Introduction
The lymphatic system serves dual roles in fluid
homeostasis and immune surveillance, which functions as a key
component of both the circulatory and immune systems. As part of
the circulatory network, it maintains tissue fluid balance by
draining ~2–4 l of protein-rich interstitial fluid on a daily basis
through specialized vessels equipped with intrinsic contractility
and unidirectional valves. Concurrently, its immune functions
facilitate antigen presentation and lymphocyte trafficking via
lymph node filtration (1).
Lymphedema, which is pathologically defined as the abnormal
accumulation of high-protein interstitial fluid (2), arises from either primary
developmental abnormalities or secondary acquired damage. Primary
lymphedema typically manifests through genetic mutations affecting
lymphatic morphogenesis (2) and is
diagnosed via combined lymphoscintigraphy findings and molecular
testing (3). Secondary forms are
identified via clinical history (including histories of surgery,
radiation or filariasis) and imaging evidence of lymphatic
obstruction, with CT/MRI indicating characteristic dermal backflow
patterns (4–6).
Although the proportion of lymphoma-associated
lymphedema in secondary lymphedema is relatively low (7), the association between lymphedema and
lymphoma exhibits distinctive clinical features, including rapid
unilateral progression, disproportionate truncal involvement and
concurrent B symptoms. For the present study, the accumulation of
11 cases over 17 years reflects both the specialization in
lymphatic disorders at Beijing Shijitan Hospital (managing
>1,200 patients with lymphedema on an annual basis) and improved
diagnostic sensitivity via advanced imaging protocols. The present
case series addresses a key literature gap, as previous reports
lacked standardized diagnostic criteria, of which only 4 out of 19
previous cases documented imaging correlates (Table I) (8–26).
These findings establish essential clinical benchmarks to
distinguish malignancy-related edema from benign edema.
 | Table I.Previous case reports of
lymphoma-associated lymphedema. |
Table I.
Previous case reports of
lymphoma-associated lymphedema.
| First author,
year | Cases, n | Patient age,
years/sex | Duration of
edema | Localization of
edema | Accompanied
symptoms | Past history | Diagnostic
findings | Histological
types | (Refs.) |
|---|
| Present study | Case 1 | 72/M | 2 months | Lower extremity | None | None | CT enhancement:
Multiple enlarged lymph nodes in the groin, pelvic cavity and
retroperitoneum | Angioimmunoblastic
T-cell lymphoma | - |
| Present study | Case 2 | 74/M | 1 month | Upper
extremity | Axillary mass | None | CT enhancement:
Multiple enlarged lymph nodes in the left axilla, with notable
delayed enhancement, splenic space- occupying lesions and multiple
lymph nodes of varying sizes in the mediastinum | Lymphoplasmacytic
lymphoma | - |
| Present study | Case 3 | 53/M | 7 months | Lower
extremity | Groin mass | None | CT: Multiple
enlarged lymph nodes in the retroperitoneum | Follicular
lymphoma | - |
| Present study | Case 4 | 41/F | 10 months | Lower
extremity | Enlargement of the
supraclavicular lymph nodes | History of
accessory breast surgery >20 years, history of hysteromyoma
>2 years, history of hysteroscopic surgery >1 year | CT: Multiple lymph
nodes on the lesser curvature of the stomach. Malignant mass in the
upper lobe of the right lung, lymph node metastasis in the right
hilum and mediastinum and multiple metastatic nodules under the
anterior chest wall | B-cell lymphoma,
unclassifiable, with features intermediate between diffuse large
B-cell lymphoma and classical Hodgkin's lymphoma | - |
| Present study | Case 5 | 62/F | 3 months | Lower
extremity | Abdominal pain and
enlargement of cervical and inguinal lymph nodes | None | CT: The root of the
mesentery, retroperitoneum to bilateral iliac masses, multiple
enlarged lymph nodes in both inguinal regions and local compression
and narrowing of the inferior vena cava. The lower end of the left
ureter is compressed and narrowed and the upper ureter and left
renal pelvis and calyx are dilated with hydronephrosis. Multiple
nodules in the spleen, considering metastatic lesions. Multiple
enlarged lymph node shadows in the upper and lower regions of both
clavicles, mediastinum and axilla, with metastatic lesions not
excluded | Follicular
lymphoma | - |
| Present study | Case 6 | 79/M | 8 months | Lower
extremity | Enlargement of
cervical lymph node | None | Ultrasound:
Bilateral cervical lymph nodes visible | Diffuse large
B-cell lymphoma | - |
| Present study | Case 7 | 52/M | 2 years | Generalized
edema | Enlargement of
lymph nodes in neck, armpit and groin and recurrent fever | None | Ultrasound:
Multiple lymph node enlargement in both inguinal regions. Bilateral
cervical lymph nodes are visible | Angioimmunoblastic
T-cell lymphoma | - |
| Present study | Case 8 | 46/M | 2 months | Lower extremity and
perineal region | Enlargement of
inguinal lymph node | None | Ultrasound:
Bilateral inguinal lymph node enlargement | Follicular
lymphoma | - |
| Present study | Case 9 | 73/F | 2 years | Lower
extremity | None | History of
endometrial cancer | CT enhancement:
Multiple small nodules in the right lung. Multiple swollen lymph
nodes in both armpits | Angioimmunoblastic
T-cell lymphoma | - |
| Present study | Case 10 | 64/M | 9 months | Lower
extremity | Enlargement of
inguinal lymph node and loss of weight | None | Null | Diffuse large
B-cell lymphoma | - |
| Present study | Case 11 | 55/M | 1 month | Lower
extremity | Enlargement of
inguinal lymph node | None | CT: A small amount
of hydrocele is present in both testicles and there is swelling in
both inguinal regions and left external iliac lymph nodes. It is
recommended to rule out tumor lesions | Diffuse large
B-cell lymphoma | - |
| Sun, 2022 | Case 12 | 69/F | 40 years | Lower
extremity | Multiple nodules of
varying sizes, the surface of some of the lesions demonstrated
erosion and exudation, with pain. | None | Null | Diffuse large
B-cell lymphoma | (8) |
| Vijaya, 2019 | Case 13 | 47/F | 47 years | Lower
extremity | Multiple ulcers and
nodules | Null | Null | Diffuse large
B-cell lymphoma | (9) |
| Sanna, 1997 | Case 14 | 84/F | 7 months | Lower
extremity | None | History of
coxarthrosis | Null | Angiotropic large
