Thyroid cancer, a leading concern among endocrine
system tumors, has demonstrated a consistent rise in global
incidence. Thyroid cancer ranked as the seventh most common
malignancy globally in 2022, with >821,000 reported cases
(1). The diverse pathological types
and clinical manifestations of thyroid cancer pose notable
challenges for both medical research and clinical management. As it
occurs within a vital organ within the human endocrine system,
thyroid gland dysfunction not only disrupts metabolic homeostasis
but also endangers overall health when compromised by cancer
progression (2).
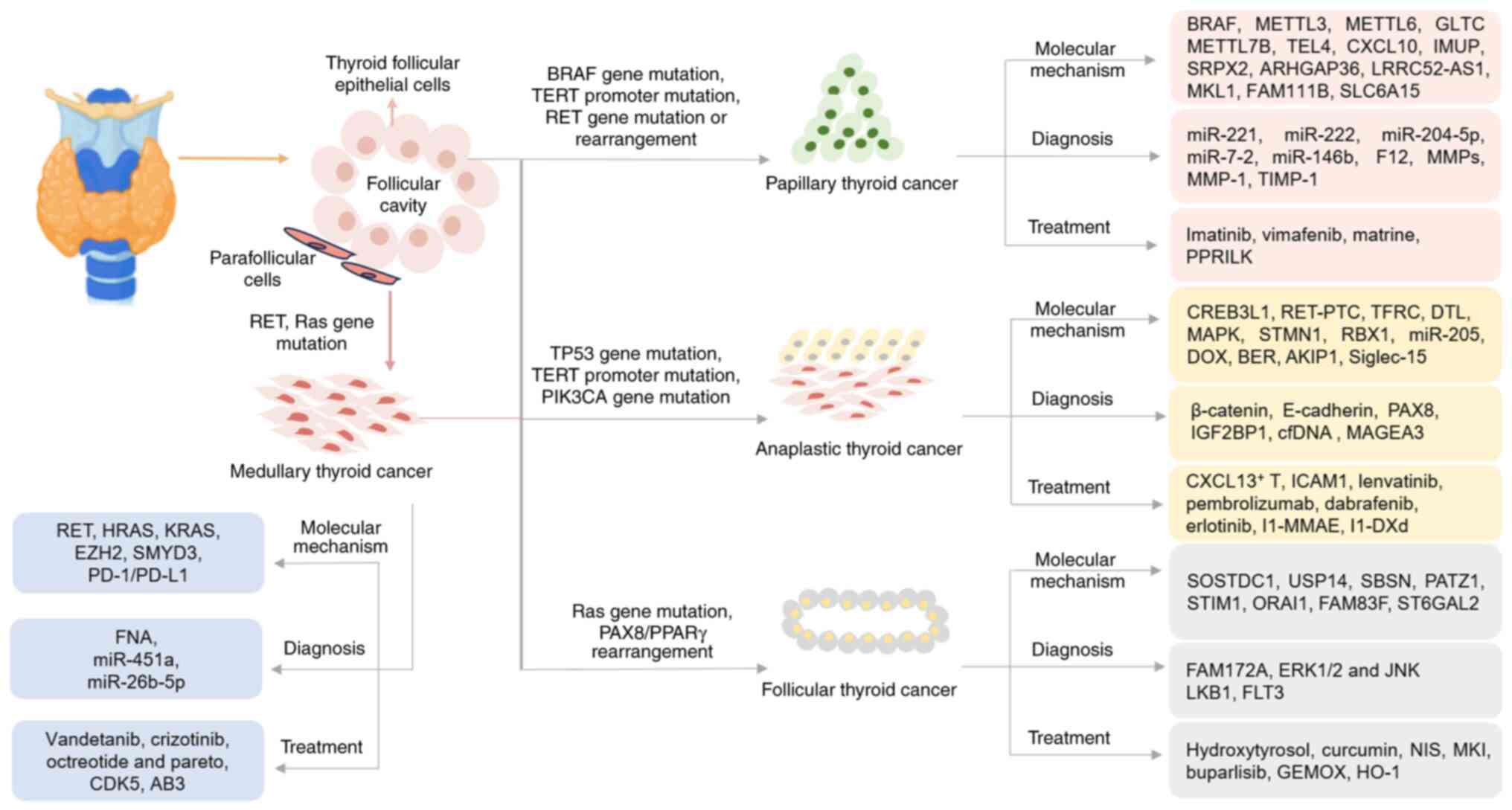
Thyroid cancer arises from follicular or
parafollicular epithelial cells, representing the most common head
and neck malignancy. Thyroid cancer can be histologically
classified into several types, including papillary thyroid
carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary
thyroid carcinoma (MTC) and anaplastic thyroid carcinoma (ATC),
with PTC being the most prevalent. The global incidence of thyroid
cancer continues to rise, particularly among high-risk groups, such
as women, individuals with genetic predispositions or those with a
family history of the disease (3).
PTC, accounting for 85–90% of thyroid malignancies,
is characterized by papillary structures and a favorable prognosis,
yet recurrence and lymph node metastasis remain clinical challenges
(4). FTC, comprising ~10% of cases,
exhibits greater invasiveness and a higher probability of distant
metastasis to the lungs and bones compared with PTC, which
necessitates precise and individualized treatment strategies
(5). MTC, a neuroendocrine
malignancy originating from calcitonin-secreting parafollicular C
cells, constitutes 1–5% of thyroid malignancies. The pathogenesis
of MTC has been significantly associated with rearranged during
transfection (RET) proto-oncogene mutations and exhibits notable
hereditary predisposition, notably mediated by gain-of-function
mutations, which necessitates comprehensive genetic counseling and
multidisciplinary approaches for optimal management (6). ATC, although accounting for only 1–2%
of all thyroid malignancies, is the most aggressive form. It is
characterized by poor differentiation, rapid progression, a poor
prognosis and limited responsiveness to current treatments
(7).
The present comprehensive review systematically
evaluates recent progress in understanding the four principal
histological subtypes of thyroid carcinoma. While PTC remains the
most extensively characterized subtype, notable knowledge gaps
persist regarding the signaling cascades and metabolic
reprogramming in FTC, MTC and ATC, as schematically illustrated in
Fig. 1. The present review
summarizes notable findings spanning molecular pathogenesis,
diagnostic biomarker development and emerging therapeutic
modalities specific to these understudied thyroid cancers.
Particular emphasis has been placed on delineating subtype-specific
molecular signatures that may inform precision diagnostics and
targeted intervention strategies. The present review aims to
establish a comprehensive framework to guide subsequent mechanistic
investigations and clinical translation in thyroid oncology.
PTC is the most prevalent form of thyroid cancer and
its incidence is rising globally. The pathogenesis of PTC is
intricate, involving a range of genetic and epigenetic alterations.
Among these, the BRAF V600E gene mutation is one of the most
frequently observed molecular events in PTC, the pooled sensitivity
of IHC for detecting BRAF V600E mutation was 96.8% [95% confidence
interval (CI): 94.1–98.3%] (8).
This mutation primarily impacts the MAPK signaling pathway,
resulting in the persistent activation of the downstream MEK
protein, which in turn promotes cell proliferation, differentiation
and tumorigenesis (9). A thorough
understanding of these molecular mechanisms not only elucidates the
pathogenesis of PTC but also identifies key molecular markers for
its diagnosis, treatment and prognostic evaluation in the
future.
PTC accounts for 85–90% of all thyroid cancer cases,
with its molecular mechanisms being both complex and diverse
(4). These mechanisms involve
various genetic mutations (such as BRAF, RAS, RET/PTC) and the
dysregulated expression of non-coding RNAs (10). Recent studies have highlighted
genes, like methyltransferase-like 3 (METTL3), METTL16, METTL7B and
transducin-like enhancer of split 4, closely associated with the
onset progression invasiveness and prognosis of PTC. Emerging
evidence identifies long non-coding RNAs (lncRNAs) among key
non-coding RNA regulators driving PTC pathogenesis (11–14).
Deciphering these molecular pathways enhances mechanistic insights
into PTC pathogenesis, while establishing essential frameworks for
early detection, therapeutic development and outcome prediction
(Fig. 2).
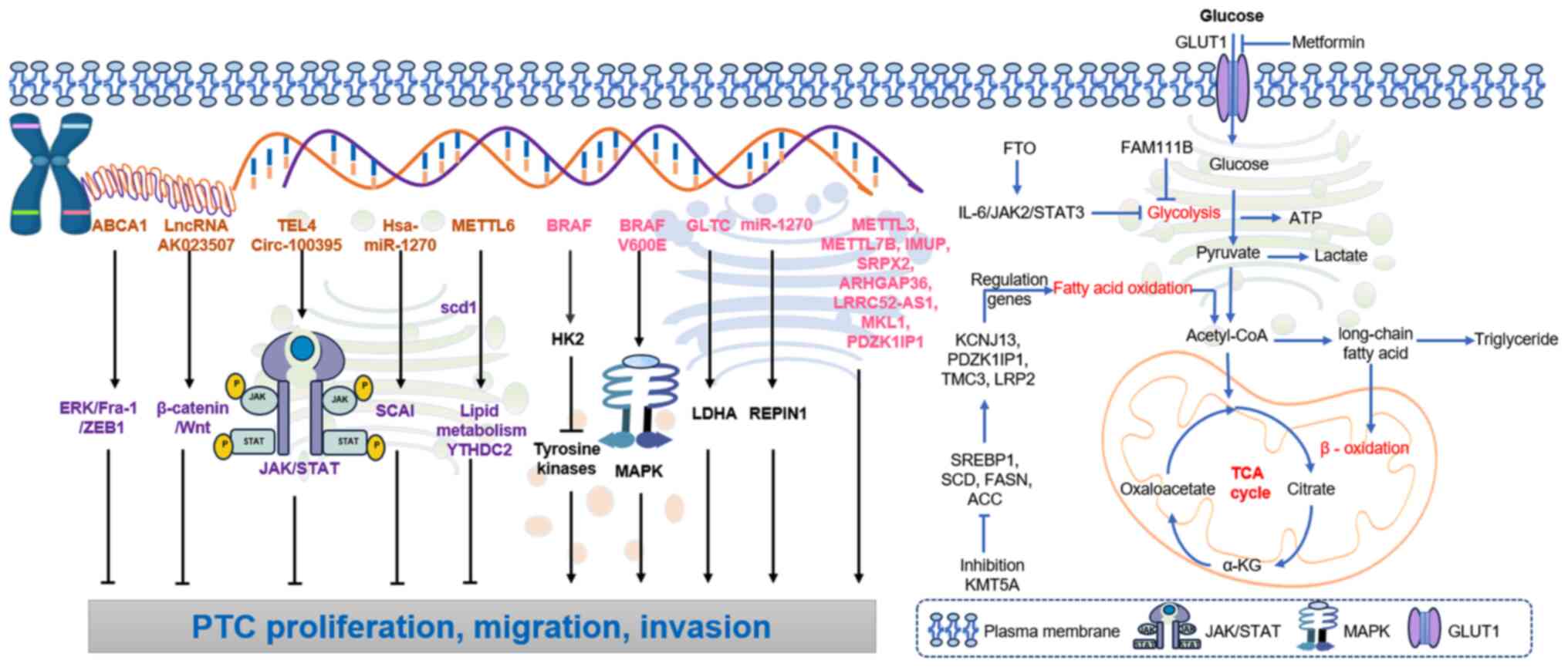
The molecular landscape of PTC is notably shaped by
mutations in various coding genes, with the BRAF gene mutation
being one of the most prevalent. Located on chromosome 7q34, BRAF
serves a key role in the Ras/Raf/MEK/ERK signaling pathway. The
BRAF V600E mutation is present in up to 90% of PTC cases and leads
to the continuous activation of the MEK protein, which promotes
cell proliferation and tumorigenesis. This mutation also
upregulates hexokinase-2 (HK2), which inhibits upstream tyrosine
kinases, thereby further contributing to PTC growth (15). The BRAF V600E mutation enhances cell
proliferation and tumor growth by activating the MAPK signaling
pathway.
Additional genes, such as METTL3, METTL6 and
METTL7B, are associated with the proliferation and migration of PTC
(11,12). Overexpression of METTL3 and METTL7B
has been shown to facilitate tumor progression, while METTL16
interacts with stearoyl-CoA desaturase 1 to activate lipid
metabolism pathways and with YT521-B homology domain-containing
protein 2, which inhibits PTC progression (13). By contrast, transducin-like enhancer
of split 4 negatively associates with the proliferation, migration
and invasion of PTC cells, while promoting the activation of the
JAK/STAT signaling pathway (14).
The potassium calcium-activated channel subfamily N
member 4 (KCNN4) gene serves an essential role in modulating the
proliferation, migration and invasion of PTC cells. Low expression
levels of KCNN4 not only hamper tumor progression but also enhance
the expression of apoptosis-related genes, which suppresses
epithelial-mesenchymal transition (EMT) (16). By contrast, the expression of PDZ
domain-containing kidney-specific protein 1-interacting protein 1
(PDZK1IP1) enhances the proliferation and migration of PTC while
inhibiting apoptosis (17).
Furthermore, C-X-C motif chemokine ligand 10 expression is
downregulated in PTC, which has been closely associated with
immunity and cellular defense mechanisms (18). C-X-C chemokine receptor type 4 gene
upregulation in PTC tissues provides a potential therapeutic target
(19). Furthermore, the
downregulation of Bcl-2-like protein 11 in PTC results in increased
microRNA (miRNA/miR)-300 expression, which influences PTC activity
and behavior (20).
Lastly, the genes immortalization-upregulated
protein (IMUP), sushi repeat-containing protein, X-linked 2
(SRPX2), ρ GTPase-activating protein 36, leucine-rich
repeat-containing protein 52 antisense RNA 1 and megakaryoblastic
leukemia 1 are positively associated with the progression of PTC.
High expression levels of these genes contributed to the
proliferation, migration and invasion of PTC cells, which suggests
their utility as potential therapeutic targets (21–25).
In glutamine-affinity PTC, the inhibition of glutamine breakdown
can reduce mitochondrial respiration, thereby inhibiting PTC
activity (26). The family with
sequence similarity 111 member B (FAM111B) motif inhibits the
glycolytic process in PTC, which in turn prevents its
proliferation, migration and invasion (27). As a tumor suppressor, solute carrier
family 6 member 15 (SLC6A15) serves a key role in inhibiting the
migration and invasion of PTC. The effect of SLC6A15 on PTC cells
was associated with intercellular adhesion molecule-1 (ICAM-1).
SLC6A15 functions as a tumor suppressor gene and may represent a
potential target for the treatment of PTC in the future (28).
Non-coding RNAs, including lncRNAs and miRNAs, are
emerging as key regulators in the pathogenesis of PTC Among the
lncRNAs, glycolysis-associated regulator of lactate dehydrogenase A
(LDHA) post-transcriptional modification 1 (GLTC), which interacts
with LDHA, serves an essential role in aerobic glycolysis and
enhances cell viability in PTC (29–31).
Elevated expression levels of GLTC in PTC tissues correlates with
more extensive metastasis, larger tumor size and worse prognosis.
Inhibition of GLTC negates the effects of K155-succinylated LDHA,
particularly on radioiodine iodine refractory, which suggests its
potential as an oncogenic target in PTC (32). Bioinformatic analyses of miRNA-mRNA
regulatory networks reveal that miR-204-5p exhibits the broadest
regulatory capacity, targeting multiple genes, including tumor
necrosis factor receptor superfamily member 12A (TNFRSF12A).
Furthermore, miR-146b, miR-204 and miR-7-2 demonstrate
stage-specific expression patterns in PTC, while elevated TNFRSF12A
and claudin-1 levels correlate with adverse prognostic outcomes in
this malignancy (23,33). The expression level of lncRNA
metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is
also upregulated in PTC, suggesting that MALAT1 may serve a
carcinogenic role and could potentially serve as a diagnostic
marker for PTC (34).
Hsa-miR-1270 is significantly upregulated in PTC
cell lines (such as TPC-1 and K1) and human PTC tumors. The
regulatory mechanism of hsa-miR-1270 in PTC appears to be primarily
associated with the suppressor of cancer cell invasion (SCAI) gene.
hsa-miR-1270 can bind to SCAI and negatively regulate the
expression of the SCAI gene in PTC cells. Downregulation of
hsa-miR-1270 expression inhibits the development of PTC cells both
in vitro and in vivo (35). By contrast, overexpression of
miR-1270 promotes the proliferation, migration and invasion of PTC
cells by targeting REPIN1 (36).
Circ-0011058 positively regulates yes-associated protein 1 (YAP1),
thereby enhancing PTC proliferation, angiogenesis and
radioresistance by acting as a sponge for miR-335-5p (37). Furthermore, miR-106a has been
implicated in the carcinogenesis of the lncRNA highly upregulated
in liver cancer (HULC). Both HULC and miR-106a are positively
associated with cell viability, proliferation, migration, invasion
capacity, and the volume and weight of PTC tumors (38). HULC overexpression enhances the
activity of the PI3K/AKT and Wnt/β-catenin signaling pathways by
upregulating the expression of miR-106a. A positive correlation
exists between the expression levels of HOXA3 and HOXA-AS2, with
HOXA-AS2 being notably expressed in the cytoplasm of PTC cells
(39). Furthermore, FOXD2-AS1
upregulates HOXA3 expression by binding to miR-15a-5p. The global
function of the HOXA-AS2/miR-15a-5p/HOXA3 axis serves a notable
role in the progression of PTC (40).
In summary, the dysregulation of both coding and
non-coding genes significantly contributes to the molecular
mechanisms of PTC. These insights not only enhance current
understanding of PTC biology but also open the door for novel
diagnostic and therapeutic strategies targeting specific genes and
pathways involved in PTC progression.
PTC involves a variety of signaling pathways that
regulate its development, proliferation and invasiveness. IMUP and
eva-1 homolog A (EVA1A), along with the key effectors of the Hippo
pathway, YAP1 and transcriptional coactivator with PDZ-binding
motif, can inhibit the proliferation, migration and invasion of PTC
cells, while also inducing apoptosis. EVA1A promotes the
progression and EMT of PTC through the Hippo signaling pathway
(41). lncRNA AK023507 inhibits the
proliferation and metastasis of PTC cells by targeting the
β-catenin/Wnt signaling pathway (42). In PTC cell lines, the
tumor-suppressive effect of Tektin-4 (TEKT4) downregulation was
associated with the silencing of the PI3K/AKT pathway. The
downregulation of TEKT4 expression inhibited cell proliferation,
colony formation, migration and invasion (43). Overexpression of circ-100395
significantly reduced aerobic glycolysis, proliferation, migration
and invasion, while downregulating the PI3K/AKT/mTOR pathway, an
effect that was reversed by the PI3K activator 740Y-P. Circ-100395
may exert an anticancer effect in PTC cells by inhibiting the
PI3K/AKT/mTOR signaling pathway (44).
SRPX2 promotes proliferation and migration of PTC.
SRPX2 serves a role in the malignant development of PTC by
activating the PI3K/AKT signaling pathway (22). ATP-binding cassette transporter A1
serves a key role in inhibiting PTC lung metastasis through the
ERK/Fos-related antigen 1/zinc finger E-box binding homeobox 1
(ZEB1) pathway (45). METTL3
inactivates the NF-κB pathway through cellular
reticuloendotheliosis viral oncogene homolog and v-rel
reticuloendotheliosis viral oncogene homolog A, regulates tumor
growth together with YT521-B homology domain-containing family
protein 2 and tumor-associated neutrophil infiltration, and serves
a key tumor suppressor role in PTC carcinogenesis, which expands
current understanding of the relationship between
N6-methyladenosine (m6A) modification and tumor microenvironment
(TME) plasticity (46).
FTO can inhibit glycolysis and the growth of PTC,
and its expression has been significantly downregulated in PTC
tissues. FTO suppresses the expression of apolipoprotein E (APOE)
through insulin-like growth factor 2 mRNA-binding protein 2
(IGF2BP2)-mediated m6A modification and may inhibit glycolytic
metabolism in PTC by regulating the IL-6/JAK2/STAT3 signaling
pathway, thereby impeding tumor growth (47). Insulin-like growth factor 1 (IGF1)
promotes the proliferation and invasion of PTC via the STAT3
signaling pathway (36,47). Key oncogenes, including leucine-rich
repeat kinase 2, solute carrier family 34 member 2, mucin 1,
forkhead box protein Q1 and keratin 19, are upregulated in
BRAF-enriched subtypes and are associated with oncogenic MAPK and
PI3K/AKT signaling pathways (48).
The present review provides a key appraisal of the
oncogenic signaling network architecture underlying PTC
pathogenesis. Emerging evidence demonstrates that a complex
interplay of mitogenic pathways, including MAPK/ERK and PI3K/AKT
cascades, coordinately regulates malignant phenotype evolution
through modulation of proliferation kinetics, metastatic
competence, apoptotic resistance and epithelial-mesenchymal
plasticity in PTC models. Particular emphasis has been placed on
delineating pathway crosstalk mechanisms that create therapeutic
vulnerabilities, with concurrent discussion of preclinical
validation strategies for molecularly targeted interventions.
Thyroid cancer is a prevalent malignant tumor within
the endocrine system, and its occurrence and progression are
closely associated with various metabolic processes. Recent studies
have demonstrated that the metabolomic profiles of serum samples
from patients with thyroid cancer differ significantly from those
of healthy adults. These differences encompass multiple metabolic
pathways, including glucose metabolism, lipid metabolism and amino
acid metabolism (49,50).
