservoir implantation (Fig. 2A) was performed and cerebrospinal
fluid examination confirmed the presence of tumor cells (Fig. 2B). Intrathecal administration of
pemetrexed (30 mg, once a month) via the Ommaya reservoir, in
combination with oral ameritinib (220 mg, once daily), led to
notable improvement in the symptoms of the patient. The treatment
regimen is ongoing and the patient receives an intrathecal
injection once a month. The timeline of the diagnosis and treatment
of the patient is displayed on the left side of Fig. S1.
Discussion
The treatment of EGFR mutations has undergone
notable advancements, with targeted therapies serving a key role in
the improvement of patient outcomes. Among the various mutations in
the EGFR gene, the E709X mutation, including the E709K mutation,
has gained attention due to its implications for treatment response
to different generations of EGFR TKIs. A comprehensive summary of
previous studies on the E709X mutation is provided in Table II.
 | Table II.All reported cases of the EGFR E709X
(E709A, E709G, E709V, E709K) and E709-T710delinsX mutation in
patients with lung adenocarcinoma treated with EGFR TKIs. |
Table II.
All reported cases of the EGFR E709X
(E709A, E709G, E709V, E709K) and E709-T710delinsX mutation in
patients with lung adenocarcinoma treated with EGFR TKIs.
| First author,
year | Age, years | Sex, M/F | Mutations | Incorporation of
other mutations | Smoker | Stage | Cancer type | Treatment | Response to TKI
(time) | PFS, months | OS, months | (Refs.) |
|---|
| Wu et al,
2011 | 61 | F |
E709-T710delinsX | No | No | IV | ADC |
Gefitiniba | SD | 5.1 | 79.0 | (24) |
|
| 65 | M |
E709-T710delinsX | No | Yes | IV | ADC | Gefitinib | PD | 0.9 | 11.1 |
|
| Wu et al,
2016 | 57 | F |
E709-T710delinsX | No | No | IV | ADC | Gefitinib | PD | 6.0 | 24.1 | (21) |
|
| 79 | M |
E709-T710delinsX | No | Yes | IV | ADC | Gefitinib | O | 6.2 | 6.2 |
|
|
| 68 | M |
E709-T710delinsX | No | Yes | IV | ADC | Gefitinib | PD | 2.3 | 29.5 |
|
|
| 59 | F | E709A | G719C | No | IV | ADC | Gefitinib | SD | 7.3 | 12.1 |
|
|
| 58 | F | E709A | G719C | No | IV | ADC |
Erlotiniba | PR | 14.9 | 29.3 |
|
|
| 76 | M | E709A | L858R | No | IV | ADC | Erlotinib | SD | 3.9 | 5.4 |
|
|
| 48 | F | E709A | L858R | No | IV | ADC | Gefitinib | PR | 13.6 | 32.0 |
|
|
| 69 | M | E709G | G719C | No | IV | ADC | Erlotinib | PD | 1.4 | 8.3 |
|
|
| 57 | F | E709G | Del exon 19 | No | IV | ADC | Gefitinib | PR | 77.4 | 104.6 |
|
|
| 85 | M | E709G | L858R | Yes | IIIB | ADC | Erlotinib | PR | 8.6 | 13.2 |
|
|
| 48/ | F | E709G | L858R | No | IV | ADC | Gefitinib | PD | 2.4 | 6.8 |
|
|
| 55 | F | E709G | L858R | No | IV | ADC | Gefitinib | PR | 18.4 | 75.3 |
|
|
| 64 | F | E709K | G719S | No | IV | ADC | Gefitinib | PR | 11.1 | 11.1 |
|
|
| 71 | M | E709K | L858R | No | IV | ADC | Gefitinib | PR | 6.5 | 6.5 |
|
|
| 69 | M | E709K | L858R | Yes | IV | ADC | Gefitinib | PR | 8.6 | 8.6 |
|
|
| 66 | M | E709V | L858R | Yes | IV | ADC | Gefitinib | PR | 9.2 | 9.5 |
|
| Ackerman et
al, 2012 | 88 | F |
E709-T710delinsX | Wild-type KRAS | No | IV | NSCLC | Erlotinib | PR (4 months) | NA | NA | (23) |
| Isaksson et