B-cell lymphoma | (10) |
| González-Vela,
2008 | Case 15 | 89/M | 7 years | Lower
extremity | Multiple
violaceous, firm, slightly infiltrated nodules | History of a right
femoropopliteal bypass | None | Diffuse large
B-cell lymphoma | (11) |
| Hills, 1993 | Case 16 | 55/M | 47 years | Lower extremity and
the right hand | Multiple firm
purplish-blue cutaneous nodules | History of a
deep-vein thrombosis of the left leg and pulmonary embolus | None | Follicular centre
cell lymphoma | (12) |
| Dargent, 2005 | Case 17 | 79/F | 28 years | Upper
extremity | A cutaneous tumor
of ~2 cm width | History of chronic
arterial hypertension, ischemic cardiomyopathy, hepatitis,
cholecystectomy, left ovariectomy, hysteropexy for cystocele
repair, varicose vein stripping and depression | None | Diffuse large
B-cell lymphoma | (13) |
| Shabbir, 2022 | Case 18 | 71/M | Several weeks | Upper
extremity | None | An extensive
history of tobacco and substance use in remission and untreated HCV
infection | A CT scan of the
RUE demonstrated cortical destruction Of the humeral head and
proximal shaft, along with infiltrative enlargement of the muscles
of the proximal RUE and marked soft tissue edema. There were also a
few enlarged axillary lymph nodes, the largest Measuring 2 cm in
short-axis. A PET scan demonstrated striking 18-FDG avidity in the
humerus and surrounding musculature (msuv 10–15), as well as two
FDG-avid axillary lymph nodes with msuv 5 and 14 | Diffuse large
B-cell lymphoma | (14) |
| Massini, 2013 | Case 19 | 45/F | Null | Upper
extremity | Purplish cutaneous
nodules, in part ulcerated and infected | Null | None | Mantle cell
lymphoma | (15) |
| Wan and Jiao,
2013 | Case 20 | 33/M | 3 years | Lower
extremity | Leg pain, flank
pain, oliguria and dark urine | Smoking
history | Vascular ultrasound
revealed segmental occlusion of inferior vena cava and
hypoechogenic lesion around abdominal aorta, bilateral sacral
arteries and right renal artery. Inguinal ultrasound found
bilateral inguinal lymphadenopathy and a hypoechoic mass in the
left inguinal region. Abdominal CT scan with contrast revealed a
retroperitoneal soft-tissue mass, which invaded the right kidney
and surrounded the abdominal aorta, inferior vena cava, superior
mesenteric artery and bilateral renal vessels (Fig. 2). Left renal pelvic and bilateral
psoas major muscles were also involved. CT urography revealed
hydronephrosis, with occlusion of right renal pelvis and right
ureter. A renal scan indicated the glomerular filtration rate to be
9.46 (left) and 65.39 ml/min (right) | Non-Hodgkin's
lymphoma of B-cell type | (16) |
| Tatnall and Mann,
1985 | Case 21 | 76/M | 3 years | Lower
extremity | Leg skin
nodules | History of prostate
cancer | Null | Non-Hodgkin's
lymphoma | (17) |
| Barki, 2020 | Case 22 | 60/M | Null | Penile scrotum | Fever, weight
loss | Null | Ultrasound imaging
indicated a thickening of the scrotum with normal scrotal contents.
CT: Bilateral upper and centrilobular pulmonary emphysema, large
lateral aortic, common iliac and left external iliac
lymphadenopathies | Hodgkin's
lymphoma | (18) |
| Paydas, 2000 | Case 23 | 63/F | Null | Upper
extremity | Skin induration and
ulceration ranging from 0.5 to 1 cm on the dorsum of her left hand
and arm | Null | Null | Diffuse large cell
lymphoma | (19) |
| Fan, 2017 | Case 24 | 56/M | 10 years | Lower
extremity | Erythema on the
right leg, multiple nodular ulcerative lesions | Null | Null | Primary cutaneous
anaplastic large cell lymphoma | (20) |
| Torres-Paoli
Sánchez, 2000, | Case 25 | 87/F | 67 years | Lower
extremity | Painful
nodules | History of
filariasis | None | Primary cutaneous
B-cell lymphoma | (21) |
| Waxman, 1984 | Case 26 | 76/M | 1.5 years | Upper
extremity | Null | Null | Null | Primary B-cell
lymphoma (?) | (22) |
| d'Amore, 1990 | Case 27 | 55/F | 30 years | Upper
extremity | Severe pain in the
left arm, a lesion of the soft tissue surrounding the upper
humerus | Null | Radiographic
studies demonstrated a large mass involving the left biceps and
triceps muscles; the underlying cortical bone was also focally
involved in this process | Primary B-cell
lymphoma | (23) |
| d'Amore, 1990 | Case 28 | 70/F | 11 years | Upper
extremity | A slowly growing,
violaceous soft-tissue nodule in the right deltoid region | Null | Null | Primary B-cell
lymphoma | (23) |
| Binjawhar,
2021 | Case 29 | 27/M | 3 years | Scrotum | None | Null | Null | Hodgkin's
lymphoma | (24) |
| Hawkins, 1980 | Case 30 | 49/M | 6 months | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 31 | 63/M | 6 weeks | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 32 | 57/F | Null | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 33 | 57/F | 1 month | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 34 | 75/F | 3 months | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 35 | 68/F | 1 day | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 36 | 56/F | Several weeks | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 37 | 64/M | 5 months | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 38 | 59/F | 3 years | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Hawkins, 1980 | Case 39 | 16/F | 1 year | Lower
extremity | Null | Null | Null | Lymphoma | (25) |
| Elgendy, 2014 | Case 40 | 60/M | Several weeks | Lower
extremity | Mild erythema,
increased warmth and moderate tenderness of the left leg | Hyperlipidaemia and
hypertension | A CT scan of the
abdomen and pelvis demonstrated left aortic lymphadenopathy and
bulky lymphadenopathies alongside the left iliac vessels, extending
to the left inguinal region with compression of the left iliac
vein | Non-Hodgkin's
lymphoma | (26) |
Case report
From May 2007 to December 2024, a cohort of 11
patients suffering from lymphedema (either induced or exacerbated
by lymphoma) received treatment at Beijing Shijitan Hospital
(Beijing, China). Following the acquisition of approval from the
institutional review board [approval no. sjtkyll-lx-2022(058)], a
retrospective analysis of these 11 patients was conducted. In the
present case report, a cohort of 11 patients diagnosed with
lymphedema underwent a series of diagnostic evaluations, including
laboratory tests (blood routine examination, blood tumor marker
examination), ultrasonography, MRI, CT and radionuclide imaging.
