Introduction
Breast cancer remains one of the most prevalent
malignancies among women globally, with triple-negative breast
cancer (TNBC) representing one of its most aggressive and
therapeutically challenging subtypes (1). TNBC accounts for approximately 15–20%
of breast cancer cases and is defined by the lack of expression of
estrogen receptor (ER), progesterone receptor (PR), and human
epidermal growth factor receptor 2 (HER2) (2,3). This
subtype is more commonly observed in younger women, particularly
those under 40 years of age, and is frequently associated with
early recurrence and high metastatic potential (3,4).
Patients with TNBC often face significant physical
and psychological burdens during treatment, including fatigue,
hepatic dysfunction, anxiety, and depressive symptoms. Supportive
strategies, such as the administration of melatonin and silymarin,
have shown beneficial effects in reducing chemotherapy-related
fatigue and toxicity (5,6). Psychological support with agents like
crocin has also been effective in mitigating distress associated
with treatment (7). In parallel,
biological markers such as cytokeratin 18 (CK18) are under
investigation as indicators of therapeutic response, helping to
personalize treatment regimens (8).
Eribulin mesylate (Halaven®), a synthetic
analog of the natural product halichondrin B, functions as a
microtubule dynamics inhibitor and has been approved for use in
patients with advanced breast cancer (9,10).
Pivotal trials such as EMBRACE (11) and Study 301 (12) have demonstrated its clinical benefit
in metastatic breast cancer, particularly among patients previously
treated with anthracycline and taxane-based regimens.
Notably, its effectiveness may be improved when used
in combination with other agents. Its efficacy may improve when
combined with synergistic agents.
Cisplatin, a platinum-based compound, induces
cytotoxicity primarily through DNA crosslinking and inhibition of
DNA repair, resulting in tumor cell apoptosis. Although it is an
established agent in TNBC therapy, its use is often constrained by
its dose-limiting toxicities (13,14).
Nevertheless, platinum-based regimens continue to hold relevance in
TNBC treatment, with recent clinical guidelines recommending
carboplatin-taxane combinations as a neoadjuvant option for
HER2-negative and TNBC cases (15,16).
In our previous investigation (17), we demonstrated the synergistic
cytotoxic potential of eribulin combined with cisplatin in TNBC
models. Building upon those findings, the present study explores
the mechanistic basis of this synergy, particularly its
relationship with autophagy-a regulated cellular process
increasingly implicated in tumor progression and therapeutic
resistance. Given the therapeutic promise of targeting autophagy in
TNBC, we hypothesized that dual treatment with eribulin and
cisplatin could potentiate antitumor effects by modulating this
pathway.
Moreover, in light of global disparities in access
to advanced cancer therapies, particularly in low- and
middle-income countries (LMICs), there is a pressing need to
develop cost-effective treatment strategies. The use of existing,
clinically approved drugs with known safety profiles, such as
eribulin and cisplatin, offers a pragmatic avenue for broadening
treatment access and improving outcomes in resource-constrained
settings (18).
Taken together, these considerations highlight the
urgent need for treatment strategies that combine mechanistic
efficacy with cost-effectiveness, particularly in the management of
TNBC.
Materials and methods
Reagents and antibodies
All cell culture reagents, including Dulbecco's
Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and
penicillin/streptomycin, were sourced from HyClone (GE Healthcare
Life Sciences, Logan, UT, USA). Trypsin-EDTA was acquired from
Gibco (Thermo Fisher Scientific, Waltham, MA, USA).
Eribulin mesylate (1 mg/vial) was generously
provided by Eisai Co., Ltd. (Tokyo, Japan), and cisplatin was
obtained from JW Pharmaceutical (Seoul, Korea). The half-maximal
inhibitory concentration (IC50) of eribulin in
MDA-MB-231 cells was determined via CCK-8 assay after 72 h of
treatment and found to be 40.12 µM. Based on this, a concentration
of 60 µM-approximately 1.5 times the IC50- was selected
for subsequent combination experiments. This supra-physiological
dosing strategy aligns with previous mechanistic synergy studies,
such as Ko et al (17) in
TNBC cells combining eribulin and cisplatin and Swami et al
(19) demonstrating similar effects
in SK-BR-3 cells, as well as the in vitro/in vivo work by
Terashima et al (20) on
EMT/MET modulation in TNBC treated with eribulin plus S-1.
PD98059 (a MAPK inhibitor) and crystal violet dye
were procured from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Primary antibodies against LC3-I/II (cat. no. 4108), phospho-ERK1/2
(Thr202/Tyr204; cat. no. 9101), and β-actin (cat. no. 4967) were
purchased from Cell Signaling Technology. Additional antibodies
against ERK (SC-94) and p62 (sc-48389) were acquired from Santa
Cruz Biotechnology (Dallas, TX, USA). Secondary antibodies
conjugated with horseradish peroxidase (anti-mouse: cat. no. 7076;
anti-rabbit: cat. no. 7074) were also from Cell Signaling
Technology. Chemiluminescence reagents (Super Signal®
West Pico) and DAPI stain were provided by Thermo Fisher. CCK-8
kits were sourced from Dojindo Molecular Technologies (Japan), and
autophagy assays (ab139484) were performed using kits from Abcam
(Cambridge, MA, USA). Annexin V-FITC apoptosis detection kits were
obtained from Koma Biotech (Seoul, Korea).
