Introduction
Small cell lung cancer (SCLC) accounts for 13–15% of
all lung cancers, of which 65–70% are extensive-stage (ES)-SCLC,
which has a high degree of malignancy and poor prognosis, with a
5-year survival rate of 5–7% (1).
Over the past few decades, platinum-based chemotherapy has been the
standard treatment for ES-SCLC, with a median survival of 8.8–10.4
months (2). However, there have
been notable breakthroughs in immunotherapy for ES-SCLC. For
example, the IMpower133 study reported that the addition of
atezolizumab [a programmed death-ligand 1 (PD-L1) inhibitor] to
chemotherapy as a first-line treatment improved the overall
survival (OS) and progression-free survival (PFS) of patients with
ES-SCLC, and enhanced the quality of life of patients (3,4). The
Caspian study reported that chemotherapy combined with durvalumab
(a PD-L1 inhibitor) for untreated patients could increase OS by 2–3
months (5,6). The Astrum-005 study reported that
chemotherapy combined with toripalimab for ES-SCLC could increase
OS to 15.4 months (7). However,
these studies did not combine chemotherapy with thoracic
radiotherapy (TRT). The trial data from the study by Kim et
al (8) identified intrathoracic
progression as the predominant pattern of treatment failure
following first-line chemo-immunotherapy in ES-SCLC. Moreover, the
study by Jeremic et al (9)
reported that chemo-radiotherapy had a synergistic antitumor effect
in the treatment of ES-SCLC. The CREST and RTOG 0937 studies
further reported that, as compared with chemotherapy alone, the
addition of TRT in the treatment plan improved the survival
advantage of patients with ES-SCLC (9–11).
Therefore, whether TRT is associated with positive synergistic
antitumor effects in the era of chemo-radiotherapy warrants further
discussion.
The present study performed a systematic review and
meta-analysis evaluating the efficacy and safety profile of
chemo-immunotherapy with and without concurrent TRT in patients
with ES-SCLC.
Materials and methods
Data search
A systematic literature search was performed across
six databases (PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/), Cochrane Library (https://www.cochranelibrary.com/), China National
Knowledge Infrastructure (https://www.cnki.net/), Wanfang (https://www.wanfangdata.com.cn/) and Chongqing
VIP Information Database (https://www.cqvip.com/) between January 1, 2018, and
March 4, 2024. The search strategy incorporated controlled
vocabulary and keywords including: ‘PD-L1’, ‘PD-1’, ‘radiotherapy’,
‘chemo-immunotherapy’, ‘ES-SCLC’, ‘immunotherapy’, ‘chemotherapy’
and ‘extensive-stage small cell lung cancer’. This retrieval
process was independently performed by two investigators.
Inclusion and exclusion criteria for
studies
The inclusion criteria were as follows: i) Patients
diagnosed with ES-SCLC through cytology or histology; ii) patients
treated with chemo-immunotherapy combined with TRT; iii) outcome
measures including OS, PFS, objective response rate and adverse
events (AE); and iv) complete, accurate and reliable data.
Moreover, the exclusion criteria were as follows: i) Reviews,
protocols, meta-analyses or letters; ii) republished articles
without new data; and iii) studies that reported outcomes but
withheld raw data or had incomplete information.
Literature screening and data
extraction
A total of two researchers independently performed
article screening and data extraction, employing cross-verification
protocols to resolve interpretive discrepancies. The extracted
information included the following: Article title; first author;
publication year; clinical trial phase; study protocol;
characteristics of the subjects included in the study and control
groups; sample size; primary outcome measures; and secondary
outcome measures.
Quality assessment of literature and
sensitivity analysis
Literature types included in the present study
consisted of cohort and single-arm studies. For cohort studies, the
quality assessment was performed using the Newcastle-Ottawa Scale
(12). For single-arm studies, the
Methodological Index for Non-Randomized Studies criteria were
applied for evaluation (13).
Furthermore, to assess the robustness of the
results, a sensitivity analysis was performed by individually
excluding each trial to evaluate its impact on the overall
outcomes. If the outcome measure included ≥10 articles, a funnel
plot was used to assess publication bias.
Data synthesis and statistical
analysis
The proportion of each endpoint with its
corresponding 95% confidence interval (CI) was pooled for each
single-arm study and visualized using forest plots. For each
dual-arm study, the hazard ratio (HR) or risk ratio (RR) with
corresponding 95% CIs were calculated. In accordance with
recommendation outlines in the Cochrane Handbook for Systematic
Reviews of Interventions (14), all
meta-analyses were performed using a random-effects model. This
approach was employed to explicitly account for the anticipated
heterogeneity in intervention effects attributable to variations
across distinct study populations and geographical settings.
Sequential trial exclusion was used in sensitivity analyses to
assess the influence of individual studies on the pooled outcomes.
Publication bias was additionally evaluated through funnel plot
analysis. The statistical analyses were performed using Stata 15
software (StataCorp LP) with Review Manager 5.4 (The Cochrane
Collaboration).
Results
Literature screening and baseline
characteristics
A total of 4,682 studies were initially screened for
inclusion. Following the removal of duplicates and the exclusion of
studies that were deemed irrelevant based on their titles and
abstracts, a total of 4,571 studies were discarded. The remaining
111 studies were subjected to a thorough review. A total of 92
studies were excluded as they did not meet the predefined
eligibility criteria. Ultimately, 19 studies were selected for
analysis, which included 10 two-arm cohort studies and 9 single-arm
studies, involving 1,577 eligible patients. The experimental group
in all studies received chemo-immunotherapy combined with TRT, and
the control group received either chemotherapy alone or
chemo-immunotherapy. The literature screening process is presented
in Fig. S1 and the basic
characteristics of the included studies are presented in Table I. All included studies were
considered to be of moderate-to-high quality (Tables SI and SII).
 | Table I.Main characteristics of the studies
included in the present meta-analysis. |
Table I.
Main characteristics of the studies
included in the present meta-analysis.
