Introduction
Immunization via vaccination not only prevents the
spread of infectious diseases in children and adults, but also
provides lifelong protection against certain diseases (1). However, whether the use of
chemotherapeutic drugs reduces vaccine efficacy remains
unclear.
Chemotherapeutic drugs kill hematopoietic cells in
the bone marrow, which can cause side-effects such as neutropenia,
erythropenia and thrombocytopenia. Due to changes in the immune
system, patients with cancer are at a higher risk of becoming
infected with pathogens than the general population, and infections
in these patients often lead to excessive morbidity and mortality
rates (2). This increased risk may
be related to a variety of causes, including cancer, chemotherapy
and poor nutrition. Although traditional chemotherapy has long been
recognized as an effective anticancer treatment, its potentially
severe side-effects are also widely recognized, including the
marked impairment of immune function (3). In patients treated with platinum-based
drugs, such as epirubicin, doxorubicin, paclitaxel and
cyclophosphamide chemotherapeutic regimens, the absolute numbers of
B-cell subsets significantly decrease between 2 and 12 weeks of
treatment (4). By contrast,
treatment with methotrexate at various doses for 6 weeks has been
shown to result in a decrease in B-cell numbers (5).
B-cells play a key role in adaptive immune
responses. These cells are activated in the germinal centers of
secondary lymphoid organs to form long-lived memory B-cells, which
differentiate into plasma cells following antigen stimulation and
produce high-affinity antibodies. The ‘Clinical Practice Guidelines
for Vaccination of Immunocompromised Hosts’ issued by the
Infectious Diseases Society of America considers that live
attenuated vaccines should be administered at least 4 weeks prior
to the start of any immunosuppressive treatment (6). In the case that radiotherapy or
chemotherapy are performed, vaccination should be terminated due to
concerns that live vaccines may cause infection (7). The present study reports the case of a
patient who was likely naturally infected with hepatitis B virus
(HBV) during chemotherapy, who recovered spontaneously and
developed high levels of anti-hepatitis Bs (anti-HBs).
Acute lymphoblastic leukemia (ALL), the disease that
the patient was originally treated for, is a malignant disease that
originates from B- or T-lymphocytes that proliferate abnormally in
the bone marrow. ALL can also invade tissues outside the bone
marrow (such as meninges, lymph nodes and peripheral blood)
(8). The treatment of ALL usually
includes four stages: i) Induction therapy; ii) consolidation
therapy; iii) enhancement therapy; and iv) targeted therapy to
prevent the relapse of central nervous system leukemia (9). During treatment, patients receive
different chemotherapeutic drugs, such as vincristine, cytarabine,
mercaptopurine, doxorubicin, methotrexate, cyclophosphamide and
L-asparaginase (10).
Case report
The patient in the present study was a 49-year-old
woman as of 2024. The vaccination history of the patient was
unknown, and the patient began to feel weak in January 2021,