Cell culture
MDA-MB-231, a human breast cancer cell line, was
obtained from the Korean Cell Line Bank. Cells were grown in DMEM
supplemented with 10% FBS, antibiotics (100 U/ml penicillin and 100
µg/ml streptomycin), sodium pyruvate (1 mM), sodium bicarbonate
(1.5 g/l), and glucose (4.5 g/l). Cultures were maintained in a
humidified 5% CO2 incubator at 37°C.
Cell viability assay
To evaluate cytotoxicity, cells were plated at 5,000
cells per well in 96-well plates and allowed to adhere overnight.
Treatments with test compounds were applied for 72 h. After
incubation, CCK-8 reagent (100 µl) was added, and absorbance at 450
nm was measured after a 2-h incubation using a microplate
reader.
Colony formation assay
Colony formation assays were conducted to evaluate
long-term proliferative capacity. MDA-MB-231 cells were seeded at
low density in 6-well plates and treated with eribulin, cisplatin,
or their combination for 14 days. Colonies were fixed with methanol
and stained with 0.5% crystal violet.
Colonies were defined as discrete clusters
containing ≥50 cells. Due to the dispersed and small-sized colonies
formed by MDA-MB-231 cells even after 14 days, manual
quantification under a microscope was technically unfeasible. Thus,
colony numbers and areas were quantified using ImageJ software
(NIH, USA) following standard image analysis steps: conversion to
8-bit grayscale, threshold adjustment, watershed segmentation, and
automated particle counting via the ‘Analyze Particles’
function.
Western blot analysis
Cells at approximately 80% confluency were treated
and harvested. Cell lysis was performed using buffer containing
Tris, NaCl, EDTA, Triton X-100, NP-40, PI, DTT, and PMSF. Lysates
were centrifuged, and protein concentrations were measured using a
BCA protein assay kit. Protein (20 µg/lane) was resolved by
SDS-PAGE and transferred onto PVDF membranes. Blots were blocked
with 5% milk in PBST and incubated with primary antibodies
overnight. HRP-conjugated secondary antibodies were applied, and
bands were visualized with enhanced chemiluminescence. Band
intensities were quantified and normalized to the respective
loading controls.
Annexin V/propidium iodide
staining
Apoptosis was quantified using an Annexin V-FITC/PI
kit as per the manufacturer's instructions. Cells treated for 72 h
were collected, stained with Annexin V-FITC and PI, and analyzed
via flow cytometry. Histogram analysis was done using Kaluza
software.
Autophagy detection assay
Autophagy flux was examined using Abcam's
fluorescent dye-based assay (ab139484), which selectively labels
autophagic compartments. Cells were cultured in 8-well chamber
slides and treated with the indicated compounds (100 µM) for the
specified time, fixed with paraformaldehyde, and stained with
autophagy-specific dyes according to the manufacturer's
instructions. Fluorescent signals were observed using a confocal
microscope and quantified with ImageJ.
Combination index
To assess drug synergy, the CI was computed using
the Chou-Talalay method with CompuSyn software (version 1.0,
Combosyn Inc., Paramus, NJ, USA). CI values were interpreted as
follows: CI <1 (synergy), CI=1 (additivity), and CI >1
(antagonism).
Statistical analysis
All experiments were independently repeated at least
three times. All statistical analyses were performed using SPSS
version 29.0 for Windows (IBM Corp.). One-way ANOVA was used with
Tukey's Honestly Significant Difference post hoc analysis. Data are
shown as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of eribulin and cisplatin on
cell viability and clonogenic growth
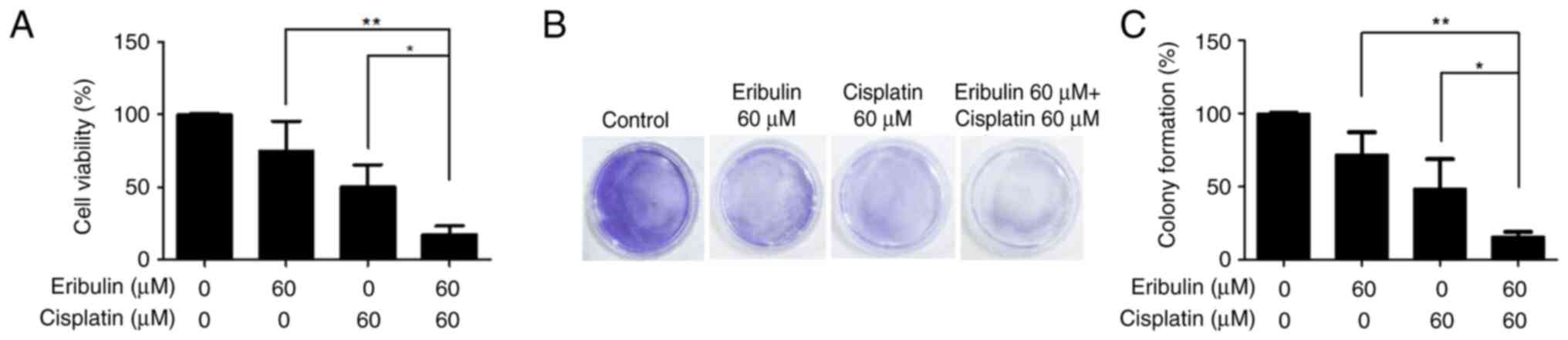
Cell viability was assessed using the CCK-8 assay
(Fig. 1A), and the synergistic
efficacy of the drug combination was further confirmed by colony
formation assays (Fig. 1B and C).