The imaging findings involved characterizations of
ultrasound-assessed lymph node size (short-axis diameter >10 mm
defined as being abnormal), vascularity and soft-tissue edema. CT
was used to evaluate lymphadenopathy (axial diameter >15 mm),
visceral/organ involvement and tumor masses. MRI was used to
characterize soft-tissue infiltration and lymphatic obstruction
patterns, as well as to differentiate between edema and neoplastic
infiltration. Additionally, bone marrow aspiration and biopsy were
performed to corroborate the diagnosis of malignancy (27).
The cohort of 11 patients included 8 men and 3
women. The distribution of lymphedema types included 1 patient with
upper extremity lymphedema, 9 patients with lower extremity
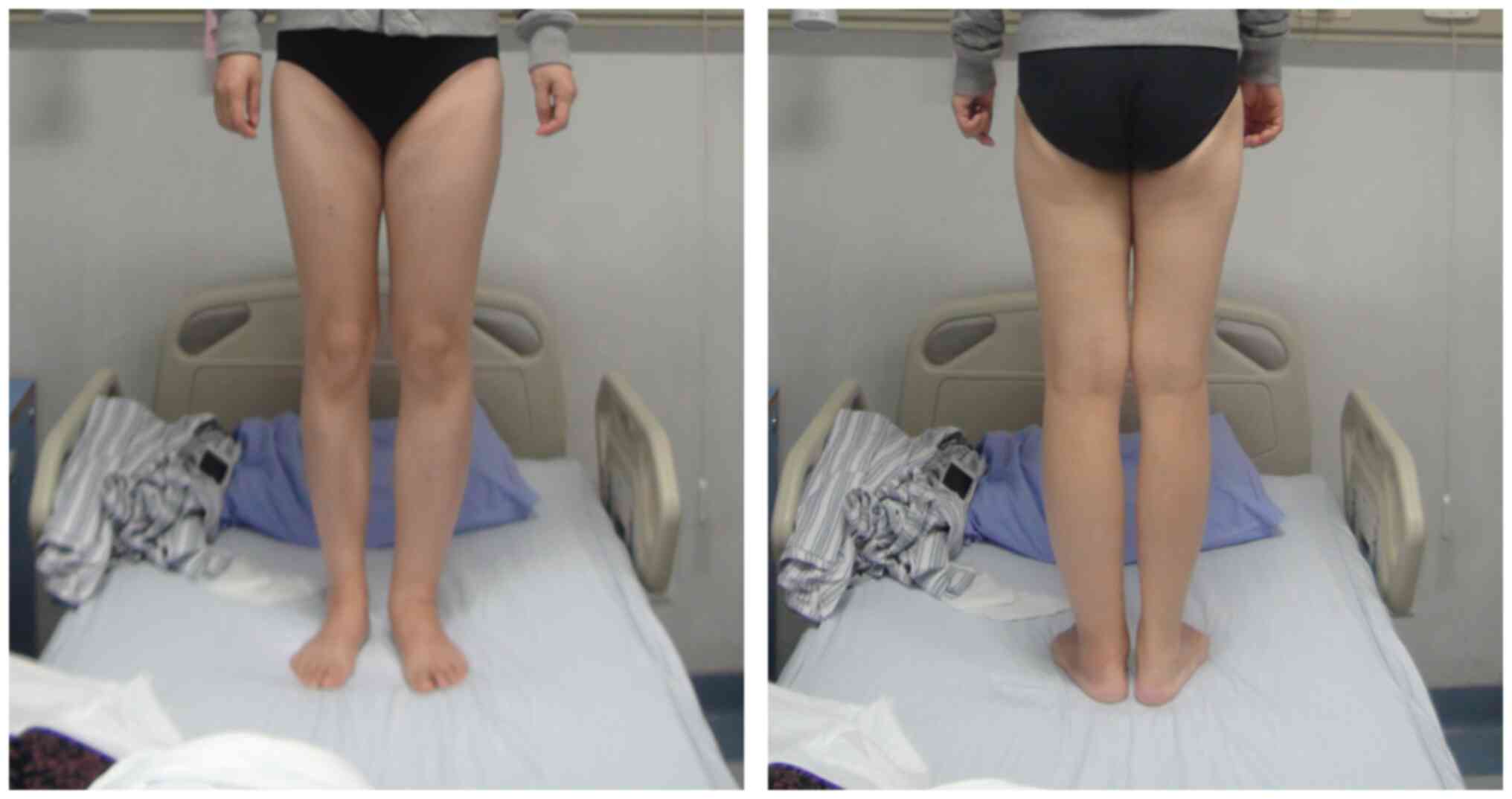
lymphedema (Fig. 1) and 1 patient
with systemic edema. The ages of the patients with lymphoma ranged
from 41 to 79 years, with a mean age of 61.0±12.5 years. The
interval between the onset of lymphedema and the subsequent
diagnosis of lymphoma varied from 1 to 24 months, with a median
duration of 7 months. Additionally, these patients presented with
various clinical symptoms, including weakness, weight loss, pain,
the presence of a mass and lymphadenopathy. A detailed summary of
the clinical features of each patient is provided in Table I.