accompanied by subcostal and lumbar pain, but did not pay attention
to it. The patient received treatment at the Chifeng Municipal
Hospital (Chifeng, China). The patient is a woman who was admitted
to the hospital for examination in March 2021 and was 46 years old
at the time. A peripheral blood examination in March, 2021,
revealed the following: i) A total white blood cell (WBC) count of
56.84×109/l (reference interval: 4-10×109/l);
ii) a lymphocyte ratio (L%) of 87.7% (reference interval: 20-40%)
and a neutrophil ratio (N%) of 6.95% (reference interval: 50-70%);
iii) a total red blood cell (RBC) count of 2.47×1012/l
(reference interval: 3.5-5.0×1012/l) and a hemoglobin
(Hb) level of 75 g/l (reference interval: 110-150 g/l); and iv) a
platelet (PLT) count of 20×109/l (reference interval:
100-300×109/l). Blood test results revealed that
primitive cells accounted for 84% of the cells (reference interval:
0%). The morphological description was that a large number of
primitive cells were observed, mature RBCs were of different sizes,
no nucleated RBCs or RBC inclusion bodies were observed and the
PLTs were relatively few. The bone marrow morphology report was
grade 1-2 myeloproliferation, mainly the abnormal proliferation of
lymphoid system cells, mature RBCs of different sizes and primitive
lymphocytes of the lymphocyte system accounting for 95%. The
primitive lymphocytes in the bone marrow smear are of different
sizes, with more nuclear chromatin, visible nucleoli, less serous
fluid, blue color, no granules, larger nuclei, and darker cytoplasm
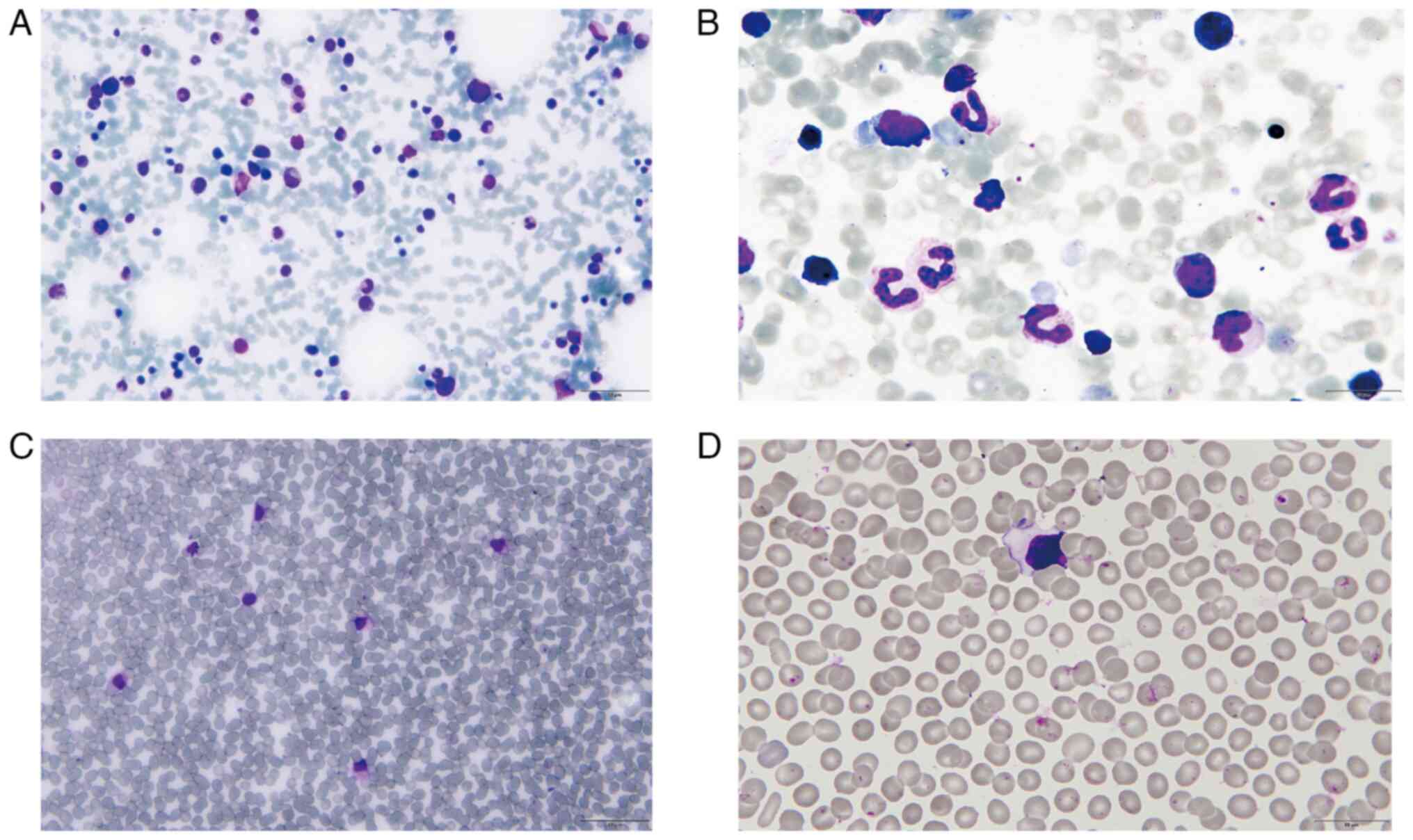
(Fig. 1A and B). Primitive
lymphocytes were also found in the peripheral blood (Fig. 1C and D). The diagnosis of ALL was
made. Immunophenotyping revealed that immature cells accounted for
91.6% of all cells expressing CD19, CD38, CD43, CD58, CD79a and
HLA-DR (human leukocyte antigen DR α chain precursor); some cells
expressed CD123, CD81, CD24, CD15, glial antigen 2 and terminal
deoxynucleotidyl transferase; there was no expression of CD45, CD5,
CD10, CD7, CD56, CD117, CD34, CD33, CD65, CD13, CD66c, CD22, CD20,
c/mκ and c/mλ. The IKAROS family zinc finger 1 mutation status was
positive.
In March 2021, the patient received 1.4
mg/m2 of vincristine, 40 mg/m2 of doxorubicin
and 1.4 mg/m2 of prednisone based on the body surface
area (m2). The aforementioned medication is called VDP
chemotherapy regimen. Bone marrow morphology analysis suggested
that the patient achieved morphological remission after 15 days of
VDP chemotherapy regimen. The day after the first administration of
VDP, the test result of HBsAg was <0.05 IU/ml (negative). One
intramuscular injection of 3,750 IU pegaspargase was given in April
2021. A further 13 days later, the patient received methotrexate
(MTX) chemotherapy at a dose of 1 g/m2 (body surface area), with a
total dose of 1.6 g. After 24 h, the MTX concentration in the blood
was 0.086 µmol/l. In May 2021, the quantitative result of HBsAg was
<0.05 IU/ml (negative). A cyclophosphamide, doxorubicin and
methotrexate (CAM) chemotherapy program was administered in June
2021. In July 2021, the patient was treated once with daunorubicin
(total dose 30 mg) and cytarabine (total dose 7.2 mg).