|
|
| Median age
(low-high) | Sex
(female/male) |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | Study type | TRT | Non-TRT | TRT | Non-TRT | Sample size
(systemic therapy/control) | Systemic
therapy | Control | Dose of CTRT | Outcome index | (Refs.) |
|---|
| Kim et al,
2024 | Retrospective |
|
| 7/32 | 10/62 | 41 (6/35) | Chemo +
atezolizumab + CTRT | Chemo +
atezolizumab | 52–66 Gy (5
patients), 24 Gy (1 patient) | OS, IPFS | (8) |
| Wu et al,
2022 | Retrospective | 64 (51–90) |
| 1/10 | 2/9 | 22 (11/11) | Chemo +
atezolizumab/durvalumab + CTRT | Chemo +
atezolizumab/durvalumab | 28–64 Gy | PFS, OS | (15) |
| Diamond et
al, 2022 | Retrospective | 66 (58–74) |
|
|
| 20 | Chemo +
atezolizumab + CTRT |
| 30–60 Gy | PFS, OS, LPFS,
DPFS, AEs | (22) |
| Fang et al,
2023 | Retrospective | 62 (58–68) | 64 (61–70) | 3/36 | 5/68 | 111 (39/62) | Chemo + ICI +
CTRT | Chemo + ICI | 30–60 Gy | PFS, OS, | (23) |
| Li et al,
2023 | Retrospective | 59 (52–65) | 63 (56–67) | 10/37 | 6/47 | 100 (47/53) | Chemo + ICI +
CTRT |
| 45–54 Gy | PFS, OS, LRFS | (24) |
| Perez et al,
2021 | Prospective | 66 (45–77) |
|
|
| 21 | Chemo + ipilimumab
+ nivolumab + CTRT |
| 3 Gy /10 f | PFS, OS, irAE | (29) |
| Longo et al,
2024 | Retrospective | 64 (60–69) | 71 (65–75) | 26/33 | 22/37 | 120 (59/61) | Chemo +
atezolizumab/ durvalumab + CTRT | Chemo +
atezolizumab/ durvalumab | 30–60 Gy | PFS, OS, AEs | (30) |
| Yao et al,
2024 | Retrospective |
|
| 21/78 | 17/81 | 197 (99/98) | Chemo + ICI +
CTRT | Chemo + ICI | 30–60 Gy | PFS, OS, AEs | (31) |
| Li et al,
2023 | Retrospective | 63 (35–84) |
| 2/31 |
| 36 | Chemo +
atezolizumab/ durvalumab + CTRT |
| 52–113 Gy | PFS, OS, AEs | (32) |
| Hoffmann et al,
2023 | Retrospective |
|
| 19/22 |
| 41 (23/18) | Chemo +
atezolizumab/durvalumab + CTRT | Chemo +
atezolizumab/ durvalumab | 3 Gy/10 f | OS | (33) |
| Welsh et al,
2020 | Prospective | 62 (37–80) |
| 13/20 |
| 33 | Chemo +
pembrolizumab + CTRT |
| 45 Gy | PFS, OS, AEs | (34) |
| Xie et al,
2023 | Retrospective | 62 (45–79) | 63 (45–90) | 10/35 | 12/61 | 118 (45/73) | Chemo + ICI +
CTRT | Chemo + ICI | 30–60 Gy | PFS, OS, AEs | (35) |
| Peng et al,
2023 | Retrospective | 63 (56–69) | 61 (55–66) | 14/43 | 13/44 | 114 (57/57) | Chemo +
atezolizumab/durvalumab + CTRT | Chemo +
atezolizumab/ durvalumab | 30–60 Gy | PFS, OS, AEs | (36) |
| Liu et al,
2022 | Retrospective | 62 (52–73) |
| 3/8 |
| 11 | Chemo +
atezolizumab/toripalimab + CTRT |
| 30–60 Gy/10–30
f | PFS, OS, AEs | (37) |
| Chen et al,
2022 | Prospective | 64 (37–75) |
| 6/25 |
| 31 | Chemo + SHR-1316 +
CTRT |
| 3 Gy/10 f or ≥2
Gy | PFS, OS, AEs | (38) |
| Gross et al,
2021 | Retrospective | 65 (40–90) |
|
|
| 244 (63/181) | Chemo + ICI +
CTRT | Chemo + ICI |
| OS | (39) |
| Daher et al,
2022 | Retrospective | 63 | 66 | 8/17 | 30/71 | 126 (25/101) | Chemo +
atezolizumab/durvalumab + CTRT | Chemo +
atezolizumab/durvalumab | 24–60 Gy | PFS, OS, AEs | (40) |
| Cai et al,
2023 | Retrospective | 41 (38–79) |
| 12/66 |
| 78 | Chemo + ICI +
CTRT |
|
| PFS, OS, ORR, DCR,
AEs | (41) |
| Meng et al,
2024 | Retrospective | 66 (50–79) |
| 6/27 |
| 33 | Chemo +
atezolizumab/durvalumab/PD-1 + CTRT |
| 40 (24–60) Gy | PFS, OS, AEs | (42) |
Overall outcomes
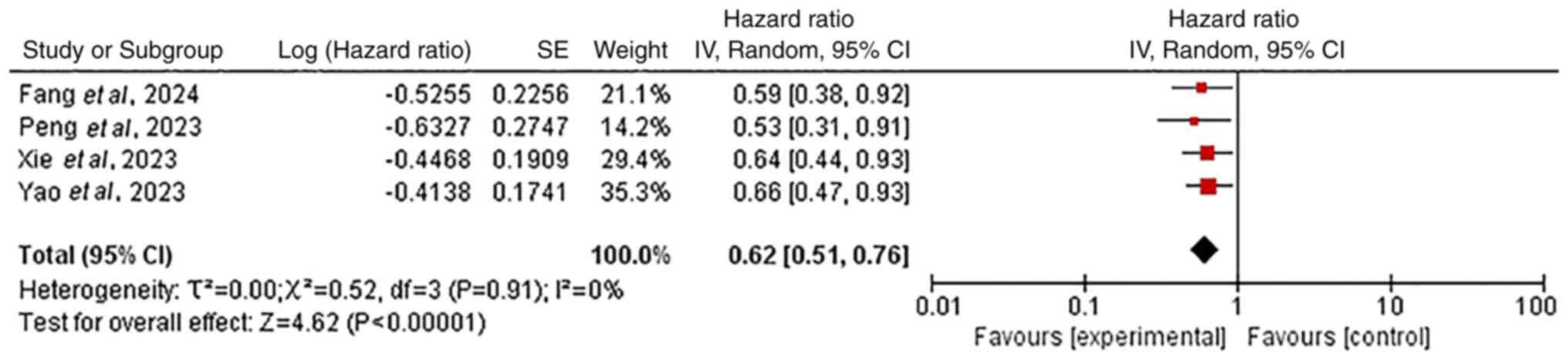
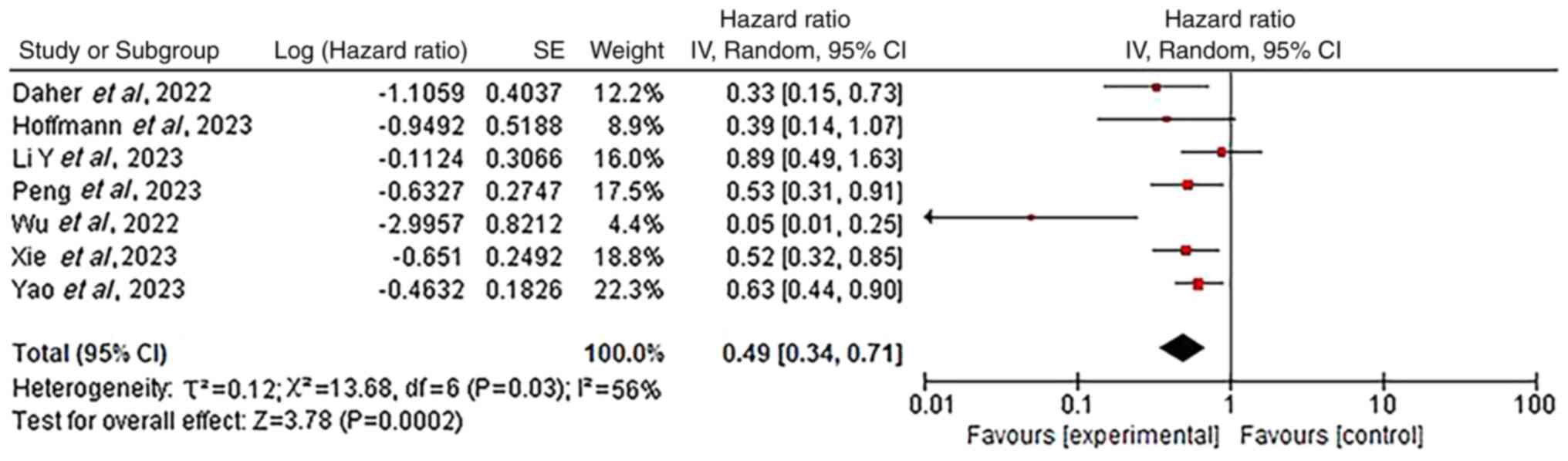
Chemo-immunotherapy with vs. without TRT was
evaluated for efficacy and safety in ES-SCLC in the present
meta-analysis. The combined approach was associated with
significantly improved survival rates compared with
chemo-immunotherapy alone. Specifically, chemo-immunotherapy with
TRT was significantly associated with improved PFS (0.62; 95% CI,
0.51–0.76; Fig. 1) and OS (0.49;
95% CI, 0.34–0.71; Fig. 2),
compared with chemo-immunotherapy alone. This indicated a
significant clinical benefit from chemo-immunotherapy combined with
TRT. Moreover, pooled analysis of specific survival data from
patients with ES-SCLC receiving chemo-immunotherapy combined with
TRT demonstrated significantly improved therapeutic outcomes. The
pooled 6-month OS was 0.92 (95% CI, 0.86–0.98), the 1-year OS was
0.72 (95% CI, 0.62–0.81) and the 18-month OS was 0.58 (95% CI,
0.43–0.73). Furthermore, the pooled 6-month PFS was 0.70 (95% CI,
0.54–0.85) and the 1-year was PFS 0.39 (95% CI, 0.27–0.51)
(Table II).
 | Table II.Summary analysis of survival time of
patients receiving chemo-immunotherapy combined with thoracic
radiotherapy. |
Table II.