In the present cohort, tumor markers were assessed
in 6 patients, with 66.7% (4 out of 6) of the patients exhibiting
abnormalities in at least one marker. The distribution of abnormal
markers included CA125 (1 patient), urinary Igκ light chain (3
patients), serum β2-microglobulin (1 patient), urinary Igλ light
chain (2 patients), serum Igκ light chain (2 patients) and urinary
β2-microglobulin (1 patient). Individual patients often presented
with multiple marker abnormalities (Table II). Additionally, the prevalence of
anemia among patients diagnosed with malignant lymphedema was 45.5%
(5 out of 11 patients). Several suspicious lesions were further
evaluated via imaging modalities, which yielded positive detection
rates of 100.0% for ultrasonography (7 out of 7 lesions), 100.0%
for CT (8 out of 8 lesions) and 100.0% for MRI (2 out of 2
lesions). Representative imaging and pathology findings from Case 4
are shown in Fig. 2. Axial chest CT
demonstrated a mass adjacent to the mediastinum involving the right
lung hilum and upper lobe. Contrast-enhanced CT revealed this mass
exhibited heterogeneous enhancement and was contiguous with a
larger mediastinal mass, confirming their connection. Whole-body
lymphoscintigraphy following foot injection showed mild left lower
limb thickening, significant tracer retention at the left foot
injection site suggesting impaired lymphatic drainage, and
non-visualization (absence of uptake) in the left inguinal, iliac
and para-aortic/lumbar nodal basins, indicating lymphatic
obstruction in these regions. Histopathological examination of the
dissected left supraclavicular lymph node (H&E stain) revealed
effacement of nodal architecture with fibrosis, scattered lymph
follicles, large cells and histiocyte-like cells. The morphology
led to the diagnosis of ‘B-cell lymphoma, unclassifiable, with
features intermediate between diffuse large B-cell lymphoma and
classical Hodgkin lymphoma’.
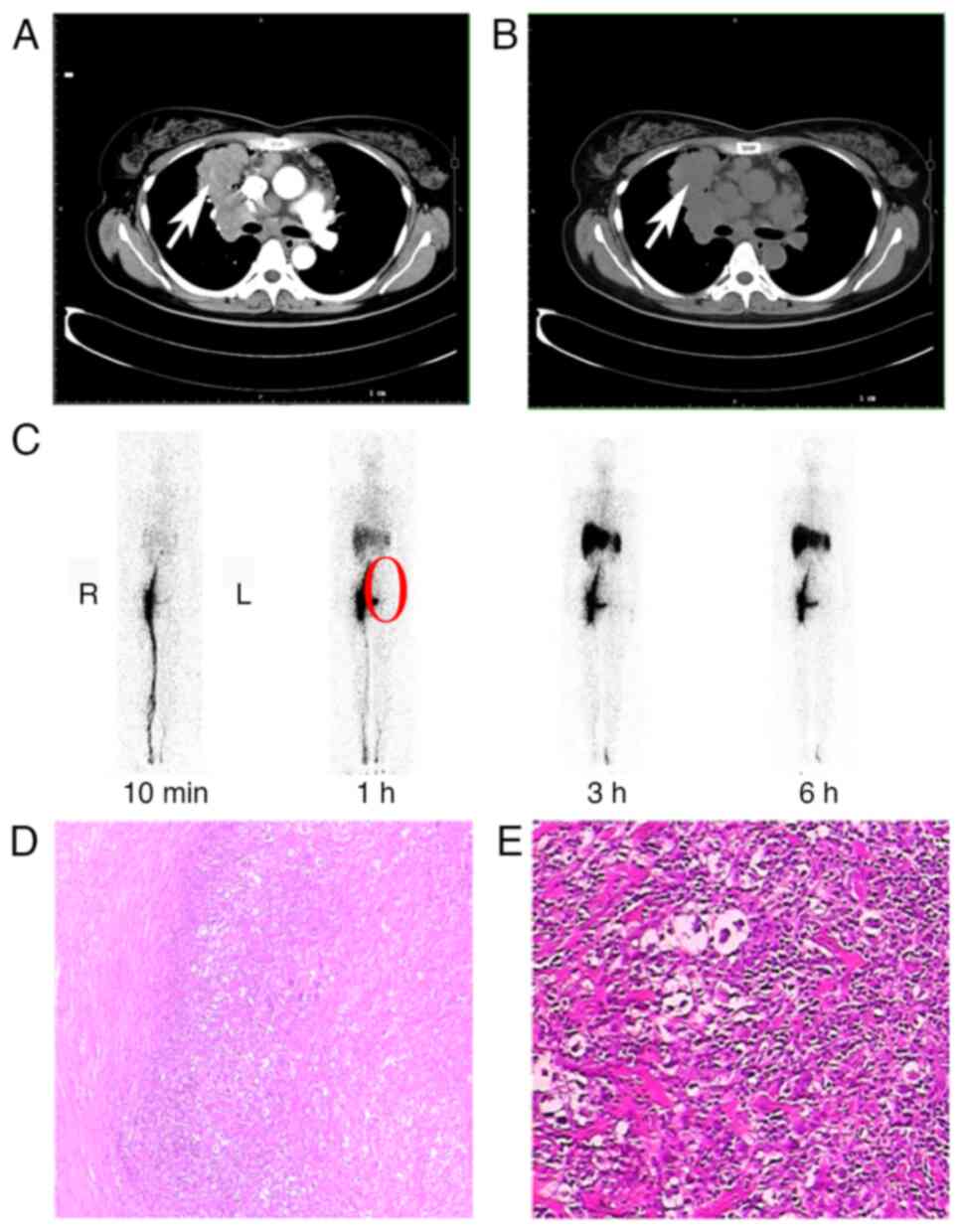 | Figure 2.Case 4. (A) Chest CT scan. This axial
CT image demonstrates a mass lesion (indicated by the white arrow)
located adjacent to the mediastinum, specifically involving the
right lung hilum and upper lobe (each mark on the scale bar
corresponds to one centimeter). (B) The contrast-enhanced axial CT
image (same level as 2A) reveals that the right hilar/upper lobe
mass (white arrow) exhibits heterogeneous (‘uneven’) enhancement
and is contiguous with a larger mediastinal mass, confirming their
connection. (Each mark on the scale bar corresponds to one
centimeter). (C) Whole-body lymphoscintigraphy image showing tracer
distribution following injection in the feet. Key findings include
the following: i) Mild thickening of the left lower limb, ii)
significant retention of the radiotracer (‘imaging agent’) at the
injection site in the left foot (suggesting impaired lymphatic
drainage), and iii) non-visualization (absence of tracer uptake) in
the left inguinal, iliac and para-aortic/lumbar lymph node basins
(highlighted by the red circle), indicating lymphatic obstruction
or dysfunction in these regions. (D and E) Pathology-left
supraclavicular lymph node: Photomicrographs of histopathological
sections from the dissected left supraclavicular lymph node (D)
magnification, ×200; (E) magnification, ×400 (H&E stain). The
morphology is diagnostic of ‘B-cell lymphoma, unclassifiable, with
features intermediate between diffuse large B-cell lymphoma and
classical Hodgkin lymphoma’. The lymph node structure is unclear,
with no subcapsular sinus observed. Fibrosis is evident and