FAM111B expression is negatively associated with
glucose uptake and inhibits the growth, migration, invasion and
glycolysis of PTC. Methylation of FAM111B mediated by DNA
(cytosine-5)-methyltransferase 3β (DNMT3B) accelerates the growth,
migration, invasion and glycolysis of PTC cells. The
estradiol/DNMT3B/FAM111B axis is a key regulator of PTC growth and
progression (27). FTO functions as
a tumor suppressor gene in PTC, inhibiting tumor glycolysis.
Research has demonstrated that FTO suppresses the expression of
APOE through IGF2BP2-mediated mRNA modification and may inhibit
glycolytic metabolism in PTC by modulating the IL-6/JAK2/STAT3
signaling pathway, thereby restraining tumor growth (51). Pyruvate carboxylase influences the
proliferation and motility of thyroid cell lines. PTC demonstrates
metabolic reprogramming characterized by enhanced tricarboxylic
acid (TCA) cycle flux that sustains oncogenic bioenergetic demands
while generating biosynthetic precursors (52). Mitochondrial oxidative metabolism is
elevated in thyroid cancer cells compared with stromal cells.
PiggyBac transposable element derived 5 (PGBD5), a gene associated
with glucose metabolism, is enriched in inflammatory response
pathways and has been correlated with high levels of immune cell
infiltration in groups sensitive to paclitaxel and anti-PD-1
treatment. Therefore, PGBD5 may serve as a therapeutic target to
inhibit the progression of PTC (53).
Emerging evidence highlights the pivotal role of
metabolic reprogramming in PTC progression, with glucose and lipid
metabolism emerging as key regulatory axes. In glucose metabolism,
pharmacological modulation using metformin suppresses HK2 and
glucose transporter 1 (GLUT1) expression, impairing glycolytic flux
in PTC models both in vitro and in vivo, which
suggests its therapeutic potential as a metabolic adjuvant
(54). This effect is
synergistically enhanced by BTB domain and CNC homology 1
knockdown, which induces metabolic inflexibility by inhibiting
mitochondrial respiration, thereby sensitizing cancer cells to
metformin-mediated cytotoxicity (55). Complementary strategies targeting
glucose transport (SGLT2 inhibitors) and mitochondrial energetics
(mitochondrial glycerol-3-phosphate dehydrogenase inhibition)
further demonstrate metabolic vulnerabilities in PTC, associating
glucose uptake restriction with oxidative stress-mediated apoptosis
and energy crisis (56,57).
Obesity-associated PTC progression associates with
METTL16-mediated lipid metabolic activation, while lysine
methyltransferase 5A (KMT5A) regulates oncogenic lipogenesis
through sterol regulatory element-binding protein 1/SCD/fatty acid
synthase (FASN) signaling, modulating redox balance and
chemotherapy response (13,58). Prognostically relevant lipid
signatures involving PDZK1IP1, transmembrane channel-like 3,
low-density lipoprotein receptor-related protein 2 and potassium
inwardly-rectifying channel subfamily J member 13 genes underscore
the clinical implications of lipid metabolism in tumor evolution
(59). Beyond canonical pathways,
metabolic crosstalk between tumors and microenvironmental
adipocytes drives local lipid restructuring, characterized by
elevated very long chain saturated fatty acids and polyunsaturated
fatty acid metabolism in peritumoral thyroid tissue (60). These findings collectively identify
metabolic plasticity, characterized by predominant aerobic
glycolysis activity, glutamine dependency and enhanced lipid
anabolism, as a hallmark feature of PTC malignancy, with both
diagnostic significance and therapeutic potential (61).
Current understanding of metabolic reprogramming in
PTC remains predominantly focused on glucose and lipid homeostasis,
with amino acid metabolism representing a key knowledge gap in PTC
pathobiology. The present review systematically examines
therapeutically exploitable mechanisms in glycolytic and lipogenic
pathways, while Table I delineates
emerging insights into amino acid metabolic networks that warrant
further mechanistic interrogation (26,51,53,58,61–101).
The paucity of comprehensive studies on glutaminolysis,
serine-glycine axis modulation and branched-chain amino acid
utilization underscores an urgent need for multi-omics
investigations to map the complete metabolic landscape of PTC.
Increased expression of Stanniocalcin 1 (STC1) in
PTC tissues has been associated with an elevated risk of lymph node
metastasis. Furthermore, elevated STC1 expression in thyroid
lesions may aid in the diagnosis of PTC, which makes it a
potentially valuable marker for predicting disease prognosis
(102). miRNAs such as miR-221,
miR-222, miR-204-5p, miR-7-2 and miR-146b have been identified as
potential biomarkers for staging PTC. Furthermore, fibronectin 1,
claudin-1, TNFRSF12A, ribosomal protein S6 kinase B1, cyclin T1,
specificity protein 1 and chromodomain helicase DNA-binding protein
4 may serve as novel prognostic biomarkers for PTC. The expression
of coagulation factor XII (F12) may influence the overall survival
(OS) of patients with PTC by modulating metabolic pathways,
suggesting that F12 could be a reliable diagnostic and prognostic
biomarker for PTC (103).
Downregulation of Alu-mediated CDKN1A/p21 transcriptional regulator
(APTR) has been associated with tumorigenesis and suggests the
potential diagnostic value of APTR in patients with PTC and ATC
(104). Lectin microarray analysis
of salivary glycoproteins in patients with PTC revealed
postoperative normalization of six lectin-binding patterns to
levels comparable with healthy volunteers. These findings highlight
the potential utility of salivary glycome profiling as a
non-invasive prognostic biomarker for PTC. Quantitative alterations
in specific lectin-reactive glycostructures may enable clinical
stratification of disease progression and therapeutic monitoring in
PTC management (55). Matrix
metalloproteinases and their inhibitors play a role in PTC cervical
metastasis, therefore high expression of matrix metalloproteinase-1
(MMP-1) and its tissue inhibitors in tumor tissue can serve as
predictive indicators for tumor metastasis (105). In PTC detection diagnosis,
immunohistochemical results demonstrated that E-cadherin
negativity, and p53 and BRAF positivity are notable risk factors,
while radioiodine refractory therapy can reduce the risk of
recurrence (106). A six-gene
diagnostic panel incorporating transient receptor potential cation
channel subfamily C member 5, teneurin transmembrane protein 1,
neural EGFL-like 2, Duchenne muscular dystrophy, solute carrier
family 35 member F3 and autism susceptibility candidate 2
demonstrated robust discriminative capacity for differentiating PTC
from benign thyroid tissues (107).
Our current routine diagnostic practice for PTC
conforms to established medical pathological standards. However,
the diagnosis in most early-stage cases is typically established
using ultrasound (108–110) and subsequently confirmed by a
series of more complex tests (111,112), such as ultrasound-guided
fine-needle aspiration biopsy for the definitive diagnosis of PTC.
Therefore, the present study summarized the relevant literature on
the diagnosis of PTC, with the aim of enhancing current
capabilities in the prevention and diagnosis of this condition.
In terms of tumor growth, the combination of
imatinib and vemurafenib resulted in nearly complete tumor
disappearance, with a reduction of ~90%. However, monotherapy was
significantly less effective in BCPAP cells expressing
platelet-derived growth factor receptor (PDGFR)α (11). Matrine has been shown to induce
apoptosis in PTC cells in vitro and inhibit tumor growth by
downregulating miR-182-5p in vivo. Matrine, a natural
product derived from marine sources, has emerged as a promising
alternative for the treatment of various types of cancer, including
exerting inhibitory effects in liver cancer and colorectal cancer.
Emerging pharmacological evidence demonstrates that Matrine exerts
potent anti-neoplastic effects in PTC through multi-modal
pro-apoptotic mechanisms (113,114). In PTC-derived TPC-1 and B-CPAP
cell models, Matrine induces mitochondrial apoptotic pathway
activation characterized by Bcl-2 suppression and caspase-3
activation cascade. Mechanistically, this alkaloid compound
mediates tumor growth inhibition via epigenetic regulation of
oncogenic miR-182-5p, establishing this miRNA as a therapeutically
actionable node in the anti-PTC activity of Matrine (115). The dual regulatory capacity of
Matrine in modulating canonical apoptotic signaling pathways and
epigenetic RNA networks establishes this alkaloid as a novel
therapeutic agent for combination therapies targeting molecular
vulnerabilities in PTC.
BRAF inhibitors (BRAFi), including vemurafenib and
dabrafenib, have demonstrated notable therapeutic efficacy in the
treatment of PTC harboring the BRAF V600E mutation (92,116).
However, the development of drug resistance is primarily attributed
to the adaptive reactivation of the MAPK signaling pathway,
exemplified by mechanisms such as neuroblastoma Ras viral oncogene
homolog mutations or the presence of BRAF splice variants that
sustain continuous ERK phosphorylation (117). Furthermore, compensatory
activation of parallel signaling pathways, notably the IGF-1
receptor (IGF-1R)-mediated PI3K/AKT pathway, contributes to
resistance (118). In this
context, Sui et al (119)
identified nerve/glial antigen 2 (NG2) as a key quaternary
structural component implicated in BRAF inhibitor resistance within
BRAF V600E-mutant PTC. NG2 modulates the activity of multiple
receptor tyrosine kinases (RTKs), including EGFR, fibroblast growth
factor receptor (FGFR), human EGFR2, insulin-like growth factor 1
receptor (IGF-1R), vascular endothelial growth factor receptor
(VEGFR) and platelet-derived growth factor receptor (PDGFR), via
the RTK signaling axis, thereby promoting resistance to BRAFi
through the activation of ERK and AKT signaling cascades. These
findings indicated that treatment approaches with triple targeted
inhibition (such as simultaneously blocking BRAF, MEK and EGFR) or
combined immunotherapies might be needed to address drug resistance
challenges in this setting (119).
While surgical resection remains the cornerstone of
PTC management, emerging multimodal strategies address key
limitations in recurrence prevention and metastatic control. Recent
advances in nano-theranostics have yielded TME-responsive platforms
such as the polypyrrole (Ppy)-poly(vinylimine)-siILK nanocomplex,
which integrates photothermal ablation with gene silencing for
synergistic anti-PTC efficacy (120). This system leverages
near-infrared-activated gelatin-stabilized Ppy for precise
photothermal conversion while exploiting pH-responsive charge
inversion to mediate lysosomal escape of siILK payloads, achieving
dual suppression of primary tumor growth and lymphatic metastasis.
Real-time visualization of tumor-specific Ppy localization further
enhances therapeutic precision.
Complementary to such technological innovations,
clinical optimization requires consideration of endocrine-metabolic
variables. Thyroglobulin antibody dynamics, modulated by iodine
homeostasis in thyroid TMEs, may serve as both prognostic
biomarkers and therapeutic adjuvants, which necessitates
personalized dietary iodine regulation during intervention
(121).
Collectively, these developments underscore the
paradigm shift toward precision multimodality in PTC care. Although
radical surgery remains primary for tumor debulking, its
combination with photothermal-gene therapy and metabolic modulation
provides a multimodal strategy against locoregional recurrence and
systemic dissemination, which are key unmet needs in advanced PTC
management.
FTC is a notable type of thyroid cancer and its
incidence exhibits distinct trends (122–124) across various regions (125). The incidence rate of FTC in the
United States increased significantly from 3.98 in 1980–1984 to
9.88 in 2005–2009. In the United States, non-Hispanic whites have
the highest incidence rate of thyroid cancer, followed by
Asian/Pacific islanders, Hispanics and non-Hispanic blacks. The
pathogenesis of FTC is highly complex, involving numerous genetic
and epigenetic alterations. Of these, mutations in the Ras gene are
among the most prevalent molecular events associated with FTC
(126), with the highest Ras
mutation rate (61.5%). Ras gene mutations primarily disrupt the
Ras-MAPK signaling pathway, which leads to the abnormal activation
of several downstream proteins, such as Raf. This disruption
results in aberrant cell proliferation, impaired differentiation
and tumor formation (127,128). Previous studies have demonstrated
that Ras gene mutations are not only associated with the
pathogenesis of FTC but that they are also closely associated with
the invasiveness, risk of recurrence and overall prognosis of the
disease (129,130).
lncRNAs may regulate the progression of FTC by
influencing cell proliferation, apoptosis, EMT and miRNA expression
(131).
1-[1-2,5-dimethyl-1H-pyrrol-3-yl]-2-pyrrolidin-1-ylethanone (IU1)
inhibits FTC proliferation and migration by targeting
ubiquitin-specific protease 14 (USP14), a deubiquitinating enzyme
that affects various cellular processes, including cell survival,
DNA repair, endoplasmic reticulum stress, endocytosis and
inflammatory responses. IU1 induces autophagy and proteasomal
stimulation in a cell type-dependent manner, increasing autophagy
in ML1 cancer cells and resulting in decreased proliferation and
migration of these cells (132).
Piperonine induces apoptosis and autophagy in human FTC cells
through the reactive oxygen species (ROS)/AKT signaling pathway
(133). Aberrant regulation of
suprabasin (SBSN) has been implicated in the development of cancer
and immune disorders. The most abundant evidence regarding SBSN
comes from cancer research. SBSN expression is the response of
cancer cells to anti-tumor T-cell activity. The role of SBSN in
adapting to stress conditions, activating pro-survival signaling
pathways and angiogenesis is often associated with carcinogenic
effects. SBSN influences tumor cell migration, proliferation,
angiogenesis, immune cell infiltration and the cancer immune cycle
(134). The infiltration of M2
macrophages and regulatory T cells (Tregs) was significantly
increased in tumor tissues with high SBSN expression. SBSN may
serve a key role in the development of thyroid cancer, tumor
dedifferentiation and immunosuppression as an important regulator
of tumor immune cell infiltration. The pox virus and zinc finger
protein/BTB and AT-hook-containing zinc finger protein 1 gene can
inhibit the malignant phenotype of thyroid follicular epithelial
cells and thyroid cancer cells, and it participates in the
dedifferentiation process of thyroid cancer (135,136).
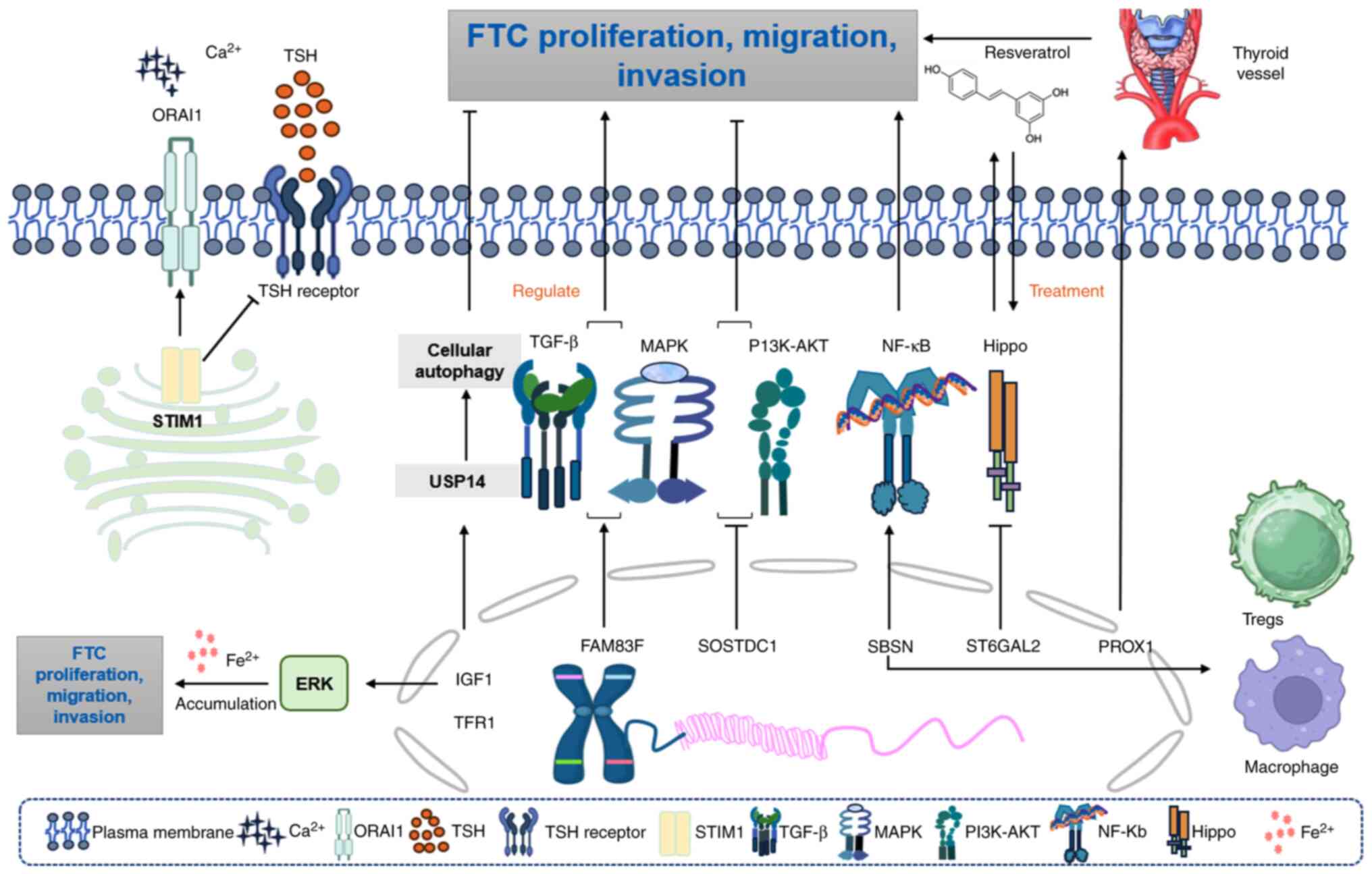
The knockdown of stromal interaction molecule 1
(STIM1) can reduce the invasion and proliferation of human FTC
cells while enhancing the expression of thyroid-specific proteins.
STIM1 and ORAI calcium release-activated calcium modulator 1
(ORAI1) calcium channels mediate store-operated calcium entry
(SOCE) and regulate various cellular functions. The presence of
STIM1 or ORAI1 enhances SOCE in thyroid cancer ML-1 cells, which
promotes invasion and the expression of primary
sphingosine-1-phosphate and VEGF-2 receptors (137,138). Compared with that in normal
tissues, STIM1 protein was upregulated in thyroid cancer tissues,
inhibiting the expression of the thyroid-stimulating hormone
receptor and thyroid-specific proteins, while increasing iodine
absorption (139).
Emerging evidence highlights key molecular drivers
in FTC pathogenesis, with dysregulated signaling networks and
intercellular communication mechanisms emerging as key therapeutic
targets (140,141). The transcription factor prospero
homeobox 1 orchestrates angiogenic programming during metastatic
dissemination through vascular remodeling mechanisms (142). Similarly, exosome-mediated
tumor-stromal crosstalk facilitates malignant progression, as
demonstrated by annexin A1-enriched vesicles from SW579 cells
inducing proliferative activation and EMT in recipient Nthy-ori3-1
cells via paracrine signaling (143).
Regarding the MAPK signaling pathway, sclerostin
domain containing 1 (SOSTDC1) inhibits the proliferation, migration
and EMT of FTC cells by inhibiting the PI3K/AKT and MAPK/ERK
signaling pathways (144). By
contrast, family with sequence similarity 83 member F upregulation
serves a pro-cancer role by cross-activating follicular cell
transformation through synergizing with the MAPK/TGFβ pathway,
which activates thyroid cell migration and causes resistance to
chemotherapy (145). Furthermore,
β-galactoside α-2,6-sialyltransferase 2 (ST6GAL2)-mediated
glycosylation perturbation inhibits Hippo tumor suppressor
signaling, establishing a dual-drug node in FTC tumorigenesis
(146). Pharmacological modulation
using resveratrol reverses ST6GAL2-Hippo axis dysfunction, which
highlights the therapeutic potential of pathway-specific inhibitors
(146). Concurrently, iron
homeostasis disruption via transferrin receptor 1 (TFR1)-dependent
ERK hyperactivation creates metabolic vulnerabilities exploitable
through iron chelation strategies (147). Cell cycle control mechanisms
further contribute to FTC pathobiology. The BRCA1/cyclin-dependent
kinase inhibitor 2C tumor suppressor axis constrains cyclin
D-CDK4/6 signaling, with CDK4 inhibitor sensitivity demonstrating
clinical potential in cyclin D-dysregulated FTC subtypes (148). Genomic landscape analyses reveal
recurrent mutations in chromatin remodelers (for example, nuclear
receptor corepressor 1), mRNA processing factors [e.g., poly(A)
binding protein cytoplasmic 1/3] and mitotic regulators (e.g., cell
division cycle 27), which suggests epigenetic vulnerabilities
(149). TME reprogramming via
fibroblast-mediated signaling amplifies FTC aggressiveness,
highlighting stromal co-targeting strategies as promising
therapeutic frontiers (150).
As a marker for FTC, family with sequence similarity
172 member A (FAM172A) promotes the development of this malignancy.
FAM172A serves a key role in the pathogenesis of FTC through the
ERK1/2 and JNK signaling pathways. The downregulation of FAM172A
can inhibit the proliferation, invasion and migration of FTC cells
via these pathways (151).