Summary analysis of survival time of
patients receiving chemo-immunotherapy combined with thoracic
radiotherapy.
| Survival | HR | 95% CI | I2,
% | P-value | (Refs.) |
|---|
| OS |
|
|
|
|
|
|
6-month | 0.92 | 0.86–0.98 | 58.60 | 0.046 | (22,32,34,41,42) |
|
1-year | 0.72 | 0.62–0.81 | 85.20 | <0.001 | (22–24,29–33,35,36,41) |
|
18-month | 0.58 | 0.43–0.73 | 71.00 | 0.032 | (23,24,36) |
| PFS |
|
|
|
|
|
|
6-month | 0.70 | 0.54–0.85 | 93.20 | <0.001 | (23,29,32,34–36,41) |
|
1-year | 0.39 | 0.27–0.51 | 86.00 | <0.001 | (23,30–32,35,36,41) |
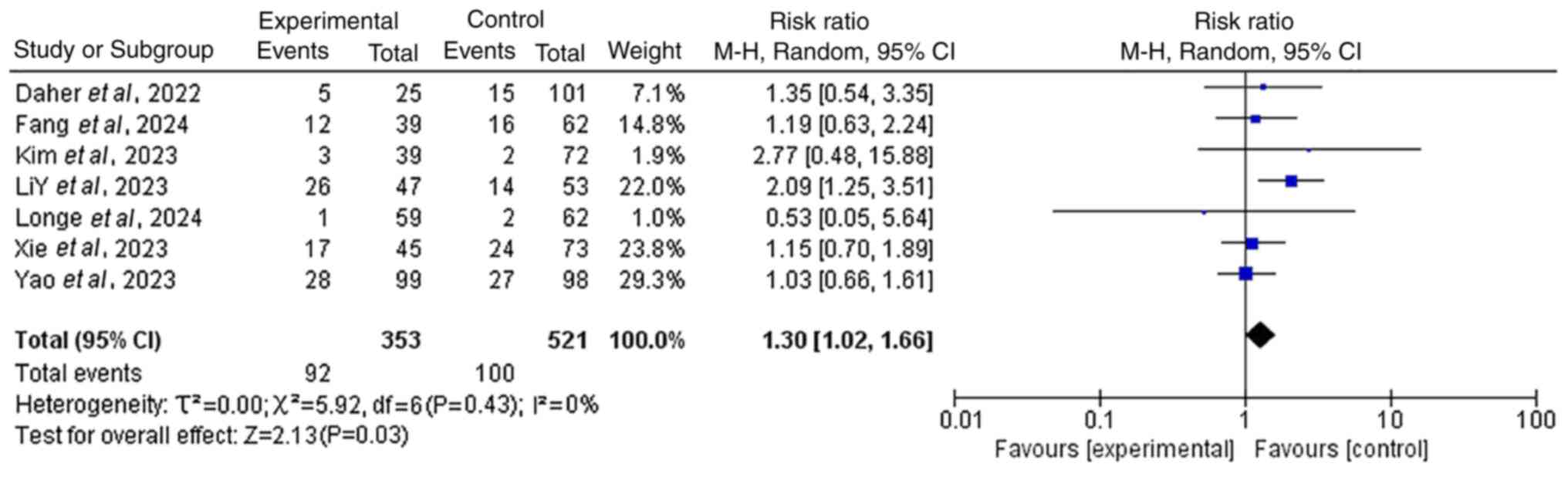
Increased incidence of grade ≥3 AEs
with combined therapy