Subsequently, 10 days later, the quantitative result of HBsAg was
<0.05 IU/ml (negative). Subsequently, 18 days later,
chemotherapy with 1.4 mg/m2 vinblastine, 40
mg/m2 daunorubicin, 800 mg/m2
cyclophosphamide and 40 mg/m2 dexamethasone (VDCD) was
administered. In August 2021, a single intramuscular injection of
high-dose MTX (total dose 2.1 g) and 3,750 IU of pegaspargase was
administered. In September 2021, the quantitative result of HBsAg
was <0.05 IU/ml (negative). In October 2021, to create
conditions for donor stem cell implantation, the patient received
0.8 mg/kg busulfan and 60 mg/kg cyclophosphamide (BUCY) regimen for
pre-autologous hematopoietic stem cell transplantation
conditioning. Hematopoietic stem cells were transfused 7 days after
the BUCY regimen was administered. In February 2022, the patient
was treated once with a regimen of 1.4 mg/m2 vindesine,
40 mg/m2 daunorubicin, 800 mg/m2
cyclophosphamide and 40 mg/m2 dexamethasone (VICP), and
3,750 IU of pegaspargase intramuscularly. for consolidation
chemotherapy. Subsequently, 10 days later, the γ-glutamyl
transferase (GGT) test result was 68 IU/l (reference interval:
<60 U/l) which indicated a marked increase.
For possible potential blood transfusion treatment,
relevant infectious markers are tested before blood transfusion.
Subsequently, 7 days later, the HBsAg quantitative level was
accidentally detected and was 0.19 IU/ml (positive) and was
followed by a peripheral blood HBV nucleic acid test; the results
revealed that no amplification occurred. In March 2022, the HBsAg
quantitative result was 12.8 IU/ml (positive); however, the
cisplatin, oxaliplatin, doxorubicin, etoposide and tegafur regimen
consolidation chemotherapy was still administered the following
day. In April 2022, the alanine aminotransferase (ALT) (reference
interval: <35 U/l) levels began to increase slightly, with a
test result of 41 IU/l, and the GGT level was 104 IU/l. In April
2022, the possibility of hepatitis B virus infection began to be
considered due to a notable increase in transaminase levels [ALT,
1,334 IU/l; aspartate aminotransferase (AST), 1,334 IU/l; and GGT,
198 IU/l] (Table I). The patient
developed jaundice and received hepatoprotective treatment with
100-200 mg magnesium isoglycyrrhizinate and 1.8-2.4 g glutathione
daily for 3 weeks. Ursodeoxycholic acid and adenosine blue butane
sulfonate (the dosage and duration of treatment are unknown) were
used for treatment simultaneously, and gradual improvements were
observed.
 | Table I.Determination of biochemical substance
content in peripheral blood. |
Table I.
Determination of biochemical substance
content in peripheral blood.
|
| Days post
admission |
|
|
|---|
|
|
|
|
|
|---|
| Biomarker name | 325 | 384 | 405 | 411 | 483 | Unit | Reference
interval |
|---|
| TP | 53.00 | 57.00 | 46.00 | 61.00 | 63.00 | g/l | 65.00-85.00 |
| ALB | 36.00 | 42.00 | 29.00 | 36.00 | 41.00 | g/l | 40.00-55.00 |
| GLO | 17.00 | 15.00 | 17.00 | 25.00 | 21.00 | g/l | 20.00-40.00 |
| A/G | 2.12 | 2.80 | 1.71 | 1.44 | 1.88 | - | 1.20-2.40 |
| PREA | 210.00 | 330.00 | 40.00 | 80.00 | 170.00 | mg/l | 180.00-350.00 |
| AST | 15.00 | 21.00 | 916.00 | 289.00 | 18.00 | IU/l | 13.00-35.00 |
| ALT | 13.00 | 41.00 | 1,334.00 | 259.00 | 12.00 | IU/l | 7.00-40.00 |
| AST/ALT | 1.15 | 0.51 | 0.69 | 1.12 | 1.53 | - | 0.25-4.4 |
| ALP | 119.00 | 93.00 | 477.00 | 230.00 | 86.00 | IU/l | 35.00-100.00 |
| GGT | 74.00 | 104.00 | 198.00 | 75.00 | 88.00 | IU/l | 7.00-45.00 |
| ADA | 10.00 | 9.00 | 138.00 | 210.00 | 6.00 | U/l | 1.00-20.00 |
VD (vincristine 1.4 mg/m2; daunorubicin
30-40 mg/m2) treatment regimen treatmentwas continued in
July 2022. In August 2022, in order to observe whether the patient
had liver damage, liver function-related biomarkers were tested,
and the results were as follows: ALT, 13.8 IU/l; AST, 17.7 IU/l;
GGT, 67 IU/l. Quantitative analysis of blood samples with hepatitis
B infection indicators on the second day showed that the HBsAg
level had decreased to <0.05 (negative) and the anti-HBs content
was 982 IU/l (Table II). The first
day of chemotherapy in March 2021, was considered as
hospitalization day 0, and some of the collected clinical test data
were counted by day to observe the results more intuitively. On day
347, HbSAg was first found to be positive and became negative on
day 524. The anti-HBs level significantly increased on day 524
(Fig. 2A and Table SI). The activities of ALT and AST
in peripheral serum can reflect the degree of liver damage, which
peaked on day 405 and then gradually decreased (Fig. 2B and Table SII). The other two indicators of
liver function, ALP and GGT, reached their highest levels on day
405 (Fig. 2C and Table SII). Peripheral blood indices (WBC,
N%, L%, Mo%, RBC, Hb and PLT) revealed irregular ‘wave-like’
changes (Fig. 2D and E; Table SIII). The disease occurrence
timeline is presented in Table
III.