al, 2020 |
| NA |
E709-T710delinsX | No | NA | IV | NA | Erlotinib | PD | 8.0 | NA | (29) |
| Sousa et al,
2020 | 66 | F |
E709-T710delinsX | No | Yes | IV | ADC | Gefitinib | PD | 3.0 | 24.0 | (30) |
|
| 46 | F |
E709-T710delinsX | No | Former heavy | IV | ADC | Erlotinib | DD | 4.0 | 26.0 |
|
|
| 57 | F |
E709-T710delinsX | No | No | IV | ADC | Erlotinib | PD | 3.0 | 18.0 |
|
| Klughammer et
al, | 50 | F |
E709-T710delinsX | No | No | Il or IV | NSCLC | Erlotinib | PD | 1.3 | 1.7 | (31) |
| 2016 | 58 | M | E709A | G719S | Yes | NA | NA | Erlotinib | SD | 253 | 509 |
|
|
| 50 | M | E709K | G719A | No | NA | NA | Erlotinib | PD | 42 | 259 |
|
| Kobayashi et
al, | 63 | M |
E709-T710delinsX | No | NA | IV | ADC | Erlotinib | SD | NA | NA | (5) |
| 2015 |
| F |
|
|
|
|
|
Afatiniba | Tumor | NA |
|
|
|
|
|
|
|
|
|
|
|
| shrinkage |
|
|
|
|
|
|
|
|
|
|
|
|
| (1 month) |
|
|
|
| lbrahim et
al, 2017 | 52 | F |
E709-T710delinsX |
| No | IV | ADC | Afatinib | 2 months | NA | NA | (32) |
| D'Haene et
al, 2019 | 57 | M |
E709-T710delinsX |
| No |
| ADC | Afatinib | PR (12
months); | 12.0 | 36.0 | (33) |
|
|
|
|
|
|
|
|
|
| PD |
|
|
|
| Martin et
al, 2019 | 60 | F |
E709-T710delinsX |
| No | IV | ADC | Erlotinib | PD | 1.0 | 3.0 | (34) |
| Wei et al,
2021 | 70 | F |
E709-T710delinsX | Amplification, | No | II | NSCLC | Afatinib | PD | 23.0 | Ongoing | (12) |
|
|
| M |
| M246_T256del,
E545K, E542K etc. |
|
|
|
Almonertiniba | SD | NA |
|
|
| Liu et al,
2022 | 30 | F | E709K | G724S, V689I | No | IV | NSCLC | Afatinib | PR (2 months) | 3 | 13.5 | (35) |
| Frega et al,
2016 | 70 | F | E709K | L833V, H835L | Yes | IV | ADC | Afatinib | PR (2 months) | 2 | Ongoing | (36) |
| Present case | 64 | M | E709K | L858R | No | IV | NSCLC |
Osimertiniba | PR (8 months) | 8 | Ongoing | - |
|
|
|
|
|
|
|
|
| Afatinib | NA | NA |
|
|
|
|
|
|
|
|
|
|
| Almonertinib | PR (>4
months) | NA |
|
|
Mutations such as E709K and L858R have demonstrated
varying responses to different generations of EGFR TKIs: i)
First-generation TKIs: Both L858R and E709K mutations exhibit
sensitivity to first-generation TKIs, although E709K generally
exhibits a slightly lower response compared with L858R. Previous
studies indicated that while first-generation TKIs are initially
effective, resistance typically develops over time (7,12,13);
ii) second-generation TKIs: Afatinib and dacomitinib, as
second-generation EGFR TKIs, irreversibly bind to the EGFR
receptor. Clinical data suggested that afatinib is particularly
effective in treating E709K mutations, offering a notable
progression-free survival (PFS) advantage. Afatinib is also highly
efficacious for L858R mutations and is often preferred due to its
potency and broader mutation coverage (5); and iii) third-generation TKIs:
Osimertinib, a third-generation TKI, is typically reserved for
patients with acquired T790M resistance mutations but has also
demonstrated efficacy in L858R mutation cases after resistance to
earlier-generation TKIs. However, the effectiveness of osimertinib
and other third-generation TKIs in the treatment of E709K mutations
remains elusive and warrants further investigation (12).