Table I summarizes the CI values
derived for the two drugs in this cell model. Single treatment with
eribulin mesylate (60 µM) decreased cell viability to 75.11±0.41%
after 72 h of exposure. Cisplatin (60 µM) single treatment showed a
reduction of 50.57±0.20%. The combination of these drugs
significantly inhibited cell viability to 17.15±0.10% (Fig. 1A). Colony-forming assays were
performed to test the ability of single cells to grow into
colonies. This drug combination synergistically inhibited colony
formation by MDA-MB-231 cells (Fig. 1B
and C).
 | Table I.CI value for the combination of
cisplatin and eribulin in MDA-MB-231 cells. |
Table I.
CI value for the combination of
cisplatin and eribulin in MDA-MB-231 cells.
| Cisplatin, µM | Eribulin, µM | CI | DRI
(cisplatin) | DRI (eribulin) |
|---|
| 20 | 10 | 2.35 | 0.74 | 0.99 |
| 40 | 20 | 3.37 | 0.39 | 1.14 |
| 60 | 60 | 0.71 | 1.39 | 3.62 |
| 140 | 140 | 0.59 | 1.68 | 2.62 |
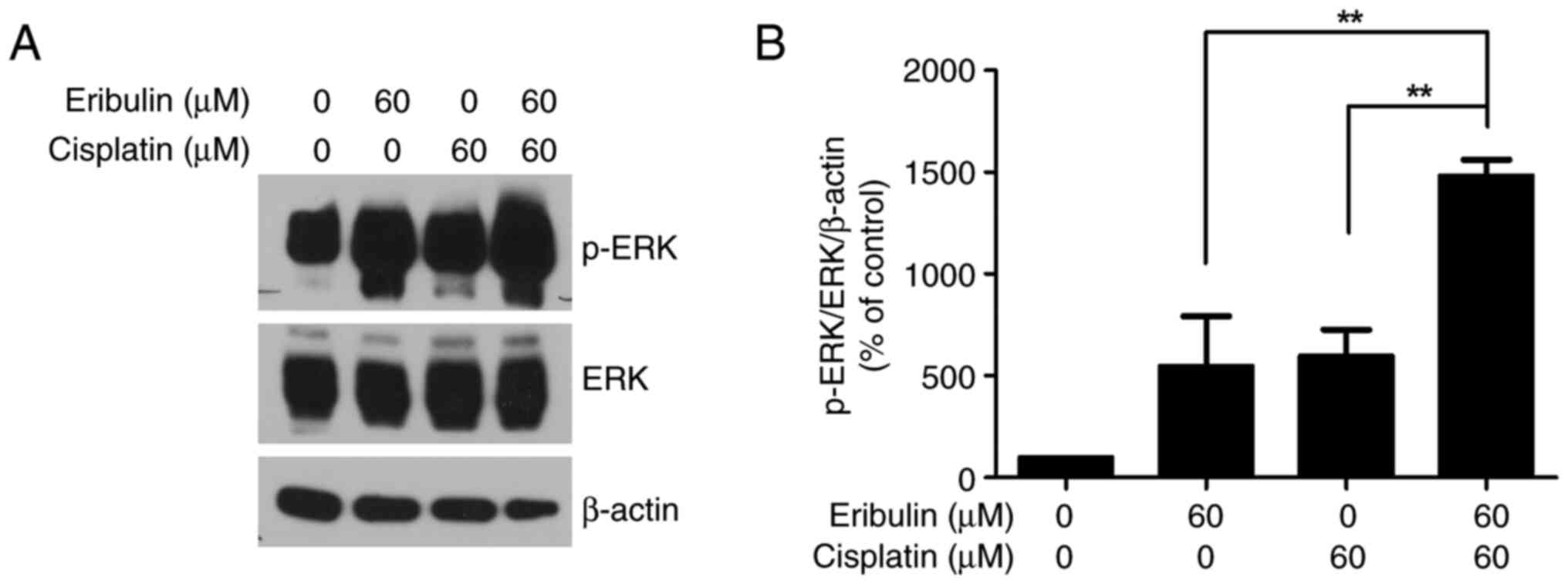
Synergistic activation of ERK1/2 by
drug combination
We observed that the ERK phosphorylation level
increased more than 5.5-fold in the experimental cells compared to
that in the control cells after the administration of 60 µM
eribulin for 72 h (Fig. 2A and B).
Moreover, 60 µM cisplatin increased ERK phosphorylation 6.0-fold
(Fig. 2A and B). In addition, when
cisplatin was added to eribulin, ERK activation increased by 14.8
times. When cisplatin was added to eribulin, ERK activation was
elevated 14.8-fold compared to the control, reflecting a 2.7- and
2.5-fold increase relative to eribulin and cisplatin treatment
alone, respectively. These findings confirmed that ERK1/2
activation increased synergistically with eribulin-cisplatin
combination.