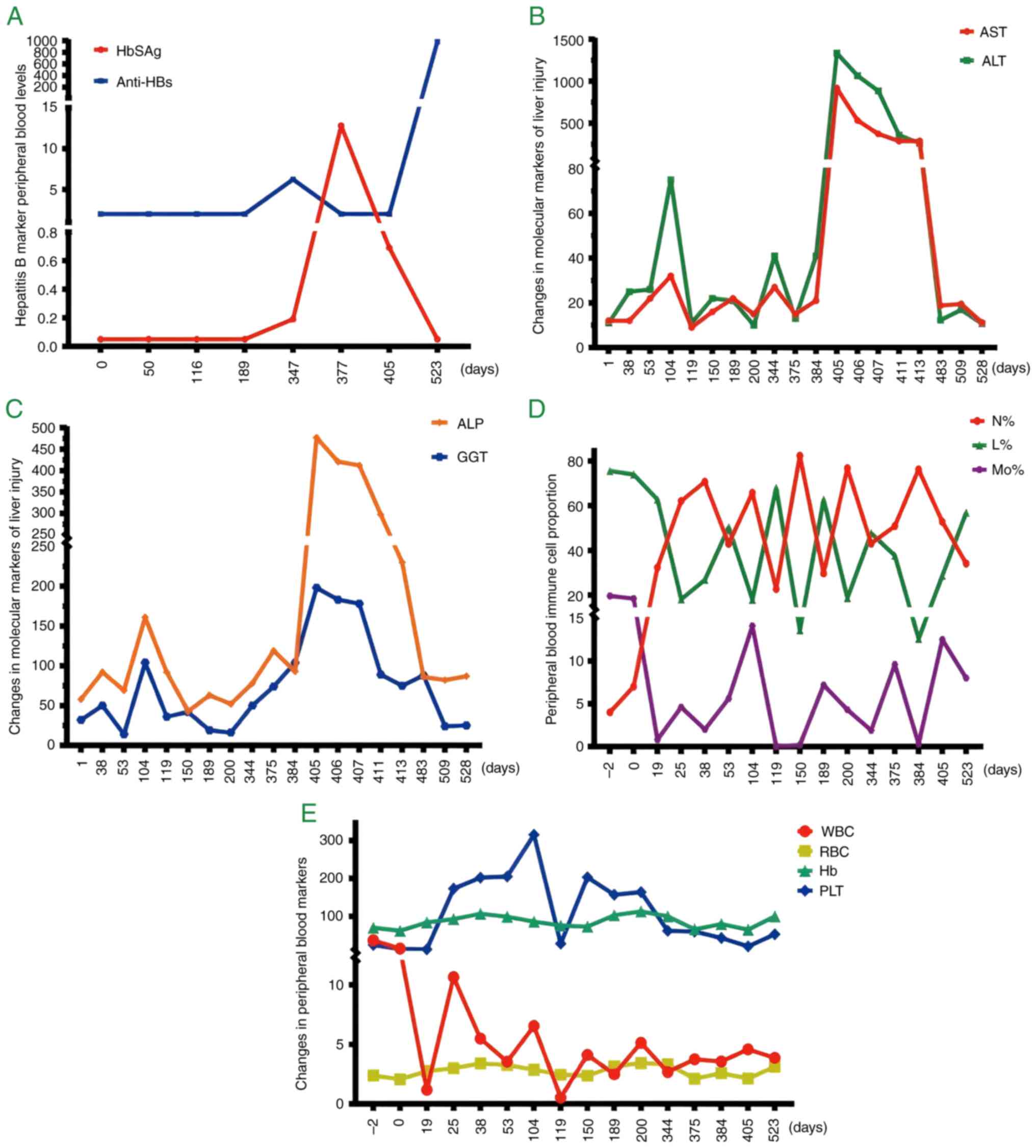 | Figure 2.Changes in trends in peripheral
blood-related clinical diagnostic markers. (A) Detection of
hepatitis B markers HBsAg (IU/ml) and anti-Hbs (IU/l). (B)
Detection of ALT and AST levels (unit, IU/l). (C) Detection of ALP
and GGT levels (unit, IU/l). (D) Proportion of major immune cells;
(E) WBC (109/l); RBC (1012/l); Hb (g/l); PLT
(109/l) content determination. HBsAg, Hepatitis B
surface antigen; anti-Hbs, anti-hepatitis B surface antibodies;
ALT, alanine aminotransferase; AST, aspartate aminotransferase;
ALP, alkaline phosphatase; GGT, γ-glutamyl transferase; N,
neutrophils; L, lymphocytes; Mo, monocytes; WBC, white blood cell;
RBC, red blood cell; Hb, hemoglobin; PLT, platelet. |
 | Table II.Changes in peripheral blood
pathogenic diagnostic markers. |
Table II.