scattered lymph follicles and nodular lymphoid tissue are visible.
In E, large cells and dry corpse-like cells are scattered within,
with some presenting as histiocyte-like. |
 | Table II.Tumor marker profiles in patients
with abnormal results. |
Table II.
Tumor marker profiles in patients
with abnormal results.
| Patient no. | CA125, U/ml
(normal, <35) | Serum β2-MG, mg/l
(normal, 1.09–2.53) | Urinary β2-MG, mg/l
(normal, <0.20) | Serum Igκ, g/l
(normal, 1.70–3.70) | Serum Igλ, g/l
(normal, 0.90–2.10) | Urinary Igκ, mg/l
(normal, <7.91) | Urinary Igλ, mg/l
(normal, <4.09) |
|---|
| 3 | - | 4.05a | 1.40a | - | - | 22.40a | 4.34a |
| 4 | 53.60a | - | - | - | - | - | - |
| 9 | - | - | - | 1.68a | - | 20.60a | 4.18a |
| 11 | - | - | - | 3.75a | - | 8.26a | - |
A total of 11 cases of lymphedema associated with
malignant tumors were pathologically verified to have originated
from the lymphatic tissue. The present cohort included 8 cases of
mature B-cell lymphoma and 3 cases of mature T-cell lymphoma, as
detailed in Table I and Fig. 2.
Furthermore, 11 patients underwent tumor and
enlarged lymph node resection biopsies. A total of 6 patients were
followed up until December 2024. Of note, 2 patients with vascular
immune T-cell lymphoma died within 1 year after diagnosis. A total
of 3 patients with diffuse large B-cell lymphoma were diagnosed and
received chemotherapy (the specific details are unknown). These
patients have survived for 7, 10 and 11 years. In addition, 1
patient with lymphoplasmacytic lymphoma has been receiving
long-term oral treatment with traditional Chinese medicine (the
specific details are unknown) and has survived for 14 years
(Table I).
Discussion
The clinical presentations of lymphoma can vary and
a notable number of cases are identified at an advanced stage,
which is primarily due to the constraints of existing diagnostic
methodologies (28). In certain
patients, lymphedema may be the initial manifestation of lymphoma,
with lymphedema representing the sole clinical manifestation of
lymphoma in certain cases. While no specific molecular biomarkers
are universally established for the diagnosis of lymphedema,
advancements in imaging and bioimpedance technologies have provided
indirect markers for the assessment of lymphatic dysfunction
(29,30). For instance, bioimpedance
spectroscopy and the tissue dielectric constant are non-invasive
tools that quantify extracellular fluid volume and detect
subclinical lymphedema with high sensitivity (31). Additionally, serum biomarkers such
as β2-microglobulin and urinary immunoglobulins (e.g., Igκ/λ light
chains) have been observed in patients with malignancy-associated
lymphedema, although these are non-specific and often linked to
underlying conditions such as lymphoma (32). Emerging research also highlights the
role of inflammatory cytokines (e.g., IL-6 and TNF-α) and
lymphangiogenic factors (such as VEGF-C/D) in lymphatic remodeling,
which may serve as potential molecular indicators in the future
(33). The current study presented
a series of lymphoma cases characterized by the presence of
lymphedema. The analysis of the present study, in conjunction with
previous studies, indicated that lymphedema could represent a
potential manifestation of lymphoma, which potentially resulted
from lymphatic or venous obstruction.
Previous studies have revealed a notable association
between lymphedema and lymphoma, with lymphomas being the most
prevalent neoplasms associated with immunodysregulation. It is
posited that abnormal lymphoid proliferation may be causally linked
to lymphatic stasis (11).