Emerging insights into FTC pathogenesis reveal paradoxical
oncogenic signaling activation within canonical tumor suppressor
pathways. Mechanistic studies demonstrated that protein kinase
A-mediated adenosine monophosphate-activated protein kinase
activation via liver kinase B1 (LKB1) phosphorylation exhibits
context-dependent tumorigenic effects, with accumulating
preclinical and clinical evidence associating LKB1 pathway
hyperactivation to FTC progression (152–154). Concurrently, genomic profiling of
thyroid malignancies identifies feline McDonough sarcoma-like
tyrosine kinase 3 domain mutations as recurrent targetable
alterations, although their functional contribution to FTC
oncogenesis and clinical utility for precision kinase inhibition
therapies remains to be fully elucidated (155).
Current therapeutic strategies for advanced FTC
encompass diverse advanced modalities, including radioactive iodine
(131I) therapy, multikinase inhibitors (MKIs) and RET/NTRK/ALK
inhibitors. These approaches target key molecular pathways and
resistance mechanisms to enhance therapeutic efficacy in refractory
cases.
Targeted therapeutic approaches primarily involve
multikinase inhibitors and PI3K pathway modulation. The treatment
of advanced FTC primarily relies on indirect evidence derived from
clinical trials of MKIs, which are associated with notable toxicity
and may adversely affect quality of life in patients. The antitumor
effect of the pan-class I PI3K inhibitor buparlisib on refractory
follicular carcinoma and poorly differentiated thyroid cancer
(PDTC) dysregulation was investigated, given that PI3K pathway
dysregulation is commonly observed in advanced FTC and PDTC, and
has been associated with tumorigenesis and disease progression. The
efficacy of buparlisib in the treatment of advanced FTC and PDTC
has been found to be limited. However, the observed reduction in
tumor growth rate may suggest that oncogenic pathways and/or escape
mechanisms are not entirely inhibited (156).
Natural compounds demonstrate antitumor effects
through redox modulation and signaling pathway regulation.
Hydroxytyrosol, a prominent phenolic compound present in olive oil,
exhibits antitumor effects attributed to its pro-oxidative
properties, as well as its capacity to inhibit cell proliferation
and promote apoptosis in various tumor cell lines, including MCF-7
and MDA-MB-231 (157). Notably,
high doses of hydroxytyrosol have been shown to induce apoptosis in
papillary and FTC cells (158).
Curcumin induces ferroptosis in FTC through heme oxygenase-1 (HO-1)
upregulation (159). Mechanistic
studies have revealed that HO-1 serves as a key mediator of
oxidative stress regulation, with its abnormal upregulation
observed in FTC vs. adjacent tissues. By contrast, elevated HO-1
levels reduce cellular viability via ferroptosis pathway activation
(160,161). Curcumin exerts dual antitumor
effects by enhancing HO-1 expression to suppress FTC proliferation
while potentiating ferroptosis-mediated cell death. These findings
position the HO-1-ferroptosis axis as a key therapeutic target in
FTC pathogenesis (162). Matrine,
a bioactive alkaloid derived from Sophora flavescens,
exhibits anti-neoplastic effects in FTC by modulating the
miR-21/phosphatase and tensin homolog deleted on chromosome ten
(PTEN)/AKT signaling axis. Mechanistic studies have demonstrated
that matrine suppresses miR-21 expression, thereby alleviating its
inhibitory effect on PTEN (163).
The subsequent upregulation of PTEN inactivates AKT
phosphorylation, which induces caspase-dependent apoptosis and
proliferation arrest in FTC-133 cells. This dual regulation of
miRNA and kinase signaling pathways highlights the therapeutic
potential of matrine in targeting FTC pathogenesis (163).
Molecular-targeted radiosensitization represents an
advanced strategy exploiting specific pathways to overcome
radioactive iodine (RAI) resistance. Sorafenib and sunitinib are
effective treatment options for delaying disease progression in
patients with RAI-refractory metastatic DTC, both demonstrating
acceptable safety profiles (164).
Furthermore, sunitinib appears to exhibit some efficacy even in
patients who experience disease progression following treatment
with sorafenib (165). Inhibition
of β-catenin expression may enhance the efficacy of radioiodine
treatment in invasive FTC cells by modulating the localization of
the sodium iodide symporter (NIS). Following the inhibition of
β-catenin expression, FTC cells that exhibit high levels of hypoxia
inducible factor-1α can be completely suppressed by radioiodine
treatment. This mechanism may be associated with the regulation of
NIS localization (166).
Chemotherapy remains an alternative for
multi-resistant cases. Conventional chemotherapy with a gemcitabine
plus oxaliplatin (GEMOX) regimen is an off-label treatment that
demonstrates some efficacy and a favorable safety profile in
advanced DTC. Dias et al (167) reported a case of metastatic FTC in
a patient who developed resistance to multiple therapies. Notably,
OS time appeared to be significantly prolonged by this chemotherapy
due to durable responses to GEMOX. In conclusion, GEMOX may be a
viable option for patients with thyroid cancer who do not respond
to MKIs.
MTC is a neuroendocrine tumor that originates from
parafollicular cells of the thyroid gland. The molecular mechanisms
underlying MTC primarily involve mutations in the RET
proto-oncogene, which is the most prevalent genetic driver of this
cancer (191). Mutations in the
RET gene result in alterations to the protein conformation in both
the inner and outer regions of cells, leading to excessive cell
proliferation and carcinogenesis. In addition to RET mutations,
alterations in the Ras gene also contribute to the pathogenesis of
MTC, particularly in cases of sporadic MTC.
Recent studies have identified novel pathogenic
genes associated with MTC, including BRAF and neurofibromatosis
type 1. These findings have enhanced current understanding of the
molecular pathological mechanisms underlying MTC. By analyzing the
genome, transcriptome, epigenome, proteome and phosphoproteome of a
substantial cohort of patients with MTC, researchers have, to the
best of our knowledge, created the first comprehensive proteomic
profile of MTC globally. This classification based on protein
expression profiles offers notable reference data for the precise
treatment of MTC (192–194).
Furthermore, the differential expression and gene
regulation of miRNA in MTC have garnered notable attention. These
studies offer potential biomarkers and therapeutic strategies for
the early diagnosis, accurate prognosis and gene therapy of MTC.
Collectively, the molecular mechanisms underlying MTC involve the
mutation and regulatory expression of multiple genes and these
findings contribute to the development of novel therapeutic
approaches and the enhancement of patient prognosis.
From a genetic perspective, RET, Harvey rat sarcoma
viral oncogene homolog (HRAS) and KRAS are the most notable genes
in MTC. Epigenetically, the development of MTC may be associated
with the methylation of the telomerase reverse transcriptase
promoter, upregulation of histone methyltransferases such as
enhancer of zeste homolog 2 and SET and murine homolog of
Max-domain containing 3, and notable fluctuations in non-coding
RNAs, including Ras association domain family member 1A (195). Unique transcriptional and
functional alterations in myeloid cells manifest prior to tumor
invasion and initiate within the bone marrow, indicating their
active role in shaping the tumor immune microenvironment (196). The PD-1/PD-L1 pathway is expressed
in patients with MTC and has been significantly correlated with
intraoperative distant metastasis, positioning it as a potential
novel target for MTC treatment (197). A comprehensive understanding of
PD-1/PD-L1 expression and its association with immunotherapy
response may provide a key foundation to address refractory
MTC.
The management of locally advanced or metastatic MTC
necessitates a thorough workup, which includes the measurement of
serum procalcitonin and carcinoembryonic antigen, assessment of
their doubling times and comprehensive imaging to evaluate the
extent of disease, aggressiveness and treatment requirements.
Recent advances in MTC diagnostics have established multimodal
approaches combining imaging guidance with molecular profiling.
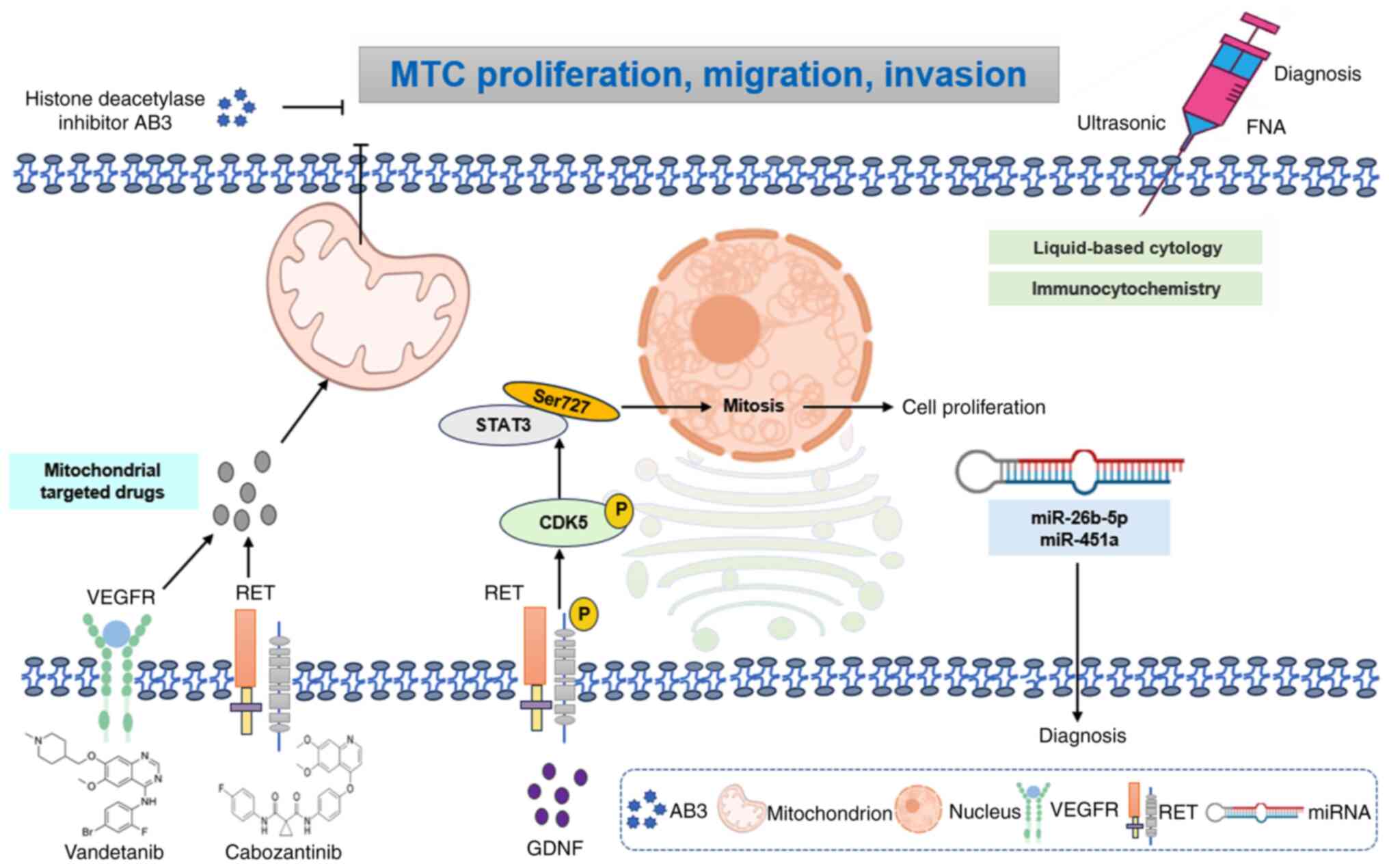
Ultrasound-guided fine-needle aspiration integrated with
liquid-based cytology and immunocytochemical analysis demonstrates
high preoperative diagnostic reliability for MTC through enhanced
cellular characterization (198).
Complementing this, circulating miRNA signatures (particularly
miR-26b-5p and miR-451a) exhibit robust diagnostic accuracy
metrics, offering non-invasive diagnostic adjuncts that synergize
with conventional techniques to enable precision risk
stratification (199). These
complementary strategies collectively advance MTC detection
paradigms by bridging cytomorphological precision with systemic
molecular biomarkers, underscoring their translational potential to
optimize clinical decision-making in thyroid oncology.
At the molecular level, mTOR pathway dysregulation
in MTC involves protein interacting with carboxyl terminus
1-mediated spliceosomal control, where its upregulation counteracts
everolimus-induced cytotoxicity via PTEN suppression and AKT-serine
(Ser)473 hyperphosphorylation, revealing novel resistance
mechanisms in neuroendocrine neoplasms (206). Concurrently, the glial cell
line-derived neurotrophic factor (GDNF)/RET/CDK5/STAT3 signaling
axis emerged as a key driver of MTC progression. Mechanistic
studies reported that GDNF-induced RET phosphorylation activates
cyclin-dependent kinase 5 (CDK5), which phosphorylates STAT3 at
Ser727 to sustain proliferative signaling (207–209). This pathway is enhanced by a
unique feature of MTC biology, such as direct RET-CDK5 interaction
(207).
These findings collectively map therapeutic
vulnerabilities across three dimensions: Neuroendocrine receptor
modulation, mTOR-AKT crosstalk and RET-kinase network targeting.
The identification of CDK5 as a RET-interacting kinase partner
particularly highlights its promise as a druggable node for
precision intervention strategies in advanced MTC management.
MTC often exhibits resistance to standard therapies,
which highlights the need for alternative treatment options. AB3, a
novel histone deacetylase inhibitor, has demonstrated efficacy in
inhibiting the proliferation of MTC cells in vitro. However,
its clinical application in vivo is hindered by poor water
solubility and stability, rapid clearance and insufficient tumor
targeting ability. Recent advancements in MTC nanotheranostics have
yielded precision-targeted delivery systems to optimize therapeutic
efficacy (210,211). Engineered single-molecule micelles
incorporating AB3 chemotherapeutic payloads with KE108
peptide-directed active targeting demonstrated enhanced
tumor-selective accumulation, achieving 2.3-fold greater
cytotoxicity in MTC models compared with free drug formulations.
This nanoplatform significantly suppresses tumor biomarker
expression while mitigating off-target effects key for patients
with comorbidities, particularly those with cardiovascular
complications requiring blood pressure stability (212). Furthermore, these micelles
demonstrated optimal anticancer effects in vivo without
notable systemic toxicity, which offers a promising strategy for
targeted therapy in MTC.
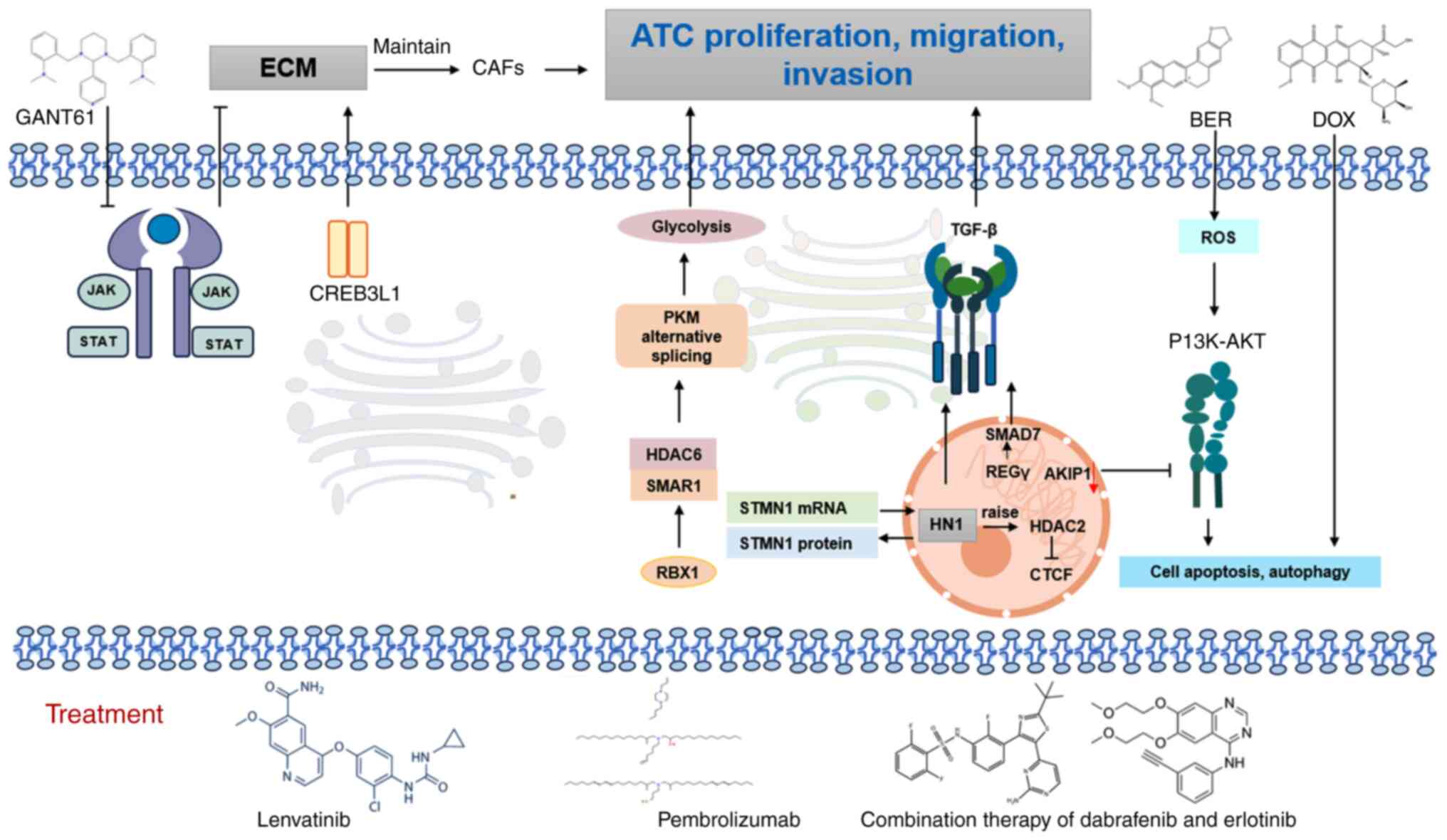
ATC is a highly aggressive endocrine malignancy
frequently presenting with extrathyroidal extension or distant
metastasis, ~70% of ATC spreads to nearby tissues such as the
trachea, esophagus and throat. The lungs, bones, and brain are also
common sites of ATC metastasis (256), although its pathogenesis remains
to be elucidated. Mechanistic studies have identified
cAMP-responsive element-binding protein 3-like 1 (CREB3L1) as a key
regulator maintaining cancer-associated fibroblast-like phenotypes
in ATC through extracellular matrix (ECM) signaling activation,
thereby remodeling TMEs to facilitate malignant progression
(257,258). The MAPK pathway, activated via
Ras/BRAF mutations or RET-PTC fusions, has been established as a
central oncogenic driver and therapeutic target for TKIs, while
secondary genomic alterations, including telomerase reverse
transcriptase (TERT) promoter and tumor protein p53 (TP53)
mutations, are correlated with advanced tumor evolution (259).
Molecular profiling analyses have revealed distinct
protein expression patterns in ATC, characterized by marked
upregulation of miRNA-regulated oncogenes, such as transferrin
receptor TFRC or CD71, and E3-ubiquitin ligase denticleless,
enabling reliable differentiation from PDTC (260). Angiogenesis and EMT are suppressed
by miR-205 through dual targeting of VEGF-A and ZEB1 (261). Epigenetic and transcriptional
regulators include sialic acid-binding Ig-like lectin 15
(Siglec-15) (STAT1/STAT3-mediated proliferation/apoptosis)
(262), CCCTC-binding
factor/hematological and neurological expressed 1 (HN1; chromatin
remodeling of thyroid differentiation genes) (263), REGγ (TGF-β activation via SMAD7
degradation) (264) and HOXD9
(PI3K/AKT-driven proliferation/EMT through miR-451a/proteasome 20S
subunit β8 axis) (265). The
miR-17-92 cluster has been implicated in differentiation blockade,
with CRISPR/caspase 9-mediated knockdown restoring
thyrocyte-specific gene expression (266).
The stepwise molecular progression from DTC to ATC
entails early MAPK pathway alterations, including BRAF V600E and
Ras mutations, with subsequent acquisition of TERT and TP53
mutations driving dedifferentiation (267).
Cellular invasion mechanisms in ATC are regulated
through multiple molecular axes: HN1 stabilizes stathmin 1 (STMN1)
mRNA and prevents its ubiquitin-mediated degradation, thereby
enhancing invasiveness (268).
RING-box protein 1 (RBX1) modulates pyruvate kinase M1/2 splicing
via scaffold/matrix attachment region binding protein 1/histone
deacetylase 6 complex disruption, promoting metastasis and aerobic
glycolysis (269) and type 2
deiodinase depletion induces cellular senescence (270).
Capsaicin exemplifies the multimodal mechanisms of
pharmacological interventions, where transient receptor potential
vanilloid 1 activation initiates mitochondrial calcium overload
culminating in apoptosis (271).
Berberine induces ROS-dependent apoptosis/autophagy via
PI3K/AKT/mTOR pathway modulation and synergizes with doxorubicin
(272). A-kinase interacting
protein 1 knockdown enhances chemosensitivity through
PI3K/AKT/β-catenin inactivation (273). Gli-antagonist 61 inhibits EMT via
AKT/mTOR and JAK/STAT3 pathway suppression (274).