Changes in peripheral blood
pathogenic diagnostic markers.
|
| Days post
admission |
|
|
|---|
|
|
|
|
|
|---|
| Marker | 189 | 189 | 377 | 524 | Unit | Reference
interval |
|---|
| HbSAg | <0.05 | 0.19 | 12.80 | <0.05 | IU/ml | Negative <0.05;
positive ≥0.05 |
| Anti-HBs | <2.00 | 6.25 | <2.00 | 982.00 | IU/l | Negative <10.0;
positive ≥10.0 |
| HBeAg | 0.10 | 0.12 | 0.97 | 0.10 | COI | Negative <1.0;
positive ≥1.0 |
| HBeAb | 1.49 | 1.40 | 1.62 | 0.01 | COI | Negative >1.0;
positive ≤1.0 |
| Anti-HBc | 2.37 | 2.11 | 0.91 | 0.01 | COI | Negative >1.0;
positive ≤1.0 |
| Anti-HBcIgM | 0.090 | 0.063 | 0.089 | - | COI | Negative <1.0;
positive ≥1.0 |
 | Table III.Timeline. |
Table III.
Timeline.
| Days post
admission | Patient notes |
|---|
| January 2021 | Fatigue and
subcostal and back pain began to occur. |
| March 2021 | Acute lymphoblastic
leukemia was diagnosed and chemotherapy was administered using the
VDP regimen. HBsAg test result <0.05 IU/ml (negative). |
| September 2021 | HBsAg test result
<0.05 IU/ml (negative). |
| October 2021 | Hematopoietic stem
cell infusion |
| February 2022 | Treated with VICP
and pegaspargase. The quantitative result of HBsAg detected
unexpectedly was 0.19 IU/ml (positive). |
| March 2022 | Chemotherapy was
performed using the COATD regimen. The quantitative result of HBsAg
was 12.8 IU/ml (positive). |
| April 2022 | Peripheral plasma
ALT levels began to increase and soon reached 1334 IU/l. The same
changes occur in AST levels. Anti-HBc-IgM levels were significantly
increased in peripheral serum. |
| July 2022 | ALT and AST levels
in peripheral plasma decreased to within the reference range. Use
VD regimen for treatment. |
| August 2022 | The HBsAg
quantitative result became <0.05 (negative), and the Anti-HBs
content was 982 IU/l. |
Discussion
The present study reports the case of an adult
patient with ALL who may have been infected with HBV during
chemotherapy. The patient subsequently produced corresponding
antibodies and successfully achieved clinical self-recovery.
The average incubation period of HBV infection is
60-90 days (11). Infants, children
<5 years of age and immunosuppressed adults usually have no
obvious clinical symptoms in the case of infection with HBV. In
adults with normal immune function, ~95% of infections will be
rapidly cleared by the immune system of the body following viral
infection (12). Immunosuppressed
patients (such as those undergoing hemodialysis and those infected
with human immunodeficiency virus) may develop chronic infection
from acute infection. Currently, there is no specific treatment
available to completely eliminate HBV from the body (13). HBsAg positivity indicates HBV
infection. In the case of an acute HBV infection, anti-HBc (IgM and
IgG subtypes) in peripheral blood can be detected 1-2 weeks later
than HBsAg (14). After a healthy
adult is infected with HBV, HBsAg usually disappears within 3-4
months and anti-HBs appear. The presence of anti-HBs usually
indicates immunity to HBV infection (15). Occult HBV infection refers to
patients who test negative for HBsAg in their peripheral blood, but
there is replication-competent viral DNA in their liver and HBV DNA
is undetectable in their serum, which poses a challenge to the
study of HBV (16). In the present
study, the use of chemotherapy did not affect the production of
anti-HBs, although there was no evidence of an occult HBV
infection. No information on the disease history of HBV in family
members was obtained from medical records of the patient.