A total of seven included studies provided data on
the incidence of grade ≥3 AEs. The results revealed that the
incidence of grade ≥3 AEs in the group receiving
chemo-immunotherapy combined with TRT was significantly higher than
that in the group receiving chemo-immunotherapy alone (RR=1.30; 95%
CI, 1.02–1.66; P<0.05), indicating that the combined treatment
had no significant impact (Fig. 3).
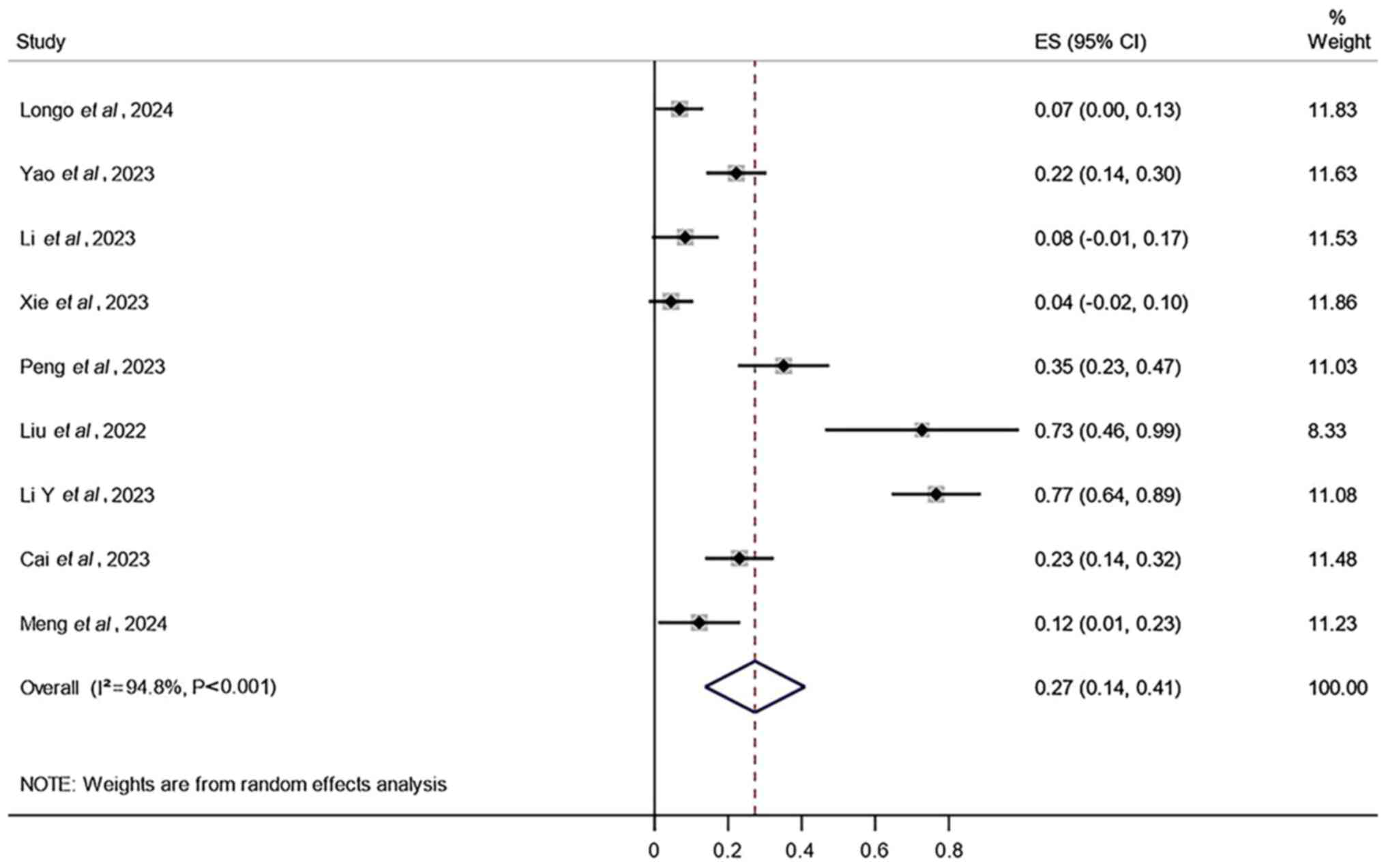
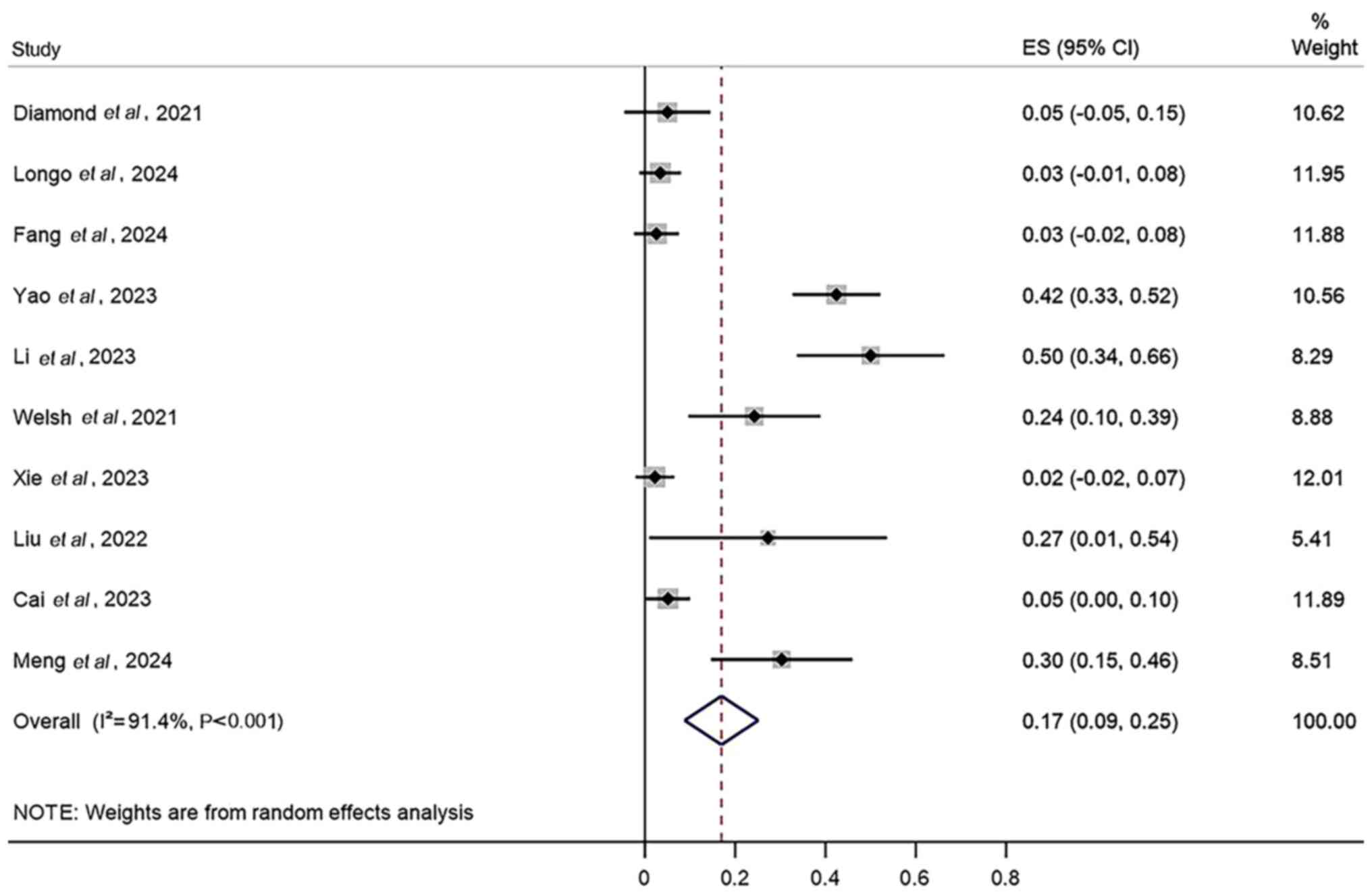
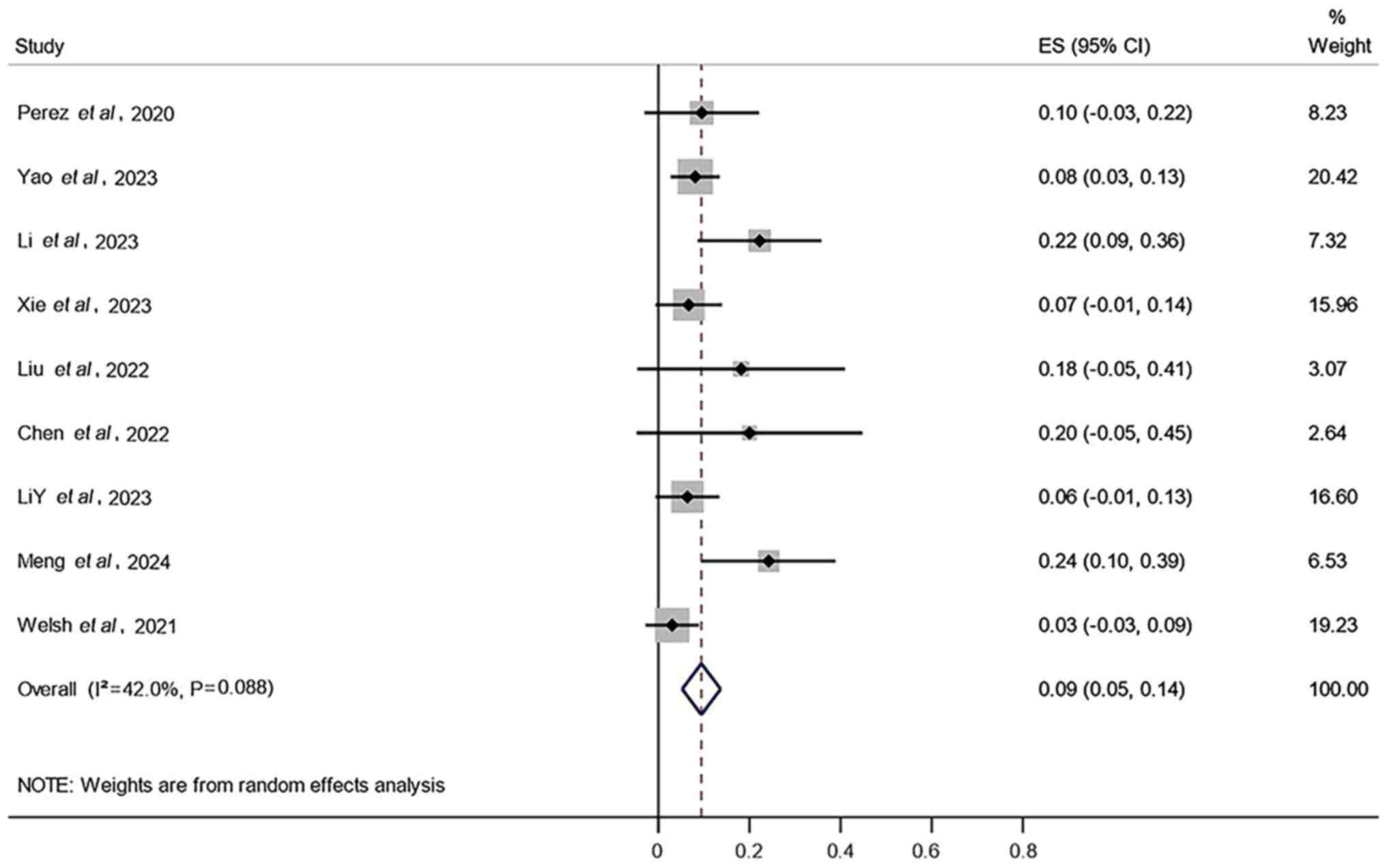
Additionally, the results demonstrated that the AEs with the
highest incidence rates in the combined treatment were radiation
pneumonitis (27%; 95% CI, 14–41%; Fig.