Previous studies reported three lung cancer cases
with dual EGFR mutations (L858R and E709K) treated using afatinib:
1 patient achieved PR and 2 patients had stable disease (SD)
(14). The E709 amino acid
alteration in the EGFR gene is relatively rare in NSCLC and is
often associated with other sensitive EGFR mutations (e.g., G719X
and L858R) (15,16). A study suggested that the E709K
mutation may be sensitive to afatinib-targeted therapy but
demonstrates reduced sensitivity to gefitinib and erlotinib
(6), a finding confirmed by a
retrospective case-control study conducted by Kuiper et al
(17). Furthermore, patients with
uncommon EGFR mutations (e.g. S768I and exon 20 insertion mutation)
(18,19) tend to have shorter PFS and overall
survival (OS) on EGFR-TKI treatment compared with those with
classic EGFR mutations (e.g. Del19 and L858R point mutation)
(20), although there is
considerable variability depending on the specific mutation.
A previous study by Kobayashi et al (5) compared the inhibitory effects of
various EGFR TKIs on Ba/F3 cells expressing the
E709K/E709_T710delinsD mutation. The results indicated that
second-generation TKIs, such as afatinib, were more potent in
inhibiting the E709K/E709_T710delinsD Ba/F3 cell line compared with
first-generation TKIs. This suggested that second-generation TKIs
may be more effective in the treatment of patients with NSCLC with
the E709_T710delinsD mutation. However, clinical evidence
confirming the efficacy of second-generation TKIs in patients with
E709_T710delinsX mutations remains sparse.
The findings of the present study align with those
of previous studies (5,8,18),
which demonstrated moderate to good responsiveness of dual EGFR
mutations (E709K and L858R) to targeted EGFR-TKIs, notably afatinib
and osimertinib. Similar to Wu et al (24), the present study observed initial
sensitivity followed by the development of resistance. By contrast,
while Kobayashi et al (5)
and Wei et al (12) reported
a notable clinical benefit with afatinib for exon 18 mutations, the
present patient exhibited limited tolerance to afatinib, which
necessitates a treatment switch to third-generation TKI
amitinib.
The novel insight provided by the present study is
the clinical efficacy and tolerability of sequential treatment
involving osimertinib, afatinib and amitinib, including
comprehensive radiotherapy and chemotherapy management strategies.
These findings suggest individualized therapeutic strategies and
highlight the importance of real-time genomic monitoring to
promptly manage resistance mechanisms. However, notable gaps
remain, particularly regarding standardized treatment protocols for
rare EGFR mutations, mechanisms underlying acquired resistance and
the optimal sequencing and combination of TKIs in clinical
practice. Future large-scale studies are key to filling these
knowledge gaps.
A notable case study reported a patient with lung
adenocarcinoma with the rare E709_T710delinsD mutation who
responded well to afatinib, which achieved a PFS of 23 months
(12). The present case highlighted
the potential of second-generation TKIs, such as afatinib, in the
management of mutations involving exon 18. Additionally, broader
analyses suggested that patients with E709K and other exon 18
mutations exhibited an improved response to afatinib or neratinib
compared with first-generation TKIs (5,25,26).
Functional studies using transfected cell models
(Ba/F3 and NIH/3T3) have demonstrated that the E709K mutation,
similar to G719X, induces oncogenic transformations. These
mutations exhibit greater sensitivity to second-generation TKIs,
such as afatinib and neratinib, compared with first-generation
TKIs. These findings underscore the importance of precise molecular
diagnostics in guiding optimal treatment strategies for patients
with rare EGFR mutations (6).
The present case included a patient with EGFR
mutations, specifically E709K and L858R, who was treated with
multiple targeted therapies, including osimertinib (8 months),
afatinib (1 month) and amitinib (ongoing). Due to the complexity of
the treatment regimen, which combined targeted therapies with
radiation for the primary lesions, it was difficult to assess the
efficacy of the treatment. However, both osimertinib (PFS, 8
months) and amitinib (ongoing therapy) demonstrated favorable
responses, which resulted in remission. The present case
highlighted the potential effectiveness of third-generation EGFR
TKIs in the treatment of the rare E709K mutation. Although
resistance ultimately developed, these treatments markedly
alleviated the condition of the patient and produced tolerable side
effects, rendering them a promising therapeutic option for similar
cases in the future.
Resistance mechanisms to third-generation EGFR TKIs,
such as osimertinib, include secondary mutations such as C797S, MET
amplification and histological transformations (e.g., small cell
transformation). The patient of the present case study eventually
demonstrated progression on osimertinib, which suggested potential
activation of such resistance pathways. Future treatment strategies
should integrate comprehensive genomic monitoring to promptly
identify and target these resistance mechanisms.