B-cells are generally known to produce secretory
antibodies during HBV infection and are a key component of the
adaptive immune response of the body (17). It is generally believed that the use
of chemotherapeutic drugs to treat tumors will have the side-effect
of suppressing bone marrow hematopoiesis, leading to a decrease in
the number of immune cells, such as B-lymphocytes and a decrease in
the ability of B-lymphocytes to respond to immune responses, such
as against viruses (18). Notably,
there is limited scientific knowledge available as to whether
chemotherapeutic drugs will affect the function of lymphoid tissue
and cell subsets, and whether they will have long-term effects on
the cellular and humoral immune responses of the body. The main
drugs used in patients with ALL in the maintenance phase are
6-mercaptopurine and MTX, which can reduce the risk of relapse;
however, they also have potentially severe toxic effects, with bone
marrow suppression being the most common side-effect (19). Therefore, it can be taken for
granted that chemotherapeutic drugs have significant adverse
effects on the immune system.
It has long been recognized that chemotherapeutic
agents can treat immune diseases. For example, the inactive
chemotherapeutic agent, cyclophosphamide, undergoes complex
metabolism in the liver to produce cytotoxic metabolites (20). Decades ago, the immunomodulatory
effects of cyclophosphamide were discovered in animal models, and
its mechanism of action is through the reduction of cell numbers
(21). In addition,
chemotherapeutic agents can deplete cells known as regulatory
T-lymphocytes (Tregs), thereby promoting local antitumor immune
responses. There has been renewed interest in the use of
cyclophosphamide to deplete human Tregs (22). As Tregs have low levels of
intracellular ATP and glutathione, they are considered to be more
susceptible to the toxic effects of cyclophosphamide than other
T-cells (23). When high-dose
corticosteroids are combined with cyclophosphamide to treat
severely active lupus nephritis, it can preferentially eliminate
naive B cells (24). Notably,
cyclophosphamide has no significant effect on class-switched memory
B cells, as these cells are normally dormant and do not proliferate
(25). Additionally,
chemotherapeutic drugs, including cyclophosphamide, doxorubicin,
methotrexate, mitomycin C, paclitaxel and vincristine, can improve
the function of antigen-presenting cells and increase the antitumor
effect of immune cells at low doses (26). Paclitaxel can also directly affect
the maturation of dendritic cells (27). Paclitaxel displays
lipopolysaccharide-mimicking activity in mice, activates TLR4, and
enhances DC activation and cytokine biosynthesis (28).
The majority of vaccination strategies for patients
with cancer involve vaccines for influenza, pneumococcal infection,
hepatitis B or recurrent herpes zoster. Experience suggests that
patients with cancer, or immunocompromised or immunosuppressed
patients may require a greater number of vaccine doses and more
vaccinations than immunocompetent individuals (29). A previous study of a recombinant
herpes zoster vaccine in patients with cancer demonstrated that the
overall immune response differed when the first dose of the vaccine
was administered prior to chemotherapy compared with when the first
dose was administered during chemotherapy (30). However, another study on a
recombinant subunit herpes zoster vaccine found that only 15% of
patients with hematological malignancies had a detectable
serological response (31).
Patients with cancer are more likely to have an
immune response following vaccination for coronavirus disease-19
than non-cancer patients. As previously demonstrated, after the
third dose of the vaccine in patients with solid tumors, the
proportion of patients with neutralization to the Omicron variant
increased from 47.8 to 88.9% (32).
It has also been found that after two doses of the mRNA vaccine in
patients with non-small cell lung cancer, the neutralization
response to the Omicron variant was 79-fold lower than that of
individuals without cancer (33).
Neutralizing antibodies are rarely detected in patients with
hematological malignancies after two doses of the Omicron vaccine;
however, neutralizing antibodies are detected in ~50% of patients
after the third dose (34).
Therefore, limited knowledge is available as regards the impact of
chemotherapy on the immune system of patients with cancer,
particularly with regards to vaccination strategies in the context
of chemotherapy.
The present study revealed that a patient
accidentally infected with HBV continued to produce protective
antibodies against HBV despite taking chemotherapy drugs to reduce
the number of lymphocytes in their blood, ultimately achieving
clinical cure. This phenomenon highlights the complexity of the
human immune system. Further understanding of the immune system is
needed to potentially produce effective strategies to enhance
antibody production in patients with HBV after vaccination.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by the Natural Science Foundation of
Inner Mongolia Autonomous Region (grant no. 2021MS08060), Inner
Mongolia Medical University Joint Project (grant nos. YKD2021LH068
and YKD2022LH072) and the Public Hospital Research Joint Fund
Science and Technology Project (grant no. 2024GLLH0990).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
LW, FZ and JH wrote the manuscript. YH, FZ and JH
provided critical comments on the manuscript content and made
substantial contributions to the interpretation of the data. LW and
YH made substantial contributions to the conception and design of
the manuscript, identified the clinical cases, assessed the
clinical data, verified the case data and reviewed the manuscript.