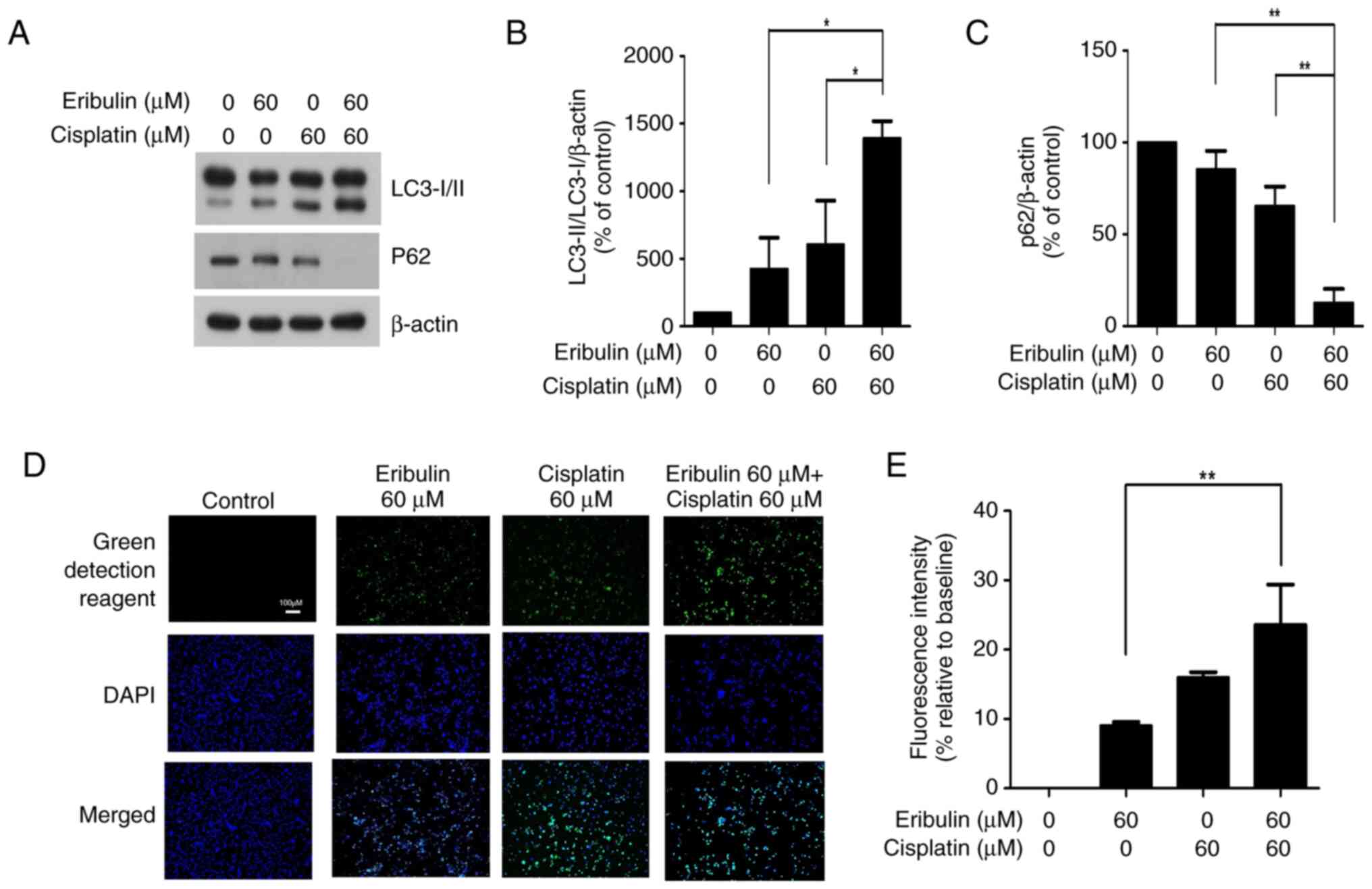
Induction of autophagy by eribulin and
cisplatin
To evaluate autophagy induction, the LC3-I/II ratio
and p62 expression were measured. As autophagy markers, the
microtubule-associated protein LC3-I/II ratio and p62 expression
were determined using western blot analysis (Fig. 3A). The LC3-I/II level increased
4.3-fold in the eribulin group compared to that in the control
group. Cisplatin alone increased the expression of these parameters
by 6.1-fold; the corresponding value for eribulin-cisplatin
combination treatment was 13.9-fold, indicating a synergistic
effect (Fig. 3B). A significant
downregulation of p62 was observed following co-treatment with
eribulin and cisplatin, suggesting promoted autophagic degradation
(Fig. 3C). The corresponding values
for eribulin alone and eribulin-cisplatin combination were 9.0 and
23.6%, respectively, indicating a significant increase in
autophagic activity in the combination group compared with the
eribulin-only group (Fig. 3D and
E). Autophagic vacuole staining showed a slight increase in
autophagic activity with either eribulin or cisplatin alone,
whereas their combination resulted in a marked enhancement,
indicating cumulative autophagic activation.