Taken together, ATC pathogenesis involves
multilevel dysregulation spanning CREB3L1-mediated ECM remodeling,
MAPK/TERT/TP53 genetic evolution and coordinated control of
invasion/metastasis by HN1/STMN1/RBX1. Therapeutic vulnerabilities
were identified in calcium signaling, redox balance, epigenetic
reprogramming and kinase cascades, which provides a molecular
framework for targeted intervention strategies.
Total residual disease remains the most key
prognostic indicator for ATC. Factors such as the capsule, marginal
status, percentage and size of the primary ATC are associated with
prognosis. Pure thyroid squamous cell carcinoma can be classified
as ATC if it shares a similar BRAF V600E genotype and prognosis.
Both BRAF-mutated and Ras-mutated ATC exhibit comparable metastatic
spread. The coexistence of BRAF or Ras mutations alongside TERT
mutations is correlated with a poor prognosis (7,275,276). Current diagnostic algorithms for
ATC incorporate three cardinal immunohistochemical features: i)
Aberrant nuclear β-catenin accumulation with membranous expression
loss, indicative of Wnt pathway activation; ii) disrupted
E-cadherin localization patterns reflecting EMT; and iii) paired
box 8 nuclear expression deficiency marking thyroid differentiation
loss, collectively serving as histopathological hallmarks for ATC
confirmation (277).
Optical imaging has been shown to effectively
visualize cellular and tissue architectures through optical
absorption, refraction and scattering properties, enabling
functional assessment across organ systems and facilitating
diagnostic applications. This modality further demonstrates
therapeutic potential for targeted and non-invasive precision
treatment in thyroid carcinoma (278). Concurrently, contrast-enhanced
CT-based radiomics analysis exhibits notable discriminative
capacity in distinguishing ATC and PDTC from DTC in patients with
advanced thyroid malignancies (279).
IGF2 mRNA-binding protein 1 (IGF2BP1) is currently
regarded as the most promising single-gene marker for ATC, followed
by melanoma-associated antigen 3 (MAGEA3), representing an
advancement over existing techniques (280), like application of single-cell
sequencing technology and epigenetic analysis. The expression
levels of IGF2BP1 and MAGEA3 can effectively differentiate ATC from
PDTC. Furthermore, IGF2BP1 has the capability to identify ATC foci
within poorly differentiated FTC. Reliable markers are key for
distinguishing this high-grade malignancy (ATC) from other types of
thyroid cancer subtypes, thereby informing surgical decisions,
treatment options and post-resection or therapeutic monitoring
strategies (280).
Circulating cell-free DNA (cfDNA) has emerged as a
key biomarker for characterizing tumor molecular profiles in ATC.
Comparative analyses have demonstrated high concordance between
cfDNA-derived genetic alterations and tumor tissue-based
next-generation sequencing results, which supports the utility of
cfDNA in dynamically monitoring actionable mutations. These
findings underscore the clinical relevance of cfDNA in guiding
therapeutic decisions and prognostic stratification in ATC
management (281).
Similarly, previous studies in non-small cell lung
cancer have demonstrated that combined radiotherapy and anti-PD-L1
treatment induces the formation of spatially adjacent synergistic
units comprising CXCL13+CD8+ T cells and
CXCL9+ macrophages, whose effector molecules, including
granzyme B and IFN-γ, directly suppress tumor growth (291,292). Similarly, in lung adenocarcinoma
associated with chronic obstructive pulmonary disease,
CXCL13+CD8+ T cells cooperate with tumor
cells exhibiting high HLA class I expression to enhance
immunotherapy response rates (293). By contrast, within clear cell
renal cell carcinoma, CXCL13+CD8+ T cells
predominantly display a terminally exhausted phenotype associated
with unfavorable clinical outcomes. The high infiltration of
CXCL13+CD8+T cells can also demonstrate the
immune escape structure of CD8+T cells, characterized by
immune damage, increased tumor promoting cells and reduced
anti-tumor factors (294). These
observations underscore that the functional role of
CXCL13+ T cells is highly contingent upon the specific
TME context, including the composition and spatial arrangement of
coexisting immune cell populations. Therefore, in ATC,
CXCL13+ T cells both promote immune activation by
augmenting antitumor responses through TLS formation and may
increase risk of malignancy due to their exhausted phenotype and
associated systemic complications.
In therapeutic investigations, combination regimens
targeting both angiogenesis and immune checkpoints, such as
lenvatinib with pembrolizumab, demonstrated favorable clinical
outcomes. A retrospective study involving 6 metastatic ATC patients
receiving combination therapy with lenvatinib and pembrolizumab and
2 PDTC patients confirmed. These regimens achieved durable complete
remissions in subsets of patients with ATC and PDTC (295). Notably, BRAF-targeted strategies
continue to evolve. A subset of patients with BRAF-mutant ATC
exhibited significant tumor regression when treated with
neoadjuvant pembrolizumab followed by BRAF/MEK inhibitor
combinations, enabling subsequent curative resection (296). Mechanistic studies further
revealed that dabrafenib combined with erlotinib synergistically
suppresses MAPK/EGFR signaling pathways, as evidenced by
downregulation of p-MEK, p-ERK and p-EGFR in preclinical models
(297). This dual inhibition
strategy overcomes monotherapy resistance and significantly reduces
tumor burden in xenograft models (298).
Clinical trials evaluating PD-1 blockade in ATC
demonstrated durable responses across BRAF mutation statuses.
Spartalizumab, an anti-PD-1 monoclonal antibody, achieved a 52.1%
1-year survival rate in patients who were PD-L1+, with
objective responses observed in both BRAF mutant and wild-type
cohorts (299). PD-L1 was
significantly upregulated in a subset of patients with advanced
ATC. Notably, PD-L1 expression in immune cells was detected in
11.1% of ATC cases (300).
Furthermore, monoclonal antibodies targeting PD-L1 have
demonstrated notable efficacy in inhibiting ATC tumor growth in
vivo (301). Siglec-15 has
emerged as a promising immunotherapeutic target in ATC, whose
function is to suppress T-cell activation by downregulating nuclear
factor of activated T cells (NFAT)1, NFAT2 and NF-κB signaling
pathways. Inhibition of Siglec-15 has been shown to enhance the
secretion of IFN-γ and IL-2 both in vitro and in vivo
(218). Furthermore, the
combination therapy of dabrafenib and trametinib has notably
transformed the treatment landscape for BRAF V600E-mutant ATC.
Despite initial responses, resistance to this targeted therapy
inevitably develops, leading to disease progression. Furthermore,
neoadjuvant BRAF-targeted therapy administered preoperatively has
demonstrated improvements in patient survival outcomes (302).
These findings underscore the potential of
immunotherapy as a backbone for multimodal treatment paradigms in
ATC. ICAM1 is a promising target for the treatment of ATC and PTC.
Two ICAM1-targeted antibody-drug conjugates (I1-MMAE and I1-DXd)
have been developed, exhibiting selective cytotoxicity against
proliferating ATC and PTC cells in vitro, while maintaining
minimal impact on non-proliferative cell populations (303). Preclinical evaluation further
demonstrated notable tumor regression in ATC and PTC xenograft
models following ADC administration (304). Similarly, a CD44-directed
nanodelivery system was engineered through tyrosine-hyaluronic
acid-polyethylenimine self-assembly, enabling simultaneous
131I/125I radiolabeling and encapsulation of p53 reactivation and
induction of massive apoptosis-1 (Prima-1), a p53 mutation-targeted
therapeutic agent. The 125I-labeled nanocomposites
achieved sustained tumor visualization and prolonged
radiosensitivity, while 131I-conjugated nanoparticles exerted
synergistic antitumor effects in ATC models through
Prima-1-mediated p53 reactivation and radiation potentiation
(305).
Recent advances in ATC therapeutics have revealed
several promising strategies. Emerging evidence suggests that
CXCL13+ T cell infiltration and nascent TLS formation
may serve as biomarkers for immunotherapy responsiveness. The
combined regimen of lenvatinib and pembrolizumab, when integrated
with BRAF/MEK inhibition, has demonstrated therapeutic efficacy in
molecularly defined ATC subsets. Notably, neoadjuvant applications
of pembrolizumab remain under clinical investigation (282,295,296). Pharmacodynamic studies have
indicated that dabrafenib-erlotinib co-administration achieves
synergistic tumor suppression through circumventing monotherapy
resistance mechanisms. PD-1 blockade via spartalizumab has induced
objective responses across diverse ATC histological variants,
including spindle cell variant, giant cell variant, and epithelioid
variant. Preclinical models further highlight the therapeutic
potential of CD44-directed nanocarriers co-delivering
131I radiotherapy and Prima-1-mediated p53 reactivation
(298,300,305). These multimodal approaches
collectively expand the therapeutic landscape for this aggressive
malignancy (Fig. 5).
In the field of thyroid cancer research, notable
progress has been made in recent years; however, several
limitations persist. Firstly, studies investigating the molecular
mechanisms and gene mutations associated with thyroid cancer have
identified the roles of various key genes (such as BRAF, RET and
Ras) (306–309). However, these studies do not fully
elucidate the occurrence and developmental processes of all types
of thyroid cancer. The intricate interactions between multiple
genetic mutations and environmental factors remain inadequately
understood, such as ionizing radiation induces DNA double strand
breaks, leading to RET/PTC rearrangements; excessive iodine can
promote BRAF V600E mutation, which increases the risk of PTC. These
poses presenting challenges in the pursuit of more effective
prevention and treatment strategies.
In the research and treatment of thyroid cancer, it
is imperative to focus on the early screening of high-risk groups,
the application of molecular markers in diagnosis and treatment,
and the development of individualized treatment strategies tailored
to the various pathological types of thyroid cancer (310). Concurrently, for advanced and
refractory cases, overcoming drug resistance and enhancing
treatment efficacy remain key areas of investigation. For instance,
in the case of highly malignant subtypes, such as ATC, treatment
options are currently limited and survival rates are dismal, which
necessitates the urgent identification of novel therapeutic targets
and strategies. Furthermore, despite the generally favorable
prognosis for DTC, the monitoring and management of recurrence and
metastasis continue to pose notable challenges in clinical practice
(311).
Targeted drug therapy has demonstrated notable
therapeutic potential in the management of thyroid cancer,
particularly for advanced or refractory cases. These therapies
inhibit tumor progression by focusing on specific molecular targets
that promote cancer cell proliferation. Common targeted drugs
include VEGFR inhibitors, such as pazopanib and sorafenib, which
primarily inhibit new angiogenesis and restrict tumor blood supply
(312–314). Furthermore, EGFR inhibitors,
including gefitinib and erlotinib (315), mainly function to inhibit tumor
cell proliferation and survival. Targeting the prevalent BRAF V600E
driver mutation in thyroid cancer subtypes, BRAFi, including
vemurafenib and dabrafenib, represent a mechanistically grounded
therapeutic strategy. Furthermore, RET inhibitors, such as
pralsetinib and selpercatinib, primarily inhibit key drivers of
MTC, such as RET gene fusion or mutation (316). Targeted therapies are
characterized by their high specificity, resulting in less impact
on normal cells and fewer side effects compared with conventional
chemotherapy; they can effectively control disease progression and
prolong progression-free survival, which makes them particularly
suitable for advanced cases that are not amenable to surgical or
radiotherapeutic interventions. However, the cost of targeted drugs
is typically high, which can impose economic burdens on patients
over the long term. Furthermore, drug resistance may develop, as
tumor cells can evolve novel mechanisms to evade treatment.
Potential side effects, including hypertension, diarrhea and rash
(317), necessitate long-term
management and continuous monitoring.
Drug resistance in BRAF V600E-mutant PTC to BRAFi
is characterized by a multifaceted mechanism encompassing
epigenetic regulation, signaling pathway reprogramming and
metabolic adaptation. For instance, Pit-Oct-Unc class 5 homeobox 1B
contributes to dabrafenib resistance by promoting stem cell-like
properties and activating the Taste 1 Receptor Member 1 signaling
axis (318). Similarly, NG2
(chondroitin sulfate proteoglycan 4) diminishes the efficacy of
BRAFi through feedback activation of the ERK/AKT pathway mediated
by multiple RTKs, including EGFR and FGFR (119). Furthermore, aberrant activation of
the JAK/STAT pathway exacerbates resistance via IFN regulatory
factor 1-driven immune evasion mechanisms, such as upregulation of
human leukocyte antigen A2 and ICAM-1 (118). Transcription factors Forkhead box
protein P2 and Src homology-2 domain-containing protein tyrosine
phosphatase 2 sustain the resistant phenotype by cross-activating
the lysophosphatidic acid receptor (LPAR)3/PI3K-AKT and MAPK/PI3K
pathways, respectively (319,320). At the metabolic level, myeloid
cell leukemia-1 inhibits apoptosis by maintaining mitochondrial
membrane stability, while resistance to ferroptosis mediated by the
arylsulfatase I-STAT3-epiregulin axis restricts the therapeutic
efficacy of sorafenib (321).
Collectively, these findings indicate that monotherapy targeting
BRAF is readily circumvented by compensatory mechanisms, which
underscores the urgent need for combinatorial therapeutic
strategies.
The mechanisms underlying drug resistance in ATC
are notably complex, frequently involving widespread dysregulation
of the transcriptome and epigenetic landscape. NOP2/Sun RNA
methyltransferase family member 2 (NSUN2)-mediated 5-methylcytosine
modification stabilizes ATP-binding cassette transporters via the
serine/arginine-rich splicing factor 6/UDP-N-acetylglucosamine
pyrophosphorylase 1 axis, thereby promoting multidrug resistance
(322). Furthermore, N-Myc
proto-oncogene (MYCN) induces paclitaxel resistance through
transcriptional reprogramming (323). Microenvironmental factors, such as
glutamate accumulation, inhibit lysophosphatidic acid receptor 1,
which results in persistent activation of the MAPK pathway and
reduced sensitivity to anlotinib (117). These interconnected mechanisms
collectively establish a ‘pan-drug resistance’ barrier, rendering
conventional chemotherapy and targeted therapies largely
ineffective in ATC.
Although BRAF/MEK inhibitors, including dabrafenib
and trametinib, have demonstrated high objective response rates
(ranging from 54 to 89%) in BRAF V600E-mutant thyroid cancer, their
long-term efficacy is compromised by the development of acquired
resistance (324,325). Combination immunotherapy
approaches, such as BRAFi combined with atezolizumab, have extended
the median survival time in patients with ATC to ~43 months;
however, the associated cumulative toxicities, including dysphoria
and fatigue, limit broader clinical application (326).
Additional challenges include histological
variability in response to targeted therapies. For instance,
lenvatinib only achieved an 11.9% 1-year survival rate in ATC
(327), while pazopanib combined
with chemoradiotherapy failed to significantly improve survival
outcomes, underscoring the limitations of VEGFR monotherapy
(219). The feasibility and safety
of metabolic interventions remains a challenge. Although melittin
combined with apatinib enhances antitumor efficacy by inducing
pyroptosis, optimizing the therapeutic-toxicity balance persists as
a key concern (220). A lack of
reliable predictive biomarkers complicates treatment. No current
biomarkers adequately forecast patient responses to NSUN2- or
MYCN-targeted therapies, which hampers patient stratification and
treatment personalization (322,323).
Future studies may continue to investigate the
molecular mechanisms underlying thyroid cancer, with particular
emphasis on key signaling pathways and gene variants in ATC. This
research will encompass the examination of oncogenes, tumor
suppressor genes, cell cycle regulatory genes and molecules that
interact with the TME. Further understanding of the molecular
heterogeneity of thyroid cancer will shift the focus of future
research towards personalized treatment strategies. This approach
can involve the selection of appropriate targeted therapies and
immunotherapy regimens based on the molecular characteristics of
individual tumors. For instance, the identification of driver
genes, such as the BRAF V600E mutation and RET/neurotrophic
tyrosine receptor kinase fusion, has informed the application of
targeted therapeutic agents (328). Concurrently, research may also
prioritize the development of novel targeted drugs and
immunotherapy techniques, as well as the integration of these
treatments with established methods such as surgery, radioiodine
therapy and TKIs to enhance treatment efficacy and improve patient
survival.
Through multi-omics studies, researchers may aim to
identify precise molecular markers of thyroid cancer, which could
potentially enhance patient stratification and risk assessment,
leading to more refined management strategies. Furthermore,
advancements in bioinformatics and artificial intelligence
technologies are expected to serve an increasingly vital role in
the diagnosis, treatment planning and prognostic evaluation of
thyroid cancer. For instance, artificial intelligence-based models
can assist in determining lymph node metastasis in DTC, thereby
improving diagnostic accuracy (329).
Research on thyroid cancer encompasses multiple
disciplines, including genetics, pathology, imaging, endocrinology
and oncology. Effectively integrating the resources and expertise
from these fields to establish a comprehensive interdisciplinary
research system is key to enhance the quality of thyroid cancer
research and its clinical applications. Clinical research must
persist in advancing efforts to validate the efficacy and safety of
novel treatment strategies and medications for patients.
Furthermore, translational research can facilitate the swift
application of laboratory findings to clinical settings, ensuring
that research outcomes benefit patients at the earliest
opportunity.
Thyroid cancer represents a highly heterogeneous
endocrine malignancy with significant differences in molecular
mechanisms, clinical presentations and therapeutic approaches
across its subtypes (PTC, FTC, MTC and ATC). This review delineates
key molecular characteristics of these four categories: PTC is
predominantly characterized by BRAF V600E mutations, FTC is
frequently associated with RAS mutations and MTC primarily
originates from RET proto-oncogene mutations, while ATC
demonstrates profound genomic instability evidenced by TP53 and
TERT promoter mutations. These molecular markers not only
facilitate disease diagnosis and prognostic stratification but also
provide foundational insights for targeted therapy development.
In diagnostic practice, integrating ultrasound,
fine-needle aspiration biopsy and molecular profiling, including
BRAF, RET and RAS mutation analysis substantially enhances
preoperative diagnostic precision. Regarding therapeutic
management, surgical resection remains the primary intervention for
localized tumors. For advanced or metastatic disease, however,
targeted therapies such as RET inhibitor selpercatinib and BRAF/MEK
inhibitor combinations alongside immunotherapy demonstrate
transformative potential. Particularly for aggressive ATC,
multimodal regimens combining surgery, radiotherapy, chemotherapy
and targeted agents may extend survival in selected patient
cohorts.
Notwithstanding substantial research advancements
in thyroid oncology, persistent challenges warrant attention.
Certain resistance mechanisms in FTC and ATC remain incompletely
elucidated. Comprehensive evaluation of long-term efficacy and
toxicity profiles for targeted therapies is imperative.
Furthermore, molecular stratification-guided personalized treatment
paradigms require validation through large-scale clinical trials.
Future investigations should prioritize novel biomarker discovery,
optimization of combination therapies and multi-omics exploration
of TME dynamics in disease progression, ultimately aiming to
enhance patient survival outcomes and quality of life.
Not applicable.
The present review was supported by grants from the Natural
Science Foundation of Shandong Province China (grant no.
ZR2023MA069), the Medical and Health Technology Development Project
of Shandong Province China (grant no. 202202050602), the Shandong
Project for Talents Introduction and development on Youth
Innovation Team of Higher Education, the Graduate Student Research
Grant from Shandong Second Medical University, Jiangyin Outstanding
Young Health Talents Project (grant no. JYOYT202310) and The Fourth
Affiliated Hospital of Nanjing Medical University 2024 Subjects
(grant no. 2024YJKT15).
Not applicable.
ZL, NW, XL and HX collected related literature and
drafted the manuscript. ZD, YL, YX, YS, TF and GW participated in
the design of the review and draft of the manuscript. All authors
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Wan Y, Li G, Cui G, Duan S and Chang S:
Reprogramming of thyroid cancer metabolism: From mechanism to
therapeutic strategy. Mol Cancer. 24:7432025. View Article : Google Scholar
|
|
3
|
Myung SK, Lee CW, Lee J, Kim J and Kim HS:
Risk factors for thyroid cancer: A hospital-based case-control
study in Korean adults. Cancer Res Treat. 49:70–78. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Li Q, Zou C, Huang Q and Chen Y:
Application and recent advances in conventional biomarkers for the
prognosis of papillary thyroid carcinoma. Front Oncol.
15:15989342025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Badulescu CI, Piciu D, Apostu D, Badan M
and Piciu A: Follicular thyroid carcinoma-clinical and diagnostic
findings in a 20-year follow up study. Acta Endocrinol (Buchar).
16:170–177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huai JX, Wang F, Zhang WH, Lou Y, Wang GX,
Huang LJ, Sun J and Zhou XQ: Unveiling new chapters in medullary
thyroid carcinoma therapy: Advances in molecular genetics and
targeted treatment strategies. Front Endocrinol (Lausanne).
15:14848152024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cleere EF, Prunty S and O'Neill JP:
Anaplastic thyroid cancer: Improved understanding of what remains a
deadly disease. Surgeon. 22:e48–e53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singarayer R, Mete O, Perrier L, Thabane
L, Asa SL, Van Uum S, Ezzat S, Goldstein DP and Sawka AM: A
systematic review and meta-analysis of the diagnostic performance
of BRAF V600E immunohistochemistry in thyroid histopathology.
Endocr Pathol. 30:201–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grabellus F, Worm K, Schmid KW and Sheu
SY: The BRAF V600E mutation in papillary thyroid carcinoma is
associated with glucose transporter 1 overexpression. Thyroid.