Additionally, the patient began chemotherapy in
April 2023, which included a combination of bevacizumab, the PP
regimen, pemetrexed chemotherapy, whole-brain radiotherapy and
continued targeted therapy with amitinib and intrathecal
injections. Xu et al (27)
also suggested that continuing EGFR-TKI therapy in combination with
bevacizumab is a rational strategy for patients with NSCLC who
experience gradual progression after initial EGFR-TKI treatment.
Clinical studies have reported that the addition of bevacizumab to
standard chemotherapy (carboplatin/paclitaxel) notably increases
survival in patients with NSCLC. In the pivotal Phase III study
(ECOG 4599), chemotherapy plus bevacizumab resulted in improved OS
(12.3 vs. 10.3 months) and longer PFS (6.2 vs. 4.5 months)
(28).
The present case underscores the value of
incorporating targeted therapies and bevacizumab into treatment
regimens for advanced NSCLC, particularly for patients with rare
mutations such as E709K, where conventional therapies may offer
limited efficacy. Further studies are warranted to optimize these
combination therapies and enhance the understanding of their
long.term impact on patient survival and quality of life.
The present case study demonstrated the complex
treatment process for a patient with advanced lung adenocarcinoma.
Through multidisciplinary collaboration, the patient received
effective symptomatic treatment and an improvement in quality of
life. The present case underscores the importance of individualized
treatment plans in oncology.
The treatment of patients with EGFR mutations,
particularly the E709X and E709_T710delinsX mutations, poses
notable challenges due to their unique properties and varying
responses to different EGFR TKIs. While first-generation TKIs
exhibit certain efficacy, second-generation TKIs, particularly
afatinib, appear to offer a more favorable therapeutic option for
patients with these mutations, the literature on E709X and
E709_T710delinsX mutations was searched and their views seem to
support this conclusion (29–36).
However, further clinical studies with larger sample sizes are
required to validate these findings and optimize treatment
strategies for these patients. Furthermore, the role of
third-generation TKIs, such as osimertinib, in the treatment of
these mutations remains to be elucidated in future research.
The E709K and L858R mutations in EGFR are key
factors in the pathogenesis and treatment response of NSCLC. While
the L858R mutation is well-characterized and commonly targeted with
established treatment protocols, the E709K mutation, although less
frequent, demonstrates promising responses to second-generation
EGFR TKIs, particularly afatinib. Ongoing research and clinical
trials are necessary to refine therapeutic strategies and improve
patient outcomes for those with these mutations. For clinicians,
understanding the specific EGFR mutation profile is essential for
the selection of the most effective targeted therapies, which
ultimately enhances both survival and quality of life for
patients.
The limited number of cases including rare or
complex mutations poses challenges for the design of large-scale
prospective trials. Therefore, translational studies and the
establishment of national and international biobanks will be key to
addressing several of the unresolved questions in this field.
In conclusion, the management of NSCLC with rare
EGFR mutations such as E709X and E709_T710delinsX remains
challenging due to their heterogeneity and limited clinical data.
This case highlights the clinical value of individualized treatment
strategies combining sequential EGFR TKIs, including afatinib and
osimertinib, and adjunct therapies such as bevacizumab and
radiotherapy. Second-generation TKIs, particularly afatinib, appear
to offer improved efficacy for exon 18 mutations compared to
first-generation TKIs, while third-generation TKIs may serve as
effective alternatives upon resistance. The integration of genomic
monitoring and multidisciplinary approaches is essential for
optimizing treatment in these complex cases. Further prospective
studies and real-world evidence are needed to establish
standardized protocols and improve outcomes for patients harboring
uncommon EGFR mutations.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the doctors who gave
their advice during the treatment of the patient: Professor Qin Wu
(Oncology Department), Professor Linfeng Wang (Neurosurgery
Department), Professor Zhibin Hu (Imaging Department) and Professor
Yongqiao Liu (Pathology Department) (The People's Hospital of
Yingcheng City, Xiaogan, China), Professor Junhong Zhang (Oncology
Department, Zhongnan Hospital, Wuhan University, Wuhan, China),
Professor Peng Zhang (Department of Oncology, Tongji Hospital,
Tongji Medical College, Huazhong University of Science and
Technology, Wuhan, China), Professor Rui Meng (Department of
Oncology, Union Hospital, Tongji Medical College, Huazhong
University of Science and Technology, Wuhan, China), Professor
Wuling Ou (Department of Oncology, Hubei Provincial Cancer
Hospital, Wuhan, China) and Professor Wei Li, Professor Lilin He,
Professor Lian Dong, Professor Rui Song and Professor Cong Chen
(Department of Oncology, The First People's Hospital of Tianmen
City, Tianmen, Hubei, China) provided consultation services such as
diagnosis and treatment. Dr Kaiyuan Diao (Guangzhou KingMed Center
for Clinical Laboratory Co., Ltd., Guangzhou, China) provided solid
tumor gene sequencing and report interpretation.