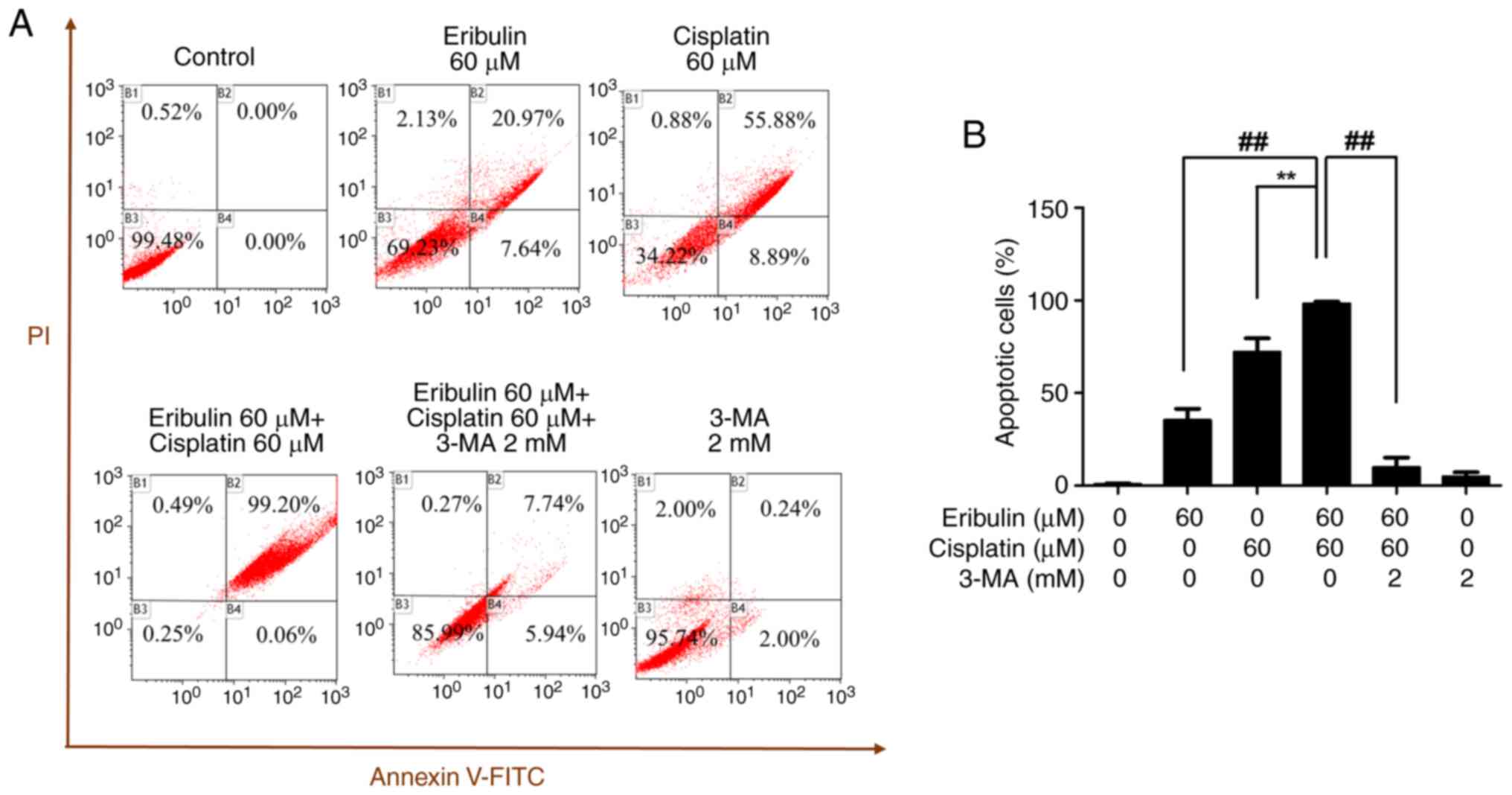
Apoptosis induced by drug combination
and its dependence on autophagy
The apoptotic activity of the combination of
eribulin and cisplatin was analyzed by flow cytometry after double
staining with annexin V and propidium iodide (Fig. 4A). The apoptotic activity levels
were 28.61, 64.77, and 99.26% when the cells were treated with
eribulin alone, cisplatin alone, or a combination thereof,
respectively. Upon co-treatment with 3-methyladenine, a known
autophagy inhibitor, the apoptotic rate induced by the
eribulin-cisplatin combination significantly decreased, suggesting
that the observed cytotoxicity is dependent on autophagic
mechanisms. When 3-methyladenine samples were treated with a
combination of eribulin and cisplatin, the apoptotic cell volume
was reduced to 13.68% compared with 99.26% in the
eribulin-cisplatin combination group (Fig. 4A and B).
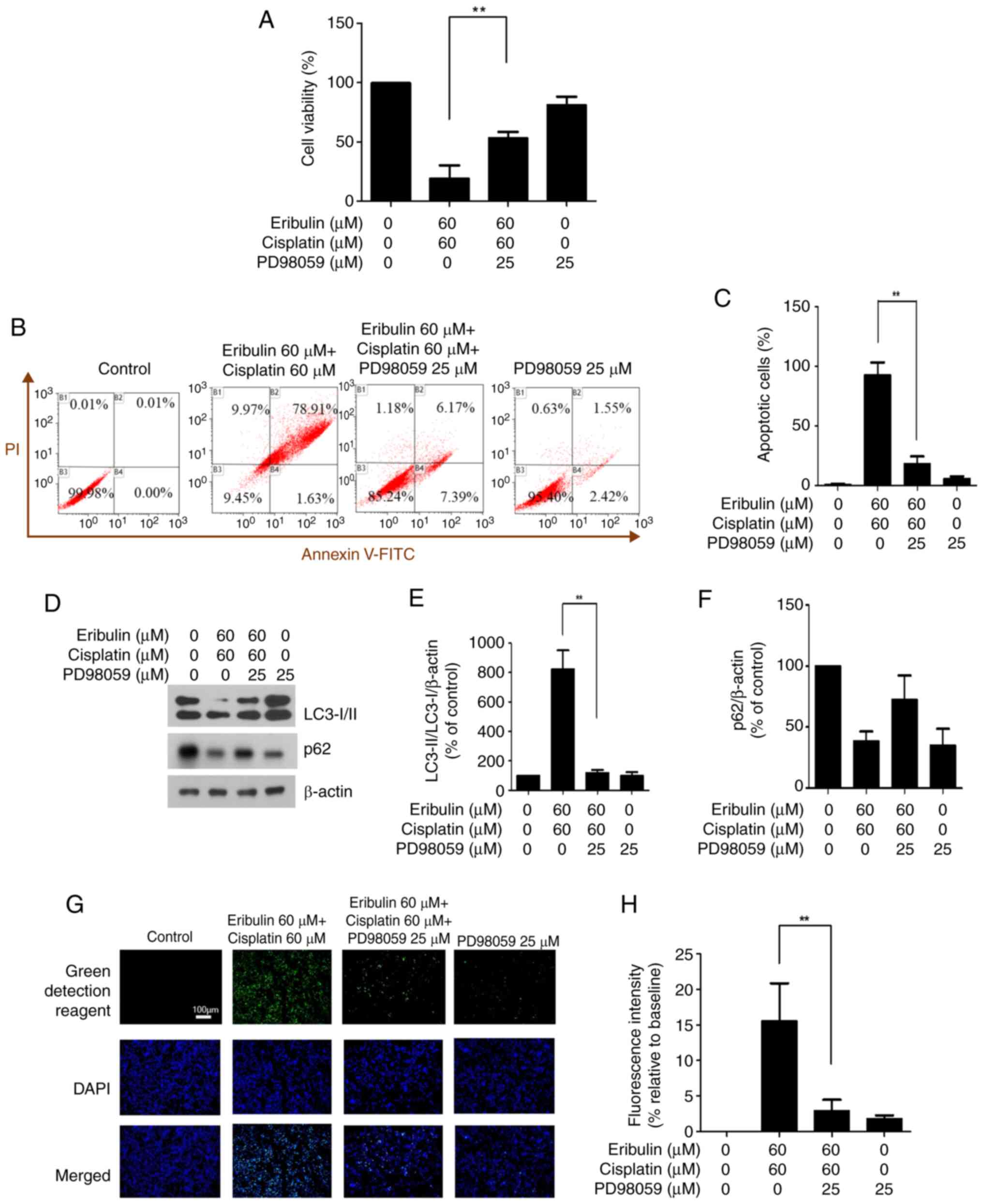
Impact of ERK inhibition on viability,
apoptosis, and autophagy
To assess the impact of ERK inhibition, cells were
pretreated with PD98059 prior to exposure to eribulin and
cisplatin, followed by a viability assessment using the CCK-8
assay. When PD98059 was combined with the two-drug combination, the
cell viability increased significantly from 33.63 to 53.37%
(Fig. 5A). Flow cytometry was used
to quantify apoptotic cells following dual labeling with Annexin V
and propidium iodide (Fig. 5B).
Compared with the eribulin and cisplatin combination group, the
apoptosis rate decreased from 92.72 to 18.01% when PD98059 was used
(Fig. 5C).
Using the control value as the reference (100%),
LC3-I/II expression was markedly increased in the eribulin plus
cisplatin group (824.99%) but was reduced to 119.30% when PD98059
was additionally administered, whereas p62 expression increased
from 38.70 to 72.59% with PD98059 treatment (Fig. 5D-F). Thus, ERK inhibition affected
the expression of autophagy-related proteins.
An autophagy assay (ab139484, Abcam) confirmed that
the levels of autophagosomes decreased when PD98059 was used in
combination with eribulin and cisplatin (Fig. 5G). Quantitative analysis of
fluorescence intensity showed consistent results, decreasing from
15.59 to 2.92% (P<0.05; Fig.
5H).
Discussion
In this study, MDA-MB-231 cells were used to
investigate the cytotoxic effect of a combination of eribulin and