4), radiation esophagitis (17%; 95% CI, 9–25%; Fig. 5) and thrombocytopenia (9%; 95% CI,
5–14%; Fig. 6).
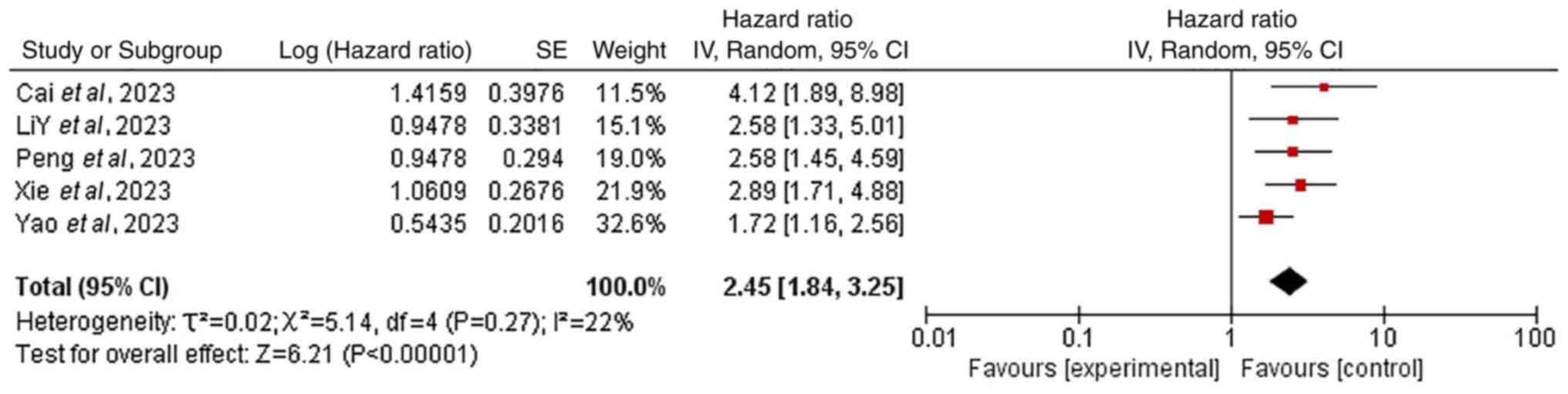
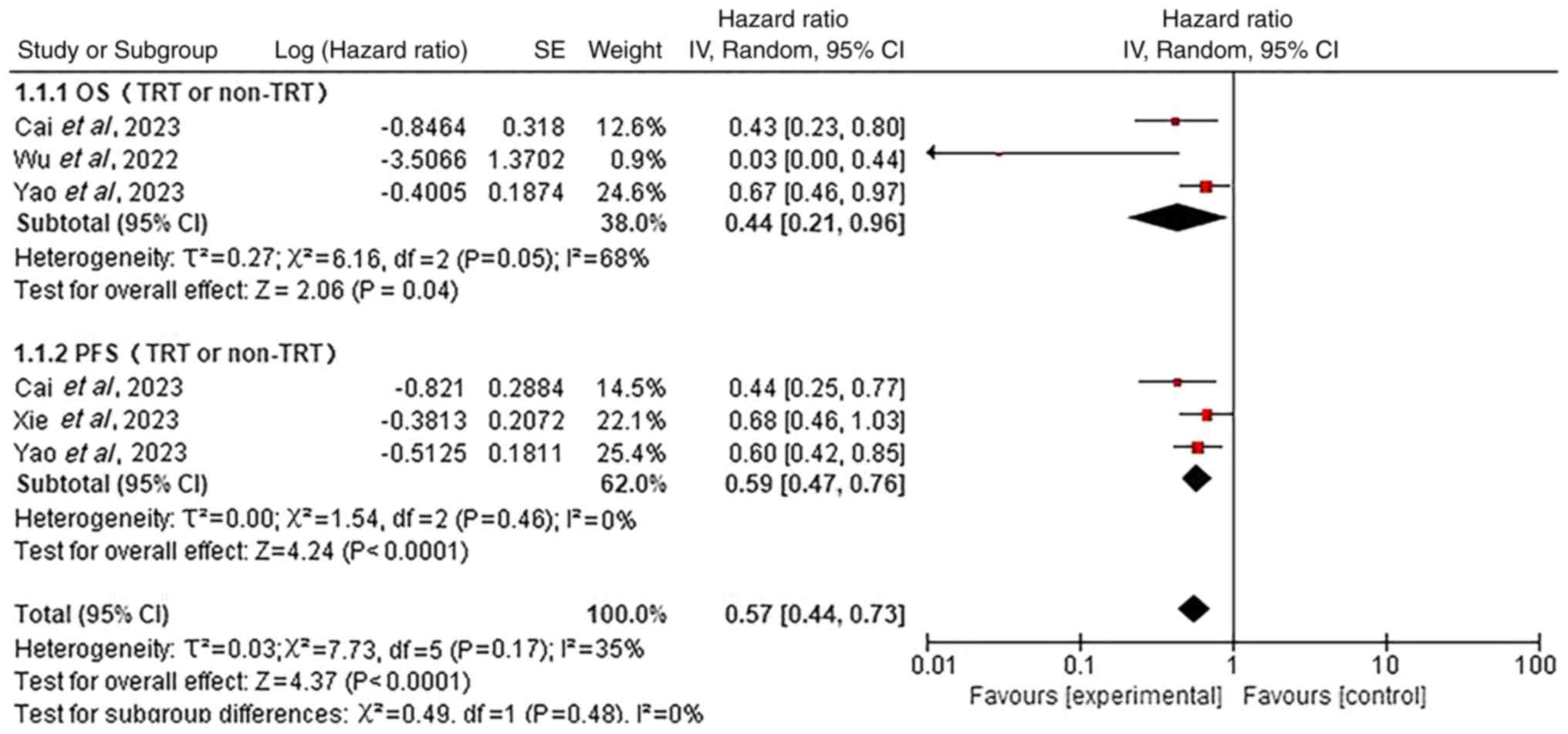
Survival disparities in prespecified
subgroups
Subgroup analyses were additionally performed to
assess the effects of pre-specified variables on OS and PFS. The
meta-analysis demonstrated that patients with ES-SCLC with baseline
liver metastases had significantly worse OS compared with patients
without liver metastases (HR=2.45; 95% CI, 1.84–3.25; P<0.05;
Fig. 7). Additionally, patients not
receiving TRT had significantly reduced OS (HR=0.44; 95% CI,
0.21–0.96; P<0.05) and PFS (HR=0.59; 95% CI, 0.47–0.76;
P<0.05) compared with TRT-treated patients (Fig. 8).