22:377–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou M, Baitei EY, Alzahrani AS, BinHumaid
FS, Alkhafaji D, Al-Rijjal RA, Meyer BF and Shi Y: Concomitant RAS,
RET/PTC, or BRAF mutations in advanced stage of papillary thyroid
carcinoma. Thyroid. 24:1256–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang N, Zhao Z, Wang Z, Ning J, Wang H,
Zhang W, Ruan X, Gao M and Zheng X: METTL3 regulates thyroid cancer
differentiation and chemosensitivity by modulating PAX8. Int J Biol
Sci. 20:3426–3441. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Wang Y, Meng X, Wang W, Duan F, Chen
S, Zhang Y, Sheng Z, Gao Y and Zhou L: METTL16 inhibits papillary
thyroid cancer tumorigenicity through
m6A/YTHDC2/SCD1-regulated lipid metabolism. Cell Mol
Life Sci. 81:812024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu J, Wang Y, Yang C, Feng Z, Huang Y,
Liu P, Chen F and Deng Z: circ-PSD3 promoted proliferation and
invasion of papillary thyroid cancer cells via regulating the
miR-7-5p/METTL7B axis. J Recept Signal Transduct Res. 42:251–260.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin J, Cai B, Lin Q, Lin X, Wang B and
Chen X: TLE4 downregulation identified by WGCNA and machine
learning algorithm promotes papillary thyroid carcinoma progression
via activating JAK/STAT pathway. J Cancer. 15:4759–4776. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wagner M, Wuest M, Lopez-Campistrous A,
Glubrecht D, Dufour J, Jans HS, Wuest F and McMullen TPW: Tyrosine
kinase inhibitor therapy and metabolic remodelling in papillary
thyroid cancer. Endocr Relat Cancer. 27:495–507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen J, Lin B, Lin L, Chen Y and Wang O:
KCNN4 is a diagnostic and prognostic biomarker that promotes
papillary thyroid cancer progression. Aging (Albany NY).
12:16437–16456. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang K, Liu S, Tian Y, Liu C, Gui Z, Yu T
and Zhang L: PDZK1 Interacting Protein 1 promotes the progression
of papillary thyroid cancer. J Clin Endocrinol Metab.
107:2449–2461. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin XJ, Lin X, Xue G, Fan HL, Wang HY, Wu
JF and Pei D: CXCL10 is a potential biomarker and associated with
immune infiltration in human papillary thyroid cancer. Biosci Rep.
41:BSR202034592021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sirakriengkrai K, Tepmongkol S, Keelawat S
and Techavijit U: Clinical association of CXCR4 in primary tumor of
papillary thyroid cancer and response to iodine-131 treatment. Nucl
Med Commun. 42:396–401. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen Y, Li X, Xie R, Chen Y, Hu X, Liu Y
and Ma H: Expression levels of MicroRNA-300/BCL2L11 in papillary
thyroid cancer and their clinical diagnostic values. Eur Surg Res.
64:342–351. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin L, Wen J, Lin B, Bhandari A, Zheng D,
Kong L, Wang Y, Wang O and Chen Y: Immortalization up-regulated
protein promotes tumorigenesis and inhibits apoptosis of papillary
thyroid cancer. J Cell Mol Med. 24:14059–14072. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo H, Liu R, Wu J, Li S, Yao W, Xu J,
Zheng C, Lu Y and Zhang H: SRPX2 promotes cancer cell proliferation
and migration of papillary thyroid cancer. Clin Exp Med.
23:4825–4834. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan T, Qiu W, Song J, Fan Y and Yang Z:
ARHGAP36 regulates proliferation and migration in papillary thyroid
carcinoma cells. J Mol Endocrinol. 66:1–10. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Xiang J, Bhandari A, Wen J, Lin B,
Kong L and Wang O: LRRC52-AS1 is associated with clinical
progression and regulates cell migration and invasion in papillary
thyroid cancer. Clin Exp Pharmacol Physiol. 47:696–702. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng X, Xu S, Pan J, Zheng J, Wang X, Yu
H, Bao J, Xu Y, Guan H and Zhang L: MKL1 overexpression predicts
poor prognosis in patients with papillary thyroid cancer and
promotes nodal metastasis. J Cell Sci. 132:jcs2313992019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Yu X, Fan C, Wang H, Wang R, Feng C
and Guan H: Targeting glutaminase-mediated glutamine dependence in
papillary thyroid cancer. J Mol Med (Berl). 96:777–790. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X, Xue C, Kang X, Jia X, Wang L,
Younis MH, Liu D, Huo N, Han Y, Chen Z, et al: DNMT3B-mediated
FAM111B methylation promotes papillary thyroid tumor glycolysis,
growth and metastasis. Int J Biol Sci. 18:4372–4387. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Li H, Liang W, Guo Y, Peng M, Ke
W, Xiao H, Guan H and Li Y: SLC6A15 acts as a tumor suppressor to
inhibit migration and invasion in human papillary thyroid cancer. J
Cell Biochem. 122:814–826. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ratti M, Lampis A, Ghidini M, Salati M,
Mirchev MB, Valeri N and Hahne JC: MicroRNAs (miRNAs) and Long
Non-coding RNAs (lncRNAs) as new tools for cancer therapy: First
steps from bench to bedside. Target Oncol. 15:261–278. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu Y, Jin L, Shi R, Li J, Wang Y, Zhang
L, Liang CZ, Narayana VK, De Souza DP, Thorne RF, et al: The long
noncoding RNA glycoLINC assembles a lower glycolytic metabolon to
promote glycolysis. Mol Cell. 82:542–554.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng X, Li S, Zeng A and Song L:
Regulatory function of glycolysis-related lncRNAs in tumor
progression: Mechanism, facts, and perspectives. Biochem Pharmacol.
229:1165112024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi L, Duan R, Sun Z, Jia Q, Wu W, Wang F,
Liu J, Zhang H and Xue X: LncRNA GLTC targets LDHA for
succinylation and enzymatic activity to promote progression and
radioiodine resistance in papillary thyroid cancer. Cell Death
Differ. 30:1517–1532. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu J, Zhang W, Zang C, Liu X, Liu F, Ge
R, Sun Y and Xia Q: Identification of key genes and miRNAs markers
of papillary thyroid cancer. Biol Res. 51:452018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Dong H, Yang Y, Qian Y, Liu J, Li
Z, Guan H, Chen Z, Li C, Zhang K, et al: Upregulation of long
noncoding RNA MALAT1 in papillary thyroid cancer and its diagnostic
value. Future Oncol. 14:3015–3022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yi T, Zhou X, Sang K, Zhou J and Ge L:
MicroRNA-1270 modulates papillary thyroid cancer cell development
by regulating SCAI. Biomed Pharmacother. 109:2357–2364. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Sun W, Hua H, Zhang J, Yu Q, Wang
J, Liu X and Dong A: Overexpression of miR-127 predicts poor
prognosis and contributes to the progression of papillary thyroid
cancer by targeting REPIN1. Horm Metab Res. 53:197–203. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Z, Wang W, Su Z, Zhang J and Cao H:
Circ_0011058 facilitates proliferation, angiogenesis and
radioresistance in papillary thyroid cancer cells by positively
regulating YAP1 via acting as miR-335-5p sponge. Cell Signal.
88:1101552021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Z, Li G, Ding C, Sun W and Zhang J:
Long non-coding RNA HULC exerts oncogenic activity on papillary
thyroid cancer in vitro and in vivo. Artif Cells Nanomed
Biotechnol. 48:326–335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia F, Chen Y, Jiang B, Du X, Peng Y, Wang
W, Huang W, Feng T and Li X: Long Noncoding RNA HOXA-AS2 promotes
papillary thyroid cancer progression by regulating
miR-520c-3p/S100A4 pathway. Cell Physiol Biochem. 50:1659–1672.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang L, Wu Z, Meng X, Chu X, Huang H and
Xu C: LncRNA HOXA-AS2 facilitates tumorigenesis and progression of
papillary thyroid cancer by modulating the miR-15a-5p/HOXA3 axis.
Hum Gene Ther. 30:618–631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin BY, Wen JL, Zheng C, Lin LZ, Chen CZ
and Qu JM: Eva-1 homolog A promotes papillary thyroid cancer
progression and epithelial-mesenchymal transition via the Hippo
signalling pathway. J Cell Mol Med. 24:13070–13080. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang M, Huang S, Zhao Y, Xie B, Hu X and
Cai Y: Novel LncRNA AK023507 inhibits cell metastasis and
proliferation in papillary thyroid cancer through β-catenin/Wnt
signaling pathway. Biochem Biophys Res Commun. 655:104–109. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng Z, Zhou X, Cai Y, Chen E, Zhang X,
Wang O, Wang Q and Liu H: TEKT4 promotes papillary
thyroid cancer cell proliferation, colony formation, and metastasis
through activating PI3K/Akt pathway. Endocr Pathol. 29:310–316.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen L, Zhuo D and Yuan H: Circ_100395
impedes malignancy and glycolysis in papillary thyroid cancer:
Involvement of PI3K/AKT/mTOR signaling pathway. Immunol Lett.
246:10–17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park JH, Myung JK, Lee SJ, Kim H, Kim S,
Lee SB, Jang H, Jang WI, Park S, Yang H, et al: ABCA1-Mediated EMT
promotes papillary thyroid cancer malignancy through the
ERK/Fra-1/ZEB1 Pathway. Cells. 12:2742023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He J, Zhou M, Yin J, Wan J, Chu J, Jia J,
Sheng J, Wang C, Yin H and He F: METTL3 restrains papillary thyroid
cancer progression via m6A/c-Rel/IL-8-mediated
neutrophil infiltration. Mol Ther. 29:1821–1837. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang L, Tan Z, Li Y, Zhang X, Wu Y, Xu B
and Wang M: Insulin-like growth factor 1 promotes proliferation and
invasion of papillary thyroid cancer through the STAT3 pathway. J
Clin Lab Anal. 34:e235312020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hong S, Xie Y, Cheng Z, Li J, He W, Guo Z,
Zhang Q, Peng S, He M, Yu S, et al: Distinct molecular subtypes of
papillary thyroid carcinoma and gene signature with diagnostic
capability. Oncogene. 41:5121–5132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Carnazza M, Quaranto D, DeSouza N,
Moscatello AL, Garber D, Hemmerdinger S, Islam HK, Tiwari RK, Li XM
and Geliebter J: The current understanding of the molecular
pathogenesis of papillary thyroid cancer. Int J Mol Sci.
26:46462005. View Article : Google Scholar
|
|
50
|
Ju SH, Song M, Lim JY, Kang YE, Yi HS and
Shong M: Metabolic reprogramming in thyroid cancer. Endocrinol
Metab (Seoul). 39:425–444. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang J, Sun W, Wang Z, Lv C, Zhang T,
Zhang D, Dong W, Shao L, He L, Ji X, et al: FTO suppresses
glycolysis and growth of papillary thyroid cancer via decreasing
stability of APOE mRNA in an N6-methyladenosine-dependent manner. J
Exp Clin Cancer Res. 41:422022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Strickaert A, Corbet C, Spinette SA,
Craciun L, Dom G, Andry G, Larsimont D, Wattiez R, Dumont JE, Feron
O, et al: Reprogramming of energy metabolism: Increased expression
and roles of pyruvate carboxylase in papillary thyroid cancer.
Thyroid. 29:845–857. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xie W, Zeng Y, Hu L, Hao J, Chen Y, Yun X,
Lin Q and Li H: Based on different immune responses under the
glucose metabolizing type of papillary thyroid cancer and the
response to anti-PD-1 therapy. Front Immunol. 13:9916562022.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shen CT, Wei WJ, Qiu ZL, Song HJ, Zhang
XY, Sun ZK and Luo QY: Metformin reduces glycometabolism of
papillary thyroid carcinoma in vitro and in vivo. J Mol Endocrinol.
58:15–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ren X, Shu J, Wang J, Guo Y, Zhang Y, Yue
L, Yu H, Chen W, Zhang C, Ma J and Li Z: Machine learning reveals
salivary glycopatterns as potential biomarkers for the diagnosis
and prognosis of papillary thyroid cancer. Int J Biol Macromol.
215:280–289. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Yang L, Mao L, Zhang L, Zhu Y, Xu
Y, Cheng Y, Sun R, Zhang Y, Ke J and Zhao D: SGLT2 inhibition
restrains thyroid cancer growth via G1/S phase transition arrest
and apoptosis mediated by DNA damage response signaling pathways.
Cancer Cell Int. 22:742022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Thakur S, Daley B, Gaskins K, Vasko VV,
Boufraqech M, Patel D, Sourbier C, Reece J, Cheng SY, Kebebew E, et
al: Metformin targets mitochondrial glycerophosphate dehydrogenase
to control rate of oxidative phosphorylation and growth of thyroid
cancer in vitro and in vivo. Clin Cancer Res. 24:4030–4043. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wen S, Luo Y, Wu W, Zhang T, Yang Y, Ji Q,
Wu Y, Shi R, Ma B, Xu M and Qu N: Identification of lipid
metabolism-related genes as prognostic indicators in papillary
thyroid cancer. Acta Biochim Biophys Sin (Shanghai). 53:1579–1589.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xu M, Sun T, Wen S, Zhang T, Wang X, Cao
Y, Wang Y, Sun X, Ji Q, Shi R and Qu N: Characteristics of lipid
metabolism-related gene expression-based molecular subtype in
papillary thyroid cancer. Acta Biochim Biophys Sin (Shanghai).
52:1166–1170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liao T, Wang YJ, Hu JQ, Wang Y, Han LT, Ma
B, Shi RL, Qu N, Wei WJ, Guan Q, et al: Histone methyltransferase
KMT5A gene modulates oncogenesis and lipid metabolism of papillary
thyroid cancer in vitro. Oncol Rep. 39:2185–2192.
2018.PubMed/NCBI
|
|
61
|
Zwara A, Hellmann A, Czapiewska M,
Korczynska J, Sztendel A and Mika A: The influence of cancer on the
reprogramming of lipid metabolism in healthy thyroid tissues of
patients with papillary thyroid carcinoma. Endocrine. 87:273–280.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Abooshahab R, Hooshmand K, Razavi SA,
Gholami M, Sanoie M and Hedayati M: Plasma metabolic profiling of
human thyroid nodules by gas Chromatography-mass spectrometry
(GC-MS)-based untargeted metabolomics. Front Cell Dev Biol.
8:3852020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ambrosi F, Righi A, Ricci C, Erickson LA,
Lloyd RV and Asioli S: Hobnail variant of papillary thyroid
carcinoma: A literature review. Endocr Pathol. 28:293–301. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Aprile M, Cataldi S, Perfetto C, Federico
A, Ciccodicola A and Costa V: Targeting metabolism by B-raf
inhibitors and diclofenac restrains the viability of BRAF-mutated
thyroid carcinomas with Hif-1α-mediated glycolytic phenotype. Br J
Cancer. 129:249–265. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Badziong J, Ting S, Synoracki S, Tiedje V,
Brix K, Brabant G, Moeller LC, Schmid KW, Fuhrer D and Zwanziger D:
Differential regulation of monocarboxylate transporter 8 expression
in thyroid cancer and hyperthyroidism. Eur J Endocrinol.
177:243–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bai N, Xia F, Wang W, Lei Y, Bo J and Li
X: CDK12 promotes papillary thyroid cancer progression through
regulating the c-myc/β-catenin pathway. J Cancer. 11:4308–4315.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen W, Fu J, Chen Y, Li Y, Ning L, Huang
D, Yan S and Zhang Q: Circular RNA circKIF4A facilitates the
malignant progression and suppresses ferroptosis by sponging
miR-1231 and upregulating GPX4 in papillary thyroid cancer. Aging
(Albany NY). 13:16500–16512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen W, Zhang T, Bai Y, Deng H, Yang F,
Zhu R, Chen Y, He Z, Zeng Q and Song M: Upregulated circRAD18
promotes tumor progression by reprogramming glucose metabolism in
papillary thyroid cancer. Gland Surg. 10:2500–2510. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chong ST, Tan KM, Kok CYL, Guan SP, Lai
SH, Lim C, Hu J, Sturgis C, Eng C, Lam PYP, et al: IL13RA2 is
differentially regulated in papillary thyroid carcinoma vs
follicular thyroid carcinoma. J Clin Endocrinol Metab.
104:5573–5584. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Davis PJ, Lin HY, Hercbergs A and Mousa
SA: Actions of L-thyroxine (T4) and tetraiodothyroacetic acid
(Tetrac) on gene expression in thyroid cancer cells. Genes (Basel).
11:7552020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fan X and Zhao Y: miR-451a inhibits cancer
growth, epithelial-mesenchymal transition and induces apoptosis in
papillary thyroid cancer by targeting PSMB8. J Cell Mol Med.
23:8067–8075. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Feng Z, Song Y, Qian J, Chen T, Yang C,
Jia L, Liu C, Liu P, Lv J and Deng Z: Differential expression of a
set of microRNA genes reveals the potential mechanism of papillary
thyroid carcinoma. Ann Endocrinol (Paris). 80:77–83. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gunjača I, Benzon B, Pleić N, Babić Leko
M, Pešutić Pisac V, Barić A, Kaličanin D, Punda A, Polašek O,
Vukojević K, et al: Role of ST6GAL1 in thyroid cancers: Insights
from tissue analysis and genomic datasets. Int J Mol Sci.
24:163342023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ha TK, Jung I, Kim ME, Bae SK and Lee JS:
Anti-cancer activity of myricetin against human papillary thyroid
cancer cells involves mitochondrial Dysfunction-mediated apoptosis.
Biomed Pharmacother. 91:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hińcza K, Kowalik A, Pałyga I, Walczyk A,
Gąsior-Perczak D, Mikina E, Trybek T, Szymonek M, Gadawska-Juszczyk
K, Zajkowska K, et al: Does the TT variant of the rs966423
polymorphism in DIRC3 affect the stage and clinical course of
papillary thyroid cancer? Cancers (Basel). 12:4232020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu H, Quan G, Yang F, Du S, Ding S, Lun Y
and Chen Q: MicroRNA-96-5p is negatively regulating GPC3 in the
metastasis of papillary thyroid cancer. SAGE Open Med.
11:205031212312057102023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hu Z, Zhao P, Zhang K, Zang L, Liao H and
Ma W: Hsa_circ_0011290 regulates proliferation, apoptosis and
glycolytic phenotype in papillary thyroid cancer via miR-1252/
FSTL1 signal pathway. Arch Biochem Biophys. 685:1083532020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jeon MJ, You MH, Han JM, Sim S, Yoo HJ,
Lee WK, Kim TY, Song DE, Shong YK, Kim WG and Kim WB: High
phosphoglycerate dehydrogenase expression induces stemness and
aggressiveness in thyroid cancer. Thyroid. 30:1625–1638. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jin L, Zheng D, Mo D, Guan Y, Wen J, Zhang
X and Chen C: Glucose-to-Lymphocyte ratio (GLR) as a predictor of
preoperative central lymph node metastasis in papillary thyroid
cancer patients with type 2 diabetes mellitus and construction of
the nomogram. Front Endocrinol (Lausanne). 13:8290092022.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kang YE, Kim JT, Lim MA, Oh C, Liu L, Jung
SN, Won HR, Lee K, Chang JW, Yi HS, et al: Association between
Circulating Fibroblast Growth Factor 21 and Aggressiveness in
Thyroid Cancer. Cancers (Basel). 11:11542019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li X, Wu Z, He J, Jin Y, Chu C, Cao Y, Gu
F, Wang H, Hou C, Liu X and Zou Q: OGT regulated O-GlcNAcylation
promotes papillary thyroid cancer malignancy via activating YAP.
Oncogene. 40:4859–4871. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li Y, Chen M, Liu C, Xia Y, Xu B, Hu Y,
Chen T, Shen M and Tang W: Metabolic changes associated with
papillary thyroid carcinoma: A nuclear magnetic resonance-based
metabolomics study. Int J Mol Med. 41:3006–3014. 2018.PubMed/NCBI
|
|
83
|
Lu J, Zhang Y, Sun M, Ding C, Zhang L,
Kong Y, Cai M, Miccoli P, Ma C and Yue X: Multi-omics analysis of
fatty acid metabolism in thyroid carcinoma. Front Oncol.
11:7371272021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mardente S, Romeo MA, Asquino A, Po A,
Gilardini Montani MS and Cirone M: HHV-6A infection of papillary
thyroid cancer cells induces several effects related to cancer
progression. Viruses. 15:21222023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nahm JH, Kim HM and Koo JS:
Glycolysis-related protein expression in thyroid cancer. Tumour
Biol. 39:10104283176959222017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Nilsson JN, Siikanen J, Hedman C, Juhlin
CC and Ihre Lundgren C: Pre-Therapeutic measurements of iodine
avidity in papillary and poorly differentiated thyroid cancer
reveal associations with thyroglobulin expression, histological
variants and Ki-67 index. Cancers (Basel). 13:36272021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Parascandolo A, Rappa F, Cappello F, Kim
J, Cantu DA, Chen H, Mazzoccoli G, Hematti P, Castellone MD,
Salvatore M and Laukkanen MO: Extracellular superoxide dismutase
expression in papillary thyroid cancer mesenchymal Stem/Stromal
cells modulates cancer cell growth and migration. Sci Rep.