DS and RY analyzed and interpreted the data. HN acquired the data.
LW and YH confirm the authenticity of all original data and take
responsibility for all aspects of the work, ensuring that any
questions regarding the accuracy or integrity of any part of the
work were appropriately investigated and resolved (according to
ICMJE regulations). All authors read and approved the final version
of the manuscript and unanimously agreed that the manuscript could
be published.
Ethics approval and consent to
participate
This case report (including images and related text)
was submitted and published in accordance with the COPE guidelines,
strictly following the Declaration of Helsinki and Chinese national
policies, and was approved by the Ethics Committee of Chifeng
Municipal Hospital (approval no. CK20250101, approved in January
2025).
Patient consent for publication
Written consent was obtained from the patient to
publish these details.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Principe DR, Kamath SD, Korc M and Munshi
HG: The immune modifying effects of chemotherapy and advances in
Chemo-immunotherapy. Pharmacol Ther. 236:1081112022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yusuf K, Sampath V and Umar S: Bacterial
infections and cancer: Exploring this association and its
implications for patients with cancer. Int J Mol Sci. 24:31102023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Terrones-Campos C, Ledergerber B, Specht
L, Vogelius IR, Helleberg M and Lundgren J: Risk of bacterial,
viral, and fungal infections in patients with solid malignant
tumors treated with curative intent radiation therapy. Adv Radiat
Oncol. 7:1009502022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goswami M, Prince G, Biancotto A, Moir S,
Kardava L, Santich BH, Cheung F, Kotliarov Y, Chen J, Shi R, et al:
Impaired B-cell immunity in patients with acute myeloid leukemia
after chemotherapy. J Transl Med. 15:1552017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zandvoort A, Lodewijk ME, Klok PA, Dammers
PM, Kroese FG and Timens W: Slow recovery of follicular B cells and
marginal zone B cells after chemotherapy: Implications for humoral
immunity. Clin Exp Immunol. 124:172–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henderson DK, Dembry LM, Sifri CD, Palmore
TN, Dellinger EP, Yokoe DS, Grady C, Heller T, Weber D, Del Rio C,
et al: Management of healthcare personnel living with hepatitis B,
hepatitis C, or human immunodeficiency virus in US healthcare
institutions. Infect Control Hosp Epidemiol. 43:147–155. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uyeki TM, Bernstein HH, Bradley JS,
Englund JA, File TM, Fry AM, Gravenstein S, Hayden FG, Harper SA,
Hirshon JM, et al: Clinical practice guidelines by the infectious
diseases society of America: 2018 update on diagnosis, treatment,
chemoprophylaxis, and institutional break management of seasonal
influenza. Clin Infect Dis. 68:e1–e47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv M, Liu Y, Liu W, Xing Y and Zhang S:
Immunotherapy for pediatric acute lymphoblastic leukemia: Recent
advances and future perspectives. Front Immunol. 13:9218942022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gavralidis A and Brunner AM: Novel
therapies for treating adult acute lymphoblastic leukemia. Curr
Hematol Malig Rep. 15:294–304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sas V, Moisoiu V, Teodorescu P, Tranca S,
Pop L, Iluta S, Pasca S, Blag C, Man S, Roman A, et al: Approach to
the adult acute lymphoblastic leukemia patient. J Clin Med.
8:11752019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghany MG, Feld JJ, Chang KM, Chan HLY, Lok
ASF, Visvanathan K and Janssen HLA: Serum alanine aminotransferase
flares in chronic hepatitis B infection: Good and bad. Lancet
Gastroenterol Hepatol. 5:406–417. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terrault NA, Lok ASF, McMahon BJ, Chang
KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH and Wong JB: Update
on the prevention, diagnosis, and treatment of chronic hepatitis B:
AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang G and Duan Z: Guidelines for
prevention and treatment of chronic hepatitis B. J Clin Transl
Hepatol. 9:769–791. 2021.PubMed/NCBI
|
|
14
|
Campos-Valdez M, Monroy-Ramírez HC,
Armendáriz-Borunda J and Sánchez-Orozco LV: Molecular mechanisms
during hepatitis B infection and the effects of the virus
variability. Viruses. 13:11672021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kramvis A, Chang KM, Dandri M, Farci P,
Glebe D, Hu J, Janssen HLA, Lau DTY, Penicaud C, Pollicino T, et
al: A roadmap for serum biomarkers for hepatitis B virus: Current
status and future outlook. Nat Rev Gastroenterol Hepatol.
19:727–745. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saitta C, Pollicino T and Raimondo G:
Occult hepatitis B virus infection: An UPdate. Viruses.