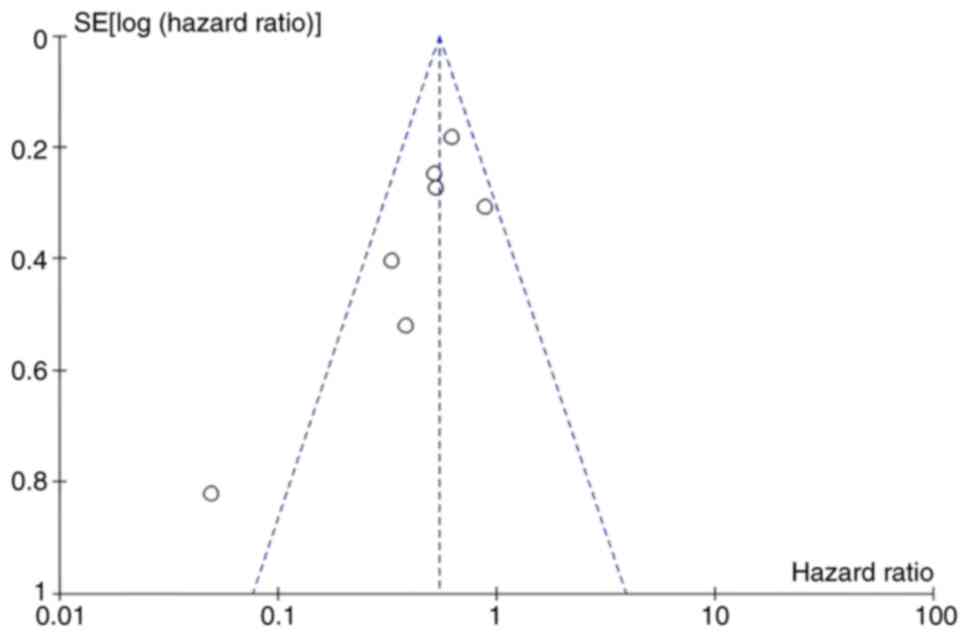
Bias and sensitivity analyses
Concurrently, a publication bias assessment and
sensitivity analysis were performed. Most included studies
exhibited a moderate risk-of-bias, primarily attributable to their
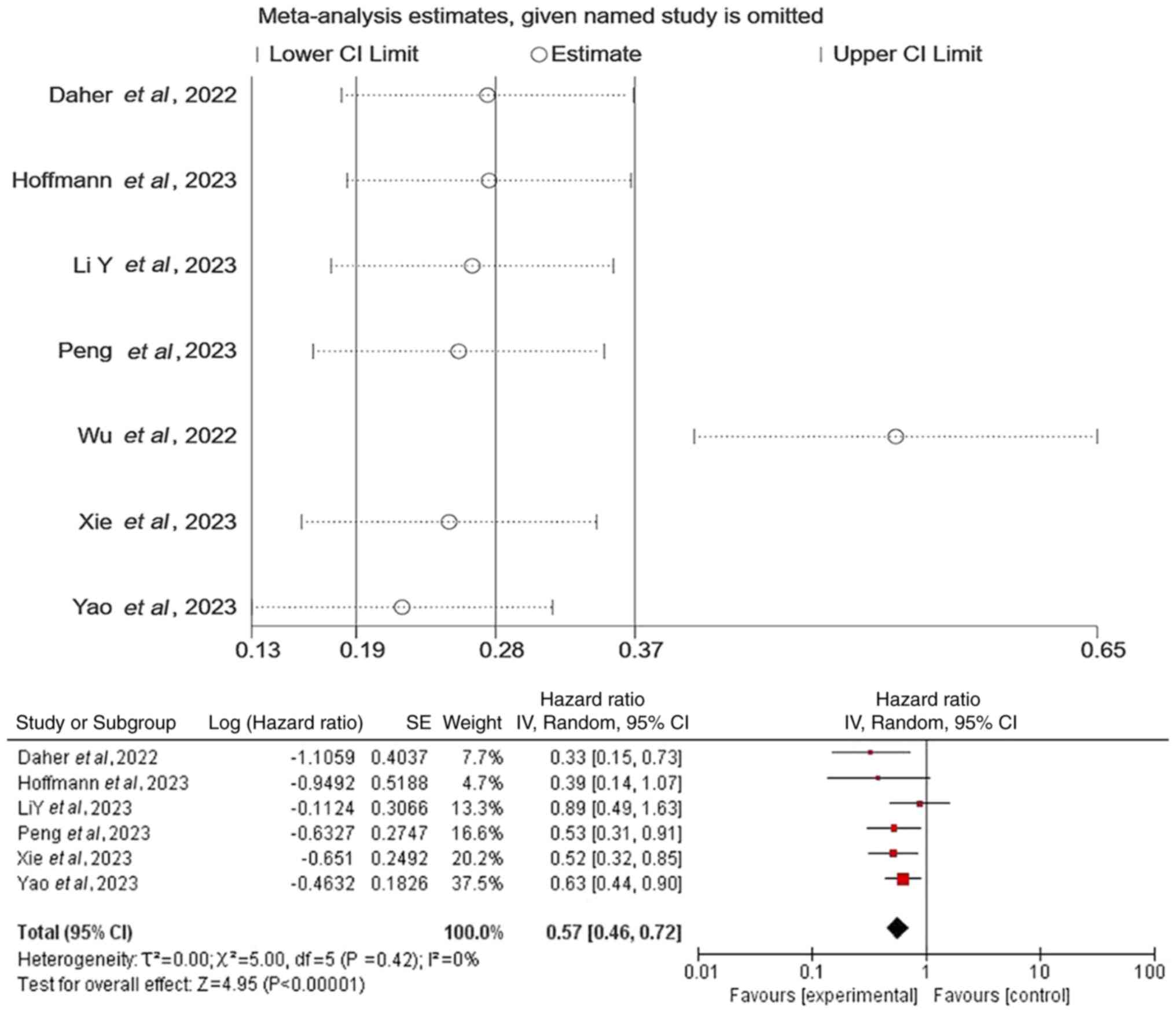
retrospective observational design (Fig. 9). Furthermore, sensitivity analysis
revealed instability in the pooled OS results. The exclusion of the
study by Wu et al (15)
significantly reduced heterogeneity (P=0.42; I2=0%),
whilst the pooled effect size remained statistically significant
(P<0.05) with unchanged overall conclusions (Fig. 10). All other outcome measures
demonstrated robust consistency.
Discussion
According to data from the American Cancer Center,
lung cancer has a high incidence and mortality rate, ranking at the
forefront among all cancers (16).
SCLC is an aggressive high-grade neuroendocrine malignancy with a
high potential for metastasis and a poor clinical prognosis. A
total of ~70% of patients are diagnosed with ES-SCLC at the time of
initial diagnosis (17,18).
Immunotherapy has made rapid progress in the field
of ES-SCLC, establishing a first-line treatment position (19). However, the application of
radiotherapy in ES-SCLC is relatively limited, with controversies
surrounding the target population, dose fractionation and timing of
its addition. Radiotherapy serves an important role in the
comprehensive treatment of SCLC (20). The phase 3 randomized controlled
trial by Slotman et al (11)
demonstrated that consolidative TRT for patients who respond to
systemic therapy has survival benefits. However, in studies such as
IMpower133 and CASPIAN, TRT was not included in the treatment
regimen (3,6). In the era of immunotherapy, the value
of TRT remains controversial. Therefore, the aim of the present
study was to further assess the efficacy and safety of adding TRT
to ES-SCLC.
The present meta-analysis included 19 relevant
studies to evaluate the efficacy and safety of the
chemo-immunotherapy combined with TRT regimen for the treatment of
ES-SCLC. The study results indicated that, compared with
chemo-immunotherapy alone, the combination with TRT significantly
prolonged OS and PFS, and was tolerable with controllable safety.
In clinical practice, the safety of treatment methods has always
been a matter of great concern. The results of the present
meta-analysis revealed a statistically significant increase in
grade ≥3 treatment-related AEs with chemo-immunotherapy + TRT
(RR=1.30; 95% CI, 1.02–1.65; P<0.05). Moreover, whilst the
adverse reactions were manageable in most cases,
radiotherapy-related complications may occur after TRT, such as
radiation-induced lung injury, radiation esophagitis and
hematological toxicity. In patients receiving chemo-immunotherapy
combined with TRT, clinicians should carefully differentiate
between radiation-induced lung injury and immune-related pneumonia,
to take appropriate treatment measures in a timely manner (21).
Notably, among the literature included in the
present study, three studies (22–24)
reported outcomes of local PFS (LPFS) and distant PFS (DPFS).