Insufficient lymphatic drainage can disrupt the normal trafficking
of lymphocytes and Langerhans cells, which are essential for the
maintenance of immunocompetence, which leads to an immunologically
susceptible state in the lymphedematous region and increases the
risks of infection and oncogenesis (34). Several theories have been proposed
to elucidate the underlying mechanisms involved in the
aforementioned process. Futrell and Myers (35) emphasized the role of the
immunological status in determining the response of animal hosts to
tumors implanted in the skin, regardless of the integrity of the
lymphatic system. Their findings revealed that, although tumor
solutions did not induce malignancy when injected into areas with
intact lymphatic vessels, malignant tumors developed when
injections occurred in regions with compromised lymphatics
(35). Furthermore, deficiencies in
the lymphatic drainage system may hinder the early detection of
tumor-specific antigens (36).
Chronic stasis can lead to alterations in the local lymphatic
protein composition, which are characterized by a decrease in the
α-2 globulin fraction and an increase in the albumin-globulin ratio
(37). This delay in protein
transport from the interstitial space to the lymphatic space may
modify the antigenic composition and/or regional immunological
competence of the tissue. The relationship between elevated
lymphocyte counts and lymphatic stasis remains complex and
context-dependent. While chronic lymphatic stasis can lead to
localized immune dysregulation, specific thresholds for lymphocyte
proliferation in lymphedema are not well-defined in the literature.
However, previous studies have suggested that persistent
CD4+ T-cell infiltration in lymphedematous tissues
contributes to chronic inflammation and fibrosis, which exacerbates
lymphatic dysfunction (38). For
instance, in cases of malignancy-related lymphedema,
tumor-associated lymphocytes may infiltrate obstructed lymphatic
pathways, although quantitative percentages are rarely reported.
Further research is warranted to establish standardized values that
associate lymphocyte counts with stasis severity. Additionally,
systemic immunodeficiency or external factors, such as potential
carcinogenic viral infections (e.g., HPV infection), may be
evaluated and utilized to further elucidate the etiology of tumors
(5,39).
In a study of 10 cases of lymphedema associated with
lymphoma, Hawkins et al (25) demonstrated that unilateral leg edema
was the sole presenting symptom in 7 cases. Similarly, Smith et
al (7) reported that among 35
cases of lymphedema attributed to neoplasms, all 8 lymphoma cases
presented with palpable inguinal lymph nodes, with 3 cases
presenting with edema as the initial manifestation of the
condition. Upon the diagnosis of lymphedema, clinicians should
maintain a heightened clinical suspicion of lymphoma as a potential
underlying cause.
In the present cohort of 11 patients, lymphedema
predominantly affected the lower extremities (9 out of 11
patients), with a mean age of 61.0±12.5 years and a median delay of
7 months between lymphedema onset and lymphoma diagnosis. The key
clinical manifestations of these patients included limb swelling,
weakness, weight loss, lymphadenopathy and pain. These findings
align with those of previous studies, such as that by Hawkins et
al (25), which reported
unilateral leg edema as the sole presenting symptom in 70% of
patients with lymphoma-associated lymphedema. Similarly, Smith
et al (7) noted that
lymphedema secondary to malignancy often manifests acutely and
asymmetrically, along with accompanying systemic symptoms such as
fatigue and weight loss. Notably, the present case report revealed
a greater proportion of lower extremity involvement (81.8%)
compared with upper limb involvement (9.1%), which is consistent
with a report by Tatnall and Mann (17), which described chronic limb
lymphedema as a predisposing factor for non-Hodgkin's lymphoma. The
present scenario contrasts with secondary lymphedema caused by
breast cancer surgery, where upper limb involvement is
predominant.
Lymphoma-induced lymphedema is frequently
misdiagnosed initially because of its nonspecific presentation. In
the present cohort, 66.7% of patients exhibited abnormal changes in
tumor markers (including alterations in CA125 and β2-microglobulin)
and 45.5% had anemia, which suggested systemic involvement.
Furthermore, imaging modalities (such as CT, MRI and
ultrasonography) achieved 100% detection rates for suspicious
lesions, which thereby underscores their diagnostic utility.
However, the case reported by González-Vela et al (11) was extremely prone to misdiagnosis:
an 89-year-old male presented with multiple cutaneous lesions on
his right limb, manifesting as chronic lymphedema. Upon skin
examination, multiple purplish red, firm, slightly infiltrated
nodules were found on his legs and instep of his foot. Biopsy of
one of the nodules revealed a diffuse large B-cell lymphoma of the
leg. CT scans of the patient's chest, abdomen, and pelvis did not
show signs of lymph node enlargement. Bone marrow aspiration and
biopsy results were normal. The patient underwent local
radiotherapy and achieved significant clinical remission. The
differentiation of lymphoma-associated lymphedema from lymphedema
of other etiologies requires a comprehensive evaluation of the
clinical presentation, imaging findings, biomarkers, pathological
mechanisms and therapeutic responses. In our research, we found
that lymphoma-induced lymphedema typically manifests with
insidious, asymmetric lower extremity swelling (81.8% of cases)
occurring over weeks to months, which is often accompanied by
systemic symptoms such as weight loss, fever or lymphadenopathy. In
addition, patients may exhibit acute-onset skin pigmentation
changes or localized neuropathic symptoms, which represent features
that are distinct from acute unilateral swelling with
tenderness/cyanosis (which occurs in venous edema) or bilateral
symmetry (which occurs in systemic causes such as heart failure).
Additionally, imaging serves a key role. Specifically, the use of
CT/MRI in lymphoma can reveal tumor-obstructed lymphatic pathways
or malignant lymphadenopathy, whereas the use of positron emission
tomography-CT can identify hypermetabolic lesions, which contrasts
the venous duplex findings of thrombosis observed in venous edema
or the hypoalbuminemia-driven fluid shifts observed with systemic
causes. Furthermore, elevated serum biomarkers (such as
β2-microglobulin and CA125) are observed in 66.7% of patients with
lymphoma, which further distinguishes lymphoma from hypoproteinemia
(low albumin levels) or a venous etiology (elevated D-dimer
levels). Pathologically, lymphoma disrupts lymphatic integrity via
direct tumor infiltration or cytokine-mediated dysfunction, which
thus creates an immunocompromised microenvironment, whereas primary
lymphedema stems from congenital lymphatic malformations.