Funding
The present study was supported by the Hubei Province Key
Laboratory of Occupational Hazard Identification and Control, Wuhan
University of Science and Technology (grant no. JF2024-Y19); the
Hubei Provincial Natural Science Foundation (grant no. 2022CFB514)
and its Key-Area R&D Program (grant no. 2022BCE067).
Availability of data and materials
The whole-genome sequencing data generated and
analyzed in the present study have been deposited in the NCBI
Sequence Read Archive (SRA) under the accession no. PRJNA1276985,
which are publicly accessible at: https://www.ncbi.nlm.nih.gov/sra/PRJNA1276985. The
data generated in the present study may be requested from the
corresponding author.
Authors' contributions
JS devised the methodology, organized laboratory
data, wrote the original draft, and edited and reviewed the
manuscript. QW, LH and LD devised the methodology, organized
medical record data, and edited and reviewed the manuscript. LC
performed imaging diagnosis, obtained the imaging data and edited
and reviewed the manuscript. HL provided organopathology and
immunohistochemical diagnosis, obtained the pathology data and
edited and reviewed the manuscript. KD obtained the molecular
pathology data, conducted the formal analysis and edited and
reviewed the manuscript. HY devised the methodology, obtained
funding for the present study and edited and reviewed the
manuscript. JS and QW confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of The First People's Hospital of Tianmen City (approval
no. 20240286). The patient provided written informed consent to the
treatment plan and genetic testing given by the attending
physician.
Patient consent for publication
The patient provided written informed consent for
the publication of data and images in the present study.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools [OpenAI.(2023). and ChatGPT-4o] were used to
improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Midha A, Dearden S and McCormack R: EGFR
mutation incidence in non small cell lung cancer of adenocarcinoma
histology: A systematic review and global map by ethnicity
(MutMapII). Am J Cancer Res. 5:2892–2911. 2015.PubMed/NCBI
|
|
3
|
Robichaux JP, Le X, Vijayan RSK, Hicks JK,
Heeke S, Elamin YY, Lin HY, Udagawa H, Skoulidis F, Tran H, et al:
Structure-based classification predicts drug response in
EGFR-mutant NSCLC. Nature. 597:732–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hama T, Yuza Y, Suda T, Saito Y, Norizoe
C, Kato T, Moriyama H and Urashima M: Functional mutation analysis
of EGFR family genes and corresponding lymph node metastases in
head and neck squamous cell carcinoma. Clin Exp Metastasis.
29:19–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi Y, Togashi Y, Yatabe Y, Mizuuchi
H, Jangchul P, Kondo C, Shimoji M, Sato K, Suda K, Tomizawa K, et
al: EGFR exon 18 mutations in lung cancer: Molecular predictors of
augmented sensitivity to afatinib or neratinib as compared with
first- or third-generation TKIs. Clin Cancer Res. 21:5305–5313.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang JC, Schuler M, Popat S, Miura S, Park
K, Passaro A, De Marinis F, Solca F, Märten A and Kim ES: Afatinib
for the treatment of non-small cell lung cancer harboring uncommon
EGFR mutations: An updated database of 1023 cases brief report.
Front Oncol. 12:8347042022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu H, Yang G, Li W, Li J, Hao X, Xing P,
Yang Y and Wang Y: EGFR exon 18 mutations in advanced non-small
cell lung cancer: A real-world study on diverse treatment patterns
and clinical outcomes. Front Oncol. 11:7134832021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borgeaud M, Parikh K, Banna GL, Kim F,
Olivier T, Le X and Addeo A: Unveiling the landscape of uncommon
EGFR mutations in NSCLC-A systematic review. J Thorac Oncol.