Although the data presentation varied and could not be pooled for
analysis, they still indicated a trend that chemo-immunotherapy
combined with TRT is beneficial for LPFS and DPFS (Table III). The present meta-analysis
combined the multivariate analyses of OS and PFS-related factors
from each group of studies. The results revealed that primary liver
metastasis is a risk factor affecting patient OS (HR=2.45; 95% CI,
1.84–3.25; P<0.05). This may be because liver metastasis weakens
the systemic efficacy of immunotherapy through macrophage-mediated
T-cell clearance (25,26). In liver metastases, multiple stromal
cells act synergistically to induce the generation of regulatory T
cells, promote T cell apoptosis and depletion, inhibit antigen
presentation, release immunosuppressive factors and remodel
metabolism, thereby creating a profoundly immunosuppressive
microenvironment that markedly restricts effector T cell activity,
leading to peripheral immune tolerance (27). By contrast, TRT is a favorable
factor for improving the prognosis of patients with ES-SCLC. This
is likely due to thoracic tumor progression being the main cause of
death in ES-SCLC (8). Even after
receiving standard systemic treatment, 75–90% of patients still
have residual thoracic lesions, and ≤90% of patients experience
intrathoracic progression within 1 year from diagnosis (28). However, TRT is still considered an
effective treatment strategy for local-regional control (8).
 | Table III.Analysis of LPFS and DPFS in patients
treated with chemo-immunotherapy combined with thoracic
radiotherapy. |
Table III.
Analysis of LPFS and DPFS in patients
treated with chemo-immunotherapy combined with thoracic
radiotherapy.
| A, LPFS |
|---|
|
|---|
| First author/s,
year | Median LPFS, HR
(95% CI; P-value) | (Refs) |
|---|
| Diamond et
al, 2022 | 11.4 months (95%
CI, 8.5–14.2; P<0.05) | (22) |
|
6-month | 94.70% |
|
|
12-month | 46.20% |
|
| Fang et al,
2023 | HR 0.30 (95% CI,
0.16–0.56; P<0.05) | (23) |
| Li et al,
2023 | HR 0.27 (95% CI,
0.13–0.53; P<0.05) | (24) |
|
| B, DPFS |
|
| First author/s,
year | Median DPFS (95%
CI; P-value) | (Refs) |
|
| Diamond et
al, 2022 | 6.7 months (95% CI,
5.8–7.5; P<0.05) | (22) |
|
6-month | 73.90% |
|
|
12-month | 14.10% |
|
However, the present study had the following
limitations: i) The 19 included articles were actually 8 single-arm
studies, 10 two-arm studies and 1 three-group study. However, only
data on chemo-immunotherapy combined with TRT and
chemo-immunotherapy alone was extracted. This may lead to
inconsistent comparator groups across studies; ii) the 19 included
articles contained a small amount of data; a small sample size
reduces statistical power, and small-sample data are susceptible to
influence from extreme values or random effects, which could
potentially influence the results of the meta-analysis; iii) it is
currently unclear what the indications are for TRT in ES-SCLC, and
there is uncertainty about whether the most suitable patient
population was selected for the study; and iv) the baseline
characteristics of the trials included in the present study varied,
and the treatment protocols differed, which may have affected the
final results.
In summary, TRT, as an effective means of local
tumor control, has demonstrated good efficacy for patients with
ES-SCLC in the current therapeutic landscape where immunotherapy is
integrated into systemic treatment, with controllable safety. The
combination of chemo-radiotherapy with TRT can enhance the survival
rate of patients with ES-SCLC, demonstrating favorable outcomes,
and the treatment is well-tolerated by patients, with manageable
AEs. However, more large-scale prospective randomized controlled
clinical trials are required to assess the optimal application
strategy of TRT in the treatment of ES-SCLC. This includes
determining the optimum timing for the introduction of TRT, the
radiation dose and the fractionation mode. In addition, research
should further explore how to effectively screen the beneficiary
population to ensure the maximization of treatment benefits.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health Industry Research
Program of Gansu Province (grant no. GSWSKY2021-057), the Lanzhou
City Talent Innovation and Entrepreneurship Project (grant no.
2021-RC-130) and the Gansu Provincial Key Talent Program (grant no.
2024RCXM17).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MJ performed data collection, statistical analysis
and manuscript writing. LS contributed to data collection and
manuscript revision. YL and JS were responsible for data
acquisition, analysis and interpretation, and confirm the
authenticity of all the raw data. SW interpreted data and designed
the study. All authors critically reviewed the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
George J, Maas L, Abedpour N, Cartolano M,
Kaiser L, Fischer RN, Scheel AH, Weber JP, Hellmich M, Bosco G, et
al: Evolutionary trajectories of small cell lung cancer under
therapy. Nature. 627:880–889. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roviello G, Zanotti L, Cappelletti MR,
Gobbi A, Senti C, Bottini A and Generali D: No advantage in
survival with targeted therapies as maintenance in patients with
limited and extensive-stage small cell lung cancer: A
literature-based meta-analysis of randomized trials. Clin Lung
Cancer. 17:334–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu SV, Reck M, Mansfield AS, Mok T,
Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J,
Califano R, Nishio M, et al: Updated overall survival and PD-L1
subgroup analysis of patients with extensive-stage small-cell lung
cancer treated with atezolizumab, carboplatin, and etoposide
(IMpower133). J Clin Oncol. 39:619–630. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldman JW, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab, with or without tremelimumab, plus
platinum–etoposide vs. platinum-etoposide alone in first-line
treatment of extensive-stage small-cell lung cancer (CASPIAN):
Updated results from a randomised, controlled, open-label, phase 3
trial. Lancet Oncol. 22:51–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide vs. platinum–etoposide in
first-line treatment of extensive-stage small-cell lung cancer
(CASPIAN): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng Y, Han L, Wu L, Chen J, Sun H, Wen
G, Ji Y, Dvorkin M, Shi J, Pan Z, Shi J, Wang X, Bai Y, Melkadze T,
Pan Y, et al: Effect of first-line serplulimab vs. placebo added to
chemotherapy on survival in patients with extensive-stage small
cell lung cancer: The ASTRUM-005 randomized clinical trial. JAMA.
328:1223–1232. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim D, Kim HJ, Wu HG, Lee JH, Kim S, Kim
TM, Kim JS and Kim BH: Intrathoracic progression is still the most
dominant failure pattern after first-line chemo-immunotherapy in
extensive-stage small-cell lung cancer: Implications for thoracic
radiotherapy. Cancer Res Treat. 56:430–441. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeremic B, Shibamoto Y, Nikolic N, Milicic
B, Milisavljevic S, Dagovic A, Aleksandrovic J and
Radosavljevic-Asic G: Role of radiation therapy in the
combined-modality treatment of patients with extensive disease
small-cell lung cancer: A randomized study. J Clin Oncol.
17:2092–2099. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gore EM, Hu C, Sun AY, Grimm DF,
Ramalingam SS, Dunlap NE, Higgins KA, Werner-Wasik M, Allen AM,
Iyengar P, et al: Randomized phase II study comparing prophylactic
cranial irradiation alone to prophylactic cranial irradiation and
consolidative extracranial irradiation for extensive-disease small
cell lung cancer (ED SCLC): NRG oncology RTOG 0937. J Thorac Oncol.
12:1561–15670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slotman BJ, van Tinteren H, Praag JO,
Knegjens JL, El Sharouni SY, Hatton M, Keijser A, Faivre-Finn C and
Senan S: Use of thoracic radiotherapy for extensive stage
small-cell lung cancer: A phase 3 randomised controlled trial.
Lancet. 385:36–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lo CK, Mertz D and Loeb M:
Newcastle-Ottawa Scale: Comparing reviewers' to authors'
assessments. BMC Med Res Methodol. 14:452014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cumpston M and Flemyng E. Chapter IV:
Updating a review [last updated August 2023]. Higgins JPT, Thomas
J, Chandler J, Cumpston M, Li T, Page MJ and Welch VA: Cochrane
handbook for systematic reviews of interventions. Version 6.5
(updated August 2024). Cochrane; 2024, www.training.cochrane.org/handbookJune
24–2024PubMed/NCBI
|
|
15
|
Wu JJ, Huang JW, Hsu KH, Huang YH, Chen
KC, Tseng JS, Yang TY and Chang GC: Thoracic radiotherapy may
improve the outcome of extensive stage small cell lung carcinoma
patients treated with first-line immunotherapy plus chemotherapy.