Therapeutically, lymphoma-associated edema responds poorly to
conventional compression therapy and requires tumor-directed
interventions (such as chemotherapy or radiotherapy), unlike venous
edema (which is anticoagulation-responsive) or primary lymphedema
(which is partially improved by using decongestive therapy).
Notably, lymphoma rarely induces systemic edema, which is a feature
that is absent in postoperative or primary lymphedema and thereby
underscores the importance of screening for malignancy in atypical
presentations (40,41).
In conclusion, based on the retrospective analysis
of 11 patients with lymphoma presenting with lymphedema at our
institution, this case series establishes that lymphedema
frequently serves as an early and occasionally isolated
manifestation of lymphoma, particularly affecting the lower
extremities with asymmetric progression. Key findings include a
median diagnostic delay of 7 months, frequent systemic biomarkers
and universal detection of malignant lesions by cross-sectional
imaging (CT/MRI/ultrasonography). Critically, lymphoma-induced
lymphedema demonstrates poor response to conventional decongestive
therapy but shows significant improvement with tumor-directed
interventions (chemotherapy/radiotherapy). These observations
underscore that unilateral lower limb edema-particularly when
acute, therapy-refractory or accompanied by unexplained serologic
abnormalities-warrants rigorous screening for occult lymphoma.
Integrating targeted imaging and biomarker assessment into the
diagnostic workflow is essential to reduce delays in lymphoma
diagnosis and initiate timely oncologic management, thereby
altering the natural history of this potentially misdiagnosed
condition.
Acknowledgements
Not applicable.
Funding
Funding for the present case report was provided by the Talent
Development Program of Beijing Shijitan Hospital Affiliated to
Capital Medical University during the 14th Five-Year Plan (grant
no. 2023LJRCSWB); the Youth Fund of Beijing Shijitan Hospital
Affiliated to Capital Medical University (grant no. 2022-q16); the
Key Project of Beijing Shijitan Hospital Affiliated to Capital
Medical University (grant no. 2024-C04); and the Haidian District
Health Development Research and Cultivation Plan of Beijing (grant
no. HP2025-04-506002).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KH, RW, WS and YS conceptualized and designed the
present case report. WS, RW and YS provided administrative support.
KH, XL, JR, CY, LZ, BL, RW, YS and WS provided the study materials
and recruited patients. KH, XL, JR, CY, LZ, BL, RW and YS collected
and assembled the data. KH, XL, JR, RW and YS performed the data
analysis and interpreted the data. KH and YS confirmed the
authenticity of all the raw data. All authors wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Beijing Shijitan Hospital,
Capital Medical University, approved the present study [approval
no. sjtkyll-lx-2022(058)], which adhered to the ethical guidelines
set forth in the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the
participating patients for the publication of the anonymized
medical images (including radiological data) and associated case
details.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davis MJ, Zawieja SD and King PD:
Transport and immune functions of the lymphatic system. Annu Rev
Physiol. 87:151–172. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rockson SG and Rivera KK: Estimating the
population burden of lymphedema. Ann NY Acad Sci. 1131:147–154.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaitelman SF, Cromwell KD, Rasmussen JC,
Stout NL, Armer JM, Lasinski BB and Cormier JN: Recent progress in
the treatment and prevention of cancer-related lymphedema. CA
Cancer J Clin. 65:55–81. 2015.PubMed/NCBI
|
|
4
|
Grada AA and Phillips TJ: Lymphedema:
Diagnostic workup and management. J Am Acad Dermatol. 77:995–1006.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruocco V, Brunetti G, Puca RV and Ruocco
E: The immunocompromised district: A unifying concept for
lymphooedematous, herpes-infected and otherwise damaged sites. J
Eur Acad Dermatol Venereol. 23:1364–1373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruocco V, Schwartz RA and Ruocco E:
Lymphedema: An immunologically vulnerable site for development of
neoplasms. J Am Acad Dermatol. 47:124–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith RD, Spittell JA and Schirger A:
Secondary lymphedema of the leg: Its characteristics and diagnostic
implications. JAMA. 185:80–82. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun L, Sun Y, Xin W, He J, Hu Y, Zhang H,
Yu J and Zhang JA: A case of CD5-positive primary cutaneous diffuse
large B-cell lymphoma, leg type secondary to chronic lymphedema. Am
J Dermatopathol. 44:179–182. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vijaya B, Narahari SR, Shruthi MK and
Aggithaaya G: A rare case of primary cutaneous diffuse large B-cell
lymphoma, leg type in a patient with chronic lymphedema of the leg.
Indian J Pathol Microbiol. 62:470–472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanna P, Bertoni F, Roggero E, Quattropani
C, Rusca T, Pedrinis E, Monotti R, Mombelli G, Cavalli F and Zucca
E: Angiotropic (intravascular) large cell lymphoma: case report and
short discussion of the literature. Tumori. 83:772–775. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
González-Vela MC, González-López MA,
Val-Bernal JF and Fernández-Llaca H: Cutaneous diffuse large B cell
lymphoma of the leg associated with chronic lymphedema. Int J
Dermatol. 47:174–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hills RJ and Ive FA: Cutaneous secondary
follicular centre cell lymphoma in association with lymphoedema
praecox. Br J Dermatol. 129:186–189. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dargent JL, Lespagnard L, Feoli F,
Debusscher L, Greuse M and Bron D: De novo CD5-positive diffuse
large B-cell lymphoma of the skin arising in chronic limb
lymphedema. Leuk Lymphoma. 46:775–780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shabbir A, Kojadinovic A, Gidfar S and
Mundi PS: Extranodal diffuse large B-cell lymphoma of bone and soft
tissue presenting with marked lymphedema and hypercalcemia. Cureus.