19:973–983. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rolfo C, Mack P, Scagliotti GV, Aggarwal
C, Arcila ME, Barlesi F, Bivona T, Diehn M, Dive C, Dziadziuszko R,
et al: Liquid biopsy for advanced NSCLC: A consensus statement from
the international association for the study of lung cancer. J
Thorac Oncol. 16:1647–1662. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Comprehensive Cancer N. NCCN
Clinical Practice Guidelines in Oncology, . Non-Small Cell Lung
Cancer. Version 2.2024. 2024, National Comprehensive Cancer
Network;
|
|
12
|
Wei Y, Cui Y, Guo Y, Li L and Zeng L: A
lung adenocarcinoma patient with a rare EGFR E709_T710delinsD
mutation showed a good response to afatinib treatment: A case
report and literature review. Front Oncol. 11:7003452021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Z, Sun J, Zhang Y, Hesilaiti N, Xia Q,
Cui H, Fan N and Xu X: Structure-guided strategies of targeted
therapies for patients with EGFR-mutant non-small cell lung cancer.
Biomolecules. 13:2102023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heigener DF, Schumann C, Sebastian M,
Sadjadian P, Stehle I, Märten A, Lüers A, Griesinger F and
Scheffler M; Afatinib Compassionate Use Consortium (ACUC), :
Afatinib in non-small cell lung cancer harboring uncommon EGFR
mutations pretreated with reversible EGFR inhibitors. Oncologist.
20:1167–1174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi Y and Mitsudomi T: Not all
epidermal growth factor receptor mutations in lung cancer are
created equal: Perspectives for individualized treatment strategy.
Cancer Sci. 107:1179–1186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuiper JL, Hashemi SM, Thunnissen E,
Snijders PJ, Grünberg K, Bloemena E, Sie D, Postmus PE, Heideman DA
and Smit EF: Non-classic EGFR mutations in a cohort of Dutch
EGFR-mutated NSCLC patients and outcomes following EGFR-TKI
treatment. Br J Cancer. 115:1504–1512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park S, Park S, Kim TM, Kim S, Koh J, Lim
J, Yi K, Yi B, Ju YS, Kim M, et al: Resistance mechanisms of EGFR
tyrosine kinase inhibitors, in EGFR exon 20 insertion-mutant lung
cancer. Eur J Cancer. 208:1142062024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu JY and Shih JY: Effectiveness of
tyrosine kinase inhibitors on uncommon E709X epidermal growth
factor receptor mutations in non-small-cell lung cancer. Onco
Targets Ther. 9:6137–6145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Xu C, Sun Y, Wang W, Li X, Liao
J, Pang L, Zeng L, Li J, Wang X, et al: Rare EGFR E709-T710delinsX:
Molecular characteristics and superior response to afatinib
treatment in NSCLC patients. Lung Cancer. 172:117–123. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ackerman AGM, Goldstein MA, Kobayashi S
and Costa DB: EGFR delE709_T710insD: A rare but potentially EGFR
inhibitor responsive mutation in non-small-cell lung cancer. J
Thorac Oncol. 7:e19–e20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY
and Yang PC: Effectiveness of tyrosine kinase inhibitors on
‘uncommon’ epidermal growth factor receptor mutations of unknown
clinical significance in non-small cell lung cancer. Clin Cancer
Res. 17:3812–3821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldman JW, Bueno AM, Dooms C, Jhaveri K,
de Miguel M, Piha-Paul SA, Unni N, Zick A, Mahipal A, Suga JM, et
al: Neratinib efficacy in patients with EGFR exon 18-mutant
non-small-cell lung cancer: Findings from the SUMMIT basket trial.
Clin Lung Cancer. 26:191–200. e1912025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Fabre E, Martin A, Chouahnia K,
Benabadji A, Matton L and Duchemann B: Successful sequential
tyrosine kinase inhibitors to overcome a rare compound of EGFR exon
18–18 and EGFR amplification: A case report. Front Oncol.
12:9188552022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Z, Teng F, Hao X, Li J and Xing P:
Bevacizumab combined with continuation of EGFR-TKIs in NSCLC beyond
gradual progression. Cancer Manag Res. 14:1891–1902. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Isaksson S, Hazem B, Jönsson M,
Reuterswärd C, Karlsson A, Griph H, Engleson J, Oskarsdottir G,
Öhman R, Holm K, et al: Clinical utility of targeted sequencing in
lung cancer: Experience from an autonomous Swedish health care
center. JTO Clin Res Rep. 1:1000132020.PubMed/NCBI
|
|
30
|
Sousa AC, Silveira C, Janeiro A, Malveiro
S, Oliveira AR, Felizardo M, Nogueira F, Teixeira E, Martins J and
Carmo-Fonseca M: Detection of rare and novel EGFR mutations in
NSCLC patients: Implications for treatment-decision. Lung Cancer.