cisplatin, both widely utilized in TNBC treatment. After 72 h of
treatment, significant cancer cell death was observed, mediated by
autophagy-dependent mechanisms involving ERK pathway activation.
These findings reveal a novel therapeutic vulnerability in
TNBC.
Eribulin, a microtubule-targeting agent derived from
the marine sponge Halichondria okadai, has demonstrated
clinical efficacy in breast cancer, particularly in taxane- and
anthracycline-resistant cases (21,22).
The EMBRACE trial notably supported its use in metastatic breast
cancer, with further analyses confirming its benefit in TNBC
(11). Previous studies have shown
that eribulin can synergize with various agents, including HDAC
inhibitors and RAF/MEK inhibitors, primarily through ERK pathway
inactivation (23,24). However, our study uniquely reveals
that eribulin induces cell death via ERK pathway activation,
representing, to our knowledge, the first report of this mechanism
in TNBC.
Cisplatin, a platinum-based chemotherapeutic, also
activates ERK and induces apoptosis across multiple carcinoma types
including cervical cancer (25),
hepatocellular carcinoma (26),
human glioma (27), and mouse
proximal tubule cancer (28).
Consistent with prior reports, our study confirms that ERK
activation persists in MDA-MB-231 cells following cisplatin
treatment, contributing to cell death. Remarkably, the combination
of eribulin and cisplatin further amplified this ERK-driven
autophagic response, leading to significantly enhanced cytotoxic
effects against TNBC cells.
TNBC is molecularly defined by the absence of
estrogen receptor (ER), progesterone receptor (PR), and HER2
overexpression, rendering it unresponsive to endocrine or
HER2-targeted therapies. This receptor-negative profile contributes
to poor prognosis and compels TNBC cells to rely on alternative
survival pathways such as the MAPK/ERK axis (29,30).
Our finding that ERK activation mediates autophagy-dependent cell
death in response to the combination therapy suggests that this
pathway-typically associated with cellular adaptation and drug
resistance-may instead represent a vulnerability in TNBC (31). Furthermore, high basal autophagic
activity, often driven by MAPK/ERK signaling, has been implicated
in TNBC progression and response to therapy, indicating that
modulation of this pathway could sensitize cells to cytotoxic
agents (32,33).