14:e220252022.PubMed/NCBI
|
|
15
|
Massini G, Hohaus S, D'Alò F, Bozzoli V,
Vannata B, Larocca LM and Teofili L: Mantle cell lymphoma relapsing
at the lymphedematous arm. Mediterr J Hematol Infect Dis.
5:e20130162013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan N and Jiao Y: Non-Hodgkin lymphoma
mimics retroperitoneal fibrosis. BMJ Case Rep.
2013:bcr20130104332013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tatnall FM and Mann BS: Non-Hodgkin's
lymphoma of the skin associated with chronic limb lymphoedema. Br J
Dermatol. 113:751–756. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barki A, Dua-Boateng P, Mhanna T, Houmaidi
AE, Souarji A and Allat Oufkir A: Penoscrotal lymphedema revealing
a lymphoma: A case report. Urol Case Rep. 33:1013082020.PubMed/NCBI
|
|
19
|
Paydaş S, Sahin B, Yavuz S and Gönlüşen G:
Postmastectomy malignant lymphoma. Leuk Lymphoma. 36:417–420. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan P, Nong L, Sun J, Liu X, Kadin ME, Li
T, Tu P and Wang Y: Primary cutaneous anaplastic large cell
lymphoma with intralymphatic involvement associated with chronic
lymphedema. J Cutan Pathol. 44:616–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torres-Paoli D and Sánchez JL: Primary
cutaneous B-cell lymphoma of the leg in a chronic lymphedematous
extremity. Am J Dermatopathol. 22:257–260. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waxman M, Fatteh S, Elias JM and Vuletin
JC: Malignant lymphoma of skin associated with postmastectomy
lymphedema. Arch Pathol Lab Med. 108:206–208. 1984.PubMed/NCBI
|
|
23
|
d'Amore ES, Wick MR, Geisinger KR and
Frizzera G: Primary malignant lymphoma arising in postmastectomy
lymphedema. Another facet of the Stewart-Treves syndrome. Am J Surg
Pathol. 14:456–463. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Binjawhar A, El-Tholoth HS, Alzayed EM and
Althobity A: Successful surgical treatment of giant scrotal
lymphedema associated with Hodgkin's lymphoma: A rare case report.
Urol Case Rep. 37:1017082021.PubMed/NCBI
|
|
25
|
Hawkins KA, Amorosi EL and Silber R:
Unilateral leg edema. A symptom of lymphoma. JAMA. 244:2640–2641.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elgendy IY and Lo MC: Unilateral lower
extremity swelling as a rare presentation of non-Hodgkin's
lymphoma. BMJ Case Rep. 2014:bcr20132024242014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi F, Zhou Q, Gao Y, Cui XQ and Chang H:
Primary splenic B-cell lymphoma, unclassifiable, with features
intermediate between diffuse large B-cell lymphoma and classical
Hodgkin lymphoma: A case report. Oncol Lett. 12:1925–1928. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sehn LH and Salles G: Diffuse large B-cell
lymphoma. N Engl J Med. 384:842–858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mozas P, Sorigué M and López-Guillermo A:
Follicular lymphoma: An update on diagnosis, prognosis, and
management. Med Clin (Barc). 157:440–448. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Merryman R, Mehtap Ö and LaCasce A:
Advancements in the management of follicular lymphoma: A
comprehensive review. Turk J Haematol. 41:69–82. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lahtinen T, Seppälä J, Viren T and
Johansson K: Experimental and analytical comparisons of tissue
dielectric constant (TDC) and bioimpedance spectroscopy (BIS) in
assessment of early arm lymphedema in breast cancer patients after
axillary surgery and radiotherapy. Lymphat Res Biol. 13:176–185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaseb H, Wang Z and Cook JR:
Ultrasensitive RNA in situ hybridization for kappa and lambda light
chains assists in the differential diagnosis of nodular
lymphocyte-predominant hodgkin lymphoma. Am J Surg Pathol.
46:1078–1083. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ge Q, Zhao L, Liu C, Ren X, Yu YH, Pan C
and Hu Z: LCZ696, an angiotensin receptor-neprilysin inhibitor,
improves cardiac hypertrophy and fibrosis and cardiac lymphatic
remodeling in transverse aortic constriction model mice. Biomed Res
Int. 2020:72568622020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Witte CL and Witte MH: Disorders of lymph
flow. Acad Radiol. 2:324–334. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Futrell JW and Myers GH Jr: Regional
lymphatics and cancer immunity. Ann Surg. 177:1–7. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parthiban R, Kaler AK, Shariff S and
Sangeeta M: Squamous cell carcinoma arising from congenital
lymphedema. SAGE Open Med Case Rep. Sep 19–2013.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rockson SG: Advances in lymphedema. Circ
Res. 128:2003–2016. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li CY, Kataru RP and Mehrara BJ:
Histopathologic features of lymphedema: A molecular review. Int J
Mol Sci. 21:25462020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gomes CA, Magalhães CB, Soares C Jr and
Peixoto Rde O: Squamous cell carcinoma arising from chronic
lymphedema: Case report and review of the literature. Sao Paulo Med
J. 128:42–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng G and Zhang J: Imaging features (CT,
MRI, MRS, and PET/CT) of primary central nervous system lymphoma in
immunocompetent patients. Neurol Sci. 40:535–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Winkelmann M, Kassube M, Rübenthaler J,
Sheikh GT and Kunz WG: Primary imaging diagnostics of lymphomas.
Radiologie (Heidelb). 65:500–507. 2025.(In German). View Article : Google Scholar : PubMed/NCBI
|