139:35–40. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klughammer B, Brugger W, Cappuzzo F,
Ciuleanu T, Mok T, Reck M, Tan EH, Delmar P, Klingelschmitt G, Yin
AY, et al: Examining treatment outcomes with erlotinib in patients
with advanced non-small cell lung cancer whose tumors harbor
uncommon EGFR mutations. J Thorac Oncol. 11:545–555. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ibrahim U, Saqib A and Atallah JP: EGFR
exon 18 delE709_T710insD mutated stage IV lung adenocarcinoma with
response to afatinib. Lung Cancer. 108:45–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D'Haene N, Le Mercier M, Salmon I, Mekinda
Z, Remmelink M and Berghmans T: SMAD4 mutation in small cell
transformation of epidermal growth factor receptor mutated lung
adenocarcinoma. Oncologist. 24:9–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin J, Lehmann A, Klauschen F, Hummel
M, Lenze D, Grohé C, Tessmer A, Gottschalk J, Schmidt B, Pau HW, et
al: Clinical impact of rare and compound mutations of epidermal
growth factor receptor in patients with non-small-cell lung cancer.
Clin Lung Cancer. 20:350–362. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Wu Y, Wu F and Hu C: Lung
adenocarcinoma harboring triple rare EGFR exon 18 mutations rapidly
developed resistance to multiple therapies. Chemotherapy.
67:248–252. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frega S, Conte P, Fassan M, Polo V and
Pasello G: A triple rare E709K and L833V/H835L EGFR mutation
responsive to an irreversible Pan-HER inhibitor: A case report of
lung adenocarcinoma treated with afatinib. J Thorac Oncol.
11:e63–e64. 2016. View Article : Google Scholar : PubMed/NCBI
|















![The percutaneous lung biopsy tissue
in the lower lobe of the left lung was observed by H&E staining
and magnifications at (A) ×50 and (B) ×400. (C) Programmed cell
death ligand 1 test result: Positive, with tumor cell
interpretation indicating 30%. Quality control demonstrated an
assessable tumor cell count of 50%. Both positive and negative
controls were successful. Combined with the results of
immunohistochemistry [(D) thyroid transcription factor-1 (+)
(nuclear staining). (E) Napsin A (+) (cytoplasmic granular
staining). (F) Cytokeratin 7 (+) (diffuse cytoplasmic staining).
(G) Ki-67:10% (labeling index). (H) P40 (−) (no nuclear staining)
(magnification, ×200)], and the pathological diagnosis was
non-small cell lung cancer, which was consistent with the type of
pulmonary adenocarcinoma. (I) Emission CT bone scintigraphy. Four
panels show whole-body bone scans from different views. Panel I
(far left, anterior view): Increased radioactivity in the parietal
bone, right shoulder joint and left humerus. Panel II (left middle,
posterior view): Increased radioactivity in the left clavicle,
multiple ribs, and T2-T4 vertebrae. Panel III (right middle,
anterior view): Increased radioactivity in the sacrum, right
sacroiliac joint, and right ilium. Panel IV (far right, posterior
view): Increased radioactivity in the bilateral hip joints and
right femur. Remaining bones and joints show normal physiological
distribution. (J) NGS results: The EGFR p.L858R mutation, with a
frequency of 15.5% in the transthoracic lung biopsy tissue. (K) NGS
results: The EGFR p.E709K mutation, with a frequency of 21.2% in
the transthoracic lung biopsy tissue. (L) Serum tumor marker
levels: A line chart depicting changes in serum CEA levels during
the course of treatment (reference range, 0–5 ng/ml). Endobronchial
ultrasound-guided transbronchial needle aspiration biopsy was
performed to obtain tissue in the lower lobe of the left lung was
observed by H&E staining at magnifications of (M) ×50 and (N)
×400. PCK, phosphoenolpyruvate carboxykinase; INSM1,
insulinoma-associated protein 1; NGS, next-generation
sequencing.](/article_images/ol/30/5/ol-30-05-15255-g00.jpg)