Autophagy, which often intersects with apoptotic
mechanisms, is increasingly recognized as a modulator of cancer
therapy response (34,35). Our data indicate that ERK activation
is not merely associated with autophagic flux but is a functional
mediator of combination-induced cell death. This positions
ERK-mediated autophagy as a viable target for combination therapy
in TNBC, particularly in the context of eribulin and cisplatin
co-treatment (36,37).
Importantly, the clinical relevance of this
dual-agent strategy extends beyond mechanistic insights. Although
targeted therapies have revolutionized TNBC management in
high-income countries, their high cost continues to restrict access
in low- and middle-income countries (LMICs) (38). Given that both eribulin and
cisplatin are already approved and relatively affordable, their
combination may offer a scalable and cost-effective solution to
mitigate global disparities in breast cancer treatment (11,17,18).
This approach is consistent with current global oncology efforts
aimed at expanding access to effective therapies in
resource-limited settings.
In LMICs, financial barriers significantly limit
access to novel targeted therapies (39). The monthly cost of immune checkpoint
inhibitors or PARP inhibitors can exceed $5,000 USD, making them
unattainable for most patients (40). In contrast, cisplatin and
eribulin-being off-patent or comparatively affordable-are
frequently listed in public health formularies. For instance, in
South Korea, the cost of eribulin treatment under the national
insurance system is approximately $1,500-$2,000 USD per cycle,
substantially lower than newer biologics [HIRA (Health Insurance
Review and Assessment Service, Korea's national health technology
assessment body)]. These economic factors reinforce the feasibility
of the eribulin-cisplatin combination, especially in regions
striving to deliver cost-effective cancer care (17,18).
In summary, this is the first report demonstrating
that eribulin alone can activate the ERK pathway to induce
autophagy-mediated cytotoxicity in TNBC. These findings highlight
ERK-mediated autophagy as a promising therapeutic target and
underscore the clinical feasibility of the eribulin-cisplatin
combination in both advanced and resource-limited settings.
Nevertheless, this study has limitations. As an
in vitro investigation, it cannot fully recapitulate the
complex tumor microenvironment. In vivo validation using
xenograft models will be necessary to confirm the translational
potential of the eribulin-cisplatin combination, particularly its
impact on ERK-mediated autophagic cell death. However, the recent
NCCN guidelines supporting platinum-based neoadjuvant regimens in
TNBC provide a clinical rationale for future studies (18).
In conclusion, our findings demonstrate that
ERK-driven autophagy mediates the synergistic cytotoxicity of
eribulin and cisplatin in TNBC. This mechanism highlights a
clinically feasible and cost-effective therapeutic strategy,
warranting further in vivo validation and clinical
development.
Acknowledgements
Not applicable.
Funding
This work was supported by Chungnam National University.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HK and JL conceived and designed the study. HK, ML,
EC and JL contributed to the design of the methodology and
performed experiments. Data analysis and validation were conducted
by HK and JL. Software tool use and visualization were performed by
HK. The experiments were performed by ML, EC and JL, with resources
provided by ML and EC. Data curation was performed by HK, ML and
EC. HK drafted the original manuscript, and JL performed critical
review and editing. Supervision and project administration were led
by JL. Funding for the project was acquired by JL. HK and JL
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID IDs: HK, 0000-0002-6621-8761; JL,
0000-0002-9345-724X; ML, 0000-0003-1732-3001; EC,
0000-0002-7281-3698.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morris GJ, Naidu S, Topham AK, Guiles F,
Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K and
Mitchell EP: Differences in breast carcinoma characteristics in
newly diagnosed African-American and Caucasian patients: A
single-institution compilation compared with the national cancer
institute's surveillance, epidemiology, and end results database.
Cancer. 110:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sedighi Pashaki A, Sheida F, Moaddab Shoar
L, Hashem T, Fazilat-Panah D, Nemati Motehaver A, Ghanbari Motlagh
A, Nikzad S, Bakhtiari M, Tapak L, et al: A randomized, controlled,
parallel-group, trial on the long-term effects of melatonin on
fatigue associated with breast cancer and its adjuvant treatments.
Integr Cancer Ther. 22:153473542311686242023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moezian GSA, Javadinia SA, Sales SS,
Fanipakdel A, Elyasi S and Karimi G: Oral silymarin formulation
efficacy in management of AC-T protocol induced hepatotoxicity in
breast cancer patients: A randomized, triple blind,
placebo-controlled clinical trial. J Oncol Pharm Pract. 28:827–835.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salek R, Dehghani M, Mohajeri SA, Talaei
A, Fanipakdel A and Javadinia SA: Amelioration of anxiety,
depression, and chemotherapy related toxicity after crocin
administration during chemotherapy of breast cancer: A double
blind, randomized clinical trial. Phytother Res. 35:5143–5153.
2021. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fazilat-Panah D, Vakili Ahrari Roudi S,
Keramati A, Fanipakdel A, Sadeghian MH, Homaei Shandiz F,
Shahidsales S and Javadinia SA: Changes in cytokeratin 18 during
neoadjuvant chemotherapy of breast cancer: A prospective study.
Iran J Pathol. 15:117–126. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swami U, Chaudhary I, Ghalib MH and Goel
S: Eribulin-a review of preclinical and clinical studies. Crit Rev
Oncol Hematol. 81:163–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mani S and Swami U: Eribulin mesilate, a
halichondrin B analogue, in the treatment of breast cancer. Drugs
Today (Barc). 46:641–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortes J, O'Shaughnessy J, Loesch D, Blum
JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V,
Delozier T, et al: Eribulin monotherapy versus treatment of
physician's choice in patients with metastatic breast cancer
(EMBRACE): A phase 3 open-label randomised study. Lancet.