7:414162017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Piana S, Zanetti E, Bisagni A, Ciarrocchi
A, Giordano D, Torricelli F, Rossi T and Ragazzi M: Expression of
NOTCH1 in thyroid cancer is mostly restricted to papillary
carcinoma. Endocr Connect. 8:1089–1096. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sekhar KR, Cyr S and Baregamian N:
Ferroptosis inducers in thyroid cancer. World J Surg. 47:371–381.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Song H, Qiu Z, Wang Y, Xi C, Zhang G, Sun
Z, Luo Q and Shen C: HIF-1α/YAP signaling rewrites Glucose/Iodine
metabolism program to promote papillary thyroid cancer progression.
Int J Biol Sci. 19:225–241. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sun Y, Gong J, Guo B, Shang J, Cheng Y and
Xu H: Association of adjuvant radioactive iodine therapy with
survival in node-positive papillary thyroid cancer. Oral Oncol.
87:152–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Valvo V, Iesato A, Kavanagh TR, Priolo C,
Zsengeller Z, Pontecorvi A, Stillman IE, Burke SD, Liu X and Nucera
C: Fine-tuning lipid metabolism by targeting
Mitochondria-associated Ac92etyl-CoA-Carboxylase 2 in BRAF(V600E)
papillary thyroid carcinoma. Thyroid. 31:1335–1358. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wu C, Ma L, Wei H, Nie F, Ning J and Jiang
T: MiR-1256 inhibits cell proliferation and cell cycle progression
in papillary thyroid cancer by targeting 5-hydroxy tryptamine
receptor 3A. Hum Cell. 33:630–640. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xia M, Wang S, Wang L, Mei Y, Tu Y and Gao
L: The role of lactate metabolism-related LncRNAs in the prognosis,
mutation, and tumor microenvironment of papillary thyroid cancer.
Front Endocrinol (Lausanne). 14:10623172023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu K and Feng Y: HOXD-AS1 is a predictor
of clinical progression and functions as an oncogenic lncRNAs in
papillary thyroid cancer. J Cell Biochem. 120:5326–5332. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yun HJ, Kim M, Kim SY, Fang S, Kim Y,
Chang HS, Chang HJ and Park KC: Effects of Anti-cancer drug
Sensitivity-related genetic differences on therapeutic approaches
in refractory papillary thyroid cancer. Int J Mol Sci. 23:6992022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zarkesh M, Zadeh-Vakili A, Akbarzadeh M,
Fanaei SA, Hedayati M and Azizi F: The role of matrix
metalloproteinase-9 as a prognostic biomarker in papillary thyroid
cancer. BMC Cancer. 18:11992018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zeng J, Ma X, Wang J, Liu R, Shao Y, Hou
Y, Li Z and Fang Y: Down-regulated HSDL2 expression suppresses cell
proliferation and promotes apoptosis in papillary thyroid
carcinoma. Biosci Rep. 39:BSR201904252019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang C, Bo C, Guo L, Yu P, Miao S and Gu
X: BCL2 and hsa-miR-181a-5p are potential biomarkers associated
with papillary thyroid cancer based on bioinformatics analysis.
World J Surg Oncol. 17:2212019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang J, Wen X, Li Y, Zhang J, Li X, Qian
C, Tian Y, Ling R and Duan Y: Diagnostic approach to thyroid cancer
based on amino acid metabolomics in saliva by ultra-performance
liquid chromatography with high resolution mass spectrometry.
Talanta. 235:1227292021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhao F, Zhu S, Fang J, Dong H and Zhu C:
Correlation of DNA methylation and lymph node metastasis in
papillary thyroid carcinoma. Head Neck. 45:1654–1662. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sengun S, Korkmaz H, Ciris M, Yüceer RO,
Boyluboy SM and Kiran M: Diagnostic and prognostic value of
Stanniocalcin 1 expression in papillary thyroid cancer. Endocrine.
78:95–103. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Luo JH, Zhang XX and Sun WH: F12 as a
reliable diagnostic and prognostic biomarker associated with immune
infiltration in papillary thyroid cancer. Aging (Albany NY).
14:3687–3704. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang K, Li C, Liu J, Li Z and Ma C:
Down-regulation of APTR and it's diagnostic value in papillary and
anaplastic thyroid cancer. Pathol Oncol Res. 26:559–565. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bumber B, Marjanovic Kavanagh M,
Jakovcevic A, Sincic N, Prstacic R and Prgomet D: Role of matrix
metalloproteinases and their inhibitors in the development of
cervical metastases in papillary thyroid cancer. Clin Otolaryngol.
45:55–62. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ali KM, Awny S, Ibrahim DA, Metwally IH,
Hamdy O, Refky B, Abdallah A and Abdelwahab K: Role of P53,
E-cadherin and BRAF as predictors of regional nodal recurrence for
papillary thyroid cancer. Ann Diagn Pathol. 40:59–65. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang Y, Song W, Li Y, Liu Z, Zhao K, Jia
L, Wang X, Jiang R, Tian Y and He X: Integrated analysis of tumor
microenvironment features to establish a diagnostic model for
papillary thyroid cancer using bulk and single-cell RNA sequencing
technology. J Cancer Res Clin Oncol. 149:16837–16850. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Boucai L, Zafereo M and Cabanillas ME:
Thyroid cancer: A review. JAMA. 331:425–435. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Levine RA: History of thyroid ultrasound.
Thyroid. 33:894–902. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Mitchell AL, Gandhi A, Scott-Coombes D and
Perros P: Management of thyroid cancer: United kingdom national
multidisciplinary guidelines. J Laryngol Otol. 130
(Suppl):S150–S160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hou ZK, Zhao J, Zhang M, Hou W, Li Y, Yang
Y, Liu Y, Ye Z, Cai Q, Wei X, et al: Preoperative identification of
papillary thyroid carcinoma subtypes and lymph node metastasis via
deep Learning-Assisted Surface-enhanced raman spectroscopy. ACS
Nano. 19:21807–21819. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Su X, Shang L, Yue C and Ma B:
Ultrasound-guided fine needle aspiration thyroglobulin in the
diagnosis of lymph node metastasis of differentiated papillary
thyroid carcinoma and its influencing factors. Front Endocrinol
(Lausanne). 15:13048322024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhao L, Zhang X and Cui S: Matrine
inhibits TPC-1 human thyroid cancer cells via the miR-21/PTEN/Akt
pathway. Oncol Lett. 16:2965–2970. 2018.PubMed/NCBI
|
|
114
|
Fu S, Ma C, Tang X, Ma X, Jing G, Zhao N
and Ran J: MiR-192-5p inhibits proliferation, migration, and
invasion in papillary thyroid carcinoma cells by regulation of
SH3RF3. Biosci Rep. 41:BSR202103422021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fu S, Zhao N, Jing G, Yang X, Liu J, Zhen
D and Tang X: Matrine induces papillary thyroid cancer cell
apoptosis in vitro and suppresses tumor growth in vivo by
downregulating miR-182-5p. Biomed Pharmacother. 128:1103272020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wen SS, Wu YJ, Wang JY, Ni ZX, Dong S, Xie
XJ, Wang YT, Wang Y, Huang NS, Ji QH, et al:
BRAFV600E/p-ERK/p-DRP1(Ser616) promotes tumor progression and
reprogramming of glucose metabolism in papillary thyroid cancer.
Thyroid. 34:1246–1259. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu B, Peng Y, Su Y, Diao C, Cha L and
Cheng R: Glutamate activates the MAPK pathway by inhibiting LPAR1
expression and promotes anlotinib resistance in thyroid cancer.
Discov Oncol. 16:10822025. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Limberg J, Egan CE, Gray KD, Singh M,
Loewenstein Z, Yang Y, Riascos MC, Al Asadi H, Safe P, El Eshaky S,
et al: Activation of the JAK/STAT pathway leads to BRAF inhibitor
resistance in BRAFV600E positive thyroid carcinoma. Mol Cancer Res.
21:397–410. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sui F, Wang G, Liu J, Yuan M, Chen P, Yao
Y, Zhang S, Ji M and Hou P: Targeting NG2 relieves the resistance
of BRAF-mutant thyroid cancer cells to BRAF inhibitors. Cell Mol
Life Sci. 81:2382024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Xu M, Zhao D, Chen Y, Chen C, Zhang L, Sun
L, Chen J, Tang Q, Sun S, Ma C, et al: Charge reversal polypyrrole
nanocomplex-mediated gene delivery and photothermal therapy for
effectively treating papillary thyroid cancer and inhibiting
lymphatic metastasis. ACS Appl Mater Interfaces. 14:14072–14086.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Pearce EN and Caldwell KL: Urinary iodine,
thyroid function, and thyroglobulin as biomarkers of iodine status.
AmJ Clin Nutr. 104 (Suppl 3):898S–901S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sørensen SM, de la Cour CD, Maltesen T,
Urbute A and Kjaer SK: Temporal trends in papillary and follicular
thyroid cancer incidence from 1995 to 2019 in adults in denmark
according to education and income. Thyroid. 32:972–982. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Aschebrook-Kilfoy B, Grogan RH, Ward MH,
Kaplan E and Devesa SS: Follicular thyroid cancer incidence
patterns in the United states 1980–2009. Thyroid. 23:1015–1021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Aschebrook-Kilfoy B, Kaplan EL, Chiu BC,
Angelos P and Grogan RH: The acceleration in papillary thyroid
cancer incidence rates is similar among racial and ethnic groups in
the United States. Ann Surg Oncol. 20:2746–2753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Liu LP, Hao JY, Pan H, Wang C and Yue P:
Mutation of RAS gene in follicular-differentiated thyroid tumors
and its significance. Zhonghua Bing Li Xue Za Zhi. 49:256–261.
2020.(In Chinese). PubMed/NCBI
|
|
127
|
Yang X and Wu H: RAS signaling in
carcinogenesis, cancer therapy and resistance mechanisms. J Hematol
Oncol. 17:1082024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Tartaglia M and Gelb BD: Disorders of
dysregulated signal traffic through the RAS-MAPK pathway:
Phenotypic spectrum and molecular mechanisms. Ann N Y Acad Sci.
1214:99–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Bahar ME, Kim HJ and Kim DR: Targeting the
RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical
studies. Signal Transduct Target Ther. 8:4552023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Takács T, Kudlik G, Kurilla A, Szeder B,
Buday L and Vas V: The effects of mutant Ras proteins on the cell
signalome. Cancer Metastasis Rev. 39:1051–1065. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
De Martino M, Esposito F, Capone M,
Pallante P and Fusco A: Noncoding RNAs in
thyroid-follicular-cell-derived carcinomas. Cancers (Basel).
14:30792022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Srinivasan V, Asghar MY, Zafar S,
Törnquist K and Lindholm D: Proliferation and migration of ML1
follicular thyroid cancer cells are inhibited by IU1 targeting
USP14: Role of proteasome and autophagy flux. Front Cell Dev Biol.
11:12342042023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lin TH, Kuo CH, Zhang YS, Chen PT, Chen
SH, Li YZ and Lee YR: Piperlongumine induces cellular apoptosis and
autophagy via the ROS/Akt signaling pathway in human follicular
thyroid cancer cells. Int J Mol Sci. 24:80482023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Pribyl M, Hodny Z and Kubikova I:
Suprabasin-A review. Genes (Basel). 108:122021.
|
|
135
|
Tan H, Wang L and Liu Z: Role of
suprabasin in the dedifferentiation of follicular epithelial
Cell-derived thyroid cancer and identification of related immune
markers. Front Genet. 13:8106812022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Iesato A, Nakamura T, Izumi H, Uehara T
and Ito KI: PATZ1 knockdown enhances malignant phenotype in thyroid
epithelial follicular cells and thyroid cancer cells. Oncotarget.
8:82754–82772. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yoshida J, Iwabuchi K, Matsui T, Ishibashi
T, Masuoka T and Nishio M: Knockdown of stromal interaction
molecule 1 (STIM1) suppresses store-operated calcium entry, cell
proliferation and tumorigenicity in human epidermoid carcinoma A431
cells. Biochem Pharmacol. 84:1592–1603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kuang CY, Yu Y, Guo RW, Qian DH, Wang K,
Den MY, Shi YK and Huang L: Silencing stromal interaction molecule
1 by RNA interference inhibits the proliferation and migration of
endothelial progenitor cells. Biochem Biophys Res Commun.
398:315–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Asghar MY, Lassila T, Paatero I, Nguyen
VD, Kronqvist P, Zhang J, Slita A, Löf C, Zhou Y, Rosenholm J and
Törnquist K: Stromal interaction molecule 1 (STIM1) knock down
attenuates invasion and proliferation and enhances the expression
of Thyroid-specific proteins in human follicular thyroid cancer
cells. Cell Mol Life Sci. 78:5827–5846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Cai X, Sun R, Yang L, Yao N, Sun Y, Zhang
G, Ge W, Zhou Y, Gui Z, Wang Y, et al: Proteomic analysis reveals
modulation of key proteins in follicular thyroid cancer
progression. Chin Med J (Engl). May 21;doi:
10.1097/CM9.0000000000003645 (Epub ahead of print).
|
|
141
|
Goenka A, Khan F, Verma B, Sinha P, Dmello
CC, Jogalekar MP, Gangadaran P and Ahn BC: Tumor microenvironment
signaling and therapeutics in cancer progression. Cancer Commun
(Lond). 43:525–561. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Rudzińska M, Mikula M, Arczewska KD, Gajda
E, Sabalińska S, Stępień T, Ostrowski J and Czarnocka B:
Transcription factor prospero Homeobox 1 (PROX1) as a potential
angiogenic regulator of follicular thyroid cancer dissemination.
Int J Mol Sci. 20:56192019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li Q, Liu W, Wang Z, Wang C and Ai Z:
Exosomal ANXA1 derived from thyroid cancer cells is associated with
malignant transformation of human thyroid follicular epithelial
cells by promoting cell proliferation. Int J Oncol. 59:1042021.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhou Q, Chen J, Feng J, Xu Y, Zheng W and
Wang J: SOSTDC1 inhibits follicular thyroid cancer cell
proliferation, migration, and EMT via suppressing PI3K/Akt and
MAPK/Erk signaling pathways. Mol Cell Biochem. 435:87–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Fuziwara CS, Saito KC, Leoni SG, Waitzberg
 FL and Kimura ET: The highly expressed FAM83F protein in
papillary thyroid cancer exerts a Pro-oncogenic role in thyroid
follicular cells. Front Endocrinol (Lausanne). 10:1342019.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Xu G, Chen J, Wang G, Xiao J, Zhang N,
Chen Y, Yu H, Wang G and Zhao Y: Resveratrol inhibits the
tumorigenesis of follicular thyroid cancer via ST6GAL2-Regulated
activation of the hippo signaling pathway. Mol Ther Oncolytics.
16:124–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Campisi A, Bonfanti R, Raciti G,
Bonaventura G, Legnani L, Magro G, Pennisi M, Russo G, Chiacchio
MA, Pappalardo F and Parenti R: Gene silencing of Transferrin-1
receptor as a potential therapeutic target for human follicular and
anaplastic thyroid cancer. Mol Ther Oncolytics. 16:197–206. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bai F, Liu X, Zhang X, Mao Z, Wen H, Ma J
and Pei XH: p18INK4C and BRCA1 inhibit follicular cell
proliferation and dedifferentiation in thyroid cancer. Cell Cycle.
22:1637–1653. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Erinjeri NJ, Nicolson NG, Deyholos C,
Korah R and Carling T: Whole-exome sequencing identifies two
discrete druggable signaling pathways in follicular thyroid cancer.
J Am Coll Surg. 226:950–959.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Fozzatti L, Alamino VA, Park S, Giusiano
L, Volpini X, Zhao L, Stempin CC, Donadio AC, Cheng SY and Pellizas
CG: Interplay of fibroblasts with anaplastic tumor cells promotes
follicular thyroid cancer progression. Sci Rep. 9:80282019.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Xu PP, Zeng S, Xia XT, Ye ZH, Li MF, Chen
MY, Xia T, Xu JJ, Jiao Q, Liu L, et al: 2020: FAM172A promotes
follicular thyroid carcinogenesis and may be a marker of FTC.
Endocr Relat Cancer. 27:657–669. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Liu JL, Mao Z, Gallick GE and Yung WK:
AMPK/ TSC2/mTOR-signaling intermediates are not necessary for
LKB1-mediated nuclear retention of PTEN tumor suppressor. Neuro
Oncol. 13:184–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Pringle DR, Vasko VV, Yu L, Manchanda PK,
Lee AA, Zhang X, Kirschner JM, Parlow AF, Saji M, Jarjoura D, et
al: Follicular thyroid cancers demonstrate dual activation of PKA
and mTOR as modeled by thyroid-specific deletion of Prkar1a and
Pten in mice. J Clin Endocrinol Metab. 99:E804–E812. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kari S, Vasko VV, Priya S and Kirschner
LS: PKA activates AMPK through LKB1 signaling in follicular thyroid
cancer. Front Endocrinol (Lausanne). 10:7692019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Borowczyk M, Szczepanek-Parulska E,
Dębicki S, Budny B, Janicka-Jedyńska M, Gil L, Verburg FA,
Filipowicz D, Wrotkowska E, Majchrzycka B, et al: High incidence of
FLT3 mutations in follicular thyroid cancer: Potential therapeutic
target in patients with advanced disease stage. Ther Adv Med Oncol.
12:17588359209075342020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Borson-Chazot F, Dantony E, Illouz F,
Lopez J, Niccoli P, Wassermann J, Do Cao C, Leboulleux S, Klein M,
Tabarin A, et al: Effect of buparlisib, a Pan-class I PI3K
inhibitor, in refractory follicular and poorly differentiated
thyroid cancer. Thyroid. 28:1174–1179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
El-Azem N, Pulido-Moran M, Ramirez-Tortosa
CL, Quiles JL, Cara FE, Sanchez-Rovira P, Granados-Principal S and
Ramirez-Tortosa M: Modulation by hydroxytyrosol of oxidative stress
and antitumor activities of paclitaxel in breast cancer. Eur J
Nutr. 58:1203–1211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Toteda G, Lupinacci S, Vizza D, Bonofiglio
R, Perri E, Bonofiglio M, Lofaro D, La Russa A, Leone F, Gigliotti
P, et al: High doses of hydroxytyrosol induce apoptosis in
papillary and follicular thyroid cancer cells. J Endocrinol Invest.
40:153–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Son Y, Lee JH, Chung HT and Pae HO:
Therapeutic roles of heme oxygenase-1 in metabolic diseases:
Curcumin and resveratrol analogues as possible inducers of heme
oxygenase-1. Oxid Med Cell Longev. 2013:6395412013. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Li R, Zhang J, Zhou Y, Gao Q, Wang R, Fu
Y, Zheng L and Yu H: Transcriptome investigation and in vitro
verification of Curcumin-induced HO-1 as a feature of ferroptosis
in breast cancer cells. Oxid Med Cell Longev. 2020:34698402020.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Kwon MY, Park E, Lee SJ and Chung SW: Heme
oxygenase-1 accelerates erastin-induced ferroptotic cell death.
Oncotarget. 6:24393–24403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Chen H, Li Z, Xu J, Zhang N, Chen J, Wang
G and Zhao Y: Curcumin induces ferroptosis in follicular thyroid
cancer by upregulating HO-1 expression. Oxid Med Cell Longev.
2023:68967902023. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Li Q, Zhang S, Wang M, Dong S, Wang Y, Liu
S, Lu T, Fu Y, Wang X and Chen G: Downregulated miR-21 mediates
matrine-induced apoptosis via the PTEN/Akt signaling pathway in
FTC-133 human follicular thyroid cancer cells. Oncol Lett.
18:3553–3560. 2019.PubMed/NCBI
|
|
164
|
Marotta V, Di Somma C, Rubino M,
Sciammarella C, Modica R, Camera L, Del Prete M, Marciello F,
Ramundo V, Circelli L, et al: Second-line sunitinib as a feasible
approach for iodine-refractory differentiated thyroid cancer after
the failure of first-line sorafenib. Endocrine. 49:854–858. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sousa Santos F, Joana Santos R and Leite
V: Sorafenib and sunitinib for the treatment of metastatic thyroid
cancer of follicular origin: A 7-year Single-Centre experience. Eur
Thyroid J. 8:262–267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lan L, Basourakos S, Cui D, Zuo X, Deng W,
Huo L, Chen L, Zhang G, Deng L, Shi B, et al: Inhibiting β-catenin
expression promotes efficiency of radioiodine treatment in
aggressive follicular thyroid cancer cells probably through
mediating NIS localization. Oncol Rep. 37:426–434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Dias D, Damásio I, Marques P, Simões H,
Rodrigues R, Cavaco BM and Leite V: Metastatic follicular thyroid
cancer with a longstanding responsiveness to gemcitabine plus
oxaliplatin. Eur Thyroid J. 12:e2202272023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Heffess CS and Thompson LD: Minimally
invasive follicular thyroid carcinoma. Endocr Pathol. 12:417–422.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Hernandez-Prera JC and Wenig BM:
RAS-mutant follicular thyroid tumors: A continuous challenge for
pathologists. Endocr Pathol. 35:167–184. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Lang BH, Lo CY, Chan WF, Lam KY and Wan
KY: Prognostic factors in papillary and follicular thyroid
carcinoma: Their implications for cancer staging. Ann Surg Oncol.