14:15042022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai Y and Yin W: Multiple functions of B
cells in chronic HBV infection. Front Immunol. 11:5822922020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heylmann D, Bauer M, Becker H, van Gool S,
Bacher N, Steinbrink K and Kaina B: Human CD4+CD25+ regulatory
T-cells are sensitive to Low-dose cyclophosphamide. PLoS One.
8:e833842013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toksvang LN, Lee SHR, Yang JJ and
Schmiegelow K: Maintenance therapy for acute lymphoblastic
leukemia: Basic science and clinical translations. Leukemia.
36:1749–1758. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Lu Y, Zhang Y, Dong J, Jiang S
and Tang Y: DHA-enriched phosphatidylserine ameliorates
cyclophosphamide-induced liver injury via regulating the gut-liver
axis. Int Immunopharmacol. 140:1128952024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khimani F, Ranspach P, Elmariah H, Kim J,
Whiting J, Nishihori T, Locke FL, Perez Perez A, Dean E, Mishra A,
et al: Increased infections and delayed CD4+ T cell but Faster B
cell immune reconstitution after Post-transplantation
cyclophosphamide compared to conventional GVHD prophylaxis in
allogeneic transplantation. Transplant Cell Ther. 27:940–948. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kondĕlková K, Vokurková D, Krejsek J,
Borská L, Fiala Z and Ctirad A: Regulatory T cells (TREG) and their
roles in immune system with respect to immunopathological
disorders. Acta Medica (Hradec Kralove). 53:73–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naicker SD, Feerick CL, Lynch K, Swan D,
McEllistrim C, Henderson R, Leonard NA, Treacy O, Natoni A, Rigalou
A, et al: Cyclophosphamide alters the tumor cell secretome to
potentiate the anti-myeloma activity of daratumumab through
augmentation of macrophage-mediated antibody dependent cellular
phagocytosis. Oncoimmunology. 10:18592632021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scurr M, Pembroke T, Bloom A, Roberts D,
Thomson A, Smart K, Bridgeman H, Adams R, Brewster A, Jones R, et
al: Low-dose cyclophosphamide induces antitumor T-cell responses,
which associate with survival in metastatic colorectal cancer. Clin
Cancer Res. 23:6771–6780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Jiang Z, Jiang Y, Ma N, Wang K and
Zhang Y: Changes in immune cell frequencies after cyclophosphamide
or mycophenolate mofetil treatments in patients with systemic lupus
erythematosus. Clin Rheumatol. 31:951–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaneno R, Shurin GV, Tourkova IL and
Shurin MR: Chemomodulation of human dendritic cell function by
antineoplastic agents at low non-cytotoxic concentrations. J Transl
Med. 7:582009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Byrd-Leifer CA, Block EF, Takeda K, Akira
S and Ding A: Role of MyD88 and TLR4 in LPS-mimetic activity of
Taxol. Eur J Immunol. 31:2448–2457. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
John J, Ismail M, Riley C, Askham J,
Morgan R, Melcher A and Pandha H: Differential effects of
paclitaxel on dendritic cell function. BMC Immunol. 11:142010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noori M, Azizi S, Abbasi Varaki F,
Nejadghaderi SA and Bashash D: A systematic review and
meta-analysis of the immune response against the first and second
doses of SARS-CoV-2 vaccines in adult patients with hematological
malignancies. Int Immunopharmacol. 110:1090462022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rubio-Viqueira B, Jung KH, Rodriguez
Moreno JF, Grande E, Marrupe Gonzalez D, Lowndes S, Puente J,
Kristeleit H, Farrugia D, McNeil SA, et al: Immunogenicity and
safety of the adjuvanted recombinant zoster vaccine in patients
with solid tumors, vaccinated before or during chemotherapy: A
randomized trial. Cancer. 125:1301–1312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pedrazzoli P, Lasagna A, Cassaniti I,
Ferrari A, Bergami F, Silvestris N, Sapuppo E, Di Maio M, Cinieri S
and Baldanti F: Vaccination for herpes zoster in patients with
solid tumors: A position paper on the behalf of the Associazione
Italiana di Oncologia Medica (AIOM). ESMO Open. 7:1005482022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei Z, He J, Wang C, Bao J, Leng T and
Chen F: Importance of booster vaccination in the context of Omicron
waves. Front Immunol. 13:9779722022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fendler A, de Vries EGE, Geurtsvan Kessel
CH, Haanen JB, Wörmann B, Turajlic S and von Lilienfeld-Toal M:
COVID-19 vaccines in patients with cancer: Immunogenicity,
efficacy, and safety. Nat Rev Clin Oncol. 19:385–401. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao L, Fu L, He Y, Li H, Song Y and Liu
S: Effectiveness and safety of COVID-19 vaccination in patients
with malignant disease. Vaccines (Basel). 11:4862023. View Article : Google Scholar : PubMed/NCBI
|