Anticancer Drugs. 33:e842–e849. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel RL, Miller KD and Wagle NS: Cancer
statistics, 2023. CA: A Cancer J Clin. 73:17–48. 2023.PubMed/NCBI
|
|
17
|
Farago AF and Keane FK: Current standards
for clinical management of small cell lung cancer. Transl Lung
Cancer Res. 7:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oronsky B, Reid TR, Oronsky A and Carter
CA: What's new in SCLC? A review. Neoplasia. 19:842–847. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrows ED, Blackburn MJ and Liu SV:
Evolving role of immunotherapy in small cell lung cancer. Semin
Cancer Biol. 86:868–874. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Zhao Y, Ma T, Shao H, Wang T, Jin S
and Liu Z: Radiotherapy for extensive-stage small-cell lung cancer
in the immunotherapy era. Front Immunol. 14:11324822023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Sheikh K, Nakajima E, Lin CT, Lee
J, Hu C, Hales RK, Forde PM, Naidoo J and Voong KR: Radiation
versus immune checkpoint inhibitor associated pneumonitis: Distinct
radiologic morphologies. Oncologist. 26:e1822–e1832. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diamond BH, Verma N, Shukla UC, Park HS
and Koffer PP: Consolidative thoracic radiation therapy after
first-line chemotherapy and immunotherapy in extensive-stage small
cell lung cancer: A multi-institutional case series. Adv Radiat
Oncol. 7:1008832022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang M, Wang L, Gu Q, Wu H, Du X and Lai
X: Efficacy and safety of thoracic radiotherapy for extensive stage
small cell lung cancer after immunotherapy in real world. Clin Exp
Metastasis. 40:423–429. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Jing W, Jing X, Sun Y, Tang X, Guo
J, Zhang Y and Zhu H: Role of consolidative thoracic radiation in
extensive-stage small-cell lung cancer with first-line
chemo-immunotherapy: A retrospective study from a single cancer
center. Discov Oncol. 14:552023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindblad KE and Lujambio A: Liver
metastases inhibit immunotherapy efficacy. Nat Med. 27:25–27. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'leary K: Liver metastases cultivate an
immune desert. Nat Rev Cancer. 21:1432021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan L, Lin Y, Fu Y and Wang J: Small cell
lung cancer with liver metastases: From underlying mechanisms to
treatment strategies. Cancer Metastasis Rev. 44:52024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slotman B, Faivre-Finn C, Kramer G, Rankin
E, Snee M, Hatton M, Postmus P, Collette L, Musat E and Senan S;
EORTC Radiation Oncology Group and Lung Cancer Group, :
Prophylactic cranial irradiation in extensive small-cell lung
cancer. N Engl J Med. 357:664–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perez BA, Kim S, Wang M, Karimi AM, Powell
C, Li J, Dilling TJ, Chiappori A, Latifi K, Rose T, et al:
Prospective single-arm phase 1 and 2 study: ipilimumab and
nivolumab with thoracic radiation therapy after platinum
chemotherapy in extensive-stage small cell lung cancer. Int J
Radiat Oncol Biol Phys. 109:425–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Longo V, Della Corte CM, Russo A, Spinnato
F, Ambrosio F, Ronga R, Marchese A, Del Giudice T, Sergi C,
Casaluce F, et al: Consolidative thoracic radiation therapy for
extensive-stage small cell lung cancer in the era of first-line
chemo-immunotherapy: Preclinical data and a retrospective study in
Southern Italy. Front Immunol. 14:12894342024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao Y, Li B, Song R, Yang L, Zou B and
Wang L: Efficacy and safety of thoracic radiotherapy in
extensive-stage small-cell lung cancer patients receiving
first-line immunotherapy plus chemotherapy: A propensity score
matched multicentre retrospective analysis. Radiat Oncol.
19:252024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Yang D, Min Y, Liao A, Zhao J, Jiang
L, Dong X, Deng W, Yu H, Yu R, Zhao J and Shi A: First-line
atezolizumab/durvalumab plus platinum–etoposide combined with
radiotherapy in extensive-stage small-cell lung cancer. BMC Cancer.
23:3182023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoffmann E, De-Colle C, Potkrajcic V,
Baumann D, Spengler W, Gani C and Utz D: Is consolidative thoracic
radiotherapy of extensive-stage small cell lung cancer still
beneficial in the era of immunotherapy? A retrospective analysis.
Strahlenther Onkol. 199:668–675. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Welsh JW, Heymach JV, Chen D, Verma V,
Cushman TR, Hess KR, Shroff G, Tang C, Skoulidis F, Jeter M, et al:
Phase I trial of pembrolizumab and radiation therapy after
induction chemotherapy for extensive-stage small cell lung cancer.
J Thorac Oncol. 15:266–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie Z, Liu J, Wu M, Wang X, Lu Y, Han C,
Cong L, Li J and Meng X: Real-world efficacy and safety of thoracic
radiotherapy after first-line chemo-immunotherapy in
extensive-stage small-cell lung cancer. J Clin Med. 12:38282023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng J, Zhang L, Wang L, Feng H, Yao D,
Meng R, Liu X, Li X, Liu N, Tan B, et al: Real-world outcomes of
PD-L1 inhibitors combined with thoracic radiotherapy in the
first-line treatment of extensive stage small cell lung cancer.
Radiat Oncol. 18:1112023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu W, Han Z, Wang J, Zhang T, Chen D,
Feng Q, Xiao Z, Lyu J, Wang X, Deng L, et al: Safety of thoracic
radiotherapy followed by PD-1/PD-L1 inhibitors after induction
therapy in extensive-stage small cell lung cancer. Chin J Radiat
Oncol. 31:236–241. 2022.
|
|
38
|
Chen D, Zou B, Meng X, Huang W, Shao Q,
Tang X, Guo J, Hu X, Zhang Y, Fu L, et al: Safety and efficacy of
SHR-1316 combined with chemotherapy and sequential chest
radiotherapy as first-line therapy for extensive-stage small cell
lung cancer (ES-SCLC): The results from a phase II single-arm
trial. J Clin Oncol. 40 (Suppl):85632022. View Article : Google Scholar
|
|
39
|
Gross AJ, Kharouta MZ, Podder TK, Choi S
and Biswas T: Role of thoracic radiotherapy in extensive stage
small cell lung cancer (ES-SCLC) in the immunotherapy era: A
national hospital-based registry analysis. Int J Radiat Oncol Biol
Phys. 111:e4662021. View Article : Google Scholar
|
|
40
|
Daher S, Allen A, Rottenberg Y, Nasrallah
H, Yosef L, Blumenfeld PA, Wollner M, Appel S, Nechushtan H,
Moskovitz M, Bar J and Zer A: 144P Real-world data of consolidative
radiotherapy for extensive stage (ES)-SCLC treated by
chemo-immunotherapy (chemo-IO). Ann Oncol. 33:S99–S100. 2022.
View Article : Google Scholar
|
|
41
|
Cai Z, Gu X, Xie J, Cheng D, Chen J, Cheng
J, Ye J and Lv T: Safety and efficacy of thoracic radiotherapy
combined with chemo-immunotherapy in patients with extensive-stage
small cell lung cancer: A multicenter retrospective analysis.
Transl Lung Cancer Res. 12:1987–2000. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng F W.: Clinical study of first-line
chemotherapy and immunotherapy combined with thoracic radiotherapy
for extensive-stage small cell lung cancer. Chin J Radiat Oncol.
33:110–115. 2024.
|