14:730–738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Levi J, Kothapalli SR, Bohndiek S, Yoon
JK, Dragulescu-Andrasi A, Nielsen C, Tisma A, Bodapati S,
Gowrishankar G, Yan X, et al: Molecular photoacoustic imaging of
follicular thyroid carcinoma. Clin Cancer Res. 19:1494–1502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Li G, Wang H, Zhong J, Bai Y, Chen W,
Jiang K, Huang J, Shao Y, Liu J, Gong Y, et al: Circulating small
extracellular vesicle-based miRNA classifier for follicular thyroid
carcinoma: A diagnostic study. Br J Cancer. 130:925–933. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Liang L, Xu J, Wang M, Xu G, Zhang N, Wang
G and Zhao Y: LncRNA HCP5 promotes follicular thyroid carcinoma
progression via miRNAs sponge. Cell Death Dis. 9:3722018.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Lin J, Qiu Y, Zheng X, Dai Y and Xu T: The
miR-199a-5p/PD-L1 axis regulates cell proliferation, migration and
invasion in follicular thyroid carcinoma. BMC Cancer. 22:7562022.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Lo CY, Chan WF, Lam KY and Wan KY:
Follicular thyroid carcinoma: The role of histology and staging
systems in predicting survival. Ann Surg. 242:708–715. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Macerola E, Proietti A, Poma AM, Vignali
P, Sparavelli R, Ginori A, Basolo A, Elisei R, Santini F and Basolo
F: Limited accuracy of Pan-trk immunohistochemistry screening for
NTRK rearrangements in Follicular-derived thyroid carcinoma. Int J
Mol Sci. 23:74702022. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Park H, Shin HC, Yang H, Heo J, Ki CS, Kim
HS, Kim JH, Hahn SY, Chung YJ, Kim SW, et al: Molecular
classification of follicular thyroid carcinoma based on TERT
promoter mutations. Mod Pathol. 35:186–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Paulsson JO, Wang N, Gao J, Stenman A,
Zedenius J, Mu N, Lui WO, Larsson C and Juhlin CC: GABPA-dependent
down-regulation of DICER1 in follicular thyroid tumours. Endocr
Relat Cancer. 27:295–308. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Pulcrano M, Boukheris H, Talbot M, Caillou
B, Dupuy C, Virion A, De Vathaire F and Schlumberger M: Poorly
differentiated follicular thyroid carcinoma: Prognostic factors and
relevance of histological classification. Thyroid. 17:639–646.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Repaci A, Salituro N, Vicennati V, Monari
F, Cavicchi O, de Biase D, Ciarrocchi A, Acquaviva G, De Leo A,
Gruppioni E, et al: Unexpected widespread bone metastases from a
BRAF K601N mutated follicular thyroid carcinoma within a previously
resected multinodular goiter. Endocr Pathol. 33:519–524. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Romitti M, Ceolin L, Siqueira DR, Ferreira
CV, Wajner SM and Maia AL: Signaling pathways in follicular
cell-derived thyroid carcinomas (review). Int J Oncol. 42:19–28.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Rossing M, Borup R, Henao R, Winther O,
Vikesaa J, Niazi O, Godballe C, Krogdahl A, Glud M, Hjort-Sørensen
C, et al: Down-regulation of microRNAs controlling tumourigenic
factors in follicular thyroid carcinoma. J Mol Endocrinol.
48:11–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Saburi S, Tsujikawa T, Miyagawa-Hayashino
A, Mitsuda J, Yoshimura K, Kimura A, Morimoto H, Ohmura G, Arai A,
Ogi H, et al: Spatially resolved immune microenvironmental
profiling for follicular thyroid carcinoma with minimal capsular
invasion. Mod Pathol. 35:721–727. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Staubitz JI, Musholt PB and Musholt TJ:
The surgical dilemma of primary surgery for follicular thyroid
neoplasms. Best Pract Res Clin Endocrinol Metab. 33:1012922019.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Stenman A, Hysek M, Jatta K, Bränström R,
Darai-Ramqvist E, Paulsson JO, Wang N, Larsson C, Zedenius J and
Juhlin CC: TERT promoter mutation spatial heterogeneity in a
metastatic follicular thyroid carcinoma: Implications for clinical
work-up. Endocr Pathol. 30:246–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Taylor T, Specker B, Robbins J, Sperling
M, Ho M, Ain K, Bigos ST, Brierley J, Cooper D, Haugen B, et al:
Outcome after treatment of high-risk papillary and non-Hürthle-cell
follicular thyroid carcinoma. Ann Intern Med. 129:622–627. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Thompson LDR: High grade differentiated
follicular Cell-derived thyroid carcinoma versus poorly
differentiated thyroid carcinoma: A clinicopathologic analysis of
41 cases. Endocr Pathol. 34:234–246. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Wenter V, Albert NL, Unterrainer M,
Ahmaddy F, Ilhan H, Jellinek A, Knösel T, Bartenstein P, Spitzweg
C, Lehner S, et al: Clinical impact of follicular oncocytic
(Hürthle cell) carcinoma in comparison with corresponding classical
follicular thyroid carcinoma. Eur J Nucl Med Mol Imaging.
48:449–460. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Yu B, Li Y, Yu X, Ai Y, Jin J, Zhang J,
Zhang Y, Zhu H, Xie C, Shen M, et al: Differentiate thyroid
follicular adenoma from carcinoma with combined ultrasound
radiomics features and clinical ultrasound features. J Digit
Imaging. 35:1362–1372. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhang H, Zhang Z, Liu X, Duan H, Xiang T,
He Q, Su Z, Wu H and Liang Z: DNA methylation haplotype block
markers efficiently discriminate follicular thyroid carcinoma from
follicular adenoma. J Clin Endocrinol Metab. 106:1011–1021. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Saltiki K, Simeakis G, Karapanou O and
Alevizaki M: Management of Endocrine Disease: Medullary thyroid
cancer: From molecular biology and therapeutic pitfalls to future
targeted treatment perspectives. Eur J Endocrinol. 187:R53–R63.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Shi X, Sun Y, Shen C, Zhang Y, Shi R,
Zhang F, Liao T, Lv G, Zhu Z, Jiao L, et al: Integrated
proteogenomic characterization of medullary thyroid carcinoma. Cell
Discov. 8:1202022. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Khan E, Hylton H, Rajan N, Bouley SJ,
Siddiqui JK, Rajasekaran S, Koshre GR, Storts H, Valenciaga A, Khan
M, et al: Proteomic profiling of medullary thyroid cancer
identifies CAPN1 as a key regulator of NF1 and RET fueled growth.
Thyroid. 35:177–187. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Navas-Carrillo D, Rodriguez JM,
Montoro-García S and Orenes-Piñero E: High-resolution proteomics
and metabolomics in thyroid cancer: Deciphering novel biomarkers.
Crit Rev Clin Lab Sci. 54:446–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Khatami F and Tavangar SM: Genetic and
epigenetic of medullary thyroid cancer. Iran Biomed J. 22:142–150.
2018.PubMed/NCBI
|
|
196
|
Rabold K, Zoodsma M, Grondman I, Kuijpers
Y, Bremmers M, Jaeger M, Zhang B, Hobo W, Bonenkamp HJ, de Wilt
JHW, et al: Reprogramming of myeloid cells and their progenitors in
patients with non-medullary thyroid carcinoma. Nat Commun.
13:61492022. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Bi Y, Ren X, Bai X, Meng Y, Luo Y, Cao J,
Zhang Y and Liang Z: PD-1/PD-L1 expressions in medullary thyroid
carcinoma: Clinicopathologic and prognostic analysis of Chinese
population. Eur J Surg Oncol. 45:353–358. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Nikas IP, Kazamias G, Vrontaki M, Rapti AS
and Mastorakis E: Medullary thyroid carcinoma diagnosed with
liquid-based cytology and immunocytochemistry. J Immunoassay
Immunochem. 43:502–515. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Besharat ZM, Trocchianesi S, Verrienti A,
Ciampi R, Cantara S, Romei C, Sabato C, Noviello TMR, Po A,
Citarella A, et al: Circulating miR-26b-5p and miR-451a as
diagnostic biomarkers in medullary thyroid carcinoma patients. J
Endocrinol Invest. 46:2583–2599. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Fugazzola L: Medullary thyroid cancer-An
update. Best Pract Res Clin Endocrinol Metab. 37:1016552023.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Almeida MQ and Hoff AO: Recent advances in
the molecular pathogenesis and targeted therapies of medullary
thyroid carcinoma. Curr Opin Oncol. 24:229–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Tomuleasa C, Tigu AB, Munteanu R, Moldovan
CS, Kegyes D, Onaciu A, Gulei D, Ghiaur G, Einsele H and Croce CM:
Therapeutic advances of targeting receptor tyrosine kinases in
cancer. Signal Transduct Target Ther. 9:2012024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Li J, Gong C, Zhou H, Liu J, Xia X, Ha W,
Jiang Y, Liu Q and Xiong H: Kinase inhibitors and Kinase-targeted
cancer therapies: Recent advances and future perspectives. Int J
Mol Sci. 25:54892024. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Patel J, Klopper J and Cottrill EE:
Molecular diagnostics in the evaluation of thyroid nodules: Current
use and prospective opportunities. Front Endocrinol (Lausanne).
14:11014102023. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Giardino E, Catalano R, Mangili F,
Barbieri AM, Treppiedi D, Elli FM, Dolci A, Contarino A, Spada A,
Arosio M, et al: Octreotide and pasireotide effects on medullary
thyroid carcinoma (MTC) cells growth, migration and invasion. Mol
Cell Endocrinol. 520:1110922021. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Bareli Y, Shimon I, Tobar A and Rubinfeld
H: PICT-1 regulates p53 splicing and sensitivity of medullary
thyroid carcinoma cells to everolimus. J Neuroendocrinol.
34:e131872022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Pozo K, Castro-Rivera E, Tan C, Plattner
F, Schwach G, Siegl V, Meyer D, Guo A, Gundara J, Mettlach G, et
al: The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell.
24:499–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Hedayati M, Zarif Yeganeh M,
Sheikholeslami S and Afsari F: Diversity of mutations in the RET
proto-oncogene and its oncogenic mechanism in medullary thyroid
cancer. Crit Rev Clin Lab Sci. 53:217–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Yue CH, Oner M, Chiu CY, Chen MC, Teng CL,
Wang HY, Hsieh JT, Lai CH and Lin H: RET regulates human medullary
thyroid cancer cell proliferation through CDK5 and STAT3
Activation. Biomolecules. 11:8602021. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kunte SC, Wenter V, Toms J, Lindner S,
Unterrainer M, Eilsberger F, Jurkschat K, Wängler C, Wängler B,
Schirrmacher R, et al: PET/CT imaging of differentiated and
medullary thyroid carcinoma using the novel SSTR-targeting peptide
[18F]SiTATE-first clinical experiences. Eur J Nucl Med
Mol Imaging. 52:900–912. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Kong Z, Li Z, Cui XY, Wang J, Xu M, Liu Y,
Chen J, Ni S, Zhang Z, Fan X, et al: CTR-FAPI PET enables precision
management of medullary thyroid carcinoma. Cancer Discov.
15:316–328. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Jaskula-Sztul R, Chen G, Dammalapati A,
Harrison A, Tang W, Gong S and Chen H: AB3-loaded and
Tumor-targeted unimolecular micelles for medullary thyroid cancer
treatment. J Mater Chem B. 5:151–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Rao SN and Smallridge RC: Anaplastic
thyroid cancer: An update. Best Pract Res Clin Endocrinol Metab.
37:1016782023. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Moreno F, Reyes C, Pineda CA, Castellanos
G, Cálix F, Calderón J and Vasquez-Bonilla WO: Anaplastic thyroid
carcinoma with unusual long-term survival: A case report. J Med
Case Rep. 16:392022. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the united states, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Fallahi P, Ferrari SM, Galdiero MR,
Varricchi G, Elia G, Ragusa F, Paparo SR, Benvenga S and Antonelli
A: Molecular targets of tyrosine kinase inhibitors in thyroid
cancer. Semin Cancer Biol. 79:180–196. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Al-Mohanna M, Alraouji NN, Alhabardi SA,
Al-Mohanna F, Al-Otaibi B, Al-Jammaz I and Aboussekhra A: The
curcumin analogue PAC has potent anti-anaplastic thyroid cancer
effects. Sci Rep. 13:42172023. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Anderson RJ, Sizemore GW, Wahner HW and
Carney JA: Thyroid scintigram in familial medullary carcinoma of
the thyroid gland. Clin Nucl Med. 3:147–151. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Ballal S, Yadav MP, Moon ES, Rösch F,
ArunRaj ST, Agarwal S, Tripathi M, Sahoo RK and Bal C:
First-in-Human experience with 177Lu-DOTAGA.(SA.FAPi)2 therapy in
an uncommon case of aggressive medullary thyroid carcinoma
clinically mimicking as anaplastic thyroid cancer. Clin Nucl Med.
47:e444–e445. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Bao L, Li Y, Hu X, Gong Y, Chen J, Huang
P, Tan Z, Ge M and Pan Z: Targeting SIGLEC15 as an emerging
immunotherapy for anaplastic thyroid cancer. Int Immunopharmacol.
133:1121022024. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Biermann K, Biersack HJ, Sabet A and
Janzen V: Alternative therapeutic approaches in the treatment of
primary and secondary dedifferentiated and medullary thyroid
carcinoma. Semin Nucl Med. 41:139–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Ceolin L, Duval M, Benini AF, Ferreira CV
and Maia AL: Medullary thyroid carcinoma beyond surgery: Advances,
challenges, and perspectives. Endocr Relat Cancer. 26:R499–R518.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Chintakuntlawar AV, Foote RL, Kasperbauer
JL and Bible KC: Diagnosis and management of anaplastic thyroid
cancer. Endocrinol Metab Clin North Am. 48:269–284. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Choi YS, Jeon MJ, Doolittle WKL, Song DE,
Kim K, Kim WB and Kim WG: Macrophage-induced carboxypeptidase A4
promotes the progression of anaplastic thyroid cancer. Thyroid.
34:1150–1162. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Contarino A, Dolci A, Maggioni M, Porta
FM, Lopez G, Verga U, Elli FM, Iofrida EF, Cantoni G, Mantovani G,
et al: Is encapsulated medullary thyroid carcinoma associated with
a better prognosis? A case series and a review of the literature.
Front Endocrinol (Lausanne). 13:8665722022. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Cristinziano L, Modestino L, Loffredo S,
Varricchi G, Braile M, Ferrara AL, de Paulis A, Antonelli A, Marone
G and Galdiero MR: Anaplastic thyroid cancer cells induce the
release of mitochondrial extracellular DNA traps by viable
neutrophils. J Immunol. 204:1362–1372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
D'Aprile S, Denaro S, Pavone AM, Giallongo
S, Giallongo C, Distefano A, Salvatorelli L, Torrisi F, Giuffrida
R, Forte S, et al: Anaplastic thyroid cancer cells reduce CD71
levels to increase iron overload tolerance. J Transl Med.
21:7802023. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Doolittle WKL, Zhao L and Cheng SY:
Blocking CDK7-mediated NOTCH1-cMYC Signaling attenuates cancer stem
cell activity in anaplastic thyroid cancer. Thyroid. 32:937–948.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Egan CE, Stefanova D, Ahmed A, Raja VJ,
Thiesmeyer JW, Chen KJ, Greenberg JA, Zhang T, He B, Finnerty BM,
et al: CSPG4 is a potential therapeutic target in anaplastic
thyroid cancer. Thyroid. 31:1481–1493. 2021.PubMed/NCBI
|
|
231
|
Harach HR and Williams ED: Glandular
(tubular and follicular) variants of medullary carcinoma of the
thyroid. Histopathology. 7:83–97. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Hu Y, Wen Q, Cai Y, Liu Y, Ma W, Li Q,
Song F, Guo Y, Zhu L, Ge J, et al: Alantolactone induces concurrent
apoptosis and GSDME-dependent pyroptosis of anaplastic thyroid
cancer through ROS mitochondria-dependent caspase pathway.
Phytomedicine. 108:1545282023. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Li C, Zhang H, Li S, Zhang D, Li J,
Dionigi G, Liang N and Sun H: Prognostic impact of inflammatory
markers PLR, LMR, PDW, MPV in medullary thyroid carcinoma. Front
Endocrinol (Lausanne). 13:8618692022. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Li Q, Zhang L, Lang J, Tan Z, Feng Q, Zhu
F, Liu G, Ying Z, Yu X, Feng H, et al: Lipid-peptide-mRNA
nanoparticles augment radioiodine uptake in anaplastic thyroid
cancer. Adv Sci (Weinh). 10:e22043342023. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Li Y: Pyrvinium pamoate can overcome
artemisinin's resistance in anaplastic thyroid cancer. BMC
Complement Med Ther. 21:1562021. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Lu L, Wang JR, Henderson YC, Bai S, Yang
J, Hu M, Shiau CK, Pan T, Yan Y, Tran TM, et al: Anaplastic
transformation in thyroid cancer revealed by single-cell
transcriptomics. J Clin Invest. 133:e1696532023. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Lu YL, Huang YT, Wu MH, Chou TC, Wong R
and Lin SF: Efficacy of adavosertib therapy against anaplastic
thyroid cancer. Endocr Relat Cancer. 28:311–324. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Ma B, Luo Y, Xu W, Han L, Liu W, Liao T,
Yang Y and Wang Y: LINC00886 negatively regulates malignancy in
anaplastic thyroid cancer. Endocrinology. 164:bqac2042023.
View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Matrone A, Gambale C, Prete A and Elisei
R: Sporadic medullary thyroid carcinoma: Towards a precision
medicine. Front Endocrinol (Lausanne). 13:8642532022. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Niccoli-Sire P, Murat A, Rohmer V, Franc
S, Chabrier G, Baldet L, Maes B, Savagner F, Giraud S, Bezieau S,
et al: Familial medullary thyroid carcinoma with noncysteine ret
mutations: Phenotype-genotype relationship in a large series of
patients. J Clin Endocrinol Metab. 86:3746–3753. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Pan Z, Bao L, Lu X, Hu X, Li L, Chen J,
Jin T, Zhang Y, Tan Z, Huang P and Ge M: IL2RA+VSIG4+
tumor-associated macrophage is a key subpopulation of the
immunosuppressive microenvironment in anaplastic thyroid cancer.
Biochim Biophys Acta Mol Basis Dis. 1869:1665912023. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R,
Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, et
al: Genetic analysis of 779 advanced differentiated and anaplastic
thyroid cancers. Clin Cancer Res. 24:3059–3068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Pusztaszeri MP, Bongiovanni M and Faquin
WC: Update on the cytologic and molecular features of medullary
thyroid carcinoma. Adv Anat Pathol. 21:26–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Rougier P, Parmentier C, Laplanche A,
Lefevre M, Travagli JP, Caillou B, Schlumberger M, Lacour J and
Tubiana M: Medullary thyroid carcinoma: Prognostic factors and
treatment. Int J Radiat Oncol Biol Phys. 9:161–169. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Shakiba E, Boroomand S, Kheradmand Kia S
and Hedayati M: MicroRNAs in thyroid cancer with focus on medullary
thyroid carcinoma: Potential therapeutic targets and
diagnostic/prognostic markers and web based tools. Oncol Res.
32:1011–1019. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Sherman EJ, Harris J, Bible KC, Xia P,
Ghossein RA, Chung CH, Riaz N, Gunn GB, Foote RL, Yom SS, et al:
Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic
thyroid cancer (NRG/RTOG 0912): A randomised, double-blind,
placebo-controlled, multicentre, phase 2 trial. Lancet Oncol.
24:175–186. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Sugarman AJ, Huynh LD, Shabro A and Di
Cristofano A: Anaplastic thyroid cancer cells upregulate
mitochondrial one-carbon metabolism to meet purine demand,
eliciting a critical targetable vulnerability. Cancer Lett.
568:2163042023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Tang J, Luo Y and Xiao L: USP26 promotes
anaplastic thyroid cancer progression by stabilizing TAZ. Cell
Death Dis. 13:3262022. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Wang X, Ying T, Yuan J, Wang Y, Su X, Chen
S, Zhao Y, Zhao Y, Sheng J, Teng L, et al: BRAFV600E restructures
cellular lactylation to promote anaplastic thyroid cancer
proliferation. Endocr Relat Cancer. 30:e2203442023. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Wu J, Liang J, Liu R, Lv T, Fu K, Jiang L,
Ma W, Pan Y, Tan Z, Liu Q, et al: Autophagic blockade potentiates
anlotinib-mediated ferroptosis in anaplastic thyroid cancer. Endocr
Relat Cancer. 30:2300362023. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Zaballos MA, Acuña-Ruiz A, Morante M,
Riesco-Eizaguirre G, Crespo P and Santisteban P: Inhibiting ERK
dimerization ameliorates BRAF-driven anaplastic thyroid cancer.
Cell Mol Life Sci. 79:5042022. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Zhang W, Ji W and Zhao X: MiR-155 promotes
anaplastic thyroid cancer progression by directly targeting SOCS1.