377:914–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaufman PA, Awada A, Twelves C, Yelle L,
Perez EA, Velikova G, Olivo MS, He Y, Dutcus CE and Cortes J: Phase
III open-label randomized study of eribulin mesylate versus
capecitabine in patients with locally advanced or metastatic breast
cancer previously treated with an anthracycline and a taxane. J
Clin Oncol. 33:594–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Xu C, Gao X and Yao Q:
Platinum-based drugs for cancer therapy and anti-tumor strategies.
Theranostics. 12:2115–2132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin C, Cui J, Peng Z, Qian K, Wu R, Cheng
Y and Yin W: Efficacy of platinum-based and non-platinum-based
drugs on triple-negative breast cancer: meta-analysis. Eur J Med
Res. 27:2012022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ko H, Lee M, Cha E, Sul J, Park J and Lee
J: Eribulin mesylate improves cisplatin-induced cytotoxicity of
triple-negative breast cancer by extracellular signal-regulated
kinase 1/2 activation. Medicina (Kaunas). 58:4572022.
|
|
18
|
Obidiro O, Battogtokh G and Akala EO:
Triple Negative breast cancer treatment options and limitations:
Future outlook. Pharmaceutics. 15:17962023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swami U, Shah U and Goel S: Eribulin in
cancer treatment. Mar Drugs. 13:5016–5058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terashima M, Sakai K, Togashi Y, Hayashi
H, De Velasco MA, Tsurutani J and Nishio K: Synergistic antitumor
effects of S-1 with eribulin in vitro and in vivo for
triple-negative breast cancer cell lines. Springerplus. 3:4172014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuznetsov G, Towle MJ, Cheng H, Kawamura
T, TenDyke K, Liu D, Kishi Y, Yu MJ and Littlefield BA: Induction
of morphological and biochemical apoptosis following prolonged
mitotic blockage by halichondrin B macrocyclic ketone analog E7389.
Cancer Res. 64:5760–5766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okouneva T, Azarenko O, Wilson L,
Littlefield BA and Jordan MA: Inhibition of centromere dynamics by
eribulin (E7389) during mitotic metaphase. Mol Cancer Ther.
7:2003–2011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ono H, Sowa Y, Horinaka M, Iizumi Y,
Watanabe M, Morita M, Nishimoto E, Taguchi T and Sakai T: The
histone deacetylase inhibitor OBP-801 and eribulin synergistically
inhibit the growth of triple-negative breast cancer cells with the
suppression of survivin, Bcl-xL, and the MAPK pathway. Breast
Cancer Res Treat. 171:43–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ono H, Horinaka M, Sukeno M, Morita M,
Yasuda S, Nishimoto E, Konishi E and Sakai T: Novel RAF/MEK
inhibitor CH5126766/VS-6766 has efficacy in combination with
eribulin for the treatment of triple-negative breast cancer. Cancer
Sci. 112:4166–4175. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guégan JP, Ezan F, Théret N, Langouët S
and Baffet G: MAPK signaling in cisplatin-induced death:
Predominant role of ERK1 over ERK2 in human hepatocellular
carcinoma cells. Carcinogenesis. 34:38–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi BK, Choi CH, Oh HL and Kim YK: Role
of ERK activation in cisplatin-induced apoptosis in A172 human
glioma cells. Neurotoxicology. 25:915–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arany I, Megyesi JK, Kaneto H, Price PM
and Safirstein RL: Cisplatin-induced cell death is EGFR/src/ERK
signaling dependent in mouse proximal tubule cells. Am J Physiol
Renal Physiol. 287:F543–F549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giltnane JM and Balko JM: Rationale for
targeting the Ras/MAPK pathway in triple-negative breast cancer.
Discov Med. 17:275–283. 2014.PubMed/NCBI
|
|
31
|
Sivaprasad U and Basu A: Inhibition of ERK
attenuates autophagy and potentiates tumour necrosis
factor-alpha-induced cell death in MCF-7 cells. J Cell Mol Med.
12:1265–1271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu S, Cao Z, Cai F, Yao Y, Chang X, Wang
X, Zhuang H and Hua ZC: ADT-OH exhibits anti-metastatic activity on
triple-negative breast cancer by combinatorial targeting of
autophagy and mitochondrial fission. Cell Death Dis. 15:4632024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han Y, Fan S, Qin T, Yang J, Sun Y, Lu Y,
Mao J and Li L: Role of autophagy in breast cancer and breast
cancer stem cells (review). Int J Oncol. 52:1057–1070.
2018.PubMed/NCBI
|
|
34
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosenfeldt MT and Ryan KM: The multiple
roles of autophagy in cancer. Carcinogenesis. 32:955–963. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gewirtz DA: The four faces of autophagy:
Implications for cancer therapy. Cancer Res. 74:647–651. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
World Health Organization, . Global breast
cancer initiative implementation framework: Assessing,
strengthening and scaling up of services for the early detection
and management of breast cancer: Executive summary. Geneva: World
Health Organization; 2023
|
|
39
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goldstein DA, Chen Q, Ayer T, Howard DH,
Lipscomb J, El-Rayes BF and Flowers CR: First- and second-line
bevacizumab in addition to chemotherapy for metastatic colorectal
cancer: A United States-based cost-effectiveness analysis. J Clin
Oncol. 33:1112–1118. 2015. View Article : Google Scholar : PubMed/NCBI
|