BMC Cancer. 19:10932019. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Zhao Q, Feng H, Yang Z, Liang J, Jin Z,
Chen L, Zhan L, Xuan M, Yan J, Kuang J, et al: The central role of
a two-way positive feedback pathway in molecular targeted
therapies-mediated pyroptosis in anaplastic thyroid cancer. Clin
Transl Med. 12:e7272022. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Zhao Z, Yin XD, Zhang XH, Li ZW and Wang
DW: Comparison of pediatric and adult medullary thyroid carcinoma
based on SEER program. Sci Rep. 10:133102020. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Alhejaily AG, Alhuzim O and Alwelaie Y:
Anaplastic thyroid cancer: Pathogenesis, prognostic factors and
genetic landscape (review). Mol Clin Oncol. 19:992023PubMed/NCBI
|
|
257
|
Zhao Y, Yu Z, Song Y, Fan L, Lei T, He Y
and Hu S: The regulatory network of CREB3L1 and its roles in
physiological and pathological conditions. Int J Med Sci.
21:123–136. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Pan Z, Xu T, Bao L, Hu X, Jin T, Chen J,
Chen J, Qian Y, Lu X, Li L, et al: CREB3L1 promotes tumor growth
and metastasis of anaplastic thyroid carcinoma by remodeling the
tumor microenvironment. Mol Cancer. 21:1902022. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Haroon Al Rasheed MR and Xu B: Molecular
alterations in thyroid carcinoma. Surg Pathol Clin. 12:921–930.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Misiak D, Bauer M, Lange J, Haase J, Braun
J, Lorenz K, Wickenhauser C and Hüttelmaier S: MiRNA deregulation
distinguishes anaplastic thyroid carcinoma (ATC) and supports
upregulation of oncogene expression. Cancers (Basel). 13:59132021.
View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Vosgha H, Ariana A, Smith RA and Lam AK:
miR-205 targets angiogenesis and EMT concurrently in anaplastic
thyroid carcinoma. Endocr Relat Cancer. 25:323–337. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Hou X, Chen C, He X and Lan X: Siglec-15
silencing inhibits cell proliferation and promotes cell apoptosis
by inhibiting STAT1/STAT3 signaling in anaplastic thyroid
carcinoma. Dis Markers. 2022:16064042022. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Pan Z, Lu X, Xu T, Chen J, Bao L, Li Y,
Gong Y, Che Y, Zou X, Tan Z, et al: Epigenetic inhibition of CTCF
by HN1 promotes dedifferentiation and stemness of anaplastic
thyroid cancer. Cancer Lett. 580:2164962024. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Jiao C, Li L, Zhang P, Zhang L, Li K, Fang
R, Yuan L, Shi K, Pan L, Guo Q, et al: REGγ ablation impedes
dedifferentiation of anaplastic thyroid carcinoma and accentuates
radio-therapeutic response by regulating the Smad7-TGF-β pathway.
Cell Death Differ. 27:497–508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Zhong Y, Yu F, Yang L, Wang Y, Liu L, Jia
C, Cai H, Yang J, Sheng S, Lv Z, et al: HOXD9/miR-451a/PSMB8 axis
is implicated in the regulation of cell proliferation and
metastasis via PI3K/AKT signaling pathway in human anaplastic
thyroid carcinoma. J Transl Med. 21:8172023. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Fuziwara CS, Saito KC and Kimura ET:
Thyroid follicular cell loss of differentiation induced by MicroRNA
miR-17-92 cluster is attenuated by CRISPR/Cas9n gene silencing in
anaplastic thyroid cancer. Thyroid. 30:81–94. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Xu B and Ghossein RA: Advances in thyroid
pathology: High grade follicular Cell-derived thyroid carcinoma and
anaplastic thyroid carcinoma. Adv Anat Pathol. 30:3–10. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Pan Z, Fang Q, Li L, Zhang Y, Xu T, Liu Y,
Zheng X, Tan Z, Huang P and Ge M: HN1 promotes tumor growth and
metastasis of anaplastic thyroid carcinoma by interacting with
STMN1. Cancer Lett. 501:31–42. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Xu D, Yu J, Yang Y, Du Y, Lu H, Zhang S,
Feng Q, Yu Y, Hao L, Shao J and Chen L: RBX1 regulates PKM
alternative splicing to facilitate anaplastic thyroid carcinoma
metastasis and aerobic glycolysis by destroying the SMAR1/HDAC6
complex. Cell Biosci. 13:362023. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Angela De Stefano M, Porcelli T, Ambrosio
R, Luongo C, Raia M, Schlumberger M and Salvatore D: Type 2
deiodinase is expressed in anaplastic thyroid carcinoma and its
inhibition causes cell senescence. Endocr Relat Cancer.
30:e2300162023. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Xu S, Cheng X, Wu L, Zheng J, Wang X, Wu
J, Yu H, Bao J and Zhang L: Capsaicin induces mitochondrial
dysfunction and apoptosis in anaplastic thyroid carcinoma cells via
TRPV1-mediated mitochondrial calcium overload. Cell Signal.
75:1097332020. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Shi XZ, Zhao S, Wang Y, Wang MY, Su SW, Wu
YZ and Xiong C: Antitumor activity of berberine by activating
autophagy and apoptosis in CAL-62 and BHT-101 anaplastic thyroid
carcinoma cell lines. Drug Des Devel Ther. 17:1889–1906. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Zheng H, Lin Q and Rao Y: A-Kinase
interacting protein 1 knockdown restores chemosensitivity via
inactivating PI3K/AKT and β-catenin pathways in anaplastic thyroid
carcinoma. Front Oncol. 12:8547022022. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Gao H, Wang W and Li Q: GANT61 suppresses
cell survival, invasion and epithelial-mesenchymal transition
through inactivating AKT/mTOR and JAK/STAT3 pathways in anaplastic
thyroid carcinoma. Cancer Biol Ther. 23:369–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Sugitani I, Miyauchi A, Sugino K, Okamoto
T, Yoshida A and Suzuki S: Prognostic factors and treatment
outcomes for anaplastic thyroid carcinoma: ATC Research Consortium
of Japan cohort study of 677 patients. World J Surg. 36:1247–1254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Califano I, Smulever A, Jerkovich F and
Pitoia F: Advances in the management of anaplastic thyroid
carcinoma: Transforming a life-threatening condition into a
potentially treatable disease. Rev Endocr Metab Disord. 25:123–147.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Kanematsu R, Hirokawa M, Tanaka A, Suzuki
A, Higuchi M, Kuma S, Hayashi T and Miyauchi A: Evaluation of
E-Cadherin and β-Catenin immunoreactivity for determining
undifferentiated cells in anaplastic thyroid carcinoma.
Pathobiology. 88:351–358. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Shao C, Li Z, Zhang C, Zhang W, He R, Xu J
and Cai Y: Optical diagnostic imaging and therapy for thyroid
cancer. Mater Today Bio. 17:1004412022. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Moon J, Lee JH, Roh J, Lee DH and Ha EJ:
Contrast-enhanced CT-based radiomics for the differentiation of
anaplastic or poorly differentiated thyroid carcinoma from
differentiated thyroid carcinoma: A pilot study. Sci Rep.
13:45622023. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Haase J, Misiak D, Bauer M, Pazaitis N,
Braun J, Pötschke R, Mensch A, Bell JL, Dralle H, Siebolts U, et
al: IGF2BP1 is the first positive marker for anaplastic thyroid
carcinoma diagnosis. Mod Pathol. 34:32–41. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Qin Y, Wang JR, Wang Y, Iyer P, Cote GJ,
Busaidy NL, Dadu R, Zafereo M, Williams MD, Ferrarotto R, et al:
Clinical utility of circulating Cell-Free DNA mutations in
anaplastic thyroid carcinoma. Thyroid. 31:1235–1243. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Han PZ, Ye WD, Yu PC, Tan LC, Shi X, Chen
XF, He C, Hu JQ, Wei WJ, Lu ZW, et al: A distinct tumor
microenvironment makes anaplastic thyroid cancer more lethal but
immunotherapy sensitive than papillary thyroid cancer. JCI Insight.
9:e1737122024.PubMed/NCBI
|
|
283
|
Ukita M, Hamanishi J, Yoshitomi H, Yamanoi
K, Takamatsu S, Ueda A, Suzuki H, Hosoe Y, Furutake Y, Taki M, et
al: CXCL13-producing CD4+ T cells accumulate in the early phase of
tertiary lymphoid structures in ovarian cancer. JCI Insight.
7:e1572152022. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Wang J, Liang Y, Xue A, Xiao J, Zhao X,
Cao S, Li P, Dong J, Li Y, Xu Z and Yang L: Intratumoral CXCL13+
CD160+ CD8+ T cells promote the formation of tertiary lymphoid
structures to enhance the efficacy of immunotherapy in advanced
gastric cancer. J Immunother Cancer. 12:e0096032024. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Han D, Lee AY, Kim T, Choi JY, Cho MY,
Song A, Kim C, Shim JH, Kim HJ, Kim H, et al: Microenvironmental
network of clonal CXCL13+CD4+ T cells and Tregs in pemphigus
chronic blisters. J Clin Invest. 133:e1663572023. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Yang Z, Tian H, Chen X, Li B, Bai G, Cai
Q, Xu J, Guo W, Wang S, Peng Y, et al: Single-cell sequencing
reveals immune features of treatment response to neoadjuvant
immunochemotherapy in esophageal squamous cell carcinoma. Nat
Commun. 15:90972024. View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Liu B, Zhang Y, Wang D, Hu X and Zhang Z:
Single-cell meta-analyses reveal responses of tumor-reactive
CXCL13+ T cells to immune-checkpoint blockade. Nat
Cancer. 3:1123–1136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Bassez A, Vos H, Van Dyck L, Floris G,
Arijs I, Desmedt C, Boeckx B, Vanden Bempt M, Nevelsteen I, Lambein
K, et al: A single-cell map of intratumoral changes during anti-PD1
treatment of patients with breast cancer. Nat Med. 27:820–832.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Lucotti S, Ogitani Y, Kenific CM, Geri J,
Kim YH, Gu J, Balaji U, Bojmar L, Shaashua L, Song Y, et al:
Extracellular vesicles from the lung pro-thrombotic niche drive
cancer-associated thrombosis and metastasis via integrin β2. Cell.
188:1642–1661.e24. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Gao SH, Liu SZ, Wang GZ and Zhou GB:
CXCL13 in cancer and other diseases: Biological functions, clinical
significance, and therapeutic opportunities. Life (Basel).
11:12822021.PubMed/NCBI
|
|
291
|
Sakai SA, Oyoshi H, Nakamura M, Taki T,
Nomura K, Hojo H, Hirata H, Motegi A, Nakamura Y, Zenkoh J, et al:
Single-cell spatial analysis with Xenium reveals anti-tumour
responses of CXCL13 + T and CXCL9+ cells after radiotherapy
combined with anti-PD-L1 therapy. Br J Cancer. Jul 16–2025.doi:
10.1038/s41416-025-03088-0 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Zhu J, Yuan Y, Wan X, Yin D, Li R, Chen W,
Suo C and Song H: Immunotherapy (excluding checkpoint inhibitors)
for stage I to III non-small cell lung cancer treated with surgery
or radiotherapy with curative intent. Cochrane Database Syst Rev.
12:CD0113002021.PubMed/NCBI
|
|
293
|
Huang Y, Liang B, Chen X, Li Z, Deng Y, Du
J, Zhong Y, Lin X, Fu J, Xie J, et al: Enhanced immunotherapy
response in lung adenocarcinoma patients with COPD: Insights into
tumor cells and immune microenvironment characteristics. Cell
Commun Signal. 23:3242025. View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Siyuan D, Han Z, Zhaopei L, Kaifeng J,
Wenbin J, Zewei W, Zhiyuan L, Ying X, Jiajun W, Yuan C, et al:
Intratumoral CXCL13+CD8+T cell infiltration determines poor
clinical outcomes and immunoevasive contexture in patients with
clear cell renal cell carcinoma. J Immunother Cancer.
9:e0018232021. View Article : Google Scholar
|
|
295
|
Dierks C, Seufert J, Aumann K, Ruf J,
Klein C, Kiefer S, Rassner M, Boerries M, Zielke A, la Rosee P, et
al: Combination of lenvatinib and pembrolizumab is an effective
treatment option for anaplastic and poorly differentiated thyroid
carcinoma. Thyroid. 31:1076–1085. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Cabanillas ME, Ferrarotto R, Garden AS,
Ahmed S, Busaidy NL, Dadu R, Williams MD, Skinner H, Gunn GB, Grosu
H, et al: Neoadjuvant BRAF- and Immune-directed therapy for
anaplastic thyroid carcinoma. Thyroid. 28:945–951. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Yang Y, Li S, Wang Y, Zhao Y and Li Q:
Protein tyrosine kinase inhibitor resistance in malignant tumors:
Molecular mechanisms and future perspective. Signal Transduct
Target Ther. 7:3292022. View Article : Google Scholar : PubMed/NCBI
|
|
298
|
Choi YS, Kwon H, You MH, Kim TY, Kim WB,
Shong YK, Jeon MJ and Kim WG: Effects of dabrafenib and erlotinib
combination treatment on anaplastic thyroid carcinoma. Endocr Relat
Cancer. 29:307–319. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
Capdevila J, Wirth LJ, Ernst T, Ponce Aix
S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto
PA, et al: PD-1 blockade in anaplastic thyroid carcinoma. J Clin
Oncol. 38:2620–2627. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Ahn S, Kim TH, Kim SW, Ki CS, Jang HW, Kim
JS, Kim JH, Choe JH, Shin JH, Hahn SY, et al: Comprehensive
screening for PD-L1 expression in thyroid cancer. Endocr Relat
Cancer. 24:97–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Cantara S, Bertelli E, Occhini R, Regoli
M, Brilli L, Pacini F, Castagna MG and Toti P: Blockade of the
programmed death ligand 1 (PD-L1) as potential therapy for
anaplastic thyroid cancer. Endocrine. 64:122–129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Hamidi S, Iyer PC, Dadu R, Gule-Monroe MK,
Maniakas A, Zafereo ME, Wang JR, Busaidy NL and Cabanillas ME:
Checkpoint inhibition in addition to Dabrafenib/Trametinib for
BRAF(V600E)-Mutated anaplastic thyroid carcinoma. Thyroid.
34:336–346. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Min IM, Shevlin E, Vedvyas Y, Zaman M,
Wyrwas B, Scognamiglio T, Moore MD, Wang W, Park S, Park S, et al:
CAR T therapy targeting ICAM-1 eliminates advanced human thyroid
tumors. Clin Cancer Res. 23:7569–7583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Zhang P, Tao C, Shimura T, Huang AC, Kong
N, Dai Y, Yao S, Xi Y, Wang X, Fang J, et al: ICAM1 antibody drug
conjugates exert potent antitumor activity in papillary and
anaplastic thyroid carcinoma. iScience. 26:1072722023. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Huang S, Zhang L, Xu M, Li C, Fu H, Huang
J, Jin X, Liang S and Wang H: Co-Delivery of 131 I and
Prima-1 by Self-Assembled CD44-Targeted Nanoparticles for
Anaplastic Thyroid Carcinoma Theranostics. Adv Healthc Mater.
10:e20010292021. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Landa I and Cabanillas ME: Genomic
alterations in thyroid cancer: Biological and clinical insights.
Nat Rev Endocrinol. 20:93–110. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–99. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Bhattacharya S, Mahato RK, Singh S, Bhatti
GK, Mastana SS and Bhatti JS: Advances and challenges in thyroid
cancer: The interplay of genetic modulators, targeted therapies,
and AI-driven approaches. Life Sci. 332:1221102023. View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Shay JW, Homma N, Zhou R, Naseer MI,
Chaudhary AG, Al-Qahtani M, Hirokawa N, Goudarzi M, Fornace AJ Jr,
Baeesa S, et al: Abstracts from the 3rd International Genomic
Medicine Conference (3rd IGMC 2015): Jeddah, Kingdom of Saudi
Arabia. 30 November-3 December 2015. BMC Genomics. 17 (Suppl
6):S4872016. View Article : Google Scholar
|
|
310
|
Guo M, Sun Y, Wei Y, Xu J and Zhang C:
Advances in targeted therapy and biomarker research in thyroid
cancer. Front Endocrinol (Lausanne). 15:13725532024. View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Sekihara K, Himuro H, Toda S, Saito N,
Hirayama R, Suganuma N, Sasada T and Hoshino D: Recent trends and
potential of radiotherapy in the treatment of anaplastic thyroid
cancer. Biomedicines. 12:12862024. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan
T, Yang W, Tian C, Miao Z, Wang T and Yang S: Small molecules in
targeted cancer therapy: Advances, challenges, and future
perspectives. Signal Transduct Target Ther. 6:2012021. View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Zhang Y, Xing Z, Liu T, Tang M, Mi L, Zhu
J, Wu W and Wei T: Targeted therapy and drug resistance in thyroid
cancer. Eur J Med Chem. 238:1145002022. View Article : Google Scholar : PubMed/NCBI
|
|
314
|
Zhang L, Feng Q, Wang J, Tan Z, Li Q and
Ge M: Molecular basis and targeted therapy in thyroid cancer:
Progress and opportunities. Biochim Biophys Acta Rev Cancer.
1878:1889282023. View Article : Google Scholar : PubMed/NCBI
|
|
315
|
Deshmukh VG, Sapkal SB, Gadekar SS and
Deshmukh V: EGFR inhibitors across generations: Progress,
challenges, and future directions. J Mol Structure. 13392025.
|
|
316
|
Chen MF, Repetto M, Wilhelm C and Drilon
A: RET Inhibitors in RET Fusion-positive lung cancers: Past,
present, and future. Drugs. 84:1035–1053. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Krajewska J, Paliczka-Cieslik E and Jarzab
B: Managing tyrosine kinase inhibitors side effects in thyroid
cancer. Expert Rev Endocrinol Metab. 12:117–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
318
|
Li J and Yu Y: POU5F1B is responsible for
the acquired resistance to dabrafenib in papillary thyroid cancer
cells with the BRAF V600E mutation. Endocrine. 87:220–233. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
319
|
Jiang S, Huang Y, Li Y, Gu Q, Jiang C, Tao
X and Sun J: Silencing FOXP2 reverses vemurafenib resistance in
BRAF(V600E) mutant papillary thyroid cancer and melanoma cells.
Endocrine. 79:86–97. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
320
|
Ma W, Tian M, Hu L, Ruan X, Zhang W, Zheng
X and Gao M: Early combined SHP2 targeting reverses the therapeutic
resistance of vemurafenib in thyroid cancer. J Cancer.
14:1592–1604. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
321
|
Cavallo MR, Yo JC, Gallant KC, Cunanan CJ,
Amirfallah A, Daniali M, Sanders AB, Aplin AE, Pribitkin EA and
Hartsough EJ: Mcl-1 mediates intrinsic resistance to RAF inhibitors
in mutant BRAF papillary thyroid carcinoma. Cell Death Discov.
10:1752024. View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Hou X, Dong Q, Hao J, Liu M, Ning J, Tao
M, Wang Z, Guo F, Huang D, Shi X, et al: NSUN2-mediated m(5)C
modification drives alternative splicing reprogramming and promotes
multidrug resistance in anaplastic thyroid cancer through the
NSUN2/SRSF6/UAP1 signaling axis. Theranostics. 15:2757–2777. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
323
|
Molteni E, Baldan F, Damante G and Allegri
L: GSK2801 reverses paclitaxel resistance in anaplastic thyroid
cancer cell lines through MYCN downregulation. Int J Mol Sci.
24:59932023. View Article : Google Scholar : PubMed/NCBI
|
|
324
|
Subbiah V, Kreitman RJ, Wainberg ZA,
Gazzah A, Lassen U, Stein A, Wen PY, Dietrich S, de Jonge MJA, Blay
JY, et al: Dabrafenib plus trametinib in BRAFV600E-mutated rare
cancers: The phase 2 ROAR trial. Nat Med. 29:1103–1112. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
325
|
Tahara M, Kiyota N, Imai H, Takahashi S,
Nishiyama A, Tamura S, Shimizu Y, Kadowaki S, Ito KI, Toyoshima M,
et al: A phase 2 study of encorafenib in combination with
binimetinib in patients with metastatic BRAF-Mutated thyroid cancer
in Japan. Thyroid. 34:467–476. 2024.PubMed/NCBI
|
|
326
|
Cabanillas ME, Dadu R, Ferrarotto R,
Gule-Monroe M, Liu S, Fellman B, Williams MD, Zafereo M, Wang JR,
Lu C, et al: Anti-programmed death ligand 1 plus targeted therapy
in anaplastic thyroid carcinoma: A nonrandomized clinical trial.
JAMA Oncol. 10:1672–1680. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
327
|
Higashiyama T, Sugino K, Hara H, Ito KI,
Nakashima N, Onoda N, Tori M, Katoh H, Kiyota N, Ota I, et al:
Phase II study of the efficacy and safety of lenvatinib for
anaplastic thyroid cancer (HOPE). Eur J Cancer. 173:210–218. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
328
|
Loo E, Khalili P, Beuhler K, Siddiqi I and
Vasef MA: BRAF V600E mutation across multiple tumor types:
Correlation between DNA-based sequencing and Mutation-specific
immunohistochemistry. Appl Immunohistochem Mol Morphol. 26:709–713.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
329
|
Cece A, Agresti M, De Falco N, Sperlongano
P, Moccia G, Luongo P, Miele F, Allaria A, Torelli F, Bassi P, et
al: Role of artificial intelligence in thyroid cancer diagnosis. J
Clin Med. 14:24222025. View Article : Google Scholar : PubMed/NCBI
|